Abstract
Bone marrow stromal stem cells (BMSCs) are adult multipotent cells, which have the potential to differentiate into cell types of mesodermal origin, namely osteocytes, adipocytes, and chondrocytes. Due to their accessibility and expansion potential, BMSCs have historically held therapeutic promise in tissue engineering and regenerative medicine applications. More recently, it has been demonstrated that not only can bone marrow stromal stem cells directly participate in tissue regeneration, but they also have the capacity to migrate to distant sites of tissue injury, where they can participate in tissue repair either directly through their differentiation or indirectly through paracrine mechanisms. Additionally, they can elicit various immunomodulatory signals, which can attenuate the inflammatory and immune responses. As such, bone marrow stromal stem cells have been explored clinically for treatment of a wide variety of different conditions including bone defects, graft-vs.-host disease, cardiovascular diseases, autoimmune diseases, diabetes, neurological diseases, and liver and kidney diseases. This review provides an overview of current clinical applications of bone marrow stromal stem cells and discusses their therapeutic properties, while also addressing limitations of their use. PubMed, Ovid, and Google Scholar online databases were searched using several keywords, including “stem cells”, “tissue engineering”, tissue regeneration” and “clinical trials”. Additionally, Clinicaltrials.gov was used to locate completed clinical trials using bone marrow derived stem cells.
Keywords: skeletal stem cells, bone marrow, tissue engineering, tissue regeneration, clinical trials, review
Introduction
In the most fundamental context, the term “stem cell” refers to an uncommitted progenitor cell having the capacity for self-renewal and differentiation into different cell types. Stem cells can be isolated from embryonic or post-natal tissues. Embryonic stem cells (ESCs) are derived from the inner cell mass of blastocysts and are characterized by unlimited proliferative capacity in an undifferentiated state. ESCs also have the potential to differentiate into cells derived from all 3 developmental germ layers (endoderm, mesoderm, or ectoderm) [1]. Post-natal and adult stem cells (ASCs) are undifferentiated cells found throughout the body and are a reservoir of precursor cells, which provide continual maintenance and repair of damaged tissues [2–6]. Mesenchymal stem cells (MSCs) are a type of ASCs, which have attracted much attention due to their potential to differentiate into cells of mesodermal origin, including osteoblasts, chondrocytes and adipocytes [7, 8]. There is evidence of in vitro MSC differentiation into cells of non-mesodermal origin, including neurons, dermis and gut epithelial cells, hepatocytes and pneumocytes; however, this capacity has not been unequivocally demonstrated in vivo [9] (Fig. 1). MSCs were first isolated from the bone marrow based on their ability to adhere to plastic culture dishes. Friedenstein et al. first described them as non-hematopoietic precursor cells with fibroblast morphology and further characterized them by their clonogenicity and multi-potency [10, 11]. In addition to bone marrow, it has more recently been recognized that MSCs can be isolated from a variety of tissues including adipose, muscle, tendon, peripheral blood, umbilical cord, dental pulp, periodontal ligament, oral mucosa and gingiva, alveolar bone, placenta, spleen, liver and thymus [4, 12–15]. Even more recently, it has been proposed that MSCs originate from pericytes, the perivascular supporting cells of blood vessels that undergo chemotaxis in areas of injury or inflammation. Following injury, released pericytes can acquire an MSC phenotype, which responds to the dynamic changes of the local microenvironment [16, 17]. The terms mesenchymal stem cells, marrow stromal fibroblastic cells, bone marrow stromal cells, stromal precursor cells, osteogenic stem cells and more recently skeletal stem cells have been used interchangeably in the literature to describe these cell populations [18].
Fig. 1.
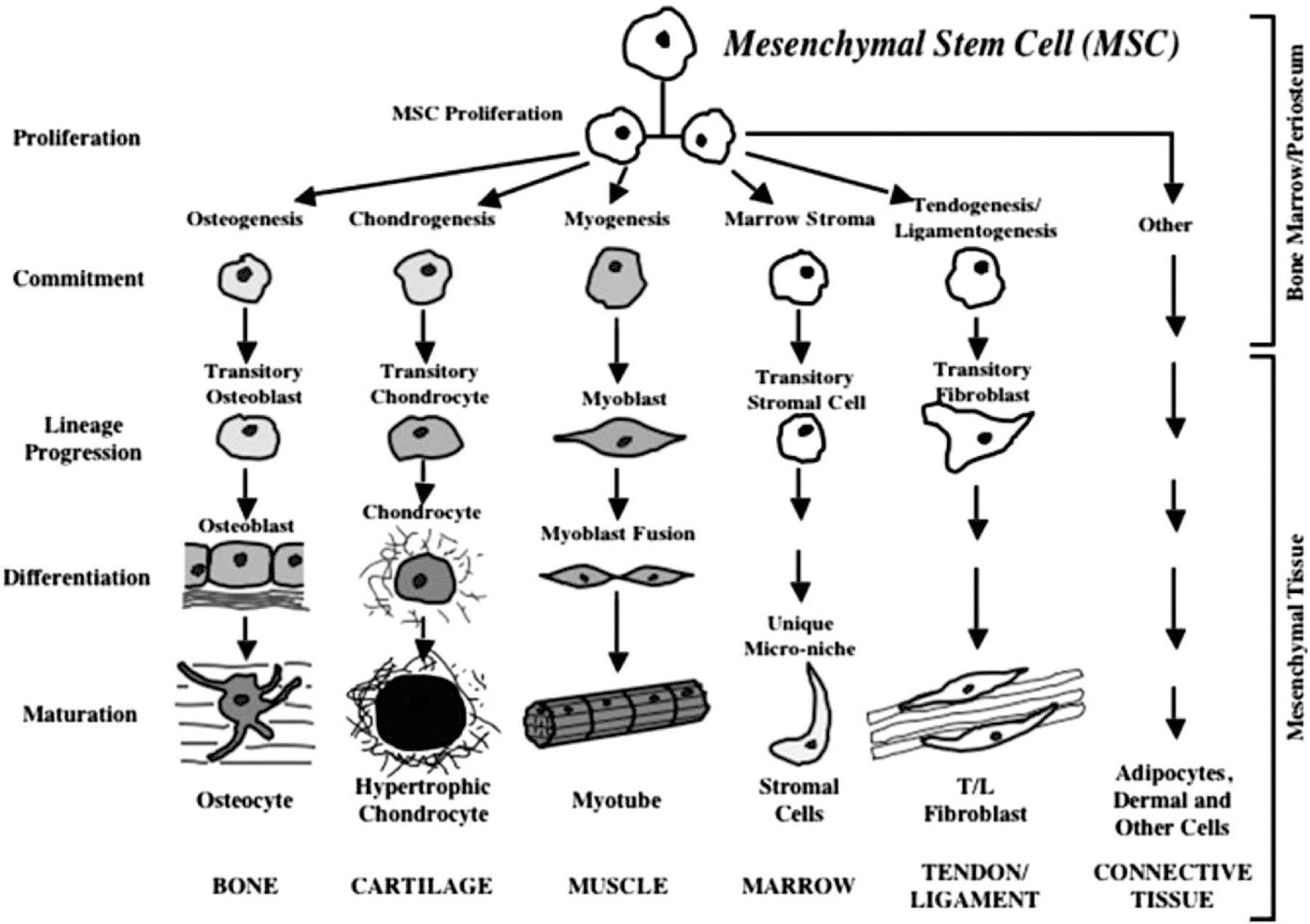
Commitment, lineage progression and differentiation of mesenchymal stem cells into different cell types [18].
Along with the terminology used to describe them, the characterization of MSCs has remained inconsistent, with different groups having different methods of isolation and ex vivo expansion of these cells. To address these issues, the International Society for Cell Therapy (ISCT) proposed 3 criteria to define human MSCs: 1) adherence to plastic; 2) positive expression of CD105, CD73 and CD90 and negative expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules; and 3) the ability to differentiate into osteoblasts, adipocytes and chondroblasts in vitro [19]. In an effort to implement a uniform terminology, the ISCT recommended the term ‘multipotent mesenchymal stromal cells’ (MSC) to describe the plastic-adherent cells isolated from the bone marrow and other tissues. However, this review will use the term bone marrow stromal stem cells (BMSSCs) in focusing discussion of therapeutic applications only on cells isolated from the bone marrow stroma [20].
In addition to their differentiation capacity, BMSSCs possess other important properties including the ability to travel or “home” to distant sites of tissue injury, interact with the immune system, and produce bioactive molecules (Fig. 2). Their ability to migrate to the site of injury (homing capacity) is based on the presence of chemokine receptors that respond to chemokine attractive gradients generated by the injured tissue [21]. These more recently identified BMSSCs properties have led to an expansion of possible therapeutic applications, where a significant role is for them to enhance the intrinsic regenerative capacity of compromised tissues through modulation of the immune response, inflammation, and the concentration of growth factors in the local microenvironment [17]. In addition to therapeutic applications BMSSCs these cells can be used to study disease through the development of disease models [7–9].
Fig. 2.
