Summary
Background
Foetal tobacco and cannabis exposure may have persistent cardio‐metabolic consequences in the offspring.
Objective
We examined the associations of maternal and paternal tobacco and cannabis use during pregnancy with offspring body fat and cardio‐metabolic outcomes.
Methods
In a population‐based prospective cohort study among 4792 mothers, fathers, and children, we assessed parental substance use by questionnaires. Childhood outcomes included body mass index (BMI), body fat, blood pressure, and lipid, glucose and insulin concentrations at 10 years.
Results
Children exposed to maternal tobacco use during pregnancy had a higher android/gynoid fat mass ratio (difference 0.22 SDS, 95% confidence interval [CI]: 0.13, 0.30), fat mass index (difference 0.20 SDS, 95% CI: 0.12, 0.28), triglyceride concentrations (difference 0.15 SDS, 95% CI: 0.04, 0.26), and a higher risk of overweight (odds ratio [OR] 1.35, 95% CI: 1.07, 1.71), compared to non‐exposed. Children exposed to maternal cannabis during pregnancy had a higher BMI (difference 0.26 SDS, 95% CI: 0.08, 0.44), android/gynoid fat mass ratio (difference 0.21 SDS, 95% CI: 0.04, 0.39), and fat‐free mass index (difference 0.24 SDS, 95% CI: 0.06, 0.41), compared to non‐exposed. The associations for paternal substance use with child cardio‐metabolic health outcomes were similar as those for maternal use.
Conclusions
Similar associations for maternal and paternal substance use during pregnancy suggest that these findings may be explained by shared family‐based social and lifestyle factors, rather than by direct foetal programming.
Keywords: body composition, cannabis, cardio‐metabolic health, child, obesity, pregnancy, smoking
1. INTRODUCTION
Adverse maternal and paternal lifestyle habits during pregnancy may have lifelong consequences for cardio‐metabolic health in offspring. 1 Developmental adaptations in response to adverse exposures may increase the susceptibility of cardiovascular disease and metabolic diseases in later life. 1 , 2 Despite many public health campaigns, maternal tobacco smoking during pregnancy remains a commonly used and modifiable factor. The adverse effects of maternal tobacco use during pregnancy on foetal development are well known. 3 , 4 , 5 In addition, the results from observational studies have suggested associations of foetal tobacco smoke exposure with obesity, cardiovascular disease and type 2 diabetes in adulthood. 6 , 7 , 8 Studies in children focused on cardiovascular risk factors showed inconsistent results. 9 , 10 , 11 , 12 , 13 These associations may be influenced by sex and ethnic differences. 9 , 14 Although less common than maternal tobacco smoking, maternal use of cannabis in pregnancy is increasing in western countries. 15 , 16 The prevalence of cannabis use in pregnant women is 7% in the United States − of which, 45% co‐use tobacco. 15 , 16 Cannabis use has been associated with reduced foetal growth. 17 Animal studies have shown that cannabis metabolites, for example, Δ9‐tetrahydrocannabinol, may affect cardiovascular and metabolic development. 18 , 19 Whether maternal cannabis use in pregnancy also has adverse cardiovascular and metabolic consequences in human offspring is not known. Importantly, observational studies on the associations of maternal tobacco and cannabis use with offspring outcomes may be confounded by family‐based social and lifestyle factors. 20 Comparison of associations between maternal and paternal substance use may provide insight on direct foetal programming effects or confounding by family‐based social and lifestyle factors. 20 Stronger associations for maternal exposure with the outcomes would suggest direct foetal programming, whereas similar or stronger associations for paternal exposures with the outcomes may suggest confounding by family‐based genetic, social and lifestyle factors.
Therefore, we examined the associations of maternal and paternal tobacco and cannabis use during pregnancy with child body mass index (BMI), body fat, blood pressure, and lipids, glucose and insulin concentrations at 10 years, in a population‐based prospective cohort study. We also compared the associations between maternal and paternal exposure to disentangle whether any association is explained by direct foetal programming or confounded by family‐based social and lifestyle factors.
2. METHODS
2.1. Study design
This study was embedded in the Generation R Study, a population‐based prospective cohort study conducted in Rotterdam, the Netherlands. 21 The study was approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam. The inclusion criteria for the pregnant women were (1) to be resident in the study area at their delivery date, (2) to have an expected date of delivery between April 2002 and January 2006, and (3) to give written informed consent. 21 The aim was to enrol mothers in pregnancy, but enrolment was possible until the birth of their child. In total, 9778 mothers were enrolled in the study. Of these mothers, 91% (n = 8879) was enrolled in pregnancy, and 71% of all fathers were included. 21 Of these, 8116 mothers had information on cannabis or tobacco and had singleton live‐born children. Cardio‐metabolic follow‐up measurements at the age of 10 years were available for 4792 (60%) children (Figure 1).
FIGURE 1.
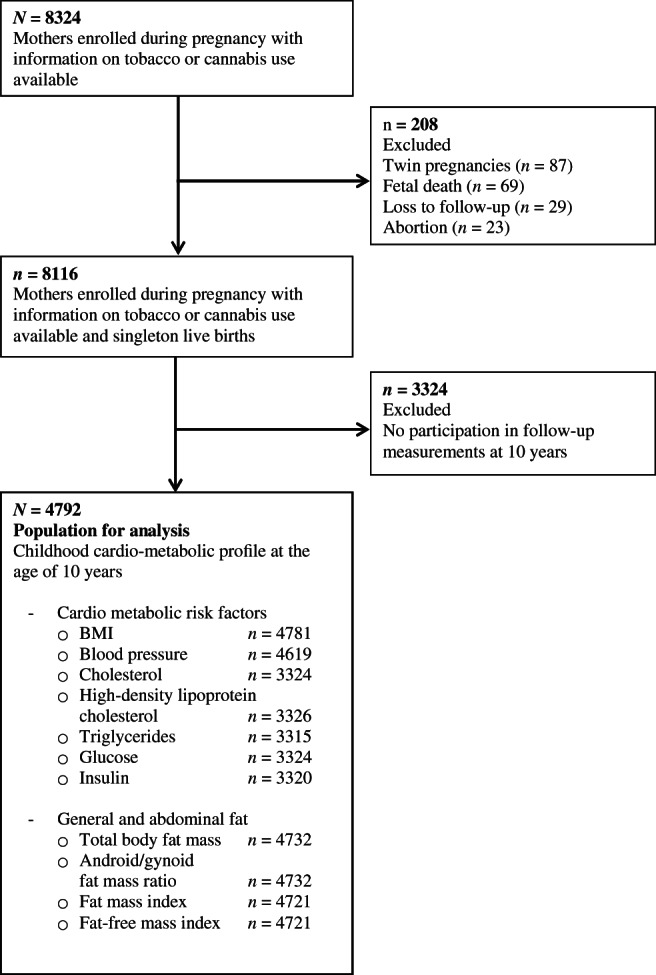
Flowchart of the study population
2.2. Foetal tobacco and cannabis exposure
As previously described, questionnaires were collected in early pregnancy (median 12.9 weeks of gestation, 25th–75th percentiles 12.1–14.5), mid‐pregnancy (median 20.4 weeks of gestation, 25th–75th percentiles 20.4–20.9), and late pregnancy (median 30.2 weeks of gestation, 25th–75th percentiles 29.9–30.8). 22 In early pregnancy, mothers were asked whether they smoked during pregnancy. Then, in mid and late pregnancy, mothers were asked whether they smoked in the last 2 months.
