Abstract
The cartilage repair and regeneration show inadequate self‐healing capability and have some complications, which are inordinate challenges in clinical therapy. Biopolymeric injectable hydrogels, a prominent type of cell‐carrier as well tissue engineering scaffolding materials, establish promising therapeutic potential of stem cell‐based cartilage‐regeneration treatment. In addition, injectable scaffolding biomaterial should have rapid gelation properties with adequate rheological and mechanical properties. In the present investigation, we developed and fabricated the macromolecular silk fibroin blended with polylysine modified chitosan polymer (SF/PCS) using thermal‐sensitive glycerophosphate (GP), which contains effective gelation ability, morphology, porosity and also has enhanced mechanical properties to induce physical applicability, cell proliferation and nutrient exchange in the cell‐based treatment. The developed and optimised injectable hydrogel group has good biocompatibility with human fibroblast (L929) cells and bone marrow‐derived mesenchymal stem cells (BMSCs). Additionally, it was found that SF/PCS hydrogel group could sustainably release TGF‐β1 and efficiently regulate cartilage‐specific and inflammatory‐related gene expressions. Finally, the cartilage‐regeneration potential of the hydrogel groups embedded with and without BMSCs were evaluated in SD rat models under histopathological analysis, which showed promising cartilage repair. Overall, we conclude that the TGF‐β1‐SF/PCS injectable hydrogel demonstrates enhanced in vitro and in vivo tissue regeneration properties, which lead to efficacious therapeutic potential in cartilage regeneration.
Keywords: articular cartilage, chitosan, hydrogel, silk fibroin, stem cells
1. INTRODUCTION
Articular cartilage is a vital part that covers the opposing site of articulate bones and helps in proper functioning of synovial joints through its biological properties of greater resiliency and function of deformability, protecting them from joint compressive loads. 1 , 2 Besides, cartilage tissues provide significant smoothness and gliding morphology to lead low friction coefficient. Generally, many people severely suffer from articular cartilage degeneration because of osteoarthritis, trauma and genetic abnormalities. 3 Most complicated issue in articular cartilage therapy is limited regeneration ability compared with other tissue regenerations and chondrocytes cell types only available in the articular cartilage tissues. Because of the decreased self‐healing capability, various surgical implantations of biomaterials have been explored to attain beneficial cartilage treatment. 4 , 5 Particularly, development of biological hydrogels is more promising to repair joint cartilage with their essential abilities of stress relaxation behaviour, which is similar to native cartilage; it could favour to load transfer and transportation of nutrients to cells. 6 , 7 Naturally, wet weights of cartilage have maximum water of approximately 80%. Hence, implantation of suitable hydrogel materials becomes a prominent option for in vitro and in situ cartilage regeneration and tissue engineering. In addition, the role of hydrogel scaffold is not only to offer microenvironment for cell attachment and proliferation nut but also to have favourable mechanical strength and stability at the defected site. 8 , 9 The novel approaches of bio‐degradable injectable hydrogel scaffolds preparations with tunable chemical and mechanical properties have great attention for biomaterial science and tissue regenerative medicine. 10 , 11 There are many requirements and difficult complications to design and fabrication of injectable hydrogel scaffolds. Specifically, as reported previously, various suitable biomaterials including organic polymers (synthetic and naturally available biopolymers) and inorganic components have been explored as effective matrices to use in tissue regeneration and repair applications. 12 , 13
Particularly, injectable polymeric hydrogels have a greater potential to promote articular cartilage regeneration because of their tailorable structural and mechanical capabilities. Importantly, injectable hydrogel system is a free‐flowing fluid when injection and instinctively changes into a semi‐solid natured system with support of body physiological changes such as pH, temperature, etc. In addition, drug molecules, growth factors and cells can be promisingly incorporated into injectable hydrogel by simple dissolution procedures. 14 , 15 As previously reported, natural polysaccharides and protein based‐biopolymers including alginate, collagen, agarose, hyaluronic acid, gelatin, silk fibroin and chitosan are popular bioactive hydrogel scaffolding materials for cartilage tissue engineering applications because of their appropriate mimicking of extracellular microenvironment. 16 , 17 , 18 , 19 Among various natural polymers, regenerated silk fibroin (SF) protein and chitosan polysaccharides have extensively been demonstrated to be outstanding candidate as injectable hydrogel scaffolding material. The fabricated silk protein hydrogel scaffolds have been reported as multifunctional tissue engineering scaffolding material with assistance of appropriate substituted materials because of its tailorable mechanical and biodegradability properties through the process of chemical cross‐linking and physical induction of β‐sheet confirmation. 20 , 21 , 22 Furthermore, SF polymeric materials can be easily processed into various forms including films, membranes, micro/nanoparticles, fibrous scaffolds and mainly hydrogel scaffolding materials. 23 Chitosan biopolymer is an abundantly available polysaccharide regenerated from complete deacetylation process of chitin, a structural component extracted from the skeletons of insects and crustaceans. 24 , 25 Importantly, chitosan‐based materials have received significant attention as a hydrogel scaffolding material because of its well‐established properties of pH sensitivity, cytocompatibility and biodegradability. Frequently, chitosan can be suggestively dissolved in acidic solutions and its viscosity nature could easily be modulated by tailoring the concentrations. 26 , 27 When chitosan solution gelated with β‐glycerophosphate, the chitosan polymer remains in solution at neutral pH and room temperature conditions, while homogeneous gelation process can be induced by increasing the heat of body physiological temperature. In the present study, β‐glycerophosphate plays an important role to achieve temperature and pH‐induced chitosan hydrogel preparation, which have low stiffness and mechanical strength because of excessive formation of hydrogen bonding and hydrophobic interactions between head‐to‐head polymeric chains, which causes consequent removal of inter‐chain electrostatic forces through amino groups neutralisation. 28 , 29 Polylysine (PL) is one of the cationic homo‐polyamides with an amide linkage between 3‐amino and a carboxy function, exhibiting a greater solubility, thermal stability and high compatibility with cells. In addition, it is a well‐known anti‐microbial agent and also has wide‐spectrum of bactericidal activity against gram‐positive and gram‐negative pathogens. Importantly, PL functionalised CS polymer may enhance the physical and chemical properties of CS with improved anti‐bacterial activity. 30 , 31
Hence, introduction of silk protein molecules into the chitosan polymers would be an effective approach to solve these problems. 32 , 33 Transforming growth factor‐β1 (TGF‐β1) is one of the pleiotropic growth factors and abundantly exists in native cartilage, which has significant regulatory potential on different cells types. It plays an effective role in bone formation, angiogenesis, neuroprotection, wound repair with capable cell proliferations and differentiations. In addition, TGF‐β1 would control the extracellular matrices production by influencing the synthesis of collagen, fibronectin and proteoglycans. Hence, it can be appearing to have noteworthy effects in regeneration of cartilage tissues. 34 , 35 , 36 As previous reports, cartilage‐regeneration process starts with the process of mesenchymal condensation, which is categorised by prominent proliferations of mesenchymal stem cells (MSCs), cell clusters formations and specifically significant differentiation into cartilage chondrocytes. Stem cell‐based cartilage therapies demonstrate low immunogenicity, multipotency with promising MSCs expansion potential in cartilage repair. 37 , 38 Different types of stem cells have been used for cartilage defect regeneration, including adipose‐derived MSCs, bone marrow‐derived MSCs and synovium‐derived MSCs. 19 , 39 , 40 The direct implantations of cell culture pellets to the defect site are hard to maintain cell diffusions, and the core of the cell pellets would affect by defect site necrosis. The main goal of the present investigation was to fabricate blended CS/SF hydrogel scaffolds to attain stem cell‐based therapeutic potential in cartilage repair. The structural stability and mechanical strength of the blended hydrogel were investigated by the quantified observations of morphology, surface roughness and compressive modulus. The cell compatibility and in vivo effectiveness were evaluated using BMSCs and SD rat models, respectively.
