Summary
To systematically review and analyze the effects of resistance‐based exercise programs on body composition, regional adiposity, and body weight in individuals with overweight/obesity across the lifespan. Using PRISMA guidelines, randomized controlled trials were searched in nine electronic databases up to December 2020. Meta‐analyses were performed using random‐effects model. One‐hundred sixteen articles describing 114 trials (n = 4184 participants) were included. Interventions involving resistance training and caloric restriction were the most effective for reducing body fat percentage (ES = −3.8%, 95% CI: −4.7 to −2.9%, p < 0.001) and whole‐body fat mass (ES = −5.3 kg, 95% CI: −7.2 to −3.5 kg, p < 0.001) compared with groups without intervention. Significant results were also observed following combined resistance and aerobic exercise (ES = −2.3% and −1.4 kg, p < 0.001) and resistance training alone (ES = −1.6% and −1.0 kg, p < 0.001) compared with no training controls. Resistance training alone was the most effective for increasing lean mass compared with no training controls (ES = 0.8 kg, 95% CI: 0.6 to 1.0 kg, p < 0.001), whereas lean mass was maintained following interventions involving resistance training and caloric restriction (ES = ~ − 0.3 kg, p = 0.550–0.727). Results were consistently observed across age and sex groups (p = 0.001–0.011). Reductions in regional adiposity and body weight measures were also observed following combined resistance and aerobic exercise and programs including caloric restriction (p < 0.001). In conclusion, this study provides evidence that resistance‐based exercise programs are effective and should be considered within any multicomponent therapy program when caloric restriction is utilized in individuals with overweight or obesity.
Keywords: body composition, obesity, resistance training
1. INTRODUCTION
Multicomponent lifestyle and therapy interventions are considered the cornerstone for the management of obesity. 1 , 2 Several guidelines recommend exercise, dietary, and behavioural interventions to improve weight loss in this population. 2 , 3 , 4 In regard to exercise interventions, aerobic exercise (i.e., activity involving large muscle groups and performed in a continuous or intermittent fashion over an extended period of time, such as cycling, swimming, jogging, or running) is recommended as the main exercise component for additional weight loss, 2 , 3 , 4 , 5 whereas resistance exercise (i.e., anabolic exercise; performing sets of repeated movements against a resistance) has been considered less critical due to insufficient evidence on the effects on reducing body weight or body mass index (BMI). 2 , 3 , 4 , 5 However, determining the effectiveness of resistance exercise is challenging due to the reliance on body weight rather than overall body composition in individuals with overweight/obesity, as resistance exercise can result in body weight increases due to the accrual of lean mass, which is highly associated with metabolic health and physical function. Although body weight and BMI are important and extensively used in clinical practice, they do not differentiate lean from fat mass or depots of adiposity (i.e., visceral vs. subcutaneous adipose tissue), underestimating the importance of these tissues for overall health. Consequently, this precludes identifying the potential use of resistance training in individuals with overweight/obesity. Moreover, despite previous systematic reviews investigating exercise and dietary effects on body composition 4 , 5 , 6 , 7 and visceral adipose tissue, 8 , 9 , 10 , 11 , 12 the specific effects of resistance exercise on fat mass and lean mass have not been investigated in depth in those overweight/obese. For instance, it is not well understood if resistance exercise, alone or combined with other exercise components and dietary interventions, results in meaningful effects on fat mass while maintaining or increasing lean mass in this population. This information may improve exercise prescription for obese individuals, increasing potential treatment options for this population.
As a result, the present study aimed to systematically review and analyze the effects of resistance‐based exercise programs (i.e., interventions including resistance exercise as one of the components) compared with no intervention control groups on body fat percentage, whole‐body and trunk fat mass, visceral and subcutaneous adipose tissue, lean mass, body weight and BMI in individuals with overweight/obesity across the lifespan. In addition, we also examined a range of possible moderators, including age at baseline, sex, and exercise modality (e.g., resistance exercise alone, or combined with different exercise or dietary interventions).
2. METHODS
2.1. Search strategy and study selection procedure
All procedures undertaken in the present study were reported in accordance with the Cochrane Back Review Group (CBRG) 13 and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement, 14 , 15 with registration at the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42020217986).
This review included randomized controlled trials evaluating the effects of resistance‐based exercise programs combined or not with nutritional programs (e.g., protein supplementation, low‐fat diet, or caloric restriction) on body fat percentage, whole‐body fat mass, trunk fat mass, visceral and subcutaneous adipose tissue and whole‐body lean mass in participants with overweight/obesity (i.e., as defined in the studies included). Studies involving children and adolescents (<18 years), young adults (≥18 to 35 years), middle‐aged adults (>35 to 59 years), and older adults (≥ 60 years) who are overweight or obese were included. The primary outcomes for this review were body fat, fat mass, trunk fat mass, visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and lean mass. Secondary outcomes were body weight and BMI. The exclusion criteria were (1) studies involving individuals with other chronic conditions such as type II diabetes or cancer because of the interaction between treatments and outcomes; (2) studies involving participants with overweight/obesity enrolled in water‐based resistance training as the only study intervention; (3) studies with interventions lasting less than 4 weeks; (4) studies comprising control groups receiving any active exercise or dietary interventions that constituted an intervention for body composition; and (5) studies written in a language other than English, Portuguese, or Spanish. This review included peer‐review published and unpublished studies. The search was conducted in CINAHL, Cochrane Library, EMBASE, LILACS, PubMed, SciELO, SportDiscus, and Web of Science databases for peer‐review published studies and MedNar, OpenGrey, and OpenThesis databases for unpublished studies. The date of the search was December 2020, with no limitation for publication date. A manual search was undertaken in the reference lists provided in all retrieved studies. Eligibility was assessed independently and evaluated in triplicate (P. L., E. R. N., and V. M. W. and P. L., R. N. B., and D. J. P. T.). The search strategy is presented in Data S1.
2.2. Data extraction
Data extraction was performed via a standardized form. For each study, details including sample size, sex, age, overweight/obesity criteria, baseline BMI, baseline body fat, experimental design (intervention groups and their respective sample sizes), resistance‐based exercise program (i.e., intervention duration, volume, and intensity), and dietary program prescription were extracted along with the outcomes of interest. In addition, retention (i.e., number of participants that completed the study) and attendance (i.e., number of sessions attended) were assessed from the studies. For the outcomes assessed, baseline and post‐intervention assessment and within‐ and between‐group mean difference were extracted in their absolute units and for the longest period of the intervention. When studies did not provide dispersion values of change such as standard deviation (SD), standard errors or 95% confidence intervals (95% CI), the SD of the change was calculated assuming a correlation of r = 0.5 between the baseline and post‐intervention assessment measures by the square root of . 16 For studies containing multiple intervention arms versus control groups, only data from those comprising resistance exercise as part of the intervention were extracted. When graphs were used instead of numerical data, the graphs were measured through their plots using a specific tool for data extraction (WebPlotDigitizer, San Francisco, CA). 17
2.3. Risk of bias assessment
The risk of bias was evaluated according to the 2nd version of the Cochrane risk‐of‐bias tool for randomized trials (RoB 2), with each assessment focused on the outcome level. 18 The six‐domain instrument includes (1) randomization process, (2) deviation from intended interventions, (3) missing outcome data, (4) measurement of the outcome, (5) selection of the reported result, and (6) overall bias. The study quality assessment for all included studies was performed independently by two reviewers (E. R. N. and V. M. W. or R. N. B. and D. J. P. T.), with disagreements resolved by a third reviewer (P. L.).
