Abstract
Intermittent pneumatic compression (IPC) is commonly used to improve peripheral circulation of the lower extremity. However, its therapeutic dosage for people with type 2 diabetes mellitus (DM) at risk for ulcers is not well established. This study explored the effect of IPC with different inflation pressures on the distal microvascular responses of the foot in people with type 2 DM. Twenty‐four subjects with and without DM were recruited. Three IPC protocols with inflation pressures of 60, 90, and 120 mmHg were applied to the foot. The foot skin blood flow (SBF) responses were measured by laser Doppler flowmetry during and after IPC interventions. Results show that all three IPC interventions significantly increased foot SBF of IPC stage in healthy subjects, but only 90 and 120 mmHg IPC significantly improved SBF in diabetic subjects. IPC with 90 and 120 mmHg showed a greater effect than 60 mmHg in both groups, but 120 mmHg IPC was more effective for diabetic subjects. This study demonstrates that 90 and 120 mmHg are effective dosages of IPC for improving blood flow in healthy people, and 120 mmHg IPC may be more suitable for people with type 2 DM.
Keywords: diabetic foot ulcer, inflation pressure, intermittent pneumatic compression, microcirculation, skin blood flow
1. INTRODUCTION
Diabetic foot ulcers are one of the most serious complications of diabetes mellitus (DM). 1 Microvascular dysfunction of the lower extremity is an important pathological factor in the development of diabetic foot ulcers. 2 , 3 Abnormal blood flow regulation, thickened capillary basement membrane, and changes in haemorheology of lower extremity microvessels cause insufficient blood supply and abnormal metabolism of foot soft tissue in people with DM, which makes foot soft tissue more vulnerable to external mechanical pressure and more prone to foot lesions. 4 , 5 Thus, improving the level of blood supply of foot soft tissue is very important to reduce the risk of diabetic foot ulcers.
Intermittent pneumatic compression (IPC) is a common clinical treatment for improving circulation and promoting ulcer healing, by exerting cyclical external compression with certain pressure, frequency, and duration on limbs. 6 , 7 , 8 , 9 Beneficial mechanisms of IPC mainly include increasing the arteriovenous pressure gradient, enhancing the production of endothelial‐diastolic substances in blood vessels, and inducing transient suspension of arteriovenous reflex. 8 , 10 , 11 Konstantinos et al applied a 5‐minute IPC stimulus with an inflation pressure of 120 mmHg with 4‐second inflation (pneumatic compression) for three cycles per minute to people with claudicating limb, in order to explore whether IPC could increase distal blood flow of lower extremities. Their results showed that IPC can improve foot skin blood flow (SBF) and may be beneficial for people with peripheral vascular disease. 12 Kavros et al studied the clinical efficacy of IPC for people with non‐healing wounds attributed to chronic critical limb ischaemia. The 18‐month intervention of IPC at an inflation pressure of 85 to 95 mmHg with 2‐second inflation for three cycles per minute, and three 2‐hour sessions per day was chosen. They found that the IPC group displayed better wound healing, limb salvage, and transcutaneous oxygen pressure level compared with the control group. 13 Pawlaczyk et al assessed the effects of IPC on postoperative oedema and skin blood flow restoration in people undergoing revascularization procedures. Two weeks of IPC treatment at 50 to 72 mmHg with 4‐second inflation for three cycles per minute was applied, and their results demonstrated that decreased local leg swelling and increased SBF and transcutaneous oxygen pressure were found in patients receiving IPC treatment. 7 These previous studies have demonstrated that IPC may be an effective intervention in improving peripheral blood circulation of lower extremities. However, the optimal IPC protocols for different clinical applications are not known. 14
Inflation pressure is an important parameter of IPC treatment which directly affects its clinical efficacy on circulation by reducing venous pressure and increasing the arteriovenous pressure gradient. 15 For people who suffer from circulatory disorders of the lower extremity because of different pathological factors, their applicable inflation pressures should be different to overcome various underlying impairments in the circulatory system. 15 Rosales‐Velderrain et al applied 30 mmHg of continuous pneumatic compression for 30 minutes to people with type 2 DM, and found that it can increase muscle blood flow of leg and improve foot sensation, but has no effect on SBF of leg. 16 Delis et al studied various applied pressures of IPC to the foot for venous emptying in the lower extremity of healthy people. Their results showed that IPC at 120 and 80 mmHg cause lower venous pressure than that at 100 and 60 mmHg, respectively; and the differences in efficacy of IPC with 140 and 120 mmHg or 100 and 80 mmHg are not significantly different. 15 Because of the chronic hyperglycaemia, people with type 2 DM suffer from impaired myogenic regulation of microvascular blood flow and have decreased SBF responses to external mechanical stimulus. 17 , 18 Many IPC studies included people with DM as subjects, but there are no therapeutic guidelines for IPC intervention for people with type 2 DM, and its applicability, optimal dosage, and physiological mechanisms for diabetic subjects are not clear. 14 In the literature, these IPC studies focused on the use of IPC to the calf or to the calf and foot for improving blood flow in the leg. Thus, the appropriate pressure of IPC intervention for improving the distal microcirculation (eg, foot SBF) in people with type 2 DM needs to be further investigated.
