Abstract
The ability to adapt to environmental and task demands while walking is critical to independent mobility outside the home and this ability wanes with age. Such adaptability requires individuals to acutely change their walking speed. Regardless of age, changes between walking speeds are common in daily life, and are a frequent type of walking adaptability. Here, we report on older and younger adults when transitioning from preferred walking speed overground to either slower or faster walking. Specifically, we evaluated biomechanical parameters prior to, during, and post transition. Individuals approached the walking speed transition similarly, independent of whether the transition was to slower or faster walking. Regardless of age or walking speed, the step during which a walking speed transition occurred was distinct from those prior- and post- transition, with on average 0.15 m shorter step lengths, 3.6° more hip flexion, and 3.3° more dorsiflexion during stance. We also found that peak hip flexion occurred 22% later, and peak hip extension (39%), knee flexion (26%), and dorsiflexion (44%) occurred earlier in stance for both typical to slower and typical to faster walking. Older adults had altered timing of peak joint angles compared with younger adults across both acceleration and deceleration conditions, indicating age-dependent responses to changing walking speed. Our findings are an important first step in establishing values for kinematics during walking speed transitions in younger and typical older adults.
Keywords: Gait, Aging, Speed transition, Mobility, Kinematics
1. Introduction
Independent mobility outside of the home is a primary contributor to quality of life (Rantakokko et al., 2013; Williams and Willmott, 2012). Walking outside of the home (community ambulation) requires complex gait adaptability in response to environmental or task demands. Adaptability consists of numerous domains – including the ability to acutely speed up (e.g., crossing a crosswalk) or slow down (e.g., navigating a crowd) (Balasubramanian et al., 2014). Adaptability is a distinguishing factor between those with mobility disability (difficulty walking a quarter of a mile or an inability to climb 10 stairs without rest (Seeman et al., 2010)) and those without (Shumway-Cook et al., 2002). As individuals age, community ambulation decreases (Shumway-Cook et al., 2007, 2002), in part due to deficits in complex gait adaptability.
Older adults (OA) exhibit different spatiotemporal gait parameters and joint angles when walking compared with younger adults (YA) (Herssens et al., 2018), contributing to the functional limitations associated with older age (Almarwani et al., 2016). OA have slower gait speed, reduced step length, increased step width, reduced peak ankle plantarflexion during stance, greater peak hip flexion, and reduced hip extension when compared with YA (Boyer et al., 2017; Hollman et al., 2011; Laufer, 2005; Voss et al., 2020). These age-associated differences have been linked to increased fall risk (Barak et al., 2006; Kerrigan et al., 2001; Verghese et al., 2009).
Some age-related differences in walking can be attributed to slower speed. The generalized effect of gait speed on spatiotemporal gait parameters and joint angles has been well established (Fukuchi et al., 2019; Kirtley et al., 1985). A meta-analysis indicated that faster gait speeds are associated with increased step length, increased peak hip flexion and increased peak ankle plantarflexion during stance (Fukuchi et al., 2019), while slower gait speeds are associated with the opposite. Slower gait speeds are also associated with wider steps (Stimpson et al., 2018), and reduced peak hip extension during stance (Fukuchi et al., 2019). Nevertheless, when gait speed is held constant, the effects of age on gait parameters are still evident (Boyer et al., 2017; Kerrigan et al., 1998).
Aging and gait speed impact lower limb kinematics during gait. OA tend to walk slower than YA, and thus the reductions in step length, range of motion in the lower limb, and increases in double support time are amplified (Boyer et al., 2017; Kang and Dingwell, 2008; Ko et al., 2010). However, a summative effect occurs with gait changes persisting regardless of walking speed (Fukuchi et al., 2019). Despite the established age and speed effects on walking parameters, the effects of walking speed transitions (i.e., walking from one speed to one faster or slower) are under-investigated.
The well-studied walk-to-run transition is typically characterized by kinematic and kinetic differences in pre-, during- and post-transition strides (Hreljac et al., 2007; Segers et al., 2013, 2006). However, the walk-to-run transition is not commonly utilized in tasks of daily living, especially by OA. Understanding transitions between different walking speeds is more applicable to experiences of community ambulation and may highlight fall risks. Indeed, clinical assessments like the Dynamic Gait Index incorporate walking speed changes to asses fall risk and balance problems (Shumway-Cook et al., 2013).
Researchers who studied acceleration during walking found that propulsive impulses increased with walking speed (Peterson et al., 2011). Knee flexor and ankle plantarflexor moment impulses were related to these propulsive impulses. However, the authors did not separate gait cycles related to the transition phases (i.e. pre-, during-, and post-speed change). In such a case, we might expect to see shorter step times, longer step lengths and increased hip flexion during and following a transition to faster walking.
Understanding walking speed transitions and how they are affected by age is an important step in understanding age-related declines in community ambulation. Here, we obtained spatiotemporal and kinematic parameters in steps prior to, during, and following transition from the preferred walking speed to either self-selected faster (normal-to-fast, NF) or slower (normal-to-slow, NS) walking in both YA and OA. We hypothesized pre-transition steps will be characterized by typical gait characteristics for the age group, post-transition steps will be characterized by changes in lower limb kinematics associated with either slower or faster speed, while during-transition steps will be different to both pre- and post-transition steps. We also hypothesized age will impact these changes, with OA showing a smaller response compared with YA (Fig. 1).
Fig. 1.
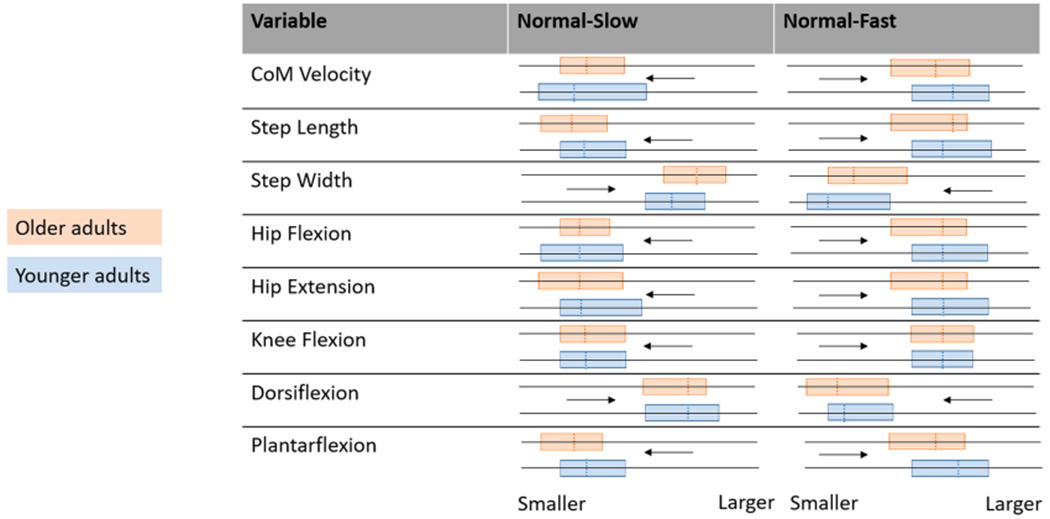
Graphical representation of hypothesized changes in spatiotemporal variables and joint angles across all phases of the transition (during-transition step shown by the dashed line) for preferred speed to slower (Normal-Slow) and preferred speed to faster (Normal-Fast) walking in younger adults (blue) and older adults (orange). Arrows indicate pre- to post- direction, while “smaller” and larger” indicate relative variable magnitude. The pre-post range in variable magnitude is highlighted by box size. Anticipated age-related differences are indicated by horizontal differences in either end of the highlighted box, which would indicate a difference in pre- or post- values across age groups, or by the dashed lines being horizontally offset, indicating the during-transition step value being different across age groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Methods
Thirty-eight healthy adults (14 younger [age: 23 ± 4 y; height: 1.70 ± 0.11 m; mass: 68.65 ± 13.58 kg; 6 male] and 24 older [age: 75 ± 4 y; height: 1.71 ± 0.10 m; mass: 76.08 ± 14.65 kg; 14 male]) provided written informed consent prior to data collection. Inclusion criteria for typical OA over the age of 70 was a Short-Physical Performance Battery score ≥ 10, and an ability to complete a 400 m walk test within 15 min. YA were included if they were between 20 and 40 years and free of medical problems that may impact walking. Participant exclusion criteria is available in supplementary material. This study was approved by the University Institutional Review Board. Reflective markers were placed according to the Plug-In-Gait marker set and recorded at 100 Hz by 16 cameras (Vicon, Oxford Metrics Inc.). Similar to the Dynamic Gait Index (Shumway-Cook et al., 2013), participants were instructed to “begin walking at your typical comfortable pace. When I tell you slow, walk at the slowest possible speed that still feels natural. When I say go, walk at your fastest safe speed without running or jogging.” Individuals performed 5 trials each of NF and NS walking over a 10 m walkway. Order of transition was randomized.
