Abstract
Traumatic brain injury (TBI) is associated with increased risk for mental health disorders, impacting post-injury quality of life and societal reintegration. TBI is also associated with deficits in psychosocial processing, defined as the cognitive integration of social and emotional behaviors, however little is known about how these deficits manifest and their contributions to post-TBI mental health.
In this pre-clinical investigation using rats, a single mild blast TBI (mbTBI) induced impairment of psychosocial processing in the absence of confounding physical polytrauma, post-injury motor deficits, affective abnormalities, or deficits in non-social behavior. Impairment severity correlated with acute upregulations of a known oxidative stress metabolite, 3-hydroxypropylmercapturic acid (3-HPMA), in urine. Resting state fMRI alterations in the acute post-injury period implicated key brain regions known to regulate psychosocial behavior, including orbitofrontal cortex (OFC), which is congruent with our previous report of elevated acrolein, a marker of neurotrauma, in this region following mbTBI. OFC of mbTBI-exposed rat demonstrated elevated mRNA expression of metabotropic glutamate receptors 1 and 5 (mGluR1/5) and injection of mGluR1/5-selective agonist in OFC of uninjured rat approximated mbTBI-induced psychosocial processing impairment, demonstrating a novel role for OFC in this psychosocial behavior. Furthermore, OFC may serve as a hotspot for TBI-induced disruption of psychosocial processing and subsequent mental health disorders.
Keywords: psychosocial, safety learning, brain injury, head trauma, blast
1. Introduction
At least 3.2 million people in the United States experience disability related to traumatic brain injury (TBI) [1]. Of all TBI-related disabilities, social and emotional dysfunction are considered the most detrimental to daily life and societal re-entry [2, 3]. TBI patients exhibit deficits in interpersonal relationships [4, 5], perceived social support [6], and emotion recognition [7]. Poor psychosocial health is related to poor functional outcome after TBI, including inability to return to work [8]. Healthy social behavior is correlated with overall mental health (reviewed in [9]), and post-TBI psychiatric diagnosis is correlated with poorer psychosocial health [10]. Congruently, TBI patients are diagnosed with psychiatric disorders at a higher rate than the general population (reviewed in [11]), particularly anxiety and mood disorders [12], which can persist years after injury [4, 12].
Despite the important role psychosocial factors play in TBI patients’ psychiatric health, little is understood about how TBI disrupts cognitive integration of social and emotional behaviors, henceforth referred to as psychosocial processing. Given the critical role psychosocial influences serve in overall mental health, disruption of psychosocial processing by TBI may contribute to long-term psychiatric symptoms after TBI. Identifying the neural substrates of psychosocial processing deficits after TBI is critical for improving post-TBI psychosocial processing impairment and related psychiatric disorders.
Often, TBI induces a variety of motor, cognitive, and emotion-like deficits which are difficult to correlate with specific injury physiology. To systematically investigate TBI effects on psychosocial processing, we use a rat model of mild blast TBI (mbTBI) with known injury distribution [13] that lacks motor deficits.
Psychosocial processing can be modeled in rats using the Social Familiarity-induced Anxiolysis (SoFiA) paradigm, which measures a rat’s ability to learn to adapt emotion-like behavior based on social cues [14–16]. In both humans and rats, familiar social cues can act as safety signals facilitating anxiolysis and fear extinction [15, 17, 18]. In SoFiA, rats learn to reduce anxiety-like behavior in the presence of a familiar conspecific, when social familiarity is established while the experimental rat is in an anxiety-like state [15]. Rats learn to integrate both anxious (emotion-like) and social inputs to produce a psychosocial behavioral output. SoFiA requires basolateral amygdala (BLA) [14] and ventromedial prefrontal cortex (vmPFC) function [15], and it has been suggested that functional circuitry between vmPFC, BLA, and orbitofrontal cortex (OFC) is essential for SoFiA [14]. In the present investigation, we hypothesized mbTBI leads to psychosocial-like deficits by disrupting structure-function relationships between the vmPFC and amygdala.
2. Materials and Methods
2.1. Animals
Male 350–450g Sprague-Dawley rats (Envigo/Harlan Laboratories, Indianapolis, IN) were individually housed on a 12-hour light/dark cycle with ad libitum food and water. Rats were handled multiple times before behavioral testing. All behavioral testing was performed between 1100–1500 each day. Procedures were conducted using protocols approved by the Purdue University IACUC (Protocol #1111000280) or Indiana University School of Medicine IACUC (Protocol #11113). Only male rats were used, as the SoFiA behavioral paradigm is not validated for female rats.
2.2. Blast Exposure
Blast exposures were performed as described previously [13, 19–23]. Briefly, animals were anesthetized with intraperitoneally injected ketamine/xylazine cocktail (80mg/kg and 10mg/kg, respectively). It should be explicitly noted that this anesthetic regimen was not utilized during functional MRI sessions (see full details in MRI methods section). Rats were then secured in a custom 10:1 driver:driven chamber length ratio (54 cm : 5.4 cm), open-ended, cylindrical (5.4 cm internal diameter) shock tube blast apparatus with custom acrylic shield body protection covering the body from the neck to the tail (simulating Kevlar vests, which raise the survivable injury threshold by preventing pulmonary and gastrointestinal injuries [24, 25], and stereotactic head fixation (Kopf Instruments; mitigating confounding effects of tertiary impact-acceleration injury). The blast shock wave was generated by using compressed nitrogen to burst a 0.25 mm thickness PET membrane resulting in a blast overpressure striking rats’ heads in a dorsoventral orientation. The dorsum of the rat skull was placed at 3.7cm from the shock tube outlet, within the 1 tube diameter range necessary to preserve ideal Friedlander wave characteristics [26]. Waveform recordings (DPX101–250 dynamic pressure transducer, Omega Eng. Inc.) re-demonstrated near-instantaneous rises to peak pressure followed by overpressure and underpressure periods consistent with classical Friedlander waveform as described in previous publications [13, 19–23]. Physical characteristics were as follows: side-on static (sensor face perpendicular to dorsum of rat skull) 150 kPa maximum overpressure, 1.25 ms overpressure duration, 20 kPa minimum underpressure; face-on dynamic (sensor face parallel to dorsum of rat skull) 160 kPa maximum overpressure, 1.75 ms overpressure duration, 5-kPa minimum underpressure. This predominantly primary blast exposure is considered mild, without inducing significant secondary (penetrating injury), tertiary (impact-acceleration injury), or quaternary (heat, radiation, chemical exposures) blast-related effects [19]. Sham rats received identical treatment including anesthesia, stereotactic head fixation, and exposure to blast noise but not the injurious shock wave.
2.3. Biochemical and Behavioral outcomes following mbTBI
2.3.1. Urine Collection and Analysis
Changes in acrolein, a known post-trauma neurotoxin, were assessed in excreted urine via its stable glutathione-reduced metabolite, 3-HPMA, in accordance with previously published methods [27–30]. Noninvasive urine collection was conducted in Blast and Sham rats on each of the 2 days prior to blast/sham exposure and daily for 4 days starting at 1 day post-injury using standard metabolic collection cages. Rats spent 4 hours per collection session in a free- roaming wire cage with water ad libitum. Urine 3-HPMA was collected and quantified as demonstrated in prior publications [28, 31]. Briefly, a solid phase extraction was used to prepare urine for elution and ensuing liquid chromatography with tandem mass spectrometry (LC/MS/MS) analysis. ENV+ cartridges (Biotage, Inc.) were used for solid phase extraction. Each cartridge was sequentially pre-conditioned with 1mL each of methanol, water, and 0.1% formic acid in water. Urine samples of 500 μL were spiked with 200 ng deuterated 3-HPMA (d3–3-HPMA, Toronto Research Chemicals Inc.) as internal control and then mixed with 500 μL ammonium formate and 10 μL undiluted formic acid, after which they were added to the ENV+ cartridge and washed 4 times with 1 mL of solution in the following order: 0.1% formic acid diluted in water (twice), 10% methanol, and 10% methanol in 90% 0.1% formic acid. Cartridges were subsequently dried with nitrogen gas for 15 minutes and then eluted three times using 2% formic acid in 600 μL methanol. Eluates were spin-dried in a vacuum evaporation centrifuge and reconstituted in 100 μL 0.1% formic acid in water prior to LC/MS/MS analysis.
LC/MS/MS was completed with an Agilent 1200 Rapid Resolution LC system coupled to an Agilent 6460 series QQQ MS system. Assuming healthy kidney function, 3-HPMA levels were normalized to urine creatinine concentrations using a commercially available creatinine assay kit (Cayman Chemical Company). Urine creatinine levels are relatively stable and commonly used to normalize measurements across variable water content due to differential hydration status of subjects [32, 33]. Creatinine standards, 10x, and 20x diluted urine samples were incubated in a 96 well plate for 20 minutes at room temperature and shielded from ambient light. Initial raw optical density was obtained using SPECTRAmax raw optical density plate reader (Molecular Devices). 5 μL of active acid solution were added to the samples and incubated on a shaker for 20 minutes at room temperature, after which final raw optical density was read. The two readings were input into the kit’s calibration curves to quantify creatinine levels in each sample, then used to normalize 3-HPMA measurements.
2.3.2. Open Field
Open Field (OF) testing is a validated measure of anxiety-like behavior in rodents [34] and was used seven days following blast/sham exposure, to evaluate baseline gross motor and non-social anxiety-like behaviors. Rats were placed in a black Plexiglas open top box with L x W x H dimensions 91.44 cm x 91.44 cm x 30.48 cm, for 5 min under dim red lighting. The data generated included anxiety-like measures of time spent in the three designated zones of the Open Field apparatus, (outer, middle and center zones) and motor measures of total distance traveled, maximum and average speeds. The OF test served as habituation to the Social Interaction testing apparatus.
2.3.3. Rotor Rod
The rotor rod is a common test to evaluate locomotor coordination and motor activity in rodents following TBI [35] was conducted following OF testing on day 7 after sham/blast exposure. Rats were placed on a custom-built laddered wheel (requiring grasping the rungs with forepaws to successfully ambulate) rotating at speed increasing steadily from 3–30RPM over 3 min. After training (3 consecutive 60+ sec runs), the test was performed 3 times per rat. Session end criteria were the rat falling or remaining stationary for one complete wheel revolution.