Therefore, this study aimed to explore the effect of IPC with different inflation pressures on the foot SBF responses in people with type 2 DM. We hypothesized that IPC with lower inflation pressure may not be effective in improving foot microcirculation, while IPC with higher inflation pressure can significantly improve SBF in people with type 2 DM. To the best of our knowledge, this is the first study investigating the effect of foot IPC on the foot SBF in people with DM. The findings of this study may provide insight on the use of foot IPC for improving foot SBF in people with type 2 DM.
2. MATERIALS AND METHODS
2.1. Participants
People with type 2 DM were recruited from hospitals. The inclusion criteria for diabetic subjects were as follows: (a) diagnosed with type 2 DM; and (b) aged between 55 and 75 years. The exclusion criteria included the following: (a) have previous history of foot ulcers or amputation, (b) have the symptoms such as redness, inflammation, lesions, and (c) were diagnosed with severe complications like peripheral neuropathy, peripheral arterial disease (ankle brachial index [ABI] < 0.9), renal disease, retinal disease, liver disease, cancer, or coronary heart disease, and have never had reconstructive vascular surgery. In order to compare and analyse the characteristics of SBF response to IPC intervention in people with DM, this study also recruited healthy subjects to establish the normal foot blood flow responses. The inclusion criteria for healthy subjects were as follows: (a) have no symptoms such as redness, inflammation, lesions, and (b) have no complications like peripheral neuropathy, peripheral arterial disease (ABI < 0.9), renal disease, retinal disease, liver disease, cancer, or coronary heart disease, and have never had reconstructive vascular surgery.
According to the preliminary data of foot SBF in five healthy subjects under three IPC tests (the paired difference of the mean and standard deviation values of SBF data in baseline and IPC stages of three IPC tests were 1.93 ± 2.08 pu, 8.76 ± 7.29 pu, 10.66 ± 9.58 pu, respectively), a sample size of 10 was estimated by the power analysis for the power of 80% at an alpha level of 0.05. Considering the balanced design and three repeated measures, a sample size of 12 was chosen. 19
This study was conducted in accordance with clinical protocols approved by the institutional review board of Affiliated Hospital of National Research Center for Rehabilitation Technical Aids. All subjects gave informed written consent prior to participation. All subjects' demographic and physiological information are shown in Table 1.
TABLE 1.
Demographic and physiological information of subjects in diabetic and healthy groups (Mean ± SD)
| Variables | Diabetic group | Healthy group | Significance |
|---|---|---|---|
| Gender (Male/Female) | 8/4 | 5/7 | / |
| Age (years) | 66.67 ± 3.94 | 22.75 ± 0.87 | P < .001 |
| Body mass index (kg/m2) | 25.99 ± 2.65 | 20.75 ± 2.24 | P < .001 |
| Systolic blood pressure (mmHg) | 125.33 ± 13.29 | 119.83 ± 9.23 | P = .198 |
| Diastolic blood pressure (mmHg) | 69.44 ± 10.08 | 71.17 ± 7.55 | P = .378 |
| Heart rate (bpm) | 72.22 ± 5.97 | 74.50 ± 10.21 | P = .312 |
| Fast blood glucose (mmol/L) | 7.37 ± 1.43 | / | / |
| Glycated haemoglobin (%) | 7.40 ± 0.47 | / | / |
| Duration of diabetes (years) | 14.78 ± 3.83 | / | / |
| Systolic blood pressure of dorsalis pedis artery (mmHg) | 137.78 ± 27.85 | 121.42 ± 14.16 | P = .021 |
| Systolic blood pressure of posterior tibial artery (mmHg) | 135.00 ± 22.64 | 119.45 ± 10.19 | P = .021 |
| Ankle brachial index | 1.13 ± 0.12 | 1.04 ± 0.09 | P = .014 |
2.2. Experimental equipment
A custom‐made air‐operated pressure device was used to deliver IPC interventions with different inflation pressures. The IPC device consists of an airbag, air pump, barometric sensor, control module, power supply, and its controlling software, as shown in Figure 1. The inflating and holding time was 4 seconds, and the deflating and holding time was 16 seconds. 12 , 20 The pressure was controlled within 5 mmHg of preset IPC pressures.
FIGURE 1.
