Abstract
Interval-counting neurons (ICNs) respond after a threshold number of sound pulses have occurred with specific intervals; a single aberrant interval can reset the counting process. Female gray treefrogs, Hyla chrysoscelis and H. versicolor, discriminate against synthetic ‘calls’ possessing a single interpulse interval 2–3 three times the optimal value, suggesting that ICNs are important for call recognition. The calls of H. versicolor consist of pulses that are longer in duration, rise more slowly in amplitude and are repeated at a slower rate than those of H. chrysoscelis. Results of recordings from midbrain auditory neurons in these species include: (1) ICNs were found in both species and their temporal selectivity appeared to result from interplay between excitation and inhibition; (2) band-pass cells in H. versicolor were tuned to slower pulse rates than those in H. chrysoscelis; (3) ICNs that were selective for slow-rise pulse shape were found almost exclusively in H. versicolor, but fast-rise-selective neurons were found in both species, and (4) band-suppression ICNs in H. versicolor showed response minima at higher pulse rates than those in H. chrysoscelis. Selectivity of midbrain ICNs for pulse rise time and repetition rate thus correlate well with discriminatory abilities of these species that promote reproductive isolation.
Keywords: Whole-cell recording, Anurans, Rise-time selectivity, Amplitude modulation, Inferior colliculus
Introduction
North American gray treefrogs comprise two cryptic species, Hyla versicolor and H. chrysoscelis. H. versicolor (tetraploid) is believed to have evolved at least three times from hybridization (i.e., allopolyploidization events) between diploid species, which include an ancestral representative of extant H. chrysoscelis and two genetically distinct chrysoscelis-like lineages that now appear to be extinct (Holloway et al. 2006). Because heterospecific matings between H. versicolor and H. chrysoscelis result in sterile triploid progeny (Johnson 1963), premating isolation mechanisms are of paramount importance. Advertisement calls of H. versicolor and H. chrysoscelis, which are the primary premating isolation mechanisms, consist of a series of pulses that differ in shape, duration and repetition rate; in H. versicolor, intrapulse amplitude rises more slowly, pulse duration is greater and pulse rate is slower (Fig. 1). Behavioral studies have shown that pulse rise time and duration are particularly salient temporal features used in call recognition by H. versicolor (Diekamp and Gerhardt 1995; Gerhardt and Schul 1999; Schul and Bush 2002). For example, females of this species strongly prefer (in dual speaker playback tests) pulses that are long in duration and have slow-rise shape; preferences for pulse rates of approximately 20 pulses/s (representing the average pulse rate for advertisement calls of this species) appear to be a byproduct of preferences for long pulse duration (Schul and Bush 2002; Gerhardt 2005). In contrast, call recognition in H. chrysoscelis is based primarily on pulse rate (Gerhardt 2005). Gray treefrogs thus provide an excellent opportunity to investigate the role of acoustic communication in speciation. An important step towards this goal is to understand how changes in sensory processing contributed to the reproductive isolation of tetraploids (i.e., H. versicolor) from their diploid counterparts (i.e., H. chrysoscelis).
Fig. 1.
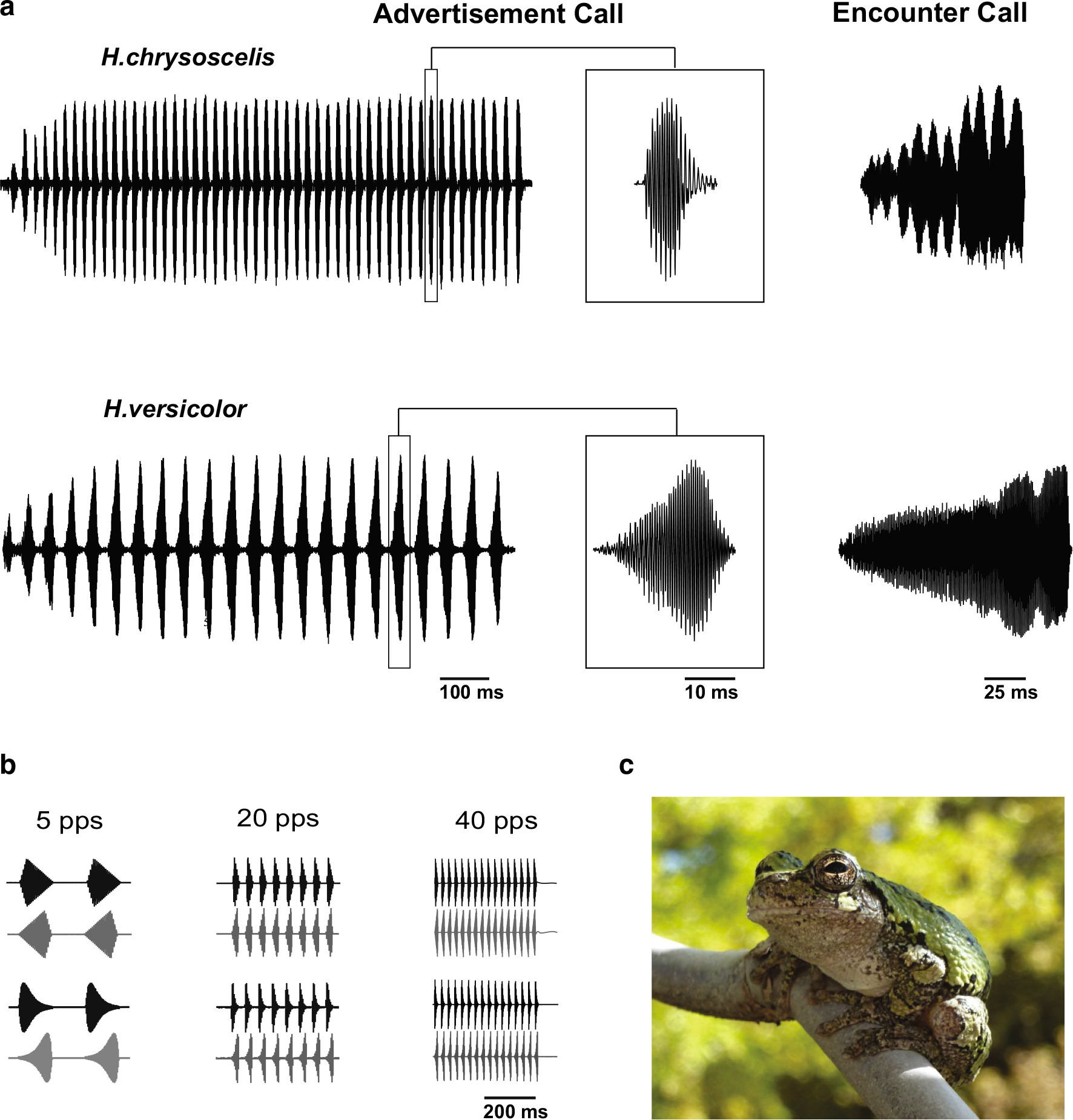
a Oscillograms of advertisement (left) and encounter (right) calls of the gray treefrog species Hyla chrysoscelis and H. versicolor and b stimuli used to investigate pulse rate and rise-time selectivity. Pulses in the advertisement calls of H. chrysoscelis and H. versicolor are repeated at rates of approximately 40–50 pulses/s and 20 pulses/s, respectively, at 20 °C. The individual pulses have fast-rise (H. chrysoscelis) or slow-rise (H. versicolor) shape. Pulses in stimuli had either linear or exponential rise–fall characteristics. c Picture of a gray treefrog; the two species of gray treefrogs are morphologically indistinguishable
Initial single-unit, extracellular recordings, aimed at identifying neural correlates of behavioral preferences in H. versicolor and H. chrysoscelis, used mainly sinusoidal amplitude modulation (SAM) to investigate temporal processing in the anuran inferior colliculus (ICanuran) (Rose et al. 1985). Results revealed the presence of the same four classes of AM selective neurons (low-pass, band-pass, high-pass and band-suppression) found in other anuran species (Rose and Capranica 1983, 1984, 1985), and that band-pass neurons in H. versicolor were on average tuned to slower AM rates than those in H. chrysoscelis (Rose et al. 1985). Results also indicated that pulses with natural shape (i.e., amplitude rise time characteristics) were more effective in eliciting spikes than were SAM signals; pulse amplitude rises more slowly and duration is greater in the natural calls of H. versicolor (Rose et al. 1985). Subsequently, Diekamp and Gerhardt (1995) reported that a higher proportion of units in the ICanuran of H. versicolor showed AM band-pass properties (i.e., tuning to AM, for stimuli that had the natural slow-rise versus fast-rise pulse shape).
More recently, studies in other anuran species have characterized ICanuran neurons using stimuli in which pulse duration and number were held constant and pulse rate varied (Alder and Rose 2000). Results suggest that most neurons can be classified as either ‘long-interval’ or ‘interval-counting’ types; duration-selective cells constitute a third class, which will not be considered further here (reviewed by Rose 2014). Long-interval cells, which can be either band-pass or low-pass to SAM, respond well to pulses that are presented at slow rates, but respond phasically or not at all to fast pulse rates (Edwards et al. 2008). Interval-counting cells, the focus of the current paper, respond only after a threshold number of pulses are presented with intervals that fall within a particular range, and a single aberrant interval can reset this integration process (Edwards et al. 2007). Behavioral studies, using calls in which a long interval has been inserted in various locations (Schwartz et al. 2010) or that differ in sequential pulse number (Velez and Bee 2011), suggest that interval-counting cells play an important role in call recognition.
To further characterize the differences in temporal processing between H. versicolor and H. chrysoscelis, we made single-unit extracellular and whole-cell recordings from ICanuran neurons in these species. With whole-cell recordings, mechanisms that underlie selectivity can be interpreted from subthreshold events associated with particular stimulus features. In the current paper, we compare the response properties of interval-counting neurons in H. versicolor and H. chrysoscelis. Specifically we investigated: (1) whether these neurons show tuning to slower pulse rates in H. versicolor; (2) whether interval tuning mechanisms are similar and ‘scalable’ between the two species, and (3) if slow-rise selectivity is exclusive to H. versicolor, and, if so, whether it appears to be a mechanistically derived (evolutionarily) trait.
Materials and methods
Animals
Animals were prepared for recording according to previously published methods (see Alder and Rose 2000), except that animals were immobilized with intramuscular injection (1 μl/g) pancuronium Br− (4 mg/ml). All procedures were approved by the University of Utah Institutional Animal Care and Use Committee. Recordings were made from 86 interval-counting neurons (ICNs) in 46 gray treefrogs. Frogs were from south and south-central Missouri, except for seven H. versicolor, which were from the northeastern lineage (Holloway et al. 2006). Either H. chrysoscelis or H. versicolor (not both species) were present at each collection site, and call characteristics were used to further establish the species identity of males. Recordings were made predominately from males, with the exception of three female H. chrysoscelis. Recordings from 72 neurons from H. versicolor and H. chrysoscelis were of sufficient duration to determine both pulse-rate tuning and rise-time selectivity; these neurons were used in statistical comparisons.
Electrode construction
Extracellular electrodes were constructed from borosilicate capillary glass (1 mm outer diameter, 0.58 mm inner diameter; #5960; A-M Systems, Everett, WA) using a Flaming-Brown-type puller (model P-97; Sutter Instruments, Novato, CA) and were pulled to resistances between 0.8 and 1.2 MΩ. These electrodes were filled with a solution of 2 M NaCl.
Whole-cell patch intracellular recordings were made, in vivo, according to methods described in detail by Rose and Fortune (1996) and Edwards et al. (2007). Patch pipettes were constructed from borosilicate capillary glass (1 mm outer diameter, 0.58 mm inner diameter; #5960; A-M Systems, Everett, WA) using a Flaming-Brown-type puller (model P-97; Sutter Instruments, Novato, CA). These electrodes had outside tip diameters of ~1–1.5 μm and had resistances between 10 and 20 MΩ. The electrode tips were back-filled with a solution (pH 7.4) consisting of (in mM) 100 potassium gluconate, 2 KCl, 1 MgCl2, 5 EGTA, 10 HEPES, 20 KOH, and biocytin (20 or 43 mM); the lower concentration of biocytin minimized unintended labeling of neurons. Biocytin was replaced by mannitol in the solution used to fill pipette shanks.
The pipette was advanced into the brain using an “inchworm” microdrive (Burleigh Instruments Model No. 6000, Fishers, NY) or a 3-axis Microdrive (Scientifica, model IVM-3000, East Sussex, England) while applying positive pressure via a 30 cc syringe to prevent clogging the tip. After reaching the recording location, the pipette was advanced in 1.5-μm increments while maintaining slight positive pressure and passing −0.1 nA square-wave pulses (500 ms) to monitor resistance. Contact with a cell was indicated by a small increase (10 %) in the voltage change that resulted from this current injection. Negative pressure was then applied to the pipette to increase the seal resistance to gigaohm levels. After a seal was formed, negative current (usually less than −0.5 nA) was manually applied to rupture the patch and achieve an intracellular recording. To label neurons, biocytin was iontophoresed (approximately +0.02–0.05 nA) and tissue was processed according to methods described previously (Alder and Rose 2000).
