Abstract
Most yeast species can ferment sugars to ethanol, but only a few can grow in the complete absence of oxygen. Oxygen availability might, therefore, be a key parameter in spoilage of food caused by fermentative yeasts. In this study, the oxygen requirement and regulation of alcoholic fermentation were studied in batch cultures of the spoilage yeast Zygosaccharomyces bailii at a constant pH, pH 3.0. In aerobic, glucose-grown cultures, Z. bailii exhibited aerobic alcoholic fermentation similar to that of Saccharomyces cerevisiae and other Crabtree-positive yeasts. In anaerobic fermentor cultures grown on a synthetic medium supplemented with glucose, Tween 80, and ergosterol, S. cerevisiae exhibited rapid exponential growth. Growth of Z. bailii under these conditions was extremely slow and linear. These linear growth kinetics indicate that cell proliferation of Z. bailii in the anaerobic fermentors was limited by a constant, low rate of oxygen leakage into the system. Similar results were obtained with the facultatively fermentative yeast Candida utilis. When the same experimental setup was used for anaerobic cultivation, in complex YPD medium, Z. bailii exhibited exponential growth and vigorous fermentation, indicating that a nutritional requirement for anaerobic growth was met by complex-medium components. Our results demonstrate that restriction of oxygen entry into foods and beverages, which are rich in nutrients, is not a promising strategy for preventing growth and gas formation by Z. bailii. In contrast to the growth of Z. bailii, anaerobic growth of S. cerevisiae on complex YPD medium was much slower than growth in synthetic medium, which probably reflected the superior tolerance of the former yeast to organic acids at low pH.
The yeast Zygosaccharomyces bailii is an important causative agent of spoilage of sweet and dry wines and other food products (6). In addition to causing undesirable properties (off-flavors, hazing), the vigorous alcoholic fermentation that occurs in spoiling foods may lead to explosion of canned and bottled foods and beverages. Z. bailii is highly tolerant to common food preservatives, high concentrations of sugars, ethanol, and organic acids, and low pH (3–5, 13, 14, 16).
The ability to ferment sugars to ethanol is widespread among yeasts (18). In principle, this property seems to indicate that the yeasts are capable of anaerobic free-energy transduction and, therefore, of growth in the absence of oxygen. However, most facultatively fermentative yeast species cannot grow under strictly anaerobic conditions (23). This fact indicates that in addition to its role in mitochondrial respiration, oxygen has other functions in cellular metabolism, probably in biosynthesis. Even Saccharomyces cerevisiae, which grows rapidly anaerobically (23), requires sterols and unsaturated fatty acids for strictly anaerobic growth, as synthesis of these compounds requires molecular oxygen (1, 2). Several factors have been proposed to explain the requirement for oxygen of most facultatively fermentative yeasts, including constraints on redox metabolism (19), mitochondrial ADP-ATP translocation (17, 24), and pyrimidine biosynthesis (9, 12).
The biosynthetic oxygen requirements of facultatively fermentative yeasts are extremely small. Consequently, special precautions (e.g., use of oxygen-resistant tubing and ultrapure nitrogen gas for sparging) are needed to minimize oxygen entry to the extent that these small oxygen requirements become apparent (23). So far, growth of the spoilage yeast Z. bailii has not been studied under rigorous oxygen limitation regimes, although growth under nonstrict anaerobiosis conditions has been reported recently (8). Regulation of growth and metabolism of Z. bailii under very severe oxygen limitation conditions is very important for determining the role of this organism in food spoilage, since canned or bottled foods and beverages are likely to be strictly anaerobic or at least very severely oxygen limited. In theory, oxygen availability may be a key parameter in alcoholic fermentation, growth, and food spoilage by Z. bailii.
The objectives of this study were to describe regulation of alcoholic fermentation in Z. bailii and to determine the oxygen requirements for growth and fermentation. We studied this yeast in aerobic and anaerobic cultures and compared it with S. cerevisiae and Candida utilis. S. cerevisiae was chosen because of its exceptionally rapid growth in anaerobic cultures (23), and C. utilis was chosen because it requires small amounts of oxygen for growth and for vigorous alcoholic fermentation (23, 25). All growth studies were performed at pH 3.0. To better mimic the nutritional environment in spoiling foods, some experiments were performed with complex medium instead of synthetic medium.
MATERIALS AND METHODS
Microorganisms and maintenance.