2. EXPERIMENTAL SECTION
2.1. Materials
The chemicals, reagents and biologic mediums were used as obtained from chemical companies, including chitosan powder (Zhejiang AoXing Biochemical Co., Ltd.) ethanol (EtOH; Aladdin Chemicals Co., Ltd.), sodium hydroxide (NaOH; Aladdin Chemicals Co., Ltd.) and acetic acid (Aladdin Chemicals Co., Ltd.). The cross‐linking agents of N‐hydroxysuccinimide (NHS) and 3‐(3‐dimethylamine‐opropyl)‐1‐ethylcarbodiimide hydrochloride (EDC.HCl) were obtained from Sigma‐Aldrich (St. Louis, MO). The biological assays and mediums including MTT assay (Sigma, USA), DMEM (Gibco, USA) and FBS (Hyclone, UT) were used for biological investigations.
2.2. Preparation of regenerated silk fibroin solution
The regenerated SF protein solution was prepared as previously described method with small modifications. 41 Briefly, silkworm glands were washed with boiling distilled water using Na2CO3 (0.2 M) thrice for 45 minutes, to remove residual gum‐like sericin particles, which is called the degumming process. After that, degummed cocoons were cut into fine pieces to extract fibroin protein as a solution. The silk fibres were dissolved in freshly prepared lithium bromide (LiBr; 9.3 M) to keep in an oven at 80°C for 1 hour. Finally, prepared colloidal form of SF solution dialysed against deionised water using benzoylated dialysis tube (MWCO: 2 kDa) and centrifuged to obtain pure SF solution without any residual particles. Meanwhile, the regenerated SF solution was stored at 4°C for further reaction process.
2.3. Preparation of polylysine modified chitosan nanoparticles
The commercially obtained chitosan powder (2 g) was dissolved in aqueous acetic acid (100 mL; 1% vol/vol) solution. Frequently, an appropriate amount of polylysine (PL) was mixed into the prepared CS solution under constant stirring for 3 hours until PL polymeric molecule dissolved methodically. Finally, the presented air bubbles were removed under pressure and PL content of the solution was neutralised with NaOH solution.
2.4. Fabrication of SF/PCS NPs injectable hydrogel scaffold
The blended SF/PL‐CS injectable hydrogel was prepared followed by previous reports with minor modifications. 5 , 42 , 43 Briefly, prepared 2 g of PL modified CS was systematically dissolved in 0.1 M HCl (10 mL). To obtain a composite form of hydrogel, an appropriate amount of RSF solution was introduced into the above‐prepared PL‐CS solution under constant stirring. After that, the prepared SF/PL‐CS mixture cooled down to 4°C and further proceeded to systemic dropwise addition of glycerophosphate solution (GP; 50 wt/vol %; 2 mL) with stirring condition at 4°C to obtain homogenous solution and obtained product labelled as SF/PCS. The different blending ratios of SF/PCS hydrogel groups were prepared with a ratio of 8:2 (SF/PCS (Group II), 5:5 (SF/PCS (Group III) and 2:8 (SF/PCS (Group IV) for the physiological, rheological and mechanical properties optimisation. Additionally, for comparison purpose, GP functionalised PCS hydrogel solution without SF was prepared and labelled as PCS (Group I).
2.5. Immobilisation of TGF‐β1 into the Injectable hydrogel
Specifically, fabricated SF/PCS injectable hydrogel was prepared to carriers transforming growth factor (TGF‐β1) peptide for the cartilage treatment. Briefly, TGF‐β1 peptide was added to PBS buffer (250 mL), and then the suspension was stirred for 5 minutes and processed with an ultrasonic processor to attain a uniformly distributed solution. After that, the prepared suspension was systematically dropwise added to the hydrogel mixture at 4°C for 15 minutes under constant stirring (1000 rpm). The TGF‐β1 immobilised hydrogel sample was lyophilised at −40°C for further morphological and physicochemical characterisation.
2.6. Characterisations of hydrogel groups
2.6.1. Structural and morphological analyses
Scanning electron microscopic method was performed to visualise the morphology of the lyophilised hydrogel by FEI Quanta 200 FEG at 7.00 keV landing E and 0.7 Torr of pressure. The infrared spectral analysis was used to prepare scaffolding materials to investigate structural behaviours and interactions between polymeric components using Nicolet 6700 ATR FT‐IR spectrometer. Each spectrum was attained at 4 cm−1 resolution and 256 scans in the spectral range of 400–4000 cm−1.
2.6.2. Porosity nature
The porosity of the lyophilised injectable hydrogel was examined through the method of liquid displacement and hexane reagent as displacement liquid. In brief, lyophilised hydrogel scaffold was carefully immersed into the hexane (known volume [V1]) under a graduated cylindrical vessel for 15 minutes, and the total volume of the hexane solution with hydrogel samples was recorded as V2. Finally, the immersed hydrogel groups were removed from the cylinder, and remaining hexane volume was recorded as V3. The porosity of the hydrogel scaffold was measured by the following formula:
2.6.3. Mechanical properties
The Young's modulus and compressive strength of the lyophilised hydrogel scaffolds were examined under Instron universal testing equipment (Ins‐5943, USA). The cylindrical‐shaped lyophilised hydrogel scaffolds were used to measure followed by F451‐91 ASTM method with minor modifications. Specifically, crosshead speed of 1 mm/min and 0.1 kN cell load were fixed for the analysis.
2.6.4. In vitro biodegradation evaluations
The degradation properties of the hydrogel scaffolds were determined by using PBS medium in the presence and absence of biologic enzymes containing 1 U/mL of protease XIV and 500 U/mL of lysozyme. The introduction of protease enzymes into PBS will be helpful to analyse the enzymatic degradation properties of the hydrogel scaffolds. The lyophilised disc‐shaped hydrogel scaffold (Wd1) was immersed into a prepared medium (PBS [pH 7.4; 0.01 M] with or without enzymes) and incubated at biological conditions (37°C). The solution was refreshed every day, and at each time point, samples were taken out and rinsed with DI water and dried. Then, it was weighed and labelled as Wd2. Finally, the degradation rate of the scaffolds was calculated by the following formula:
2.7. Rheological characterisation
The rheological properties of the developed hydrogel were evaluated under strain‐controlled TA rheometer (AR‐2000ex) using non‐porous stainless steel and parallel plates of 25 mm diameter. The strain amplitude was optimised to confirm that determinations were used in a linear viscoelastic region. Hence, elastic modulus (G′) and viscous modulus (G″) were independent of the results of strain amplitude. Accurately, 1.5 mL of the prepared hydrogel was put into the sample holder for the analysis, and greater than 1000 μm of gap was always stretched to the hydrogel group relaxed until equilibrium. All the measurements were conducted at different temperatures from 25°C to 45°C at a rate of 1°C/min. For dependence of isothermal frequency, G′ and G″ were determined in the range of 0.1 to 100 Hz at constant strain (1%) and temperature (37°C).
