Summary
Postbariatric loss of muscle tissue could negatively affect long‐term health due to its role in various bodily processes, such as metabolism and functional capacity. This meta‐analysis aimed to unravel time‐dependent changes in the magnitude and progress of lean body mass (LBM), fat‐free mass (FFM), and skeletal muscle mass (SMM) loss following bariatric surgery. A systematic literature search was conducted in Pubmed, Embase, and Web of Science. Fifty‐nine studies assessed LBM (n = 37), FFM (n = 20), or SMM (n = 3) preoperatively and ≥1 time points postsurgery. Random‐effects meta‐analyses were performed to determine pooled loss per outcome parameter and follow‐up time point. At 12‐month postsurgery, pooled LBM loss was −8.13 kg [95%CI −9.01; −7.26]. FFM loss and SMM loss were −8.23 kg [95%CI −10.74; −5.73] and −3.18 kg [95%CI −5.64; −0.71], respectively. About 55% of 12‐month LBM loss occurred within 3‐month postsurgery, followed by a more gradual decrease up to 12 months. Similar patterns were seen for FFM and SMM. In conclusion, >8 kg of LBM and FFM loss was observed within 1‐year postsurgery. LBM, FFM, and SMM were predominantly lost within 3‐month postsurgery, highlighting that interventions to mitigate such losses should be implemented perioperatively.
Keywords: bariatric surgery, fat‐free mass, lean body mass, skeletal muscle mass
1. INTRODUCTION
Bariatric surgery induces an average weight loss of 32% of preoperative weight within two years postsurgery. 1 This weight loss does not only exclusively consists of fat mass loss but also includes loss of muscle mass. 2 , 3 Muscle mass can be expressed by various terms, such as fat‐free mass (FFM), lean body mass (LBM), and skeletal muscle mass (SMM). These terms should not be used interchangeably, since the exact components are different (see Figure 1). A two‐compartment model divides the body into fat mass and FFM, in which FFM contains bone tissue, water, organ tissue, and the skeletal muscles. LBM, as measured by three‐compartment models, is defined as total body mass minus fat mass and bone mineral content. SMM only contains the dry weight of skeletal muscles and is estimated via segmental analysis of muscle volume by whole body MRI. Because skeletal muscle tissue is the main component of LBM and FFM, they are often used as a surrogate marker for SMM.
FIGURE 1.
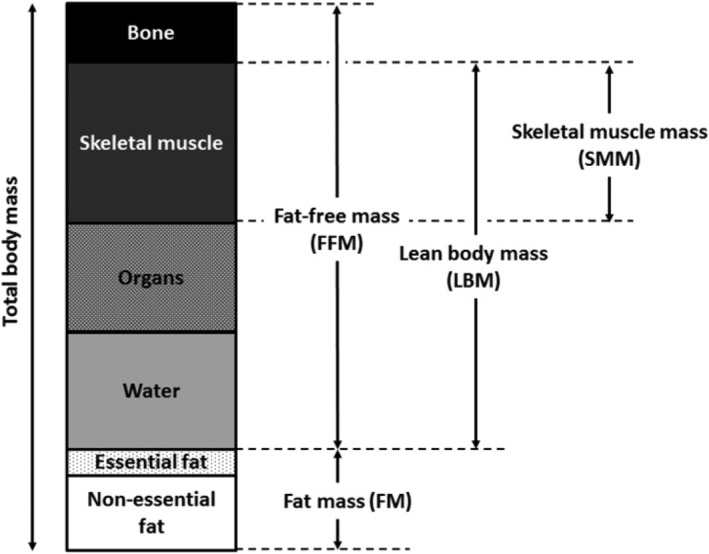
The components of fat‐free mass, lean body mass, and skeletal muscle mass according to the definitions used by magnetic resonance imaging (MRI), dual energy X‐ray absorptiometry (DXA), or computed tomography (CT) scans. Please note that proportions of the components are an approximation; exact proportions may vary across individuals
Muscle tissue is essential for a healthy metabolism, bone (re)modeling, thermoregulation, and preservation of functional capacity and can also function as a storage for glycogen, fat, and protein. 4 A substantial loss of muscle tissue can, therefore, result in a decreased basal metabolism, functional impairment, and poorer quality of life. 5 , 6 , 7 Furthermore, recent findings suggest that postbariatric FFM loss plays a role in energy balance regulation by increasing appetite, which may ultimately lead to weight regain. 8 , 9 , 10 Therefore, loss of muscle tissue may negatively impact the long‐term success of bariatric surgery. Previous studies suggested that muscle tissue is predominantly lost within 6 months postbariatric surgery. 2 , 11 However, the exact amounts of loss that can be expected in each postoperative phase remain unclear. This makes it difficult for clinicians to recognize excessive amounts of LBM, FFM, and SMM loss. More insight into the expected ranges of postbariatric LBM, FFM, and SMM loss could help to identify patients who may benefit from additional care on muscle mass preservation. Furthermore, more insight into the time‐dependent progress of LBM, FFM, and SMM loss will help to define the most optimal time window to counteract such loss in these patients.
Computed tomography (CT) and magnetic resonance imaging (MRI) are the gold standards to assess body composition in vivo, because they are not affected by hydration status. 12 Likewise, dual energy X‐ray absorptiometry (DXA) is considered a good alternative to evaluate body composition, because of its high precision and reproducibility. 13 However, postbariatric studies that use DXA, CT, or MRI have either small sample sizes or few longitudinal measurements, making it difficult to determine the magnitude and progress of muscle mass loss over time. Combining findings from these studies may yield information on the expected ranges of postbariatric muscle mass loss over time. Therefore, the present systematic review and meta‐analysis aimed to unravel time‐dependent changes in the magnitude and progress of LBM, FFM, and SMM loss measured by DXA, CT or MRI following bariatric surgery.
2. METHODS
2.1. Search strategy
This systematic review was registered in PROSPERO (number CRD42020150511) and performed using the Preferred Reporting Items for Systematic reviews and Meta‐Analysis statement 2015 (PRISMA). The databases of PubMed, Embase, and Web of Science were systematically searched for eligible articles up to March 15, 2021. The following search strategy was used: Bariatric surgery AND (DXA OR ((CT OR MRI) AND (Lean mass OR FFM OR Muscle mass)). The extensive search strategy with adaptations for each database is added in supporting information Table S1.
2.2. Definitions of body composition parameters
This study included three body composition parameters: LBM, FFM, and SMM. Since these terms cannot be used interchangeably due to differences in components (see Figure 1), all analyses were performed for LBM, FFM and SMM separately.
2.3. Study selection
Three reviewers independently screened all titles and abstracts for eligibility. Thereafter, the same three reviewers assessed the remaining studies in full text to determine whether the study could be included in our meta‐analysis. Studies were deemed eligible if they conformed to the predetermined inclusion and exclusion criteria (Table 1). First, all subjects should be ≥18 years old, have a BMI ≥ 35 kg/m2, and have undergone a bariatric procedure. Bariatric procedures included Roux‐en‐Y gastric bypass surgery (RYGB), sleeve gastrectomy (SG), biliopancreatic diversion (BPD), and adjustable gastric band procedures. Studies with temporary restrictive procedures (e.g., intragastric balloon) were excluded. Furthermore, studies that used ≥1 of the following outcomes—amount (kg) of LBM, FFM, or SMM measured with whole body DXA, CT, or MRI—were included. Studies with other measurement techniques; segmental analysis of DXA, CT, and MRI; or studies that exclusively reported outcomes relative to body weight (e.g., %FFM) were excluded. Finally, studies required longitudinal data on body composition with one preoperative measurement and at least one postoperative measurement. Small ranges in postoperative measurement were allowed; however, studies with >3‐month difference within one postoperative measurement point (e.g., mean follow‐up: 18 months [range 12 to 24 months]) were also excluded. When separate studies had overlapping study populations, only the study with most observations was included. In case of more than one outcome measure within overlapping populations (e.g., Study 1: FFM (n = 30) and Study 2: FFM (n = 25) and SMM (n = 25)), the secondary outcome of the study with less observations was also included. Disagreement between reviewers was resolved through consensus.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Population | |
| ‐ Human subjects | ‐ Animal studies |
| ‐ Bariatric procedure (including RYGB, SG, BPD, adjustable gastric banding, gastric bypass) | ‐ Gastrectomy for other medical reasons (not focused on weight loss) |
| ‐ All subjects ≥18 years old | ‐ Abdominal liposuction |
| ‐ Mean BMI ≥ 35 kg/m2 | ‐ Other severe diseases: cancer, lung diseases, kidney diseases, gastrointestinal diseases, cardiovascular diseases or immunodeficiency diseases (except for obesity‐related diseases such as diabetes mellitus type 2, hypertension, arthrosis and sleep apnoea) |
| Study design | |
| ‐ Observational studies | ‐ Cross‐sectional studies |
| ‐ Longitudinal measurements (including a preoperative measurement and ≥1 postoperative measurement) | ‐ Review articles |
| ‐ Control groups of randomized controlled trials | ‐ Intervention groups of randomized controlled trials |
| ‐ Ranges >3 months within one postoperative measurement point | |
| Measurements | |
| ‐ Whole body DXA scan | ‐ BIA |
| ‐ Whole body CT | ‐ Bod Pod |
| ‐ Whole body MRI | |
| Outcome | |
| ‐ Lean body mass / lean mass /lean tissue | ‐ %FFM (percentage of body weight) |
| ‐ Fat‐free mass | |
| ‐ Skeletal muscle mass/muscle mass | |
| Other | |
| ‐ English language | ‐ Abstract only |
| ‐ Full text available | ‐ Conference proceedings |
|
‐ Study protocols ‐ Letter to the editor |
|
| ‐ Case reports |
Abbreviations: BIA, bioelectrical impedance analysis; BMI, body mass index; BPD, biliopancreatic diversion; CT, computed tomography; DXA, dual energy X‐ray absorptiometry; FFM, fat‐free mass; MRI, magnetic resonance imaging; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
2.4. Data extraction
Data were extracted with the use of a predetermined data extraction file. One reviewer recorded all pre‐operative and postoperative data on LBM, FFM, and SMM and corresponding standard deviations (SD) or standard error of the mean (SEM). Furthermore, other information was extracted, including year of publication; country; age; sex; preoperative BMI; preoperative LBM, FFM, or SMM mass; type of surgery; and weight loss per time point. In eight studies, data on body composition or weight loss were reported in figures; therefore, data were extracted using GetData Graph Digitizer software (version 2.26). When viable information was missing, data were requested from the authors by email (n = 24 studies; authors provided requested information in n = 5 studies).
