Abstract
Wounds continue to be of a global concern. Therefore, a more focussed, evidence‐based approach to wound assessment and management is required. The WOUND COMPASS™ Clinical Support App (CSA) is designed to support the health care professional with wound assessment and management at the point of care. This real‐world pilot study aimed to determine the utility of the CSA during routine wound management, in multiple care settings. A non‐interventional, real‐world pilot programme of the CSA was conducted at four sites. Patients received routine wound management. The CSA was programmed to replicate the site's formulary for evidence‐based wound management. Anonymised pre‐ and post‐pilot clinician opinion surveys on useability and impact of the CSA were collected and reported. Wound Specialists (n = 7 [100%]) and Non‐Wound Specialists (NWS) (n = 58 [82%]) indicated that competence and confidence in wound assessment were enhanced with use of the CSA (100%; 82%). Furthermore, practice variation was reduced because of a greater compliance to their local formulary (n = 7 [100%]; 79% [54%]). This real‐world pilot shows the positive impact of the CSA, and the improvements that can be potentially realised via reduction in practice variation, improvement in NWSs confidence when managing wounds and increased formulary compliance.
Keywords: clinical support app, formulary compliance, practice variation, wound management
1. INTRODUCTION
Wounds and their management are a hugely important health care issue, with estimates suggesting a prevalence of chronic wounds of 1.67 per 1000 population (95% confidence interval: 0.83‐2.80). 1 Furthermore, it has been reported that approximately 2% of all hospitalised patients, globally, have a chronic wound. 2 Guest et al 3 have illuminated the problem of wounds, both acute and chronic, for the UK National Health Service (NHS), identifying in 2012 to 2013 that there were 2.2 million patients living with a wound, equivalent to 4.5% of the adult population at the time. An update of this work in 2017 to 2018 showed that there were an estimated 3.8 million patients with a wound, equivalent to 7% of the adult population, indicating a stark increase of 1.6 million wound patients. 4 A similar rise in wound prevalence has been identified by Yao et al, 2 where among hospitalised individuals the prevalence of chronic wounds rose (57.1%) from 16.8 per 1000 in 2014, to 26.4 per 1000 in 2018.
Of importance is the impact that wounds have on the individual, with many studies focussing specifically on the influence on health‐related quality of life. Olsson et al 5 undertook a systematic review of the literature and identified that individuals with chronic wounds had significantly lower mean scores across all SF‐36 domains (measure of health) when compared to the control groups without wounds. Of significance is that pain and reduced mobility were the main problem areas for patients with wounds, 5 a finding also highlighted by others. For example, Hopkins et al 6 report that patients with pressure ulcers experience endless intractable pain which is frequently exacerbated by equipment and treatments employed to manage the wound. From an acute wound perspective, those with a surgical site infection (SSI) also experience profound physical, psychological, social, spiritual, and economic effects. 7 SSIs have also been found to be an independent predictor of mortality, particularly among the elderly where there is a 4‐fold increased risk of death when compared with their matched counterparts. 8 More generally, those with an SSI are at 2 to 11 times higher risk of death compared with surgical patients without an SSI. 9 Furthermore, 38% to 77% of deaths in those with SSI are directly related to infection. 9 The impact of the COVID‐19 pandemic has also directly impacted wound care, where data are now becoming available, including reports of a 40% decrease in wound centre visits in 2020, compared with the same period in 2019. 10 If patients are unable or not willing to access wound management facilities as a result of the pandemic, ultimately this will cause wound deterioration and increase morbidity and mortality.
Given the substantial and rising prevalence of wounds, it comes as no surprise that management of these wounds consumes a significant proportion of health care expenditure. For example, estimates from one region in Denmark suggest that the total annual costs of treatment, including hospitalisation, were approximately 1.6% to 1.8% of total budget for the hospitals and 1.5% to 2.4% for the municipalities. 11 In Ireland, the total health care cost of wound care for 2017 was estimated at €629 064 198, at an average cost of €3941 per patient, accounting for 5% of total public health expenditure. 12 In the United States, Nussbaum et al, 13 identified that the total Medicare spending for all wound types ranged from $28.1 to $96.8 billion, which included three tiered estimates defined as low‐range, mid‐range and upper‐bound. When infection costs were included, the most expensive were surgical wounds ($11.7, $13.1, and $38.3 billion, respectively), and diabetic foot ulcers ($6.2, $6.9, and $18.7 billion, respectively). The work of Guest et al, 3 provides insight into the impact of non‐healing wounds on the UK NHS, where the annual cost of managing wounds that healed was estimated to be £2.1 billion, contrasting with £3.2 billion for the 39% of wounds that did not heal within the study year. Importantly, the authors therefore conclude that the patient care cost of an unhealed wound was a mean 135% more than that of a healed wound.
Wounds and their associated problems are a substantial and ‘snowballing’ health care concern, significantly impacting negatively on the individual and on scarce health care resources. 5 , 7 The concern is that despite the longevity of our understanding of the pathological processes involved in wound repair, and the large array of treatment modalities available, many wounds remain unhealed, thereby compounding the challenges outlined. 14 The fundamentals of care involve accurate and ongoing patient and wound assessment to identify pertinent information needed to make a correct diagnosis and from there to plan an effective care plan for treatment. 15 However, despite availability of these gold standard principles, Guest et al, 3 identified substantial deviations from agreed standards, with 30% of patients in their study lacking a differential diagnosis. Further, Guest et al, 3 note that assessment of peripheral perfusion is a pre‐requisite for planning leg ulcer and diabetic foot ulcer management. However, in their study just 15% of patients with a leg or foot ulcer had a Doppler ankle brachial pressure index documented in the clinical notes, of which 75% were prescribed some form of compression therapy. Additionally, they report that dressings and bandages were regularly altered at random for most patients, indicating health care practitioner confusion and as a result, a lack of concordance with prescribed treatment plans. 3 These findings are not unique, indeed, work by McCaughan et al, 16 concurs, with patients interviewed in their study expressing dissatisfaction with a perceived lack of continuity and consistency of care in relation to wound management. Therefore, a more focussed approach to the assessment and management of patients with wounds is needed to be sustainably imbedded into clinical practice.
