Abstract
Background and Objectives
Coronavirus disease 2019 (COVID-19) has spread rapidly worldwide. Saudi Arabia was significantly impacted by COVID-19. In March 2021, 381,000 cases were reported with 6539 deaths. This study attempts to quantify the impact of remdesivir on healthcare costs in Saudi Arabia, in terms of intensive care unit admissions, mechanical ventilation, and death prevention.
Methods
A forecasting model was designed to estimate the impact of remdesivir on the capacity of intensive care units and healthcare costs with patients requiring low flow oxygen therapy. The forecasting model was applied in the Saudi context with a 20-week projection between 1 February and 14 June, 2021. Model inputs were collected from published global and Saudi literature, available forecasting resources, and expert opinions. Three scenarios were assumed: the effective pandemic infection rate (Rt) remains at 1, the Rt increases up to 1.2, and the Rt declines from 1 to 0.8 over the study period.
Results
The model estimated that the use of remdesivir in hospitalized patients, in the optimistic and pessimistic scenarios, could prevent between 1520 and 3549 patient transfers to intensive care units and mechanical ventilation, prevent between 815 and 1582 deaths, and make potential cost savings between $US154 million and $US377 million owing to the reduction in intensive care unit capacity, respectively.
Conclusions
The treatment with remdesivir may improve patient outcomes and reduce the burden on healthcare resources during this pandemic.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-022-01177-z.
Key Points
| Remdesivir-based treatment in patients requiring low-flow oxygen can reduce the burden on healthcare facilities. |
| The introduction of remdesivir for the treatment of coronavirus disease 2019, in patients requiring low-flow oxygen, can generate important cost savings for hospitals. |
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) causes the respiratory illness designated as coronavirus disease 2019 (COVID-19). The worldwide COVID-19 pandemic put significant pressure on national healthcare systems resulting in a high social and economic impact [1, 2]. As of 16 May, 2022, Saudi Arabia has recorded 759,856 cases and 9118 deaths due to COVID-19 [3]. With an estimated 13% of all cases resulting in hospitalizations, this has placed a substantial additional burden on the Saudi Arabian healthcare system [4] despite the early and active prevention measures adopted by the Saudi Arabian government [5].
A substantial number of patients who are hospitalized for COVID-19 require resource-intensive and expensive treatment in intensive care units (ICUs) with or without the support of mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO) [6]. Given that ICUs have limited capacity and are required to treat numerous severe pathologies, effective therapies for COVID-19 offer the opportunities to free up ICU capacity for the treatment of other diseases and improve the management of emergency units in hospitals.
Remdesivir (Veklury®) is an antiviral drug developed by Gilead Sciences, Inc. and has been conditionally or fully approved for use in hospitalized patients with COVID-19 in more than 50 countries, including by the US Food and Drug Administration (full approval granted October 2020 [7]), and conditional approval in the European Medicines Agency in June 2020 [8]. Typically, remdesivir is indicated for hospitalized patients with COVID-19 with pneumonia, though there is variation by agency on whether patients must also be receiving oxygen support. The Ministry of Health of Saudi Arabia issued guidelines that state that severe or critical patients with COVID-19 are eligible for treatment with remdesivir [6]. On 1 September, 2021, the Saudi Food and Drug Administration approved remdesivir by granting it marketing authorization [9].
The pivotal, randomized, double-blind, placebo-controlled clinical trial, ACTT-1, enrolled 1062 patients [10]. The results of the trial demonstrated that treatment with remdesivir significantly reduced the time to recovery in adults who were hospitalized with COVID-19 by a median of 5 days, with a rate ratio of 1.29 (95% confidence interval 1.12–1.49; p < 0.001) [10]. In addition, patients treated with remdesivir showed a reduced need for new oxygen support and a 43% reduction in the incidence of MV or ECMO use. In a sub-group analysis, patients requiring low-flow oxygen had a statistically significant 70% reduction in mortality compared with placebo (95% confidence interval 0.14–0.64) [10]. The objective of this study was to investigate the potential impact of treating hospitalized patients with COVID-19 on low-flow oxygen with remdesivir on healthcare resource use and costs in Saudi Arabia by using an epidemiologic and health economic model.
Materials and Methods
Study Design and Model Structure
This study utilizes a previously published epidemiologic model [11, 12] to estimate the potential impact of remdesivir administration on hospital resource use and costs in Saudi Arabia. A targeted literature review for Saudi Arabia-specific data as well as the elicitation of local clinical opinion was conducted to ensure the model reflected the local healthcare context.
