Abstract
Purpose
The purpose of this study was to investigate the genetic and clinical characteristics of eyes shut homolog (EYS)-associated retinitis pigmentosa (RP).
Methods
This was a retrospective cross-sectional observational study of 36 patients with EYS-associated autosomal recessive RP (arRP).
Results
The gene sequencing results revealed that c.6416G>A (p.Cys2139Tyr) and c.7228+1G>A were the two most predominant variants in our cohort and that variants near the C-terminus, which contains alternating laminin and epidermal growth factor (EGF) domains, accounted for the majority of the allele counts (58 of a total of 72) and relative allele frequencies (81%). Over half of the patients presented with pericentral-type RP (n = 19, 60%), which frequently occurred in combination with macular lesions (n = 10, 52%). Patients having both variants within the alternating laminin and EGF domains near the C-terminus had a more severe disease progression (average 0.045 logMAR increase per year) than those having one variant in the N-terminus and the other in the C-terminus (average 0.001 logMAR increase per year).
Conclusions
Pericentral RP was the major phenotype in patients with EYS-associated arRP. There was also a statistically significant relationship between the location of the variants and the severity of the disease.
Translational Relevance
This study may aid patients with EYS-associated arRP to predict future vision acuity based on their genetic and clinical features.
Keywords: eyes shut homolog (EYS), genotype–phenotype correlation, inherited retinal disease, pericentral retinitis pigmentosa, retinitis pigmentosa (RP)
Introduction
Retinitis pigmentosa (RP), an inherited retinal disease that afflicts an estimated 1 in 4000 individuals worldwide, is the primary cause of visual disability in young people. The disease is characterized initially by the progressive degeneration of rods, resulting in night blindness and a restricted visual field, and subsequently by a substantial loss in visual acuity later in life once the cones are also affected. The most common inheritance pattern of the disease is autosomal recessive, which accounts for 50% to 60% of all patients with RP, whereas autosomal dominant and X-linked inheritance occur in only 30% to 40% and 5% to 15% of patients, respectively.1 Currently, there are over 60 causal genes of autosomal recessive RP (arRP) registered on the Retinal Information Network (RetNet) database, demonstrating the genetic heterogeneity of the disease.
The eyes shut homolog (EYS) gene, which spans over 2 Mb of genomic DNA with 44 exons and encodes a 3165-amino-acid protein, is the largest eye-specific gene identified to date.2,3 The human EYS protein was initially found to be the ortholog of the Drosophila protein spacemaker (spam), which has an essential role in the morphogenesis of photoreceptors in insects.3 Previous studies have shown that EYS is located in the connecting cilium of the photoreceptor and that its deficiency causes mislocalization of the outer segment proteins and degeneration of the photoreceptors in zebrafish, thus further supporting its importance in maintaining the retinal architecture.4–6 With a reported prevalence of 7% to 32.8% in different populations worldwide, EYS gene is recognized as one of the common disease-causing gene of arRP7 and is the major cause in the Taiwanese population.8 As the gene size of EYS exceeds the 4.7 kb carrying capacity of adeno-associated viral (AAV) vectors, EYS-associated RP cannot be treated by AAV gene therapy. The potential alternative would be gene editing through a base editor, such as the clustered regularly interspaced short palindrome repeats (CRISPR)-associated protein 9 (Cas9) system. Therefore, the identification and genetic characterization of the EYS variants would be important for the development of therapies for EYS-associated arRP.9
According to the Leiden Open Variation Database, there are currently over 600 reported variants of the EYS gene. The genetic spectrum of EYS also differs widely, as some variants are predominantly found in certain populations.10 There is also heterogeneity in the clinical manifestations of the EYS variants, which exhibit differences in disease progression7 and phenotype.11 The phenotypic spectrum of EYS-associated diseases even extends to cone-rod dystrophy12,13 and macular dystrophy.13 Given the genetic and clinical heterogeneity of these variants, subgroup analysis might help in elucidating the causes behind these differences. Therefore, this study was carried out to investigate the detailed genetic and clinical characteristics of a Taiwanese cohort of patients with EYS-associated arRP.
Methods
Subjects
From July 2015 to June 2020, 36 patients with EYS-associated arRP, each of whom carried at least 2 EYS variants, were recruited from 31 families enrolled in the Taiwan Inherited Retinal Degeneration Project. The patients were evaluated at the Department of Ophthalmology, National Taiwan University Hospital. All patients with RP and cone-rod dystrophy were diagnosed clinically by retinal specialists on the basis of clinical symptoms, family history, and fundus imaging findings. The patients’ clinical information and electroretinogram, color fundus photography, optical coherence tomography (OCT), and fundus autofluorescence (FAF) imaging results were studied retrospectively. The visual acuity of each patient was determined using the Snellen chart and the results were converted to the logarithm of the minimum angle of resolution (logMAR) scale for analysis according to previous studies.14,15 The visual acuity measures “counting fingers,” “hand movement,” “light perception,” and “no light perception” were assigned logMAR values of 1.9, 2.3, 2.8, and 2.9, respectively.