Stimulus generation and delivery
Acoustic stimuli were constructed using Tucker Davis Technologies (Alachua, FL) System III hardware and custom-made software (Alder and Rose 2000). Stimuli were presented free field in an audiometric room maintained at 20 °C, the temperature at which behavioral experiments have been performed in these species (Gerhardt 2008; Gerhardt and Schul 1999). The speaker was situated 0.5 m from the animal and contralateral to the recording site. Search stimulus carrier frequencies were systematically varied from 150 to 2200 Hz with modulation frequencies (sinusoidal AM, SAM) ranging from 10 to 100 Hz. The best excitatory carrier frequency (BEF) and threshold of each neuron were determined as in Alder and Rose (2000); threshold was defined as the sound level at which a neuron produced at least one spike in response to at least 50 % of the presentations of the preferred stimulus. For neurons in the present study, the range of thresholds was 37–84 dB SPL with a median of 62 dB SPL. The BEF of each neuron was used in generating pulse stimuli. The details regarding how the different pulse shapes were generated (for SAM, natural AM, and variable duty-cycle stimuli) are described in Alder and Rose (2000). Stimuli used to characterize pulse-rate selectivity were 400–500 ms in duration and consisted of pulses of constant duty-cycle (50 or 100 %) at rates that varied from 5–80 pulses/s. Stimuli used to characterize rise-time selectivity consisted of pulses with rise times that approximated those of the H. chrysoscelis and H. versicolor advertisement calls. Pulses were generated by increasing the amplitude so that the maximum was reached at either 20 % (fast-rise) or 80 % (slow-rise) of the duration of the pulse. Both linear and exponential amplitude rise-time/fall-time functions were generated (Fig. 1b) and produced similar responses in the neurons tested. Stimulus amplitude was held at approximately 10–20 dB above threshold (Alder and Rose 2000). When possible, interval-counting characteristics were further assessed with additional stimuli presented at the optimal rise-time and pulse-rate for that neuron. To determine the threshold pulse-number of interval-counting neurons, stimuli of the preferred pulse-rate were generated beginning with one pulse and then the number of pulses per stimulus was increased until firing was consistently induced. To assess the duration of the interval necessary to reset intervalcounting, a pulse-train containing double the sub-threshold number of pulses for each neuron was generated. The middle interval of this pulse-train was then gradually increased until a decrease in the response to the stimulus was observed.
Neurophysiological measurements and statistics
Recordings were digitized at 10 kHz (power 1401, Cambridge Electronic Design, Cambridge, UK) and stored as data files using Spike-2 software, also from the same supplier. Analyses were performed using acquired and custom Spike-2 programs. Band-pass ICNs generally did not spike in response to pulse rates well below their best rate. The decline in response at pulse rates above the best rate varied across cells, and a decrease of at least 25 % was used as a criterion for band-pass classification. ICNs were classified as high-pass if they showed increasing spike counts for pulse rates >60 pulses/s (the high end of the biologically relevant range). Neurons were classified as band-suppression if there was 50 % (or greater) reduction in the response level for mid-pulse rates relative to lower or higher pulse rates.
To make population-level comparisons between ICNs in H. chrysoscelis and H. versicolor, we averaged the number of spikes produced in response to at least three presentations of the same stimulus per neuron. For each of 72 ICNs from which we collected sufficient pulse rate and rise-time selectivity data, rise-time and ‘call’ selectivity indices were calculated. Rise-time selectivity was calculated using the following formula:
| (1) |
where SR and FR were the average number of spikes in response to slow-rise and fast-rise pulses of the preferred pulse rate. Thus, negative values indicate fast-rise selectivity and positive values indicate slow-rise selectivity. The ‘call’ selectivity index was calculated based on the responses to stimuli that had pulse rate and pulse rise-time characteristics of the advertisement calls of H. versicolor and H. chrysoscelis using the following formula:
| (2) |
Where was the highest spike average for slow-rise pulses between 10 and 20 pulses/s (characteristic of H. versicolor, Hv), and was the highest spike average for fast-rise pulses between 40 and 60 pulses/s (characteristic of H. chrysoscelis, Hch).
A t test was used to analyze the pulse rate minima of band-suppression neurons for the two species; Kolmogorov–Smirnov (best pulse rate selectivity) and Mann–Whitney U tests (rise-time and ‘call’ selectivity) were used for comparisons between data sets that did not meet the assumption of normality. A signed-rank test was used on the ‘call’ selectivity indices to test for positive or negative biases in neural responses, which correspond to selectivity for ‘versicolor-like’ versus ‘chrysoscelis-like’ stimuli (see Eq. 2 above).
Results
Extracellular (n = 28) and whole-cell patch (n = 58) recordings were made from ICNs in the auditory midbrain of 26 Hyla versicolor (44 cells) and 20 H. chrysoscelis (42 cells). Labeled neurons were predominantly in the principal and magnocellular nuclei; the proportionality was approximately 2:1. We first present representative extracellular recordings to show general features of ICNs and how they differ between these species. We then present whole-cell recordings from neurons that represent the range of response characteristics observed. These recordings show stimulus-related, subthreshold changes in membrane potential that were used to make mechanistic comparisons between these neurons and ICNs recorded from other anuran species. Finally, we combined data from extracellular and whole-cell recordings to analyze species differences in the temporal selectivity of midbrain neurons. We show that ICNs in H. versicolor are generally tuned to slower pulse rates than those in H. chrysoscelis and that slow-rise selectivity is exclusive to H. versicolor. Although pulse rate tuning differed between the two species, in both cases selectivity appeared to result from interplay between excitation and inhibition, i.e., the shift in tuning seen in H. versicolor appears to result from differential scaling of existing mechanisms.
General features of interval-counting neurons in H. versicolor and H. chrysoscelis
Extracellular recordings
Single-unit recordings from the ICanuran of H. chrysoscelis and H. versicolor revealed interval-counting neurons similar to those recorded previously in Rana pipiens and Pseudacris (formerly Hyla) regilla (Alder and Rose 1998, 2000; Edwards et al. 2007). Based on responses to stimuli shown in Fig. 1b, interval-counting neurons in gray treefrogs could be classified as band-pass/high-pass (or band-suppression (n = 18) (Figs. 2, 3) or band-suppression (n = 7) (Fig. 4). Band-pass neurons (n = 9) recorded from H. chrysoscelis showed best pulse rates (pulse rate that elicited maximal response) that ranged from 30 to 60 pulses/s (median 50 pulses/s). The representative band-pass/high-pass cell found in H. chrysoscelis (Fig. 2) showed little or no spikes to pulse rates below approximately 30 pulses/s, and the response level peaked at 50–60 pulses/s. This steep response level vs. pulse rate relation is characteristic of this neuron type. Response latency decreased markedly for 50 vs. 40 pulses/s but, in both cases, spiking stopped approximately 50–60 ms after the stimuli ended (Fig. 2b). This differential response onset time is consistent with an integration process, i.e., interval counting contributing to response latency, not simply conduction time. Consistent with this interpretation, this neuron did not respond to stimuli that had five pulses (Fig. 2c, upper trace), or less, delivered with optimal intervals (20 ms between onsets of successive pulses). At least 10 pulses were required for the cell to respond on 50 % or more of the stimulus presentations (Fig. 2c). Response levels over the biologically important 5–60 pulses/s range were highly similar for fast-rise and slow-rise pulses; only a slight preference for fast-rise pulses was observed (Fig. 2a).
Fig. 2.
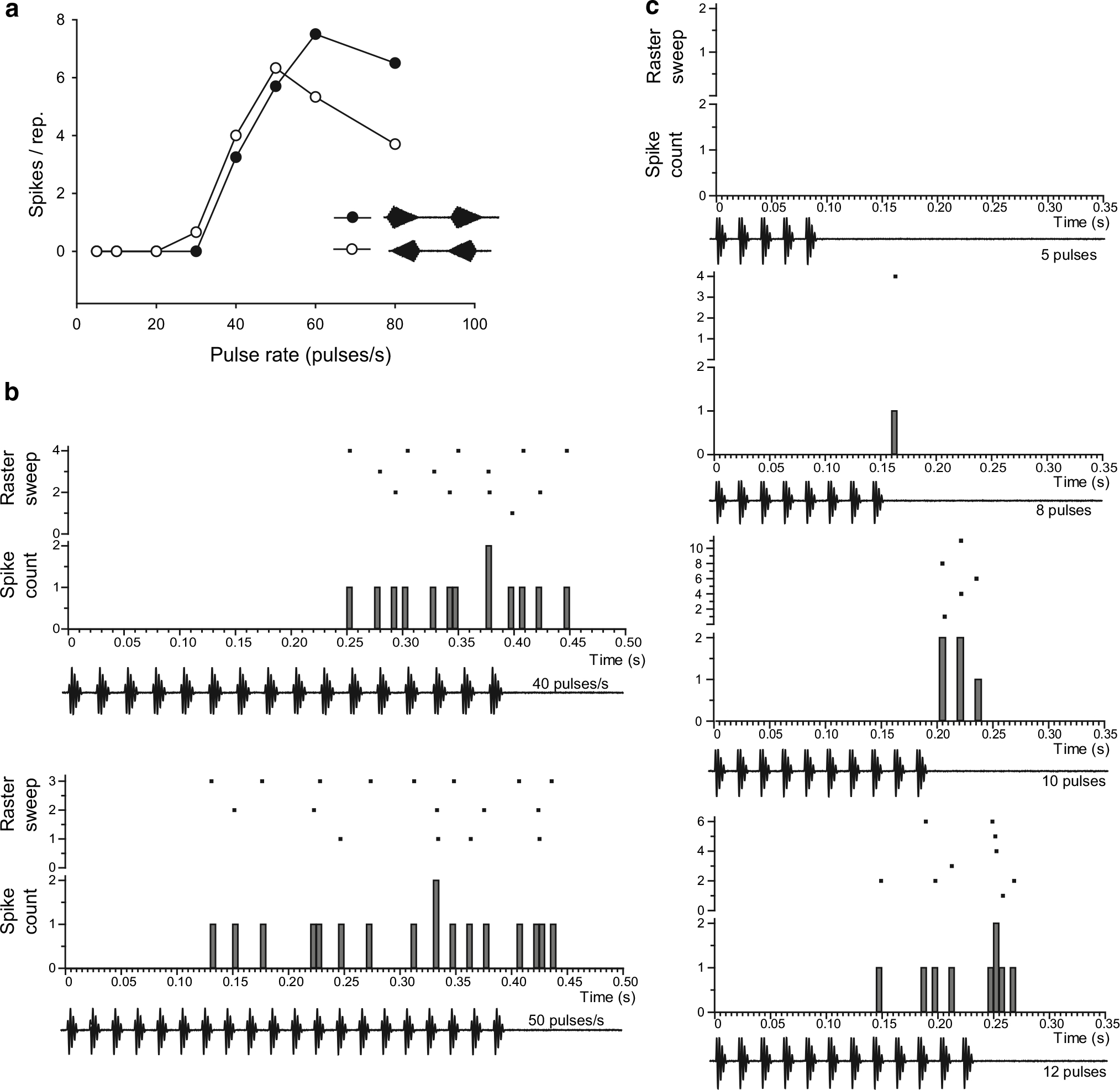
Extracellularly recorded responses of an interval-counting neuron in H. chrysoscelis. a Average spike count per presentation of stimuli that differed in pulse rate and pulse shape (20 % rise:80 % fall, closed circles, or 80 % rise:20 % fall, open circles). b Peri-stimulus-time histograms (PSTHs) and raster plots of spikes in response to stimuli in which pulses had fast (20 %) rise and were presented at 40 or 50 pulses/s, with 50 % duty cycle (on and off periods are equal duration). c PSTHs and raster plots of responses to stimuli that consisted of 5, 8, 10 or 12 pulses. Carrier frequency (BEF) = 425 Hz, 62 dB SPL
Fig. 3.

Extracellularly recorded responses of an interval-counting neuron in H. versicolor. a Average spike count per presentation of stimuli that differed in pulse rate and pulse shape (20 % rise:80 % fall, closed circles, or 80 % rise:20 % fall, open circles); stimulus amplitude was either 60 dB SPL (grey lines) or 78 dB SPL (black lines). b Peri-stimulus-time histograms (PSTHs) and raster plots of spikes in response to stimuli that consisted of fast-rise pulses (20 %) and were presented at 10 or 15 pulses/s, with 50 % duty cycle; stimulus amplitude was either 60 dB SPL (upper two panels) or 72 dB SPL (lower panel). c PSTHs and raster plots of responses to stimuli that consisted of 2, 3 or 4 pulses, repeated with intervals of 75 ms (15 pulses/s), upper 3 panels; with 75 ms intervals, 3 pulses were required to elicit spikes on at least 50 % of the stimulus presentations. Bottom panel shows the effects of increasing the duration of the middle interval to 125 ms (grey) or 150 ms (black). Carrier frequency (BEF) = 280 Hz
Fig. 4.
