Abstract
Introduction:
The prevalence of cardiometabolic disease following spinal cord injury is known to be high. However, it is unknown whether engaging in high-intensity exercise, which is advocated by recent guidelines, is beneficial or feasible for these individuals.
Design:
Combination of a randomized controlled trial and an open label intervention study of functional electrical stimulation legs plus arms rowing.
Setting:
Outpatient academic rehabilitation hospital.
Participants:
Forty individuals with spinal cord injury, with ASIA impairments scales A-D and neurological levels of injury C1-T12.
Intervention:
Six months of high-intensity, hybrid functional electrical stimulation rowing.
Main Outcome Measures:
Change in VO2max, prevalence of cardiometabolic disease, serum lipids, and insulin resistance.
Results:
Individuals averaged 42.1 ± 22.0 minutes of hybrid-functional electrical stimulation rowing a week over an average of 1.69 sessions per week over the 6 months of intervention. This amounted to an average of 170.9 ± 100 km rowed, at a mean heart rate of 82.7% of individualized maximum. Only one of 40 individuals met current exercise guidelines for the full 6 months. VO2max increased significantly (p<0.001), yet prevalence of cardiometabolic disease did not significantly change (decrease from 22.5% to 20%, p=0.70). A1C did significantly decrease over this time (p=0.01), though serum lipids and fasting glucose/insulin levels were unchanged. In exploratory subanalyses assessing individuals injured less than or greater than 12 months, those with more chronic injuries decreased their triglyceride:HDL ratio (p=0.04), a marker of cardiac mortality. Stratifying by neurological level of injury, individuals with paraplegia had worsened LDL (p=0.02) and total cholesterol:HDL ratio (p=0.04) over the six-month intervention.
Conclusions:
Sustained high-intensity, exercise with hybrid functional electrical stimulation rowing does not decrease prevalence of cardiometabolic disease after spinal cord injury.
Keywords: cardiovascular disease, metabolic disease, cardiometabolic disease, spinal cord injury
Introduction:
Following chronic spinal cord injury (SCI), cardiovascular disease is the leading source of mortality.1,2 Metabolic disease further has been postulated to occur nearly three times more often in this population.3 Together, cardiovascular and metabolic disease, termed cardiometabolic disease, has been estimated to effect over 60% of individuals with chronic SCI.4 This disease state has significant health consequences and has been linked to increased rates of myocardial infarction, stroke, sexual dysfunction, and mortality.5 While cardiometabolic disease has classically been perceived as a problem associated with chronic SCI, new research has demonstrated high rates of cardiometabolic disease in individuals with subacute SCI at discharge from acute inpatient rehabilitation,6 driven by increased rates of obesity and depressed levels of high density lipoprotein (HDL) cholesterol.
In individuals without SCI, participation in an exercise program is one of the primary interventions to address cardiometabolic disease. Exercise results in progressive weight loss,7 improved lipid metabolism,8,9 greater insulin sensitivity,10 and augmented blood pressure control.11 However, paralysis due to SCI often limits the amount of available muscle that can be actively recruited to elicit these known exercise benefits. To overcome this, functional electrical stimulation (FES) of paralyzed muscles has been employed to recruit additional muscle mass when volitional control is lacking. When this direct stimulation is performed time-synced with voluntary upper body exercise, high-intensity aerobic exercise is possible with accompanying improved gains in aerobic capacity and lean muscle mass after SCI.12
Specific exercise guidelines have been created for individuals with SCI aimed at decreasing cardiometabolic disease.13 Under these guidelines, 30 minutes of moderate to vigorous exercise three times per week is recommended to decrease risk of cardiometabolic disease for those with chronic SCI. Compiled from expert consensus and extrapolations from large populations of uninjured individuals, these guidelines have not been assessed as to their actual benefit. The primary objective of this study was to assess the effects of high-intensity, whole-body, exercise on the prevalence of cardiometabolic disease in a cohort of individuals with SCI. Clinically, now knowing that cardiometabolic disease gains a strong foothold at time of discharge from acute inpatient rehabilitation,6 this study looks to address the question of how to mitigate this risk.
Methods:
This study included individuals with SCI who trained for six months with hybrid FES rowing. Individuals were included who participated in the active treatment group of a prospective randomized controlled trial of this exercise intervention (NCT02139436) or were part of an open label exercise intervention using the same modality for older individuals with chronic SCI. This combined method was chosen to maximize clinical applicability of study findings. Applicable IRB approval was attained.