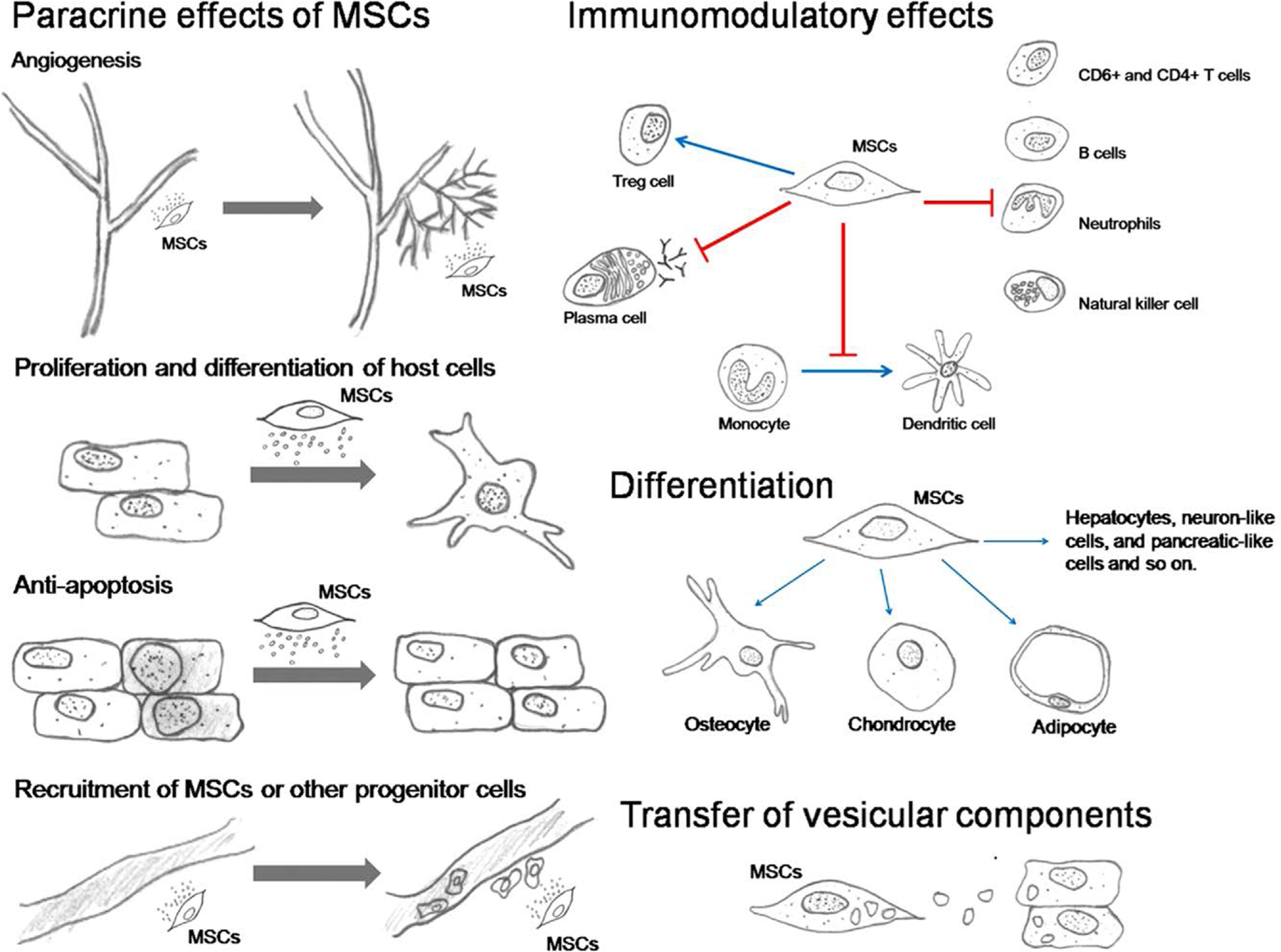
MSCs travel or “home” to distant sites of tissue injury where they exert their paracrine effects namely, the stimulation of angiogenesis, protection of other cells from apoptosis, recruitment of host MSCs or other progenitor cells and stimulation of their proliferation and differentiation. The immunomodulatory effects of MSC consist of inhibiting the proliferation and activity of neutrophils, NK cells, B cells, and T cells, preventing the maturation of monocytes into dendritic cells, suppressing plasma cell immunoglobulin production but stimulating proliferation of regulatory T cells. MSCs have the potential to differentiate into multiple cell types and transfer vesicles containing mRNA, miRNA, proteins, and perhaps mitochondria to the host cells [98].
The identification and characterization of various stem cell populations provide exciting opportunities to develop novel therapeutic strategies for tissue regeneration in the context of tissue engineering and regenerative medicine [1, 10]. This review summarizes the properties and possible mechanisms of action of BMSSCs and outlines the current clinical data for the usage of these cells in tissue engineering and regenerative medicine, as well as their potential therapeutic role in various diseases.
Therapeutic Applications of BMSSCs
Tissue engineering and regenerative medicine
Tissue engineering emerged as the “ideal” therapy for tissue loss or organ failure. The classical tissue engineering approach is based upon 3 critical components: cells capable of populating tissue to substitute and generate novel tissue, biomimetic scaffolds, and environmental signals (i. e., growth factors) [11]. Biomimetic scaffolds act as matrices to provide a 3 dimensional microenvironment in which cells can proliferate and differentiate and ultimately through which tissue can develop. Biocompatibility, controlled degradation rate, physical properties and porosity for cell in-growth, diffusion of nutrients and neovascularization, are all key variables to consider in scaffold design [12]. These biodegradable scaffolds are subsequently seeded with cells which ultimately create their own extracellular matrix [13]. More recent strategies have emerged, which use 3-dimensional imaging technologies to create “customized” scaffolds that can be tailored to highly variable defects and are manufactured to fit the tissue defects with more precision [14].
The ideal cell type to incorporate into a tissue-engineering schema is one that is easy to harvest, proliferative, non-immunogenic and has the capacity to differentiate into the specific cell type for the tissue to be regenerated. Due to ethical and legislative issues associated with the clinical use of ESCs, most clinical stem cell therapies focus on ASCs particularly those isolated from the bone marrow (BMSSCs). An integral part of the tissue engineering approach involves ex vivo expansion of cells over a period of time in either static or dynamic culture systems. Perfusion bioreactors are used in most dynamic systems and are advantageous because they promote the transportation of nutrients, oxygen, and metabolites in the scaffold interior [15, 16].
Based on their capacity to differentiate into cells of mesodermal origin, BMSSCs have been widely used in the field of tissue engineering for re-building damaged or diseased mesenchymal tissues, such as bone and cartilage [17] (Table 1). Furthermore, BMSSCs secrete a broad spectrum of bioactive molecules, which can act in a paracrine or autocrine manner and provide a robust regenerative microenvironment for a variety of injured adult tissues (Fig. 3). This property of BMSSCs is referred to as the ‘trophic’ activity of BMSSCs and it makes these cells important mediators for regenerative processes. [18] (Table 2). The terms “tissue engineering” and regenerative medicine” have been used interchangeably and even though they are based on different mechanisms, there is no clear distinction between them. In fact, the coupling of both tissue engineering and these trophic activities is necessary to regenerate tissues that are functionally integrated into the human body.
Table 1.
Clinical studies using bone marrow stromal stem cells for tissue engineering.
| Musculoskeletal Conditions | |||||||
|---|---|---|---|---|---|---|---|
| Reference | No. of patients | Study design | Indication | Source | Administration | Outcome | Follow-up |
| Quarto et al. 2001 [23] | 3 | Case series | Large bone defects | Autologous | Macroporous HA scaffolds | Callous formation, good integration with host bone, no complications | 15–27 months |
| Kawate et al. 2006 [29] | 3 | Case series | Steroid-induced osteonecrosis of the femoral head | Autologous | β-TCP carrier | Arrest of osteonecrosis progression, early bone regeneration, not indicated in cases with severe preoperative collapse | 34 months |
| Marcacci et al. 2007 [31] | 4 | Case series | Bone diaphysis defects | Autologous | Porous HA ceramic scaffolds | No major complications, integration of the implants, functional recovery of the limb | 6–7 years |
| Aoyama et al. 2014 [32] | 10 | Prospective, open-labeled, proof-of-concept clinical trial | Idiopathic osteonecrosis of the femoral head | Autologous | β-TCP carrier in combination with vascularized bone grafts | Safety, increased bone volume, improvement in clinical scores | 24 months |
| Hernigou et al. 2014 [33] | 60 | Case control study | Total hip arthroplasty | Autologous | Allograft carrier | Better radiographic graft union rates, less allograft resorption, less failures as compared with allograft alone | 12 years |
| Bolland et al. 2006 [34] | 6 | Cohort study | Hip replacement | Autologous | Morselized allograft carrier | Higher shear strength | 4 weeks |
| Behnia et al. 2012 [37] | 3 | Case series | Alveolar cleft defect | Autologous | HA/TCP polygonal 3 mm cubes mixed with PDGF and SSCs | 51.3 % fill of the bone defect | 3 months |
| Meijer et al. 2008 [36] | 6 | Case series | Intra-osseous defects | Autologous | HA porous scaffold | No orthotropic bone formation | 15 months |
| Kaigler et al. 2013 [38] | 24 | Phase 1/2 RCT | Alveolar bone defects | Autologous | Absorbable gelatin sponge (Gelfoam) | Accelerated alveolar bone regeneration, reduced need for secondary bone grafting | 1 year |
| Rajan et al. 2014 [39] | 1 | Case report | Jawbone deficiencies following injury | Autologous | β-TCP | 80 % regeneration of the bone defect, functional and aesthetic rehabilitation of the patient with implants | 16 months |
| Kaigler et al. 2015 [40] | 30 | Phase 1/2 RCT | Bone deficiencies of the maxillary sinus | Autologous | β-TCP | Accelerated bone regeneration, enhanced bone density | 16 months |
| Wakitani et al. 2011 [43] | 41 | Case series | Cartilage repair | Autologous | Type I bovine collagen sheet | Neither tumors nor infections were observed | 75 months |
| Nejadnik et al. 2010 [44] | 72 | Cohort study | Cartilage repair | Autologous | Cell sheets | SSCs as effective as chondrocytes for cartilage repair | 24 months |
RCT: Randomized controlled trial; HA: Hydroxyapatite; β-TCP: Beta-tricalcium phosphate; iv: Intravenous; ip: Intraperitoneally; sc: Subcutaneously; MI: Myocardial infarction; LV: Left ventricular; ALS: Amyotrophic lateral sclerosis; MS: Multiple sclerosis; GVHD: Graft vs. host disease; CR: Complete response; PR: Partial response; MR: Mixed response; GI: Gastrointestinal; SLE: Systemic lupous erythematosus
Fig. 3.