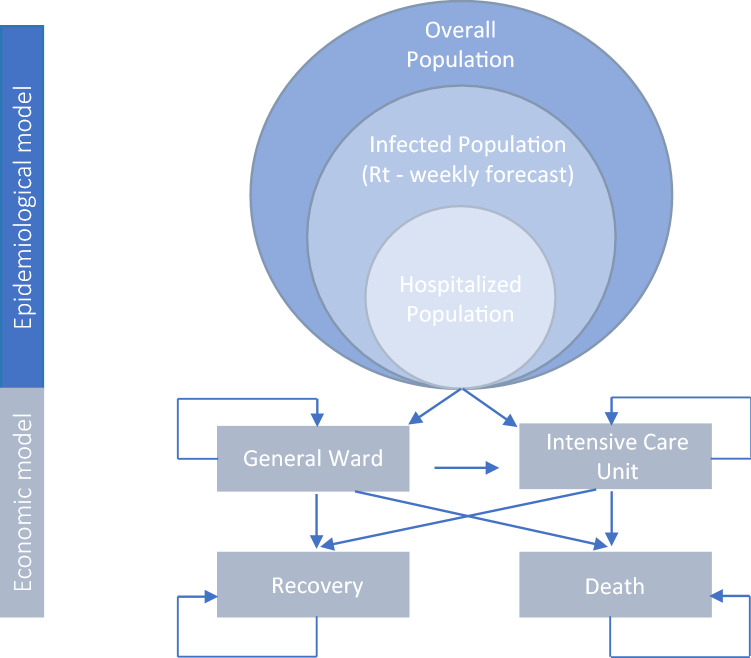
The model (Fig. 1) has two stages:
-
A weekly epidemiological estimation of the infection rate (Rt) in Saudi Arabia, including the estimated number of:
(a) patients infected with COVID-19 requiring hospitalization; and
(b) those requiring a stay in the ICU.
An economic model that estimates the subsequent direct costs to the health system because of hospitalization from COVID-19, comparing a cohort of patients on low-flow oxygen who receive remdesivir plus standard of care to a cohort of patients who receive standard of care alone.
Fig. 1.
Model structure. Rt infection rate. Graphic adapted from Ruggeri et al. [11, 12]
Epidemiological Model
The first stage of the model estimates the development of the COVID-19 epidemic and is modeled over a 20-week period in order to allow comparison to previously published studies [11, 12]. The model utilizes published data on Rt based on real observations between 1 February and 22 March, 2021, [13] and subsequently we modeled three potential scenarios (see Table 1):
A base case, where the Rt remains at 1.
Scenario 1: an ‘optimistic’ scenario where the weekly Rt decreases to 0.8 over the 20-week period.
Scenario 2: a ‘pessimistic’ scenario where the weekly Rt increases to 1.2.
Table 1.
Infection rate (Rt) over 20 weeks
| Week | Starting date | Rt | Source | |
|---|---|---|---|---|
| Scenario 1 | Scenario 2 | |||
| 1 | 1 February | 1 | 1 | [13] |
| 2 | 8 February | 0.9 | 0.9 | [13] |
| 3 | 15 February | 1 | 1 | [13] |
| 4 | 22 February | 1.05 | 1.05 | [13] |
| 5 | 1 March | 1.1 | 1.1 | [13] |
| 6 | 8 March | 1 | 1 | [13] |
| 7 | 15 March | 1.1 | 1.1 | [13] |
| 8 | 22 March | 1.15 | 1.15 | [13] |
| 9 | 29 March | 1.15 | 1.15 | [13] |
| 10 | 5 April | 1.15 | 1.15 | [13] |
| 11 | 12 April | 1.2 | 1 | Experts’ opinion |
| 12 | 19 April | 1.2 | 0.97 | Experts’ opinion |
| 13 | 26 April | 1.2 | 0.96 | Experts’ opinion |
| 14 | 3 May | 1.2 | 0.95 | Experts’ opinion |
| 15 | 10 May | 1.2 | 0.93 | Experts’ opinion |
| 16 | 17 May | 1.2 | 0.91 | Experts’ opinion |
| 17 | 24 May | 1.2 | 0.87 | Experts’ opinion |
| 18 | 31 May | 1.2 | 0.85 | Experts’ opinion |
| 19 | 7 June | 1.2 | 0.83 | Experts’ opinion |
| 20 | 14 June | 1.2 | 0.8 | Experts’ opinion |
These scenarios were developed alongside clinical experts from Saudi Arabia (see Expert Validation for details on the selection of experts and opinion elicitation) to take into account specific Saudi Arabia epidemic circumstances (e.g., Ramadan festivities from April to May 2021 in the pessimistic scenario and government vaccination campaigns in the optimistic scenario). The estimation of COVID-19 hospitalization rate was taken from published data [13]. The model also estimated the mortality rate in the non-hospitalized infected population using data from the literature [14–19].
