Abstract
Many patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) will not respond to platinum-containing salvage chemotherapy. Predicting treatment failure earlier could help clinicians minimize chemotherapy toxicities for non-responders in favor of other treatments. We conducted a pilot study where 2 early PET/CTs were obtained on days 4 (D4) and 21 (D21) of cycle 1 (C1) of salvage therapy for DLBCL. Twenty-five patients were enrolled and have evaluable data. Ten (40%) had an unplanned therapy change after C1 and before end-of-treatment (EOT) evaluation due to treatment failure on early PET/CT as interpreted by the treating physician. Early PET/CT response at D4 or D21 was not associated with EOT response in evaluable patients. Disease specific survival was longer for patients with a persistent response on both D4 and D21 (p=0.042). Early PET/CT may predict salvage chemotherapy failure and could inform future clinical trials investigating early therapy change to non-chemotherapy treatments.
Keywords: Diffuse large B-cell lymphoma, positron emission tomography/computed tomography, end of treatment, real-time response assessment, salvage chemotherapy
Introduction
Patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) after frontline treatment have a poor prognosis,1 particularly if they had refractory disease,2 with median overall survival of less than one year. Standard of care salvage treatment for fit patients with R/R DLBCL is platinum-containing chemotherapy, followed by high dose chemotherapy and autologous stem cell transplant (ASCT) for patients responsive to salvage therapy.3 Since the approval of the anti-CD20 antibody rituximab, two widely used regimens employed in first salvage with intent to transplant have been rituximab, ifosfamide, carboplatin, and etoposide (R-ICE)4 and rituximab, dexamethasone, cytarabine, and cisplatin (R-DHAP)5. The CORAL randomized study found these two regimens achieved similar outcomes with a response rate of 63%, 50% ASCT rate, and a 3-year progression free survival of 53% in transplanted patients. Common toxicities included cytopenias necessitating transfusion, infection with or without neutropenia, and renal injury.6
Thus, despite receiving aggressive cytotoxic chemotherapy associated with significant toxicity, only half of patients will be able to proceed with an ASCT, and half of those patients will relapse after transplant with the current chemotherapy-based treatment paradigm. Patients refractory to salvage platinum-containing chemotherapy have a low likelihood of success with ASCT; these patients now have access to anti-CD19 chimeric antigen receptor T-cell therapy (CART19), a third-line non-chemotherapy treatment option that leads to long term remissions in up to half of patients.7–9
Though baseline factors at time of relapse such as time to first relapse and international prognostic index (IPI) were associated with end-of-treatment (EOT) response and survival,6 it is unknown whether real time markers captured early during salvage platinum-containing chemotherapy can predict these outcomes. The 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) scan is the gold standard for response evaluation in aggressive lymphomas10 and is used after salvage chemotherapy for DLBCL to assess a patient’s candidacy for high-dose chemotherapy with ASCT.11 An interim PET/CT (iPET) is often obtained after 2–4 cycles of frontline immunochemotherapy in DLBCL to evaluate for early response.12 PET/CT response is interpreted by visual assessment with the five-point scale (5-PS) and Lugano classification in routine practice,13,14 though a semiquantitative method that calculates the relative reduction in maximum standardized uptake value (ΔSUVmax) may be a more objective measurement of the iPET that reduces false positives.15,16 Risk-adapted approaches with therapy intensification in response to a positive iPET have to date not improved patient outcomes.17,18 These studies only intensified iPET positive patients to more aggressive cytotoxic chemotherapy regimens, but a similar approach with newly approved non-chemotherapy treatments may prove more effective.19
Very early PET scans within 1–7 days after initiation of DLBCL therapy are feasible to obtain and can demonstrate immediate reductions in 18F-flurodeoxyglucose uptake.20,21 iPET after cycle 1 (C1) of frontline immunochemotherapy for DLBCL yielded similar prognostic value to iPET after cycle 2.22
No prior study has specifically investigated the feasibility and utility of early prognostic evaluation with functional imaging in the R/R setting. We hypothesized that early PET/CT scans could identify refractoriness to salvage chemotherapy. Here, we report the results of an investigator initiated single-institution prospective pilot study (NCT02405078) where 2 early PET/CTs were obtained on approximately days 4 (D4) and 21 (D21) of C1 of salvage platinum-containing chemotherapy. We hypothesized specifically that early PET/CT response could successfully predict EOT response. Response by visual assessment and ΔSUVmax on early PET/CTs was correlated with the primary outcome of EOT response after 2–3 total cycles of platinum-containing chemotherapy as well as long-term survival, including in patients who underwent an early therapy change after C1.
Methods
Patients and treatment
Adult patients aged 18 years or older with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 with histologically confirmed DLBCL, primary mediastinal B-cell lymphoma (PMBCL), or transformed indolent lymphoma (TIL) relapsed from or refractory to at least one prior treatment were eligible for enrollment. Patients were required be suitable candidates for platinum-containing chemotherapy with intent to proceed to high dose chemotherapy and ASCT. Salvage regimens were chosen by the treating physician and were planned to be administered approximately every 21 days. Treating physicians were not blinded to early PET/CT results and were able to make treatment changes based on these results before EOT but were encouraged not to over interpret ambiguous results. Patients categorized as “responders” by EOT PET/CT could proceed with high dose chemotherapy and ASCT.