2.4. Statistical analysis
For the meta‐analysis, the pooled effect estimates from body fat percentage, fat mass, trunk fat mass, lean mass, body weight, and BMI were obtained and expressed as mean difference (MD) of baseline to the final assessment of the intervention versus control group. For VAT and SAT, results were expressed as standardized mean difference (SMD) due to the different units reported in the studies included. Meta‐analyses were conducted for overall studies with subgroup analyses provided for (1) age groups (i.e., children/adolescents, young adults, middle‐aged adults, older adults); (2) sex (i.e., female, male, mixed participants); and (3) exercise modality (e.g., resistance training, combined resistance and aerobic exercise, resistance training + caloric restriction). Furthermore, to avoid overestimating the weight of a study by entering it multiple times in the overall effect analyses, effects of different exercise groups were combined when reported/presented in the same study, 19 whereas they were considered independent in subgroup analysis for intervention modality effects. Calculations were performed using a random‐effects model with the DerSimonian and Laird method. 20 Statistical significance was assumed when the MD or SMD effect was below an α level of p ≤ 0.05. Effect sizes (ES) for SMD results were according to Cohen with values of 0.0 to <0.5 indicating small, values of 0.51 to <0.8 indicating medium, and values ≥0.8 indicating large effects. 21
Statistical heterogeneity was assessed using the Cochran Q test. A threshold p‐value of 0.1 and values greater than 50% in I 2 were considered indicative of high heterogeneity. 19 We examined heterogeneity using the package “dmetar” from R (function find. Outlier; R Core Team, 2020) by omitting studies in which the confidence intervals did not overlap the estimated pooled effect. Publication bias was explored by contour‐enhanced funnel plots and Egger's test, 22 and if necessary, trim‐and‐fill computation was used to estimate the effect of publication bias on the interpretation of results. 23 Analyses were conducted using the package “meta” from R (R Core Team, 2020) and Review Manager (RevMan) software from the Cochrane Collaboration (version 5.4, Copenhagen: The Nordic Cochrane Centre). Results and tables presented for the outcome measures are after sensitivity analysis procedure adjustments.
3. RESULTS
Four‐thousand seven‐hundred and fifty‐six studies were retrieved from our search, with 2737 potential records retained for screening after duplicate removals. After excluding 1080 records due to their irrelevance to the research question, 1657 were considered eligible for full‐text assessment (Figure 1). A total of 116 articles 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 describing 114 independent trials were included in this systematic review and meta‐analysis with 23 articles examining children or adolescents, 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 30 articles examining young adults, 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 38 articles examining middle‐aged adults, 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 and 25 articles examining older adults who are overweight or obese. 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139
FIGURE 1.
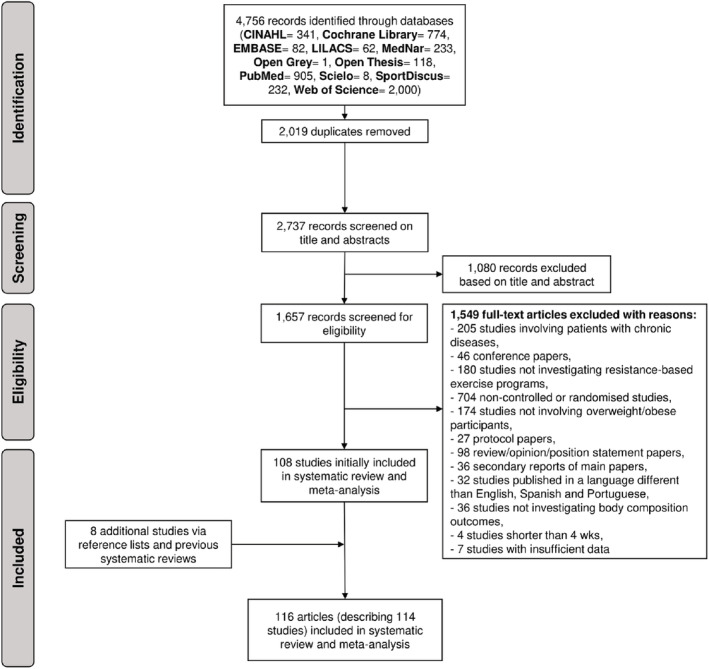
Flow chart of study selection process
A total of 4184 participants with overweight/obesity were included in this systematic review, involving 878 children/adolescents [median age = 14.8 years (interquartile range [IQR]): 11.8 to 15.4 years), median BMI = 30.1 kg·m2 (IQR: 26.4 to 33.4), and body fat percentage = 36.7% (IQR: 34.1 to 43.2%)], 658 young adults [median age = 23.6 years (IQR: 21.9 to 27.2), median BMI = 29.6 kg·m2 (IQR: 26.9 to 31.4), and body fat percentage = 31.7% (IQR: 28.1 to 37.4)], 1416 middle‐aged adults [median age = 47.0 years (IQR: 39.9 to 52.4), median BMI = 30.4 kg·m2 (IQR: 28.7 to 32.7), and body fat percentage = 38.5% (IQR: 32.0 to 44.7)] and 1232 older adults [median age = 67.3 years (IQR: 64.1 to 68.9), median BMI = 29.6 kg·m2 (IQR: 28.1 to 31.7), and body fat percentage = 37.4% (IQR: 35.3 to 42.7)].
In summary, most studies included resistance training alone (56 out of 114 studies, 49.1%), followed by combined resistance and aerobic exercise (51 out of 114 studies, 44.7%), combined resistance and aerobic exercise + caloric restriction (8 out of 114 studies, 7.0%), and resistance training + caloric restriction (6 out of 114 studies, 5.3%). Regarding exercise prescription characteristics, the mean intervention duration was 14.6 ± 11.0 weeks (range: 4 to 96 weeks) with frequency ranging from 1 to 5 sessions per week. Information about resistance training volume was reported by 75 studies (65.8%) and ranged from 20 to 165 weekly resistance exercise sets, whereas resistance training peak intensity was reported by 64 studies (56.1%) and ranged from 20% to 97% of one‐repetition maximum (1‐RM). The characteristics of the individual studies are presented in Tables S1 to S4.
3.1. Risk of bias
High risk of bias was observed in 70 out of 98 studies (71.4%) examining body fat percentage, 37 out of 62 studies (59.7%) examining fat mass, 6 out of 15 studies (40.0%) examining VAT, 3 out of 8 studies (37.5%) examining SAT, and 43 out of 70 studies (61.4%) examining lean mass. For body weight and BMI, 23 out of 98 studies (23.5%) had a high risk of bias in the overall risk of bias assessment. The individual risk of bias assessment for children/adolescents, young adults, middle‐aged adults, and older adults are presented in Tables S5 to S8.