2.5. Assessment of risk of bias in included studies
The reporting quality of each included study was assessed using the STROBE Statement, 14 which facilitates critical appraisal and interpretation of observational research. The checklist consists of 22 items relating to title, abstract, introduction, methods, and results and discussion sections of articles. A total of 34 points could be received; however, not all items were applicable in every study. Therefore, quality scores were calculated both as total of points and as percentage of the applicable items. Subsequently, total scores were divided into three categories: <60%, 60–80%, and ≥80%.
2.6. Data synthesis
Follow‐up time points were divided into five categories: <3‐month, ≥3‐ to <6‐month, ≥6‐ to 9‐month, 12‐month, and 18‐ to ≤36‐month postsurgery. If two follow‐up measurements of one study fell within one time category (e.g., both 24‐ and 36‐month postsurgery), only the first time point was included in the meta‐analysis. In studies with multiple study arms (e.g., Roux‐en‐Y gastric bypass vs. SG), all study arms were separately included in statistical analyses. Since loss of muscle tissue is highly dependent on weight loss, the proportional loss within total weight loss (=%loss/WL) was estimated. First, study‐specific %loss/WL was calculated at each time point, by dividing the mean LBM, FFM or SMM loss by the mean weight loss times 100. Then, a weight factor was applied based on the sample size of each study. Subsequently, the weighted average %loss/WL was calculated per time point. Although the %loss/WL is not suitable for meta‐analysis since group level calculations may not reflect individual results and group level SEs are lacking, this outcome gives insight into the relative amount of postbariatric LBM, FFM, and SMM loss.
2.7. Statistical analysis
A random‐effects model (specified a priori) was used to account for possible heterogeneity between studies and to determine the overall effect (i.e., amounts of LBM loss, FFM loss, and SMM loss in kilograms). For each outcome measure, a meta‐analysis was performed to estimate the pooled effect size per follow‐up visit in terms of the mean difference with its corresponding 95%CI. Furthermore, Cochrane's Q statistic and I 2 were calculated to assess the degree of heterogeneity across studies. Whereas the Q statistic indicates significant heterogeneity at P < 0.10, the I 2 characteristic reflects the percentage of the observed between‐study variability. An I 2 > 50% is considered as substantial heterogeneity. 15 Forest plots were generated to illustrate the study‐specific effect sizes with 95%CI. For the subanalysis, type of surgery was added to the model as additional fixed effect to determine the difference in LBM, FFM, and SMM loss between procedures, adjusted for time. Studies that included ≥2 procedures but did not distinguish their results for each type were excluded. Mean differences with respect to the reference group with corresponding 95%CI were determined for all possible comparisons. Additionally, forest plots for LBM, FFM, and SMM loss per bariatric procedure were generated, in which either the 12‐month timepoint or the latest available timepoint was included per study. Finally, potential differences in proportional loss (=%loss/WL) between procedures were assessed by a one‐way ANOVA. Statistical analyses were performed using R Statistical Software (version 3.6.2), and the script was added as a supporting information. Statistical significance was assumed at P < 0.05.
3. RESULTS
The systematic search resulted in 302 eligible articles in PubMed, 507 articles in Embase, and 315 articles in Web of Science. After removal of duplicates and elimination based on the selection criteria, 59 studies were included in risk and bias assessment and quantitative analyses (Figure 2), with 37 studies assessing LBM, 11 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 20 studies assessing FFM 22 , 25 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , and 3 studies assessing SMM. 71 , 72 , 73 The subanalysis on type of surgery was performed on 52 studies. Included studies were published from 2000 to 2021.
FIGURE 2.
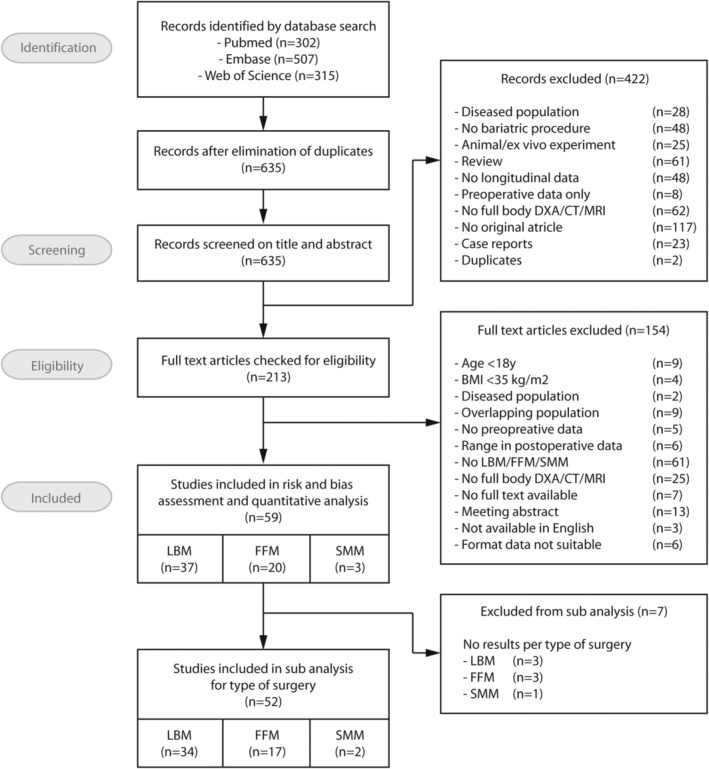
Preferred Reporting Items for Systematic reviews and Meta‐Analysis (PRISMA) flowchart of search strategy outcomes and screening process. CT, computed Tomography; DXA, dual energy X‐ray absorptiometry; FFM, fat‐free mass; LBM, lean body mass; MRI, magnetic resonance imaging; SMM, skeletal muscle mass
3.1. Cohort characteristics
An overview of general characteristics of the included studies is displayed in Table 2. In total, data were aggregated from 2270 individuals with a mean BMI of 44.2 kg/m2 (range 37.79 to 51.20 kg/m2). Mean LBM was 58.2 kg (range 49 to 69 kg), mean FFM was 63.1 kg (range 55 to 89 kg), and mean SMM was 27.1 kg (range 22 to 37 kg). Main continent of origin of the studies was Europe (n = 38), followed by North America (n = 12), Asia (n = 5), South America (n = 3), and Australia (n = 1). In 35 studies both sexes were included, whereas 22 studies exclusively included females. Most studies included only one type of surgery: RYGB (n = 27), adjustable gastric band (n = 6), SG (n = 7), BPD (n = 5), whereas 14 studies examined the effect of ≥2 procedures. Outcomes were predominantly measured by DXA (n = 56), whereas only three studies used MRI.
TABLE 2.
Study characteristics of the included studies (n = 59)
| Study | Country | N | Sex (M/F) | Age (years) | BMI (kg/m2) | Muscle mass (kg) | Surgery type | Timepoints | Methods | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Arhire (2018) | Romania | 75 | 13/62 | 42.1 ± 11.5 | 45.15 ± 6.78 | 65.58 ± 12.42 | SG | pre, 6 months, 12 months | DXA | LBM |
| Bazzocchi (2015) | Italy | 41 | 0/41 | 40.6 ± 10.0 | 42.6 ± 6.6 | 53.37 ± 11.15 | RYGB | pre, 3 months, 6 months, 12 months | DXA | LBM |
| Beckman (2017) | USA | 32 | 0/32 | 47 ± 12 | 48 ± 7 | 61 ± 10 | RYGB | pre, 6–9 months, 12 months | DXA | LBM |
| Bellicha (2019) | France | 45 | 0/45 | 43 [38–51] | 42.6 [40.0–45.5] | 56.4 [51.8–61.2] | RYGB | pre, 6 months | DXA | LBM |
| Blom‐Høgestøl (2020) | Norway | 34 | 13/21 | 45.2 ± 9.7 | 40.9 ± 3.5 | 61.1 ± 11.2 | RYGB | pre, 12 months | DXA | LBM |
| Bojsen‐Møller (2015) | Denmark | 9 | 3/6 | 32 [29–46] | 39.2 (35.2, 43.3) | 64 (57, 71) | RYGB | pre, 3 months | DXA | FFM |
| Brzozowska (2020) | Australia | 39 | 12/27 |
RYGB: 51.1 ± 7.6 SG: 51 ± 11BAND: 42.3 ± 13.1 |
RYGB: 42.3 ± 7.7SG: 42.6 ± 5.3BAND: 37.3 ± 4.5 | RYGB: 56.1 ± 11SG: 59.7 ± 8.1BAND: 51.3 ± 6.7 | RYGB (n = 7), SG (n = 21), BAND (n = 11) | Pre, 6 months, 12 months, 24 months, 36 months | DXA | LBM |
| Busetto (2000) | Italy | 6 | 0/6 | 38 to 42 | 42.6 ± 1.1 | 65.4 ± 1.3 | BAND | pre, 8 weeks, 24 weeks | MRI | FFM |
| Calleja‐Fernández (2015) | Spain | 46 | 7/39 | 45.43 ± 9.56 | 45.77 ± 5.10 |
LBM: 53.13 ± 9.30 FFM: 56.27 ± 8.58 |
BPD | pre, 12 months | DXA | LBM, FFM |
| Carrasco (2009) | Chile | 42 | 0/42 | 37.7 ± 9.6 | 45.0 ± 4.3 | 58.9 ± 5.8 | RYGB | pre, 6 months, 12 months | DXA | FFM |