2.8. In vitro analyses
2.8.1. In vitro cell compatibility on the hydrogel scaffolds
In vitro cell cytotoxicity of the prepared injectable hydrogel scaffold was performed on the cell lines of BMSCs and human fibroblast (L929) for 5 days as previous protocols with minor modifications. 31 , 44 The hydrogel extract was prepared by adding hydrogel (0.5 g/mL) with distilled water and incubated at 37°C for 24 hours. The hydrogel extract was sterilised and added into the concentrated culture media (RPMI‐1640; Gibco) containing calf serum (6 vol%; Gibco) to obtain clear hydrogel (100%) extract. The selected cell lines of BMSCs and L929 were independently cultured onto 96‐well plates at 5000 cell numbers per well with the addition of mentioned culture media and incubated at humidified conditions at 37°C/5% CO2 for 12 hours. After that, different sample extracts were replaced with culture media of the cells and incubated for 48 hours and observed cell viability using MTT assay method as previous reports. 45 Briefly, after completion of incubated time, the hydrogel treated cell culture media was removed and washed with fresh RPMI‐1640 media. Then, MTT solution (10 μL; 5 mg/mL) was added to the treated culture of each well. Finally, SDS (100 μL; 10 wt%) and HCl (0.01 M) mixed solution was added to the MTT treated culture after 4 hours and incubated for 12 hours. The quantitative cell survival rate was measured by optical density using a microplate reader at a wavelength of 570 nm. Meanwhile, cell viability of main composited hydrogel was observed in different concentrations (0, 12.5, 25, 50, 100, 200 μg/mL) at humidified conditions of 37°C/5% CO2 for 48 hours. The cell proliferations morphology and proliferation rate were evaluated by the same protocol as mentioned above for different time periods (1, 3 and 5 days). The morphology of the BMSCs and L929 cells were observed by optical microscopic images.
2.8.2. BMSCs encapsulated hydrogel vesicle
The prepared blended hydrogel solution was taken into a glass‐bottom dish and loaded with cell suspension (2 × 106 cells/mL) in proper biological conditions. Then, the culture medium was added into the glass‐dish and incubated under the suitable humidified incubation condition (37°C, 5% CO2) with proper replacement of culture media for every 2 to 3 days. After 12 days, the proliferation of the BMSCs into hydrogel was observed by CLSM microscopic technique before use for in vivo analyses.
2.8.3. Gene expressions by RT‐qPCR
The TRIZOL Reagent (Invitrogen) was used to homogenising all RNA isolated from cells presented in the hydrogel group and then Qiagen RNeasy Plus Mini Kit was used for RNA purification. After that, reverse transcription process was performed via SuperScript cDNA synthesis kit (VILO; Invitrogen) as systematically followed by manufactures instructions. The Applied Biosystems manufactured SYBR Green Reaction Mix and thermocycler (StepOne‐Plus) was used to perform real‐time PCR. Finally, the levels of gene expressions (Sox 9, col II & X, MMP‐13) and aggrecan were quantitatively observed and graphed using GraphPad Prism software. All the observed values were normalised to RPL13A ribosomal protein by using the C T method.
2.9. In vivo effect of hydrogel groups on cartilage defect
In vivo cartilage repair potential of the developed hydrogel groups in the presence and absence of BMSCs were used using SD rat articular cartilage defect model, and all the animal protocols were approved by Institutional Animal Research Committee of the Nanjing Medical University, PR China. To examine the cartilage repair therapeutic potential of hydrogel groups with BMSCs transplantations, SD rats (Male; 160–200 g; n = 24) were randomly divided into four groups and even six animals assigned per group as follows; Group (i) control (saline) (n = 6), Group (ii) SF/PCS hydrogel (n = 6), Group (iii) TGF‐β1‐SF/PCS (n = 6) and Group (iv) BMSCs@ TGF‐β1‐SF/PCS (n = 6). The respective grouped samples were systematically injected into the defect site with proper surgical procedures. After 6 and 12 weeks, all the rats were sacrificed with anaesthesia (overdose), and treated cartilage joint was harvested methodically for further analyses. The harvested specimens were fixed with formalin (10%) for 1 day, and then EDTA (15%) was used for the decalcification process. After that, decalcified samples were fixed onto a piece of paraffin film (5 μm) section. Finally, the samples were stained with haematoxylin and eosin (H & E) and Masson's trichrome (MTS) staining and visualised with Olympus microscopic technique with charge‐coupled device camera.
2.10. Statistical analysis
The microscopic analyses were observed by triple investigate persons who were completely blinded to the groups. All quantitative analysis data are presented as mean (±) standard deviation. The statistical differences of the individual data groups were performed via one‐way ANOVA and Tukey's post‐analysis. The analysis of data was assessed through GraphPad Prism software, and statistical significance was considered with a P value of less than .05 (P > .05) (Figure 1).
FIGURE 1.

Schematic illustration of BMSCs and TGF‐β1 encapsulated SF/PCS injectable hydrogel for cartilage repair application
3. RESULTS AND DISCUSSION
The chemical structural composition, phase purity and crystalline behaviours of the prepared hydrogel scaffolds and bare SF and CS components were examined by FT‐IR and XRD analysis techniques (Figure 2). As shown in Figure 2A, SF/PCS hydrogel displays an absorption band of amide stretching vibrations at 1732 cm−1, which was the increasing intensity with the addition of GP and EDC/NHS cross‐linking agent demonstrating that formation of successful chemical bonding between SF and CS polymeric molecules. The characteristic peaks at the regions of 1541 and 1652 cm−1 corresponded to the absorption of random coil and α‐helix, respectively, in the blended SF/PCS hydrogel group, which was reduced compared with the pure SF group. Meanwhile, well‐defined peak was observed at ranges between 1633 and 1640 cm−1 is attributed to the amide I band, which demonstrates the stable β‐folding structure of SF molecules. This observation confirmed that the blended SF/PCS hydrogel group with GP component had been prominently modified from the unstable random coil and α‐helix structure of SF to an effective and stable β‐folding structure of fibroin blended with PCS polymeric network. 46 Besides, XRD analysis (Figure 2B) of CS hydrogel group displayed notably weak characteristic diffraction bands at 10.12°, 19.34° and 21.54°, and bare SF and SF/PCS blended hydrogel displayed a characteristic peak at 2ϴ = 20.8. The results of the XRD pattern investigation demonstrated that blended SF/PCS have a higher degree of crystalline property and formation of a stable β‐folding structure with increasing intensity when compared with the bare CS and SF materials. Consequently, blending of CS molecules could support the crystalline behaviour of SF by modulating random curls into the highly stable β‐folding structure, which promotes hydrogels' structural stability. 47
FIGURE 2.
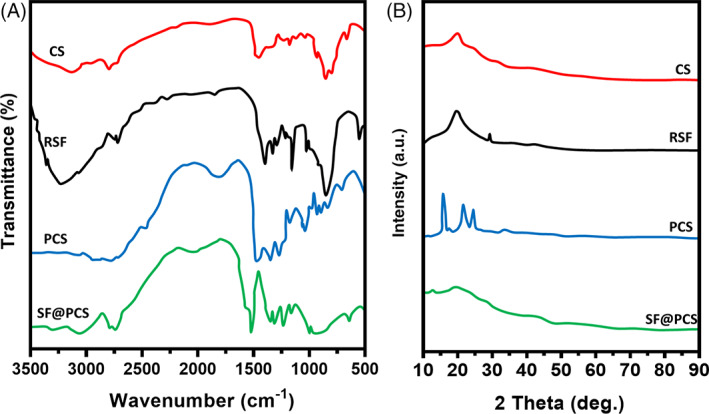
The structural interactions and component phase purity of CS, RSF, PCS and SF/PCS were evaluated by FT‐IR (A) and XRD (B) spectroscopic analyses
The morphological microstructure of the lyophilised hydrogel groups was investigated by scanning electron microscopic technique, which exhibits well‐distributed and interconnected porous surface as shown in Figure 3A. The results demonstrate that incorporation of GP solution and blending ratios of SF and PCS have been significantly influenced by the structure of the hydrogel network and rapidly affected the morphological nature and microstructure of the SF/PCS hydrogel scaffolds. Furthermore, the morphology of the hydrogel was strongly connected to the optimised proportion of SF and PCS polymers at suitable concentrations, which was used in the hydrogel preparation method. The prepared SF/PCS hydrogel scaffold with optimal composition had a favourable average porous size of 38.45 ± 3.55 μm (Figure 3B) that would be greatly suitable for cell compatibility, adhesion and proliferations, meanwhile support effective metabolite and nutrient diffusion to the cells.