The TIME (Tissue, Infection or Inflammation, Moisture, Edge) principle was a first step in addressing the failure to adopt a systematic approach to wound management, and since its inception, TIME has been widely integrated into research and practice. 15 A limitation of TIME, however, was that it focused primarily on the wound, and although acting as a guide for Wound Bed Preparation (WBP), a more holistic framework was needed. In answering this need, a group of experts developed the TIME Clinical Decision Support Tool (CDST). 15 This model, consisting of A, B, C, D and E, takes health professionals though assessment, use of a Multidisciplinary Team (MDT) and control systemic disorders, to the decision on the treatment plan and an evaluation of outcomes. 15 However, the publication of the TIME CDST alone does not necessarily mean that it will be used in practice, dissemination is fundamental to enhance awareness of its existence. 17
Gordon et al 18 argue that digital technology has the potential to enhance evidence‐based practice, and as such may be a useful addition to strategies for information dissemination. Therefore, a further development of the TIME CDST is the WOUND COMPASS™ Clinical Support App (CSA), a proprietary mobile application, designed, and developed by Smith and Nephew (Hull, UK), with the intention to support the health‐care professional with wound assessment and management. The CSA is not classified as a medical device. As such, the CSA supports wound assessment and guides appropriate selection of wound products from the clinical product formulary (bespoke or generalisable using TIME 15 at the point of care and across multiple settings; acute, community and homecare. The CSA consists of three functions: (a) a digitised adaptation of the TIME wound assessment tool; (b) a product formulary specified by the user's Wound Care Specialist and (c) contextual guidance to support the user along the wound assessment journey. Figure 1 details selected screenshots from the CSA.
FIGURE 1.
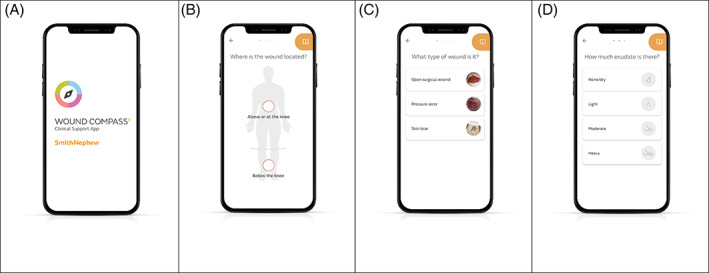
Screenshots from the Clinical Support App (CSA). (A) the opening screen of the CSA. (B) wound location selection screen with option of ‐ above or below the knee. (C) type of wound selection screen. (D) exudate category selection screen
This real‐world pilot study aimed to determine the utility of the CSA by Wound Specialists and Non‐Wound Specialists during routine wound management in multiple care settings, across two countries. Furthermore, the clinician opinion and feedback during use of the CSA will enable the assessment of the impact on wound management practice variation, Non‐Wound Specialists confidence when treating wounds and formulary compliance.
2. METHODS
2.1. Design, setting, and sample
This pilot employed a real‐world observational design, conducted across four centres, three in the United Kingdom and one in the United States, from April 2021 until September 2021. Each centre's wound management treatment formulary was programmed into the CSA prior to the commencement of the pilot, then reviewed and approved by each site's Wound Specialist. The resultant wound assessment output from the CSA was therefore in alignment with standard of care at each site. Patients with skin tears, pressure ulcers/injuries, open surgical wounds, venous leg ulcers, arterial ulcers, and diabetic foot ulcers; receiving treatment and management at the pilot sites were eligible to be managed using the CSA. No patient data were stored in the CSA and the CSA was not linked to each site's Electronic Health Records (EHR). The CSA provided the clinician with an option for an output (PDF) of the wound assessment and treatment recommendations, which could then be uploaded or filed within the patient's medical notes. Ethical committee and Institutional Review Board (IRB) committee approval was not required for this pilot programme, as standard of care was not changed. Each clinical site had the option of running an 8 or 12‐week pilot programme of the CSA.
2.2. Data collection
Before the CSA pilot, each site's participating clinicians were asked to complete a pre‐pilot survey, which captured patient volume, use of wound assessment tools, confidence around wound assessment, accessing the hospital formulary, compliance with the formulary and use of digital technology.
On completion of the pre‐survey, each site was trained on the use of the CSA. The CSA was made available for use on both Apple and Android mobile devices. The participants used their personal phones or their site's own provided mobile devices at the discretion of site. Smith and Nephew, the app designer and developer, did not provide mobile devices for use. On conclusion of the pilot, the Wound Specialists and NWS were asked to complete a second anonymous survey on their experiences of using the CSA.
The surveys were designed by Smith and Nephew, CSA developer, with the objective to collect HCP's opinion and experience of wound assessment, practise variation and formulary compliance prior and post use of the CSA. The survey questions were designed specifically to capture opinion and experience and not designed to measure an outcome, therefore did not undergo validation. Two types of survey were designed: one for the Wound Specialist, defined as the lead nurse for the site who designs and maintains the wound management protocols and formulary, and one for the Non‐Wound Specialists (NWS), defined as the team of nurses who provide routine patient care (including providing wound management). Both the pre and post pilot surveys, across both groups were anonymous. Table 1 details how each question answer option was quantified in terms of a given percentage allocated to the answer option. The surveys were designed to be multiple choice, with HCPs advised to select either a single answer or all options that apply to them. There were also options to provide free text, as part of their answers.
TABLE 1.