Gait events were identified from marker trajectories with custom code, detecting foot contact as defined in De Asha et al. (2012) and foot off as in Fellin et al. (2010). Data were filtered with a zero-lag low-pass Butterworth filter with 10 Hz cut-off, and the dynamic Plug-In Gait Model was used to obtain YXZ Cardan joint angles and whole body center of mass (CoM) position. Forward CoM velocity was calculated and plotted in Matlab (Mathworks Inc.). Within a user-selected range covering the period in which there was a substantial change in CoM velocity (exceeding normal cyclical variation), the transition point was defined as the point that immediately preceded the shift in forward CoM velocity (for NF trials, a trough; for NS trials, a peak; Fig. 2). Each step was analyzed with respect to the transition point and classified. When a step occurred prior to the transition point, it was considered pre-transition; when a step occurred following the transition point, it was considered post-transition. If the transition point occurred during a step, it was considered during-transition (Fig. 2). For each step, the variables outlined in Table 1 were obtained, and all pre-transition steps were averaged together, as were all post-transition steps and during-transition steps within a participant.
Fig. 2.
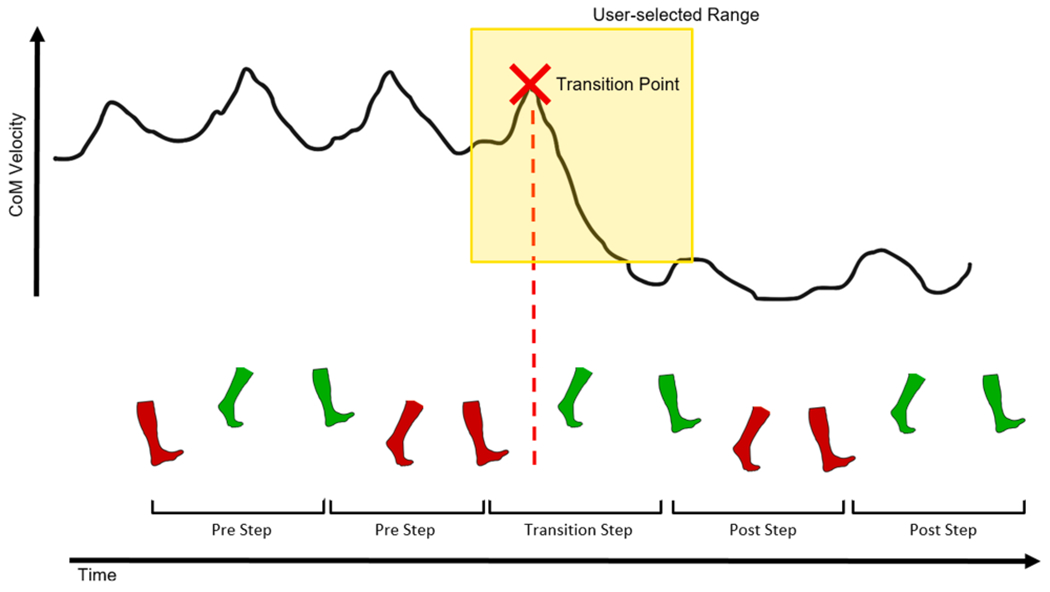
Schematic of procedure to determine the point of transition. Forward center of mass (CoM) velocity trace with graphical representation of user-selected range (yellow box) and mathematically identified transition point (red cross), with corresponding left (green) and right (red) steps depicted. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Spatiotemporal and joint angle definitions.
| Variable | Definition |
|---|---|
| Stance limb | The limb whose heel strike starts a gait cycle: e.g. if a gait cycle was right foot contact to right foot contact, the right limb would be the stance limb and the left would be the opposite. |
| Spatiotemporal Parameters | |
| Mean center of mass (CoM) velocity | Forward velocity of the center of mass averaged over the duration of the step. |
| Step length | The average anterior-posterior distance from stance limb heel marker to the opposite limb heel marker between the time from opposite limb heel strike to stance limb toe off. |
| Step width | The average mediolateral distance from stance limb heel marker to opposite limb heel marker between the time from opposite limb heel strike to stance limb toe off. |
| Joint Angles | |
| Stance limb hip flexion | Peak hip flexion angle (positive values) on the stance limb, during the stance phase (incorporating both double and single support). |
| Stance limb hip extension | Peak hip extension angle (negative values) on the stance limb, during the stance phase (incorporating both double and single support). |
| Stance limb knee flexion | Peak knee flexion angle (positive values) on the stance limb, during the stance phase (incorporating both double and single support). |
| Stance limb ankle plantarflexion | Peak ankle plantarflexion angle (negative values) on the stance limb, during the stance phase (incorporating both double and single support). |
| Stance limb ankle dorsiflexion | Peak ankle dorsiflexion angle (positive values) on the stance limb, during the stance phase (incorporating both double and single support). |
| Timing of Joint Angles | |
| Timing of peak hip flexion | Stance phase was normalized to 100%, and time at which peak hip flexion occurs is reported as percentage of stance phase |
| Timing of peak hip extension | Stance phase was normalized to 100%, and time at which peak hip extension occurs is reported as percentage of stance phase |
| Timing of peak knee flexion | Stance phase was normalized to 100%, and time at which peak knee flexion occurs is reported as percentage of stance phase |
| Timing of peak plantarflexion | Stance phase was normalized to 100%, and time at which peak plantarflexion occurs is reported as percentage of stance phase |
| Timing of peak dorsiflexion | Stance phase was normalized to 100%, and time at which peak dorsiflexion occurs is reported as percentage of stance phase |
Statistical Analysis:
Two repeated-measures mixed MANOVAs were run (1: NF, 2: NS) with a within-subjects factor (phase: pre-, during-, or post-transition) and a between-subjects factor (group: YA or OA). α was set at the level of 0.05 but was adjusted with Dunn-Bonferroni corrections for multiple comparisons, including 95% confidence interval adjustments. All statistical analyses were performed in SPSS (IBM, version 26). For meaningful interpretation, we provide partial eta squared (ηp2) values for effect sizes: small effect ηp2 = 0.01, medium effect ηp2 = 0.06, large effect ηp2 ≥ 0.14 (Cohen, 1988).
3. Results
Normal-to-slower walking:
As anticipated, both YA and OA had the fastest CoM velocity occurring pre-transition and reducing to the slowest velocity at post-transition (Table 2, all p < 0.001, ηp2 = 0.879). Both groups had least variability (coefficient of variation – CV) of CoM velocity pre-transition compared with during- and post-phases (Table 2, all p ≤ 0.013).