Z. bailii ISA 1307, originally isolated from a continuous-production plant that produced sparkling wine (26), was obtained from the Culture Collection of the Instituto Superior de Agronomia (Lisbon, Portugal); S. cerevisiae CEN.PK113-7D (20) was obtained from P. Kötter (J.-W. Goethe University, Frankfurt, Germany); and C. utilis (anamorph of Pichia jadinii) CBS 621 was obtained from the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. Stock cultures were stored in glycerol (30%, vol/vol) at −80°C, while working cultures were maintained on YPD agar plates at 30°C.
Growth media.
Defined synthetic medium (22) and complex YPD medium (11) were used for growth experiments. The media were supplemented with glucose (2%, wt/vol) as a carbon and energy source. For anaerobic growth in synthetic media, Tween 80 and ergosterol were added at concentrations of 420 and 10 mg · liter−1, respectively (21).
Inocula.
Inocula for aerobic growth experiments were prepared by pregrowing the yeasts in shake flasks with a headspace-to-culture volume ratio of 4. To prepare inocula for anaerobic growth experiments, the headspace-to-culture volume ratio was reduced to 0.25 to ensure that growth was oxygen limited. Moreover, the inocula used for anaerobic cultures were flushed with pure nitrogen gas for 30 min before inoculation of the fermentors. All shake flask cultures were incubated in an orbital shaker at 200 rpm at 30°C.
Batch cultivation in fermentors.
Aerobic and anaerobic batch cultures were grown at 30°C in 7-liter laboratory fermentors (Applikon, Schiedam, The Netherlands) with stirrer speeds of 800 and 600 rpm, respectively. The initial working volume of each culture was 4 liters. The pH was maintained at 3.0 ± 0.1 by automatic addition of KOH (2.0 M) via an ADI 1030 biocontroller. Aerobic cultures were flushed with air (4 liters · min−1) by using a Brooks 5850S mass flow controller. The dissolved oxygen concentration, as measured with a Mettler-Toledo polarographic oxygen probe, remained more than 30% of air saturation throughout all aerobic growth experiments. Anaerobic cultures were flushed with certified pure nitrogen gas (Air Products, Waddinxveen, The Netherlands) containing less than 5 ppm of oxygen at a flow rate of 0.5 liter · min−1. To minimize oxygen diffusion (23), the tubing used for the entire fermentor setup was Norprene tubing (Cole-Parmer, Vernon Hills, Ill.). The off-gas was cooled with a condenser (2°C). The O2 and CO2 concentrations in the off-gas were analyzed with an ADC 7000 gas analyzer (ADC BioScientific Ltd., Hoddesdon, United Kingdom).
Anaerobic cultivation in serum flasks.
Anaerobic cultures in 30-ml serum flasks capped with butyl rubber septa were grown on 20 ml of the synthetic medium used for fermentor cultivation. When present, uracil was added at a concentration of 20 mg · liter−1. Oxygen was removed by seven cycles of evacuation (down to a pressure of 0.2 × 105 Pa) and refilling with argon gas (up to a pressure of 1.8 × 105 Pa). After inoculation with a syringe, the flasks were incubated at 30°C at 1.8 × 105 Pa. Samples were withdrawn with a 1-ml syringe at regular time intervals.
Determinations of culture dry weight.
The dry weights of cells in culture samples were determined by using predried and preweighed nitrocellulose filters (pore size, 0.45 μm). After removal of the medium by filtration, the filters were washed with demineralized water, dried in a Sharp type R-4700 microwave oven for 20 min at 360 W, and reweighed. Parallel samples varied by less than 1%.
Metabolite analysis.
The concentrations of glucose, ethanol, acetate, glycerol, and uracil in culture supernatants were determined by high-pressure liquid chromatography (7).
Preparation of cell extracts.
Samples were collected from the reactors between the fourth and fifth generations after inoculation. Cells were harvested by centrifugation (10 min, 10,000 × g) and washed twice with 10 mM potassium phosphate buffer (pH 7.5) containing 2 mM EDTA. Each sample was concentrated and stored at −20°C. Before disruption, the samples were thawed, washed, and resuspended in 100 mM potassium buffer (pH 7.5) containing 1 mM dithiothreitol and 2 mM MgCl2. Extracts were prepared by sonication at 0°C for 7 min (0.5-min intervals) with a Measuring & Scientific Equipment Ltd. sonicator (150-W output; 7-μm peak-to-peak amplitude). Unbroken cells and debris were removed by centrifugation (20 min, 47,000 × g, 4°C). Each supernatant was used as a cell extract.
Enzyme assays.