| Chen (2021) | China | 49 | 29/20 | 28 [23.5–35.0] | 40.0 ± 5.4 | 60.7 ± 11.9 | SG | Pre, 6 months, 12 months | DXA | LBM |
| Ciangura (2010) | France | 42 | 0/42 | 39.5 ± 11.6 | 44.6 ± 6.1 | 61.5 ± 7.8 | RYGB | pre, 3 months, 6 months, 12 months | DXA | LBM |
| Clements (2011) | USA | 16 | 1/15 | 46 ± 7.5 | 43.6 ± 4.2 | 54 ± 8.1 | RYGB | pre, 2 weeks, 8 weeks | DXA | LBM |
| Cole (2017) | USA | 5 | 0/5 | 47.2 ± 10.9 | 48.8 ± 9.7 | LBM: 63.5 ± 13.2FFM: 66.4 ± 13.9 | RYGB | pre, 6 weeks, 6 months, 12 months, 9y | DXA | LBM, FFM |
| Coupaye (2005) | France | 36 | 0/36 | 42.7 ± 8.7 | 47.2 ± 8.5 | 54.4 ± 7.1 | BAND | pre, 12 months | DXA | LBM |
| Coupaye (2007) | France | 32 | 0/32 | 42.6 ± 8.4 | 45.5 ± 6.4 | 25.7 ± 4.6 | BAND | pre, 12 months | DXA | SMM |
| Davidson (2018) | USA | 93 | 14/79 | 44.2 ± 11.6 | Male: 44.7 ± 4.1Female: 45.7 ± 4.1 | RYGB: 25.3 ± 3.3SG: 28.6 ± 4.8BAND: 23.6 ± 4.0 RYGB: 36.8 ± 3.3 | RYGB (n = 72), SG (n = 11), BAND (n = 10) | pre, 12 months, 24 months, 60 months | MRI | SMM |
| Diniz‐Sousa (2020) | Portugal | 20 | 4/16 | 46.5 ± 8.5 | 46.1 ± 4.2 | 53 (51.7, 54.5) | RYGB, SG | Pre, 1 month, 6 months, 12 months | DXA | LBM |
| Faucher (2019) | France | 279 | 64/215 | G1: 63.7 ± 2.7G2: 42.6 ± 9.7 | G1: 44.5 ± 5.1 G2: 44.2 ± 5.4 | G1: 58.6 ± 9.4G2: 61.4 ± 10.9 | RYGB, SG | pre, 3 months, 6 months, 12 months | DXA | LBM |
| Favre (2018) | Switzerland | 44 | 0/44 | G1: 37.2 ± 9.5 G2: 38.5 ± 11.2 G3: 44.9 ± 3.8 | G1: 42.5 ± 4.4G2: 41.7 ± 3.5G3: 39.9 ± 6.4 | G1: 49.08 ± 6.96G2: 50.46 ± 6.92G3: 56.22 ± 5.57 | RYGB | pre, 12 months | DXA | LBM |
| Fjeldborg (2015) | Denmark | 31 | 12/19 | 43.9 ± 7.7 | 42.3 ± 4.7 | 67.1 ± 12.8 | RYGB | pre, 12 months | DXA | LBM |
| Garrapa (2005) | Italy | 15 | 4/11 | 32.5 ± 3.8 | 42.2 ± 0.9 | 55.98 (3.02) | BAND | pre, 6 months | DXA | FFM |
| Hayashi (2017) | Japan | 14 | 4/10 | G1: 43.3 ± 7.3G2: 64.6 ± 0.5 | G1: 45.1 ± 7.5G2:43.2 ± 4.8 | G1: 63.4 ± 10.8G2: 37.9 ± 32.9 | SG | pre 12 months | DXA | LBM |
| Hirsch (2020) | USA | 21 | NR | 43.7 ± 10.7 | 51.2 ± 13.7 | 59.4 ± 9.2 | RYGB, SG | Pre, 3 weeks, 12 weeks, 24 weeks | DXA | FFM |
| Jacobsen (2013) | Denmark | 12 | 3/9 | NR | 39.2 ± 1.8 | NR | RYGB | pre, 3 months | DXA | FFM |
| Johnson (2017) | USA | 25 | 0/25 | 48 ± 10 | 46.6 ± 6.8 | 52.3 ± 11.6 | RYGB | pre, 6 months, 12 months | DXA | FFM |
| Jorsal (2020) | Denmark | 20 | 5/15 | 47 [39–50] | 43.4 [39.7–47.0] | 57.4 [52.2–66.4] | RYGB | Pre, 3 months | DXA | FFM |
| Kayser (2017) | France | 59 | 0/59 | RYGB: 37.3 ± 1.9BAND: 34.5 ± 1.6 | RYGB: 46.5 ± 1.0BAND: 43.6 ± 0.7 | RYGB: 58.9 (1.2)BAND: 55.5 (1.4) | RYGB (n = 37), BAND (n = 22) | pre, 1 month, 3 months | DXA | FFM |
| Kenngott (2019) | Germany | 31 | 13/18 | 46.4 ± 9.7 | 45.2 ± 6.5 | 22.5 ± 4.7 | RYGB (n = 11), SG (n = 20) | pre, 3 months, 12 months | MRI | SMM |
| Khoo (2014) | USA | 30 | 10/20 | 49.6 ± 1.4 | 43.4 ± 0.8 | 63.0 ± 20.5 | RYGB | pre, 6 months, 12 months | DXA | FFM |
| Kim (2020) | USA | 44 | 10/34 | 45 ± 12 | 44 ± 7 | 62 ± 12 | RYGB | pre, 6 months | DXA | LBM |
| Legro (2012) | USA | 29 | 0/29 | 34.5 ± 4.3 | 49 ± 7 | 57 ± 5 | RYGB | pre, 1 month, 3 months, 6 months, 12 months, 24 months | DXA | LBM |
| Lubrano (2004) | Italy | 45 | 17/28 | 19 to 49 | 47.7 ± 6.4 | 62.3 ± 11.1 | RYGB | pre, 1 month, 3 months, 6 months, 9 months, 12 months, 24 months, 36 months | DXA | LBM |
| Maïmoun (2019) | France | 30 | 5/25 | 40.9 ± 15.1 | 41.9 ± 4.5 | 59.8 ± 8.9 | SG | pre, 1 months, 12 months | DXA | LBM |
| Marengo (2017) | Spain | 38 | 0/38 | 46.3 ± 8.2 | 42.91 ± 3.62 | 54.97 ± 4.83 | RYGB | pre, 12 months, 36 months | DXA | LBM |
| Matos (2020) | Brazil | 17 | 0/17 | 41.2 ± 11 | 42.0 ± 3.6 | 57.2 ± 11.1 | RYGB | Pre, 6 months | DXA | LBM |
| Mingrone (2002) | Italy | 46 | 15/31 | 30 to 45 | Male: 48.0 ± 5.4Female: 48.3 ± 6.3 | Male: 88.7 ± 8.1Female: 59.3 ± 5.6 | BPD | pre, 12 months | DXA | FFM |
| Moehlecke (2017) | Brazil | 30 | 5/25 | 43 ± 12 | 49 (9) | 80 (4) | RYGB | pre, 6 months | DXA | FFM |
| Moizé (2013) | Spain | 50 | 9/41 | RYGB: 43.0 ± 2.0SG: 45.0 ± 2.9 | RYGB: 45.5 ± 0.6SG: 47.2 ± 1.0 | RYGB: 53.5 (1.6)SG: 59.6 (2.2) | RYGB (n = 25), SG (n = 25) | pre, 4 months, 12 months | DXA | LBM |
| Nielsen (2021) | Denmark | 41 | 6/35 | 39.6 ± 9.5 | 40.8 ± 1.2 | 68.0 (1.4) | RYGB, SG | Pre, 6 months, 18 months | DXA | FFM |
| Olbers (2006) | Sweden | 83 | NR | NR | RYGB: 42.3 ± 4.5BAND: 42.6 ± 4.2 | RYGB: 54.9 ± 8.9BAND: 56.3 ± 9.1 | RYGB (n = 37), BAND (n = 46) | pre, 12 months | DXA | LBM |
| Oppert (2018) | France | 22 | 0/22 | 43.9 ± 10.7 | 43.6 ± 6.2 | 55.6 ± 8.4 | RYGB | pre, 6 months | DXA | LBM |
| Rabl (2014) | USA | 20 | 4/16 | RYGB: 47.4 ± 8.7BAND: 49.0 ± 10.7 | RYGB: 48.4 ± 6.8BAND: 44.3 ± 5.0 | RYGB: 61.9 ± 15.4BAND: 59.0 ± 7.6 | RYGB, BAND | pre, 2 weeks, 6 months | DXA | LBM |
| Raffaelli (2015) | Italy | 8 | 0/8 | 34 ± 4 | 51.2 ± 7.95 | 68.47 ± 21.26 | BPD | pre, 6 months | DXA | FFM |
| Sajoux (2019) | Spain | 39 | 2/37 | 40.8 ± 10.4 | 45.6 ± 6.2 | 56.7 ± 9.9 | RYGB (n = 15), BPD (n = 15), SG (n = 19) | pre, 2–3 months, 4–6 months | DXA | FFM |
| Savastano (2010) | Italy | 45 | 0/45 | 35.3 ± 9.1 | 42.1 ± 4.1 | 60.1 ± 6.1 | BAND | pre, 6 months, 12 months | DXA | FFM |
| Sergi (2003) | Italy | 6 | 0/6 | 38 to 42 | 42.8 ± 1.0 | 55.2 ± 2.4 | BAND | pre, 2 months, 6 months | DXA | FFM |
| Tacchino (2003) | Italy | 101 | 0/101 | 41 ± 8 | 45.5 ± 7.7 | 58.0 ± 6.6 | BPD | pre, 2 months, 6 months, 12 months, 24 months | DXA | LBM |
| Talalaj (2020) | Poland | 155 | 38/117 | 42.0 ± 10.5 | 43.9 ± 5.6 | 64.5 ± 10.6 | SG | pre, 12 months | DXA | LBM |
| Tamboli (2010) | USA | 29 | 4/25 | 43.8 ± 9.6 | 46.3 ± 5.5 | 62.9 ± 10.2 | RYGB | pre, 6 months, 12 months | DXA | LBM |
| Tan (2016) | Singapore | 22 | 6/13 | 40.6 ± 2.1 | 38.8 ± 1.3 | 56.56 (2.23) | RYGB (n = 10), SG (n = 12) | pre, 12 months | DXA | FFM |
| Turcotte (2019) | Canada | 16 | 5/11 | 41.6 ± 8.8 | 49.4 ± 5.6 | 69.5 ± 16.1 | BPD | pre, 3 months, 12 months | DXA | FFM |
| Vatier (2012) | France | 86 | 19/67 | 42.3 ± 10.3 | 48.1 ± 5.9 | 68.8 ± 12.8 | RYGB | pre, 6 months, 12 months | DXA | LBM |
| Vaurs (2015) | France | 114 | 21/93 | 39.6 ± 11.7 | 43.3 ± 5.4 | 57.0 ± 10.5 | RYGB (n = 70), SG (n = 44) | pre, 3 months, 12 months | DXA | LBM |
| Vilarrasa (2011) | Spain | 59 | 0/59 | 46.0 ± 8.2 | 43.9 ± 4.2 | 54.1 ± 5.5 | RYGB | pre, 12 months, 36 months | DXA | LBM |
| Von Scholten (2017) | Denmark | 19 | 5/14 | 40 ± 9.3 | 41 ± 6 | 66.2 ± 12.2 | RYGB | pre, 6 months | DXA | LBM |
| Werling (2015) | Sweden | 6 | 0/6 | 41.1 (28.8 to 50) | 41.4 (39.1 to 44.8) | 55.9 (47.5 to 59.3) | RYGB | pre, 10 days, 3 months, 20 months | DXA | LBM |
| Zhang X (2018) | China | 128 | 48/80 | 32.23 ± 10.52 | 39.66 ± 6.23 | 56.20 ± 11.81 | SG | pre, 6 months | DXA | LBM |
| Zhang Y (2017) | China | 37 | 18/19 | G1: 42.71 ± 14.13G2: 29.04 ± 5.85 | G1: 37.79 ± 4.87G2: 40.56 ± 4.54 | G1: 55.29 ± 11.02G2: 62.92 ± 12.12 | SG | pre, 3 months | DXA | LBM |
Note: Data are displayed as mean ± SD, mean (SE), median [IQR 25%–75%], mean (LCI, UCI), or as (minimum to maximum).
