Abstract
Purpose
This study aimed to characterize 252 consecutive patients with an indication for major emergency abdominal surgery including patients not proceeding to surgery (No-Lap). Patients who do not proceed to major emergency abdominal surgery and their clinical outcomes are not well characterized in the existing literature. Triage criteria may vary between centers, potentially impacting reported outcomes.
Methods
A single-center prospective observational study in a high-volume Danish surgical center including 252 patients presenting with an indication for major emergent abdominal surgery was conducted from the 15th of October 2020 to the 15th of August 2021. The primary outcome was to estimate the prevalence of No-Lap patients.
Results
Overall, 21 patients (8.3%) of our total study cohort did not proceed to surgery. These patients were significantly older, more comorbid with higher ASA scores, poorer performance status, and were more likely to have bowel ischemia. Poor functional performance and surgeons’ consideration of futile intervention were the main reasons for deferring surgery in all 21 patients. Overall, 30-day mortality was 95% for the No-LAP cohort, 9% for the LAP cohort, and 16% for the whole cohort, respectively.
Conclusions
The No-LAP group selection process could be one of the main determinants of reported postoperative outcomes. Prospective international multi-center studies to characterize the entire cohort of patients eligible for emergency laparotomy including the No-LAP population are needed, as large variations in triage criteria and culture seem to exist.
Trial registration Retrospectively registered.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00068-022-02052-4.
Keywords: Surgery, Perioperative medicine, Mortality, Frailty, Patient selection
Background
Acute high-risk abdominal surgery (AHA) surgery, defined as major emergency abdominal pathology requiring emergency laparotomy or laparoscopy can be lifesaving but carries a considerable risk of poor outcomes and may be deemed futile in high-risk patients. International data indicate that there potentially are significant variations in triage criteria [1–6]. The decision to operate frail patients presenting with major emergency abdominal pathology is challenging and is affected by multiple factors including patient characteristics, surgeon’s experience, intuition, cultural differences in healthcare systems, the patients’ beliefs and values, and shared decision making is crucial in the surgical referral process [1, 7–11]. Although several studies have assessed outcomes following major emergent abdominal surgery in frail patients, and different mortality and morbidity prediction systems have been developed to support clinical decision making [12, 13], only one prospective study has prospectively reported outcomes for patients who fulfill criteria for surgery but do not proceed to surgery (The No-LAP population) [12]. This recent single-center study showed that the No-LAP population represented 32% of all patients meeting the criteria for emergency laparotomy/laparoscopy according to the NELA (National Emergency Laparotomy Audit) criteria [14]. While this population is prevalent in daily clinical practice, they remain uncharacterized in current studies and audits. The lack of data complicates comparison of outcomes for the whole cohort presenting with an indication for major emergency abdominal surgery. This challenges rational conclusions and comparisons to be drawn on outcomes including mortality and effects of patient care pathways. This may as well impede establishment of evidence-based guidelines to define futility and guide decision-making in surgery [6].
We aimed to characterize the entire population fulfilling criteria for AHA surgery in a single high-volume Danish surgical center, with a well-established patient pathway, to evaluate potential differences in surgical triage culture, as well as the impact of preoperative decision-making on overall outcomes for the whole population.
Methods
Approvals
Permission for the data acquisition for the study was granted by the Center for Regional Development, Capital Region of Denmark (ID no. H-20036076) The Regional Ethics committee had no objections to the study (no. H-20036076) and did not require informed consent from the patients as the study was purely observational. The study was reported according to The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [15].