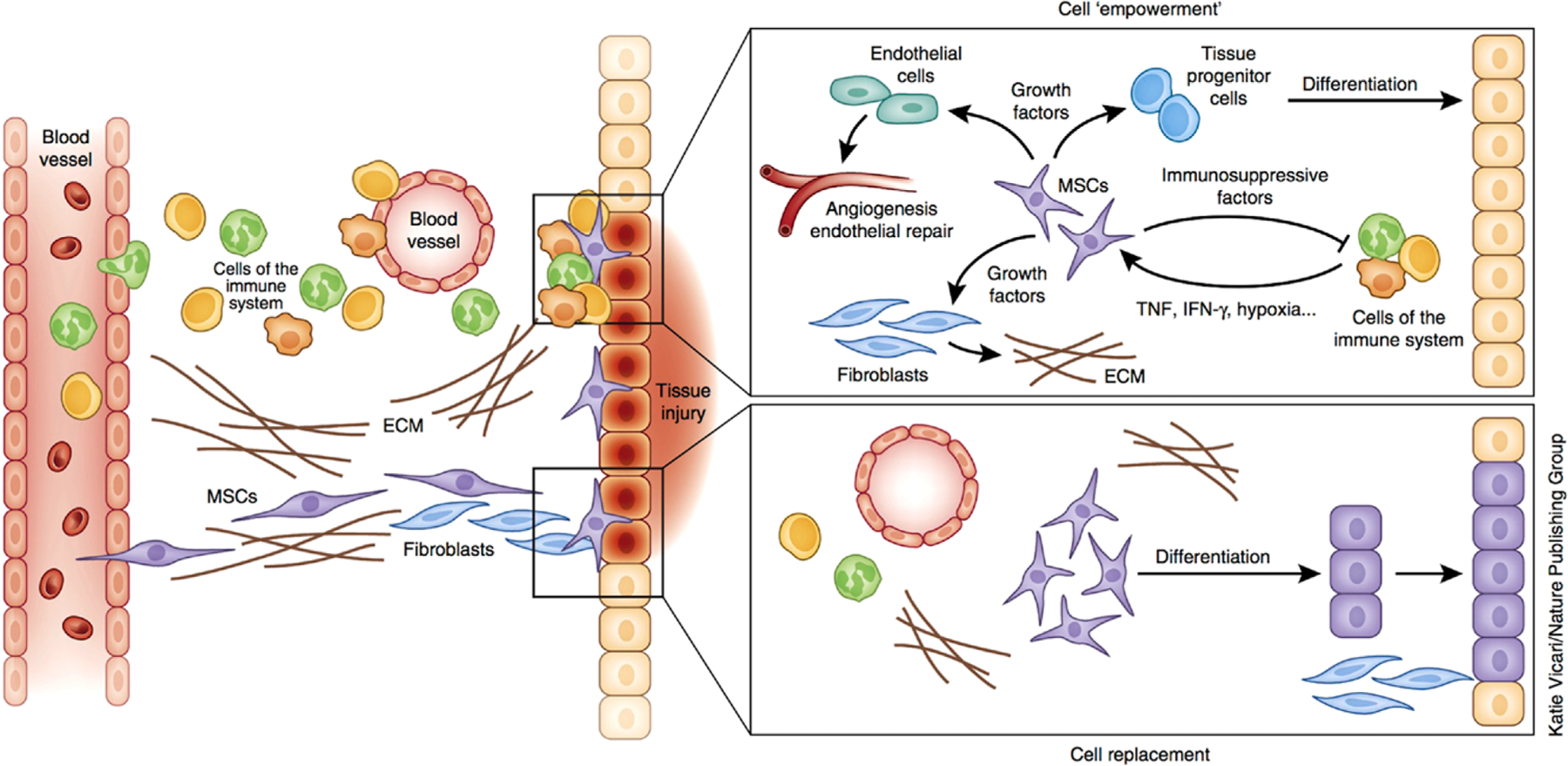
Modes of SSC-based tissue regeneration: cell replacement vs. trophic activity. SSCs migrate to the site of tissue injury and differentiate into functional cells to replace damaged cells. Besides their differentiation capacity, SSCs can enhance the intrinsic regenerative capacity of the tissues through the modulation of the immune system and the influence of released cytokines on the microenvironment. The concerted action of these factors and cells facilitates tissue repair through angiogenesis, remodeling of the extracellular matrix (ECM) and the differentiation of tissue progenitor cells [99].
Table 2.
Clinical studies using bone marrow stromal stem cells for regenerative medicine.
| Musculoskeletal Conditions | |||||||
|---|---|---|---|---|---|---|---|
| Reference | No. of patients | Study design | Indication | Source | Administration | Outcome | Follow-up |
| Kitoh et al. 2007 [25] | 46 | Case control | Distraction osteogenesis of the long bones | Autologous | Combination of SSCs and PRP injected into the distracted callus | Reduced treatment period and associated complications, accelerated new bone formation | NK |
| Horwitz et al. 2002 [27] | 6 | Case series | Osteogenesis imperfecta | Allogeneic | Infusion | No toxicity, engraftment in bone, marrow stroma, and skin, acceleration of growth | 3–6 months |
| Cahill et al. 2007 [28] | 1 | Case report | Infantile hypophosphatasia | Bone fragments from donor father and cultured osteoblasts | ip, sc, iv infusion | Improved skeletal mineralization, no premature loss of deciduous teeth | 7 years |
| Wong et al. 2013 [42] | 56 | Phase II RCT | Cartilage repair (knees) | Autologous | Intra-articular injection | Improved clinical and radiologic outcomes | 2 years |
| Cardiovascular Diseases | |||||||
| Rodrigo et el. 2013 [45] | 9 | Case series | Acute MI | Autologous | Intramyocardial injection | No adverse events, improvement of LV function similar to percutaneous coronary intervention | 5 years |
| Lee et al. 2014 [46] | 58 | Phase I-II RCT | Acute MI | Autologous | Intracoronary injection | No significant adverse events, modest improvement in LV ejection fraction | 6 months |
| Chen et al. 2004 [47] | 69 | Phase II RCT | Acute MI | Autologous | Intracoronary injection | Safe, improved LV function | 6 months |
| Katritsis et al. 2005 [48] | 22 | Cohort study | Recent and old anteroseptal MIs | Autologous | Intracoronary infusion | Safe, improved myocardial contractility | 4 months |
| Hare et al. 2009 [49] | 53 | Phase I-II RCT | Acute MI | Allogeneic (Prochymal) | Intravenous | Safe, increased LV ejection fraction and led to reverse remodeling | 6 months |
| Williams et al. 2011 [50] | 8 | Case series | Ischemic cardiomyopathy | Autologous | Intramyocardial injection | Improved regional contractility of a chronic myocardial scar | 1 year |
| Mathiasen et al. 2012 [51] | 60 | Phase II RCT | Chronic ischemic heart failure | Autologous | Intramyocardial injection | Confirms the results from previous openlabeled clinical trials | 1 year |
| Ascheim et al. 2014 [52] | 30 | Phase I-II RCT | End-stage heart failure | Allogeneic | Intramyocardial injection | Safe, increased successful temporary wean off device | 12 months or until cardiac transplant |
| Heldman et al. 2014 [53] | 65 | Phase I-II RCT | Ischemic cardiomyopathy | Autologous | Transendocardial injection | No treatment-emergent serious adverse events, improved regional myocardial function, reduced infarct size | 1 year |
| Karantalis et al. 2014 [54] | 6 | Case series | Chronic ischemic left ventricular dysfunction secondary to MI | Autologous | Transepicardial delivery | Increased LV ejection fraction and decreased scar mass | 18 months |
| Hare et al. 2012 [55] | 31 | Phase I-II randomized comparison | Ischemic cardiomyopathy | Autologous and allogeneic | Transendocardial injection | Both were safe and improved patient functional capacity, quality of life, and ventricular remodeling | 13 months |
| Chin et al. 2011 [57] | 10 | Case series | Severe dilated cardiomyopathy (ischemic and non-ischemic) | Autologous | Intramyocardial and intracoronary injection | Safe, improvement in left ventricular parameters, scar reduction | 1 year |
| Mushtaq et al. 2014 [58] | 36 | Phase I-II randomized pilot study | Non-ischemic dilated cardiomyopathy | Autologous and allogeneic | Transendocardial injection | Designed to compare allogeneic vs. autologous mesenchymal stem cell therapy | 1 year |
| Mathiasen et al. 2013 [59] | 31 | Non-randomized prospective phase I-II clinical trial | Severe stable coronary artery disease and refractory angina | Autologous | Intramyocardial injection | Clinical improvements in exercise time, angina class, weekly number of angina attacks and use of nitroglycerine, reduced hospital admissions, long term safety | 3 years |
| Henry et al. 2014 [56] | 61 | Phase II RCT | Ischemic and non-ischemic cardiomyopathy | Autologous | Minithoracotomy catheter injection, intramyocardial injection | Ixmyelocel-T intramyocardial injection reduced major adverse cardiovascular events only in patients with ischemic dilated cardiomyopathy | 1 year |
| Liver and Kidney Diseases | |||||||
| Amer et al. 2011 [61] | 40 | Phase I-II RCT | End-stage liver failure due to hepatitis C | Autologous | Intrasplenic and intrahepatic administration | Safety, short-term efficacy, no difference between intrasplenic and intrahepatic groups | 6 months |
| El-Ansary et al. 2012 [62] | 25 | Phase II clinical trial | Hepatitis C induced liver cirrhosis | Autologous | iv infusion | Partial improvement of liver function tests (elevation of prothrombin and serum albumin levels, decline of elevated bilirubin) | 6 months |
| Jang et al. 2014 [63] | 12 | Phase II clinical trial | Alcoholic cirrhosis | Autologous | Injection through the hepatic artery | No side effects, histological improvement of hepatic fibrosis | 12 weeks |
| El-Ansary et al. 2012 [65] | 30 | Case series | Chronic kidney disease | Autologous | Intravenous infusion | Reduction of serum creatinine, elevation of creatinine clearance levels | 6 months |
| Diabetes | |||||||
| Carlsson et al. 2015 [70] | 20 | Phase I-II RCT | Type 1 diabetes | Autologous | Intravenous infusion | No side effects, preserved β-cell function | 1 year |
| Bhansali et al. 2014 [71] | 10 | Phase I-II RCT | Type 2 diabetes | Autologous | Injection into the superior pancreaticoduodenal artery | Increase in glucagon-stimulated C-peptide, decrease in insulin requirement | 1 year |
| Lu et al. 2011 [73] | 41 | Phase I-II RCT | Diabetic critical limb ischemia and foot ulcer | Autologous | Intramuscular injection | Well tolerated, increased lower limb perfusion and foot ulcer healing | 24 weeks |
| Neurological Diseases | |||||||
| Slavin et al. 2008 [77] | 12 | Case series | MS, ALS | Autologous | Intrathecal and iv injection | Safe procedure, no major risks | 1 year |
| Oh et al. 2015 [78] | 7 | Phase I trial | ALS | Autologous | Intrathecal injection | No serious adverse events of repeated intrathecal injections of autologous SSCs | 1 year |
| Wang et al. 2013 [79] | 52 | Open-labeled, self-controlled trial | Cerebral palsy | Autologous | Intrathecal or intraparenchymal infusion | No side effects, improved motor function | 18 months |
| Chen et al. 2013 [80] | 60 | Phase I-II controlled clinical trial | Cerebral palsy | Autologous | Injection into the subarachnoid cavity | No adverse events, increase in motor function | 6 months |
| Venkataramana et al. 2010 [81] | 7 | Case series | Parkinson’s disease | Autologous | Transplantaion into the sublateral ventricular zone by stereotaxic surgery | No serious adverse events, subjective improvement in facial expression, gait, and freezing episodes | 10–36 months |
| Pal et al. 2009 [82] | 30 | Case series | Spinal cord injury/paraplegia | Autologous | Lumbar puncture | No serious adverse events | 1–3 years |
