Summary
Background
Non-alcoholic fatty liver disease (NAFLD) is a common chronic liver disease, and among the non-invasive tests, controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) have shown better diagnostic performance in NAFLD. This meta-analysis aimed to evaluate the performance of CAP and LSM for assessing steatosis and fibrosis in NAFLD.
Methods
We searched the PubMed, Web of Science, Cochrane Library, and Embase databases for relevant articles published up to February 13th, 2022, and selected studies that met the inclusion and exclusion criteria, and evaluated the quality of evidence. Then we pooled sensitivity (SE), specificity (SP), and area under receiver operating characteristic (AUROC) curves. A random effect model was applied regardless of heterogeneity. Meta-regression analysis and subgroup analysis were performed to explore heterogeneity, and Fagan plot analysis was used to evaluate clinical utility. This meta-analysis was completed in Nanjing, Jiangsu and registered on PROSPERO (CRD42022309965).
Findings
A total of 10537 patients from 61 studies were included in our meta-analysis. The AUROC of CAP were 0·924, 0·794 and 0·778 for steatosis grades ≥ S1, ≥ S2 and = S3, respectively, and the AUROC of LSM for detecting fibrosis stages ≥ F1, ≥ F2, ≥ F3, and = F4 were 0·851, 0·830, 0·897 and 0·925, respectively. Subgroup analysis revealed that BMI ≥ 30 kg/m² had lower accuracy for diagnosing S ≥ S1, ≥ S2 than BMI<30 kg/m². For the mean cut-off values, significant differences were found in CAP values among different body mass index (BMI) populations and LSM values among different regions. For diagnosing S ≥ S1, ≥ S2 and = S3, the mean CAP cut-off values for BMI ≥ 30 kg/m² were 30·7, 28·2, and 27·9 dB/m higher than for BMI < 30 kg/m² (P = 0·001, 0·001 and 0·018, respectively). For diagnosing F ≥ F2 and = F4, the mean cut-off values of Europe and America were 0·96 and 2·03 kPa higher than Asia (P = 0·027, P = 0·034), respectively. In addition, the results did not change significantly after sensitivity analysis and the trim and fill method to correct for publication bias, proving that the conclusions are robust.
Interpretation
The good performance of CAP and LSM for the diagnosis of mild steatosis (S ≥ S1), advanced liver fibrosis (F ≥ F3), and cirrhosis (F = F4) can be used to screen for NAFLD in high-risk populations. Of note, the accuracy of CAP for the detection of steatosis in patients with obesity is reduced and requires specific diagnostic values. For LSM, the same diagnostic values can be used when the appropriate probes are selected based on BMI and the automated probe selection tool. The performance of CAP and LSM in assessing steatosis in patients with obesity, moderate to severe steatosis, and low-grade fibrosis should be further validated and improved in the future.
Funding
The study was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Keywords: Controlled attenuation parameter (CAP), Liver stiffness measurement (LSM), Diagnostic accuracy, Non-alcoholic fatty liver disease (NAFLD), Non-alcoholic steatohepatitis (NASH), Meta-analysis
Research in context.
Evidence before this study
In recent years, research has found that among the non-invasive tests, controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) using Fibroscan® equipment exhibited high accuracy in quantifying steatosis and fibrosis in patients with NAFLD. However, although CAP and LSM have been used to evaluate steatosis and fibrosis in many studies, the results were inconsistent, especially for the population with obesity and different probes. Detailed pooled estimates of the accuracy of CAP and LSM for assessing steatosis and fibrosis in NAFLD are needed. We searched the PubMed, Web of Science, Cochrane Library, and Embase databases for relevant articles published up to February 13th 2022. Then we pooled diagnostic indexes to analyze the diagnostic accuracy of CAP and LSM and calculated mean cut-offs. Meta-regression analysis and subgroup analysis were performed to explore heterogeneity according to the cut-off value, BMI, probe type, and region, and Fagan plot analysis was used to evaluate clinical utility.
Added value of this study
According to our meta-analysis results, overall, the diagnostic performance of CAP decreased with the severity of steatosis while the diagnostic performance of LSM increased with the aggravation of fibrosis. Subgroup analysis showed that CAP was less accurate in patients with obesity, the mean CAP cut-off values for BMI ≥ 30 kg/m² were significantly higher than for BMI < 30 kg/m². However, for LSM, the accuracy was similar between different subgroups. The lower LSM values obtained with the XL probe in patients with obesity were partially offset by the higher LSM values generated in patients with obesity, resulting in an insignificant difference in LSM values.
Implications of all the available evidence
Considering the good performance of CAP and LSM for the diagnosis of mild steatosis (S ≥ S1), advanced liver fibrosis (F ≥ F3), and cirrhosis (F = F4), they can be used to screen for NAFLD in high-risk populations. For the population with obesity, higher CAP diagnostic values and more accurate tools for non-invasive detection of steatosis are needed, while the same LSM diagnostic values can be used for patients with obesity when selecting the appropriate probe based on BMI and the automated probe selection tool. The performance of Fibroscan in detecting steatosis in patients with obesity, moderate to severe steatosis, and low-grade fibrosis needs to be further explored and improved.
Alt-text: Unlabelled box
Introduction
With the prevalence of obesity and type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD) has become the largest chronic liver disease in developed countries,1 and in the general population, the estimated prevalence of NAFLD is 25·24%.2 Nonalcoholic steatohepatitis (NASH) is the inflammatory subtype of NAFLD. With time, NASH can progress to cirrhosis, hepatocellular carcinoma (HCC), and end-stage liver disease.1,3 Besides, growing evidence shows that NAFLD is a multisystem disease, affecting extra-hepatic organs and regulatory pathways, with an increasing risk of T2DM, cardiovascular (CVD), and chronic kidney disease (CKD).4 Therefore, it is necessary for the early diagnosis of NAFLD and to take steps to prevent its progression.
So far, liver biopsy is still regarded as the gold standard for NAFLD diagnosis.1 However, it is limited by invasiveness, cost, sampling error,5 and procedure-related complications.6 Thus, there is an urgent need to develop reliable tools for noninvasive diagnosis of NAFLD/NASH.
In recent years, the use of transient elastography (TE) with Fibroscan® equipment (Echosens, Paris, France) to obtain controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) has been seen as a promising tool for noninvasive quantifying hepatic steatosis and fibrosis, respectively,7,8 and showed low failure (3·2%), high reliability (> 95%) rates, and high reproducibility.9 Besides, the development of the XL probe for patients with obesity has solved the limitations of the clinical application of CAP and LSM.10, 11, 12 Studies found unreliable measurements were independently associated with body mass index (BMI) ≥ 30 kg/m², while 83% of patients (BMI ≥ 30 kg/m²) who could not be measured with the M probe could be measured with the XL probe.10 In patients with obesity, due to thicker subcutaneous fat, the skin-liver capsule distance (SCD) is more than 25 mm, and the XL probe can increase the detection depth (35-75 mm vs M: 25-65 mm) to improve the measurement success rate. In addition, the new version of the FibroScan equipment includes an automatic probe selection tool that measures SCD and suggests which probe to use (M: SCD < 25 mm, XL: SCD ≥ 25 mm). In clinical practice, physicians can select probes based on BMI and the automated probe selection tool.13 However, as the use of CAP and LSM became more popular, results began to diverge, particularly regarding differences in diagnostic accuracy and cut-off values between different BMI populations and between different probes. Earlier, several meta-analyses had discussed the accuracy of CAP or LSM alone in NAFLD patients,14,15 but few studies were included, with only nine studies, which might lead to relatively limited conclusions.
Hence, we did this meta-analysis with the aim of comprehensively evaluating the performance of CAP and LSM for assessing steatosis and fibrosis in NAFLD/NASH.
Methods
Search strategy and selection criteria
This meta-analysis was conducted following the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) guidelines.16 The protocol for this meta-analysis was registered with PROSPERO (CRD42022309965) https://www.crd.york.ac.uk/PROSPERO/#recordDetails.
We searched the PubMed, Web of Science, Cochrane Library, and Embase databases for relevant articles published up to February 13th, 2022. Our search strategy consisted of MeSH terms and entry terms with no restrictions on the language of the articles. We also scanned the reference lists of eligible articles for additional eligible articles that were not retrieved during the literature search.
For example, in PubMed, we searched (“Non-alcoholic Fatty Liver Disease” OR “nonalcoholic fatty liver disease” OR “fatty liver” OR “nonalcoholic fatty liver disease*” OR “fatty liver*” OR “NAFLD” OR “nonalcoholic steatohepatiti*” OR “NASH” OR “steatohepatiti*” OR “liver steatos*” AND “transient elastography” OR “TE” OR “controlled attenuation parameter” OR “CAP” OR “liver stiffness” OR “LSM” OR “FibroScan” AND “diagnos*” OR “assess*” OR “detect*” OR “qualif*” OR “discriminat*” OR “distin*” OR “different*” OR “predict*”). The details of the search strategy are presented in supplementary materials (Table S1).
The inclusion criteria were as follows: (1) Patients: adult NAFLD/NASH patients; (2) Reference standards: liver biopsy was used as the gold standard for the diagnosis of NAFLD/NASH; (3) Index test: steatosis and fibrosis were assessed by using CAP and LSM of Fibroscan® equipment (Echosens, Paris, France); (4) Steatosis and fibrosis staging: steatosis was staged according to the NASH Clinical Research Network scoring system.17 Fibrosis was staged from F0 to F4 according to the Kleiner score.17 If fibrosis was assessed according to other scoring systems, data were transformed according to Goodman's method18 to unify results on the liver fibrosis staging; (5) Studies that provided sensitivity (SE), specificity (SP), sample size or enough information to get true positive (TP), false positive (FP), true negative (TN) and false negative (FN); (6) Studies included at least 20 patients to obtain good reliability.