Patients
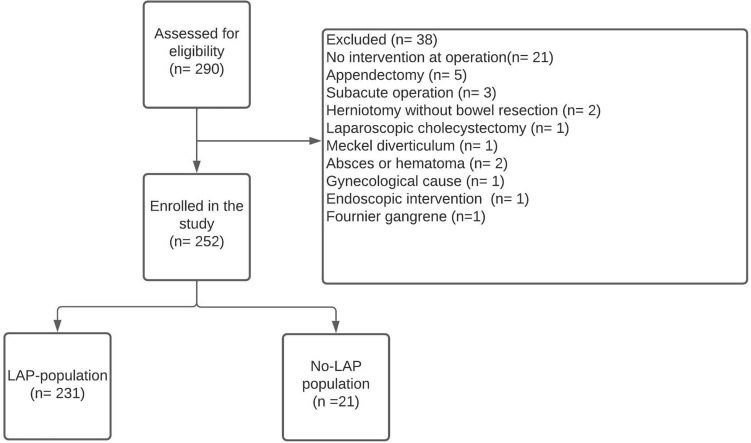
This study was a single-center prospective observational cohort study and included all adult patients (≥ 18 years) eligible for acute-high risk abdominal surgery (AHA) defined as emergency laparoscopy or laparotomy at the Gastro Unit, Hvidovre University Hospital, Hvidovre, Denmark from October 15th, 2020 to August 15th, 2021, due to the following conditions: (Perforated viscus, intestinal obstruction, intestinal ischemia, intraabdominal bleeding, emergency reoperations after elective surgery due to a post-operative complication e.g. intraabdominal bleeding, obstructive ileus, intestinal ischemia or anastomotic leakage). These patients are characterized as AHA (Acute High-risk Abdominal surgery) patients. Patients undergoing the following procedures were excluded: trauma, appendectomies, cholecystectomies, negative diagnostic laparoscopies/laparotomies, herniotomies without bowel resection, internal herniation after Roux-en-Y gastric bypass, gynecological, urogenital or vascular pathology requiring surgery and subacute surgery (surgery planned within 48 h) (Fig. 1). We focused on patients undergoing AHA surgery defined as laparotomy or laparoscopy for bowel obstruction, ischemia, and perforation as this patient group is well-established in previous international research including reports from the NELA group who have similar inclusion and exclusion criteria as our study [12]. This patient group differs substantially from patients presenting with e.g. appendicitis and internal hernia both in terms of baseline demographics (age, comorbidity) and from a physiologic point of view due to an increased surgically induced stress response, increased surgical complexity as well as a high incidence of frailty [6, 16]. Data have shown that this patient group is the primary contributor to morbidity and mortality in the general surgical population yet variation in clinical management and outcomes exist [2, 4].
Fig. 1.
Flowchart of patients included in the present study
The Gastrounit at Hvidovre University Hospital serves an area of 515 000 inhabitants with approximately 3–400 AHA operations annually [2]. All patients suspected to be an AHA-patient undergo a well-defined pre-operative care bundle protocol, which includes early preoperative administration of high-dose intravenous broad-spectrum antibiotics, placement of a nasogastric tube and urinary catheter, preoperative blood sample collection, blood and urinary culture analyses, arterial blood gas evaluation and performance of early contrast-enhanced abdominal computed tomography (CT), followed by early multidisciplinary evaluation by a consultant anesthesiologist and consultant surgeon [2].
Post-operatively, AHA-patients are either admitted directly to the intensive care unit (ICU) or to a perioperative unit based on the patients general condition and surgical APGAR score [17]. Perioperative unit stay length is determined by the surgical APGAR score. Patients are discharged from the perioperative unit to a specialized subunit in our surgical department where bedside rounds are caried daily by a dedicated team of acute care surgeons (senior consultants/consultants). The AHA patients follow an enhanced recovery after surgery (ERAS)-program including multimodal analgesia, early mobilization and oral nutrition, early removal of drains, nasogastric tubes and urinary catheters, lung physiotherapy and follow specific discharge criteria.
The “No-LAP” population was defined as patients who fulfilled the AHA criteria for surgery but did not proceed to surgery. In case of refrainment from surgery, the reasons for the decision were registered. The decisions were categorized before the start of the study and were assessed by the attending surgeon as either patients’ choice of declining surgery; surgery likely to be futile either due to poor patient fitness or advanced malignancy with low life expectancy.
Screening of potential NoLap patients was performed daily by the study team and all physicians at our surgical department were informed about the study.
When a patient was considered a NoLAP candidate the doctor on call contacted the study team. The study team did not guide decision-making as the study was purely observational.
Overall the process of shared decision making in our cohort followed the following steps:
The patient undergoes clinical evaluation by a surgeon who may find indications for further investigations e.g., CT.
The surgeon finds indications for surgical treatment based on clinical findings and imaging test results e.g., CT suspecting a major emergency abdominal condition.
The surgeon assesses whether surgery can be offered by taking the patient’s individualized risk into account (ASA, performance status, comorbidities)
The surgeon informs the patient about the established diagnosis, and treatment options are discussed including risks and benefits of surgery vs. non-surgical treatment.
The surgeon and the patient reach a mutual decision.
In all steps (a-e) the patient may choose to refuse surgical treatment [10, 11].