FIGURE 3.

The morphological and interconnected structure of prepared hydrogel groups with different blending ratios were observed and visualised by SEM analysis (A). Porosity (B), swelling ratio (C) and water uptake ratio (D) of different hydrogel groups were presented
Generally, prepared hydrogels applied for the tissue engineering application, specifically in the treatment of cartilage repair, should be stable and maintain respective microenvironment in the defect site for the cell adhesion and have the ability to effective tissue regeneration properties. The swelling properties of the prepared blended hydrogel samples after being immersed into the PBS medium at suitable biological conditions (37°C) for 24 hours as shown in Figure 3C. The observed results exhibited blended hydrogel group (IV) has greater equilibrated swelling rate and water uptake ability in the percentage of 94.80% and 90.76%, respectively, indicating that blended hydrogel has prominent suitability water‐holding capability. Frequently, when the incorporation of GP with EDC‐NHS cross‐linking agents was gradually increased swelling ratio, which also supported the formation of effective blended hydrogels. As described above, the distribution of GP and increasing concentration of CS molecules into the blending hydrogel have improved cross‐linking density, suitable porous size and stable network, which would induce the water diffusion into hydrogels. 42
The favourable and improved mechanical strength of the hydrogel is important for the tissue engineering application because hydrogel is required to maintain their structural integrity and also to withstand host responding forces by surrounding tissues. 48 Hence, stress‐stain curve, compressive strength and compressive modulus were investigated for the developed hydrogel groups, as exhibited in Figure 4. It was distinguished that the changing blending ratios of the polymeric groups significantly influenced their mechanical properties, as shown in diagrams. Predominantly, compared with Group (I), the blended hydrogel of SF/PCS (Group IV) exhibited outstanding improvement in compressive elastic modulus and compressive strength because of effective cross‐linking density of GP and CS molecules as displayed in Figure 4B,C.
FIGURE 4.
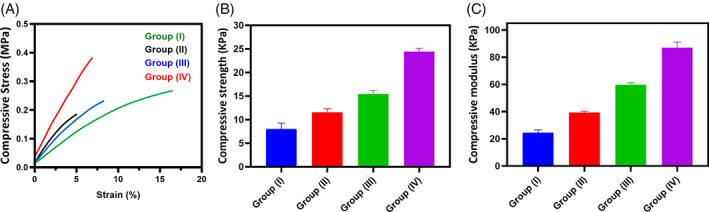
The evaluations of compressive stress‐strain curve (A), compressive strength (D) and compressive modulus (C) of the blended SF/PCS hydrogel groups were exhibited to show mechanical ability and suitability of the scaffolding material
Naturally, the optimised composition of the thermal‐sensitive injectable hydrogel is a main factor to maintain their gelation and injectability properties with physiological temperature, as shown in Figure 5. The results exhibited that increasing concentration of GP into the SF/PCS blended hydrogel has greatly influenced gelation time, elastic modulus (G′) and viscous modulus (G″) behaviours at neutral pH value. The SF/PCS and SF/PCS/GP(i) hydrogel groups exhibited weak elastic modulus (G′) and viscous modulus (G″) when increasing temperature from 25°C to 37°C and juncture of G′ and G″ having a G′ < G″ feature, signifying their viscous ability. The G′ and G″ curves of hydrogel groups of SF/PCS/GP (ii) and SF/PCS/GP (iii) have noted that smaller gap between them when compared with other groups, which confirms that increasing concentration of GP molecules would improve the elasticity of the blended hydrogel group. The data exhibited that incipient gelling temperature (T i ) of the prepared hydrogel groups was differentiated depending on the amount of GP incorporated into the hydrogel. The T i of the SF/PCS/GP group was about 35°C, which exhibits higher than that of other hydrogel groups, demonstrating that the increasing concentrated incorporation of GP have well‐maintained and permitting their gelling temperature to be closer to the physiological temperature. The results presented in Figure 1 is also supported that SF/PCS/GP (ii) and SF/PCS/GP (iii) have reduced gelation time when compared with the SF/PCS/GP (i) hydrogel group because of the influences of higher content of thermosensitive GP molecules. These observed rheological results established that increasing suitable concentration of GP greatly influenced their gelation time and were significantly able to form gel when reaching physiological temperature. 24
FIGURE 5.
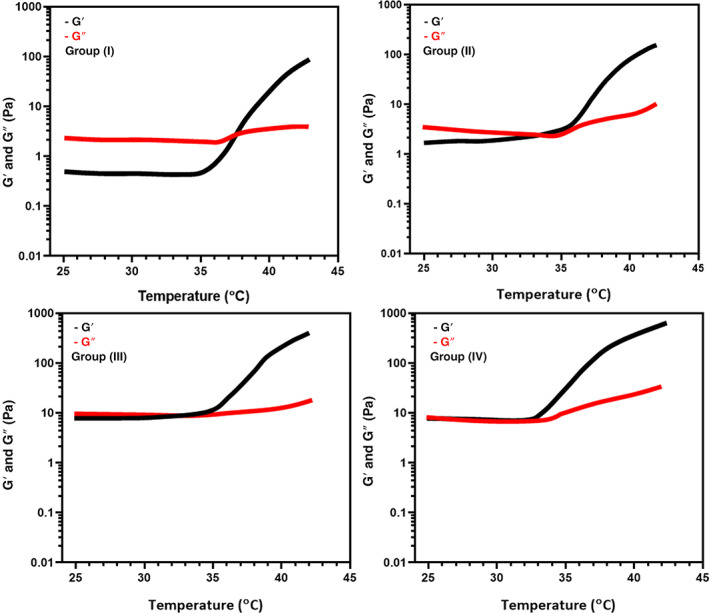
The rheological measurements (storage modulus and loss modulus) of the prepared hydrogel with different blending ratios in different temperatures from 25°C to 45°C
The biodegradation property of the prepared injectable hydrogel groups is very prominent and highly related to the activity in tissue engineering applications. The suitable speed of biodegradation of the implanted scaffolds would favour drug/cell delivery and new tissue formation. In the present study, the degradation ability of the hydrogel groups was investigated by using biological PBS solution in the presence and absence of protease XIV and lysozyme, which may be similar to the physiological conditions of human body fluid, as shown in Figure 6A,B. The SF/PCS scaffold without GP has low biodegradation properties with weight remaining of 85.3% and 90.5% in the PBS medium with and without protease enzyme, respectively, for 8 weeks. Nonetheless, SF/PCS injectable hydrogel groups with GP have significant biodegradation rate of 20.63%, 60.6%, 52.6% and 43.7% for SF/PCS Group I, II, III and IV, respectively, and groups respectively in PBS medium with protease enzyme, which has higher degradation behaviour compared with the PBS medium without protease enzyme. These observations have strongly supported to confirm the incorporation of GP influenced to biodegradation of SF/PCS blended hydrogel groups. In addition, the degradation behaviour of the SF/PCS hydrogel could be tunable by adjusting the concentration of GP based on application purpose. Therefore, the favourable in vitro degradation ability of the injectable hydrogel materials in the PBS with protease enzyme could be prominently used to expect in vivo biodegradability and confirms that the hydrogel material can gradually disappear from the body after affecting its function. 27
FIGURE 6.