HCP opinion survey question and answer options with quantifiable definition using percentages to quantify the response within the context of the question
| Survey term | Definition using percentage to quantify response |
|---|---|
| ‘Very’/‘All of the time’/‘All’ | 100% |
| ‘Sufficiently’/‘Some of the time’/‘Most of the time’/‘Most’ | Greater than 50% |
| ‘Moderately’/‘Rarely’/‘Barely’/‘Some’ | Less than 50% |
| ‘Not at all’/‘Never’ | 0% |
Note: An example question is stated below:
- Very (100%).
- Sufficiently (Greater than 50%).
- Barely (Less than 50%).
- Not at all (0%).
2.3. Data analysis
The surveys were designed and captured using the Snap Surveys™ tool and the data were analysed and reported using SAS 9.4. All results were reported as a multi‐centre aggregate. Categorical variables were summarised with frequencies and percentages. Free text provided in the surveys were grouped into categories and reported.
3. RESULTS
3.1. Pre‐pilot survey
A total of seven Wound Specialists and 116 Non‐Wound Specialists (NWSs) completed the pre‐pilot survey and received training and access to the CSA. Table 2 details the number of participants per site and the length of the pilot phase, and Table 3 details the responses to the pre‐pilot survey.
TABLE 2.
Number of participating clinicians by site at the start of the CSA pilot
| Country | Site name | Treatment setting | Number of Wound Specialists responses (n = 7) | Number of Non‐Wound Specialists responses (n = 116) | Length of pilot to be conducted (weeks) |
|---|---|---|---|---|---|
| UK | North East London Foundation Trust (NELFT) | Community | 1 | 29 | 8 |
| UK | Southern Health NHS Foundation Trust | Community | 1 | 22 | 12 |
| UK | Medway NHS Foundation Trust | Acute | 3 | 45 | 8 |
| US | Brookdale Home Health | Homecare | 2 | 20 | 12 |
TABLE 3.
Pre‐pilot survey; Wound Specialists (n = 7) and NWS (n = 116)
| Survey question | Wound Specialists (n = 7) n (%) | Non‐Wound Specialists (n = 116) n (%) |
|---|---|---|
| In a typical day, approximately, what percentage of your time is utilised in managing patients with wounds? | ||
| 0% to 25% | 2 (29) | 21 (18) |
| 26% to 50% | 0 (0) | 33 (28) |
| 51% to 75% | 0 (0) | 44 (38) |
| 76% to 100% | 5 (71) | 18 (16) |
| In a typical day, approximately, how many patients to do you treat? | ||
| 0 to 5 | 2 (29) | 15 (13) |
| 6 to 10 | 3 (43) | 75 (65) |
| 11 to 14 | 1 (14) | 20 (17) |
| 15 to 19 | 1 (14) | 3 (3) |
| >20 | 0 (0) | 3 (3) |
| In a typical day, approximately how long does a wound management treatment visit/appointment take with a patient? | ||
| Less than 15 minutes | 0 (0) | 7 (6) |
| 15 to 30 minutes | 1 (14) | 53 (46) |
| 30 to 60 minutes | 5 (71) | 50 (44) |
| >60 minutes | 1 (14) | 5 (4) |
| How do you assess a wound? | ||
| Use my facility's clinical decision‐making protocol | 2 (29) | 78 (68) |
| Use TIME assessments | 4 (57) | 30 (26) |
| Other wound assessment tools | 1 (14) | 33 (29) |
| Experience and training | 6 (86) | 92 (80) |
| How do you decide what to do to that wound? | ||
| Use my facility's clinical decision‐making protocol | 3 (43) | 76 (66) |
| Experience and training | 7 (100) | 99 (85) |
| Published literature | 4 (57) | 24 (21) |
| Public online resources (eg, Google) | 0 (0) | 10 (9) |
| How do you select a wound treatment product? | ||
| Follow ordering system | 2 (29) | 39 (34) |
| Consult wound specialist | Not Applicable | 74 (64) |
| Find the closest product to hand/available | 0 (0) | 15 (13) |
| Experience and training | 2 (29) | 88 (77) |
| Do you use apps/digital management systems currently for wound management? | ||
| Yes | 2 (29) | 11 (10) |
| No | 5 (71) | 105 (91) |
| Are you provided with devices, such as mobile phones/tablets or do you use your own? | ||
| Issued mobile phone device | 3 (43) | 65 (58) |
| Issued tablet device | 4 (57) | 47 (42) |
| Use my own mobile phone device or tablet device | 0 (0) | 15 (13) |
| How well do you adopt/embrace technology? | ||
| Very well | 4 (57) | 67 (58) |
| On occasions where it helps me | 3 (43) | 38 (33) |
| When I have too | 0 (0) | 9 (8) |
| Badly – will avoid | 0 (0) | 2 (2) |
3.1.1. Wound assessment
Wound Specialists
Of the Wound Specialists surveyed, 71% (n = 5) spent from 76% to 100% of their time managing patients with wounds, and 43% (n = 3) reported seeing 6 to 10 wound patients per day, with 71% (n = 5) reporting an approximate visit/appointment time of 30 to 60 minutes. In terms of how they completed wound assessments, the most selected options were ‘experience and training’ (86%, n = 6) and ‘use TIME assessment’ (57%, n = 4). Participants were asked how wound treatment was decided, all reported using ‘experience and training’ (100%, n = 7), in addition to either ‘published literature’ (57%, n = 4) or the ‘use of facility's clinical decision‐making protocol’ (43%, n = 3).
The selection of wound treatment products was ‘by experience and training’ for all participants (100%, n = 7), with two also identifying that they used their facility's ordering system (29%, n = 2). The use of apps and digital management systems for wound management was limited, with five (71%) participants reporting no use, however, all advised that their teams were provided with either a mobile phone device (43%, n = 3), or a tablet device (57%, n = 4). Finally, in terms of how well they adopted/embraced use of technology, all reported either ‘Very Well’ (57%, n = 4) or ‘On occasion where it helps me’ (43%, n = 3).
