Abstract
Mechanical force plays a pivotal role in the pathogenesis of hypertrophic scar (HTS). Dermal fibroblasts and myofibroblasts are the key cells involved in HTS. Myofibroblasts in HTS possess different biochemical and biophysical characteristics by which myofibroblasts are often distinguished from fibroblasts. The role of mechanotransducers outside the nucleus in the pathogenesis of HTS has been reported in many studies. However, the role of Nesprin‐2 in HTS is not clear. Hence, we aim to construct a cell model of HTS and explore the role of Nesprin‐2 in this process. Myofibroblasts and fibroblasts were isolated from HTS and healthy skin tissues of the same patient. Fibroblasts were exposed to cyclic stretch with 10% magnitude and a frequency of 0.1 Hz for 3 days, 5 days, and 7 days, respectively. After the cell model was confirmed, fibroblasts transfected with siRNA targeting human Nesprin‐2 were exposed to cyclic stretch. The mechanical behaviour and biochemical reaction of the dermal fibroblasts were analysed. The stretched fibroblasts at day 5 showed the same mechanotransductive and biochemical features as unstretched myofibroblasts. Mechanical strain could induce the myofibroblasts differentiation and a cell model of HTS was established successfully at day 5. The expressions of lamin A/C, alpha‐smooth muscle actin, transforming growth factor beta 1, and collagen type I in fibroblasts were reduced by the silencing of Nesprin‐2. Mechanical strain could induce the myofibroblasts differentiation and silencing of Nesprin‐2 could block the mechanical stimulation of terminal myofibroblasts differentiation. Nesprin‐2 might be a potential target to treat the HTS.
Keywords: cyclic stretch, differentiation, hypertrophic scar, myofibroblasts, Nesprin
1. INTRODUCTION
Even with ongoing research in the field of abnormal wound healing and scarring, the pathogenesis underlying the formation of hypertrophic scar (HTS) remains largely unknown. It is well known to plastic surgeons that wounds with high tension always heal with HTS. Mechanical strain is deemed to play an important role in the process of HTS formation, although the explicit molecular mechanisms are still not clear enough. 1 , 2 Dermal fibroblasts and myofibroblasts are considered to be the pivotal cells involved in HTS. 3 , 4 Myofibroblasts in HTS have some specific characteristics like high expressions of alpha‐smooth muscle actin (a‐SMA), type I collagens, and transforming growth factor (TGF), by which myofibroblasts are often distinguished from normal skin fibroblasts.
The family of integrins are transmembrane glycoprotein receptors that can mediate cell‐matrix or cell‐cell communications. 5 Integrins can sense the change of mechanical forces outside of the cells and induce the organisation of cytoskeleton, such as focal adhesion kinase (FAK), p130cas, and actin filaments. Thus, in turn, the mechanical signal can be transferred into internal cellular structures and the nucleus. During the process of mechanical signal transmission through the nuclear envelope, the linker of nucleoskeleton and cytoskeleton (LINC) complex is the pivotal messenger. 6 The LINC complex contains two protein domains: the conserved C‐terminal KASH (Klarsicht/ANC‐1/Syne Homology) domain and SUN (Sad1p, UNC‐84) domain. The proteins of C‐terminal KASH, spanning the outer nucleus membrane, are considered to be Nesprins (nuclear envelope spectrin‐repeat proteins). 7 , 8
Nesprins, a family of nuclear membrane proteins, are composed of four members including Nesprin‐1G, ‐2G, ‐3, and ‐4. 9 Nesprin‐2, the predominant protein expressed in skin, contains two domains: two N‐terminal calponin homology domains and C‐terminal KASH domain. Nesprins interact with the cytoskeleton components in the cytoplasm via its N‐terminal domains. Its C‐terminal KASH domain also spans the perinuclear space and interacts with SUN 1/2 proteins anchored to the inner nuclear membrane. 10 Lamin A and C (lamin A/C) are intermediate filament proteins that form a meshwork underneath the inner nuclear membrane. 11 Nesprins, SUN proteins, and lamin A/C interact with each other, which can maintain nuclear architecture, regulate the organisation of chromosomes, transcription, cell cycle progression, and nuclear migration. 12 , 13
Compared with their wild‐type counterparts, the Nesprin‐2 knockout mice show delayed wound healing, although they are viable and healthy. 14 Because of abnormal focal adhesion and actin localisation, fibroblasts in the Nesprin‐2 knockout mice generate fewer mechanical forces compared with wild‐type fibroblasts, which brings forward an explanation for the delay in wound healing. 15 On the other hand, the decreased tension induced by Nesprin‐2 knockout might affect the formation and progression of HTS.
