Abstract
Lepidopteran insects provide important model systems for innate immunity of insects, particularly for cell biology of hemocytes and biochemical analyses of plasma proteins. Caterpillars are also among the most serious agricultural pests, and understanding of their immune systems has potential practical significance. An early response to infection in lepidopteran larvae is the activation of hemocyte adhesion, leading to phagocytosis, nodule formation, or encapsulation. Plasmatocytes and granular cells are the hemocyte types involved in these responses. Infectious microorganisms are recognized by binding of hemolymph plasma proteins to microbial surface components. This “pattern recognition” triggers phagocytosis and nodule formation, activation of prophenoloxidase and melanization and the synthesis of antimicrobial proteins that are secreted into the hemolymph. Many hemolymph proteins that function in such innate immune responses of insects were first discovered in lepidopterans. Microbial proteinases and nucleic acids released from lysed host cells may also activate lepidopteran immune responses. Hemolymph antimicrobial peptides and proteins can reach high concentrations and may have activity against a broad spectrum of microorganisms, contributing significantly to clearing of infections. Serine proteinase cascade pathways triggered by microbial components interacting with pattern recognition proteins stimulate activation of the cytokine Spätzle, which initiates the Toll pathway for expression of antimicrobial peptides. Aproteinase cascade also results in proteolytic activation of phenoloxidase and production of melanin coatings that trap and kill parasites and pathogens. The proteinases in hemolymph are regulated by specific inhibitors, including members of the serpin superfamily. New developments in lepidopteran functional genomics should lead to much more complete understanding of the immune systems of this insect group.
INTRODUCTION
Moth larvae have proven to be extremely useful for experiments providing insights on the innate immune systems of insects. Many hemolymph proteins with immune functions were first studied in caterpillars by biochemical methods.1–11 Lepidopteran larvae have also been important for experiments aimed at characterizing immune functions of insect hemocytes.12 Much of the research on lepidopteran immunity has made use of large moth species, including the tobacco hornworm, Manduca sexta and wild silkmoths such as Hyalophora cecropia. The domestic silkmoth, Bombyx mori has also provided significant discoveries about immunity in moths and with the advantage of a sequenced genome,13 it continues to serve as an important model organism. The wax moth, Galleria mellonella, was one of the earliest species used for research in insect immunity14,15 and is the subject of much current research. In addition to these laboratory model species, research on immunity is also underway to investigate the immune systems of moths whose larvae are among the most destructive agricultural pests worldwide, particularly including species in the family Noctuidae. In comparison, there has been relatively little research on immunity in butterflies, perhaps because they can be more difficult to rear or due to lower agricultural impact.
In this review, we describe selected developments in research on innate immunity in lepidopteran insects, with an emphasis on aspects discovered through biochemical and cell biological approaches and potential new insights that may be gained from functional genomics methods. Several recent reviews with a focus on lepidopteran immunity are available.16–23
HEMOCYTES
With a large hemolymph volume in many lepidopteran larvae and pupae, it is possible to collect 104–106 hemocytes from a single individual, making feasible the use of cell biological techniques such as flow cytometry and cell sorting that have advanced mammalian immunology. The most abundant hemocyte types typically described in Lepidopteran larvae are granular cells and plasmatocytes, which are capable of adhesion and phagocytosis.12 Nonadherant hemocyte types include oenocytoids, which synthesize prophenoloxidase (proPO), and spherule cells, whose functions are poorly understood. Monoclonal antibodies are very useful reagents for distinguishing lepidopteran hemocyte populations based on antigenicity rather than morphology,24–26 which can vary considerably, especially for plasmatocytes. Two subpopulations of plasmatocytes have been distinguished based on monoclonal antibody markers in Pseudoplusia includens26 and in M. sexta,27 which may indicate different stages in differentiation or functional specialization. The composition of hemocyte populations varies through larval development.28,29 Hematopoesis in lepidopteran larvae28,31 and the embryonic origin granular cells and plasmatocytes30 have been described.
Granular cells and plasmatocytes function in immunity through responses that involve adherence of cells to foreign surfaces or to other hemocytes.22,32 Both granular cells and plasmatocytes can be phagocytic and in different lepidopteran species either cell type may be the predominant contributor to phagocytosis as a defense.12 A hyperphagocytic cell type, very large cells capable of phagocytosing 500 bacteria, has been described in M. sexta.33 These cells are morphologically similar to a neuroglian-positive subpopulation of plasmatocytes, which can act as a focus for attachment of hemocytes to foreign surfaces34 and perhaps are the same hemocyte type.
Adhesion of granular cells and plasmatocytes leads to two related responses to infection: nodule formation, in which hemocytes cluster to entrap aggregated microorganisms, and encapsulation, in which hemocytes form a multi-layered cellular capsule around a larger eukaryotic parasite.12 Adhesion of hemocytes to injured body wall also probably helps to seal wounds to prevent bleeding.32 Cytokines have been identified in lepidopterans, which promote these hemocyte functions. A hemocyte chemotactic peptide from Pseudaletia separata stimulates directed movement and aggregation of hemocytes.35 This peptide is structurally similar to lepidopteran cytokines called ENF peptides, which have multiple biological activities, including the stimulation of plasmatocyte adhesion and spreading, reduced bleeding and the stimulation of oenocytoid lysis to release proPO.36–40 RNAi results indicate that the M. sexta plasmatocyte spreading peptide from this family promotes hemocyte nodule formation as a protective response to bacterial infection.41 The active ENF peptides are produced by proteolytic processing of a larger protein present in hemolymph.36,37 In M. sexta hemolymph, a serine proteinase with trypsin-like specificity is responsible for this activation, but has not yet been identified due to its instability (Kanost et al, unpublished results). Eicosanoids such as prostaglandins can also simulate hemocytes to aggregate to form nodules.42,43 Prostaglandins can also elicit the lysis of oenocytoids, releasing proPO into the plasma.44 ProPO is activated by plasma proteinases and participates in formation of melanin, which coats nodules and encapsulated objects. This response is discussed in more detail below.
Hemocyte attachment during encapsulation and nodulation is mediated by cell surface adhesion proteins. Lepidopteran hemocytes have cell surface integrins, which function as adhesion molecules.45–50 Plasmatocytes of M. sexta express a specific integrin that is required for efficient encapsulation.47,50 The adhesive properties of this hemocyte-specific integrin derive at least in part from binding to neuroglian and a tetraspanin on neighboring hemocytes.49,51
RECOGNITION OF MICROORGANISMS
Plasma proteins that bind to components on the surface of microorganisms are a key component of the innate immune system of insects. Such proteins stimulate responses including phagocytosis and activation of proteinase signaling cascades. Some of these “pattern recognition” proteins were first discovered in silkworms and then found to occur in immune systems of other insect groups, whereas others appear to be unique to lepidopterans.
Hemolin is a 48 kDa plasma protein first identified in Hyalophora cecropia52,53 and Manduca sexta.54,55 Hemolin is composed of four I-set immunoglobulin (Ig) domains commonly found in cell adhesion proteins of vertebrates and invertebrates. Hemolin also exists in other lepidopteran species including B. mori, Hyphantria cunea, Lymantria dispar, Antheraea pernyi, Antheraea mylitta, Plutella xylostella, Samia cynthia.13,56–61 Hemolin has not been identified in isects from any other order, suggesting that it may have evolved after the split of the Lepidoptera from other insect groups. Hemolin shares structural features with neuroglian,62 a transmembrane Ig-domain protein located on the surface of Manduca glial and neuronal cells63 and the developing embryonic prothoracic gland,64 as well as a subpopulation of M. sexta plasmatocytes.34,51 Hemolin expression is induced by bacteria or their surface components injected into the hemocoel. In H. cecropia, this transcriptional activation is controlled via an intronic enhancer that contains a κB motif.65 Developmental and hormonal signals (e.g., 20-hydroxyecdysone) also affect hemolin production.58,66,67 It has been speculated that hemolin may have an antiviral function.68 Baculovirus exposure up-regulates hemolin transcription in A. pernyi,69 but not in B. mori, Helicoverpa zea, or Heliothis virescens.13,70 A possible role for hemolin in antiviral responses remains to be established. On the other hand, several lines of evidence support the idea that hemolin functions in immune responses to bacterial infection. Hemolin binds to bacterial lipopolysaccharide (LPS) and lipoteichoic acid71,72 and associates with hemocytes.55,73,74 The horseshoe-shape structure of hemolin suggests that one or more of its Ig domains may interact through domain swapping with Ig domains of cell adhesion molecules such as neuroglian on hemocyte surfaces.75 Interaction of hemolin with molecules on the surface of bacteria and with hemocyte membranes suggests that it may bring microorganisms to hemocyte surfaces, promoting phagocytosis or nodule formation. RNAi knockdown of hemolin expression in M. sexta larvae significantly reduced phagocytosis and nodulation of E. coli.76
Peptidoglycan recognition proteins (PGRPs) associate with bacterial peptidoglycans through a conserved domain homologous to T4 bacteriophage lysozyme.77 PGRPs were first discovered in lepidopterans. B. mori PGRP-S1 was isolated from plasma of silkworm larvae as a 19 kDa protein that binds to Micrococcus luteus peptidoglycan and triggers the proPO activation system.8 Molecular cloning of PGRPs from Trichoplusia ni,10 B. mori,13,78 M. sexta,79–81 G. mellonella,82 S. cynthia,83,84 P. xylostella,60 and Ostrinia nubilalis85 suggests that multiple PGRPs are present in every lepidopteran species. In addition to binding to peptidoglycan, these proteins may hydrolyze peptidoglycan if the residues for Zn2+-binding and amidase activity are present.86 The B. mori genome has six short (S) and six long (L) PGRP genes: S3 through S6 are putative amidases; L1 and L4 possess a transmembrane region; L6 is likely cytosolic.13 Constitutive expression of PGRP-S1 is up-regulated in larvae after injection of Enterobacter cloacae.78 E. coli or Bacillus subtilis treatment increases the mRNA levels for PGRP-S1, -S2 and -S5, whereas Staphylococcus aureus injection enhances transcription of B. mori PGRP-S1, -S5, -L1, but not -S2.13 Microbe-induced transcription of PGRPs also occurs in other lepidopteran species.10,79,82,84 The T. ni, S. cynthia and M. sexta PGRPs bind to Bacillus meso-diaminopimelic acid-type peptidoglycans and M. luteus Lys-type peptidoglycans.10,83,87 This M. sexta PGRP can stimulate proPO activation.88 Knockdown of M. sexta PGRP1 synthesis increased larval susceptibility to infection by Photorhabdus luminescens.89,90 So far, the level of knowledge on structures and functions of moth PGRPs is significantly less than for Drosophila PGRPs.91,92 Furthermore, molecular details of how peptidoglycan binding by PGRPs promotes the activation of serine proteinases involved in proPO and spätzle activation are unknown.
Insect β-1,3-glucan recognition proteins (βGRPs) and Gram-negative bacteria binding proteins (GNBPs) are a family of ~55 kDa plasma proteins with an amino-terminal glucan-binding domain and a carboxyl-terminal region similar to β-1,3-glucanases. B. mori βGRP1, M. sexta βGRP1 and βGRP2 and Plodia interpunctella βGRP bind to β-1,3-glucans and to bacteria and stimulate the proPO activation cascade.9,93–97 The amino-terminal domain of B. mori βGRP1 adopts an Ig-like β-sandwich fold and residues have been identified that may form a glucan-binding site.98 M. sexta βGRP1 and βGRP2 gene expression in fat body is differentially regulated: βGRP1 is constitutively expressed, whereas βGRP2 transcripts become highly abundant in the early wandering stage prior to pupation or after an immune challenge.94,95 M. sexta βGRP2 is also present in cuticle of wandering stage larvae.95 Binding of βGRP1 or βGRP2 to curdlan and M. sexta hemolymph proteinase-14 precursor (proHP14) stimulates autoactivation of HP14 to initiate an immune proteinase cascade leading to proPO activation.99,100 B. mori GNBP binds to E. cloacae but not Bacillus licheniformis or curdlan.101 An orthologous M. sexta GNBP recognizes LPS and laminarin (a β-1,3-glucan with β-1,6 branches) and initiates melanization (Y. Wang and H. Jiang, unpublished results). An active glucanase related in sequence to the GRPs was isolated from midgut extract of Helicoverpa armigera larvae. It hydrolyzes β-1,3-glucan but not β-1,4-glucan or glucans with mixed β-1,3 and β-1,4 linkages102 and probably functions as a digestive enzyme.