Abbreviations: BAND, adjustable gastric banding; BPD, biliopancreatic diversion; DXA, dual‐energy x‐ray absorptiometry; FFM, fat‐free mass; G1/G2/G3, group 1, 2, or 3 (when data are split based on patient characteristics); LBM, lean body mass; MRI, magnetic resonance imaging; NR, not reported. RYGB, Roux‐en‐Y gastric bypass; SG, sleeve Gastrectomy; SMM, skeletal muscle mass.
3.2. Quality assessment of reporting
Fifty‐nine studies were included for risk and bias assessment. The mean ± SD score on the STROBE checklist was 24.3 ± 2.4, reflecting 80.0 ± 7.7% of the maximal achievable score. One study scored below <60%, 26 studies scored between 60% and 80%, and 32 studies scored >80% (supporting information Figure S1). A sensitivity analysis of our main outcome measures after exclusion of the study with a score <60% did not yield different results. Study‐specific STROBE scores were included in supporting information Table S2.
3.3. Magnitude and progress of muscle mass loss
LBM loss, FFM loss, and SMM loss were displayed in forest plots (Figures 3, 4, 5, respectively). At 12‐month postsurgery, meta‐analyses showed pooled losses of −8.13 kg LBM [95%CI −7.26; −9.01], −8.23 kg FFM [95%CI −5.73; −10.74], and −3.18 kg SMM [95%CI −0.71; −5.64]. Although absolute SMM loss is lower compared with LBM and FFM loss, relative SMM loss with respect to preoperative SMM was equal to relative FFM and LBM loss (SMM: 13% vs. FFM 13% vs. 13% LBM). The estimated proportional loss with respect to weight loss at 12‐month postsurgery was comparable with 23.4%, 20.8%, and 8.2% loss/WL for LBM, FFM, and SMM, respectively. The studies (n = 8) that assessed LBM both at 12 and 18–36 months showed a mean loss of −1.29 ± 1.0 kg LBM between these timepoints, suggesting a more stabilized LBM after 12 months. On the contrary, all studies showed greatest declines in LBM, FFM, and SMM between the preoperative and first follow‐up measurement, after which a more gradual decrease occurred. Patients lost −4.45 kg LBM [95%CI −6.21; −2.70] within 3‐month postsurgery, accounting for 55% of the 12‐month LBM loss. A similar pattern was seen for FFM, in which the FFM loss within <3 months (−4.25 kg [95%CI −6.30 to −2.20]) reflected 52% of 12‐month FFM loss and SMM loss at 3–6 months (−2.10 kg [95%CI −4.22 to 0.02]) reflected 66% of 12‐month SMM loss. Study‐specific changes in LBM, FFM, and SMM over time are displayed in supporting information Figure S2.
FIGURE 3.
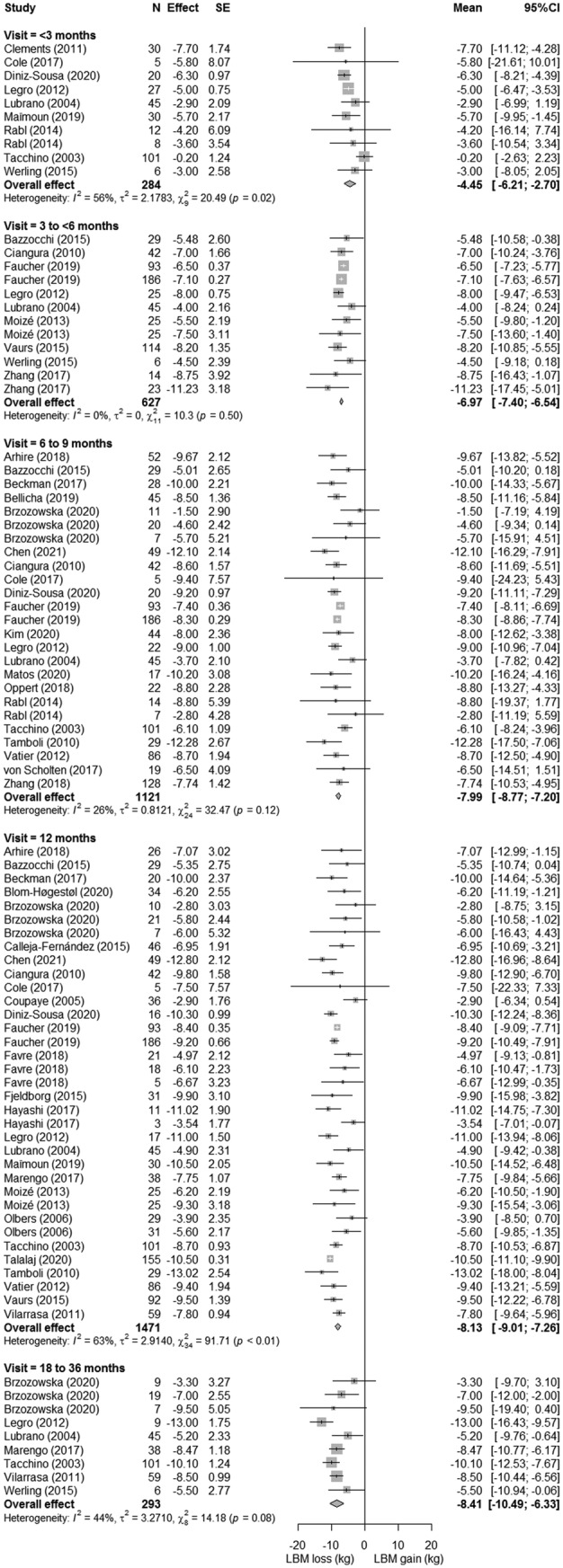
Forest plots of lean body mass loss with respect to preoperative measures. The effect size (mean difference between preoperative and postoperative measure) and 95% confidence interval for individual studies and the pooled estimate per time point are depicted. Mean follow‐up time was 1.1 ± 0.6 months for <3 months, 3.2 ± 0.4 months for 3 to 6 months, 6.1 ± 0.3 months for 6 to 9 months, 12 ± 0 months for 12 months, and 26.2 ± 5.7 months for 18 to 36 months
FIGURE 4.
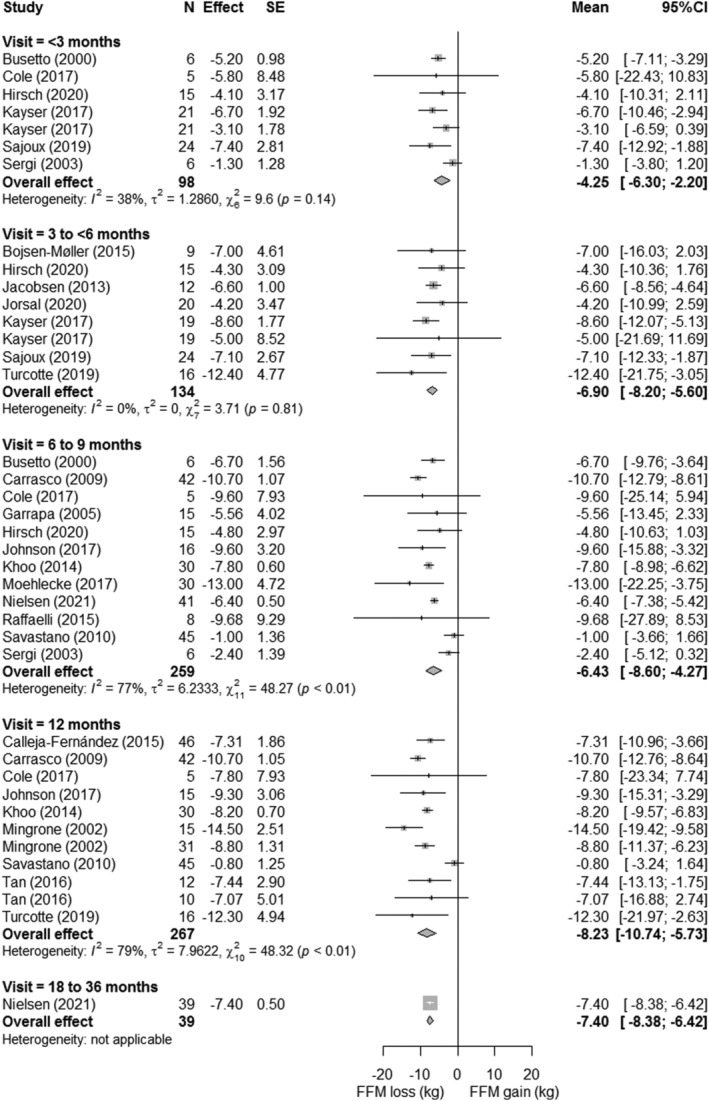
Forest plots of fat‐free mass loss with respect to preoperative measures. The effect size (mean difference between preoperative and postoperative measure) and 95% confidence interval for individual studies and the pooled estimate per time point are depicted. Mean follow‐up time was 1.5 ± 0.8 months for <3 months, 3.3 ± 0.7 months for 3 to 6 months, 6 ± 0 months for 6 to 9 months, 12 ± 0 months for 12 months, and 18 ± 0 months for 18 to 36 months
FIGURE 5.
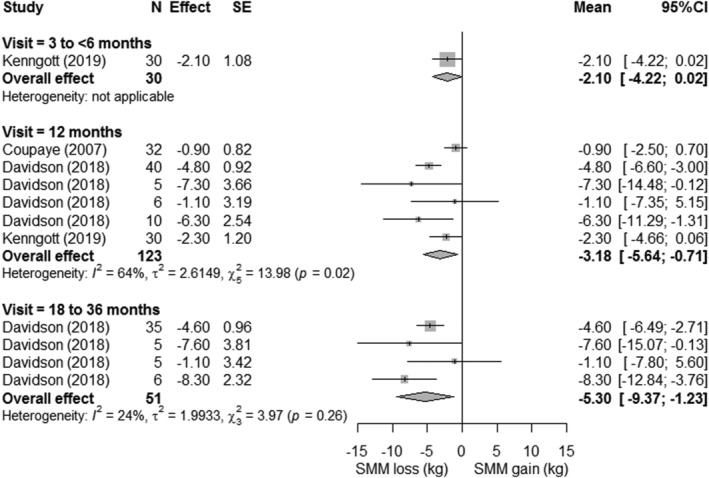
Forest plots of skeletal muscle mass loss with respect to preoperative measures. The effect size (mean difference between preoperative and postoperative measure) and 95% confidence interval for individual studies and the pooled estimate per time point are depicted. Mean follow‐up time was 3 ± 0 months for 3 to 6 months, 12 ± 0 months for 12 months, and 24 ± 0 months for 18 to 36 months
3.4. Muscle mass loss per surgery type
Forest plots of LBM, FFM, and SMM loss per bariatric procedure were displayed in supporting information Figures S3–S5. The comparison of LBM, FFM, and SMM loss between different types of surgery adjusted for time is illustrated in Figure 6. Adjustable gastric band procedures showed a lower LBM loss of −3.1 kg [95%CI −5.9, −0.3], −4.2 kg [95%CI −6.6, −1.8], and −5.5 kg [95%CI −8.1, −2.9] compared with BPD, RYGB, and SG procedures, respectively (all P < 0.05). Similar effects were seen for FFM and SMM, in which the adjustable gastric band showed significantly smaller decreases in FFM compared with RYGB and BPD and smaller decreases in SMM with respect to RYGB and SG. However, the procedures induced differences in total body weight loss as well, in which the greatest weight loss was found for BPD (43.5 ± 9.8 kg), followed by RYGB (38.6 ± 5.2 kg), SG (33.2 ± 6.1 kg) and BAND (22.4 ± 6.1 kg). When considering LBM, FFM, and SMM loss with respect to total body weight loss, no significant differences in the proportional loss were observed across bariatric surgery types (supporting information Figure S6).