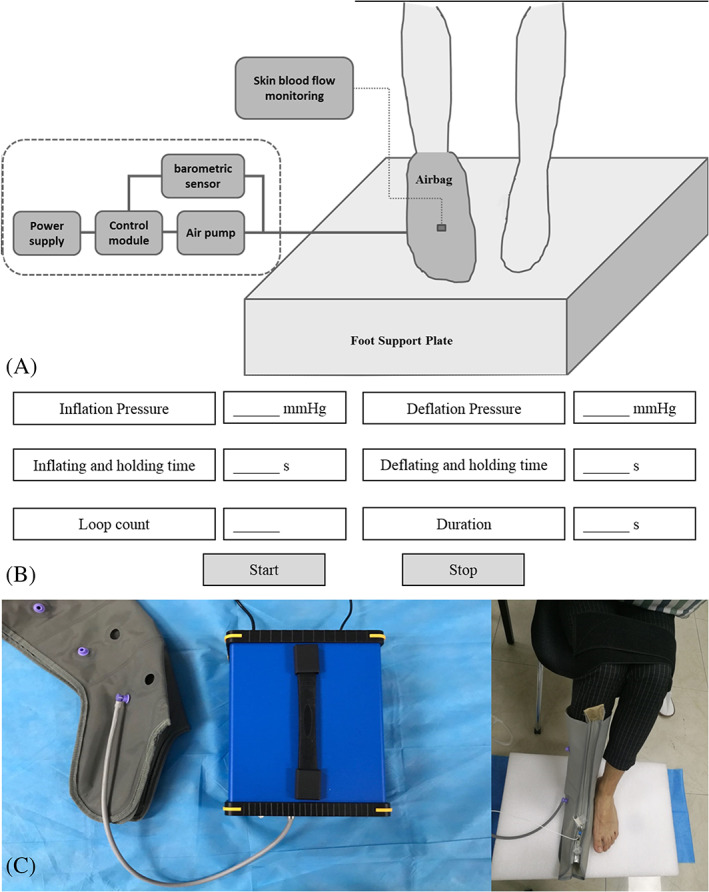
Experimental set‐up for delivering intermittent pneumatic compression (IPC) interventions. (A) Experimental concept map, (B) software interface for adjusting IPC parameters, and (C) pictures of IPC equipment and skin blood flow measurement
The laser Doppler flowmetry (PeriFlux 5000, 407, Perimed, Stockholm, Sweden) was used to non‐invasively measure SBF (blood flow in the network of capillaries within 1 mm of the skin) of the subjects' foot. In order to ensure the reliability of SBF data collection, the probe has been calibrated before each measurement and was attached to the skin surface with an adhesive tape to limit movement artefacts during the tests. In order to reduce the effect of inter‐subject variations in tissue hardness on SBF measurement, the dorsal foot (the middle region of the second and third metatarsals, 2 cm from the toes) was chosen to reflect the microvascular responses to IPC intervention. 7
2.3. Procedures
Before the test, subjects were asked to rest for at least 30 minutes in a room at a temperature of 24 ± 2°C to acclimate to the room environment. 18 Subjects were instructed to relax the lower limbs in a sitting position.
During the test, each subject's dorsal SBF of the right foot was measured for 5 minutes (Baseline stage); then, one of three IPC interventions was randomly applied to the subject's right foot for 9 minutes, and the SBF was also continuously recorded (IPC stage); after the IPC intervention, the SBF was continuously recorded for 5 minutes (recovery stage). A 30‐minute washout period was allowed for subjects to rest, and then the process was repeated with the remaining two IPC interventions.
For achieving a better effect of veins emptying without causing arteries collapse, the inflation pressure of three different IPC interventions was set as 60, 90, and 120 mmHg, with the inflation time of 4 seconds for three cycles per minute.
2.4. Data analyses
This study analysed the variations of SBF during baseline, IPC, and recovery stages and its change percentages during IPC and recovery stages in three IPC tests to compare the microvascular responses to IPC stimulus with different inflation pressures in the foot of diabetic and healthy subjects.
In the baseline or recovery stage, the mean values of SBF within the 5‐minute test periods were calculated. In the IPC stage, the abnormal SBF data caused by the inflation and deflation of pump were eliminated, and the SBF data in the inflation period (IPC_I), deflation period (IPC_D), and inflation‐deflation period (IPC_ID) in each cycle were extracted, and their corresponding average values during IPC stage were calculated for analysis. An illustration of analysed SBF parameters in one IPC cycle during the IPC stage is shown in Figure 2.
FIGURE 2.
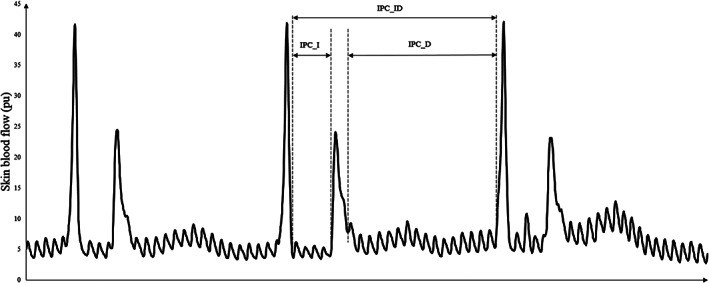
Illustration of analysed skin blood flow parameters in one intermittent pneumatic compression (IPC) cycle during the IPC stage
The change percentages of SBF were calculated for IPC and recovery stages according to Equation (1).
| (1) |
where ‘s’ represents SBF parameters in IPC or recovery stage.
2.5. Statistical analyses
The Shapiro‐Wilk test was used to test the normality of the SBF parameters of all subjects. The paired t test or Wilcoxon matched pairs signed‐rank test (based on Shapiro‐Wilk's normality test) was used to compare the difference in SBF variations between IPC stage and baseline stage, recovery stage and baseline stage; a repeated measure anova test or Friedman test was used to compare the difference in SBF responses among three IPC tests; an independent t test or Mann‐Whitney U test was used to compare the difference in SBF responses between diabetic and healthy subjects. A statistical significance level of .05 was used. All statistical analyses were performed in SPSS (Version 20.0, IBM, Armonk, New York).