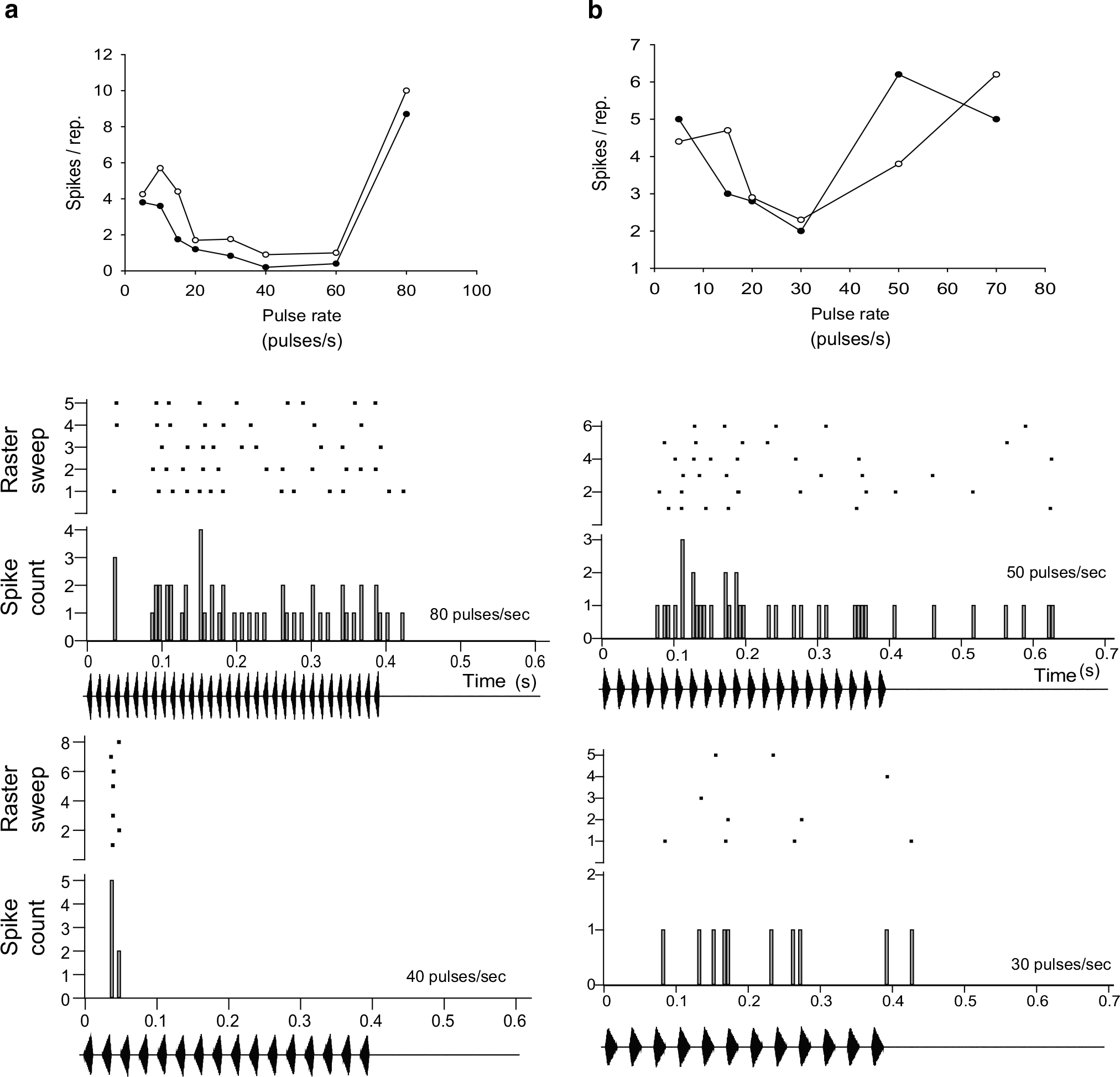
Extracellularly recorded responses from band-suppression neurons in a H. versicolor and b H. chrysoscelis; responses to 20 %-rise pulses (closed symbols) or 80 %-rise pulses (open symbols). PSTHs and raster plots are shown, as in Figs. 2 and 3. Carrier frequencies (BEF) = a 2200 Hz, 80 dB SPL; b 2200 Hz, 73 dB SPL
Extracellular recordings were made from 14 intervalcounting neurons in H. versicolor. Band-pass cells (n = 6) in this species had best pulse rates that ranged from 10 to 50 pulses/s (median 30 pulses/s). Thus, in this species, ICNs were recorded that showed tuning to relatively slow pulse rates, an atypical property for neurons of this type. The exemplar shown in Fig. 3 exhibited band-pass tuning to approximately 15 pulses/s. Pulse-rate tuning and response levels were largely independent of pulse shape (i.e., amplitude rise time) for stimuli that were approximately 9 dB above the cell’s threshold of 51 dB SPL (Fig. 3a, grey lines). At a sound level of 78 dB SPL, however, the peak response to stimuli consisting of fast-rise pulses was strongly attenuated and shifted to 10 pulses/s (Fig. 3a, black lines, filled circles). For slow-rise pulses at this stimulus amplitude (Fig. 3a, black lines, open circles), the response level of this cell at 15 pulses/s was comparable to those observed for the lower amplitude stimuli. However, first-spike latency increased and spike density was greater at the higher stimulus amplitude (Fig. 3b, middle and lower panels). Similar to the neuron from H. chrysoscelis shown in Fig. 2, response latency also generally increased for less effective stimuli in H. versicolor (e.g., compare responses to 10 and 15 pulses/s at same amplitude in Fig. 3b, top and middle panels). Thus it was surprising that first-spike latency increased despite a strong overall level of response to the higher-amplitude, 15 pulses/s stimuli. This increased first-spike latency suggests that the initial stimulus pulses primarily elicit inhibition. This cell required at least 3 pulses, delivered at 15 pulses/s, to respond (Fig. 3c, top two panels), and response levels were similar for stimuli in which pulses had slow-rise characteristics or fast-rise (not shown) characteristics (1.0 and 1.3 spikes/repetition, respectively). A sequence of four pulses, presented at inter-onset intervals of 75 ms, elicited strong responses on each stimulus presentation (Fig. 3c, 3rd panel). Increasing the middle interval to 125 ms resulted in a reduction in the strength of the response and a middle interval of 150 ms almost completely reset this interval-counting process (Fig. 3c, bottom panel, grey and black traces).
Examples of interval-counting neurons that show band-suppression pulse-rate selectivity are shown in Fig. 4. Band-suppression neurons respond to slow pulse rate stimuli, provided that individual pulses are of sufficient duration (Edwards and Rose 2003; Leary et al. 2008). For stimuli such as those depicted in Fig. 1b, pulse duty cycle (the proportion of the pulse interval that sound is present) is held constant, and, thus, pulse duration increases as pulse rate decreases. The band-suppression neuron recorded in H. versicolor (Fig. 4a) showed a broad response minimum over the 40–60 pulses/s range, which encompasses the pulse rates of advertisement calls for western populations of H. chrysoscelis (Gerhardt 2005). Over most of the range in pulse rate tested, this cell also responded slightly more strongly for slow-rise pulses (Fig. 4a). Responses to 80 pulse/s stimuli were mostly sustained, but showed a ‘pause’ between a variable onset spike and the onset of the sustained response component in 3 of the 5 stimulus presentations (Fig. 4a, middle panel). In contrast, only the onset response was observed for 40 pulses/s stimuli (Fig. 4a, lower panel). The pause in firing could result from delayed, transient inhibition to the cell, or be a temporal property of its excitatory input. Intracellular recordings from band-suppression neurons in other anuran species indicate that a balance of excitation and inhibition shapes responses, with excitation being favored at fast pulse rates and inhibition favored at intermediate rates (Leary et al. 2008; Rose 2014). The band-suppression neuron recorded from H. chrysoscelis showed a response minimum at approximately 30 pulses/s and no consistent preference for pulse shape was observed (Fig. 4b). First-spike latency for the 30 pulses/s stimulus was generally greater than that for a 50 pulses/s stimulus (Fig. 4b, compare lower and middle panels), as was observed for band-pass or high-pass ICNs.
Thus, extracellular recordings demonstrate that intervalcounting neurons exist in the ICanuran of gray treefrogs, and some are highly similar in their response properties to those that have been recorded in other anuran species. In H. versicolor, however, interval-counting neurons were recorded that showed tuning to comparatively slow pulse rates and selectivity for slow-rise pulses. In other species, interval-counting neurons typically show band-pass or high-pass selectivity for mid- to fast-pulse rates, and this selectivity emerges from interplay between time-dependent excitation and inhibition (reviewed in Rose 2014). To determine whether interval-counting and interval selectivity in gray treefrogs is mechanistically similar to that in other species, and to provide some insight into how selectivity for slow-rise pulses arises, we made whole-cell patch recordings from midbrain neurons in H. versicolor and H. chrysoscelis.
Whole-cell recordings
For interval-counting neurons in H. chrysoscelis that showed temporal selectivity similar to that displayed in Fig. 2 (i.e., band-pass or high-pass selectivity for pulse rate, with maximum responses in the 40–60 pulses/s range), whole-cell recordings revealed interplay between excitation and inhibition much like that found for intervalcounting neurons in other species that have been studied (Edwards et al. 2007; Rose et al. 2011). For the exemplar shown in Fig. 5a, pulses presented at a rate of 5 pulses/s elicited primarily inhibition (hyperpolarizations). At pulse rates greater than approximately 20 pulses/s, however, stimuli elicited an initial hyperpolarization which then was progressively reversed, leading to depolarization and spikes. This rate-dependent shift in the balance of excitation and inhibition with successive pulses is one of the hallmarks of interval-counting neurons (Rose 2014). The best pulse rate for this neuron was 50 pulses/s, and slowrise and fast-rise pulses were equally effective (Fig. 5a). Response characteristics of this general type were observed in just one of the 31 whole-cell recordings made from H. versicolor (Fig. 5b). This cell showed mainly inhibition for 10 pulses/s stimuli. At pulse rates of 30–60 pulses/s, however, strong depolarization followed initial hyperpolarization, with maximal spike rate occurring at 40 pulses/s (Fig. 5b). Importantly, this neuron did not show selectivity for slow-rise pulses; such selectivity would preclude strong responses to pulse rates in the 40–60 pulses/s range because the short-duration pulses in such stimuli have shorter rise times.
Fig. 5.
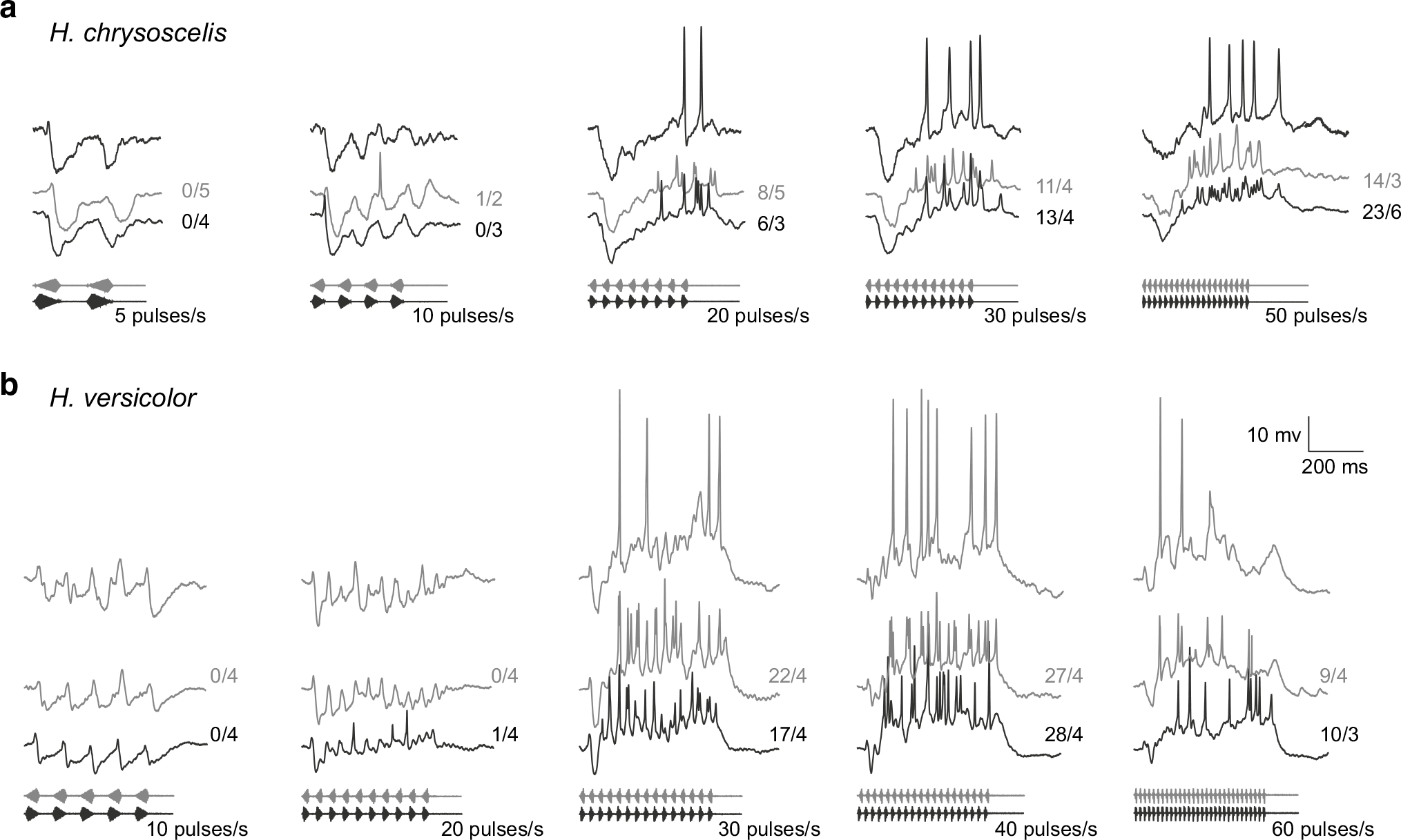
Whole-cell recordings from interval-counting neurons from H. chrysoscelis (a) and H. versicolor (b) that showed selectivity for pulse rates typical of H. chrysoscelis advertisement calls. Top traces responses to single presentations of the stimuli shown; middle and lower traces in each panel are averaged responses, hence spike height is smaller. Responses to 80 %-rise pulses (grey) and 20 %-rise pulses, along with spike counts over number of presentations of each stimulus are shown. Carrier frequencies (BEF) = a 270 Hz, 71 dB SPL; b 250 Hz; 58 dB SPL
We now turn to whole-cell recordings from ICNs that are more representative of those recorded from H. versicolor but unique in many respects (e.g., slow-rise selectivity, tuned to slow pulse rates) from ICNs found in other anuran species. Figure 6 shows whole-cell recordings from three interval-counting neurons in H. versicolor that showed tuning to pulse rates characteristic of the advertisement call (15–20 pulses/s) of this species. These cells represent the range of selectivity for slow-rise pulses that was observed. The neuron that showed greatest slowrise selectivity (Fig. 6a) failed to respond with spikes to fast-rise pulses at any pulse rate tested. Hyperpolarizations were observed at the slowest (5 pulses/s) and fastest (40–50 pulses/s) pulse rates tested. At pulse rates of 10–20 pulses/s, slow-rise pulses elicited EPSPs that temporally summated to produce supra-threshold depolarizations. Successive fast-rise pulses, however, elicited depolarizations that, at 10 pulses/s, decreased in amplitude. Further, these EPSPs occurred on a background of slow hyperpolarization, suggesting a build-up of inhibition. Fast-rise pulses that were presented at 20 pulses/s resulted in a maximal depolarization of approximately 4 mV, compared to at least 25 mV for slow-rise pulses. The neuron shown in Fig. 6b also responded selectively to slow-rise pulses; across the range of pulse rates tested, only one spike was triggered with a fast rise time stimulus (at 10 pulses/s). In this case, the large differential in spiking for slow- vs. fast-rise pulses at 15 pulses/s resulted mainly from ‘thresholding processes’; the threshold for spike production was slightly below the depolarization reached in response to slow-rise pulses, and above (less negative membrane potential) the maximum depolarization for fast-rise pulses. The case shown in Fig. 6c showed band-pass selectivity for pulse rates in the 15–20 pulses/s range, but exhibited similar levels of response for slow-rise and fast-rise pulses. Thus, sharp band-pass tuning to slow pulse rates could be observed in H. versicolor even for interval-counting neurons that showed no slow-rise selectivity.
