Abstract
Open-system electronic nicotine delivery systems (ENDS) permit modifications to device characteristics such as power, potentially increasing nicotine and toxicant delivery. Limiting liquid nicotine concentration may carry unintended consequences by prompting users to increase device power to increase nicotine delivery. This study examined the abuse liability of ENDS across nicotine concentration and power settings. In a clinical laboratory study, n = 19 exclusive ENDS users and n = 13 dual ENDS/cigarette users, aged 21–55 completed four Latin-square ordered conditions that varied by liquid nicotine concentration (10 mg/ml [low], 30 mg/ml [high]) and device power (15 watts [low], 30 watts [high]), that were followed by a fifth own brand (OB) condition. A progressive ratio task (PRT) using bar presses to earn ENDS puffs was used to assess abuse liability and compare between conditions using mixed effects linear regressions. The low nicotine/high watt condition was associated with a significantly higher number of bar presses and puffs earned relative to the OB ENDS, high nicotine/high watt, and high nicotine/low watt conditions (p < .05). Findings appeared to be driven largely by exclusive ENDS users; most comparisons were not significant among dual users. Participants worked significantly harder for puffs of low nicotine/high watt ENDS, highlighting previous findings that suggest limiting liquid nicotine concentration without addressing power settings may be insufficient to reduce the abuse liability of ENDS.
Keywords: electronic nicotine delivery systems, abuse liability, progressive ratio task, nicotine concentration, device power
Electronic nicotine delivery systems (ENDS) represent a tobacco product class consisting of a power source, a heating element, and a tank or reservoir containing a liquid solution consisting of solvents, flavorings, and nicotine (Breland et al., 2017; Hiler et al., 2017). Open-system ENDS permit modifications to device characteristics such as liquid nicotine concentration and power, potentially increasing nicotine and toxicant delivery (Chen et al., 2016; Talih et al., 2021). Nicotine is the dependence-inducing drug in tobacco products and facilitates ongoing use of tobacco products (Benowitz, 2010). Nicotine concentration in ENDS liquids typically ranges from 0 mg/ml to 36 mg/ml (Breland et al., 2017) but can reach higher concentration in some products (e.g., those containing nicotine salts; Talih et al., 2019). To address nicotine’s dependence-inducing effects and related public health concerns, the European Union (EU) has limited the nicotine concentration in ENDS liquids to 20 mg/ml through its Tobacco Products Directive (TPD), as have Iceland, Israel, Moldova, Saudi Arabia, and the United Kingdom (Bagley, 2014; Costa et al., 2014; European Commission, 2014; Government of Canada, 2021). Canada is also considering adopting a 20 mg/ml nicotine concentration limit for ENDS liquids (Government of Canada, 2021).
Limiting liquid nicotine concentration may lead to incomplete suppression of nicotine abstinence symptoms among users who then may be motivated to manipulate other features in open-system ENDS to increase nicotine delivery, including device power. Device power, assessed in wattage (W) is contingent on the voltage (V) of the power source and the resistance of the heating element which is assessed in ohms (Ω; Breland et al., 2017). Increasing the power of a device by altering one or more of its characteristics increases the amount of liquid solution aerosolized by the device. Thus with a given nicotine-containing solution, increased power is associated with increased nicotine yield, that is, the amount of nicotine that leaves the mouthpiece after puffing (Talih et al., 2015). Closed systems (e.g., cigalike devices) often have lower power than open systems (e.g., tank style devices; Breland et al., 2017); overall, ENDS currently available on the market generally range from 8 to 200 W (Rudy et al., 2017).
Because of the modifiable characteristics of open-system ENDS, limiting nicotine concentration without addressing other device-related factors implicated in nicotine delivery is problematic as users can modify device settings to change nicotine delivery, thus circumventing the regulatory intent in markets with open-system ENDS (Eissenberg et al., 2021). Previous clinical lab work among ENDS users demonstrates how varying ENDS power (40.5 W or 13.5 W) and liquid nicotine concentration (3 or 8 mg/ml) influences acute effects under directed and ad lib use procedures (Hiler et al., 2020). Findings here reinforced that increasing ENDS power increased nicotine delivery even with low nicotine concentration liquid. During ad lib use, participants also took more puffs and consumed more liquid during low nicotine concentration conditions (3 mg/ml). This finding is consistent with other clinical lab work where ENDS users of higher-powered devices used ENDS liquids with lower nicotine concentrations but consumed more liquid overall (Wagener et al., 2017). The effect of device power and liquid nicotine concentration on liquid consumption is concerning as users may be inadvertently increasing their exposure to chemicals found in ENDS aerosol, including carbonyl compounds such as formaldehyde (Talih et al., 2021).
Findings to date suggest that limiting nicotine concentration alone may be an insufficient regulatory approach to mitigate the harm potential of ENDS in markets where open-system devices are readily available. However, more information is needed on how liquid nicotine concentration and device power settings in combination influence the abuse liability, or the reinforcing value, of ENDS. The progressive ratio task (PRT) presents one method for measuring abuse liability of a drug by quantifying the degree to which an individual is willing to work to obtain this drug (Perkins et al., 1994). The PRT assesses a participant’s motivation to work for a reinforcer by requiring an escalating number of tasks/work to be performed to earn additional reinforcement. This task also captures the participant’s “breaking point,” that is, the “price” (or “work requirement”) at which the participant ceases to work for the reinforcer (Hodos, 1961). A substantial body of research in nonhuman animals has used the PRT to investigate the abuse liability of a spectrum of drugs, including cocaine, heroin, methylphenidate, and secobarbital (e.g., Duvauchelle et al., 1998; Griffiths et al., 1975). Research with human subjects is available as well; past literature has used the PRT as an index of abuse liability for drugs including, but not limited to methylphenidate and d-amphetamine, heroin, and caffeine (Comer et al., 1999; Griffiths et al., 1989; Stoops et al., 2004).
The PRT has also been previously used in the context of tobacco use. An in-lab within-subjects study evaluated the reinforcing effects of nicotine among smokers of combustible cigarettes by comparing the reinforcing value of nicotine-containing cigarettes to those of denicotinized cigarettes, as indexed by earning cigarette puffs via plunger pulls (Shahan et al., 1999). In order to obtain participants’ peak response rate and the breakpoint, the PR schedule was not reset but instead increased after each session, in order to be continued with greater response requirements in the next session (Shahan et al., 1999). When the two products were presented to participants one at a time on a PR schedule that increased across sessions, participants exerted equal effort to earn either product (Shahan et al., 1999). However, when the two cigarette types were presented concurrently, participants worked significantly harder to earn puffs off the nicotine-containing cigarette (Shahan et al., 1999). Some research suggests that nicotine deprivation influences motivation to work for cigarette puffs. For example, an in-lab, within-subjects study utilizing a PRT indexed with space bar presses found that nicotine-abstinent smokers worked harder for cigarette puffs relative to their nonnicotine-deprived counterparts (Rusted et al., 1998).