To the best of our knowledge, no evidence is reported that the formation of HTS is related to Nesprin‐2 in humans. In the present study, we constructed a cell model of HTS induced by cyclic stretch and found that silencing of Nesprin‐2 with small interfering RNA (siRNA) delayed the differentiation of myofibroblasts.
2. MATERIALS AND METHODS
2.1. Isolation and expansion of fibroblasts and myofibroblasts
Healthy skin tissues and paired HTS tissues were obtained from three patients who underwent operations to remove HTS tissues and repair the defects with full‐thickness skin graft in the Department of Burn and Plastic Surgery, the Affiliated Hospital of Qingdao University. The data of patients are shown in Table 1. Every biopsy was about 5 mm in diameter. It was easy to obtain the tissue biopsy because the size of the skin graft was always bigger than that of the defect. After the purpose and procedure of this study were informed, all patients signed the written informed consent. All the protocols were approved by the institutional review boards of the Affiliated Hospital of Qingdao University, Qingdao, China.
TABLE 1.
The data of patients providing the hypertrophic scar (HTS) and healthy skin tissues
| Patients | Gender | Age (years) | Location of HTS tissues | Location of healthy skin tissues (skin graft) | Size of a biopsy |
|---|---|---|---|---|---|
| No. 1 | male | 27 | right elbow | Right groin | 5 mm in diameter |
| No. 2 | female | 37 | right wrist | Inferior abdominal wall | |
| No. 3 | female | 18 | Face | Left upper arm |
Myofibroblasts and fibroblasts were isolated from HTS and healthy skin tissues with an explant outgrowth culture system, 16 respectively. After the primary cells were harvested, they were sub‐cultured and expanded in α‐minimum essential medium (Corning, USA) with 10% fetal bovine serum (Gibco, USA) at 37°C and 5% CO2. The fifth‐ to eighth‐generation cells were used for all the following experiments.
2.2. Establishment of myofibroblast model by cyclic stretch and siRNA transfection
All stretch experiments were performed using Flexcell Tension Plus system equipped with 25‐mm BioFlex Loading Station (FX‐4000 T, Flex‐cell International Incorporation, USA). Briefly, cells were seeded onto six‐well flexible‐bottomed culture plates at a density of 3 × 104 cells/cm2 and incubated overnight in α‐MEM medium containing 10% fetal bovine serum at 37°C under 5% CO2. When the cells were 70% to 90% confluent, the medium was replenished completely. Then, the six‐well culture plates were transferred onto a multi‐channel tension‐loading bioreactor and exposed to cyclic stretch with 10% magnitude and a frequency of 0.1 Hz for 3 days, 5 days, 7 days, respectively. At every given time, the cells were harvested for the follow‐up analysis.
After the cell model of HTS was confirmed, fibroblasts transfected with siRNA targeting human Nesprin‐2 were exposed to cyclic stretch to detect the role of Nesprin‐2 in the mechanotransduction process.
Each experiment was performed in triplicate.
2.3. Cell proliferation assay
At each time point after cyclic stretch, the cells in six‐well culture plates were washed twice with phosphate‐buffered saline (PBS) (Solarbio, China). Then, 200 μL of CCK‐8 assay reagents from the Cell Counting Kit‐8 (Dojindo Molecular Technologies, Japan) were added and incubated for additional 3 hours. After the supernatant from six‐well culture plates was collected, the optical density (OD) at a wavelength of 450 nm was obtained with an absorbance microplate reader (Tecan Trading AG, Swiss).
2.4. Immunofluorescence assay
Immunofluorescence analysis was carried out at days 3, 5, and 7. Firstly, the culture medium was removed, the cells were washed thrice with PBS and fixed with 4% paraformaldehyde for 15 minutes. Secondly, the cells were permeabilised using 0.3% Triton X‐100 for 10 minutes. After being washed thrice with PBS again, the cells were locked in 5% bovine serum albumin (BSA) for 1 hour. Thirdly, the cells were incubated with rabbit anti‐human α‐SMA primary antibodies (# ab5694, Abcam, UK) overnight at 4°C. Then the cells were washed thrice with PBS and incubated with goat anti‐rabbit fluorescent secondary antibodies (# A23220, Abbkine, USA). In addition, nuclei were stained using DAPI (Solarbio, China). At last, images were obtained under a fluorescence microscope (Olympus, Japan).