Fig. 6.
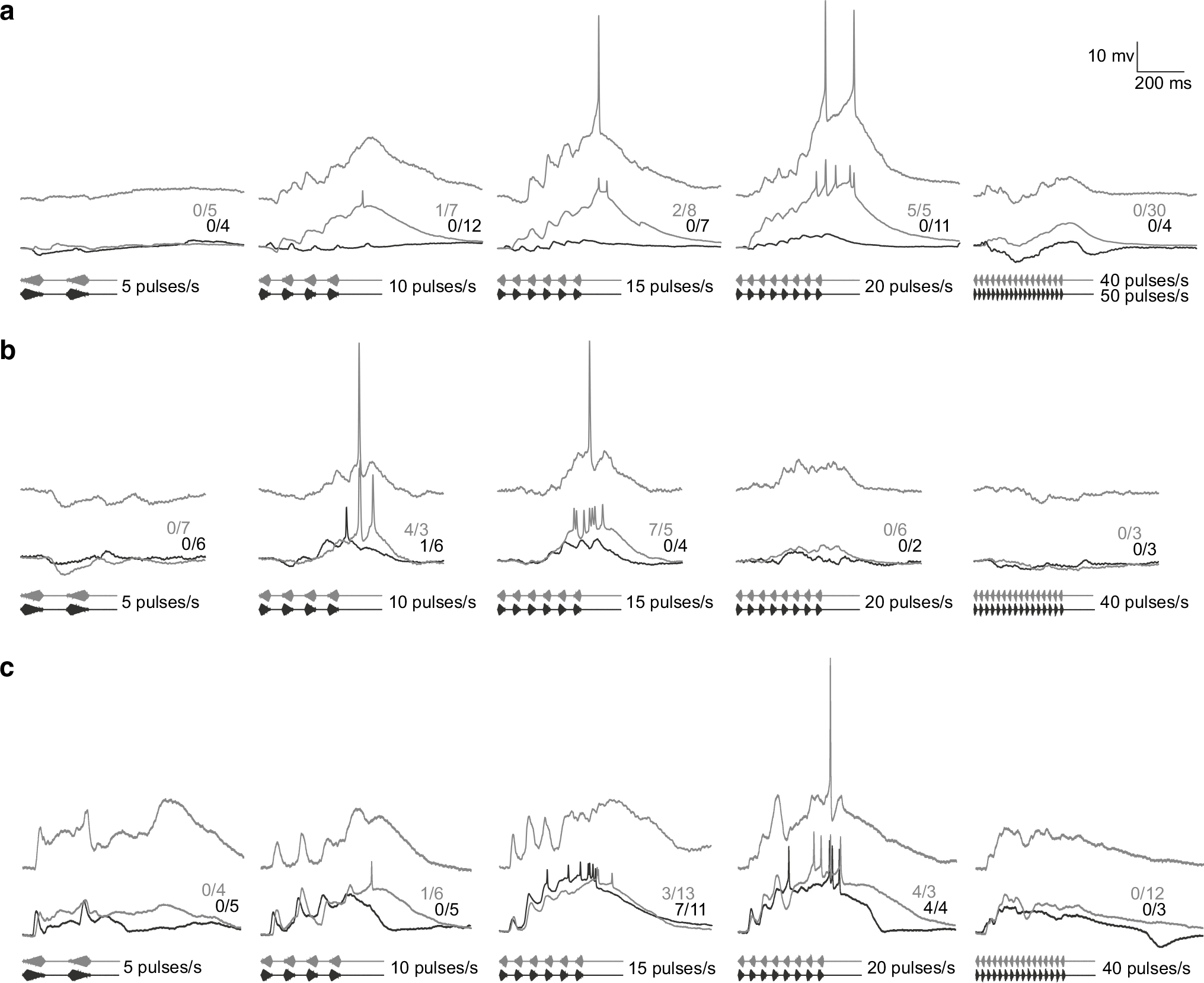
Whole-cell recordings from interval-counting neurons in H. versicolor that show tuning to pulse rates characteristic of the advertisement call of this species. As in Fig. 5, traces are membrane-potential fluctuations in response to single presentations of the stimuli shown, or averaged responses; the number of spikes and stimulus presentations are shown. The recordings (a–c) represent the range of selectivity for slow-rise pulses, from strong (a) to nonselective (c) Carrier frequencies (BEFs) = a 1200 Hz, 81 dB SPL; b 160 Hz, 69 dB SPL; c 225 Hz (BEF), 61 dB SPL
We now turn to the subthreshold events that underlie interval counting and how intervals that are longer than optimal reset the counting process. We show that inhibition appears to be important for interval counting and selectivity. Figure 7 shows averaged whole-cell recordings from an interval-counting neuron in H. versicolor that was tuned to 20 pulses/s. Over the range of 5–60 pulses/s, interplay between excitation and inhibition (evidenced as hyperpolarizations) was clear, and the latter largely dominated responses at all rates except 20 pulses/s (Fig. 7a). At this pulse rate, this balance shifted, from inhibition for initial pulses to excitation for pulses near the end of the stimulus. Single pulses elicited a small depolarization, followed by a prominent hyperpolarization (IPSP) (Fig. 7b). Additional pulses, presented at 20 pulses/s, elicited depolarizations that appeared to summate as inhibition decreased (note that depolarization peaks approximately 150–175 ms after the end of sequences of 4–6 pulses, with apparently no offset inhibition/hyperpolarization, Fig. 7b). Approximately 10 pulses were required to elicit spikes in at least 50 % of the stimulus presentations. Presenting eight pulses elicited spikes on approximately one-third of the stimulus presentations, and increasing the middle interval in this pulse sequence from 50 to 150 ms reset the counting process (Fig. 7c). For 100 and 150 ms middle intervals, the first pulse after the gap elicited IPSPs that were 7.3 and 10.4 mV, respectively. These IPSPs were smaller than the IPSPs elicited by the first pulse in a sequence (12 mV), suggesting that inhibition decreased during the pulse train.
Fig. 7.
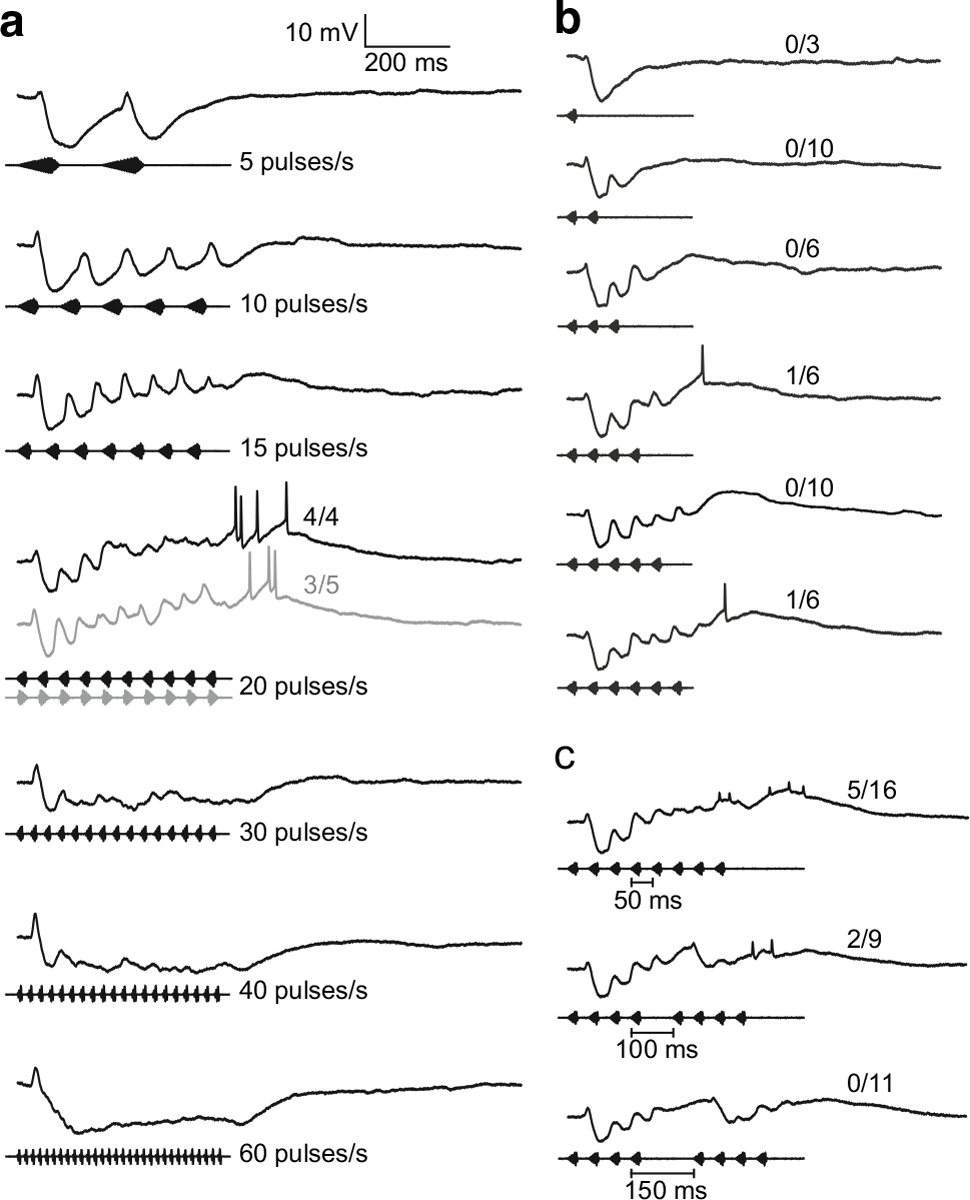
Whole-cell recordings from an interval-counting neuron in H. versicolor in response to a pulse rates from 5–60 pulses/s; b stimuli having 1–6 pulses; c stimuli in which the middle interval of a sequence of 8 pulses was increased from 50–150 ms, in steps of 50 ms. Carrier frequency (BEF) = 250 Hz, 73 dB SPL
Whole-cell recordings were made from six intervalcounting neurons in H. versicolor that showed high-pass selectivity for pulse rate; high-pass selectivity is somewhat unexpected considering that pulse rates in the advertisement calls of H. versicolor are below 25 pulses/s (see Fig. 1). Interestingly, however, four of these cells showed slow-rise selectivity, which either greatly attenuated or completely abolished responses to stimuli characteristic of H. chrysoscelis advertisement calls, e.g., fast-rise pulses, 40–50 pulses/s. Recordings from three of these cases are shown in Fig. 8. The cell presented in the top panels (a, b) showed selectivity for slow-rise pulses at 40 pulses/s, but whole-cell recordings revealed that depolarizing responses were only slightly larger for slow-rise vs. fast-rise pulses. Nevertheless, differences in depolarizations were large enough to result in an approximately fivefold greater spike count for slow-rise pulses at 40 pulses/s, but not at higher rates. For the other two neurons, slow-rise selectivity was more robust across pulse rates (Fig. 8c–f). Whole-cell recordings revealed that fast-rise pulses, across the pulse rates characteristic of the advertisement calls of these species, elicited primarily hyperpolarizations; depolarization was observed for slow-rise pulses. This slow-rise selectivity was critical, therefore, for ensuring that stimuli having similar pulse rates to that of H. chrysoscelis advertisement calls did not elicit spikes in these cells.