Non‐Wound Specialists (NWSs)
For the NWS's, 28% (n = 33), and 38% (n = 44), spent 26% to 50% and 51% to 75% of their time managing patients with wounds, respectively. Further, 65% (n = 75) reported seeing 6 to 10 wound patients per day, with 46% (n = 53) and 44% (n = 50) reporting an approximate visit/appointment time of 15 to 30 minutes and 30 to 60 minutes, respectively. In terms of how the NWSs conducted wound assessments, 80% (n = 92) reported ‘experience and training’, and 68% (n = 78) reported ‘use of my facilities pre‐determined wound assessment’.
Most of the NWS respondents reported that wound treatment was decided upon using ‘experience and training’ (85%, n = 99), or use of ‘facility's clinical decision‐making protocol’ (66%, n = 76), with a smaller percentage reporting use of ‘published literature’ (21%, n = 24), and ‘public online resources’ (9%, n = 10). Regarding how they select treatments for patients wounds, most reported using ‘experience and training’ (77%, n = 88), as well as ‘consulting their Wound Specialist’ (64%, n = 74). In addition, 13% (n = 15) reported that the selection of products for wound treatment would be based on ‘ease of access’.
Overall, NWS did not (91%, n = 105) report use of apps or digital management systems for wound management. A total of 58% (n = 65) were issued with a mobile device, while 42% (n = 47) were issued a tablet device. In addition, 13% (n = 15) also reported using their own mobile phone, or tablet device, for other elements of their daily work. Finally, in terms of adoption/embracement of technology, most responded ‘very well’ (58%, n = 67) or ‘on occasion where it helps me’ (33%, n = 83). However, 8% (n = 9) and 2% (n = 2) reported that they would adopt/embrace technology ‘when I have to’ or they would ‘avoid it’, respectively.
3.1.2. Practice variation
Wound Specialists and Non‐Wound Specialists (NWSs)
Almost all the NWSs (95%, n = 108) reported that they conducted a wound assessment at ‘each dressing change’. To assess the issues faced in wound assessment, both groups were asked what they found challenging. Apart from the identification of the signs and symptoms of biofilm, specialists consistently reported all other elements, such as identification of the aetiology and wound type, wound location, tissue type, signs and symptoms of infection, exudate management and wound depth as being more challenging than that reported by the NWSs (Figure 2).
FIGURE 2.
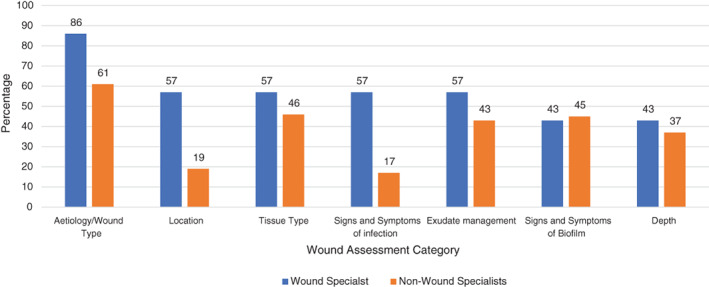
Categories of wound assessment found most challenging* in pre‐pilot survey by the Wound Specialists (n = 7) and Non‐Wound Specialists (n = 116). *Wound Specialists were asked the following question in relation to how challenging their team found wound assessment: ‘Which parts of the assessment are most challenging for your team?’
The two groups were also asked to give their opinions on how confident they felt conducting wound assessment and selecting the correct wound management product. The wound specialists were asked in the context of the teams they manage, with five (71%) identifying they were ‘moderately confident’ in their teams conducting wound assessments. For product selection, they were ‘very’ (29%, n = 2) and ‘moderately’ (71%, n = 5) confident in their teams. When asked if they were requested to give assistance to their teams, the responses were ‘some of the time’ (57%, n = 4) and ‘rarely’ (43%, n = 3).
For the NWSs, the most common responses to how confident they felt in conducting wound assessment were, ‘very’ (23%, n = 27) and ‘sufficiently’ (62%, n = 72), with one NWS indicating that they were ‘not at all’ confident. Regarding their confidence in selection of wound care products, the responses were as follows: ‘very’ (18%, n = 21), ‘sufficiently’ (60%, n = 69), ‘moderately’ (22%, n = 25) and ‘not at all’ (1%, n = 1). When asked if they requested support from their wound specialist, the responses were ‘some of the time’ (47%, n = 54) and ‘rarely’ (43%, n = 49), with 9% (n = 10) reporting that they requested wound specialist support ‘all of the time’.
3.1.3. Formulary compliance
Wound Specialists
As the Wound Specialists at each site are responsible for establishing the wound product formulary, they were asked if their teams adhered to their site's formulary. The responses were as follows: ‘very’ (43%, n = 3), ‘sufficiently’ (29%, n = 2) and ‘moderately’ (29%, n = 2). The ability to check the adherence to the local formulary was identified as being possible for 86% (n = 6) of the Wound Specialists and they made corrections on wound product choices by their teams ‘some of the time’ (71%, n = 5) or ‘rarely’ (43%, n = 2).
3.2. Post Pilot Survey
3.2.1. Use of the CSA
As part of the pilot programme, application analytics were available to determine the volume of usage and length of time a wound assessment required to be completed in the CSA; over the pilot period there were 443 assessments logged using the CSA and the mean time to perform a wound assessment was 88 seconds.
At the conclusion of the pilot phase, each of the participating clinicians were again asked to complete a survey, this time exploring the impact the CSA had on their confidence in wound assessment, their wound management practice and formulary compliance. Table 4 details the number of Wound Specialists and NWSs who responded. Because of employee sick leave, clinician re‐deployment as a result the COVID‐19 pandemic and clinicians leaving the healthcare service, there was a 32% reduction (n = 33) in the participating NWSs, in the post pilot phase, however, all 7 Wound Specialists responded. Table 5 outlines the participants responses.