2.5. Fluorescent real‐time polymerase chain reaction (RT‐PCR)
At any given time, total RNA was extracted from cells using TRIzol reagent (Ambion, USA), according to manufacturer's recommendations. After being treated with Dnase (Santa Cruz, USA), 1 μg of extracted RNA from each sample was transcribed into cDNA with Oligo‐dT primers using an avian myeloblastosis virus (AMV) RNA polymerase chain reaction (PCR) Kit (TaKaRa, China). Then, fluorescent quantitative (FQ)‐PCR assay was performed on an ABI‐7500 quantitative PCR System (Life Technologies, USA).
All primers that we used for amplifying target genes are shown in Table 2(designed and synthesised by Shanghai Sangon Biotech). Human glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as an endogenous reference gene. During expression analysis, the levels of mRNA expression were reported as relative values (ΔΔCT), and each sample was amplified in triplicate.
TABLE 2.
List of primer pairs used for quantitative reverse transcription PCR
| Gene Sequences (5′‐3′) | ||
|---|---|---|
| Integrin β1 | F: ACAATGGAGAGTGCGTCTGC | R: CGTTGCTGGCTTCACAAGTA |
| a‐SMA | F: CAATGTCCCTGCCATGTACGTC | R: GGCAGGGCATAGCCTTCATAGA |
| Nesprin‐2 | F: CAGTCCTTACAACTCCTGGACAC | R: GACTGATTCTCCTACCCACAGAC |
| Lamin A/C | F: AGCCCCCAGAACTGCAGCATCATGTAA | R: TTACATGATGCTGCAGTTCTGGGGGCT |
| TGF β1 | F: ATTCCTGGCGATACCTCAG | R: AAGGCGAAAGCCCTCAAT |
| COL1A1 | F: CCTGGAAAGAATGGAGATGATG | R: ATCCAAACCACTGAAACCTCTG |
| GAPDH | F: TCACCATCTTCCAGGAGCGA | R: CACAATGCCGAAGTGGTCGT |
Abbreviations: a‐SMA, alpha‐smooth muscle actin; COL1A1, collagen type I, α 1 chain; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; TGFβ1, transforming growth factor beta 1.
2.6. Protein contents in culture supernatant
At each time point, the conditioned supernatant from the individual culture was collected, and protein contents assay for TGF β1, collagen type I was carried out with commercial enzyme‐linked immunosorbent assay (ELISA) kits (R&D Systems, USA), according to the manufacturer's instruction.
2.7. Western blot analysis with cells lysate
At any given time after loading cyclic stretch, cultures of fibroblasts were harvested and lysed with cell lysis buffer containing l mmol/L phenylmethyl sulfonylfluoride (PMSF). After being resolved by standard sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS‐PAGE), cell lysates were electroblotted onto a polyvinylidene fluoride (PVDF) (Millipore, USA) membrane. Then, the membrane was incubated with different primary antibodies including rabbit anti‐human α‐smooth muscle actin primary antibodies (# ab5694, Abcam, UK), rabbit anti‐human integrin β1 (# ab52971, Abcam, UK), rabbit anti‐human lamin A/C (# ab108595, Abcam, UK), and rabbit anti‐human Nesprin‐2 (# ab217057, Abcam, UK). Next, an anti‐rabbit horseradish peroxidase‐conjugated secondary antibody (# CW01035, CWbiotech, China) was added. Following the supplier's instructions, chemiluminescent detection of proteins was performed with enhanced chemiluminescence (ECL) detection kit (GE Healthcare Amersham, China). The relative intensities of immunoreactive protein bands were quantified by scanning densitometry using ImageJ program inspired by NIH Image at Bethesda, Maryland.
2.8. Statistical analysis
IBM SPSS v23 statistical software was used to analyse all the data, and the results were expressed as means ± standard deviation. A two‐tailed Student's t test was used to compare the difference between the two groups and one‐way ANOVA analysis of variance was performed across the groups. At last, P values less than 0.05 were considered significant.
3. RESULTS
3.1. Cyclic stretch induced differentiation of myofibroblasts from fibroblasts
3.1.1. Cell proliferation
After being exposed to cyclic stretch with 10% magnitude for 3 days, 5 days, and 7 days, respectively, cultured fibroblasts presented a significant improvement of the proliferation ability in a strain time‐dependent manner. The peak effect appeared in cells at day 7. The proliferation ability of stretched fibroblasts (SF) at day 5 was significantly higher than that of SF at day 3 and that of unstretched fibroblasts (USF) at day 5 (all P < 0.05, n = 3). There were no differences for the proliferation ability between SF and unstretched myofibroblasts (USM) at day 5 (P > 0.05, n = 3) (Figure 1A).
FIGURE 1.