2.3.4. SI Testing
Social Interaction (SI) testing was completed as described previously [36, 37]. This SI test is a validated measure for anxiety-like behavior [38]. Rats were taken in their home cages to a dimly red-lit staging area located just outside the behavioral testing room for a minimum of 30 min prior to the SI test. The SI test consisted of simultaneously placing the experimental rat and an age, weight and sex-matched partner rat into the OF apparatus for 5 min. The apparatus was cleaned thoroughly between each animal. SI tests were recorded from above and scored using ODlog for Mac OS X version 2.6.1 (Macropod Software). Scoring was done by an observer blinded to the treatment (Blast vs Sham), and behavior was scored according to the amount of time the test rat initiated non-aggressive physical contact or investigation (as previously described in detail [15]). Partner-initiated contact was not scored, as it has been demonstrated that partner rat SI time and anxiety state do not influence experimental rat SI time [14, 15]. No incidences of aggression were observed during any of the SI testing sessions. Partner rats were used a maximum of 2 times per day and were paired with one Blast and one Sham rat each day, tests being separated by at least 30 min.
2.3.5. Bright Light Challenge
The Bright Light Challenge (BLC) procedure has been previously described in detail [15] and is a validated anxiogenic stimulus for rats undergoing SI testing [39]. Briefly, rats are placed in dimly lit (red lighting) environment for at least 30 min prior to behavioral testing/challenge. Just prior to testing, rats were brought into the testing room that was also dimly lit with red lighting. Immediately following the placement of the experimental and partner rats into the testing arena, the BLC was initiated by abruptly switching from the dim red lighting in the room to bright white lighting by turning on the lights of the room with additional overhanging lights aimed at the open field arena. The brightness of the lights was 488 lux at the level of the rats’ faces at the midpoint of the box. The lights remained on for the entire 5-minute testing session and were turned off as soon as the 5 min session concluded to resume dim red light conditions.
2.3.6. SI-Habituation Training to Measure Social Familiarity-induced Anxiolysis (SoFiA)
Twenty-four (cohort 1) or forty-eight (cohort 2) hours after baseline SI testing, SoFiA acquisition was measured through the SI-habituation (SI-hab) testing paradigm as described previously [14, 15]. This paradigm consists of performing the SI test as described above except the test rat is paired with the same partner conspecific each day for 6 consecutive daily SI sessions. The SI-hab sessions were all performed under BLC conditions.
2.3.7. Social Recognition Test
The Social Recognition (SR) or discriminatory task is a validated measure of social memory in rodents [40]. Experimental subjects were given a SR) test to verify the test rat’s ability to distinguish the familiar partner from a novel partner, a foundational memory component underpinning SoFiA. The 2 min SR test was conducted in the same OF apparatus, now bi-partitioned with two inserts (plastic vertical bars) enclosing conspecifics in opposing corners and a test rat freely moving in the center. One novel and one familiar conspecific were randomly assigned to each corner in a counterbalanced fashion. Testing was performed under BLC conditions after blast/sham, as opposed to under dim red light after intracranial injection. Amount of time the test rat spent in the familiar or novel conspecific zone was quantified. Zones extended from the partitions to the diagonal midline of the OF box. The familiar conspecific was the same partner used for SI-hab training.
2.3.8. Tail Suspension Test
The Tail Suspension Test (TST), a validated measure of despair-like behavior in rodents, was performed similarly to a previous protocol [41]. TST consists of wrapping rats’ tails in protective cloth tape, then suspending the tail via duct tape from a horizontal bar 4ft above ground for 5 min. Time spent immobile was quantified by ANY-maze software.
2.4. Localizing neurotrauma following mbTBI exposure: seed-based resting state fMRI
2.4.1. T2-weighted and Resting State Functional Magnetic Resonance Imaging
T2-weighted anatomical MRI was performed on a separate cohort of rats at pre-blast, 24 hours post-injury, and 1-week post-injury. A separate cohort of rats was necessary for logistics purposes as well as to prevent unmeasurable effects of sedation, additional animal handling, and long experimental times on subsequent behavioral and biochemical assays in the primary animal cohorts. Rats were secured in the scanner with a custom 3D-printed MRI-compatible stereotaxic apparatus, where nosecone isoflurane was continuously administered (0.1 – 0.5%, SomnoSuite Kent Scientific) with dexmedetomidine sedation (subcutaneous 0.03 mg/kg bolus then continuous 0.03 mg/kg/h infusion), preserving cortical networks for fMRI as has been previously described [42]. Respiration rate (30–40 breaths/minute) and body temperature (36–37 ° C) were continuously monitored, recorded, and maintained via minor anesthetic and warming surface adjustments. Tuning, matching, localization, B0 adjustment, and an ellipsoid mapshim encompassing only brain tissue were performed for each animal.
T2-weighted anatomical magnetic resonance imaging (MRI) data were acquired on a 7 Tesla scanner (Bruker Biosystems) with Paravision 6.0.1 software. Probe (RF RES 300 1H 112/086 QSN TO AD, Bruker Biosystems), gradient coil (BA-GA12SHP BC 70/30), and surface coil (RF SUC 300 1H R BR QSN RO AD, Bruker Biosystems) were consistent for all acquisitions. High-resolution T2-weighted images were acquired with 240 × 120 × 90 voxels at 0.15 mm × 0.15 mm (in-plane) × 0.3 mm (slice thickness) in interleaved coronal slices. Echo time (TE) 11.34 ms, repetition time (TR) 9979 ms, flip angle (FA) 90°, RARE factor eight, six averages, and one repetition.
Resting-state functional magnetic resonance imaging (rs-fMRI) was performed; this protocol has been demonstrated to reveal the brain’s intrinsic functional networks [43]. rs-fMRI was performed on the same rats used for T2 imaging above at pre-blast, 24 hours post-injury, and 1-week post-injury in the same session immediately following T2 image acquisition. This protocol has been demonstrated to reveal the brain’s intrinsic functional networks Six sequential rs-fMRI scans were performed per subject per time point using 600 repetition 2-D single-shot gradient echoplanar imaging (EPI) sequence (TR = 1 s, TE = 15 ms, FA 55°, slice thickness 1mm, in-plane resolution 0.5×0.5 mm2).
The fMRI images underwent slice-time correction (slicetimer), motion correction (3dvolreg for inter-volume motion, retroicor for respiratory/cardiac activity), and EPI distortion correction with normalization to Waxholm Space Atlas (WHS) space cortical surface segmentation by nonlinear intra- and inter-subject T2 image co-registration (flirt & fnirt) using Analysis of Functional Neuroimages (AFNI) software and custom Matlab scripts [44, 45]. Detrending was performed by 2nd-order polynomial function regression and bandpass filtering for pulse and respiration. Mean, standardized signal variance was subtracted. Spatial smoothing utilized 3-D Gaussian kernel (0.5mm full width at half maximum) to minimize spurious nearest neighbor correlations. Population-averaged seed-based correlation analysis was performed with custom Matlab scripts using vmPFC or amygdala as seed regions.
An adapted version of the Worsley et. al. [46] spatial extent method with Gaussian smoothing to correct for voxel-wise spurious nearest neighbor correlations while preserving region-wise non-spurious relationships was incorporated into the image processing pipeline. Traditionally, a conservative α of 0.01 is recommended as a global statistical threshold when this processing method is incorporated, however we elected to be even more conservative given the relatively small number of subjects and chose an α of 0.005 as the significance level.
2.5. Contributions of glutamatergic signaling in OFC to mbTBI-induced psychosocial processing impairment
2.5.1. Taqman® Low Density Array Gene Expression Analysis
15 days after blast/sham exposure, rats were sacrificed and brains processed for RT-PCR as previously described [47]. Briefly, OFC were dissected from 300 μm frontal cortex sections and placed in lysis buffer. RNA was extracted and converted to cDNA, which was transferred into Taqman® Low Density Array (TLDA) microarrays (Thermo-Fisher Scientific) consisting of 96 primers for endogenous control genes and genes related to GABA/glutamate receptors. Expression of all GABA/glutamate-related genes were normalized to endogenous control genes. Endogeneous control genes included Actb, Hprt1, and Rplp2, which were selected using geNorm (Biogazelle, Gent, Belgium) from the following: S18, Actb, CAMK2, Gapdh, Hprt1, Rplp2, and Ubc.
2.5.2. Stereotaxic Surgery and Microinjections
Isoflurane-anesthetized rats were implanted with bilateral guide cannulae (Plastics One) at +3.2mm anteroposterior, +/− 2 mm mediolateral, and −4.8 mm dorsoventral to bregma [48], then fitted with dummy cannulae and protective cap and given at least 4 days to recover, receiving four injections of 0.03 mg/kg buprenorphine subcutaneously every 12 hours for pain. For intracranial microinjection, protective caps and dummy cannulae were removed and a bilateral injector cannula was inserted.. Small 33-gauge injector cannula that protrude 1 mm beyond the guide cannula into the OFC were used to minimize damage to OFC tissue from repeated injections. Injectors were connected to 10 μL syringes, which administered 50 μM (S)-3,5-Dihydroxyphenylglycine (DHPG) (Tocris Bioscience) or 0.9% saline vehicle of a total volume of 0.5 μL per side at 0.25 μL/min. Injectors remained in place for 1 min following injection. Caps and dummy cannulae were replaced and rats returned to home cage. Behavior testing commenced 30 minutes after microinjection and preceded SI-hab training daily.
2.5.3. Injection Site Confirmation
Isoflurane-anesthetized rats were intracranially injected via the same guide cannulae with 0.5 μL per side of diluted Normal Donkey Serum (Abcam) in 0.9% saline at 0.25 μL/min, followed by transcardial perfusion. Perfused brains were sliced, 30 μm coronally, and collected in 4 parallel series. For visualization of injection sites, one series of sectioned frontal cortex from each rat was processed using immunohistochemistry for donkey serum similar to procedures described in [49]. Briefly, slices were thoroughly washed and blocked for at least one hour in an incubation solution (PBS containing 0.1% bovine serum albumin and 0.4% triton-X-100). Then, slices were incubated with biotinylated goat anti-donkey secondary antibody (Thermo-Fisher) 1:500 dilution in incubation solution for one hour, followed by washing and exposure to avidin-biotin-horseradish peroxidase (ABC-Elite, 1:1000 dilution in PBS; Vector Laboratories). Complexes were visualized by a 15 min exposure to chromogen solution containing 0.02% 3,3’-diaminobenzidine tetrahydrochloride (DAB, Sigma). The reaction was terminated by extensive washing in PBS, then slices were mounted on cover-slipped glass slides and visualized under light microscope. All visualized injection sites were of expected size without excessive damage to the surrounding OFC tissue.