C-type lectins (CTLs) from animals are a large group of carbohydrate-recognition molecules that bind ligands in a calcium-dependent manner.103 B. mori LPS-binding protein (LBP or CTL20),104,105 Hyphantria cunea Hdd15,106 and M. sexta immulectin-1107 were among the first C-type lectins identified in plasma of lepidopteran larvae. They all contain two carbohydrate-recognition domains. CTLs with this dual-domain structure also include: M. sexta immulectins-2, -3 and -4,108–110 B. mori MBP (CTL10),111 immulectin (CTL11),112 LEL-1 and -2,113 CTL19, CTL21,13 and H. armigera Ha-lectin.114 GenBank currently contains more than 30 additional similar CTL cDNA sequences from eleven other lepidopteran species in five families. The only other dual-domain CTL identified in any other insect species is TcCTL3115 from a beetle, Tribolium castaneum. Hence, such tandem domain CTL genes appear to be fairly unique to Lepidoptera and their emergence and expansion may have occurred early in evolution of this insect group. Expression of at least some of these CTL genes is induced microbial infection.106,108–110,114
Many of the lepidopteran CTLs bind to bacterial LPS and some to lipoteichoic acid.105,106,108–110 They can cause agglutination of bacteria and yeast,107,109–111 presumably due to each of the molecule’s two carbohydrate-binding domains binding to carbohydrates on the surface of adjacent microbial cells. This activity most often requires the presence of calcium. Such clustering of microorganisms may result in more efficient clearance of pathogens by hemocytes through nodule formation.105 Experiments have demonstrated that M. sexta immulectin-2 enhances clearance of Serratia marcesens,116 and suppression of immulectin-2 expression by RNA interference reduced larval survival of a Photorhabdus infection.90 In addition, the immulectins can promote proPO activation and melanin deposition at the surface of objects encapsulated by hemocytes,107,108,110,117,118 which may also promote killing of pathogens and parasites.
Lipophorins are insect hemolymph proteins that transport lipids between tissues.119 The lipophorin particle contains two protein subunits, apolipophorin-I and apophorin-II. When the neutral lipid load is high, an exchangeable plasma protein, apolipophorin-III (apoLpIII) also associates with low density lipophorin to cover hydrophobic surfaces. In lepidopterans, apoLpIII concentration in hemolymph is generally much lower in larvae than in adults, where it functions in transporting lipids from fat body to flight muscles during prolonged flight. Lipophorins and apoLpIII have been implicated in several defense mechanisms.32 Lipophorin appears to be involved in hemolymph clotting in at least some species,20 and may in this way participate in physical trapping of invading microorganisms. There may also be an association of proteins from the melanization cascade with lipophorin or other clotting components, which could contribute to defense.120–122 The affinity of lipophorin and apoLpIII for hydrophobic ligands is consistent with their reported binding of bacterial LPS and lipoteichoic acids,123–128 and the partitioning of these microbial membrane lipids into complexes with lipophorin or apoLpIII may contribute to reducing their toxicity to insect hosts. Lipophorin and apoLpIII have been reported to stimulate other humoral or cellular immune responses in G. mellonella,129–133 and research toward understanding molecular mechanisms underlying such observations is needed.
Pathogenic bacteria and fungi produce proteinases to utilize lepidopteran host proteins as a source of nutrients and to degrade immunity-related defense molecules such as antimicrobial peptides. Thermolysin-like metalloproteinases associated with entomopathogenic bacteria and fungi are essential virulence factors.134–137 However, the presence of microbial proteinases may also be recognized as a signal of infection and stimulate immune defenses in the host. Evidence for sensing of microbial proteinases and their regulation during innate immune responses has been reported from G. mellonella.138 Thermolysin is a potent activator of the serine proteinase cascade that controls proPO activation leading to melanization. Thus, this virulence factor also directly triggers an immune response. In addition, the activity of microbial metalloproteinases within the body of G. mellonella generates peptide fragments that strongly elicit the synthesis of antimicrobial peptides.139 The immune-stimulatory peptides were identified as collagen IV fragments containing the RGD/RGE motif, which bind to integrins of immune-compentent hemocytes.140 The stimulation of two innate immune responses, proPO activation and antimicrobial peptide synthesis, in response to microbial metalloproteinases provides evidence that lepidopteran innate immune responses include reaction to danger-associated molecules produced by microbial virulence factors.141 Furthermore, G. mellonella hemolymph contains an inducible metalloproteinase inhibitor (IMPI), the only specific peptidic inhibitor of thermolysin-like metalloproteinases reported to date from any animal.142 The IMPI gene encodes two distinct metalloproteinase inhibitors that putatively contribute to the regulation of metalloproteinases associated with invading pathogens.143,144
Nucleic acids released from damaged or necrotic cells form another danger signal to enhance insect immune responses. Injection of synthetic oligonucleotides induced attacin expression in B. mori larvae.145 Co-injection of purified host nucleic acids with heat-inactivated P. luminescens into G. mellonella larvae synergistically elevated the level of antimicrobial activity, reduced the total number of hemocytes (a consequence of the attachment of immune-competent cells to tissues during cellular responses) and prolonged the survival of insects infected by P. luminescens.146 DNA and RNA released from damaged cells may interact with lipophorin to trigger clot formation and entrap invading pathogens in hemolymph. In Pseudaletia separata, nucleic acids as well as cytoplasmic proteins (e.g., proPO) are released from oenocytoids through cell lysis induced by the growth-blocking peptide.40
ANTIMICROBIAL PEPTIDES AND PROTEINS
Many insect antimicrobial plasma proteins were first identified and isolated from lepidopteran hemolymph. Expression of antimicrobial peptides and proteins is often induced by microbial infection, with strongest expression usually occurring in fat body, although hemocytes also contribute to the pool of antimicrobial peptides secreted into hemolymph plasma.147 Antimicrobial peptides are also expressed in the midgut of lepidopteran prepupae and secreted into the lumen, perhaps as a prophylaxis against infection during metamorphosis.148 Antimicrobial peptides can also be expressed in extraembryonic tissues of lepidopteran eggs, providing protection against infection for the developing embryo.149
Lysozyme, the first antimicrobial protein reported from insects, was identified more than forty years ago in G. mellonella150 and, like other insect lysozymes, shares structural similarity with C-type (chicken) lysozyme.151 Its activity against Gram-positive bacteria has been attributed to its ability to degrade cell wall peptidoglycan by hydrolysis of the β-1,4 linkages between N-acetylglucosamine and N-acetylmuramic acid residues.2 Besides moderate activity against Gram-negative bacteria,152–154 lepidopteran lysozyme also exhibits antifungal activity.155 Lyozyme also appears to negatively regulate activation of proPO in M. sexta.156
Insects produce a variety of amphipathic peptides with antimicrobial activity attributed to their ability to damage cell membranes of pathogens. The first antimicrobial peptide from insects, isolated from the hemolymph of the silkmoth H. cecropia, was named cecropin.4 Families of cecropin genes have now been found in many lepidopteran (and dipteran) species. Thirteen cecropin genes were identified in the B. mori genome.13 Cecropins are typically ~4 kDa basic peptides, which have an amphipathic α-helical structure. The moricins constitute another group of amphipathic α-helical antimicrobial peptides,157,158 first discovered in B. mori.159 Nine moricin genes are present in the B. mori genome.13 Eight moricin homologs identified in G. mellonella have activity against Gram-negative and Gram-positive bacteria, as well as against yeast and filamentous fungi.160
Lepidoptera possess glycine-rich AMPs (attacins and gloverins) and proline-rich AMPs (lebocins). H. cecropia has two 20 kDa attacin isoforms, an acidic and a basic attacin, with 80% sequence identity.5,161 The B. mori genome also contains two attacin genes,13 and attacin cDNAs have now been cloned from many lepidopteran species. Treatment of E. coli with H. cecropia attacins leads to an increase in outer-membrane permeability, preceding any increase in inner-membrane permeability by at least one generation time. Inhibition of outer-membrane protein synthesis is achieved on the transcriptional level and triggered by binding of attacin to the cell surface without entering the inner membrane or the cytoplasm. Primary binding occurs on LPS, explaining why basic attacin is more active against E. coli than the acidic form. Another family of proline-rich AMPs, the gloverins, appears to exert a similar mechanism of inhibition of outer-membrane protein synthesis.162 Expression and evolution of four B. mori gloverin genes have been investigated,163,164 and gloverins have been studied in several other lepidopteran species,16,165–168 but no homologs have been identified to date in insects from other orders. A family of proline-rich AMPs called lebocins has been characterized in lepidopterans.16,147,169–173 A somewhat puzzling aspect of this family is that the 3.5 kDa active lebocin peptide is processed from a larger precursor and in some members of the family, the amino-terminal pro-region of the protein is conserved in sequence, but the carboxyl-terminal sequence corresponding to the original antimicrobial peptide is not, suggesting that the pro-region may have a function not yet discovered.
AMPs stabilized by intramolecular disulfide bonds are widely distributed in insects. Those with three or four disulfide bonds are commonly referred to as insect defensins because of overall structural similarities to mammalian α and β defensins.174 Insect defensins can be grouped into peptides with an α-helix/β-sheet mixed structure and peptides forming triple-stranded antiparallel β-sheets. Defensin-like AMPs with antibacterial and antifungal activities from several lepidopteran species have been investigated.175–180 Two cysteine-rich defensin-like peptides from G. mellonella specifically inhibit growth of filamentous fungi.179,180 A group of atypical defensin-like peptide named x-tox identified in G. mellonella and two Spodoptera species is characterized by imperfectly conserved tandem repeats of cysteine-stabilized αβ motifs, the structural scaffold characteristic of invertebrate defensins and scorpion toxins.181–183 They are induced upon activation of the immune system but lack detectable antimicrobial activity, suggesting that they may have an immune function yet to be discovered.
EXTRACELLUAR AND INTRACELLULAR SIGNAL TRANSDUCTION STIMULATING ANTIMICROBIAL PEPTIDE SYNTHESIS
Genetic investigations in Drosophila have revealed three major immune signaling pathways (Toll, Imd-JNK and JAK-STAT) that are conserved in mammals.184 Genomic analyses suggest similar pathways exist in other holometabolous insects, including B. mori.13 Additional bioinformatic and experimental evidence described below supports the existence of functional Toll and Imd pathways in lepidopterans.
There are fourteen Toll-like receptor genes in the silkworm genome: six in group A with Drosophila Toll and eight in group B with Drosophila 18-wheeler.13 BmToll and BmToll2 are group B genes highly expressed in ovary and their transcripts become more abundant in fat body after injection of microorganisms,185 suggesting possible involvement in embryonic development and immune response. BmToll3, BmToll4, BmToll9 and BmToll10 mRNA levels in fat body also increase after injection of some microbes. In M. sexta, a Toll-like receptor is present in hemocytes, fat body, epidermis, midgut and Malpighian tubules.186. Its mRNA level increased in hemocytes, but not in fat body, after injection of microorganisms.
Spätzle, the ligand which activates Toll, has been identified and functionally characterized in B. mori and M. sexta.187,188 Spätzle is synthesized as an inactive precursor, proSpätzle, which is secreted as a disulfide-linked homodimer into the hemolymph and requires proteolytic processing to form the active Toll ligand. Expression of proSpätzle is significantly greater in M. sexta hemocytes than in fat body.188 In B. mori, expression was detected primarily in fat body and midgut, but hemocytes were not tested.187 B. mori and M. sexta proSpätzle proteins are only ~20% identical to Drosophila proSpätzle, but have slightly greater similarity (~26% identity) in the carboxyl-terminal 108 residues corresponding to the active form of Drosophila Spätzle known to bind to Toll. Recombinant B. mori and M. sexta Spätzle were active when injected into larvae, inducing fat body expression of attacins, cecropins, gloverin, moricin and lebocin in B. mori187 and attacin, cecropin, moricin and hemolin in M. sexta, with corresponding strong induction of plasma antimicrobial activity.188 Howevever, injection of proSpätzle had little effect, indicating the need for proteolytic activation of this cytokine in response to infection.
Hemolymph proteinase-8 (HP8) is a clip-domain proteinase demonstrated to activate proSpätzle in M. sexta by specific cleavage to produce the carboxyl-terminal 108 residue fragment, as a disulfide-linked homodimer.188 The Drosophila proteinases most similar to HP8 are Easter and Spätzle processing enzyme, both of which function to activate proSpätzle.189 Injection of active HP8 into larvae stimulates expression of attacin, cecropin, gloverin and moricin and elevates plasma antibacterial activity, consistent with a role for HP8 as an activator of proSpätzle.189 HP8 is present in plasma as a zymogen, proHP8 and is activated by another clip-domain proteinase, HP6, an apparent ortholog of Drosophila Persephone. Injection of recombinant HP6 also promoted expression of antimicrobial peptides in larvae.189 ProHP6 is activated in plasma exposed to bacteria or the β-1,3-glucan curdlan, but a hemolymph proteinase responsible for activation of HP6 has not been identified yet. It is apparent that recognition of microbial pattern molecules triggers activation of a proteinase cascade to generate the cytokine Spätzle, leading to expression of a suite of antimicrobial peptides as an innate immune response in this moth (Fig. 1).
Figure 1.