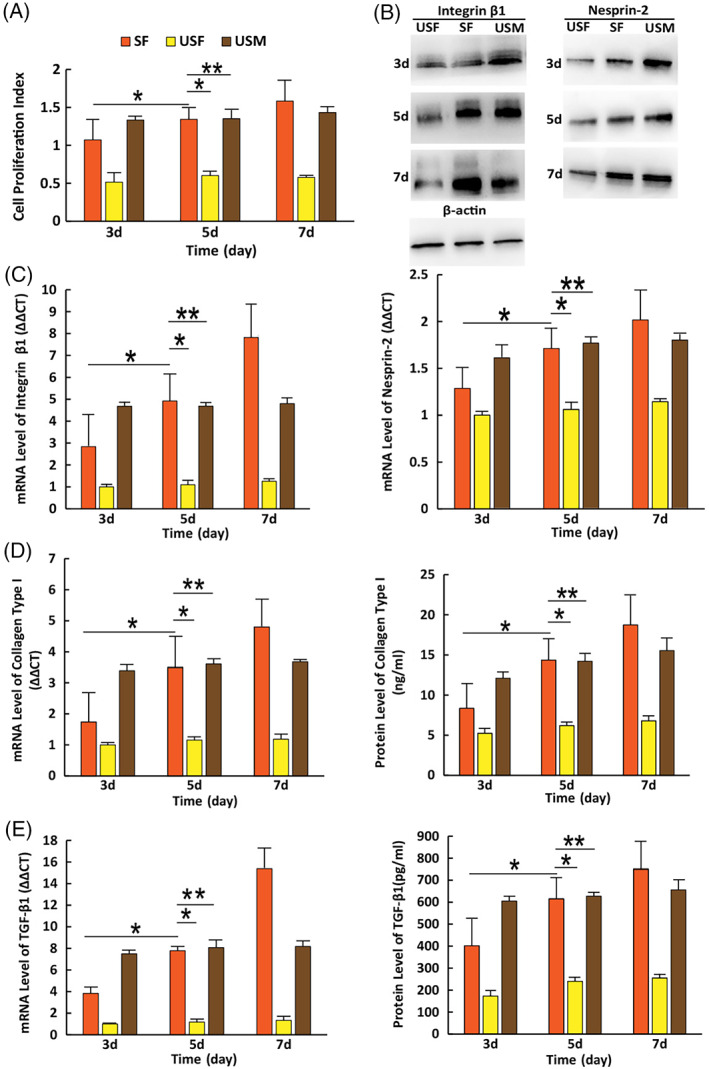
Reactions of cells to cyclic stretch at different time. (A) Cell proliferation ability. (B) The protein levels of integrin β1 and Nesprin‐2 detected by western blotting. (C) The mRNA expression levels of integrin β1 and Nesprin‐2 detected by RT‐PCR. (D) The mRNA expression and protein levels of collagen type I detected by RT‐PCR and ELISA. (E) The mRNA expression and protein levels of TGF‐β1 detected by RT‐PCR and ELISA. (i) SF: Stretched Fibroblasts; (ii) USF: unstretched fibroblasts; (iii) USM: unstretched myofibroblasts. Error bar: ±SD (n = 3); *P < 0.05; **P > 0.05
3.1.2. The expression of integrin β1, α‐SMA, and Nesprin‐2 of cells
The expression levels of integrin β1, α‐SMA, and Nesprin‐2 monitored by mRNA and protein productions increased in a strain time‐dependent manner in all stretched cells. The SF at day 7 produced the highest levels of mRNA expression for integrin β1, α‐SMA, and Nesprin‐2 among all the groups. Their expression levels in the SF at day 5 were as high as those in the USM (P > 0.05, n = 3) (Figure 1C), (Figure 2B).
FIGURE 2.