2.6. Statistics
All statistical tests were performed in Prism 8.0 software (GraphPad). Multiple day testing (behavior and urine) were performed using 2-way repeated measures analysis, with main factors being treatment group (Sham vs Blast exposure or Drug vs Control) and day of testing as a repeated main factor. Post-hoc tests were performed as follows: within analysis compared to control condition (e.g. SI-hab day 1 vs other days) were performed using Dunnett’s test; between treatment groups and within comparisons under different conditions analyses were performed using Fisher’s LSD tests. In all cases where Fisher’s LSD was used as post-hoc, they were protected by appropriate significant main effect or interaction in the ANOVA test. To evaluate changes in rs-fMRI correlations, the Fisher’s r-to-z transform was applied to all images followed by a voxel-wise paired t-test. Significance levels were set at p < 0.05. Treatment group sizes were based on a priori power analyses, found to be N=5–7 per group, which is similar to previous experiments conducted in our lab. Data was examined for outliers and normality using the ROUT Method (Q set to 5%) and Shapiro-Wilk Normality Tests, respectively.
3. Results
3.1. Biochemical and behavioral outcomes following mbTBI
3.1.1. Blast exposure resulted in mbTBI
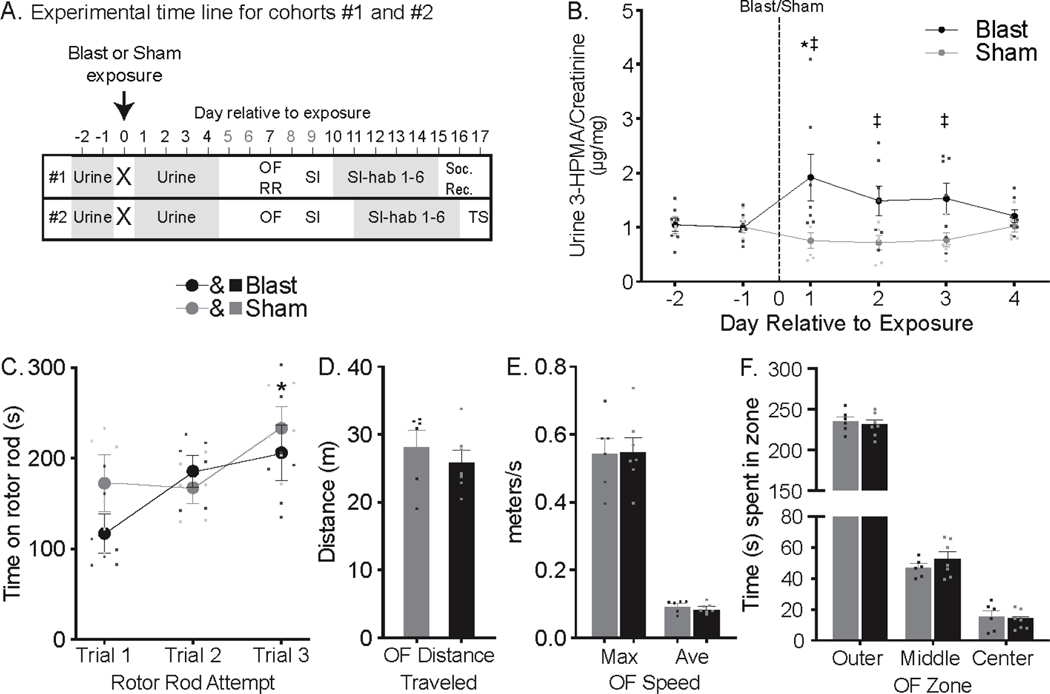
The protocol and timeline used for Experiment 1 is summarized in Figure 1a. Animals underwent testing in two separate cohorts in order to optimize time windows during which daily behavioral testing occurred. Cohorts were tested at different times during the year, and data from each cohort was analyzed separately. Testing procedures were identical between cohorts with any exceptions noted in the text. For all results of Experiment 1, cohort 1 (n=6 sham, n=7 blast) is referenced unless otherwise indicated as cohort 2 (n=9/group). Urine was collected from all rats and analyzed for 3-HPMA, a stable glutathione-reduced metabolite of acrolein, which is a known marker of oxidative stress and neurotrauma [19]. Blast exposure significantly increased urine 3-HPMA levels (Two-way repeated measures ANOVA, exposure main effect F5,55=15.54, P=0.0023 & exposure X day interaction F5,55=2.65, P=0.0322), compared to pre-blast levels on post exposure day 1 and compared to sham levels on days 1–3 (Dunnett’s p=0.0094 and Fisher’s LSD p ≤ 0.0132, respectively; Figure 1b) in cohort 1 animals. Cohort 2 animal urine analysis for 3-HPMA levels was performed but the data were discarded due to irregularities noted in the internal control deuterated 3-HPMA measurements not matching the applied 200 ng/sample (eventually discovered to be due to a faulty LC/MS/MS machine column). The injury severity in this study, as in prior reports using the same model and exposure conditions [13, 19–23], is considered mild based on the absence of acute motor deficits. Consistent with prior work, one week following blast exposure rats displayed no motor deficits relative to sham rats in rotor rod, where time on the rod increased in both groups equally in subsequent trials (main effect of trial F2,22=5.043, p=0.0157; Figure 1c) or open field (OF) tests [unpaired t-test1,11 t=0.859, p=0.409 (distance); t=0.088, p=0.932 (speed max), t=0.843, p=0.417 (speed avg), Figure 1d]. Blast rats also did not display changes in baseline anxiety-like behavior compared to sham rats in OF test as measured by time spent in outer, middle, or center zones (Figure 1e). Cohort 2 blast rats likewise demonstrated sham-equivalent motor and anxiety-like behaviors in OF (Supp Figure 1).
Figure 1: Blast exposure resulted in mbTBI.
(A) Experiment 1 timeline for cohorts #1 (top row) and #2 (bottom row). X indicates exposure to either sham (n = 6, 9 in cohort #1 & #2 respectively) or blast (n = 7, 9 in cohorts #1 & #2 respectively), followed by experimental tests and day relative to exposure they were performed. Behavior tests were delayed to 1 week post-injury to allow for animal recovery from acute injury and to prevent the interference of urine collection on behavioral test outcomes. The main test of interest, SI-hab, was delayed to start 9–10 days post-injury as baseline behavioral measures (OF, SI) and acclimation to test environments were necessary to complete prior to SI-hab. Behavior tests: OF=Open Field, RR=Rotor Rod, SI=Social Interaction, SI-Hab=SI-habituation, Soc. Rec.=Social Recognition and TS=Tail Suspension. (B) Presented here are mean±SEM μg of 3-HPMA per mg creatinine (a marker of neurotrauma) from daily urine samples from cohort #1 including Sham (gray circles) and blast (black circles) exposed rats. Urine 3-HPMA was increased in blast rats on post- injury day 1 compared to pre-injury (* Dunnett’s p=0.0094) and compared to sham rat on post-injury days 1–3 (‡ Fisher’s LSD p≤0.0132). Blast rats did not demonstrate motor deficits. (c) On rotor rod, blast rats performed comparably to sham rats, where time increased in both groups equally in subsequent trials (main effect of trial F2,22=5.043, p=0.0157). Presented here are mean±SEM time on rotor rod for cohort #1. (* indicates significant increase compared to trial 1 for both group). (d, e and f) Presented here are mean±SEM for total distance traveled (d), maximum and average speed (e) and time spent in outer, middle and center zones (f) of cohort #1 sham (gray bars) and blast (black bars) rats during 5 min open field test. No differences were observed between sham and blast rats in any of the OF measures.
3.1.2. mbTBI resulted in selective deficits in psychosocial-like processing
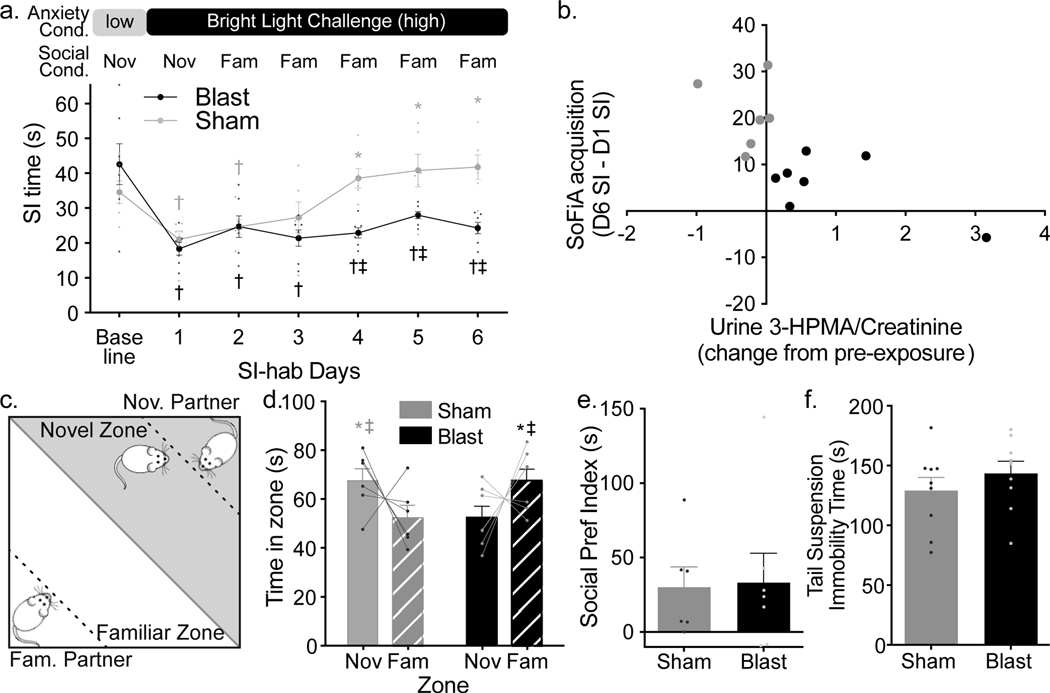
Starting at 9 days following sham or blast exposure, anxiety-like behavior was assessed using the social interaction (SI) test followed by assessment of psychosocial processing using the SI habituation training (SI-hab), which entails repeated SI testing (SI-hab days 1–6) using the same conspecific partner rat and anxiogenic Bright Light Challenge (BLC) conditions (Figure 2a, top). Sham and blast rats had equal anxiety-like responses under dim light conditions (baseline) and to initial anxiogenic challenge (SI-hab day 1), but their anxiety-like behavioral response to social familiarity differed across SI-hab (Two-way repeated measures ANOVA exposure X day interaction F6,66=5.281, P=0.0002; Figure 2a, bottom). Specifically, all rats had similar baseline SI times, which were significantly reduced in response to BLC on SI- hab day 1 (Fisher’s LSD p≤0.038). SI time reduction was unaffected by social familiarity in blast rats, and SI times remained significantly lower than baseline across all SI-hab days (Fisher’s LSD p≤0.037). Conversely, SI time in sham rats increased with social familiarity, where SI times on SI-hab days 4–6 were no longer reduced compared to their baseline and were significantly increased compared to SI-hab day 1, (Dunnett’s p≤0.0051) and compared to blast rats (Fisher’s LSD p≤0.005). Thus, sham rats acquired SoFiA and blast rats failed to acquire SoFiA. This selective blast-induced SoFiA impairment was replicated in a different group of rats (Supplemental Figure 2). SoFiA acquisition values (calculated as difference in SI time between last and first SI-hab session) had a significant inverse correlation with the change in urine 3-HPMA levels 24 hours after injury; greater increases in urine 3-HPMA corresponded to lower SoFiA acquisition values (Pearson r=−0.682, p=0.0102, Figure 2b). Please note Cohort 2 animals began the SI-hab protocol at 2 days following baseline testing whereas Cohort 1 began the SI-hab protocol at 1 day following baseline testing. This difference in timing was due to equipment availability, and we have previously found that having 1 vs. 2 days between baseline and SoFiA testing day 1 does not impact SoFiA acquisition (unpublished data).