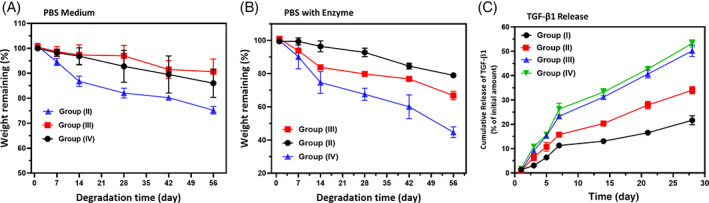
In vitro biodegradation analysis (%) of blended hydrogel groups in PBS medium without (A) and with (B) enzymes (protease XIV and Lysozyme) and (C) examination of TGF‐β1 releasing ability from hydrogel groups
In vitro cytocompatibility is one of the significant biological factors to investigate the suitability of implant material for biomedical purposes. Importantly, implant materials for tissue engineering applications should not show any toxicity to the tissue site normal human cells. As previously reported studies, the preparation of implanted materials using SF and CS components would not show any toxicity and might enhance the cell compatibility with improved cell growth and proliferation. 34 , 43 , 49 In the present study, in vitro cell survival of prepared hydrogel groups was used on two different kinds of cells (BMSCs and L929) using MTT assay for 24 hours of incubation (Figure 7). The presented results confirmed that all hydrogel groups have cell survival rate between 85% and 100% for L929 cells, and BMSCs have ranged between 90% and 100%, demonstrating that incorporation of GP with higher concentration does not provide toxicity to the cells. The cell viability (%) reduction differences between control and treated samples by >30% was represented as a more toxic effect according to ISO 10993‐5 in vitro cytotoxicity standard. Therefore, all the hydrogel groups have no toxicity on BMSCs and L929 cell lines. Additionally, the main sample of implantation TGF‐β1‐Group (IV) was performed on respective cell lines (BMSCs and L929) in different concentrations (0, 50, 100, 150, 200 and 250 μg/mL), exhibited that little toxicity with reasonable cell compatibility with increasing concentrations, indicating that prepared injectable hydrogel group greatly suitable for cell loading and delivery into the defect site for tissue engineering applications. Figure 7A exhibits the respective fluorescence microscopic images of BMSCs cell growth on the different hydrogel surfaces. As presented in images, the BMSCs were proliferated and adhered well onto the hydrogel surfaces and exhibited typical and elongated morphology when increasing incubation days to 14 days. Specifically, TGF‐β1@Group (IV) hydrogel group has greater cell proliferation ability compared with the other hydrogel groups, demonstrating that incorporation of TGF‐β1 would provide essential components for cell proliferation and differentiation. Biocompatibility is very important for prepared hydrogel scaffolds, which are fabricated for cellular delivery and bioengineered tissue regeneration applications. The improved cell compatibility properties of the blended SF/PCS hydrogel groups might be mainly because of the high hydrophilic ability and existence of RGD sequences of silk fibroin polymeric groups. In addition, large number of cationic sites presented CS molecules blended to SF network would influence the cell adhesion and proliferation on hydrogel groups because of the favourable electrostatic interactions between negatively charged groups of cell and positively charged amino groups of CS polymer. As previously reported, SF/CS blended hydrogel has been demonstrated for the greater cell survival ability and uniform cell distributions because of the directional flow of media that replaces nutrients and supports cell proliferation that resulted in existing surface of the scaffold. The cell compatibility results of the present investigation demonstrated that blended hydrogel groups have enhanced cell survival proliferation of BMSCs, which may be because of the amended hydrophilicity of the blended hydrogel samples. In addition, previous reports established that blended hydrogels have played outstanding therapeutic potential in cartilage tissue engineering applications through the introduction of stem cells and their polymeric components with exceeding swollen network, allowing required nutrients and biological factors to cell growth. The fabricated SF/CS blended hydrogel groups have extraordinary cell compatibility and chemistry versatility to facilitate BMSCs cell adhesion, proliferation and matrix production.
FIGURE 7.

In vitro qualitative (A) and quantitative (B and C) evaluations of cell compatibility of hydrogel groups on BMSCs and L929 fibroblast cell lines incubated for 24 hours; (A) Fluorescence microscopic observation of BMSCs and L929 proliferations after 24 hours incubation with different hydrogel groups, (Scale bar = 20 μm) (B) quantitative observation of cell survival rate (%) treated with different hydrogel groups under MTT assay and (C) cell survival rate (%) treated with increasing concentrations of TGF‐β1@SF/PCS hydrogel after 24 hours incubation time
The prepared SF/PCS blended hydrogels with GP are capable of indorsing and preserving the matrix deposition and cell phenotype in vitro and in vivo after implantation. The presentations of TGF‐β1 and BMSCs into the hydrogel greatly prompted the up‐regulation of cartilage‐specific gene expressions that have been established in previously reported works. 34 , 50 The gene expression levels of BMSCs loaded with hydrogels were seeded in a chondrogenic differentiation medium, and gene expression levels were examined at different post‐encapsulation time periods (14 and 28 days) under qRT‐PCR method (Figure 8). The results exhibited that the developed hydrogel groups have significant up‐regulation of cartilage‐specific genes, including aggrecan (AGG), collagen type II (COL II) and SOX‐9. The SOX‐9 transcription factor is a well‐known early stage chondrogenic marker, and its expression level was significantly increased for TGF‐β1@Group (IV) and BMSCs/TGF‐β1@ Group (IV) when compared with the chondrogenic model (control). As previous reports, SOX‐9 is a vital transcription factor, which has been greatly influenced in the mechanism of chondrogenesis and also involved in condensation of mesenchymal cells and inhibition of cartilage hypertrophy. Additionally, increased expressions of SOX‐9 would be activation to different genes of chondrocytes proliferation, including COL II, COL IX, COL XI and AGG. 50 , 51 The expression levels of COL II and AGG were also increased suggestively, demonstrating the considerable gene‐related matrix production and effective cartilage‐specific ECM deposition. Importantly, increasing inflammation is a general complication for articular cartilage defect patients. Hence, producing lower expressions of inflammation‐related genes (IL‐1β and IL‐6) could make possibilities to abridge in vivo cartilage degradation because increasing expressions of IL‐6 would play a key role in cartilage catabolism. The results exhibited that the expression levels of inflammation‐related factors (IL‐1β and IL‐6) were considerably down‐regulated when compared with the control group after 14 and 28 days of incubation. Overall, observed results established that BMSCs embedded TGF‐β1@Group (IV) hydrogel has up‐regulated functions in cartilage‐related genes and down‐regulated activity in inflammation‐based genes, which confirmed that prepared hydrogel can be greatly suitable for the cartilage‐regeneration treatment.
FIGURE 8.