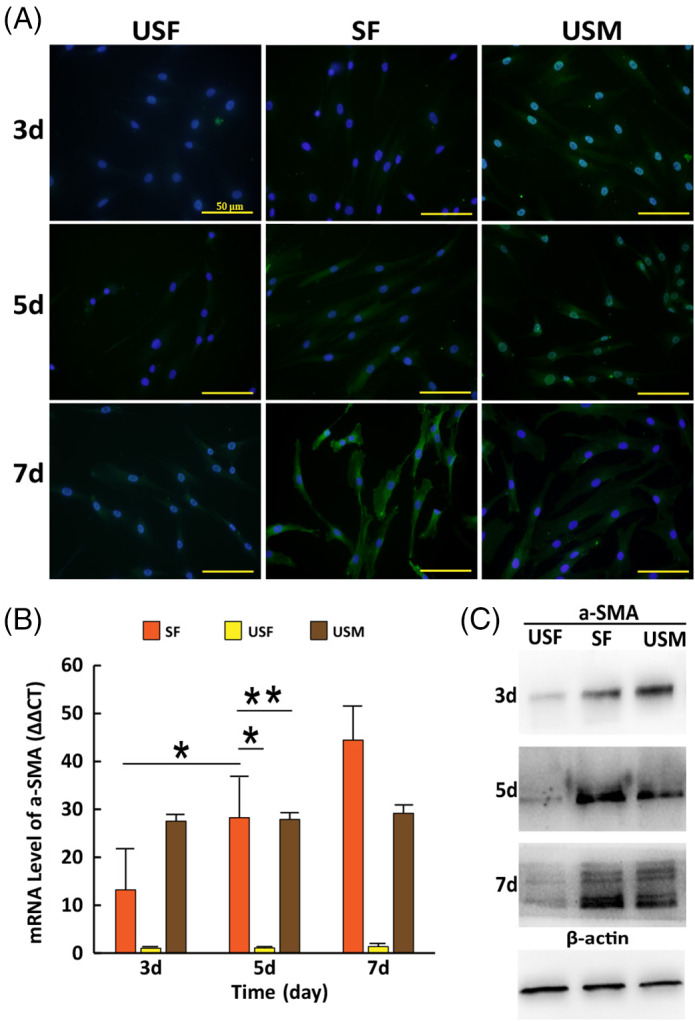
Detection of α‐SMA expression in cells at different time. (A) α‐SMA expression (Green) and nuclei staining (Blue) detected by immunofluorescence assay. Scale bars: 50 μm. (B) The mRNA expression levels of α‐SMA detected by RT‐PCR. (C) The protein levels of α‐SMA detected by western blotting. (i) SF: stretched fibroblasts; (ii) USF: unstretched fibroblasts; (iii) USM: unstretched myofibroblasts. Error bar: ±SD (n = 3); *P < 0.05; **P > 0.05
The changing trends of protein levels of integrin β1, α‐SMA, and Nesprin‐2 were the same as their mRNA levels (Figure 1B), (Figure 2C).
α‐SMA stain was clearly present in SF at days 5 and 7 and USM at days 3, 5, and 7, negligible stain in SF at day 3, and USF at days 3, 5, and 7 (Figure 2A).
3.1.3. The expression of TGF‐β1 and collagen type I of cells
The SF at day 5 presented significantly higher mRNA levels of TGF‐β1 and collagen type I than those at day 3 (all, P < 0.05, n = 3). Their expression levels in SF at day 5 were as high as those in USM (all, P > 0.05, n = 3). The mechanical stretch had the same effects on the protein levels of TGF‐β1 and collagen type I as it did on their mRNA levels. (Figure 1D,E).
3.2. Silencing of Nesprin‐2 delayed the differentiation of myofibroblasts induced by cyclic stretch
The results of the above experiments showed that cyclic stretch could induce the phenotype switch of cells and fibroblasts exposed to cyclic stretch were transformed into myofibroblasts at day 5. Fibroblasts transfected with siRNA targeting Nesprin‐2 were exposed to cyclic stretch to detect the phenotype switch of cells at day 5.
3.2.1. Cell proliferation
The proliferation ability of SF transfected with siRNA (SF + siRNA) was lower than that in SF and USM at day 5 (P < 0.05, n = 3) (Figure 3A).
FIGURE 3.
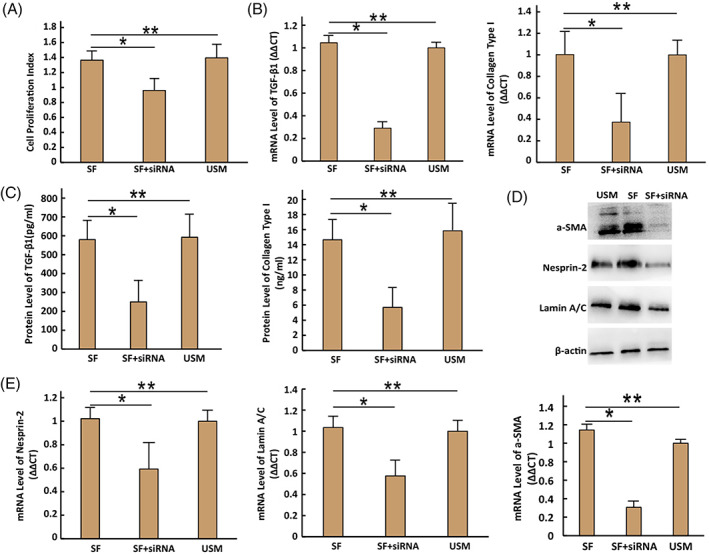
Reactions of cells to cyclic stretch with 10% magnitude at day 5. (A) Cell proliferation ability. (B) The mRNA expression levels of TGF‐β1 and collagen type I detected by RT‐PCR. (C) The protein levels of TGF‐β1 and collagen type I detected by ELISA. (D) The protein levels of a‐SMA, Nesprin‐2, and lamin A/C detected by western blotting. (E) The mRNA expression of a‐SMA, Nesprin‐2, and lamin A/C detected by RT‐PCR. (i) SF: stretched fibroblasts; (ii) SF + siRNA: SF transfected with siRNA; (iii) USM: unstretched myofibroblasts. Error bar: ±SD (n = 3); *P < 0.05; **P > 0.05
3.2.2. The expression of α‐SMA, Nesprin‐2, and lamin A/C of cells
The mRNA expression levels of α‐SMA, Nesprin‐2 and lamin A/C in SF + siRNA was lower than those in SF and USM at day 5 (all, P < 0.05, n = 3). The silencing of Nesprin‐2 had the same effect on their protein levels as it did on the mRNA levels (Figure 3D,E).