Enzyme assays were performed immediately after the cell extracts were prepared. Spectrophotometric assays were carried out at 340 nm and 30°C. In all assays, the proportionality of the initial reaction velocity and the amount of cell extract added to the reaction mixture was verified. One unit of enzyme activity was defined as the quantity of the enzyme that catalyzed conversion of 1 μmol of substrate · min−1 under the assay conditions used. The results presented below are averages based on three independent experiments. Enzymes were assayed by using previously described procedures (10). For alcohol dehydrogenase (EC 1.1.1.1) the assay mixture contained 50 mM glycine-KOH (pH 9.0) and 1 mM NAD+, and the reaction was started with 100 mM ethanol. For glucose-6-phosphate dehydrogenase (EC 1.1.1.49) the assay mixture contained 50 mM Tris hydrochloride buffer (pH 8.0), 15 mM MgCl2, and 0.4 mM NADP+, and the reaction was started with 5 mM glucose 6-phosphate. For pyruvate decarboxylase (EC 4.1.1.1) the assay mixture contained 40 mM imidazole hydrochoride buffer (pH 6.5), 5 mM MgCl2, 0.2 mM thiamine pyrophosphate, 0.15 mM NADH, and 88 U of alcohol dehydrogenase (Boehringer, Mannheim, Germany), and the reaction was started with 50 mM pyruvate. The protein concentrations in cell extracts were determined by the Lowry method. Dried bovine serum albumin (catalog no. A-6003; Sigma Chemical Co., St. Louis, Mo.) was used as a standard.
RESULTS
Growth and alcoholic fermentation in aerobic cultures.
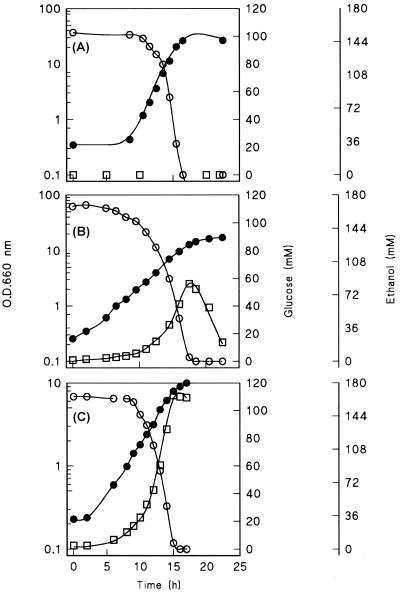
To characterize regulation of alcoholic fermentation in Z. bailii ISA 1307 and to obtain a reference for anaerobic growth experiments, we first studied growth on glucose and metabolite formation in aerobic batch cultures grown on a synthetic medium. As many of the foodstuffs and beverages (including wines) in which Z. bailii is encountered have a low pH, these fermentor cultures were grown at pH 3.0. Growth of Z. bailii was compared with growth of two reference organisms, S. cerevisiae CEN.PK113-7D and C. utilis CBS 621, which were grown under identical conditions. S. cerevisiae is known to exhibit vigorous alcoholic fermentation in aerobic, glucose-grown cultures, whereas C. utilis does not exhibit aerobic alcoholic fermentation. Although previous studies of these yeasts were performed mainly at pH 5.0 (20–22, 23, 25), the same behavior was observed at pH 3.0 (Fig. 1 and Table 1).
FIG. 1.
Optical densities at 660 nm (O.D.660 nm) (●) and concentrations of glucose (○) and ethanol (□) in aerobic, pH-controlled batch cultures of C. utilis (A), Z. bailii (B), and S. cerevisiae (C). Cultures were grown at pH 3.0 on a synthetic medium (22) supplemented with 20 g of glucose per liter. In all experiments, the dissolved oxygen concentration remained more than 30% of air saturation. Data from two independent replicate experiments differed by less than 10%.
TABLE 1.