Table 2.
Mean ± SE (95% CI) and coefficient of variation (CV) for spatiotemporal variables in older adults (OA), younger adults (YA), for pre-, during-, and post- transition phases for normal-slow (NS) and normal-fast (NF) conditions.
| Normal Slow |
Normal Fast |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Pre | During | Post | Pre | During | Post | |
| CoM Velocity (m/s) | OA | Mean ± SE (95% CI) | 1.21 ± 0.04 ([1.13 1.29])† | 1.05 ± 0.04 ([0.97 1.13])^† | 0.8 ± 0.04 ([0.73 0.88])^ | 1.23 ± 0.04 ([1.15 1.32])† | 1.26 ± 0.05 ([1.17 1.36])† | 1.65 ± 0.06 ([1.53 1.77])^ |
| CV ± SE (95% CI) | 0.06 ± 0.01 ([0.05 0.06])† | 0.11 ± 0.01 ([0.09 0.12])^† | 0.12 ± 0.01 ([0.1 0.15])^ | 0.04 ± 0.01 ([0.03 0.05]) | 0.06 ± 0.01 ([0.04 0.07])† | 0.08 ± 0.01 ([0.07 0.09])^ | ||
| YA | Mean ± SE (95% CI) | 1.24 ± 0.05 ([1.13 1.34])† | 1.05 ± 0.05 ([0.96 1.15])^† | 0.84 ± 0.05 ([0.74 0.94])^ | 1.21 ± 0.05 ([1.11 1.32])† | 1.33 ± 0.06 ([1.22 1.45])^† | 1.81 ± 0.07 ([1.66 1.95])^ | |
| CV ± SE (95% CI) | 0.05 ± 0.01 ([0.04 0.06])† | 0.11 ± 0.01 ([0.09 0.13])^† | 0.1 ± 0.02 ([0.07 0.13])^ | 0.04 ± 0.01 ([0.02 0.05])† | 0.08 ± 0.01 ([0.06 0.09])^† | 0.07 ± 0.01 ([0.06 0.09])^ | ||
| Step Length (m) | OA | Mean ± SE (95% CI) | 0.67 ± 0.01 ([0.64 0.7])† | 0.52 ± 0.01 ([0.49 0.54])^† | 0.56 ± 0.02 ([0.53 0.6])^ | 0.67 ± 0.01 ([0.64 0.7])† | 0.52 ± 0.01 ([0.49 0.54])^† | 0.72 ± 0.02 ([0.69 0.76])^ |
| CV ± SE (95% CI) | 0.04 ± 0.01 ([0.01 0.06])† | 0.05 ± 0.03 ([0.01 0.11])† | 0.1 ± 0.02 ([0.05 0.14])^ | 0.03 ± 0.01 ([0.02 0.04])† | 0.05 ± 0.01 ([0.04 0.06])*^† | 0.08 ± 0.01 ([0.06 0.09])^ | ||
| YA | Mean ± SE (95% CI) | 0.66 ± 0.02 ([0.62 0.7])† | 0.5 ± 0.02 ([0.47 0.53])^† | 0.54 ± 0.02 ([0.49 0.58])^ | 0.66 ± 0.02 ([0.63 0.7])† | 0.5 ± 0.02 ([0.47 0.54])^† | 0.77 ± 0.02 ([0.73 0.81])^ | |
| CV ± SE (95% CI) | 0.06 ± 0.01 ([0.03 0.09])† | 0.13 ± 0.04 ([0.05 0.21])^† | 0.13 ± 0.03 ([0.07 0.19])^ | 0.03 ± 0.01 ([0.02 0.04])† | 0.08 ± 0.01 ([0.06 0.09])*^† | 0.07 ± 0.01 ([0.05 0.08])^ | ||
| Step Width (m) | OA | Mean ± SE (95% CI) | 0.08 ± 0.01 ([0.07 0.1])† | 0.1 ± 0.01 ([0.09 0.11])^ | 0.1 ± 0.01 ([0.09 0.11])^ | 0.08 ± 0.01 ([0.07 0.1]) | 0.1 ± 0.01 ([0.09 0.11])^† | 0.08 ± 0.01 ([0.07 0.1]) |
| CV ± SE (95% CI) | 0.37 ± 0.03 ([0.31 0.43])† | 0.21 ± 0.03 ([0.16 0.26])^ | 0.25 ± 0.04 ([0.18 0.32])^ | 0.39 ± 0.05 ([0.3 0.49])* | 0.25 ± 0.02 ([0.21 0.29])*^† | 0.46 ± 0.03 ([0.39 0.52])*^ | ||
| YA | Mean ± SE (95% CI) | 0.1 ± 0.01 ([0.08 0.11]) | 0.1 ± 0.01 ([0.09 0.11]) | 0.1 ± 0.01 ([0.09 0.12]) | 0.1 ± 0.01 ([0.08 0.12]) | 0.11 ± 0.01 (0.1 [0.13])† | 0.1 ± 0.01 ([0.08 0.11]) | |
| CV ± SE (95% CI) | 0.29 ± 0.04 ([0.22 0.36]) | 0.19 ± 0.03 ([0.12 0.25])† | 0.3 ± 0.04 ([0.21 0.39]) | 0.23 ± 0.06 ([0.11 0.35])* | 0.13 ± 0.02 ([0.08 0.17])*† | 0.3 ± 0.04 ([0.22 0.38])* | ||
Indicates significant between-subjects difference (p < 0.05).
Indicates significantly different from within-subjects pre-transition values (p < 0.05).
Indicates significantly different from within-subjects post-transition values (p < 0.05).
The during-transition step was the shortest for both groups, and longest steps occurred pre-transition (Table 2, all p < 0.001, ηρ2 = 0.986). Both OA and YA had greater step length CV post-transition compared with pre- (Table 2, all p ≤ 0.022).
OA had narrower pre-transition steps than either during- or post-transition (Table 2, all p ≤ 0.00, ηp2 = 0.174), while YA did not alter step width (Table 2, all p ≥ 0.398). OA had greatest step width CV pre-transition compared with during- and post-transition phases (Table 2, all p ≤ 0.009), whereas YA had greater step width CV post-transition compared with during-transition (Table 2, p = 0.013).
Hip flexion angle was significantly different at all phases for both groups, with greatest hip flexion during-transition (Table 3, all p ≤ 0.047, ηp2 = 0.725). Both groups produced hip flexion later in the during-transition step compared with other steps (Table 4, all p < 0.001, ηp2 = 0.798). OA produced hip flexion later than YA in the during-transition step (Table 4, p = 0.01, ηp2 = 0.168). Hip flexion timing CV was different between age groups (Table 4, p = 0.034, ηp2 = 0.115), although not significantly different across phases (all p ≥ 0.055).
Table 3.