Data collection
Study data were collected and managed using REDCap electronic data capture tools hosted at Gastrounit, Copenhagen University Hospital Hvidovre [18]. The following data were collected: Age, Sex, American Society of Anesthesiologist (ASA) score, patient comorbidities, Eastern Cooperative Oncology Group (ECOG) performance score [19], indication for surgery, preoperative radiology and findings; charge of deciding surgeon; baseline blood tests including creatinine, eGFR, CRP, hemoglobin, serum albumin, and arterial blood gas were also registered.
Primary and secondary outcomes
The primary endpoint for this study was to estimate the prevalence of No-LAP patients in a Danish single-center setting. Secondary outcomes included 30 and 90-days mortality for the LAP and No-Lap populations, and overall cohort mortality.
Statistical analysis
Continuous data are presented as median with interquartile range (IQR) and categorical data as number and percentage. When comparing groups, Fisher's exact test was used for categorical variables and the Mann–Whitney test for continuous variables. Kaplan–Meier curves were computed to estimate survival probabilities for the No-LAP and the LAP group. The difference in survival time between the two groups was assessed by the log-rank test. All tests were two-tailed and were conducted at a 5% significance level and 95% confidence intervals (CI) were estimated when appropriate.
All analyses were performed with R statistical software 4.1.2 [20]
Power analysis
A sample size estimation was carried out based on the No-LAP population incidence of 32% reported by McIlveen et al. [14]. We wished to power the study to detect a relative deviation of more than 25% in the incidence of NoLap, compared to the previously reported No-LAP population. Using an alpha-cut-off value of 5% and a beta-cut-off of 20%, a sample size of 253 patients in total, including NoLap patients, was required.
The study was performed with monthly patients counts, so that the study would be terminated on the first full month after the required 250 patients were included. Study termination was therefore assessed on a month-by-month basis pending inclusion of at least 250 patients.
Results
Patient characteristics
A total of 252 patients with a median age of 68 years were included in the study (Fig. 1) & Online Supporting Information Table S1. Overall, 21 (8.3%), 95%CI (5.5%-12.4%) patients of our study cohort did not proceed to surgery (NoLAP-patients). The most prevalent indications for surgery were intestinal obstruction, intestinal perforation, and intestinal ischemia which accounted for 57%, 30%, and 6% respectively (Table 1). Patients who did not proceed to emergency surgery were significantly older, more comorbid with higher ASA scores, poor performance status, and were more likely to present with bowel ischemia in contrast to patients who underwent surgery (LAP-population) (Table 1). The No-LAP-patients presented with significantly lower albumin and eGFR levels, and higher CRP, and lactate values as compared with the LAP-population preoperatively. Lower median hemoglobin levels were also observed in the No-LAP group; however, it did not reach statistical significance p = 0.076 (Table 1).
Table 1.
Baseline patient characteristics and mortality outcomes
| All patients N = 252 |
NoLAP-population N = 21 |
LAP-population N = 231 |
P-value | |
|---|---|---|---|---|
| Age, years, (median, IQR) | 68 (53–78) | 76 (73–83) | 66 (52–76) | < 0.001 |
| Male, n (%) | 127 (50) | 10 (48) | 117 (51) | 0.8 |
|
ASA-score I II III IV V |
21 (8.3) 101 (40) 105 (42) 22 (8) 3 (1.2) |