3. RESULTS
The variations of foot SBF in diabetic subjects and healthy subjects during baseline, IPC and recovery stages are shown in Figure 3. For diabetic subjects, the SBF did not increase under 60 mmHg IPC test (P > .05), but showed a significant increase in IPC_D (P = .08) and IPC_ID (P = .023) under 90 mmHg IPC test, and in IPC_I (P = .041), IPC_D (P = .008) and IPC_ID (P = .008) under 120 mmHg IPC test. No significant increase in SBF during recovery stage was found in all three IPC tests. For healthy subjects, there was a significant increase in SBF of IPC_I, IPC_D, and IPC_ID compared with basal SBF in all three IPC tests (P < .05).
FIGURE 3.
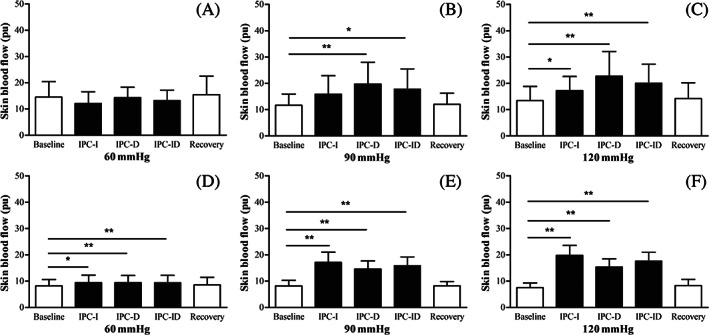
Foot skin blood flow (SBF) of diabetic subjects (A‐C) and healthy subjects (D‐F) in baseline, IPC, and recovery stages. * indicates there is a significant difference in SBF between the two corresponding stages, *P < .05, **P < .01. IPC_I: mean value of SBF in inflation periods during IPC stage; IPC_D: mean value of SBF in deflation periods during IPC stage; IPC_ID: mean value of SBF in inflation and deflation periods during IPC stage. IPC, intermittent pneumatic compression
The foot SBF percentage changes of IPC_I, IPC_D, IPC_ID in diabetic subjects and healthy subjects under three IPC tests are shown in Figure 4. For diabetic subjects, SBF of IPC_I (P = .021) and IPC_ID (P = .024) under 120 mmHg IPC test was significantly greater than that in 60 mmHg IPC test; and the SBF of IPC_D under 90 mmHg IPC test was significantly greater than that in 60 mmHg IPC test (P = .043). For healthy subjects, SBF of IPC_I, IPC_D, IPC_ID under both 90 mmHg (IPC_I: P = .013, IPC_D: P = .001, IPC_ID: P = .013) and 120 mmHg (IPC_I: P < .001, IPC_D: P = .001, IPC_ID: P < .001) IPC tests were significantly greater than that in 60 mmHg IPC test.
FIGURE 4.
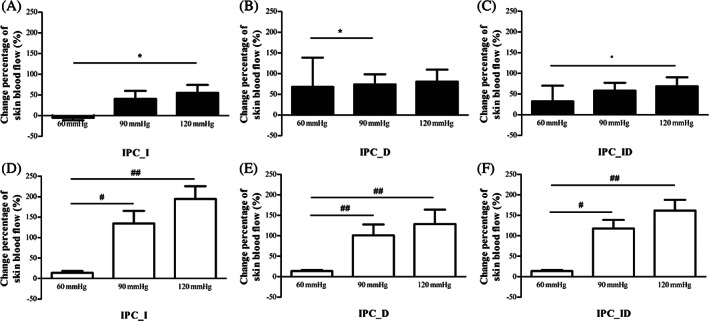
Change percentages of foot skin blood flow (SBF) in diabetic subjects (A‐C) and healthy subjects (D‐F) in three intermittent pneumatic compression (IPC) interventions with inflation pressure of 60, 90, 120 mmHg during baseline, IPC, and recovery stages. * indicates a significant difference in SBF percentage changes among three IPC tests in diabetic subjects; *P < .05. # indicates a significant difference in SBF percentage changes among three IPC tests in healthy subjects; # P < .05, ## P < .01
As shown in Figure 5, the SBF of IPC_I in healthy subjects was significantly greater than that in diabetic subjects under all three IPC tests (60 mmHg: P = .017, 90 mmHg: P = .008, 120 mmHg: P = .001), and the SBF of IPC_ID in healthy subjects was significantly greater than that in diabetic subjects under 60 mmHg (P = .028) and 120 mmHg (P = .013) IPC tests.
FIGURE 5.