| Zhang et al. 2008 [83] | 7 | Case series | Traumatic brain injury | Autologous | Direct application during cranial surgery, iv infusion | No toxicity, significantly improved neurologic function | 6 months |
HA: Hydroxyapatite; β-TCP: Beta-tricalcium phosphate; iv: Intravenous; ip: Intraperitoneally; sc: Subcutaneously; MI: Myocardial infarction; LV: Left ventricular; ALS: Amyotrophic lateral sclerosis; MS: Multiple sclerosis; GVHD: Graft vs. host disease; CR: Complete response; PR: Partial response; MR: Mixed response; GI: Gastrointestinal; SLE: Systemic lupous erythematosus NK: Not known
Musculoskeletal conditions
Bone is a metabolically active tissue which continuously undergoes the process of resorption and formation through highly coordinated processes controlled by multiple factors [19–21]. When subjected to trauma or damage, bone has the innate self-limiting capacity to repair small fractures and non- “critical-sized” defects. However, “critical-size” bone defects (above a certain size) cannot heal without intervention and in addition to surgery, therapies often include use of growth factors and specialized biomaterials. In this context, the application of BMSSCs for engineering and regenerating bone has been extensively investigated [17].
Tissue engineering and regenerative medicine strategies used to treat non-union and critical-sized segmental defects have included the use of bone marrow aspirates, bone marrow concentrates, and ex vivo expanded BMSSCs [22–24]. Over 30 years ago, early clinical studies reported on the percutaneous injection of autologous bone marrow aspirate, as a minimally invasive method to stimulate fracture repair in cases of delayed union and non-union [22]. Since this time, autogenous bone marrow injection has shown efficacy in the treatment of simple bone cysts and other orthopedic bone healing disturbances including osteonecrosis of the femoral head and non-union fractures [24, 25].
In lieu of using whole bone marrow aspirates and bone marrow concentrates, the ability to isolate BMSSCs from bone marrow has enabled the ex vivo expansion of BMSSCs for their clinical transplantation. Transplantation of these cells with or without the addition of growth factors has been used to induce bone regeneration. A retrospective study of 46 patients who underwent distraction osteogenesis of the long bones reported that local injection of ex vivo expanded BMSSCs in conjunction with platelet rich plasma (PRP) resulted in faster healing and fewer complications as compared to controls [25]. Recently, the intra-articular injection of autologous ex-vivo expanded BMSSCs on a patient suffering from chronic knee osteoarthritis resulted in improved walking ability and decreased pain 2 months after transplantation. At 6 and 12 months post-transplantation the MRI revealed an extension of the repaired tissue over subchondral bone, eliminating the need of hospitalization or surgery [26]. Although the clinical evidence is still limited, infusion of allogeneic BMSSCs has been shown to be beneficial in treating genetic bone disorders, such as osteogenesis imperfecta and hypophosphatasia in children [27, 28]. It has been suggested that cultured BMSSCs may fail to engraft into bone after intravenous infusion due to loss of adhesion molecules and loss of self-renewal ability [28]. However, even a low level of engraftment has demonstrated to be adequate to produce clinically measurable benefits [27].
Besides the local injection of BMSSCs into the site of injury, other methods of cell transplantation include the use of various carriers such as demineralized bone matrix or hydroxyapatite scaffolds [22, 23]. Ex vivo expanded BMSSCs loaded onto absorbable scaffolds have been successfully used in the treatment of idiopathic osteonecrosis of the femoral head, “critical-sized” long bone defects and spinal fusion [29–32]. The application of BMSSCs has also been investigated in 60 patients requiring revision of total hip arthroplasty. Allografts loaded with autologous BMSSCs and transplanted into the osteolytic lesion intra-operatively show better radiographic graft union rates and less allograft resorption compared to allografts alone. This group also demonstrated better mechanical stability and there was no need for revision of the procedure over the course of 12 years of follow-up [33]. Autologous BMSSCs have also been reported to enhance biomechanical properties of impacted allografts. BMSSCs seeded onto allografts survive the impaction process, continue to differentiate and proliferate with an osteoblastic phenotype, and lead to enhanced mechanical properties of these grafts at 4 weeks [34].
Beyond the scope of orthopedics, the treatment of craniofacial bone defects arising from congenital malformations, disease or injury still presents many challenges. In the last decade, numerous clinical studies have emerged introducing the use of BMSSCs as a promising alternative approach for the reconstruction of these defects. Warnke et al. reported successful repair of an extended mandibular discontinuity defect by growth of a custom bone transplant inside the latissimus dorsi muscle of an adult male patient [35]. Several randomized controlled clinical trials have investigated autologous cell therapy for reconstruction of jaw defects, maxillary sinus augmentation, and bone defects resulting from alveolar clefts [36–40]. Using heterogeneous mixed cell populations containing BMSSCs, the clinical outcomes of these studies demonstrated enhanced and accelerated healing and regeneration of bone as compared to standard-of-care controls, yielding better bone quality and less need for secondary and revision grafting surgeries. Variability in clinical outcomes between patients was mainly attributed to patient-to-patient variability in the autologous cell populations which were transplanted [40].
The mechanism by which new bone can be engineered is complex and may not depend solely on the action of BMSSCs. It has recently been proposed that CD34 + hematopoietic stem cells play a pivotal role using paracrine and autocrine cellular crosstalk signaling to up regulate other bone marrow stem cells, resulting in significant bone regeneration within the microenvironment of the graft. Marx et al. demonstrated that the combination of CD34 + cells with a concentration of at least 200/ml with BMSSCs and recombinant human-BMP2 (rh-BMP2), seeded in a scaffold, achieved significantly more bone regeneration after 6 months in patients with large continuity defects in the mandible as compared to BMSSCs alone or lower concentrations of CD34 + cells [41].
In addition to bone tissue engineering and regeneration, BMSSCs have also been employed for the engineering and regeneration of cartilage tissue. The local infusion of BMSSCs in knee joints has been used to treat cartilage defects. A randomized controlled clinical trial including 56 patients with 2 years follow-up examined the intra-articular injection of autologous BMSSCs 3 weeks after surgery in knees with cartilage defects and found that this treatment led to improvement of clinical and radiologic outcomes [42]. Furthermore, since cartilage is an avascular tissue, cartilage tissue engineering has had success in that these approaches do not face the main challenge associated with engineering other tissues, which is the need to establish a concomitant blood supply to support the engineered tissue. Transplantation of autologous BMSSCs in collagen type I gel for joint cartilage repair has demonstrated excellent safety long-term after 11 years follow-up in 41 patients [43]. Another study compared the transplantation of cell sheets of autologous chondrocytes to cell sheets of autologous BMSSCs in 72 patients with articular cartilage defects and resulted in similar clinical outcomes for both groups at 2 years, with less cost and donor site morbidity for the BMSSC group [44].