Specific growth rates, biomass yields, and metabolic fluxes in aerobic and anaerobic cultures of Z. bailii, C. utilis, and S. cerevisiaea
| Medium | Conditions | Organism | Specific growth rate (h−1) | Biomass yield (g of biomass · g of glucose−1) | Metabolic fluxes (mmol · g of biomass−1 · h−1)
|
||
|---|---|---|---|---|---|---|---|
| Glucose | Ethanol | Glycerol | |||||
| Defined | Aerobic | Z. bailii | 0.25 ± 0.02 | 0.29 ± 0.01 | 4.7 ± 0.1 | 3.5 ± 0.1 | 0.10 ± 0.01 |
| C. utilis | 0.53 ± 0.02 | 0.53 ± 0.01 | 5.5 ± 0.1 | <0.01 | <0.01 | ||
| S. cerevisiae | 0.29 ± 0.01 | 0.12 ± 0.01 | 13 ± 1 | 19 ± 4 | 0.09 ± 0.06 | ||
| Anaerobic | Z. bailii | 0.03–0.08b | 0.07 | 1.9–6.4 | 2.6–9.4 | 0.3–1.1 | |
| C. utilis | 0.01–0.03b | 0.05 | 1.1–3.3 | 1.6–4.6 | 0.2–0.6 | ||
| S. cerevisiae | 0.27 ± 0.01 | 0.10 ± 0.01 | 15 ± 1 | 24 ± 1 | 2.7 ± 0.3 | ||
| Complex YPD | Anaerobic | Z. bailii | 0.09 ± 0.01 | 0.09 ± 0.01 | 5.6 ± 0.2 | 9.5 ± 0.9 | |
| S. cerevisiae | 0.17 ± 0.01 | 0.07 ± 0.004 | 13 ± 1 | 22 ± 1 | |||
Cells were grown on 20 g of glucose per liter in defined and complex YPD media in pH-controlled fermentors (pH 3.0). The data for aerobic Z. bailii and S. cerevisiae cultures are from the respirofermentative parts of the growth curves. Unless indicated otherwise, data are averages ± standard deviations based on duplicate independent experiments.
Range calculated along the linear growth curve (μ = dx/xdt, where μ is the specific growth rate and dx/dt is constant). Data obtained in three independent experiments differed by less than 10%.
Throughout growth of Z. bailii, the dissolved oxygen concentration remained more than 30% of air saturation. Nevertheless, this yeast produced substantial amounts of ethanol during aerobic growth (Table 1 and Fig. 1). At pH 3.0, the specific growth rate of Z. baillii was almost as high as that of S. cerevisiae. However, its specific rate of ethanol production was five- to sixfold lower than that of S. cerevisiae. As a result of the larger contribution of respiration to glucose dissimilation in Z. bailii, its biomass yield was higher than that of S. cerevisiae (Table 1). C. utilis, which exhibited completely respiratory metabolism, produced the highest biomass yield on glucose of the three yeasts (Table 1).
Anaerobic growth in synthetic medium.
The results described above indicate that Z. bailii can grow rapidly on synthetic medium in aerobic cultures. To examine growth and alcoholic fermentation in anaerobic cultures, the synthetic medium was supplemented with Tween 80 and ergosterol. These compounds are known to be required for anaerobic growth of S. cerevisiae (1, 2), the only yeast presently known to exhibit rapid anaerobic growth (23).
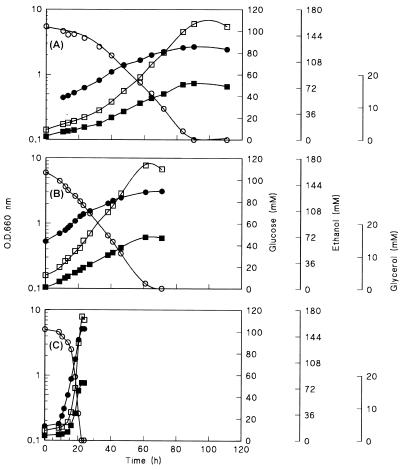
In the present study, fermentor cultures were sparged with ultrapure nitrogen to minimize the dissolved oxygen concentration. When this protocol was used, all three yeasts were able to grow. Ethanol and glycerol were the main fermentation products (Fig. 2 and Table 1). Consistent with previous studies, S. cerevisiae exhibited rapid growth, whereas C. utilis grew very poorly (23). For Z. bailii, anaerobic growth was much slower than growth under aerobic conditions (Table 1). Although the ethanol and glycerol yields on glucose were identical for the three yeasts studied, the specific production rates were much lower for Z. bailii and C. utilis, which is consistent with their much lower specific growth rates. Indeed, the amounts of biomass produced per amount of ATP generated during dissimilation were approximately the same for the three yeasts (data not shown), suggesting that significant uncoupling of growth and energy metabolism did not occur in Z. bailii and C. utilis.
FIG. 2.
Optical densities at 660 nm (O.D.660 nm) (●) and concentrations of glucose (○), ethanol (□), and glycerol (■) in anaerobic, pH-controlled batch cultures of C. utilis (A), Z. bailii (B), and S. cerevisiae (C). Cultures were grown at pH 3.0 on a synthetic medium (22) containing Tween 80 and ergosterol and supplemented with 20 g of glucose per liter. Fermentors were sparged with nitrogen gas to minimize oxygen diffusion into the cultures. Data from two independent replicate experiments differed by less than 10%.