0 2 (9.5) 14 (67) 4 (19) 1 (4.8) |
22 (9.1) 99 (43) 91 (39) 18 (7.8) 2 (0.9) |
0.001 |
| WHO Performance status > 2, n (%) | 22 (8.7) | 8 (38) | 14 (6.1) | < 0.001 |
| Past medical history, n (%) | ||||
| Former intraabdominal surgery | 132 (52) | 10 (47) | 122(53) | 0.5 |
| Comorbidity (n %) | 98 (84) | 21 (100) | 77 (80) | 0.023 |
|
Diabetes IDDM NIDDM |
18 (7.1) 9 (3.6) |
0 3 (14) |
18 (7.8) 6 (2) |
0.3 |
| Lung disease | 46 (18) | 8 (38) | 38 (16) | 0.033 |
| Neurological diseases including cerebrovascular diseases | 31 (12) | 5 (24) | 26 (11) | 0.4 |
| Nephropathy | 13 (5) | 2 (9.5) | 11 (4.7) | 0.2 |
| Dementia | 6 (2.4) | 1 (4.8) | 5 (2.2) | 0.4 |
| Malignancy (active or previous) | 53 (21) | 10 (48) | 43(19) | 0.003 |
| Hypertension | 85 (34) | 9 (43) | 76 (33) | 0.4 |
| Atrial flutter | 26 (10) | 4 (19) | 22 (9.6) | 0.2 |
| Heart failure | 6 (2.38) | 0 | 6 (2.6) | > 0.9 |
| Ischemic heart disease | 23 (9.2) | 3 (14) | 20 (8.7) | 0.4 |
| Liver cirrhosis | 7 (2.8) | 2 (9.5) | 5 (2.2) | 0.11 |
| Blood results at admission, median (IQR) | ||||
| Albumin, (g/L) | 32 (26–37) | 24 (20–32) | 33 (27–36) | 0.002 |
| eGFR mL/min/(1.73m2) | 78 (52–90) | 48 (27–83) | 78 (56–90) | 0.004 |
| Potassium (mmmol/L) | 3.9 (3.6–4.2) | 4.20 (3.7–4.3) | 3.9 (3.6–4.1) | 0.10 |
| Creatinine (mmmol/L) | 82 (64–110) | 98 (64–193) | 80 (64–103) | 0.087 |
| Sodium (mmmol/L) | 137 (134–140) | 136.5 (131–140) | 137 (134–140) | 0.6 |
| CRP (mg/L) | 53 (9–160) | 145 (62–222) | 48 (8–140) | 0.015 |
| Hemoglobin (mmmol/L) | 8.10 (7.10–9.10) | 7.25 (6.6–8.2) | 8.2 (7.1–9.1) | 0.076 |
| WBC (WBC × 109/L) | 10.7 (8.7–14.3) | 10 (7–18) | 11 (9–14) | 0.6 |
| Platelets. (Platelets × 109/L) | 295 (234–294) | 266 (224–386) | 266 (224–386) | 0.9 |
| Lactate > 2 mmol/L | 18 (7.1) | 7 (33) | 11 (4.8) | < 0.001 |
| Indications for surgery n (%) | ||||
| Intestinal obstruction | 145 (57) | 5 (24) | 140 (60) | < 0.001 |
| Intestinal perforation | 76 (30) | 8 (38) | 68 (29) | 0.7 |
| Intestinal ischemia | 15 (6) | 8 (38) | 7 (3) | < 0.001 |
| Anastomotic leakage | 6 (2.4) | 0 | 6 (2.6) | < 0.001 |
| Bleeding | 6 (2.4) | 0 | 6 (2.6) | < 0.001 |
| Iatrogenic bowel perforation after primary surgery | 4 (1.5) | 0 | 4(1.7) | < 0.001 |
| Mortality, n (%) | ||||
| 30-day mortality | 41 (16) | 20 (95) | 21 (9) | < 0.001 |
| 90-day mortality | 53 (21) | 21 (100) | 32 (14) | < 0.001 |
IQR, interquartile range; ASA, American Society of Anesthesiology; WHO, World Health Organization; IDDM, Insulin-dependent diabetes mellitus; NIDDM, non-insulin-dependent diabetes mellitus; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; WBC, white blood cell count
Reasons for deferral from surgery
Five patients (24%) abstained from surgical treatment before surgical risk evaluation (step c)due to self-perceived poor performance status and subjective consideration of surgical futility. One of the 5 patients considered advanced malignancy with low life expectancy as a major reason to refrain from surgery (Table 2). In all five patients, the surgeons agreed with the decision. The remaining 16 patients underwent surgical risk evaluation (step a–e). Surgery was considered futile by the surgeons in all 16 patients due to very poor expected outcomes. Advanced malignancy with low life expectancy was the main reason to defer surgery in 8 of these 16 patients (50%), while very low functional level was the main indication in the remainder. All 16 patients agreed upon the decision (Table 2). In all NoLap-patients, the deciding surgeon was either a senior consultant or consultant (Table 2).
Table 2.