Economic Model
The second stage of the model uses a Markov dynamic-cohort cost-effectiveness model [11, 12] with a 1-week cycle and a 20-week time horizon. New individuals are added to the model for each cycle based on the Rt for that cycle. The model is made up of four mutually exclusive health states: hospitalized (general ward, with or without non-invasive oxygen support), ICU (with MV or ECMO), recovery (discharge), and death. Hospitalized individuals can stay hospitalized, move into the ICU, recover, or die. For individuals admitted directly into the ICU, they can stay in the ICU, recover, or die. No discount rate was applied given the short time horizon.
The model compares patients on low-flow oxygen who are treated with remdesivir plus standard of care versus those on low-flow oxygen on standard of care alone. The outcomes of interest included the number of days in hospital (ward and ICU), deaths, and associated hospital costs. Estimates of the number of ICU patients requiring MV or ECMO as well as the length of hospital/ICU stay were provided by the experts. Estimates of the mortality rate for hospitalized patients were derived from the literature [14–19]. To estimate the comparative impact of remdesivir on patients who receive low-flow oxygen, efficacy data from the pivotal phase III trial [10] were used to model the potential reduction in time to recovery, disease progression, and mortality (see Table 2).
Table 2.
Parameter model input
| Parameter | Base case | Distribution | Source |
|---|---|---|---|
| Mortality rate, general infected | 3.5% | Beta | Mathematical average [17, 18] |
| Mortality rate, hospitalized population | 10% | Beta | Mathematical average [17, 18] |
| Percent starting in ward | 96% | Beta | [13] |
| Percent starting in ICU | 4% | Beta | [13] |
| Percent of patients requiring low-flow O2 | 55% | Beta | [13] |
| Percent of patients requiring MV/ECMO | 23% | Beta | [10] |
| Hazard ratio, time to recovery (low-flow patients) | 1.32 | Beta | [10] |
| Relative reduction in progression to ICU | 30% | Beta | [10] |
| Hazard ratio, mortality (low-flow patients) | 0.30 | Beta | [10] |
| Remdesivir treatment | 5 days (6 vials) | Gamma | Assumption: from clinical practice |
| Hospital ward stay | 12 days | Gamma | [17, 18] |
| ICU stay, non MV/ECMO | 5 days | Gamma | Expert opinion |
| ICU stay, MV/ECMO | 13 days | Gamma | Expert opinion |
| Hospital stay, patients who die | 24.7 days | Gamma | [15, 17, 18] |
| Hospital ward per day | $US1666 | Deterministic | [15] |
| ICU non-MV per day | $US2536 | Deterministic | [15] |
| ICU MV per day | $US2990 | Deterministic | [15] |
| Remdesivir (per vial) | $US390 | Deterministic | Gilead Sciences, Inc. |
ECMO extra corporeal membrane oxygenation, ICU intensive care unit, MV mechanical ventilation
Data on the use of healthcare resources, specifically on general ward stay, were sourced from the local literature [17, 18]. The ICU length of stay (stratified by the need for MV/ECMO or not) was provided by clinical expert opinion. For patients requiring an ICU stay, it was assumed that they would spend at least some time on a general ward. This length of stay estimate was provided by clinical expert opinion. For the remdesivir arm, it was assumed that 5 days of therapy was provided in line with the guidelines. Direct medical costs for the cost per day of the hospital ward and the ICU (for non-MV and MV) were taken from Khan et al. [15] and are based on the year of 2020. The cost of remdesivir was set to be $US390 per vial [20]. Table 2 provides an overview of the model inputs.