The exclusion criteria were as follows: (1) Animal experiments, review, conference abstracts, case reports and meta-analyses; (2) Patients with morbid obesity: BMI>40 kg/m²; (3) Full-text or sufficient data could not be extracted.
Studies from the database were managed using EndNote X9 software to remove duplicate articles. The included articles based on inclusion and exclusion criteria were screened by FQ and Y-JZ, who worked independently. Disagreement was discussed with another author (L-LX) and subsequently resolved via consensus.
The quality of eligible articles was independently assessed by two investigators (FQ, Y-JZ) with the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) checklist19 by Review Manager Version 5.4.1, and any disagreement was resolved by the third investigator (L-LX). The QUADAS-2 checklist included four parts: patient selection, index test, reference standard, and flow and timing. Each signaling question was judged as “yes” or “no” or “unclear”. The risk of bias and concern about applicability in each study were judged as “high” or “low”, or “unclear”. Concern about applicability assessment does not apply in the flow and timing domain.
Extracted research information includes: (1) Background information: first author, publication year, country, study design, sample size, age, sex, BMI, and probe type; (2) Diagnostic parameters: cut-off values, area under the receiver operating characteristic (AUROC) curves, SE, SP, TP, FP, TN, FN. Then we constructed a diagnostic 2∗2 contingency table. If the same original article contains multiple groups of available data, it would be divided into multiple independent studies for data extraction. The process was performed independently by FQ and Y-JZ. Any disagreements were resolved by discussion and consensus.
Data analysis
To analyze the diagnostic accuracy of CAP and LSM in patients with NAFLD/NASH, the summary SE, SP, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with their 95% confidence interval (95% CI) and AUROC with standard errors (SE) were calculated based on TP, FP, FN, and TN. Further, we drew forest plots and constructed summary receiver operating characteristic (SROC) curves.
The threshold effect was analyzed by Spearman correlation coefficient. Cochran's Q statistic and I2 statistic were used to quantitatively evaluate the heterogeneity of studies. When Cochran's Q Statistic showed P ≤ 0·10, it was considered to show significant heterogeneity. Studies with I² of 0-25%, 25-75%, and > 75% were considered to have low, moderate, and high heterogeneity, respectively. A random effect model was applied regardless of heterogeneity. Meta-regression analyses were performed to determine the source of heterogeneity. According to the cut-off value, BMI, probe type, and region, we conducted subgroup analyses. The groups were as follows: cut-off value ≥ median value and < median value; BMI < 30 kg/m² and ≥ 30 kg/m²; M and XL probe, Europe and America (EUR-USA) and Asia.
Moreover, according to an estimated prevalence of 25% of NAFLD in the general population, we used Fagan plot analysis to evaluate the pre-test probabilities of 25% and corresponding post-test probabilities in clinical utility. Besides, sensitivity analysis was performed to evaluate the robustness of the results, and Deeks’ funnel plot asymmetry test was used to investigate publication bias, and a P value < 0·05 indicated a significant publication bias. The trim and fill method was conducted to rectify the funnel plot asymmetry caused by publication bias.20 All statistical analyses were done with Meta-Disc Version 1.4 and Stata Version 16.0. The results were considered significant when P < 0·05.
Role of the funding source
The funding source was used for article processing charges. Beyond that, the funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Results
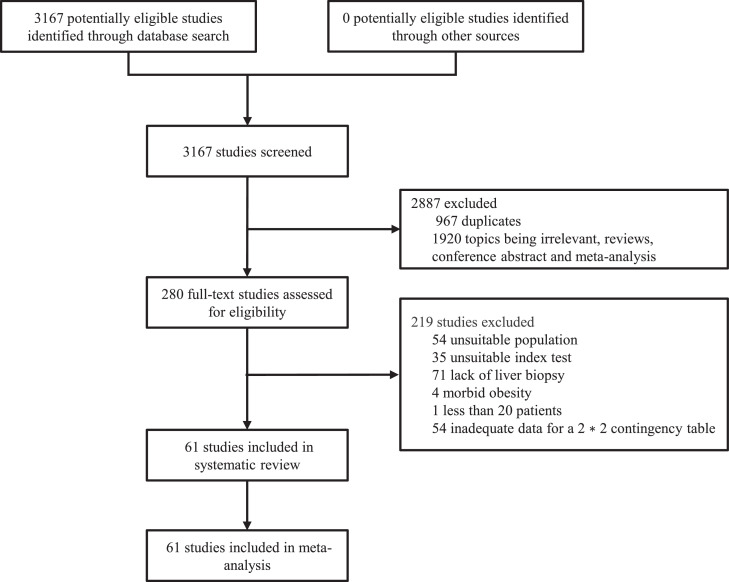
Through database searches, 3167 records (PubMed: 1068; EMBASE: 715; Cochrane Library: 501; Web of Science: 1384) were found. After removing duplicates, 2200 records remained, and 1920 records were excluded after browsing titles and abstracts. The remaining 280 records were evaluated in full text, and 219 articles were excluded. Finally, 61 articles were selected for the meta-analysis. The specific screening process is shown in Figure 1.
Figure 1.
Study selection.
A total of 10537 patients from 61 articles were included. The characteristics of the 61 included articles are shown in supplementary materials (Table S2). In these articles, 10 articles21, 22, 23, 24, 25, 26, 27, 28, 29, 30 assessed the accuracy of CAP for diagnosing and staging steatosis in NAFLD/NASH, 39 articles31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 evaluated the accuracy of LSM for diagnosing and staging fibrosis in NAFLD/NASH, and 12 articles70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 evaluated both. Most of the included studies were of good quality. The details are shown in the supplementary materials (Figure S1a and b).
14 studies reported the diagnostic accuracy of CAP for detecting S ≥ S1. The mean cut-off value and range was 268.5 (233·5-304) dB/m. The summary SE and SP of 14 studies were 0·84 (95% CI: 0·83-0·86, Figure S2a) and 0·86 (95%: 0·81-0·90, Figure S2b), respectively. The pooled PLR, NLR and DOR were 4·72 (95%: 3·10-7·17, Figure S2c), 0·16 (95% CI: 0·11-0·22, Figure S2d) and 32·97 (95% CI: 17·40-62·48, Figure S2e), respectively. Finally, the summary AUROC was 0·924 (SE = 0·019, Figure S2f). Similarly, 26 and 25 studies reported the diagnostic accuracy of CAP for detecting S ≥ S2 and = S3 (Figure S3a-f, S4a-f), respectively. For the accuracy of LSM in detecting F ≥ F1, ≥ F2, ≥ F3, and = F4, we pooled 16, 44, 59, and 32 studies, respectively (Figure S5a-f, S6a-f, S7a-f, S8a-f). The detailed summarized diagnostic index data are shown in Table 1, corresponding heterogeneity is shown in Table S4, and the mean cut-off values are shown in Tables 2 and 3.
Table 1.
Diagnostic accuracy of CAP and LSM in NAFLD/NASH.