Maternal information on cannabis use was collected using a combination of self‐reports and urinalysis. In early pregnancy, mothers indicated whether they used cannabis before and/or during pregnancy, and whether they continued using cannabis after becoming aware of their pregnancy. 23 Mothers also reported the frequency of use (daily, weekly or monthly). Urine samples were available in a subset of the cohort and were collected in three trimesters, and the first available sample was used for urinalysis of the cannabis metabolite, 11‐nor‐Δ9‐THC‐9‐COOH, which were analysed using DRI® Cannabinoid Assay (Microgenics) with a cut‐off value of 50 μg/L as recommended by the manufacturer and the Substance Abuse and Mental Health Security Agency. Agreement between the self‐reported cannabis use and urinalysis was 0.77 (Yule's Y). 23
Maternal smoking during pregnancy was categorized into three groups as follows, excluding mother's cannabis users: no, until pregnancy was known (first trimester only) and continued smoking. In addition, a second categorization was performed given that cannabis use is often used in combination with tobacco. 16 We combined the information on maternal tobacco and cannabis use and categorized in four non‐overlapping groups: no (included women that quit smoking tobacco until pregnancy was known), cannabis before pregnancy, cannabis during pregnancy (in combination with tobacco) and continued tobacco use during pregnancy (without cannabis).
Paternal information on tobacco and cannabis use during pregnancy was assessed by both maternal reports and self‐reports during the first trimester of pregnancy, without specifying an exact period. The inter‐rater agreement between maternal and self‐report was high (Cohen's kappa cannabis use = 0.83, p < 0.001 and Cohen's kappa tobacco use = 0.86, p < 0.001). We used maternal reports because this information was available for more children as fewer fathers completed questionnaires (n = 4453).
Furthermore, the maternal and paternal frequency of smoking was also available and was categorized into three categories (no smoking, less than 5 per day, and more and equal than 5 per day).
2.3. Childhood body fat measurements and cardio‐metabolic risk score
Information on child anthropometrics, body composition, and cardio‐metabolic health was obtained at the median age of 9.7 years (95% range: 9.4–10.7). We measured the children's height and weight, without shoes and heavy clothing. We calculated BMI as total body weight in kilogram (kg) divided by height squared in meter (m2). BMI standard deviation score (SDS) was adjusted for sex and age according to Dutch reference growth curves (Growth Analyzer 4.0; Dutch Growth Research Foundation, Rotterdam, Netherlands). 24 We also created a categorical variable for childhood BMI (underweight, normal weight, overweight and obesity) according to the International Obesity Task Force cut‐offs. 25 For the analysis, we combined the overweight and obesity groups, hereafter only referred to as the overweight group. Total, android, and gynoid body fat mass were measured using a dual‐energy X‐ray absorptiometry (DXA) scanner (iDXA; General electrics, Lunar, Madison, Wisconsin, USA). Then, we calculated the android/gynoid fat mass ratio. 26 Childhood body fat mass is strongly influenced by the height of the child. We created index variables of body fat measurements independent of height, by using optimal adjustment estimated by log–log regression analysis. 27 We calculated fat mass index (FMI) and fat‐free mass index (FFMI) (total fat mass was divided by height at ‘4’ exponential, and fat‐free mass by height at ‘2’ exponential). 27
Systolic blood pressure and diastolic blood pressure were measured at the right brachial artery, four times with an interval of 1 min using the validated automatic sphygmomanometer Datascope Accutorr Plus. 28 We calculated mean systolic and diastolic blood pressure values using the last three blood pressure measurements to reduce measurement error. Non‐fasting venous blood samples were obtained to measure total cholesterol (mmol/L), high‐density lipoprotein cholesterol (HDL) (mmol/L), triglycerides (mmol/L), glucose (mmol/L) concentrations using enzymatic methods (Cobas 8000, Roche, Almere, the Netherlands) and insulin (pmol/L) concentrations using electrochemiluminescence immunoassay on the E411 module (Roche, Almere, the Netherlands). For the clustering of cardio‐metabolic risk factors, we defined whether there were any of three or more following components: android fat mass ≥ 75th percentile, systolic or diastolic blood pressure ≥ 75th percentile, triglycerides ≥75th percentile or HDL cholesterol ≤25th percentile and insulin ≥75th percentile. 29 We used android fat mass as percentage of total body fat mass as a proxy for waist circumference because waist circumference was not available.
We additionally examined the association with a continuous composite cardio‐metabolic score based on using standardized residuals (z‐score) (details in Methods S1). 30
2.4. Covariates
Potential covariates were selected based on previous literature and presented as a directed acyclic graphic (Figure S1). 7 , 8 , 10 , 15 , 16 , 31 , 32 , 33 Information on parental age, education, ethnicity, pre‐pregnancy BMI and alcohol use during pregnancy was obtained from self‐report questionnaires. Information on education and ethnicity were categorized according to the classification of Netherlands Statistics. 34 , 35 Maternal alcohol use was categorized as never drank, drank until pregnancy, and continued drinking during pregnancy. Like paternal smoking and cannabis use, paternal alcohol use was based on maternal report with a high inter‐rater (Cohen's kappa alcohol use = 0.80, p < 0.001). Paternal anthropometric measurements were assessed at enrolment. Height and weight were measured without shoes and heavy clothing, and BMI was calculated. Maternal psychopathology score was assessed with the Brief Symptom Inventory (BSI), a validated self‐reported measure of 53‐items covering a spectrum of psychopathology symptoms. 36 Child sex was extracted from medical records.
2.5. Statistical analysis
First, we showed descriptive statistics of the study population and performed non‐response analyses by comparing children with and without follow‐up measurements at years using chi‐squared for categorical and Student's t‐test or Mann–Whitney U tests for continuous variables. Second, we used linear regression models to analyse the associations of foetal tobacco and cannabis exposure with offspring body composition and cardio‐metabolic outcomes at 10 years, and used logistic regression to analyse the associations with risks of overweight and clustering of cardio‐metabolic risk in offspring. We used two models for the analysis. The basic model was adjusted for child sex and age. The confounder model was additionally adjusted for maternal age, education, ethnicity, alcohol use, psychopathology score, and pre‐pregnancy BMI. We tested the statistical interaction terms between parental tobacco and cannabis with child sex and with maternal ethnicity to examine potential differential associations. 9 , 14 In this article, we presented analyses for the full group in the main tables. Also, in the supplementary information, we showed the results for boys and girls separately and for Dutch mothers only. Analyses among the other ethnic subgroups were not possible because of the low numbers of the various ethnic subgroups. In addition, in the paternal tobacco and cannabis use models, we adjusted for paternal variables (age, ethnicity, alcohol use and BMI) instead of maternal variables. Finally, we examined the associations of foetal tobacco and cannabis exposure with the continuous composite cardio‐metabolic score in order to capture potential subtle differences cardio‐metabolic health. Not normally distributed outcomes measures (android/gynoid fat mass ratio, and insulin and triglycerides concentrations) were log‐natural transformed. To enable comparison of effect estimates, we constructed SDS of outcomes. Missing information on the covariates was between 0% and 10.2%, with the exception of maternal pre‐pregnancy BMI (14%), psychopathology score (15.9%) and paternal BMI (20.9%). To avoid the bias of complete case analyses, we used multiple imputation to impute missing information of the covariates in 25 datasets, using the mice package. 37 We repeated all analyses among complete cases only and observed similar associations (data not shown).