Sensitivity Analysis
To investigate uncertainty around the parameter estimates, both one-way and probabilistic sensitivity analyses were conducted for both stages of the model. When possible, the standard deviation of the estimates from the literature was used. If this was not possible, values were adjusted by ± 30%. Table 1 in the Electronic Supplementary Material (ESM) provides an overview of the variables included in the probabilistic sensitivity analysis as well as the distributions utilized.
Expert Input Validation
In order to ensure that the models fit the Saudi Arabia context, an expert panel of local physicians was formed to validate the model structure and local information. Three experts were selected according to their real-world clinical experience caring for patients with COVID-19 as well as their expertise with the Saudi Arabia health system and hospital management. Once chosen, the experts participated in a one-to-one structured interview to elicit views on the model structure and inputs, as well as potential variations specific to the Saudi Arabia context. The second phase involved a joint interview where the results from a targeted literature review were provided for their validation as well as reaching a consensus on the Rt evolution scenarios. This research employed structured interviews [21, 22], where participants were provided with clear objectives at the beginning of the interview. The structured questions allowed the elicitation of information on specific issues and themes surrounding key model inputs and assumptions. Standard methods of synthesizing the interview data were used [21].
Results
Population
In the base case, the model estimated that there was a total of 178,405 people who were infected with COVID-19 in the time period. Of those, 27,438 were people admitted to hospital with 10,027 requiring the ICU. Table 3 presents the findings of the epidemiological model, which provided the population for the economic model.
Table 3.
Results of the epidemiological model
| Population | Overall |
|---|---|
| Number of infected | |
| Base case | 178,405 |
| Scenario 1 | 109,087 |
| Scenario 2 | 247,724 |
| Number requiring hospitalization | |
| Base case | 27,438 |
| Scenario 1 | 16,942 |
| Scenario 2 | 37,934 |
| Number requiring ICU at baseline | |
| Base case | 10,027 |
| Scenario 1 | 5966 |
| Scenario 2 | 14,088 |
| Number of deaths, without RDV | |
| Base case | 1712 |
| Scenario1 | 1164 |
| Scenario2 | 2260 |
ICU intensive care unit, RDV remdesivir
Comparative Outcomes
When investigating the impact of treating patients requiring low-flow oxygen with remdesivir, the model estimated substantially lower ICU use across all three scenarios when patients were treated with remdesivir. Table 4 summarizes the overall admissions to ICU for the three scenarios and by treatment arm. Figure 2 presents the weekly ICU admissions, over 20 weeks, for the base case, Scenario 1, and Scenario 2. In the base case, there were a total of 7491 ICU admissions over the modeled period for the group treated with remdesivir, compared with 10,027 in the standard of care group, a difference of 2535. For Scenario 1, there was a difference of 1520 and for Scenario 2, there was a difference of 3549 admissions. In terms of mortality, the use of remdesivir in the low-flow oxygen population resulted in 1199 fewer deaths than standard of care alone.
Table 4.
Outcomes by treatment arm
| Outcome | SoC | SoC + RDV | Difference |
|---|---|---|---|
| Total admissions to ICU | |||
| Base case | 10,027 | 7491 | – 2535 |
| Scenario 1 | 5966 | 4445 | – 1520 |
| Scenario 2 | 14,088 | 10,538 | – 3549 |
| Total number of deaths | |||
| Base case | 1712 | 513 | – 1199 |
| Scenario 1 | 1164 | 349 | – 815 |
| Scenario 2 | 2260 | 678 | – 1582 |
ICU intensive care unit, RDV remdesivir, SoC standard of care
Fig. 2.
Predicted weekly intensive care unit (ICU) admissions for the three scenarios in the 20-week time horizon: the base case (A), Scenario 1 (B), and Scenario 2 (C). Yellow bars representing standard of care (SoC); orange bars representing SoC plus remdesivir (RDV)
Cost Effectiveness
The use of remdesivir resulted in both a reduction in the number of ICU admissions and in the rates of mortality and was less costly, resulting in remdesivir plus standard of care being dominant over standard of care regardless of the scenario. Table 5 provides a summary of the deterministic cost results.
Table 5.