| Category | Subgroup | Case (n) | Sample (n) | SE (95% CI) | SP (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUROC (SE*) |
|---|---|---|---|---|---|---|---|---|---|
| S≥S1 | 14 | 2138 | 0·84 (0·83-0·86) | 0·86 (0·81-0·90) | 4·72 (3·10-7·17) | 0·16 (0·11-0·22) | 32·97 (17·40-62·48) | 0·924 (0·019) | |
| Probe type | M | 6 | 621 | 0·92 (0·90-0·94) | 0·91 (0·84-0·96) | 6·58 (3·08-14·08) | 0·09 (0·07-0·13) | 84·87 (37·71-191·01) | 0·969 (0·011) |
| XL | 2 | 176 | 0·93 (0·89-0·97) | 0·89 (0·52-1·00) | 5·77 (1·34-24·81) | 0·08 (0·04-0·15) | 73·55 (11·54-468·99) | / | |
| BMI | < 30 | 5 | 564 | 0·92 (0·90-0·95) | 0·91 (0·83-0·96) | 6·98 (2·97-16·43) | 0·09 (0·06-0·13) | 88·20 (35·61-218·50) | 0·970 (0·011) |
| ≥30 | 9 | 1574 | 0·82 (0·80-0·84) | 0·82 (0·75-0·88) | 3·72 (2·57-5·38) | 0·21 (0·15-0·28) | 18·64 (11·25-30·89) | 0·883 (0·018) | |
| Cut-off value | ≥ median | 8 | 1539 | 0·82 (0·80-0·84) | 0·83 (0·75-0·88) | 3·91 (2·43-6·31) | 0·18 (0·13-0·26) | 21·17 (10·86-41·26) | 0·887 (0·018) |
| <median | 6 | 599 | 0·90 (0·87-0·92) | 0·90 (0·82-0·95) | 6·09 (3·00-12·35) | 0·12 (0·06-0·25) | 59·42 (22·71-155·45) | 0·950 (0·022) | |
| Region | Europe and America | 7 | 1242 | 0·79 (0·76-0·81) | 0·80 (0·72-0·86) | 3·43 (2·32-5·08) | 0·26 (0·21-0·33) | 16·63 (10·44-26·28) | 0·876 (0·019) |
| Asia | 7 | 896 | 0·92 (0·90-0·94) | 0·93 (0·87-0·97) | 10·24 (5·42-19·38) | 0·10 (0·08-0·12) | 119·49 (53·97-264·53) | 0·969 (0·010) | |
| S≥S2 | 26 | 3414 | 0·79 (0·78-0·81) | 0·64 (0·62-0·67) | 2·28 (1·96-2·65) | 0·30 (0·26-0·36) | 8·61 (6·39-11·59) | 0·794 (0·023) | |
| Probe type | M | 12 | 1449 | 0·82 (0·79-0·85) | 0·69 (0·65-0·73) | 2·80 (2·09-3·75) | 0·24 (0·18-0·32) | 13·74 (8·29-22·76) | 0·850 (0·031) |
| XL | 4 | 357 | 0·85 (0·80-0·90) | 0·52 (0·44-0·59) | 1·68 (1·31-2·17) | 0·24 (0·11-0·55) | 7·22 (3·19-16·31) | 0·707 (0·054) | |
| BMI | < 30 | 12 | 1260 | 0·85 (0·82-0·88) | 0·65 (0·61-0·69) | 2·60 (1·91-3·55) | 0·20 (0·13-0·29) | 15·29 (8·83-26·49) | 0·848 (0·037) |
| ≥30 | 14 | 1978 | 0·76 (0·74-0·79) | 0·64 (0·61-0·67) | 2·06 (1·79-2·36) | 0·37 (0·32-0·43) | 5·83 (4·52-7·52) | 0·771 (0·017) | |
| Cut-off value | ≥ median | 13 | 2079 | 0·76 (0·73-0·78) | 0·63 (0·60-0·67) | 2·03 (1·79-2·29) | 0·38 (0·34-0·43) | 5·66 (4·64-6·90) | 0·770 (0·016) |
| <median | 13 | 1135 | 0·86 (0·84-0·89) | 0·66 (0·62-0·70) | 2·74 (1·97-3·81) | 0·19 (0·13-0·29) | 16·75 (9·32-30·11) | 0·861 (0·034) | |
| Region | Europe and America | 9 | 1560 | 0·77 (0·75-0·80) | 0·64 (0·60-0·68) | 2·21 (1·79-2·73) | 0·35 (0·28-0·45) | 7·03 (4·47-11·08) | 0·743 (0·048) |
| Asia | 14 | 1456 | 0·81 (0·78-0·84) | 0·65 (0·61-0·68) | 2·37 (1·85-3·04) | 0·27 (0·20-0·36) | 10·20 (6·35-16·37) | 0·822 (0·032) | |
| S=S3 | 25 | 3549 | 0·75 (0·72-0·78) | 0·62 (0·60-0·64) | 2·18 (1·91-2·49) | 0·41 (0·36-0·48) | 6·00 (4·50-8·01) | 0·778 (0·020) | |
| Probe type | M | 13 | 1773 | 0·75 (0·71-0·79) | 0·63 (0·60-0·65) | 2·17 (1·82-2·58) | 0·37 (0·28-0·50) | 6·89 (4·25-11·16) | 0·792 (0·035) |
| XL | 4 | 357 | 0·79 (0·67-0·88) | 0·63 (0·57-0·68) | 2·10 (1·53-2·90) | 0·34 (0·21-0·56) | 6·72 (3·41-13·23) | 0·817 (0·049) | |
| BMI | < 30 | 12 | 1260 | 0·76 (0·70-0·81) | 0·63 (0·60-0·66) | 2·13(1·82-2·50) | 0·42 (0·32-0·54) | 5·71 (3·80-8·58) | 0·755 (0·033) |
| ≥30 | 12 | 1965 | 0·78 (0·74-0·81) | 0·62 (0·59-0·64) | 2·31 (1·83-2·91) | 0·39 (0·33-0·46) | 6·87 (4·44-10·64) | 0·810 (0·023) | |
| Cut-off value | ≥ median | 11 | 1782 | 0·71 (0·66-0·75) | 0·69 (0·67-0·72) | 2·62 (2·06-3·33) | 0·42 (0·33-0·52) | 7·17 (4·31-11·93) | 0·797 (0·029) |
| <median | 14 | 1767 | 0·81 (0·77-0·85) | 0·55 (0·53-0·58) | 1·91 (1·66-2·20) | 0·39 (0·32-0·48) | 4·84 (3·64-6·44) | 0·754 (0·032) | |
| Region | Europe and America | 8 | 1531 | 0·72 (0·68-0·76) | 0·61 (0·58-0·64) | 2·24 (1·69-2·97) | 0·46 (0·38-0·55) | 5·01 (3·20-7·84) | 0·767 (0·029) |
| Asia | 13 | 1535 | 0·76 (0·70-0·81) | 0·63 (0·60-0·66) | 2·02 (1·77-2·30) | 0·44 (0·36-0·55) | 4·99 (3·62-6·88) | 0·744 (0·030) | |
| F≥F1 | 16 | 2264 | 0·73 (0·71-0·75) | 0·80 (0·76-0·83) | 3·40 (2·74-4·20) | 0·29 (0·23-0·36) | 12·82 (9·20-17·86) | 0·851 (0·016) | |
| Probe type | M | 10 | 1369 | 0·77 (0·75-0·80) | 0·80 (0·76-0·84) | 3·67 (3·00-4·48) | 0·27 (0·21-0·35) | 14·81 (10·84-20·24) | 0·863 (0·013) |
| XL | 1 | 57 | / | / | / | / | / | / | |
| BMI | < 30 | 10 | 1384 | 0·78 (0·75-0·80) | 0·79 (0·74-0·83) | 3·37 (2·63-4·31) | 0·27 (0·21-0·35) | 14·09 (9·90-20·04) | 0·859 (0·014) |
| ≥30 | 6 | 880 | 0·66 (0·62-0·70) | 0·81 (0·76-0·86) | 3·56 (2·29-5·53) | 0·32 (0·21-0·49) | 11·31 (5·90-21·68) | 0·842 (0·039) | |
| Cut-off value | ≥ median | 9 | 1661 | 0·71 (0·68-0·73) | 0·83 (0·79-0·86) | 4·00 (3·26-4·92) | 0·30 (0·23-0·40) | 13·67 (10·17-18·38) | 0·864 (0·014) |
| <median | 7 | 603 | 0·80 (0·76-0·84) | 0·71 (0·64-0·78) | 2·66 (1·93-3·66) | 0·27 (0·18-0·40) | 11·54 (5·51-24·19) | 0·831 (0·054) | |
| Region | Europe and America | 5 | 810 | 0·65 (0·61-0·69) | 0·80 (0·74-0·85) | 3·28 (1·89-5·70) | 0·33 (0·20-0·54) | 11·24 (4·73-26·70) | 0·844 (0·053) |
| Asia | 11 | 1454 | 0·77 (0·75-0·80) | 0·80 (0·75-0·84) | 3·60 (2·96-4·38) | 0·27 (0·22-0·34) | 14·37 (10·62-19·44) | 0·860 (0·013) | |
| F≥F2 | 44 | 6186 | 0·76 (0·75-0·78) | 0·73 (0·72-0·75) | 3·18 (2·69-3·75) | 0·33 (0·29-0·37) | 11·06 (8·90-13·74) | 0·830 (0·011) | |
| Probe type | M | 22 | 3308 | 0·77 (0·74-0·79) | 0·72 (0·70-0·74) | 3·07 (2·47-3·81) | 0·32 (0·26-0·38) | 11·04 (8·24-14·80) | 0·838 (0·015) |
| XL | 6 | 647 | 0·82 (0·77-0·86) | 0·66 (0·60-0·71) | 2·55 (1·54-4·24) | 0·38 (0·30-0·49) | 6·48 (4·35-9·66) | 0·798 (0·026) | |
| BMI | < 30 | 21 | 2787 | 0·78 (0·76-0·80) | 0·73 (0·71-0·75) | 3·08 (2·46-3·85) | 0·31 (0·26-0·37) | 10·96 (8·16-14·71) | 0·836 (0·015) |