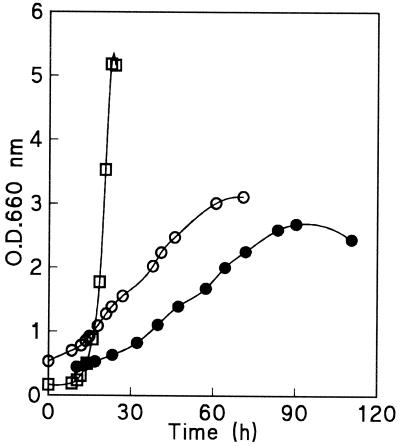
Completely anaerobic growth conditions are notoriously difficult to achieve in laboratory fermentors (23). The fact that in the anaerobic growth experiments the dissolved oxygen concentrations in the fermentors remained below the detection limit does not exclude the possibility that there was a small but significant flux of oxygen into the cultures. Therefore, it is conceivable that the slow growth of Z. bailii and C. utilis might have been due to slow leakage of oxygen into the fermentors. If a constant oxygen leakage rate were balanced by consumption of the oxygen by the biomass, linear rather than exponential growth kinetics would be expected. Indeed, analysis of the anaerobic growth curves revealed that linear growth occurred in both the Z. bailii and C. utilis cultures (Fig. 3). For both species an initial exponential growth phase was observed (Fig. 2 and 3). This initial exponential growth phase might reflect carryover of oxygen-requiring biosynthetic intermediates with the inoculum or, alternatively, greater oxygen availability in the early phases of growth.
FIG. 3.
Comparison of the anaerobic growth curves of C. utilis (●), Z. bailii (○), and S. cerevisiae (□). Optical densities at 660 nm (O.D.660 nm) are plotted on a linear axis. Cultures were grown anaerobically on a synthetic medium (see the legend to Fig. 2).
To estimate the rate of oxygen leakage into the fermentors, dissolved oxygen concentrations were monitored in the absence of cells after the nitrogen supply to an anaerobic fermentor was switched off. There was a slow linear increase in the amount of dissolved oxygen in the fermentor, which could be rapidly restored to a value below the detection limit by restoring the nitrogen flow. The rate of oxygen entry calculated from these experiments was 0.3 μmol of O2 · h−1. This slow oxygen leakage was insignificant compared to the estimated rate of oxygen inflow resulting from contamination of the nitrogen gas that was used to flush the fermentors. With an estimated oxygen content of 5 ppm (the maximum oxygen concentration in the nitrogen gas according to the manufacturer), ca. 6 μmol of O2 · h−1 entered the fermentors via this route.
Anaerobic growth in complex YPD medium.
Z. bailii is a well-known spoilage yeast in foods and beverages. In addition to being anaerobic or severely oxygen limited, these environments are typically very complex in terms of nutritional composition. Consequently, the experiments with synthetic medium, described above have limited relevance to food spoilage. We therefore investigated growth and alcoholic fermentation in complex YPD medium.
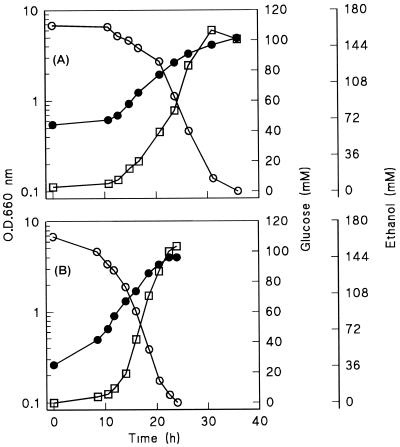
In complex YPD medium, anaerobic cultures of Z. bailii exhibited exponential growth kinetics. Apparently, growth in complex YPD medium was not limited by oxygen leakage (Fig. 4A). This suggests that components of YPD medium could alleviate a biosynthetic requirement for oxygen that limited growth on synthetic medium. In the facultatively fermentative yeast Pichia stipitis, uracil biosynthesis has been identified as a key oxygen-requiring biosynthetic process (12). We therefore tested whether addition of uracil stimulated anaerobic growth of Z. bailii on synthetic medium with Tween 80 and ergosterol in serum flask cultures. As observed in the anaerobic fermentor cultures, growth in these anaerobic cultures was slow and linear. After 100 h of incubation, neither the residual glucose concentrations nor the optical densities of cultures grown with uracil differed from those of reference cultures that did not contain uracil.
FIG. 4.