The study was approved by the Research Ethics Committee of the National Taiwan University Hospital (institutional review board [IRB] No.: 201408082RINC). Informed consent was obtained from all patients and local ethics committee of National Taiwan University Hospital (NTUH). Authors reporting experiments on humans confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Genetic Analysis
Blood samples were obtained from the patients, and genomic DNA was then extracted from the peripheral blood mononuclear cells using a DNA extraction kit. The DNA was then subjected to capture-based next-generation sequencing targeting 212 inherited retinal disease-associated genes (Supplementary Digital Content 1). The target genes were selected from the RetNet database, Online Mendelian Inheritance in Man (OMIM) database (https://www.ncbi.nlm.nih.gov/omim), and published studies (PubMed search query: hereditary retinal dystrophy). The detailed methods of genetic testing are described in our previously published paper.8
Phenotype Grouping Based on Fundus Autofluorescence Imaging Findings
On the basis of the distribution of involved retinal areas on FAF imaging, all patients were classified into four morphological subtypes: typical, pericentral, diffuse, and sectoral. The typical type is defined by the degeneration of the photoreceptor or retinal pigment epithelium, occurring initially in the mid-periphery and then moving pericentrally, with a distinct border between the abnormal/periphery and normal/macular regions. In the pericentral type, the degeneration starts in the near-periphery around the vascular arcades, and the far-periphery usually remains intact and better preserved than that of the typical type.16 The ring scotoma region of the pericentral type usually has an outer diameter of between 5 degrees and 30 degrees.17 The diffuse type is similar to the typical type but without the apparent border. The sectoral type is defined if only one or two quadrants are affected. Any type of RP with an isolated macular lesion is characterized by an additional macular lesion, presenting as a hyperautofluorescent ring or patches of atrophy.
Genotype Grouping Based on Variant Location
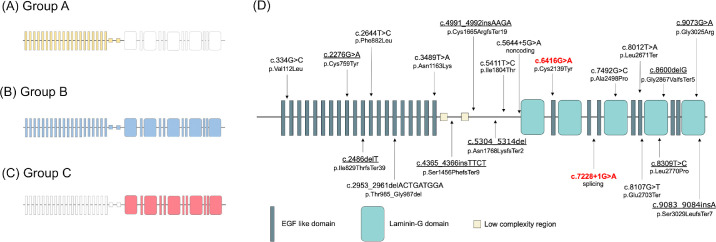
To assess the influence of the variant location on the clinical presentation, the patients were classified into three groups (Fig. 1A–C): group A patients have both variants in the N-terminus (c.1-c.5647), group B patients have one variant in the N-terminus and the other in the C-terminal laminin G containing domain (c.5647-c.9495), and group C patients have both variants in the C-terminus (c.5647-c.9495).
Figure 1.
Schematic of the structure of the eyes shut homolog (EYS) gene. (A, B, C) Graphical representation of the classification of the genotype groups. The shaded areas represent the regions where variants are located in each group. (A) In the group A patients, the nucleotide positions of all variants are located in the N-terminus (c.1-c.5647; shown as a yellow-shaded area). (B) In the group B patients, they have one variant in the N-terminus and the other in the C-terminal laminin G containing domain (c.5647-c.9495; shown as a blue-shaded area). (C) In the group C patients, the nucleotide positions of all variants are located in the C terminal laminin G containing domain (shown as a red-shaded area). (D) Schematic of the EYS gene structure with the locations of the variants found in this study. Nine novel variants are underlined; the two most predominant variants in our cohort are highlighted in red.
Statistical Analysis
The descriptive results in this study are expressed as the mean ± standard deviation or the median with interquartile range (IQR), depending on the normality of distribution for continuous variables and number and percentage for discrete variables. Comparison among groups was assessed using the Kruskal–Wallis test. Multiple and univariate linear regression models were developed for the prediction of visual acuity. All data were analyzed using R studio (version 4.0.3). Results with P values of less than 0.05 were considered statistically significant.
Results
Demographics
Of the 36 patients enrolled in this study, 18 (50%) were men and 18 (50%) were women. The median age at first visit was 43 years (range = 18–78 years, IQR = 20 years). The median age at symptom onset was 25 years (range = 12–63 years, IQR = 26 years). The mean follow-up period, defined by the length of time between the first and last visits, was 2 years (IQR = 3 years). Most of the patients in this cohort were diagnosed with arRP (35/36, 97%), whereas the remaining patient was afflicted with cone-rod dystrophy (1/35, 3%). The detailed genotype and phenotype results are summarized in Table 1.
Table 1.