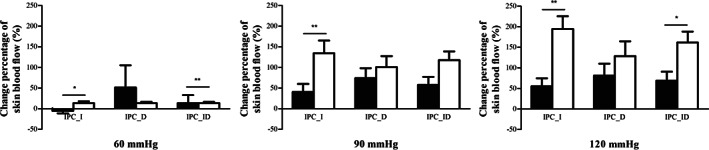
Difference in the change percentages of foot skin blood flow (SBF) in diabetic subjects (black bars) and healthy subjects (white bars) in three intermittent pneumatic compression (IPC) interventions. * indicates a significant difference in SBF percentage changes between diabetic subjects and healthy subjects in each IPC test; *P < .05, **P < .01
4. DISCUSSION
This study explored the effect of IPC with different inflation pressures on the foot SBF responses in people with and without type 2 DM. The results demonstrate that, for diabetic subjects, only IPC with inflation pressure of 90 and 120 mmHg can significantly increase foot SBF during the IPC intervention, and are more effective in improving foot SBF responses than 60 mmHg; for healthy subjects, all IPC interventions with three inflation pressures can significantly increase foot SBF, and IPC with 90 and 120 mmHg also show a greater effect than 60 mmHg. Moreover, the SBF responses to IPC intervention are stronger in healthy subjects than diabetic subjects.
When IPC is applied on the foot, the external mechanical pressure causes the emptying of plantar venous plexus and the increase of arteriovenous pressure gradients; the decreased venous pressure induces a transient suspension of the arteriovenous response and reduces peripheral vascular resistance; the shear stress and cyclic strain exerted by external compression from the IPC system cause the production of endothelial vasodilators. 15 , 21 , 22 , 23 Thus, it is helpful to improve vascular function and enhance blood flow. During the inflation and its holding period, the external pressure acts on the muscle tissue and blood vessels, which increase the arteriovenous pressure gradients, and subsequently enhance the instantaneous flow velocity and blood flow. During the deflation and its holding period, the augmented blood flow is mainly attributed to the active vasodilatation and hyperaemic responses after the increase in arteriovenous pressure gradients and ischaemia caused by compression of IPC. 14 , 19
The results in this study showed that the foot SBF in diabetic subjects did not increase under 60 mmHg IPC, but had a significant enhancement under 90 mmHg IPC and 120 mmHg IPC. Moreover, the increment percentages of SBF parameters under 90 and 120 mmHg IPC tests are significantly greater than the corresponding parameters under 60 mmHg IPC (Figure 4). The foot venous pressure in an average‐sized man is approximately 60 mmHg in the sitting position. 15 , 24 Thus, IPC with an inflation pressure of 60 mmHg may provide an insufficient effect on venous emptying due to vascular sclerosis caused by diabetes and energy loss caused by the dissemination of compression energy in soft tissues. 2 , 15 , 25 Taradaj et al compared the efficacy of IPC interventions with different inflation pressures on people with venous lymphatic ulcers in the lower extremities. This study found that the effect of 120 mmHg IPC on the elimination of oedema was better than 60 mmHg IPC intervention. Taradaj et al pointed out that IPC with too low pressure (less than 80 mmHg) may have no effect on lymphatic pressure. They also mentioned that subjects recruited in their study were relatively adaptable to the compression pressure of 120 mmHg without any discomfort pain. 26 Delis et al studied the effects of IPC interventions with different inflation pressures on venous emptying. Their study found that the effects of 120 mmHg IPC and 80 mmHg IPC on the reduction of venous pressure were better than 100 mmHg IPC and 60 mmHg IPC, respectively. The authors indicated that the greater IPC inflation pressure, the better venous emptying. 15 Alvarez et al also pointed out that IPC with an inflation pressure of 120 mmHg is a safe and effective therapy for people with peripheral vascular disease and severe lower limb ischaemia. 27 The findings in previous studies were consistent with the results of this study. Higher compression pressure is needed for improving foot circulation compared with other parts (like leg) because of inherent structure and relatively small blood storage. 28 Especially for people with diabetes, IPC with lower inflation pressure may produce a weak effect due to the stiffened soft tissue and vascular sclerosis. 4 , 29 Thus, IPC with an inflation pressure of 120 mmHg may be more suitable for people with type 2 DM to improve distal microcirculation. Compared with the characteristics of diabetic SBF responses, in addition to 90 mmHg IPC and 120 mmHg IPC, 60 mmHg IPC could also increase SBF parameters in the foot of healthy subjects. Moreover, IPC interventions with 90 and 120 mmHg show a greater impact. The results imply that both IPC interventions with inflation pressure of 90 or 120 mmHg are suitable for improving foot blood flow in healthy people. The treatment efficacy of IPC is related to the treatment regimen, individual physiological characteristics, and severity of disease. 30 Although IPC has been proven to be effective in improving foot circulation in healthy and diabetic groups, there are still few subjects who did not display positive microvascular responses to the IPC intervention.