Individuals were older than 18 years of age, had SCI (American Spinal Injury Association Impairment Scale (AIS) A-D),14 and had neurological levels of injury at or above T12 (Table 1). All individuals had ≥ 4/5 strength in at least one group of elbow flexors to allow them to volitionally pull within the exercise equipment and were screened to ensure they were free from medical conditions that might preclude safe exercise.15 Each individual completed acute inpatient rehabilitation and was at least three months post injury prior to enrollment. None of the individuals participated in regular exercise training of any intensity (defined as at least 30 min, three times a week) following their SCI.
Table 1:
Demographics for individuals included in this study.
| Hybrid-FES Rowing (n=40) | |
|---|---|
| Sex | 6 F/ 34 M |
| Age | 34.1 ± 12.4 years |
| NLI* C1-C8 | 21 |
| T1-T6 | 11 |
| T7-T12 | 7 |
| AIS** A | 19 |
| B | 8 |
| C | 5 |
| D | 2 |
| Mean time since injury | 41.4 ± 87.4 months |
| BMI | 25.1 ± 4.6 kg/ m2 |
NLI=neurological level of injury. AIS= American Spinal Injury Association Impairment Scale. BMI=body mass index. All measures are at time of enrollment.
n=39 with known NLI
n=34 with known AIS
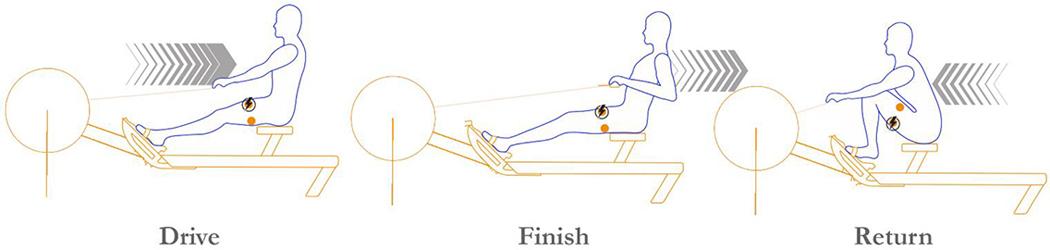
To maximize the amount of exercise individuals were able to engage in, a hybrid FES rowing program was implemented. This has been previously described,12 but briefly enrolled individuals underwent 6 months of training with volitional arm pull and FES to their quadriceps and hamstrings to assist in drive/finish and return phases respectively (Figure 1).
Figure 1:
Schematic of hybrid functional electrical stimulation rowing with time-locked stimulation  occurring at corresponding phase of rowing stroke.
occurring at corresponding phase of rowing stroke.
Training occurred with a goal of 2–3 sessions per week at a goal heart rate of over 75% of maximum, quantified during baseline VO2max testing using the hybrid-FES rowing. Exercise and amount of aerobic benefit were quantified through total number of kilometers rowed, minutes spent rowing, average heart rate (expressed as percentage of maximum), and the change in VO2max from baseline to 6-month time points.
To quantify cardiometabolic disease, body weight (kg), height (m), and laboratory tests (A1C, free insulin, fasting glucose, LDL, HDL, total cholesterol, triglycerides) were recorded at both baseline and following the exercise intervention at 6 months. Resting, supine blood pressure was recorded at each time point and averaged over 5 minutes of continuous recording for all of those who participated in the formal randomized controlled trial (Finapres, Ohmeda Medical, calibrated for accuracy compared to Dinamap Dash 2000, GE). For those who were part of the open label exercise trial, resting blood pressures were attained with a manual cuff at baseline and 6 months. From fasting laboratory values, insulin resistance was defined as either a free glucose of > 100 mg/dL or a HOMA-2 score indicating age/sex specific insulin resistance.16 Per convention,17 cardiometabolic disease was defined as having ≥ 3 of the following risk factors
Obesity as defined as SCI-specific BMI > 22 kg/m2,18
Insulin resistance,
Triglycerides > 150mg/dL,
HDL < 40 mg/dL (males) or < 50 mg/dL (females),
Systolic blood pressure > 130 mmHg or diastolic blood pressure > 85 mmHg.