FIGURE 6.
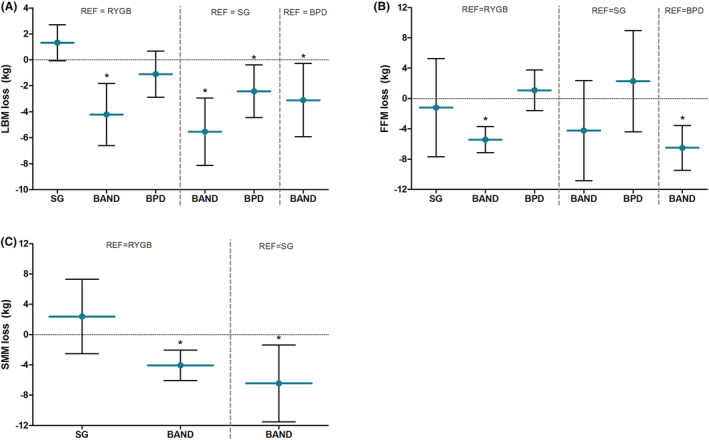
Differences in lean body mass loss (A), fat‐free mass loss (B), and skeletal muscle mass loss (C) in kilograms between bariatric procedures adjusted for time‐effects. Blue horizontal lines reflect the mean difference with respect to the reference group, and the error bars reflect the corresponding lower and upper limit of the 95%CI. A positive value on the y‐axis reflects a greater loss compared with the reference group, whereas a negative value reflects a smaller loss than the reference group. BAND, adjustable gastric band operation; BPD, biliopancreatic diversion; RYGB, Roux‐en‐Y Gastric bypass; SG, sleeve gastrectomy; * P < 0.05 with respect to reference group
3.5. Heterogeneity
At 12‐month postsurgery, substantial heterogeneity in the amount of LBM, FFM, and SMM loss was observed across studies (supporting information Figure S7). For LBM, this resulted in a pooled effect range from −2.8 to −13.0 kg (I 2 = 63%; Q = 91.71; P < 0.01). Likewise, large pooled effect ranges were found for FFM (range −0.8 to −14.5 kg; I 2 = 77%; Q = 48.32; P < 0.01) and SMM (range −0.9 to −7.3 kg; I 2 = 64%; Q = 13.98; P = 0.02). Furthermore, the relatively large standard errors and 95%CIs of individual studies suggest some heterogeneity in LBM, FFM, and SMM loss within studies as well.
4. DISCUSSION
The present work is the first meta‐analysis to assess the magnitude and progress of postbariatric LBM, FFM, and SMM loss and the impact of different bariatric procedures on such losses. We found a considerable loss of LBM and FFM at 12‐month postsurgery, which was predominantly lost within 3‐month postsurgery and continuously decreases up to 6 months. Substantial heterogeneity was present across studies, which highlights the need for personalized care among postbariatric patients. Furthermore, these findings suggest that perioperative care programs should actively monitor changes in body composition and that interventions to limit muscle mass loss should start immediately after (or even before) surgery.
A previous review on changes in FFM during weight loss showed FFM losses of 17.5% to 31.3% of total weight loss after bariatric surgery, dependent on the procedure. However, only 16 studies were available at the time and no pooled meta‐analysis, adjustments for time of follow‐up, or statistical tests between procedures could be performed. Our meta‐analysis demonstrated over 8‐kg FFM and LBM loss within 1‐year postbariatric surgery, which reflected 21% and 22% of total body weight loss, respectively. In comparison, weight loss by a 12‐week low‐caloric diet of 800–1000 kcal/day resulted in 1.5‐kg FFM loss (16% loss/WL), whereas another 12‐week isocaloric diet with 25% restriction of habitual diet resulted in 2.1‐kg LBM loss (23% loss/WL). 71 , 72 Although bariatric surgery induced greater amounts of FFM and LBM loss, proportional loss is quite similar to dietary interventions on the long term. This suggests a strong relation between weight loss and FFM or LBM loss, in which higher weight loss automatically results in greater FFM or LBM loss. However, higher proportional FFM losses of 30–33%loss/WL are observed within 3‐month postbariatric surgery, which suggests that excessive FFM loss predominantly occurs shortly after surgery.
Although benefits of weight loss may outweigh the burden of muscle mass loss in the early‐postoperative phase, excessive loss of muscle tissue may particularly be detrimental on the long term, because of its role in various bodily processes, such as functional capacity, bone strength, and metabolic health. A previous meta‐analysis showed a decline of −1.95 kcal in resting energy expenditure (REE) per kg FFM loss after bariatric surgery. 74 This decline in REE after bariatric surgery is greater than REE declines by dietary interventions, 75 but this difference was annulled by adjusting for changes in body composition. Still, 29% of bariatric patients showed greater declines in REE than can be explained by changes in body composition, which is indicative of adaptive thermogenesis. 75 This may be caused by altered metabolic activity of fat mass and vital organs. 76 , 77 A lower REE is not necessarily problematic, since REE was found to regulate energy intake and appetite control in weight‐stable subjects. 78 However, this relation between weight loss associated REE and energy intake and appetite control has not been confirmed for patients with severe obesity. Moreover, recent findings suggest that a higher proportional FFM loss during weight loss may enhance the drive to eat, 10 which could still predispose weight regain and thus comprise long‐term treatment outcomes. Moreover, predictive score of sarcopenia (i.e., degenerative loss of muscle strength, muscle quantity and quality, and low physical performance that occurs with aging or immobility) based on sex‐specific SMM index increased from 8% to 32% within 1‐year postbariatric surgery. 79 When accompanied by muscle strength loss, this would increase the risk for frailty, functional disability, mortality, and cardiometabolic diseases. 80 , 81 Co‐existence of sarcopenia and obesity (i.e., sarcopenic obesity) is considered even more harmful, since the negative effects of low SMM and high fat mass may potentiate each other. 82 For this reason, it would be valuable to include clinical outcomes, such as physical rehabilitation, muscle strength, and muscle function to determine the impact of bariatric surgery on long‐term health.
The first postoperative weeks are most crucial to limit LBM and FFM loss, since >50% of the total loss occurs within 3‐month postsurgery. This rapid loss shortly after surgery is probably multifactorial. First, dietary protein intake is found to decrease to approximately 30 g/day at 1 month postsurgery. 83 , 84 This decrease in protein intake is likely caused by an overall restriction in dietary intake (i.e., only 500–800 kcal/day), which makes it difficult to achieve the recommended postbariatric protein intake of at least 60 g/day or 1.1 g/kg ideal body weight. 85 Second, muscle protein synthesis (MPS) functions via a dose–response relationship with protein intake, at which a dose of 20–40 g/meal leads to the most optimal response. 86 However, these larger portion sizes are not well tolerated by postbariatric patients, potentially leading to suboptimal anabolic responses. Third, the body does not store protein, despite its essential function throughout various bodily structures and processes. For this reason, a regular and sufficient protein intake is required. However, in periods of protein deprivation (such as first postoperative weeks), unused muscle tissue is broken down to compensate for caloric restriction and to acquire amino acids for other processes. 87 Fourth, periods of muscle unloading are known to cause loss of muscle tissue, which is likely to occur as clinical guidelines discourage exercise (except walking and daily life activities) and prohibit lifting weight up to six weeks postbariatric surgery. 85 Together, these factors make postbariatric patients very susceptible for large amounts of muscle mass loss in the acute postoperative phase.
Our data present substantial heterogeneity in the magnitude of LBM, FFM, and SMM loss across studies. The subanalysis regarding bariatric procedures suggests that heterogeneity is mainly driven by type of surgery, in which adjustable gastric band procedures induced approximately 3 to 6 kg less LBM and FFM loss compared with other bariatric procedures. Nevertheless, weight loss is also lower after adjustable gastric band surgery. 88 Proportional loss was similar across procedures, which suggests that no bariatric procedure is more detrimental for LBM, FFM, or SMM than others. Furthermore, variation in magnitude of weight loss may also cause heterogeneity between and within studies. This hypothesis was confirmed by strong correlations between weight loss and LBM loss (N = 73, r = 0655, P < 0.001), FFM loss (N = 31, r = 0.639, P < 0.001), and SMM loss (N = 9, r = 0.854, P = 0.003). Future studies are therefore encouraged to report proportional LBM, FFM, or SMM loss, in order to compare loss of muscle tissue between individuals. Other preoperative factors are known to affect postbariatric muscle mass loss, such as body composition, gender, ethnicity, age, thyroid function and prevalence of diabetes, and growth hormone deficiency. 2 , 89 , 90 , 91 , 92 Postoperative factors such as protein intake and exercise levels may also contribute to the interindividual variation that was observed. 93 It is likely that the observed residual heterogeneity is caused by a combination of preoperative and postoperative factors. Unfortunately, we could not untangle their individual contributions due to the absence of this information in included studies and lack of stratified analyses. We therefore recommend future studies to consider these parameters in their analyses and perform stratified analyses to elucidate the exact impact of these factors on muscle mass loss. Taken together, the heterogeneity between and within studies calls for a more personalized approach in the battle against postbariatric LBM, FFM, and SMM loss.