While cigarettes have been the subject of these previous investigations, comparably little human subjects research has investigated the reinforcing value of ENDS on use behaviors using the PRT paradigm. Previous preclinical work has tested the reinforcing value of various constituents of smoke and different tobacco products, including ENDS aerosol using PR schedules of reinforcement with mixed results regarding the contribution of nonnicotine chemicals of ENDS aerosol (Marusich et al., 2019; Harris et al., 2020). Clinical research using a within-subjects design compared the reinforcing value of flavored versus unflavored puffs from a nicotine-containing ENDS, where participants worked for flavored ENDS puffs on a PR schedule while nonflavored ENDS puffs remained on a fixed ratio schedule (Audrain-McGovern et al., 2016). Participants were willing to work harder for flavored ENDS puffs relative to nonflavored ENDS puffs (Audrain-McGovern et al., 2016). Another clinical lab study assessed the abuse liability of ENDS with and without nicotine, a nicotine inhaler, and an own brand (OB) cigarette in cigarette smokers naive to ENDS using a different behavioral task, the multiple-choice procedure (MCP; Maloney et al., 2019). The outcome indexing reinforcing value (i.e., crossover point) for the nicotine-containing ENDS was significantly greater than the nicotine inhaler, not significantly different from the nonnicotine-containing ENDS, and significantly lower than OB cigarette smoking. Given the appeal of highly modifiable ENDS to novel and established ENDS users (Barrington-Trimis et al., 2020) the effects of varying liquid nicotine concentration in combination with different ENDS device power settings on ENDS abuse liability deserves investigation.
The aim of the present study was to investigate how combinations of liquid nicotine concentrations and device power settings influence users’ performance on the PRT using an in-lab, within-subjects design. Participants in this study were either exclusive ENDS users or dual users of combustible cigarettes and ENDS. Responses to tobacco product or market modifications may differ between dual and exclusive ENDS users—potentially due to differences in their risk profiles (Ali et al., 2016), the appeal of substitute products (Quisenberry et al., 2017), or motivations for ENDS use (Harrell et al., 2015) between these two groups. Consequently, understanding if and how responses to ENDS regulations differ between dual and exclusive ENDS users is important to projecting the real-world impact of proposed regulations limiting nicotine concentration. We hypothesized that the abuse liability of ENDS as indexed by the PRT would be decreased by lowering nicotine content, but this decrease would be offset by increasing device power. Results of this work were aimed to predict how ENDS users may respond to regulations limiting liquid nicotine concentration in markets where open-system ENDS are available.
Method
Sample
The institutional review board (IRB) at Virginia Commonwealth University approved our study titled “Effects of E-Cigarette Power and Nicotine Content in Dual Users and Vapers” (HM20012696). Potential participants were recruited by IRB-approved advertisements (e.g., flyers posted in community locations, social media) posted in the Richmond, VA area, and word-of-mouth. Participants who completed all sessions could receive up to $500.
Informed consent was obtained from all participants, and the screening was conducted by research assistants. Sixty-six participants consented to the full study, 47 were enrolled, 36 completed all study activities, and 32 participants were included in the present analyses. Included participants were 19 exclusive ENDS users and 13 users of cigarettes and ENDS (“dual users”), aged 21–55. Exclusive ENDS users reported daily ENDS use (≥3 mg/ml nicotine) and no past month cigarette use. Dual users reported either (a) daily use of ENDS (≥3 mg/ml nicotine) and some day use of tobacco cigarettes (≥3 days per week) or (b) someday use of ENDS (i.e., ≥3 mg/ml nicotine ≥3 days per week) and daily use of tobacco cigarettes. Both groups had a semiquantitative urine cotinine result of “positive” at screening (at least 200 ng/mL; urine cotinine cassette test). Eligible participants were healthy, as defined by no self-reported diagnoses or current medical conditions including, but not limited to, conditions of the heart, lungs, kidney, liver, respiratory system, or immune system. Eligible participants also did not self-report diagnosed and current psychiatric conditions or related treatment (e.g., psychotropic medication use). Participants who were pregnant or breastfeeding, reported past month use of illicit drugs, reported >15 days out of the last 30 of marijuana use, or reported >25 days out of the past 30 for alcohol use were excluded. Those who intended to quit tobacco/nicotine products in the next 30 days were also excluded and referred to treatment resources. The study is registered on clinicaltrials.gov (NCT03830892).
Study Design and Materials
Following in-person screening participants completed five sessions spaced at least 48 hr apart after having abstained from tobacco products for 12 hr prior to the start of each session. The first four single-blind sessions were Latin-square ordered and involved the use of one condition-specific ENDS each; the fifth and final session was an OB ENDS session for all conditions. The four ENDS conditions varied in liquid nicotine concentration (below the EU TPD limit: 10 mg/ml; above TPD limit: 30 mg/ml) and device power (15 vs. 30 watts): (a) low nicotine (10 mg/ml)/low watt (15 watts), (b) low nicotine (10 mg/ml)/high watt (30 watts), (c) high nicotine (30 mg/ml)/low watt (15 watts), (d) high nicotine (30 mg/ml)/high watt (30 watts). Device power levels were selected based on previous work (Hiler et al., 2020; Wagener et al., 2017). Of note, while a nicotine concentration of 30 mg/ml may be perceived as high, liquids that exceed this concentration are common in today’s marketplace (Breland et al., 2017), particularly those that are nicotine salt-based as was used in this study (e.g., 5% JUUL pod, 69 mg/ml; Talih et al., 2019). The OB ENDS condition served as a positive control, benchmarking abuse liability to facilitate comparisons of ENDS nicotine content and device power settings.
All liquids were labeled as 30% propylene glycol (PG)/70% vegetable glycerin (VG) and purchased locally (AVAIL Vapor, Richmond, VA). Nicotine content was protonated (i.e., salt form) and confirmed by an independent laboratory prior to use. The ENDS device was a variable watt KangerTech Subox Mini-C with a KangerTech Subtank Mini-C (3 ml capacity) and KangerTech SSOCC Nichrome Coil (0.5 ohm; all KangerTech, Shenzhen, China). Coil resistance was measured prior to use in all sessions. Liquids were available in four flavors (tobacco [Jamestown], menthol [Arctic Blast], dessert [Rift], or fruit [Mardi Gras]). At screening, participants were allowed to sample 0 mg/ml liquid at the same PG/VG ratio using the same ENDS materials at 30 W and choose one liquid flavor for use during the experimental ENDS sessions.
For the OB session, exclusive ENDS users used their OB ENDS device and liquid (prepurchased by the study staff), and dual users were randomized to use either their OB ENDS device/liquid (n = 13) or tobacco cigarette (n = 4). Importantly, due to a small sample size attributable to Coronavirus disease (COVID-19) precipitating lab closures, dual users randomized to use their tobacco cigarette in the OB session were not included in these analyses. The results reported here only present findings for those dual users that used their OB ENDS during the OB session. Thus, the final sample size of the study was 32 (19 exclusive ENDS users and 13 dual users). While this report focuses exclusively on the results from one behavioral assessment of abuse liability, the PRT, during the course of each session participants also completed drug purchase tasks (DPT; Reed et al., 2020) and a MCP (Griffiths et al., 1993).
Measures
The PRT was used to assess abuse liability. This task initially required participants to press the space bar on a computer four times to earn one puff of the session-specific tobacco product, which was provided immediately. For each subsequent puff, the PRT required the participant to “work” twice as hard, that is, after each reward (a single puff) the work required to earn another puff was doubled, such that the second puff required eight space bar presses, the third puff required 16 space bar presses, and so on for each successive puff (Rusted et al., 1998; Willner et al., 1995). The task ended after the participant failed to press the space bar for a period of 5 min (Perkins et al., 2002; Rusted et al., 1998; Willner et al., 1995). The principal outcome measures from this task were the total number of responses entered (i.e., number of space bar presses the participant entered) and the total number of reinforcers earned (i.e., number of ENDS puffs the participant earned during the course of the task). The PRT was administered using a computer program written in QBasic (available upon request).