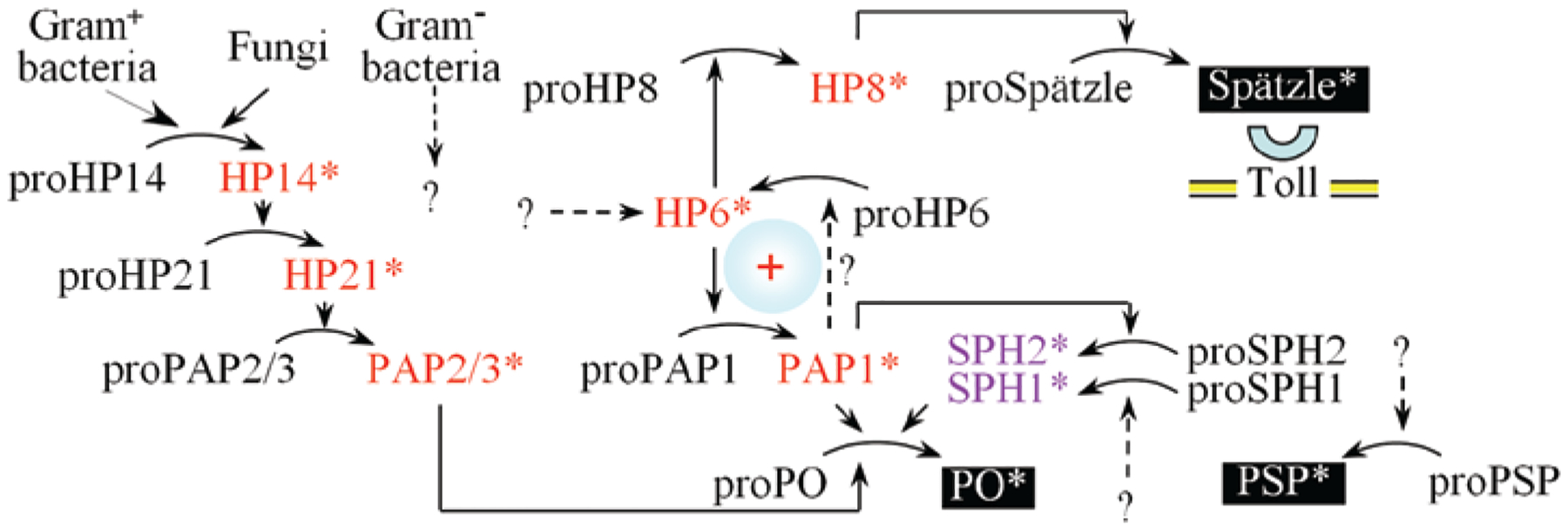
A current model of the hemolymph proteinase system in M. sexta larvae. An initiation proteinase precursor, proHP14, is autoactivated in response to Gram-positive bacterial or fungal infection. HP14 activates proHP21; HP21 activates proPAP2 or proPAP3; PAP2 or PAP3 then cleaves proPO to form active PO in the presence of SPH1 and SPH2. Activation of proPO can also be catalyzed by PAP1 when the high Mr SPH complex is present simultaneously. PAP1 also activates proSPH2 directly and can indirectly lead to proHP6 activation. HP6, whose direct activator is unknown, cleaves proPAP1 and proHP8. PAP1 and HP6 form a positive feedback loop, in which PAP1 indirectly stimulates activation of HP6. HP8 activates Spätzle to induce antimicrobial peptide synthesis via Toll receptor. Active proteins, including HPs, PAPs, SPHs, PO, Spätzle and plasmatocyte-spreading peptide (PSP), are labeled “*” and unknown HPs are marked with “?”.
Two rel-family transcription factors participate in immunity-related gene expression in B. mori.190–192 BmRel encodes, via alternative splicing, RelA and RelB, which are orthologous to Drosophila Dorsal. BmRelish also encodes two splicing isoforms, Relish1 and Relish2. RelA activates the lebocin-4 gene strongly and an attacin gene weakly.190 RelB, lacking the first 52 residues of RelA, activates the attacin gene strongly and other genes to a lesser extent. The Rel homology domain in RelA and RelB binds specifically to κB sites in attacin and lebocin-4 genes. In transgenic silkworms whose BmRel expression is knocked down, expression of antibacterial peptide genes fails to be induced by M. luteus. Knockdown of BmRelish expression abolishes antimicrobial peptide production elicited by E. coli.191 Intact Relish1 and Relish2 do not activate promoters of B. mori attacin, cecropin B1, lebocin-3 and lebocin-4 genes. Removal of the ankyrin repeats in Relish1 is necessary for its transcriptional activation of antibacterial peptide genes. However, Relish2, which lacks the repeats and the transactivation domain, serves as a dominant negative factor to suppress the function of active Relish1. Relish1 binds to the κB sites in attacin and cecropin B1 genes, while the sites for activating lebocin-4 promotor differ between Relish1 active form and RelA. Meso-diaminopimelic acid- and Lys-type peptidoglycans can stimulate differential expression of antimicrobial peptide genes in the silkworm, which is affected by the level, binding affinity and transactivation activity of Relishes and Rels.192 Relish1 is inhibited by its own ankyrin repeats and RelA and RelB are negatively regulated by B. mori Cactus.193 Cactus interacts with the DNA-binding domain in the Rels but not with Relish1 or Relish2. Taken together, these data strongly suggest that the Toll and Imd pathways are functional in the silkworm to regulate immunity-related gene expression.
PROPHENOLOXIDASE ACTIVATION SYSTEM
PO-catalyzed quinone and melanin formation is a universal response in arthropods for killing and entrapping pathogens or parasites.194,195 PO in insect hemolymph has tyrosinase-like activities, including o-hydroxylation of monophenols and oxidation of o-diphenols to quinones.18 Tyrosine, DOPA and dopamine are substrates in insect hemolymph that contribute to PO-catalyzed quinone formation and subsequent melanin synthesis. Tyrosine hydroxylase and dopa decarboxylase are upregulated in fat body after injection of bacteria and probably contribute to provision of hemolymph dopamine for the innate immune response.80,196–198 Oxidation of dopamine by PO leads to production of 5,6-dihydroxyindole, which has antimicrobial activity toward bacteria and fungi.199 PO is produced as a zymogen that requires a specific proteolytic cleavage to gain activity. This regulatory mechanism protects the host insect from potentially harmful effects of the reactive chemicals produced by PO, as the enzyme is activated only when elicited by wounding or infection. Understanding of insect PO and its activation was pioneered through detailed biochemical investigations with B. mori,11 and the M. sexta model system is providing new insights into lepidopteran PO function and regulation.17,19
ProPO from B. mori and M. sexta exists in plasma as a heterodimer of two related subunits, each ~80 kDa.200,201 Insect proPO sequences are related to arthropod hemocyanins, and copper-binding motifs in the two groups of proteins are conserved.202 ProPO is synthesized constitutively by oenocytoids.197,201,203 ProPOs lack secretion signal peptides and are released from oenocytoids by lysis of the cells.40,44 Activation of the proPO zymogen requires cleavage of a conserved Arg-Phe bond about 50 residues from the amino-terminus.11,18 The crystal structure of the M. sexta proPO heterodimer suggests that the proteolysis between Arg51 and Phe52 induces a conformational change to dislodge a specific Phe residue in each subunit and open up the active site for substrate binding.204 The active site contains a canonical type-3 di-nuclear copper center, with each copper ion coordinated by three conserved His residues. Glu395 of the subunit-2 may act as a general base to deprotonate monophenols, a key step in the o-hydroxylation of tyrosine by PO.
Extracellular serine proteinase pathways have evolved in animals to stimulate rapid responses to tissue damage, pathogen invasion, or physiological cues.205 A few eliciting molecules, via specific recognition and cascade amplification, lead to sequential proteolytic activation of a large number of pathway components within minutes. This type of proteinase pathway results in activation of proPO in response to infection. Many of the proteinases that function in such cascade pathways in arthropods contain a carboxyl-terminal serine proteinase domain similar trypsin or chymotrypsin and one or two amino-terminal clip domains, which have likely regulatory functions.206 The proteinases from lepidopterans known to activate proPO and most of the proteinases upstream in the activation pathway are clip domain proteinases. Fifteen clip domain proteinase genes were identified in the B. mori genome,13 and fourteen such enzymes are expressed in fat body or hemocytes of M. sexta.207
In lepidopterans, proPO activating proteinases (PAPs) have been well characterized in M. sexta208–211 and B. mori.212 They are present in hemolymph as zymogens at low concentration in naïve larvae, and their expression in fat body is upregulated in response to injection of bacteria. M. sexta PAP1 contains a single clip domain, whereas PAP2 and PAP3 and B. mori proPO activating enzyme each contain two clip domains. The solution structure of the region of PAP2 containing the dual clip domains suggests a potential proPO-binding site, a bacteria-interacting region and a surface for activator/adaptor docking in each domain.213 Purified M. sexta PAP1, PAP2 and PAP3 do not efficiently generate active PO activity, even after a significant amount of proPO is cleaved at Arg51, without the presence of protein cofactors from hemolymph, identified as serine proteinase homologs (SPHs). SPHs also contain clip domains but lack proteinase activity due to substitution of the active site Ser residue with Gly.214,215 M. sexta SPH1 and SPH2 also require proteolytic processing to gain function, which leads to their assembly into the active, high Mr cofactor required in the reaction with proPO and PAP to generate high levels of PO activity.215,216 This interesting interaction, which does not seem to be required for the silkworm proPO activating enzyme,212 is not well understood and requires further investigation.
The M. sexta proPO activation system includes at least four other serine proteinases (Fig. 1). An initiatiing hemolymph proteinase (HP14) contains five low-density lipoprotein receptor class A repeats, one Sushi domain, one Wonton domain and one proteinase catalytic domain.217 Adding recombinant proHP14 to larval plasma greatly enhances proPO activation in response to M. luteus. The HP14 proenzyme, with its first domain truncated, was isolated from plasma of larvae injected with bacteria.99 After incubation with β-1,3-glucan and βGRP1 or βGRP2, the proHP14 was converted to a two-chain active form, which significantly enhanced plasma proPO activation. The activation of proHP14 results from an autoactivation cleavage after Leu387, occurring when proHP14 interacts with β-1,3-glucan and βGRP. Characterization of individual domains and truncation mutants of HP14 showed that the amino-terminal regulatory region of HP14 participates in the specific binding of microbial polysaccharides and βGRP1.100 Proteins orthologous to M. sexta HP14 also function at the top of proteinase cascades in immune responses of Drosophila and a beetle, Tenebrio molitor.218,219
HP14 activates a clip domain proteinase HP21, which can then activate proPAP2 and proPAP3,220,221 resulting in activation of proPO. ProPAP1, which differs from proPAP2 and proPAP3 in having only one clip domain, is activated by HP6.189 HP6 also functions in the proSpätzle activation pathway,188,189 providing cross-talk between these two immune cascades in M. sexta. Addition of active PAP1 to hemolymph stimulates the proteolytic activation of HP6, HP8, SPH1 and SPH2.222 PAP1 directly activates proSPH2, but processing of the other precursors is probably indirect, depending on other plasma factors. Consequently, a minute amount of PAP1 added to plasma from naïve larvae stimulates a remarkably high level of PO activity in a short period of time, as a result of a positive feedback loop (Fig. 1). Some gaps in this pathway still need to be filled. To date, it is not clear which HP generates active HP6, leading to both PAP1-mediated melanization and HP8-mediated Toll pathway activation. The proteinase which activates SPH1 has not been identified and the possible involvement of HPs in Gram-negative bacteria-induced defense responses is not yet well understood.
INHIBITORY REGULATION OF HEMOLYMPH PROTEINASES BY SERPINS
Immune responses can produce molecules that are harmful to the host. Serine proteinases and the molecules they activate have potentially toxic effects. Proteinase inhibitors of different families can exist constitutively at relatively high levels in plasma of naïve insects and may also be produced in response to physiological or pathological stimuli.223 Serpins are ~50 kDa proteins, many of which are irreversible inhibitors and key modulators of immune proteinase pathways.224 Serpins occur as plasma proteins in vertebrates and invertebrates. They have been purified and studied by molecular cloning from lepidopteran insects including B. mori,225,226 M. sexta,7,227–232 H. cunea,56 Mythimna unipuncta,233 and Mamestra configurata.234 The B. mori genome contains 34 serpin genes, which have been analyzed with regard to molecular evolution of this gene family.13,235 Serpin biochemical and physiological functions in lepidopterans have been characterized most extensively in M. sexta.17,236
The M. sexta serpin-1gene encodes twelve protein isoforms, each having the same amino-terminal 336 residues and a variable region consisting of the carboxyl-terminal ~40 residues, including the reactive center loop that interacts with a serine proteinase during an inhibition reaction. The variable region is produced by mutually exclusive alternative splicing of twelve different versions of the ninth exon of the gene,237,238 resulting in a group of serpin proteins with diverse inhibitory selectivity.227 Serpin genes that employ alternative splicing at the same position to generate multiple serpin isoforms have been studied in other moth species. These include B. mori serpin genes 1 (3 isoforms) and 28 (4 isoforms),235 M. configurata serpin-1 (9 isoforms),239 and Choristoneura fumiferana serpin-1 (at least 4 isoforms).240 This mechanism for expanding serpin functional diversity, first discovered in Lepidoptera, been observed in other insect orders and in nematodes.241
Physiological functions have been identified for a few of the M. sexta serpin-1 isoforms. Serpin-1A, -1E and -1J can inhibit HP8, and serpin-1J appears to be a physiologically relevant regulator of HP8 activity during immune responses87 (An, Ragan, Kanost, unpublished results). Serpin-1J also inhibits all three PAPs to regulate proPO activation210,216 (Jiang, unpublished data). Serpin-1I can inhibit HP14.99 Serpin-1K was identified in hemolymph in a compex with a midgut chymotrypsin,87 suggesting a potential role for serpin-1 proteins in protection from digestive proteinases that escape into the hemocoel.