Mean ± SE (95% CI) and coefficient of variation (CV) for lower limb peak joint sagittal angles in older adults (OA) and younger adults (YA), for pre-, during-, and post-transition phases for normal slow and normal fast conditions. Positive values indicate hip flexion, knee flexion, and dorsiflexion. Negative values indicate hip extension and plantarflexion.
| Normal Slow |
Normal Fast |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Pre | During | Post | Pre | During | Post | |
| Peak Hip Flexion (°) | OA | Mean ± SE (95% CI) | 2.2 ± 0.7 ([0.7 3.7])† | 4.5 ± 0.8 ([36])^† | 2.8 ± 0.8 ([1.2 4.4])^ | 2.1 ± 0.8 ([0.6 3.7])† | 5.4 ± 0.8 ([3.8 6.9])^† | 0.7 ± 0.8 ([−0.9 2.3])^ |
| CV ± SE (95% CI) | 0.5 ± 0.6 ([−0.7 1.8]) | 0.6 ± 0.2 ([0.2 0.9]) | −0.1 ± 0.6 ([−1.3 1.1]) | 0.1 ± 0.6 ([−1.1 1.3]) | 0.7 ± 1.8 ([−3.0 4.3]) | −0.4 ± 4.7 ([−9.9 9.1]) | ||
| YA | Mean ± SE (95% CI) | 0.5 ± 0.9 ([−1.3 2.4])† | 5.0 ± 1.0 ([3.0 7.0])^† | 1.9 ± 1.0 ([−0.1 3.9])^ | 0.6 ± 1.0 ([−1.4 2.5])† | 5.2 ± 0.9 ([3.3 7.1])^† | −1.5 ± 1.0 ([−3.5 0.4])^ | |
| CV ± SE (95% CI) | −1.3 ± 0.8 ([−2.9 0.2]) | 0.2 ± 0.2 ([−0.2 0.6]) | 0.3 ± 0.8 ([−1.3 1.9]) | 0.3 ± 0.7 ([−1.2 1.8]) | −3.2 ± 2.2 ([−7.7 1.3]) | −12.3 ± 5.8 ([−24–0.5]) | ||
| Peak Hip Extension (°) | OA | Mean ± SE (95% CI) | −4.7 ± 0.8 ([−6.3–3.1]) | −5.4 ± 0.8 ([−7.1–3.7])† | −4.7 ± 0.8 ([−6.4–3]) | −4.6 ± 0.8 ([−6.2–3]) | −5.4 ± 0.9 ([−7.2–3.7])† | −4.6 ± 0.8 ([−6.2–3.0]) |
| CV ± SE (95% CI) | 0.1 ± 0.6 ([−1.1 1.3]) | −0.2 ± 1.9 ([−4.1 3.6]) | −0.4 ± 6.9 ([−14.3 13.5]) | −0.2 ± 0.4 ([−0.9 0.5]) | −8.4 ± 6.0 ([−20.6 3.8]) | −0.8 ± 0.4 ([−1.6–0.1]) | ||
| YA | Mean ± SE (95% CI) | −5.9 ± 1.0 ([−7.9–3.8]) | −5.9 ± 1.1 ([−8–3.7]) | −5.5 ± 1.1 ([−7.6–3.3]) | −5.9 ± 1.0 ([−7.9–3.9]) | −5.9 ± 1.1 ([−8.1–3.8]) | −6.3 ± 1.0 ([−8.3–4.3]) | |
| CV ± SE (95% CI) | 0.3 ± 0.7 ([−1.2 1.8]) | 2.7 ± 2.4 ([−2.2 7.6]) | −12.7 ± 8.7 ([−30.3 4.9]) | −1.0 ± 0.5 ([−1.9–0.1]) | −0.4 ± 7.5 ([−15.6 14.7]) | −0.2 ± 0.5 ([−1.2 0.7]) | ||
| Peak Knee Flexion (°) | OA | Mean ± SE (95% CI) | 14.2 ± 1.6 ([10.9 17.5]) | 17.0 ± 1.6 ([13.8 20.2])^† | 15.3 ± 1.7 ([11.9 18.7]) | 14.1 ± 1.9 ([10.3 18.0]) | 14.4 ± 1.9 ([10.6 18.2])† | 12.0 ± 1.8 ([8.4 15.6]) |
| CV ± SE (95% CI) | 0.6 ± 0.4 ([−0.2 1.3]) | 0.3 ± 0.1 ([0.1 0.4])* | 0.1 ± 0.2 ([−0.2 0.4])* | 0.3 ± 0.1 ([0.2 0.5]) | 0.4 ± 0.1 ([0.2 0.6]) | 0.6 ± 0.7 ([−0.9 2.1]) | ||
| YA | Mean ± SE (95% CI) | 11.8 ± 2.1 ([7.6 16.0]) | 15.7 ± 2.0 ([11.6 19.7])^† | 13.7 ± 2.1 ([9.4 18.0]) | 13.0 ± 2.4 ([8.2 17.8]) | 13.1 ± 2.3 ([8.4 17.8])† | 10.5 ± 2.2 ([6.0 15.0]) | |
| CV ± SE (95% CI) | 0.0 ± 0.5 ([−1.0 0.9]) | 0.5 ± 0.1 ([0.3 0.7])* | 0.8 ± 0.2 ([0.4 1.2])* | 0.4 ± 0.1 ([0.2 0.6]) | 0.4 ± 0.1 ([0.2 0.6]) | −0.8 ± 0.9 ([−2.6 1]) | ||
| Peak Plantarflexion (°) | OA | Mean ± SE (95% CI) | −0.9 ± 1.4 ([−3.7 1.9]) | −1.8 ± 1.6 ([−5.0 1.4]) | −0.1 ± 1.2 ([−2.5 2.4]) | −0.5 ± 1.3 ([−3.1 2.1]) | −2.1 ± 1.4 ([−5.0 0.8]) | −1.6 ± 1.4 ([−4.5 1.3]) |
| CV ± SE (95% CI) | 0.3 ± 0.9 ([−1.5 2.1]) | −0.6 ± 0.9 ([−2.4 1.2]) | 1.2 ± 1.3 ([−1.5 3.9]) | 0.9 ± 1.0 ([−1.2 3.0]) | −0.7 ± 0.4 ([−1.5 0.1]) | 1.3 ± 1.2 ([−1.1 3.6]) | ||
| YA | Mean ± SE (95% CI) | −4.1 ± 1.8 ([−7.7–0.5]) | −7.0 ± 2.0 ([−11.1–2.9])^† | −3.5 ± 1.5 ([−6.6–0.4]) | −3.5 ± 1.6 ([−6.7–0.3]) | −6.1 ± 1.8 ([−9.7–2.5])^ | −4.4 ± 1.8 ([−8.0–0.8]) | |
| CV ± SE (95% CI) | −2 ± 1.1 ([−4.3 0.3]) | −0.2 ± 1.1 ([−2.5 2.1]) | 2.0 ± 1.7 ([−1.4 5.4]) | −0.5 ± 1.3 ([−3.1 2.1]) | −0.8 ± 0.5 ([−1.8 0.1]) | −0.4 ± 1.4 ([−3.3 2.5]) | ||
| Peak Dorsiflexion (°) | OA | Mean ± SE (95% CI) | 2.0 ± 1.1 ([−0.3 4.3])* | 5.5 ± 1.0 ([3.5 7.5])*^† | 2.7 ± 1.0 ([0.7 4.8])* | 2.4 ± 1.1 ([0.1 4.6]) | 5.4 ± 1.4 ([2.6 8.1])^† | 1.8 ± 1.1 ([−0.4 4.1]) |
| CV ± SE (95% CI) | 0.3 ± 0.9 ([−1.5 2.2]) | 0.3 ± 0.2 ([−0.1 0.7]) | 0.7 ± 0.4 ([−0.1 1.4]) | 1.8 ± 1.8 ([−1.8 5.4]) | −0.4 ± 0.7 ([−1.7 0.9]) | −0.3 ± 1.4 ([−3.2 2.5]) | ||
| YA | Mean ± SE (95% CI) | −1.7 ± 1.4 ([−4.6 1.1])* | 1.6 ± 1.3 ([−0.9 4.2])*^† | −1.0 ± 1.3 ([−3.6 1.6])* | −1.0 ± 1.4 ([−3.9 1.8]) | 2.5 ± 1.7 ([−0.9 5.9])^† | −1.1 ± 1.4 ([−3.9 1.8]) | |
| CV ± SE (95% CI) | 0.2 ± 1.2 ([−2.1 2.6]) | 0.4 ± 0.3 ([−0.1 0.9]) | 0.3 ± 0.4 ([−0.6 1.2]) | −0.6 ± 2.2 ([−5.1 3.9]) | 0.9 ± 0.8 ([−0.7 2.5]) | 2.7 ± 1.7 ([−0.8 6.3]) | ||
Indicates significant between-subjects difference (p < 0.05).