3.2.3. The expression of TGF‐β1 and collagen type I
The SF + siRNA at day 5 produced significantly lower levels of collagen type I and TGF‐β1 than those in SF and USM at day 5 (all, P < 0.05, n = 3). The silencing of Nesprin‐2 affected the expression of mRNA levels of TGF‐β1 and collagen type I in the same way as that of their protein levels (Figure 3B,C).
4. DISCUSSION
Myofibroblast, an aberrant phenotype of normal skin fibroblast, is believed to be the vital protagonist in the formation of HTS. The presence of a‐SMA starts the activation program of myofibroblast in an intracellular mechanical feedback loop. 17 , 18 Expression of a‐SMA is always used to define myofibroblast differentiation in experimental conditions. 19 In the present study, we constructed a cell model of HTS induced by cyclic stretch to explore the mechanisms of hypertrophic scarring.
Previous studies show that cells' response to external stress depends on the applied strain magnitude and duration primarily but not frequency. Although large numbers of studies try to explore the optimal stretching magnitude, it is still not clear what kind of stretch would induce the differentiation of myofibroblasts from fibroblasts. In our previous study, we found that facial skin fibroblasts imposed with 10% magnitude cyclic stretch showed a maximised mechanical and biochemical reaction. 16 To explore the mechanical behaviour of dermal fibroblasts from different sites of human body, the fibroblasts from scalp, anterior chest, suprapubic, axilla, and planta of human bodies were cultured and detected in vitro. Cyclic stretch with 0%, 5%, 10%, 15%, and 20% magnitudes, at a frequency of 0.1 Hz, was imposed on the cells for 48 hours, and thereafter, the cells' reaction was monitored. The results showed that the cyclic stretch with an optimum magnitude could lead to biochemical reactions inside the fibroblasts in the greatest degree, and the optimum magnitude might be in the range of 10% to 15%. 20 Additionally, the cultured skin fibroblasts from different anatomical sites of human bodies reacted to mechanical stretch with a certain magnitude in the same way.
In the present study, fibroblasts had been exposed to cyclic stretch at 10% magnitudes for different days, and the differentiation of cells was monitored. In our previous study, we found that cultured cells obtained from HTS produced high expression level of α‐SMA no matter whether they were exposed to cyclic stretch or not, by which they could be defined as myofibroblasts. 21 According to the expression level of α‐SMA in USM, we can figure out whether the stretched healthy skin fibroblasts were transformed into myofibroblasts or not. To decrease the individual differences, SF, USF, and USM were obtained from healthy skin tissues and paired HTS tissues of the same human body. Besides α‐SMA, the expression levels of integrin β1, Nesprin‐2, TGF‐β1, and collagen type I in SF increased in a strain time‐dependent manner. Their expression levels in SF at day 5 were as high as those in USM. The SF at day 5 showed the same mechanotransductive and biochemical features as USM, which indicates the fibroblasts had been transformed into myofibroblasts and a cell model of HTS was established successfully. The phenotype transformation of myofibroblasts could be triggered by mechanical stretch without inflammatory factors, which is consistent with the previous reports. 22 , 23
Mechanical stretch can significantly enhance the integrin β1, α‐SMA, Nesprin‐2, and lamin A/C expression inside the cells. The role of mechanotransducer outside the nucleus in the pathogenesis of HTS, including integrin β1 and some cytoskeletons, has been reported in many studies. 20 , 24 , 25 However, the role of Nesprin‐2 in hypertrophic scarring is not clear.