Cardiovascular diseases
Because of their trophic properties related to angiogenesis (Fig. 2), promising results of early preclinical studies have opened the door to numerous clinical trials investigating the safety and efficacy of cell-based therapies in patients with cardiovascular diseases. In patients with acute myocardial infarction (MI), transplantation of autologous BMSSCs with intracoronary injection and intramyocardial injection at different time intervals (up to 21 days) following MI showed evidence of safety and feasibility and improvement of left ventricular function at 6 months and 5 years, respectively [45, 46]. On the other hand, Chen et al. demonstrated that the intracoronary injection of autologous BMSSCs in 69 patients within 18 days after acute MI resulted in significantly improved left ventricular function at 6 months as compared to control [47]. Another study on 22 patients with recent and old anteroseptal MIs combined this approach with delivery of endothelial progenitors and demonstrated safety along with improvement in myocardial contractility after 4 months [48].
Though timeframes for delivery following MI vary according to different therapeutic protocols, a major limitation associated with the use of autologous BMSSCs is that the ex vivo cell expansion time can range from 2 to 5 weeks; thus, this precludes the acute therapeutic use of autologous BMSSCs within a short time of MI [45]. As a result, clinical trials have evaluated the feasibility of “off-the shelf” cell therapies using allogeneic BMSSCs in acute MI patients. Feasibility and safety have been demonstrated in early Phase 1 clinical trials where intravenous injection of allogenic BMSSCs was deemed safe in patients following acute MI [49].
In addition to acute therapy, treatment of chronic cardiovascular conditions has been evaluated for patients with chronic ischemic cardiomyopathy. In several studies with these applications, the clinical outcomes have yielded reverse remodeling, antifibrotic effects, neoangiogenesis and improved regional contractility of the myocardial scar after approximately 1 year [50–54]. In the treatment of another chronic condition involving left ventricular dysfunction, a Phase 1/2 study including 31 patients compared the safety and efficacy of autologous vs. allogeneic BMSSCs delivery. The findings demonstrated that relative to safety, both treatments were associated with low rates of serious adverse events. Regarding efficacy, both treatments favorably affected patient functional capacity, quality of life, and ventricular remodeling at 13 months post-intervention [55]. Additionally through transendocardial stem cell injections in patients with chronic ischemic cardiomyopathy this approach revealed that more significant scar size reduction and ventricular functional responses occur at the sites of stem cell injection as compared to remote sites. Another phase 2 clinical trial evaluated the effects of an autologous ex-vivo expanded BMSSC product (Ixmyelocel-T) in 61 patients with ischemic and non-ischemic dilated cardiomyopathy. The results of the study demonstrated that Ixmyelocel-T intramyocardial injection reduced major adverse cardiovascular events and improved symptoms in patients with ischemic dilated cardiomyopathy but not in patients with non-ischemic dilated cardiomyopathy [56]. Whether it be intramyocardial, intracoronal, or transendocardial administration of autologous BMSSCs in cases of chronic heart disease, has demonstrated safety and improved clinical outcomes in parameters of left ventricular function [57, 58].
Additionally, it has been suggested that BMSSC treatment not only improves function and clinical symptoms, but also slows down disease progression. A clinical trial assessing safety and efficacy of intramyocardial injection of autologous BMSSCs in 31 patients with severe stable coronary artery disease and refractory angina showed sustained clinical effects, reduced hospital admissions for cardiovascular disease and excellent safety 3 years post-treatment [59].
Liver and kidney diseases
Though a few preclinical studies demonstrated that human bone marrow derived BMSSCs can differentiate into hepatocyte-like cells, most therapeutic approaches for liver and kidney regeneration aim to exploit the trophic properties of BMSSCs [60]. In fact, several clinical trials have reported that BMSSC administration via intravenous, intrasplenic, or intrahepatic portal resulted in clinical improvement, as well as improvement in serum markers of patients with cirrhosis or liver failure due to hepatitis, at 6 months post-treatment [61, 62]. One study provided histologic evidence of improvement of hepatic fibrosis after administration of autologous BMSSCs in 12 patients with alcoholic cirrhosis [63].
Preclinical models of acute and chronic renal failure suggest that BMSSCs promote recovery of renal function and regeneration of damaged renal tissue following intravenous injection [64]. Magnetic resonance imaging shows that BMSSCs home to areas of glomerular damage. Based on these promising preclinical findings, a pilot clinical trial on 30 patients with chronic kidney disease was conducted where intravenous injection of BMSSCs showed improved renal function as measured by serum creatinine and creatinine clearance levels before and after BMSSC injection at 1, 3, and 6 months post-treatment [65]. Larger controlled clinical trials are needed to assess the efficacy and safety profile of BMSSCs in liver and kidney diseases.
Diabetes
The treatment of type 1 diabetes for those with end-stage organ failure is limited by the shortage of donor organs for transplantation and the immunosuppressive regimen the patient has to follow for many years post-transplantation. As such, more recent therapeutic approaches have focused on novel ways to generate insulin-producing cells with the use of embryonic stem cells and various adult stem cells, such as pancreatic stem cells, liver stem cells and BMSSCs [66, 67]. In addition to their ability to home to the damaged tissue, BMSSCs have shown the in vitro capacity to differentiate into insulin-producing cells [68]. If this holds true clinically, it would provide a much needed source of islet cells for β-cell replacement in treating diabetes mellitus [69]. Initial trials have emerged implementing BMSSCs in the treatment of type 1 and type 2 diabetes and early results have been encouraging.
A small pilot clinical trial with 20 adult patients investigating autologous BMSSC-based treatment in newly diagnosed type 1 diabetes reported preserved β-cell function and no side effects in the treatment group 1 year post-treatment [70]. Another prospective, randomized controlled clinical trial evaluated the efficacy and safety of autologous bone marrow-derived stem cell transplantation in 10 patients with type 2 diabetes. The findings of this study include an increase in glucagon-stimulated C-peptide (fragment of proinsulin) in treated cases compared to controls along with a decrease in supportive insulin therapy at the 1 year follow-up [71].
Another significant problem for patients with diabetes is the management of non-healing ulcers, one of the most chronic, common, and difficult to treat complications of diabetes [72]. A clinical trial compared the application of BMSSCs to bone marrow derived mononuclear cells for the treatment of diabetic limb ischemia and foot ulcer in 41 patients. At the 6 month follow-up, BMSSCs were better tolerated and more effective than bone marrow derived mononuclear cells for increasing lower limb perfusion and promoting foot ulcer healing in diabetic patients [73].
Neurological diseases
Regenerative approaches to neurological diseases include BMSSC-based therapies in which cells are delivered via intracerebral or intrathecal injection. Upon transplantation into the brain, BMSSCs promote endogenous neuronal growth, decrease apoptosis, reduce levels of free radicals, encourage synaptic connection from damaged neurons and regulate inflammation, primarily through paracrine actions [74]. Experimental studies in rats have shown that human BMSSCs injected intravenously successfully migrate into the brain and selectively concentrate around the injury site in a dose-dependent manner [75, 76]. The mechanisms responsible for the migration of stem cells into the brain are not clear. It has been speculated that disruption of the blood-brain barrier may facilitate selective entry of BMSSCs into the ischemic brain compared with the non-ischemic contralateral cerebral tissue. In addition, the fact that approximately 20 % of microglia is thought to originate from the marrow could also further explain the possible mechanism of migration of BMSSCs into the brain [75].
Amyotrophic lateral sclerosis (ALS), a neurodegenerative disease, is characterized by progressive loss of motor neurons, which ultimately leads to muscle atrophy, weakness, and immobility. Standard of care treatment of this disease aims to meet 2 goals: first, to eliminate the self-reactive lymphocytes and prevent the de novo development of self-reactivity; second, to achieve neuronal regeneration with re-myelinization of the affected neurons. Two Phase 1 clinical trials with 1 year follow-ups indicate that intrathecal and intravenous injection of autologous BMSSCs is a safe therapeutic strategy for ALS [77, 78].
Cerebral palsy (CP) comprises a group of disorders that are associated with chronic motor disability in children. Autologous BMSSC transplantation was found to be safe and to improve motor function in 52 children with cerebral palsy at 1 month, 6 months, and 18 months post-transplantation [79]. These results were corroborated by another study on 60 patients with moderate to severe cerebral palsy, which investigated a novel treatment where autologous BMSSCs were induced to differentiate into neural stem cell-like cells, which were then injected into the subarachnoid cavity. The motor functions of the patients significantly improved at 3 and 6 months as compared to controls, while no adverse effects were observed [80].
Parkinson’s disease is another neurological condition where BMSSC transplantation has been investigated. Through stereotaxic surgery, BMSSCs were transplanted into the sublateral ventricular zone in 7 patients and after 1 and 3 years, no adverse events were reported deeming this as a safe approach. Additionally, improvement in unified Parkinson’s disease rating scale (UPDRS) and subjective symptoms led to a decrease in the dosage of anti-Parkinson medications in a study [81]. The same group performed another study on 30 patients suffering from spinal cord injury and reported that intra spinal lumbar injection of autologous BMSSCs was safe and resulted in moderate recovery in the sensory and motor levels at 1–3 years post-treatment, if the treatment was performed within 6 months of the injury [82]. Using a similar approach, direct application followed by intravenous injection of BMSSCs in 7 patients with traumatic brain injury demonstrated no immediate or delayed toxicity combined with an improvement in neurologic function within the 6-month follow-up period [83].