Genotype and Phenotype of the Patients With EYS-Associated Autosomal Recessive Retinitis Pigmentosa
| Visual Acuity at First Visit (LogMAR) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID | Patient ID | Gender | Diagnosis | Nucleotide Change | Protein Variants | Inheritance Pattern | Age at First Visit (Years) | Age at Symptom Onset (Years)* | OD | OS | Genotype Group |
| F1 | P1 | M | RP | c.7228+1G>A(;)8012T>A | p.(?)(;)(Leu2671Ter) | AR | 21 | 16 | 0.22 | 0.05 | C |
| P2 | M | RP | c.7228+1G>A(;)8012T>A | p.(?)(;)(Leu2671Ter) | AR | 18 | 16 | 0.15 | 0.15 | C | |
| F2 | P3 | M | RP | c.[6416G>A];[6416G>A] | p.[Cys2139Tyr];[Cys2139Tyr] | AR | 71 | 50 | 2.80 | 2.80 | C |
| F3 | P4 | F | RP | c.[6416G>A];[7228+1G>A] | p.[Cys2139Tyr];[?] | AR | 37 | 15 | 0.40 | 0.40 | C |
| P5 | F | RP | c.[6416G>A];[7228+1G>A] | p.[Cys2139Tyr];[?] | AR | 49 | N.A. | 0.10 | 0.22 | C | |
| P6 | M | RP | c.[6416G>A];[7228+1G>A] | p.[Cys2139Tyr];[?] | AR | 47 | N.A. | 0.82 | 0.82 | C | |
| F4 | P7 | M | RP | c.6416G>A(;)7228+1G>A | p.(Cys2139Tyr)(;)(?) | AR | 59 | 50 | 1.30 | 1.30 | C |
| F5 | P8 | M | RP | c.[4991_4992insAAGA];[5644+5G>A] | p.[Cys1665ArgfsTer19];[?] | AR | 41 | 30 | 0.30 | 0.40 | A |
| F6 | P9 | M | RP | c.334G>C(;)7228+1G>A | p.(Val112Leu)(;)(?) | AR | 77 | 50 | 0.22 | 0.15 | B |
| F7 | P10 | M | CRD | c.3489T>A(;)8107G>T | p.(Asn1163Lys)(;)(Glu2703Ter) | AR | 32 | 30 | 0.15 | 0.40 | B |
| F8 | P11 | F | RP | c.4365_4366insTTCT(;)9083_9084insA | p.(Ser1456PhefsTer9)(;)(Ser3029LeufsTer7) | AR | 58 | 48 | 0.40 | 0.30 | B |
| F9 | P12 | F | RP | c.[6416G>A];[7228+1G>A] | p.[Cys2139Tyr];[?] | AR | 46 | 28 | 1.70 | 0.70 | C |
| F10 | P13 | F | RP | c.[6416G>A];[6416G>A] | p.[Cys2139Tyr];[Cys2139Tyr] | AR | 37 | 21 | 0.10 | 0.10 | C |
| F11 | P14 | M | RP | c.6416G>A(;)7228+1G>A | p.(Cys2139Tyr)(;)(?) | AR | 37 | 16 | 0.10 | 0.00 | C |
| P15 | F | RP | c.6416G>A(;)7228+1G>A | p.(Cys2139Tyr)(;)(?) | AR | 36 | 16 | 0.52 | 0.22 | C | |
| F12 | P16 | F | RP | c.7492G>C(;)8107G>T | p.(Ala2498Pro)(;)(Glu2703Ter) | AR | 64 | 45 | 1.70 | 1.70 | C |
| F13 | P17 | M | RP | c.6416G>A(;)7492G>C | p.(Cys2139Tyr)(;)(Ala2498Pro) | AR | 55 | 45 | 1.30 | 0.52 | C |
| F14 | P18 | M | RP | c.6416G>A(;)7492G>C | p.(Cys2139Tyr)(;)(Ala2498Pro) | AR | 63 | 15 | 2.28 | 2.28 | C |
| F15 | P19 | F | RP | c.[8600del];[8600del] | p.[Gly2867ValfsTer5];[Gly2867ValfsTer5] | AR | 36 | 21 | 0.22 | 0.30 | C |
| F16 | P20 | F | RP | c.5304_5314del(;)6416G>A | p.(Asn1768LysfsTer2)(;)(Cys2139Tyr) | AR | 64 | 21 | 0.40 | 0.40 | B |
| P21 | M | RP | c.5304_5314del(;)6416G>A | p.(Asn1768LysfsTer2)(;)(Cys2139Tyr) | AR | 60 | 43 | 0.52 | 0.52 | B | |
| F17 | P22 | F | RP | c.2644T>C(;)5411T>C | p.(Phe882Leu)(;)(Ile1804Thr) | AR | 41 | 38 | 0.30 | 1.00 | A |
| F18 | P23 | M | RP | c.5644+5G>A(;)8107G>T | p.(?)(;)(Glu2703Ter) | AR | 54 | 25 | 1.30 | 1.30 | B |
| F19 | P24 | F | RP | c.[7228+1G>A];[7228+1G>A] | p.[?];[?] | AR | 41 | 12 | 0.82 | 1.30 | C |
| F20 | P25 | M | RP | c.6416G>A(;)9073G>A | p.(Cys2139Tyr)(;)(Gly3025Arg) | AR | 42 | 21 | 0.80 | 0.70 | C |
| F21 | P26 | M | RP | c.6416G>A(;)8012T>A | p.(Cys2139Tyr)(;)(Leu2671Ter) | AR | 40 | 20 | 0.40 | 0.52 | C |
| F22 | P27 | F | RP | c.6416G>A(;)8107G>T | p.(Cys2139Tyr)(;)(Glu2703Ter) | AR | 49 | N.A. | 1.00 | 0.82 | C |
| F23 | P28 | F | RP | c.6416G>A(;)8012T>A | p.(Cys2139Tyr)(;)(Leu2671Ter) | AR | 28 | N.A. | 0.10 | 0.05 | C |
| F24 | P29 | F | RP | c.[6416G>A];[6416G>A] | p.[Cys2139Tyr];[Cys2139Tyr] | AR | 56 | N.A. | 2.28 | 0.82 | C |
| F25 | P30 | F | RP | c.2486delT(;)6416G>A | p.(Ile829ThrfsTer39)(;)(Cys2139Tyr) | AR | 68 | N.A. | 1.00 | 1.30 | B |
| F26 | P31 | F | RP | c.6416G>A(;)8012T>A | p.(Cys2139Tyr)(;)(Leu2671Ter) | AR | 43 | N.A. | 0.40 | 0.40 | C |