Fig. 8.
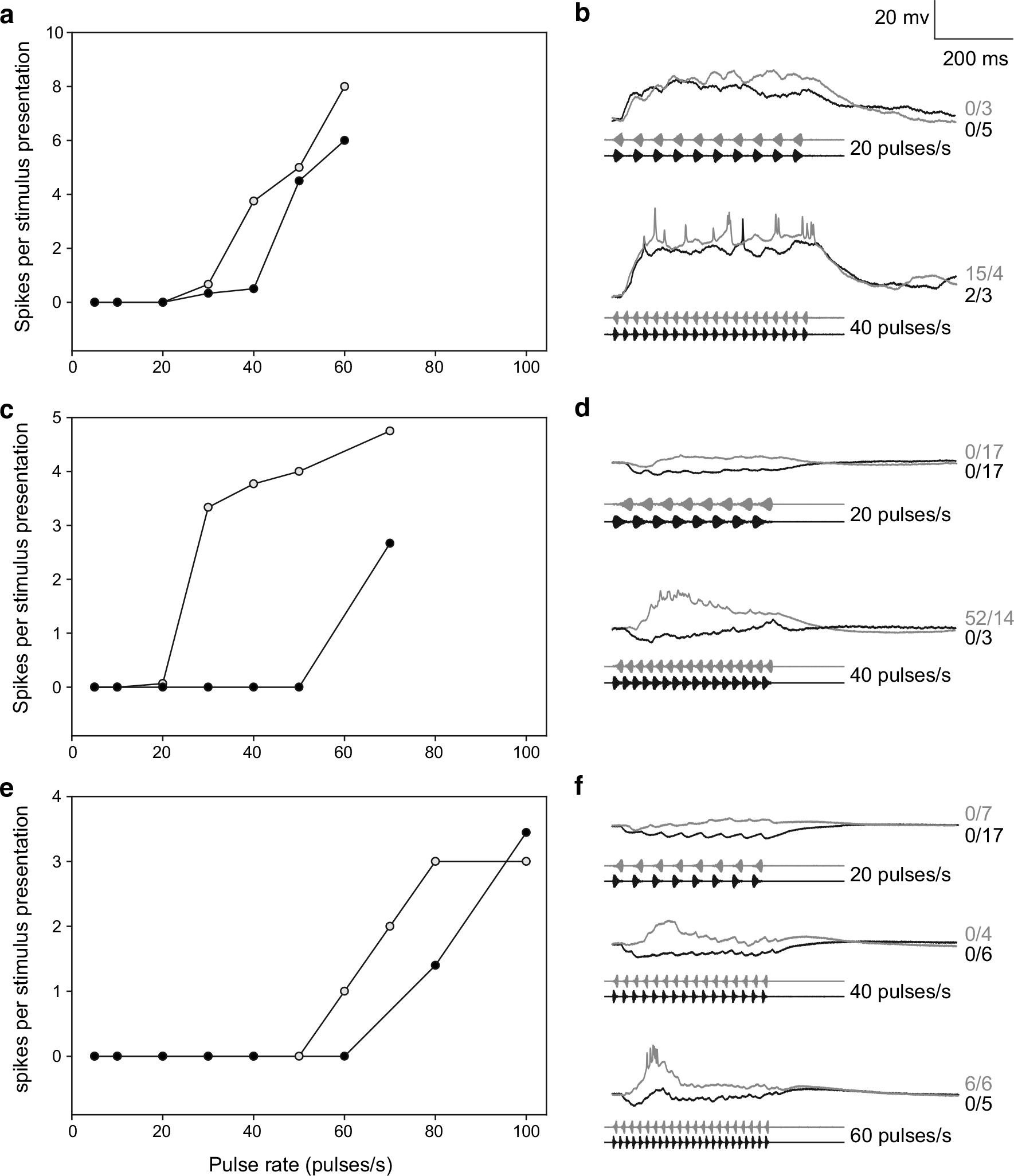
High-pass neurons in H. versicolor that show slow-rise selectivity. a, c, e Spike rate vs. pulse rate functions for three neurons that showed high-pass selectivity and responded more strongly to slowrise pulses over a cell-specific range of pulse rates. b, d, f Averaged whole-cell recordings of responses, from the same neurons shown in a, c, e, to stimuli in which slow-rise or fast-rise pulses were repeated at 20 or 40 pulses/s. Carrier frequencies (BEFs) = 800 Hz, 73 dB SPL (a, b); 1150 Hz, 81 dB SPL (c, d); 1150 Hz, 84 dB SPL (e, f)
Whole-cell recordings were made from 22 band-suppression neurons (n = 12 in H. chrysoscelis, n = 10 in H. versicolor). As with extracellular recordings, these cells showed a response minimum that was situated between regions of stronger responses at lower and higher pulse-rates (representative cases are shown in Fig. 9). These cases depict that the response minimum of band-suppression cells spanned a larger range and was centered at a higher pulse rate for H. versicolor (Fig. 9a, b) relative to H. chrysoscelis (Fig. 9c, d). At slow pulse rates, both neurons responded with greater depolarizations and spike activity for slow-rise pulses. For pulse rates that were least effective, depolarization was greatest at stimulus onset and offset (middle panels, Fig. 9b, d). The decrease in depolarization between these maxima underlies the diminished responses of neurons to intermediate pulse rates, i.e., their band-suppression properties, and is likely due to inhibition.
Fig. 9.
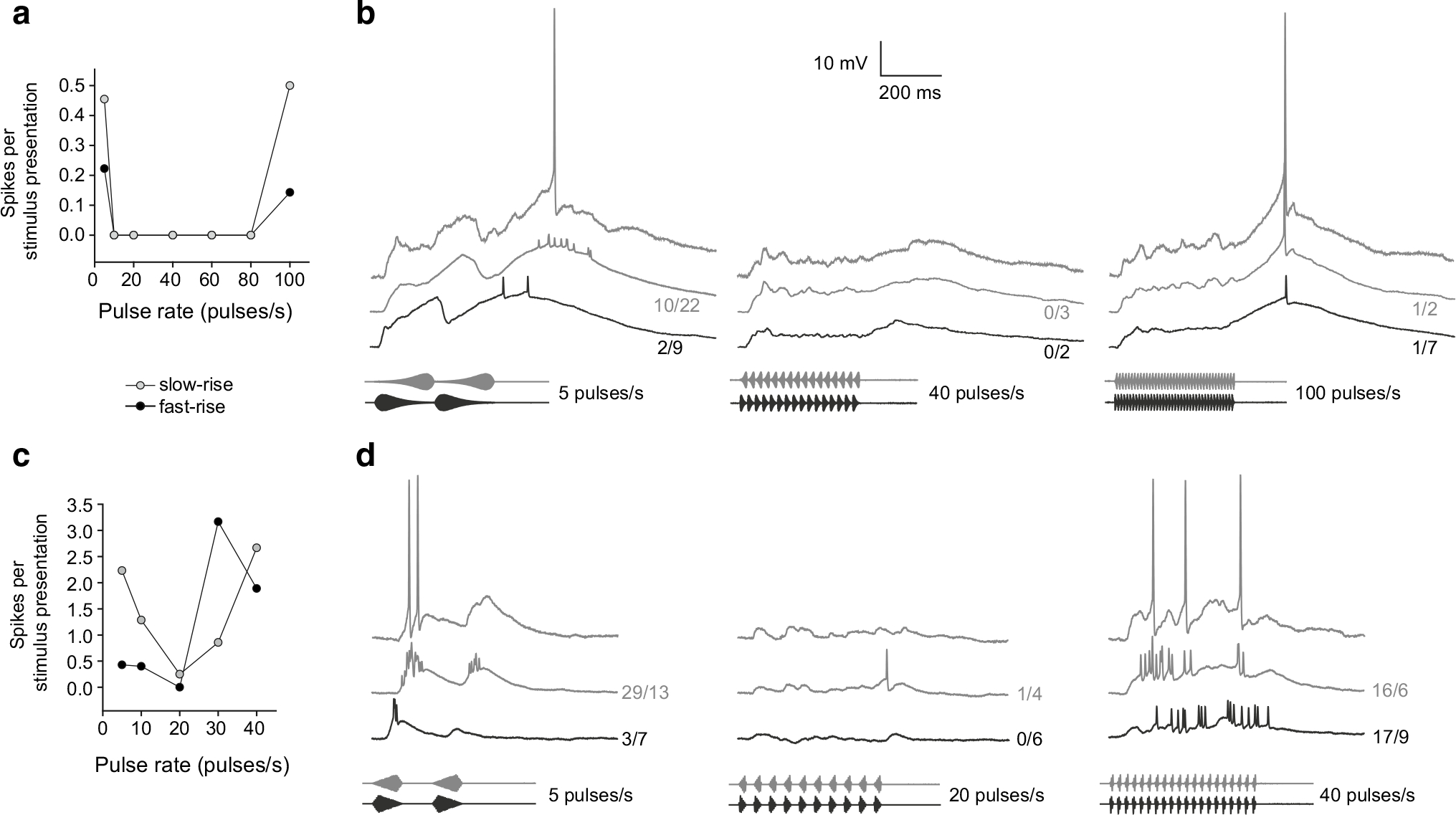
Pulse-rate selectivity functions (a, c) and whole-cell recordings (b, d) from band-suppression interval-counting neurons from H. versicolor and H. chrysoscelis, respectively. As in previous figures, upper traces in panels b and d are recordings of membrane potential responses to single presentations of the stimulus shown; other traces are averaged recordings. Responses to pulses that have slow-rise (80 %) or fast-rise (20 %) shape are shown in grey and black, respectively. Carrier frequencies (BEFs) = a 225 Hz, 81 dB SPL; c 275 Hz, 73 dB SPL
Population analyses
Spike rate data from extracellular and whole-cell recordings were combined to further examine species differences in selectivity for pulse rate, pulse shape and their interaction. In general, analyses of these data show clear evidence of species-specific temporal selectivity. Band-pass intervalcounting neurons in H. versicolor showed tuning to slower pulse rates than those recorded from H. chrysoscelis (K–S test, p < 0.05, Fig. 10a); cells that responded in a high-pass fashion were not included in this analysis. Peaks in this histogram occurred at pulse-rate regions that encompass pulse rates typical of advertisement calls of these species (i.e., pulse rates of advertisement calls are approximately 20 pulses/s in H. versicolor and 40–50 pulses/s in H. chrysoscelis at approximately 20 °C). In this analysis, the tuning value for each cell is the pulse rate, with either fast-rise or slow-rise pulse shape, that elicited the greatest number of spikes per stimulus presentation. Band-suppression neurons also showed species-specificity in their pulse rate selectivity (Fig. 10b). As shown in Figs. 4 and 9, when tested with stimulus regimes in which pulse duty cycle was held constant (pulse duration changes with pulse rate, Fig. 1), these neurons exhibited a prominent response minimum at intermediate pulse rates. This response ‘suppression’ point (the mid-value in the band of lowest response) was lower in H. chrysoscelis (20 pulses/s) vs. H. versicolor (29 pulses/s) (t test, p < 0.01, Fig. 10b). Pulse-number thresholds were highly similar between neurons in the two species (mean 3.8 pulses/s for H. chrysoscelis, mean 4.2 pulses/s for H. versicolor).
Fig. 10.