Total cholesterol:HDL and Triglycerides:HDL ratios were further calculated as these have both been linked to increased mortality (ratios greater than 4.5 and 3.0 respectively)19,20 and have been demonstrated to be elevated following SCI.6
Statistical analysis
Descriptive statistics were calculated for demographics and mean baseline values. Paired t-tests analyzed baseline to 6-month changes for all individuals with regard to A1C (%), fasting glucose, HOMA-2, LDL, HDL, total cholesterol, triglycerides, supine blood pressure, total cholesterol:HDL, triglycerides:HDL, and the number of cardiometabolic risk factors. As exact time points for metrics varied slightly among individuals, all recorded metrics were plotted and fit with a polynomial equation. Normalized endpoints at 180 days were used for data visualization only to account for this variance. Values are presented as means +/− standard deviation. Given the multiple comparisons, a Bonferroni correction was applied with a p-value of less than 0.005 treated as statistically significant. Exploratory sub-analyses were performed to assess the effects of hybrid-FES rowing on individuals based upon neurological level of injury (paraplegia vs tetraplegia) and time since injury (dichotomized as less than or greater than 12 months).
Results:
Forty individuals participated in 6 months of Hybrid-FES rowing as part of this study (Table 1). This included 30 who were part of the randomized controlled trial (age less than 40, less than 24 months since injury) and 10 older individuals or with more chronic SCI in the open label intervention.
At baseline, cardiometabolic disease was present in 22.5% of all enrolled individuals. Obesity (BMI >22 kg/m2 per SCI-specific guidelines)17,21 was present in 70% of individuals, while insulin resistance was noted in only 12.5% of individuals. Hypertriglyceridemia was present in 25% of individuals at baseline, while low HDL was seen in 47.5% (Table 2). Pre-hypertension was found in 12.5% of individuals.
Table 2:
Changes in cardiometabolic lab work with 6 months of Hybrid-FES rowing
| Hybrid-FES rowing (n=40) | ||||
|---|---|---|---|---|
| Pre-FES | 6 months post FES | Mean relative change | P-value | |
| A1C | 5.3 ± 0.4% | 5.2 ± 0.5% | −3.3 ± 6.8% | 0.01 |
| Total Cholesterol | 168.6 ± 30.9 | 174.2 ± 34.5 | +0.8 ± 12.1% | 0.11 |
| Triglycerides | 124.0 ± 95.2 | 115.8 ± 82.2 | −11.8 ± 29.9% | 0.12 |
| HDL | 44.5 ± 14.0 | 45.1 ±13.1 | +0.4± 12.0% | 0.48 |
| LDL | 101.1± 28.0 | 105.9 ± 30.5 | +1.6 ± 16.5% | 0.08 |
| Fasting glucose | 81.7 ± 11.2 | 83.5 ± 12.3 | +1.5 ± 8.2% | 0.10 |
| HOMA-2 | 1.30 ± 1.6 | 1.4 ± 1.7 | +7.0 ± 52.9% | 0.18 |
| TC:HDL | 4.1 ± 1.4 | 4.2 ± 1.6 | +0.5 ± 13.6% | 0.39 |
| >4.5 | 35% | 40% | 0.52 | |
| Trig:HDL | 3.4 ± 3.6 | 3.1 ± 3.2 | −11.3 ± 34.7% | 0.07 |
| >3.0 | 35% | 22.5% | 0.10 | |
At baseline, mean VO2max was 18.1 ± 6.1 ml/kg. Over the 6 months of intervention, individuals with SCI, utilizing hybrid-FES, rowed an average of 50.1 ± 16.1 times for a total of 42.1 ± 22.0 minutes per week (average 1.69 sessions per week). On average, these individuals rowed 170.9 ± 100 kilometers over the course of the 6 months, averaging 82.7% of their individual maximum heart rates. Only one of the forty individuals (2.5%) averaged the prescribed 30 minutes of aerobic exercise three times a week for the full duration of the study.