Baseline patient and disease characteristics
Baseline characteristics were captured at time of confirmation of relapsed or refractory disease. Time to first progression was calculated from original diagnosis date. Cell of origin (COO) classification as germinal center B-cell (GCB) or non-GCB was determined by Hans algorithm.23 Double-hit lymphoma (DHL) was defined as DLBCL with a rearrangement of MYC with a concurrent rearrangement of BCL2 and/or BCL6 by fluorescence in situ hybridization. Double expressor lymphoma (DEL) was defined as positivity by immunohistochemical staining for MYC and BCL2.
PET/CT
All patients underwent a standard PET/CT scan prior to starting salvage chemotherapy. Early and EOT PET/CTs were performed at the same institution using standardized techniques. Early PET/CTs were performed on approximately D4 and D21 of cycle 1, +/− 2 days. PET/CT scans were reviewed by an experienced nuclear medicine physician, and tumor SUVmax, 5-PS, and Lugano response were recorded. ΔSUVmax was calculated as previously described15 for the D4 and D21 early PET/CTs. The most avid tumor used to calculate SUVmax could differ in location between scans. A ΔSUVmax cutoff of >50% was chosen to define a “response” by semiquantitative analysis. Though a cutoff of 66% for ΔSUVmax has been found to be optimal across several studies investigating the iPET after two cycles of frontline immunochemotherapy for DLBCL,15,16,24 a more conservative, lower threshold of 50% was felt to be more appropriate for scans obtained after just one cycle of therapy.22 The EOT PET/CT (obtained at the conclusion of 2–3 total cycles of therapy) was categorized as “responder” (complete response, CR, or partial response, PR) or “non-responder” (stable disease, SD, or progressive disease, PD) by Lugano criteria for the primary outcome.14
Statistics
In this pilot study exploring the utility of early PET/CT, descriptive statistics defined baseline characteristics and treatment response/outcomes. Fisher’s exact test was used to evaluate the association between EOT response and other categorical variables. Kaplan-Meier method was used to estimate time-to-event endpoints. Log-rank test was used to evaluate the difference in time-to-event outcomes between patient groups. Progression free survival (PFS) was defined as time from start of salvage treatment to the first occurrence of progression, relapse, or death due to any cause. Disease specific survival (DSS) was defined as time from start of salvage treatment to death secondary to lymphoma. A two-sided P-value of <0.05 was considered statistically significant. Statistical software used included SAS 9.4 (SAS, Cary, NC), S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA), and R 4.0.2 (R Core Team, Vienna, Austria).
Results
A total of 32 patients were enrolled in the study, 25 of whom had early PET/CTs obtained and were evaluable. Of the remaining 7, the reasons for missing early PET/CT evaluation included logistical difficulties with scheduling (4), acute disease or treatment complications (2), and withdrawal of consent (1). The 25 patients were treated with platinum-containing chemotherapy between 2/5/2016 and 10/30/2018 and were included in the analysis, with data cutoff as of 2/29/2020. Their baseline characteristics at time of relapse and treatment decisions are described in Table 1.
Table 1.
Baseline patient characteristics and treatment patterns
| Characteristic | All patients (n=25) |
|---|---|
| Age, median (range), y | 61 (25 – 82) |
| Sex, male | 15 (60) |
| LDH | |
| > ULN | 18 (72) |
| ≤ ULN | 7 (28) |
| Stage | |
| I | 9 (36) |
| II | 1 (4) |
| III | 2 (8) |
| IV | 13 (52) |
| ECOG PS | |
| 0–1 | 22 (88) |
| 2 | 3 (12) |
| Extranodal sites | |
| 0–1 | 17 (68) |
| >1 | 8 (82) |
| IPI | |
| 0 | 2 (8) |
| 1–2 | 12 (48) |
| 3–4 | 11 (44) |
| COO | |
| GCB | 17 (68) |
| Non-GCB | 8 (32) |
| MYC status | |
| DHL | 8 (32) |
| DEL | 5 (20) |
| Negative | 12 (48) |
| Histology | |
| DLBCL* | 18 (72) |
| PMBCL | 2 (8) |
| TIL | 5 (20) |
| Frontline therapy | |
| R-EPOCH-like | 9 (36) |
| R-CHOP-like | 16 (64) |
| Prior lines of therapy | |
| 1 | 19 (76) |
| 2 | 5 (20) |
| 3 | 1 (4) |
| Median time to 1st progression (range), mo | 5.8 (1.8 – 77.7) |
| Salvage regimen | |
| R-DHAP | 11 (44) |
| R-ICE | 9 (36) |
| R-GDP | 3 (12) |
| R-hyperC | 2 (8) |
| Outcome after cycle 1 | |
| Continue sample therapy | 12 (48) |
| Changed therapy | 10 (40) |
| Discontinue treatment | 3 (12) |
| Definitive therapy | |
| ASCT | 7 (28) |
| AlloSCT | 2 (8) |
| CART19 | 7 (28) |
including high-grade B-cell lymphomas
LDH, lactate dehydrogenase; ULN, upper limit of normal; ECOG, eastern cooperative oncology group performance status; IPI, international prognostic index; COO, cell-of-origin; GCB, germinal center B-cell; DHL, double-hit lymphoma; DEL, double-expressor lymphoma; DLBCL, diffuse large B-cell lymphoma; PMBCL, primary mediastinal B-cell lymphoma; TIL, transformed indolent lymphoma; ASCT, autologous stem cell transplant; alloSCT, allogeneic stem cell transplant, CART19, anti-CD19 chimeric antigen receptor T-cell therapy
Ten (40%) patients had a therapy change after C1 and before EOT evaluation due to clinically relevant early treatment failure or progression (4 SD and 6 PD) based on early PET/CT result as interpreted by the treating physician. Twelve (48%) continued with a second cycle of the same regimen, and another 3 (8%) discontinued therapy in favor of supportive measures. No patients changed or stopped therapy because of toxicity. Sixteen (64%) patients were evaluable for EOT response by PET/CT, of which nine (56%) achieved an EOT response and 7 (44%) did not. Seven (28%) patients did not have an EOT PET/CT due to early progression. One patient missing an EOT response underwent apheresis for ASCT prior to the EOT PET/CT timepoint after the early PET/CTs identified a CR, and another patient had their EOT response evaluated with CT-imaging only, demonstrating a CR. No baseline characteristics were associated with EOT response (supplemental Table 1). Of the 10 patients who had a therapy change due to early PET/CT findings, 6 had an EOT PET/CT performed after completion of the subsequent therapy, and 4 responded to subsequent therapy.