Cost-effectiveness outcomes
| Outcome | SoC | SoC + remdesivir | Difference |
|---|---|---|---|
| Total cost for hospital ward patients, $US | |||
| Base case | 252,578,717 | 203,964,078 | −48,614,639 |
| Scenario 1 | 288,505,171 | 232,944,906 | −5560,265 |
| Scenario 2 | 645,976,423 | 523,403,092 | −122,573,331 |
| Total cost for ICU, $US | |||
| Base case | 202,803,340 | 150,967,278 | −51,836,061 |
| Scenario 1 | 231,932,306 | 172,818,955 | −59,113,351 |
| Scenario 2 | 547,620,706 | 409,638,921 | −37,981,784 |
| Total cost for patients who died, $US | |||
| Base case | 77,517,030 | 23,255,109 | −54,261,921 |
| Scenario 1 | 85,905,704 | 25,771,711 | −60,133,992 |
| Scenario 2 | 166,729,155 | 50,018,746 | −116,710,408 |
| Total costs, $US | |||
| Base case | 532,899,088 | 378,186,466 | −154,712,622 |
| Scenario 1 | 606,343,182 | 431,535,572 | −174,807,610 |
| Scenario 2 | 1,360,326,285 | 983,060,760 | −377,265,524 |
ICU intensive care unit, SoC standard of care
Sensitivity Analysis
One-way deterministic sensitivity analyses indicated that the model was most sensitive to Rt values, the subsequent number of ICU admissions, mortality rates, overall hospitalization rate, and the relative risk of mortality when treated with remdesivir. Despite being sensitive to these factors, the model results consistently demonstrated substantial cost savings (see Figs. 1–3 of the ESM). The results of the probabilistic sensitivity analysis show that over 93% of simulations result in remdesivir plus standard of care being dominant over standard of care alone, regardless of the outcome (see Figs. 3, 4).
Fig. 3.
Cost-effectiveness plane: relationship between incremental costs and avoided deaths
Fig. 4.
Cost-effectiveness plane: relationship between incremental costs and incremental intensive care units (ICUs) [mechanical ventilation]
Discussion
This analysis has shown that in Saudi Arabia, remdesivir plus standard of care has the potential to reduce healthcare resource use, mortality, and costs when compared with standard of care alone across a range of plausible local epidemiological scenarios. The Markov dynamic-cohort model is a simplification of the reality, and it allows an estimate of the epidemiologic situation on a 20-week time horizon. The model hypothesizes four health states (general ward, ICU, recovery, and death) where patients can move from one state to another (except for death) with a certain transition probability defined for each state, obtaining the weekly number of admissions to each state. The modeled cost savings arise primarily because of the data from Beigel et al. [10], which reported a reduction in the number of patients needing to move into the ICU as well as a statistically significant reduction in mortality in patients receiving low-flow oxygen support. Similar to other countries around the world, the costs of stay in the ICU are substantially higher than standard hospitalization (45% higher in the case of Saudi Arabia) and therefore any therapies, which can potentially reduce the need and/or the length of stay in an ICU, can result in substantial cost savings.
This study is subject to some limitations. The model scenarios were based on forecasting derived from expert clinical opinion that took into account the situation in Saudi Arabia, but uncertainty in these assumptions remain. Additionally, we recognize that the use of the Rt index does have some limitations [23], it is a firmly established methodology and allows for generalizability and comparability across studies. In addition, the information on many of the inputs was taken from a targeted literature review, which may mean that relevant data were missed. The model could potentially benefit from testing the clinical inputs using data from other studies as the model currently relies on clinical effectiveness data for remdesivir from one phase III trial, ACTT-1 [10]. While this was a pivotal trial upon which regulatory approval was granted, additional sources of data such as trial meta-analyses or real-world data may strengthen this analysis. A recently published meta-analysis and findings of the SOLIDARITY trial show that in those patients who were receiving oxygen support (the modeled population in this study) at admission saw a decrease in mortality and were at a lower risk of progressing to needing MV [24]. Finally, the model does not take into account adverse events for either treatment (remdesivir nor standard of care) arm. This is because of the relatively low rates of serious treatment-related adverse events reported in Beigel et al. [10] but the exclusion of adverse events may result in a small impact on both the costs and benefits. Despite these limitations, the sensitivity analyses conducted demonstrated that the results were robust overall. In conclusion, a strength of this model is that it can be easily adjusted to a narrower context, for instance regional, and can be continuously updated with the most recent data available. Hence, the model can be a useful decision-making tool in times of crisis, such as the COVID-19 pandemic.