A putative orthologous group of serpins including M. sexta serpin-3,229 B. mori serpin-3,235 and an H. cunea serpin56 are synthesized in response to infection and form a clade with Drosophila serpin-27A and Anopheles gambiae and Aedes aegypti serpin-2, which have immune regulatory functions.235 M. sexta serpin-3 contains a reactive site sequence (Asn-Lys-Phe-Gly) highly similar to the proteolytic activation site (Asn-Arg-Phe-Gly) in both proPO subunits. By mimicking these natural substrates, serpin-3 acts as an efficient inhibitor of all three PAPs.229 M. sexta serpin-4 and serpin-5230 are closely related to each other and form a clade with B. mori serpins 4, 5, 7, 8, 14, 31 and 32.235 Serpin-4 suppresses proPO activation by inhibiting HPs upstream of the PAPs, such as HP1, HP6 and HP21, while serpin-5 forms complexes with HP1 and HP6.231 M. sexta and B. mori serpin-6 are apparent orthologs. M. sexta serpin-6 can inhibit PAP3 to block proPO activation and it also inhibits HP8 to potentially regulate the Toll pathway.242
LEPIDOPTERAN IMMUNE RESPONSES TO DIFFERENT TYPES OF INFECTION
Herbivorous lepidopteran caterpillars consume enormous amounts of plant diet and are capable of increasing their body weight up to 20% per day. Plant leaves harbor microbial communities, which enter the alimentary canal with the ingested food. The midgut of caterpillars can sense bacterial contamination of the diet and trigger immune responses, which are accompanied with life history tradeoffs.243,244 Bacteria can also naturally enter and infect lepidopterans through wounds. Phagocytosis by granular hemocytes or plasmatocytes, depending on the species is probably the earliest response,245 and when numbers of bacteria are relatively low, can efficiently clear infection, particularly with bacteria of low virulence. Larger numbers of bacteria lead to hemocyte aggregation and formation of hemocyte nodules, probably aided by plasma pattern recognition proteins that agglutinate bacteria. Activation of lepidopteran hemocytes to become adhesive involves cytokines from the ENF family245 and eicosanoid signaling.42 Hemocyte nodules often become melanized, as products of PO polymerize to form a melanin coat around the aggregated hemocytes and bacteria. This response also generates quinones and reactive oxygen species that may help to kill the entrapped bacteria. The humoral response, synthesis of antibacterial peptides, occurs more slowly than the initial hemocyte response, requiring several hours before significant concentrations of antimicrobial molecules accumulate. This broad-spectrum antibacterial activity, comprised of a mixture of different antibacterial peptides, is an effective protective response that can last up to a few days.
Entomopathogenic fungi can invade insect hosts directly via their sclerotized chitinous integument. Penetration and lateral growth within the inner part of the integument is achieved by joint action of physical pressure and secreted enzymes among which proteinases play a predominant role.246 Most, if not all, entomopathogenic fungi develop in the hemocoel as cells known as protoplasts or hyphal bodies, which lack a fully developed cell wall. The absence of typical fungal cell wall components such as β-1,3-glucan may allow these fungi to evade the host immune surveillance. However, hyphal bodies of the entomopathogenic fungus Metarhizium anisopliae are ingested by plasmatocytes in G. mellonella during an early phase of infection,247 even though they lack β-1,3-glucan on their surface. Ingested hyphal bodies are not killed, but propagate and grow within phagocytic vacuoles of the plasmatocytes, which are likely occupied as a vehicle for dispersal within the hemocoel. Survival of hyphal bodies within the hemocytes as well as overcoming of multicellular encapsulation have been attributed to fungal secondary metabolites (toxins), such as destruxins and cyclosporins which suppress cellular and humoral responses within the infected hosts.248,249 Similar to bacteria, fungal cells are recognized, phagocytosed or, if too large or too numerous, encapasulated by hemocytes in the hemcoel of Lepidoptera.250 Eicosanoids have been implicated as mediators in cellular antifungal defense.251 The lepidopteran antifungal response also encompasses proPO activation, production of reactive oxygen species,252 and synthesis of potent antifungal peptides including cecropins253 and gallerimycin.254 However, the humoral responses upon infection with parasitic fungi such as Beauveria bassiana is different from that observed after challenge with bacteria.255
Larger eukaryotic parasites such as nematodes and parasitic wasps provoke hemocytic encapsulation and melanization,245 but little is understood about molecular mechanisms for recognizing such parasites as foreign.256 Successful parasites are able to disrupt or suppress the host insect’s immune response. Entomopathogenic nematodes have a mutualistic relationship with virulent bacterial pathogens of insects, in which the bacteria produce virulence factors that disable cellular and humoral immune responses of their insect host.257 Parasitoid wasps that use lepidopteran larvae as hosts inject venom and accessory fluid components that disrupt the host immune system. In braconid and ichneumonid parasitoid wasps, this adaptation includes the injection of polydnaviruses, which infect host hemocytes and express immunity-disrupting proteins, but do not replicate.258 Among these are gene products which cause apoptosis of hemocytes, disrupt signal transduction pathways in the humoral immune response and block melanization.259–261
Some aspects of immunity to viral infection in lepidopterans are now becoming understood.262 Baculoviruses are the most commonly studied viral pathogens of these insects. These viruses enter the larva by first infecting the gut epithelial cells. A response that can protect caterpillars from these infections is apoptosis of infected midgut cells. Infected cells die before viral replication can be completed, thus preventing spread of the virus to other cells or tissues.263 Baculoviruses encode gene products that inhibit caspases responsible for initiating apoptosis, allowing the infection cycle to proceed.263 Hemocytic encapsulation of infected tracheal cells is another immune response to baculoviruses that has been observed in lepidopterans.262 In addition, hemolymph PO is correlated with virucidal activity toward baculoviruses,264 and thus, the proPO activation cascade may help protect against baculoviral infection.
CONCLUSION
Lepidopteran insects have some important advantages as model systems for immunological research, including a depth of knowledge developed so far and the availability of large hemolymph samples from individual insects for studies of hemocytes and plasma proteins. With the recent exception of B. mori, studies on moths and butterflies have been hampered by a lack of genomic information, which would facilitate proteomics investigations and also lead to more ready identification of candidate genes for experimental study. This situation is likely to change dramatically in the next few years, as it is anticipated that genome sequences for several additional lepidopteran species will soon become available. Transgenic technology for silkworms is now well developed and may yield new fundamental information on immunity and perhaps strains with improved disease resistance. Furthermore, more complete understanding of lepidopteran immune responses could lead to future developments of enhanced strategies for regulating insect pest populations through use of specific pathogens and parasites.
ACKNOWLEDGEMENTS
Research in the authors’ laboratories was supported by NIH grants GM58634 (HJ) and GM41247 (MRK) and by grants from the Deutsche Forschungsgemeinschaft (AV) VI 219/3-1.
REFERENCES
- 1.Faye I, Pye A, Rasmuson T et al. Insect immunity II. Simultaneous induction of antibacterial activity and selective synthesis of some hemolymph proteins in diapausing pupae of Hyalophora cecropia and Samia cynthia. Infect Immun 1975; 12:1426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powning RF, Davidson WJ. Studies on the insect bacteriolytic enzymes-II. Some physical and enzymatic properties of lysozyme from haemolymph of Galleria mellonella. Comp Biochem Physiol 1976; 55:221–28. [DOI] [PubMed] [Google Scholar]
- 3.Hultmark D, Steiner H, Rasmuson T et al. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem 1980; 106:7–16. [DOI] [PubMed] [Google Scholar]
- 4.Steiner H, Hultmark D, Engström A et al. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981; 292:246–48. [DOI] [PubMed] [Google Scholar]
- 5.Hultmark D, Engström A, Andersson K et al. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J 1983; 2:571–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida H, Ochai M, Ashida M. β-1,3-Glucan receptor and peptidoglycan receptor are present as separate entities within insect prophenoloxidase activating system. Biochem Biophys Res Commun 1986; 114:1177–83. [DOI] [PubMed] [Google Scholar]
- 7.Kanost MR, Prasad SV, Wells MA. Primary structure of a member of the serpin superfamily of proteinase inhibitors from an insect, Manduca sexta. J Biol Chem 1989; 264:965–72. [PubMed] [Google Scholar]
- 8.Yoshida H, Kinoshita K, Ashida M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J Biol Chem 1996; 271:13854–60. [DOI] [PubMed] [Google Scholar]
- 9.Ochiai M, Ashida M. Purification of a β-1,3-glucan recognition protein in the prophenoloxidase system from hemolymph of the silkworm, Bombyx mori. J Biol Chem 1998; 263:12056–62. [PubMed] [Google Scholar]
- 10.Kang D, Liu G, Lundström A et al. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci USA 1998; 95:10078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashida M, Brey PT. Recent advances on the research of the insect prophenoloxidase cascade. In: Brey PT Hultmark D, eds. Molecular Mechanisms of Immune Responses in Insects. London:Chapman and Hall, 1998:135–72. [Google Scholar]
- 12.Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol 2002; 32:1295–309. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Ishibashi J, Fujita K et al. A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem Mol Biol 2008; 38:1087–110. [DOI] [PubMed] [Google Scholar]
- 14.Stevens JM. Bactericidal activity of the blood of actively immunized wax moth larvae. Canadian J Microbiol 1962; 8:491–9. [Google Scholar]
- 15.Powning RF, Davidson WJ. Studies on the insect bacteriolytic enzymes-II. Some physical and enzymatic properties of lysozyme from haemolymph of Galleria mellonella. Comp Biochem Physiol 1976; 55:221–28. [DOI] [PubMed] [Google Scholar]
- 16.Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev 2004; 198:97–105. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H The biochemical basis of antimicrobial responses in Manduca sexta. Insect Science 2008; 15:53–66. [Google Scholar]
- 18.Kanost MR, Gorman MJ. Phenoloxidases in insect immunity. In: Beckage NE ed. Insect Immunology. San Diego: Elsevier, 2008:69–96. [Google Scholar]
- 19.Ragan EJ, An C, Jiang H et al. Roles of hemolymph proteins in antimicrobial defences of Manduca sexta. In: Insect Infection and Immunity. Reynolds S, Rolff J eds. Oxford University Press, 2009:34–48. [Google Scholar]
- 20.Dushay MS. Insect hemolymph clotting. Cell Mol Life Sci 2009; 66:2643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilcinskas A Lepidopterans as model mini-hosts for human pathogens and as a source for peptide antibiotics. In: In: Goldsmith M and Marec F, eds. Molecular Biology and Genetics of the Lepidoptera Boca Raton:CRC Press, 2010:293–305. [Google Scholar]
- 22.Kanost MR, Nardi JB. Innate immune responses of Manduca sexta. In: Goldsmith M and Marec F, eds. Molecular Biology and Genetics of the Lepidoptera Boca Raton:CRC Press, 2010:271–291. [Google Scholar]
- 23.Mukherjee K, Altincicek B, Hain T et al. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol. 2010; 76:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullett H, Ratcliffe NA, Rowley AF. The generation and characterization of anti-insect blood cell monoclonal antibodies. J. Cell Sci 1992; 105:93–100. [Google Scholar]