3.2. Body fat percentage and whole‐body fat mass
Resistance‐based exercise programs resulted in significant reductions in body fat percentage (number of studies [k] = 89, ES = −2.2%, 95% CI: −2.4 to −2.0%) and whole‐body fat mass (k = 52, ES = −1.6 kg, 95% CI: −1.9 to −1.3 kg) (Table 1). These effects were consistent across children/adolescents (ES = −2.1%, 95% CI: −2.8 to −1.3% and ES = −1.9 kg, 95% CI: −2.9 to −0.8 kg), young adults (ES = −2.7%, 95% CI: −3.7 to −1.7% and ES = −1.0 kg, 95% CI: −1.4 to −0.5 kg), middle‐aged adults (ES = −2.4%, 95% CI: −2.5 to −2.3% and ES = −1.2 kg, 95% CI: −2.1 to −0.4 kg), and older adults (ES = −1.9%, 95% CI: −2.4 to −1.4% and ES = −1.7 kg, 95% CI: −2.3 to −1.2 kg). Results are presented for body fat percentage and whole‐body fat mass across the lifespan before sensitivity analysis procedure adjustments in Figure 2 and Figure 3, respectively. In addition, significant effects were observed in studies involving female (ES = −2.4%, 95% CI: −2.5 to −2.3% and ES = −1.0 kg, 95% CI: −1.3 to −0.7 kg), males (ES = −2.8%, 95% CI: −3.4 to −2.2% and ES = −2.6 kg, 95% CI: −3.8 to −1.4 kg), and mixed participants (ES = −1.7%, 95% CI: −2.1 to −1.2% and ES = −2.0 kg, 95% CI: −2.6 to −1.5 kg). The most effective exercise modality for reducing body fat percentage was resistance training + caloric restriction, with changes of −3.8% (95% CI: −4.7 to −2.9%). For reducing fat mass, both resistance training + caloric restriction and combined resistance and aerobic exercise + caloric restriction were the most effective with changes of −5.1 kg (95% CI: −6.3 to −3.8 kg) and −5.3 kg (95% CI: −7.2 to −3.5 kg), respectively (Table 1). Results were also significant for studies prescribing combined resistance and aerobic exercise + caloric restriction (ES = −3.0%), combined resistance and aerobic exercise + healthy diet (ES = −2.3%), combined resistance and aerobic exercise (ES = −2.3%) and resistance training alone (ES = −1.6%) on body fat percentage (p < 0.001), and combined resistance and aerobic exercise (ES = −1.4 kg) and resistance training alone (ES = −1.0 kg) on fat mass (p < 0.001) (Table 1). Forest plots for each exercise modality before sensitivity analysis procedure adjustments are presented in Figures S1 and S2.
TABLE 1.
Overall and subgroup analyses of resistance‐based exercise effects on body fat percentage and whole‐body fat mass in participants who are overweight or obese
| Random effect meta‐analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|
| k | ES | 95% CI | p‐value | Q | I 2 | p‐value | |
| Body fat percentage, % | |||||||
| Overall effect | 99 | −2.3 | −2.7 to −1.9 | <0.001 | 651.2 | 85% | <0.001 |
| Without outlier c | 89 | −2.2 | −2.4 to −2.0 | <0.001 | 95.6 | 8% | 0.273 |
| Age | |||||||
| Children/adolescent | 19 | −2.1 | −2.8 to −1.3 | <0.001 | 26.4 | 32% | 0.090 |
| Young adults c | 26 | −2.7 | −3.7 to −1.7 | <0.001 | 24.2 | 0% | 0.507 |
| Middle‐aged adults c | 27 | −2.4 | −2.5 to −2.3 | <0.001 | 19.7 | 0% | 0.805 |
| Older adults | 19 | −1.9 | −2.4 to −1.4 | <0.001 | 36.3 | 51% | 0.006 |
| Sex | |||||||
| Female c | 45 | −2.4 | −2.5 to −2.3 | <0.001 | 41.1 | 0% | 0.599 |
| Male c | 25 | −2.8 | −3.4 to −2.2 | <0.001 | 35.3 | 32% | 0.064 |
| Mixed c | 20 | −1.7 | −2.1 to −1.2 | <0.001 | 17.0 | 0% | 0.593 |
| Exercise modality a | |||||||
| RET c | 45 | −1.6 | −1.9 to −1.2 | <0.001 | 46.8 | 6% | 0.360 |
| RET + Caloric restriction | 3 | −3.8 | −4.7 to −2.9 | <0.001 | 0.4 | 0% | 0.817 |
| COMB c | 40 | −2.3 | −2.7 to −1.9 | <0.001 | 45.2 | 14% | 0.229 |
| COMB + Caloric restriction | 6 | −3.0 | −4.1 to −1.8 | <0.001 | 4.8 | 0% | 0.439 |
| COMB + Healthy diet | 2 | −2.3 | −2.8 to −1.8 | <0.001 | 1.6 | 38% | 0.203 |
| Fat mass, kg | |||||||
| Overall effect | 63 | −2.1 | −2.7 to −1.6 | <0.001 | 219.0 | 72% | <0.001 |
| Without outlier c | 52 | −1.6 | −1.9 to −1.3 | <0.001 | 39.5 | 0% | 0.879 |
| Age | |||||||
| Children/adolescent | 13 | −1.9 | −2.9 to −0.8 | <0.001 | 20.8 | 42% | 0.053 |
| Young adults c | 14 | −1.0 | −1.4 to −0.5 | <0.001 | 11.9 | 0% | 0.540 |
| Middle‐aged adults c | 14 | −1.2 | −2.1 to −0.4 | 0.003 | 17.8 | 27% | 0.166 |
| Older adults c | 17 | −1.7 | −2.3 to −1.2 | <0.001 | 22.7 | 30% | 0.121 |
| Sex | |||||||
| Female c | 26 | −1.0 | −1.3 to −0.7 | <0.001 | 23.3 | 0% | 0.558 |
| Male | 15 | −2.6 | −3.8 to −1.4 | <0.001 | 22.1 | 37% | 0.076 |
| Mixed c | 17 | −2.0 | −2.6 to −1.5 | <0.001 | 20.8 | 23% | 0.186 |
| Exercise modality b | |||||||
| RET c | 33 | −1.0 | −1.4 to −0.7 | <0.001 | 22.0 | 0% | 0.908 |
| RET + Caloric restriction | 5 | −5.1 | −6.3 to −3.8 | <0.001 | 8.5 | 53% | 0.074 |
| RET + Low‐sugar diet | 2 | 0.2 | −1.7 to 2.0 | 0.880 | 0.1 | 0% | 0.782 |
| RET + Protein supplementation | 2 | −0.7 | −3.4 to 2.1 | 0.640 | 0.0 | 0% | 0.889 |
| COMB c | 22 | −1.4 | −2.0 to −0.8 | <0.001 | 38.3 | 45% | 0.012 |
| COMB + Caloric restriction | 7 | −5.3 | −7.2 to −3.5 | <0.001 | 23.6 | 75% | <0.001 |
Abbreviations: COMB, combined resistance and aerobic exercise; ES, effect size; I 2, percentage of variation across studies that is due to heterogeneity; k, number of studies; Q, Cochran's Q test of heterogeneity; RET, resistance training.