This model was originally built as part of a wider project to estimate the impact of potential changes in epidemiology on the cost effectiveness of alternative treatments for different pathologies, testing its ability to adapt to different realities. The model was originally developed for the Italian context [11] and was later adapted for the Portuguese context, reporting similar findings to this study. Although this paper has the limitation of using a methodology that was already published, it provides an overview of how the model could work in contexts with different economic structures, emergency management protocols, and epidemiological courses (e.g., Middle East vs Europe).
In addition, several other countries have used similar models to estimate the impact of treating patients with remdesivir on healthcare resource use and overall costs to the healthcare system [25–28]. The findings from the Italian and Portuguese studies are similar to what was found with this model, indicating that these results can be generalizable across geographies experiencing similar infection and hospitalization rates.
Conclusions
This study indicates that the use of remdesivir in patients requiring low-flow oxygen reduces the burden on healthcare facilities and provides important cost savings for Saudi Arabia hospitals.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This research was funded by Gilead Sciences, United Arab Emirates.
Conflict of interest
This project was funded in part by an unrestricted grant from Gilead Ltd. to UniCamillus Medical University of Rome. The authors report grant support from Gilead Ltd. during the conduct of the study. Three co-authors in the paper have labor contractual relationships with Gilead Ltd. Matteo Ruggeri has not received any fee or reimbursement for participating in the study and writing the article. The vision expressed in this paper is the one of the authors and does not represent any involvement of the bodies or authorities of affiliation.
Ethics approval
No ethics approval was needed as the examined population was purely hypothetical, and no patients were involved in the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All the data supporting the findings of this study are available within the article and its supplementary materials.
Code availability
Not applicable.
Author contributions
MR conceptualization and methodology, software, and data curation; AS fundraising and project management; SC software, data curation, and writing (original draft preparation); BA validation; AA validation; JJ supervision, review, and editing; SK supervision, review, and editing; CH supervision; and TAM validation. All authors have read and agreed to the published version of the manuscript.
References
- 1.Havrlant D, Darandary A, Muhsen A. Early estimates of the impact of the COVID-19 pandemic on GDP: a case study of Saudi Arabia. Appl Econ. 2021;53(12):1317–1325. doi: 10.1080/00036846.2020.1828809. [DOI] [Google Scholar]
- 2.International Monetary Fund (IMF). IMF reports and publications by country: gross domestic product, constant prices. https://www.imf.org/en/Data. Accessed 23 Apr 2021.
- 3.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus pandemic (COVID-19). https://ourworldindata.org. Accessed 16 May 2022.
- 4.Algaissi AA, Alharbi NK, Hassanain M, Hashem AM. Preparedness and response to COVID-19 in Saudi Arabia: building on MERS experience. J Infect Public Health. 2020;13(6):834–838. doi: 10.1016/j.jiph.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alahmari AA, Khan AA, Elganainy A, Almohammadi EL, Hakawi AM, Assiri AM, et al. Epidemiological and clinical features of COVID-19 patients in Saudi Arabia. J Infect Public Health. 2021;14(4):437–443. doi: 10.1016/j.jiph.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health (MoH). Saudi MoH protocol for patients suspected of/confirmed with COVID-19. MoH. 2021. https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf. Accessed 15 Apr 2022.
- 7.US Food and Drug Administration (FDA). EUA approval. Reissued Oct. 22nd, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. Accessed 2 Nov 2021.
- 8.European Medicines Agency (EMA). Veklury (remdesivir): an overview of Veklury and why it is authorised in the EU. EMA/676677. 23 June, 2020. https://www.ema.europa.eu/en/documents/overview/veklury-epar-medicine-overview_en.pdf. Accessed 23 Apr 2021.
- 9.Saudi Food & Drug Authority (SFDA). 2021. https://www.sfda.gov.sa/en/drugs-list. Accessed 27 Nov 2021.
- 10.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19: final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri M, Signorini A, Drago C, Rosiello F, Marchetti M. Modello di stima dei costi sanitari e della capacity delleterapie intensive in Italia nel trattamento di pazienti affetti da COVID-19: valutazione dell’impatto di remdesivir. AboutOpen. 2020;7(1):95–102. doi: 10.33393/abtpn.2020.2213. [DOI] [Google Scholar]
- 12.Ruggeri M, Signorini A, Caravaggio S, Rua J, Luìs N, Braz S, et al. Estimation model for healthcare costs and intensive care units access for Covid-19 patients and evaluation of the effects of remdesivir in the Portuguese context: hypothetical study. Clin Drug Investig. 2022;42:345–354. doi: 10.1007/s40261-022-01128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epiforecast. 2021. https://www.epiforecasts.io/. Accessed 22 Mar 2021.