- 25.Willott E, Trenczek T, Thrower LW et al. Immunochemical identification of insect hemocyte populations: monoclonal antibodies distinguish four major hemocyte types in Manduca sexta. Eur J Cell Biol 1994; 65:417–23. [PubMed] [Google Scholar]
- 26.Gardiner EM, Strand MR. Monoclonal antibodies bind distinct classes of hemocytes in the moth Pseudoplusia includens. J Insect Physiol 1999; 45:113–126. [DOI] [PubMed] [Google Scholar]
- 27.Nardi JB, Pilas B, Bee CM et al. Neuroglian-positive plasmatocytes of Manduca sexta and the initiation of hemocyte attachment to foreign surfaces. Dev Comp Immunol 2006; 30:447–62. [DOI] [PubMed] [Google Scholar]
- 28.Gardiner EM, Strand MR. Hematopoiesis in larval Pseudoplusia includens and Spodoptera frugiperda. Arch Insect Biochem Physiol 2000; 43:147–64. [DOI] [PubMed] [Google Scholar]
- 29.Beetz S, Holthusen TK, Koolman J et al. Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol 2008; 67:63–75. [DOI] [PubMed] [Google Scholar]
- 30.Nardi JB. Embryonic origins of the two main classes of hemocytes—granular cells and plasmatocytes—in Manduca sexta. Dev Genes Evol 2004; 214:19–28. [DOI] [PubMed] [Google Scholar]
- 31.Nardi JB, Pilas B, Ujhelyi E et al. Hematopoietic organs of Manduca sexta and hemocyte lineages. Dev Genes Evol 2003; 213(10):477–91. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt O, Söderhäll K, Theopold U et al. Role of adhesion in arthropod immune recognition. Annu Rev Entomol 2010; 55:485–504. [DOI] [PubMed] [Google Scholar]
- 33.Dean P, Potter U, Richards EH et al. Hyperphagocytic haemocytes in Manduca sexta. J Insect Physiol 2004; 50:1027–36. [DOI] [PubMed] [Google Scholar]
- 34.Nardi JB, Pilas B, Bee CM et al. Neuroglian-positive plasmatocytes of Manduca sexta and the initiation of hemocyte attachment to foreign surfaces. Dev Comp Immunol 2006; 30:447–62. [DOI] [PubMed] [Google Scholar]
- 35.Nakatogawa S, Oda Y, Kamiya M et al. A novel peptide mediates aggregation and migration of hemocytes from an insect. Curr Biol 2009; 19:779–85. [DOI] [PubMed] [Google Scholar]
- 36.Clark KD, Pech LL, Strand MR. Isolation and identification of a plasmatocyte-spreading peptide from the hemolymph of the lepidopteran insect Pseudoplusia includens. J Biol Chem 1997; 272:23440–7. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Jiang H, Kanost MR. Biological activity of Manduca sexta paralytic and plasmatocyte spreading peptide and primary structure of its hemolymph precursor. Insect Biochem Mol Biol 1999; 29:1075–86. [DOI] [PubMed] [Google Scholar]
- 38.Strand MR, Hayakawa Y, Clark KD. Plasmatocyte spreading peptide (PSP1) and growth blocking peptide (GBP) are multifunctional homologs. J Insect Physiol 2000; 46:817–24. [DOI] [PubMed] [Google Scholar]
- 39.Volkman BF, Anderson ME, Clark KD et al. Structure of the insect cytokine peptide plasmatocyte-spreading peptide 1 from Pseudoplusia includens. J Biol Chem 1999; 274:4493–6. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto Y, Oda Y, Uryu M et al. Insect cytokine growth-blocking peptide triggers a termination system of cellular immunity by inducing its binding protein. J Biol Chem 2003; 278:38579–85. [DOI] [PubMed] [Google Scholar]
- 41.Eleftherianos I, Xu M, Yadi H et al. Plasmatocyte-spreading peptide (PSP) plays a central role in insect cellular immune defenses against bacterial infection. J Exp Biol 2009; 212(Pt 12):1840–8. [DOI] [PubMed] [Google Scholar]
- 42.Stanley D. Prostaglandins and other eicosanoids in insects: biological significance. Annu Rev Entomol 2006; 51:25–44. [DOI] [PubMed] [Google Scholar]
- 43.Shrestha S, Kim Y. Various eicosanoids modulate the cellular and humoral immune responses of the beet armyworm, Spodoptera exigua. Biosci Biotechnol Biochem 2009; 73:2077–84. [DOI] [PubMed] [Google Scholar]
- 44.Shrestha S, Kim Y. Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm Spodoptera exigua. Insect Biochem Mol Biol 2008; 38:99–112. [DOI] [PubMed] [Google Scholar]
- 45.Lavine MD, Strand MR. Haemocytes from Pseudoplusia includens express multiple alpha and beta integrin subunits. Insect Mol Biol 2003; 12:441–52. [DOI] [PubMed] [Google Scholar]
- 46.Wiegand C, Levin D, Gillespie J et al. Monoclonal antibody MS13 identifies a plasmatocyte membrane protein and inhibits encapsulation and spreading reactions of Manduca sexta hemocytes. Arch Insect Biochem Physiol 2000; 45:95–108. [DOI] [PubMed] [Google Scholar]
- 47.Levin DM, Breuer LN, Zhuang S et al. A hemocyte-specific integrin required for hemocytic encapsulation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol 2005; 35:369–80. [DOI] [PubMed] [Google Scholar]
- 48.Nardi JB, Zhuang S, Pilas B et al. Clustering of adhesion receptors following exposure of insect blood cells to foreign surfaces. J Insect Physiol 2005; 51:555–64. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang S, Kelo L, Nardi JB. An integrin-tetraspanin interaction required for cellular innate immune responses of an insect, Manduca sexta. J Biol Chem 2007; 282:22563–72. [DOI] [PubMed] [Google Scholar]
- 50.Zhuang S, Kelo L, Nardi JB et al. Multiple alpha subunits of integrin are involved in cell-mediated responses of the Manduca immune system. Dev Comp Immunol 2008; 32:365–79. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang S, Kelo L, Nardi JB et al. Neuroglian on hemocyte surfaces is involved in homophilic and heterophilic interactions of the innate immune system of Manduca sexta. Dev Comp Immunol 2007; 31:1159–67. [DOI] [PubMed] [Google Scholar]
- 52.Andersson K, Steiner H. Structure and properties of protein P4, the major bacteria-inducible protein in pupae of Hyalophora cecropia. Insect Biochem 1987; 17:133–40. [Google Scholar]
- 53.Sun SC, Lindstöm I, Boman HG et al. Hemolin: an insect immune protein belonging to the immunoglobulin superfamily. Science 1990; 250:1729–32. [DOI] [PubMed] [Google Scholar]
- 54.Ladendorff NE, Kanost MR. Isolation and characterization of bacteria induced protein P4 from hemolymph of Manduca sexta. Arch Insect Biochem Physiol 1990; 15:33–41. [DOI] [PubMed] [Google Scholar]
- 55.Ladendorff NE, Kanost MR. Bacteria-induced protein P4 (hemolin) from Manduca sexta: a member of the immunoglobulin superfamily which can inhibit hemocyte aggregation. Arch Insect Biochem Physiol 1991; 18:285–300. [DOI] [PubMed] [Google Scholar]
- 56.Shin SW, Park SS, Park DS et al. Isolation and characterization of immune-related genes from the fall webworm, Hyphantria cunea, using PCR-based differential display and subtractive cloning. Insect Biochem Mol Biol 1998; 28:827–37. [DOI] [PubMed] [Google Scholar]
- 57.Lee KY, Horodyski FM, Valaitis AP et al. Molecular characterization of the insect immune protein hemolin and its high induction during embryonic diapause in the gypsy moth, Lymantria dispar. Insect Biochem Mol Biol 2002; 32:1457–67. [DOI] [PubMed] [Google Scholar]
- 58.Li W, Terenius O, Hirai M et al. Cloning, expression and phylogenetic analysis of hemolin, from the Chinese oak silk moth, Antheraea pernyi. Dev Comp Immunol 2005; 29:853–64. [DOI] [PubMed] [Google Scholar]
- 59.Gandhe AS, Arunkumar KP, John SH et al. Analysis of bacteria-challenged wild silkmoth, Antheraea mylitta (Lepidoptera) transcriptome reveals potential immune genes. BMC Genomics 2006; 7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eum JH, Seo YR, Yoe SM et al. Analysis of the immune-inducible genes of Plutella xylostella using expressed sequence tags and cDNA microarray. Dev Comp Immunol 2007; 31:1107–20 [DOI] [PubMed] [Google Scholar]
- 61.Bao Y, Yamano Y, Morishima I. Induction of hemolin gene expression by bacterial cell wall components in eri-silkworm, Samia cynthia ricini. Comp Biochem Physiol Part B 2007; 146:147–51. [DOI] [PubMed] [Google Scholar]
- 62.Faye I, Kanost MR. Function and regulation of hemolin. In: Brey PT, Hultmark D eds. Molecular Mechanisms of Immune Responses in Insects. London: Chapman and Hall, 1998:173–88. [Google Scholar]
- 63.Gibson NJ, Tolbert LP. Activation of epidermal growth factor receptor mediates receptor axon sorting and extension in the developing olfactory system of the moth Manduca sexta. J Comp Neurol 2006; 495:554–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen CL, Lampe DJ, Robertson HM et al. Neuroglian is expressed on cells destined to form the prothoracic glands of Manduca embryos as they segregate from surrounding cells and rearrange during morphogenesis. Dev Biol 1997; 181:1–13. [DOI] [PubMed] [Google Scholar]
- 65.Roxström-Lindquist K, Lindström-Dinnetz I, Olesen J et al. An intron enhancer activates the immunoglobulin-related hemolin gene in Hyalophora cecropia. Insect Mol Biol 2002; 11:505–15. [DOI] [PubMed] [Google Scholar]
- 66.Yu XQ, Kanost MR. Developmental expression of Manduca sexta hemolin. Arch Insect Biochem Physiol 1999; 42:198–212. [DOI] [PubMed] [Google Scholar]
- 67.Roxström-Lindquist K, Assefaw-Redda Y, Rosinska K et al. 20-hydroxyecdysone indirectly regulates hemolin gene expression in Hyalophora cecropia. Insect Mol Biol 2005; 14:645–52. [DOI] [PubMed] [Google Scholar]
- 68.Terenius O Hemolin-A lepidopteran anti-viral defense factor? Dev Comp Immunol 2008; 32:311–6. [DOI] [PubMed] [Google Scholar]
- 69.Hirai M, Terenius O, Li W et al. Baculovirus and dsRNA induce hemolin, but no antibacterial activity in Antheraea pernyi. Insect Mol Biol 2004; 13:399–405. [DOI] [PubMed] [Google Scholar]
- 70.Terenius O, Popham HJ, Shelby KS. Bacterial, but not baculoviral infections stimulate hemolin expression in noctuid moths. Dev Comp Immunol 2009; 33:1176–85. [DOI] [PubMed] [Google Scholar]
- 71.Daffre S, Faye I. Lipopolysaccharide interaction with hemolin, an insect member of the Ig-superfamily. FEBS Lett 1997; 408:127–30. [DOI] [PubMed] [Google Scholar]
- 72.Yu XQ, Kanost MR. Binding of hemolin to bacterial lipopolysaccharide and lipoteichoic acid. An immunoglobulin superfamily member from insects as a pattern-recognition receptor. Eur J Biochem 2002; 269:1827–34. [DOI] [PubMed] [Google Scholar]
- 73.Zhao L, Kanost MR. In search of a function for hemolin, a hemolymph protein from the immunoglobulin superfamily. J Insect Physiol 1996; 42:73–9. [Google Scholar]
- 74.Bettencourt R, Lanz-Mendoza H, Lindquist KR et al. Cell adhesion properties of hemolin, an insect immune protein in the Ig superfamily. Eur J Biochem 1997; 250:630–7. [DOI] [PubMed] [Google Scholar]
- 75.Su XD, Gastinel LN, Vaughn DE et al. Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science 1998; 281:991–5. [DOI] [PubMed] [Google Scholar]
- 76.Eleftherianos I, Gokcen F, Felfoldi G et al. The immunoglobulin family protein hemolin mediates cellular immune responses to bacteria in the insect Manduca sexta. Cellular Microbiol 2007; 9:1137–47. [DOI] [PubMed] [Google Scholar]
- 77.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs). Genome Biol 2006; 7:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ochiai M, Ashida M. A pattern recognition protein for peptidoglycan. Cloning the cDNA and the gene of the silkworm, Bombyx mori. J Biol Chem 1999; 274:11854–8. [DOI] [PubMed] [Google Scholar]
- 79.Yu XQ, Zhu YF, Ma C et al. Pattern recognition proteins in Manduca sexta plasma. Insect Biochem Mol Biol 2002; 32:1287–93. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y, Johnson T, Kanost MR. Identification of differentially expressed genes in the immune response of the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol 2003; 33:541–59. [DOI] [PubMed] [Google Scholar]
- 81.Zou Z, Najar F, Wang Y et al. Pyrosequence analysis of expressed sequence tags for Manduca sexta hemolymph proteins involved in immune responses. Insect Biochem Mol Biol 2008; 38:677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seitz V, Clermont A, Wedde M et al. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol 2003; 27:207–15. [DOI] [PubMed] [Google Scholar]
- 83.Onoe H, Matsumoto A, Hashimoto K et al. Peptidoglycan recognition protein (PGRP) from eri-silkworm, Samia cynthia ricini; protein purification and induction of the gene expression. Comp Biochem Physiol B 2007; 147:512–19. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto K, Mega K, Matsumoto Y et al. Three peptidoglycan recognition protein (PGRP) genes encoding potential amidase from eri-silkworm, Samia cynthia ricini. Comp Biochem Physiol B Biochem Mol Biol 2007; 148:322–8. [DOI] [PubMed] [Google Scholar]