Optical densities at 660 nm (O.D. 660 nm) (●) and concentrations of glucose (○) and ethanol (□) in anaerobic, pH-controlled batch cultures of Z. bailii (A) and S. cerevisiae (B). Cultures were grown at pH 3.0 on complex YPD medium containing 20 g of glucose per liter (11). Cultures were sparged with nitrogen gas to minimize oxygen diffusion into the cultures. Data from two independent replicate experiments differed by less than 10%.
Anaerobic growth experiments with S. cerevisiae in complex YPD medium (at pH 3.0) revealed that (Fig. 4B) both the growth rate and the biomass yield of S. cerevisiae were substantially lower than the values for corresponding cultures in synthetic medium. In the case of C. utilis, anaerobic growth in complex YPD medium even resulted in a high frequency of dead cells (data not shown).
Regulation of alcoholic fermentation.
We assayed the specific activities of glucose-6-phosphate dehydrogenase, alcohol dehydrogenase, and pyruvate decarboxylase under anaerobic and aerobic conditions (Table 2). The specific activity of glucose-6-phosphate dehydrogenase, a key enzyme in biosynthesis, did not differ significantly in cells growing in the presence of oxygen and in cells growing in the absence of oxygen. An increase in the activity of alcohol dehydrogenase was observed under anaerobic conditions in Z. bailii and S. cerevisiae but not in C. utilis. Furthermore, in C. utilis the activity of pyruvate decarboxylase increased under anaerobic conditions, whereas no significant induction of this key fermentative enzyme was observed in anaerobic cultures of Z. bailii and S. cerevisiae.
TABLE 2.
Specific activities of glucose-6-phosphate dehydrogenase, pyruvate decarboxylase, and alcohol dehydrogenase in cell extracts from aerobic and anaerobic batch cultures of Z. bailii, C. utilis, and S. cerevisiaea
| Conditions | Organism | Sp act (U · mg of protein−1)
|
||
|---|---|---|---|---|
| Glucose-6-phosphate dehydrogenase | Pyruvate decarboxylase | Alcohol dehydrogenase | ||
| Aerobic | Z. bailii | 0.31 ± 0.02b | 0.73 ± 0.03 | 0.86 ± 0.03 |
| C. utilis | 0.85 ± 0.03 | 0.14 ± 0.01 | 0.84 ± 0.02 | |
| S. cerevisiae | 0.37 ± 0.01 | 1.2 ± 0.1 | 1.6 ± 0.15 | |
| Anaerobic | Z. bailii | 0.41 ± 0.01 | 0.95 ± 0.04 | 6.1 ± 0.25 |
| C. utilis | 0.63 ± 0.01 | 0.47 ± 0.03 | 0.97 ± 0.05 | |
| S. cerevisiae | 0.31 ± 0.06 | 1.3 ± 0.06 | 4.9 ± 0.18 | |
Cultures were grown in defined medium in pH-controlled fermentors at pH 3.0.
Average ± standard deviation based on the results obtained from two independent cultures.
DISCUSSION
We evaluated the ability of Z. bailii to grow under anaerobic conditions. In synthetic medium supplemented with ergosterol and an unsaturated fatty acid, growth of Z. bailii was very slow and linear, implying that Z. bailii is unable to grow or grows extremely poorly in synthetic medium in the complete absence of oxygen. The poor anaerobic growth of Z. bailii is not unusual. In an examination of 77 facultatively fermentative strains belonging to all known yeast genera, only 18 strains exhibited significant anaerobic growth, and S. cerevisiae was the only yeast that exhibited a specific growth rate greater than 0.05 h−1 (23). A definitive answer to the question of whether Z. bailii can grow at all in synthetic medium under completely anaerobic conditions (however slowly) will require extensive modifications in the fermentor setup. In particular, traces of oxygen must be removed from the nitrogen gas stream that is used for sparging, and the cause(s) of oxygen leakage or diffusion into the fermentors must be identified and eliminated.
The experiments performed with complex YPD medium indicated that in the context of the role of Z. bailii as a food spoilage organism, the (in)ability of this yeast to grow anaerobically in synthetic media is an academic matter. The rapid growth under anaerobic (or severely oxygen-limited) conditions in complex YPD medium, compared to the very slow growth in synthetic medium, indicates that oxygen availability is unlikely to limit cell proliferation and alcoholic fermentation by Z. bailii in food spoilage situations. Therefore, even the most stringent measures to prevent oxygen entry into canned or bottled foods and beverages are unlikely to decrease the risk of spoilage caused by Z. bailii.