| F27 | P32 | M | RP | c.2276G>A(;)6416G>A | p.(Cys759Tyr)(;)(Cys2139Tyr) | AR | 63 | 63 | 0.15 | 0.40 | B |
| F28 | P33 | M | RP | c.7492G>C(;)8309T>C | p.(Ala2498Pro)(;)(Leu2770Pro) | AR | 31 | 24 | 0.15 | 0.40 | C |
| F29 | P34 | M | RP | c.6416G>A(;)8107G>T | p.(Cys2139Tyr)(;)(Glu2703Ter) | AR | 50 | N.A. | 0.30 | 0.70 | C |
| F30 | P35 | F | RP | c.2953_2961delACTGATGGA(;)5644+5G>A | p.(Thr985_Gly987del)(;)(?) | AR | 46 | 30 | 0.22 | 0.70 | A |
| F31 | P36 | F | RP | c.6416G>A(;)7228+1G>A | p.(Cys2139Tyr)(;) | AR | 57 | N.A. | 1.00 | 0.70 | B |
EYS, eyes shut homolog gene; RP, retinitis pigmentosa; CRD, cone-rod dystrophy; AR, autosomal recessive; N.A., not available; OD, oculus dextrus; OS, oculus sinister.
Some data are not available owing to self-reporting.
EYS Variants and In Silico Molecular Genetic Analysis
Capture-based next-generation sequencing of the DNA from the patients identified 20 variants distributed throughout the entire EYE gene sequence (Fig. 1D), of which 9 were novel variants. The 2 most predominant variants in this cohort were c.6416G>A (p.Cys2139Tyr) and c.7228+1G>A (Table 2), with relative allele frequencies of 36.11% and 18.06%, respectively. With regard to the distribution of the variants, 11 were located near the N-terminus before the beginning of the first laminin G domain, whereas 9 were located near the C-terminus that contained alternating laminin and epidermal growth factor (EGF) domains. Although there were more variants near the N-terminus, the variants near the C-terminus accounted for the majority of the allele counts (58 of a total of 72) and relative allele frequencies (81%). The top five variants with the highest relative allele frequencies were also all located near the C-terminus. The results of the detailed in silico analysis of the 20 variants are shown in Supplementary Digital Content 2.
Table 2.
Results of the Genetic Analysis of the 20 EYS Variants
| No. | Variant (Nucleotide Change; Amino Acid Change) | Variant Type | Allele Count | Relative Allele Frequency | ACMG Classification |
|---|---|---|---|---|---|
| 1 | c.6416G>A (p.Cys2139Tyr) | Missense | 26 | 36.11% | Likely pathogenic |
| 2 | c.7228+1G>A | Splicing | 13 | 18.06% | Pathogenic |
| 3 | c.8012T>A (p.Leu2671Ter) | Nonsense | 5 | 6.94% | Pathogenic |
| 4 | c.8107G>T (p.Glu2703Ter) | Nonsense | 5 | 6.94% | Pathogenic |
| 5 | c.7492G>C (p.Ala2498Pro) | Missense | 4 | 5.56% | Uncertain significance |
| 6 | c.5644+5G>A | Noncoding | 3 | 4.17% | Uncertain significance |
| 7 | c.8600delG (p.Gly2867ValfsTer5) | Frameshift | 2 | 2.78% | Likely pathogenic |
| 8 | c.5304_5314del (p.Asn1768LysfsTer2) | Frameshift | 2 | 2.78% | Pathogenic |
| 9 | c.4991_4992insAAGA (p.Cys1665ArgfsTer19) | Frameshift | 1 | 1.39% | Likely pathogenic |
| 10 | c.334G>C (p.Val112Leu) | Missense | 1 | 1.39% | Uncertain significance |
| 11 | c.3489T>A (p.Asn1163Lys) | Missense | 1 | 1.39% | Pathogenic |
| 12 | c.9083_9084insA (p.Ser3029LeufsTer7) | Frameshift | 1 | 1.39% | Likely pathogenic |
| 13 | c.4365_4366insTTCT (p.Ser1456PhefsTer9) | Frameshift | 1 | 1.39% | Likely pathogenic |
| 14 | c.2644T>C (p.Phe882Leu) | Missense | 1 | 1.39% | Uncertain significance |
| 15 | c.5411T>C (p.Ile1804Thr) | Missense | 1 | 1.39% | Uncertain significance |
| 16 | c.9073G>A (p.Gly3025Arg) | Missense | 1 | 1.39% | Uncertain significance |
| 17 | c.2486delT (p.Ile829ThrfsTer39) | Frameshift | 1 | 1.39% | Likely pathogenic |
| 18 | c.2276G>A (p.Cys759Tyr) | Missense | 1 | 1.39% | Uncertain significance |
| 19 | c.8309T>C (p.Leu2770Pro) | Missense | 1 | 1.39% | Uncertain significance |
| 20 | c.2953_2961delACTGATGGA (p.Thr985_Gly987del) | In-frame | 1 | 1.39% | Likely pathogenic |
c.6416G>A (p.Cys2139Tyr) and c.7228+1G>A were the most prevalent variants in our cohort.