TABLE 4.
Number of participating clinicians, by site, at the end of the pilot
| Country | Site name | Treatment setting | Number of Wound Specialists responses (n = 7) | Number of Non‐Wound Specialists responses (n = 71) | Length of pilot completed (weeks) |
|---|---|---|---|---|---|
| UK | North East London Foundation Trust (NELFT) | Community | 1 | 13 | 8 |
| UK | Southern Health NHS Foundation Trust | Community | 1 | 19 | 12 |
| UK | Medway NHS Foundation Trust | Acute | 3 | 28 | 8 |
| US | Brookdale Home Health | Homecare | 2 | 11 | 12 |
TABLE 5.
Post‐pilot survey; Wound Specialists (n = 7) and Non‐Wound Specialists (n = 71)
| Survey question | Wound Specialists (n = 7); n (%) | Non‐wound specialist (n = 71); n (%) |
|---|---|---|
| Have you been using the CSA? | ||
| Yes | 7 (100) | 71 (100) |
| No | 0 (0) | 0 (0) |
| Have you enjoyed using the CSA? | ||
| Yes | 7 (100) | 63 (89) |
| No | 0 (0) | 8 (11) |
| Would you recommend the CSA? | ||
| Yes | 7 (100) | 63 (90) |
| No | 0 (0) | 7 (10) |
| How disappointed would you be if we took it away? | ||
| Very | 4 (57) | 21 (30) |
| Moderately | 3 (43) | 34 (48) |
| Barely | 0 (0) | 10 (14) |
| Not at all | 0 (0) | 6 (9) |
| Did it make your team's/your wound assessments easier/better? | ||
| Yes | 6 (86) | 59 (83) |
| No | 1 (14) | 12 (17) |
| On what proportion of wounds assessments did you use the CSA? | ||
| All | 3 (43) | 14 (20) |
| Most | 2 (29) | 25 (35) |
| Some | 1 (14) | 32 (45) |
| Not at all | 1 (14) | 0 (0) |
| Over time do you think your facility/service will use the CSA more, less or the same? | ||
| More than during the pilot | 6 (86) | 30 (42) |
| Same as during the pilot | 1 (14) | 34 (48) |
| Less than during the pilot | 0 (0) | 7 (10) |
| How has your team's/your confidence to perform a wound assessment changed? | ||
| Increased | 4 (57) | 41 (59) |
| Stayed the same | 3 (43) | 29 (41) |
| Decreased | 0 (0) | 0 (0) |
| How has your team's/your confidence to select the right product changed? | ||
| Increased | 4 (57) | 48 (68) |
| Stayed the same | 3 (43) | 23 (32) |
| Decreased | 0 (0) | 0 (0) |
| Do you believe the CSA helped to improve your team's/your competence and confidence when managing wounds? | ||
| Yes | 7 (100) | 58 (82) |
| No | 0 (0) | 13 (18) |
| Do you think your teams/you are more compliance to pathway and formulary guidance through using the CSA? | ||
| Yes | 7 (100) | 54 (79) |
| No | (0) | 14 (21) |
| Did you/your teams store the outputs of the assessments in the patients' records? | ||
| All of the time | 0 (0) | 12 (17) |
| Most of the time | 2 (29) | 21 (30) |
| Rarely | 14 (1) | 12 (17) |
| Never | 4 (57) | 12 (17) |
| Do you/your teams believe you needed more information about selected products such as application guides? | ||
| Yes | 3 (43) | 32 (45) |
| No | 4 (57) | 39 (55) |
Wound Specialists
All the respondents used the CSA during the pilot phase (100%, n = 7) and 43% (n = 3) and 29% (n = 2) reported that ‘All’ or ‘Most’ of assessments were carried out using the CSA, respectively. Furthermore, all indicated that they enjoyed having the app within their facility/service (100%, n = 7), and would recommend the use of the CSA (100%, n = 7). In terms of responses to the question ‘how disappointed would you be if the CSA was removed from their facility/service’, 57%, (n = 4) indicated they would be ‘very disappointed’ with 43%, (n = 3) indicating they would be ‘sufficiently disappointed’. In addition, 67%, (n = 4) indicated their teams would be ‘very disappointed’, and 33% (n = 2), would be ‘sufficiently disappointed’ if the CSA was removed from their facility/service. A total of 86% (n = 6) Wound Specialists agreed that the CSA made wound assessment easier/better.
Fifty seven percent (n = 4) reported that their confidence in their team's ability to perform wound assessment had increased, with 43% (n = 3) reporting their confidence ‘stayed the same’. Similarly, 57% (n = 4) reported their confidence in their team's ability to select the right wound product had increased, with 43% (n = 3) reporting that it ‘stayed the same’ (43%, n = 3). Twenty‐nine percent (n = 2) reported a decrease in the number of times they were requested to provide specialist assistance in wound assessments, with 71% (n = 5) reporting that the number of requests stayed the same. The Wound Specialists were asked their opinion if a change in the number of requests from their teams for assistance was ‘good’, ‘bad’ or ‘indifferent’; 43% (n = 3) indicated that this change was ‘good’, while the remaining respondents, 57% (n = 4) answered ‘indifferent’, suggesting a lack of opinion on the number of times their teams requested assistance with wound assessment. However, anonymised free text feedback from the Wound Specialist's was provided in response to this question: ‘it means they (NWS) felt more confident with their selections’ and ‘they (NWS) consulted me less often because they had increase confidence in the assessment’.