Immunomodulation
As was previously mentioned, under pathological conditions such as tissue injury, BMSSCs migrate to the site of damage, where they exert their effects (Table 3). Tissue damage is usually accompanied by pro-inflammatory factors, produced by both innate and adaptive immune responses, to which BMSSCs are known to respond. In fact, BMSSCs have significant immunosuppressive effects by inhibiting several cells of the immune system including T-lymphocytes, B-cells, dendritic cells and natural killer cells [84]. The interactions of BMSSCs with the immune cells result in attenuated inflammatory responses and the promotion of anti-inflammatory pathways (Fig. 2, 4). BMSSCs reduce secretion of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interferon γ (IFN-γ) and increase production of anti-inflammatory interleukin-10 (IL-10) and interleukin-4 (IL-4) [85]. Moreover, the low or complete lack of MHC Class I, Class II and co-stimulatory molecules on BMSSCs render them non-immunogenic, supporting the concept that transplantation of allogeneic BMSSCs does not require immunosupression of the host [86].
Table 3.
Clinical studies using bone marrow stromal stem cells for immunomodulation.
| Reference | Study design | No. of patients | Indication | Source | Administration | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|
| Le Blanc et al. 2004 [87] | Case report | 1 | Acute GVHD | Allogeneic (from mother) | iv injection | Patient alive without disease | 1 year |
| Prasad et al. 2011 [89] | Case series | 12 | Refractory acute GVHD | Allogeneic, premanufactured (Prochymal) | iv injection | No treatment-related serious adverse events,58 % CR, 17 % PR, 25 % MR. Complete resolution of GI symptoms in 75 % | 1–3 years |
| Slavin et al. 2008 [77] | Case series | 12 | MS, ALS | Autologous | Intrathecal and iv injection | Safe procedure, no major risks | 1 year |
| Yamout et al. 2010 [91] | Case series | 10 | MS | Autologous | Intrathecal injection | Clinical but no radiologic improvement | 1 year |
| Connick et al. 2012 [92] | Case series | 10 | Secondary progressive MS | Autologous | Intravenous infusion | No serious adverse events, improvement in visual endpoints | 6 months |
| Duijvestein et al. 2010 [93] | Case series | 10 | Refractory Crohn’s disease | Autologous | iv injection | Safe, reduction in Crohn’s disease activity index (CDAI) | 6 weeks |
| Carrion et al. 2010 [94] | Case series | 2 | SLE | Autologous | iv infusion | No adverse effects, but no modification of initial disease activity | 14 weeks |
| Liang et al. 2010 [95] | Case series | 15 | Refractory SLE | Allogeneic (donors from patients’ families) | iv infusion | No serious adverse events, decreased disease activity, improvement in serological markers, stabilization of renal function | 3–36 months |
HA: Hydroxyapatite; β-TCP: Beta-tricalcium phosphate; iv: Intravenous; ip: Intraperitoneally; sc: Subcutaneously; MI: Myocardial infarction; LV: Left ventricular; ALS: Amyotrophic lateral sclerosis; MS: Multiple sclerosis; GVHD: Graft vs. host disease; CR: Complete response; PR: Partial response; MR: Mixed response; GI: Gastrointestinal SLE: Systemic lupous erythematosus
Fig. 4.
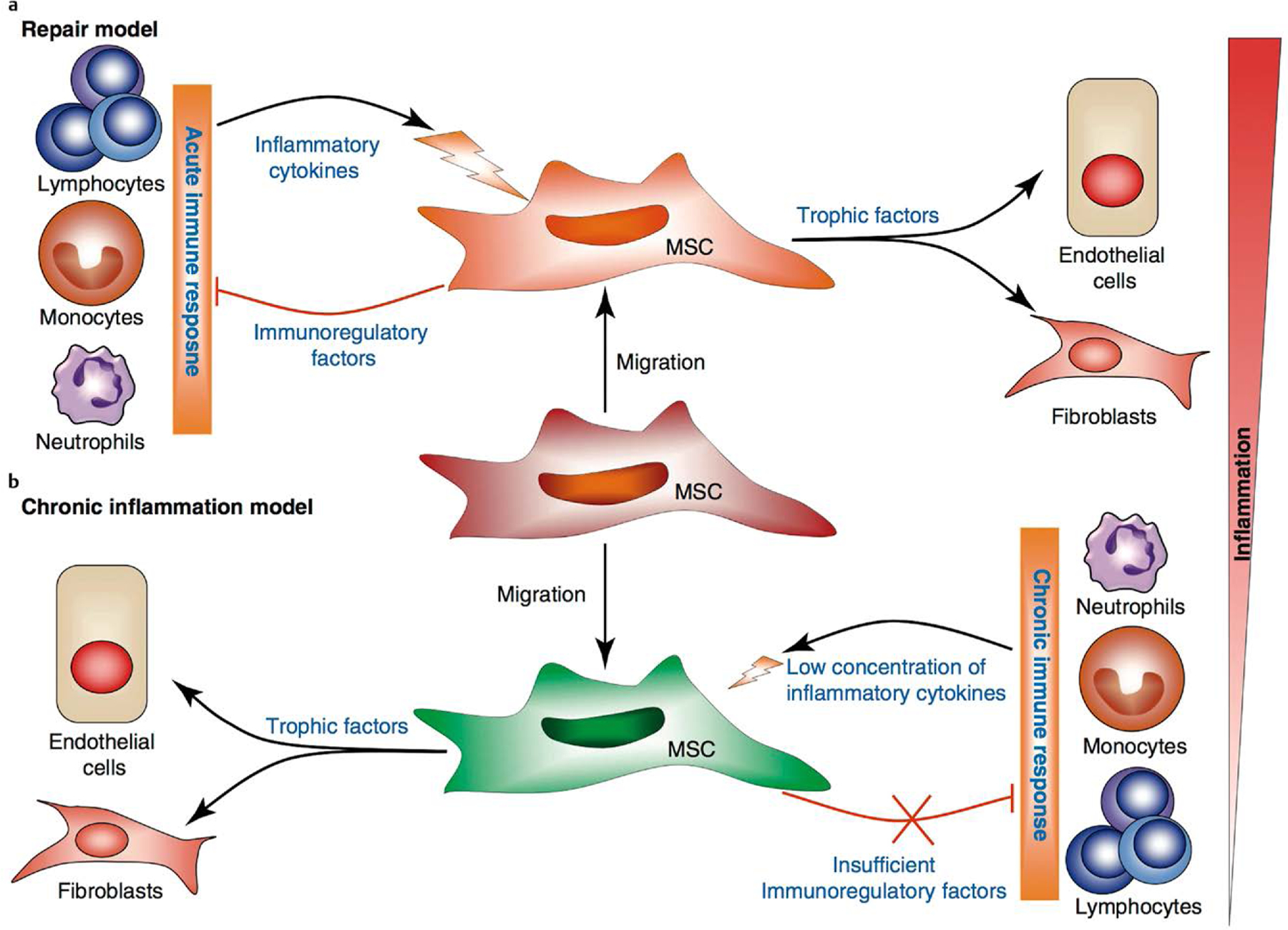
Immunomodulatory function of SSCs. SSCs are recruited to sites of tissue damage in response to local inflammatory cytokines produced by activated immune cells. SSC activation leads to the production of immunosuppressive factors and trophic factors. Depending on the types of immune responses (acute vs. chronic inflammation), SSCs may either attenuate the inflammatory response and lead to tissue repair, or maintain a persistent chronic inflammatory response, leading to fibrosis and deformation of tissue architecture [84].
It has to be noted, however, that the migration of BMSSCs to distant sites of tissue injury, the suppression of uncontrolled immune responses, and the production bioactive molecules are well-orchestrated events, which all contribute to tissue repair.
Immunomodulatory conditions
The immunomodulatory properties of BMSSCs make them a useful tool for the treatment of autoimmune diseases. The first clinical case report of successful use of BMSSCs in this context was associated with the treatment of severe steroid-refractory acute graft vs. host disease (GVHD) in a 9-year-old boy with acute lymphoblastic leukemia [87]. The successful outcome of this case served as a foundation for BMSSC therapy to be investigated extensively in the treatment and prevention of steroid-refractory GVHD. Although numerous clinical trials have confirmed that allogeneic transplantation of BMSSC is a safe and efficacious treatment for pediatric patients, the results have been mixed when testing this approach in adults [88]. There is currently one commercially available, FDA-approved, ex vivo expanded allogeneic BMSSC product, Prochymal®, for the treatment of steroid-refractory GVHD in children [89]. Twelve pediatric patients with severe steroid-refractory acute GVHD were treated with intravenous infusion of Prochymal and followed-up for 1–3 years. The treatment was well tolerated, no serious adverse events were seen and 9 out of 12 patients experienced complete resolution of gastro-intestinal symptoms [89].
The application of BMSSCs has recently been investigated as an alternative to hematopoietic stem cell transplantation for the treatment of multiple sclerosis (MS) [74]. MS is considered an autoimmune disease, characterized by chronic progressive demyelination and inflammation of the central nervous system, which leads to physical and cognitive impairment. Even though it has been suggested that there are differences in mesenchymal stem cell cytokine profiles between MS patients and healthy donors, clinical trials on MS patients treated with autologous BMSSCs have provided evidence of safety with no serious adverse events or improvements in some of the clinical manifestations of the disease [77, 90–92].