Nicotine abstinence symptoms also were assessed using an adapted form of the Minnesota Nicotine Withdrawal Scale (13 items; Hughes & Hatsukami, 1986) and measured using a Visual Analog Scale anchored at 0 = Not at all and 100 = Extremely, before and after each behavioral measure (e.g., the PRT).
Procedure
Recruitment and In-Person Screening
Potentially eligible individuals were identified after responding to an online/phone prescreening survey. These individuals were then asked to attend an in-person screening to confirm eligibility criteria. Participants also completed other measures to assess tobacco-related risk perceptions, discounting, and risk-taking behavior. Liquid flavor preference was also assessed (as described above).
Clinical Laboratory Session Structure
The structure for each session is depicted in Figure 1. At the start of each session, 12-hr abstinence from nicotine/tobacco was biochemically verified by assessing exhaled carbon monoxide (CO; Vitalograph, Lenexa, Kansas, USA). CO cutoffs were set at ≤10 ppm for dual users and ≤7 ppm for exclusive ENDS users as CO is a byproduct of cigarette use not ENDS, and previous research has identified higher CO in dual users than in exclusive ENDS users (Carroll et al., 2018). Participants were asked about any physical symptoms (e.g., cough, shortness of breath, chest pain, nausea, fatigue, and fever) they had experienced since their last visit (used to determine relative safety to continue in study). Thirty minutes after session onset, participants sampled the session-specific product (two puffs; Audrain-McGovern et al., 2016) followed by a 1-hr rest period. This rest period was used to induce nicotine abstinence prior to conducting any behavioral assessments. After the rest period, subjective measures were completed and then either PRT or the DPT/MCP tasks were completed. The order of these abuse liability tasks (PRT-DPT/MCP or DPT/MCP-PRT) was counterbalanced across participants and sessions to control for the effects of completing one task set prior to the other. Following the first counterbalanced abuse liability task, participants completed a second subjective assessment which was followed by another 1-hr rest period to reestablish nicotine abstinence. After this second 1-hr rest period, participants completed a third subjective assessment followed by the second counterbalanced abuse liability task and one final set of subjective measures.
Figure 1.
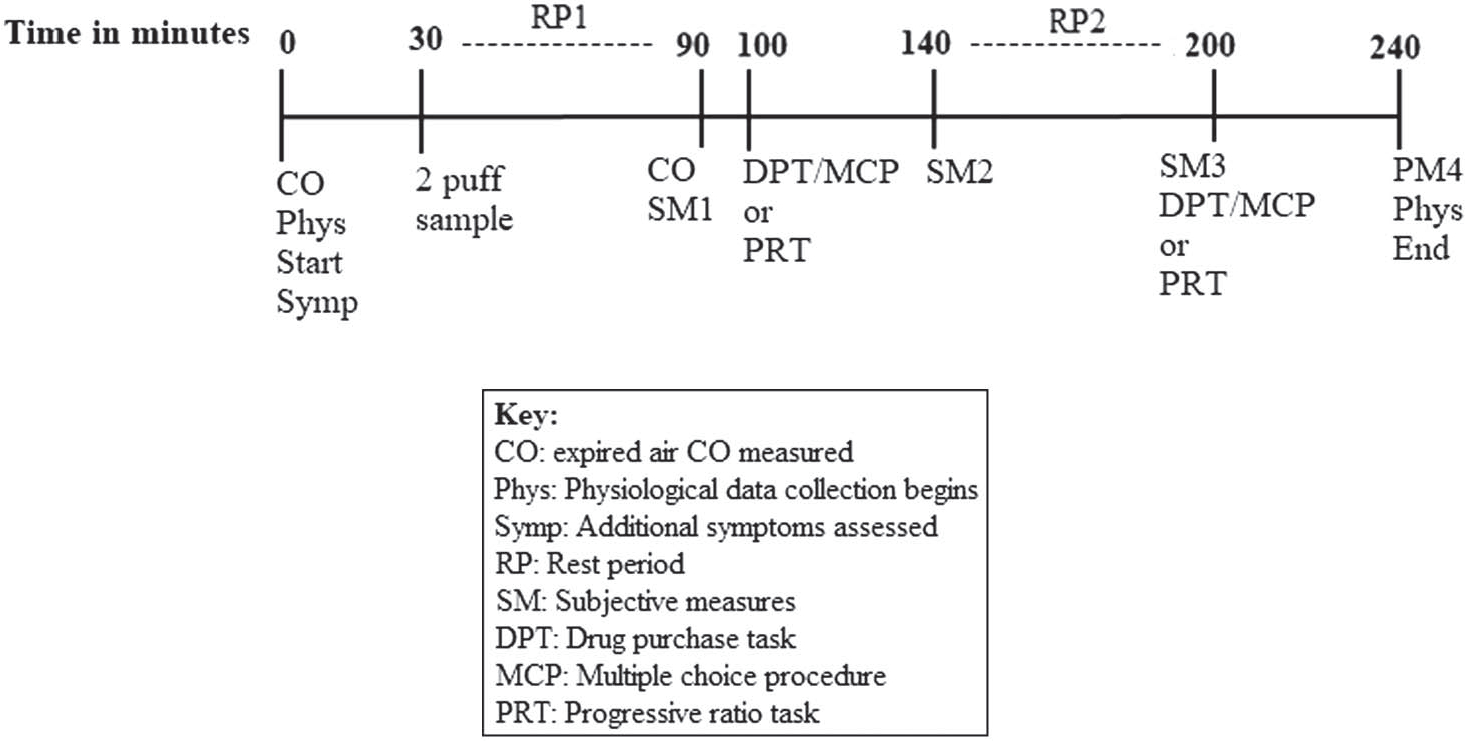
Clinical Laboratory Session Timeline
We examined whether task order affected the primary PRT outcomes (responses entered and reinforcers earned) initially using t-tests. Across conditions, participants who completed the PRT first (Nsessions = 77) during sessions earned significantly more reinforcers (Mean [M] = 5.26, Standard Deviation [SD] = 1.58, vs. M = 4.48 [SD = 1.77] reinforcers earned, p = .004) and entered significantly more responses (M = 252.75 [SD = 261.80] vs. M = 170.42 [SD = 209.42] bar presses, p = .029) compared to participants randomized to complete the PRT second (Nsessions = 83; see Table S1). However, we did not expect that task order would systematically alter our primary conclusions as task order was counterbalanced across conditions (confirmed using a chi-squared test finding no difference in task order across conditions; χ2 = 0.65, p = .96).
To provide further context on how the experimental design may have affected indices of abuse liability, we also examined how nicotine abstinence symptoms changed following the PRT as well as the 1-hr waiting period that occurred between the first and second abuse liability task assessments as validity checks (see Supplemental Results and Table S2 for details). Results suggest that (a) reinforcers earned during the PRT suppressed nicotine abstinence symptoms and (b) the 1-hr rest period between tasks induced nicotine abstinence symptoms.