Reasons for deferring No-lap patients’ surgery
| Reason why patient was considered No-lap patient, n (%) | Patients abstaining from surgical treatment before surgical risk evaluation (n = 5) |
|---|---|
| Patient considered their performance status too low to undergo surgery | 4 (80) |
| Advanced malignancy with low life expectancy considered by the patient and the surgeon as the main cause to defer from surgery | 1 (20) |
| Patients deferred from surgery after surgical risk evaluation (n = 16) | |
| Surgeon considered surgery to be futile due to poor performance status | 8 (50) |
| Surgeon considered advanced malignancy with low life expectancy as a leading cause to defer from surgery | 8 (50) |
| Charge of deciding surgeon, n (%) | |
| Senior consultant or consultant | 21 (100) |
Mortality
All No-LAP patients but one (95%), CI (77.3%–99.1%), died within 30-days after admission. In this case, a 95-year-old woman, with a previous medical history including a cerebral stroke, autoimmune hepatitis (active prednisolone treatment), and a colon cancer 10 years before admission which she refused adjuvant chemotherapy for—was admitted with severe epigastric pain and fever. A CT scan was performed showing free air around the duodenum suspecting a perforated duodenal ulcer. After a thorough evaluation, the patient and the surgeon agreed upon deferral from surgery. Conservative management was initiated with a proton pump inhibitor and antibiotics and the patient showed good clinical response and was discharged 21 days after admission. She later died of natural causes after 42 days.
The 30-day mortality for the LAP-cohort was 9%, CI (6.4%–13.0%) and the overall mortality for the entire cohort, including NoLap patients was 16%, CI (12.2%–21.3%).
All No-LAP patients (100%), CI (84.5%–100%), were dead 90 days after admission in contrary to the LAP-population where 32 patients (13.85%), CI (9.98–18.9) died p < 0.001. The overall 90-day mortality rate for the total study cohort was 21%, CI(16.4%-26.4%) (Table 1). The LAP cohort had better survival during the whole study period than the NoLap cohort p-value for log-rank test < 0.001 (Fig. 2).
Fig. 2.
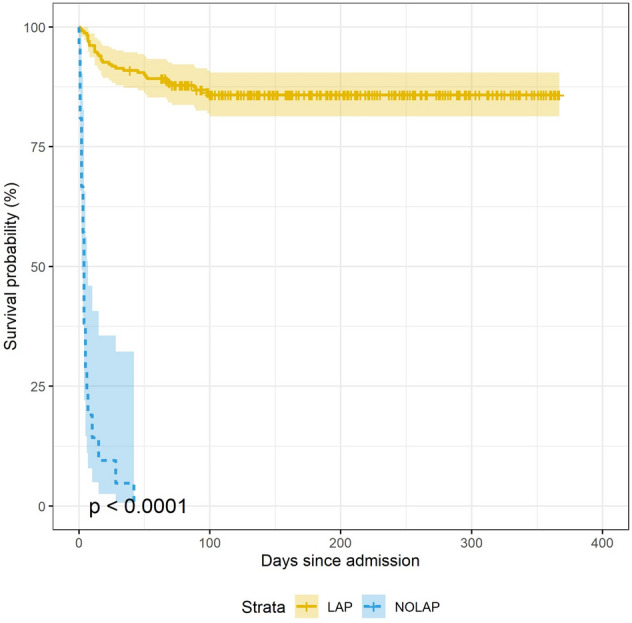
Kaplan–Meier survival curves showing survival probabilities for the LAP vs No-LAP group
Discussion
This prospective, single-center study aimed to characterize and report clinical outcomes for a consecutive cohort of patients eligible for major emergent abdominal surgery including patients who did not proceed to major emergent abdominal surgery (No-LAP population) in a Danish center with an established care pathway.
The study revealed that 8.3% of our total patient cohort did not proceed to surgery in comparison to the 32% reported by McIlveen et al. [14]. In our cohort, all but one No-LAP patient (95%) died within a month after admission while approximately 37% of the No-LAP patients in the study by McIlveen et al. were alive 30 days after admission [14] The 30-day mortality rate for the LAP-cohort was 9% in our cohort vs. 12.6% in the study by McIlveen et al. However, the 30-day mortality rate for the whole cohort was 16% in our study versus 29% in the study by McIlveen et al. [14].
