Abstract
Background
Shoulder injury related to vaccine administration (SIRVA) occurs when an intramuscular deltoid injection is administered into the shoulder joint. This observational study describes clinical features in 16 patients with SIRVA following Coronavirus 2019 (COVID-19) vaccination who presented to chiropractic, orthopedic, and physiotherapy clinics in Hong Kong between January 1, 2021, and January 1, 2022.
Material/Methods
Adults age ≥18 with new-onset shoulder pain and imaging-confirmed shoulder pathology were retrospectively identified from 35 clinics. Patient demographics and clinical and vaccination details were extracted from the electronic medical record. Shoulder injury was determined by correlating clinical and imaging features.
Results
Of 730 patients with shoulder pain, 16 SIRVA cases (mean age, 49±10 years, 75% female) were identified; (12/16, 75%) of patients received the Pfizer-BioNTech vaccine while (4/16, 25%) received Sinovac-CoronaVac. The most common diagnosis was adhesive capsulitis (10/16, 63%), followed by bursitis (3/16, 19%) and supraspinatus tear (3/16, 19%). Mean symptom onset was 3.5±2.5 days post-vaccination, and always occurred after the 2nd or 3rd vaccination, involving reduced shoulder range of motion (ROM). Mean baseline pain was 8.1±1 (out of 10). All patients received conservative care (eg, exercise, manual therapies). At 3-month follow-up, mean pain reduced to 2.4±1.4; all patients had normal shoulder ROM.
Conclusions
In the past 2 years, millions of intramuscular COVID-19 vaccinations have been administered. It is important that clinicians are aware of SIRVA as a cause of new symptoms of shoulder injury and should ask the patient about recent vaccinations, including for COVID-19.
Keywords: COVID-19 Vaccine, Injection Site Reaction, Shoulder Pain
Background
Millions of doses of the Coronavirus 2019 (COVID-19) vaccine have been widely administered with efficacy in combating the COVID-19 pandemic [1]. In Hong Kong, the location of the current study, 65% of the population had received at least 1 dose of the COVID-19 vaccine by January 1, 2022 [2]. While local vaccine injection site reactions of pain, redness, and swelling have been well described, these symptoms are generally not serious and are self-limited [3]. Conversely, a potentially more problematic shoulder injury related to vaccine administration (SIRVA) has seldom been described in the literature in relation to COVID-19 vaccination.
SIRVA is defined as shoulder pain and decreased range of motion after receiving a vaccine intended for intramuscular delivery in the upper arm [4,5]. This condition is becoming recognized as a potential vaccine-related adverse effect and has been most often associated with influenza vaccine administration [4,5]. One potential cause of SIRVA is accidental injection into the subdeltoid bursa, causing bursitis, tendinitis, and/or capsulitis [4].
According to a recent systematic review, SIRVA typically occurs in middle-aged adults (median age 51) and tends to affect females (73% of cases) [5]. The most common diagnoses include bursitis, adhesive capsulitis, and rotator cuff tear [4,5]. SIRVA is typically treated conservatively, which leads to full recovery in 3–56% of patients [5]. Unfortunately, there are limited data regarding the optimal treatment strategy for SIRVA and typical duration of symptoms [4,5].
However, to the best of our knowledge, only 20 cases of SIRVA after COVID-19 vaccination have been reported according to a PubMed search on April 14, 2022 using the terms “shoulder injury related to vaccine administration” and “COVID” [6–14]. Given this limited number of cases, little is known about the clinical features and risk factors for SIRVA after COVID-19 vaccination. Based on the limited data, it appears that SIRVA may occur independent of the type of COVID-19 vaccine used [6–14]. Further, among published cases, adhesive capsulitis and bursitis appear to be more common, with rotator cuff tear being uncommon [6–14].