A total of 24 patients had a PET/CT performed on D4 and 22 on D21. One patient did not have their baseline PET/CT images available so ΔSUVmax could not be calculated. On D4, 14 (58%) patients achieved a response (CR 2 [8%], PR 12 [50%]) by Lugano classification and 10 (43%) patients had a ΔSUVmax >50%. On D21, 7 (32%) patients achieved a response and 5 (24%) patients had a ΔSUVmax >50%. Six (26%) patients achieved a response on both D4 and D21 and 4 (18%) patients achieved a ΔSUVmax >50% on both D4 and D21. Median ΔSUVmax on D4 and D21 were 45% and 26% (decrease from baseline), respectively.
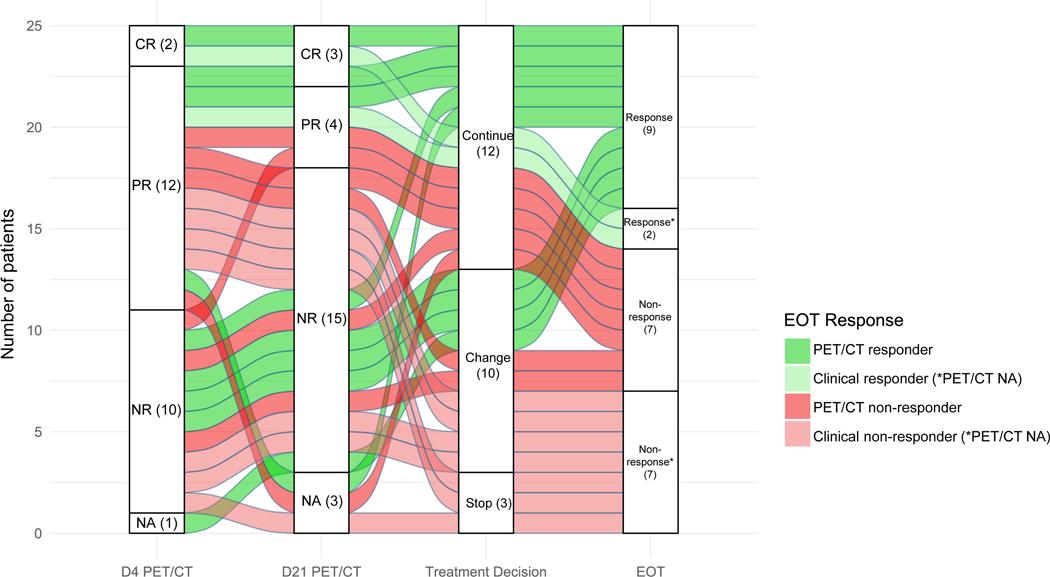
In comparing response at D4 with response at D21, we identified that the 2 patients with a CR at D4 maintained their CR at D21. Of the 12 partial responders at D4, 1 patient improved to a CR, 3 maintained a PR, and 6 experienced PD by D21 (2 missing D21 PET/CT and none with SD). Of the 10 non-responders at D4, 1 patient improved to a PR and 8 experienced persistent non-response (1 missing D21 PET/CT). A significant number of partial responders at D4 lost that response by D21, but it was unlikely for a non-responder at D4 to achieve a response by D21. Notably, 4/8 persistent non-responders would eventually go on to achieve an EOT response (3 underwent an early therapy change and 2 received an ASCT). The evolution of early PET/CT responses stratified by treatment change and EOT response is depicted for all patients (Figure 1) and for an individual patient (Figure 2). No early PET/CT metric was statistically associated with EOT response (Table 2).
Figure 1.