Current literature is scarce with only one prospective study reporting outcomes for this patient population [8, 14]. In our study, the No-Lap population was predominantly characterized by elderly patients with poor performance status, severe comorbidities, hypoalbuminemia, elevated lactate, and creatinine levels, and those likely to have bowel ischemia at admission. These features also appeared to be common in the study by McIlveen et al. [14] and have also been described in patients who had undergone futile surgery despite extreme preoperative risk [5, 21, 22]. While all No-Lap patients but one died within 30-days after admission, early mortality within the first 30 days was also observed in the LAP cohort, however, to a lesser extent. A continuous increase in mortality was observed in the LAP cohort until 90 days after admission where the mortality seems to reach a steady state (Fig. 2).This may indicate potential variation in patient related characteristics and physiological adaptation to the surgical stress response in late survivors vs. non-survivors.
The results of our study, and the results presented by McIlveen et al. indicate potential large differences in pre-operative decision-making which may have an immense effect on patient related outcomes [23]. Decision-making in emergency abdominal surgery is challenging due to a large heterogeneity in patient-related factors i.e. surgical pathology and physiology (perforation vs. intestinal obstruction, presence of frailty, comorbidities, and age) [6]. Other well-established factors affecting decision making include surgeons' clinical and operative experience, perception of risks and benefits of operative and non-operative treatment, external pressure to operate i.e. from patients or their relatives, and culture [1, 7, 9, 23, 24].
The potential variation in pre-operative decision-making between our study and the study presented by McIlveen et al. seems to be large and raises several issues. First, from an ethical point of view, if patients suitable for major emergency surgery are deselected from surgery due to cultural variations in health care systems, unnecessary excessive mortality can be expected. Second, these variations may hamper the development of evidence-based guidelines, as the comparison of research outcomes and evaluation of different patient care pathways are based on operated patients, thus making it challenging to establish objective criteria to define surgical futility. Third excessive surgery to patients with low life expectancy is futile and causes both unnecessary suffering and waste scarce surgical resources.
While the main strength of the current study is the prospective design with the inclusion of a high number of consecutive patients some limitations need to be addressed. We conducted a single-center study which may limit the extrapolation of our findings, as different centers may have different demographic characteristics, center volume, and triage. It is reasonable that the conduction of the study may have led to increased awareness about the No-LAP patients which may have led to different decision-making during the study period.
Moreover, the COVID-19 pandemic and the associated lockdowns have affected surgical health care services globally and should be considered as a potential limitation when interpreting the results of our study [25]
COVID-19 may have had an impact on the incidence of patients presenting with major emergency abdominal pathology as some patients may have died at home/nursing home facilities instead of admission to surgical wards potentially biasing mortality outcomes. During the study period, there was a period with lockdown and cancellation of elective non-cancer surgery which may have led to fewer reoperations for complications during the study period. The average number of patients undergoing AHA surgery at our institution was 23.1 during the study period versus 26.4 during the same monthly period pre-Covid dates (15th October 2018–15th August 2019), indicating a 14% decrease. While this decrease could be due to reduced patient intake secondary to Covid-19 restrictions, it could also be due to fewer complications secondary to elective surgery. However, it should be noted that complications secondary to elective surgery have been shown to have lower mortality than primary emergency surgery [26]. During the COVID-19 pandemic the hospital resources (Operating theaters for emergency surgery and Intensive care resources including bed availability) for AHA-patients were not affected. The lower number of patients undergoing surgery during the inclusion period could be due to an increase in the number of NoLap patients secondary to the present study.
In the era of shared decision-making, proper identification of the frail surgical patient and early evaluation of whether surgical intervention is deemed futile, is of paramount importance, to avoid futile care which seems to be common [5]. Nevertheless, a complex clinical and ethical dilemma that every surgeon and anesthesiologist will face regularly, and probably face more frequently, as life expectancy and the need for emergency surgery is on the rise globally [16]. Preoperative decision-making must be a multidisciplinary assessment process where the surgeon and the anesthesiologists address the morbidity and mortality risks associated with care concerning the patient’s pathology, comorbidities, functional status, and wishes adequately to avoid futile care [27].
There is an obvious need for prospective multi-center studies to characterize the whole population of patients eligible for major emergency abdominal surgery including the No-LAP population. Moreover to establish a standardized report form for these decisions allowing comparisons to be made, and evidence-based guidelines for futility to be established, hopefully increasing quality of care for these patients.
Final results from an ongoing large multicenter, UK-based prospective cohort study aiming to characterize the No-LAP patients are awaited [28]. While this study may show potential regional and cultural differences between centers in the UK it will not show potential international cultural differences. This can be assessed by a prospective international multicenter cohort study in the future although it will require a standardization of cohort definitions and outcome measures.