A thorough shoulder examination is requisite for diagnosis of SIRVA, and includes inspection, palpation, and range of motion testing [4]. Specialized provocative tests are of uncertain utility in suspected cases of SIRVA. Patients with SIRVA often have tenderness at the injection site and a global reduction in shoulder range of motion affecting all planes of motion [4]. MRI may be useful to evaluate for soft-tissue pathology [4], and can demonstrate findings consistent with inflammation [5]. In the current study, clinical diagnoses of SIRVA based on pain and limited range of motion were compared to and supported by imaging findings.
Despite an increase in overall awareness of SIRVA, few studies have evaluated SIRVA associated with COVID-19 vaccination. Therefore, this observational clinical study describes the presentation and clinical features of SIRVA in 16 patients following COVID-19 vaccination who presented to chiropractic, orthopedic, and physiotherapy clinics in Hong Kong between January 1, 2021, and January 1, 2022.
Material and Methods
Study Design
The Ethics Committee of the Chiropractic Doctors Association of Hong Kong approved of this study (Causeway Bay, Hong Kong; IRB ID: CDA20221202). Written informed consent for patient information and images to be published was provided by the patient(s) or a legally authorized representative. Data for patients presenting with new shoulder pain were retrospectively collected by querying an electronic medical records system shared between 35 affiliated chiropractic, orthopedic, and physiotherapy clinics in Hong Kong. Data were extracted in March through April 2022 with a search window of January 1, 2021, to January 1, 2022.
Participants
Inclusion criteria were patients age ≥18 years with a new concern of shoulder pain, a history of COVID-19 vaccination within 1 month preceding shoulder pain onset, and shoulder pathology confirmed via imaging. Exclusion criteria were a history of influenza vaccination within 1 year of shoulder pain onset, shoulder injury within 1 year preceding shoulder pain onset, shoulder pain in the context of an inconsistent injection site (shoulder pain contralateral to injection site or in a non-shoulder region), radiological features of severe, recent, and/or pre-existing shoulder pathology or non-SIRVA injury including shoulder fracture, tumor, and severe degenerative disease. In addition, patients in which the vaccination history could not be confirmed were excluded. The study flowchart is shown in Figure 1.
Figure 1.
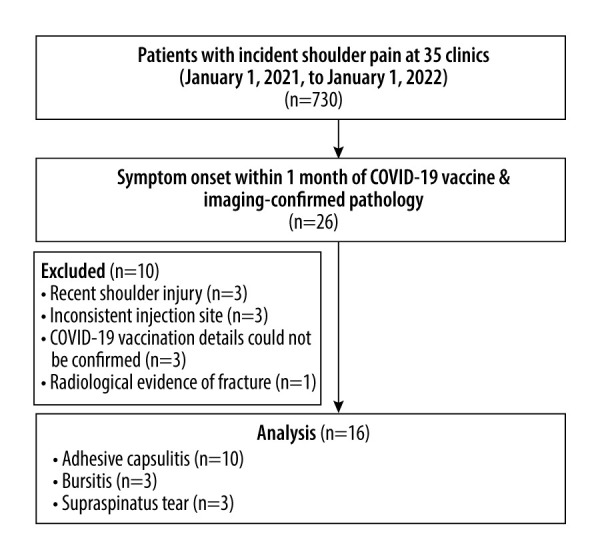
Identification of patients with shoulder pain secondary to COVID-19 vaccine administration. Abbreviations: Coronavirus disease 2019 (COVID-19); magnetic resonance imaging (MRI). Figure created by Eric CP Chu using Microsoft Word Version 2205.
Variables
Data regarding sex, age, laterality of shoulder affected, vaccine dose, duration between vaccine administration and shoulder pain onset, type of vaccine (Pfizer-BioNTech or Sinovac-CoronaVac), active shoulder range of motion, pain severity (using a visual analog scale of 0–10; 10 being most severe), and type of treatment (medication, steroid injection, manual therapy, exercise therapy, or surgery) were extracted from the electronic medical record. The primary shoulder injury diagnosis (adhesive capsulitis, subacromial-subdeltoid bursitis, or rotator cuff tear) was determined by correlating the clinical and imaging features.