Exercise modalities excluded due to insufficient evidence for body fat percentage: RET + Ginger supplementation, ES = −4.9% (95% CI: −13.4 to 3.6); RET + Green tea, ES = −12.4% (95% CI: −15.3 to −9.5); RET + Protein supplementation, ES = −0.8% (95% CI: −3.1 to −1.6); COMB + Amino acids, ES = −0.3% (95% CI: −2.5 to 1.9); COMB + Caffeine supplementation, ES = −0.6% (95% CI: −3.4 to 2.1); COMB + Caloric restriction + Protein supplementation, ES = −2.7% (95% CI: −5.5 to 0.1); COMB + Fatty acids, ES = −1.2% (95% CI: −5.9 to 3.5); COMB + Isoflavones supplementation, ES = −2.0% (95% CI: −4.9 to 0.9); COMB + Protein supplementation, ES = −2.1% (95% CI: −3.2 to −1.0).
Exercise modalities excluded due to insufficient evidence for fat mass: RET + Ginger supplementation, ES = −3.1 kg (95% CI: −10.2 to 4.0); RET + Green tea, ES = −11.7 kg (95% CI: −15.3 to −8.1); COMB + Amino acids, ES = −0.2 kg (95% CI: −2.4 to 2.0); COMB + Caffeine supplementation, ES = 0.3 kg (95% CI: −4.9 to 5.5); COMB + Caloric restriction + Protein supplementation, ES = −3.8 kg (95% CI: −8.7 to 1.1); COMB + Fatty acids, ES = −1.8 kg (95% CI: −7.0 to 3.4); COMB + Healthy diet, ES = −2.0 kg (95% CI: −3.4 to −0.6); COMB + Isoflavones supplementation, ES = 1.1 kg (95% CI: −1.9 to 4.1); COMB + Low‐sugar diet, ES = −1.8 kg (95% CI: −3.0 to −0.6); COMB + Protein supplementation, ES = −2.3 kg (95% CI: −3.4 to −1.2).
Adjustment after omitting studies in which the confidence intervals did not overlap the estimated pooled effect.
FIGURE 2.
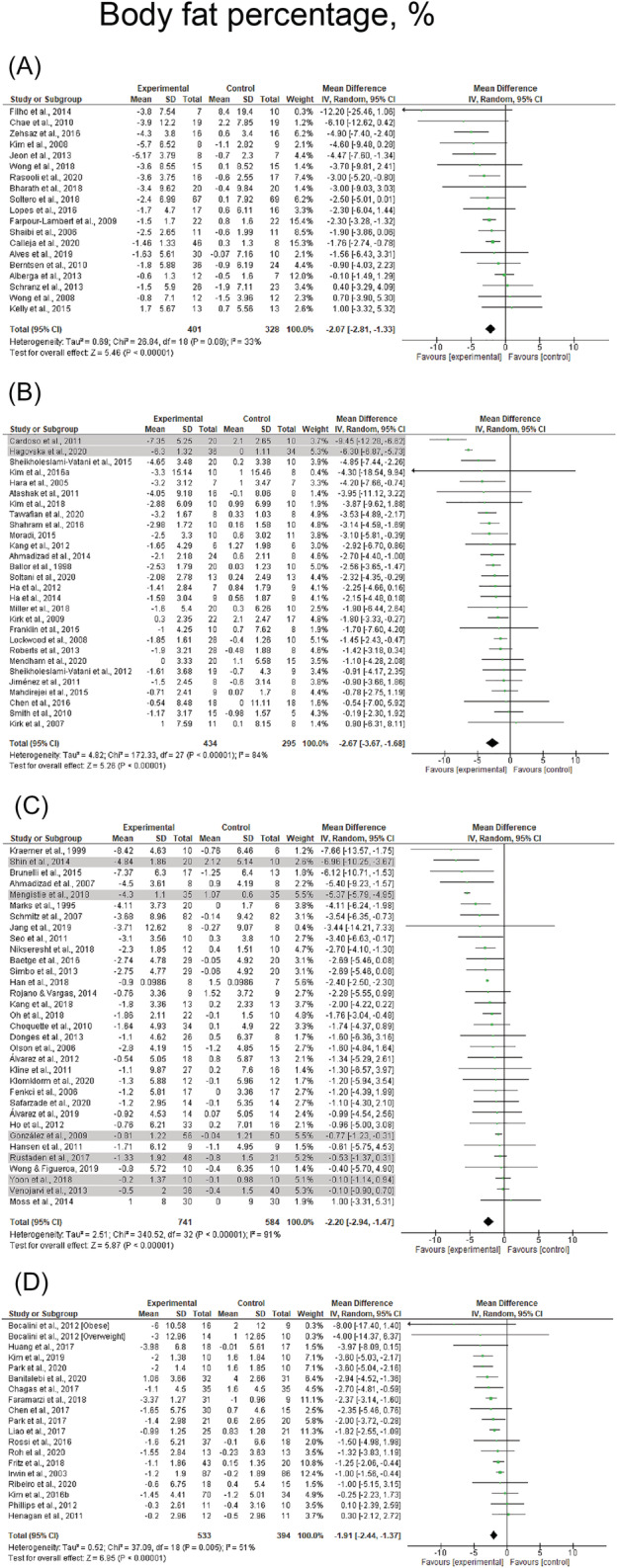
Mean difference effects of resistance‐based exercise compared with control on body fat percentage in children/adolescents (A), young adults (B), middle‐aged adults (C), and older adults with overweight/obesity (D). Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis; studies deemed outliers are highlighted in gray
FIGURE 3.
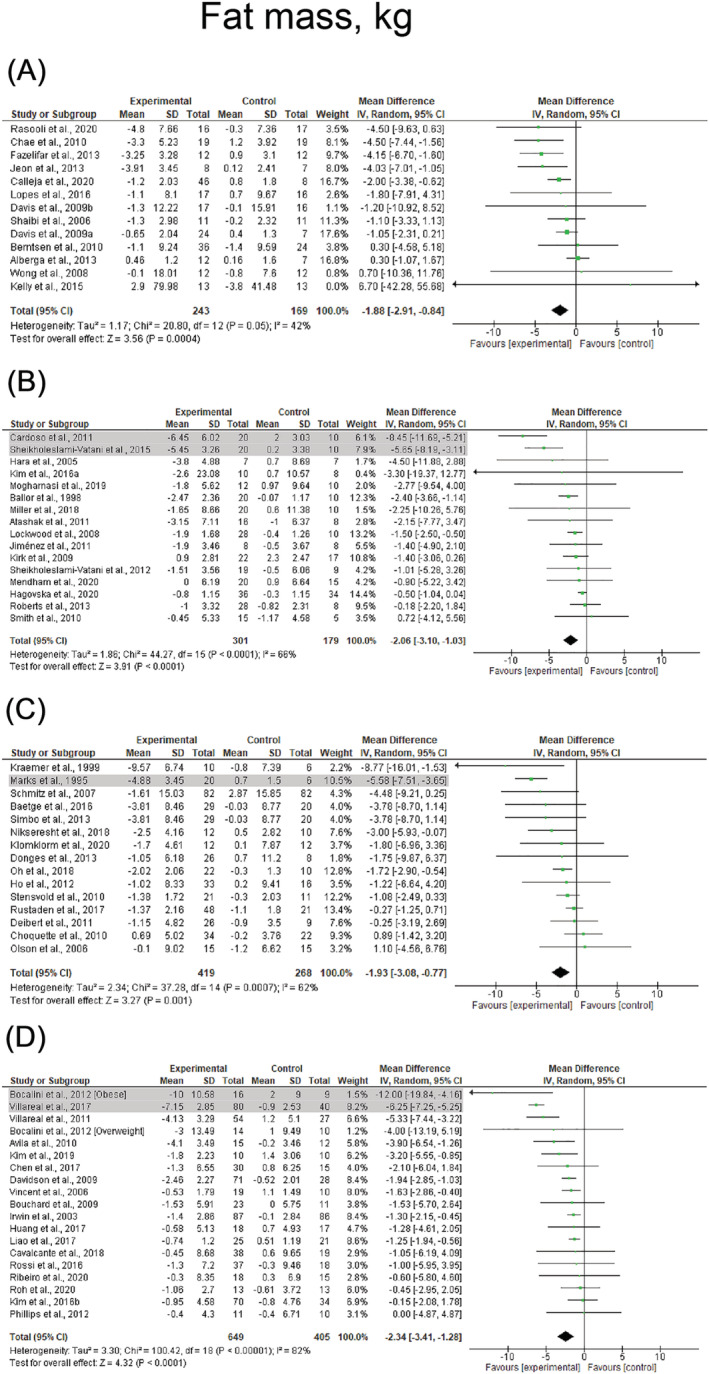
Mean difference effects of resistance‐based exercise compared with control on whole‐body fat mass in children/adolescents (A), young adults (B), middle‐aged adults (C), and older adults with overweight/obesity (D). Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis; studies deemed outliers are highlighted in gray
Heterogeneity ranged from I 2 = 0% to 8% after removing outliers. 33 , 54 , 66 , 73 , 77 , 84 , 85 , 95 , 98 , 102 , 105 , 109 , 115 , 121 , 122 , 131 No evidence of publication bias was identified in body fat percentage or whole‐body fat mass (τ = −1.8 to 0.4, p = 0.069–0.690).