Experimental results also showed that the increment percentages of SBF parameters in healthy subjects are greater than that in diabetic subjects, which is mainly due to the poor vasodilatory function caused by diabetes. Diabetic subjects recruited in this study were older and had a greater BMI than healthy subjects, which may affect the production of endothelial vasodilators and reduce the SBF responses to the stimulation of cyclic shear stress associated with IPC compression. 31 , 32 , 33 Moreover, the systolic blood pressures of dorsalis pedis artery and posterior tibial artery and ABI in diabetic subjects were also greater than that of healthy subjects, which indicates sclerosis and poor elasticity of the vessels in the lower extremities of diabetic subjects. One of the studies of Delis et al showed that the change percentage of SBF under IPC intervention in healthy people is greater than that in claudicated people. 12 They pointed out that the greater SBF increment in healthy limbs may be due to a lower basal blood flow level at the sitting position, because healthy people with normal autonomic sympathetic reflex have a larger arterial blood capacity than claudicated people. 34 , 35 This may contribute to the significant difference in blood flow between the two groups, because a lower basal SBF was shown in healthy subjects than diabetic subjects (P = .016).
Peripheral neuropathy is one of the most prevalent complications of diabetes, 36 and may increase the risk of diabetic foot ulcers by affecting neurovascular regulation. 4 Neuropathy causes the opening of arteriovenous shunts and reduces blood flow in the nutritive capillary bed. 37 , 38 Moreover, it also weakens nerve axon reflex and impedes microvascular vasodilation, resulting in the decrease of blood flow supply. 39 , 40 , 41 , 42 Thus, the foot microvascular responses to IPC in diabetic people with neuropathy may be different from diabetic people without neuropathy. Future studies may need to examine the IPC regimen tested in this study in people with diabetes and neuropathy to assess the efficacy of using IPC to improve peripheral circulation.
Delis et al observed the duration and amplitude decay of the inflow enhancement in the lower extremity of claudicated people under 5‐minute IPC intervention. They found that the physiologic mechanisms of IPC are active for 0 to 20 seconds after IPC therapy, the endothelial vasodilatation is effective for 20 to 35 seconds, and the effect of arteriovenous pressure gradient lasts for 50 seconds. 19 The results of the study of Delis et al imply that the effect of IPC on blood flow responses is instantaneous (or a fast response). In this study, the applied IPC could increase foot SBF during the IPC stage but not the recovery stage, which may be due to the low dose of applied IPC and higher venous pressure in the sitting position.
The venous pressure of foot and the arterial systolic blood pressure at the ankle is approximately 60 and 170 mmHg in the sitting position, respectively. 15 For better emptying of veins without affecting arteries, compression pressure between arterial and venous pressure (65‐120 mmHg) is often used in commercial IPC devices. 14 , 43 Thus, 60, 90, and 120 mmHg were tested in this experiment to preliminarily explore the optimum inflation pressure of IPC for people with type 2 DM. Moreover, the study of Gaskell and Parrott reported that the refill time of venous blood in the foot is approximately 16 to 20seconds, and the higher compression frequency would not provide additional benefit. 44 The inflating and deflating parameters (inflation time of 4 seconds and deflation time of 16 seconds) selected in this study provide sufficient time and effective frequency to allow the refill of foot veins. 22 , 45
There are some limitations to this study that should be noted. Firstly, this study mainly explored the transient effects of IPC with different inflation pressures on the SBF responses of diabetic foot. Its effect on the arterial flow and venous emptying, as well as its long‐term intervention effects for people with type 2 DM, need to be investigated in future studies. Secondly, the inflation and deflation time at 4 seconds/16 seconds, 10 seconds/20 seconds, 15 seconds/30 seconds are commonly used parameters in the IPC interventions. The intervention duration in this study was set as 9 minutes (27 cycles), in order to facilitate the comparison in future research on IPC with the same intervention duration (9 minutes) but different inflation and deflation times (4 seconds/16 seconds, 10 seconds/20 seconds, 15 seconds/30 seconds).
5. CONCLUSIONS
This study explored the effects of IPC with three inflation pressures on the foot SBF responses in people with and without type 2 DM, in order to determine suitable inflation pressures of IPC as a therapy for improving microcirculation of the foot and reducing the risk of diabetic foot ulcers in people with type 2 DM. The findings of this study indicate that all IPC interventions with 60, 90, and 120 mmHg can increase the foot SBF responses in healthy people, but only 90 and 120 mmHg are effective in improving foot microcirculation in people with type 2 DM.
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported.
ACKNOWLEDGEMENTS
The authors thank all subjects who participated in this study. This research was funded by the National Natural Science Foundation of China [grant number 11902089, 11672027, U20A20390, 11827803].
Ren W, Duan Y, Jan Y‐K, et al. Effect of intermittent pneumatic compression with different inflation pressures on the distal microvascular responses of the foot in people with type 2 diabetes mellitus. Int Wound J. 2022;19(5):968-977. doi: 10.1111/iwj.13693
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 11902089, 11672027, 11827803, U20A20390
Contributor Information
Fang Pu, Email: pufangbme@buaa.edu.cn.