Data Preparation and Analysis
Chi-square tests and Fisher’s exact tests were used to identify differences in baseline characteristics between exclusive and dual users. For each PRT outcome, differences between experimental ENDS conditions and the OB ENDS condition were assessed using mixed effects linear regressions with robust standard errors, a flexible and efficient approach (Gueorguieva & Krystal, 2004) that has been used in other studies comparing the abuse liability of tobacco products (Barnes et al., 2017; Bono et al., 2020; Karelitz & Perkins, 2021). Pairwise differences between each experimental ENDS condition were then assessed with Wald tests. Predicted means (PM) and robust standard errors (SE) from these models are presented to facilitate comparisons between conditions, although due to our model structure and balanced data, PM are identical to sample means. Analyses were conducted using the entire sample as well as stratified by cigarette smoking status. Additionally, as repeated measures analysis of variance (ANOVA) represents a common alternative estimation approach to mixed effects linear regressions in within-subjects laboratory studies, results of the main effects and global tests of significance were compared between the two approaches and were found to be consistent.
In sensitivity analyses, independent t-tests examined the potential influence of three ENDS characteristics on PRT responses: Liquid flavor chosen for experimental conditions, concordance of flavor chosen for experimental conditions and OB liquid flavor, and nicotine concentration and watt of OB ENDS (categorized as “lower” nicotine/“higher” watt or “higher” nicotine/“lower” watt; see Supplemental Materials). To examine the influence of task order, we also conducted a sensitivity analysis controlling for task order. All analyses were conducted in Stata version 15.1 (Statacorp, College Station, Texas, USA) and used a significance threshold of p < .05.
Results
Characteristics
Of the 32 included participants, 19 were exclusive ENDS users and 13 were dual users (see Table 1). Participants were, on average, 28 years old. Most participants were men (62%), White (66%), had received at least some college education (78%), and used ENDS every day (94%). Chi-square tests and Fisher’s exact tests indicated that exclusive ENDS users and dual users did not differ significantly on most demographic or tobacco use characteristics, including ENDS dependence levels (p > .05).
Table 1.
Characteristics of Dual Cigarette and ENDS Users and Exclusive ENDS Users (N = 32)
| Characteristic | Full sample N = 32 | Dual users N = 13 | Exclusive users N = 19 | p value |
|---|---|---|---|---|
|
| ||||
| Demographics and substance use | ||||
| Age (years), M (SD) | 28.1 (7.2) | 29.0 (6.8) | 27.5 (7.5) | .577 |
| Gender (female), N (%) | 12 (37.5) | 6 (46.2) | 6 (31.6) | .403 |
| Race, N (%) | .637* | |||
| White | 21 (65.6) | 8 (61.5) | 13 (68.4) | |
| Black/African American | 6 (18.8) | 3 (23.1) | 3 (15.8) | |
| Asian | 2 (6.3) | 1 (7.7) | 1 (5.3) | |
| Middle Eastern | 1 (3.1) | 1 (7.7) | 0 (0.0) | |
| Multiple races | 2 (6.3) | 0 (0.0) | 2 (10.5) | |
| Hispanic/Latino, N (%) | 2 (6.3) | 1 (7.7) | 1 (5.3) | .655* |
| Education, N (%) | .803* | |||
| High school graduate/GED | 7 (21.9) | 2 (15.4) | 5 (26.3) | |
| Some college | 17 (53.1) | 8 (61.5) | 9 (47.4) | |
| College graduate or higher | 8 (25.0) | 3 (23.1) | 5 (26.3) | |
| Past 30-day alcohol use (days, N = 31), M (SD) | 5.6 (5.0) | 2.8 (3.2) | 7.3 (5.3) | .013 |
| Past 30-day cannabis use (days, N = 24), M (SD) | 1.6 (3.1) | 1.3 (3.5) | 1.8 (3.0) | .719 |
| ENDS characteristics | ||||
| ENDS use per week (days), M (SD) | 6.9 (0.5) | 6.7 (0.8) | 7 (0.0) | .082 |
| ENDS history (months), M (SD) | 22.9 (25.2) | 15.2 (21.7) | 28.2 (26.5) | .155 |
| Penn State ENDS dependence score, M (SD) | 11.6 (3.7) | 11.3 (4.1) | 11.7 (3.5) | .754 |
| PROMIS-E, M (SD) | 8.6 (3.2) | 8.8 (2.9) | 8.4 (3.4) | .716 |
| Own brand liquid primary flavor, N (%) | .151* | |||
| Tobacco | 2 (6.3) | 1 (7.7) | 1 (5.3) | |
| Menthol | 4 (12.5) | 2 (15.4) | 2 (10.5) | |
| Fruit | 17 (53.1) | 9 (69.2) | 8 (42.1) | |
| Dessert | 9 (28.1) | 1 (7.7) | 8 (42.1) | |
| Flavor chosen for experiment, N (%) | .529* | |||
| Tobacco | 3 (9.4) | 0(0) | 3 (15.8) | |
| Menthol | 9 (28.1) | 4 (30.8) | 5 (26.3) | |
| Fruit | 14 (43.8) | 7 (53.9) | 7 (36.8) | |
| Dessert | 6 (18.8) | 2 (15.4) | 4 (21.1) | |
| Flavor concordance (concordant)b, N (%) | 22 (68.8) | 9 (69.2) | 13 (68.4) | .636* |
| OB ENDS power and nicotine concentration, N (%)a | .633* | |||
| “Higher” nicotine/“lower” watt | 15 (46.9) | 7 (53.9) | 8 (42.1) | |
| “Lower” nicotine/“higher” watt | 15 (46.9) | 6 (46.2) | 9 (47.4) | |
| Other | 2 (6.3) | 0 (0) | 2 (10.5) | |
| Cigarette characteristics | ||||
| Menthol preference (menthol), N (%) | — | 8 (61.5) | — | — |
| Cigarettes per day (cigarettes), M (SD) | — | 10.2 (11.9) | — | — |
| Smoking history (months), M (SD) | — | 80.5 (96.6) | — | — |
| Age of smoking initiation (years), M (SD) | — | 17.8 (1.9) | — | — |
| FTND summary score—Cigarette, M (SD) | — | 2.8 (3.1) | — | — |
Note. GED = tests of general educational development; ENDS = electronic nicotine delivery systems; OB = own brand; PROMIS= patient-reported outcomes measurement information system nicotine dependence items for E-Cigarettes; FTND = fagerstrom test for nicotine dependence. Dual cigarette and ENDS users reported daily use of ENDS (≥3 mg/mL nicotine) or tobacco cigarettes (any amount) AND someday use (≥3 days/week) of ENDS (≥3 mg/mL nicotine) or tobacco cigarettes (any amount) for the past 3 months or longer. Exclusive ENDS users report daily use of ENDS (≥1 mL of ENDS liquid or approximately 1 pod or cartomizer per day; ≥3 mg/mL nicotine) or the past 3 months or longer and no cigarette use.
“Lower” nicotine was defined as ≤10 mg/mL while “highef’ nicotine was defined as >10 mg/mL. Devices at >16 watts were considered “highef’ watt and devices at ≥16 watts were considered “lowef ’ watt.
Liquid flavor concordance is defined as choosing the same flavor category (tobacco, menthol, fruit, or dessert) for liquid used in the experimental ENDS conditions as used in the OB ENDS condition.
p values not marked with an asterisk are derived from chi-squared tests or t-tests examining differences between dual users and exclusive ENDS users; p values marked with an asterisk indicate that Fisher’s exact test was used due to cell sizes ≤5.