In conjunction with the recognition of muscle mass loss as unfavorable consequence of bariatric surgery is enhancing, the research on this topic is expanding as well. Studies aiming for muscle mass preservation mainly focus on protein intake and (resistance) exercise, because of their essential role in protein synthesis. Whereas protein supplementation and exercise are often proposed as potential interventions, evidence of their effectiveness in muscle mass preservation during postbariatric weight loss is still scarce. Protein is known to increase satiety and enhance weight loss, 94 , 95 but additional protein intake via supplementation or high‐protein diets does not preserve LBM loss. 96 Likewise, studies incorporating (resistance) exercise training in postbariatric care show inconclusive results on muscle mass loss. 97 , 98 As both sufficient protein and exercise levels are required to optimally stimulate MPS, studies with combined approaches are warranted. Furthermore, certain other topics should be further addressed in future research. First, segmental analyses of postbariatric muscle mass should be performed to examine the impact of regional losses on health risks. Second, it should be determined how much of the FFM loss consists of preoperative excess fat mass and excess FFM. 99 Third, the magnitude of muscle tissue loss at which long‐term health substantially decreases should be further addressed in order to develop evidence‐based guidelines for clinical practice. Finally, there is evidence that muscle myostatin expression (i.e., a muscle growth inhibitor) is declined after bariatric surgery, suggesting that myostatin might be a natural regulator of muscle size in conditions of caloric restriction. 100 , 101 It is however unknown to which extent changes in myostatin contribute to muscle mass preservation.
One limitation of this meta‐analysis is that studies rarely report their exact definition of FFM or LBM and that DXA algorithms used to quantify body composition parameters were lacking. Due to interchangeable use of FFM and LBM, it could be possible that some data were stratified incorrectly. On the other hand, we were primarily interested in time‐dependent within‐study comparisons, so outcomes are likely less affected by this limitation. More clarity about underlying algorithms in DXA and more precise definitions of LBM and FFM could improve generalizability and harmonization of findings in future studies and meta‐analyses.
5. CONCLUSION
In conclusion, bariatric surgery induces 8 kg of LBM loss within 1‐year postsurgery. The most optimal time window to intervene are the first weeks postsurgery, since 55% of LBM loss is lost within 3 months. Although adjustable gastric band procedures showed less absolute LBM, FFM, and SMM loss, proportional loss was similar compared with other procedures. Future studies should focus on identifying patients with high risk for excessive loss of muscle tissue and on optimization of protein intake (e.g., protein source, timing, and tolerance) and exercise guidelines (e.g., type, volume, intensity and tolerance) in the first postoperative months. These insights could support the development of evidence‐based guidelines to limit postbariatric muscle mass loss, with feasible and effective interventions specifically for the bariatric population.
CONFLICT OF INTEREST
No conflict of interest was declared.
Supporting information
Figure S1. STROBE reporting quality per subitem and total score. Bars reflect the number of studies that score within each category (<60%, 60–80% ≥ 80%). Percentages are based on the points divided by the amount of applicable items within each subitem. T&A = title and abstract, In = Introduction, Me = methods, Re = results, Dis = discussion, Fun = funding.
Figure S2. Time‐dependent changes in lean body mass (2A), fat‐free mass (2B) and skeletal muscle mass (2C) up to 12‐months post‐surgery for each individual study arm.
Figure S3. Forest plots of lean body mass loss with respect to preoperative measures for each bariatric procedure. The effects size (mean differences between preoperative and postoperative measure) and 95% CI for individual studies and the pooled estimate per bariatric procedure are depicted. For each study, either the 12‐month follow‐up timepoint or the latest available timepoint was included for analysis. BAND = adjustable gastric band operation, BPD = biliopancreatic diversion, RYGB = Roux‐en‐Y gastric bypass, SG = sleeve gastrectomy, N = number of subjects, FU = follow up timepoint in months.
Figure S4. Forest plots of fat‐free mass loss with respect to preoperative measures for each bariatric procedure. The effects size (mean differences between preoperative and postoperative measure) and 95% CI for individual studies and the pooled estimate per bariatric procedure are depicted. For each study, either the 12‐month follow‐up timepoint or the latest available timepoint was included for analysis. BAND = adjustable gastric band operation, BPD = biliopancreatic diversion, RYGB = Roux‐en‐Y gastric bypass, SG = sleeve gastrectomy, N = number of subjects, FU = follow up timepoint in months.
Figure S5. Forest plots of skeletal muscle mass loss with respect to preoperative measures for each bariatric procedure. The effects size (mean differences between preoperative and postoperative measure) and 95% CI for individual studies and the pooled estimate per bariatric procedure are depicted. For each study, either the 12‐month follow‐up timepoint or the latest available timepoint was included for analysis. BAND = adjustable gastric band operation, BPD = biliopancreatic diversion, RYGB = Roux‐en‐Y gastric bypass, SG = sleeve gastrectomy, N = number of subjects, FU = follow up timepoint in months.
Figure S6. Proportional loss with respect to weight loss for lean body mass (LBM, panel A), fat‐free mass (FFM, panel B) and skeletal muscle mass (SMM, panel C). Blue lines reflect the pooled effect and the error bars reflect the corresponding lower and upper limit of the 95%CI. RYGB = Roux‐en‐Y Gastric bypass, SG = sleeve gastrectomy, BAND = adjustable gastric band operation, BPD = biliopancreatic diversion. No significant differences in proportional muscle loss between the different bariatric procedures were found for any of the outcome measures.
Figure S7. Waterfall plot for the 12‐month loss of LBM (dark blue), FFM (light blue) and SMM (white). Bars reflect individual study arms and are ranked from the largest effect to the smallest effect. The red dotted line reflects the pooled loss, as calculated by the meta‐analysis.
Table S1 Extensive search strategy for all key words adapted for each database.
Table S2 STROBE total and subscale scores per individual study (n = 59).
Data S1. Supporting information
Data S2. Supporting information
Nuijten MAH, Eijsvogels TMH, Monpellier VM, Janssen IMC, Hazebroek EJ, Hopman MTE. The magnitude and progress of lean body mass, fat‐free mass, and skeletal muscle mass loss following bariatric surgery: A systematic review and meta‐analysis. Obesity Reviews. 2022;23(1):e13370. doi: 10.1111/obr.13370
REFERENCES
- 1. Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219‐234. [DOI] [PubMed] [Google Scholar]
- 2. Nuijten MAH, Monpellier VM, Eijsvogels TMH, Janssen IMC, Hazebroek EJ, Hopman MTE. Rate and determinants of excessive fat‐free mass loss after bariatric surgery. Obes Surg. 2020;30(8):3119‐3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davidson LE, Yu W, Goodpaster BH, et al. Fat‐free mass and skeletal muscle mass five years after bariatric surgery. Obesity (Silver Spring). 2018;26(7):1130‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475‐482. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham JJ. Body composition as a determinant of energy expenditure: A synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54(6):963‐969. [DOI] [PubMed] [Google Scholar]
- 6. Faria SL, Kelly E, Faria OP. Energy expenditure and weight regain in patients submitted to Roux‐en‐Y gastric bypass. Obes Surg. 2009;19(7):856‐859. [DOI] [PubMed] [Google Scholar]
- 7. Van Venrooij LM, Verberne HJ, De Vos R, Borgmeijer‐Hoelen MM, Van Leeuwen PA, De Mol BA. Postoperative loss of skeletal muscle mass, complications and quality of life in patients undergoing cardiac surgery. Nutrition. 2012;28(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 8. Grannell A, De Vito G, Murphy JC, Le Roux CW. The influence of skeletal muscle on appetite regulation. Expert Rev Endocrinol Metab. 2019;14(4):267‐282. [DOI] [PubMed] [Google Scholar]
- 9. Flack KD, Hays HM, Moreland J. The consequences of exercise‐induced weight loss on food reinforcement. A randomized controlled trial. PLoS ONE. 2020;15(6):e0234692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turicchi J, O'Driscoll R, Finlayson G, et al. Associations between the proportion of fat‐free mass loss during weight loss, changes in appetite, and subsequent weight change: results from a randomized 2‐stage dietary intervention trial. Am J Clin Nutr. 2020;111(3):536‐544. [DOI] [PubMed] [Google Scholar]
- 11. Ciangura C, Bouillot J‐L, Lloret‐Linares C, et al. Dynamics of change in total and regional body composition after gastric bypass in obese patients. Obesity (Silver Spring, Md). 2010;18:760‐765. [DOI] [PubMed] [Google Scholar]
- 12. Fosbol MO, Zerahn B. Contemporary methods of body composition measurement. Clin Physiol Funct Imaging. 2015;35(2):81‐97. [DOI] [PubMed] [Google Scholar]
- 13. Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: Old evidence and new insights. Eur Radiol. 2020;30(4):2199‐2208. [DOI] [PubMed] [Google Scholar]
- 14. Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Ann Intern Med. 2007;147(8):W163‐W194. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arhire LI, Mihalache L, Padureanu SS, et al. Changes in bone mineral parameters after sleeve gastrectomy: Relationship with ghrelin and plasma adipokine levels. Acta Endocrinol (Bucharest, Romania: 2005). 2018;14:498‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bazzocchi A, Ponti F, Cariani S, et al. Visceral fat and body composition changes in a female population after RYGBP: A two‐year follow‐up by DXA. Obes Surg. 2015;25(3):443‐451. [DOI] [PubMed] [Google Scholar]
- 18. Beckman LM, Boullata JI, Fisher PL, Compher CW, Earthman CP. Evaluation of lean body weight equation by dual‐energy x‐ray absorptiometry measures. JPEN J Parenteral Enteral Nutr. 2017;41(3):392‐397. [DOI] [PubMed] [Google Scholar]
- 19. Bellicha A, Ciangura C, Roda C, Torcivia A, Portero P, Oppert J‐M. Changes in cardiorespiratory fitness after gastric bypass: Relations with accelerometry‐assessed physical activity. Obes Surg. 2019;29(9):2936‐2941. [DOI] [PubMed] [Google Scholar]