- 85.Coates BS, Sumerford DV, Hellmich RL et al. Mining an Ostrinia nubilalis midgut expressed sequence tag (EST) library for candidate genes and single nucleotide polymorphisms (SNPs). Insect Mol Biol 2008; 17:607–20. [DOI] [PubMed] [Google Scholar]
- 86.Mellroth P, Karlsson J, Steiner H. A scavenger function for a Drosophila peptidoglycan recognition protein. J Biol Chem 2003; 278:7059–64. [DOI] [PubMed] [Google Scholar]
- 87.Ragan EJ. Immune-related protein complexes and serpin-1 isoforms in Manduca sexta plasma. PhD Dissertation, Kansas State University. 2008. [Google Scholar]
- 88.Sumathipala N. Expression, purification and characterization of peptidoglycan recognition protein 1 from Manduca sexta (L.). MS Thesis, Oklahoma State University. 2009. [Google Scholar]
- 89.Eleftherianos I, Marokhazi J, Millichap PJ et al. Prior infection of Manduca sexta with nonpathogenic Escherichia coli elicits immunity to pathogenic Photorhabdus luminescens: roles of immune-related proteins shown by RNA interference. Insect Biochem Mol Biol 2006; 36:517–25. [DOI] [PubMed] [Google Scholar]
- 90.Eleftherianos I, Millichap PJ, ffrench-Constant RH et al. RNAi suppression of recognition protein mediated immune responses in the tobacco hornworm Manduca sexta causes increased susceptibility to the insect pathogen Photorhabdus. Dev Comp Immunol 2006; 30:1099–107. [DOI] [PubMed] [Google Scholar]
- 91.Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol 2007; 5:264–77. [DOI] [PubMed] [Google Scholar]
- 92.Charroux B, Rival T, Narbonne-Reveau K et al. Bacterial detection by Drosophila peptidoglycan recognition proteins. Microbes Infect 2009; 11:631–636. [DOI] [PubMed] [Google Scholar]
- 93.Ochiai M, Ashida M. A pattern-recognition protein for β-1,3-glucan: the binding domain and the cDNA cloning of β-1,3-glucan recognition protein from the silkworm, Bombyx mori. J Biol Chem 2000; 275:4995–5002. [DOI] [PubMed] [Google Scholar]
- 94.Ma C, Kanost MR. A β-1,3-glucan recognition protein from an insect, Manduca sexta, agglutinates microorganisms and activates the phenoloxidase cascade. J Biol Chem 2000; 275:7505–14. [DOI] [PubMed] [Google Scholar]
- 95.Jiang H, Ma C, Lu ZQ et al. β-1,3-glucan recognition protein-2 (βGRP-2) from Manduca sexta: an acute-phase protein that binds β-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem Mol Biol 2004; 34:89–100. [DOI] [PubMed] [Google Scholar]
- 96.Fabrick JA, Baker JE, Kanost MR. cDNA cloning, purification, properties and function of a beta-1,3-glucan recognition protein from a pyralid moth, Plodia interpunctella. Insect Biochem Mol Biol 2003; 33:579–94. [DOI] [PubMed] [Google Scholar]
- 97.Fabrick JA, Baker JE, Kanost MR. Innate immunity in a pyralid moth: functional evaluation of domains from a β-1,3-glucan recognition protein. J Biol Chem 2004; 279:26605–11. [DOI] [PubMed] [Google Scholar]
- 98.Takahasi K, Ochiai M, Horiuchi M et al. Solution structure of the silkworm βGRP/GNBP3 N-terminal domain reveals the mechanism for β-1,3-glucan-specific recognition. Proc Natl Acad Sci USA 2009; 106:11679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y, Jiang H. Interaction of β-1,3-glucan with its recognition protein activates hemolymph proteinase 14, an initiation enzyme of the prophenoloxidase activation system in Manduca sexta. J Biol Chem 2006; 281:9271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Jiang H. Binding properties of the regulatory domains in Manduca sexta hemolymph proteinase-14, an initiation enzyme of the prophenoloxidase activation system. Dev Comp Immunol 2010; 34:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee WJ, Lee JD, Kravchenko VV et al. Purification and molecular cloning of an inducible gram-negative bacteria-binding protein from the silkworm, Bombyx mori. Proc Natl Acad Sci USA 1996; 93:7888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pauchet Y, Freitak D, Heidel-Fischer HM et al. Immunity or digestion: glucanase activity in a glucan-binding protein family from Lepidoptera. J Biol Chem 2009; 284:2214–24. [DOI] [PubMed] [Google Scholar]
- 103.Vasta GR, Quesenberry M, Ahmed H et al. C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev Comp Immunol 1999; 23,401–20. [DOI] [PubMed] [Google Scholar]
- 104.Koizumi N, Morozumi A, Imamura M et al. Lipopolysaccharide-binding proteins and their involvement in the bacterial clearance from the hemolymph of the silkworm Bombyx mori. Eur J Biochem 1997; 248:217–24. [DOI] [PubMed] [Google Scholar]
- 105.Koizumi N, Imai Y, Morozumi A et al. Lipopolysaccharide-binding protein of Bombyx mori participates in a hemocyte-mediated defense reaction against Gram-negative bacteria. J Insect Physiol 1999; 45:853–9. [DOI] [PubMed] [Google Scholar]
- 106.Shin SW, Park DS, Kim SC et al. Two carbohydrate recognition domains of Hyphantria cunea lectin bind to bacterial lipopolysaccharides through O-specific chain. FEBS Lett 2000; 467:70–4. [DOI] [PubMed] [Google Scholar]
- 107.Yu XQ, Gan H, Kanost MR. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem Mol Biol 1999; 29:585–97. [DOI] [PubMed] [Google Scholar]
- 108.Yu XQ, Kanost MR. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to Gram-negative bacteria. J Biol Chem 2000; 275:37373–81. [DOI] [PubMed] [Google Scholar]
- 109.Yu XQ, Tracy ME, Ling E et al. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem Mol Biol 2005; 35:285–95. [DOI] [PubMed] [Google Scholar]
- 110.Yu XQ, Ling E, Tracy ME et al. Immulectin-4 from the tobacco hornworm Manduca sexta binds to lipopolysaccharide and lipoteichoic acid. Insect Mol Biol 2006; 15:119–28. [DOI] [PubMed] [Google Scholar]
- 111.Watanabe A, Miyazawa S, Kitami M et al. Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B. mori hemolymph. J Immunol 2006; 177:4594–604. [DOI] [PubMed] [Google Scholar]
- 112.Kim SR, Lee KS, Kim I et al. cDNA sequence of a novel immulectin homologue from the silkworm, Bombyx mori. Int J Indust Entomol 2003; 6:99–102. [Google Scholar]
- 113.Takase H, Watanabe A, Yoshizawa Y et al. Identification and comparative analysis of three novel C-type lectins from the silkworm with functional implications in pathogen recognition. Dev Comp Immunol 2009; 33:789–800. [DOI] [PubMed] [Google Scholar]
- 114.Chai LQ, Tian YY, Yang DT et al. Molecular cloning and characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev Comp Immunol 2008; 32:71–83. [DOI] [PubMed] [Google Scholar]
- 115.Zou Z, Evans J, Lu Z et al. Comparative genome analysis of the Tribolium immune system. Genome Biol 2007; 8:R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu XQ, Kanost MR. Manduca sexta lipopolysaccharide-specific immulectin-2 protects larvae from bacterial infection. Dev Comp Immunol 2003; 27:189–96. [DOI] [PubMed] [Google Scholar]
- 117.Yu XQ, Kanost MR. Immulectin-2, a pattern recognition receptor that stimulates hemocyte encapsulation and melanization in the tobacco hornworm, Manduca sexta. Dev Comp Immunol 2004; 28:891–900. [DOI] [PubMed] [Google Scholar]
- 118.Ling E, Yu XQ. Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev Comp Immunol 2006; 30:289–99. [DOI] [PubMed] [Google Scholar]
- 119.Van der Horst DJ, Roosendaal SD, Rodenburg KW. Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol Cell Biochem. 2009; 326:105–19. [DOI] [PubMed] [Google Scholar]
- 120.Li D, Scherfer C, Korayem AM et al. Insect hemolymph clotting: evidence for interaction between the coagulation system and the prophenoloxidase activating cascade. Insect Biochem Mol Biol 2002; 32:919–28. [DOI] [PubMed] [Google Scholar]
- 121.Ma G, Hay D, Li D et al. Recognition and inactivation of LPS by lipophorin particles. Dev Comp Immunol 2006; 30:619–26. [DOI] [PubMed] [Google Scholar]
- 122.Rahman MM, Ma G, Roberts HL et al. Cell-free immune reactions in insects. J Insect Physiol 2006; 52:754–62. [DOI] [PubMed] [Google Scholar]
- 123.Kato Y, Motoi Y, Taniai K et al. Lipopolysaccharide-lipophorin complex formation in insect hemolymph: a common pathway of lipopolysaccharide detoxification both in insects and in mammals. Insect Biochem Mol Biol 1994; 24:547–55. [DOI] [PubMed] [Google Scholar]
- 124.Dunphy G, Halwani A. Haemolymph proteins of larvae of Galleria mellonella detoxify endotoxins of the insect pathogenic bacteria Xenhorabdus nematophilus (Enterobacteriaceae). J Insect Physiol 1997; 43:1023–9. [DOI] [PubMed] [Google Scholar]
- 125.Halwani AE, Niven DF, Dunphy GB. Apolipophorin-III and the interactions of lipoteichoic acids with the immediate immune responses of Galleria mellonella. J Invertebr Pathol 2000; 76:233–41. [DOI] [PubMed] [Google Scholar]
- 126.Pratt CC, Weers PM. Lipopolysaccharide binding of an exchangeable apolipoprotein, apolipophorin III, from Galleria mellonella. Biol Chem 2004; 385:1113–9. [DOI] [PubMed] [Google Scholar]
- 127.Kim HJ, Je HJ, Park SY et al. Immune activation of apolipophorin-III and its distribution in hemocyte from Hyphantria cunea. Insect Biochem Mol Biol 2004; 34:1011–23. [DOI] [PubMed] [Google Scholar]
- 128.Leon LJ, Idangodage H, Wan CP et al. Apolipophorin III: lipopolysaccharide binding requires helix bundle opening. Biochem Biophys Res Commun 2006; 348:1328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weisner A, Losen S, Kopacek P et al. Isolated apolipophorin III from Galleria mellonella stimulates the immune reaction of this insect. J Insect Physiol 1997; 43:383–91. [DOI] [PubMed] [Google Scholar]
- 130.Halwani AE, Dunphy GB. Apolipophorin-III in Galleria mellonella potentiates hemolymph lytic activity. Dev Comp Immunol 1999; 23:563–70. [DOI] [PubMed] [Google Scholar]
- 131.Iimura Y, Ishikawa H, Yamamoto K et al. Hemagglutinating properties of apolipophorin III from the hemolymph of Galleria mellonella larvae. Arch Insect Biochem Physiol 1998; 38:119–25. [DOI] [PubMed] [Google Scholar]
- 132.Halwani AE, Niven DF, Dunphy GB. Apolipophorin-III in the greater wax moth, Galleria mellonella (Lepidoptera: Pyralidae). Arch Insect Biochem Physiol 2001; 48:135–43. [DOI] [PubMed] [Google Scholar]
- 133.Whitten MM, Tew IF, Lee BL et al. A novel role for an insect apolipoprotein (apolipophorin III) in β-1,3-glucan pattern recognition and cellular encapsulation reactions. J Immunol 2004; 172:2177–85. [DOI] [PubMed] [Google Scholar]
- 134.St. Leger RJ, Bidochka MJ, Roberts DW. Isoforms of the cuticle-degrading Pr1 proteinase and production of a metalloproteinase by Metarhizium anisopliae. Arch Biochem Biophys 1994; 313:1–7. [DOI] [PubMed] [Google Scholar]
- 135.Fedhila S, Nel P, Lereclus D. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J Bacteriol. 2002; 184:3296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Held KG, LaRock CN, D’Argenio DA et al. A metalloprotease secreted by the insect pathogen Photorhabdus luminescens induces melanization. Appl Environ Microbiol 2007; 73(23):7622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qazi SS, Khachatourians GG. Hydrated conidia of Metarhizium anisopliae release a family of metalloproteases. J Invertebr Pathol 2007; 95:48–59. [DOI] [PubMed] [Google Scholar]
- 138.Griesch J, Wedde M, Vilcinskas A. Recognition and regulation of metalloproteinase activity in the haemolymph of Galleria mellonella: a new pathway mediating induction of humoral immune responses. Insect Biochem Mol Biol 2000; 30:461–72. [DOI] [PubMed] [Google Scholar]
- 139.Altincicek B, Linder M, Linder D et al. Microbial metalloproteinases mediate sensing of invading pathogens and activate innate immune responses in the lepidopteran model host Galleria mellonella. Infect Immun 2007; 75:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Altincicek B, Berisha A, Mukherjee K et al. Identification of collagen IV derived danger/alarm signals in insect immunity by nanoLC-FTICR MS. Biological Chemistry 2009; 390:1303–11. [DOI] [PubMed] [Google Scholar]
- 141.Matzinger P The danger model: a renewed sense of self. Science 2002; 296:301–05. [DOI] [PubMed] [Google Scholar]
- 142.Vilcinskas A, Wedde M. Inhibition of Beauveria bassiana proteases and fungal development by inducible protease inhibitors in the haemolymph of Galleria mellonella larvae. Biocontrol Sci Technol 1997; 7:591–601. [Google Scholar]
- 143.Clermont A, Wedde M, Seitz V et al. Cloning and expression of an inhibitor against microbial metalloproteinases from insects (IMPI) contributing to innate immunity. Biochem J 2004; 382:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wedde M, Weise C, Nuck C et al. The insect metallo-proteinase inhibitor gene of the lepidopteran Galleria mellonella encodes two distinct inhibitors. Biol Chem 2007; 388:119–27. [DOI] [PubMed] [Google Scholar]