| ≥30 | 20 | 2894 | 0·76 (0·74-0·78) | 0·74 (0·72-0·77) | 3·60 (2·67-4·86) | 0·32 (0·27-0·39) | 12·51 (8·75-17·89) | 0·849 (0·016) | |
| Cut-off value | ≥ median | 23 | 3502 | 0·73 (0·70-0·75) | 0·78 (0·76-0·80) | 3·57 (2·95-4·32) | 0·34 (0·28-0·40) | 13·10 (9·39-18·27) | 0·854 (0·017) |
| <median | 19 | 2544 | 0·81 (0·79-0·83) | 0·66 (0·63-0·68) | 2·55 (2·06-3·15) | 0·31 (0·26-0·38) | 8·70 (6·63-11·41) | 0·816 (0·017) | |
| Region | Europe and America | 16 | 2713 | 0·74 (0·71-0·76) | 0·76 (0·73-0·78) | 3·25 (2·57-4·11) | 0·34 (0·29-0·41) | 10·44 (7·59-14·37) | 0·832 (0·017) |
| Asia | 21 | 2254 | 0·75 (0·73-0·78) | 0·79 (0·76-0·81) | 3·58 (2·85-4·50) | 0·32 (0·26-0·39) | 13·39 (9·17-19·55) | 0·854 (0·017) | |
| F≥F3 | 59 | 11976 | 0·83 (0·82-0·84) | 0·79 (0·78-0·80) | 4·32 (3·78-4·93) | 0·21 (0·18-0·25) | 22·70 (17·58-29·31) | 0·897 (0·010) | |
| Probe type | M | 28 | 5436 | 0·80 (0·78-0·82) | 0·80 (0·79-0·81) | 4·55 (3·84-5·39) | 0·23 (0·19-0·28) | 19·70 (15·30-25·35) | 0·886 (0·010) |
| XL | 5 | 566 | 0·70 (0·63-0·77) | 0·81 (0·76-0·84) | 3·56 (2·49-5·10) | 0·31 (0·17-0·54) | 13·03 (5·86-28·95) | 0·837 (0·025) | |
| BMI | < 30 | 27 | 4337 | 0·81 (0·78-0·83) | 0·83 (0·82-0·85) | 4·83 (4·27-5·47) | 0·24 (0·21-0·28) | 21·70 (16·97-27·74) | 0·894 (0·008) |
| ≥30 | 29 | 7134 | 0·84 (0·83-0·86) | 0·77 (0·75-0·78) | 4·00 (3·28-4·89) | 0·17 (0·13-0·23) | 27·04 (17·34-42·15) | 0·917 (0·017) | |
| Cut-off value | ≥ median | 25 | 3417 | 0·79 (0·76-0·81) | 0·84 (0·83-0·86) | 5·08 (4·27-6·05) | 0·20 (0·15-0·27) | 26·72 (18·40-38·81) | 0·905 (0·011) |
| <median | 32 | 8419 | 0·84 (0·83-0·85) | 0·77 (0·76-0·78) | 3·73 (3·16-4·40) | 0·22 (0·18-0·26) | 19·08 (13·57-26·83) | 0·889 (0·015) | |
| Region | Europe and America | 27 | 7629 | 0·82 (0·81-0·84) | 0·78 (0·76-0·79) | 4·09 (3·40-4·93) | 0·23 (0·18-0·29) | 19·27 (13·39-27·73) | 0·885 (0·015) |
| Asia | 22 | 2616 | 0·86 (0·83-0·88) | 0·83 (0·81-0·84) | 4·82 (3·93-5·90) | 0·19 (0·16-0·24) | 28·08 (19·11-41·25) | 0·911 (0·012) | |
| F=F4 | 32 | 4594 | 0·83 (0·79-0·86) | 0·85 (0·84-0·86) | 6·37 (5·17-7·85) | 0·17 (0·12-0·25) | 36·63 (25·11-53·45) | 0·925 (0·010) | |
| Probe type | M | 16 | 2286 | 0·82 (0·77-0·86) | 0·86 (0·84-0·87) | 7·19 (5·13-10·06) | 0·17 (0·10-0·29) | 31·40 (20·66-47·72) | 0·921 (0·012) |
| XL | 5 | 566 | 0·72 (0·60-0·81) | 0·87 (0·83-0·90) | 6·51 (3·87-10·95) | 0·20 (0·06-0·67) | 20·79 (10·22-42·27) | 0·910 (0·026) | |
| BMI | < 30 | 17 | 2178 | 0·85 (0·80-0·89) | 0·88 (0·86-0·89) | 7·39 (5·76-9·47) | 0·14 (0·07-0·26) | 42·55 (27·67-65·44) | 0·937 (0·010) |
| ≥30 | 13 | 2235 | 0·80 (0·75-0·85) | 0·82 (0·81-0·84) | 5·08 (3·64-7·10) | 0·21 (0·12-0·35) | 27·07 (14·61-50·18) | 0·911 (0·022) | |
| Cut-off value | ≥ median | 17 | 2577 | 0·77 (0·72-0·82) | 0·87 (0·86-0·89) | 6·83 (5·38-8·68) | 0·21 (0·13-0·33) | 30·66 (20·34-46·22) | 0·925 (0·011) |
| <median | 14 | 1929 | 0·90 (0·85-0·93) | 0·83 (0·81-0·85) | 5·99 (4·15-8·64) | 0·17 (0·11-0·25) | 43·82 (21·30-90·12) | 0·939 (0·017) | |
| Region | Europe and America | 12 | 2016 | 0·85 (0·80-0·89) | 0·82 (0·80-0·84) | 5·24 (3·66-7·52) | 0·18 (0·11-0·30) | 33·02 (16·87-64·60) | 0·925 (0·020) |
| Asia | 15 | 1521 | 0·95 (0·90-0·98) | 0·88 (0·86-0·89) | 7·72 (5·72-10·43) | 0·11 (0·06-0·19) | 83·70 (43·88-159·63) | 0·959 (0·010) |
Abbreviations: NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; CAP: controlled attenuation parameter; LSM: liver stiffness measurement; BMI: body mass index; SE: sensitivity; SP: specificity; PLR: positive likelihood ratio; NLR: negative likelihood ratio; DOR: diagnostic odds ratio; AUROC: area under the receiver operating characteristic curve; 95% CI: 95% confidence interval; SE*: standard errors; NA: not applicable.
Table 2.
Mean cut-off and range of CAP in NAFLD/NASH.
| Category | Subgroup | Case (n) | Sample (n) | Mean Cut-off (range) (dB/m) | D value (dB/m) | P value |
|---|---|---|---|---|---|---|
| S≥S1 | 14 | 2138 | 268·5 (233·5-304) | |||
| Probe type | M | 6 | 621 | 254·4 (233·5-275) | 23·6 | 0·091 |
| XL | 2 | 176 | 278·0 (271-285) | |||
| BMI | < 30 | 5 | 564 | 251·2 (233·5-275) | 30·7 | 0·001 |
| ≥30 | 9 | 1574 | 281·9 (261-304) | |||
| Cut-off value | ≥ median | 8 | 1539 | 285·6 (271-304) | 34·3 | < 0·001 |
| <median | 6 | 599 | 251·3 (233·5-266) | |||
| Region | Europe and America | 7 | 1242 | 274·8 (233·5-304) | 11·2 | 0·313 |
| Asia | 7 | 896 | 263·6 (236-285) | |||
| S≥S2 | 26 | 3414 | 288·0 (245-331) | |||
| Probe type | M | 12 | 1449 | 282·9 (263-313·5) | 1·7 | 0·861 |
| XL | 4 | 357 | 281·3 (273-291) | |||
| BMI | < 30 | 12 | 1260 | 272·8 (245-313·5) | 28·2 | 0·001 |
| ≥30 | 14 | 1978 | 301·0 (266-331) | |||
| Cut-off value | ≥ median | 13 | 2079 | 307·4 (289-331) | 38·8 | < 0·001 |
| < median | 13 | 1135 | 268·6 (245-285) | |||
| Region | Europe and America | 9 | 1560 | 297·6 (245-331) | 16·7 | 0·109 |
| Asia | 14 | 1456 | 280·9 (258-331) | |||
| S=S3 | 25 | 3549 | 313·1 (245-366) | |||
| Probe type | M | 13 | 1773 | 311·6 (267-366) | 15·7 | 0·322 |
| XL | 4 | 357 | 327·3 (302-355) | |||
| BMI | < 30 | 12 | 1260 | 298·9 (245-337) | 27·9 | 0·018 |
| ≥30 | 12 | 1965 | 326·8 (267-366) | |||
| Cut-off value | ≥ median | 11 | 1782 | 339·6 (320-366) | 47·3 | < 0·001 |
| < median | 14 | 1767 | 292·3 (245-312) | |||
| Region | Europe and America | 8 | 1531 | 312·5 (245-345) | 6·3 | 0·629 |
| Asia | 13 | 1535 | 306·2 (267-355) |
Abbreviations: NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; CAP: controlled attenuation parameter; BMI: Body Mass Index.
Table 3.
Mean cut-off and range of LSM in NAFLD/NASH.
