Abstract
Background
Gas‐related symptoms (GRS) are common in the general population (GPop) and among patients with disorders of gut‐brain interactions but there is no patient‐reported outcome evaluating these symptoms and their impact on daily life. We have previously developed a 43‐item intestinal gas questionnaire (IGQ). The aim of the present study is to perform a psychometric validation of this instrument.
Methods
Participants (119 from the GPop and 186 irritable bowel syndrome (IBS) patients) were recruited from 3 countries (UK, Spain, France). IBS patients fulfilled ROME IV criteria with an IBS severity score between 150 and 300. Participants completed the IGQ, the functional Digestive Disorders Quality of Life (FDDQL), and the EQ‐5D. A subgroup (n = 90) repeated the IGQ completion after 7 days on paper or electronically.
Results
From the original IGQ questionnaire, 26 items were deleted because of poor performance. Confirmatory factorial analysis on the remaining 17 items (7 symptom and 10 impact items) yielded a 6‐factor structure accounting for 67% of the variance for bloating (6 items), flatulence (3), belching (2), bad breath (2), stomach rumbling (2), and difficult gas evacuation (2). Global score (0‐100) was worse among IBS vs GPop (40 ± 15 vs 33 ± 17; p = 0.0016). At the second visit, the intraclass correlation coefficient of IGQ scores was between 0.71 and 0.86 (n = 67) for test‐retest reliability and 0.61‐0.87 (n = 64) for equivalence between electronic and paper versions of IGQ.
Conclusion
The IGQ available in paper and electronic versions in 3 languages is a robust instrument for capturing and measuring GRS and their impact on daily life.
Keywords: gas‐related symptoms, general population, IBS, patient‐reported outcomes, psychometrics, quality of life
Intestinal Gas Quesitonnaire (IGQ): a new tool to measure Gas‐Related Symptoms and their impact on daily life.

1. INTRODUCTION
Gas‐related symptoms (GRS) such as bloating, borborygmi/stomach rumbling, or flatulence are common complaints in irritable bowel syndrome (IBS) 1 , 2 , 3 and in general population (GPop). 4 , 5 They affect daily life, 1 , 4 , 5 , 6 and each of us has experienced how flatulence or bad breath can affect social interactions. 7 , 8 GRS fluctuate from day‐to‐day influenced by triggers such as food and beverages. 9 , 10 The passage of gas from the anus up to 20 times a day is considered normal 11 as is occasional belching after meals. 12 Indeed, each subject has a unique perception of what he or she considers normal or abnormal. The Rome Foundation (https://theromefoundation.org/) has defined a series of diagnostic criteria for what are known as the functional gastrointestinal (GI) disorders, more recently named as disorders of brain‐gut interaction 13 but unfortunately, there are no internationally agreed criteria for what constitutes a normal or abnormal amount of GRS, and no validated, objective ways of capturing the extent of the problem. 14 , 15 , 16
Previous studies showed that a 4‐item digestive symptom frequency questionnaire (DSFQ) assessing 3 GRS (bloating, flatulence, and rumbling stomach) was sensitive to detect significant changes in response to a probiotic in 2 randomized studies in GPop reporting mild GI discomfort. 17 , 18 However, the DSFQ is too short to capture the full experience of subjects with regard to GRS, especially as one, out of the 4 items, refers to abdominal pain, which is outside the scope of the conceptual framework we aimed to create.
Our research program aimed to develop an intestinal gas questionnaire (IGQ) which could assess GRS and their impact in a consistent manner to be used in for the purpose of clinical trials as well as in clinical practice.
In an initial study, IBS patients and subjects from GPop complaining of GRS were interviewed simultaneously in 3 countries (UK, France, and Spain) and a conceptual framework for the pilot version of IGQ, measuring both GRS and their impact on daily life, was created. Similar concepts were identified for both IBS patients and GPop subjects. This 43‐item pilot IGQ consisted of a 24‐hour recall symptom diary assessing 7 GRS using 17 items and a 7‐day recall questionnaire assessing the impact of those symptoms on daily life, using 26 items. The 7 GRS were as follows: abdominal distension, abdominal pressure/feeling bloated, flatulence, belching, bad breath, stomach rumbling, and difficult gas evacuation. 19
The aim of the current work was to perform a psychometric validation and to develop a shorter IGQ version with the selection of final items and factor structure. This aim was carried out in samples of GPop and IBS patients in UK, France, and Spain.
2. METHODS
2.1. Participants
Subjects from GPop and IBS patients according to Rome IV, 13 complaining of GRS were included in the study. Two questionnaires were used for the selection of participants. First, a simple symptom screening tool (SST), which assessed the frequency of four GRS (bloating, flatulence, belching, and stomach rumbling) in the past month (from 0 = never to 5 = every day), with a global score ranging from 0 to 20. 19 Second, the IBS‐SSS questionnaire 20 which measured intensity and frequency of abdominal pain, bloating/distension, dissatisfaction with bowel habit, and interference on daily life (score range: 0‐500). IBS patients had to have a score of 4 or greater for at least one symptom on the SST; and an IBS‐SSS between 75 and 300 indicating mild to moderate IBS severity. GPop subjects did not fulfill IBS Rome IV criteria, had a score of 4 or greater for at least one symptom on the SST and had a regular stool frequency (ie, 3‐21 bowel movements per week).
Exclusion criteria for all participants were as follows: (1) Recent (last 2 weeks) change in diet or intake of potentially flatulogenic compounds (fiber, lactulose); (2) Organic gastrointestinal disease; (3) Other functional gastrointestinal disorder defined by Rome IV criteria, especially functional dyspepsia; (4) Any severe and progressive disease (eg, depression, cancer, uncontrolled diabetes, and rheumatoid arthritis); (5) Any severe psychiatric disorder (eg, acute episode of schizophrenia or bipolar disorder). An additional exclusion criterion for GPop subjects was any treatment for diarrhea or constipation. All participants were required to have cognitive and linguistic ability to complete several self‐administration questionnaires, and a BMI > 18.5 and <30.0 kg/m2.
Patients were offered to participate in the study by their attending physicians in 3 centers (Wythenshawe Hospital, Neurogastroenterology Unit, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK; University Hospital Vall d’Hebron, Digestive System Research Unit, Barcelona, Spain; AP‐HP, Louis‐Mourier Hospital, Gastroenterology Department, France). Participants from the GPop were recruited by public advertisement in the medical centers and by proxy.
2.2. Study design and procedure
This study had a cross‐sectional part for all participants and a longitudinal test‐retest component for a subset of them (30%). All participants completed paper version questionnaires: IGQ, specific quality of life FDDQL, and generic health status EQ‐5D. Participants in the test‐retest component completed at first visit the IGQ in paper or electronic version and paper version of the FDDQL and the EQ‐5D. At the second visit (one week later ± 2 days), investigators checked if participants had any new medication since last visit, and participants completed IGQ in electronic or paper, the IBS‐SSS questionnaire (for IBS patients) and global Gastrointestinal (GI) Well‐Being scale in paper. The order of administration of IGQ in paper and electronic at both visits was randomized.
2.3. Measures
Six questionnaires were used for the study as follows: pilot IGQ, IBS‐SSS, SST, FDDQL, EQ‐5D, and global gastrointestinal well‐being scale. IBS‐SSS and SST were used as inclusion criteria and for known‐group validity of IGQ. FDDQL and EQ‐5D were used for convergent/divergent validity. Global GI Well‐Being scale classified participants as unchanged, worse or improved to assess test‐retest and paper‐electronic reliability among unchanged participants.
2.3.1. IGQ
IGQ results from a previous qualitative research analysis. 19 The 43‐item pilot IGQ consists of a 24‐hour recall symptom diary assessing 7 GRS (17 items) and a 7‐day recall questionnaire which assesses the impact of those symptoms on 12 domains (26 items). The 7 GRS are as follows: abdominal distension, abdominal pressure/feeling bloated, flatulence, belching, bad breath, stomach rumbling, and difficult gas evacuation. The 12 impact domains are as follows: clothing, emotional, diet, cognitive function, physical appearance, work, sexual life, physical activity, social life, sleep, activities of daily living, and partner relationship. 19 Answer options are 0‐10 numerical and Likert scales.