We applied Bonferroni correction to take multiple testing into account, so we divided the α = 0.05 by three categories of outcomes (body composition, blood pressure, metabolic outcomes), setting the statistical significance as two‐sided p < 0.017. All statistical analyses were performed using R statistical software, version 3.6.3 (R Foundation for Statistical Computing).
3. RESULTS
3.1. Subject characteristics and non‐response analysis
Table 1 shows the study population characteristics. Of all mothers, 24.1% and 2.5% used tobacco and cannabis during pregnancy, respectively. Of all fathers, 42.8% and 9.6% used tobacco and cannabis, respectively. Median child BMI was 17.0 kg/m2 (range 95% 14.0–24.9), with 18.7% being overweight. Tables S1 and S2 show the characteristics according to tobacco and cannabis categorization are provided. Non‐response analyses showed that participating mothers were slightly older, more often had European origin, had a higher education, had a lower psychopathology score, and less often used tobacco and cannabis during pregnancy compared to non‐participating mothers (Table S3).
TABLE 1.
Subject characteristics (N = 4792)
| Maternal characteristic | |
|---|---|
| Age, years, mean (SD) | 30.8 (4.9) |
| Ethnicity | |
| Dutch (%) | 56.9 |
| Non‐Dutch Non‐Western (%) | 31.1 |
| Non‐Dutch Western (%) | 12.0 |
| Educational level | |
| None/Primary (%) | 8.0 |
| Secondary (%) | 42.9 |
| Higher (%) | 49.1 |
| Pre‐pregnancy body mass index, kg/m2, median (95% range) | 22.6 (18.1–34.9) |
| Psychopathology score, median (95% range) | 0.15 (0–1.36) |
| Maternal alcohol use | |
| Never drank in pregnancy (%) | 43.5 |
| Drank until pregnancy was known (%) | 13.9 |
| Continued drinking (%) | 42.6 |
| Maternal tobacco use | |
| Never smoked in pregnancy (%) | 75.9 |
| Smoked until pregnancy was known (%) | 8.8 |
| Continued smoking in pregnancy (%) | 15.3 |
| Maternal cannabis use | |
| No use (%) | 94.9 |
| Cannabis before pregnancy (%) | 2.6 |
| Cannabis during pregnancy (%) | 2.5 |
| Paternal characteristics | |
|---|---|
| Age, years, mean (SD) | 33.4 (5.8) |
| Ethnicity | |
| Dutch (%) | 58.1 |
| Non‐Dutch Non‐Western (%) | 32.0 |
| Non‐Dutch Western (%) | 9.9 |
| Alcohol use, yes (%) | 77.9 |
| Tobacco use, yes (%) | 42.8 |
| Cannabis use, yes (%) | 9.6 |
| Body mass index, kg/m2, median (95% range) | 25.1 (19.6–32.9) |
| Child characteristics | |
|---|---|
| Female sex, yes (%) | 50.5 |
| Age, years, mean (SD) | 9.8 (0.3) |
| Weight, kilograms, median (95% range) | 34 (25.2–53.8) |
| Height, centimetres, mean (SD) | 141.6 (6.7) |
| Body mass index, kg/m2, median (95% range) | 17.0 (14.0–24.9) |
| Body composition | |
| Total fat mass, kg, median (95% range) | 8.5 (4.5–22.2) |
| Android/gynoid fat mass ratio, median (95% range) | 0.24 (0.15–0.49) |
| Fat‐free mass, kg, median (95% range) | 25.3 (19.1,33.9) |
| Blood pressure | |
| Systolic, mmHg, mean (SD) | 103.2 (7.9) |
| Diastolic, mmHg, mean (SD) | 58.6 (6.4) |
| Lipid concentrations | |
| Total cholesterol, mmol/L, mean (SD) | 4.31 (0.66) |
| HDL cholesterol, mmol/L, mean (SD) | 1.48 (0.34) |
| Triglycerides, mmol/L, median (95% range) | 0.98 (0.42–2.62) |
| Insulin, pmol/L, median (95% range) | 176.7 (35.7–646.4) |
| Glucose, mmol/L, mean (SD) | 5.2 (0.9) |
| Overweight, yes (%) | 18.7 |
| Cardio‐metabolic clustering risk, yes (%) | 9.5 |
Note: Values are presented as means (SD), medians (95% range) or percentages. There were no missing data on these variables as they were imputed using multiple imputation methods. There were no missing data on these variables as they were imputed using multiple imputation methods.
Abbreviations: HDL, high‐density lipoprotein; SD, standard deviation.
3.2. Parental tobacco and cannabis exposure and childhood body fat outcomes at 10 years
Maternal tobacco smoking in the first trimester only was not associated with childhood BMI nor body composition (Table 2). Compared to non‐exposed children, those exposed to maternal continued smoking during pregnancy had a higher android/gynoid fat mass ratio (difference 0.22 SDS, 95% confidence interval [CI] 0.13–0.30), a higher fat mass index (difference 0.20 SDS, 95% CI 0.12–0.28) and a higher risk of overweight (odds ratio [OR] 1.35, 95% CI 1.07–1.71). Dose–response association displayed the highest effect estimates in children whose mothers continued smoking ≥5 cigarettes per day (Table 2). Maternal cannabis use before pregnancy was not associated with childhood body fat outcomes. As compared to non‐exposed children, those exposed to maternal cannabis use during pregnancy had a higher BMI (difference 0.26 SDS, 95% CI 0.08–0.44), a higher android/gynoid fat mass ratio (difference 0.21 SDS, 95% CI 0.04–0.39), and a higher fat‐free mass index (difference 0.24 SDS, 95% CI 0.06–0.41) (Table 2). Dose–response analyses displayed the highest effect estimates in children whose mothers used daily cannabis (data not shown). No associations with fat mass index were observed (Table 2). We also observed largely similar associations of maternal and paternal tobacco/cannabis use with offspring outcomes (Table 2).
TABLE 2.