In vitro quantitative investigations of gene expression levels of different blended concentrations of hydrogel groups under RT‐qPCR method; The chondrogenesis gene expression level of SOX 9, Aggrecan and COL II (cartilage‐specific genes), IL‐1β and IL‐6 (inflammatory genes) were examined at 2 and 4 weeks of post‐encapsulation
The therapeutic potential of the injectable hydrogels scaffold in cartilage regeneration was investigated by the injection of partial‐thickness cartilage defect on knees of SD rat models. The treated animal models did not express any complications such as inflammation and rejection responses during postoperative periods. Additionally, the incised skin sites were healed rapidly, and all models endured in healthy condition. Then, we examined the treated models to show cartilage‐regeneration capability of the injectable hydrogel under microscopic histological observations and histological score assessments. The qualitative and quantitative assessments of treated cartilage defects were measured by histological observations and markings of regeneration percentages, respectively, which was supportive of demonstrating repair efficiency of implant substitutes as shown in Figures 9, 10, 11. The H&E staining (Figure 9) and MTS staining (Figure 10) observations of BMSCs loaded TGF‐β1@Group (IV) hydrogel group exhibited freshly formed cartilage cells and significantly distributed on the defect sites when compared with the control group at 6 weeks of postoperative period. The defect groups of developed hydrogel group with and without BMSCs could display some chondrocyte cells. Additionally, it is interesting to observe that BMSCs loaded TGF‐β1@ Group (IV) hydrogel group effectively regrows cartilage layer on the defect site and as well exhibited repaired hyaline cartilage with the subchondral bone. Consequently, the control group had limited repair ability, and controlled fibrocartilage cells appeared after 6 weeks of postoperative period. Furthermore, it is worth observing that the BMSCs embedded TGF‐β1@ Group (IV) hydrogel group provide an effective regeneration of subchondral bone, chondrocytes and superior junctions between natural cartilage and newly formed regenerated cartilage at 12 weeks of postoperative period. The histopathological results of H&E and MTS staining confirmed that blended hydrogel group with BMSCs delivery exhibited a suitable cell MSCs carrier and provided successful regeneration ability to cartilage defect repair.
FIGURE 9.
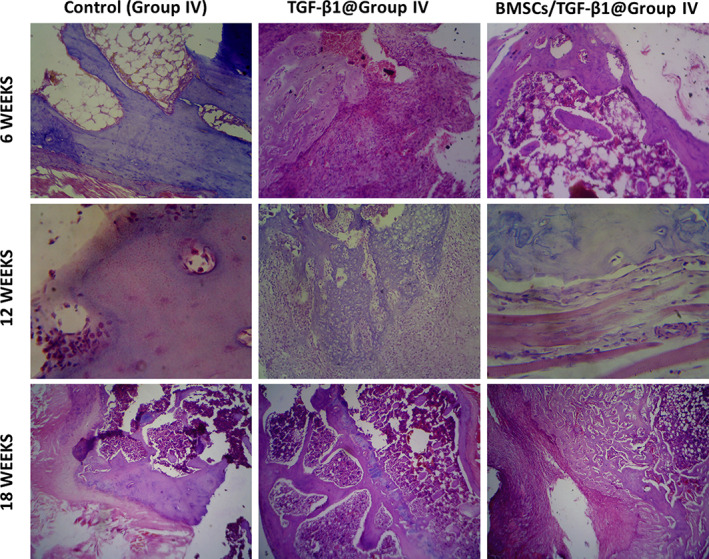
Histological (H&E staining) observation of in vivo cartilage defect treated with BMSCs and TGF‐β1 embedded SF/PCS hydrogel groups at 6, 12 and 18 weeks of postoperative period; Magnification = ×40
FIGURE 10.

Histological (MTS) observation of in vivo cartilage defect treated with BMSCs and TGF‐β1 embedded SF/PCS hydrogel groups at 6, 12 and 18 weeks of postoperative period; Magnification = ×40
FIGURE 11.
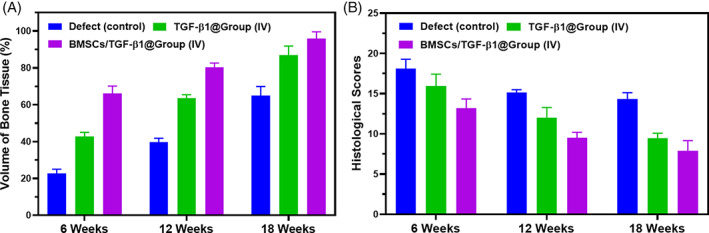
Quantitative data analysis of in vivo analyses; (A) volume of freshly formed bone tissue and (B) histopathological score for regenerated tissue of different treatment groups at 6, 12 and 18 weeks of postoperative period; Magnification = ×40
In addition, the volume of newly formed cartilage and subchondral bone were quantitatively analysed, as shown in Figure 11. The percentage of newly formed cartilage of TGF‐β1@ Group (IV) and BMSCs loaded TGF‐β1@ Group (IV) hydrogel groups were 45.34% and 54.34 at 6 weeks post‐implantation period. The bone volume percentages of these treated TGF‐β1@ Group (IV) hydrogel groups with and without BMSCs were promisingly progressed to 97.87% and 80.89% after 12 weeks of post‐implantation, which regenerated the maximum area of the defect site compared with the control group (Figure 11A). The measurement of the histological score was examined by different features including surface regularity, cell morphology and matrix staining, integration of donor with host cartilage tissue, defect filling and reconstruction of subchondral bone. The histological score value of BMSCs loaded TGF‐β1@Group (IV) hydrogel group was decreased when increasing postoperative time, which demonstrates developed hydrogel groups have superior histological score and favourable performance than the control group as exhibited in Figure 11B. Furthermore, there are no statistical differences between TGF‐β1@ Group (IV) and BMSCs loaded TGF‐β1@ Group (IV) groups. Andrew M.A et al., reported that human ASC‐encapsulated CS/SF blended hydrogel acts as cytoprosthetic hybrid and has effective stem cells differentiation, which provides support to cutaneous wound healing applications. Jingjing wu et al, demonstrated that hydroxyapatite‐loaded CS/SF injectable hydrogel has therapeutic potential with injectability, improved mechanical characteristics and degradation tolerance. Nandana et al., blended SF/CS hydrogel, which has strong in vitro chondrogenesis support of MSCs and greater cell attachment, proliferation and differentiation, reported enhanced cartilage repair. Parinita et al, reported that human MSCs encapsulated CS/SF with glucosamine was successfully established for cartilage construct with suitable hMSCs differentiation to chondrocytes of cartilage‐regeneration treatment. The observed results demonstrated that the hydrogel scaffolding materials with and without BMSCs have played a prominent role in articular cartilage defect repair. Additionally, the developed hydrogel structure may improve survival, and effective carrier of the BMSCs could greatly influence the chondrogenic differentiation. Meanwhile, the hydrogel was suitably degraded along with cell delivery to the defect site and favourably accepted by the body microenvironment, and a new cartilage formed instead of the hydrogel.
4. CONCLUSION
In summary, we established the suitability of SF blended polylysine‐chitosan injectable hydrogel scaffold using glycerophosphate as a thermal‐sensitive agent for effective stem cell carrier in the articular cartilage‐regeneration treatment. This novel approach of biopolymeric injectable hydrogel scaffold might be valuable to provide therapeutic potential in cartilage defect repair and regeneration of cartilage layer through minimally invasive way. The blended hydrogel scaffolds with different blending ratios have prominent advantages, including well‐organised porous structure, smooth morphology, enhanced rheological and mechanical properties and suitable biodegradability behaviours. BMSCs cultured on the injectable hydrogel could effectively proliferate cells into their favourable microenvironment and also provide survival ability of the cells. Then, the regeneration ability of the TGF‐β1@ Group (IV) hydrogel loaded with BMSCs was examined using histological observation of partial‐thickness cartilage defect in an SD rat model. Overall, the results demonstrated that the blended hydrogel with TGF‐β1 and BMSCs could be a promising substrate for articular cartilage repair.