ACMG, American College of Medical Genetics and Genomics.
Phenotype Distribution of the EYS-Associated arRP Cohort
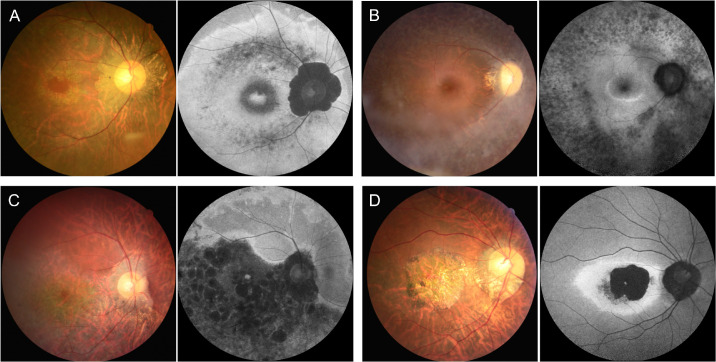
Based on the phenotypic classification, the majority of our patients were classified as having pericentral-type RP (n = 19, 60%), and over half of them also had combined macular lesions (n = 10, 52%; Fig. 2). Three patients were classified with typical-type RP (10%) and two with sectoral-type RP (6%), whereas one patient had diffuse-type RP (3%) and another had cone-rod dystrophy (3%). Six other patients (19%) already had end-stage disease and were therefore not amenable to classification.
Figure 2.
Representative cases of different phenotypic subtypes in our cohort with eyes shut homolog (EYS)-associated autosomal recessive retinitis pigmentosa (arRP), based on the distribution of the involved retinal areas on fundus autofluorescence (FAF) imaging. There was high phenotypic heterogeneity among our patients. (A) A 51-year-old woman (patient ID: P27) classified with pericentral-type RP with macular involvement. FAF imaging showed the hypoautofluorescent (hypo-AF) lesion surrounded by two concentric hyper-AF bands interiorly and exteriorly along the vascular arcades, with sparing of the far periphery. There was also an increased AF signal in the fovea region. (B) A 37-year-old woman (patient ID: P13) classified with typical-type RP. FAF imaging showed an extensive hypo-AF signal along and beyond the vessel arcade. (C) A 43-year-old man (patient ID: P25) classified with inferior sectoral RP. FAF imaging showed a hypo-AF inferior sectoral lesion. (D) A 35-year-old man (patient ID: P10) classified with cone-rod dystrophy. FAF imaging showed a hyper-AF ring inside the vessel arcade, with a near absence of autofluorescence within.
Disease Progression According to Different Variant Locations
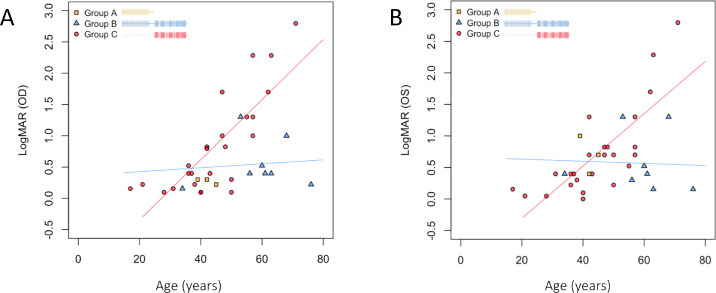
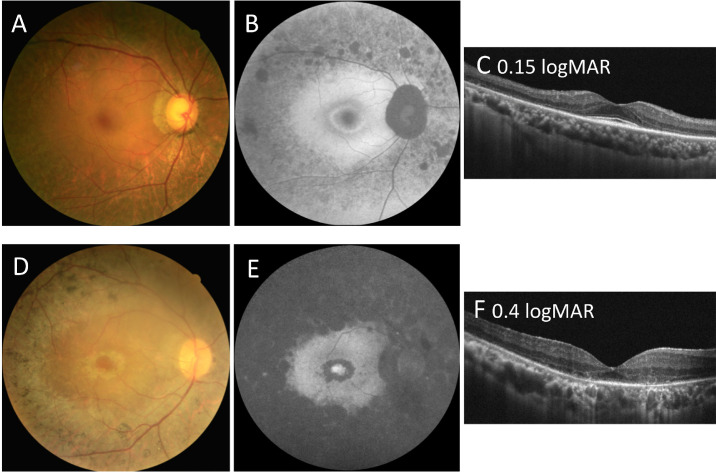
To investigate the correlation between variant location and clinical presentation, the progression of visual loss, age at symptom onset, and age at first visit were compared among groups A, B, and C, which comprised 3 (8%), 8 (22%), and 25 (69%) patients, respectively. As the most frequent alleles were located near the C-terminus, which contains alternating laminin and EGF domains, the majority of our patients were in groups B and C. Both the age of symptom onset and the age at first visit were much lower in group C (mean = 21 and 43 years, respectively) than in group B (mean = 43 and 63 years, respectively), with the difference in age at first visit between these two groups being statistically significant (P = 0.031). The progression of visual acuity was analyzed by estimating the visual acuity (i.e. the oculus dextrus) as a function of other variables, including age, gender, genotype subgroup, and other baseline measurements. After performing stepwise selection and using P = 0.05 as a cutoff for both selection and elimination, only age and genotype subgroup remained as the predictors in our final model, giving an overall R2 value of 0.528 (Supplementary Digital Content 3). The linear models fitted for the genotype subgroups (Fig. 3) revealed group C (patients having all variants in the C-terminus) to have a more severe disease progression than group B (patients having one variant in the N-terminus and the other in the C-terminus). The increase in logMAR visual acuity in group C was an average of 0.045 logMAR per year, whereas that in group B was an average of 0.001 logMAR per year. Linear regression was not fitted for group A because of the small sample