BMSSC based therapies have been evaluated for treatment of other autoimmune conditions and the limited clinical trials conducted so far show variability in their results. A phase I clinical trial performed in 10 Crohn’s disease patients who were followed short-term, for 6 weeks, reported that intravenous administration of autologous bone marrow derived MSCs was safe and feasible [93]. However, the clinical outcomes were mixed with some patients showing decrease in Crohn’s disease activity index score; and some patients requiring surgery due to disease worsening. Another important finding of the study was that BMSSCs isolated from patients with Crohn’s disease showed similar morphology, phenotype, growth potential and immunomodulatory capacity compared to BMSSCs from healthy donors [93]. In the treatment of another debilitating inflammatory condition, systemic lupus erythematosus (SLE), a case series reported that the intravenous infusion with allogeneic BMSSCs was safe but showed limited effects on disease activity and clinical manifestations of the disease at the 4 month follow-up [94]. On the other hand, allogeneic BMSSC transplantation has been shown to provide short-term benefit in treatment of 15 patients with SLE refractory to conventional treatment options [95]. BMSSC therapy has also attracted attention as a therapeutic approach for rheumatic diseases. However, only preclinical studies have explored the therapeutic potential of these therapies in the treatment of rheumatoid arthritis reporting controversial results, hence no clinical trials have been conducted to date [96]. (Table 3)
Current Challenges and Conclusions
Although there is clinical evidence to support the incorporation of BMSSCs into tissue engineering and regenerative protocols, several challenges remain to make these approaches more feasible. Key issues are associated with difficulties in isolation and characterization of stem cells, as well as the establishment of cell expansion conditions that meet regulatory standards and lead to consistent and well characterized cell populations [97]. Fundamentally, there is still a need for better understanding of the mechanisms involved in stem cell differentiation and how these cells interact with the variable regenerative and healing micro-environments in which they are used. Even with increased knowledge of stem cell behavior and properties, cost effective means of procuring and manufacturing them need to be developed. Additionally, sophisticated yet more simplified and practical materials and scaffolds will also need to be commercialized to deliver BMSSCs in the appropriate manner.
Despite the increase in the number of clinical studies using BMSSC based therapies over the last decade, the bulk of the evidence includes case reports, case series and small phase 1/2 clinical trials which have demonstrated limited but beneficial effects in various human disease contexts. More encouraging is the fact that regardless of the condition being treated, route of delivery, or delivery vehicle used, strong evidence exists in the clinical studies reported to date that autologous and allogeneic transplantation of BMSSCs is safe. Although companies world-wide now manufacture stem cells and are evaluating their use in cell-based therapies, there are no FDA approved stem cell products using BMSSCs at present. If these therapies are to be approved for widespread use in the variety of clinical scenarios outlined in this review, larger, multi-centered clinical trials are needed with longer, more consistent follow-ups.
Acknowledgements
The authors would like to acknowledge NIDCR/NIH (R56-DE-025097) for support of this work.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–1147 [DOI] [PubMed] [Google Scholar]
- 2.Young HE, Black AC Jr. Adult stem cells. Anat Rec A Discov Mol Cell Evol Biol 2004; 276: 75–102 [DOI] [PubMed] [Google Scholar]
- 3.Young HE, Duplaa C, Katz R, Thompson T, Hawkins KC, Boev AN, Henson NL, Heaton M, Sood R, Ashley D, Stout C, Morgan JH 3rd, Uchakin PN, Rimando M, Long GF, Thomas C, Yoon JI, Park JE, Hunt DJ, Walsh NM, Davis JC, Lightner JE, Hutchings AM, Murphy ML, Boswell E, McA-bee JA, Gray BM, Piskurich J, Blake L, Collins JA, Moreau C, Hixson D, Bowyer FP 3rd, Black AC Jr. Adult-derived stem cells and their potential for use in tissue repair and molecular medicine. J Cell Mol Med 2005; 9: 753–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronthos S, Akintoye SO, Wang CY, Shi S. Bone marrow stromal stem cells for tissue engineering. Periodontology 2000 2006; 41: 188–195 [DOI] [PubMed] [Google Scholar]
- 5.Kim EH, Heo CY. Current applications of adipose-derived stem cells and their future perspectives. World J Stem Cell 2014; 6: 65–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa BR, Parreira RC, Fonseca EA, Amaya MJ, Tonelli FM, Lacerda SM, Lalwani P, Santos AK, Gomes KN, Ulrich H, Kihara AH, Resende RR. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry A 2014; 85: 43–77 [DOI] [PubMed] [Google Scholar]
- 7.Collins M Fibrous Dysplasia: A Model Disease for the Study of cAMP in Bone. Horm Metab Res 2012; 44: A-14 [Google Scholar]
- 8.Bahn RS. Current Insights into the Pathogenesis of Graves’ Ophthalmopathy. Horm Metab Res 2015; 47: 773–778 [DOI] [PubMed] [Google Scholar]
- 9.Rahmig S, Bornstein SR, Chavakis T, Jaeckel E, Waskow C. Humanized mouse models for type 1 diabetes including pancreatic islet transplantation. Horm Metab Res 2015; 47: 43–47 [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Lozano P, Rajan P. Stem cells as in vitro models of disease. Curr Stem Cell Res Ther 2007; 2: 280–292 [DOI] [PubMed] [Google Scholar]
- 11.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999; 354: Si32–Si34 [DOI] [PubMed] [Google Scholar]
- 12.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci 2004; 4: 743–765 [DOI] [PubMed] [Google Scholar]
- 13.Furth ME, Atala A, Van Dyke ME. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 2007; 28: 5068–5073 [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Choi B, Wu B, Lee M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013; 5: 045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv Q, Deng M, Ulery BD, Nair LS, Laurencin CT. Nano-ceramic composite scaffolds for bioreactor-based bone engineering. Clin Orthop Relat Res 2013; 471: 2422–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temple JP, Yeager K, Bhumiratana S, Vunjak-Novakovic G, Grayson WL. Bioreactor cultivation of anatomically shaped human bone grafts. Meth Mol Biol (Clifton, NJ) 2014; 1202: 57–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinert AF, Rackwitz L, Gilbert F, Noth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cell Transl Med 2012; 1: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213: 341–347 [DOI] [PubMed] [Google Scholar]
- 19.Bo T, Zhu JM. Low levels of free testosterone correlated with bone mineral densities of femoral necks in aged healthy Shanghainese men. Horm Metab Res 2014; 46: 697–701 [DOI] [PubMed] [Google Scholar]
- 20.Dumic-Cule I, Draca N, Luetic AT, Jezek D, Rogic D, Grgurevic L, Vukicevic S. TSH prevents bone resorption and with calcitriol synergistically stimulates bone formation in rats with low levels of calciotropic hormones. Horm Metab Res 2014; 46: 305–312 [DOI] [PubMed] [Google Scholar]
- 21.Hind K, Jones B, King RF. Re: bone: an acute buffer of plasma sodium during exhaustive exercise? Horm Metab Res 2014; 46: 819–820 [DOI] [PubMed] [Google Scholar]
- 22.Connolly JF. Clinical use of marrow osteoprogenitor cells to stimulate osteogenesis. Clinical orthopaedics and related research 1998; 355 (Suppl): S257–S266 [DOI] [PubMed] [Google Scholar]
- 23.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Eng J Med 2001; 344: 385–386 [DOI] [PubMed] [Google Scholar]
- 24.Hendrich C, Franz E, Waertel G, Krebs R, Jager M. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev 2009; 1: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone 2007; 40: 522–528 [DOI] [PubMed] [Google Scholar]
- 26.Mehrabani D, Mojtahed Jaberi F, Zakerinia M, Hadianfard MJ, Jalli R, Tanideh N, Zare S. The Healing Effect of Bone Marrow-Derived Stem Cells in Knee Osteoarthritis: A Case Report. World J Plast Surg 2016; 5: 168–174 [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA 2002; 99: 8932–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahill RA, Wenkert D, Perlman SA, Steele A, Coburn SP, McAlister WH, Mumm S, Whyte MP. Infantile hypophosphatasia: transplantation therapy trial using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab 2007; 92: 2923–2930 [DOI] [PubMed] [Google Scholar]
- 29.Kawate K, Yajima H, Ohgushi H, Kotobuki N, Sugimoto K, Ohmura T, Kobata Y, Shigematsu K, Kawamura K, Tamai K, Takakura Y. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Org 2006; 30: 960–962 [DOI] [PubMed] [Google Scholar]
- 30.Neen D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine 2006; 31: E636–E640 [DOI] [PubMed] [Google Scholar]
- 31.Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 2007; 13: 947–955 [DOI] [PubMed] [Google Scholar]
- 32.Aoyama T, Goto K, Kakinoki R, Ikeguchi R, Ueda M, Kasai Y, Maekawa T, Tada H, Teramukai S, Nakamura T, Toguchida J. An exploratory clinical trial for idiopathic osteonecrosis of femoral head by cultured autologous multipotent mesenchymal stromal cells augmented with vascularized bone grafts. Tissue Eng B Rev 2014; 20: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernigou P, Pariat J, Queinnec S, Homma Y, Flouzat Lachaniette CH, Chevallier N, Rouard H. Supercharging irradiated allografts with mesenchymal stem cells improves acetabular bone grafting in revision arthroplasty. Int Orthop 2014; 38: 1913–1921 [DOI] [PubMed] [Google Scholar]
- 34.Bolland BJ, Partridge K, Tilley S, New AM, Dunlop DG, Oreffo RO. Biological and mechanical enhancement of impacted allograft seeded with human bone marrow stromal cells: potential clinical role in impaction bone grafting. Regener Med 2006; 1: 457–467 [DOI] [PubMed] [Google Scholar]
- 35.Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmoller M, Russo PA, Bolte H, Sherry E, Behrens E, Terheyden H. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004; 364: 766–770 [DOI] [PubMed] [Google Scholar]
- 36.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials 2008; 29: 3053–3061 [DOI] [PubMed] [Google Scholar]
- 37.Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg 2012; 40: 2–7 [DOI] [PubMed] [Google Scholar]