3.3. Trunk fat mass, visceral adipose tissue, and subcutaneous adipose tissue
Regarding the different depots of adiposity, VAT (k = 13, ES = −0.4 SMD, 95% CI: −0.5 to −0.2) and SAT (k = 9, ES = −0.4 SMD, 95% CI: −0.5 to −0.2) were significantly reduced following resistance‐based exercise programs (Table 2). Studies assessed VAT by magnetic resonance imaging (MRI), 32 , 74 , 83 , 101 , 118 bioelectrical impedance analysis (BIA), 73 , 98 , 105 , 107 computerized tomography (CT), 82 , 93 , 115 and dual‐energy X‐ray absorptiometry (DXA), 71 , 113 whereas SAT was assessed by MRI, 32 , 74 , 101 , 118 CT, 82 , 93 , 115 and ultrasound. 68 Significant changes in trunk fat mass were not observed (k = 7, ES = −0.4 kg, 95% CI: −1.1 to 0.2 kg, p = 0.219). Results were maintained for studies examining VAT in middle‐aged adults (ES = −0.3 SMD, 95% CI: −0.6 to −0.1) and older adults (ES = −0.5 SMD, 95% CI: −0.9 to −0.1), and studies examining SAT in older adults (−0.5 SMD, 95% CI: −0.9 to −0.1). Results are presented before sensitivity analysis procedure adjustments in Figure 4. Studies involving females (ES = −0.3 SMD, 95% CI: −0.5 to −0.1) presented significant reductions in VAT, whereas SAT was significantly reduced in studies involving females (ES = −0.3 SMD, 95% CI: −0.5 to −0.1) and mixed participants (ES = −0.6 SMD, 95% CI: −0.9 to −0.2). Combined resistance and aerobic exercise was the most effective intervention for reducing both VAT (ES = −0.7 SMD, 95% CI: −1.2 to −0.2) and SAT (ES = −0.5 SMD, 95% CI: −0.9 to −0.2), although ES were considered small‐to‐moderate (Table 2). Results were also significant for studies prescribing resistance training alone (ES = ~ − 0.4 SMD, p = 0.002–0.003). Heterogeneity was I 2 = 0% after removing two studies which were considered outliers in the VAT analysis, 73 , 105 with no evidence of publication bias (τ = −0.7 to −0.2, p = 0.822–0.930). Forest plots for each exercise modality before sensitivity analysis procedure adjustments are presented in Figure S3.
TABLE 2.
Overall and subgroup analyses of resistance‐based exercise effects on trunk fat mass, visceral adipose tissue and subcutaneous adipose tissue in participants who are overweight or obese
| Random effect meta‐analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|
| k | ES | 95% CI | p‐value | Q | I 2 | p‐value | |
| Trunk fat mass, kg | |||||||
| Overall effect | 7 | −0.4 | −1.1 to 0.2 | 0.219 | 1.3 | 0% | 0.970 |
| Without outlier | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Age | |||||||
| Children/adolescent | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Young adults | 1 | −0.3 | −1.4 to 0.8 | ‐ | ‐ | ‐ | ‐ |
| Middle‐aged adults | 2 | −1.0 | −2.8 to 0.9 | 0.308 | 0.1 | 0% | 0.772 |
| Older adults | 4 | −0.3 | −1.2 to 0.5 | 0.431 | 0.8 | 0% | 0.841 |
| Sex | |||||||
| Female | 5 | −0.4 | −1.2 to 0.4 | 0.346 | 0.9 | 0% | 0.919 |
| Male | 1 | −0.3 | −1.4 to 0.8 | ‐ | ‐ | ‐ | ‐ |
| Mixed | 1 | −1.3 | −4.2 to 1.6 | ‐ | ‐ | ‐ | ‐ |
| Exercise modality a | |||||||
| RET | 3 | −0.5 | −1.5 to 0.5 | 0.298 | 0.5 | 0% | 0.776 |
| COMB | 3 | −0.3 | −1.3 to 0.7 | 0.577 | 0.1 | 0% | 0.963 |
| Visceral adipose tissue, SMD | |||||||
| Overall effect | 15 | −0.7 | −1.1 to −0.3 | <0.001 | 88.5 | 84% | <0.001 |
| Without outlier c | 13 | −0.4 | −0.5 to −0.2 | <0.001 | 12.0 | 0% | 0.446 |
| Age | |||||||
| Children/adolescent | 1 | −0.3 | −1.0 to 0.4 | ‐ | ‐ | ‐ | ‐ |
| Young adults | 3 | 0.8 | −2.1 to 0.5 | 0.221 | 23.0 | 91% | <0.001 |
| Middle‐aged adults c | 7 | −0.3 | −0.6 to −0.1 | 0.005 | 5.5 | 0% | 0.485 |
| Older adults | 3 | −0.5 | −0.9 to −0.1 | 0.011 | 5.1 | 61% | 0.080 |
| Sex | |||||||
| Female c | 7 | −0.3 | −0.5 to −0.1 | <0.001 | 4.7 | 0% | 0.582 |
| Male | 1 | −0.3 | −1.1 to 0.5 | ‐ | ‐ | ‐ | ‐ |
| Mixed c | 5 | −0.4 | −0.8 to −0.0 | 0.032 | 6.3 | 36% | 0.179 |
| Exercise modality b | |||||||
| RET | 6 | −0.4 | −0.6 to −0.1 | 0.002 | 2.5 | 0% | 0.772 |
| RET + Caloric restriction | 2 | −0.5 | −1.2 to 0.2 | 0.142 | 0.0 | 0% | 0.932 |
| COMB c | 9 | −0.7 | −1.2 to −0.2 | 0.005 | 40.3 | 80% | <0.001 |
| Subcutaneous adipose tissue, SMD | |||||||
| Overall effect | 9 | −0.4 | −0.5 to −0.2 | <0.001 | 7.5 | 0% | 0.485 |
| Without outlier | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Age | |||||||
| Children/adolescent | 1 | −0.6 | −1.3 to 0.1 | ‐ | ‐ | ‐ | ‐ |
| Young adults | 2 | −0.2 | −0.7 to 0.4 | 0.475 | 0.0 | 0% | 0.936 |
| Middle‐aged adults | 3 | −0.3 | −0.5 to 0.0 | 0.067 | 0.1 | 0% | 0.965 |
| Older adults | 3 | −0.5 | −0.9 to −0.1 | 0.011 | 5.1 | 61% | 0.080 |
| Sex | |||||||
| Female | 4 | −0.3 | −0.5 to −0.1 | 0.003 | 1.0 | 0% | 0.808 |
| Male | 1 | −0.2 | −1.0 to 0.6 | ‐ | ‐ | ‐ | ‐ |
| Mixed | 4 | −0.6 | −0.9 to −0.2 | 0.004 | 3.9 | 24% | 0.269 |
| Exercise modality | |||||||
| RET | 6 | −0.3 | −0.6 to −0.1 | 0.003 | 2.3 | 0% | 0.813 |
| COMB | 6 | −0.5 | −0.9 to −0.2 | 0.002 | 9.7 | 49% | 0.084 |
Abbreviations: COMB, combined resistance and aerobic exercise; ES, effect size; I 2, percentage of variation across studies that is due to heterogeneity; k, number of studies; Q, Cochran's Q test of heterogeneity; RET, resistance training; SMD, standardized mean difference.