Indicates significantly different from within-subjects pre-transition values (p < 0.05).
Indicates significantly different from within-subjects post-transition values (p < 0.05).
Table 4.
Mean ± SE (95% CI) and coefficient of variation (CV) for timing of peak joint angles normalized across the stance phase in older adults (OA) and younger adults (YA), for pre-, during-, and post- transition phases for normal slow and normal fast conditions.
| Normal Slow |
Normal Fast |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Pre | During | Post | Pre | During | Post | |
| Time of Peak Hip Flexion (% stance) | OA | Mean ± SE (95% CI) | 10.5 ± 1.7 ([7.0 14.0]) | 38.2 ± 2.2 ([33.8 42.6])*^† | 9.8 ± 1.3 ([7.1 12.5]) | 11.0 ± 1.3 ([8.4 13.6]) | 34.2 ± 2.5 ([29.1 39.3])*^† | 13.3 ± 2.0 ([9.3 17.3]) |
| CV ± SE (95% CI) | 0.3 ± 0.1 ([0.2 0.4]) | 0.4 ± 0.0 ([0.3 0.4]) | 0.4 ± 0.1 ([0.3 0.5]) | 0.3 ± 0.1 ([0.2 0.4]) | 0.3 ± 0.0 ([0.2 0.3]) | 0.3 ± 0.1 ([0.2 0.5]) | ||
| YA | Mean ± SE (95% CI) | 8.2 ± 2.2 ([3.8 12.7]) | 26.1 ± 2.7 ([20.6 31.7])*^† | 6.1 ± 1.7 ([2.7 9.6]) | 8.0 ± 1.6 ([4.8 11.2])† | 23.1 ± 3.1 ([16.8 29.4])*^† | 12.8 ± 2.4 ([7.9 17.7])^ | |
| CV ± SE (95% CI) | 0.1 ± 0.1 ([0.0 0.3]) | 0.3 ± 0.1 ([0.2 0.4]) | 0.3 ± 0.1 ([0.2 0.4]) | 0.2 ± 0.1 ([0.0 0.4]) | 0.2 ± 0.0 ([0.1 0.3]) | 0.4 ± 0.1 ([0.2 0.5]) | ||
| Time of Peak Hip Extension (% stance) | OA | Mean ± SE (95% CI) | 97.9 ± 1.4 ([95.1 100.7]) | 60.3 ± 3.7 ([52.7 67.8])^† | 97.6 ± 1.7 ([94.2 101]) | 98.3 ± 0.9 ([96.4 100.1]) | 50.2 ± 3.8 ([42.5 58.0])*^† | 97.4 ± 1.8 ([93.9 101.0]) |
| CV ± SE (95% CI) | 0.0 ± 0.0 ([0.0 0.1]) | 0.6 ± 0.1 ([0.4 0.7])^† | 0.0 ± 0.0 ([0.0 0.1]) | 0.0 ± 0.0 ([0.0 0.1]) | 0.4 ± 0.1 ([0.3 0.6])^† | 0.06 ± 0.03 ([−0.01 0.12]) | ||
| YA | Mean ± SE (95% CI) | 99.9 ± 1.7 ([96.4 103.4]) | 68.3 ± 4.7 ([58.7 77.8])^† | 99.7 ± 2.1 ([95.4 104]) | 100.0 ± 1.1 ([97.7 102.3]) | 63.4 ± 4.7 ([53.8 73.0])*^† | 97.5 ± 2.2 ([93.1 101.9]) | |
| CV ± SE (95% CI) | 0.0 ±0.0 ([−0.1 0.1]) | 0.4 ± 0.1 ([0.1 0.1])^† | 0.0 ± 0.0 ([−0.1 0.1]) | 0.0 ± 0.0 ([−0.1 0.1]) | 0.2 ± 0.1 ([0.0 0.4]) | 0.0 ± 0.0 ([0.0 0.1]) | ||
| Time of Peak Knee Flexion (% stance) | OA | Mean ± SE (95% CI) | 85.7 ± 5.1 ([75.4 96]) | 62.2 ± 4.5 ([53.0 71.5])^† | 91.7 ± 4.7 ([82.1 101.2]) | 84.5 ± 5.5 ([73.4 95.7]) | 51.5 ± 3.9 ([43.5 59.5])^† | 79.5 ± 5.6 ([68.2 90.8]) |
| CV ± SE (95% CI) | 0.2 ± 0.1 ([0.1 0.4]) | 0.2 ± 0.1 ([0.1 0.4]) | 0.1 ± 0.1 ([0.0 0.3]) | 0.2 ± 0.1 ([0.1 0.4]) | 0.3 ± 0.1 ([0.2 0.4]) | 0.2 ± 0.0 ([0.1 0.4]) | ||
| YA | Mean ± SE (95% CI) | 80.5 ± 6.5 ([67.5 93.6]) | 65.3 ± 5.8 ([53.7 77])^† | 81.8 ± 6.0 ([69.6 93.9]) | 81.2 ± 6.8 ([67.4 95.0]) | 57.5 ± 4.9 ([47.6 67.3])^† | 81.3 ± 6.9 ([67.3 95.3]) | |
| CV ± SE (95% CI) | 0.4 ± 0.1 ([0.2 0.6]) | 0.3 ± 0.1 ([0.2 0.5]) | 0.4 ± 0.1 ([0.2 0.5]) | 0.3 ± 0.1 ([0.1 0.4]) | 0.3 ± 0.1 ([0.2 0.5]) | 0.4 ± 0.1 ([0.2 0.6]) | ||
| Time of Peak Plantarflexion (% stance) | OA | Mean ± SE (95% CI) | 34.3 ± 5.2 ([23.7 44.9])* | 29.0 ± 3.9 ([21.0 37.0]) | 45.1 ± 5.1 ([34.8 55.4])* | 34.1 ± 5.4 ([23.1 45.1])* | 35.8 ± 4.0 ([27.7 43.9]) | 29.4 ± 4.0 ([21.2 37.6]) |
| CV ± SE (95% CI) | 0.5 ± 0.1 ([0.3 0.7]) | 0.8 ± 0.1 ([0.6 1.1]) | 0.5 ± 0.1 ([0.4 0.7]) | 0.5 ± 0.1 ([0.3 0.7]) | 0.7 ± 0.1 ([0.4 0.9]) | 0.6 ± 0.1 ([0.4 0.7]) | ||
| YA | Mean ± SE (95% CI) | 58.9 ± 6.6 ([45.4 72.3])* | 22.7 ± 5.0 ([12.6 32.7])^† | 64.1 ± 6.4 ([51.1 77.1])* | 59.1 ± 6.7 ([45.5 72.7])*† | 24.0 ± 4.9 ([14.0 34.0])^ | 40.4 ± 5.0 ([30.2 50.5]) | |
| CV ± SE (95% CI) | 0.6 ± 0.1 ([0.4 0.8]) | 1.0 ± 0.2 ([0.7 1-3])† | 0.5 ± 0.1 ([0.3 0.7]) | 0.5 ± 0.1 ([0.2 0.7]) | 0.8 ± 0.2 ([0.5 1.2]) | 0.6 ± 0.1 ([0.4 0.8]) | ||
| Time of Peak Dorsiflexion (% stance) | OA | Mean ± SE (95% CI) | 80.9 ± 5.3 ([70.1 91.7])*† | 26.7 ± 4.0 ([18.7 34.8])^† | 70.4 ± 6.0 ([58.3 82.5])* | 82.1 ± 6.4 ([69.2 95.1]) | 28.4 ± 4.2 ([19.8 37.0]^† | 85 ± 4.2 ([76.5 93.5]) |
| CV ± SE (95% CI) | 0.2 ± 0.1 ([0.1 0.4])* | 0.3 ± 0.0 ([0.2 0.4]) | 0.4 ± 0.1 ([0.2 0.6])* | 0.2 ± 0.1 ([0.0 0.3])* | 0.3 ± 0.1 ([0.2 0.4]) | 0.3 ± 0.1 ([0.1 0.4]) | ||
| YA | Mean ± SE (95% CI) | 51.7 ± 6.7 ([38.1 65.3])* | 30.4 ± 5.0 ([20.2 40.6])^ | 46.2 ± 7.5 ([30.9 61.4])* | 51.6 ± 7.9 ([35.6 67.6])† | 27.9 ± 5.2 ([17.2 38.5])^† | 84.3 ± 5.2 ([73.7 94.8])^ | |
| CV ± SE (95% CI) | 0.6 ± 0.1 ([0.5 0.8])* | 0.4 ± 0.1 ([0.2 0.5])^† | 0.9 ± 0.1 ([0.6 1.1])* | 0.5 ± 0.1 ([0.3 0.7])* | 0.3 ± 0.1 ([0.2 0.5]) | 0.3 ± 0.1 ([0.1 0.5]) | ||
Indicates significant between-subjects difference (p < 0.05).