size. Two patients were selected from groups B and C for a more detailed comparison. The patient from group B (patient ID: P32; 63 years of age) had a relatively normal visual acuity of 0.15 logMAR and relatively preserved FAF and OCT imaging findings. By contrast, the patient from group C (patient ID: P26; 40 years of age) already had mild visual impairment with a visual acuity of 0.4 logMAR, a near absence of autofluorescence in the FAF image, and a loss of the ellipsoid zone line in the OCT image (Fig. 4, see Table 1). As c.7228+1G>A and c.6416G>A are the two most predominant variants in group C, we have done a further within-group analysis to decide whether their presence would skew group C to a more severe disease progression. However, we did not find a relationship between the number of alleles from either c.7228+1G>A or c.6416G>A and the disease severity in terms of onset age, age at examination, or the rate of visual acuity decline in group C (Supplementary Fig. S1). These results imply that if the variants occur near the C-terminus region containing alternating laminin and EGF domains, the disease course will be more severe than that resulting from other variants in other locations.
Figure 3.
Comparison of the progression of logMAR visual acuity among patients with autosomal recessive retinitis pigmentosa carrying eyes shut homolog (EYS) gene variants at different locations. (A, B) Visual acuity of the right (A) and left eyes (B) at the first visit, presented as a function of age. Every point represents an individual patient in our cohort. The blue and red lines are the linear regression lines for groups B and C, respectively. (A) The coefficients for age in groups B and C are 0.0031 and 0.048, respectively. (B) The coefficients for age in groups B and group C are –0.001 and 0.041, respectively. The group C patients, who have variants near the C-terminus, had a more severe disease progression than the group B patients, who have one variant near the N-terminal domain and the other in the C-terminal laminin G containing domain (c.5647-c.9495). OD, right eye; OS, left eye.
Figure 4.
Comparison of fundal imaging findings among patients with autosomal recessive retinitis pigmentosa carrying eyes shut homolog (EYS) gene variants at different locations. (A, B, C) Color fundus photograph and fundus autofluorescence (FAF) and optical coherence tomography (OCT) imaging results for a 63-year-old man (patient ID: P32) carrying the c.2276G>A and c.6416G>A variants, showing a relatively normal visual acuity of 0.15 logMAR. He was classified as group B for the genetic subgroup and as pericentral-type RP for the phenotypic subtype. (B) FAF imaging showing the hypo-AF signal along the vascular arcades and hyper-AF circular patches inside the retinal arcade with a perifoveal hyper-AF ring. The far periphery is relatively spared. (C) OCT imaging showed the thinning of the outer retinal area and shortening of the ellipsoid zone line. The central macula region is relatively preserved. (D, E, F) Color fundus photograph and FAF and OCT imaging results for a 40-year-old man (patient ID: P26) carrying the c.6416G>A and c.8012T>A variants, showing mild visual impairment of 0.4 logMAR. He was classified as group C for the genetic subgroup and as typical-type RP for the phenotypic subtype. (E) FAF imaging showing the near absence of autofluorescence outside, but hyper-AF circular patches inside, the retinal arcade. There was also an increased AF signal in the fovea region. (F) OCT imaging showed the thinning of the outer retinal area and the loss of a discernible ellipsoid zone line.