Figure 2: mbTBI produced selective psychosocial-like deficits.
(a, top) Schematic of social familiarity-induced anxiolysis (SoFiA) paradigm. (a, bottom) SI times (mean±SEM) for sham (n=6, gray throughout) and blast rats (n=7, black throughout) at baseline and during 6 SI-habituation days (cohort #1). Blast rats demonstrated SoFiA deficit compared to sham (Two-way repeated measures ANOVA exposure X day interaction F6,66=5.281, P=0.0002). SI times for blast and sham rats reduced from baseline during bright light challenge (BLC) on SI-hab day1 († = Fisher’s LSDp≤0.038). Sham rats acquired SoFiA as SI time increased compared to SI-hab day 1 on SI-hab days 4–6 (* Dunnett’s p≤0.0051). Blast rats’ SI time remained below baseline across all SI-hab days († Fisher’s LSD p≤ 0.037) and compared to sham rats on SI-hab days 4–6 (‡ Fisher’s LSD p≤0.005). (b) SoFiA acquisition value (difference in SI time between last and first SI-hab days) inversely correlated with change in urine 3-HPMA levels between pre-injury and post-injury day 1 (Pearson r=−0.682, p=0.0102). (c) Schematic of social recognition (SR) test. (d) Mean±SEM time spent in novel (solid bars) or familiar (striped bars) conspecific zones for sham and blast rats. Both groups distinguished novel vs. familiar conspecifics, but blast exposure induced differential social familiarity responses (2-way ANOVA, exposure X zone interaction F1,22=9.924, P=0.0046). Sham rats (n=6) spent more time in the novel zone compared to blast rats (n=7) and the familiar zone (Fisher’s LSD, ‡ p=0.045 and * p=0.037 respectively). Blast rats spent more time in the familiar zone compared to sham rats and the novel zone (Fisher’s LSD, ‡ p=0.029 and * 0.037 respectively). (e) Mean±SEM social preference index (absolute difference in time spent between conspecifics zones) for blast and sham rats. (f) Blast and sham rats (cohort #2, n = 9/group) demonstrate equivalent time immobile (mean±SEM) in tail suspension test.
At 24 hours following the last SI session, blast and sham rats were assessed for their ability to remember the familiar conspecific using a social recognition (SR) test (Figure 2c). Both sham and blast rats differentiated a familiar conspecific from a novel conspecific by spending significantly different amounts of time with the novel compared to the familiar conspecific (2-way ANOVA, exposure X zone interaction F1,22=9.924, P=0.0046), indicative of intact social memory (Figure 2d). It is worth noting that the overall memory effect in each group were modest and not displayed by every rat, however the overall pattern of zone selection was opposite in sham compared to blast rats. Sham rats spent more time with the novel conspecific (expected rodent behavior [50]), blast rats demonstrated equivalently greater time spent with the familiar conspecific (Figure 2d,e). Sham rats spent significantly more time in the novel conspecific zone, compared to the familiar zone and compared to blast rats (Fisher’s LSD p=0.045, p=0.037 respectively) while blast rats spent significantly more time in the familiar conspecific zone compared to the novel zone and compared to sham rats (Fisher’s LSD p=0.029, p=0.037 respectively). Collectively, these data suggest an intact ability to discriminate between conspecifics, but an altered mechanism of social processing in blast-injured compared to sham rats. Time spent in the novel zone correlates with change in 3-HPMA (Pearson’s r=0.517, p(one-tailed)=0.0353, number of pairs =13) (data not shown). Additionally, time spent in the novel zone correlates with SoFiA acquisition determined as change in SI time from end of SI-hab training to SI-hab day 1 (Pearson’s r=0.6581, p=0.0145) (data not shown). In rats used in the replicated SoFiA study, sham and blast rats displayed equivalent immobility time in the tail suspension test (TST) 24 hours after the last SI-hab session (Figure 2f), further supporting selective psychosocial-like processing deficit following mbTBI exposure.
3.2. Localizing neurotrauma following mbTBI: seed-based resting state fMRI
3.2.1. mbTBI incited acute seed-based resting state fMRI alterations
Our initial hypothesis regarding blast-induced alterations in vmPFC-amygdala structure and function, based on prior work demonstrating these regions are critical for SoFiA [14, 15], was not borne out on initial analysis of post-mortem brain tissue of the rats from cohorts 1 and 2. Western blotting (cohort 1) of excised vmPFC and bilateral amygdala tissue was negative for expected increases in acrolein-protein adducts or inflammatory markers (not shown). Similarly, immunohistochemistry (cohort 2) demonstrated no change in neuronal cell counts (NeuN staining), microglial morphology (Iba1 staining), or overall neuroinflammatory changes (Iba1 and GFAP staining) – all not shown. It is of note that these post-mortem analyses were performed on tissue collected 18 days after sham/blast exposure.
An additional group of rats were studied with rs-fMRI at pre-injury, 24 hour post-injury, and 1 week post-injury time points (n=3; 6 repetitions/subject/time point). T2-weighted anatomical images collected in parallel did not demonstrate gross abnormalities on qualitative inspection (Supp Figure 3). For rs-fMRI data analysis, the vmPFC or bilateral amygdala were used as seed regions due to their well-elucidated roles in SoFiA [14, 15] and histologic integrity following mbTBI in this model (lack of oxidative stress, inflammation, cell death, or significant mechanical loading) [13].
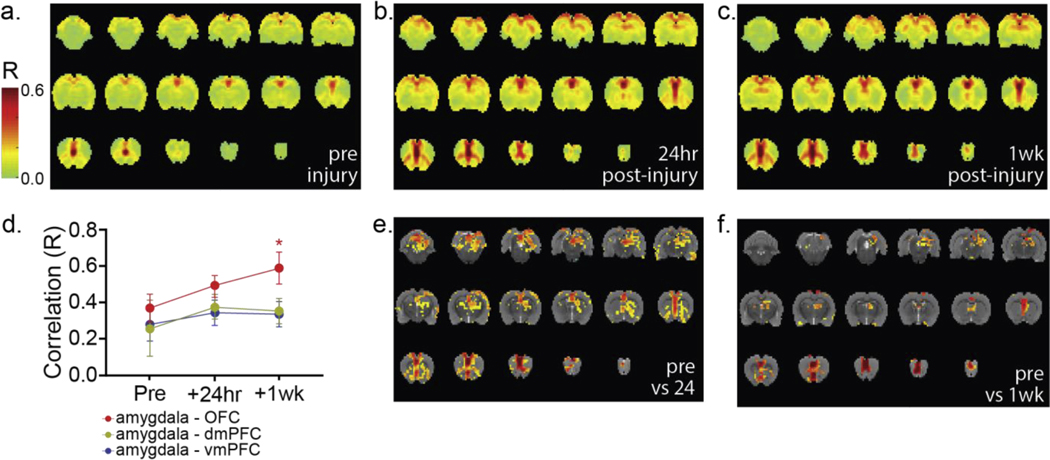
The organized functional network containing the vmPFC, amygdala, and OFC was well-demonstrated at all time points, remained intact post-injury, and was well-isolated from background neural activity using the vmPFC as the seed (Figure 3a-c). Significant subregional voxel-wise increases in correlated functional activity were observed in each region of interest after blast exposure compared to pre-injury imaging [voxel-wise paired t-test, (Figure 3e) t=3.94 – 15.96, p=0.005 – 4.75×10−8, (Figure 3f) t=4.46 – 11.81, p=0.005 – 5.72×10−6]. Many voxel-wise increases in correlated functional connectivity were transient, returning to baseline levels at 1 week post-injury. However, region-wide trends of increased correlated functional activity observed between the amygdala and OFC at 24 hours persisted and were significant at 1 week post-injury (Fig 3d, nested 1-way ANOVA, F2,6=12.09, p=0.0079). Such changes suggest functional reorganization within network regions rather than between-region damage since post-injury hypo-connectivity between network member regions was not observed. This is consistent with our previously reported auditory evoked potential recordings [21], which suggested intact inter-regional signal conduction but altered signal processing within network member regions.
Figure 3: Localizing neurotrauma following mbTBI exposure: seed-based resting state fMRI.
We assessed rs-fMRI connectivity using the vmPFC as the seed region at (a) pre-blast, (b) post-injury day 1, and (c) 1-week post-injury in the same animals (n = 3; 6 repetitions/animal/time point). No major changes in gross network architecture were observed (a-c). Some increased connectivity was observed between network member regions, but all regions observed at pre-blast imaging remained part of the functional network at both post-injury time points. (d) Increases in region-wise (aggregate of all voxels in each region) correlated functional activity (nested 1-way ANOVA, mean±SD) between the amygdala and prefrontal cortical regions including the vmPFC (F 2,6=1.241, P=0.3541), dmPFC (F2,6=1.413, p=0.3142), and OFC (F2,6=12.09, p=0.0079) were observed across time points. OFC – amygdala correlated functional activity was significantly increased, compared to preinjury at the 1 week postinjury time point, while the remaining tracts did not differ significantly from pre-injury levels. Interestingly, at both (e) 24 hours post-injury (voxel-wise paired t-test, t=3.94 – 15.96, p=0.005 – 4.75×10−8) and (f) 1 week post-injury (voxel-wise paired t-test, t=4.46 – 11.81, p=0.005 – 5.72×10−6), rs-fMRI correlated functional activity assessed via intra-regional, voxel-wise analysis demonstrated subregional variation with significant differences at both post-injury time points in all regions of interest. In (e,f), all colored voxels have p≤0.005. Color bar at left applies to panels (a-c).