Anaerobic growth of S. cerevisiae was impaired in the presence of complex YPD medium components (Fig. 2C and 4B). This observation is probably related to the low pH at which the fermentations were carried out, since shake flask studies at pH 5 to 6 consistently yield similar or higher specific growth rates on complex YPD medium (data not shown). Most likely, the presence of weak acids in yeast extract and/or peptone led to dissipation of the plasma membrane pH gradient in the S. cerevisiae cultures (15, 22). Alternatively, such inhibitory compounds may have been products of yeast metabolism in complex YPD medium. Growth inhibition by complex-medium components was not observed with Z. bailii, which supports the view that resistance to organic compounds and acidic environments is the predominant factor that allows growth and metabolic activity of Z. bailii in oxygen-limited or anaerobic food spoilage environments.
At present, the biochemical background underlying the (in)ability of facultatively fermentative yeasts to grow anaerobically is incompletely understood. Some authors have implicated dihydroorotate dehydrogenase (in S. cerevisiae encoded by the URA1 gene), a key enzyme in pyrimidine biosynthesis, as a pivotal enzyme in anaerobic growth (9). In several non-Saccharomyces yeasts, dihydroorotate dehydrogenase is linked to the mitochondrial respiratory chain and operates only under aerobic (or oxygen-limited) conditions. In contrast, the S. cerevisiae enzyme is soluble and can donate its electrons to fumarate (9). Indeed, an ability to grow under anaerobic conditions can be conferred to P. stipitis by transformation with the S. cerevisiae URA1 gene (12). Since addition of uracil to synthetic media did not have positive effect on anaerobic growth of Z. bailii, pyrimidine biosynthesis is not the only reason for the poor anaerobic growth of Z. bailii in synthetic medium. Nevertheless, the great difference in the specific growth rates of anaerobic Z. bailii cultures grown on synthetic and complex media, combined with the rapid aerobic growth on synthetic medium, indicates that complex YPD medium contains one or more compounds specifically required for anaerobic growth. Identification of these compounds should lead to a deeper understanding of the anaerobic physiology of yeasts and, provided that cellular uptake or metabolism of the compounds can be inhibited by food grade substances, may be highly relevant for controlling food spoilage.
ACKNOWLEDGMENTS
We thank Ton van Maris and Carole Raftery for critically reading the manuscript and our colleagues in Delft and Braga for stimulating discussions.
Fernando Rodrigues was the recipient of a fellowship from PRAXIS XXI, and this study was supported by a research grant from the Fundação para a Ciência e Tecnologia, Portugal (contract PRAXIS XXI P/AGR/11135/98).
REFERENCES
- 1.Andreasen A A, Stier T J B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Comp Physiol. 1953;41:23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen A A, Stier T J B. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Comp Physiol. 1954;43:271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- 3.Arneborg N, Vespersen L, Jakobsen M. Individual cells of Saccharomyces cerevisiae and Zygosaccharomyces bailii exhibit different short-term intracellular pH responses to acetic acid. Arch Microbiol. 2000;174:125–128. doi: 10.1007/s002030000185. [DOI] [PubMed] [Google Scholar]
- 4.Cole M B, Keenan M H J. Effects of weak acids and external pH on the intracellular pH of Zygosaccharomyces bailii, and its implications in weak-acid resistance. Yeast. 1987;3:23–32. [Google Scholar]
- 5.Fernandes L, Côrte-Real M, Loureiro V, Loureiro-Dias M C, Leão C. Glucose respiration and fermentation in Zygosaccharomyces bailii and Saccharomyces cerevisiae express different sensitivity patterns to ethanol and acetic acid. Lett Appl Microbiol. 1997;25:249–253. doi: 10.1046/j.1472-765x.1997.00214.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleet G. Spoilage yeasts. Crit Rev Biotechnol. 1992;12:1–44. doi: 10.3109/07388559209069186. [DOI] [PubMed] [Google Scholar]