| Category | Subgroup | Case (n) | Sample (n) | Mean Cut-off (range) (kPa) | D value (kPa) | P value |
|---|---|---|---|---|---|---|
| F≥F1 | 16 | 2264 | 6·67 (5·3-8·6) | |||
| Probe type | M | 10 | 1369 | 6·63 (5·3-7·5) | / | / |
| XL | 1 | 57 | / | |||
| BMI | < 30 | 10 | 1384 | 6·47 (5·3-7·5) | 0·54 | 0·242 |
| ≥ 30 | 6 | 880 | 7·01 (5·9-8·6) | |||
| Cut-off value | ≥ median | 9 | 1661 | 7·30 (6·7-8·6) | 1·43 | < 0·001 |
| < median | 7 | 603 | 5·87 (5·3-6·3) | |||
| Region | Europe and America | 5 | 810 | 6·44 (5·3-8·6) | 0·34 | 0·491 |
| Asia | 11 | 1454 | 6·78 (5·9-7·68) | |||
| F≥F2 | 44 | 6186 | 7·61 (5-11) | |||
| Probe type | M | 22 | 3308 | 7·82 (5-11) | 0·87 | 0·197 |
| XL | 6 | 647 | 6·95 (5-8·9) | |||
| BMI | < 30 | 21 | 2787 | 7·62 (5-11) | 0·72 | 0·126 |
| ≥30 | 20 | 2894 | 8·33 (5-11) | |||
| Cut-off value | ≥ median | 23 | 3502 | 8·94 (7·7-11) | 2·25 | < 0·001 |
| < median | 19 | 2544 | 6·70 (5-7·6) | |||
| Region | Europe and America | 16 | 2713 | 7·66 (6·2-9·8) | 0·96 | 0·027 |
| Asia | 21 | 2254 | 8·62 (6·7-11) | |||
| F≥F3 | 59 | 11976 | 9·75 (7·1-13·6) | |||
| Probe type | M | 28 | 5436 | 9·91 (7·1-13·6) | 0·67 | 0·355 |
| XL | 5 | 566 | 9·24 (7·2-11·5) | |||
| BMI | < 30 | 27 | 4337 | 9·54 (7·2-12) | 0·09 | 0·814 |
| ≥30 | 29 | 7134 | 9·63 (6·7-13·6) | |||
| Cut-off value | ≥ median | 25 | 3417 | 10·7 (9·8-13·6) | 2·09 | < 0·001 |
| < median | 32 | 8419 | 8·65 (6·7-9·75) | |||
| Region | Europe and America | 27 | 7629 | 9·46 (6·7-12·5) | 0·49 | 0·257 |
| Asia | 22 | 2616 | 9·95 (7·1-13·6) | |||
| F=F4 | 32 | 4594 | 12·91 (7·9-17·5) | |||
| Probe type | M | 16 | 2286 | 13·26 (9·5-17·5) | 1·00 | 0·454 |
| XL | 5 | 566 | 12·26 (7·9-15) | |||
| BMI | < 30 | 17 | 2178 | 13·12 (7·9-17·5) | 0·47 | 0·645 |
| ≥30 | 13 | 2235 | 12·65 (6·9-16·1) | |||
| Cut-off value | ≥ median | 17 | 2577 | 14·78 (13·1-17·5) | 4·34 | < 0·001 |
| < median | 14 | 1929 | 10·44 (6·9-12·4) | |||
| Region | Europe and America | 12 | 2016 | 11·94 (6·9-16·1) | 2·03 | 0·034 |
| Asia | 15 | 1521 | 13·97 (11-17·5) |
Abbreviations: NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; LSM: liver stiffness measurement; BMI: Body Mass Index; NA: not applicable.
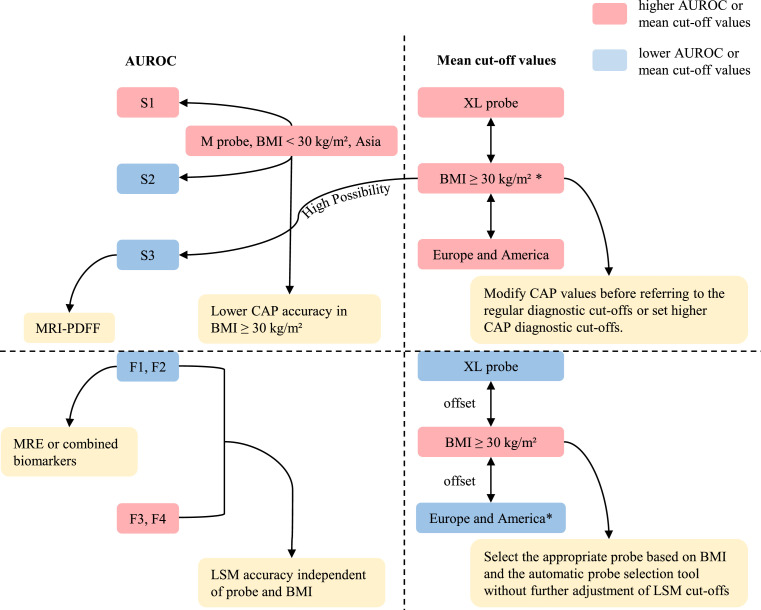
Meta-regression analyses were performed according to covariates including cut-off value, BMI, probe type, study design, and region. The results showed that BMI (P = 0·0252), cut-off value (P = 0·0313), probe type (P = 0·0304), and region (P = 0·0068) might be the possible sources of heterogeneity (Table S3). Furthermore, we conducted subgroup analyses according to the probe type, BMI, cut-off value, and region. We found that M probe, BMI < 30 kg/m², low cut-off values, and Asia groups showed higher AUROC in detecting S ≥ S1 and ≥ S2, while for diagnosing S = S3, F ≥ F1, F ≥ F2, ≥ F3, and = F4, the AUROC of different probe types, BMI, cut-off values, and regions were similar (Table 1, Figure 2).
Figure 2.
Diagnostic performance of CAP and LSM, mean cut-off values, and influencing factors.
Furthermore, we also compared the mean cut-off values between different subgroups. We found that there were significant differences in CAP values among different BMI populations and LSM values among different regions. For diagnosing S ≥ S1, ≥ S2, and = S3, the mean cut-off values of BMI ≥ 30 kg/m² were 30·7, 28·2, and 27·9 dB/m higher than BMI < 30 kg/m² (P = 0·001, 0·001 and 0·018), respectively. For diagnosing F ≥ F2 and = F4, the mean cut-off values of EUR-USA were 0·96 and 2·03 kPa higher than Asia (P = 0·027, P = 0·034), respectively. Interestingly, there were also differences, although not significant, that seemed to follow a pattern: higher mean CAP values for XL probes and EUR-USA groups, and higher mean LSM values for M probes and BMI ≥ 30 kg/m² groups. Specifically, for diagnosing S ≥ S1 and = S3, the mean cut-off values of XL probe were 23·6 and 15·7 dB/m higher than M probe, respectively. For diagnosing S ≥ S1, ≥ S2 and = S3, the mean cut-off values of EUR-USA were 11·2, 16·7, and 6·3 dB/m higher than Asia. Moreover, for diagnosing F ≥ F2, ≥ F3, and = F4, the mean cut-off values of M probe were 0·87, 0·67 and 1·00 kPa higher than XL probe, respectively. For diagnosing F ≥ F1, ≥ F2, and ≥ F3, the mean cut-off values of high BMI were 0·54, 0·72, and 0·09 kPa higher than low BMI, respectively (Tables 2 and 3, Figure 2).
To investigate the clinical utility of CAP and LSM for hepatic steatosis grading and liver fibrosis staging, respectively. We evaluated the pre-test probabilities of 25% and the corresponding post-test probabilities. For diagnosing S ≥ S1, the Fagan plot analysis revealed a PLR and NLR of 11 and 0·09, respectively. A positive CAP value demonstrated a 78% probability of correct diagnosis and a negative CAP value demonstrated a 3% probability of wrong diagnosis (Figure S2g). For diagnosing S ≥ S2 and S = S3, the Fagan plot analysis revealed a PLR and NLR of 2 and 0·26, 2 and 0·36, respectively, and the positive and negative post-test probability were 44% and 8%, 43% and 11%, respectively (Figure S3g, S4g). For diagnosing F ≥ F1, F ≥ F2, F ≥ F3, and F = F4, the Fagan plot analysis revealed a PLR and NLR of 4 and 0·28, 3 and 0·29, 5 and 0·18, 8 and 0·11, respectively, and the positive and negative post-test probability were 55% and 9%, 52% and 9%, 61% and 6%, 72% and 4%, respectively (Figures S5-8g).
In addition, goodness-of-fit and bivariate normality analyses showed that the results were moderately robust to detect steatosis and fibrosis at each stage (Figure S9-15a and b). Furthermore, we used influence analysis and outlier detection to identify relevant outliers (Figure S9-15c and d). After we excluded the relevant outliers, we found no significant changes in the overall results (Table S5), also suggesting that the results are reliable. We used Deeks’ funnel plot asymmetry test to assess the publication bias. There was no evidence of publication bias among included studies in diagnosing S ≥ S1 (P = 0·58, Figure S2h), S ≥ S2 (P = 0·09, Figure S3h), F ≥ F1 (P = 0·26, Figure S5h) and F ≥ F3 (P = 0·71, Figure S7h). However, publication bias was found in diagnosing S ≥ S3 (P = 0·00, Figure S4h), F ≥ F2 (P = 0·03, Figure S6h) and F = F4 (P = 0·03, Figure S8h). The results of the trim and fill method showed that for diagnoses S ≥ S3, F ≥ F2, and F = F4, after adding 11, 15, and 12 studies, respectively, there was no significant asymmetry in the filled funnel plots, indicating no publication bias (Figures S4i, S6i and S8i), and no significant changes in the pooled effect values and 95% CI (all P = 0·000), indicating that the results were stable.
Discussion
In this meta-analysis, we found that the AUROC of CAP were 0·924, 0·794 and 0·778 for S ≥ S1, ≥ S2 and = S3, respectively, and the AUROC of LSM for detecting F ≥ F1, ≥ F2, ≥ F3, and = F4 were 0·851, 0·830, 0·897 and 0·925, respectively. From the above data, we could conclude that, overall, the diagnostic performance of CAP decreased with the severity of steatosis, while the diagnostic performance of LSM increased with the aggravation of fibrosis. Fagan plot analysis also showed similar results in clinical utility. This result was also consistent with previous meta-analyses about the diagnostic performance of CAP and LSM in NAFLD/NASH.14,15 Although ultrasound is the main method for examining NAFLD, it is not sensitive enough to detect steatosis with liver fat content less than 20%,82 for which CAP has good accuracy. Current EASL and Asia-Pacific guidelines recommend screening for NAFLD in high-risk populations (e.g., obesity, diabetes),3,83 and CAP is a good option for detecting mild steatosis in this population. In addition, long-term fatty liver may also progress to NASH, liver fibrosis and then advanced liver disease. The good performance of LSM in the diagnosis of advanced liver fibrosis and cirrhosis may be a viable alternative to liver biopsy.