- 20. Blom‐Høgestøl IK, Mala T, Kristinsson JA, Brunborg C, Gulseth HL, Eriksen EF. Changes in bone quality after Roux‐en‐Y gastric bypass: A prospective cohort study in subjects with and without type 2 diabetes. Bone. 2020;130:115069‐115069. [DOI] [PubMed] [Google Scholar]
- 21. Brzozowska MM, Tran T, Bliuc D, et al. Roux‐en‐Y gastric bypass and gastric sleeve surgery result in long term bone loss. Int J Obes (Lond). 2021;45:235‐246. [DOI] [PubMed] [Google Scholar]
- 22. Calleja‐Fernández A, Pintor‐de‐la‐Maza B, Diez‐Rodríguez R, et al. Relationship between diet and body composition after biliopancreatic diversion. Obes Surg. 2015;25(11):2093‐2099. [DOI] [PubMed] [Google Scholar]
- 23. Chen X, Zhang C, Li J, Liu W, Zhang J, Zhou Z. Effects of laparoscopic sleeve gastrectomy on bone mineral density and bone metabolism in Chinese patients with obesity. Diabetes Metab Syndr Obes. 2020;13:4095‐4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clements RH, Saraf N, Kakade M, Yellumahanthi K, White M, Hackett JA. Nutritional effect of oral supplement enriched in beta‐hydroxy‐beta‐methylbutyrate, glutamine and arginine on resting metabolic rate after laparoscopic gastric bypass. Surg Endosc. 2011;25(5):1376‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole AJ, Kuchnia AJ, Beckman LM, et al. Long‐term body composition changes in women following Roux‐en‐Y gastric bypass surgery. JPEN J Parenteral Enteral Nutr. 2017;41(4):583‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coupaye M, Bouillot J‐L, Coussieu C, et al. One‐year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obes Surg. 15:827‐833. [DOI] [PubMed] [Google Scholar]
- 27. Diniz‐Sousa F, Veras L, Boppre G, et al. The effect of an exercise intervention program on bone health after bariatric surgery: A randomized controlled trial. J Bone Miner Res. 2021;36(3):489‐499. [DOI] [PubMed] [Google Scholar]
- 28. Faucher P, Aron‐Wisnewsky J, Ciangura C, et al. Changes in body composition, comorbidities, and nutritional status associated with lower weight loss after bariatric surgery in older subjects. Obes Surg. 2019;29(11):3589‐3595. [DOI] [PubMed] [Google Scholar]
- 29. Favre L, Marino L, Roth A, et al. The reduction of visceral adipose tissue after Roux‐en‐Y gastric bypass is more pronounced in patients with impaired glucose metabolism. Obes Surg. 2018;28(12):4006‐4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fjeldborg K, Pedersen SB, Møller HJ, et al. Intrahepatic fat content correlates with soluble CD163 in relation to weight loss induced by Roux‐en‐Y gastric bypass. Obesity (Silver Spring, Md). 2015;23:154‐161. [DOI] [PubMed] [Google Scholar]
- 31. Hayashi A, Maeda Y, Takemoto M, et al. Outcomes of laparoscopic sleeve gastrectomy in elderly obese Japanese patients. Geriatr Gerontol Int. 2017;17(11):2068‐2073. [DOI] [PubMed] [Google Scholar]
- 32. Kim TY, Shoback DM, Black DM, et al. Increases in PYY and uncoupling of bone turnover are associated with loss of bone mass after gastric bypass surgery. Bone. 2020;131:115115‐115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Legro RS, Dodson WC, Gnatuk CL, et al. Effects of gastric bypass surgery on female reproductive function. J Clin Endocrinol Metab. 2012;97(12):4540‐4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lubrano C, Cornoldi A, Pili M, et al. Reduction of risk factors for cardiovascular diseases in morbid‐obese patients following biliary‐intestinal bypass: 3 years' follow‐up. Int J Obes Relat Metab Disord. 2004;28(12):1600‐1606. [DOI] [PubMed] [Google Scholar]
- 35. Maïmoun L, Lefebvre P, Aouinti S, et al. Acute and longer‐term body composition changes after bariatric surgery. Surg Obes Relat Dis. 2019;15(11):1965‐1973. [DOI] [PubMed] [Google Scholar]
- 36. Marengo AP, Guerrero Pérez F, San Martín L, et al. Is trabecular bone score valuable in bone microstructure assessment after gastric bypass in women with morbid obesity? Nutrients. 2017;9(12):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matos OR, Ruthes EMP, Malinowski AKC, et al. Changes in bone mass and body composition after bariatric surgery. Gynecol Endocrinol. 2020;36(7):578‐581. [DOI] [PubMed] [Google Scholar]
- 38. Moizé V, Andreu A, Rodríguez L, et al. Protein intake and lean tissue mass retention following bariatric surgery. Clin Nutr (Edinburgh, Scotland). 2013;32:550‐555. [DOI] [PubMed] [Google Scholar]
- 39. Olbers T, Bjorkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux‐en‐y gastric bypass and laparoscopic vertical banded gastroplasty—A randomized clinical trial. Ann Surg. 244:715‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oppert J‐M, Bellicha A, Roda C, et al. Resistance training and protein supplementation increase strength after bariatric surgery: A randomized controlled trial. Obesity (Silver Spring, Md). 2018;26:1709‐1720. [DOI] [PubMed] [Google Scholar]
- 41. Rabl C, Rao MN, Schwarz J‐M, Mulligan K, Campos GM. Thermogenic changes after gastric bypass, adjustable gastric banding or diet alone. Surgery. 2014;156(4):806‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tacchino RM, Mancini A, Perrelli M, et al. Body composition and energy expenditure: relationship and changes in obese subjects before and after biliopancreatic diversion. Metab: Clin Exp. 2003;52(5):552‐558. [DOI] [PubMed] [Google Scholar]
- 43. Tałałaj M, Bogołowska‐Stieblich A, Wąsowski M, et al. The influence of laparoscopic sleeve gastrectomy on body composition and fat distribution in obese Caucasian men and women. Obes Surg. 2020;30(10):3974‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tamboli RA, Hossain HA, Marks PA, et al. Body composition and energy metabolism following Roux‐en‐Y gastric bypass surgery. Obesity (Silver Spring, Md). 2010;18:1718‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vatier C, Henegar C, Ciangura C, et al. Dynamic relations between sedentary behavior, physical activity, and body composition after bariatric surgery. Obes Surg. 2012;22(8):1251‐1256. [DOI] [PubMed] [Google Scholar]
- 46. Vaurs C, Diméglio C, Charras L, Anduze Y, Chalret du Rieu M, Ritz P. Determinants of changes in muscle mass after bariatric surgery. Diabetes Metab. 2015;41(5):416‐421. [DOI] [PubMed] [Google Scholar]
- 47. Vilarrasa N, San José P, García I, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3‐year follow‐up. Obes Surg. 2011;21(4):465‐472. [DOI] [PubMed] [Google Scholar]
- 48. Von Scholten BJ, Persson F, Svane MS, Hansen TW, Madsbad S, Rossing P. Effect of large weight reductions on measured and estimated kidney function. BMC Nephrol. 2017;18(1):52‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Werling M, Fändriks L, Olbers T, et al. Roux‐en‐Y gastric bypass surgery increases respiratory quotient and energy expenditure during food intake. PLoS ONE. 2015;10(6):e0129784‐e0129784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang X, Zhu C, Gao J, et al. Gender difference in the relationship between serum uric acid reduction and improvement in body fat distribution after laparoscopic sleeve gastrectomy in Chinese obese patients: A 6‐month follow‐up. Lipids Health Dis. 2018;17(1):288‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Zhu C, Wen X, et al. Laparoscopic sleeve gastrectomy improves body composition and alleviates insulin resistance in obesity related acanthosis nigricans. Lipids Health Dis. 2017;16(1):209‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bojsen‐Møller KN, Jacobsen SH, Dirksen C, et al. Accelerated protein digestion and amino acid absorption after Roux‐en‐Y gastric bypass. Am J Clin Nutr. 2015;102(3):600‐607. [DOI] [PubMed] [Google Scholar]
- 53. Busetto L, Tregnaghi A, Bussolotto M, et al. Visceral fat loss evaluated by total body magnetic resonance imaging in obese women operated with laparascopic adjustable silicone gastric banding. Int J Obes Relat Metab Disord. 2000;24(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 54. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1):41‐46. [DOI] [PubMed] [Google Scholar]
- 55. Garrapa GGM, Canibus P, Gatti C, et al. Changes in body composition and insulin sensitivity in severely obese subjects after laparoscopic adjustable silicone gastric banding (LASGB). Med Sci Monit: Int Med J Exp Clin Res. 2005;11(11):CR522‐CR528. [DOI] [PubMed] [Google Scholar]
- 56. Hirsch KR, Blue MNM, Trexler ET, Ahuja S, Smith‐Ryan AE. Provision of ready‐to‐drink protein following bariatric surgery: An evaluation of tolerability, body composition, and metabolic rate. Clin Nutr (Edinburgh, Scotland). 2021;40(4):2319‐2327. [DOI] [PubMed] [Google Scholar]
- 57. Jacobsen SH, Bojsen‐Møller KN, Dirksen C, et al. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose‐tolerant individuals. Diabetologia. 2013;56(10):2250‐2254. [DOI] [PubMed] [Google Scholar]
- 58. Johnson AJ, Matthie JR, Kuchnia A, et al. Evaluation of Advanced bioimpedance spectroscopy models for measuring body composition in healthy adults (NHANES 1999‐2004) and those undergoing massive weight loss following Roux‐en‐Y gastric bypass surgery. BRASPEN J. 2017;32:193‐202. [PMC free article] [PubMed] [Google Scholar]
- 59. Jorsal T, Christensen MM, Mortensen B, et al. Gut mucosal gene expression and metabolic changes After Roux‐en‐Y gastric bypass surgery. Obesity. 2020;28:2163‐2174. [DOI] [PubMed] [Google Scholar]
- 60. Kayser BD, Lhomme M, Dao MC, et al. Serum lipidomics reveals early differential effects of gastric bypass compared with banding on phospholipids and sphingolipids independent of differences in weight loss. Int J Obes (2005). 2017;41:917‐925. [DOI] [PubMed] [Google Scholar]
- 61. Khoo CM, Chen J, Pamuklar Z, Torquati A. Effects of Roux‐en‐Y gastric bypass or diabetes support and education on insulin sensitivity and insulin secretion in morbidly obese patients with type 2 diabetes. Ann Surg. 2014;259(3):494‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mingrone G, Greco AV, Giancaterini A, Scarfone A, Castagneto M, Pugeat M. Sex hormone‐binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis. 2002;161(2):455‐462. [DOI] [PubMed] [Google Scholar]
- 63. Moehlecke M, Andriatta Blume C, Rheinheimer J, Trindade MRM, Crispim D, Leitão CB. Early reduction of resting energy expenditure and successful weight loss after Roux‐en‐Y gastric bypass. Surg Obes Related Dis. 2017;13(2):204‐209. [DOI] [PubMed] [Google Scholar]
- 64. Nielsen MS, Alsaoodi H, Hjorth MF, Sjodin A. Physical activity, sedentary behavior, and sleep before and after bariatric surgery and associations with weight loss outcome. Obes Surg. 2021;31(1):250‐259. [DOI] [PubMed] [Google Scholar]
- 65. Raffaelli M, Iaconelli A, Nanni G, et al. Effects of biliopancreatic diversion on diurnal leptin, insulin and free fatty acid levels. Br J Surg. 2015;102(6):682‐690. [DOI] [PubMed] [Google Scholar]