- 145.Kim I, Kim SH, Lee YS et al. Immune stimulation in the silkworm, Bombyx mori L., by CpG oligodeoxy-nucleotides. Arch Insect Biochem Physiol 2004; 55:43–8. [DOI] [PubMed] [Google Scholar]
- 146.Altincicek B, Stötzel S, Wygrecka M et al. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation and prolong survival upon infection in insects. J Immunol 2008; 181:2705–12. [DOI] [PubMed] [Google Scholar]
- 147.Lavine MD, Chen G, Strand MR. Immune challenge differentially affects transcript abundance of three antimicrobial peptides in hemocytes from the moth Pseudoplusia includens. Insect Biochem Mol Biol 2005; 35:1335–46. [DOI] [PubMed] [Google Scholar]
- 148.Dunn PE, Bohnert TJ, Russell V. Regulation of antibacterial protein synthesis following infection and during metamorphosis of Manduca sexta. Ann N Y Acad Sci 1994; 712:117–30. [DOI] [PubMed] [Google Scholar]
- 149.Gorman MJ, Kankanala P, Kanost MR. Bacterial challenge stimulates innate immune responses in extra-embryonic tissues of tobacco hornworm eggs. Insect Mol Biol 2004; 13:19–24. [DOI] [PubMed] [Google Scholar]
- 150.Mohrig W, Messner B. Immunreaktionen bei Insekten. I. Lysozyme als grundlegender antibakterieller Faktor im humoralen Abwehrgeschehen. Biol Zentralbl 1968; 87:439–47. [Google Scholar]
- 151.Jolles J, Schoentgen F, Croizier G et al. Insect lysozymes from three species of Lepidoptera: Their structural relatedness to the C (chicken) type lysozyme. J Mol Evol 1979; 14:267–71. [DOI] [PubMed] [Google Scholar]
- 152.Yu KH, Kim KN, Lee JH et al. Comparative study on characteristics of lysozymes from the hemolymph of three lepidopteran larvae, Galleria mellonella, Bombyx mori, Agrius convolvuli. Dev Comp Immunol 2002; 26:707–13. [DOI] [PubMed] [Google Scholar]
- 153.Chapelle M, Girard PA, Cousserans F et al. Lysozymes and lysozyme-like proteins from the fall armyworm, Spodoptera frugiperda. Mol Immunol 2009; 47:261–9. [DOI] [PubMed] [Google Scholar]
- 154.Wang WX, Wang YP, Deng XJ et al. Molecular and functional characterization of a c-type lysozyme from the Asian corn borer, Ostrinia furnacalis. J Insect Sci 2009; 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vilcinskas A, Matha V. Effect of the entomopathogenic fungus Beauveria bassiana on humoral immune response of Galleria mellonella larvae (Lepidoptera: Pyralidae). Eur J Entomol 1997; 94:461–72. [Google Scholar]
- 156.Rao XJ, Ling E, Yu XQ. The role of lysozyme in the prophenoloxidase activation system of Manduca sexta: an in vitro approach. Dev Comp Immunol 2010; 34:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hemmi H, Ishibashi J, Hara S et al. Solution structure of moricin, an antibacterial peptide, isolated from the silkworm Bombyx mori. FEBS Lett 2002; 518:33–8. [DOI] [PubMed] [Google Scholar]
- 158.Dai H, Rayaprolu S, Gong Y et al. Solution structure, antibacterial activity and expression profile of Manduca sexta moricin. J Pept Sci 2008; 14:855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hara S, Yamakawa M. Moricin, a novel antibacterial peptide family isolated from the silkworm, Bombyx mori. Biochem J 1995; 310:651–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brown S, Howard A, Kasprzak AB et al. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleria mellonella. Insect Biochem Mol Biol 2008; 38:201–12. [DOI] [PubMed] [Google Scholar]
- 161.Kockum K, Faye I, Hofsten PV et al. Insect immunity. Isolation and sequence of two cDNA clones corresponding to acidic and basic attacins from Hyalophora cecropia. EMBO J 1984; 3:2071–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Axén A, Carlsson A, Engström A et al. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur J Biochem 1997; 247:614–619. [DOI] [PubMed] [Google Scholar]
- 163.Mrinal N, Nagaraju J. Intron loss is associated with gain of function in the evolution of the gloverin family of antibacterial genes in Bombyx mori. J Biol Chem 2008; 283:23376–87. [DOI] [PubMed] [Google Scholar]
- 164.Kawaoka S, Katsuma S, Daimon T et al. Functional analysis of four Gloverin-like genes in the silkworm, Bombyx mori. Arch Insect Biochem Physiol 2008; 67:87–96. [DOI] [PubMed] [Google Scholar]
- 165.Mackintosh JA, Gooley AA, Karuso PH et al. A gloverin-like antibacterial protein is synthesized in Helicoverpa armigera following bacterial challenge. Dev Comp Immunol 1998; 22:387–99. [DOI] [PubMed] [Google Scholar]
- 166.Lundström A, Liu G, Kang D et al. Trichoplusia ni gloverin, an inducible immune gene encoding an antibacterial insect protein. Insect Biochem Mol Biol 2002; 32:795–801. [DOI] [PubMed] [Google Scholar]
- 167.Seitz V, Clermont A, Wedde M et al. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol 2003; 27:207–15. [DOI] [PubMed] [Google Scholar]
- 168.Wang Q, Liu Y, He HJ et al. Immune responses of Helicoverpa armigera to different kinds of pathogens. BMC Immunol 2010; 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hara S, Yamakawa M. A novel antibacterial peptide family isolated from the silkworm, Bombyx mori. Biochem J 1995; 310:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Furukawa S, Taniai K, Ishibashi J et al. A novel member of lebocin gene family from the silkworm, Bombyx mori. Biochem Biophys Res Commun 1997; 238:769–74. [DOI] [PubMed] [Google Scholar]
- 171.Liu G, Kang D, Steiner H. Trichoplusia ni lebocin, an inducible immune gene with a downstream insertion element. Biochem Biophys Res Commun 2000; 269:803–7. [DOI] [PubMed] [Google Scholar]
- 172.Bao Y, Yamano Y, Morishima I. A novel lebocin-like gene from eri-silkworm, Samia cynthia ricini, that does not encode the antibacterial peptide lebocin. Comp Biochem Physiol B Biochem Mol Biol 2005; 140:127–31 [DOI] [PubMed] [Google Scholar]
- 173.Rayaprolu S, Wang Y, Kanost MR et al. Functional analysis of four processing products from multiple precursors encoded by a lebocin-related gene from Manduca sexta. Dev Comp Immunol 2010; 34:638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bulet P, Stöcklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev 2004; 198:169–84. [DOI] [PubMed] [Google Scholar]
- 175.Lamberty M, Ades S, Uttenweiler-Joseph S et al. Insect immunity. Isolation from the lepidopteran Heliothis virescens of a novel insect defensin with potent antifungal activity. J Biol Chem 1999; 274:9320–6. [DOI] [PubMed] [Google Scholar]
- 176.Lamberty M, Caille A, Landon C et al. Solution structures of the antifungal heliomicin and a selected variant with both antibacterial and antifungal activities. Biochemistry 2001; 40:11995–2003. [DOI] [PubMed] [Google Scholar]
- 177.Mandrioli M, Bugli S, Saltini S et al. Molecular characterization of a defensin in the IZD-MB-0503 cell line derived from immunocytes of the insect Mamestra brassicae (Lepidoptera). Biol Cell 2003; 95:53–7. [DOI] [PubMed] [Google Scholar]
- 178.Volkoff AN, Rocher J, d’Alençon E et al. Characterization and transcriptional profiles of three Spodoptera frugiperda genes encoding cysteine-rich peptides. A new class of defensin-like genes from lepidopteran insects? Gene 2003; 319:43–53. [DOI] [PubMed] [Google Scholar]
- 179.Schuhmann B, Seitz V, Vilcinskas A et al. Cloning and expression of gallerimycin, an antifungal peptide expressed in immune response by the greater wax moth, Galleria mellonella. Arch Insect Biochem Physiol 2003; 53:125–33. [DOI] [PubMed] [Google Scholar]
- 180.Lee YS, Yun EK, Jang WS et al. Purification, cDNA cloning and expression of an insect defensin from the great wax moth, Galleria mellonella. Insect Mol Biol 2004; 13:65–72. [DOI] [PubMed] [Google Scholar]
- 181.Seitz V, Clermont A, Wedde M et al. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol 2003; 27:207–15. [DOI] [PubMed] [Google Scholar]
- 182.Girard P, Boublik Y, Wheat CW et al. X-tox: an atypical defensin derived family of immune-related proteins specific to Lepidoptera. Dev Comp Immunol 2008; 32:575–84. [DOI] [PubMed] [Google Scholar]
- 183.Destoumieux-Garzón D, Brehelin M, Bulet P et al. Spodoptera frugiperda X-tox protein, an immune related defensin rosary, has lost the function of ancestral defensins. PLoS One 2009; 4(8):e6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Ann Rev Immunol 2007; 25:697–743. [DOI] [PubMed] [Google Scholar]
- 185.Cheng TC, Zhang YL, Liu C et al. Identification and analysis of Toll-related genes in the domesticated silkworm, Bombyx mori. Dev Comp Immunol 2008; 32:464–75. [DOI] [PubMed] [Google Scholar]
- 186.Ao JQ, Ling E, Yu XQ. A Toll receptor from Manduca sexta is in response to Escherichia coli infection. Mol Immunol 2008; 45:543–52. [DOI] [PubMed] [Google Scholar]
- 187.Wang Y, Cheng T, Rayaprolu S et al. Proteolytic activation of pro-spätzle is required for the induced transcription of antimicrobial peptide genes in lepidopteran insects. Dev Comp Immunol 2007; 31:1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.An C, Jiang H, Kanost MR. Proteolytic activation and function of the cytokine Spätzle in innate immune response of a lepidopteran insect, Manduca sexta. FEBS J 2010; 277:148–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.An C, Ishibashi J, Ragan EJ et al. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J Biol Chem 2009; 284:19716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tanaka H, Yamamoto M, Moriyama Y et al. A novel Rel protein and shortened isoform that differentially regulate antibacterial peptide genes in the silkworm Bombyx mori. Biochim Biophys Acta 2005; 1730:10–21. [DOI] [PubMed] [Google Scholar]
- 191.Tanaka H, Matsuki H, Furukawa S et al. Identification and functional analysis of Relish homologs in the silkworm, Bombyx mori. Biochim Biophys Acta 2007; 1769:559–68. [DOI] [PubMed] [Google Scholar]
- 192.Tanaka H, Sagisaka A, Nakajima Y et al. Correlation of differential expression of silkworm antimicrobial peptide genes with different amounts of rel family proteins and their gene transcriptional activity. Biosci Biotechnol Biochem 2009; 73:599–606. [DOI] [PubMed] [Google Scholar]
- 193.Furukawa S, Tanaka H, Ishibashi J et al. Functional characterization of a cactus homolog from the silkworm Bombyx mori. Biosci Biotechnol Biochem 2009; 73:2665–70. [DOI] [PubMed] [Google Scholar]
- 194.Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem Mol Biol 2005; 35:443–59. [DOI] [PubMed] [Google Scholar]
- 195.Cerenius L, Lee BL, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 2008; 29:263–71. [DOI] [PubMed] [Google Scholar]
- 196.Noguchi H, Tsuzuki S, Tanaka K et al. Isolation and characterization of a dopa decarboxylase cDNA and the induction of its expression by an insect cytokine, growth-blocking peptide in Pseudaletia separata. Insect Biochem Mol Biol 2003; 33:209–17. [DOI] [PubMed] [Google Scholar]
- 197.Gorman MJ, An C, Kanost MR. Characterization of tyrosine hydroxylase from Manduca sexta. Insect Biochem Mol Biol 2007; 37:1327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hashimoto K, Yamano Y, Morishima I. Induction of tyrosine hydroxylase gene expression by bacteria in the fat body of eri-silkworm, Samia cynthia ricini. Comp Biochem Physiol B 2008; 149:501–6. [DOI] [PubMed] [Google Scholar]
- 199.Zhao P, Li J, Wang Y et al. Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem Mol Biol 2007; 37:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yasuhara Y, Koizumi Y, Katagiri C et al. Reexamination of properties of prophenoloxidase isolated from larval hemolymph of the silkworm Bombyx mori. Arch Biochem Biophys 1995; 320:14–23. [DOI] [PubMed] [Google Scholar]
- 201.Jiang H, Wang Y, Ma C et al. Subunit composition of pro-phenol oxidase from Manduca sexta: molecular cloning of subunit proPO-p1. Insect Biochem Mol Biol 1997; 27:835–50. [DOI] [PubMed] [Google Scholar]
- 202.Burmester T. Origin and evolution of arthropod hemocyanins and related proteins. J Comp Physiol B 2002; 172:95–107. [DOI] [PubMed] [Google Scholar]
- 203.Iwama R, Ashida M. Biosynthesis of prophenoloxidase in hemocytes of larval hemolymph of the silkworm, Bombyx mori. Insect Biochem 16:547–55. [Google Scholar]