Analysis of the free text feedback given was conducted to ascertain the impact the CSA had on their teams and on wound care delivery. Figure 3 details the themes of the feedback given, quantified into categories. All (100%, n = 7) reported the CSA helped improve their teams' competence and confidence when managing wounds, and that there was greater compliance with the local formulary. The number of corrections to wound assessments did not change during the CSA pilot for 86% (n = 6) of the Wound Specialists. However, one reported a decrease, illustrated by the following free text: ‘those using the CSA did not need further consultations/clarifications’. One of the wound specialists provided feedback regarding the positive impact of introducing technology such as CSA, noting that; ‘new technology that will aid staff in a visual manner will always be positive’.
FIGURE 3.
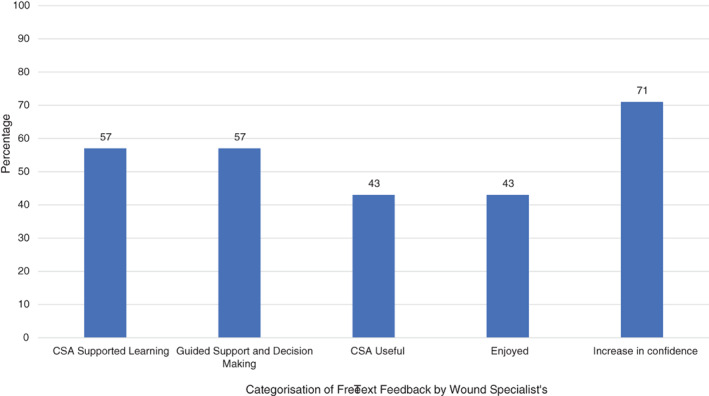
Post‐pilot categorisation of free‐text feedback by Wound Specialists (n = 7) on use of the CSA
Non‐Wound Specialists (NWSs)
Of the NWSs surveyed, 89% (n = 63) enjoyed having the CSA within their facility/service and would recommend the app. Eighty three percent (n = 58) reported that the CSA was easy to use and agreed the CSA made wound assessments easier/better. When asked their response to if the CSA was to be removed from their facility/service, 43% (n = 34) indicated they would be ‘sufficiently disappointed’ and 30% (n = 21) indicated they would be ‘very disappointed’. In terms of how often they used the CSA for wound assessments; 20% (n = 14) indicated ‘all’, 35% (n = 25) ‘most’ and 45% (n = 32) responded ‘some’. The NWS were asked to give further information related to this question, with the most common themes to explain the variability in use being access to a mobile device, clinician confident in wound assessment without need for supporting tool, busy workload and forgot about use of the app.
Further analysis of the free text feedback given by the NWSs was conducted to ascertain the impact the CSA had on wound care delivery during the pilot. Figure 4 details the themes of the feedback, quantified into categories. Fifty nine percent (n = 41) indicated that their confidence in their ability to perform wound assessment had increased, with the remaining participants reporting their confidence ‘stayed the same’. Regarding the selection of wound care products, 68% (n = 48) reported that their confidence had increased, with the remaining participants reporting their confidence ‘stayed the same’.
FIGURE 4.
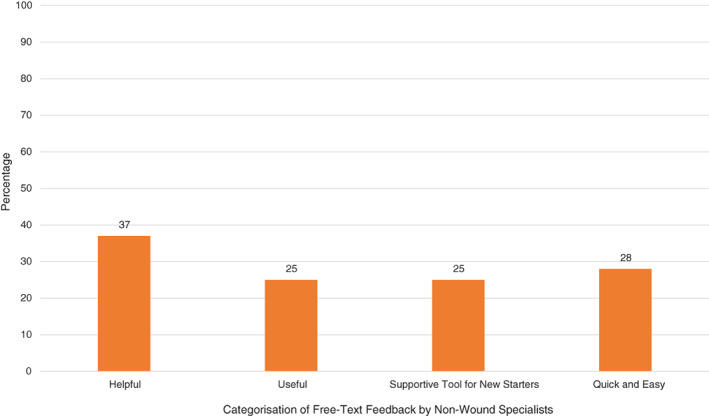
Post‐pilot categorisation of free‐text feedback by Non‐Wound Specialists (n = 71) on use of the CSA
Overall, 82% (n = 58) reported the CSA helped improve their competence and confidence when managing wounds, with the remaining participants indicating that their competence and confidence remained the same. When asked about the impact the CSA had on local pathway and formulary guidance compliance, 79% (n = 54) reported that the CSA helped them in being more compliant, with 60% (n = 42) reporting that their wound specialist had ‘rarely’ or ‘never’ had to make product choice changes.
4. DISCUSSION
4.1. Current practice and challenges
Most of the Wound Specialists reported treating between 6 and 10 patients a day, which mirrored what was reported by the NWSs. Only six NWSs reported seeing between 15 to 19 (n = 3, 3%) and >20 patients a day (n = 3, 3%), compared with one Wound Specialist who reported treating 15 to 19 patients and zero reporting treating >20 patients per day. For the NWS, the time spent caring for patients with wounds varied more widely than the Wound Specialists, with most spending between 26% and 75% of their time, whereas most of the Wound Specialists reported spending 76% to 100% of their time caring for patients with wounds.
Given these figures, it is interesting to reflect on the areas of wound assessment identified as being challenging for both groups. For example, 86% of the Wound Specialists reported that their teams found assessment of the aetiology and wound type challenging, compared with 61% of the NWS. Further, 57% of the Wound Specialist reported their teams found identifying the signs and symptoms of wound infection challenging, compared with 17% of the NWS. McCluskey and McCarthy 19 found a statistically significantly positive association between the numbers of wounds treated per week by the participants in their study, and self‐assessed competence. Thus, this may explain the differences between the opinion‐based reporting by Wound Specialists and the NWS in this reported real‐world pilot. However, Dutton et al, 20 identified that Wound Specialists spend much of their time caring for patients with complex needs, therefore, it is not surprising that the Wound Specialists in this current pilot found many elements of wound assessment more challenging for their teams, as they are often the escalation point for complex cases. Furthermore, McCluskey and McCarthy 19 also identified that education in wound care was associated with increased perception of self‐assessed competence. Education and training enhance reflection skills and thus self‐awareness, 21 therefore the specialist who is dealing with more complex patients, may be more reflective of current practice and thus more insightful as to the elements that are found to be challenging for clinicians.