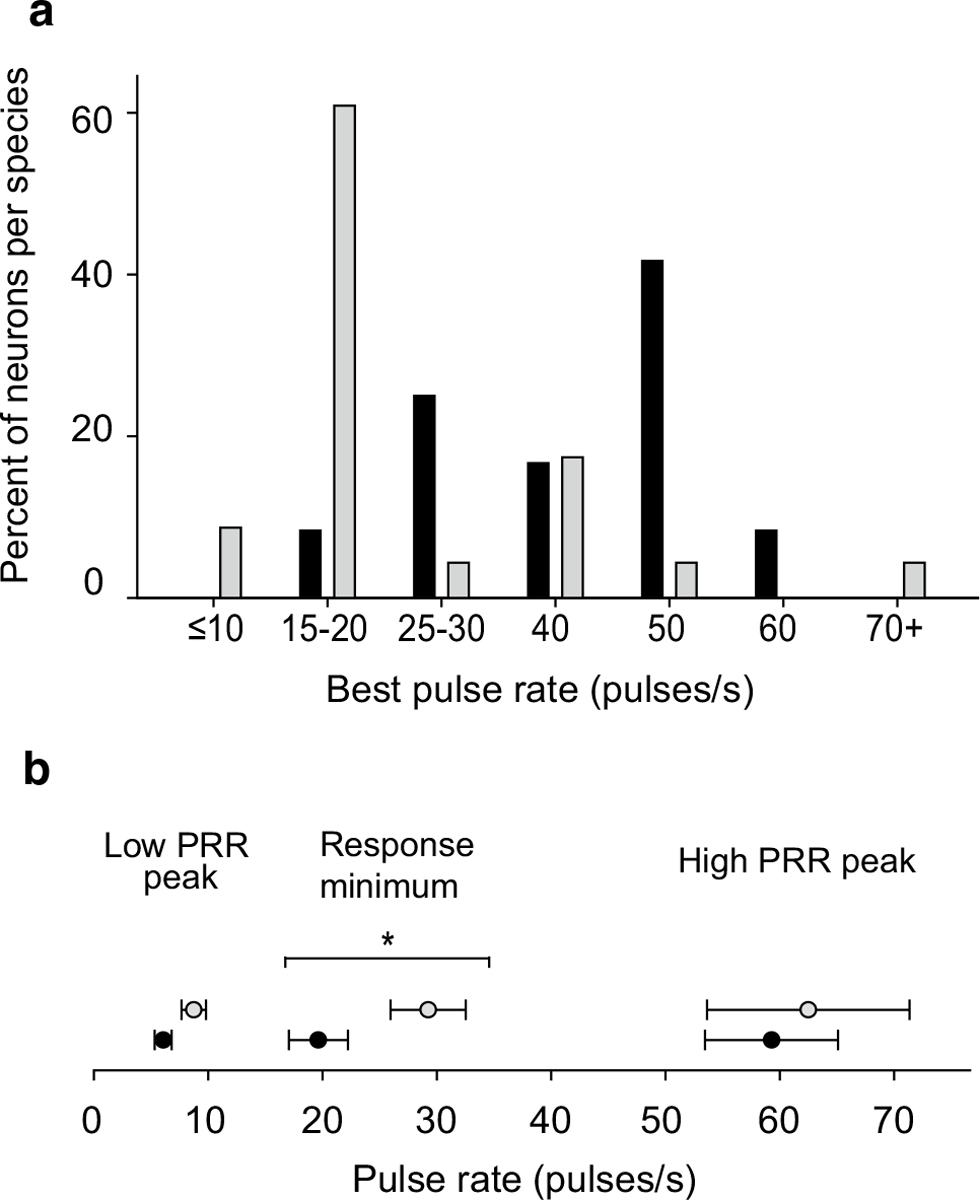
Species-specificity of pulse rate selectivity for band-pass (a) and band-suppression (b) interval-counting neurons. a Histogram of best pulse rates for neurons recorded from H. versicolor (open bars) and H. chrysoscelis (closed bars); values are for pulse shape that elicited strongest responses. b Mean pulse rates and standard error of response maxima within slow and fast pulse rate ranges, and response minima (middle set of points) for band-suppression neurons of H. versicolor (open symbols) and H. chrysoscelis (closed symbols)
In seven of the recordings made from H. versicolor, we tested whether increasing the middle interval of a series of optimally spaced pulses would decrease spiking, and the duration that is sufficient for resetting the interval-counting process. In six of the seven H. versicolor neurons increasing the middle interval by an average of 185 % decreased the average spiking rate to below 50 % of the response to a uniform-interval stimulus; one H. versicolor neuron did not reset.
Selectivity of ICNs for pulse shape is shown in Fig. 11a. Pulse-shape selectivity was quantified as the difference in spike rate for slow-rise pulses (80 % rise:20 % fall) vs. fast-rise pulses (20 % rise:80 % fall), normalized by dividing the difference by the total spike count for both (see Eq. 1). The maximum selectivity measured across the pulse rates tested was then plotted. Values of 1.0 and −1.0 indicate that the neuron responded exclusively to slow-rise or fast-rise pulses, respectively. As expected from behavioral studies, strong selectivity for slow-rise pulses was commonly observed for ICNs in H. versicolor, but rarely in H. chrysoscelis (Fig. 11a). However, unexpectedly, approximately equal numbers of fast-rise and slow-rise neurons were found in H. versicolor. Overall selectivity for pulse shape was much weaker for interval-counting neurons in H. chrysoscelis. Selectivity for slow-rise pulse shape was significantly greater for H. versicolor than H. chrysoscelis neurons (Mann–Whitney U, p < 0.05).
Fig. 11.
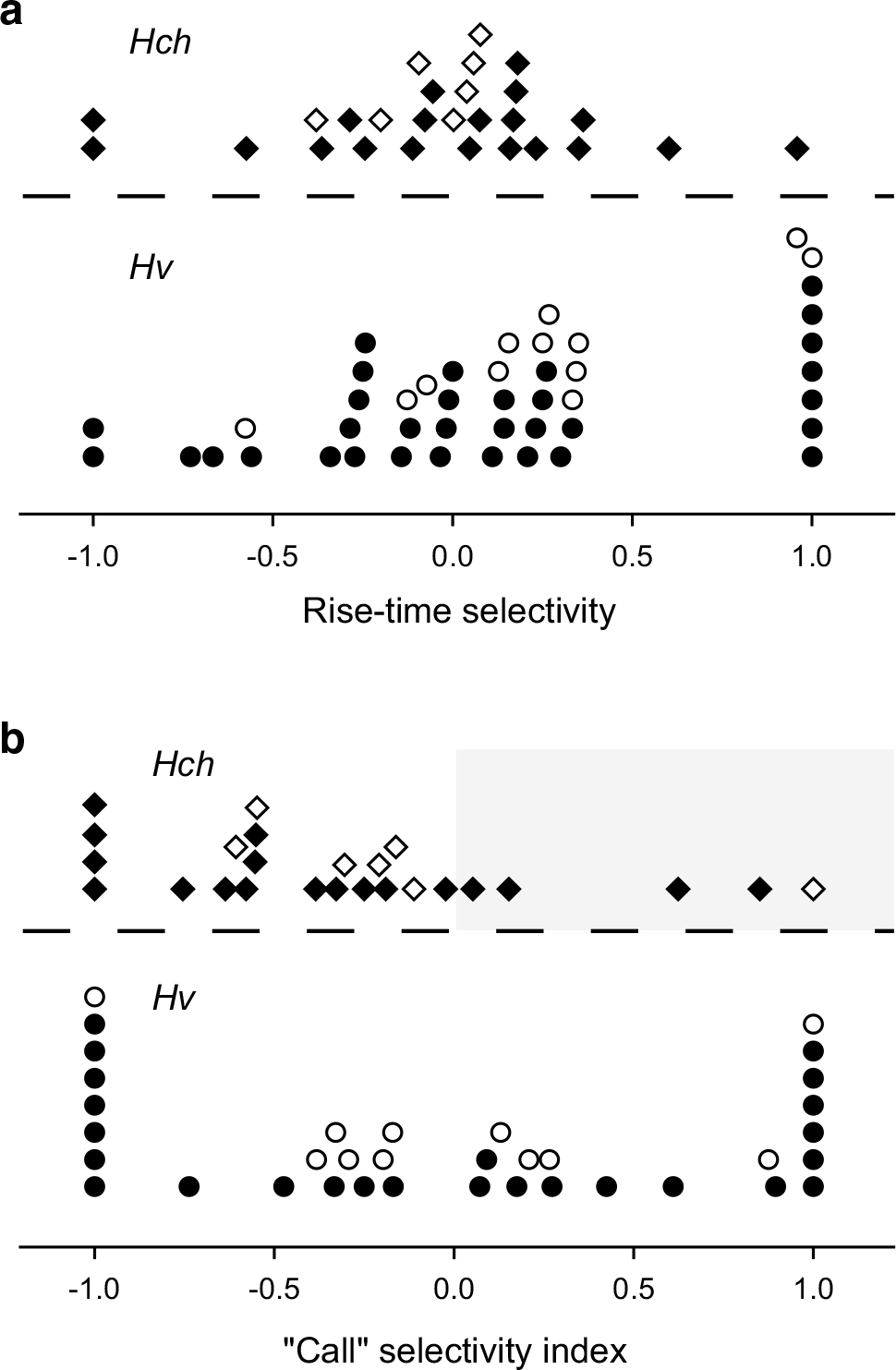
Species-specificity of selectivity for pulse shape alone (a) or pulse shape and rate combined (b). Rise-time selectivity ranges from +1.0 to −1.0 (response to only slow-rise pulses, or fast-rise pulses, respectively); responses were those measured at the cell’s best pulse rate, hence band-suppression neurons were not included in this analysis. The call selectivity index reflects the relative response level of band-suppression (open symbols) and all other interval-counting neurons (closed symbols) for stimuli that have temporal properties characteristic of the advertisement calls of H. versicolor (slow-rise pulses, 10–20 pulses/s) and H. chrysoscelis (fast-rise pulses, 40–60 pulses/s). Values in the lower and upper sectors of each plot are from neurons in H. versicolor and H. chrysoscelis, respectively. Shaded region (b) highlights that few neurons in H. chrysoscelis responded preferentially to stimuli with temporal properties characteristic of H. versicolor advertisement calls
Because both pulse rate and pulse shape influenced responses of ICNs, we next addressed the question of principal behavioral relevance: to what extent do ICNs discriminate in their firing rate between stimuli that have temporal structure characteristic of H. versicolor advertisement calls (slow-rise pulses, 10–20 pulses/s) or H. chrysoscelis (fast-rise pulses, 40–60 pulses/s)? To quantify this differential, we calculated a ‘call selectivity’ index (see Eq. 2). Exclusive response to the stimulus that represents the temporal properties of H. versicolor advertisement calls results in a call-selectivity index of 1.0; an index of −1.0 reflects exclusive response to H. chrysoscelis-type stimulus (Fig. 11b). As expected, many (50 %) of ICNs in H. versicolor showed selectivity for temporal features characteristic of its advertisement call (positive call-selectivity index values), including nine cases (25 %) that were highly selective (values >0.8); in contrast, only five H. chrysoscelis cells (20 %) had positive index values, with strong selectivity in just two cases (Fig. 11b, shaded portion of upper panel). Selectivity was significantly biased towards negative index values (H. chrysoscelis-like) (signed-rank test, p < 0.025). Most surprising was that selectivity for H. chrysoscelis temporal properties was as common and strong as that for H. versicolor-type selectivity, i.e., many neurons in H. versicolor responded more strongly to the stimulus in which fast-rise pulses were repeated at 40–60 pulses/s. This latter result is consistent with the (unexpected) finding that many interval-counting neurons in H. versicolor show selectivity for fast-rise pulses (Fig. 11a). Also, six cells in H. versicolor showed high-pass selectivity for pulse rate. Finally, one neuron was recorded from H. versicolor that was not included in these analyses because it showed band-pass selectivity for slow-rise pulses (Fig. 12a, open symbols) and band-suppression selectivity for fast-rise pulses (filled symbols). Whole-cell recordings revealed that both fast-rise and slow-rise pulses elicited relatively sustained depolarizations, but for fast-rise pulses the amplitude was subthreshold for spike initiation (Fig. 12b, middle traces). Responses were comparable at 5 pulses/s, apparently because the absolute rise time (to peak amplitude) was equal to that of the 80 % rise pulses at 20 pulses/s, i.e., rise times in both cases were 20 ms. For the 60 pulses/s stimuli (fast and slow-rise pulses), a phasic depolarization triggered a burst of spikes. The amplitude of the underlying depolarization then decreased to approximately 40 % of its peak value; spike counts per stimulus presentation were, therefore, lower than in the case of the 20 pulses/s stimulus (slow-rise), which showed a sustained response.
Fig. 12.
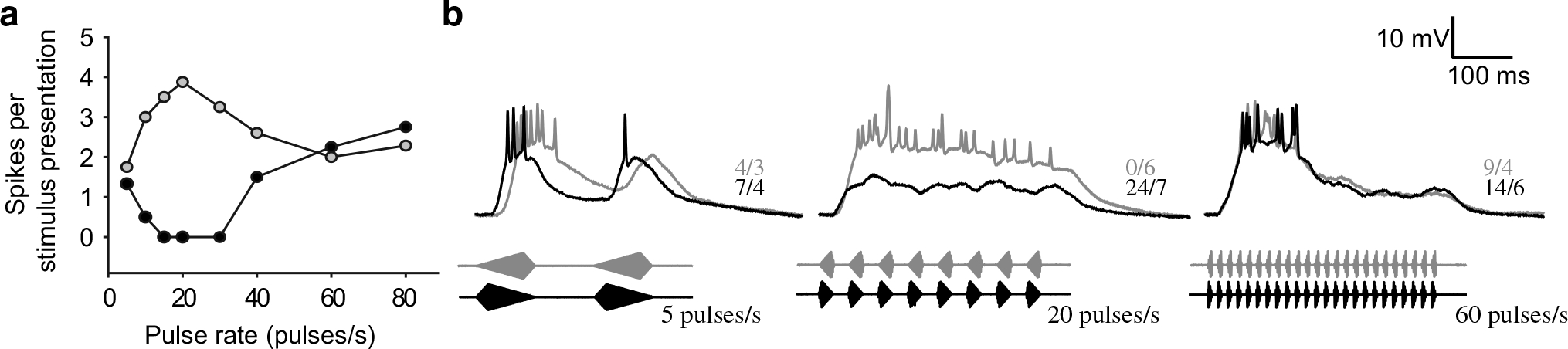
Pulse-rate selectivity depends on pulse shape. This H. versicolor interval-counting neuron had different pulse rate tuning for slow-rise pulses versus fast-rise pulses. a Pulse rate tuning curves showing average number of spikes per stimulus presentation. b Averaged membrane potential traces from presentations of slow-rise (grey) or fast-rise (black) pulses at rates of 5, 20 and 60 pulses/s
The calls of both species have spectral peaks at approximately 1.1 and 2.2 kHz. Interval-counting neurons might, therefore, be expected to have spectral tuning near these frequencies. We found, however, that many ICNs had low BEFs (Fig. 13). Thus, it appears that the central nervous system processes that underlie temporal selectivity are not uniquely associated with neurons tuned to frequencies represented in the advertisement calls.
Fig. 13.