Results from the UK-based multicenter study may, however, raise questions about which departments should manage these complex surgical patients if potential variation in decision making and mortality-related outcomes between low-volume and high volume surgical centers are found [28]. Future studies should focus on objective assessment tools to identify patients in whom surgery may not be beneficial.
Moreover, reporting of study outcomes including reporting outcomes for the No-LAP population should be standardized to make comparison feasible in future research.
Conclusions
There is an unmet need for prospective international multi-center studies to characterize the entire cohort of patients presenting with major pathology warranting acute high-risk abdominal surgery including the patients who do not proceed to surgery (No-LAP population), as large variations in triage criteria and culture probably exist and seem to impact outcome measures.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (DOCX 14 KB) Table S1: Monthly number of patients included during the study period.
Acknowledgements
Not applicable.
Abbreviations
- AHA
Acute high-risk abdominal surgery
- NELA
National Emergency Laparotomy Audit
- ASA
American Society of Anesthesiologists score
- ECOG
Eastern Cooperative Oncology Group
- COVID-19
Coronavirus disease 2019
- UK
United Kingdom
Author contributions
Study concept and design (MLL, NBF). Acquisition of data (ME, MLL, MC, KLH). Analysis and interpretation of data (ME, MLL, MC, KLH, NBF). Drafting of the manuscript (ME, MLL, MC, KLH, NBF). Critical revision of the manuscript for important intellectual content (ME, MLL, MC, KLH, NBF). Statistical analysis (ME). Study supervision (MLL, NBF). All authors read and approved the final manuscript.
Funding
The study received no external funding.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
ME, MLL, MC, KL and NB have no competing interests to declare.
Ethics approval and consent to participate
Permission for the data acquisition for the study was granted by the Center for Regional Development, Capital Region of Denmark (ID no. H-20036076) The Regional Ethics committee had no objections to the study (no. H-20036076) and did not require informed consent from the patients as the study was purely observational.
Authors information
ME: Specialist Trainee in Surgery. MLL: Senior consultant Emergency General Surgeon and Clinical Associate Professor. MC: Intern, Ph.D. student. KLH: Clinical research nurse. NBF: Senior consultant in Anaesthesia and Professor.
Consent for publication
Not applicable.
References
- 1.Markar SR, Vidal-Diez A, Holt PJ, Karthikesalingam A, Hanna GB. An international comparison of the management of gastrointestinal surgical emergencies in octogenarians-England Versus United States: a national population-based cohort study. Ann Surg. 2021;273:924–932. doi: 10.1097/SLA.0000000000003396. [DOI] [PubMed] [Google Scholar]
- 2.Tengberg LT, Bay-Nielsen M, Bisgaard T, et al. Multidisciplinary perioperative protocol in patients undergoing acute high-risk abdominal surgery. Br J Surg. 2017;104:463–471. doi: 10.1002/bjs.10427. [DOI] [PubMed] [Google Scholar]
- 3.Vester-Andersen M, Lundstrom LH, Moller MH, Waldau T, Rosenberg J, Moller AM. Mortality and postoperative care pathways after emergency gastrointestinal surgery in 2904 patients: a population-based cohort study. Br J Anaesth. 2014;112:860–870. doi: 10.1093/bja/aet487. [DOI] [PubMed] [Google Scholar]
- 4.Saunders DI, Murray D, Pichel AC, Varley S, Peden CJ. Variations in mortality after emergency laparotomy: the first report of the UK emergency laparotomy network. Br J Anaesth. 2012;109:368–375. doi: 10.1093/bja/aes165. [DOI] [PubMed] [Google Scholar]
- 5.Chiu AS, Jean RA, Resio B, Pei KY. Early postoperative death in extreme-risk patients: a perspective on surgical futility. Surgery. 2019;166:380–385. doi: 10.1016/j.surg.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Foss NB. Emergency laparotomy success – optimisation or triage? Anaesthesia. 2020;75:1289–1292. doi: 10.1111/anae.15114. [DOI] [PubMed] [Google Scholar]
- 7.Hendra L, Hendra T, Parker SJ. Decision-making in the emergency laparotomy: a mixed methodology study. World J Surg. 2019;43:798–805. doi: 10.1007/s00268-018-4849-6. [DOI] [PubMed] [Google Scholar]
- 8.Broughton KJ, Aldridge O, Pradhan S, Aitken RJ. The perth emergency laparotomy audit. ANZ J Surg. 2017;87:893–897. doi: 10.1111/ans.14208. [DOI] [PubMed] [Google Scholar]