4.2. Confidence in wound management
Following use of the CSA, participants were asked to identify if they felt that their own or their team's competence and confidence in wound management and formulary compliance had improved. All the Wound Specialists and more than 70% of the NWS indicated that competence and confidence (100%, n = 7; 82%, n = 58 respectively) were enhanced; along with greater compliance to the local formulary (100%, n = 7; 79%, n = 54 respectively). This is an important finding, as at its essence this was a key aim of the CSA.
Dugdall and Watson 22 stress that care delivered to patients is very much influenced by the individual characteristic of the nurse, with appropriate knowledge for practice being essential. As such, the authors argue that more specific training in wound management should lead to enhanced care delivery to the patient. However, given the ever‐changing demographic profile of users of the health services, with more critically ill and complex patients needing care, there is a corresponding challenge in ensuring that nurses are equipped with the most up‐to‐date information they need to practice effectively. 23 None the less, access to high‐ quality guidance is essential for the practicing clinician, as this provides reassurance pertaining to best practice in this clinical field. 17
This is where the CSA can have a significant impact, in that it standardises the approach to assessment and product usage and is bespoke to each facility/department/service. The impact of standardised wound management on practice variation and non‐wound specialist confidence has been demonstrated through earlier work using the TIME CDST. 24 Further to this having an app, such as CSA, at point of care to support clinicians as the wound is assessed in real time is ‘helpful’, ‘useful’, ‘quick and easy to use’, could reduce the time spent, changing the focus to quality of time over quantity of time on assessment. Ultimately, with the goal of improving clinical, patient and economic outcomes.
4.3. Use of the CSA
All of the participating Wound Specialists used the CSA, were happy with it, would recommend its use to others and would be ‘very’ or ‘moderately’ disappointed if they no longer had access to it. Concurrently, most of the NWS expressed similar feedback: ‘very disappointed’ 30%, n = 21; ‘moderately disappointed’ 48%, n = 34; to the Wound Specialists, however there were a proportion who felt differently: ‘barely disappointed’ 14%, n = 10; ‘not at all disappointed’ 9%, n = 6. Further elaboration by these participants provided some insights into the reasons for this, namely, lack of access to a mobile device, busy workload or they forgot about using the app.
These findings are not unique to this current pilot, indeed, a national survey in Australia by Eley et al, 25 identified similar trends. In their survey of 10 000 nurses, the top‐cited barriers to the adoption of technology in practice were work demands, access to computers and lack of support. Further work by Cho et al (2021) 26 also provides some potential explanations for the findings in the current pilot of CSA. The authors identified that understanding the factors that result in resistant behaviours towards technology is of great importance, and a statistically significant influencing variable is ‘resistance to change’. To ensure fuller adoption of the technology into practice, due consideration needs to be given to identifying the perceived factors that may impede or enhance the introduction among all staff. 27 This is an area for future research of the CSA given that the current data shows the positive impact of the CSA, and the improvements that can be realised via reduction in practice variation, improvement in Non‐Wound Specialist (NWS) confidence and increase in formulary compliance. Further research options, including the use of quality improvement methodology to show the impact of the CSA at the patient level through improved clinical and economic outcomes, as well as the useability of the app to ultimately help clinicians manage workload, can be considered. The wider picture of adherence to formulary is that it may reduce cost, however this pilot was not designed to capture an economic outcome. None the less, the need to capture health economic outcomes to show treatment and product cost effectiveness is critically important and future work on the CSA should not only incorporate clinical outcomes but economic as well.
5. CONCLUSION
This real‐world pilot shows the positive impact of the CSA, and the improvements that can be potentially realised via reduction in practice variation, improvement in clinician confidence when managing wounds and increasing compliance to product formulary. The impact of the CSA on clinical and economic outcomes was not a component of this pilot, however, future research should explore the impact.
6. LIMITATIONS
This was an opinion‐based survey, so respondents may not necessarily want to report that they find areas of wound assessment challenging.
The complexity of the cases the teams are seeing will vary, as well as current pressure with the pandemic may mean resources are limited.
The 32% loss in NWS from start of pilot to completion has reduced the dataset and sample population for the post‐ pilot survey. Thus, there will have been CSA users that will not have been able to feedback on their experiences of the app – positively or negatively.
Clinical and economic outcomes was not a component of this real‐world pilot, however, future research should explore this impact to ascertain the impact when using the CSA in routine wound management.
CONFLICT OF INTEREST
No funding (monetary or wound care medical devices) was provided by Smith and Nephew for the conduct of this real‐world pilot programme. The Health care professional authors, at each pilot site, received a consultancy fee for their time in participating in the development and writing of this manuscript.
ACKNOWLEDGEMENTS
The authors thank all their teams for participating in this real‐world pilot. The authors thank Mr Tim Styche and Miss Jennifer Gale at Smith and Nephew for managing the Snap Surveys™ portal, which enabled the collection of the survey responses and producing the data reports.