Discussion
Two EYS Variants With High Allele Frequency in Our Cohort
The genetic results revealed c.6416G>A (p.Cys2139Tyr) and c.7228+1G>A to be the two most prevalent variants in our cohort. Both of them as well as other reported variants appear to occur mostly in the Asian population.18,19 The recurrent variant c.6416G>A appears to be especially prevalent in the Chinese population, with one study reporting its presence in 8 of 18 Chinese patients with EYS-associated RP.18 Although c.7228+1G>A has also been reported in other Chinese patients, it occurs less frequently in this population than c.6416G>A.20
In our study, although eight patients from five families harbored the same compound heterozygous variants (c.6416G>A and c.7228+1G>A), they displayed phenotypic variability despite all having the same genotype. The reported age at symptom onset ranged from 15 to 50 years, and the disease progression was also variable. One patient (patient ID: P14) had a normal visual acuity of 0.1 and 0.0 logMAR in the right and left eyes, respectively, at the age of 40 years, whereas another patient (patient ID: P4) already had mild visual impairment with a visual acuity of 0.4 logMAR in both eyes at the age of 36 years (see Table 1). Intrafamilial phenotypic variability in terms of both age of symptom onset and fundus imaging findings among patients with the same genotype has also been previously reported.21
Genotype–Phenotype Correlation
Based on our results, the location of the EYS variants appeared to be related to the severity of the disease. Patients with all variants located near the C-terminus (group C) had an average increase of 0.045 logMAR per year, whereas patients contained variants in both near N-terminus and near C-terminus region (group B) had an average increase of 0.001 logMAR per year. The progression rate of group C is faster than the 0.015 logMAR increase per year reported in a previous study,13 whereas that of group B is much slower. Variants near the C-terminus were observed to lead to more deleterious effects than those near the N-terminus. It was also suggested in another study that the location of the EYS variant may affect the patterns on FAF imaging, as variants occurring in positions closer to the C-terminus were more likely to present with hyperautofluorescent rings on the FAF image.11 Whether macular involvement is the cause behind the disease severity related to the hyperautofluorescent rings requires further investigation. Nonetheless, these results highlight the importance of the region near the C-terminus for proper functioning of the EYS protein. The alternating laminin and EGF domains in the C-terminus region suggest the interaction of EYS with other binding proteins.6 The binding of EYS with matriglycans via the laminin G domain is also required for its localization to connecting cilia.22 Therefore, patients harboring EYS truncating variants are also expected to have a more severe disease progression, as was shown in one study.7 However, although the patients in our cohort harboring EYS truncating variants did seem to have an earlier age of symptom onset and earlier age at first visit, the data differences were not statistically significant. Adding grouping based on truncating variants as an additional predictor for visual acuity also did not make a statistically significant improvement to the prediction. It may also be possible that our sample size was not large enough to detect significant results.
Pericentral Type of EYS-Associated Autosomal Recessive Retinitis Pigmentosa
As described above, the phenotype of EYS-associated arRP in our cohort was predominantly of the pericentral type. Although previous studies of EYS-associated arRP did not further distinguish the pericentral from the typical types in their cohorts, on the basis of the FAF images provided, we found that the majority of this disease in other cohorts was also of the pericentral type.11,23 To date, it is still unclear why pericentral-type EYS-associated arRP is predominant. Recent studies have revealed that EYS may be important for the localization of the outer segment proteins (e.g. rhodopsin, opsin-1 short-wave-sensitive 1 [opn1sw1], guanine nucleotide-binding protein G[I]/G[S]/G[T] subunit beta-3 [GNB3], and peripherin-2) and the maintenance of the actin filaments in the photoreceptors. Hence, EYS protein deficiency or dysfunction would cause outer segment protein mislocalization and structural disruption, resulting in the death of the photoreceptors.4 However, the photoreceptors are supposed to be equally affected, as all of them would possess affected EYS protein. Most of the FAF images from our patients had concentric hyperautofluorescent or hypoautofluorescent rings, indicating that the variation in photoreceptor degeneration might not be associated with EYS alone. As the progression of cell death follows a specific pattern, the microenvironment of the retina might also play a major role. Interestingly, the initial region of atrophy (5 degrees to 30 degrees) not only surrounded the arcade vessels but also corresponded to where the rod photoreceptors reach their highest density (10 degrees to 20 degrees).24 It can be hypothesized that the affected EYS proteins may cause the photoreceptors to become more susceptible to the stress that these regions contain.
It is interesting to note that another disease, hydroxychloroquine retinopathy, coincidently shares some of the same characteristics as the disease of our cohort. Recent studies have found that hydroxychloroquine retinopathy in the Asian population may develop predominantly with the pericentral pattern rather than the classic bull's-eye pattern.25,26 In our cohort, EYS-associated arRP presented with mostly pericentral patterns, a trend that increased in prevalence among populations in the eastern parts of the world. The mechanisms behind the pericentral pattern of EYS-associated arRP and hydroxychloroquine retinopathy are still unclear, and further studies are needed to clarify whether both diseases share some of the same mechanistic pathways.
Limitations of This Study
This study had several limitations. First, as this is a retrospective study, some missing data could have led to biased results. In particular, as patients presented to this hospital with different stages of the disease, a small number of patients were already in the end stage of the disease and could not be classified into a specific phenotypic pattern. Second, the sample size may not be large enough to obtain significant results regarding other predictors. Third, as more severe alleles within a disease-causing gene also may be observed more often in clinical practice, it is possible that potential ascertainment bias may arise. Finally, because all patients were Taiwanese, the phenotype distribution or other findings may not apply to other ethnicities. Nonetheless, this is the first study to highlight the importance of genetic defects located in alternating laminin and EGF domains and pericentral disease progression as being the main characteristics of EYS-associated RP. Future studies with a longer follow-up period and larger cohort size may provide more definitive insights into this disease.
Conclusion
In conclusion, this is the first detailed genetic and clinical analyses of a cohort of patients with EYS-associated arRP in Taiwan. Our results demonstrated the genetic and clinical heterogeneity of the disease, the predominance of the pericentral phenotype in the Taiwanese cohort, and the statistically significant relationship between the location of the variants and the severity of the disease.