2.3.2. IBS‐SSS
IBS‐Severity Scoring System (IBS‐SSS) consists of 4 VAS measuring abdominal pain intensity and frequency during the last 10 days, bloating, dissatisfaction with bowel habit, and interference with life. The maximum score is 500, 20 it is used to classify IBS symptoms as mild (<75), moderate (75‐299), and severe ≥ 300.
2.3.3. SST
The Symptom Screening Tool (SST) assesses the frequency of 4 GRS (bloating, flatulence, belching, and stomach rumbling) in the past month (from 0 = never to 5 = every day), with a global score ranging from 0 to 20 (worse symptoms) (19, adapted from 17 to 31).
2.3.4. FDDQL
The Functional Digestive Disorders Quality of Life questionnaire (FDDQL) assesses specifically the quality of life questionnaire validated for IBS and Functional Dyspepsia with 43 items with a 2‐week recall period and 8 domains. The 8 domains are as follows: daily activities, anxiety, diet, sleep, discomfort, health perception, control of disease, and impact of stress. Domains and global scores range from 0 to 100 (best quality of life). 6
2.3.5. EQ‐5D
The EuroQol EQ‐5D‐5L generic health index, comprises 5 items assessing mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression on 5‐point scale and a 100‐point visual analog scale (100 being best health status). 21 EQ‐5D index value from 0 to 1 (best health status) has been calculated using UK reference norms.
2.3.6. Global GI Well‐Being scale
The global GI Well‐Being scale is a single item assessing the overall change of GRS on a 3‐point Likert scale (improved, unchanged, worse) (adapted from 21).
2.4. Statistical methods
2.4.1. Factor structure
Exploratory Factor Analysis (EFA) was used to assess the dimensionality of IGQ. The factorability of the sample intercorrelation matrix was assessed using Bartlett’s test of sphericity and the Kaiser‐Meyer‐Olkin criteria. Factors extraction relied on the principal component‐based method with varimax rotation to help factor interpretation. The number of factors was based on a compromise between Kaiser’s rule (eigenvalue above 1) and parallel analysis (analysis of scree plots of randomized data matrices). Items loading higher than 0.4 on a given factor were considered as reliable indicator of that latent trait, unless cross‐loadings occurred in which case a decision based on item content has been made on whether an item should be kept or not. For factorial analyses, the 12 items with 0‐10 option scale were recorded to a 5‐point scale to be consistent with other items and answers “I did not have…,” “my tummy did not…,” and “not applicable” were recoded as “not at all.”
2.4.2. Item reduction
Distribution of item responses in each category, mean ± SD, cumulated proportions for the 2 extreme response categories, inter‐item correlations and item‐scale correlation were used to identify items with (1) floor or ceiling effect above 60%, (2) high response variance, (3) high inter‐item correlation ≥0.75, (4) low factor loading and/or cross‐loading, and (5) issue in item‐scale correlation (low correlation with its own scale <0.4 or correlation >0.4 with another scale). Thresholds were lowered for deletion if an item cumulated issues on several properties. The item reduction process took account also relevance of items to the dimensions they purported to capture. If a pair of highly similar items satisfied retention criteria, the item deemed to be less conceptually relevant was deleted. A confirmatory factor analysis was performed on the final items retained. 22
2.4.3. Scale consistency
Internal consistency was calculated on item scores of each dimension using Cronbach’s alpha (with 95% bootstrap CI), 23 in conjunction with multi‐trait scaling analysis from which scale‐specific scaling success (coherence of inter‐item correlation within and between scales) is derived. 24 Basically, for each item its Pearson’s correlation with all other items was computed: for every pair of items belonging to the same scale, a correlation higher than 0.30 was expected while correlation between items from different scales should be lower than 0.30. Correlation coefficients were tested at a 5% FWER (family‐wise error rate)‐corrected level, using Bonferroni’s method.
2.4.4. Reliability of the measurement instrument
For each scale, the standard error of measurement (SEM) has been determined based on Cronbach’s alpha and standard deviation (SD), following the relation SEM = SD × √(1‐R), where R is the reliability defined as the value of Cronbach’s alpha. 25
2.4.5. Recoding of items and scoring
For construction of scores, Item scores were recoded to 0‐100 (worse symptom/impact). Dimension scores were computed by the mean of sum of item scores, and a global score as the mean of sum of dimension scores.
2.4.6. Convergent/divergent validity
Convergent/divergent validity was assessed using FDDQL, as a specific measure of health‐related quality of life related to functional digestive disorders, and EQ‐5D questionnaire, as a generic measure of health status. Linear correlations between scale scores were computed to evaluate the degree to which domains of those questionnaires matched or not those in the newly developed IGQ questionnaire. It was expected that correlation will be higher with FDDQL and especially it Discomfort dimension, than with EQ‐5D.
2.4.7. Discriminant validity
Different proxies were used to check the capacity of IGQ scores to discriminate sub‐groups according to (1) gender, (2) IBS patients vs. general population, (3) IBS subtypes, (4) severity of IBS based on IBS‐SSS, and (5) Frequency of GRS based on SST. Standard parametric tests and Pearson’s correlation were used, with a 5% alpha level.
2.4.8. Test‐retest reliability and validation of IGQ electronic version
A subset of 90 participants was used to validate the IGQ electronic form and to assess the temporal stability of scores at an interval of one week. Participants during the 2nd IGQ completion were classified as unchanged, improved, or worse, according to the global GI Well‐Being rating of change scale. Test‐retest reliability and equivalence of the electronic vs paper version were assessed among unchanged participants using intraclass correlation coefficient (ICC) and Wilcoxon signed‐rank test for paired samples for comparison of scores. The ICC ranges from 0 to 1 with a recommended minimal level of 0.70 for group comparison. 26 The remaining participants (ie, improved or worse) were used as a preliminary study of the responsiveness of IGQ.
2.5. Sample size and randomization scheme
It was estimated that a total of 300 participants were required as factorial analysis was used to uncover the factor structure of a newly developed questionnaire in three countries with (1) a ratio 0.6:0.4 of participants with IBS diagnosis and general population; (2) 100 participants by country to ensure reliable item statistics; and (3) including a test‐retest study with validation of the electronic version. This corresponds to a subjects‐to‐variables ratio (STV) above 10 and it offers the best compromise in terms of economic cost (subject recruitment, questionnaire administration, debriefing) and statistical efficiency. 27
A subset of 90 was required to complete two IGQ within a mean 7‐day interval for test‐retest and validation of electronic vs. paper version. The order of IGQ administration in paper vs. electronic was randomized. A block size of 6 randomization list, stratified by center, was generated to allocate participants to one of the two sequence order (A = electronic first or B = paper first). Ninety participants allowed for at least 36 participants to report being unchanged on the global GI Well‐Being scale. Considering a theoretical reliability of 0.8, a sample size of 36 individuals allowed to verify if the ICC is greater than 0.7 with 95% confidence. 28 , 29
All analyses were done using the open‐source R 3.6.3 statistical software. 30
2.6. Regulatory and ethical requirements of the study
The IGQ validation study, adding only patient‐reported questionnaires (PRO) and clinician‐reported questionnaires, was classified as an observational study. Approval from Ethics Committees has been obtained in the 3 countries: North West—Greater Manchester West, n°17/NW/0004; Comité Etico de investigación clínica del hospital universitari Vall d’Hebron, Barcelona, n°PR(AG)340/2016; CPP IdF IV, Paris, n°2016/42NI. According to each national law for this type of study, a written consent was required for Spain and UK and a “no opposition” for France. The study has been registered on clinicaltrials.gov (NCT03002584).