Moore ZEH, Aynge GE, Carr CG, et al. A Clinical Support App for routine wound management: reducing practice variation, improving clinician confidence and increasing formulary compliance. Int Wound J. 2022;19(5):1263‐1275. doi: 10.1111/iwj.13868
Funding information Smith and Nephew
DATA AVAILABILITY STATEMENT
Author elects to not share data
REFERENCES
- 1. Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta‐analysis of observational studies. Ann Epidemiol. 2019;29:8‐15. doi: 10.1016/j.annepidem.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 2. Yao Z, Niu J, Cheng B. Prevalence of chronic skin wounds and their risk factors in an inpatient hospital setting in Northern China. Adv Skin Wound Care. 2020;33(9):1‐10. doi: 10.1097/01.ASW.0000694164.34068.82 [DOI] [PubMed] [Google Scholar]
- 3. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that different wound types impose on the UK's National Health Service. Int Wound J. 2017;14(2):322‐330. doi: 10.1111/iwj.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK's National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10:e045253. doi: 10.1136/bmjopen-2020-045253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114‐125. doi: 10.1111/wrr.12683 [DOI] [PubMed] [Google Scholar]
- 6. Hopkins A, Dealey C, Bale S, Defloor T, Worboys F. Patient stories of living with a pressure ulcer. J Adv Nurs. 2006;56:345‐353. doi: 10.1111/j.1365-2648.2006.04007.x [DOI] [PubMed] [Google Scholar]
- 7. Avsar P, Patton D, Ousey K, Blackburn J, O'Connor T, Moore Z. The impact of surgical site infection on health‐related quality of life: a systematic review. Wound Manag Prev. 2021;67(6):10‐19. [PubMed] [Google Scholar]
- 8. Anderson DJ, Podgorny K, Berríos‐Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605‐627. doi: 10.1086/676022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaye KS, Anderson DJ, Sloane R, et al. The effect of surgical site infection on older operative patients. J Am Geriatr Soc. 2009;57(1):46‐54. doi: 10.1111/j.1532-5415.2008.02053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rogers LC, Armstrong DG, Capotorto J, et al. Wound centre without walls: the new model of providing care during the COVID‐19 pandemic. Wounds. 2020;32(7):178‐185. [PMC free article] [PubMed] [Google Scholar]
- 11. Gottrup F, Henneberg E, Trangbæk R, Bækmark N, Zøllner K, Sørensen J. Point prevalence of wounds and cost impact in the acute and community setting in Denmark. J Wound Care. 2013;22(8):413‐414, 416, 418–22. doi: 10.12968/jowc.2013.22.8.413 [DOI] [PubMed] [Google Scholar]
- 12. Gillespie P, Carter L, McIntosh C, Gethin G. Estimating the health‐care costs of wound care in Ireland. J Wound Care. 2019;28(6):324‐330. doi: 10.12968/jowc.2019.28.6.324 [DOI] [PubMed] [Google Scholar]
- 13. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. doi: 10.1016/j.jval.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 14. Ferris AE, Harding KG. Are chronic wounds a feature of frailty? Br J Gen Pract. 2020;70(694):256‐257. doi: 10.3399/bjgp20X709829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore Z, Dowsett C, Smith G, et al. TIME CDST: an updated tool to address the current challenges in wound care. J Wound Care. 2019;28(3):154‐161. doi: 10.12968/jowc.2019.28.3.154 [DOI] [PubMed] [Google Scholar]
- 16. McCaughan D, Sheard L, Cullum N, Dumville J, Chetter I. Patients' perceptions and experiences of living with a surgical wound healing by secondary intention: A qualitative study. Int J Nurs Stud. 2018;77:29‐38. doi: 10.1016/j.ijnurstu.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grimshaw JM, Schunemann HJ, Burgers J et al. Disseminating and Implementing Guidelines: Article 13 in Integrating and Coordinating Efforts in COPD Guideline Development. An official ATS/ERS workshop. 2012. [DOI] [PubMed]
- 18. Gordon WJ, Landman A, Zhang H, Bates DW. Beyond validation: getting health apps into clinical practice. Npj Digit Med. 2020;3:14. doi: 10.1038/s41746-019-0212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCluskey P, McCarthy G. Nurses' knowledge and competence in wound management. Wounds. 2012;8(2):37‐47. [Google Scholar]
- 20. Dutton M, Chiarella M, Curtis K. The role of the wound care nurse: an integrative review. Br J Community Nurs. 2014;19(Supplement 3):S39‐S40, S42‐7. doi: 10.12968/bjcn.2014.19.sup3.s39 [DOI] [PubMed] [Google Scholar]
- 21. Bulman C, Lathlean J, Gobbi M. The concept of reflection in nursing: qualitative findings on student and teacher perspectives. Nurse Educ Today. 2012;32(5):e8‐e13. doi: 10.1016/j.nedt.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 22. Dugdall H, Watson R. What is the relationship between nurses' attitude to evidence‐based practice and the selection of wound care procedures? J Clin Nurs. 2009;18:1442‐1450. [DOI] [PubMed] [Google Scholar]
- 23. Fawaz M, Hamdan‐Mansour A. Tass Ai, challenges facing nursing education in the advanced healthcare environment. Int J Africa Nurs Sci. 2018;9:105‐110. doi: 10.1016/j.ijans.2018.10.005 [DOI] [Google Scholar]
- 24. Woo K. Using the new T.I.M.E. clinical decision support tool to promote consistent holistic wound management and eliminate variation in practice: part 3 at the west part healthcare Centre, chronic care and rehabilitation hospital. CANA. 2019;10(3):48‐55. [Google Scholar]
- 25. Eley R, Fallon T, Soar J, Buikstra E, Hegney D. Barriers to use of information and computer technology by Australia's nurses: a national survey. J Clin Nurs. 2009;18(8):1151‐1158. doi: 10.1111/j.1365-2702.2008.02336.x [DOI] [PubMed] [Google Scholar]
- 26. Cho Y, Kim M, Choi M. Factors associated with nurses' user resistance to chance of electronic health records systems. BMC Med Inform Decis Mak. 2021;21(1):218. doi: 10.1186/s12911-021-01581-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Veer AJ, Fleuren MA, Bekkema N, Francke AL. Successful implementation of new technologies in nursing care: a questionnaire survey of nurse‐users. BMC Med Inform Decis Mak. 2011;11:67. doi: 10.1186/1472-6947-11-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Author elects to not share data