- 204.Li Y, Wang Y, Jiang H et al. Crystal structure of Manduca sexta prophenoloxidase provides insights into the mechanism of type-3 copper enzymes. Proc Natl Acad Sci USA 2009; 106:17001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Krem MM, Di Cera E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci 2002; 27:67–74. [DOI] [PubMed] [Google Scholar]
- 206.Jiang H, Kanost MR. The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol 2000; 30:95–105. [DOI] [PubMed] [Google Scholar]
- 207.Jiang H, Wang Y, Gu Y et al. Molecular identification of a bevy of serine proteinases in Manduca sexta hemolymph. Insect Biochem Mol Biol 2005; 35:931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jiang H, Wang Y, Kanost MR. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: a bacteria-inducible protein similar to Drosophila Easter. Proc Natl Acad Sci USA 1998; 95:12220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jiang H, Wang Y, Yu XQ et al. Prophenoloxidase-activating proteinase-2 from hemolymph of Manduca sexta. A bacteria-inducible serine proteinase containing two clip domains. J Biol Chem 2003; 278:3552–61. [DOI] [PubMed] [Google Scholar]
- 210.Jiang H, Wang Y, Yu XQ et al. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: a clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem Mol Biol 2003; 33:1049–60. [DOI] [PubMed] [Google Scholar]
- 211.Gupta S, Wang Y, Jiang H. Purification and characterization of Manduca sexta prophenoloxidase-activating proteinase-1 (PAP-1), an enzyme involved in insect immune responses. Protein Exp Purif 2005a; 39:261–8. [DOI] [PubMed] [Google Scholar]
- 212.Satoh D, Horii A, Ochiai M et al. Prophenoloxidase-activating enzyme of the silkworm, Bombyx mori: purification, characterization and cDNA cloning. J Biol Chem 1999; 274:7441–53. [DOI] [PubMed] [Google Scholar]
- 213.Huang R, Lu Z, Dai H et al. The solution structure of clip domains from Manduca sexta prophenoloxidase activating proteinase-2. Biochemistry 2007; 46:11431–9. [DOI] [PubMed] [Google Scholar]
- 214.Yu XQ, Jiang H, Wang Y et al. Nonproteolytic serine proteinase homologs involved in phenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol 2003; 33:197–208. [DOI] [PubMed] [Google Scholar]
- 215.Wang Y, Jiang H. Prophenoloxidase (proPO) activation in Manduca sexta: an analysis of molecular interactions among proPO, proPO-activating proteinase-3 and a cofactor. Insect Biochem Mol Biol 2004; 34:731–42. [DOI] [PubMed] [Google Scholar]
- 216.Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem Mol Biol 2005; 35:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ji C, Wang Y, Guo X et al. A pattern recognition serine proteinase triggers the prophenoloxidase activation cascade in the tobacco hornworm, Manduca sexta. J Biol Chem 2004; 279:34101–6. [DOI] [PubMed] [Google Scholar]
- 218.Buchon N, Poidevin M, Kwon HM et al. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci USA 2009; 106:12442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Roh KB, Kim CH, Lee H et al. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J Biol Chem 2009; 284:19474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang Y, Jiang H. Reconstitution of a branch of the Manduca sexta prophenoloxidase activation cascade in vitro: snake-like hemolymph proteinase 21 (HP21) cleaved by HP14 activates prophenoloxidase-activating proteinase-2 precursor. Insect Biochem Mol Biol 2007; 37:1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gorman MJ, Wang Y, Jiang H et al. Manduca sexta hemolymph proteinase 21 activates prophenoloxidase-activating proteinase 3 in an insect innate immune response proteinase cascade. J Biol Chem 2007b; 282:11742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang Y, Jiang H. A positive feedback mechanism in the Manduca sexta prophenoloxidase activation system. Insect Biochem Mol Biol 2008; 38:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kanost MR. Serine proteinase inhibitors in arthropod immunity. Dev Comp Immunol 1999; 23:291–301. [DOI] [PubMed] [Google Scholar]
- 224.Gettins PG. Serpin structure, mechanism and function. Chem Rev 2002; 102:4751–804. [DOI] [PubMed] [Google Scholar]
- 225.Sasaki T, Kobayashi K. Isolation of two novel proteinase inhibitors from hemolymph of silkworm larva, Bombyx mori: comparison with human serum proteinase inhibitors. J Biochem 1984; 95:1009–17. [DOI] [PubMed] [Google Scholar]
- 226.Sasaki T Patchwork-structure serpins from silkworm (Bombyx mori) larval hemolymph. Eur J Biochem 1991; 202:255–61. [DOI] [PubMed] [Google Scholar]
- 227.Jiang H, Kanost MR. Characterization and functional analysis of 12 naturally occurring reactive site variants of serpin-1 from Manduca sexta. J Biol Chem 1997; 272:1082–7. [DOI] [PubMed] [Google Scholar]
- 228.Gan H, Wang Y, Jiang H et al. A bacteria-induced, intracellular serpin in granular hemocytes of Manduca sexta. Insect Biochem Mol Biol 2001; 31:887–98. [DOI] [PubMed] [Google Scholar]
- 229.Zhu Y, Wang Y, Gorman MJ et al. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J Biol Chem 2003b; 278:46556–64. [DOI] [PubMed] [Google Scholar]
- 230.Tong Y, Kanost MR. Manduca sexta serpin-4 and serpin-5 inhibit the prophenol oxidase activation pathway: cDNA cloning, protein expression and characterization. J Biol Chem 2005; 280:14923–31. [DOI] [PubMed] [Google Scholar]
- 231.Tong Y, Jiang H, Kanost MR. Identification of plasma proteases inhibited by Manduca sexta serpin-4 and serpin-5 and their association with components of the prophenol oxidase activation pathway. J Biol Chem 2005; 280:14932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang Y, Jiang H. Purification and characterization of Manduca sexta serpin-6: a serine proteinase inhibitor that selectively inhibits prophenoloxidase-activating proteinase-3. Insect Biochem Mol Biol 2004; 34, 387–95. [DOI] [PubMed] [Google Scholar]
- 233.Cherqui A, Cruz N, Simões N. Purification and characterization of two serine protease inhibitors from the hemolymph of Mythimna unipuncta. Insect Biochem Mol Biol 2001; 31:761–9. [DOI] [PubMed] [Google Scholar]
- 234.Chamankhah M, Braun L, Visal-Shah S et al. Mamestra configurata serpin-1 homologs: cloning, localization and developmental regulation. Insect Biochem Mol Biol 2003; 33:355–69. [DOI] [PubMed] [Google Scholar]
- 235.Zou Z, Zhao P, Weng H et al. A comparative analysis of serpin genes in the silkworm genome. Genomics 2009; 93:367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kanost MR. Serpins in a Lepidopteran insect, Manduca sexta. In: Silverman GA, Lomas DA eds. The Serpinopathies: molecular and cellular aspects of serpins and their disorders. Hackensack, NJ: World Scientific Publishing. 2007:229–42. [Google Scholar]
- 237.Jiang H, Wang Y, Kanost MR. Mutually exclusive exon use and reactive center diversity in insect serpins. J Biol Chem 1994; 269:55–8. [PubMed] [Google Scholar]
- 238.Jiang H, Wang Y, Huang Y et al. Organization of serpin gene-1 from Manduca sexta: evolution of a family of alternate exons encoding the reactive site loop. J Biol Chem 1996; 271:28017–23. [DOI] [PubMed] [Google Scholar]
- 239.Hegedus DD, Erlandson M, Baldwin D et al. Differential expansion and evolution of the exon family encoding the Serpin-1 reactive centre loop has resulted in divergent serpin repertoires among the Lepidoptera. Gene 2008; 418:15–21. [DOI] [PubMed] [Google Scholar]
- 240.Zheng YP, He WY, Béliveau C et al. Cloning, expression and characterization of four serpin-1 cDNA variants from the spruce budworm, Choristoneura fumiferana. Comp Biochem Physiol B Biochem Mol Biol 2009; 154:165–73. [DOI] [PubMed] [Google Scholar]
- 241.Krüger O, Ladewig J, Köster K et al. Widespread occurrence of serpin genes with multiple reactive centre-containing exon cassettes in insects and nematodes. Gene 2002; 29:97–105. [DOI] [PubMed] [Google Scholar]
- 242.Zou Z, Jiang H. Manduca sexta serpin-6 regulates immune serine proteinases PAP-3 and HP8. cDNA cloning, protein expression, inhibition kinetics and function elucidation. J Biol Chem 2005; 280:14341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Freitak D, Wheat CW, Heckel DG et al. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol 2007; 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Freitak D, Heckel DG, Vogel H. Bacterial feeding induces changes in immune-related gene expression and has trans-generational impacts in the cabbage looper (Trichoplusia ni). Front Zool 2009a; 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Strand MR. Insect hemocytes and their role in immunity. In: Beckage NE, ed. Insect Immunology. San Diego, CA: Academic Press. 2008:25–47. [Google Scholar]
- 246.St. Leger RJ, Charnley AK, Cooper RM. Cuticle-degrading enzymes of entomopathogenic fungi: synthesis in culture on cuticle. J Invertebr Pathol 1986; 48:85–95. [Google Scholar]
- 247.Vilcinskas A, Matha V, Götz P. Effects of the entomopathogenic fungus Metarhizium anisopliae and its secondary metabolites on morphology and cytoskeleton of plasmatocytes isolated from Galleria mellonella. J Insect Physiol 1997; 43:1149–59. [DOI] [PubMed] [Google Scholar]
- 248.Vilcinskas A, Götz P. Parasitic fungi and their interactions with the insect immune system. Adv Parasitol 1999; 43:267–13. [Google Scholar]
- 249.Fiolka MJ. Immunosuppressive effect of cyclosporin A on insect humoral immune response. J Invertebr Pathol 2008; 98:287–92. [DOI] [PubMed] [Google Scholar]
- 250.Gillespie J, Bailey A, Cobb B et al. Fungal elicitors of insect immune responses. Arch Insect Biochem Physiol 2000; 44:49–68. [DOI] [PubMed] [Google Scholar]
- 251.Lord JC, Anderson S, Stanley DW. Eicosanoids mediate Manduca sexta cellular response to the fungal pathogen Beauveria bassiana: a role for the lipoxygenase pathway. Arch Insect Biochem Physiol 2002; 51:46–54. [DOI] [PubMed] [Google Scholar]
- 252.Bergin D, Reeves E, Renwick J et al. Superoxide production in Galleria mellonella hemocytes: Identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun 2005; 73:4161–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ekengren S, Hultmark D. Drosophila cecropin as an antifungal agent. Insect Biochem Mol Biol 1999; 29(11):965–72. [DOI] [PubMed] [Google Scholar]
- 254.Langen G, Imani J, Altincicek B et al. Transgenic expression of gallerimycin, a novel antifungal insect defensin from the greater wax moth Galleria mellonella, confers resistance against pathogenic fungi in tobacco. Biol Chem 2006; 387:549–57. [DOI] [PubMed] [Google Scholar]
- 255.Wojda I, Kowalski P, Jakubowicz T. Humoral immune response of Galleria mellonella larvae after infection by Beauveria bassiana under optimal and heat-shock conditions. J Insect Physiol 2009; 55:525–31. [DOI] [PubMed] [Google Scholar]
- 256.Schmidt O Insect immune recognition and suppression. In: Beckage NE, ed. Insect Immunology. San Diego, CA: Academic Press. 2008; 271–294. [Google Scholar]
- 257.Clarke DJ. Photorhabdus: a model for the analysis of pathogenicity and mutualism. Cell Microbiol 2008; 10:2159–67. [DOI] [PubMed] [Google Scholar]
- 258.Beckage NE. Parasitoid polydnaviruses and insect immunity. In: Beckage NE, ed. Insect Immunology. San Diego, CA: Academic Press. 2008; 243–270. [Google Scholar]
- 259.Suderman RJ, Pruijssers AJ, Strand MR. Protein tyrosine phosphatase-H2 from a polydnavirus induces apoptosis of insect cells. J Gen Virol 2008; 89:1411–20. [DOI] [PubMed] [Google Scholar]
- 260.Thoetkiattikul H, Beck MH, Strand MR. Inhibitor kappaB-like proteins from a polydnavirus inhibit NF-kappaB activation and suppress the insect immune response. Proc Natl Acad Sci USA 2005; 102:11426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lu Z, Beck MH, Wang Y et al. The viral protein Egf1.0 is a dual activity inhibitor of prophenoloxidase-activating proteinases 1 and 3 from Manduca sexta. J Biol Chem 2008; 283:21325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sparks WO, Bartholomay LC, Bonning BC. Insect immunity to viruses. Beckage, NE Parasitoid polydnaviruses and insect immunity. In: Beckage NE, ed. Insect Immunology. San Diego, CA: Academic Press. 2008; 209242–270. [Google Scholar]
- 263.Clem RJ. Baculoviruses and apoptosis: a diversity of genes and responses. Curr Drug Targets 2007; 8:1069–74. [DOI] [PubMed] [Google Scholar]
- 264.Shelby KS, Popham HJ. Plasma phenoloxidase of the larval tobacco budworm, Heliothis virescens, is virucidal. J Insect Sci 2006; 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