3. RESULTS
3.1. Participant demographics
A total of 305 participants (186 IBS patients and 119 GPop subjects) were recruited by the 3 countries from February 2017 to April 2018 (Table 1). Mean age was 42 ± 14 years, and 69% were women. There was no difference between IBS and GPop except for sex ratio, with more women among IBS group (87% IBS‐C, 61% IBS‐D, 77% IBS‐M) (Table 1). Fifty‐six percent had a university education, 70% were working full or part time, and 48% were living as a family.
TABLE 1.
Participant demographic and IBS and GRS characteristics
| Results by population | Results by country | |||||
|---|---|---|---|---|---|---|
| Results expressed as mean ± SD (Min‐Max) or % | All (305) | General population (GPop) (119) | IBS (186) | UK (105) 39 GP/66 IBS | FR (100) 41 GP/59 IBS | SP (100) 39 GP/61 IBS |
| Women | 68.9% | 58.8% | 75.3% | 72.4% | 64% | 70% |
| Age (years) | 41.7 ± 13.6 (18‐74) | 44.1 ± 14 (19‐73) | 40.1 ± 13.1 (18‐74) | 39.7 ± 13.7 (19‐74) | 40.5 ± 13.3 (19 ‐72) | 44.9 ± 13.3 (18‐73) |
| BMI (score) | 24.2 ± 3.2 (16.8‐29.9) | 24.8 ± 3.1 (16.8a‐29.8) | 23.8 ± 3.1 (18.5‐29.9) | 24.6 ± 3 (18.9‐29.9) | 23.5 ± 3.4 (16.8‐29.8) | 24.5 ± 3.1 (18.7‐29.9) |
| Duration of GRS (years) | 8.4 ± 8.3 (0.2‐50)b | 8.3 ± 8.3 (0.2‐50) | 8.4 ± 8.3 (0.5‐50) | 10.6 ± 9.4 (1‐50) | 6.9 ± 7.6 (0.2‐50) | 7.5 ± 7.2 (0.5‐35) |
| Duration of IBS (years) | 9.3 ± 8.6 (0.5‐50) | 12.4 ± 9.9 (2‐50) | 7.3 ± 6.7 (1‐30) | 7.9 ± 7.8 (0.5‐35) | ||
| IBS Subtype | ||||||
| IBS‐C | 68 (36.6%) | 26 (39.4%) | 20 (33.9%) | 22 (36.1%) | ||
| IBS‐D | 61 (32.8%) | 19 (28.8%) | 23 (39.0%) | 19 (31.1%) | ||
| IBS‐M | 57 (30.6%) | 21 (31.8%) | 16 (27.1%) | 20 (32.8%) | ||
| IBS‐SSS (score) for IBS | 251.9 ± 41.2 (138‐303)d | 261.7 ± 40.2 (140‐300) | 248.8 ± 37.5 (138‐303)d | 244.3 ± 44.1 (146‐302)d | ||
| Number of bowel movements per week | ||||||
| IBS | 10.5 ± 9 (1‐50) | 12.1 ± 11.6 (1‐50) | 10 ± 7.5 (1‐42) | 9.3 ± 6.5 (1‐30) | ||
| GP | 8.9 ± 4.6 (3‐21) | 9 ± 4.5 (3‐21) | 9.7 ± 5.3 (3‐21) | 8.1 ± 3.9 (3‐21) | ||
| SST (score) | 14.4 ± 3.4 (5‐20) | 13.8 ± 3.5 (5‐20) | 14.9 ± 3.2 (6‐20) | 14.2 ± 3.2 (6‐20) | 15.1 ± 3.6 (6‐20) | 14 ± 3.3 (5‐20) |
| FDDQL (Global score) | 50.8 ± 16.9 (12.6‐92.9) | 59.3 ± 18.7 (12.6‐92.9) | 45.4 ± 13 (15.6‐77.1) | 57.4 ± 18.6 (19.2‐92.6) | 43.9 ± 14.4 (12.6‐92.9) | 50.9 ± 14.4 (21.3‐79.7) |
| EQ‐5D Index | 0.75 ± 0.20 (0.1‐1)c | 0.80 ± 0.20 (0.12‐1) | 0.72 ± 0.19 (0.12‐1) | 0.81 ± 0.16 (0.25‐1) | 0.62 ± 0.23 (0.12‐1) | 0.81 ± 0.14 (0.33‐1) |
Abbreviations: BMI, Body Mass Index; FDDQL, Functional Digestive Disorders Quality of Life; GRS, Gas‐Related Symptoms; IBS, Irritable Bowel Syndrome; IBS‐SSS, Irritable Bowel Syndrome Severity Scoring System; SST, Symptom Screening Tool.
1 subject had a BMI inferior to the threshold 18.5 requested by the protocol.
n = 304.
n = 303.
2 patients had a IBS‐SSS just over 300.
3.2. Description of IBS and GRS characteristics
Mean duration of IBS was 9.3 ± 8.6 years with a mean IBS‐SSS of 252 ± 41. Mean presence of GRS was 8.4 ± 8.3 years. The FDDQL global score was lower (worse) among IBS patients (45.4 ± 13.0) compared to the GPop group (59.3 ± 18.7), and the mean SST score was higher (worse) among IBS (14.9 ± 3.2) compared to GPop (13.8 ± 3.5) (Table 1).
The mean SST score was higher among IBS compared to GPop for 3 symptoms: bloating (4.2 ± 1.0 vs 3.6 ± 1.5), excessive flatulence (4.3 ± 0.8 vs 4.1 ± 1.1), and rumbling stomach (3.7 ± 1.3 vs 3.0 ± 1.9). GPop had a higher score for belching (3.0 ± 1.9 vs 2.6 ± 1.9). On the total sample, belching had the lowest mean score (ie, less frequent) compared to the 3 other SST symptoms. A similar proportion of GPop subjects and IBS patients reported having at least one episode of excessive flatulence during the last month, that is, over 98%. Fewer GPop subjects reported at least one episode of bloating (93.3% vs 98.4%), and of rumbling stomach (85.7% vs 94.1%). A higher proportion of GPop subjects reported at least one episode of belching during the last month (81.5% vs 76.3%).
Based on the initial pool of IGQ symptom items, the proportion of GPop subjects not reporting bloating, abdominal distension, or stomach rumbling over the last 24 h was higher compared to IBS patients, the largest difference being for difficult gas evacuation (50.9% GPop vs 19.9%). A similar proportion of GPop subjects and IBS patients reported no bad breath or belching episode over the last 24 h.
There were only 6 IBS patients (3.2%) with a possible history of post‐infectious IBS. IBS patients were regular consulters for their IBS symptoms by more than 52%. In contrast, more than 71% of GPop subjects were not regular consulters for their GRS symptoms.
3.2.1. Treatment
IBS patients were taking prescribed or OTC gastrointestinal treatments (more than 2 weeks before inclusion) more often than GPop subjects: antispasmodic (18.3% vs 3.4%), antidiarrheal (5.4% vs 0%), laxative (15.1% vs 0%), bloating remedies (3.2% vs 0%), anti‐flatulents (3.2% vs 0.8%), PPI or H2 blocker (11.8% vs 8.4%). A similar proportion of participants was taking antacids: 2.2% (IBS) vs 2.5% (GPop).
3.2.2. Co‐morbidities
Forty‐seven (39.5%) GPop subjects had 1 or more co‐morbidities with a total of 67 co‐morbidities and 55 (29.6%) IBS patients had a total of 72 co‐morbidities. Mood disorder (16.5%), hypertension (15.8%), rheumatologic conditions (eg, osteoarthritis) (10.1%), lung disease (eg, asthma, COPD) (7.9%), and controlled diabetes (5.8%) were the most frequent reported co‐morbidities in the whole group. While IBS patients reported more frequent mood disorder, GPop subjects reported more frequent hypertension and accounted for all diabetes cases.
3.2.3. Single completion/test‐retest
Among the 305 participants, 215 completed the questionnaires once (131 IBS, 84 GPop) and 90 (55 IBS, 35 GPop) agreed to participate in test‐retest one week apart with a very similar distribution (GPop, IBS, and IBS subtypes) across the 3 countries (29 UK, 30 FR, 31 SP).