Associations of maternal and paternal tobacco and cannabis use with childhood body fat outcomes at age 10 years
| Tobacco only | Body mass index (SDS) a (n = 4252) | Android‐gynoid ratio (SDS) a (n = 4208) | Fat mass index (SDS) a (n = 4199) | Fat‐free mass index (SDS) a (n = 4199) | Overweight b (n = 4252) |
|---|---|---|---|---|---|
| Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | OR (95% CI) | |
| Maternal use | |||||
| No | Reference | Reference | Reference | Reference | Reference |
| First trimester only | 0.02 (−0.09, 0.13) | 0.11 (0.00, 0.21)* | −0.02 (−0.12, 0.08) | 0.03 (−0.07, 0.13) | 1.16 (0.85, 1.60) |
| Continued | 0.16 (0.07, 0.25)** | 0.22 (0.13, 0.30)** | 0.20 (0.12, 0.28)** | 0.07 (−0.01, 0.16) | 1.35 (1.07, 1.71)** |
| <5 per day | 0.10 (−0.02, 0.23) | 0.13 (0.00, 0.25)* | 0.11 (0.00, 0.22) | 0.04 (−0.08, 0.16) | 1.13 (0.81, 1.60) |
| ≥5 per day | 0.21 (0.10, 0.33)** | 0.29 (0.18, 0.40)** | 0.28 (0.18, 0.38)** | 0.10 (−0.01, 0.21) | 1.54 (1.15, 2.07)** |
| Paternal use | |||||
| No | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.13 (0.06, 0.19)** | 0.17 (0.11, 0.23)** | 0.15 (0.09, 0.20)** | 0.03 (−0.03, 0.09) | 1.30 (1.09, 1.55)** |
| <5 per day | 0.07 (−0.02, 0.15) | 0.08 (0.00, 0.17) | 0.06 (−0.02, 0.13) | 0.02 (−0.06, 0.10) | 1.10 (0.85, 1.44) |
| ≥5 per day | 0.16 (0.09, 0.24)** | 0.22 (0.15, 0.29)** | 0.21 (0.14, 0.27)** | 0.03 (−0.03, 0.10) | 1.40 (1.15, 1.72)** |
| Cannabis | Body mass index (SDS) a (n = 4781) | Android‐gynoid ratio (SDS) a (n = 4732) | Fat mass index (SDS) a (n = 4721) | Fat‐free mass index (SDS) a (n = 4721) | Overweight b (n = 4781) |
|---|---|---|---|---|---|
| Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | OR (95% CI) | |
| Maternal use | |||||
| No | Reference | Reference | Reference | Reference | Reference |
| Cannabis before | 0.08 (−0.10, 0.25) | 0.01 (−0.16, 0.18) | 0.03 (−0.13, 0.19) | 0.15 (−0.02, 0.32) | 1.25 (0.75, 2.08) |
| Cannabis during | 0.26 (0.08, 0.44)** | 0.21 (0.04, 0.39)** | 0.14 (−0.02, 0.30) | 0.24 (0.06, 0.41)** | 1.33 (0.82, 2.16) |
| Continued tobacco only | 0.17 (0.08, 0.25)** | 0.21 (0.13, 0.30)** | 0.21 (0.13, 0.28)** | 0.07 (−0.01, 0.15) | 1.34 (1.07, 1.69)** |
| Paternal use | |||||
| No | Reference | Reference | Reference | Reference | Reference |
| Cannabis use | 0.12 (0.03, 0.22)** | 0.12 (0.02, 0.22)** | 0.08 (−0.01, 0.17) | 0.17 (0.08, 0.27)** | 1.29 (0.99, 1.69) |
Note: Confounder parental models were adjusted for maternal age, maternal education, maternal ethnicity, maternal alcohol use, maternal psychopathology score, pre‐pregnancy body mass index (BMI), child sex and child age. *p‐value <0.05, **p‐value <0.017 (Bonferroni corrected values for multiple testing).
Abbreviation: Android‐gynoid ratio (Android/gynoid fat mass ratio).
Values represent regression coefficients (difference) and 95% confidence interval (95% CI) from linear regression models that reflects the differences in childhood outcomes standard deviation score (SDS) for maternal or paternal tobacco and/or cannabis use during pregnancy, compared to the reference group.
Values are odds ratio (OR) and 95% CI from logistic regression models that reflect the risk of childhood overweight and obesity for maternal and paternal tobacco and/or cannabis use during pregnancy, compared to the reference group.
3.3. Parental tobacco and cannabis exposure and childhood cardio‐metabolic risk factors at 10 years
First‐trimester maternal tobacco was not associated with childhood cardio‐metabolic outcomes (Table 3). Compared to children of mothers who did not use tobacco during pregnancy, children exposed to maternal continued tobacco use during pregnancy had higher triglyceride concentrations (difference 0.15 SDS, 95% CI 0.04–0.26). Also, children exposed to continued smoking during pregnancy ≥5 cigarettes per day had a higher systolic blood pressure (difference 0.15 SDS, 95% CI 0.03–0.26) and a higher risk of cardio‐metabolic clustering (OR 1.59, 95% CI 1.09, 2.32) (Table 3). We did not observe associations of maternal tobacco use with cholesterol and glucose outcomes (Table 3). The association of maternal continued tobacco use during pregnancy with diastolic blood pressure was explained by family‐based social and lifestyle factors (data not shown). No associations of maternal cannabis use with childhood cardio‐metabolic outcomes were observed (Table 3). We observed largely similar associations of maternal and paternal tobacco/cannabis use with offspring outcomes (Table 3).
TABLE 3.