Nesprins, SUN proteins, and lamin A/C interact with each other, which plays a pivotal role in the process of mechanical signal transmission through the nuclear membrane. Lamin A/C can incorporate with chromatin and transcription factors and affect their properties. 26 We found that the cyclic stretch could up‐regulate the expression of Nesprin‐2 and lamin A/C in fibroblasts. However, the expression of lamin A/C was reduced after the Nesprin‐2 was silenced, which indicated the mechanical signals from the extracellular matrix were less efficiently transmitted to the nucleus.
On the other hand, the expression of α‐SMA, TGF‐β1, and collagen type I in fibroblasts was also reduced by the silencing of Nesprin‐2. TGF‐β1 is the major cytokine inducing myofibroblast differentiation. 27 , 28 Cyclic stretch promoted the expression of TGF‐β1 in SF. Mechanical stretch can cooperate with the TGF‐β1 signalling pathway to regulate a‐SMA expression and induce myofibroblast differentiation. 23 We speculate that the disruption of the LINC complex not only down‐regulate the expression of α‐SMA directly, but through TGF‐β1 signalling pathway, and finally impaired the mechanical stimulation of terminal myofibroblasts differentiation.
Mutations in nuclear envelope proteins such as amins and emerin can bring on some human diseases like laminopathies or nuclear envelopathies, progeria, neuropathy, and lipodystrophy to cardiac and muscular dystrophies. 29 , 30 However, the Nesprin‐2 knockout mice always are viable and healthy 14 , 31 and exhibit delayed skin wound closure. 32 Woychek A et al provided a mechanistic explanation that Nesprin‐2G knockout fibroblasts exhibited reduced migration, changes in focal adhesion composition, and reduced ability to generate traction forces. 15 We speculate the reduced ability to generate traction forces is related to the lower expression of α‐SMA.
Liver fibrosis results from excessive deposition of extracellular matrix and formation of scar tissue, which is similar to the pathology of HTS of skin. The latest report shows that disruption of the Nesprin might be a therapeutic strategy to liver stiffness through targeting the stiffness‐mediated intracellular mechanical tensions. 33
In summary, mechanical strain could induce the differentiation of myofibroblasts from fibroblasts, and silencing of Nesprin‐2 could block the mechanical stimulation of terminal myofibroblasts differentiation. Nesprin‐2 might be a potential target to treat the HTS.
CONFLICT OF INTEREST
The authors report no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the Provincial Natural Science Foundation of Shandong, China (No. ZR2017MH083) and (No. ZR2020MH183).
Xu Q, Miao Y, Ren J, et al. Silencing of Nesprin‐2 inhibits the differentiation of myofibroblasts from fibroblasts induced by mechanical stretch. Int Wound J. 2022;19(5):978-986. doi: 10.1111/iwj.13694
Funding information Provincial Natural Science Foundation of Shandong, China, Grant/Award Numbers: ZR2017MH083, ZR2020MH183
Contributor Information
Xia Cai, Email: caixia72@163.com.
Zhiguo Wang, Email: qyfywzg@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tsai CH, Ogawa R. Keloid research: current status and future directions. Scars Burn Heal. 2019;5:2059513119868659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yagmur C, Akaishi S, Ogawa R, Guneren E. Mechanical receptor‐related mechanisms in scar management: a review and hypothesis. Plast Reconstr Surg. 2010;126(2):426‐434. [DOI] [PubMed] [Google Scholar]
- 3. Sarrazy V, Billet F, Micallef L, Coulomb B, Desmoulière A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10‐s15. [DOI] [PubMed] [Google Scholar]
- 4. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: An update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81‐94. [DOI] [PubMed] [Google Scholar]
- 5. Nolte MA, Nolte‐'t Hoen ENM, Margadant C. Integrins control vesicular trafficking; new tricks for old dogs. Trends Biochem Sci. 2021;46(2):124‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318(5855):1408‐1412. [DOI] [PubMed] [Google Scholar]