Nevertheless, some studies reported that multiple confounding factors would affect the performance of CAP and LSM, such as fasting conditions,84,85 probe type,63 liver transaminases,42,86,87 extrahepatic cholestasis,88 waist circumference,45 and obesity.42,89 Besides, studies found that steatosis could influence LSM86,90 and fibrosis could also influence CAP,91 indicating that LSM and CAP values might influence each other.79 Our subgroup analyses showed that M probe, low BMI, low cut-off values, and Asia had higher AUROC in detecting S ≥ S1 and ≥ S2, suggesting better diagnostic accuracy. Such results do not seem to be coincidental since Asians have a lower BMI than Europeans and Americans, most of which can be successfully measured with the M probe, and these factors are interlinked and all seem to indicate a higher diagnostic accuracy of CAP in BMI < 30 kg/m² population (Figure 2). Similar conclusion was reached in a previous study: CAP correlated extremely well with actual liver fat percentage in NAFLD patients with BMI < 28 kg/m2, especially < 25 kg/m2.91 The reason for the decreased accuracy of CAP in patients with obesity may come from the thicker subcutaneous adipose tissue, so would increasing the probing depth using the XL probe improve accuracy? The results of the subgroup analysis seem to disprove this conjecture. Therefore, it is reasonable to speculate that, in addition to affecting the measurement by increasing the SCD, excessive subcutaneous fat itself interferes with ultrasound attenuation. In addition, patients with obesity are more likely to have severe steatosis, just as the overall analysis showed that CAP accuracy decreases with increasing steatosis, which increases its diagnostic uncertainty and requires further exploration and improvement in the use of CAP in populations with obesity and severe steatosis. In this case, magnetic resonance imaging-based proton density fat fraction (MRI-PDFF) may be a better option than CAP, which quantifies steatosis more accurately.92,93 In a meta-analysis, the AUROC for MRI-PDFF diagnosis of S ≥ S1, ≥ S2 and = S3 was 0·97, 0·91 and 0·90, respectively.92
Notably, biomarkers are also commonly used non-invasive tests that have shown diagnostic value for fibrosis. A meta-analysis summarized four biomarkers: fibrosis-4 index (FIB-4), NAFLD fibrosis score (NFS), aspartate aminotransferase to platelets ratio index (APRI) and BARD score in the diagnosis of F ≥ F3 with AUROC of 0·84, 0·84, 0·77, and 0·76, respectively.94 Our analysis showed that the AUROC of LSM for diagnosing F ≥ F3 was 0·897, higher than these biomarkers. However, these biomarkers are cheaper, simpler, and more accessible than LSM due to the limitations of equipment cost and place of use. In primary health care, their use is more advantageous. This is also what the current NAFLD guidelines recommend.3 However, for people at high risk of cirrhosis, such as NAFLD, we recommend choosing LSM with higher accuracy. More importantly, our subgroup analysis showed that the accuracy was similar between different BMI, different probes, and different regions. In addition, some studies have shown that combining LSM with FIB-4 or NFS can improve diagnostic accuracy.52,56,95 Another FibroMeter vibration-controlled transient elastography (FM-VCTE) model, which combines LSM with biochemical results (platelet count, α2-macroglobulin, urea, prothrombin index, transaminases), also improves the diagnostic accuracy of fibrosis in NAFLD.36,47 In one study, for the diagnosis of F ≥ F3, the AUROC of LSM was 0·922, while the AUROC of FM-VCTE improved to 0·968.36 For the diagnosis of F ≥ F1, the combination of LSM with FIB-4 or NFS increased the AUROC of using LSM alone from 0·855 to 0·886 and 0·871, respectively,56 whereas FM-VCTE increased the positive predictive value for the diagnosis of F ≥ F2,47 which seems to compensate for the lack of accuracy of LSM in the diagnosis of low-grade fibrosis (F1-2) to a certain extent. However, there are relatively few clinical studies and no relevant meta-analyses, and more studies are needed to verify this. The evidence for the superiority of magnetic resonance elastography (MRE) in the diagnosis of low-grade fibrosis appears to be stronger. For the diagnosis of F ≥ F2, a single study showed an AUROC of 0·91 for MRE, higher than 0·82 for LSM,93 while a meta-analysis showed an AUROC of 0·91 for MRE, also higher than 0·83 for LSM (Figure 2).96
In addition, many studies have also reported that CAP and LSM values might be affected by confounding factors. Hence, we compared the mean cut-off values between different subgroups. We found that there were significant differences in CAP values among different BMI populations. For diagnosing S ≥ S1, ≥ S2 and = S3, the mean CAP cut-off values for BMI ≥ 30 kg/m² were 30·7, 28·2, and 27·9 dB/m higher than for BMI < 30 kg/m², respectively. Interestingly, this had also been confirmed in previous studies. One study found that high BMI was significantly related to an increase in CAP.97 Another study recalculated the CAP values according to variable BMI, suggesting that when BMI is within 20-25kg/m2, for every unit below 25 kg/m2, 4·4 dB/m is added; and when BMI is within 25-30 kg/m2, 4·4 dB/m is subtracted for every unit above 25 kg/m2.98 Besides, among different probes and different regions, the CAP values of XL probes and EUR-USA were slightly higher than those of M probes and Asian. Although these differences were not statistically significant, considering the higher prevalence of obesity in the EUR-USA populations and the higher use of the XL probe in the population with obesity, it may exacerbate the overestimation of steatosis in patients with obesity in clinical diagnosis. Therefore, setting higher diagnostic cut-offs for patients with obesity than the routine is needed, but unfortunately, there are no specific diagnostic criteria for this population, or, as mentioned earlier, modifying CAP value for patients with obesity before referring to the regular diagnostic cut-offs (Figure 2).
Moreover, significant differences were found in LSM values among different regions. For diagnosing F ≥ F2 and = F4, the mean cut-off values of EUR-USA were 0·96 and 2·03 kPa higher than Asia, respectively. The mean cut-off values were also slightly higher in Europe-USA than in Asia for diagnoses F ≥ F1 and ≥ F3. This is an interesting finding, and the smaller skeleton and narrower rib space in Asians may be responsible for the higher liver stiffness measurements. There have been a number of studies on the differences in LSM values between different probes, and most agreed that the LSM values obtained using XL probes are lower than those obtained using M probes,10,62,63,71,77,99 and the guideline summarized that the mean LSM values obtained by XL probes were 1·5 kPa lower than those obtained by M probes (range: 0·8–2·3 kPa).100 Our analysis results also remained consistent with these studies. As to whether BMI will affect LSM values, the results are not yet uniform. Some believe that it does not,31,49 and some believe that it does.41,42,50,51,62,63,77 In our study, the mean cut-off values for BMI ≥ 30 kg/m² were higher than those for BMI < 30 kg/m². However, it is noteworthy that none of the differences between the different probes and BMI populations were statistically significant, indicating that LSM values appear to be independent of probe type and BMI. The XL probe is mostly used in patients with obesity, and its lower LSM values are partially offset by higher LSM values in patients with obesity, which may be the reason for the insignificant difference in LSM values.62 Therefore, when selecting the appropriate probe according to BMI and the automatic probe selection tool in clinical use, the same LSM diagnostic values can be used without further adjustment (Figure 2).13,62
Not long ago, a meta‐analysis had investigated the performance of CAP and LSM, but its population involved alcoholic liver disease patients, pediatric patients, and patients with morbid obesity.101 Studies found that cirrhosis caused by NASH had higher LSM values than cirrhosis caused by chronic hepatitis C,102 and LSM values should be selected based on different diseases.35,41 Besides, children and adult NAFLD patients had different histopathological features,103 and Fibroscan did not accurately predict steatosis or fibrosis in comparison to histology in morbidly patients with obesity.104 There were also meta-analyses that discussed the accuracy of CAP or LSM alone in NAFLD patients,14,15 but they included only nine articles, which also led to relatively limited conclusions. Therefore, we included only adult NAFLD/NASH patients, collecting all relevant literature as much as possible. Furthermore, to explore heterogeneity and influence factors, we did meta-regression analysis and subgroup analysis to further analyze the effects of the population with obesity and different probes on CAP and LSM. As we know, this was the first meta-analysis to evaluate the performance of both CAP and LSM for assessing steatosis and fibrosis in adult NAFLD/NASH patients.
Even so, there were some limitations to our work. First, the interval between liver biopsy and index test was unclear or more than 3 months in some included studies, which might increase the risk of bias. Second, partial summary results had great heterogeneity. Although meta-regression analysis and subgroup analysis explained some of the sources of heterogeneity, there were still some that they couldn't explain. Third, due to insufficient data, we could only calculate the mean cut-off values, but could not get the optimal cut-off values. Finally, although we considered the effect of BMI, probe, and region on diagnosis, we were unable to extract sufficient data for other confounding factors such as gender, transaminases, diabetes, and the effect of liver fibrosis on CAP, for further analysis.