Associations of maternal and paternal tobacco and cannabis use with childhood cardio‐metabolic risk factors at age 10 years
| Tobacco only | Systolic blood pressure (SDS) a (n = 4109) | Diastolic blood pressure (SDS) a (n = 4109) | Total cholesterol (SDS) a (n = 2959) | HDL cholesterol (SDS) a (n = 2960) | Triglyceride (SDS) a (n = 2950) | Glucose (SDS) a (n = 2958) | Cardio‐metabolic clustering b (n = 2862) |
|---|---|---|---|---|---|---|---|
| Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | OR (95% CI) | |
| Maternal use | |||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| First trimester only | −0.06 (−0.17, 0.05) | −0.02 (−0.13, 0.09) | 0.05 (−0.08, 0.18) | 0.02 (−0.11, 0.15) | 0.09 (−0.04, 0.22) | 0.01 (−0.13, 0.14) | 0.87 (0.56, 1.36) |
| Continued | 0.09 (0.00, 0.18) | 0.08 (−0.01, 0.17) | 0.07 (−0.04, 0.18) | −0.07 (−0.18, 0.04) | 0.15 (0.04, 0.26)** | 0.02 (−0.09, 0.13) | 1.31 (0.96, 1.78) |
| <5 per day | 0.02 (−0.11, 0.15) | 0.01 (−0.12, 0.14) | 0.08 (−0.07, 0.23) | −0.09 (−0.24, 0.06) | 0.11 (−0.04, 0.26) | 0.02 (−0.13, 0.17) | 0.99 (0.62, 1.57) |
| ≥5 per day | 0.15 (0.03, 0.26)** | 0.14 (0.02, 0.25)* | 0.05 (−0.09, 0.20) | −0.06 (−0.20, 0.08) | 0.19 (0.04, 0.33)** | 0.02 (−0.12, 0.17) | 1.59 (1.10, 2.32)** |
| Paternal use | |||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | |
| Yes | 0.04 (−0.03, 0.10) | 0.03 (−0.03, 0.09) | 0.05 (−0.03, 0.12) | −0.02 (−0.09, 0.06) | 0.06 (−0.02, 0.13) | 0.00 (−0.08, 0.08) | 1.16 (0.92, 1.47) |
| <5 per day | 0.00 (−0.09, 0.09) | 0.02 (−0.07, 0.11) | 0.02 (−0.08, 0.13) | −0.05 (−0.15, 0.06) | 0.10 (−0.01, 0.20) | −0.13 (−0.24, −0.02)** | 1.10 (0.78, 1.53) |
| ≥5 per day | 0.06 (−0.01, 0.14) | 0.04 (−0.04, 0.12) | 0.07 (−0.02, 0.15) | 0.00 (−0.08, 0.09) | 0.03 (−0.06, 0.12) | 0.08 (−0.01, 0.17) | 1.20 (0.93, 1.56) |
| Cannabis | Systolic blood pressure (SDS) a (n = 4619) | Diastolic blood pressure (SDS) a (n = 4619) | Total cholesterol (SDS) a (n = 3324) | HDL cholesterol (SDS) a (n = 3326) | Triglyceride (SDS) a (n = 3315) | Glucose (SDS) a (n = 3324) | Cardio‐metabolic clustering b (n = 3212) |
|---|---|---|---|---|---|---|---|
| Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | OR (95% CI) | |
| Maternal use | |||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Cannabis before | 0.01 (−0.17, 0.19) | 0.06 (−0.12, 0.24) | −0.09 (−0.30, 0.12) | 0.08 (−0.13, 0.29) | −0.14 (−0.35, 0.07) | −0.05 (−0.26, 0.17) | 0.55 (0.23, 1.31) |
| Cannabis during | 0.16 (−0.03, 0.35) | 0.02 (−0.17, 0.21) | 0.00 (−0.23, 0.23) | −0.14 (−0.37, 0.08) | −0.03 (−0.26, 0.20) | 0.02 (−0.21, 0.25) | 1.74 (0.94, 3.22) |
| Continued tobacco only | 0.10 (0.01, 0.19)* | 0.08 (−0.01, 0.17) | 0.06 (−0.05, 0.17) | −0.09 (−0.20, 0.01) | 0.16 (0.05, 0.27)** | 0.00 (−0.11, 0.11) | 1.33 (0.99, 1.80) |
| Paternal use | |||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Cannabis use | 0.04 (−0.07, 0.14) | 0.02 (−0.08, 0.12) | 0.02 (−0.10, 0.15) | 0.07 (−0.05, 0.20) | −0.04 (−0.16, 0.08) | −0.07 (−0.19, 0.05) | 0.95 (0.65, 1.37) |
Note: Confounder parental models were adjusted for maternal age, maternal education, maternal ethnicity, maternal alcohol use, maternal psychopathology score, pre‐pregnancy body mass index (BMI), child sex and child age. *p‐value <0.05, **p‐value <0.017 (Bonferroni corrected values for multiple testing).
Abbreviation: HDL, high‐density lipoprotein cholesterol.
Values represent regression coefficients (difference) and 95% confidence interval (95% CI) from linear regression models that reflects the differences in childhood outcomes standard deviation score (SDS) for maternal or paternal tobacco and/or cannabis use during pregnancy, compared to the reference group.
Values are odds ratio (OR) and 95% CI from logistic regression models that reflect the risk of childhood cardio‐metabolic clustering risk for maternal and paternal tobacco and/or cannabis use during pregnancy, compared to the reference group.
3.4. Supplementary analysis
Overall, the associations of foetal tobacco and cannabis exposure with the body fat outcomes tended to be somewhat stronger among girls than among boys (Tables S4). The associations of foetal tobacco exposure with blood pressure and lipids concentrations tended to be somewhat stronger among boys, and with glucose outcomes tended to be somewhat stronger among girls (Table S5 and S6). Girls whose parents used cannabis during pregnancy had a higher fat mass index, a higher risk of overweight, higher insulin concentrations, and a higher risk of cardio‐metabolic clustering (Tables S4, S5 and S6).
In addition, we also observed statistical interactions of maternal ethnicity with android/gynoid fat mass ratio, fat‐free mass index, and triglycerides concentrations. The analyses among only children of Dutch mothers showed similar results as in the full study population, but the effect sizes tended to be stronger (Tables S7 and S8). The p‐values for interaction are shown in Supplementary Tables S4–S8. Furthermore, the adjustment for paternal variables instead of maternal variables did not change the results (data not shown). Finally, children exposed to continued maternal smoking during pregnancy had a higher continuous composite cardio‐metabolic z‐score, as compared to those non‐exposed to continued maternal smoking during pregnancy. The effect estimate was strongest in those exposed to more than five cigarettes per day (Table S9).
4. DISCUSSION
In this population‐based prospective birth cohort study, we observed that not only maternal but also paternal tobacco use during pregnancy was associated with an adverse body fat and cardio‐metabolic profile in children. The associations of parental cannabis use during pregnancy with an adverse body fat and cardio‐metabolic profile were stronger in girls than in boys. Similar associations for maternal and paternal tobacco and cannabis use with childhood cardio‐metabolic health factors in offspring suggest that these associations may not be solely explained through direct foetal programming, but by shared family‐based social and lifestyle factors.
4.1. Interpretation main findings
Foetal exposure to tobacco and cannabis may affect foetal growth and the cardio‐metabolic health in offspring. 1 , 2 Worldwide, up to 25% of all pregnant women continue to use tobacco during pregnancy. 33 While many women who smoke attempt to quit smoking, only 20%–30% successfully quit. 38 Findings from previous studies suggest that foetal tobacco exposure negatively influences cardio‐metabolic health in childhood and adulthood. 6 , 7 , 8 , 9 , 10 , 13 , 32 , 39 However, the impact of foetal cannabis exposure on childhood cardio‐metabolic health is not known yet. 15 , 16 The current study was specifically focused on the associations of both foetal tobacco and cannabis exposure with offspring body fat and cardio‐metabolic outcomes at 10 years. It is well known that observational studies on the associations of maternal lifestyle‐related factors with childhood outcomes may be confounded by shared family‐based genetic, social and lifestyle factors. 20 Comparison of associations between maternal and paternal substance use may provide insight on direct foetal programming effects or confounding by family‐based social and lifestyle factors. 20
The results from a previous meta‐analysis showed that children from mothers who continued tobacco smoking during pregnancy had a higher risk of overweight and obesity in early childhood, adolescence and adulthood. 13 , 40 Previous studies also observed that paternal tobacco use was associated with higher offspring body fat measures and risks of overweight and obesity. 9 , 13 , 40 , 41 , 42 A meta‐analysis among 109 838 children aged 4–18 years showed that the effect estimates for the association of maternal tobacco use during pregnancy with offspring obesity risk were slightly higher than the effect estimates for paternal tobacco use, but similar effect estimates and overlapping confidence intervals were observed with household smoking. 40 In addition, another meta‐analysis of 106 601 children aged 5–10 years reported that the effect estimates for the associations of maternal tobacco use on childhood overweight risk were slightly higher than for paternal tobacco use, but the risk increased significantly when both parents used tobacco. 13 These large meta‐analyses are important, but had limited information about detailed cardio‐metabolic outcomes. Also, no information on cannabis use was available.