Indicates significantly different from within-subjects pre-transition values (p < 0.05).
Indicates significantly different from within-subjects post-transition values (p < 0.05).
Hip extension did not differ for YA, yet OA had less hip extension when walking slower post-transition than during transition (Table 3, p < 0.001, ηp2 = 0.068). Both groups produced hip extension earlier in the during-transition step compared with both pre- and post-transition steps (Table 4, all p < 0.001, ηp2 = 0.765). Hip extension timing CV was greatest during transition for both groups (all p ≤ 0.016), and there were no significant differences between groups at any phase (all p ≥ 0.153).
Knee flexion was greatest at the during-transition step for both OA and YA (all p ≤ 0.008, ηp2 = 0.299), but was not different between pre- and post-transition phases (all p ≥ 0.231). All adults produced knee flexion earlier in the stance phase of the during-transition step compared with other phases (Table 4, all p ≤ 0.021, ηp2 = 0.495). OA had reduced variability in peak knee flexion angle compared with YA in both during- (p = 0.027, Table 3) and post-transition (p = 0.013, Table 3) phases.
YA had increased plantarflexion during-transition than either pre- or post-transition phases (Table 3, all p ≤ 0.011, ηp2 = 0.248), while OA did not change. OA produced plantarflexion earlier than YA for both pre- and post-transition phases (Table 4, all p ≤ 0.026, ηp2 = 0.132).
Both OA and YA had increased dorsiflexion during-transition steps (Table 3, all p < 0.001, ηp2 = 0.622), and pre- and post-transition phases were not different. OA were more dorsiflexed than YA during all phases (Table 3, all p ≤ 0.045, ηp2 = 0.126), and produced peak dorsiflexion later pre- and post-transition (Table 4, all p ≤ 0.016, ηp2 = 0.138). YA produced peak dorsiflexion in the during-transition significantly earlier than pre-transition (Table 4, p = 0.014). YA had the lowest dorsiflexion timing CV during-transition (all p ≤ 0.035), while OA had no differences. OA had less peak dorsiflexion timing CV than YA both pre- and post-transition (Table 4, all p ≤ 0.002, ηp2 = 0.335).
Normal-to-faster walking:
CoM velocity in OA during-transition and pre-transition steps was significantly slower than post-transition (Table 2, all p < 0.001, ηρ2 = 0.992). YA showed incremental increases, with fastest CoM velocity occurring post-transition (Table 2, all p < 0.001). Post-transition velocity CV was greater than other phases for OA (all p ≤ 0.023, ηp2 = 0.384), while YA saw lowest velocity CV during pre-transition phase (all p ≤ 0.002). There was a significant phase * group interaction for velocity CV (p = 0.007, ηp2 = 0.166), but none of the phases were significant between age group (all p ≥ 0.091).
For both groups, during-transition step length was shortest, (Table 2, all p < 0.001, ηp2 = 0.989). OA had reduced step length CV than YA during-transition (p = 0.004, ηp2 = 0.223), and saw step length CV greatest post-transition and least variable during-transition (all p < 0.001, ηp2 = 0.858).
OA took wider during-transition steps compared with other phases (Table 2, all p < 0.001, ηp2 = 0.264), yet YA only saw an increase in during-transition step width compared to post-transition steps (p = 0.011). Step width CV was consistently greater in OA than YA (all p ≤ 0.043, ηp2 = 0.226), with OA showing the least variability during-transition (all p ≤ 0.003, ηp2 = 0.388). YA were less variable during-transition than post-transition (p < 0.001).
There were large, significant effects of phase on hip flexion (Table 3, p < 0.001, ηp2 = 0.734), knee extension (p = 0.004, ηp2 = 0.143), plantarflexion (p < 0.001, ηp2 = 0.204), and dorsiflexion (p < 0.001, ηp2 = 0.722). However, age did not alter the pattern.
During-transition steps had the greatest hip flexion compared to pre- and post-transition for both OA and YA (Table 3, all p < 0.001). OA had significantly less hip extension post-transition than during-transition step (Table 3, p = 0.035) but no other phases were significantly different. Both YA and OA had greater knee flexion during-transition compared with post-transition (Table 3, all p ≤ 0.035). OA saw no difference in plantarflexion, but YA had less plantarflexion pre-transition compared with during-transition (Table 3, p = 0.024) with no other phases different. Phase had no effect on joint angle CV (all p ≥ 0.159), although there was an age effect in hip flexion CV (p = 0.041, ηp2 = 0.111).
OA produced hip flexion (Table 4, p = 0.009, ηp2 = 0.121) in the during-transition step and dorsiflexion (Table 4, p = 0.005, ηp2 = 0.188) in the pre-transition steps later than YA. Hip extension (Table 4, p = 0.037, ηp2 = 0.096) in the during-transition step and plantarflexion (Table 4, p = 0.006, ηp2 = 0.211) in the pre-transition steps occurred earlier in stance for OA than YA. For both groups, hip flexion occurred later in the during-transition step than either pre- or post-transition (all p ≤ 0.11). During-transition step hip extension (all p < 0.001) and knee flexion (all p ≤ 0.003) occurred earlier than other phases for both groups. Hip extension timing CV was greater during-transition compared with both pre- and post-transition (all p < 0.001, ηp2 = 0.408) for OA. YA produced plantarflexion later in pre-transition steps than either during- or post-transition (all p < 0.001), while OA did not change. Dorsiflexion occurred earlier during-transition than pre- and post- for both YA (all p < 0.001) and OA (all p ≤ 0.009). Peak dorsiflexion timing CV was less in OA than YA pre-transition (p = 0.008, ηp2 = 0.160), and YA produced dorsiflexion earlier pre-transition than post-transition (p < 0.001).
4. Discussion
Here, we investigated walking speed transitions in YA and OA and found the step during which the speed transition occurred was distinct from steps prior and following transition, in agreement with our initial hypothesis. For NS and NF conditions, both groups had significantly shorter step lengths, more hip flexion, and increased dorsiflexion during the transition step. Peak hip flexion occurred later, while peak hip extension, knee flexion, and dorsiflexion occurred earlier during stance of the during-transition step. The during-transition step for both OA and YA was narrower for the NF condition, while YA increased plantarflexion earlier in stance for the NS condition.