Magnetic resonance imaging (MRI) and shoulder radiographs were interpreted by board-certified medical radiologists. Subacromial and subdeltoid bursitis were defined as distended fluid-filled structures between the deltoid muscle, acromion, and supraspinatus and infraspinatus tendons on MRI [14]. Rotator cuff tears were assessed using fat-suppressed, intermediate-weighted/gradient echo sequences, in which the hyperintense signal region within the tendon frequently corresponded to fluid on T2-weighted imaging [12].
Diagnosis of adhesive capsulitis was based limited active and passive range of motion and supported by evidence on MRI. Signs of capsulitis on MRI included the subcoracoid triangle sign, joint capsule thickening, coracohumeral ligament thickening >4 millimeters, T2 hyperintensity of the inferior glenohumeral ligament, and soft-tissue thickening within the rotator muscles or biceps anchor [13].
Statistical Analysis
Descriptive statistics were utilized to calculate percentages for categorical variables and standard deviations for continuous variables.
Results
Participants
A total of 730 patients presented with shoulder pain at the 35 included clinics during the study time window, and 26 patients reported that their symptoms began within 1 month following COVID-19 vaccine administration. A total of 26 shoulder MRIs and 6 shoulder radiographic series were analyzed. Sixteen patients met the inclusion criteria while 10 were excluded (Figure 1). No patients had a history of COVID-19 infection preceding their vaccination or developed COVID-19 during the study time window. Included patients were of Asian ethnicity. Three of 16 (19%) of the patients had significant medical comorbidities, including diabetes and/or cardiovascular disease, while no patients had thyroid disease, autoimmune disease, cancer, or Parkinson’s disease.
Descriptive Data
Of the included patients, 12/16 (75%) were women and 4/16 (25%) were men. At the time of injury, the mean patient age was 49±10 years (Table 1). The mean onset of symptoms was 3.5±2.5 days after vaccination. All patients presented with reduced active shoulder range of motion (Table 2). The baseline mean shoulder pain severity was 8.1±0.8. The injection site and shoulder pain were on the left in 13/16 (81)% of patients and right in 3/16 (19)%. Regarding COVID-19 vaccine type, 12/16 (75%) of patients were administered Pfizer-BioNTech while 4/16 (25%) received Sinovac-CoronaVac.
Table 1.
Cases of shoulder injury related to Coronavirus 2019 vaccine administration.
| Patient | Sex | Age | Laterality | Dose | Vaccine type | Onset after vaccination (days) | Diagnosis | Treatment | Pain severity | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3-months | |||||||||
| 1 | F | 42 | L | 2 | P | 0 | Capsulitis | MT | 7 | 0 |
| 2 | F | 50 | L | 2 | P | 7 | Capsulitis | Rx+EX | 8 | 2 |
| 3 | F | 62 | L | 3 | P | 1 | Capsulitis | EX | 9 | 1 |
| 4 | F | 70 | L | 3 | P | 7 | Capsulitis | MT | 7 | 2 |
| 5 | F | 62 | L | 3 | S | 4 | Bursitis | MT | 8 | 4 |
| 6 | F | 51 | R | 3 | S | 1 | Supraspinatus tear | EX | 8 | 3 |
| 7 | M | 40 | L | 3 | P | 3 | Supraspinatus tear | EX | 9 | 5 |
| 8 | F | 65 | L | 2 | P | 7 | Supraspinatus tear | MT | 8 | 4 |
| 9 | F | 42 | L | 2 | S | 0 | Bursitis | MT | 8 | 3 |
| 10 | M | 43 | R | 3 | S | 1 | Bursitis | MT | 9 | 2 |
| 11 | M | 41 | L | 3 | P | 2 | Capsulitis | EX | 8 | 4 |
| 12 | F | 40 | L | 2 | P | 3 | Capsulitis | EX | 9 | 3 |
| 13 | M | 52 | R | 3 | P | 5 | Capsulitis | MT | 7 | 2 |
| 14 | F | 40 | L | 2 | P | 3 | Capsulitis | MT | 7 | 1 |
| 15 | F | 41 | L | 2 | P | 7 | Capsulitis | Rx+EX | 8 | 3 |
| 16 | F | 43 | L | 2 | P | 5 | Capsulitis | Rx+MT | 9 | 0 |
EX – exercise therapy; F – female; L – left; M – male; MT – manual therapy; P – Pfizer-BioNTech; Rx – prescription medication; R – right; S – Sinovac-CoronaVac.