3.3. Number of participants analyzed, missing data, recoding of items
The rate of missing data on the IGQ was 0.16%: 7 participants among 305 had 1 or more IGQ missing answers (2 participants had 7 and 8 missing answers) at single or first completion, resulting in 21 missing answers. Missing data concerned 14 items of the IGQ questionnaire, but no one exceeded 2 missing answers. Thus, 302 to 305 participants were analyzed depending of items included in the factorial analyses and in correlation computation.
3.4. Item analyses
The first factor analysis (FA) (n = 304) performed on 43‐item IGQ pilot questionnaire yielded a clear 6‐factor structure, the first 2 factors being, respectively, impact of bloating (Eigenvalue: 14.18) and bloating symptom (4.74), the 3rd gathering difficult gas evacuation and stomach rumbling (2.96), and the 3 last each capturing a single symptom, that is, bad breath (2.76), flatulence (2.52), and belching (2.10). Other findings were that (1) Abdominal pressure/feeling bloated and subjective abdominal distension (“looking big”) did not project on different factors; (2) Items of a given symptom and items about its impact projected on the same factor.
The factor structure yielded few cross‐loadings and factor loading was always superior for the expected factor than for another. There was only one item (n°I18 flatulence impact: “did you avoid certain food or drinks to avoid getting wind?”) which did not project on its factor but on the “bloating impact” factor (factor loading = 0.42). Bloating items tended to project on 2 factors among IBS patients, but globally the factor structure was similar among GPop and IBS. Although globally the structure remained robust and similar across the 3 countries, the structure in the Spanish sample was slightly less clear.
3.5. Item reduction
Following a meeting of the scientific committee (OC, MD, NP, BC, FA, PW) to which data were submitted, 23 items (10 symptom and 14 impact items) were deleted for one or several reasons: high floor effect (n = 9), high inter‐item correlation over 0.74 (n = 11), low factor loading and/or cross‐loading (n = 3), issue in the item‐scale correlation (low correlation with its own scale or correlation over 0.4 with another scale) (n = 3), issue in the content validity (relevance, importance or wording of the concept, or preference between 2 items similar in concept) (n = 15). The final FA (n = 305) on the 17 remaining items (7 symptom and 10 impact items) yielded a clear 6‐factor structure explaining 67% of the variance with, respectively, bloating (BL, 6 items), flatulence (FL, 3), belching (BE, 2), bad breath (BB, 2), stomach rumbling (SR, 2), and difficult gas evacuation (DGE, 2) (Table 2).
TABLE 2.
Item statistics aggregated by dimension for the reduced IGQ questionnaire
| Total population (n = 305) | Eigenvalue | Cumulative variance | Factor loading | Item correlation within scale | Item correlation between scales | Cronbach’s alpha (95% CI) | SEM |
|---|---|---|---|---|---|---|---|
| Bloating (6 items) | 3.33 | 0.20 | 0.51‐0.87 | 0.31‐0.70 | 0.02‐0.35 | 0.85 (0.82 ; 0.88) | |
| Flatulence (3) | 1.86 | 0.31 | 0.58‐0.85 | 0.43‐0.63 | 0.02‐0.33 | 0.76 (0.71 ; 0.81) | |
| Belching (2) | 1.65 | 0.40 | 0.71‐0.99 | 0.66 | 0.05‐0.26 | 0.80 (0.75 ; 0.84) | |
| Bad breath (2) | 1.61 | 0.50 | 0.76‐0.96 | 0.67 | 0.02‐0.27 | 0.81 (0.76 ; 0.86) | |
| Stomach rumbling (2) | 1.57 | 0.59 | 0.63‐0.96 | 0.63 | 0.08‐0.32 | 0.78 (0.72 ; 0.83) | |
| Difficult gas evacuation (2) | 1.30 | 0.67 | 0.67‐0.78 | 0.55 | 0.02‐0.35 | 0.71 (0.63 ; 0.79) | |
| All 17 items | 0.84 (0.81 ; 0.87) | 6.44 | |||||
| All items (except bloating) (11) | 0.77 (0.72 ; 0.81) | 6.16 |
Confidence interval for Cronbach’s alpha computed by bootstrap. SEM: The standard error of measurement (SEM) has been determined based on Cronbach’s alpha and standard deviation (S), following the relation SEM = S√(1‐R), where R is the reliability defined as the value of Cronbach’s alpha.
Abbreviation: IGQ, Intestinal Gas Questionnaire.
3.6. Confirmatory factor analysis
A confirmatory FA was performed on 302 participants with no missing data (whole sample, GPop, IBS, and 3 countries). Comparative Fit Index (CFI) was over 0.9 and Root Mean Square Error of Approximation (SRMR) and Standardized Root Mean Residual (RMSEA) were lower than 0.08 for most models indicating good‐fit statistics (Table 3).
TABLE 3.
Confirmatory analysis of the 17 IGQ items retained
| Whole sample | GPop | IBS | UK | FR | SP | |
|---|---|---|---|---|---|---|
| Comparative Fit Index (CFI) | 0.923 | 0.918 | 0.913 | 0.940 | 0.905 | 0.879 |
| RMSEA | 0.068 | 0.075 | 0.067 | 0.064 | 0.073 | 0.092 |
| SRMR | 0.053 | 0.053 | 0.070 | 0.068 | 0.084 | 0.068 |
Parameters for good fit of models. Cut‐off for good fit, Comparative Fit Index (CFI ≥ 0.9), Root Mean Square Error of Approximation (RMSEA < 0.08), Standardized Root Mean Square Residual (SRMR < 0.08).
Abbreviations: GPop, General Population; IBS, Irritable Bowel Syndrome.
3.7. Inter‐item correlation within and between scales
Inter‐item Pearson’s correlation levels between the 17 remaining IGQ items ranged from 0.02 to 0.70 (Table 2). All items belonging to their respective dimensions were correlated over 0.30. Among the 136 possible pairs between the 17 IGQ items, 6 pairs of items not belonging to the same dimension correlated over 0.30 (maximum: 0.35). Thus, the scaling success was 96%.
3.8. Scale consistency
Cronbach’s alpha for IGQ dimensions ranged from 0.71 (2 items on difficult gas evacuation) to 0.85 (6 items on bloating). Cronbach’s alpha on all 17 items was 0.84. Logically Cronbach’s alpha of dimensions with few items (ie, 2 or 3) is slightly lower, but still over 0.7 (Table 2).
3.9. Reliability of the measurement instrument
If applying a one‐SEM value for defining the Minimal Important Difference (MID), then MID could be set at 6 for IGQ global score (Table 2).
3.10. Scoring
Score of each of the 6 GRS dimensions was computed as well as a global score. As bloating dimension was relatively independent from the other GRS dimensions, a global score except bloating dimension was also computed. Scores range from 0 to 100 (worst symptom or impact).
Correlation between IGQ dimension scores
All IGQ dimensions correlated with the global score from 0.54 to 0.67. The different dimensions (BL, FL, BE, BB, SR, DGE) were at most moderately correlated between them (ranging from 0.09 between BB and DGE to 0.37 between BL and DGE), confirming the relative independence of the 6 GRS symptom and impact dimensions of IGQ.
Distribution of scores
The mean IGQ scores range between 25.9 ± 25.6 (bad breath) and 50.0 ± 25.0 (flatulence), with a mean global score of 37.2 ± 15.9 (Table 4).
TABLE 4.