Yubo Fan, Email: yubofan@buaa.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kavitha KV, Tiwari S, Purandare VB, Khedkar S, Bhosale SS. Choice of wound care in diabetic foot ulcer: a practical approach. World J Diabetes. 2014;5(4):546‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenman RL, Panasyuk S, Wang X, et al. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. 2005;366(9498):1711‐1717. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Lazzarini PA, Mcphail SM, Netten J, Armstrong DG, Pacella RE. Global disability burdens of diabetes‐related lower‐extremity complications in 1990 and 2016. J Diabetes Care. 2020;43(5):dc191614. [DOI] [PubMed] [Google Scholar]
- 4. Chao CYL, Cheing GLY. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes‐Metab Res Rev. 2009;25(7):604‐614. [DOI] [PubMed] [Google Scholar]
- 5. Kabbani M, Rotter R, Busche M, et al. Impact of diabetes and peripheral arterial occlusive disease on the functional microcirculation at the plantar foot. Plast Reconstr Surg. 2013;1(7):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eze AR, Comerota AJ, Cisek PL, et al. Intermittent calf and foot compression increases lower extremity blood flow. Am J Surg. 1996;172(2):130‐134. [DOI] [PubMed] [Google Scholar]
- 7. Pawlaczyk K, Gabriel M, Urbanek T, et al. Effects of intermittent pneumatic compression on reduction of postoperative lower extremity Edema and normalization of foot microcirculation flow in patients undergoing arterial revascularization. Med Sci Monit. 2015;21(2015):3986‐3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labropoulos N, Wierks C, Suffoletto B. Intermittent pneumatic compression for the treatment of lower extremity arterial disease: a systematic review. J Vasc Med. 2002;7(2):141‐148. [DOI] [PubMed] [Google Scholar]
- 9. Moran PS, Teljeur C, Harrington P, Ryan M. A systematic review of intermittent pneumatic compression for critical limb ischaemia. J Vasc Med. 2015;20(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 10. Oresanya L, Mazzei M, Bashir R, et al. Systematic review and meta‐analysis of high‐pressure intermittent limb compression for the treatment of intermittent claudication. J Vasc Surg. 2018;67(2):620‐628. [DOI] [PubMed] [Google Scholar]
- 11. Delis KT, Nicolaides AN, Wolfe JHN. Peripheral sympathetic autoregulation in arterial calf inflow enhancement with intermittent pneumatic compression. Eur J Vasc Endovasc Surg. 2001;22(4):317‐325. [DOI] [PubMed] [Google Scholar]
- 12. Delis KT, Husmann MJW, Nicolaides AN, Wolfe JH, Cheshire NJ. Enhancing foot skin blood flux in peripheral vascular disease using intermittent pneumatic compression: controlled study on claudicants and grafted arteriopaths. World J Surg. 2002;26(7):861‐866. [DOI] [PubMed] [Google Scholar]
- 13. Kavros SJ, Delis KT, Turner NS, et al. Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18‐month follow‐up. J Vasc Surg. 2008;47(3):543‐549. [DOI] [PubMed] [Google Scholar]
- 14. Sheldon RD, Roseguini BT, Laughlin MH, Newcomer SC. New insights into the physiologic basis for intermittent pneumatic limb compression as a therapeutic strategy for peripheral artery disease. J Vasc Surg. 2013;58(6):1688‐1696. [DOI] [PubMed] [Google Scholar]
- 15. Delis KT, Azizi ZA, Stevens RJG, Wolfe JHN, Nicolaides AN. Optimum intermittent pneumatic compression stimulus for lower‐limb venous emptying. Eur J Vasc Endovasc Surg. 2000;19(3):261‐269. [DOI] [PubMed] [Google Scholar]
- 16. Rosales‐Velderrain A, Padilla M, Choe CH, Hargens AR. Increased microvascular flow and foot sensation with mild continuous external compression. Physiol Rep. 2013;1(7):e00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jan YK, Shen S, Foreman RD, Ennis WJ. Skin blood flow response to locally applied mechanical and thermal stresses in the diabetic foot. Microvasc Res. 2013;89:40‐46. [DOI] [PubMed] [Google Scholar]
- 18. Ren W, Pu F, Luan H, et al. Effects of local vibration with different intermittent durations on skin blood flow responses in diabetic people. Front Bioeng Biotechnol. 2019;7(2019):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delis KT, Knaggs AL. Duration and amplitude decay of acute arterial leg inflow enhancement with intermittent pneumatic leg compression: an insight into the implicated physiologic mechanisms. J Vasc Surg. 2005;42(4):717‐725. [DOI] [PubMed] [Google Scholar]
- 20. Rifkind JM, Nagababu E, Dobrosielski DA, et al. The effect of intermittent pneumatic compression of legs on the levels of nitric oxide related species in blood and on arterial function in the arm. J Nitric Oxide: Biol Chem. 2014;40:117‐122. [DOI] [PubMed] [Google Scholar]
- 21. Tan XL, Qi WN, Gu XS, Urbaniak JR, Chen LE. Intermittent pneumatic compression regulates expression of nitric oxide synthases in skeletal muscles. J Biomech. 2006;39(13):2430‐2437. [DOI] [PubMed] [Google Scholar]