3.3. Contributions of glutamatergic signaling in OFC to mbTBI-induced psychosocial processing impairment
3.3.1. Expression of GABA-/Glutamate-related genes elevated in OFC following mbTBI
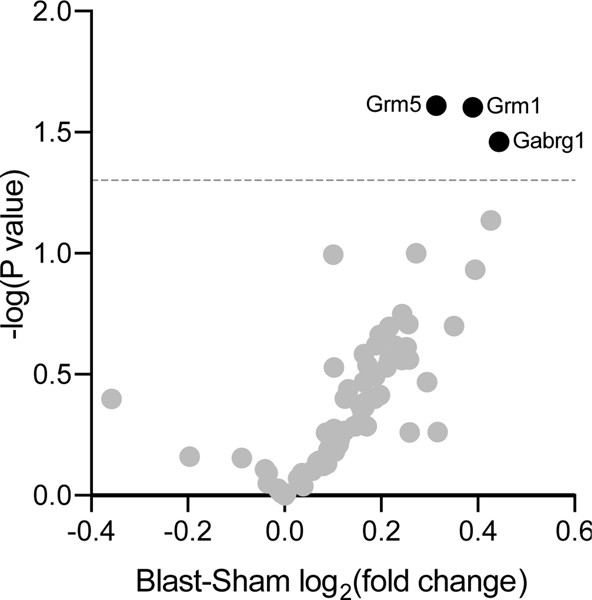
GABA-/glutamate-related gene expression was measured in OFC of a separate cohort of blast vs. sham exposed rats (n=6/group) using a custom TaqMan Low Density Array (described in [47]). 15 days after sham or mbTBI exposure (corresponding with the end of SI-hab testing window), rats were sacrificed, tissue processed for RT-PCR, then GABA-/glutamate-related gene expression assay was run (Supp Figure 4). Relative expression of only 3 of the 87 genes assayed were significantly different between groups, based on analysis criteria established in [47] (Figure 4). Expression of Grm1, Grm5 [metabotropic glutamate receptors 1 and 5 (mGluR1/5)] and Gabrg1 (GABA type A receptor gamma1 subunit) were significantly elevated in blast rats relative to sham (t-test p=0.0249, p=0.0246 and p=0.0346 respectively).
Figure 4: Expression of GABA- and Glutamate-related genes in OFC following mbTBI vs. sham exposure.
Presented here is a volcano plot of GABA and glutamate related gene expression from the OFC of blast rats relative to sham rats (n=6/group). Mean relative expression are plotted on the x-axis and corresponding p-values from t-test (sham vs blast) are plotted on the y-axis. Blast lead to significant increased expression of Grm1, Grm5, and Gabrg1 compared to sham (t-test p=0.0249, p=0.0246 and p=0.0346, respectively). Grm1 = Metabotropic Glutamate receptor 1, Grm5 = Metabotropic Glutamate receptor 5, and Gabrg1 = GABA A receptor gamma subunit 1.
3.3.2. Injection of mGluR1/5 selective agonist into OFC of uninjured rats approximated mbTBI-induced psychosocial-like processing deficit
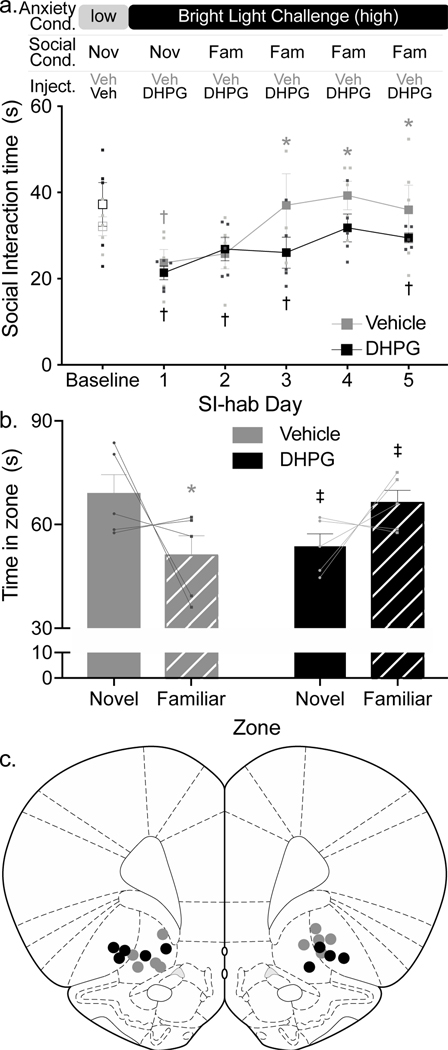
To assess OFC mGluR1/5 involvement in SoFiA acquisition, exposure-naïve rats (no sham or mbTBI) received bilateral intracranial injections of selective mGluR1/5 agonist, Dihydroxyphenylglycine (DHPG, 50 μM), or saline vehicle (n=5/group) into OFC 30 min prior to SI-hab days 1–5. DHPG injection attenuated SoFiA acquisition compared to vehicle injection (Two-way repeated measures ANOVA drug X day interaction F5,40 = 2.681, P=0.0351, Figure 5a). Vehicle-injected rats acquired SoFiA with SI times significantly greater than SI-hab day 1 on social training days 3–5 (Dunnett’s p≤0.0069), while DHPG-injected rats only had a transient increase in SI time compared to social training day 1 on day 4 (Dunnett’s p=0.0277).
Figure 5: Injection of DHPG into OFC of uninjured rats partially recapitulated mbTBI-induced psychosocial-like processing deficit.
(a) Presented here are mean±SEM SI times for rats injected with mGluR1/5 agonist, DHPG (black squares, n = 5) or vehicle (gray squares, n = 5), into OFC. Rats with DHPG injections prior to SI-hab sessions demonstrated attenuated SoFiA compared to rats injected with vehicle (Two-way repeated measures ANOVA drug X day interaction F5,40 = 2.681, P=0.0351). Vehicle rats had increased SI time on SI-hab days 3–5 compared to SI-hab day 1 (*Dunnett’s p≤0.0069). DHPG rats displayed a transient increase in SI time on SI-hab day 4 compared to SI-hab day 1 (* Dunnett’s p=0.0277). SI times of vehicle-injected rats were lower than baseline only on SI-hab day 1 (Fisher’s LSD p=0.024), while DHPG-injected rats’ SI time was lower than baseline during all SI=hab days except day 4 (Fisher’s LSD p≤0.039). (b) Presented here are mean±SEM of time spent in zone of a novel conspecific (solid bars) or familiar conspecific (striped bars) in vehicle- (gray bars) or DHPG-(black bars) treated rats. Drug treatment differentially affected the time spent in zones (2-way ANOVA, drug X zone interaction F1,16=10.37, P=0.0054). Vehicle, but not DHPG rats spent more time in the novel zone, compared to the familiar zone (* Fisher’s LSD, p=0.0169). DHPG treatment lead to differential response compared to vehicle treatment with DHPG-injected rats spending significantly less time in the zone of a novel conspecific and significantly more time in the zone of a familiar conspecific, compared to vehicle rats (‡ Fisher’s LSD p=0.037 and p=0.037, respectively). (c) Injection sites for all rats (gray circles, vehicle; black circles, DHPG). Injections were located at the approximate locations shown between anteroposterior coordinates +4.20mm and +3.00mm relative to bregma.
Furthermore, SI-times of vehicle-injected rats were significantly lower than baseline only on SI-hab day 1 (Fisher’s LSD p=0.024) while DHPG-injected rats’ SI-time were significantly lower than baseline during all SI-hab days except day 4 (Fisher’s LSD p≤0.039). To determine if DHPG injection into OFC also recapitulated mbTBI-induced aberrant social recognition response, these rats underwent SR testing following injections of DHPG or vehicle into OFC. Rats injected with DHPG demonstrated social memory deficit, while vehicle-injected rats demonstrated intact social memory (Two-way ANOVA drug X zone interaction F1,16=10.37, P=0.0054) (Figure 5b). Vehicle-injected rats spent significantly more time near the novel conspecific (Fisher’s LSD, p=0.0169) while DPHG-injected rats displayed a trend towards increased time spent with the familiar conspecific. Similar to behavior observed by blast exposed rats, DHPG-injected rats, compared to vehicle-injected rats, spent significantly more time in familiar zone and significantly less time in the novel zone compared to vehicle-injected rats (Fisher’s LSD p=0.037 and p=0.037, respectively). It is notable that again not every rat demonstrated this behavioral pattern despite statistical significance. Injection sites were confirmed to be within OFC between anteroposterior coordinates +4.20mm and +3.00mm relative to bregma (Figure 5c). All reported data was confirmed to have no outliers as determined by the ROUT Method (Q set to 5%). All reported data passed Shapiro-Wilk Normality Tests.
4. Discussion
4.1. mbTBI-induced oxidative stress correlated with psychosocial processing deficits
The established mbTBI model [13, 19] resulted in expected, transient 3-HPMA elevation in urine. 3-HPMA is a stable metabolite of acrolein [33], and elevation of acrolein is associated with neurotrauma and oxidative stress [13, 19, 33]. Oxidative stress elevations in the brain are widely reported in rodent mbTBI, including our work [13, 19], and are considered a key secondary injury pathway in the acute to subacute post-injury period [51–54]. In addition, oxidative stress has been independently associated with psychopathology, which is frequently reported in TBI patients [55, 56]. 3-HPMA levels in urine at 24 hours post-injury inversely correlated with SoFiA acquisition during days 9–15 post-injury. Clinical evidence supports this because intrinsic antioxidant capacity can predict neurofunctional recovery after TBI [57]. 3-HPMA and oxidant-antioxidant measures present novel early surrogate biomarker targets for predicting later-onset psychosocial impairment after TBI. In addition, treatment strategies to help the brain combat oxidative stress in the acute post-injury period may help prevent delayed post-TBI sequelae, including mental health abnormalities. Some preclinical evidence already suggests early interventions to reduce oxidative stress may provide benefits after mild TBI, including blast TBI [53, 54, 58].