Distribution of IGQ scores among GP subjects and IBS patients
| Total (n = 305) | Total (n = 305)* | GPop (n = 119)** | IBS (n = 186)*** | p‐Valuea |
|---|---|---|---|---|
| Global score (GS) | 37.17 ± 15.87 | 33.47 ± 16.92 | 39.54 ± 14.72 | 0.0016 |
| Global score (except BL) | 35.60 ± 16.44 | 33.10 ± 17.54 | 37.20 ± 15.54 | 0.0385 |
| Bloating (BL) | 44.76 ± 23.91 | 35.17 ± 23.70 | 50.87 ± 22.02 | p < 0.0001 |
| Flatulence (FL) | 50.02 ± 24.98 | 45.67 ± 26.73 | 52.80 ± 23.43 | 0.018 |
| Belching (BE) | 32.85 ± 30.05 | 33.26 ± 31.54 | 32.58 ± 29.13 | 0.85 |
| Bad breath (BB) | 25.92 ± 25.61 | 28.34 ± 26.02 | 24.36 ± 25.29 | 0.19 |
| Stomach rumbling (SR) | 32.97 ± 26.39 | 28.80 ± 28.44 | 35.63 ± 24.71 | 0.0325 |
| Difficult gas evacuation (DGE) | 36.43 ± 25.87 | 29.41 ± 25.55 | 40.93 ± 25.12 | 0.0001 |
Scores range from 0‐100 (worst symptom or impact). Due to a few missing data, the number of patients analyzed varies depending of the dimension scores: *n = 302‐305, **n = 118‐119, ***n = 184‐186.
Abbreviations: GPop, General Population; IBS, Irritable Bowel Syndrome.
Comparison between GPop and IBS (t‐test).
Scores distribution across countries
IGQ scores were generally higher among French participants, except for SR and DGE. IGQ global score was 39.59 ± 15.48 in French sample compared to 36.03 ± 14.81 in UK and 35.97 ± 17.15 in Spain (p = 0.18). For most IGQ dimension scores, differences were not statistically significant across countries, except for BB: 30.98 ± 27.75 in French sample, compared to 24.83 ± 24.85 in UK, and 22.05 ± 23.55 in Spain (p = 0.041).
3.11. Convergent/divergent validity
Correlation between IGQ and specific FDDQL questionnaire
The highest correlation (r) levels were between IGQ bloating and global scores, with FDDQL Discomfort (DT) and global (GS) scores, r ranging from 0.57 to 0.75. Other moderate correlation of these 2 IGQ scores with FDDQL Diet (DI), Daily activities (DA), Anxiety (AN), and Sleep (SL) dimension scores ranged from 0.38 to 0.56. FDDQL Discomfort was the most correlated dimension with other IGQ dimensions (FL, r = 0.46; SR, r = 0.43; DGE, r = 0.44). The least correlated IGQ dimension with FDDQL was bad breath, with a correlation level not exceeding 0.22 (Table not shown).
Correlation between IGQ and generic EQ‐5D health status questionnaire
As expected, correlation levels were lower ranging from no (0.01) to moderate correlation (0.53) between IGQ and EQ‐5D. The highest correlation was between IGQ bloating and EQ‐5D Pain/Discomfort (r = 0.53). EQ‐5D Self‐care item was the least correlated with IGQ scores (r near 0 and sometimes negative). The highest correlation level between IGQ global score and EQ‐5D was with Pain/Discomfort (r = 0.43). Similar findings were noted with the VAS Health Status and for the Index, with a correlation at 0.39 between IGQ global score and EQ‐5D VAS health status and EQ‐5D Index (Table not shown).
3.12. Discriminant validity
Comparison of IGQ scores according to gender
Mean IGQ scores tended to be worse among women compared to men (BB, SR, DGE), the difference being statistically significant for bloating with a mean difference over 18 points (50.4 ± 22.2 vs. 32.2 ± 22.8, p < 0.0001). Accordingly, global score tended to be worse among women (38.4 ± 15.5 vs. 34.5 ± 16.4, p = 0.058) (Table not shown).
Comparison of IGQ scores between IBS patients and subjects from general population
Mean IGQ scores were statistically worse for 4 (BL, FL, SR, DGE) of the 6 GRS symptoms among IBS patients compared to GPop. The highest mean difference was over 15 points for bloating (50.9 ± 22.0 vs. 35.2 ± 23.7, p < 0.0001). The second largest difference over 11 points was with difficult gas evacuation (40.9 ± 25.1 vs. 29.4 ± 25.6, p = 0.0001). Similarly, IGQ global score was worse among IBS patients (39.5 ± 14.7 vs. 33.5 ± 16.9, p = 0.0016) (Table 4).
Comparison of IGQ scores among the 3 IBS subtypes
IGQ global scores were similar across the 3 IBS subtypes. However, BL score was statistically (p = 0.0195) higher (worse) among IBS‐C (56.1 ± 18.7) compared to IBS‐M (50.6 ± 23.2) and even more to IBS‐D (45.2 ± 23.2). IBS‐D patients tended to have higher scores for flatulence and stomach rumbling, and IBS‐M patients tended to have higher scores for belching and bad breath. Only comparison for bloating scores reached statistical significance (Table not shown).
Comparison of IGQ scores according to IBS‐SSS severity score among IBS patients
There was moderate and statistical (p < 0.0001) association between IBS‐SSS and IGQ global (r = 0.346) and stomach rumbling (r = 0.226) scores, that is, patients with higher severity score on the IBS‐SSS had higher IGQ scores. Correlation levels were lower although still significant between IBS‐SSS and bloating, flatulence, difficult gas evacuation, and bad breath scores. The lowest correlation was with belching (Table 5). All IGQ scores got worse (higher) across the 4 categories of IBS‐SSS score (138‐150, 151‐200, 201‐250, and >251), except for belching. Largest differences between the 2 extreme IBS‐SSS categories were with bloating (>27 points), difficult gas evacuation (>26), stomach rumbling (>24), and flatulence (>21) (Table 6). The mean IGQ global score across the 4 IBS‐SSS categories was, respectively, 23.97 ± 11.90 (n = 5), 30.91 ± 13.43 (n = 21), 36.26 ± 13.84 (n = 55), and 43.74 ± 13.99 (n = 105) (ANOVA, p < 0.0001), with a difference between the 2 extreme IBS‐SSS categories approaching 20 points.
TABLE 5.
Correlation levels between IGQ scores and IBS‐SSS and Symptom Screening Tool (SST)
| IBS‐SSS IBS patients (n = 186)* | SST Total sample (n = 305)** | |||
|---|---|---|---|---|
| IBS patients (n = 186)* | r | p‐Value | r | p‐Value |
| Global score (GS) | 0.346 | <0.0001 | 0.610 | <0.0001 |
| Global score (except BL) | 0.326 | <0.0001 | 0.587 | <0.0001 |
| Bloating (BL) | 0.249 | 0.001 | 0.402 | <0.0001 |
| Flatulence (FL) | 0.215 | 0.003 | 0.302 | <0.0001 |
| Belching (BE) | 0.116 | 0.116 | 0.541 | <0.0001 |
| Bad breath (BB) | 0.171 | 0.02 | 0.180 | 0.002 |
| Stomach rumbling (SR) | 0.266 | <0.0001 | 0.464 | <0.0001 |
| Difficult gas evacuation (DGE) | 0.246 | 0.001 | 0.298 | <0.0001 |
Due to a few missing items, some of the correlation values are calculated on *184 or 185 patients and **302 to 304 subjects.
Abbreviation: IBS‐SSS, Irritable Bowel Syndrome Severity Scoring System.
TABLE 6.
Comparison of scores according to 4 categories of IBS‐SSS severity score
| IBS‐SSS (n = 186) mean ± SD | [138‐150] (n = 5) | [151‐200] (n = 21) | [201‐250] (n = 55)* | [251‐303] (n = 105)** | ANOVA p value |
|---|---|---|---|---|---|
| Global score (GS) | 23.97 ± 11.90 | 30.91 ± 13.43 | 36.26 ± 13.84 | 43.74 ± 13.99 | <0.0001 |
| Global score (except BL) | 23.23 ± 12.19 | 28.75 ± 14.75 | 33.75 ± 14.84 | 41.41 ± 14.81 | 0.0001 |
| Bloating (BL) | 27.67 ± 17.73 | 41.67 ± 16.32 | 47.72 ± 22.74 | 55.44 ± 21.49 | 0.0016 |
| Flatulence (FL) | 34.67 ± 14.26 | 46.51 ± 22.44 | 51.06 ± 20.20 | 55.84 ± 25.01 | 0.086 |
| Belching (BE) | 34.50 ± 11.10 | 24.29 ± 26.89 | 29.14 ± 29.15 | 35.99 ± 29.88 | 0.27 |
| Bad breath (BB) | 9.50 ± 18.57 | 13.81 ± 20.38 | 23.91 ± 23.23 | 27.45 ± 26.88 | 0.072 |
| Stomach rumbling (SR) | 17.50 ± 19.20 | 22.86 ± 21.38 | 30.77 ± 25.13 | 41.60 ± 23.65 | 0.0007 |
| Difficult gas evacuation (DGE) | 20.00 ± 32.60 | 36.31 ± 24.34 | 33.86 ± 22.78 | 46.55 ± 24.79 | 0.0027 |
Due to a few missing items, some of the means are calculated on *54 and **104 patients.