Following 6 months of hybrid-FES rowing, VO2max improved a mean of 2.2 ± 2.7 ml/kg (p<0.001). Prevalence of cardiometabolic disease decreased from 22.5% to 20% (p=0.70 compared to baseline), with two individuals who initially met criteria for CMD no longer meeting criteria and one individual initially without CMD, meeting criteria at 6 months. BMI increased slightly from a mean of 25.1 ± 4.6 kg/m2 to 25.4 ± 4.7 kg/m2 (p=0.27 compared to baseline), with prevalence of obesity increasing from 70% to 77.5%. Prevalence of insulin resistance decreased from 12.5% to 10.3% following the 6 months of exercise, with hypertriglyceridemia decreasing from 25% to 20% and low HDL decreasing from 47.5% to 40%. Pre-hypertension decreased in prevalence from 12.5% to 5%. Overall, the 6-month intervention led to decreases in the absolute number of risk factors in 10 individuals, with 25 individuals experiencing no change, and 5 individuals gaining additional risk factors. There were no statistically significant baseline contributors to who would decrease, increase, or remain constant in their number of CMD risk factors (considering age, sex, time since injury, baseline BMI, baseline blood pressures, baseline lab values, number of rowing sessions per week, kilometers rowed, percentage of maximum heart rate averaged, or changes in VO2max).
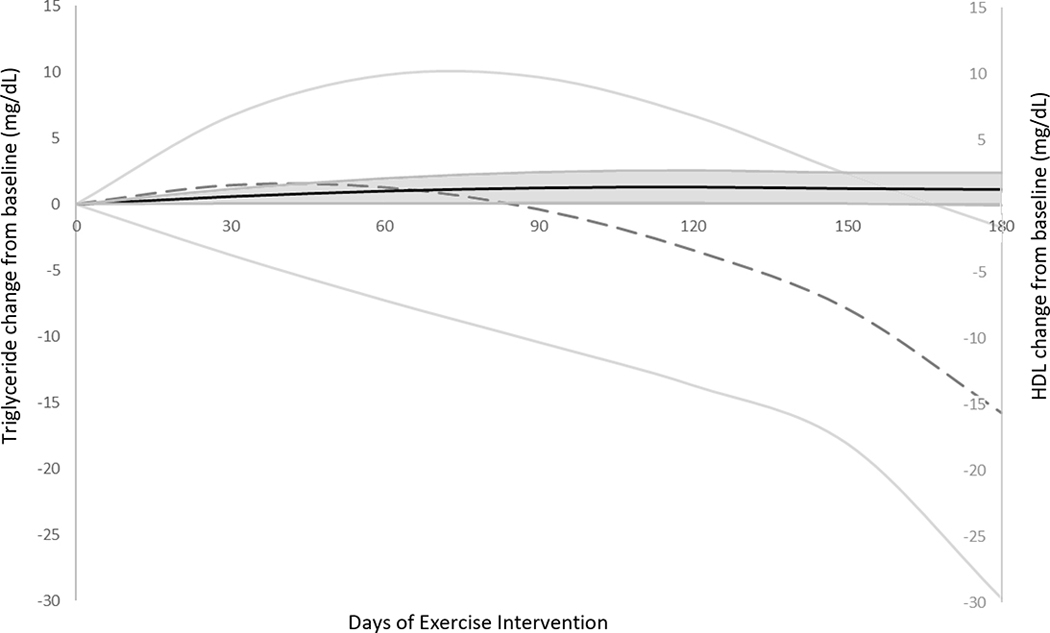
Triglycerides generally decreased after the exercise intervention (Figure 2), though not to a statistically significant level (p=0.12). Changes in triglycerides were poorly correlated with number of rowing sessions per week, total kilometers rowed, or gains in VO2max (all R2<0.12). While some individuals experienced increases in HDL with exercise (range −15 to +17 mg/dL, Figure 2), increases were similarly poorly correlated with exercise training load such as number of rowing sessions per week, total kilometers rowed, or gains in VO2max (all R2<0.07, Figure 2). Mean LDL increased during the 6-month intervention, though increases were poorly correlated with all markers of exercise (all R2<0.05).
Figure 2:
Changes in triglycerides  and HDL
and HDL  from baseline to 6 months of hybrid functional electrical stimulation rowing with standard deviation error bars.
from baseline to 6 months of hybrid functional electrical stimulation rowing with standard deviation error bars.
Total cholesterol to HDL ratio was suprathreshold (>4.5) in 35% of individuals at baseline, which increased in both the absolute value of the ratio and percentage of individuals surpassing threshold after the 6-months of exercise (Table 2). Triglyceride to HDL ratio decreased in both absolute value and prevalence with the 6-months of intervention. Analyzing this further, decreases is this ratio were poorly correlated with minutes rowed per week, sessions per week, kilometers rowed, or gains in VO2max over the 6 months (all R2<0.05).