Early PET/CT responses stratified by treatment decision and EOT response. Each flow represents an individual patient and is colored according to their end-of-treatment response. Patients continued the same therapy, changed to a new therapy, or stopped therapy for supportive measures after cycle 1 of salvage treatment. PET/CT non-response includes stable disease and progressive disease.
*Patients missing an EOT PET/CT were categorized as clinical responders or non-responders here. Of the 2 clinical responders, 1 underwent early apheresis and autologous stem cell transplant before EOT after achieving a CR on D4 and D21 PET/CTs and 1 achieved a CR at EOT by CT imaging. Of the 7 clinical non-responders, 3 discontinued therapy after cycle 1 for supportive measures and 4 changed therapy after cycle 1 but had clinical or radiographic (CT) evidence of non-response to their next therapy.
CR: complete response; PR: partial response; NR: non-response; NA: not available; D: day; PET/CT: positron emission tomography/ computed tomography; EOT: end-of-treatment
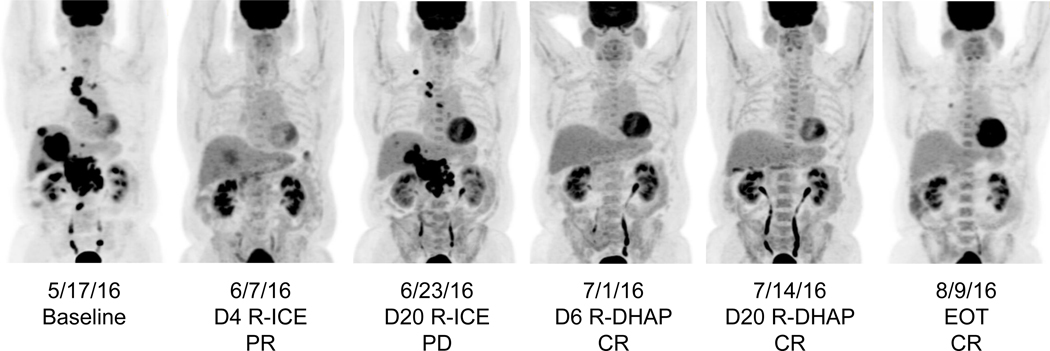
Figure 2.
Serial early PET/CT images from an individual patient who underwent early therapy change. This patient achieved a partial response by D4 of R-ICE salvage immunochemotherapy, however developed progressive disease by D20. They were immediately switched to R-DHAP and demonstrated a persistent complete response on a second set of early PET/CTs. After a second cycle of R-DHAP, they were still in a complete response (end-of-therapy). This patient proceeded to receive an autologous stem cell transplant.
D: day; R-ICE: rituximab, ifosfamide, carboplatin, etoposide; PR: partial response; PD: progressive disease; R-DHAP: rituximab, dexamethasone, cytarabine, cisplatin; CR: complete response; EOT: end-of-therapy
Table 2.
Detailed early PET/CT metrics and association with EOT response
| Early PET/CT result | EOT responder (n=9)† | EOT non-responder (n=7)† | P – value | |
|---|---|---|---|---|
| D4 Visual | 24 | |||
| Response (CR + PR) | 14 (58) | 4 (50) | 4 (57) | 1.0 |
| Non-response (SD + PD) | 10 (42) | 4 (50) | 3 (43) | |
| D4 ΔSUVmax | 23 | |||
| >50% | 10 (43) | 5 (71) | 2 (29) | 0.29 |