Table 2.
Active shoulder range of motion.
| Patient | Baseline | 3-month follow-up | ||||
|---|---|---|---|---|---|---|
| Flexion | Abduction | External rotation | Flexion | Abduction | External rotation | |
| 1 | 100 | 85 | 35 | 160 | 150 | 70 |
| 2 | 90 | 80 | 25 | 150 | 130 | 85 |
| 3 | 100 | 85 | 30 | 160 | 150 | 80 |
| 4 | 90 | 80 | 20 | 150 | 130 | 70 |
| 5 | 80 | 80 | 20 | 160 | 125 | 70 |
| 6 | 70 | 75 | 30 | 140 | 130 | 65 |
| 7 | 80 | 80 | 20 | 130 | 120 | 60 |
| 8 | 70 | 75 | 20 | 140 | 130 | 70 |
| 9 | 90 | 75 | 35 | 150 | 125 | 85 |
| 10 | 85 | 80 | 35 | 160 | 130 | 80 |
| 11 | 60 | 85 | 30 | 140 | 120 | 70 |
| 12 | 80 | 70 | 35 | 130 | 130 | 70 |
| 13 | 85 | 85 | 40 | 140 | 125 | 85 |
| 14 | 70 | 75 | 35 | 140 | 130 | 80 |
| 15 | 80 | 85 | 35 | 130 | 120 | 70 |
| 16 | 100 | 85 | 30 | 160 | 150 | 90 |
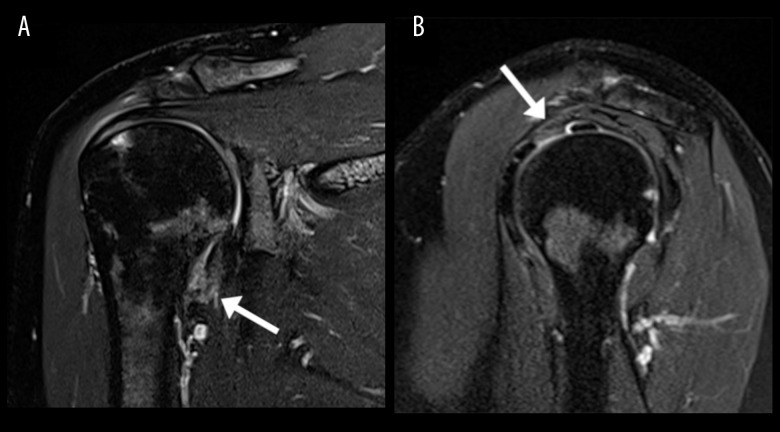
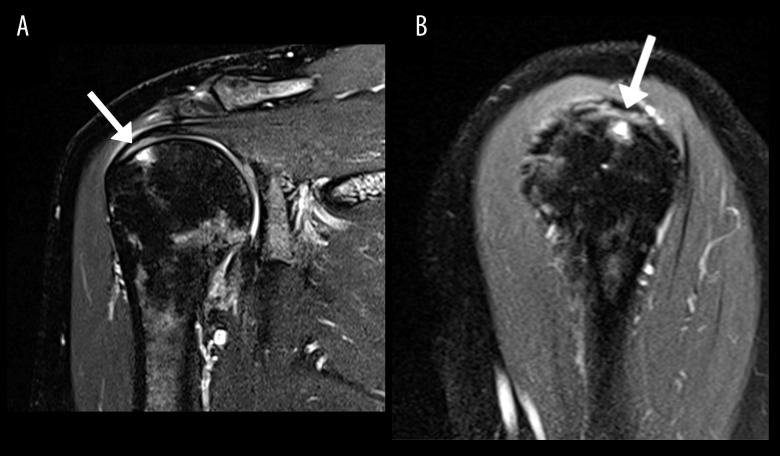
A primary diagnosis of adhesive capsulitis was most common, occurring in 10/16 (63%) of patients (Figure 2). Subacromial-subdeltoid bursitis and rotator cuff tear (Figure 3) each occurred in 3/16 (19%) of patients. All patients with rotator cuff tear had a supraspinatus tear. No other relevant shoulder pathologies (eg, labrum tear) were identified.