Exploratory analysis comparing those with injuries prior to 12 months at time of enrollment (n=14) to those injured greater than 12 months (n=26) demonstrated similar neurological levels of injury, AIS, baseline BMI, age, systolic blood pressure, and diastolic blood pressure. Similar amounts of exercise were performed (sessions per week, meters rowed, minutes logger per week, and percentage of maximum HR achieved, p>0.14). Notably, VO2max increased to a smaller degree in the more chronically injured group (more acute injuries increasing from 18.56 ± 5.2 ml/kg to 22.61 ± 5.9 ml/kg compared to 17.81 ± 6.6 ml/kg to 18.99 ± 7.3 ml/kg in the more chronically injured group). Prevalence of CMD in the acute group was 14% at baseline and was unchanged after the 6 months of exercise. The number of CMD risk factors (mean of 1.36) was similarly unchanged in this more acute group. In the subgroup injured greater than 12 months, CMD was present in 27% of individuals at baseline, which deceased to 23% after the 6 months of exercise (p=0.66). The number of CMD risk factors (mean 1.85) at baseline decreased to a mean of 1.62 at the end of the intervention. BMI increased a mean of 0.63 ± 1.2 kg/m2 in individuals injured less than 12 months and increased a mean of 0.13 ± 1.9 kg/m2 in individuals injured greater than 12 months over the 6 months. Further changes in lab values are described in Table 3.
Table 3:
Changes in cardiometabolic lab work with 6 months of Hybrid-FES rowing for both individuals with SCI less than or greater than 12 months in duration.
| SCI < 12 months (n=14) | SCI > 12 months (n=26) | |||||
|---|---|---|---|---|---|---|
| Pre-FES | 6 months post FES | P-value | Pre-FES | 6 months post FES | P-value | |
| A1C | 5.3 ± 0.5 | 5.0 ± 0.7 | 0.02 | 5.3 ± 0.8 | 5.2 ± 0.3 | 0.33 |
| Total Cholesterol | 167.5 ± 28.7 | 176.6 ± 35.9 | 0.19 | 169.2 ± 32.5 | 172.9 ± 34.3 | 0.35 |
| Triglycerides | 106.6 ± 70.2 | 106.7 ± 59.5 | 0.99 | 133.3 ± 106.4 | 120.6 ± 92.9 | 0.08 |
| HDL | 43.4 ± 10.9 | 44.1 ± 11.8 | 0.70 | 45.0 ± 15.7 | 45.6 ± 14.0 | 0.54 |
| LDL | 102.9 ± 29.0 | 111.2 ± 35.0 | 0.15 | 100.1 ± 28.0 | 103.1 ± 28.2 | 0.02 |
| Fasting glucose | 79.7 ± 12.1 | 82.5 ± 16.5 | 0.15 | 82.8 ± 10.8 | 84.0 ± 9.6 | 0.34 |
| HOMA-2 | 1.2 ± 1.9 | 1.5 ± 2.2 | 0.11 | 1.3 ± 1.4 | 1.3 ± 1.4 | 0.65 |
| TC:HDL | 4.1 ± 1.4 | 4.3 ± 1.6 | 0.34 | 4.1 ± 1.4 | 4.1 ± 1.6 | 0.74 |
| >4.5 | 29% | 43% | 0.24 | 38% | 38% | 1.0 |
| Trig:HDL | 2.9 ± 2.9 | 2.9 ± 3.0 | 0.31 | 3.7 ± 4.0 | 3.2 ± 3.4 | 0.04 |
| >3.0 | 29% | 14% | 0.24 | 38% | 27% | 0.23 |
Dichotomizing individuals to those who had tetraplegia (n=21) vs paraplegia (n=18) demonstrated no significant baseline differences in time since injury, AIS, or BMI. Age tended to be slightly different between groups (tetraplegia group = 32.7 ± 2.7 years vs paraplegia = 34.2 ± 2.6). Resting systolic blood pressure was lower in individuals with tetraplegia (111.1 ± 13.8 vs 119.6 ± 11.5), though diastolic pressure was similar (69.8 ± 11.3 vs 69.6 ± 10.6). Individuals with tetraplegia and paraplegia rowed a similar number of sessions per week (1.64 ± 0.5 vs 1.70 ± 0.5), minutes per week (37.1 ± 18.8 vs 47.2 ± 25.1,), rowed similar total kilometers (145.6 ± 87.6 vs 192.3 ± 106.9), achieved similar percentage maximum heart rate averaged (82.0 ± 7.1% vs 83.3 ± 7.0%), and though individuals with tetraplegia tended to increase VO2max less (1.44 ± 2.6 ml/kg vs 3.06 ± 2.59 ml/kg).