| ≤50% | 13 (57) | 2 (29) | 5 (71) | |
| D4 ΔSUVmax, median (range) | 45 (−76 – 89)* | 57 | 37 | 0.37 |
| D21 Visual | 22 | |||
| Response (CR + PR) | 7 (32) | 3 (38) | 2 (33) | 1.0 |
| Non-response (SD + PD) | 15 (68) | 5 (62) | 4 (67) | |
| D21 ΔSUVmax | 21 | |||
| >50% | 5 (24) | 3 (43) | 1 (17) | 0.56 |
| ≤50% | 16 (76) | 4 (57) | 5 (83) | |
| D21 ΔSUVmax, median (range) | 26 (−140 – 89)* | 42 | 29 | 0.94 |
| Response on D4 & D21 | 23 | |||
| Y | 6 (26) | 3 (38) | 1 (17) | 0.58 |
| N | 17 (74) | 5 (62) | 5 (83) | |
| ΔSUVmax >50% on D4 & D21 | 22 | |||
| Y | 4 (18) | 3 (43) | 0 (0) | 0.19 |
| N | 18 (82) | 4 (57) | 6 (100) | |
positive number represents decrease in SUVmax
Excludes patients who did have early PET/CTs performed but were missing EOT PET/CT evaluation
PET/CT, positron emission tomography/computed tomography; EOT, end-of-treatment; D4, day 4; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; SUVmax, maximum standardized uptake value; D21, day 21
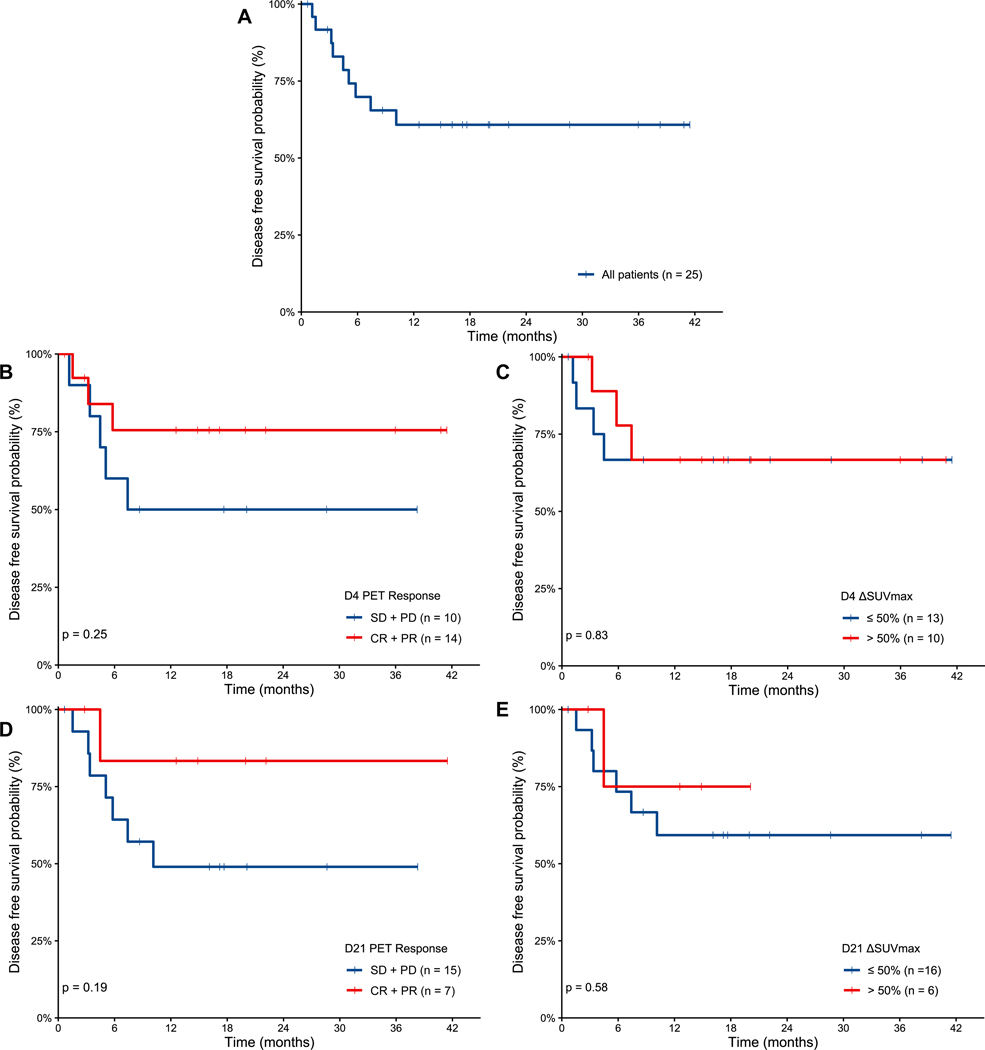
Median follow up was 19.7 months (range 0.7–40.9) from start of platinum-containing chemotherapy. Median PFS was 2.7 months. PFS was shorter for patients with an ECOG PS of > 1 (p=0.004), double-hit lymphoma (p=0.011), TIL (p=0.002), and who did not receive R-ICE or R-DHAP (p=0.011) (supplementary Figure 1).
A total of 11 patients died, 9 from progressive lymphoma and 2 from complications of transplant. Median DSS was not reached and was 61% at 24 months (Figure 3A). DSS was shorter for patients with an ECOG PS > 1 (p=0.002), international prognostic index ≥3 (p=0.046), and who did not receive R-ICE or R-DHAP (p=0.006) (supplementary Figure 2). There was no difference in DSS based on the response individually on D4 or D21 PET/CT, by visual assessment (Figure 3B, D) or ΔSUVmax (Figure 3C, E).
Figure 3.
Disease specific survival from start of salvage immunochemotherapy for all patients (A) and according to response by visual assessment and ΔSUVmax cutoff of 50% on PET/CT from D4 (B-C) and D21 (D-E), respectively.
D: day; PET: positron emission tomography; SD: stable disease; PD: progressive disease; CR: complete response; PR: partial response; SUVmax: maximum standardized uptake value.