In the current study, we compared the associations of maternal and paternal use to disentangle between support for direct foetal programming effects, genetic confounding or confounding by family‐based social and lifestyle factors. 20 We observed that both maternal and paternal continued tobacco use during pregnancy were associated with a higher risk of overweight, and higher body fat measures. However, only maternal continued smoking during pregnancy was associated with higher triglycerides concentrations, a higher systolic blood pressure, and a higher risk of cardio‐metabolic clustering in the offspring. The current study is a follow‐up study of several previous reports from the Generation R cohort. We previously reported that the 4‐year‐old offspring of mothers who continued smoking during pregnancy had a higher BMI and an increased risk of obesity, as compared to those whose mothers did smoke during pregnancy. 32 Paternal smoking was not associated with risk of obesity in offspring. 32 A previous follow‐up study in the same cohort among children aged 6 years observed that the effects estimates for the associations of maternal and paternal smoking with body fat outcomes were similar. 9 Another study reported that children aged 6 years whose mothers smoked more than 10 cigarettes per day during pregnancy had a higher diastolic blood pressure and a higher fractional shortening of left ventricular outflow, as compared to those who did not smoke during pregnancy. 10 The difference between these previous studies and the current study could be explained by the changes in body size and proportion that occur with childhood development. The association of adverse foetal exposure with childhood or adult outcomes might differ across the life course. An observational study among 9424 children aged 0–10 years in the United Kingdom (UK) reported that maternal smoking during pregnancy, but not paternal smoking, was associated with a higher ponderal index until aged 2 years. However, both maternal and paternal smoking during pregnancy were associated with childhood BMI, and the effect estimates were similar for fathers and mothers. 42 Another study in the same cohort in the UK reported similar associations of both maternal and paternal smoking with childhood body fat outcomes, consistent with the findings of the current study. 41
Also, previous results from the Nurse Health Study II (NHS) suggested that both maternal and paternal smoking (more than 15 cigarettes per day) during pregnancy were associated with an increased risk of hypertension and type 2 diabetes in their adult daughters. 7 , 8 These findings suggest that the associations of maternal and paternal smoking during pregnancy with offspring outcomes may differ across the life course. We observed that maternal education was the strongest confounder in the observed associations and explained the largest part of the difference between the basic and adjusted model. Differences in early childhood may be the result of direct foetal programming according to previous studies, whereas at later age, the differences may be the results of social and family‐based risk shared risk factors. We used paternal tobacco smoking or cannabis use as negative control for the associations of maternal smoking or cannabis use exposure with childhood outcomes. This approach is generally being used. 43 A potential limitation of this approach is that paternal tobacco smoking or cannabis use might still have direct biological effects through sperm and subsequent foetal programming. However, previous studies showed that the associations of paternal smoking or passive maternal smoking with childhood outcomes are much weaker as compared to those for maternal smoking. 13 To the best of our knowledge, the current study is the first that examined the associations of maternal and paternal cannabis use during pregnancy with childhood cardio‐metabolic outcomes in offspring. We observed that both maternal and paternal cannabis use during pregnancy are associated with an adverse body fat distribution. These associations tended to be stronger among girls than among boys. Previous findings from a study in rats showed that cannabis exposure during foetal life led to alterations of endocrine pancreatic in the development of impaired glucose tolerance, and aberrant insulin response in adult females only. 19 Foetal cannabis exposure may disrupt the complex signalling of the endocannabinoid system, charging of the regulating metabolism and appetite. 44 , 45 However, similar to tobacco use, the presence of associations for both maternal and paternal cannabis use with adverse body fat outcomes and cardio‐metabolic outcomes suggests that the associations are explained by confounding by family‐based social and lifestyle factors rather than direct foetal programming mechanisms.
Clearly, previous studies and our results demonstrating associations of maternal tobacco and cannabis use during pregnancy with a wide range of adverse outcomes for mothers and their unborn child, which should be without any doubt clear enough to discourage tobacco and cannabis use before, during and after pregnancy. 5 , 13 , 16 , 17 Our findings provide an important perspective in the needed to prevent smoking in women and men in reproductive age. Further studies are needed to assess critical periods for tobacco and cannabis exposure effects on childhood development.
4.2. Strengths and limitations
The strengths of our study were a prospective design, large sample size, information of a large number of potential confounders, assessment of both maternal and paternal tobacco and cannabis use and information about a wide range of outcomes. However, some limitations need to be discussed. First, we had a follow‐up response of about 60%. We may have selectively missed women at risk for tobacco and cannabis use. Second, the assessment of tobacco and cannabis use by questionnaire may have introduced misclassification. Parents may tend to underreport their tobacco and/or cannabis use as a socially disapproved behaviour, potentially causing an underestimation of the observed associations. However, previous studies have reported a high correlation between cotinine concentrations and self‐reported smoking. 46 In addition, urinalysis showed that self‐reported information was in agreement with cannabis metabolites. 23 Third, the fasting time before blood sampling was limited to 30 min; thus, our samples were considered non‐fasting. 47 Also, the blood samples were collected at different time‐points during the day depending on the time of the study visit. Since glucose and insulin levels shift easily during the day and are sensitive toward carbohydrate intake, this may lead to non‐differential misclassification of children underestimating the observed effect estimates. Conversely, the non‐fasting sampling of lipid levels is superior to fasting in predicting cardio‐metabolic events. 48 So, the lipids levels may be not influenced by the non‐fasting state. Finally, we used information about multiple confounders and were able to compare maternal and paternal exposure to address familial confounding. Nevertheless, as in any observational study, residual confounding might still be present.
5. CONCLUSION
In conclusion, our findings suggest that both maternal tobacco and cannabis use during pregnancy are associated with adverse body fat and cardio‐metabolic profiles in their offspring. However, similar associations between maternal and paternal tobacco and cannabis use during pregnancy with offspring cardio‐metabolic health outcomes suggest that these findings are explained by genetic or family‐based social and lifestyle factors, rather than direct foetal programming.
CONFLICT OF INTEREST
No conflict of interest was declared.
Supporting information
Table S1. Descriptive statistics according to tobacco use.
Table S2. Descriptive statistics according to cannabis and tobacco use.
Table S3. Non‐response analysis.
Figure S1. Directed acyclic graphic of the study.
Table S4. Associations of maternal and paternal tobacco and cannabis use during pregnancy with childhood body fat outcomes at age 10 years stratified for child sex.