Despite 6 months of high-intensity exercise, neither group decreased their prevalence of cardiometabolic disease to a significant degree (p>0.58 for both) and laboratory values were largely unchanged (Table 4). The exception to this were increases in both LDL and insulin resistance (via HOMA-2) in the sub-group with paraplegia. There was a tendency for decreases in the triglyceride to HDL ratio in individuals with tetraplegia.
Table 4:
Changes in cardiometabolic lab work with 6 months of Hybrid-FES rowing for both individuals with tetraplegia and paraplegia.
| Tetraplegia (n=21) | Paraplegia (n=18) | |||||
|---|---|---|---|---|---|---|
| Pre-FES | 6 months post FES | P-value | Pre-FES | 6 months post FES | P-value | |
| A1C | 5.3 ± 0.3 | 5.1 ± 0.4 | 0.08 | 5.3 ± 0.5 | 5.2 ± 0.5 | 0.19 |
| Total Cholesterol | 164.5 ± 31.3 | 166.2 ± 36.5 | 0.72 | 172.6 ± 31.5 | 182.8 ± 31.5 | 0.07 |
| Triglycerides | 109 ± 55.7 | 97.6 ± 37.5 | 0.13 | 145.1 ± 127.2 | 138.3 ± 113.5 | 0.39 |
| HDL | 41.7 ± 8.6 | 43.6 ± 9.5 | 0.12 | 46.2 ± 17.6 | 45.3 ± 15.5 | 0.54 |
| LDL | 101.8 ± 31.2 | 102.6 ± 35.7 | 0.83 | 100.3 ± 25.5 | 110.4 ± 24.4 | 0.02 |
| Fasting glucose | 81.7 ± 10.4 | 83..1 ± 10.1 | 0.15 | 81.6 ± 12.7 | 83.9 ± 15.0 | 0.30 |
| HOMA-2 | 1.5 ± 1.5 | 1.4 ± 1.5 | 0.74 | 1.1 ± 1.7 | 1.4 ± 2.0 | 0.03 |
| TC:HDL | 4.1 ± 1.2 | 4.0 ± 1.1 | 0.26 | 4.2 ± 1.6 | 4.6 ± 1.9 | 0.04 |
| >4.5 | 38% | 38% | 1.0 | 33% | 44% | 0.32 |
| Trig:HDL | 2.8 ± 1.8 | 2.4 ± 1.1 | 0.10 | 4.2 ± 4.9 | 4.0 ± 4.5 | 0.38 |
| >3.0 | 33% | 14% | 0.06 | 39% | 33% | 0.63 |
The one individual who met SCI-specific recommendations for aerobic exercise13 had T4 AIS A paraplegia, injured 28 months prior, logged an average of 110 minutes per week on the hybrid-FES rower for 28 weeks (442.3km) at an average of 86% of his maximum heart rate. He improved his VO2max from 23.4 ml/kg to 28.8 ml/kg. At baseline he had cardiometabolic disease with obesity, hypertriglyceridemia, and low HDL. After 28 weeks of the high-intensity hybrid-FES rowing, these risk factors all persisted, and he continued to meet criteria for cardiometabolic disease.