- 66. Sajoux I, Lorenzo PM, Gomez‐Arbelaez D, et al. Effect of a very‐low‐calorie ketogenic diet on circulating myokine levels compared with the effect of bariatric surgery or a low‐calorie diet in patients with obesity. Nutrients. 2019;11(10):2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Savastano S, Belfiore A, Di Somma C, et al. Validity of bioelectrical impedance analysis to estimate body composition changes after bariatric surgery in premenopausal morbidly women. Obes Surg. 2010;20(3):332‐339. [DOI] [PubMed] [Google Scholar]
- 68. Sergi G, Lupoli L, Busetto L, et al. Changes in fluid compartments and body composition in obese women after weight loss induced by gastric banding. Ann Nutr Metab. 2003;47(3‐4):152‐157. [DOI] [PubMed] [Google Scholar]
- 69. Tan HC, Khoo CM, Tan MZ‐W, et al. The effects of sleeve gastrectomy and gastric bypass on branched‐chain amino acid metabolism 1 year after bariatric surgery. Obes Surg. 2016;26(8):1830‐1835. [DOI] [PubMed] [Google Scholar]
- 70. Turcotte A‐F, Grenier‐Larouche T, Ung R‐V, et al. Effects of biliopancreatic diversion on bone turnover markers and association with hormonal factors in patients with severe obesity. Obes Surg. 2019;29(3):990‐998. [DOI] [PubMed] [Google Scholar]
- 71. Coupaye M, Bouillot J‐L, Poitou C, Schutz Y, Basdevant A, Oppert J‐M. Is lean body mass decreased after obesity treatment by adjustable gastric banding? Obes Surg. 2007;17(4):427‐433. [DOI] [PubMed] [Google Scholar]
- 72. Davidson LE, Yu W, Goodpaster BH, et al. Fat‐free mass and skeletal muscle mass five years after bariatric surgery. Obesity (Silver Spring, Md). 2018;26:1130‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kenngott HG, Nickel F, Wise PA, et al. Weight loss and changes in adipose tissue and skeletal muscle volume after laparoscopic sleeve gastrectomy and Roux‐en‐Y gastric bypass: A prospective study with 12‐month follow‐up. Obes Surg. 2019;29(12):4018‐4028. [DOI] [PubMed] [Google Scholar]
- 74. Lamarca F, Melendez‐Araujo MS, Porto de Toledo I, Dutra ES, de Carvalho KMB. Relative energy expenditure decreases during the first year after bariatric surgery: A systematic review and meta‐analysis. Obes Surg. 2019;29(8):2648‐2659. [DOI] [PubMed] [Google Scholar]
- 75. Muller MJ, Bosy‐Westphal A. Adaptive thermogenesis with weight loss in humans. Obesity (Silver Spring). 2013;21(2):218‐228. [DOI] [PubMed] [Google Scholar]
- 76. Browning MG. Methodologic considerations in the evaluation of adaptive thermogenesis. Am J Clin Nutr. 2016;103(3):952‐953. [DOI] [PubMed] [Google Scholar]
- 77. Muller MJ, Wang Z, Heymsfield SB, Schautz B, Bosy‐Westphal A. Advances in the understanding of specific metabolic rates of major organs and tissues in humans. Curr Opin Clin Nutr Metab Care. 2013;16(5):501‐508. [DOI] [PubMed] [Google Scholar]
- 78. Hopkins M, Blundell JE. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clin Sci (Lond). 2016;130(18):1615‐1628. [DOI] [PubMed] [Google Scholar]
- 79. Voican CS, Lebrun A, Maitre S, et al. Predictive score of sarcopenia occurrence one year after bariatric surgery in severely obese patients. PLoS ONE. 2018;13(5):e0197248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta‐analysis. Exp Gerontol. 2020;131:110801. [DOI] [PubMed] [Google Scholar]
- 81. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: A systematic review and meta‐analysis. PLoS ONE. 2017;12(1):e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ciudin A, Simo‐Servat A, Palmas F, Barahona MJ. Sarcopenic obesity: A new challenge in the clinical practice. Endocrinol Diabetes Nutr. 2020;67(10):672‐681. [DOI] [PubMed] [Google Scholar]
- 83. Giusti V, Theytaz F, Di Vetta V, Clarisse M, Suter M, Tappy L. Energy and macronutrient intake after gastric bypass for morbid obesity: A 3‐y observational study focused on protein consumption. Am J Clin Nutr. 2016;103(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 84. Bertoni L, Valentini R, Zattarin A, et al. Assessment of protein intake in the first three months after sleeve gastrectomy in patients with severe obesity. Nutrients. 2021;13(3):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tabesh MR, Maleklou F, Ejtehadi F, Alizadeh Z. Nutrition, physical activity, and prescription of supplements in pre‐ and post‐bariatric surgery patients: A practical guideline. Obes Surg. 2019;29(10):3385‐3400. [DOI] [PubMed] [Google Scholar]
- 86. Macnaughton LS, Wardle SL, Witard OC, et al. The response of muscle protein synthesis following whole‐body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep. 2016;4(15):e12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Daniels P, Burns RD, Brusseau TA, et al. Effect of a randomised 12‐week resistance training programme on muscular strength, cross‐sectional area and muscle quality in women having undergone Roux‐en‐Y gastric bypass. J Sports Sci. 2018;36(5):529‐535. [DOI] [PubMed] [Google Scholar]
- 88. Kang JH, Le QA. Effectiveness of bariatric surgical procedures: A systematic review and network meta‐analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hunter GR, Bryan DR, Borges JH, David Diggs M, Carter SJ. Racial differences in relative skeletal muscle mass loss during diet‐induced weight loss in women. Obesity (Silver Spring). 2018;26:1255‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zheng X, Cheng Q, Long J, et al. Prevalence of low lean mass in patients with adult growth hormone deficiency with or without low‐dose growth hormone therapy. Clin Endocrinol (Oxf). 2019;90(6):834‐841. [DOI] [PubMed] [Google Scholar]
- 91. Stangierski A, Ruchala M, Krauze T, Moczko J, Guzik P. Treatment of severe thyroid function disorders and changes in body composition. Endokrynol Pol. 2016;67(4):359‐366. [DOI] [PubMed] [Google Scholar]
- 92. Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cava E, Yeat NC, Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8(3):511‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Faria SL, Faria OP, Buffington C, De Almeida CM, Ito MK. Dietary protein intake and bariatric surgery patients: A review. Obes Surg. 2011;21(11):1798‐1805. [DOI] [PubMed] [Google Scholar]
- 95. Lopes Gomes D, Moehlecke M, Lopes da Silva FB, Dutra ES, D'Agord Schaan B, Baiocchi de Carvalho KM. Whey protein supplementation enhances body fat and weight loss in women long after bariatric surgery: A randomized controlled trial. Obes Surg. 2017;27(2):424‐431. [DOI] [PubMed] [Google Scholar]
- 96. Romeijn MM, Holthuijsen DDB, Kolen AM, et al. The effect of additional protein on lean body mass preservation in post‐bariatric surgery patients: A systematic review. Nutr J. 2021;20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bellicha A, Ciangura C, Poitou C, Portero P, Oppert JM. Effectiveness of exercise training after bariatric surgery—A systematic literature review and meta‐analysis. Obes Rev. 2018;19(11):1544‐1556. [DOI] [PubMed] [Google Scholar]
- 98. Morales‐Marroquin E, Kohl HW 3rd, Knell G, De la Cruz‐Munoz N, Messiah SE. Resistance training in post‐metabolic and bariatric surgery patients: A systematic review. Obes Surg. 2020;30(10):4071‐4080. [DOI] [PubMed] [Google Scholar]
- 99. Hwaung P, Bosy‐Westphal A, Muller MJ, et al. Obesity tissue: Composition, energy expenditure, and energy content in adult humans. Obesity (Silver Spring). 2019;27(9):1472‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc. 2011;43(10):1828‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Milan G, Dalla Nora E, Pilon C, et al. Changes in muscle myostatin expression in obese subjects after weight loss. J Clin Endocrinol Metab. 2004;89(6):2724‐2727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. STROBE reporting quality per subitem and total score. Bars reflect the number of studies that score within each category (<60%, 60–80% ≥ 80%). Percentages are based on the points divided by the amount of applicable items within each subitem. T&A = title and abstract, In = Introduction, Me = methods, Re = results, Dis = discussion, Fun = funding.
Figure S2. Time‐dependent changes in lean body mass (2A), fat‐free mass (2B) and skeletal muscle mass (2C) up to 12‐months post‐surgery for each individual study arm.
Figure S3. Forest plots of lean body mass loss with respect to preoperative measures for each bariatric procedure. The effects size (mean differences between preoperative and postoperative measure) and 95% CI for individual studies and the pooled estimate per bariatric procedure are depicted. For each study, either the 12‐month follow‐up timepoint or the latest available timepoint was included for analysis. BAND = adjustable gastric band operation, BPD = biliopancreatic diversion, RYGB = Roux‐en‐Y gastric bypass, SG = sleeve gastrectomy, N = number of subjects, FU = follow up timepoint in months.
Figure S4. Forest plots of fat‐free mass loss with respect to preoperative measures for each bariatric procedure. The effects size (mean differences between preoperative and postoperative measure) and 95% CI for individual studies and the pooled estimate per bariatric procedure are depicted. For each study, either the 12‐month follow‐up timepoint or the latest available timepoint was included for analysis. BAND = adjustable gastric band operation, BPD = biliopancreatic diversion, RYGB = Roux‐en‐Y gastric bypass, SG = sleeve gastrectomy, N = number of subjects, FU = follow up timepoint in months.
Figure S5. Forest plots of skeletal muscle mass loss with respect to preoperative measures for each bariatric procedure. The effects size (mean differences between preoperative and postoperative measure) and 95% CI for individual studies and the pooled estimate per bariatric procedure are depicted. For each study, either the 12‐month follow‐up timepoint or the latest available timepoint was included for analysis. BAND = adjustable gastric band operation, BPD = biliopancreatic diversion, RYGB = Roux‐en‐Y gastric bypass, SG = sleeve gastrectomy, N = number of subjects, FU = follow up timepoint in months.
Figure S6. Proportional loss with respect to weight loss for lean body mass (LBM, panel A), fat‐free mass (FFM, panel B) and skeletal muscle mass (SMM, panel C). Blue lines reflect the pooled effect and the error bars reflect the corresponding lower and upper limit of the 95%CI. RYGB = Roux‐en‐Y Gastric bypass, SG = sleeve gastrectomy, BAND = adjustable gastric band operation, BPD = biliopancreatic diversion. No significant differences in proportional muscle loss between the different bariatric procedures were found for any of the outcome measures.
Figure S7. Waterfall plot for the 12‐month loss of LBM (dark blue), FFM (light blue) and SMM (white). Bars reflect individual study arms and are ranked from the largest effect to the smallest effect. The red dotted line reflects the pooled loss, as calculated by the meta‐analysis.
Table S1 Extensive search strategy for all key words adapted for each database.
Table S2 STROBE total and subscale scores per individual study (n = 59).
Data S1. Supporting information
Data S2. Supporting information