As expected for NS, gait speed decreased at each phase. This was accompanied by reductions in step length, although the shortest step was the one where the speed transition occurred. Reduced step lengths are associated with higher braking forces (Martin and Marsh, 1992). While we did not collect braking forces, this reduced length during speed transitions may drive the observed deceleration (Peterson et al., 2011). In the NF condition, we observed an average increase in gait speed of 0.49 m/s with increased step length, although the during-transition step persisted in having the shortest step length. In this case, the shorter step may allow for a shorter ground contact time which can increase propulsive impulses (Peterson et al., 2011). The during-transition step was narrower for both OA and YA in the NF condition, distinct from steps before and after the speed change. Reduced step width decreases stability, and our observations are similar to those previously reported (Hak et al., 2012). While Hak and colleagues observed an increase in step width (and shorter steps) when responding to a walking perturbation, they concluded local stability reduces in response to a perturbation, like a change in walking speed.
Spatiotemporal findings appear to be a by-product of joint angle changes. We hypothesized stance limb hip extension would reduce over time in the NS condition, but it did not change. However, during-transition there was increased hip flexion, knee flexion, and dorsiflexion.
Increased flexion combined with altered step length could be contributing to increased energy absorption in the joints (e.g., Gordon et al., 1980), leading to the observed deceleration. In the NF condition, YA increased plantarflexion in the during-transition step by an average of 2.6°, although OA did not. Hip flexion and dorsiflexion increased during-transition when compared to post-transition values, while hip extension also increased for OA. This is unexpected as increased hip flexion and dorsiflexion would increase braking force (Lieberman et al., 2015), and increased walking speed can be effected through reducing braking force (Peterson et al., 2011). Increased joint flexion may be indicative of a preparation for propulsion at the hip and ankle, which could explain the similar walking speed for pre- and during-phases in OA.
In the NF condition we saw hip extension occurring 40% earlier in the stance phase in the transition step, and an insignificant increase in peak hip extension, yet this is not coordinated with ankle plantarflexion timing. Acceleration during running is partially achieved through coordinated extension of the ankle, knee and hip joints creating a stiffer lower limb (Hewit et al., 2011). Walking is not expected to follow identical patterns to running. Hip extension, knee flexion, and dorsiflexion occurred much earlier in stance of the during-transition step for both age groups, suggesting the control strategy does alter when changing speed. In NS, we observed a delay in the time to peak hip flexion in the during-transition step, while hip extension and knee flexion occurred earlier. Joint kinematic timing may be a contributor to changing walking speed. Indeed, the timing of peak joint angles, moments, and powers have been implicated in walk-to-run transitions (Diedrich and Warren, 1995; Pan et al., 2021; Seay et al., 2006), thus further analysis of joint work and electromyographic analysis of the lower limb muscles is necessary.
We observed age differences in both NS and NF conditions. In NS, OA had more dorsiflexion at all phases and produced less knee flexion during- and post-transition compared with YA, similar to Monaco et al., (2009). OA rely on more proximal joints for forward propulsion (DeVita and Hortobagyi, 2000) and our results suggest OA may have less energy absorption capacity at more distal joints. During-transition, OA produced peak hip flexion later than YA, possibly indicating a delayed response to energy absorption strategies to elicit braking. OA produced peak plantarflexion earlier and dorsiflexion later than YA at both pre- and post- transition phases, suggesting an age-associated difference in ankle kinematics during walking that disappears when actively decelerating. In NF, OA produced hip flexion later and hip extension earlier than YA. These findings further support OA may be driving acceleration from the hip.
An important limitation to consider is that gait speed can affect the variables we measured (Fukuchi et al., 2019), and we did not control for walking speed in our analysis. However, walking speed was not different between age groups at any phase and the step during which a speed transition occurs was distinct from those either pre- or post-transition, regardless of acceleration or deceleration. To ensure individuals were not approaching the anticipated transition differently between conditions, we ran a paired t-test on the pre-transition steps. There were no differences in pre-transition variables between NS and NF, regardless of age group (all p ≥ 0.176), suggesting the approach to accelerating or decelerating walking speed was similar.
Prior research has shown women have different gait patterns to men, especially as they age (Ko et al., 2011). Despite being underpowered to investigate sex differences, we find distinct differences between NS and NF conditions at the three phases across both age-groups, thus we do not anticipate the inclusion of more than one sex to affect our results.
Our approach to identify the transition point was based on change in velocity. In subsequent data collections, we implemented a marker display when the verbal cue to change speed was given. In post-hoc analysis of these trials, we used both the marker and the change in velocity approach to define the transition point. Consistently, the step during which the transition point occurred was 1 step (0.26–0.48 s) earlier with the marker compared with the change in velocity. It takes time to process an audio cue to change behavior and this could be an interesting future direction of this work.
We looked solely at peak joint angles and their timings during the stance phase. While we found clear age- and phase-differences in walking speed transitions using this approach, an analysis of angle curves may elucidate further differences of interest.
The ability to speed up or slow down gait speed in response to environmental and task demands is vital to independent community ambulation. Here, we have shown that the step during a speed transition is distinct from those steps prior- and following-transition for both YA and OA. While we anticipate gait speed transitions may highlight mobility deficits, more research is needed to see if this approach is sensitive in individuals with mobility disability and underlying movement disorders. The present study is an important first step in establishing normative lower limb kinematics for gait speed transitions in younger and older adults.
Acknowledgements
We would like to thank the participants for their participation, and acknowledge the hard work of our undergraduate team for their assistance in processing the collected data. This work was funded by the National Institute of Health (U01AAG061389).
Footnotes
CRediT authorship contribution statement
Francesca E. Wade: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Grace K. Kellaher: Writing – review & editing, Investigation. Sarah Pesquera: Writing – review & editing, Investigation. Sidney T. Baudendistel: Writing – review & editing, Methodology, Investigation. Arkaprava Roy: Writing – review & editing, Formal analysis. David J. Clark: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition. Rachael D. Seidler: Writing – review & editing, Funding acquisition. Daniel P. Ferris: Writing – review & editing, Funding acquisition. Todd M. Manini: Writing – review & editing, Project administration, Funding acquisition. Chris J. Hass: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Availability of supporting data
Data will be made available upon request.