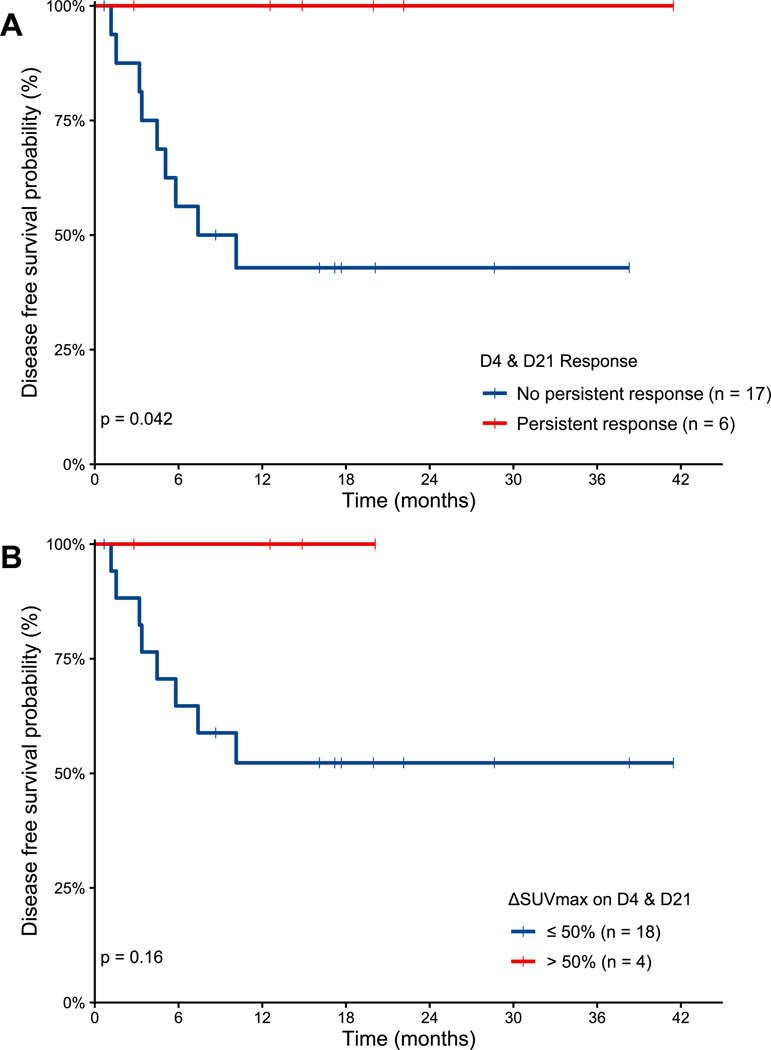
However, DSS was longer for patients who had a persistent response on both D4 and D21 compared to patients without a persistent response, 100 vs. 47% at 24 months (p=0.042, Figure 4A). Patients who had a persistent response experienced a longer median time to first progression compared to patients without a persistent response (38.3 versus 5.4 months respectively, p=0.027). No other baseline characteristics were significantly different between the two groups (data not shown). Of the 6 patients with a persistent response, 4 had a late first relapse (range 23.1–78.9 months after diagnosis) and 2 had an early first relapse (1.9–9.0 months). Thus, a persistent response on early PET/CTs during platinum-containing chemotherapy was predictive of excellent long-term outcomes even if patients experienced early relapse after frontline therapy. Of the 6 patients with a persistent response, 4 patients received ASCT, one CART19 because of sub-optimal depth of response at EOT, and one other treatments because a repeat PET/CT performed after EOT and before planned ASCT demonstrated subsequent PD. The DSS benefit was not statistically significant when comparing patients who had a ΔSUVmax >50% on both D4 and D21 with those who did not (p=0.16) (Figure 4B).
Figure 4.
Disease specific survival from start of salvage immunochemotherapy for patients according to persistent response on D4 and D21 by visual assessment (A) and ΔSUVmax cutoff of 50% (B).
A total of 7 patients not eligible for ASCT eventually received CART19. Three patients received CART19 due to an unsatisfactory early PET/CT result resulting in treatment change prior to completion of 2 cycles of platinum-containing chemotherapy, and 2 were alive at data censoring. The remaining 4 patients who eventually received CART19 did not have a treatment change based upon early PET/CT results and were all alive at data censoring. Three received CART19 after their EOT PET/CT demonstrated non-response, and 1 achieved a response at EOT but relapsed after ASCT and received CART19 at that time. In comparison, of patients who underwent an early therapy change due to an unsatisfactory early PET/CT result and did not receive CART19, only 1 of 7 was alive at data censoring. Long-term OS was similar for patients who were able to obtain an ASCT versus CART19 (supplementary figure 3).
Discussion
This prospective pilot study is the first of its kind to investigate early PET/CTs during salvage therapy for R/R DLBCL. We identified that early PET/CTs on D4 and D21 can reveal early disease progression and/or treatment failure during the first cycle, prompting unplanned change of therapy in 40% of patients and discontinuation of therapy in 8% of patients. No early PET/CT metrics, either based on visual assessment or semiquantitative analysis by ΔSUVmax, were associated with the primary endpoint of EOT response.
Response on either D4 or D21 individually was not associated with EOT response or DSS, but a persistent response on both D4 and D21 was predictive of excellent long-term DSS. This suggests that overall chemosensitivity can be determined during C1 of platinum-containing chemotherapy. Patients who failed to achieve a response on either D4 or D21 conversely had significantly worse survival outcomes. Achieving a response on D4 but experiencing progression of lymphoma by D21 in comparison to D4 was common and likely reflected a “kinetic failure” or early disease progression where tumor growth outpaced the temporary cytoreduction achieved earlier in the cycle. The decrease in median ΔSUVmax from 45% to 26% from D4 to D21 may be due to this phenomenon as well. Of the 12 patients with PR on D4, half had PD on the D21 scan which would have not been detected without early scans. Conversely, only 1 out of 10 patients with a non-response on D4 subsequently achieved a response by D21, suggesting that chemorefractoriness to salvage immunochemotherapy can be predicted very early in the cycle. A persistent ΔSUVmax >50% on both D4 and D21 may not have been predictive of survival because of small numbers or due to variation in scans.