Exercise modalities excluded due to insufficient evidence for trunk fat mass: COMB + Amino acids, ES = 0.0 kg (95% CI: −1.2 to 1.2); COMB + Fatty acids, ES = −1.3 kg (95% CI: −4.2 to 1.6); COMB + Isoflavones, ES = −0.9 kg (95% CI: −4.0 to 2.2).
Exercise modalities excluded due to insufficient evidence for visceral adipose tissue: COMB + Caloric restriction, ES = −0.2 SMD (95% CI: −1.3 to 0.9); COMB + Fatty acids, ES = −0.0 SMD (95% CI: −0.8 to 0.8).
Adjustment after omitting studies in which the confidence intervals did not overlap the estimated pooled effect.
FIGURE 4.
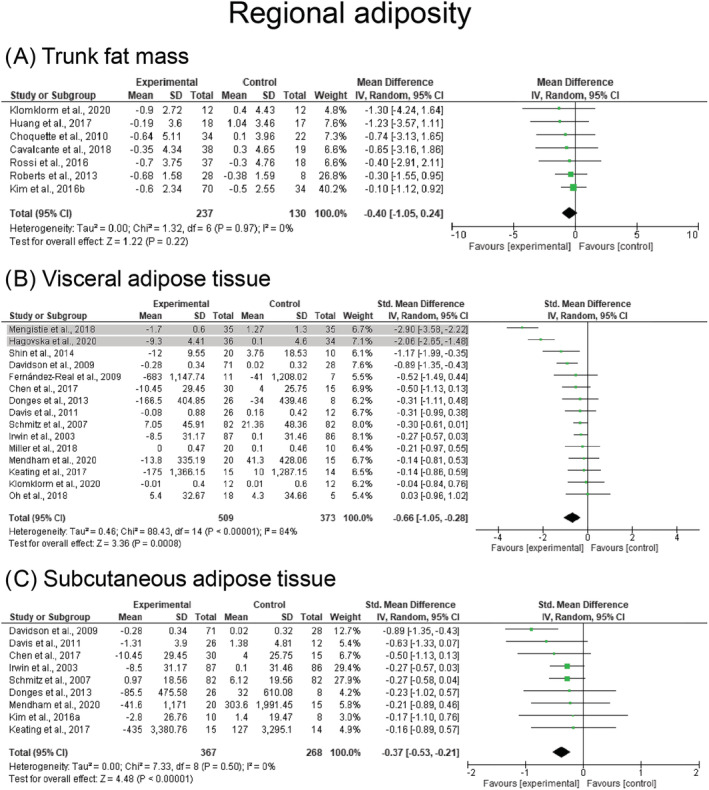
Mean difference effects of resistance‐based exercise compared with control on trunk fat mass (A), visceral adipose tissue (B), and subcutaneous adipose tissue (C) in participants who are overweight or obese participants. Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis; studies deemed outliers are highlighted in gray
3.4. Lean mass
Resistance‐based exercise programs resulted in significant increases in lean mass (k = 67, ES = 0.7 kg, 95% CI: 0.5 to 0.8 kg) (Table 3). These effects were consistent across the lifespan with significant results observed in children/adolescents (ES = 0.8 kg, 95% CI: 0.4 to 1.1 kg), young adults (ES = 1.4 kg, 95% CI: 0.9 to 1.9 kg), middle‐aged adults (ES = 0.3 kg, 95% CI: 0.1 to 0.6 kg), and older adults (ES = 0.8 kg, 95% CI: 0.6 to 1.1 kg). Results are presented across the lifespan before sensitivity analysis procedure adjustments in Figure 5. Significant and similar results were also observed for studies involving females and mixed participants (ES = 0.6–0.8 kg, p < 0.001). Resistance training alone and combined resistance and aerobic exercise were the most effective for increasing lean mass with changes of 0.8 kg (95% CI: 0.6 to 1.0 kg) and 0.6 kg (95% CI: 0.3 to 0.9 kg), respectively (Table 3). Changes in lean mass were not observed following resistance training + caloric restriction (ES = −0.2 kg, p = 0.727), resistance training + low‐sugar diet (ES = 1.2 kg, p = 0.143), and combined resistance and aerobic exercise + caloric restriction (ES = −0.3 kg, p = 0.550) (Table 3). Heterogeneity was I 2 = 0% after removing four studies considered outliers in the analyses. 54 , 59 , 73 , 131 Publication bias was not observed (τ = 0.4, p = 0.687). Forest plots for each exercise modality before sensitivity analysis procedure adjustments are presented in Figure S4.
TABLE 3.