Effects of ENDS Power and Nicotine Concentrations
Participants earned significantly more reinforcers (i.e., ENDS puffs) during the low nicotine/high watt condition (predicted mean [PM] = 5.50, standard error [SE] = 0.28) than the OB ENDS (PM = 4.78, SE = 0.27), high nicotine/high watt (PM = 4.66, SE = 0.27), high nicotine/low watt (PM = 4.50, SE = 0.33), and low nicotine/low watt (PM = 4.84, SE = 0.30) conditions (p < .05, see Figure 2A). Similarly, the low nicotine/high watt condition was associated with significantly more responses (bar presses; PM = 303.53, SE = 55.80) relative to the OB ENDS (PM = 176.50, SE = 31.11, p < .01), high nicotine/high watt (PM = 199.56, SE = 45.88, p < .05), and high nicotine/low watt conditions (PM = 170.88, SE = 32.27, p < .001; see Figure 2B).
Figure 2.
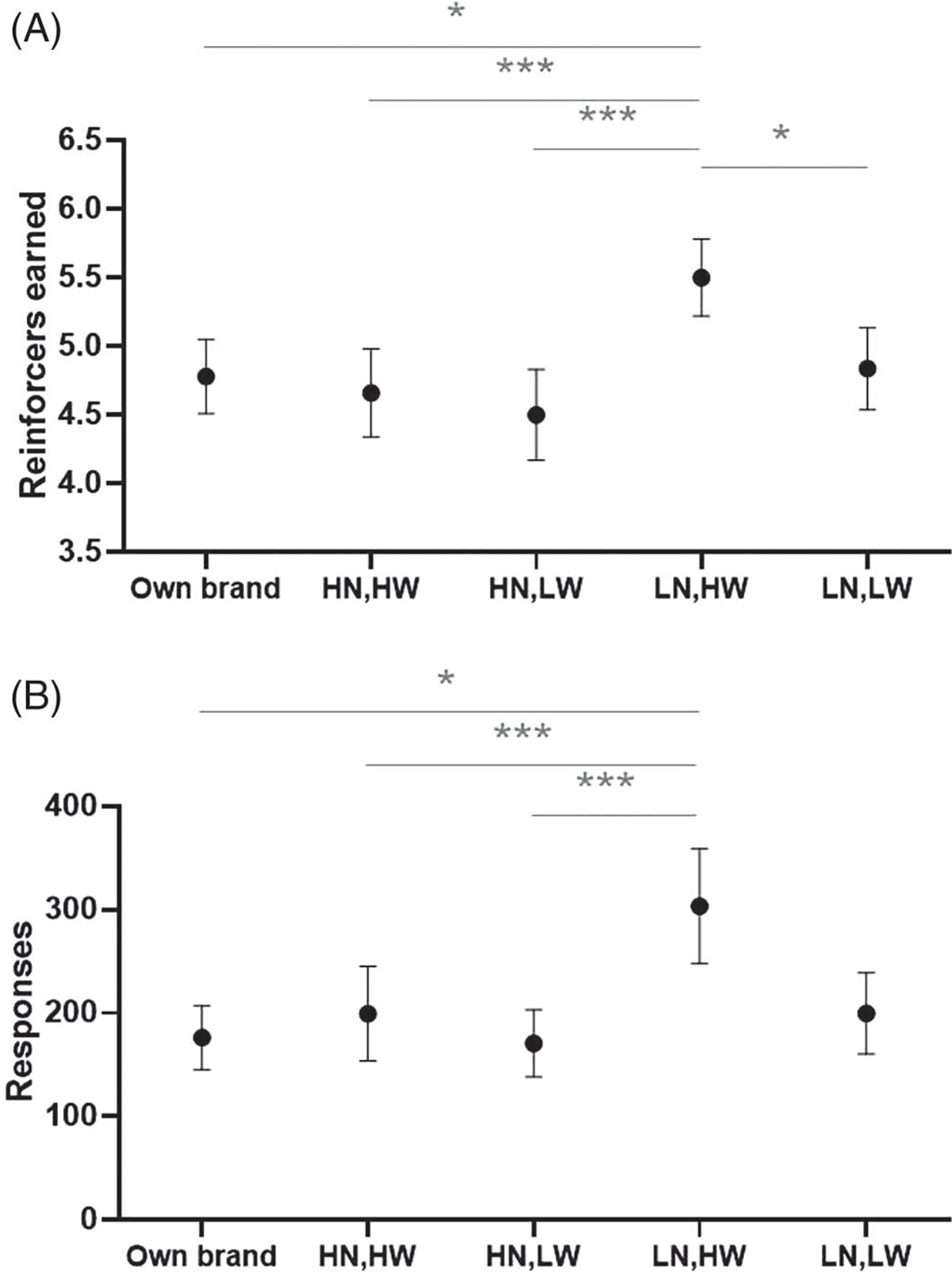
Reinforcers Earned And Responses Entered Across Conditions
Note. ENDS = electronic nicotine delivery systems. Predicted mean (standard error) number of reinforcers earned (Panel A) and number of responses entered (Panel B) in the progressive ratio task, by ENDS nicotine concentration and wattage (N = 32). Significant differences between two experimental conditions are indicated by a horizontal bar notated with * (p < .05) or *** (p < .001).
Stratified analyses revealed potential differences between dual users and exclusive ENDS users. Both groups earned significantly more reinforcers in the PRT when using the low nicotine/high watt ENDS relative to the high nicotine/high watt ENDS (p < .05, see Table S3). However, exclusive ENDS users also earned more reinforcers (PM = 5.58, SE = 0.38) and entered more responses (PM = 331.16, SE = 79.04) in the low nicotine/high watt condition compared with the high nicotine/low watt condition (reinforcers earned: PM = 4.57, SE = 0.41, p < .01; responses entered: PM = 174.95, SE = 43.63, p < .01) and the OB ENDS condition (reinforcers earned: PM = 4.94, SE = 0.35, p < .05; responses entered: PM = 198.95, SE = 45.19, p < .05).
Sensitivity Analyses
When comparing participants who chose tobacco or menthol-flavored liquid for experimental ENDS conditions who with those chose fruit- or dessert-flavored liquid, no differences in the number of reinforcers earned or responses entered were observed (p > .05, see Table S4). Over two-thirds of participants chose a flavor to use in the experimental ENDS conditions that was concordant with their OB liquid flavor (e.g., fruit-flavored liquid used in experimental ENDS conditions and fruit-flavored liquid used in OB ENDS condition). Similarly, no significant differences in PRT outcomes emerged between those who chose a flavor for experimental ENDS conditions that was concordant with the flavor used in their OB ENDS condition and those who did not. Participants’ OB ENDS also varied by type (i.e., open vs. closed systems), brand, device power setting (i.e., watts), and liquid nicotine concentration. However, all but two participants’ OB ENDS fell into one of two broad categories: “higher” nicotine/“lower” watt or “lower” nicotine/“higher” watt. No difference between these two OB ENDS categories for PRT outcomes in the experimental conditions were observed (see Table S5). Lastly, the pattern of results including differences between conditions was not sensitive to task order effects (when included as a covariate) as compared to the unadjusted results (see Table S6). Together, these findings suggest that PRT performance was not affected by liquid flavor, OB ENDS liquid nicotine concentration and power setting, or task order.
Discussion
More investigative efforts are needed to clarify whether the public health intent of liquid nicotine concentration limits imposed by regulators, as in the EU TPD, can be subverted via increasing ENDS device power to increase nicotine delivery. We tested how pairing of ENDS liquid solutions varying in nicotine concentration with different device power settings influences a product’s abuse liability among dual users and exclusive ENDS users. For this examination, we utilized the PRT, a sensitive measure previously used to quantify the effort smokers were willing to exert in order to earn cigarette puffs (e.g., Rusted et al., 1998).