Our study was not designed to be interventional so we can only generate hypotheses regarding the utility of early therapy change after one cycle of therapy based on unsatisfactory early PET/CT results. Clinicians were not blinded to these results and were not disallowed from utilizing them in treatment decision making, and arguably it may have been unethical to withhold this information if progression or mixed response was identified. The primary endpoint of EOT response was thus confounded by an early therapy change in 6 (38%) of 16 patients with EOT data evaluable, which could explain why early PET/CT measures were not associated with EOT response.
Three randomized studies comparing salvage chemotherapy followed by ASCT with CART19 in the second-line setting have completed accrual,25,26 but currently patients are only eligible for CART19 after at least two prior lines of therapy. A confirmatory study designed to investigate the benefit of shortening exposure to chemotherapy in the second-line to just one cycle for non-responders in favor of non-chemotherapy treatments such as CART19 could help avoid unnecessary toxicity, symptomatic and organ-threatening disease progression, and therapy-induced lymphopenia that may prevent successful manufacturing of CAR T-cells.27
There were some limitations of this pilot study. Relatively small patient numbers led to some associations not reaching statistical significance. Ideally, the treating physicians would have been blinded to the early PET/CT results, however we considered this unethical to deprive potentially clinically meaningful information. Thus, the decision to change therapy early was not done in a randomized fashion, making direct comparisons between patients who underwent therapy change versus those who did not complicated by selection bias. The use of rituximab may have caused immune-mediated inflammation leading to false positive early PET/CT scans28 that could not be excluded without repeat tumor biopsies, however this is unlikely given the rarity of early SD/PD evolving into CR/PR. The study population had a higher incidence of high-risk disease features, including primary refractory disease and a significant fraction with double-hit lymphoma. Early PET/CTs may be less predictive of outcomes in a more generalizable patient population with better prognostic features and sensitivity to initial chemotherapy. A minority of patients with variant histology (PMBCL or TIL) and with very late relapses after primary therapy were included in the study; though their standard treatment is the same, their underlying disease biology and early radiographic response may differ.
In conclusion, we identified that performing early PET/CTs during the first cycle of salvage therapy in R/R DLBCL in this pilot study was feasible and could demonstrate real-time evidence of chemotherapy resistance. A durable early response was associated with chemosensitivity at the end of therapy and predicted excellent long-term outcomes. If CART19 therapy remains limited to the third-line setting, a larger confirmatory study investigating dynamic risk assessment during salvage therapy for R/R DLBCL is warranted. Because of the logistical challenges of performing two early PET/CT scans during the first cycle, performing just one at D21 would be more feasible and still capture patients experiencing “kinetic failures” of chemotherapy. Such a study should also incorporate blood-based biomarkers such as circulating tumor DNA29,30 and randomize patients to early CART19 or continuation of immunochemotherapy to demonstrate the utility of early therapy change.
Supplementary Material
Funding
This research is supported in part by the MD Anderson NIH/NCI Cancer Center Support Grant under award number P30 CA016672.
HHC is a consultant for Sage Evidence-Based Medicine & Practice Institute. RS has received research support from Rafael Pharmaceuticals and Seattle Genetics. PS is a consultant for Roche-Genentech and has received research support from AstraZeneca/Acerta. LJN has received honorarium from ADC Therapeutics, BMS, Bayer, Epizyme, Genentech, Gilead/Kite, Janssen, Morphosys, Novartis, Pfizer, TG Therapeutics; research support from BMS, Caribou Bioscience, Epizyme, Genentech, Gilead/Kite, IGM Biosciences, Janssen, Novartis, Pfizer, TG Therapeutics. SSN has received honorarium from Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, and Unum Therapeutics; research support from Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences, and Acerta; and royalties from Takeda Pharmaceuticals. CRF is a consultant for Abbvie, Bayer, BeiGene, Celgene, Denovo Biopharma, Genetech/Roche, Genmab, Gilead, Karyopharm, Pharmacyclics/Janssen, SeaGen, and Spectrum and has received research support from 4D, Abbvie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, Celgene, Cellectis, EMD, Gilead, Genentech/Roche, Guardant, Iovance, Jannssen Pharmaceutical, Kite, Morphosys, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Welcome Fund, Eastern Cooperative Oncology Group, NCI, V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research. JW is a consultant for Gilead/Kite, BMS, Novartis, Morphosys, ADC Therapeutics, Genentech, Iksuda, IMV, Umoja, Abbvie, Amgen, and has received research support from Gilead/Kite, BMS, Novartis, Morphosys, Genentech, Abbvie, Janssen, Curis, AstraZeneca, and Janssen.
Footnotes
Disclosure of conflicts of interest
Authors not listed had no disclosures to report.
Clinical trial information
This trial was registered on Clinicaltrials.gov (NCT02405078).