Figure 2.
Imaging features of adhesive capsulitis. Magnetic resonance image (MRI) of the right shoulder; patient 13. On the coronal T2 fat-suppressed sequence (A) the arrow indicates thickening of the inferior joint capsule with hyperintense T2 signal. On the sagittal T2 fat-suppressed sequence (B) the arrow indicates thickening and mild hyperintense signal at the rotator interval.
Figure 3.
Imaging features of supraspinatus rotator cuff tear. Magnetic resonance image (MRI) of the right shoulder; patient 6. In the coronal T2 fat-suppressed image (A) the arrow points the articular side of a partial tear of the supraspinatus tendon, involving 50% of the tendon thickness. Also noted is underlying marrow edema at the greater tuberosity of the humerus. In the sagittal T2 fat-suppressed image (B), the arrow again points to the articular side of the partial thickness tear of the supraspinatus tendon, further confirming 50% of tendon thickness involvement.
Outcome Data
Most patients (13/16, 81%) received manual therapy or exercise therapy for treatment of shoulder pain as provided by a chiropractor or physiotherapist. A minority of patients (3/16, 19%) additionally received medications to treat their shoulder pain, these being nonsteroidal anti-inflammatory drugs (NSAIDs) and muscle relaxants. All patients completed between 10 and 20 treatments over the course of 3 months, based on a typical treatment plan for shoulder disorders utilized among the affiliated clinics. No patients underwent surgery or steroid injection.
After 3 months of conservative treatment, patients were re-assessed as part of routine clinical care. The average shoulder range of motion improved on flexion (83° to 146°), abduction (80° to 131°), and 90-degree external rotation (30° to 75°), such that each patient went from an abnormal range at baseline to within the normative range (Table 2) [15]. Mean pain severity reduced to 2.4±1.4, with 12/16 (75%) of patients attaining a mild pain severity (≤3 of 10) at 3-month follow-up.
Discussion
The current study is the largest reported series of SIRVA related to COVID-19 vaccination. This study provides limited evidence that this condition typically occurs in females within approximately 1 week after COVID-19 vaccination, presents with pain, limited shoulder range of motion, and radiologic features of capsulitis, bursitis, or, less commonly, rotator cuff tear. We also found that SIRVA related to COVID-19 vaccine administration responds positively to conservative care, with pain mostly subsiding within 3 months and recovery of range of motion.
In the current series, SIRVA after COVID-19 vaccination was more common in women (12/16, 75%). After synthesizing the sex of patients in the current and the previously published 20 cases (36 total), the overall female proportion of this condition remains high at 72% [6–14]. This is also concordant with previous research that identified that females have a greater risk of adverse events related to COVID-19 vaccination in general [16]. The finding of current series that symptoms began within 1 week after vaccination is also similar to prior studies in which onset typically occurred between the same day and 10 days of COVID-19 vaccination [6–10,12–14], with only 1 case reporting a 2-week delay to symptom onset [9].
All currently reported cases of SIRVA occurred after the 2nd or 3rd dose of the vaccine, a finding which is inconsistent among previously published cases [6–14]. While more patients (12/16, 75%) with SIRVA were administered the Pfizer-BioNTech COVID-19 vaccine in the current series, this was also inconsistent among previous cases [6–14]. The most common shoulder pathology involved in the current series was adhesive capsulitis (10/16, 63%), with subacromial and subdeltoid bursitis and rotator cuff tear being less common. While all of these conditions have been previously reported in prior SIRVA cases [6–14], it is difficult to compare their relative frequency given differences in diagnostic methods and study design.