4.2. mbTBI resulted in psychosocial processing deficits
Cumulatively, when applied repeatedly over time, the SI-hab protocol measures SoFiA, which is a form of safety learning characterized as learned reduction in anxiety-like behavior in the presence of a familiar conspecific. Safety learning is critical for the identification of and reaction to situations requiring fear/anxiety responses and is therefore critical for organism survival (reviewed in [59, 60]). Impaired safety learning can lead to an overgeneralization of fear and tonic fear expression despite the lack of an active fear stimulus, implicating safety learning deficits in PTSD, depression, and chronic anxiety [61–63]. Social support from a conspecific can mitigate behavioral and autonomic anxiety-like responses to threat in both rodents (as social buffering) [64, 65] and humans [66, 67]. Furthermore, social support is positively correlated with improved therapeutic outcomes for patients with anxiety, depression, and PTSD [68, 69], and social support derived from the patient-therapist alliance is a key element of psychotherapy [66, 67, 70].
Blast exposure resulted in aberrant responses to social familiarity. Blast rats failed to acquire SoFiA, defined as the ability to reduce anxiety-like behavior via repeated presence of a familiar conspecific under anxiogenic conditions. In contrast, sham rats acquired SoFiA comparably to previous studies [14, 15]. Although we have previously shown this mbTBI model results in elevated oxidative stress marker in rat hippocampus [13], in the present SR test blast rats showed no deficit in differentiation of novel and familiar conspecifics, suggesting intact social discriminant memory. Rather, while sham rats spent greater time near the novel conspecific (consistent with expected rodent behavior [71]), blast rats spent comparably greater time near the familiar conspecific. Together, these findings suggest selective deficits in psychosocial processing following mbTBI, in which gross social drive is intact while innate and learned behavioral responses to social cues in the setting of an anxiogenic stimulus may be altered. Deficits in social, affective, and cognitive behaviors have been variably reported in other animal studies of mild TBI [72]. Contrary to our findings, mice receiving mild controlled impact TBI demonstrate altered social drive but no changes in social novelty preference, even when these mice were also exposed to repeated traumatic stress modeling a PTSD-like condition [72]. Our findings of psychosocial processing deficits may therefore be specifically related to the blast mechanism of injury, or unique neurobehavioral changes resulting from acute anxiogenic stimuli.
The SoFiA model may measure the positive effect of social support on mental health [16]. Using social cues to reduce anxiety-like behavior is a form of safety learning and is consistent with the positive role social support plays in overall mental health (for review, see [73]). Reduced perception of social support has been reported by blast-injured veterans [74] and is an independent variable impacting overall life satisfaction and recovery after TBI [75] that is positively correlated with improved therapeutic outcomes for patients with anxiety, depression, and PTSD [68, 69, 76]. Additionally, there are reports of difficulty establishing the psychotherapeutic alliance with TBI patients, which relies heavily on perceptions of social support [77].
Regarding behavioral interpretation, we must acknowledge the possible influence of blast-induced auditory impairments, which have been reported after our and other models of mbTBI that would encompass rats’ anxiety-related ultrasonic vocalizations (USV) near 22 kHz [21, 78–80]. We suspect that USV audibility is not a major causal factor leading to SoFiA impairments due to the relatively minor hearing threshold shifts in our mbTBI model [21], and the fact that olfactory systems are likely more critical for social behavior in rats than auditory systems. The possibility of TBI-induced photophobia should be recognized; however, we are reassured that TBI-exposed rats did not experience a significantly different response to the BLC because their anxiety-like behavior upon first BLC exposure was comparable to sham rats.
4.3. Putative loci and mechanisms underlying mbTBI-induced psychosocial processing deficits
Impaired behaviors following TBI are most commonly reported in the executive, emotional, and attentional cognitive domains, which rely on intact PFC function and its inhibitory control over other regions [2, 81–84]. The ventromedial PFC (vmPFC) and basolateral amygdala (BLA) are important for SoFiA acquisition and expression [14, 15]. Surprisingly, these structures do not demonstrate oxidative stress elevations [13], histologic evidence of neuronal death or inflammation (not shown), or disrupted network/regional-scale connectivity in this mbTBI model. Tonic amygdalar drive (measured as baseline anxiety behavior) did not appear to be altered and vmPFC-amygdala correlated functional activity was intact or increased post-blast. Consistent with these observations, a parallel biochemical and in silico research effort by our group in the same mbTBI model showed that vmPFC and bilateral amygdala were relatively biomechanically spared [13]. However, the orbitofrontal cortex (OFC) was found to be both biochemically vulnerable (increased acrolein-protein adducts) and mechanically compromised [13].
Paradoxically, mbTBI increased resting state functional connectivity between the OFC, vmPFC, and amygdala at 24-hour and 1-week post-injury time points. While significantly increased resting state connectivity was observed on a sub-regional, voxel-wise basis in each region of interest, significant region-wide increased correlated resting state functional activity was observed exclusively between the OFC and amygdala bilaterally. Notably, the sample size used for the presented fMRI study was small, however we are confident with our findings from this small sample size due to each animal undergoing repeated sessions sufficient to generate six samples of functional connectivity per subject and condition. Additionally, we examined the correlation coefficient (as the measurement of functional connectivity) from individual samples and observed a consistent change (or lack thereof) in functional connectivity before and after injury. Additionally, although this protocol is valid to reveal the brain’s intrinsic functional networks [43] it is desirable to acquire resting state fMRI in awake animals [85]. However, fMRI in awake animals is challenging and rarely performed in practice. It requires extended periods of behavioral training to acclimate animals to both the MRI environment and positional restraint procedures, which was incompatible with the present study design.
The aforementioned normality in structure, function, and mechanical sparing of the amygdala led us to deduce a new hypothesis that bilateral OFC were the principle functionally aberrant loci underlying the rs-fMRI findings. Congruently, we have demonstrated acute post-mild mbTBI oxidative stress elevations and mechanical forces above tissue injury thresholds affecting the OFC in this mbTBI model previously [13].
In both rats and humans, OFC injury reduces social contact and increases aberrant social behavior [86–88]. OFC functions as a value-based decision gate involved in initiation or avoidance of social behavior and cue-associated reversal learning [89–91]. OFC may regulate social valuation and its disruption may impair use of social cues to learn safety. OFC has been implicated in extinction learning [92], a paradigm similar to safety learning, and our lab has previously indicated OFC as a likely critical contributor to SoFiA acquisition [14, 15].
Neuronal projections between the vmPFC-OFC-amygdala regions remain largely intact after mbTBI in this model. This observation is consistent with our prior report of altered evoked potential amplitudes but unaffected latencies in this model, which suggested dysfunctional intra-regional processing but intact inter-regional signal conduction [21]. Furthermore, large- scale cell death in any of these regions is unlikely, a notion consistent with unchanged NeuN+ cell counts in the vmPFC and amygdala (not shown). We hypothesized that functional excitatory/inhibitory imbalance could alter OFC subregional processing in the absence of cell death. Strengthened resting state connectivity within the network of interest could result from post-injury aberrant glutamatergic/GABAergic signaling, as these primary regulators of excitatory/inhibitory tone are known to affect the BOLD signal with incompletely understood region-wise variability [93].
Exploring this hypothesis, we identified selective increased expression of excitatory class I metabotropic glutamate receptors (mGluRs) genes Grm1 and Grm5 and GABA receptor subunit gene Gabrg1 in OFC following mbTBI. Gabrg1 is enriched in limbic resting state networks [94], suggesting a possible role in observed TBI-induced resting state network alterations. Class I mGluRs contribute to TBI pathophysiology [95], are upregulated in the presence of oxidative stress, and protect against accumulation of oxidative stress mediators [96]. We hypothesize mechanical injury or oxidative stress within OFC following mbTBI [13] may increase expression of Grm1 and Grm5, driving excess excitatory signaling in the OFC–vmPFC–BLA network, disrupting psychosocial processing.
Supporting this, we demonstrate that selective agonism of mGluR1/5 receptors in OFC attenuates SoFiA acquisition in exposure-naïve rats. Class I mGluRs regulate learning and memory (for review, see [97]) and could contribute to SoFiA learning. We chose to pursue recapitulation of mGluR1/5-mediated dysregulation in exposure-naïve rats first before reversal of TBI-mediated dysregulation through mGluR1/5 antagonism because we suspected antagonism would additionally dysregulate OFC functionality. We suspect mGluR1/5 antagonism would disrupt OFC functionality in sham/control rats as well. Given the importance of mGluR1/5-mediated signaling, we propose in future directions that pharmacologically stabilizing glutamate function, rather than antagonizing, may normalize aberrant neuronal signaling pathways in OFC following mbTBI exposure, facilitating SoFiA acquisition.
Importantly, mGluR1/5 agonism is not an exact replication of Grm1 and Grm5 upregulation found in mbTBI-exposed rats, and applying agonist did not completely mirror mbTBI-induced deficits in psychosocial processing. Rats receiving mGluR1/5 agonist demonstrated SoFiA attenuation and impaired social memory, while mbTBI-exposed rats completely failed to acquire SoFiA and demonstrated altered conspecific preference but intact memory. Notably, these data exhibit significant inter-subject variability suggesting this data must be interpreted with caution. Other brain regions and incompletely understood intraregional complexities likely contribute to mbTBI-induced psychosocial deficits. Anterior (agranular) insular cortex (AIC) shares significant reciprocal connectivity with OFC, vmPFC, and amygdala [98]. Bilateral acute oxidative stress elevations in this mbTBI model [13] occur in OFC and AIC, which co-activates with the amygdala during social-emotional paradigms [99]. Post-mbTBI acrolein elevations were reported [13] in bilateral regions containing ventral hippocampi which, via connections with BLA, can impact social behaviors [100].
While specific OFC subregions were not parcellated in the present investigation, most rats received injections in the ventral and lateral subregions, suggesting the present data is congruent with these regions’ predicted roles in cognitive flexibility, reversal learning, and reinforcer devaluation [101, 102]. Furthermore, rats receiving injection in VO versus LO did not differ significantly in SoFiA acquisition or SR outcomes (data not shown).
4.4. Conclusions
In summary, we report selective psychosocial processing impairments in rats following mbTBI in the absence of major cognitive, motor, or affective confounders. Deficit severity inversely correlated with urine oxidative stress marker 3-HPMA and was associated with subregional functional connectivity alterations in a network containing the vmPFC, OFC, and amygdala bilaterally, as well as region-wide increased correlated resting state functional activity between the bilateral OFC and amygdala. Furthermore, mbTBI resulted in elevated expression of mGluR1/5 in OFC and selective mGluR1/5 agonist injected into OFC of exposure-naïve animals approximated mbTBI-induced psychosocial impairment. This mbTBI model serves as a unique avenue to explore psychosocial processing circuitry, at the level of both brain region and neuronal substrate.