- 7.Flikweert M T, van Dijken J P, Pronk J T. Metabolic responses of pyruvate decarboxylase-negative Saccharomyces cerevisiae to glucose excess. Appl Environ Microbiol. 1997;63:3399–3404. doi: 10.1128/aem.63.9.3399-3404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leyva J S, Manrique M, Prats L, Loureiro-Dias M C, Peinado J M. Regulation of fermentative CO2 production by the food spoilage yeast Zygosaccharomyces bailii. Enzyme Microb Technol. 1999;24:270–275. [Google Scholar]
- 9.Nagy M, Lacroute F, Thomas D. Divergent evolution of pyrimidine biosynthesis between anaerobic and aerobic yeasts. Proc Natl Acad Sci USA. 1992;89:8966–8970. doi: 10.1073/pnas.89.19.8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postma E, Verduyn C, Scheffers W A, van Dijken J P. Enzymic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman F, Fink G R, Hicks J B. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 12.Shi N Q, Jeffries T W. Anaerobic growth and improved fermentation of Pichia stipitis bearing a URA1 gene from Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1998;50:339–345. doi: 10.1007/s002530051301. [DOI] [PubMed] [Google Scholar]
- 13.Sousa M J, Miranda L, Côrte-Real M, Leão C. Transport of acetic acid in Zygosaccharomyces bailii: effects of ethanol and their implications on the resistance of the yeast to acid environments. Appl Environ Microbiol. 1996;62:3152–3157. doi: 10.1128/aem.62.9.3152-3157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sousa M J, Rodrigues F, Côrte-Real M, Leão C. Mechanisms underlying the transport and intracellular metabolism of acetic acid in the presence of glucose in the yeast Zygosaccharomyces bailii. Microbiology. 1998;144:665–670. doi: 10.1099/00221287-144-3-665. [DOI] [PubMed] [Google Scholar]
- 15.Stratford M, Anslow P A. Comparison of the inhibitory action on Saccharomyces cerevisiae of weak-acid preservatives, uncouplers, and medium-chain fatty acids. FEMS Microbiol Lett. 1996;142:53–58. doi: 10.1111/j.1574-6968.1996.tb08407.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomas D S, Davenport R R. Zygosaccharomyces bailii—a profile of characteristics and spoilage activities. Food Microbiol. 1985;2:157–275. [Google Scholar]
- 17.Trézéguet V, Zeman I, David C, Lauquin G J M, Kolarov J. Expression of the ADP/ATP carrier encoding genes in aerobic yeasts; phenotype of an ADP/ATP carrier deletion mutant of Schizosaccharomyces pombe. Biochim Biophys Acta. 1999;1410:229–236. doi: 10.1016/s0005-2728(98)00180-7. [DOI] [PubMed] [Google Scholar]
- 18.van Dijken J P, van den Bosch E J, Hermans L, de Miranda L, Scheffers W A. Alcoholic fermentation by ‘non-fermenting’ yeasts. Yeast. 1986;2:123–127. doi: 10.1002/yea.320020208. [DOI] [PubMed] [Google Scholar]
- 19.van Dijken J P, Scheffers W A. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev. 1986;32:199–224. [Google Scholar]
- 20.van Dijken J P, Bauer J, Brambilla L, Duboc P, Francois J M, Gancedo C, Giuseppin M L F, Heijnen J J, Hoare M, Lange H C, Madden E A, Niederberger P, Nielsen J, Parrou J L, Petit T, Porro D, Reuss M, van Riel N, Rizzi M, Steensma H Y, Verrips C T, Vindelov J, Pronk J T. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb Technol. 2000;26:706–714. doi: 10.1016/s0141-0229(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 21.Verduyn C, Postma E, Scheffers W A, van Dijken J P. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol. 1990;59:49–63. doi: 10.1099/00221287-136-3-395. [DOI] [PubMed] [Google Scholar]
- 22.Verduyn C, Postma E, Scheffers W A, van Dijken J P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- 23.Visser W, Batenburg-van der Vegte W, Scheffers W A, van Dijken J P. Oxygen requirements of yeasts. Appl Environ Microbiol. 1990;56:3785–3792. doi: 10.1128/aem.56.12.3785-3792.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visser W, van der Baan A A, Batenburg-van der Vegte W, Scheffers W A, Krämer R, van Dijken J P. Involvement of mitochondria in the assimilatory metabolism of anaerobic Saccharomyces cerevisiae cultures. Microbiology. 1994;140:3039–3046. doi: 10.1099/13500872-140-11-3039. [DOI] [PubMed] [Google Scholar]
- 25.Weusthuis R A, Visser W, Pronk J T, Scheffers W A, van Dijken J P. Effects of oxygen limitation on sugar metabolism in yeasts: a continuous culture study of the Kluyver effect. Microbiology. 1995;140:703–715. doi: 10.1099/00221287-140-4-703. [DOI] [PubMed] [Google Scholar]
- 26.Wium H, Malfeito-Ferreira M, Loureiro V, Aubyn S. A rapid characterization of yeast contaminants associated with sparkling wine production. Ind Bevand. 1990;19:504–506. [Google Scholar]