Table S5. Associations of maternal and paternal tobacco and cannabis use during pregnancy with childhood blood pressure and metabolic outcomes at age 10 years stratified for child sex.
Table S6. Associations of maternal and paternal tobacco and cannabis use during pregnancy with childhood cardio‐metabolic risk factors at age 10 years stratified for child sex.
Table S7. Associations of maternal and paternal tobacco and cannabis use with childhood body fat outcomes only for children at age 10 years of Dutch mothers.
Table S8. Associations of maternal and paternaltobacco and cannabis use with childhood cardio‐metabolic risk factors only for children at age 10 years of Dutch mothers.
Methods S1. Calculation of the continuous composite cardio‐metabolic risk z‐score.
Table S9. Associations of maternal and paternal tobacco and cannabis use with childhood continuous composite cardio‐metabolic z‐score at 10 years.
ACKNOWLEDGEMENTS
Kim N. Cajachagua‐Torres was involved conception, study design, data analysis and interpretation, literature research, generation of tables and figures, and writing of the manuscript. Vincent W.V. Jaddoe and Hanan El Marroun were involved in conception, study design, data acquisition, data analysis and interpretation. Irwin K.M. Reiss and Susana Santos were involved in the interpretation of data. All authors critically reviewed the manuscript and had final approval of the submitted version.
The general design of the Generation R Study was supported by Erasmus Medical Center, Erasmus University Rotterdam, the Netherlands, the Organization for Health Research and Development (ZonMw) and the Ministry of Health, Welfare and Sport. Vincent W.V. Jaddoe received funding from a Consolidator Grant from the European Research Council (ERC‐2014‐CoG‐648 916). Hanan El Marroun was supported by Stichting Volksbond Rotterdam, the Dutch Brain Foundation (De Hersenstichting, Project Number GH2016.2.01) and the NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (Grant Number 27853), and the European Union's Horizon 2020 Research and Innovation Program (LifeCycle, Grant Agreement 733 206). Kim N. Cajachagua‐Torres was supported by Peruvian Scholarship (547‐2018‐SERVIR). The study sponsors had no role in the study design, analysis and interpretation of data, and writing of the manuscript, or in the decision to submit the manuscript for publication. The authors gratefully acknowledge the contribution of parents and their children, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Cajachagua‐Torres KN, El Marroun H, Reiss IKM, Santos S, Jaddoe VWV. Foetal tobacco and cannabis exposure, body fat and cardio‐metabolic health in childhood. Pediatric Obesity. 2022;17(3):e12863. doi: 10.1111/ijpo.12863
Funding information European Research Council, Grant/Award Number: ERC‐2014‐CoG‐648916; European Union's Horizon 2020 Research and Innovation Program, Grant/Award Number: 733206; NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation, Grant/Award Number: 27853; Peruvian Scholarship, Grant/Award Number: 547‐2018‐SERVIR; Stichting Volksbond Rotterdam, the Dutch Brain Foundation, Grant/Award Number: GH2016.2.01
REFERENCES
- 1. Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733‐1736. doi: 10.1126/science.1095292 [DOI] [PubMed] [Google Scholar]
- 2. Bakker H, Jaddoe VWV. Cardiovascular and metabolic influences of fetal smoke exposure. Eur J Epidemiol. 2011;26(10):763‐770. doi: 10.1007/s10654-011-9621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaddoe VW, Verburg BO, de Ridder MA, et al. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epidemiol. 2007;165(10):1207‐1215. doi: 10.1093/aje/kwm014 [DOI] [PubMed] [Google Scholar]
- 4. Roza SJ, Verburg BO, Jaddoe VW, et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur J Neurosci. 2007;25(3):611‐617. doi: 10.1111/j.1460-9568.2007.05393.x [DOI] [PubMed] [Google Scholar]
- 5. Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125‐S140. doi: 10.1080/14622200410001669187 [DOI] [PubMed] [Google Scholar]
- 6. Harris HR, Willett WC, Michels KB. Parental smoking during pregnancy and risk of overweight and obesity in the daughter. Int J Obes. 2013;37(10):1356‐1363. doi: 10.1038/ijo.2013.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaddoe VW, de Jonge LL, van Dam RM, et al. Fetal exposure to parental smoking and the risk of type 2 diabetes in adult women. Diabetes Care. 2014;37(11):2966‐2973. doi: 10.2337/dc13-1679 [DOI] [PubMed] [Google Scholar]
- 8. de Jonge LL, Harris HR, Rich‐Edwards JW, et al. Parental smoking in pregnancy and the risks of adult‐onset hypertension. Hypertension. 2013;61(2):494‐500. doi: 10.1161/hypertensionaha.111.200907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durmus B, Heppe DH, Taal HR, et al. Parental smoking during pregnancy and total and abdominal fat distribution in school‐age children: the Generation R Study. Int J Obes. 2014;38(7):966‐972. doi: 10.1038/ijo.2014.9 [DOI] [PubMed] [Google Scholar]
- 10. Taal HR, de Jonge LL, van Osch‐Gevers L, et al. Parental smoking during pregnancy and cardiovascular structures and function in childhood: the Generation R Study. Int J Epidemiol. 2013;42(5):1371‐1380. doi: 10.1093/ije/dyt178 [DOI] [PubMed] [Google Scholar]
- 11. Nordenstam F, Norman M, Wickström R. Blood Pressure and Heart Rate Variability in Preschool Children Exposed to Smokeless Tobacco in Fetal Life. Article. J Am Heart Assoc. 2019;8(21):e012629. doi: 10.1161/jaha.119.012629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Power C, Atherton K, Thomas C. Maternal smoking in pregnancy, adult adiposity and other risk factors for cardiovascular disease. Atherosclerosis. 2010;211(2):643‐648. doi: 10.1016/j.atherosclerosis.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 13. Philips EM, Santos S, Trasande L, et al. Changes in parental smoking during pregnancy and risks of adverse birth outcomes and childhood overweight in Europe and North America: An individual participant data meta‐analysis of 229,000 singleton births. PLoS Med. 2020;17(8):e1003182. doi: 10.1371/journal.pmed.1003182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Hoog MLA, van Eijsden M, Stronks K, Gemke RJBJ, Vrijkotte TGM. Ethnic differences in cardiometabolic risk profile at age 5‐6 years: the ABCD study. PLoS ONE. 2012;7(8):e43667. doi: 10.1371/journal.pone.0043667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volkow ND, Han B, Compton WM, McCance‐Katz EF. Self‐reported Medical and Nonmedical Cannabis Use Among Pregnant Women in the United States. JAMA. 2019;322(2):167‐169. doi: 10.1001/jama.2019.7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215(4):506 e1‐e7. doi: 10.1016/j.ajog.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 17. Gunn JK, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta‐analysis. BMJ Open. 2016;6(4):e009986. doi: 10.1136/bmjopen-2015-009986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woods SC. Role of the Endocannabinoid System in Regulating Cardiovascular and Metabolic Risk Factors. Am J Med. 2007;120(3 Suppl 1):S19‐S25. doi: 10.1016/j.amjmed.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 19. Gillies R, Lee K, Vanin S, et al. Maternal exposure to Δ9‐tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod Toxicol. 2020;94:84‐91. doi: 10.1016/j.reprotox.2020.04.070 [DOI] [PubMed] [Google Scholar]