Abbreviation: IBS‐SSS, Irritable Bowel Syndrome Severity Scoring System.
Comparison of IGQ scores according to the Symptom Screening Tool (SST) score
There was high association between most of IGQ scores (eg, 0.61 for IGQ global score) and the 4‐item SST. The lowest correlation was with bad breath (Table 5). All IGQ scores got statistically worse (higher) across the 3 categories of SST score (5‐10, 11‐15, and 16‐20). Largest differences between the 2 extreme SST categories were with belching (>36 points), stomach rumbling (>34), bloating (>25), and flatulence. 31 The mean IGQ global score across the 3 SST categories was, respectively, 22.04 ± 10.39 (n = 41), 33.43 ± 12.70 (n = 140), and 46.52 ± 15.00 (n = 124) (ANOVA, p < 0.0001), with a difference between the 2 extreme SST categories over 24 points. (Figure 1).
FIGURE 1.
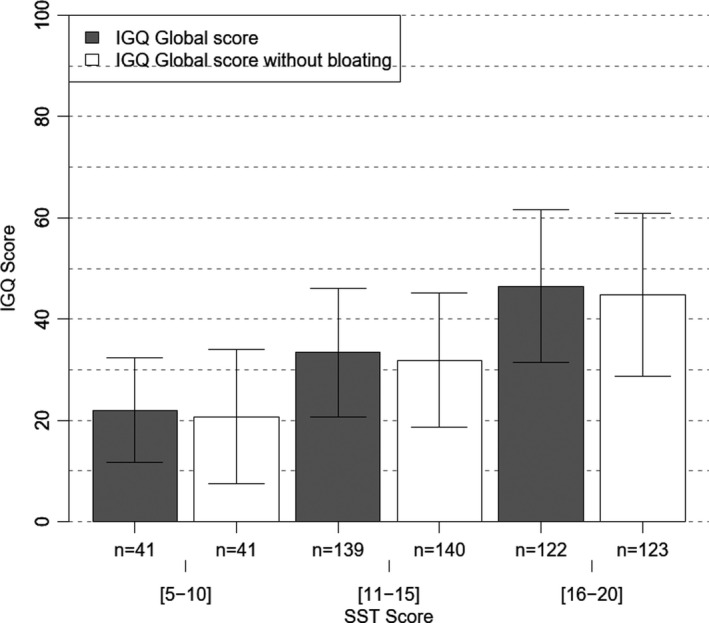
IGQ scores according to 3 categories of Symptom Screening Tool (SST) severity score
3.13. Test‐retest reliability & validation of electronic version
90 participants (55 IBS, 35 GPop) completed twice IGQ. 67 perceived themselves as “unchanged” on the global GI Well‐Being scale and their data were analyzed for the reliability testing of IGQ. 22 perceived as improved or worse and their data were analyzed for exploring responsiveness, and 1 subject did not complete the global GI Well‐Being scale. The mean interval between the 2 completions was 7.32 days ± 0.88 (SD) (min‐max: 6‐11). None of the IGQ scores differed statistically between V1 and V2 among the 67 “unchanged” participants, for example, mean IGQ global score was 34.84 ± 14.21 at V1 and 35.02 ± 15.26 at V2. ICC ranged from 0.71 to 0.86 across the different IGQ dimension scores and reached 0.85 (95% CI 0.78; 1) for the global score (Table 7).
TABLE 7.
Comparison of IGQ mean scores between V1 and V2 and Intraclass Correlation (ICC)
| “unchanged” subjects on Global GI Well‐Being scale (n = 67) | V1 mean ± SD | V2 mean ± SDa | p Valueb | ICC (95% CI)b |
|---|---|---|---|---|
| Global score (GS) | 34.84 ± 14.21 | 35.02 ± 15.26 | 0.989 | 0.85 (0.78 ; 1) |
| Global score (except BL) | 33.85 ± 15.14 | 33.99 ± 15.56 | 0.909 | 0.82 (0.75 ; 1) |
| Bloating (BL) | 39.80 ± 22.27 | 40.00 ± 20.94 | 0.746 | 0.86 (0.76 ; 1) |
| Flatulence (FL) | 47.64 ± 23.49 | 46.26 ± 25.02 | 0.728 | 0.79 (0.69 ; 1) |
| Belching (BE) | 33.36 ± 27.07 | 37.57 ± 27.90 | 0.498 | 0.80 (0.71 ; 1) |
| Bad breath (BB) | 20.67 ± 23.08 | 20.30 ± 21.48 | 0.651 | 0.82 (0.74 ; 1) |
| Stomach rumbling (SR) | 30.07 ± 25.24 | 31.08 ± 27.43 | 0.721 | 0.79 (0.68 ; 1) |
| Difficult gas evacuation (DGE) | 37.50 ± 22.93 | 36.94 ± 25.14 | 0.736 | 0.71 (0.51 ; 1) |
During the 2nd completion, there were FL missing items for 1 patient preventing to calculate the corresponding FL score and the Global scores.
Wilcoxon rank test for paired samples and ICC concerned 67 subjects except for FL, and Global scores where 66 subjects could be analyzed.
Sixty‐four “unchanged” participants were analyzed for the correlation between IGQ paper and electronic version (3 participants were not able to complete the IGQ electronic version, but completed instead paper version). None of the IGQ scores differed statistically between electronic and paper completion of IGQ, for example, mean IGQ global score was, respectively, 34.52 ± 14.35 and 34.07 ± 15.09. ICC ranged from 0.79 (SR) to 0.87 (BL), except for difficult gas evacuation with an ICC at 0.61. ICC of the global score was 0.82 (95% CI 0.71; 1).
3.14. Responsiveness
10 and 12 participants reported to be, respectively, improved and worse on the global GI Well‐Being scale 7 days after first completion of IGQ. IGQ global score was reduced by a median of 10.4 points (min‐max: ‐23.9‐2.6) among improved participants and was increased by 3.12 points (min‐max: 3.9‐8.3) among 12 participants who perceived themselves as worse.
4. DISCUSSION
Our study indicates that IGQ is a robust instrument for capturing and measuring GRS and their impact on daily life; the final 17‐item questionnaire, available in paper and electronic version, has good psychometric properties. The pilot IGQ questionnaire with 43 items covering 7 symptoms and their impact on various aspects of daily life 19 was reduced to 17 items covering 6 dimensions. Each dimension contains items about one GRS, over a 24‐hour recall period, and its impact on daily life over a 7‐day recall period. The similar structure yielded by factorial analysis confirms that IGQ is valid for both the GPop and IBS, and for English, French, and Spanish cultures.
There were sound correlation levels between the concepts measured by IGQ and those measured by the specific FDDQL and the generic EQ‐5D questionnaires, confirming the convergent/divergent validity of IGQ. Moderate to high correlation was found between IGQ and FDDQL dimensions scores. The highest correlation was between IGQ bloating score and FDDQL Discomfort (DT) dimension score (r = 0.75) which is consistent with the content of their items.
The two most severe IGQ scores observed in our validation study were flatulence and bloating both in GPop and IBS. By comparison, Tielemans et al found that the 3 most frequent symptoms in a large survey of 16,758 questionnaires completed by Dutch adult general population were bloating (63%), borborygmi (60%), and flatulence (71%) 4 but they did not use a specific questionnaire for the assessment of gas‐related symptoms.