Supplementary Material
Highlights.
Mild blast traumatic brain injury (mbTBI) generates a specific psychosocial learning behavior deficit in the absence of confounding motor, affective, or other non-social behavioral changes.
Behavioral impairment correlated with a known oxidative stress metabolite, 3-hydroxypropylmercapturic acid (3-HPMA)
Post-injury resting state fMRI demonstrated altered connectivity in a network containing bilateral vmPFC, OFC, and amygdala, as well as region-wide increased correlated resting state functional activity between the bilateral OFC and amygdala
OFC of blast-injured rat brains expressed elevated mRNA levels of metabotropic glutamate receptors 1 and 5 (mGluR1/5) and injection of mGlur1/5-selective agonist in OFC of uninjured rat brain approximated blast-induced psychosocial behavior deficit
Funding acknowledgements
Research reported in this publication was supported by National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, and National Center for Advancing Translational Sciences, of the National Institutes of Health under award numbers R01MH106568 to WT, R01MH104402 to ZL, R21 NS090244-01 to RS and UL1TR002529 to AS. This work was also supported in part by the Indiana State Department of Health (Grant No. 204200 to R.S. & 19920 to W.T.), and Indiana Clinical and Translational Sciences Institute through Collaboration in Biomedical Translational Research (CBR/CTR) Pilot Program Grant (Grant No. RR025761 to RS & WT).
Disclosures
Riyi Shi is a co-founder of Neuro Vigor, a company developing novel drug treatments and diagnostic approaches for neurodegenerative diseases and neurotrauma. Nicholas Race is a board member of the same company. Anantha Shekhar has received research grants from Eli Lilly, Takeda, Astra Zeneca and Johnson & Johnson to support preclinical and clinical programs. He is co-founder of Anagin Inc. an IU biotech company. The present work has not used any nor has any conflict with any of the above funding. The remaining authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevention C.f.D.C.a., Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. 2015, Centers for Disease Control and Prevention: Atlanta, GA. [DOI] [PubMed] [Google Scholar]
- 2.Spikman JM, et al. , Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. J Neurotrauma, 2012. 29(1): p. 101–11. [DOI] [PubMed] [Google Scholar]
- 3.Morton MV and Wehman P, Psychosocial and emotional sequelae of individuals with traumatic brain injury: a literature review and recommendations. Brain Inj, 1995. 9(1): p. 81–92. [DOI] [PubMed] [Google Scholar]
- 4.Ponsford JL, et al. , Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J Neurotrauma, 2014. 31(1): p. 64–77. [DOI] [PubMed] [Google Scholar]
- 5.Pugh MJ, et al. , Traumatic Brain Injury Severity, Comorbidity, Social Support, Family Functioning, and Community Reintegration Among Veterans of the Afghanistan and Iraq Wars. Arch Phys Med Rehabil, 2018. 99(2S): p. S40–S49. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy ML, et al. , Self-reported psychosocial health among adults with traumatic brain injury. Arch Phys Med Rehabil, 2006. 87(7): p. 953–61. [DOI] [PubMed] [Google Scholar]
- 7.May M, et al. , Social Behavior and Impairments in Social Cognition Following Traumatic Brain Injury. J Int Neuropsychol Soc, 2017. 23(5): p. 400–411. [DOI] [PubMed] [Google Scholar]
- 8.Struchen MA, et al. , Relation of executive functioning and social communication measures to functional outcomes following traumatic brain injury. NeuroRehabilitation, 2008. 23(2): p. 185–98. [PubMed] [Google Scholar]
- 9.Kawachi I. and Berkman LF, Social ties and mental health. J Urban Health, 2001. 78(3): p. 458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draper K, Ponsford J, and Schonberger M, Psychosocial and emotional outcomes 10 years following traumatic brain injury. J Head Trauma Rehabil, 2007. 22(5): p. 278–87. [DOI] [PubMed] [Google Scholar]
- 11.Ponsford J, Alway Y, and Gould KR, Epidemiology and Natural History of Psychiatric Disorders After TBI. J Neuropsychiatry Clin Neurosci, 2018: p. appineuropsych18040093. [DOI] [PubMed]
- 12.Alway Y, et al. , A prospective examination of Axis I psychiatric disorders in the first 5 years following moderate to severe traumatic brain injury. Psychological Medicine, 2016. 46: p. 1331–1341. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Gonzalez D, et al. , Cognition based bTBI mechanistic criteria; a tool for preventive and therapeutic innovations. Sci Rep, 2018. 8(1): p. 10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truitt WA, et al. , From anxiety to autism: spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology (Berl), 2007. 191(1): p. 107–18. [DOI] [PubMed] [Google Scholar]
- 15.Lungwitz EA, et al. , The role of the medial prefrontal cortex in regulating social familiarity-induced anxiolysis. Neuropsychopharmacology, 2014. 39(4): p. 1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumdar S, et al. , Animal models to investigate social support induced anxiety reductions, in Neurobiology of abnormal emotion and motivated behaviors: Integrating animal and human research, Sangha S. and Foti D, Editors. 2018, Academic Press. p. 225–240. [Google Scholar]
- 17.Hornstein EA and Eisenberger NI, A Social Safety Net: Developing a Model of Social- Support Figures as Prepared Safety Stimuli. Current Directions in Psychological Science, 2018. 27(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 18.Mikami K, et al. , Social buffering enhances extinction of conditioned fear responses in male rats. Physiol Behav, 2016. 163: p. 123–8. [DOI] [PubMed] [Google Scholar]
- 19.Walls MK., et al., Structural and biochemical abnormalities in the absence of acute deficits in mild primary blast-induced head trauma. J Neurosurg, 2015. [DOI] [PMC free article] [PubMed]
- 20.Song S, et al. , A Wireless Intracranial Brain Deformation Sensing System for Blast-Induced Traumatic Brain Injury. Sci Rep, 2015. 5: p. 16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Race N, et al. , Differences in post-injury auditory system pathophysiology after mild blast and non-blast acute acoustic trauma. J Neurophysiol, 2017: p. jn 00710 2016. [DOI] [PMC free article] [PubMed]
- 22.Acosta G, et al. , Acrolein-mediated alpha-synuclein pathology involvement in the early post-injury pathogenesis of mild blast-induced Parkinsonian neurodegeneration. Molecular and Cellular Neuroscience, 2019. [DOI] [PMC free article] [PubMed]
- 23.Shi R. and Race N, Mild Blast-Induced Traumatic Brain Injury Model, in Animal Models of Acute Neurological Injury. 2019, Springer. p. 367–378. [Google Scholar]
- 24.Rafaels K, et al. , Survival risk assessment for primary blast exposures to the head. Journal of neurotrauma, 2011. 28(11): p. 2319–2328. [DOI] [PubMed] [Google Scholar]
- 25.Bass CR, Rafaels KA, and Salzar RS, Pulmonary injury risk assessment for short-duration blasts. Journal of Trauma and Acute Care Surgery, 2008. 65(3): p. 604–615. [DOI] [PubMed] [Google Scholar]
- 26.Newman AJ and Mollendorf JC, The Peak Overpressure Field Resulting From Shocks Emerging From Circular Shock Tubes. Journal of Fluids Engineering, 2010. 132(8): p. 081204. [Google Scholar]
- 27.Carmella SG, et al. , Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmosphericpressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol, 2007. 20(7): p. 986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckert E, Drexler H, and Goen T, Determination of six hydroxyalkyl mercapturic acids in human urine using hydrophilic interaction liquid chromatography with tandem mass spectrometry (HILIC-ESI-MS/MS). J Chromatogr B Analyt Technol Biomed Life Sci, 2010. 878(27): p. 2506–14. [DOI] [PubMed] [Google Scholar]
- 29.Schettgen T, Musiol A, and Kraus T, Simultaneous determination of mercapturic acids derived from ethylene oxide (HEMA), propylene oxide (2-HPMA), acrolein (3-HPMA), acrylamide (AAMA) and N,N-dimethylformamide (AMCC) in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom, 2008. 22(17): p. 2629–38. [DOI] [PubMed] [Google Scholar]
- 30.Parent RA, et al. , Metabolism and distribution of [2,3–14C]acrolein in Sprague-Dawley rats. II. Identification of urinary and fecal metabolites. Toxicol Sci, 1998. 43(2): p. 110–20. [DOI] [PubMed] [Google Scholar]
- 31.Chen C-H, et al. , Development of a Mass Spectrometry Sampling Probe for Chemical Analysis in Surgical and Endoscopic Procedures. Analytical Chemistry, 2013. 85(24): p. 11843–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan W, et al. , Development and validation of a direct LC-MS-MS method to determine the acrolein metabolite 3-HPMA in urine. J Chromatogr Sci, 2010. 48(3): p. 194–9. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, et al. , Determination of Urine 3-HPMA, a Stable Acrolein Metabolite in a Rat Model of Spinal Cord Injury. J Neurotrauma, 2013. 30(15): p. 1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choleris E, et al. , A detailed ethological analysis of the mouse open ®eld test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neuroscience and Biobehavioral Reviews, 2001. 25: p. 235–260. [DOI] [PubMed] [Google Scholar]
- 35.Hamm RJ, et al. , The Rotarod Test: An Evaluation of Its Effectiveness in Assessing Motor Deficits Following Traumatic Brain Injury. Journal of Neurotrauma, 1994. 11(2): p. 187–196. [DOI] [PubMed] [Google Scholar]
- 36.Sanders S. and Shekhar A, Regulation of anxiety by GABA A receptors in the rat amygdala. Pharmacology Biochemistry and Behavior, 1995. 52(4): p. 701–706. [DOI] [PubMed] [Google Scholar]
- 37.Shekhar A. and Katner J, Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacology Biochemistry and Behavior, 1995. 50(2): p. 253–258. [DOI] [PubMed] [Google Scholar]
- 38.File S, The validation of animal tests of anxiety--pharmacological implications. Polish Journal of Pharmacology and Pharmacy, 1984. 36(5): p. 505–512. [PubMed] [Google Scholar]
- 39.File SE and Hyde JRG, Can social interaction be used to measure anxiety? Br. J. Pharmac, 1978. 62: p. 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thor DH and Holloway WR, Social memory of the male laboratory rat. Journal of Comparative and Physiological Psychology, 1982. 96(6): p. 1000–1006. [Google Scholar]
- 41.Chermat R, et al. , Adaptation of the tail suspension test to the rat. Journal de pharmacologie, 1985. 17(3): p. 348–350. [PubMed] [Google Scholar]
- 42.Weber R, et al. , A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage, 2006. 29(4): p. 1303–1310. [DOI] [PubMed] [Google Scholar]