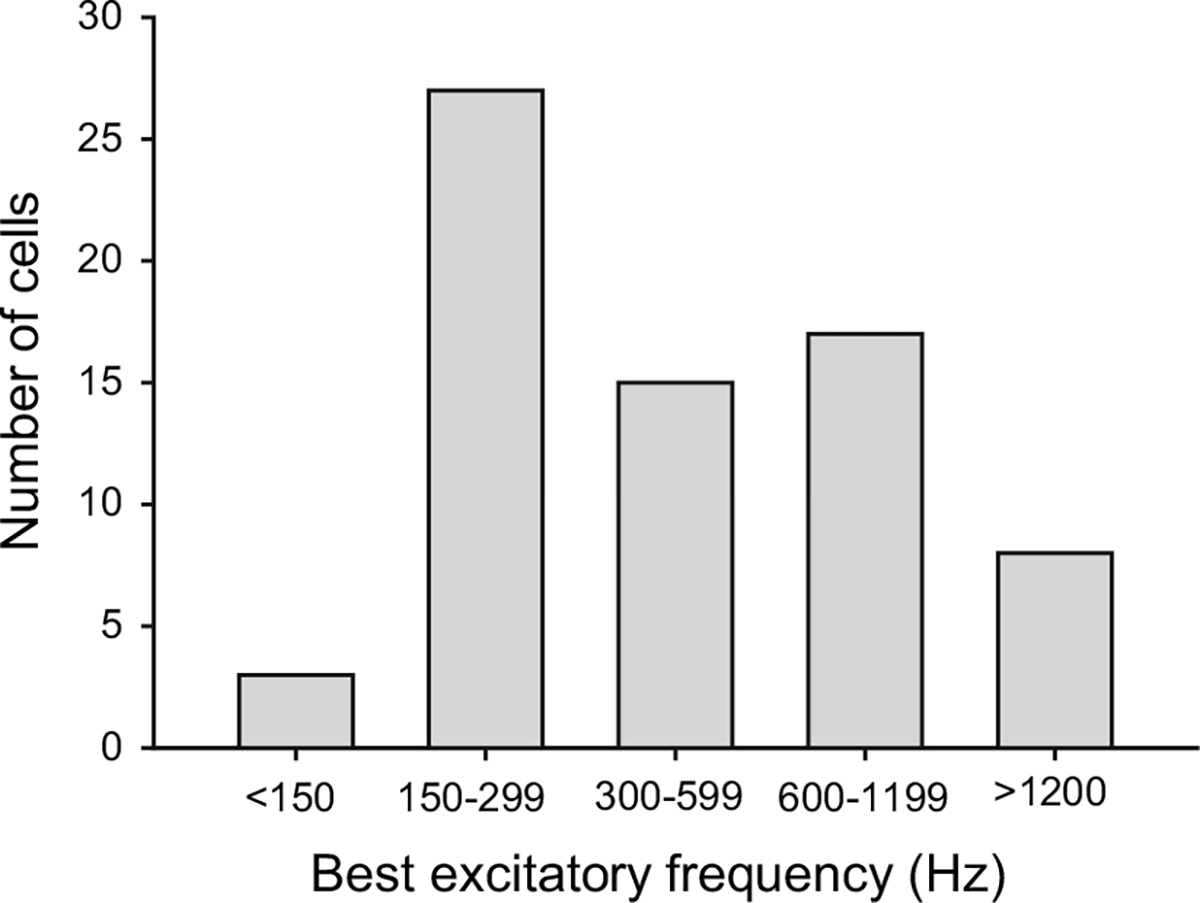
Histogram showing the number of interval-counting neurons that had best excitatory frequencies (spectral tuning) in the ranges shown. Best excitatory frequency was defined as the carrier frequency that elicited a threshold level of response at the lowest sound pressure level (Alder and Rose 2000)
Discussion
The mechanisms that underlie the abilities of organisms to discriminate between call types that differ primarily in temporal structure are poorly understood. In the case of gray treefrogs (H. versicolor and H. chrysoscelis), such discriminatory abilities are important in species isolation. Advertisement calls of gray treefrogs differ markedly in pulse duration and shape, and the rate at which pulses are repeated. Previous work has shown differences in the temporal selectivity of ‘AM band-pass’ ICanuran neurons in these two species (Rose et al. 1985), but the distinction between ‘long-interval’ vs. ‘intervalcounting’ neuron types was unknown at that time. In the present study, we have shown that many intervalcounting neurons in the ICanuran of H. versicolor exhibit tuning to slow pulse rates (≈20 pulses/s) characteristic of their advertisement calls. Across recordings, band-pass cells in H. versicolor were tuned to slower pulse rates than those in H. chrysoscelis. In addition, ICNs that were selective for slow-rise pulse shape were found almost exclusively in H. versicolor, but fast-rise-selective neurons were found in both species. Selectivity for pulse rate and pulse shape combined to produce a strong species difference in responses for stimuli that had versicolor-like vs. chrysoscelis-like temporal properties. This finding is consistent with results of earlier recordings showing that more neurons were temporally selective for stimuli that incorporated temporal properties of natural calls than was the case for sinusoidal AM stimuli (Diekamp and Gerhardt 1995).
We also recorded band-suppression neurons in both species and found that the response minimum (suppression point) was shifted to slower pulse rates in H. chrysoscelis relative to H. versicolor. With this shift in selectivity, band-suppression neurons in H. chrysoscelis respond minimally to pulse rates typical of H. versicolor calls (≈20 pulses/s) and, in most cases, show strong responses to pulse rates of 40–50 pulses/s, characteristic of the H. chrysoscelis advertisement call. We would expect these neurons to respond well to advertisement calls and encounter calls of this species (Fig. 1). Additional studies, using natural calls, are needed to test this hypothesis.
Relations to previous work
Several types of interval-selective neurons have been identified in anurans. In the species studied thus far, ‘long-interval neurons’ respond well to pulses that are repeated at slow rates, and respond phasically or not at all to fast pulse rates. Because these cells respond phasically to individual pulses, they show band-pass tuning to sinusoidal AM, or ‘constant duty-cycle’ stimuli such as those displayed in Fig. 1. Interval-counting neurons, on the other hand, generally show selectivity for faster pulse rates (short intervals between successive pulse onsets). Because the pulse rate seen in H. versicolor advertisement calls (20 pulses/s) is comparatively slow, it might be expected that long-interval neurons would be used for recognition. Behavioral studies, however, indicate that interval-counting neurons are actually used for advertisement call recognition in this species; a single long interval, embedded in a series of optimally timed pulses, decreases the attractiveness of the ‘call’ (Schwartz et al. 2010; Henderson and Gerhardt 2013). These data are consistent with our neurophysiological evidence that the tuning of interval-counting neurons in this species is ‘rescaled’, relative to H. chrysoscelis, to slower pulse rates. Thus, interval-counting neurons are likely to play an important role in enabling these animals to discriminate between species-specific calls.
Behavioral studies have shown that the advertisement calls of anurans can act as a strong premating isolation mechanism. For example, in two-choice phonotaxis experiments, females of various anuran species strongly prefer their conspecific advertisement call over those of the other species (Gerhardt 2001). Because H. versicolor and H. chrysoscelis advertisement calls are spectrally nearly identical, recognition is expected to be based on temporal properties. Indeed, behavioral studies have shown that H. chrysoscelis uses primarily pulse rate to discriminate between calls of conspecifics and heterospecifics. For H. versicolor, however, pulse duration and rise time are also important parameters for call recognition (Gerhardt and Schul 1999; Schul and Bush 2002); pulse rates of approximately 10–20 pulses/s are effective in eliciting phonotaxis, provided pulse duration exceeds approximately 25 ms. In two-choice phonotaxis experiments, H. versicolor females strongly prefer (i.e., all choose) 25 ms pulses that have 20 ms rise and 5 ms fall times over time-reversed pulses (5 ms rise, 20 ms fall) (Diekamp and Gerhardt 1995). In single-speaker experiments, however, where attractiveness of a stimulus is based on computed ‘attractiveness’ scores (Schul and Bush 2002), fast-rise pulses elicit significant phonotaxis in H. versicolor, but are not as effective as slow-rise pulses. These findings suggest that an approximately twofold difference in attractiveness may be sufficient to underlie the exclusive choice of synthesized ‘calls’ that have slow-rise vs. fast-rise pulses in the two-choice discrimination studies. In the present study, we found strong selectivity for slow-rise pulses in H. versicolor but not in H. chrysoscelis. In addition, we found some evidence that slow-rise selectivity was amplitude-dependent (e.g., Fig. 3); however, additional recordings are needed in which rise-time selectivity is evaluated over a large range of sound amplitudes. Interestingly, selectivity for fast-rise pulses was also observed in H. versicolor, and the relative proportion of neurons that showed pulse-shape selectivity ranging from −1.0 to <0.4 (strong fast-rise selectivity to moderate slow-rise selectivity) was quite similar between the two species. The finding that some neurons in H. chrysoscelis show rather strong fast-rise selectivity at first glance appears at odds with the generally accepted conclusion that H. chrysoscelis discriminates between calls based on pulse rate, not shape. However, it is important to remember that female H. chrysoscelis actually do show preference for fast-rise pulses provided pulse duration is at least 50 % greater than that typical of their advertisement calls (Gerhardt 2005). Slow-rise selectivity is particularly well developed for some interval-counting neurons in H. versicolor, which could underlie the strong preference for slow-rise pulses that has been seen in two-choice behavioral tests. Also, the positive phonotaxis of female H. versicolor for stimuli that consist of fast-rise pulses is consistent with our finding that the majority of interval-counting neurons show rather weak selectivity for pulse shape; the activity of these cells, along with those that show appreciable fast-rise selectivity, could account for the single-speaker behavioral results. A second, but not mutually exclusive, hypothesis is that fast-rise selectivity might be a necessary component in generating selectivity for slow-rise pulses. For example, these cells might inhibit the slow-rise selective interval-counting neurons. The latter hypothesis would be supported if neurons that are fast-rise selective are GABAergic; physiologically characterized neurons could be immunolabeled for GABA and biocytin to test this possibility.
When both pulse rate and pulse rise time are considered in the selective responses of interval-counting neurons, very few H. chrysoscelis neurons respond well to versicolor-like stimuli, which is likely due to their pulse-rate tuning. In contrast, a large proportion of H. versicolor neurons were highly selective, but split evenly between chrysoscelis- and versicolor-like stimuli. While highly positive ‘call’ selectivity can be explained by strong slow-rise selectivity, the H. versicolor neurons with highly negative ‘call’ selectivity values (see Eq. 2) are likely a consequence of sharp tuning to chrysoscelis-typical rates (as in Fig. 5b). The presence, in H. versicolor of neurons that prefer chrysoscelis-like stimuli is surprising. One possible explanation is to permit recognition and avoidance of heterospecifics, thereby preventing mismatings. However, this scenario is qualitatively inconsistent with the behavioral finding that some H. versicolor females will approach H. chrysoscelis advertisement calls, given no other choice (Gerhardt and Doherty 1988; Schul and Bush 2002). Clearly our finding that the selectivity of ICNs was split evenly between chrysoscelis- and versicolor-like stimuli contrasts with the strong preferences of female H. versicolor for their conspecific call. Another possible resolution of this paradox is that ICNs tuned to fast pulse rates inhibit those tuned to slow pulse rates, thereby functioning to sharpen selectivity for the advertisement call. Finally, behavioral decisions may not be based on the activity of ICNs alone; other classes of temporally selective cells also play roles in behavioral decisions. Specifically, ‘long-interval’ neurons (Alder and Rose 2000; Edwards et al. 2008), which are also found in H. versicolor (unpublished observations), respond preferentially to slow pulse rates, including that characteristic of the advertisement calls. It is highly unlikely that our H. versicolor males were misclassified, as they were collected in areas that were devoid of H. chrysoscelis and identified from the calls that they produced.
How do neural mechanisms of pulse-rate selectivity for ICNs in these species relate to mechanistic information gleaned from behavioral studies? Phonotaxis studies have shown that H. chrysoscelis approach calls only if they have at least 6–9 sequential pulses, presented at fast rates (Velez and Bee 2011). This result is consistent with our finding that ICNs in this species can have pulse-number thresholds in this range; for example, the neuron shown in Fig. 2 required at least 9–10 pulses to respond reliably. Similarly, H. versicolor females approach experimental calls that have six or more pulses (Bush et al. 2002). For ICNs, an average of approximately four pulses was required to elicit spikes on most stimulus presentations. Behavioral pulse-number thresholds appear, therefore, to be slightly greater than mean values for ICNs. This disparity might indicate that a critical level of activity within the population of ICNs must be reached before behavioral responses are elicited. Consistent with this hypothesis, stronger phonotaxis has been observed in these studies for calls in which the number of pulses exceeds the threshold value.