Our results indicated participants worked to earn more ENDS puffs (i.e., reinforcers) in the low nicotine/high watt condition relative to other conditions including the low nicotine/low watt, high nicotine (either watt), and OB ENDS conditions. These differences suggest that abuse liability for ENDS products with low nicotine concentration liquid may be increased when they are paired with high device power. This pattern of results also highlights how limiting nicotine concentration without accounting for other device-related factors, as has been done in the EU (TPD, ≤20 mg/ml; Bagley, 2014; Costa et al., 2014), may be insufficient to reduce ENDS abuse potential. In fact, regulations that ignore device power may have the inadvertent effect of potentially exposing users to increased levels of toxicants due to increased ENDS liquid consumption when using lower nicotine concentration liquids (Dawkins et al., 2018; Hiler et al., 2020). Despite these concerns, efforts resembling the TPD in the EU are underway in the U.S. and elsewhere (Government of Canada, 2021; Nedelman, 2019).
Participants did not work harder to obtain puffs from their OB ENDS or the high nicotine conditions compared to other conditions. This finding was unexpected as one might assume preferred ENDS and/or those with higher nicotine concentration to result in more effective nicotine delivery and therefore greater reinforcing capability. One explanation may be that the nicotine delivery profile of these conditions actually may have induced nicotine-related satiety more quickly following earned puffs during the PRT compared to the low nicotine/high watt condition. On the other hand, participants did not work harder to obtain puffs of the low nicotine/low watt ENDS, which was presumed to have less effective nicotine delivery than all other ENDS on a per-puff basis, suggesting that the lower willingness to work observed in this condition was not a function of reaching nicotine-related satiety. Instead, the low nicotine/low watt ENDS may have been such a poor reinforcer that participants did not consider it worth the effort required for the PRT. While this study did not assess the pharmacokinetic profile of nicotine and wattage conditions, following study completion we used a published web-based mathematical model (Talih et al., 2017) to determine their nicotine flux (i.e., nicotine emitted from the mouth-end of the ENDS per second): low nicotine/low watt = 30.3 μg/s, low nicotine/high watt = 60.6 μg/s, high nicotine/low watt = 90.6 μg/s, and high nicotine/high watt = 181.2 μg/s. These differences in calculated nicotine flux align with the idea that the low nicotine/low watt condition was less reinforcing than the low nicotine/high watt condition—the product closest to cigarettes in nicotine emitted per second (51.4–90.0 μg/s; Armitage et al., 1988)—while conditions with higher nicotine concentrations, regardless of wattage, may lead to quicker nicotine satiety. Regulation that balances the relative risks of ENDS nicotine delivery and liquid consumption will require additional study to promote optimal public health benefit.
These findings and others (Audrain-McGovern et al., 2016; Hiler et al., 2020) support the idea that the nicotine delivery profile and associated nicotine abstinence symptom suppression are not the only characteristics related to ENDS abuse liability. Consistent with the present study’s result, a high power ENDS (40.5 W) paired with 3 mg/ml liquid nicotine was rated as significantly more pleasant and satisfying by experienced ENDS users relative to when paired with higher nicotine concentration liquid (8 mg/ml) despite higher nicotine delivery observed for the latter condition (Hiler et al., 2020). Preferences for the low nicotine (3 mg/ml)/high watt (40.5 W) condition (Hiler et al., 2020) were hypothesized to be related to greater visible aerosol production, positive sensations of the aerosol produced, and less “throat hit” self-reported by participants. While these attributes were unassessed in the present study, they, along with the nicotine flux profile, may also help explain the greater abuse liability observed for the low nicotine/high watt condition. Nonetheless, this study offers new evidence suggesting that reductions in abuse liability due to limiting nicotine concentration may be offset by user-controlled device characteristics, like power settings, in markets where open-system devices are available (as observed among ENDS users in the EU; Smets et al., 2019).
When stratified by cigarette smoking status, effects were consistent for both groups between the low nicotine/high watt and high nicotine/high watt conditions, but differences between the low nicotine/high watt and other conditions were only observed for exclusive ENDS users. This finding suggests exclusive ENDS users were more sensitive to condition-related differences compared to dual users. While these groups were similar in terms of baseline characteristics, ENDS dependence, and OB ENDS liquid/device power, there may have been other unassessed factors that influenced responsiveness. In addition, there was considerable variability within each user group for OB ENDS liquid flavors, nicotine concentration, and device characteristics. One potential explanation is that exclusive ENDS users were more sensitive to the ENDS nicotine content and/or aerosol characteristics due to their sole use of this inhaled tobacco product. Past research has suggested that dual users perceive their OB ENDS as significantly less rewarding relative to their OB cigarettes and report less craving suppression after use (St. Helen et al., 2020). Future work should recruit larger and more heterogeneous groups of ENDS users to assess how ENDS use history and product preferences influence measures of abuse liability.
In terms of sensitivity analyses, nonsignificant findings related to flavor selected may not be surprising given participants mostly chose their study product flavor to match their OB ENDS liquid flavor; given broad availability of flavors on the ENDS market, it is likely that their usual liquid flavor is already the flavor most reinforcing to them. The lack of significant effects noted between OB ENDS nicotine/power categories suggests that familiarity with a “lower” nicotine/“higher” watt ENDS product (e.g., SMOK Mag Baby, VOOPOO Alpha One) did not influence condition-related effects. Individuals who preferred “higher” nicotine/“lower” watt ENDS (e.g., JUUL, Suorin Drop) displayed a similar results pattern with more reinforcers earned and more responses entered for the low nicotine/high watt condition.
Our findings also have implications for the design of future research using multiple reinforced behavioral tasks in the same session. We found that across all conditions, those who completed the PRT first worked significantly harder and earned significantly more puffs relative to participants who completed the PRT second. Although task order did not appear to influence our primary conclusions regarding differences between ENDS conditions, the significant differences in experimental outcomes by task order support the idea of holding task order constant to reduce the influence of participant fatigue and/or nicotine satiety. Relatedly, completion of the PRT resulted in significant declines in self-reported ratings of “Urges to use an e-cigarette” and “Irritability/frustration/anger” in all conditions except for the low nicotine/low watt condition for the latter item. These effects suggest that despite differences in hypothesized nicotine delivery profile all conditions produced measurable nicotine abstinence symptom suppression (as in Dawkins et al., 2018; Hiler et al., 2020). The 1-hr rest period used to induce nicotine abstinence symptoms between tasks was also effective increasing these two self-reported nicotine abstinence symptoms. Future work using the PRT should include measures such as these to help understand the role of nicotine satiety and abstinence symptomology on task responding.