- 9.Nally DM, Sørensen J, Valentelyte G, et al. Volume and in-hospital mortality after emergency abdominal surgery: a national population-based study. BMJ Open. 2019;9:e032183. doi: 10.1136/bmjopen-2019-032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinkunas LA, Klipowicz CJ, Carlisle EM. Shared decision making in surgery: a scoping review of patient and surgeon preferences. BMC Med Inform Decis Mak. 2020;20:1–14. doi: 10.1186/s12911-020-01211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truglio-Londrigan M, Slyer JT, Singleton JK, Worral PS. A qualitative systematic review of internal and external influences on shared decision-making in all health care settings. JBI Database System Rev Implement Rep. 2014;12:121–194. doi: 10.11124/jbisrir-2014-1414. [DOI] [PubMed] [Google Scholar]
- 12.Eugene N, Oliver CM, Bassett MG, et al. Development and internal validation of a novel risk adjustment model for adult patients undergoing emergency laparotomy surgery: the National Emergency Laparotomy Audit risk model. Br J Anaesth. 2018;121:739–748. doi: 10.1016/j.bja.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Oliver CM, Walker E, Giannaris S, Grocott MPW, Moonesinghe SR. Risk assessment tools validated for patients undergoing emergency laparotomy: a systematic review. Br J Anaesth. 2015;115:849–860. doi: 10.1093/bja/aev350. [DOI] [PubMed] [Google Scholar]
- 14.McIlveen EC, Wright E, Shaw M, et al. A prospective cohort study characterising patients declined emergency laparotomy: survival in the ‘NoLap’ population. Anaesthesia. 2020;75:54–62. doi: 10.1111/anae.14839. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Torrance A, Powell S, Griffiths E. Emergency surgery in the elderly : challenges and solutions. Emerg Surg. 2015;89:55–68. doi: 10.2147/OAEM.S68324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar Score for Surgery. J Am Coll Surg. 2007;204:201–208. doi: 10.1016/j.jamcollsurg.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103206. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oken MM, Creech RH, Davis TE. Toxicology and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;6:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team (2020). R: A language and environment for statistical computing. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria., 2020. www.R-project.org/.
- 21.Resio BJ, Chiu AS, Zhang Y, Pei KY. Characterization of high mortality probability operations at national surgical quality improvement program hospitals. JAMA Surg. 2020;155:85–88. doi: 10.1001/jamasurg.2019.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal G, Broughton KJ, Williams LJ, Peden CJ, Quiney N. Early postoperative death in patients undergoing emergency high-risk surgery: towards a better understanding of patients for whom surgery may not be beneficial. J Clin Med. 2020;9:1–10. doi: 10.3390/jcm9051288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis SS, Babidge WJ, McCulloch GAJ, Maddern GJ. Fatal flaws in clinical decision making. ANZ J Surg. 2019;89:764–768. doi: 10.1111/ans.14955. [DOI] [PubMed] [Google Scholar]
- 24.Morris RS, Ruck JM, Conca-Cheng AM, Smith TJ, Carver TW, Johnston FM. Shared decision-making in acute surgical illness: the surgeon’s perspective. J Am Coll Surg. 2018;226:784–795. doi: 10.1016/j.jamcollsurg.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Nepogodiev D, Omar OM, Glasbey JC, et al. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cihoric M, Tengberg LT, Foss NB, Gögenur I, Tolstrup M-B, Bay-Nielsen M. Functional performance and 30-day postoperative mortality after emergency laparotomy—a retrospective, multicenter, observational cohort study of 1084 patients. Perioperat Med. 2020;9:1–11. doi: 10.1186/s13741-020-00143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivarajah V, Walsh U, Malietzis G, Kontovounisios C, Pandey V, Pellino G. The importance of discussing mortality risk prior to emergency laparotomy. Updat Surg. 2020;72:859–865. doi: 10.1007/s13304-020-00756-z. [DOI] [PubMed] [Google Scholar]
- 28.Price A, Mclennan E, Boyle J. Group on behalf of the E study 535 ELF 2: defining the denominator elf study group. Age Ageing. 2021;50:1–4. doi: 10.1093/ageing/afab117.01. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1 (DOCX 14 KB) Table S1: Monthly number of patients included during the study period.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.