Discriminant capacity is supported by comparisons of IGQ scores according to several proxies. Mean IGQ scores were statistically worse among IBS patients compared to GPop for 4 of the 6 GRS symptoms (bloating, flatulence, stomach rumbling, and difficult gas evacuation). This appears consistent as these symptoms are frequently associated with IBS or altered bowel movements 32 while belching and bad breath are not specific of IBS and are experienced commonly in the general population. 33 Considering IBS subtypes, bloating score was statistically worse among IBS‐C compared to IBS‐M or IBS‐D. This higher prevalence of bloating among IBS‐C and IBS‐M patients over IBS‐D has been previously reported. 1 , 34 Indeed, bloating can be the most prevalent bothersome symptom in IBS‐C. 35 All IGQ scores, except belching, got worse (higher) across the 4 categories of IBS‐SSS score. Furthermore, there is a higher association between most of the IGQ scores (up to 0.61 for IGQ global score) and the 4‐item SST. The largest differences between the 2 extreme SST categories were observed in the 4 symptoms measured by both questionnaires (ie, bloating, flatulence, belching, and stomach rumbling). Interestingly, mean IGQ scores tend to be worse among women compared to men. The literature confirms that in a variety of situations this problem is perceived as worse and affects quality of life more in women. 4 , 36 , 37
The reliability of IGQ over time and the equivalence of the electronic and paper versions is supported by the high ICC values that are over the recommended threshold. 26
According to ROME IV criteria, functional abdominal bloating and distension (FABD) is now defined as a subjective feeling of increased abdominal fullness/pressure associated or not with a measurable increase in abdominal girth defined as distension. 38 , 39 We acknowledge that our study did not confirm the subjective perception of GRS in general and particularly abdominal distension by an objective marker. Indeed, several studies have suggested different underlying mechanisms for feeling bloated vs. objective abdominal distension. However, the techniques used to quantify the distension such as CT scan are not part of routine and would have not been appropriate or feasible in our study. Moreover, the correlation between intra‐abdominal gas contents and bloating perception is repeatedly reported as poor. 15 , 16 Only in IBS‐C does the severity of abdominal bloating seem to correlate with the degree of abdominal distension, suggesting that the pathophysiology is likely to be different between subtypes of bowel habit. 40
During the test‐retest, and while subjects were globally considered to be unchanged on the GI Well‐Being scale at the second visit compared to the first, the ICC did not reach 0.80 for some of the IGQ dimension scores (ie, flatulence, stomach rumbling, and difficult gas evacuation). One explanation is that the day‐to‐day fluctuation of these GRS can be affected by even very small changes in the diet 41 and the large intra‐ and inter‐variability among subjects. 42 A shorter interval between the two completions, such as one day, would possibly have yielded higher correlation levels for these highly fluctuating symptoms. However, ICC of global score is high at 0.85, and a shorter interval than 7 days would have been inconsistent with the 7‐day recall period used for capturing the impact of symptoms on daily life.
Further studies are needed to confirm the ability of IGQ to detect changes in response to different interventions such as diet or probiotics in the general population. However, these preliminary results suggest that IGQ is sensitive to change over time as IGQ global score gets better (lower by a median of 10 points) among subjects reporting being improved on the global GI Well‐Being scale. This change may be put in perspective with the minimal important difference (MID) calculated for the global score based on the one‐SEM value (ie, around 6). A one‐SEM criterion is among the recommended approaches for MID. 43 , 44 A change higher than one SEM is likely to reflect a true change in individual status rather than a measurement error.
5. CONCLUSION
This study confirms the excellent psychometric properties of a new measure of GRS, in terms of validity and reliability which are in line with FDA recommendations. 45 The IGQ may be a useful tool in surveys, looking at the prevalence of digestive symptoms in different sets of the population and in clinical trials to assess the efficacy of treatments or nutriments aimed at relieving gas‐related symptoms.
CONFLICT OF INTEREST
The university research team of MD and OC received funding from Danone Research to perform the study. Institutions of PW, FA, and BC received funding from the research team as investigator center. Others do not report conflict of interest within this study.
AUTHOR CONTRIBUTION
OC, MD, PW, and FA contributed to the study design. SA, BL, DA, JS, BC, FA, and PW recruited participants and collected data. FT, OC, and PB monitored and analyzed the data. OC, MD, NP, BC, FA, and PW were members of the scientific committee. As such, they took decisions in the reduction of items process. OC and MD wrote the first draft version of the manuscript. All other authors reviewed and approved the final version.
USER AGREEMENTS FOR USE OF QUESTIONNAIRES
User agreements for use of questionnaires were obtained: EQ‐5D (January 23, 2017), IBS‐SSS (December 28, 2016). No need for user agreement for the ROME criteria (email of April 14, 2017), the SST and global GI well‐being (no owner) and for the FDDQL (own by Olivier Chassany).
ACKNOWLEDGEMENTS
The authors thank all the patients and subjects who participated in this study.
We thank Mrs Doryssemma Tchatat, Clinical study technician at Hôpital Louis‐Mourier (Colombes, France).
Duracinsky M, Archbold S, Lobo B, et al. The Intestinal Gas Questionnaire (IGQ): Psychometric validation of a new instrument for measuring gas‐related symptoms and their impact on daily life among general population and irritable bowel syndrome. Neurogastroenterology & Motility. 2022;34:e14202. 10.1111/nmo.14202
Funding information
This Investigator Sponsored Study has been funded by Danone Research. Danone did not interfere with the analysis and interpretation of data. The work was supported in part by the Spanish Ministry of Economy and Competitiveness (Dirección General de Investigación Científica y Técnica, SAF 2016‐76648‐R). Ciberehd is funded by the Instituto de Salud Carlos III
References
- 1. Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastorenterol Hepatol. 2009;7:68–72. [DOI] [PubMed] [Google Scholar]
- 2. Houghton LA, Whorwell PJ. Towards a better understanding of abdominal bloating and distension in functional gastrointestinal disorders. Neurogastroenterol Motil. 2005;17(4):500–511. [DOI] [PubMed] [Google Scholar]
- 3. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. [DOI] [PubMed] [Google Scholar]
- 4. Tielemans MM, Jaspers Focks J, van Rossum LG , Eikendal T, Jansen JB, Laheij RJ, van Oijen MG . Gastrointestinal symptoms are still prevalent and negatively impact health‐related quality of life: a large cross‐sectional population based study in The Netherlands. PLoS One. 2013;8:e69876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tuteja AK, Talley NJ, Joos SK, Tolman KG, Hickam DH. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol. 2008;103(5):1241–1248. [DOI] [PubMed] [Google Scholar]
- 6. Chassany O, Marquis P, Scherrer B, Read NW, Finger T, Bergmann JF, Fraitag B, Geneve J, Caulin C. Validation of a specific quality of life questionnaire in functional digestive disorders (FDDQL). Gut. 1999;44:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kinberg S, Stein M, Zion N, Shaoul R. The gastrointestinal aspects of halitosis. Can J Gastroenterol. 2010;24(9):552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Jongh A , van Wijk AJ , Horstman M, de Baat C . Self‐perceived halitosis influences social interactions. BMC Oral Health. 2016;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright‐McNaughton M, Ten Bokkel Huinink S, Frampton CMA, McCombie AM, Talley NJ, Skidmore PML, Gearry RB. Measuring diet intake and gastrointestinal symptoms in irritable bowel syndrome: Validation of the food and symptom times diary. Clin Transl Gastroenterol. 2019;10(12):e00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holmes BA, Habi‐Rachedi F, Trotin B, Paineau D, Guyonnet D, Rondeau P, Flourié B, Whelan K. Dietary patterns, digestive symptoms, and health‐related quality of life in women reporting minor digestive symptoms. Nutrition. 2017;35:132–138. [DOI] [PubMed] [Google Scholar]
- 11. Levitt MD, Furne J, Olsson S. The relation of passage of gas and abdominal bloating to colonic gas production. Ann Intern Med. 1996;124:422–444. [DOI] [PubMed] [Google Scholar]
- 12. Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–1392. [DOI] [PubMed] [Google Scholar]
- 13. Drossman DA, Hasler WL. Rome IV‐functional GI disorders: Disorders of Gut‐brain interaction. Gastroenterology. 2016;150(6):1257–1261. [DOI] [PubMed] [Google Scholar]
- 14. Obekli T, Akyuz F, Akyuz U, Arici S, İliaz R, Gokturk S, Evirgen S, Cavus B, Karaca C, Demir K, Besisik F, Kaymakoglu S. Belching in irritable bowel syndrome: An impedance study. J Neurogastroenterol Motil. 2017;23(3):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Accarino A, Perez F, Azpiroz F, Quiroga S, Malagelada J. Abdominal distention results from caudo‐ventral redistribution of contents. Gastroenterology. 2009;136(5):1544–1551. [DOI] [PubMed] [Google Scholar]
- 16. Seo AY, Kim N, Oh DH. Abdominal bloating: pathophysiology and treatment. J Neurogastroenterol Motil. 2013;19(4):433–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyonnet D, Schlumberger A, Mhamdi L, Jakob S, Chassany O. Fermented milk containing Bifidobacterium lactis DN‐173 010 improves gastrointestinal well‐being and digestive symptoms in women reporting minor digestive symptoms: a randomised, double‐blind, parallel, controlled study. British J Nutrition. 2009;102:1654–1662. [DOI] [PubMed] [Google Scholar]
- 18. Marteau P, Guyonnet D, Lafaye de Micheaux P, Gelu S. A randomized, double‐blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I‐2494 in healthy women reporting minor digestive symptoms. Neurogastroenterol Motil. 2013;25(4):331–e252. [DOI] [PubMed] [Google Scholar]
- 19. Chassany O, Tugaut B, Marrel A, Guyonnet D, Arbuckle R, Duracinsky M, Whorwell P, Azpiroz F. The Intestinal Gas Questionnaire (IGQ): a new instrument for measuring gas‐related symptoms and their impact on daily life. Neurogastroenterol Motil. 2015;27:885–898. [DOI] [PubMed] [Google Scholar]
- 20. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 21. EuroQol EQ‐5Q‐5L . http://www.euroqol.org/ (Accessed on January 11, 2021).