- 14.Al-Omari A, Alhuqbani WN, Zaidi ARZ, Al-Subaie MF, AlHindi AM, Abogosh AK, et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: a descriptive cross-sectional study. J Infect Public Health. 2021;13:1639–1644. doi: 10.1016/j.jiph.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AA, AlRuthia Y, Balkhi B, Alghadeer SM, Temsah M-H, Althunayyan SM, Alsofayan YM. Survival and estimation of direct medical costs of hospitalized COVID-19 patients in the Kingdom of Saudi Arabia. Int J Environ Res Public Health. 2020;17(20):7458. doi: 10.3390/ijerph17207458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badreddine SA, Zammo M, Elhosiny AA, Alhomsy MW, Aldabbagh Y, Mansouri AS, et al. Clinical course and outcome of 395 Covid 19 patients admitted to one hospital in Jeddah, Saudi Arabia. Int J Infect Dis Ther. 2021;5(4):118–126. [Google Scholar]
- 17.Alharthy A, Aletreby W, Faqihi F, Balhamar A, Alaklobi F, Alanezi K, et al. Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidemiol Glob Health. 2021;11(1):98–104. doi: 10.2991/jegh.k.200928.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AlSulaiman KA, Aljuhani O, Eljaaly K, Alharbi AA, Al Shabasy AM, Alsaeedi AS, et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–187. doi: 10.1016/j.ijid.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alqahtani AM, AlMalki ZS, Alalweet RM, Almazrou SH, Alanazi AS, Alanazi MA, et al. Assessing the severity of illness in patients with coronavirus disease in Saudi Arabia: a retrospective descriptive cross-sectional study. Front Public Health. 2020;8:593256. doi: 10.3389/fpubh.2020.593256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilead Sciences, Inc. An open letter from Daniel O’Day, Chairman & CEO, Gilead Sciences. 29 June, 2020. https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/an-open-letter-from-daniel-oday-chairman--ceo-gilead-sciences. Accessed 20 Apr 2021.
- 21.Gubrium JF, Holstein JA. Handbook of interview research: context & method. Thousand Oaks: Sage Publications, Inc.; 2001. [Google Scholar]
- 22.Patton MQ. How to use qualitative methods in evaluation. Centre for Study of Evaluation, University of California, Los Angeles, California, USA. Thousand Oaks: Sage Publications, Inc.; 1987.
- 23.Gostic KM, McGough L, Baskerville EB, Abbott S, Joshi K, Tedijanto C, et al. Practical considerations for measuring the effective reproductive number. Rt. Open Access. 2020 doi: 10.1371/journal.pcbi.1008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Solidarity Trial Consortium Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Open Access. 2022;399(10399):P1941–P1953. doi: 10.1016/S0140-6736(22)00519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeck J, Jakobs F, Kron A, Franz J, Cornely OA, Kron F. A cost of illness study of COVID-19 patients and retrospective modelling of potential cost savings when administering remdesivir during the pandemic “first wave” in a German tertiary care hospital. Infection. 2022;50(1):191–201. doi: 10.1007/s15010-021-01685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Béraud G, Timsit JF, Leleu H. Remdesivir as a tool to relieve hospital care systems stressed by COVID-19: a modelling study on bed resources and budget impact. medRxiv. 2021. 10.1101/2021.02.24.21252355. [DOI] [PMC free article] [PubMed]
- 27.Jo Y, Jamieson L, Edoka I, Long L, Silal S, Pulliam JRC, et al. Cost-effectiveness of remdesivir and dexamethasone for COVID-19 treatment in South Africa. medRxiv. 2020. 10.1101/2020.09.24.20200196(Update in: Open Forum Infect Dis. 2021;8(3):ofab040 PMID: 32995824; PMCID: PMC7523165). [DOI] [PMC free article] [PubMed]
- 28.Oksuz E, Malhan S, Gonen MS, Kutlubay Z, Keskindemirci Y, Jarrett J, et al. Cost-effectiveness analysis of remdesivir treatment in COVID-19 patients requiring low-flow oxygen therapy: payer perspective in Turkey. Adv Ther. 2021 doi: 10.1007/s12325-021-01874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.