From phonotaxis experiments, Schul and Bush (2002) postulated that the shift in preference to relatively slow pulse rates (H. versicolor) could result from a mechanistically novel selectivity for pulses that rise slowly in amplitude and have long duration, as opposed to altering the parameters of an existing mechanism. They concluded that the evolution of signal structure preference in gray treefrogs does not exclusively fit either ‘classical sexual selection’ or ‘receiver bias’, e.g., sensory exploitation (Ryan and Rand 1993), models of signal and preference evolution. Our recordings provide direct evidence for novel neural selectivity for slow-rise pulse shape in H. versicolor. Some ICNs in H. versicolor showed strong responses and tuning to approximately 20 pulses/s, provided that pulses had slow-rise characteristics; pulses with the same duration that had fast-rise properties were much less effective. This subset of strongly slow-rise selective neurons in H. versicolor were not found in H. chrysoscelis, and therefore do not seem to be a result of a shift in selectivity, but a novel feature in H. versicolor. However, we also found evidence in H. versicolor for quantitative changes in an existing mechanism, i.e., tuning for pulse rate per se. We recorded ICNs that showed little or no rise-time selectivity, yet were also tuned to slow pulse rates. Further, the cell in Fig. 3, which was selective for slow-rise pulses at high, but not at low, stimulus amplitudes, showed tuning to 15 pulses/s in both cases. In whole-cell recordings, neurons showed minimal depolarization, and no spikes, for pulse rates of 5 pulses/s, which for constant-duty-cycle stimuli, consisted of long-duration pulses. Thus, tuning to approximately 20 pulses/s cannot be attributed exclusively to selectivity for pulse duration. Interplay between rate-dependent inhibition and excitation appears to also underlie this tuning, as has been seen in other anuran species (Edwards et al. 2007; Rose et al. 2011) and for pulse-rate selective neurons in the midbrain of electric fish (Carlson 2009; George et al. 2011; Baker and Carlson 2014). This tuning appears, therefore, to be mechanistically similar for both H. chrysoscelis and H. versicolor but ‘rescaled’ (shifted) in the allopolyploid H. versicolor. In addition, we found some H. versicolor neurons that were tuned to 40–50 pulses/s, which may represent ‘remnants of the ancestral condition’ (‘ghosts of biases past’ Ryan and Rand 1993) that, as mentioned earlier, possibly function in a ‘lateral inhibitory’ manner to sharpen tuning of ICNs for pulse rates characteristic of H. versicolor advertisement calls (~20 pulses/s). Thus, both conserved mechanisms (found in the diploid H. chrysoscelis ancestor) and novel mechanisms (found in the tetraploid H. versicolor descendent) contribute to selectivity for slow pulse rates.
As mentioned earlier, a single long interval, embedded in a series of optimal intervals, can reset the interval-counting process, and decrease the attractiveness of calls to females. Recent behavioral studies of H. versicolor have shown that adding a trailing, but not preceding, appendage (e.g., tone burst) to a call that is interrupted in this manner can rescue its attractiveness (Henderson and Gerhardt 2013). For interval-counting neurons in H. versicolor that are selective for slow pulse rates, we found that appreciable depolarization can be seen even 200 ms after stimulus offset (e.g., Fig. 6). In contrast, interval-counting neurons that respond best at fast pulse rates generally show hyperpolarization over this time frame (Edwards et al. 2007; Rose et al. 2011). The onset of a trailing appendage would be expected to elicit additional excitation, but also inhibition that would increase in strength as the time between stimulus offset and appendage onset increases (e.g., Fig. 7). Thus, an appendage that occurs just 50 ms after the offset of a series of pulses (as was used in the aforementioned behavioral experiments) should elicit predominantly excitation, which could then summate with depolarization resulting from the pulse train. It is interesting that a leading appendage is relatively ineffective in rescuing the behavioral response to a ‘gap call’. A long-duration tone burst is an effective stimulus for band-suppression neurons (Leary et al. 2008). Thus, if these neurons contributed to phonotactic behavior, gap calls that are preceded with a long-duration appendage should be attractive to females. These results imply that band-suppression neurons may play little role in advertisement call detection in H. versicolor; preliminary recordings indicate, however, that aggressive (‘encounter’) calls (Fig. 1) are effective stimuli for these neurons. Activation of band-suppression neurons may, however, underlie the enhancement of call attractiveness by long-duration appendages in H. chrysoscelis (Seeba et al. 2010).
Evolutionary implications
Tetraploid H. versicolor is believed to have evolved via allopolyploidy events on at least three occasions from hybridization between ancestral diploids, including representatives of extant H. chrysoscelis and two genetically distinct H. chrysoscelis-like lineages that appear to be extinct (Holloway et al. 2006). Matings between diploids and tetraploids result in triploids that are largely sterile, thus mechanisms to avoid mismating are expected. Behavioral studies have shown that the advertisement calls serve as a strong premating isolation mechanism; in two-choice phonotaxis experiments, females strongly prefer their conspecific advertisement call over that of the other species (Gerhardt and Doherty 1988). Further behavioral analyses have shown that discrimination is based on analysis of temporal properties of these calls, consistent with the property that H. versicolor and H. chrysoscelis advertisement calls are spectrally nearly identical (Gerhardt 2001, 2005).
The question arises, therefore, as to how tetraploid ancestors of H. versicolor avoided mating with diploid parental species. One hypothesis is that polyploidy directly caused changes in the neural networks that control vocal motor pattern generation and sensory processing of temporal information. In this scenario, polyploids produced calls in which pulses are longer in duration and rise-time, and repeated at slower rates; on the sensory side, the temporal tuning of interval-selective neurons may have been shifted to slower rates as a result of polyploidization. If substantial sensory–motor changes accompanied polyploidy, the initial tetraploids may have been immediately able to differentiate between their calls and those of diploids. In support of this hypothesis, behavioral selectivity for highly conserved temporal features such as slow-rise pulses is highly similar across H. versicolor lineages (HC Gerhardt, personal communication). Recent studies of lab-generated autotriploids, created by cold-shocking eggs of (diploid) H. chrysoscelis (Keller and Gerhardt 2001; Tucker and Gerhardt 2012) provide additional support for this hypothesis. Male autotriploids produced calls in which pulse rates were approximately 13 % slower than characteristic for calls of diploid H. chrysoscelis (Keller and Gerhardt 2001), and female autotriploids showed preference for some synthetic stimuli representative of the calls of autotriploid males (Tucker and Gerhardt 2012). Future neurophysiological studies of autopolyploids should provide insight into the changes in neural processing of temporal information associated with polyploidy per se. Of particular interest is whether pronounced shifts in selectivity for rise time and pulse rate, as seen in H. versicolor, are observed in autopolyploids. This result would support the hypothesis that both an evolutionary novelty (slow-rise selectivity) and quantitative changes in existing mechanisms (pulse rate tuning) resulted from polyploidy. Further differentiation in call parameters could then result from classical selection (against hybridization with diploids).
Acknowledgments
We thank H.C. Gerhardt, J. Schwartz and their colleagues for providing the animals used in this study. We also thank Stephen Odom and Caleb Herrick for assisting in experiments and data processing. This work was supported by a grant from NIDCD.
Abbreviations
- ICN
Interval-counting neuron
- ICanuran
Anuran inferior colliculus
- AM
Amplitude modulation
- SAM
Sinusoidal amplitude modulation
- SPL
Sound pressure level in decibels (dB), re 2 × 10−5 N/m2
- EPSP
Excitatory postsynaptic potential
- IPSP
Inhibitory postsynaptic potential
- BEF
Best excitatory frequency
Contributor Information
Gary J. Rose, Department of Biology, University of Utah, 257 South 1400 East Rm 204, Salt Lake City, UT 84112, USA
Jessica L. Hanson, Department of Biology, University of Utah, 257 South 1400 East Rm 204, Salt Lake City, UT 84112, USA
Christopher J. Leary, Department of Biology, University of Utah, 257 South 1400 East Rm 204, Salt Lake City, UT 84112, USA University of Mississippi, Oxford, MS 38677, USA.
Jalina A. Graham, Department of Biology, University of Utah, 257 South 1400 East Rm 204, Salt Lake City, UT 84112, USA
Rishi K. Alluri, Department of Biology, University of Utah, 257 South 1400 East Rm 204, Salt Lake City, UT 84112, USA
Gustavo A. Vasquez-Opazo, Department of Biology, University of Utah, 257 South 1400 East Rm 204, Salt Lake City, UT 84112, USA
References
- Alder TB, Rose GJ (1998) Long-term temporal integration in the anuran auditory system. Nat Neurosci 1:519–523 [DOI] [PubMed] [Google Scholar]
- Alder TB, Rose GJ (2000) Integration and recovery processes contribute to the temporal selectivity of neurons in the midbrain of the northern leopard frog, Rana pipiens. J Comp Physiol A 186:923–937 [DOI] [PubMed] [Google Scholar]
- Baker CA, Carlson BA (2014) Short-term depression, temporal summation, and onset inhibition shape interval tuning in midbrain neurons. J Neurosci 34:14272–14287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush SL, Gerhardt HC, Schul J (2002) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14 [Google Scholar]
- Carlson BA (2009) Temporal-pattern recognition by single neurons in a sensory pathway devoted to social communication behavior. J Neurosci 29:9417–9428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekamp B, Gerhardt HC (1995) Selective phonotaxis to advertisement calls in the grey treefrog Hyla versicolor: behavioral experiments and neurophysiological correlates. J Comp Physiol A 177:173–190 [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Rose GJ (2003) Interval-integration underlies amplitude modulation band-suppression selectivity in the anuran midbrain. J Comp Physiol A 189:907–914 [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Leary CJ, Rose GJ (2007) Counting on inhibition and rate-dependent excitation in the auditory system. J Neurosci 27:13384–13392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Leary CJ, Rose GJ (2008) Mechanisms of long-interval selectivity in midbrain auditory neurons: roles of excitation, inhibition, and plasticity. J Neurophysiol 100:3407–3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AA, Lyons-Warren AM, Ma X, Carlson BA (2011) A diversity of synaptic filters are created by temporal summation of excitation and inhibition. J Neurosci 31:14721–14734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt HC (2001) Acoustic communication in two groups of closely related treefrogs. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ (eds) Advances in the study of behavior, vol 30. Academic Press, New York, pp 99–167 [Google Scholar]
- Gerhardt HC (2005) Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution 59:395–408 [PubMed] [Google Scholar]
- Gerhardt HC (2008) Phonotactic selectivity in two cryptic species of gray treefrogs: effects of differences in pulse rate, carrier frequency and playback level. J Exp Biol 211:2609–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt HC, Doherty JA (1988) Acoustic communication in the gray treefrog Hyla versicolor: evolutionary and neurobiological implications. J Comp Physiol A 162:261–278 [Google Scholar]
- Gerhardt HC, Schul J (1999) A quantitative analysis of behavioral selectivity for pulse rise-time in the gray treefrog, Hyla versicolor. J Comp Physiol A 185:33–40 [DOI] [PubMed] [Google Scholar]
- Henderson JJ, Gerhardt HC (2013) Restoration of call attractiveness by novel acoustic appendages in grey treefrogs. Anim Behav 86:537–543 [Google Scholar]
- Holloway AK, Cannatella DC, Gerhardt HC, Hillis DM (2006) Polyploids with different origins and ancestors form a single sexual polyploid species. Am Nat 167:E88–E101 [DOI] [PubMed] [Google Scholar]
- Johnson C (1963) Additional evidence of sterility between call-types in the Hyla versicolor complex. Copeia 1963:139–143 [Google Scholar]
- Keller MJ, Gerhardt HC (2001) Polyploidy alters advertisement call structure in gray treefrogs. Proc R Soc B 268:341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary CJ, Edwards CJ, Rose GJ (2008) Midbrain auditory neurons integrate excitation and inhibition to generate duration selectivity: an in vivo whole-cell patch study in anurans. J Neurosci 28:5481–5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GJ (2014) Time computations in anuran auditory systems. Front Physiol 5:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Capranica RR (1983) Temporal selectivity in the central auditory system of the leopard frog. Science 219:1087–1089 [DOI] [PubMed] [Google Scholar]
- Rose G, Capranica RR (1984) Processing amplitude-modulated sounds by the auditory midbrain of two species of toads: matched temporal filters. J Comp Physiol A 154:211–219 [Google Scholar]
- Rose GJ, Capranica RR (1985) Sensitivity to amplitude modulated sounds in the anuran auditory nervous system. J Neurophysiol 53:446–465 [DOI] [PubMed] [Google Scholar]
- Rose GJ, Fortune ES (1996) New techniques for making whole-cell recordings from CNS neurons in vivo. Neurosci Res 26:89–94 [DOI] [PubMed] [Google Scholar]
- Rose GJ, Brenowitz EA, Capranica RR (1985) Species specificity and temperature dependency of temporal processing by the auditory midbrain of two species of treefrogs. J Comp Physiol A 157:763–769 [DOI] [PubMed] [Google Scholar]
- Rose GJ, Leary CJ, Edwards CJ (2011) Interval-counting neurons in the anuran auditory midbrain: factors underlying diversity of interval tuning. J Comp Physiol A 197:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Rand AS (1993) Sexual selection and signal evolution: the ghost of biases past. Proc R Soc B 340:187–195 [Google Scholar]
- Schul J, Bush SL (2002) Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc R Soc B 269:1847–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JJ, Huth K, Hunce R, Lentine B (2010) Effect of anomalous pulse timing on call discrimination by females of the gray treefrog (Hyla versicolor): behavioral correlates of neurobiology. J Exp Biol 213:2066–2072 [DOI] [PubMed] [Google Scholar]
- Seeba F, Schwartz JJ, Bee MA (2010) Testing an auditory illusion in frogs: perceptual restoration or sensory bias? Anim Behav 79:1317–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MA, Gerhardt HC (2012) Parallel changes in mate-attracting calls and female preferences in autotriploid tree frogs. Proc R Soc B 279:1583–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez A, Bee MA (2011) Dip listening and the cocktail party problem in grey treefrogs: signal recognition in temporally fluctuating noise. Anim Behav 82:1319–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]