CONFLICT OF INTEREST
There are no conflicts of interest for the present study.
ACKNOWLEDGEMENT
This study was funded by the Changzhou Innovation Team Introduction Project (XK201603 and XK201805), China.
Zheng D, Chen T, Han L, et al. Synergetic integrations of bone marrow stem cells and transforming growth factor‐β1 loaded chitosan nanoparticles blended silk fibroin injectable hydrogel to enhance repair and regeneration potential in articular cartilage tissue. Int Wound J. 2022;19(5):1023-1038. doi: 10.1111/iwj.13699
Dong Zheng, Tong chen, and Long Han contributed equally to this work.
Funding information Changzhou Innovation Team Introduction Project, Grant/Award Numbers: XK201805, XK201603
Contributor Information
Yuji Wang, Email: 13775221377@139.com.
Nanwei Xu, Email: xunanwei1963@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D‐engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011;32:8927‐8937. 10.1016/j.biomaterials.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen W, Li C, Peng M, Xie B, Zhang L, Tang X. Autologous nasal chondrocytes delivered by injectable hydrogel for in vivo articular cartilage regeneration. Cell Tissue Bank. 2018;19:35‐46. 10.1007/s10561-017-9649-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan LP, Oliveira JM, Oliveira AL, Caridade SG, Mano JF, Reis RL. Macro/microporous silk fibroin scaffolds with potential for articular cartilage and meniscus tissue engineering applications. Acta Biomater. 2012;8:289‐301. 10.1016/j.actbio.2011.09.037 [DOI] [PubMed] [Google Scholar]
- 4. Cipriani F, Krüger M, De Torre IG, et al. Cartilage regeneration in Preannealed silk elastin‐like co‐Recombinamers injectable hydrogel embedded with mature chondrocytes in an ex vivo culture platform. Biomacromolecules. 2018;19:4333‐4347. 10.1021/acs.biomac.8b01211 [DOI] [PubMed] [Google Scholar]
- 5. Bhardwaj N, Nguyen QT, Chen AC, Kaplan DL, Sah RL, Kundu SC. Potential of 3‐D tissue constructs engineered from bovine chondrocytes/silk fibroin‐chitosan for in vitro cartilage tissue engineering. Biomaterials. 2011;32:5773‐5781. 10.1016/j.biomaterials.2011.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pourjavadi A, Doroudian M, Ahadpour A, Azari S. Injectable chitosan/κ‐carrageenan hydrogel designed with Au nanoparticles: a conductive scaffold for tissue engineering demands. Int J Biol Macromol. 2019;126:310‐317. 10.1016/j.ijbiomac.2018.11.256 [DOI] [PubMed] [Google Scholar]
- 7. Dong R, Zhao X, Guo B, Ma PX. Self‐healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl Mater Interfaces. 2016;8:17138‐17150. 10.1021/acsami.6b04911 [DOI] [PubMed] [Google Scholar]
- 8. Huang J, Jiang X. Injectable and degradable pH‐responsive hydrogels via spontaneous amino‐yne click reaction. ACS Appl Mater Interfaces. 2018;10(1):361‐370. 10.1021/acsami.7b18141 [DOI] [PubMed] [Google Scholar]
- 9. Liu X, Yang Y, Li Y, et al. Integration of stem cell‐derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430‐4438. 10.1039/c7nr00352h [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Allardyce BJ, Rajkhowa R, et al. 3D printing of silk particle‐reinforced chitosan hydrogel structures and their properties. ACS Biomater Sci Eng. 2018;4:3036‐3046. 10.1021/acsbiomaterials.8b00804 [DOI] [PubMed] [Google Scholar]
- 11. Chouhan D, Lohe TU, Samudrala PK, Mandal BB. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds. Adv Healthc Mater. 2018;7:1‐15. 10.1002/adhm.201801092 [DOI] [PubMed] [Google Scholar]
- 12. Gong Z, Yang Y, Ren Q, Chen X, Shao Z. Injectable thixotropic hydrogel comprising regenerated silk fibroin and hydroxypropylcellulose. Soft Matter. 2012;8:2875‐2883. 10.1039/c2sm06984a [DOI] [Google Scholar]
- 13. Lee EJ, Kang E, Kang SW, Huh KM. Thermo‐irreversible glycol chitosan/hyaluronic acid blend hydrogel for injectable tissue engineering. Carbohydr Polym. 2020;244:116432. 10.1016/j.carbpol.2020.116432 [DOI] [PubMed] [Google Scholar]
- 14. Hu J, Chen B, Guo F, et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J Mater Sci Mater Med. 2012;23:711‐722. 10.1007/s10856-011-4533-y [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Cao J, Han S, et al. ECM based injectable thermo‐sensitive hydrogel on the recovery of injured cartilage induced by osteoarthritis. Artif Cells Nanomed Biotechnol. 2018;46:152‐160. 10.1080/21691401.2018.1452752 [DOI] [PubMed] [Google Scholar]
- 16. Wang Q, Chen D. Synthesis and characterization of a chitosan based nanocomposite injectable hydrogel. Carbohydr Polym. 2016;136:1228‐1237. 10.1016/j.carbpol.2015.10.040 [DOI] [PubMed] [Google Scholar]
- 17. Hong H, Seo YB, Kim DY, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. 10.1016/j.biomaterials.2019.119679 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Hsu YH, Huang APH, Hsu SH. Semi‐interpenetrating polymer network of Hyaluronan and chitosan self‐healing hydrogels for central nervous system repair. ACS Appl Mater Interfaces. 2020;12:40108‐40120. 10.1021/acsami.0c11433 [DOI] [PubMed] [Google Scholar]
- 19. Tsai CC, Kuo SH, Lu TY, Cheng NC, Shie MY, Yu J. Enzyme‐cross‐linked gelatin hydrogel enriched with an articular cartilage extracellular matrix and human adipose‐derived stem cells for hyaline cartilage regeneration of rabbits. ACS Biomater Sci Eng. 2020;6:5110‐5119. 10.1021/acsbiomaterials.9b01756 [DOI] [PubMed] [Google Scholar]
- 20. Grabska‐Zielińska S, Sionkowska A, Carvalho Â, Monteiro FJ. Biomaterials with potential use in bone tissue regeneration‐collagen/chitosan/silk fibroin scaffolds cross‐linked by EDC/NHS. Materials. 2021;14:1‐21. 10.3390/ma14051105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ribeiro VP, da Silva Morais A, Maia FR, et al. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018;72:167‐181. 10.1016/j.actbio.2018.03.047 [DOI] [PubMed] [Google Scholar]
- 22. Chen W, Xu Y, Li H, et al. Tanshinone IIA delivery silk fibroin scaffolds significantly enhance articular cartilage defect repairing via promoting cartilage regeneration. ACS Appl Mater Interfaces. 2020;12:21470‐21480. 10.1021/acsami.0c03822 [DOI] [PubMed] [Google Scholar]
- 23. Luo J, Zhang H, Zhu J, et al. 3‐D mineralized silk fibroin/polycaprolactone composite scaffold modified with polyglutamate conjugated with BMP‐2 peptide for bone tissue engineering. Colloids Surfaces B Biointerfaces. 2018;163:369‐378. 10.1016/j.colsurfb.2017.12.043 [DOI] [PubMed] [Google Scholar]