Limitations
Our study had several limitations, including that the study was stopped prematurely due to the COVID-19 pandemic. A larger study sample may have yielded additional statistical power to detect other significant findings including possible differences between OB cigarettes, OB ENDS, and nicotine content/power setting conditions among dual users. Testing additional nicotine doses and wattages could also yield more fine-grained insights regarding the interaction of nicotine concentration and device power. Additionally, a single study product was available to participants on a PRT schedule during each session. Extending the PRT task by making one product available on a PRT schedule while offering another at a fixed ratio schedule could have provided closer approximation to tobacco markets by assessing how exclusive ENDS and dual users substitute between their OB and session-specific products as one becomes harder to obtain (e.g., due to tobacco product regulations). By not controlling for puff duration/volume during the PRT, we introduced another source of variability between and within participants. Experienced ENDS users typically perform longer puffs resulting in greater volume (Hiler et al., 2017). Greater puff volume allows for more nicotine to be delivered to the user which in turn can produce more effective suppression of abstinence symptoms (Hiler et al., 2017). It is possible that less experienced participants may have engaged in compensatory puffing while more experienced ENDS users could have obtained nicotine more effectively from the study products. Differences in puff volume also may have occurred as a result of participants adjusting to different nicotine concentration/power combinations during each condition. Future work also may consider pretesting conditions using a fixed ratio schedule to assess the number of puffs needed by a product to provide satiety or satisfaction. Lastly, the rapidly escalating PR schedule used in our study may have discouraged participants from working for reinforcers who may have continued to perform presses if the reinforcers had been available on a PR schedule that escalated more slowly. Thus, our PR schedule may have been less sensitive to participants’ potentially ongoing motivation to earn puffs than an alternative PR schedule could have been.
Conclusions
Regulatory steps have been taken to curb the abuse liability of ENDS, including limiting nicotine concentration. However, initial evidence suggests restricting nicotine concentration without regulating device power may be insufficient given compensatory puffing and subsequent self-administration of increased amounts of harmful toxicants (Dawkins et al., 2018; Wagener et al., 2017). Our study adds to this emerging body of research and finds participants’ willingness to work for puffs of ENDS liquid concentrations below current TPD limits could be increased by increasing device power. While more evidence is needed, regulatory efforts concerned with the abuse liability of ENDS should consider regulation of other device characteristics in tandem with liquid nicotine concentration in markets with open-system devices or consider restricting sales of ENDS to closed-system devices.
Supplementary Material
Public Health Significance.
Several countries have current policy in place or are considering limiting the nicotine concentration found in the liquid solution of electronic nicotine delivery systems (ENDS) to decrease their abuse liability. This study suggests that limiting liquid nicotine concentration without regulating ENDS device power may be insufficient as abuse liability for low nicotine ENDS increases if paired with greater device power.
Acknowledgments
Funding was provided by the National Institute on Drug Abuse of the National Institutes of Health (NIH) and the Center for Tobacco Products of the U.S. Food and Drug Administration (FDA; grant number U54DA036105). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA. The only role of the funding source was the provision of financial support. We have no conflicts of interest to disclose. All authors have significantly contributed to the manuscript; the manuscript has been read and approved by all authors and is not under review elsewhere. We would like to acknowledge the Virginia Commonwealth University Center for the Study of Tobacco Products External Advisory Board members David Ashley, Eric Donny, Melissa Harrell, and Suchitra Krishnan-Sarin for their helpful feedback on the initial findings derived from this study. We also thank Rebecca Lester-Scholtes, Jessica Presley, and Megan Underwood for their contributions to the study. Our study was preregistered at clinicaltrials.gov (NCT03830892). Preliminary findings of this study have been reported at the annual Tobacco Centers of Regulatory Science (TCORS) meeting in 2020 and at the annual meeting of the Society for Research on Nicotine and Tobacco (SRNT) in 2021.
Cosima Hoetger played lead role in writing of original draft and equal role in writing of review and editing. Rose S. Bono played lead role in formal analysis and visualization and equal role in writing of original draft and writing of review and editing. Augustus M. White played equal role in writing of original draft and writing of review and editing. Andrew J. Barnes played equal role in conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation and writing of review and editing. Caroline O. Cobb played equal role in conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation and writing of review and editing.
References
- Ali M, Gray TR, Martinez DJ, Curry LE, & Horn KA (2016). Risk profiles of youth single, dual, and poly tobacco users. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 18(7), 1614–1621. 10.1093/ntr/ntw028 [DOI] [PubMed] [Google Scholar]
- Armitage AK, Alexander J, Hopkins R, & Ward C (1988). Evaluation of a low to middle tar/medium nicotine cigarette designed to maintain nicotine delivery to the smoker. Psychopharmacology, 96(4), 447–453. 10.1007/BF02180022 [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, & Wileyto EP (2016). The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug and Alcohol Dependence, 166, 263–267. 10.1016/j.drugalcdep.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley JH (2014). EU Tobacco Products Directive trumps debate on regulation of electronic cigarettes. British Medical Journal, 349, Article g5897. 10.1136/bmj.g5897 [DOI] [PubMed] [Google Scholar]
- Barnes AJ, Bono RS, Lester RC, Eissenberg TE, & Cobb CO (2017). Effect of flavors and modified risk messages on e-cigarette abuse liability. Tobacco Regulatory Science, 3(4), 374–387. 10.18001/TRS.3.4.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Yang Z, Schiff S, Unger J, Cruz TB, Urman R, Cho J, Samet JM, Leventhal AM, Berhane K, & McConnell R (2020). E-cigarette product characteristics and subsequent frequency of cigarette smoking. Pediatrics, 145(5), Article e20191652. 10.1542/peds.2019-1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (2010). Nicotine addiction. The New England Journal of Medicine, 362(24), 2295–2303. 10.1056/NEJMra0809890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono RS, Cobb CO, Wall CS, Lester RC, Hoetger C, Lipato T, Guy MC, Eissenberg T, Bickel WK, & Barnes AJ (2020). Behavioral economic assessment of abuse liability for Black & Mild cigar flavors among young adults. Experimental and Clinical Psychopharmacology. Advance online publication. 10.1037/pha0000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, & Eissenberg T (2017). Electronic cigarettes: What are they and what do they do? Annals of the New York Academy of Sciences, 1394(1), 5–30. 10.1111/nyas.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll DM, Wagener TL, Peck JD, Brame LS, Thompson DM, Stephens LD, Campbell JE, & Beebe LA (2018). Biomarkers of exposure in ENDS users, smokers, and dual users of American Indian descent. Tobacco Regulatory Science, 4(2), 3–15. 10.18001/TRS.4.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhuang Y-L, & Zhu S-H (2016). E-Cigarette design preference and smoking cessation: A U.S. population study. American Journal of Preventive Medicine, 51(3), 356–363. 10.1016/j.amepre.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, & Fischman MW (1999). Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology, 143(4), 327–338. 10.1007/s002130050956 [DOI] [PubMed] [Google Scholar]