- 7. Holt I, Fuller HR, Lam LT, et al. Nesprin‐1‐alpha2 associates with kinesin at myotube outer nuclear membranes, but is restricted to neuromuscular junction nuclei in adult muscle. Sci Rep. 2019;9(1):14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouzid T, Kim E, Riehl BD, et al. The LINC complex, mechanotransduction, and mesenchymal stem cell function and fate. J Biol Eng. 2019;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Curr Opin Cell Biol. 2011;23(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 10. Arsenovic PT, Ramachandran I, Bathula K, et al. Nesprin‐2G, a component of the nuclear LINC complex, is subject to myosin‐dependent tension. Biophys J. 2016;110(1):34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saez A, Herrero‐Fernandez B, Gomez‐Bris R, Somovilla‐Crespo B, Rius C, Gonzalez‐Granado JM. Lamin a/C and the immune system: one intermediate filament. Many Faces Int J Mol Sci. 2020;21(17):6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hieda M. Implications for diverse functions of the LINC complexes based on the structure. Cell. 2017;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrés V, González JM. Role of A‐type Lamins in signaling, transcription, and chromatin organization. J Cell Biol. 2009;187(7):945‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lüke Y, Zaim H, Karakesisoglou I, et al. Nesprin‐2 giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J Cell Sci. 2008;121(11):1887‐1898. [DOI] [PubMed] [Google Scholar]
- 15. Woychek A, Jones JCR. Nesprin‐2G knockout fibroblasts exhibit reduced migration, changes in focal adhesion composition, and reduced ability to generate traction forces. Cytoskeleton. 2019;76(2):200‐208. [DOI] [PubMed] [Google Scholar]
- 16. Kuang RX, Wang ZG, Xu QC, Cai X, Liu T. Exposure to varying strain magnitudes influences the conversion of Normal skin fibroblasts into hypertrophic scar cells. Ann Plast Surg. 2016;76(4):388‐393. [DOI] [PubMed] [Google Scholar]
- 17. Talele NP, Fradette J, Davies JE, Kapus A, Hinz B. Expression of alpha‐smooth muscle Actin determines the fate of mesenchymal stromal cells. Stem Cell Rep. 2015;4(6):1016‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tondeleir D, Lambrechts A, Müller M, et al. Cells lacking beta‐Actin are genetically reprogrammed and maintain conditional migratory capacity. Mol Cell Proteomics. 2012;11(8):255‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darby I, Skalli O, Gabbiani G. Alpha‐smooth muscle Actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Investig. 1990;63(1):21‐29. [PubMed] [Google Scholar]
- 20. Xu QC, Kuang RX, Wei SQ, Kang Q, Wang JJ, Wang ZG. Analysis of mechanical behavior of dermal fibroblasts obtained from various anatomical sites in humans. Ann Plast Surg. 2017;79(5):438‐443. [DOI] [PubMed] [Google Scholar]
- 21. An Y, Hao RA, Kuang RX, Hu CN, Chen L, Wang ZG. Effect of low‐amplitude mechanical strain in inducing the redifferentiation of myofibroblasts. J Qingdao Univ. 2021;57(3):432‐436. [Google Scholar]
- 22. Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146‐155. [DOI] [PubMed] [Google Scholar]
- 23. Walker M, Godin M, Pelling AE. Mechanical stretch sustains myofibroblast phenotype and function in microtissues through latent TGF‐β1 activation. Integr Biol (Camb). 2020;12(8):199‐210. [DOI] [PubMed] [Google Scholar]
- 24. Angelini A, Trial J, Ortiz‐Urbina J, Cieslik KA. Mechanosensing dysregulation in the fibroblast: a hallmark of the aging heart. Ageing Res Rev. 2020;63:101150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Massou S, Nunes Vicente F, Wetzel F, et al. Cell stretching is amplified by active Actin remodeling to deform and recruit proteins in mechanosensitive structures. Nat Cell Biol. 2020;22(8):1011‐1023. [DOI] [PubMed] [Google Scholar]
- 26. Perepelina K, Klauzen P, Kostareva A, Malashicheva A. Tissue‐specific influence of Lamin a mutations on notch signaling and osteogenic phenotype of primary human mesenchymal cells. Cell. 2019;8(3):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton‐Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, Long X, Liu W, et al. Type I collagen induces mesenchymal cell differentiation into myofibroblasts through YAP‐induced TGF‐β1 activation. Biochimie. 2018;150:110‐130. [DOI] [PubMed] [Google Scholar]
- 29. Cartwright S, Karakesisoglou I. Nesprins in health and disease. Semin Cell Dev Biol. 2014;29:169‐179. [DOI] [PubMed] [Google Scholar]
- 30. Dutta S, Bhattacharyya M, Sengupta K. Changes in the nuclear envelope in Laminopathies. Adv Exp Med Biol. 2018;1112:31‐38. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Xu R, Zhu B, et al. Syne‐1 and Syne‐2 play crucial roles myonuclear anchorage and motor neuron innervation. Development. 2007;134(5):901‐908. [DOI] [PubMed] [Google Scholar]
- 32. Rashmi RN, Eckes B, Glöckner G, et al. The nuclear envelope protein Nesprin‐2 has roles in cell proliferation and differentiation during wound healing. Nucleus. 2012;3(2):172‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guixé‐Muntet S, Ortega‐Ribera M, Wang C, et al. Nuclear deformation mediates liver cell mechanosensing in cirrhosis. JHEP Rep. 2020;2(5):100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