References
- 1.Farooq U, Maurer MJ, Thompson CA, et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol. 2017;179(1):50–60. doi: 10.1111/bjh.14813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305 [DOI] [PubMed] [Google Scholar]
- 4.Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103(10):3684–3688. doi: 10.1182/blood-2003-11-3911 [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Geyer SM, Kurtin PJ, et al. Salvage chemotherapy with rituximab DHAP for relapsed non-Hodgkin lymphoma: A phase II trial in the North Central Cancer Treatment Group. Leuk Lymphoma. 2008;49(6):1074–1080. doi: 10.1080/10428190801993470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 9.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 10.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32(27):3048–3058. doi: 10.1200/JCO.2013.53.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terasawa T, Dahabreh IJ, Nihashi T. Fluorine‐18‐Fluorodeoxyglucose Positron Emission Tomography in Response Assessment Before High‐Dose Chemotherapy for Lymphoma: A Systematic Review and Meta‐Analysis. Oncologist. 2010;15(7):750–759. doi: 10.1634/theoncologist.2010-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CH M.Interim PET-CT in the management of diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Progr. 2012;2012. doi: 10.1182/ASHEDUCATION-2012.1.397 [DOI] [PubMed] [Google Scholar]
- 13.Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on interim-PET scan in lymphoma. In: Leukemia and Lymphoma. Vol 50. Leuk Lymphoma; 2009:1257–1260. doi: 10.1080/10428190903040048 [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: The lugano classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C, Itti E, Haioun C, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48(10):1626–1632. doi: 10.2967/jnumed.107.042093 [DOI] [PubMed] [Google Scholar]
- 16.Itti E, Meignan M, Berriolo-Riedinger A, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: Comparison between Deauville criteria and ΔsUVmax. Eur J Nucl Med Mol Imaging. 2013;40(9):1312–1320. doi: 10.1007/s00259-013-2435-6 [DOI] [PubMed] [Google Scholar]
- 17.Moskowitz CH, Schöder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28(11):1896–1903. doi: 10.1200/JCO.2009.26.5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dührsen U, Müller S, Hertenstein B, et al. Positron emission tomography-guided therapy of Aggressive Non-Hodgkin Lymphomas (PETAL): A multicenter, randomized phase III trial. J Clin Oncol. 2018;36(20):2024–2034. doi: 10.1200/JCO.2017.76.8093 [DOI] [PubMed] [Google Scholar]
- 19.Neelapu SS, Dickinson M, Ulrickson ML, et al. Interim Analysis of ZUMA-12: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) as First-Line Therapy in Patients (Pts) With High-Risk Large B Cell Lymphoma (LBCL). Blood. 2020;136(Supplement 1):49–49. doi: 10.1182/blood-2020-134449 [DOI] [Google Scholar]
- 20.Yamane T, Daimaru O, Satoshi Ito, et al. Decreased 18 F-FDG Uptake 1 Day After Initiation of Chemotherapy for Malignant Lymphomas. Vol 45.; 2004. [PubMed] [Google Scholar]
- 21.Mayerhoefer ME, Raderer M, Jaeger U, et al. Ultra-early response assessment in lymphoma treatment: [18F]FDG PET/MR captures changes in glucose metabolism and cell density within the first 72 hours of treatment. Eur J Nucl Med Mol Imaging. 2018;45(6):931–940. doi: 10.1007/s00259-018-3937-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan L, Kreissl MC, Su L, et al. Prognostic analysis of interim 18 F-FDG PET/CT in patients with diffuse large B cell lymphoma after one cycle versus two cycles of chemotherapy. Eur J Nucl Med Mol Imaging. 2019;46(2):478–488. doi: 10.1007/s00259-018-4198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545 [DOI] [PubMed] [Google Scholar]
- 24.Casasnovas RO, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118(1):37–43. doi: 10.1182/blood-2010-12-327767 [DOI] [PubMed] [Google Scholar]
- 25.Oluwole OO, Bishop MR, Gisselbrecht C, et al. ZUMA-7: A phase 3 randomized trial of axicabtagene ciloleucel (Axi-Cel) versus standard-of-care (SOC) therapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL). J Clin Oncol. 2018;36(15_suppl):TPS7585-TPS7585. doi: 10.1200/jco.2018.36.15_suppl.tps7585 [DOI] [Google Scholar]
- 26.Westin J, Bishop M, Flinn I, et al. BELINDA: A Phase 3 Study Evaluating the Safety and Efficacy of Tisagenlecleucel versus Standard of Care in Adult Patients with Relapsed/Refractory Aggressive B-Cell Non-Hodgkin Lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19:S270–S271. doi: 10.1016/j.clml.2019.07.197 [DOI] [Google Scholar]
- 27.Roddie C, O’Reilly M, Dias Alves Pinto J, Vispute K, Lowdell M. Manufacturing chimeric antigen receptor T cells: issues and challenges. Cytotherapy. 2019;21(3):327–340. doi: 10.1016/j.jcyt.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 28.Han HS, Escalón MP, Hsiao B, Serafini A, Lossos IS. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol. 2009;20(2):309–318. doi: 10.1093/annonc/mdn629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol. 2018;36(28):2845–2853. doi: 10.1200/JCO.2018.78.5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtz DM, Esfahani MS, Scherer F, et al. Dynamic Risk Profiling Using Serial Tumor Biomarkers for Personalized Outcome Prediction. Cell. 2019;178(3):699–713.e19. doi: 10.1016/j.cell.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.