- Costa H, Gilmore AB, Peeters S, McKee M, & Stuckler D (2014). Quantifying the influence of the tobacco industry on EU governance: Automated content analysis of the EU Tobacco Products Directive. Tobacco Control, 23(6), 473–478. 10.1136/tobaccocontrol-2014-051822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Cox S, Goniewicz M, McRobbie H, Kimber C, Doig M, & Kośmider L (2018). “Real-world” compensatory behaviour with low nicotine concentration e-liquid: Subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction, 113(10), 1874–1882. 10.1111/add.14271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvauchelle CL, Sapoznik T, & Kornetsky C (1998). The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacology, Biochemistry, and Behavior, 61(3), 297–302. 10.1016/S0091-3057(98)00098-7 [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Soule E, Shihadeh A, & the CSTP Nicotine Flux Work Group. (2021). “Open-System” electronic cigarettes cannot be regulated effectively. Tobacco Control, 30(2), 234–235. 10.1136/tobaccocontrol-2019-055499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. (2014). Questions & Answers: New rules for tobacco products. https://ec.europa.eu/commission/presscorner/detail/en/MEMO_14_134
- Government of Canada. (2021). Consultation—Proposed concentration of nicotine in vaping products regulations. https://www.canada.ca/en/health-canada/programs/consultation-proposed-concentration-nicotine-vaping-products-regultions.html
- Griffiths RR, Bigelow GE, & Liebson IA (1989). Reinforcing effects of caffeine in coffee and capsules. Journal of the Experimental Analysis of Behavior, 52(2), 127–140. 10.1901/jeab.1989.52-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Findley JD, Brady JV, Dolan-Gutcher K, & Robinson WW (1975). Comparison of progressive-ratio performance maintained by cocaine, methylphenidate and secobarbital. Psychopharmacology, 43(1), 81–83. 10.1007/BF00437619 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, & Mumford GK (1993). Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behavioural Pharmacology, 4(1), 3–13. 10.1097/00008877-199302000-00001 [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, & Krystal JH (2004). Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry, 61(3), 310–317. 10.1001/archpsyc.61.3.310 [DOI] [PubMed] [Google Scholar]
- Harrell PT, Simmons VN, Piñeiro B, Correa JB, Menzie NS, Meltzer LR, Unrod M, & Brandon TH (2015). E-cigarettes and expectancies: Why do some users keep smoking? Addiction, 110(11), 1833–1843. 10.1111/add.13043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Smethells JR, Palumbo M, Goniewicz M, & LeSage MG (2020). Comparison of the relative abuse liability of electronic cigarette aerosol extracts and nicotine alone in adolescent rats: A behavioral economic analysis. International Journal of Environmental Research and Public Health, 17(3), Article 860. 10.3390/ijerph17030860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler M, Breland A, Spindle T, Maloney S, Lipato T, Karaoghlanian N, Shihadeh A, Lopez A, Ramôa C, & Eissenberg T (2017). Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: Influence of liquid nicotine concentration and user experience. Experimental and Clinical Psychopharmacology, 25(5), 380–392. 10.1037/pha0000140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler M, Karaoghlanian N, Talih S, Maloney S, Breland A, Shihadeh A, & Eissenberg T (2020). Effects of electronic cigarette heating coil resistance and liquid nicotine concentration on user nicotine delivery, heart rate, subjective effects, puff topography, and liquid consumption. Experimental and Clinical Psychopharmacology, 28(5), 527–539. 10.1037/pha0000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W (1961). Progressive ratio as a measure of reward strength. Science, 134(3483), 943–944. 10.1126/science.134.3483.943 [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289–294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Karelitz JL, & Perkins KA (2021). Acute subjective sensory perceptions predict relative reinforcing effects of smoked nicotine. Addictive Behaviors, 117, Article 106835. 10.1016/j.addbeh.2021.106835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney SF, Breland A, Soule EK, Hiler M, Ramôa C, Lipato T, & Eissenberg T (2019). Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Experimental and Clinical Psychopharmacology, 27(5), 443–454. 10.1037/pha0000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Wiley JL, Silinski MA, Thomas BF, Meredith SE, Gahl RF, & Jackson KJ (2019). Comparison of cigarette, little cigar, and waterpipe tobacco smoke condensate and e-cigarette aerosol condensate in a self-administration model. Behavioural Brain Research, 372, Article 112061. 10.1016/j.bbr.2019.112061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelman M (2019). New bill aims to cap nicotine levels in e-cigarettes. https://www.cnn.com/2019/10/07/health/vaping-nicotine-cap-krishnamoorthi-bn/index.html
- Perkins K, Jacobs L, Sanders M, & Caggiula A (2002). Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology, 163(2), 194–201. 10.1007/s00213-002-1168-1 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, & Fonte C (1994). Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacology, Biochemistry, and Behavior, 47(1), 107–112. 10.1016/0091-3057(94)90118-X [DOI] [PubMed] [Google Scholar]
- Quisenberry AJ, Koffarnus MN, Epstein LH, & Bickel WK (2017). The Experimental Tobacco Marketplace II: Substitutability and sex effects in dual electronic cigarette and conventional cigarette users. Drug and Alcohol Dependence, 178, 551–555. 10.1016/j.drugalcdep.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DD, Naudé GP, Salzer AR, Peper M, Monroe-Gulick AL, Gelino BW, Harsin JD, Foster RNS, Nighbor TD, Kaplan BA, Koffarnus MN, & Higgins ST (2020). Behavioral economic measurement of cigarette demand: A descriptive review of published approaches to the cigarette purchase task. Experimental and Clinical Psychopharmacology, 28(6), 688–705. 10.1037/pha0000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy AK, Leventhal AM, Goldenson NI, & Eissenberg T (2017). Assessing electronic cigarette effects and regulatory impact: Challenges with user self-reported device power. Drug and Alcohol Dependence, 179, 337–340. 10.1016/j.drugalcdep.2017.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted JM, Mackee A, Williams R, & Willner P (1998). Deprivation state but not nicotine content of the cigarette affects responding by smokers on a progressive ratio task. Psychopharmacology, 140(4), 411–417. 10.1007/s002130050783 [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, & Badger GJ (1999). Comparing the reinforcing efficacy of nicotine containing and denicotinized cigarettes: A behavioral economic analysis. Psychopharmacology, 147(2), 210–216. 10.1007/s002130051162 [DOI] [PubMed] [Google Scholar]
- Smets J, Baeyens F, Chaumont M, Adriaens K, & Van Gucht D (2019). When less is more: Vaping low-nicotine vs. high-nicotine e-liquid is compensated by increased wattage and higher liquid consumption. International Journal of Environmental Research and Public Health, 16(5), Article 723. 10.3390/ijerph16050723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Helen G, Nardone N, Addo N, Dempsey D, Havel C, Jacob P III, & Benowitz NL, (2020). Differences in nicotine intake and effects from electronic and combustible cigarettes among dual users. Addiction, 115(4), 757–767. 10.1111/add.14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Fillmore MT, & Rush CR (2004). Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. Journal of Psychopharmacology (Oxford, England), 18(4), 534–543. 10.1177/026988110401800411 [DOI] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, & Shihadeh A (2015). Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 17(2), 150–157. 10.1093/ntr/ntu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Salman R, El-Hage R, Karaoghlanian N, El-Hellani A, Baassiri M, Jaroudi E, Eissenberg T, Saliba N, & Shihadeh A (2017). Transport phenomena governing nicotine emissions from electronic cigarettes: model formulation and experimental investigation. Aerosol Science and Technology, 51(1), 1–11. 10.1080/02786826.2016.1257853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Salman R, El-Hage R, Karam E, Karaoghlanian N, El-Hellani A, Saliba N, Eissenberg T, & Shihadeh A (2021). Might limiting liquid nicotine concentration result in more toxic electronic cigarette aerosols? Tobacco Control. 30(3), 348–350. https://tobaccocontrol.bmj.com/content/tobaccocontrol/early/2020/06/09/tobaccocontrol-2019-055523.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Salman R, El-Hage R, Karam E, Karaoghlanian N, El-Hellani A, Saliba N, & Shihadeh A (2019). Characteristics and toxicant emissions of JUUL electronic cigarettes. Tobacco Control, 28(6), 678–680. 10.1136/tobaccocontrol-2018-054616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, & Queimado L (2017). Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco Control, 26(e1), e23–e28. 10.1136/tobaccocontrol-2016-053041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Hardman S, & Eaton G (1995). Subjective and behavioural evaluation of cigarette cravings. Psychopharmacology, 118(2), 171–177. 10.1007/BF02245836 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