Overall and subgroup analyses of resistance‐based exercise effects on lean mass in participants who are overweight or obese
| Random effect meta‐analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|
| k | ES | 95% CI | p‐value | Q | I 2 | p‐value | |
| Lean mass, kg | |||||||
| Overall effect | 71 | 0.9 | 0.4 to 1.4 | 0.001 | 636.4 | 89% | <0.001 |
| Without outlier b | 67 | 0.7 | 0.5 to 0.8 | <0.001 | 40.9 | 0% | 0.994 |
| Age | |||||||
| Children/adolescent | 15 | 0.8 | 0.4 to 1.1 | <0.001 | 10.4 | 0% | 0.731 |
| Young adults b | 15 | 1.4 | 0.9 to 1.9 | <0.001 | 10.1 | 0% | 0.755 |
| Middle‐aged adults | 18 | 0.3 | 0.1 to 0.6 | 0.009 | 6.9 | 0% | 0.985 |
| Older adults b | 20 | 0.8 | 0.6 to 1.1 | <0.001 | 9.7 | 0% | 0.960 |
| Sex | |||||||
| Female b | 33 | 0.6 | 0.4 to 0.9 | <0.001 | 6.7 | 0% | 0.999 |
| Male | 17 | 0.5 | −0.1 to 1.0 | 0.087 | 5.4 | 0% | 0.994 |
| Mixed b | 17 | 0.8 | 0.4 to 1.2 | <0.001 | 28.1 | 43% | 0.031 |
| Exercise modality a | |||||||
| RET | 34 | 0.8 | 0.6 to 1.0 | <0.001 | 20.8 | 0% | 0.951 |
| RET + Caloric restriction | 5 | −0.2 | −1.2 to 0.8 | 0.727 | 7.6 | 47% | 0.108 |
| RET + Low‐sugar diet | 2 | 1.2 | −0.4 to 2.7 | 0.143 | 0.1 | 0% | 0.761 |
| COMB b | 28 | 0.6 | 0.3 to 0.9 | <0.001 | 17.2 | 0% | 0.927 |
| COMB + Caloric restriction | 7 | −0.3 | −1.4 to 0.8 | 0.550 | 28.4 | 79% | <0.001 |
Abbreviations: COMB, combined resistance and aerobic exercise; ES, effect size; I 2, percentage of variation across studies that is due to heterogeneity; k, number of studies; Q, Cochran's Q test of heterogeneity; RET, resistance training.
Exercise modalities excluded due to insufficient evidence for lean mass: RET + Ginger supplementation, ES = 3.0 kg (95% CI: −22.0 to 28.0); RET + Green tea, ES = 8.9 kg (95% CI: 6.1 to 11.7); RET + Protein supplementation, ES = 0.8 kg (95% CI: −5.6 to 7.2); COMB + Caffeine supplementation, ES = 2.0 kg (95% CI: −6.8 to 10.7); COMB + Caloric restriction + Protein supplementation, ES = −0.3 kg (95% CI: −4.4 to 3.7); COMB + Fatty acids, ES = −0.6 kg (95% CI: −5.1 to 3.9); COMB + Healthy diet, ES = 0.6 kg (95% CI: −0.5 to 1.7); COMB + Isoflavones supplementation, ES = 1.1 kg (95% CI: −1.9 to 4.1); COMB + Low‐sugar diet, ES = 0.6 kg (95% CI: −0.7 to 1.9); 95% CI, 95% confidence interval; COMB + Protein supplementation, ES = 0.8 kg (95% CI: −0.3 to 1.9).
Adjustment after omitting studies in which the confidence intervals did not overlap the estimated pooled effect.
FIGURE 5.
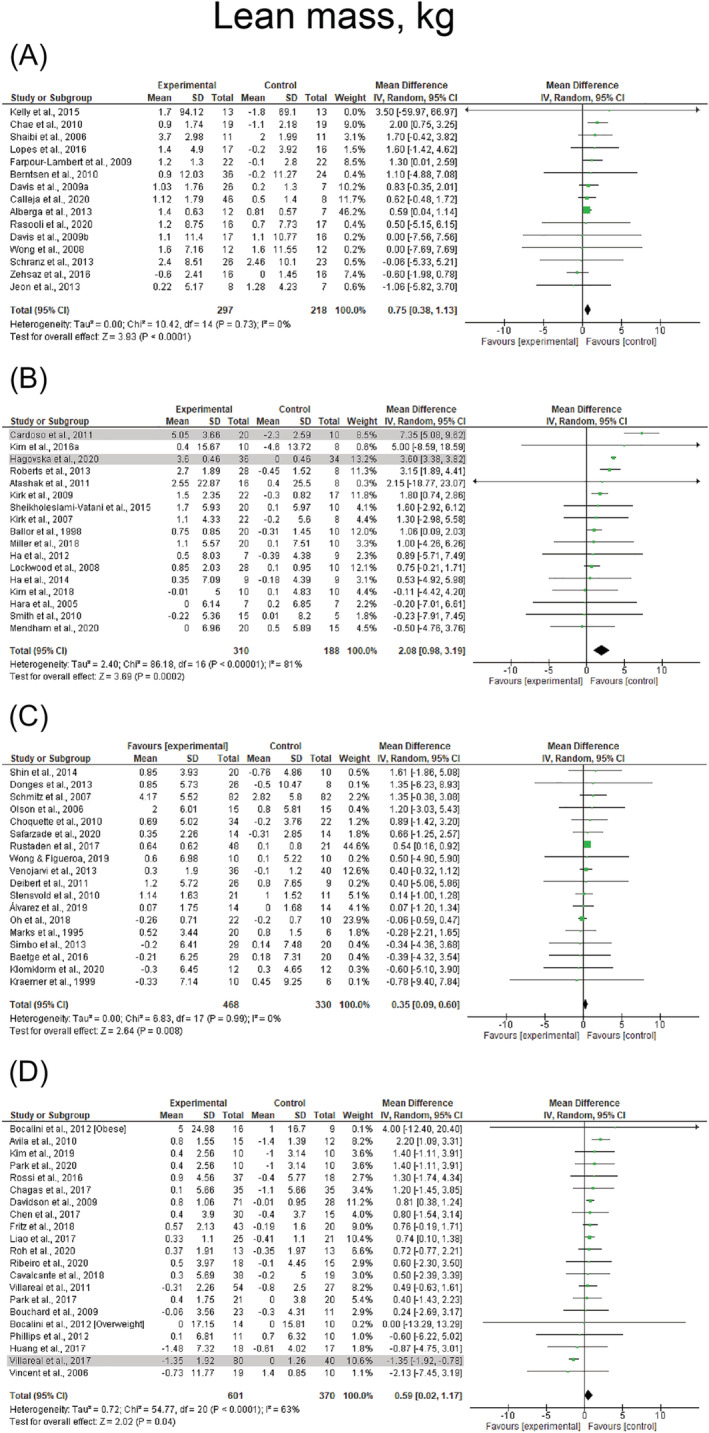
Mean difference effects of resistance‐based exercise compared with control on lean mass in children/adolescents (A), young adults (B), middle‐aged adults (C), and older adults with overweight/obesity (D). Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis; studies deemed outliers are highlighted in gray
3.5. Body weight and body mass index
Reductions in body weight (k = 93, ES = −1.6 kg, 95% CI: −1.9 to −1.3 kg) and BMI (k = 74, ES = −0.6 kg·m2, 95% CI: −0.7 to −0.5 kg·m2) were observed following resistance‐based exercise programs (Table 4). Resistance‐based exercise programs resulted in significant reductions in children/adolescents (ES = −1.1, 95% CI: −2.2 to −0.0), young adults (ES = −1.3 kg, 95% CI: −2.0 to −0.6 and ES = −0.4 kg·m2, 95% CI: −0.8 to −0.0 kg·m2), middle‐aged adults (ES = −0.5 kg, 95% CI: −1.0 to −0.1 kg and ES = −0.5 kg·m2, 95% CI: −0.8 to −0.2 kg·m2), and older adults (ES = −1.8 kg, 95% CI: −2.3 to −1.2 kg and ES = −0.6 kg·m2, 95% CI: −0.9 to −0.4 kg·m2), whereas changes in BMI were not observed in children/adolescents (ES = 0.3 kg·m2, p = 0.163). Results are presented for body weight and BMI across the lifespan before sensitivity analysis procedure adjustments in Figure 6 and Figure 7, respectively. Studies involving female, male, and mixed participants presented significant reductions in body weight and BMI (p ≤ 0.001–0.032). Resistance training + caloric restriction and combined resistance and aerobic exercise + caloric restriction were the most effective for reducing body weight with changes of −5.3 kg (95% CI: −7.6 to −3.0 kg) and −5.6 kg (95% CI: −7.8 to −3.4), respectively. Results were also significant for studies prescribing combined resistance and aerobic exercise (ES = −1.9 kg, p < 0.001). Combined resistance and aerobic exercise + caloric restriction was the most effective for reducing BMI (ES = −1.2 kg·m2, 95% CI: −1.8 to −0.6 kg·m2), whereas results were also significant for studies prescribing combined resistance and aerobic exercise (ES = −0.7 kg·m2, p < 0.001). Heterogeneity was I 2 = 0% after removing studies which were considered outliers in body weight and BMI analyses. 27 , 33 , 38 , 59 , 73 , 77 , 84 , 95 , 101 , 102 , 105 , 131 No effect of publication bias was observed (τ = −0.7 to −0.1, p = 0.161–0.472). Forest plots for each exercise modality before sensitivity analysis procedure adjustments are presented in Figures S5 and S6.