Supplementary Material
Acknowledgments
Disclosure J.-E. Lo, None; C.-Y. Cheng, None; C.-H. Yang, None; C.-M. Yang, None; Y.-C. Chen, None; Y.-S. Huang, None; P.-L. Chen, None; T.-C. Chen, None
References
- 1. Hartong DT, Berson EL, Dryja TP.. Retinitis pigmentosa. Lancet. 2006; 368: 1795–1809. [DOI] [PubMed] [Google Scholar]
- 2. Collin RWJ, Littink KW, Klevering BJ, et al.. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet. 2008; 83: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abd El-Aziz MM, Barragan I, O'Driscoll CA, et al.. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 2008; 40: 1285–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu Z, Hu X, Liu F, et al.. Ablation of EYS in zebrafish causes mislocalisation of outer segment proteins, F-actin disruption and cone-rod dystrophy. Sci Rep. 2017; 7: 46098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Messchaert M, Dona M, Broekman S, et al.. Eyes shut homolog is important for the maintenance of photoreceptor morphology and visual function in zebrafish. PLoS One. 2018; 13: e0200789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu M, Liu Y, Li J, et al.. Eyes shut homolog is required for maintaining the ciliary pocket and survival of photoreceptors in zebrafish. Biol Open. 2016; 5: 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwanami M, Oshikawa M, Nishida T, Nakadomari S, Kato S.. High prevalence of mutations in the EYS gene in Japanese patients with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012; 53: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 8. Chen T-C, Huan D-S, Lin C-W, et al.. Genetic characteristics and epidemiology of inherited retinal degeneration in Taiwan. NPJ Genom Med. 2021; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fry LE, McClements ME, MacLaren RE.. Analysis of Pathogenic Variants Correctable With CRISPR Base Editing Among Patients With Recessive Inherited Retinal Degeneration. JAMA Ophthalmol. 2021; 139: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hosono K, Ishigami C, Takahashi M, et al.. Two novel mutations in the EYS gene are possible major causes of autosomal recessive retinitis pigmentosa in the Japanese population. PLoS One. 2012; 7: e31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sengillo JD, Lee W, Nagasaki T, et al.. A Distinct Phenotype of Eyes Shut Homolog (EYS)-Retinitis Pigmentosa Is Associated With Variants Near the C-Terminus. Am J Ophthalmol. 2018; 190: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bandah-Rozenfeld D, Littink KW, Ben-Yosef T, et al.. Novel null mutations in the EYS gene are a frequent cause of autosomal recessive retinitis pigmentosa in the Israeli population. Invest Ophthalmol Vis Sci. 2010; 51: 4387–4394. [DOI] [PubMed] [Google Scholar]
- 13. Pierrache LHM, Messchaert M, Thiadens AAHJ, et al.. Extending the Spectrum of EYS-Associated Retinal Disease to Macular Dystrophy. Invest Ophthalmol Vis Sci. 2019; 60: 2049–2063. [DOI] [PubMed] [Google Scholar]
- 14. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M.. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg Visual Acuity Test. Invest Ophthalmol Vis Sci. 2006; 47: 1236–1240. [DOI] [PubMed] [Google Scholar]
- 15. Grover S, Fishman GA, Anderson RJ, et al.. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. 1999; 106: 1780–1785. [DOI] [PubMed] [Google Scholar]
- 16. Comander J, Weigel-DiFranco C, Maher M, et al.. The Genetic Basis of Pericentral Retinitis Pigmentosa—A Form of Mild Retinitis Pigmentosa. Genes (Basel). 2017; 8: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karali M, Testa F, Brunetti-Pierri R, et al.. Clinical and Genetic Analysis of a European Cohort with Pericentral Retinitis Pigmentosa. Int J Mol Sci. 2019; 21: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao X, Cao Y, Chen S, et al.. Whole exome sequencing reveals novel EYS mutations in Chinese patients with autosomal recessive retinitis pigmentosa. Mol Vis. 2019; 25: 35–46. [PMC free article] [PubMed] [Google Scholar]
- 19. Iwanami M, Oishi A, Ogino K, et al.. Five major sequence variants and copy number variants in the EYS gene account for one-third of Japanese patients with autosomal recessive and simplex retinitis pigmentosa. Mol Vis. 2019; 25: 766–779. [PMC free article] [PubMed] [Google Scholar]
- 20. Gu S, Tian Y, Chen X, Zhao C.. Targeted next-generation sequencing extends the phenotypic and mutational spectrums for EYS mutations. Mol Vis. 2016; 22: 646–657. [PMC free article] [PubMed] [Google Scholar]
- 21. Abd El-Aziz MM, O'Driscoll CA, Kaye RS, et al.. Identification of novel mutations in the ortholog of Drosophila eyes shut gene (EYS) causing autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010; 51: 4266–4272. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Yu M, Shang X, et al.. Eyes shut homolog (EYS) interacts with matriglycan of O-mannosyl glycans whose deficiency results in EYS mislocalization and degeneration of photoreceptors. Sci Rep. 2020; 10: 7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cundy O, Broadgate S, Halford S, et al.. Genetic and clinical findings in an ethnically diverse retinitis pigmentosa cohort associated with pathogenic variants in EYS. Eye (Lond)., 2021; 35: 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silverstein LD. Foundations of Vision, by Brian A. Wandell, Sinauer Associates, Inc., Sunderland, MA, 1995. xvi + 476 pp., hardcover $49.95. Color Res Application. 1996; 21: 142–144. [Google Scholar]
- 25. Lee DH, Melles RB, Jo SG, et al.. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015; 122: 1252–1256. [DOI] [PubMed] [Google Scholar]
- 26. Melles RB, Marmor MF.. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015; 122: 110–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.