- 22. Kalodiki E, Giannoukas AD. Intermittent pneumatic compression (IPC) in the treatment of peripheral arterial occlusive disease (PAOD) ‐ a useful tool or just another device? Eur J Vasc Endovasc Surg. 2007;33(3):309‐310. [DOI] [PubMed] [Google Scholar]
- 23. Roseguini BT, Soylu SM, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP‐1 expression in skeletal muscle. Am J Physiol‐Heart C. 2010;298(6):H1991–H2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol. 1949;1(9):649‐662. [DOI] [PubMed] [Google Scholar]
- 25. Zimny S, Dessel F, Ehren M, Pfohl M, Schatz H. Early detection of microcirculatory impairment in diabetic patients with foot at risk. Diabetes Care. 2001;24(10):1810‐1814. [DOI] [PubMed] [Google Scholar]
- 26. Ponikowska IGK, Szczepanowski A. Intermittent pneumatic compression therapy in lower leg venous – lymphatic disorders. Balneol Pol. 1997;39(1–2):87‐93. [Google Scholar]
- 27. Alvarez OM, Wendelken ME, Markowitz L, Comfort C. Effect of high‐pressure, intermittent pneumatic compression for the treatment of peripheral arterial disease and critical limb ischemia in patients without a surgical option. Wounds. 2015;27(11):293‐301. [PubMed] [Google Scholar]
- 28. Gardner AM, Fox RH. The venous footpump: influence on tissue perfusion and prevention of venous thrombosis. Ann Rheum Dis. 1992;51(10):1173‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun J‐H, Cheng BK, Zheng Y‐P, Huang Y‐P, Leung JY, Cheing GL. Changes in the thickness and stiffness of plantar soft tissues in people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2011;92(9):1484‐1489. [DOI] [PubMed] [Google Scholar]
- 30. Morris RJ. Intermittent pneumatic compression ‐ systems and applications. J Med Eng Technol. 2008;32(3):179‐188. [DOI] [PubMed] [Google Scholar]
- 31. Shiogai Y, Stefanovska A, Mcclintock P. Nonlinear dynamics of cardiovascular ageing. J Phys Rep. 2010;488(2–3):51‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jan Y‐K, Struck BD, Foreman RD, Robinson C. Wavelet analysis of sacral skin blood flow oscillations to assess soft tissue viability in older adults. Microvasc Res. 2009;78(2):162‐168. [DOI] [PubMed] [Google Scholar]
- 33. Kraemer‐Aguiar LG, de Miranda ML, Bottino DA, et al. Increment of body mass index is positively correlated with worsening of endothelium‐dependent and independent changes in forearm blood flow. Front Physiol. 2015;6(2015):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sumner DS. Essential haemodynamic principles. In: Rutherford RB, ed. Vascular Surgery. Philadelphia, Pennsylvania: Saunders; 1995:18‐44. [Google Scholar]
- 35. Sumner DS. Haemodynamics in the lower limb. In: Myers KA, Nicobides AN, Sumner DS, eds. Lower Limb Ischaemia. Nicosia: Med‐Orion; 1997:1‐29. [Google Scholar]
- 36. Feldman EL, Callaghan BC, Pop‐Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):42‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiernsperger NF. In defense of microvascular constriction in diabetes. Clin Hemorheol Microcirc. 2001;25(2):55‐62. [PubMed] [Google Scholar]
- 38. Hoffman RP, Sinkey CA, Kienzle MG, Anderson EA. Muscle sympathetic nerve activity is reduced in IDDM before overt autonomic neuropathy. Diabetes. 1993;42(3):375‐380. [DOI] [PubMed] [Google Scholar]
- 39. Hamdy O, Abou‐Elenin K, LoGerfo FW, Horton ES, Veves A. Contribution of nerve‐axon reflex‐related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care. 2001;24(2):344‐349. [DOI] [PubMed] [Google Scholar]
- 40. Uccioli L, Monticone G, Durola L, et al. Autonomic neuropathy influences great toe blood pressure. Diabetes Care. 1994;17(4):284‐287. [DOI] [PubMed] [Google Scholar]
- 41. Caselli A, Rich J, Hanane T, Uccioli L, Veves A. Role of C‐nociceptive fibers in the nerve axon reflex‐related vasodilation in diabetes. Neurology. 2003;60(2):297‐300. [DOI] [PubMed] [Google Scholar]
- 42. Arora S, Pomposelli F, LoGerfo FW, Veves A. Cutaneous microcirculation in the neuropathic diabetic foot improves significantly but not completely after successful lower extremity revascularization. J Vasc Surg. 2002;35(3):501‐505. [DOI] [PubMed] [Google Scholar]
- 43. Lurie F, Scott V, Yoon HC, Kistner RL. On the mechanism of action of pneumatic compression devices: combined magnetic resonance imaging and duplex ultrasound investigation. J Vasc Surg. 2008;48(4):1000‐1006. [DOI] [PubMed] [Google Scholar]
- 44. Gaskell P, Parrott JC. The effect of a mechanical venous pump on the circulation of the feet in the presence of arterial obstruction. Surg Gynecol Obstet. 1978;146(4):583‐592. [PubMed] [Google Scholar]
- 45. Delis KT, Nicolaides AN, Labropoulos N, Stansby G. The acute effects of intermittent pneumatic foot versus calf versus simultaneous foot and calf compression on popliteal artery hemodynamics: a comparative study. J Vasc Surg. 2000;32(2):284‐292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