- 20. Santos S, Zugna D, Pizzi C, Richiardi L. Sources of confounding in life course epidemiology. J Dev Orig Health Dis. 2019;10(3):299‐305. doi: 10.1017/S2040174418000582 [DOI] [PubMed] [Google Scholar]
- 21. Jaddoe VWV, van Duijn CM, Franco OH, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739‐756. doi: 10.1007/s10654-012-9735-1 [DOI] [PubMed] [Google Scholar]
- 22. Jaddoe VWV, Mackenbach JP, Moll HA, et al. The Generation R Study: Design and cohort profile. Eur J Epidemiol. 2006;21(6):475‐484. doi: 10.1007/s10654-006-9022-0 [DOI] [PubMed] [Google Scholar]
- 23. El Marroun H, Tiemeier H, Jaddoe VW, et al. Agreement between maternal cannabis use during pregnancy according to self‐report and urinalysis in a population‐based cohort: the Generation R Study. Eur Addict Res. 2011;17(1):37‐43. doi: 10.1159/000320550 [DOI] [PubMed] [Google Scholar]
- 24. Fredriks AM, van Buuren S, Wit JM, Verloove‐Vanhorick SP. Body index measurements in 1996‐7 compared with 1980. Arch Dis Child. 2000;82(2):107‐112. doi: 10.1136/adc.82.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240‐1243. doi: 10.1136/bmj.320.7244.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helba M, Binkovitz LA. Pediatric body composition analysis with dual‐energy X‐ray absorptiometry. Pediatr Radiol. 2009;39(7):647‐656. doi: 10.1007/s00247-009-1247-0 [DOI] [PubMed] [Google Scholar]
- 27. Wells JC, Cole TJ. steam As. Adjustment of fat‐free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26(7):947‐952. doi: 10.1038/sj.ijo.0802027 [DOI] [PubMed] [Google Scholar]
- 28. Wong SN, Tz Sung RY, Leung LC. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Press Monit. 2006;11(5):281‐291. doi: 10.1097/01.mbp.0000209082.09623.b4 [DOI] [PubMed] [Google Scholar]
- 29. Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119(4):628‐647. doi: 10.1161/CIRCULATIONAHA.108.191394 [DOI] [PubMed] [Google Scholar]
- 30. Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17‐17. doi: 10.1186/1475-2840-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El Marroun H, Tiemeier H, Jaddoe VW, et al. Demographic, emotional and social determinants of cannabis use in early pregnancy: the Generation R study. Drug Alcohol Depend. 2008;98(3):218‐226. doi: 10.1016/j.drugalcdep.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 32. Durmus B, Kruithof CJ, Gillman MH, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R Study. Am J Clin Nutr. 2011;94(1):164‐171. doi: 10.3945/ajcn.110.009225 [DOI] [PubMed] [Google Scholar]
- 33. Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta‐analysis. Lancet Glob Health. 2018;6(7):e769‐e776. doi: 10.1016/s2214-109x(18)30223-7 [DOI] [PubMed] [Google Scholar]
- 34. Statistics Netherlands . Allochtonen in Nederland 2004. Centraal Bureau voor de Statistiek; 2004. [Google Scholar]
- 35. Statistics Netherlands . Standaard Onderwijsindeling 2003. Centraal Bureau voor de Statistiek; 2004. [Google Scholar]
- 36. Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595‐605. doi: 10.1017/S0033291700048017 [DOI] [PubMed] [Google Scholar]
- 37. van Buuren S, Groothuis‐Oudshoorn K. Mice: multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):1‐67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 38. Bruin JE, Gerstein HC, Holloway AC. Long‐term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116(2):364‐374. doi: 10.1093/toxsci/kfq103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rayfield S, Plugge E. Systematic review and meta‐analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2017;71(2):162‐173. doi: 10.1136/jech-2016-207376 [DOI] [PubMed] [Google Scholar]
- 40. Riedel C., Schonberger K., Yang S., Koshy G., Chen Y.‐C., Gopinath B., Ziebarth S., von Kries R. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared with paternal smoking‐‐a meta‐analysis. International Journal of Epidemiology. 2014;43(5):1593‐1606. doi: 10.1093/ije/dyu150 [DOI] [PubMed] [Google Scholar]
- 41. Leary SD, Smith GD, Rogers IS, Reilly JJ, Wells JCK, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity (Silver Spring). 2006;14(12):2284‐2293. doi: 10.1038/oby.2006.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Howe LD, Matijasevich A, Tilling K, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. Int J Epidemiol. 2012;41(3):722‐732. doi: 10.1093/ije/dys025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102(2):245‐256. doi: 10.1111/j.1742-7843.2007.00191.x [DOI] [PubMed] [Google Scholar]
- 44. Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27(1):73‐100. doi: 10.1210/er.2005-0009 [DOI] [PubMed] [Google Scholar]
- 45. Cajachagua‐Torres KN, Jaddoe VWV, de Rijke YB, et al. Parental cannabis and tobacco use during pregnancy and childhood hair cortisol concentrations. Drug Alcohol Depend. 2021;225:108751. doi: 10.1016/j.drugalcdep.2021.108751 [DOI] [PubMed] [Google Scholar]
- 46. McDonald SD, Perkins SL, Walker MC. Correlation between self‐reported smoking status and serum cotinine during pregnancy. Addict Behav. 2005;30(4):853‐857. doi: 10.1016/j.addbeh.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 47. Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243‐1264. doi: 10.1007/s10654-016-0224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Langsted A, Nordestgaard BG. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. 2019;51(2):131‐141. doi: 10.1016/j.pathol.2018.09.062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Descriptive statistics according to tobacco use.
Table S2. Descriptive statistics according to cannabis and tobacco use.
Table S3. Non‐response analysis.
Figure S1. Directed acyclic graphic of the study.
Table S4. Associations of maternal and paternal tobacco and cannabis use during pregnancy with childhood body fat outcomes at age 10 years stratified for child sex.
Table S5. Associations of maternal and paternal tobacco and cannabis use during pregnancy with childhood blood pressure and metabolic outcomes at age 10 years stratified for child sex.
Table S6. Associations of maternal and paternal tobacco and cannabis use during pregnancy with childhood cardio‐metabolic risk factors at age 10 years stratified for child sex.
Table S7. Associations of maternal and paternal tobacco and cannabis use with childhood body fat outcomes only for children at age 10 years of Dutch mothers.
Table S8. Associations of maternal and paternaltobacco and cannabis use with childhood cardio‐metabolic risk factors only for children at age 10 years of Dutch mothers.
Methods S1. Calculation of the continuous composite cardio‐metabolic risk z‐score.
Table S9. Associations of maternal and paternal tobacco and cannabis use with childhood continuous composite cardio‐metabolic z‐score at 10 years.