- 22. Suhr D. Exploratory or confirmatory factor analysis? In Statistics and Data Analysis. 2006, from http://www2.sas.com/proceedings/sugi31/200‐31.pdf. (Accessed on January 11, 2021)
- 23. Bliese PD. Within‐group, agreement, non‐independence, and reliability: Implications for data aggregation and analysis. In Klein KJ, Kozlowski SW, eds. Multilevel Theory, Research, and Methods in Organizations. San Francisco, CA: Jossey‐Bass, Inc; 2000:349‐381. [Google Scholar]
- 24. Fayers PM, Machin D. Quality of Life: The assessment, analysis and interpretation of patient‐reported outcomes, 2nd ed. Wiley‐Blackwell; 2007. [Google Scholar]
- 25. Nunnally JC, Bernstein IH. Psychometric theory, 3rd ed. New York: McGraw‐Hill Inc; 1994. [Google Scholar]
- 26. Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, Lenderking WR, Cella D, Basch E, ISPOR ePRO Task Force . Recommendations on evidence needed to support measurement equivalence between electronic and paper‐based patient‐reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health. 2009;12:419–429. [DOI] [PubMed] [Google Scholar]
- 27. Beavers A, Lounsbury J, Richards J, Huck S, Skolits G, Esquivel S. Practical considerations for using exploratory factor analysis in educational research. Pract Assess Res Evaluation. 2013;18:1‐13. Available at: https://scholarworks.umass.edu/cgi/viewcontent.cgi?article=1303&context=pare (Accessed on January 11, 2021). [Google Scholar]
- 28. Duracinsky M, Lalanne C, Goujard C, Herrmann S, Cheung‐Lung C, Brosseau JP, Schwartz Y, Chassany O. Electronic versus paper‐based assessment of health‐related quality of life specific to HIV disease: Reliability study of the PROQOL‐HIV questionnaire. J Med Internet Res. 2014;16(4):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gwaltney CJ, Shields AL, Shiffman S. Equivalence of electronic and paper‐and‐pencil administration of patient‐reported outcome measures: a meta‐analytic review. Value Health. 2008;11:322–333. [DOI] [PubMed] [Google Scholar]
- 30. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R‐project.org/. [Google Scholar]
- 31. Guyonnet D, Naliboff B, Rondeau P, Mayer E, Chassany O. Gastrointestinal well‐being in subjects reporting mild gastrointestinal discomfort: characteristics and properties of a global assessment measure. Br J Nutrition. 2013;110:1263–1271. [DOI] [PubMed] [Google Scholar]
- 32. Spiegel BM, Bolus R, Agarwal N, Sayuk G, Harris LA, Lucak S, Esrailian E, Chey WD, Lembo A, Karsan H, Tillisch K, Talley J, Chang L. Measuring symptoms in the irritable bowel syndrome: development of a framework for clinical trials. Aliment Pharmacol Ther. 2010;32:1275–1291. [DOI] [PubMed] [Google Scholar]
- 33. Patel P, Bercik P, Morgan DG, Bolino C, Pintos‐Sanchez MI, Moayyedi P, Ford AC. Irritable bowel syndrome is significantly associated with somatisation in 840 patients, which may drive bloating. Aliment Pharmacol Ther. 2015;41(5):449–458. [DOI] [PubMed] [Google Scholar]
- 34. Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96(12):3341–3347. [DOI] [PubMed] [Google Scholar]
- 35. Kanazawa M, Miwa H, Nakagawa A, Kosako M, Akiho H, Fukudo S. Abdominal bloating is the most bothersome symptom in irritable bowel syndrome with constipation (IBS‐C): a large population‐based Internet survey in Japan. Biopsychosoc Med. 2016;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender‐related differences in IBS symptoms. Am J Gastroenterol. 2001;96(7):2184–2193. [DOI] [PubMed] [Google Scholar]
- 37. Adeyemo MA, Spiegel BM, Chang L. Meta‐analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment Pharmacol Ther. 2010;32(6):738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malagelada JR, Accarino A, Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017;112(8):1221. [DOI] [PubMed] [Google Scholar]
- 39. Lacy BE, Cangemi D, Vazquez‐Roque M. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2020;19(2):219‐231.e1. [DOI] [PubMed] [Google Scholar]
- 40. Houghton LA, Lea R, Agrawal A, Reilly B, Whorwell PJ. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003–1010. [DOI] [PubMed] [Google Scholar]
- 41. Le Nevé B, de la Torre AM , Tap J, Derrien M, Cotillard A, Barba E, Mego M, Nieto Ruiz A, Hernandez‐Palet L, Dornic Q, Faurie JM, Butler J, Merino X, Lobo B, Batet FP, Accarino A, Pozuelo M, Manichanh C, Azpiroz F. A fermented milk product with B. Lactis CNCM I‐2494 and lactic acid bacteria improves gastrointestinal comfort in response to a challenge diet rich in fermentable residues in healthy subjects. Nutrients. 2020;12(2):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winham DM, Hutchins AM. Perceptions of flatulence from bean consumption among adults in 3 feeding studies. Nutr J. 2011;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wyrwich KW. Minimal important difference thresholds and the standard error of measurement: is there a connection? J Biopharm Stat. 2004;14(1):97–110. [DOI] [PubMed] [Google Scholar]
- 44. Woaye‐Hune P, Hardouin JB, Lehur PA, Meurette G, Vanier A. Practical issues encountered while determining minimal clinically important difference in patient‐reported outcomes. Health Qual Life Outcomes. 2020;18(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Food and Drug Administration: Guidance for industry . Patient‐reported outcome measures: use in medical product development to support labeling claims. 2009. Available at: https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/patient‐reported‐outcome‐measures‐use‐medical‐product‐development‐support‐labeling‐claims (Accessed on January 11, 2021)