Discussion:
Cardiometabolic disease is a significant problem following SCI, with this population at high risk due to limited availability to engage in sustained whole-body exercise- the first line intervention many physicians would recommend.22 Here we demonstrate that even with increased amount of muscle mass able to be recruited using FES and despite significant aerobic gains in VO2max following an exercise-based intervention, prevalence of cardiometabolic disease remains largely unchanged. While the weekly duration of exercise did not meet current guidelines, aerobic gains on the order of 12%, as seen here with this intervention, are still commonly associated with improvements in serum lipids in individuals without SCI.23,24 This does not appear to be true for individuals with SCI. Previous studies in individuals with SCI have shown that CMD is driven largely by high BMI and depressed HDL. In uninjured populations, exercise can improve BMI7 and increase HDL9. Unfortunately, BMI increased and HDL did not significantly change in our study population over the 6 months of exercise. Further, in those who did experience improvements in their cardiometabolic health, this was not related to amount of exercise performed, suggesting some individuals may be primed to succeed with minimal interventions and others may put in enormous effort with minimal gain. A priori identification of those who will improve cardiometabolic health with exercise will be important moving forward.
Expert consensus guidelines for addressing CMD following SCI recommend moderate to vigorous exercise 3 time per week for 30 minutes.13 While all participants met the moderate to vigorous criteria (mean HR of 83% maximum), only one participant (2.5% of the study population) was able to sustain an average 90 minutes a week for the 6 months. As the population who chose to enroll in this study were likely self-selected for those who would like to perform exercise, overall adherence to such exercise guidelines may be lower. Reflecting this likely self-selection, our baseline prevalence of CMD was lower noted in previous literature. Notably, exercise guidelines do discuss them as being for individuals with injuries greater than 12 months. In our exploratory sub-analysis looking at time since injury, those injured greater than 12 months were able to decrease their triglyceride:HDL ratio to a notable degree, though the prevalence of CMD did not change. It is possible that greater than 6 months of exercise or pairing exercise with diet modification or pharmacotherapy is needed to achieve meaningful changes in CMD, though this requires further, nuanced evaluation.
Individuals injured less than 12 months at enrollment did not change their incidence of CMD, which was low at baseline. The case could be made that sustained exercise is needed to prevent chronic development of CMD, particularly as it relates to addressing insulin resistance. However, studies of individuals with subacute SCI point to primarily BMI and depressed HDL as drivers of early CMD following SCI. BMI increased in this population and HDL was unchanged, despite significant aerobic gains (mean increase in VO2max of 4.05 ml/kg, p<0.001 compared to baseline). It is unclear if sustaining high-intensity exercise for a longer time period would stave off CMD, though such cumulative aerobic gains are unlikely.25 Further, as 97.5% of the study population was unable to sustain the guidelines for frequency/duration of exercise for the 6 months, goals of extending this intensity beyond 6 months may be challenging.
Further exploring who might be optimal for such an exercise program, baseline characteristics such as neurological level of injury did not affect outcomes. Comparing individuals with tetraplegia to those with paraplegia, the paraplegia group was unsurprisingly able to do more work, tending to have greater gains in VO2max. Given their lower neurological level of injury, one could further argue that these individuals proportionately have less effect from SCI compared to those with tetraplegia. However, even with increased amount of work and less effects of SCI, these individuals still made minimal meaningful gains.
Limitations
As noted, exercise compliance over the 6 months rarely met the recommended three times per week for 30 minutes. As such, full assessment of how these guidelines perform for individuals with SCI is not possible. Our exercise intervention, utilizing hybrid-FES rowing, does require specialized exercise equipment and, in many cases for those with SCI, assistance transferring into the equipment. While this allowed whole-body muscle recruitment, the barriers of travel and setup may have limited the frequency of participants exercising. Future, home-based, equipment that would similarly recruit large amounts of paralyzed muscle and achieve VO2max gains would be welcomed. Finally, while we did not demonstrate significant changes in cardiometabolic disease with our intervention, a larger number of enrolled participants may have detected a difference, though this may or may not have been clinically significant.
Conclusions
Sustained high-intensity, whole-body exercise with hybrid functional electrical stimulation rowing does not decrease prevalence of cardiometabolic disease after spinal cord injury.