- 24. Xu Y, Li Y, Chen Q, Fu L, Tao L, Wei Y. Injectable and self‐healing chitosan hydrogel based on imine bonds: design and therapeutic applications. Int J Mol Sci. 2018;19:2198. 10.3390/ijms19082198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang S, Qian Z, Liu D, Wen N, Xu J, Guo X. Integration of C‐type natriuretic peptide gene‐modified bone marrow mesenchymal stem cells with chitosan/silk fibroin scaffolds as a promising strategy for articular cartilage regeneration. Cell Tissue Bank. 2019;20:209‐220. 10.1007/s10561-019-09760-z [DOI] [PubMed] [Google Scholar]
- 26. Ma X, Wu G, Dai F, et al. Chitosan/polydopamine layer by layer self‐assembled silk fibroin nanofibers for biomedical applications. Carbohydr Polym. 2021;251:117058. 10.1016/j.carbpol.2020.117058 [DOI] [PubMed] [Google Scholar]
- 27. Ngoenkam J, Faikrua A, Yasothornsrikul S, Viyoch J. Potential of an injectable chitosan/starch/β‐glycerol phosphate hydrogel for sustaining normal chondrocyte function. Int J Pharm. 2010;391:115‐124. 10.1016/j.ijpharm.2010.02.028 [DOI] [PubMed] [Google Scholar]
- 28. Cho J, Heuzey MC, Bégin A, Carreau PJ. Physical gelation of chitosan in the presence of β‐glycerophosphate: the effect of temperature. Biomacromolecules. 2005;6:3267‐3275. 10.1021/bm050313s [DOI] [PubMed] [Google Scholar]
- 29. Crompton KE, Goud JD, Bellamkonda RV, et al. Polylysine‐functionalised thermoresponsive chitosan hydrogel for neural tissue engineering. Biomaterials. 2007;28:441‐449. 10.1016/j.biomaterials.2006.08.044 [DOI] [PubMed] [Google Scholar]
- 30. Li YN, Ye QQ, Hou WF, Zhang GQ. Development of antibacterial ε‐polylysine/chitosan hybrid films and the effect on citrus. Int J Biol Macromol. 2018;118:2051‐2056. 10.1016/j.ijbiomac.2018.07.074 [DOI] [PubMed] [Google Scholar]
- 31. Wang R, Zhu J, Jiang G, et al. Forward wound closure with regenerated silk fibroin and polylysine‐modified chitosan composite bioadhesives as dressings. ACS Appl Bio Mater. 2020;3(11):7941‐7951. 10.1021/acsabm.0c01064 [DOI] [PubMed] [Google Scholar]
- 32. Agrawal P, Pramanik K. Enhanced chondrogenic differentiation of human mesenchymal stem cells in silk fibroin/chitosan/glycosaminoglycan scaffolds under dynamic culture condition. Differentiation. 2019;110:36‐48. 10.1016/j.diff.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 33. Grabska‐Zielińska S, Sionkowska A, Reczyńska K, Pamuła E. Physico‐chemical characterization and biological tests of collagen/silk fibroin/chitosan scaffolds cross‐linked by dialdehyde starch. Polymers. 2020;12:372. 10.3390/polym12020372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Liu Y, Guo Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF‐β1 and BMP‐2. Arthritis Res Ther. 2021;23:1‐11. 10.1186/s13075-020-02382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elisseeff J, McIntosh W, Fu K, Blunk T, Langer R. Controlled‐release of IGF‐I and TGF‐β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098‐1104. 10.1016/S0736-0266(01)00054-7 [DOI] [PubMed] [Google Scholar]
- 36. Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF‐β1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095‐7103. 10.1016/j.biomaterials.2005.05.083 [DOI] [PubMed] [Google Scholar]
- 37. Wu YY, Jiao YP, Xiao LL, et al. Experimental study on effects of adipose‐derived stem cell‐seeded silk fibroin chitosan film on wound healing of a diabetic rat model. Ann Plast Surg. 2018;80:572‐580. 10.1097/SAP.0000000000001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Legendre F, Ollitrault D, Gomez‐Leduc T, et al. Enhanced chondrogenesis of bone marrow‐derived stem cells by using a combinatory cell therapy strategy with BMP‐2/TGF‐β1, hypoxia, and COL1A1/HtrA1 siRNAs. Sci Rep. 2017;7:3406. 10.1038/s41598-017-03579-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li J, Huang Y, Song J, et al. Cartilage regeneration using arthroscopic flushing fluid‐derived mesenchymal stem cells encapsulated in a one‐step rapid cross‐linked hydrogel. Acta Biomater. 2018;79:202‐215. 10.1016/j.actbio.2018.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun AX, Lin H, Fritch MR, et al. Chondrogenesis of human bone marrow mesenchymal stem cells in 3‐dimensional, photocrosslinked hydrogel constructs: effect of cell seeding density and material stiffness. Acta Biomater. 2017;58:302‐311. 10.1016/j.actbio.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bessa PC, Balmayor ER, Hartinger J, et al. Silk fibroin microparticles as carriers for delivery. Tissue Eng Part C. 2010;16:937‐945. [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Liu J, Shi Y, Wan Y. Rheological, mechanical and degradable properties of injectable chitosan/silk fibroin/hydroxyapatite/glycerophosphate hydrogels. J Mech Behav Biomed Mater. 2016;64:161‐172. 10.1016/j.jmbbm.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 43. Bhardwaj N, Kundu SC. Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends. Biomaterials. 2012;33:2848‐2857. 10.1016/j.biomaterials.2011.12.028 [DOI] [PubMed] [Google Scholar]
- 44. Antich C, de Vicente J, Jiménez G, et al. Bio‐inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020;106:114‐123. 10.1016/j.actbio.2020.01.046 [DOI] [PubMed] [Google Scholar]
- 45. Shen X, Zhang Y, Gu Y, et al. Sequential and sustained release of SDF‐1 and BMP‐2 from silk fibroin‐nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials. 2016;106:205‐216. 10.1016/j.biomaterials.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 46. Li J, Zhou Y, Chen W, et al. A novel 3D in vitro tumor model based on silk fibroin/chitosan scaffolds to mimic the tumor microenvironment. ACS Appl Mater Interfaces. 2018;10:36641‐36651. 10.1021/acsami.8b10679 [DOI] [PubMed] [Google Scholar]
- 47. Li DW, Lei X, He FL, et al. Silk fibroin/chitosan scaffold with tunable properties and low inflammatory response assists the differentiation of bone marrow mesenchymal stem cells. Int J Biol Macromol. 2017;105:584‐597. 10.1016/j.ijbiomac.2017.07.080 [DOI] [PubMed] [Google Scholar]
- 48. Li J, Chen G, Xu X, et al. Advances of injectable hydrogel‐based scaffolds for cartilage regeneration. Regen Biomater. 2019;6:129‐140. 10.1093/rb/rbz022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Altman AM, Yan Y, Matthias N, et al. IFATS collection: human adipose‐derived stem cells seeded on a silk fibroin‐chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27:250‐258. 10.1634/stemcells.2008-0178 [DOI] [PubMed] [Google Scholar]
- 50. Fathi‐Achachelouei M, Keskin D, Bat E, Vrana NE, Tezcaner A. Dual growth factor delivery using PLGA nanoparticles in silk fibroin/PEGDMA hydrogels for articular cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2020;108:2041‐2062. 10.1002/jbm.b.34544 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Yu J, Ren K, Zuo J, Ding J, Chen X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules. 2019;20:1478‐1492. 10.1021/acs.biomac.9b00043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