- 43.Lu H, et al. , Rat brains also have a default mode network. Proc Natl Acad Sci U S A, 2012. 109(10): p. 3979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glasser MF, et al. , The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage, 2013. 80: p. 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papp EA, et al. , Waxholm Space atlas of the Sprague Dawley rat brain. NeuroImage, 2014. 97: p. 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worsley KJ, et al. , A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow & Metabolism, 1992. 12(6): p. 900–918. [DOI] [PubMed] [Google Scholar]
- 47.Truitt WA, et al. , Ethanol and nicotine interaction within the posterior ventral tegmental area in male and female alcohol-preferring rats: evidence of synergy and differential gene activation in the nucleus accumbens shell. Psychopharmacology (Berl), 2015. 232(3): p. 639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paxinos G. and Watson C, The rat brain in stereotaxic coordinates. 5th ed. 2004: Academic Press. [DOI] [PubMed] [Google Scholar]
- 49.Truitt WA, et al. , Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience, 2009. 160(2): p. 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engelmann M, Wotjak CT, and Landgraf R, Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav, 1995. 58(2): p. 315–21. [DOI] [PubMed] [Google Scholar]
- 51.Cho HJ, et al. , Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience, 2013. 253: p. 9–20. [DOI] [PubMed] [Google Scholar]
- 52.Readnower RD, et al. , Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res, 2010. 88(16): p. 3530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du X, et al. , Effects of antioxidant treatment on blast-induced brain injury. PLoS One, 2013. 8(11): p. e80138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ewert DL, et al. , Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hearing research, 2012. 285(1): p. 29–39. [DOI] [PubMed] [Google Scholar]
- 55.Miller SC, et al. , Risk for broad-spectrum neuropsychiatric disorders after mild traumatic brain injury in a cohort of US Air Force personnel. Occupational and environmental medicine, 2015: p. oemed-2014–102646. [DOI] [PubMed]
- 56.Orlovska S, et al. , Head injury as risk factor for psychiatric disorders: a nationwide register-based follow-up study of 113,906 persons with head injury. American journal of psychiatry, 2014. 171(4): p. 463–469. [DOI] [PubMed] [Google Scholar]
- 57.Shohami E., et al., Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional outcome. Journal of Cerebral Blood Flow & Metabolism, 1997. 17(10): p. 1007–1019. [DOI] [PubMed] [Google Scholar]
- 58.Abdul-Muneer PM, et al. , Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med, 2013. 60: p. 282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong E, et al. , Learning not to fear: neural correlates of learned safety. Neuropsychopharmacology, 2014. 39(3): p. 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christianson JP, et al. , Inhibition of fear by learned safety signals: a mini-symposium review. Journal of Neuroscience, 2012. 32(41): p. 14118–14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jovanovic T, et al. , Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 2012. 62(2): p. 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollak DD, et al. , An animal model of a behavioral intervention for depression. Neuron, 2008. 60(1): p. 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollak DD, et al. , A translational bridge between mouse and human models of learned safety. Annals of medicine, 2010. 42(2): p. 127–134. [DOI] [PubMed] [Google Scholar]
- 64.Kiyokawa Y, et al. , A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behavioural brain research, 2014. 267: p. 189–193. [DOI] [PubMed] [Google Scholar]
- 65.Kiyokawa Y, Takeuchi Y, and Mori Y, Two types of social buffering differentially mitigate conditioned fear responses. European Journal of Neuroscience, 2007. 26(12): p. 3606–3613. [DOI] [PubMed] [Google Scholar]
- 66.Eisenberger NI, et al. , Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences, 2011. 108(28): p. 11721–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin DJ, Garske JP, and Davis MK, Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. 2000, American Psychological Association. [PubMed] [Google Scholar]
- 68.Dour HJ, et al. , Perceived social support mediates anxiety and depressive symptom changes following primary care intervention. Depression and anxiety, 2014. 31(5): p. 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Southwick SM, Vythilingam M, and Charney DS, The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu. Rev. Clin. Psychol, 2005. 1: p. 255–291. [DOI] [PubMed] [Google Scholar]
- 70.Baldwin SA, Wampold BE, and Imel ZE, Untangling the alliance-outcome correlation: Exploring the relative importance of therapist and patient variability in the alliance. Journal of consulting and clinical psychology, 2007. 75(6): p. 842. [DOI] [PubMed] [Google Scholar]
- 71.van der Kooij MA and Sandi C, Social memories in rodents: methods, mechanisms and modulation by stress. Neurosci Biobehav Rev, 2012. 36(7): p. 1763–72. [DOI] [PubMed] [Google Scholar]
- 72.Ojo JO, et al. , Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Frontiers in behavioral neuroscience, 2014. 8: p. 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orff HJ, et al. , Sleep disturbance, psychiatric, and cognitive functioning in veterans with mild to moderate traumatic brain injury. Journal of Sleep Disorders: Treatment and Care, 2016. 2015.
- 74.Seidl JNT, et al. , Factors related to satisfaction with life in veterans with mild traumatic brain injury. Rehabilitation psychology, 2015. 60(4): p. 335. [DOI] [PubMed] [Google Scholar]
- 75.Carter GC, et al. , Comprehensive review of factors implicated in the heterogeneity of response in depression. Depress Anxiety, 2012. 29(4): p. 340–54. [DOI] [PubMed] [Google Scholar]
- 76.Coan JA., Schaefer HS, and Davidson RJ, Lending a hand: social regulation of the neural response to threat. Psychol Sci, 2006. 17(12): p. 1032–9. [DOI] [PubMed] [Google Scholar]
- 77.Judd D. and Wilson S, Psychotherapy with brain injury survivors: An investigation of the challenges encountered by clinicians and their modifications to therapeutic practice. Brain Injury, 2005. 19(6): p. 437–449. [DOI] [PubMed] [Google Scholar]
- 78.Luo H, et al. , Blast‐Induced tinnitus and spontaneous firing changes in the rat dorsal cochlear nucleus. Journal of neuroscience research, 2014. 92(11): p. 1466–1477. [DOI] [PubMed] [Google Scholar]
- 79.Luo H, et al. , Blast-induced tinnitus and spontaneous activity changes in the rat inferior colliculus. Neuroscience letters, 2014. 580: p. 47–51. [DOI] [PubMed] [Google Scholar]
- 80.Mao JC, et al. , Blast-induced tinnitus and hearing loss in rats: behavioral and imaging assays. Journal of neurotrauma, 2012. 29(2): p. 430–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spikman JM, et al. , Early Computed Tomography Frontal Abnormalities Predict Long-Term Neurobehavioral Problems But Not Affective Problems after Moderate to Severe Traumatic Brain Injury. Journal of neurotrauma, 2016. 33(1): p. 22–28. [DOI] [PubMed] [Google Scholar]
- 82.van der Horn HJ, et al. , Brain networks subserving emotion regulation and adaptation after mild traumatic brain injury. Journal of neurotrauma, 2016. 33(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 83.van der Horn HJ, et al. , Brain network dysregulation, emotion, and complaints after mild traumatic brain injury. Human brain mapping, 2016. 37(4): p. 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spikman JM, et al. , Deficits in facial emotion recognition indicate behavioral changes and impaired self-awareness after moderate to severe traumatic brain injury. PloS one, 2013. 8(6): p. e65581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang Z, King J, and Zhang N, Uncovering intrinsic connectional architecture of functional networks in awake rat brain. Journal of Neuroscience, 2011. 31(10): p. 3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beer JS, et al. , Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of cognitive neuroscience, 2006. 18(6): p. 871–879. [DOI] [PubMed] [Google Scholar]
- 87.Kolb B. and Nonneman AJ, Frontolimbic lesions and social behavior in the rat. Physiology & Behavior, 1974. 13(5): p. 637–643. [DOI] [PubMed] [Google Scholar]
- 88.Cicerone KD and Tanenbaum LN, Disturbance of social cognition after traumatic orbitofrontal brain injury. Archives of clinical neuropsychology, 1997. 12(2): p. 173–188. [PubMed] [Google Scholar]
- 89.Machado CJ and Bachevalier J, The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behavioral neuroscience, 2006. 120(4): p. 761. [DOI] [PubMed] [Google Scholar]
- 90.Sul JH, et al. , Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron, 2010. 66(3): p. 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McAlonan K. and Brown VJ, Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural brain research, 2003. 146(1): p. 97–103. [DOI] [PubMed] [Google Scholar]
- 92.Sarlitto MC, Foilb AR, and Christianson JP, Inactivation of the ventrolateral orbitofrontal cortex impairs flexible use of safety signals. Neuroscience, 2018. 379: p. 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drew PJ, Vascular and neural basis of the BOLD signal. Current opinion in neurobiology, 2019. 58: p. 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson KM, et al. , Gene expression links functional networks across cortex and striatum. Nat Commun, 2018. 9(1): p. 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lyeth BG, et al. , Group I metabotropic glutamate antagonist reduces acute neuronal degeneration and behavioral deficits after traumatic brain injury in rats. Exp Neurol, 2001. 169(1): p. 191–9. [DOI] [PubMed] [Google Scholar]
- 96.Sagara Y. and Schubert D, The activation of metabotropic glutamate receptors protects nerve cells from oxidative stress. The Journal of Neuroscience, 1998. 18(17): p. 6662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mukherjee S. and Manahan-Vaughan D, Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology, 2013. 66: p. 65–81. [DOI] [PubMed] [Google Scholar]
- 98.Öngür D. and Price J, The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral cortex, 2000. 10(3): p. 206–219. [DOI] [PubMed] [Google Scholar]
- 99.Mutschler I, et al. , Functional organization of the human anterior insular cortex. Neuroscience letters, 2009. 457(2): p. 66–70. [DOI] [PubMed] [Google Scholar]
- 100.Felix-Ortiz AC and Tye KM, Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci, 2014. 34(2): p. 586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Izquierdo A, Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. Journal of Neuroscience, 2017. 37(44): p. 10529–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jennings JH, et al. , Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature, 2019. 565(7741): p. 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.