This case series suggests that SIRVA related to COVID-19 vaccination has a favorable prognosis. Despite the initial severe symptoms, loss of range of motion, and imaging-confirmed shoulder pathology in each case, each patient recovered a normal range of motion, and most patients had a mild pain severity within 3-month follow-up. These results are comparable to those of previous similar SIRVA cases, which reported a range of recovery from 1 week to 3 months [7,9,10,12–14]. In contrast, the most common diagnosis in this series, adhesive capsulitis, typically takes 1–3 years to resolve when unrelated to vaccine administration [17].
It is important for healthcare professionals to be aware of the risk factors for SIRVA and take the necessary precautions to avoid this injury. The distance of the subdeltoid bursa from the acromion ranges from 3.0 to 6.0 centimeters (cm), while the depth from the skin to the subdeltoid bursa ranges from 0.8 to 1.6 cm [18]. Accordingly, a 2.5-cm needle or even a shorter 1.6-cm needle may penetrate the bursa if misplaced. Because the subdeltoid and subacromial bursas are connected, irritation can spread proximally, causing tendinitis and capsulitis [18]. Therefore, it is conceivable that COVID-19 vaccine injections could be introduced into the subdeltoid bursa and cause inflammation and bursitis.
Suggested by this series as well as a prior case [7], SIRVA related to COVID-19 vaccination may occasionally involve a rotator cuff tear. We suggest 2 hypotheses that may explain this occurrence. First, a patient may have a pre-existing asymptomatic rotator cuff tear and when vaccinated, resultant synovial inflammation could trigger acute shoulder pain. Second, inadvertent injection into the subacromial space may lead to bursa inflammation related to antigen-antibody complex formation [4]. It is possible that this inflammation would then predispose patients to a rotator cuff tear during normal activities of daily living.
Common treatments for SIRVA include physical therapy, pain medications such as nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgery in some cases [4]. In the current series, conservative treatments were employed, and no patients received injections or underwent surgery. Manual therapies such as gua sha were applied to the shoulder in many cases of the current series, as this treatment may help mobilize soft tissue and inhibit pain-promoting substances and neuronal responses [19].
Limitations
There are several limitations to this study. First, its retrospective nature may increase the chance of bias. For example, there could be recall bias in the sense that patients’ self-report of the onset of shoulder symptoms was inaccurate. Further, cases of SIRVA after COVID-19 administration could have been missed by the medical record query if shoulder injuries were not coded or documented accurately by clinicians. Second, there was selection bias because only patients with shoulder pathology confirmed by imaging were considered; therefore, the study is not representative of a broader population with pain but no radiological findings. Third, there was diagnosis bias as patients were assessed by different clinicians and imaging was interpreted by different radiologists. Fourth, a record of the exact site of vaccine administration in terms of distance from the acromion was not available in most cases, which prevented a definitive conclusion that improper vaccine administration was causative of each case of SIRVA. Fifth, although this series revealed that the rate of SIRVA was low among patients presenting with shoulder pain after COVID-19 vaccination (16 of 730; 2%), the overall prevalence of this condition among vaccinated individuals could not be determined from the current study design. Sixth, in each case, the type of provider administering the vaccine was not clear given this relied on patients’ self-report. However, in most cases this was likely a nurse, and in some cases, this may have been a physician. In Hong Kong, pharmacists do not typically administer vaccines. Finally, because the natural history of SIRVA related to COVID-19 vaccination is not well described, it is possible patients would have recovered without treatment in a similar time frame.
Conclusions
In the past 2 years, millions of intramuscular COVID-19 vaccinations have been administered. It is important that clinicians are aware of SIRVA as a cause of new symptoms of shoulder injury and should ask the patient about recent vaccinations, including for COVID-19.
Footnotes
Conflict of interest: None declared
Research Ethics and Patient Consent
The study was conducted according to the guidelines of the Declaration of Helsinki. The Ethics Committee of the Chiropractic Doctors Association of Hong Kong approved of this study (Causeway Bay, Hong Kong; IRB ID: CDA20221202). Written informed consent for patient information and images to be published was provided by the patient(s) or a legally authorized representative.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part
Financial support: None declared
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19) [Internet] Our World Data. 2020. [cited 2022 Apr 13]. Available from: https://ourworldindata.org/covid-vaccinations.