In conclusion, the diagnostic performance of CAP decreased with the severity of steatosis while the diagnostic performance of LSM increased with the aggravation of fibrosis. In terms of CAP, its good performance for the diagnosis of mild steatosis can be used to screen for NAFLD in high-risk groups. Note, its low accuracy in patients with obesity and the significant differences in mean cut-off values across different BMI populations suggest the need for special diagnostic values in the population with obesity. All these make its wide application in NAFLD, where the proportion of people with obesity is much higher, a practical problem. In NAFLD, it has high accuracy in diagnosing advanced fibrosis and cirrhosis. Combination with other noninvasive biomarkers seems to improve the diagnosis of F2, and the same diagnostic criteria can be used in the population with obesity when selecting appropriate probes. However, so far, the evidence for noninvasive tests related to NAFLD is still limited, and the future role of CAP and LSM as potential noninvasive alternatives to liver biopsy in the assessment of steatosis and fibrosis should be further validated and improved, especially for the assessment of steatosis in patients with obesity, moderate to severe steatosis, and low-grade fibrosis.
Contributors
Y-TC and X-QZ conceived and designed the study. FQ, Y-JZ and L-LX contributed to data collection. Y-TC, YC and X-QZ conducted the data analysis and interpretation. Y-TC and YC drafted the initial manuscript. X-QZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Data sharing statement
The data analyzed during the current systematic review and meta-analysis is available from the corresponding author on reasonable request.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101547.
Appendix. Supplementary materials
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 4.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 6.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the group of epidemiology of the French association for the study of the liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 7.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Piazzolla VA, Mangia A. Noninvasive diagnosis of NAFLD and NASH. Cells. 2020;9 doi: 10.3390/cells9041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuppalanchi R, Siddiqui MS, Van Natta ML, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–144. doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich-Rust M, Hadji-Hosseini H, Kriener S, et al. Transient elastography with a new probe for obese patients for non-invasive staging of non-alcoholic steatohepatitis. Eur Radiol. 2010;20:2390–2396. doi: 10.1007/s00330-010-1820-9. [DOI] [PubMed] [Google Scholar]
- 11.de Lédinghen V, Wong VW, Vergniol J, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J Hepatol. 2012;56:833–839. doi: 10.1016/j.jhep.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 12.de Lédinghen V, Hiriart JB, Vergniol J, Merrouche W, Bedossa P, Paradis V. Controlled attenuation parameter (CAP) with the XL Probe of the Fibroscan®: a comparative study with the M Probe and Liver Biopsy. Dig Dis Sci. 2017;62:2569–2577. doi: 10.1007/s10620-017-4638-3. [DOI] [PubMed] [Google Scholar]
- 13.Berger A, Shili S, Zuberbuhler F, et al. Liver stiffness measurement with fibroscan: use the right probe in the right conditions! Clin Transl Gastroenterol. 2019;10 doi: 10.14309/ctg.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pu K, Wang Y, Bai S, et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:51. doi: 10.1186/s12876-019-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease–the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 16.McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–U104. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470–1476. doi: 10.1111/jgh.12557. [DOI] [PubMed] [Google Scholar]
- 22.Darweesh SK, Omar H, Medhat E, et al. The clinical usefulness of elastography in the evaluation of nonalcoholic fatty liver disease patients: a biopsy-controlled study. Eur J Gastroenterol Hepatol. 2019;31:1010–1016. doi: 10.1097/meg.0000000000001365. [DOI] [PubMed] [Google Scholar]
- 23.de Lédinghen V, Wong GL, Vergniol J, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:848–855. doi: 10.1111/jgh.13219. [DOI] [PubMed] [Google Scholar]
- 24.Friedrich-Rust M, Romen D, Vermehren J, et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325–e331. doi: 10.1016/j.ejrad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Harry S, Lai LL, Nik Mustapha NR, et al. Volumetric liver fat fraction determines grade of steatosis more accurately than controlled attenuation parameter in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18:945–953.e2. doi: 10.1016/j.cgh.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Karlas T, Petroff D, Garnov N, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar M, Rastogi A, Singh T, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013;28:1194–1201. doi: 10.1111/jgh.12134. [DOI] [PubMed] [Google Scholar]
- 28.Runge JH, Smits LP, Verheij J, et al. MR spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology. 2018;286:547–556. doi: 10.1148/radiol.2017162931. [DOI] [PubMed] [Google Scholar]
- 29.Shalimar Kumar R, Rout G, et al. Body mass index-based controlled attenuation parameter cut-offs for assessment of hepatic steatosis in non-alcoholic fatty liver disease. Indian J Gastroenterol. 2020;39:32–41. doi: 10.1007/s12664-019-00991-2. [DOI] [PubMed] [Google Scholar]
- 30.Shao CX, Ye J, Dong Z, et al. Steatosis grading consistency between controlled attenuation parameter and MRI-PDFF in monitoring metabolic associated fatty liver disease. Ther Adv Chronic Dis. 2021;12 doi: 10.1177/20406223211033119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther. 2016;44:366–379. doi: 10.1111/apt.13710. [DOI] [PubMed] [Google Scholar]
- 32.Aykut UE, Akyuz U, Yesil A, et al. A comparison of fibrometer™ NAFLD score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Scand J Gastroenterol. 2014;49:1343–1348. doi: 10.3109/00365521.2014.958099. [DOI] [PubMed] [Google Scholar]
- 33.Boursier J, Guillaume M, Leroy V, et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol. 2019;71:389–396. doi: 10.1016/j.jhep.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570–578. doi: 10.1016/j.jhep.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Chang PE, Hartono JL, Ngai YL, Dan YY, Lim KB, Chow WC. Optimal liver stiffness measurement values for the diagnosis of significant fibrosis and cirrhosis in chronic liver disease in Singapore. Singapore Med J. 2019;60:532–537. doi: 10.11622/smedj.2018156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dincses E, Yilmaz Y. Diagnostic usefulness of FibroMeter VCTE for hepatic fibrosis in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:1149–1153. doi: 10.1097/MEG.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 37.Ergelen R, Akyuz U, Aydin Y, Eren F, Yilmaz Y. Measurements of serum procollagen-III peptide and M30 do not improve the diagnostic accuracy of transient elastography for the detection of hepatic fibrosis in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:667–671. doi: 10.1097/MEG.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 38.Ergelen R, Yilmaz Y, Asedov R, et al. Comparison of Doppler ultrasound and transient elastography in the diagnosis of significant fibrosis in patients with nonalcoholic steatohepatitis. Abdom Radiol (NY) 2016;41:1505–1510. doi: 10.1007/s00261-016-0699-6. [DOI] [PubMed] [Google Scholar]
- 39.Forsgren MF, Nasr P, Karlsson M, et al. Biomarkers of liver fibrosis: prospective comparison of multimodal magnetic resonance, serum algorithms and transient elastography. Scand J Gastroenterol. 2020;55:848–859. doi: 10.1080/00365521.2020.1786599. [DOI] [PubMed] [Google Scholar]
- 40.Furlan A, Tublin ME, Yu L, Chopra KB, Lippello A, Behari J. Comparison of 2D shear wave elastography, transient elastography, and MR elastography for the diagnosis of fibrosis in patients with nonalcoholic fatty liver disease. AJR Am J Roentgenol. 2020;214:W20–W26. doi: 10.2214/AJR.19.21267. [DOI] [PubMed] [Google Scholar]
- 41.Gaia S, Carenzi S, Barilli AL, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54:64–71. doi: 10.1016/j.jhep.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Hanafy AS, Seleem WM, El-Kalla F, AbdAlkhalik Basha M, Abd-Elsalam S. Efficacy of a non-invasive model in predicting the cardiovascular morbidity and histological severity in non-alcoholic fatty liver disease. Diabetes Metab Syndr. 2019;13:2272–2278. doi: 10.1016/j.dsx.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 43.Jafarov F, Kaya E, Bakir A, Eren F, Yilmaz Y. The diagnostic utility of fibrosis-4 or nonalcoholic fatty liver disease fibrosis score combined with liver stiffness measurement by fibroscan in assessment of advanced liver fibrosis: a biopsy-proven nonalcoholic fatty liver disease study. Eur J Gastroenterol Hepatol. 2020;32:642–649. doi: 10.1097/MEG.0000000000001573. [DOI] [PubMed] [Google Scholar]
- 44.Kumar R, Rastogi A, Sharma MK, et al. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig Dis Sci. 2013;58:265–274. doi: 10.1007/s10620-012-2306-1. [DOI] [PubMed] [Google Scholar]
- 45.Lee MS, Bae JM, Joo SK, et al. Prospective comparison among transient elastography, supersonic shear imaging, and ARFI imaging for predicting fibrosis in nonalcoholic fatty liver disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leong WL, Lai LL, Nik Mustapha NR, et al. Comparing point shear wave elastography (ElastPQ) and transient elastography for diagnosis of fibrosis stage in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35:135–141. doi: 10.1111/jgh.14782. [DOI] [PubMed] [Google Scholar]
- 47.Loong TC, Wei JL, Leung JC, et al. Application of the combined FibroMeter vibration-controlled transient elastography algorithm in Chinese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2017;32:1363–1369. doi: 10.1111/jgh.13671. [DOI] [PubMed] [Google Scholar]
- 48.Lupsor M, Badea R, Stefanescu H, et al. Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2010;19:53–60. [PubMed] [Google Scholar]
- 49.Mahadeva S, Mahfudz AS, Vijayanathan A, Goh KL, Kulenthran A, Cheah PL. Performance of transient elastography (TE) and factors associated with discordance in non-alcoholic fatty liver disease. J Dig Dis. 2013;14:604–610. doi: 10.1111/1751-2980.12088. [DOI] [PubMed] [Google Scholar]