Funding details:
National Institute of Health, R01HL117037
Footnotes
Declaration of interest: The authors report no declarations of interest
References:
- 1.Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury Results from a national population health survey. Neurology. 2013;81(8):723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers J, Lee M, Kiratli, J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–152. [DOI] [PubMed] [Google Scholar]
- 3.Nash MS, Tractenberg RE, Mendez AJ, et al. Cardiometabolic syndrome in people with spinal cord injury/disease: guideline-derived and nonguideline risk components in a pooled sample. Arch Phys Med Rehabil. 2016;97(10):1696–1705. [DOI] [PubMed] [Google Scholar]
- 4.Nash MS, Mendez AJ. A guideline-driven assessment of need for cardiovascular disease risk intervention in persons with chronic paraplegia. Arch Phys Med Rehabil. 2007;88(6):751–757. [DOI] [PubMed] [Google Scholar]
- 5.Govindarajan G, Whaley-Connell A, Mugo M, Stump C, Sowers JR. The cardiometabolic syndrome as a cardiovascular risk factor. Am J Med Sci. 2005;330(6):311–318. [DOI] [PubMed] [Google Scholar]
- 6.Solinsky R, Betancourt L, Marino R, et al. Stakeholder perceptions and clinical assessments of cardiometabolic disease after spinal cord injuries and disorders. Top Spinal Cord Inj Rehabil. 2019;25:122–123. [Google Scholar]
- 7.Schjerve IE, Tyldum GA, Tjønna AE, et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci (Lond). 2008;115:283–293. [DOI] [PubMed] [Google Scholar]
- 8.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014. Aug 1;48(16):1227–34. [DOI] [PubMed] [Google Scholar]
- 9.Badrov MB, Wood KN, Lalande S, et al. Effects of 6 Months of Exercise-Based Cardiac Rehabilitation on Autonomic Function and Neuro-Cardiovascular Stress Reactivity in Coronary Artery Disease Patients. J Am Heart Assoc. 2019;8(17):e012257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjønna AE, Lee SJ, Rognmo O, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molmen-Hansen HE, Stolen T, Tjønna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19:151–160. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JA, Picard G, Widrick JJ. Aerobic capacity with hybrid FES rowing in spinal cord injury: comparison with arms-only exercise and preliminary findings with regular training. PM&R. 2011;3:817–824. [DOI] [PubMed] [Google Scholar]
- 13.Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal cord. 2018;56(4):308–321. [DOI] [PubMed] [Google Scholar]
- 14.Kirshblum S, Waring W. Updates for the international standards for neurological classification of spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25(3):505–517. [DOI] [PubMed] [Google Scholar]
- 15.Pescatello LS. American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 9th Edition. Philadelphia, PA; Wolter Kluwer/Lippincott Williams & Wilkins Health; 2014 [Google Scholar]
- 16.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes care. 1998;21(12):2191–2192. [DOI] [PubMed] [Google Scholar]
- 17.Nash MS, Groah SL, Gater DR Jr, et al. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury: Clinical Practice Guideline for Health Care Providers Top Spinal Cord Inj Rehabil. 2018;24(4):379–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laughton GE, Buchholz AC, Martin Ginis KA, et al. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–762. [DOI] [PubMed] [Google Scholar]
- 19.Lemieux I, Lamarche B, Couillard C, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161(22):2685–2692. [DOI] [PubMed] [Google Scholar]
- 20.Marotta T, Russo BF, Ferrara LA. Triglyceride-to-HDL-cholesterol Ratio and Metabolic Syndrome as Contributors to Cardiovascular Risk in Overweight Patients. Obesity. 2010;18(8):1608–1613. [DOI] [PubMed] [Google Scholar]
- 21.Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab. 2011;36(1):107–114. [DOI] [PubMed] [Google Scholar]
- 22.Phillips SA, Mahmoud AM, Brown MD, Haus JM. Exercise interventions and peripheral arterial function: implications for cardio-metabolic disease. Prog Cardiovasc Dis. 2015;57(5):521–534. [DOI] [PubMed] [Google Scholar]
- 23.Gaesser GA, Rich RG. Effects of high-and low-intensity exercise training on aerobic capacity and blood lipids. Med Sci Sport Exer. 1984;16(3):269–74. [PubMed] [Google Scholar]
- 24.Leaf DA, Parker DL, Schaad DO. Changes in VO2max, physical activity, and body fat with chronic exercise: effects on plasma lipids. Med Sci Sport Exer. 1997;29(9):1152. [DOI] [PubMed] [Google Scholar]
- 25.Vivodtzev I, Picard G, Cepeda FX, Taylor JA. Acute Ventilatory Support During Whole-Body Hybrid Rowing in Patients With High-Level Spinal Cord Injury: A Randomized Controlled Crossover Trial. Chest. 2019. e-publication [DOI] [PMC free article] [PubMed] [Google Scholar]