- 3.Ramos CL, Kelso JM. “COVID Arm”: Very delayed large injection site reactions to mRNA COVID-19 vaccines. J Allergy Clin Immunol Pract” Elsevier. 2021;9:2480–81. doi: 10.1016/j.jaip.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesel BB, Keeling LE. Shoulder injury related to vaccine administration. J Am Acad Orthop Surg. 2021;29:732–39. doi: 10.5435/JAAOS-D-21-00021. [DOI] [PubMed] [Google Scholar]
- 5.MacMahon A, Nayar SK, Srikumaran U. What do we know about shoulder injury related to vaccine administration? An updated systematic review. Clin Orthop Relat Res. 2022;480(7):1241–50. doi: 10.1097/CORR.0000000000002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantarelli Rodrigues T, Hidalgo PF, Skaf AY, Serfaty A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: A case of shoulder injury related to vaccine administration (SIRVA) Skeletal Radiol. 2021;50:2293–97. doi: 10.1007/s00256-021-03803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonsri P, Chuaychoosakoon C. Combined subacromial-subdeltoid bursitis and supraspinatus tear following a COVID-19 vaccination: A case report. Ann Med Surg. 2021;69:102819. doi: 10.1016/j.amsu.2021.102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuaychoosakoon C, Parinyakhup W, Tanutit P, et al. Shoulder injury related to Sinovac COVID-19 vaccine: A case report. Ann Med Surg. 2021;68:102622. doi: 10.1016/j.amsu.2021.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honarmand AR, Mackey J, Hayeri R. Shoulder injury related to vaccine administration (SIRVA) following mRNA COVID-19 vaccination: Report of 2 cases of subacromial-subdeltoid bursitis. Radiol Case Rep. 2021;16:3631–34. doi: 10.1016/j.radcr.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakajima K, Miyata A, Kato S, et al. Calcific tendinitis of the shoulder induced by an mRNA vaccine for COVID-19: A case report. Mod Rheumatol Case Rep. 2022 doi: 10.1093/mrcr/rxac006. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wharton BR, Doan KC, Wolcott ML. Shoulder injury related to COVID-19 vaccine administration: A case report. JSES Rev Rep Tech. 2022;2(2):178–81. doi: 10.1016/j.xrrt.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuen WLP, Loh SYJ, Wang DB. SIRVA (Shoulder Injury Related to Vaccine Administration) following mRNA COVID-19 vaccination: Case discussion and literature review. Vaccine. 2022;40:2546. doi: 10.1016/j.vaccine.2022.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow JCK, Koles SL, Bois AJ. Shoulder injury related to SARS-CoV-2 vaccine administration. CMAJ. 2022;194:E46–49. doi: 10.1503/cmaj.211162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahu D, Shetty G. Frozen shoulder after COVID-19 vaccination. JSES Int. 2022 doi: 10.1016/j.jseint.2022.02.013. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellenbecker TS. Clinical examination of the shoulder. 1st edition. St Louis, Mo: Saunders; 2004. [Google Scholar]
- 16.Green MS, Peer V, Magid A, et al. Gender differences in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines (Basel) 2022;10:233. doi: 10.3390/vaccines10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le HV, Lee SJ, Nazarian A, Rodriguez EK. Adhesive capsulitis of the shoulder: Review of pathophysiology and current clinical treatments. Shoulder Elbow. 2017;9:75–84. doi: 10.1177/1758573216676786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25:585–87. doi: 10.1016/j.vaccine.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Chu ECP, Wong AYL, Sim P, Krüger F. Exploring scraping therapy: Contemporary views on an ancient healing – a review. J Fam Med Prim Care. 2021;10:2757. doi: 10.4103/jfmpc.jfmpc_360_21. [DOI] [PMC free article] [PubMed] [Google Scholar]