- 50.Myers RP, Elkashab M, Ma M, Crotty P, Pomier-Layrargues G. Transient elastography for the noninvasive assessment of liver fibrosis: a multicentre Canadian study. Can J Gastroenterol. 2010;24:661–670. doi: 10.1155/2010/153986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petta S, Di Marco V, Camma C, Butera G, Cabibi D, Craxi A. Reliability of liver stiffness measurement in non-alcoholic fatty liver disease: the effects of body mass index. Aliment Pharmacol Ther. 2011;33:1350–1360. doi: 10.1111/j.1365-2036.2011.04668.x. [DOI] [PubMed] [Google Scholar]
- 52.Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2015;35:1566–1573. doi: 10.1111/liv.12584. [DOI] [PubMed] [Google Scholar]
- 53.Petta S, Wong VWS, Cammà C, et al. Serial combination of noninvasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with nonalcoholic fatty liver disease. Dig Liver Dis. 2017;49:e2. doi: 10.1016/j.dld.2017.01.007. [DOI] [Google Scholar]
- 54.Seki K, Shima T, Oya H, Mitsumoto Y, Mizuno M, Okanoue T. Assessment of transient elastography in Japanese patients with non-alcoholic fatty liver disease. Hepatol Res. 2017;47:882–889. doi: 10.1111/hepr.12829. [DOI] [PubMed] [Google Scholar]
- 55.Shi YW, Wang QY, Zhao XY, et al. Non-obese patients with nonalcoholic fatty liver disease may use a lower liver stiffness cut-off to assess fibrosis stages. J Dig Dis. 2020;21:279–286. doi: 10.1111/1751-2980.12868. [DOI] [PubMed] [Google Scholar]
- 56.Shima T, Sakai K, Oya H, et al. Diagnostic accuracy of combined biomarker measurements and vibration-controlled transient elastography (VCTE) for predicting fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2020;55:100–112. doi: 10.1007/s00535-019-01626-1. [DOI] [PubMed] [Google Scholar]
- 57.Staufer K, Halilbasic E, Spindelboeck W, et al. Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United European Gastroenterol J. 2019;7:1113–1123. doi: 10.1177/2050640619865133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taibbi A, Petta S, Matranga D, et al. Liver stiffness quantification in biopsy-proven nonalcoholic fatty liver disease patients using shear wave elastography in comparison with transient elastography. Ultrasonography. 2021;40:407–416. doi: 10.14366/usg.20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tapper EB, Challies T, Nasser I, Afdhal NH, Lai M. The performance of vibration controlled transient elastography in a US cohort of patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;111:677–684. doi: 10.1038/ajg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tovo CV, Villela-Nogueira CA, Leite NC, et al. Transient hepatic elastography has the best performance to evaluate liver fibrosis in non-alcoholic fatty liver disease (NAFLD) Ann Hepatol. 2019;18:445–449. doi: 10.1016/j.aohep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Troelstra MA, Witjes JJ, van Dijk AM, et al. Assessment of imaging modalities against liver biopsy in nonalcoholic fatty liver disease: the Amsterdam NAFLD-NASH cCohort. J Magn Reson Imaging. 2021 doi: 10.1002/jmri.27703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong VW, Irles M, Wong GL, et al. Unified interpretation of liver stiffness measurement by M and XL probes in non-alcoholic fatty liver disease. Gut. 2019;68:2057–2064. doi: 10.1136/gutjnl-2018-317334. [DOI] [PubMed] [Google Scholar]
- 63.Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–1871. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 64.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 65.Yang X, Chang X, Wu S, et al. Performance of liver stiffness measurements obtained with FibroScan is affected by glucose metabolism in patients with nonalcoholic fatty liver disease. Lipids Health Dis. 2021;20 doi: 10.1186/s12944-021-01453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 67.Yoneda M, Mawatari H, Fujita K, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2008;40:371–378. doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 68.Pathik P, Ravindra S, Ajay C, Prasad B, Jatin P, Prabha S. Fibroscan versus simple noninvasive screening tools in predicting fibrosis in high-risk nonalcoholic fatty liver disease patients from western India. Ann Gastroenterol. 2015;28:281–286. [PMC free article] [PubMed] [Google Scholar]
- 69.Cassinotto C, Boursier J, de Lédinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 70.Cardoso AC, Cravo C, Calçado FL, et al. The performance of M and XL probes of FibroScan for the diagnosis of steatosis and fibrosis on a Brazilian nonalcoholic fatty liver disease cohort. Eur J Gastroenterol Hepatol. 2020;32:231–238. doi: 10.1097/MEG.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 71.Chan WK, Nik Mustapha NR, Wong GLH, Wong VWS, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017;5:76–85. doi: 10.1177/2050640616646528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 73.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637. doi: 10.1053/j.gastro.2015.11.048. e7. [DOI] [PubMed] [Google Scholar]
- 74.Lee HW, Park SY, Kim SU, et al. Discrimination of nonalcoholic steatohepatitis using transient elastography in patients with nonalcoholic fatty liver disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee JI, Lee HW, Lee KS. Value of controlled attenuation parameter in fibrosis prediction in nonalcoholic steatohepatitis. World J Gastroenterol. 2019;25:4959–4969. doi: 10.3748/wjg.v25.i33.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mikolasevic I, Domislovic V, Klapan M, et al. Accuracy of controlled attenuation parameter and liver stiffness measurement in patients with non-alcoholic fatty liver disease. Ultrasound Med Biol. 2021;47:428–437. doi: 10.1016/j.ultrasmedbio.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 77.Oeda S, Takahashi H, Imajo K, et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol. 2020;55:428–440. doi: 10.1007/s00535-019-01635-0. [DOI] [PubMed] [Google Scholar]
- 78.Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152:598–607.e2. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petta S, Wong VW, Cammà C, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145–1155. doi: 10.1002/hep.28843. [DOI] [PubMed] [Google Scholar]
- 80.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–163.e2. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trowell J, Alukal J, Zhang T, et al. How good are controlled attenuation parameter scores from fibroscan to assess steatosis, NASH, and fibrosis? Dig Dis Sci. 2021;66:1297–1305. doi: 10.1007/s10620-020-06269-4. [DOI] [PubMed] [Google Scholar]
- 82.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 83.Wong VW, Chan WK, Chitturi S, et al. Asia-pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 84.Berzigotti A, De Gottardi A, Vukotic R, et al. Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS One. 2013;8:e58742. doi: 10.1371/journal.pone.0058742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kjaergaard M, Thiele M, Jansen C, et al. High risk of misinterpreting liver and spleen stiffness using 2D shear-wave and transient elastography after a moderate or high calorie meal. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cournane S, Browne JE, Fagan AJ. The effects of fatty deposits on the accuracy of the Fibroscan® liver transient elastography ultrasound system. Phys Med Biol. 2012;57:3901–3914. doi: 10.1088/0031-9155/57/12/3901. [DOI] [PubMed] [Google Scholar]
- 87.Petroff D, Blank V, Newsome PN, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185–198. doi: 10.1016/S2468-1253(20)30357-5. [DOI] [PubMed] [Google Scholar]
- 88.Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–1723. doi: 10.1002/hep.22577. [DOI] [PubMed] [Google Scholar]
- 89.Petta S, Wai-Sun Wong V, Bugianesi E, et al. Impact of obesity and alanine aminotransferase levels on the diagnostic accuracy for advanced liver fibrosis of noninvasive tools in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2019;114:916–928. doi: 10.14309/ajg.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 90.Petta S, Maida M, Macaluso FS, et al. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology. 2015;62:1101–1110. doi: 10.1002/hep.27844. [DOI] [PubMed] [Google Scholar]
- 91.Fujimori N, Tanaka N, Shibata S, et al. Controlled attenuation parameter is correlated with actual hepatic fat content in patients with non-alcoholic fatty liver disease with none-to-mild obesity and liver fibrosis. Hepatol Res. 2016;46:1019–1027. doi: 10.1111/hepr.12649. [DOI] [PubMed] [Google Scholar]
- 92.Gu Q, Cen L, Lai J, et al. A meta-analysis on the diagnostic performance of magnetic resonance imaging and transient elastography in nonalcoholic fatty liver disease. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13446. [DOI] [PubMed] [Google Scholar]
- 93.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637. doi: 10.1053/j.gastro.2015.11.048. E7. [DOI] [PubMed] [Google Scholar]
- 94.Xiao GQ, Zhu SX, Xiao X, Yan LN, Yang JY, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 95.Petta S, Wong VWS, Camma C, et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther. 2017;46:617–627. doi: 10.1111/apt.14219. [DOI] [PubMed] [Google Scholar]
- 96.Selvaraj EA, Mozes FE, Jayaswal ANA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2021;75:770–785. doi: 10.1016/j.jhep.2021.04.044. [DOI] [PubMed] [Google Scholar]
- 97.de Lédinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–1031. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 98.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 99.Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199–208. doi: 10.1002/hep.24624. [DOI] [PubMed] [Google Scholar]
- 100.Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version) Ultraschall Med. 2017;38:E16–E47. doi: 10.1055/s-0043-103952. [DOI] [PubMed] [Google Scholar]
- 101.Cai C, Song X, Chen X, et al. Transient elastography in alcoholic liver disease and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Can J Gastroenterol Hepatol. 2021;2021 doi: 10.1155/2021/8859338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 104.Al Juboori A, Diaz-Arias A, Nasser M, et al. The utility of liver elastography in evaluation of liver fibrosis in obese patients undergoing bariatric surgery. Hepatology. 2018;68:975A. doi: 10.1002/hep.30257. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.