- 38.Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, Tarle SA, Bartel RL, Giannobile WV. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transpl 2013; 22: 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajan A, Eubanks E, Edwards S, Aronovich S, Travan S, Rudek I, Wang F, Lanis A, Kaigler D. Optimized cell survival and seeding efficiency for craniofacial tissue engineering using clinical stem cell therapy. Stem Cell Transl Med 2014; 3: 1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, Wang F, Lanis A, Giannobile WV. Bone Engineering of Maxillary Sinus Bone Deficiencies Using Enriched CD90 + Stem Cell Therapy: A Randomized Clinical Trial. J Bone Miner Res 2015; 30: 1206–1216 [DOI] [PubMed] [Google Scholar]
- 41.Marx RE, Harrell DB. Translational research: The CD34 + cell is crucial for large-volume bone regeneration from the milieu of bone marrow progenitor cells in craniomandibular reconstruction. Int J Oral Maxillofac Impl 2014; 29: e201–e209 [DOI] [PubMed] [Google Scholar]
- 42.Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthros-copy 2013; 29: 2020–2028 [DOI] [PubMed] [Google Scholar]
- 43.Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, Kuroda R, Kurosaka M, Yoshiya S, Hattori K, Ohgushi H. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regener Med 2011; 5: 146–150 [DOI] [PubMed] [Google Scholar]
- 44.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sport Med 2010; 38: 1110–1116 [DOI] [PubMed] [Google Scholar]
- 45.Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Ca negieter SC, Roelofs H, Al Younis I, Dibbets-Schneider P, Fibbe WE, Zwaginga JJ, Bax JJ, Schalij MJ, Beeres SL, Atsma DE. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res 2013; 6: 816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Kwon W, Hong IS, Lee K, Kwan J, Park KS, Choi D, Jang YS, Hong MK. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci 2014; 29: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004; 94: 92–95 [DOI] [PubMed] [Google Scholar]
- 48.Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, Voridis EM, Papamichail M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv 2005; 65: 321–329 [DOI] [PubMed] [Google Scholar]
- 49.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr., Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am College Cardiol 2009; 54: 2277–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 2011; 108: 792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathiasen AB, Jorgensen E, Qayyum AA, Haack-Sorensen M, Ekblond A, Kastrup J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial). Am Heart J 2012; 164: 285–291 [DOI] [PubMed] [Google Scholar]
- 52.Ascheim DD, Gelijns AC, Goldstein D, Moye LA, Smedira N, Lee S, Klodell CT, Szady A, Parides MK, Jeffries NO, Skerrett D, Taylor DA, Rame JE, Milano C, Rogers JG, Lynch J, Dewey T, Eichhorn E, Sun B, Feldman D, Simari R, O’Gara PT, Taddei-Peters WC, Miller MA, Naka Y, Bagiella E, Rose EA, Woo YJ. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014; 129: 2287–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 2014; 311: 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res 2014; 114: 1302–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012; 308: 2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henry TD, Traverse JH, Hammon BL, East CA, Bruckner B, Remmers AE, Recker D, Bull DA, Patel AN. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circ Res 2014; 115: 730–737 [DOI] [PubMed] [Google Scholar]
- 57.Chin SP, Poey AC, Wong CY, Chang SK, Tan CS, Ng MT, Chew KH, Lam KH, Cheong SK. Intramyocardial and intracoronary autologous bone marrow-derived mesenchymal stromal cell treatment in chronic severe dilated cardiomyopathy. Cytotherapy 2011; 13: 814–821 [DOI] [PubMed] [Google Scholar]
- 58.Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, Heldman AW, Hare JM. Rationale and design of the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (the POSEIDON-DCM study): a phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. J Cardiovasc Transl Res 2014; 7: 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathiasen AB, Haack-Sorensen M, Jorgensen E, Kastrup J. Autotrans-plantation of mesenchymal stromal cells from bone-marrow to heart in patients with severe stable coronary artery disease and refractory angina–final 3-year follow-up. Int J Cardiol 2013; 170: 246–251 [DOI] [PubMed] [Google Scholar]
- 60.Snykers S, De Kock J, Rogiers V, Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cell (Dayton, Ohio) 2009; 27: 577–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol 2011; 23: 936–941 [DOI] [PubMed] [Google Scholar]
- 62.El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev 2012; 8: 972–981 [DOI] [PubMed] [Google Scholar]
- 63.Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, Park HJ, Park SY, Kim BR, Kim JW, Soo Kim H, Kwon SO, Choi EH, Kim YM. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int 2014; 34: 33–41 [DOI] [PubMed] [Google Scholar]
- 64.Hauger O, Frost EE, van Heeswijk R, Deminiere C, Xue R, Delmas Y, Combe C, Moonen CT, Grenier N, Bulte JW. MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology 2006; 238: 200–210 [DOI] [PubMed] [Google Scholar]
- 65.El-Ansary M, Saadi G, Abd El-Hamid SM. Mesenchymal stem cells are a rescue approach for recovery of deteriorating kidney function. Nephrology (Carlton, Vic) 2012; 17: 650–657 [DOI] [PubMed] [Google Scholar]
- 66.Seissler J, Schott M. Generation of insulin-producing beta cells from stem cells–perspectives for cell therapy in type 1 diabetes. Horm Metab Res 2008; 40: 155–161 [DOI] [PubMed] [Google Scholar]
- 67.Theil A, Wilhelm C, Guhr E, Reinhardt J, Bonifacio E. The relative merits of cord blood as a cell source for autologous T regulatory cell therapy in type 1 diabetes. Horm Metab Res 2015; 47: 48–55 [DOI] [PubMed] [Google Scholar]
- 68.Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, Li L, Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol 2009; 50: 1174–1183 [DOI] [PubMed] [Google Scholar]
- 69.Xie QP, Huang H, Xu B, Dong X, Gao SL, Zhang B, Wu YL. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differ Res Biol Divers 2009; 77: 483–491 [DOI] [PubMed] [Google Scholar]
- 70.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015; 64: 587–592 [DOI] [PubMed] [Google Scholar]
- 71.Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, Sachdeva N, Sharma RR, Marwaha N, Khandelwal N. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant 2014; 23: 1075–1085 [DOI] [PubMed] [Google Scholar]
- 72.Dash SN, Dash NR, Guru B, Mohapatra PC. Towards reaching the target: clinical application of mesenchymal stem cells for diabetic foot ulcers. Rejuv Res 2014; 17: 40–53 [DOI] [PubMed] [Google Scholar]
- 73.Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 74.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regener Med 2010; 5: 933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001; 32: 1005–1011 [DOI] [PubMed] [Google Scholar]
- 76.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003; 53: 697–702 discussion 702–693 [DOI] [PubMed] [Google Scholar]
- 77.Slavin S, Kurkalli BG, Karussis D. The potential use of adult stem cells for the treatment of multiple sclerosis and other neurodegenerative disorders. Clin Neurol Neurosurg 2008; 110: 943–946 [DOI] [PubMed] [Google Scholar]
- 78.Oh KW, Moon C, Kim HY, Oh SI, Park J, Lee JH, Chang IY, Kim KS, Kim SH. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cell Transl Med 2015; 4: 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Cheng H, Hua R, Yang J, Dai G, Zhang Z, Wang R, Qin C, An Y. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: a preliminary clinical study. Cytotherapy 2013; 15: 1549–1562 [DOI] [PubMed] [Google Scholar]
- 80.Chen G, Wang Y, Xu Z, Fang F, Xu R, Wang Y, Hu X, Fan L, Liu H. Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy. J Translat Med 2013; 11: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK, Totey SM. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Translat Res 2010; 155: 62–70 [DOI] [PubMed] [Google Scholar]
- 82.Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, Dixit A, Rauthan A, Murgod U, Totey S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy 2009; 11: 897–911 [DOI] [PubMed] [Google Scholar]
- 83.Zhang ZX, Guan LX, Zhang K, Zhang Q, Dai LJ. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy 2008; 10: 134–139 [DOI] [PubMed] [Google Scholar]
- 84.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012; 33: 136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822 [DOI] [PubMed] [Google Scholar]
- 86.Myers TJ, Granero-Molto F, Longobardi L, Li T, Yan Y, Spagnoli A. Mesenchymal stem cells at the intersection of cell and gene therapy. Expert Opin Biol Ther 2010; 10: 1663–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441 [DOI] [PubMed] [Google Scholar]
- 88.Introna M, Rambaldi A. Mesenchymal stromal cells for prevention and treatment of graft-versus-host disease: successes and hurdles. Curr Opin Organ Transplant 2015; 20: 72–78 [DOI] [PubMed] [Google Scholar]
- 89.Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 2011; 17: 534–541 [DOI] [PubMed] [Google Scholar]
- 90.Mazzanti B, Aldinucci A, Biagioli T, Barilaro A, Urbani S, Dal Pozzo S, Amato MP, Siracusa G, Crescioli C, Manuelli C, Bosi A, Saccardi R, Massacesi L, Ballerini C. Differences in mesenchymal stem cell cytokine profiles between MS patients and healthy donors: implication for assessment of disease activity and treatment. J Neuroimmunol 2008; 199: 142–150 [DOI] [PubMed] [Google Scholar]
- 91.Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol 2010; 227: 185–189 [DOI] [PubMed] [Google Scholar]
- 92.Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 2012; 11: 150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 2010; 59: 1662–1669 [DOI] [PubMed] [Google Scholar]
- 94.Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D, Monckeberg G, Figueroa FE. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus 2010; 19: 317–322 [DOI] [PubMed] [Google Scholar]
- 95.Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis 2010; 69: 1423–1429 [DOI] [PubMed] [Google Scholar]
- 96.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Int Med 2013; 28: 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monsarrat P, Vergnes JN, Planat-Benard V, Ravaud P, Kemoun P, Sensebe L, Casteilla L. An Innovative, Comprehensive Mapping and Multiscale Analysis of Registered Trials for Stem Cell-Based Regenerative Medicine. Stem Cell Transl Med 2016; 5: 826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res 2015; 48: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 2014; 15: 1009–1016 [DOI] [PubMed] [Google Scholar]