References
- Almarwani M, VanSwearingen JM, Perera S, Sparto PJ, Brach JS, 2016. Challenging the motor control of walking: Gait variability during slower and faster pace walking conditions in younger and older adults. Arch. Gerontol. Geriatr 66, 54–61. 10.1016/j.archger.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Balasubramanian CK, Clark DJ, Fox EJ, 2014. Walking Adaptability after a Stroke and Its Assessment in Clinical Settings. Stroke Res. Treatm 2014, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Wagenaar RC, Holt KG, 2006. Gait Characteristics of Elderly People With a History of Falls: A Dynamic Approach. Phys. Ther 86, 1501–1510. 10.2522/ptj.20050387. [DOI] [PubMed] [Google Scholar]
- Boyer KA, Johnson RT, Banks JJ, Jewell C, Hafer JF, 2017. Systematic review and meta-analysis of gait mechanics in young and older adults. Exp. Gerontol 95, 63–70. 10.1016/j.exger.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic, New York, NY. [Google Scholar]
- De Asha AR, Robinson MA, Barton GJ, 2012. A marker based kinematic method of identifying initial contact during gait suitable for use in real-time visual feedback applications. Gait Posture 36, 650–652. 10.1016/j.gaitpost.2012.04.016. [DOI] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T, 2000. Age causes a redistribution of joint torques and powers during gait. J. Appl. Physiol 88, 1804–1811. 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Diedrich FJ, Warren WH, 1995. Why change gaits? Dynamics of the walk-run transition. J. Exp. Psychol. Hum. Percept. Perform 21, 183–202. 10.1037/0096-1523.21.1.183. [DOI] [PubMed] [Google Scholar]
- Fellin RE, Rose WC, Royer TD, Davis IS, 2010. Comparison of methods for kinematic identification of footstrike and toe-off during overground and treadmill running. J. Sci. Med. Sport 13, 646–650. 10.1016/j.jsams.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi CA, Fukuchi RK, Duarte M, 2019. Effects of walking speed on gait biomechanics in healthy participants: a systematic review and meta-analysis. Syst. Rev 8, 153. 10.1186/s13643-019-1063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Robertson E, Winter DA, 1980. Mechanical energy generation, absorption and transfer amongst segments during walking. J. Biomech 13, 845–854. 10.1016/0021-9290(80)90172-4. [DOI] [PubMed] [Google Scholar]
- Hak L, Houdijk H, Steenbrink F, Mert A, van der Wurff P, Beek PJ, van Dieën JH, 2012. Speeding up or slowing down?: Gait adaptations to preserve gait stability in response to balance perturbations. Gait Posture 36, 260–264. 10.1016/j.gaitpost.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Herssens N, Verbecque E, Hallemans A, Vereeck L, Van Rompaey V, Saeys W, 2018. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 64, 181–190. 10.1016/j.gaitpost.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Hewit J, Cronin J, Button C, Hume P, 2011. Understanding Deceleration in Sport. Strength Cond. J 33, 47–52. 10.1519/SSC.0b013e3181fbd62c. [DOI] [Google Scholar]
- Hollman JH, McDade EM, Petersen RC, 2011. Normative spatiotemporal gait parameters in older adults. Gait Posture 34, 111–118. 10.1016/j.gaitpost.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreljac A, Imamura RT, Escamilla RF, Edwards WB, 2007. When does a gait transition occur during human locomotion? J. Sports Sci. Med 6, 36–42. [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB, 2008. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J. Biomech 41, 2899–2905. 10.1016/j.jbiomech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan DC, Lee LW, Collins JJ, Riley PO, Lipsitz LA, 2001. Reduced hip extension during walking: Healthy elderly and fallers versus young adults. Arch. Phys. Med. Rehabil 82, 26–30. 10.1053/apmr.2001.18584. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ, 1998. Biomechanical gait alterations independent of speed in the healthy elderly: Evidence for specific limiting impairments. Arch. Phys. Med. Rehabil 79, 317–322. 10.1016/S0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirtley C, Whittle MW, Jefferson RJ, 1985. Influence of walking speed on gait parameters. J. Biomed. Eng 7, 282–288. 10.1016/0141-5425(85)90055-X. [DOI] [PubMed] [Google Scholar]
- Ko S, Tolea MI, Hausdorff JM, Ferrucci L, 2011. Sex-specific differences in gait patterns of healthy older adults: Results from the Baltimore Longitudinal Study of Aging. J. Biomech 44, 1974–1979. 10.1016/j.jbiomech.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S-U, Hausdorff JM, Ferrucci L, 2010. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: results from the Baltimore longitudinal study of ageing. Age Ageing 39 (6), 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer Y, 2005. Effect of Age on Characteristics of Forward and Backward Gait at Preferred and Accelerated Walking Speed. J. Gerontol. Ser. A: Biol. Sci. Med. Sci 60, 627–632. 10.1093/gerona/60.5.627. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Warrener AG, Wang J, Castillo ER, 2015. Effects of stride frequency and foot position at landing on braking force, hip torque, impact peak force and the metabolic cost of running in humans. J. Exp. Biol 218, 3406–3414. 10.1242/jeb.125500. [DOI] [PubMed] [Google Scholar]
- Martin PE, Marsh AP, 1992. Step length and frequency effects on ground reaction forces during walking. J. Biomech 25, 1237–1239. 10.1016/0021-9290(92)90081-B. [DOI] [PubMed] [Google Scholar]
- Monaco V, Rinaldi LA, Macrì G, Micera S, 2009. During walking elders increase efforts at proximal joints and keep low kinetics at the ankle. Clin. Biomech 24, 493–498. 10.1016/j.clinbiomech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Pan J, Zhang S, Li L, 2021. Paired nonlinear behavior of active and passive joint torques associated with preparation for walk-to-run gait transition. J. Electromyogr. Kinesiol 57, 102527 10.1016/j.jelekin.2021.102527. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Kautz SA, Neptune RR, 2011. Braking and propulsive impulses increase with speed during accelerated and decelerated walking. Gait Posture 33, 562–567. 10.1016/j.gaitpost.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantakokko M, Mänty M, Rantanen T, 2013. Mobility Decline in Old Age. Exerc. Sport Sci. Rev 41, 19–25. 10.1097/JES.0b013e3182556f1e. [DOI] [PubMed] [Google Scholar]
- Seay JF, Haddad JM, van Emmerik REA, Hamill J, 2006. Coordination Variability around the Walk to Run Transition during Human Locomotion. Mot. Control 10, 178–196. 10.1123/mcj.10.2.178. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS, 2010. Disability Trends Among Older Americans: National Health and Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am. J. Public Health 100, 100–107. 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers V, Aerts P, Lenoir M, De Clercq D, 2006. Spatiotemporal characteristics of the walk-to-run and run-to-walk transition when gradually changing speed. Gait Posture 24, 247–254. 10.1016/j.gaitpost.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Segers V, De Smet K, Van Caekenberghe I, Aerts P, De Clercq D, 2013. Biomechanics of spontaneous overground walk-to-run transition. J. Exp. Biol 10.1242/jeb.087015. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Guralnik JM, Phillips CL, Coppin AK, Ciol MA, Bandinelli S, Ferrucci L, 2007. Age-Associated Declines in Complex Walking Task Performance: The Walking InCHIANTI Toolkit: AGING AND COMPLEX WALKING TESTS. J. Am. Geriatr. Soc 55, 58–65. 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM, 2002. Environmental Demands Associated With Community Mobility in Older Adults With and Without Mobility Disabilities. Phys. Ther 82, 670–681. 10.1093/ptj/82.7.670. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Taylor CS, Matsuda PN, Studer MT, Whetten BK, 2013. Expanding the Scoring System for the Dynamic Gait Index. Phys. Ther 93, 1493–1506. 10.2522/ptj.20130035. [DOI] [PubMed] [Google Scholar]
- Stimpson KH, Heitkamp LN, Horne JS, Dean JC, 2018. Effects of walking speed on the step-by-step control of step width. J. Biomech 68, 78–83. 10.1016/j.jbiomech.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, Wang C, 2009. Quantitative Gait Markers and Incident Fall Risk in Older Adults. J. Gerontol. Ser. A: Biol. Sci. Med. Sci 64A, 896–901. 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S, Joyce J, Biskis A, Parulekar M, Armijo N, Zampieri C, Tracy R, Palmer AS, Fefferman M, Ouyang B, Liu Y, Berry-Kravis E, O’Keefe JA, 2020. Normative database of spatiotemporal gait parameters using inertial sensors in typically developing children and young adults. Gait Posture 80, 206–213. 10.1016/j.gaitpost.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G, Willmott C, 2012. Higher levels of mobility are associated with greater societal participation and better quality-of-life. Brain Inj. 26, 1065–1071. 10.3109/02699052.2012.667586. [DOI] [PubMed] [Google Scholar]