TABLE 4.
Overall and subgroup analyses of resistance‐based exercise effects on body weight and body mass index in participants who are overweight or obese
| Random effect meta‐analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|
| k | ES | 95% CI | p‐value | Q | I 2 | p‐value | |
| Body weight, kg | |||||||
| Overall effect | 103 | −1.8 | −2.6 to −1.0 | <0.001 | 815.8 | 88% | <0.001 |
| Without outlier c | 93 | −1.6 | −1.9 to −1.3 | <0.001 | 60.5 | 0% | 0.996 |
| Age | |||||||
| Children/adolescent | 21 | −1.1 | −2.2 to −0.0 | 0.043 | 33.4 | 40% | 0.031 |
| Young adults c | 23 | −1.4 | −2.1 to −0.8 | <0.001 | 10.5 | 0% | 0.981 |
| Middle‐aged adults c | 33 | −0.6 | −1.0 to −0.1 | 0.021 | 30.4 | 0% | 0.549 |
| Older adults c | 20 | −1.7 | −2.2 to −1.2 | <0.001 | 14.6 | 0% | 0.748 |
| Sex | |||||||
| Female c | 48 | −1.4 | −1.9 to −0.9 | <0.001 | 31.7 | 0% | 0.958 |
| Male | 26 | −1.1 | −2.1 to −0.1 | 0.032 | 32.9 | 24% | 0.133 |
| Mixed c | 23 | −1.2 | −1.8 to −0.6 | <0.001 | 29.6 | 26% | 0.128 |
| Exercise modality a | |||||||
| RET c | 50 | −0.1 | −0.5 to 0.3 | 0.511 | 30.1 | 0% | 0.985 |
| RET + Caloric restriction | 6 | −5.3 | −7.6 to −3.0 | <0.001 | 17.0 | 71% | 0.005 |
| RET + Low‐sugar diet | 2 | 2.7 | 1.1 to 4.3 | 0.001 | 0.2 | 0% | 0.676 |
| COMB c | 44 | −1.9 | −2.5 to −1.3 | <0.001 | 65.1 | 34% | 0.017 |
| COMB + Caloric restriction | 8 | −5.6 | −7.8 to −3.4 | <0.001 | 36.7 | 81% | <0.001 |
| COMB + Healthy diet | 2 | −3.1 | −7.1 to 0.9 | 0.127 | 3.1 | 68% | 0.078 |
| Body mass index, kg·m 2 | |||||||
| Overall effect | 83 | −0.6 | −0.9 to −0.3 | <0.001 | 396.2 | 79% | <0.001 |
| Without outlier c | 74 | −0.6 | −0.7 to −0.5 | <0.001 | 33.7 | 0% | 0.999 |
| Age | |||||||
| Children/adolescent | 18 | −0.3 | −0.8 to 0.1 | 0.163 | 30.7 | 45% | 0.022 |
| Young adults c | 22 | −0.5 | −0.8 to −0.2 | 0.003 | 19.3 | 0% | 0.563 |
| Middle‐aged adults c | 27 | −0.5 | −0.8 to −0.2 | 0.001 | 37.8 | 31% | 0.064 |
| Older adults | 14 | −0.6 | −0.8 to −0.4 | <0.001 | 3.2 | 0% | 0.997 |
| Sex | |||||||
| Female c | 37 | −0.4 | −0.6 to −0.2 | <0.001 | 24.5 | 0% | 0.928 |
| Male c | 19 | −0.5 | −0.8 to −0.2 | <0.001 | 15.5 | 0% | 0.630 |
| Mixed c | 21 | −0.5 | −0.7 to −0.2 | <0.001 | 21.8 | 8% | 0.351 |
| Exercise modality b | |||||||
| RET | 43 | −0.1 | −0.3 to 0.1 | 0.209 | 25.6 | 0% | 0.978 |
| RET + Caloric restriction | 2 | −2.1 | −3.8 to −0.4 | 0.017 | 0.2 | 0% | 0.622 |
| RET + Low‐sugar diet | 2 | 1.6 | 1.0 to 2.1 | <0.001 | 0.5 | 0% | 0.497 |
| RET + Protein supplementation | 2 | −0.0 | −1.3 to 1.3 | 0.980 | 0.0 | 0% | 0.879 |
| COMB c | 36 | −0.7 | −0.9 to −0.6 | <0.001 | 31.6 | 0% | 0.632 |
| COMB + Caloric restriction | 3 | −1.2 | −1.8 to −0.6 | <0.001 | 1.1 | 0% | 0.588 |
Abbreviations: COMB, combined resistance and aerobic exercise; ES, effect size; I 2, percentage of variation across studies that is due to heterogeneity; k, number of studies; Q, Cochran's Q test of heterogeneity; RET, resistance training.
Exercise modalities excluded due to insufficient evidence for body weight: RET + Green tea, ES = 1.7 kg (95% CI: −3.0 to 6.4); RET + Protein supplementation, ES = 0.1 kg (95% CI: −8.3 to 8.5); COMB + + Caloric restriction + Protein supplementation, ES = −4.1 kg (95% CI: −11.7 to 3.4); COMB + Fatty acids, ES = −1.5 kg (95% CI: −8.7 to 5.7); COMB + Isoflavones supplementation, ES = −0.2 kg (95% CI: −8.0 to 7.6); COMB + Low‐sugar diet, ES = −0.5 kg (95% CI: −2.0 to 1.0); COMB + Protein supplementation, ES = −1.5 kg (95% CI: −3.7 to 0.7).
Exercise modalities excluded due to insufficient evidence for body mass index: RET + Ginger supplementation, ES = −0.4 kg·m2 (95% CI: −7.3 to 6.5); RET + Green tea, ES = 1.6 kg·m2 (95% CI: 0.2 to 3.3); COMB + Fatty acids, ES = −0.6 kg·m2 (95% CI: −3.1 to 1.9); COMB + Isoflavones supplementation, ES = −0.1 kg·m2 (95% CI: −2.2 to 2.0).
Adjustment after omitting studies in which the confidence intervals did not overlap the estimated pooled effect.
FIGURE 6.
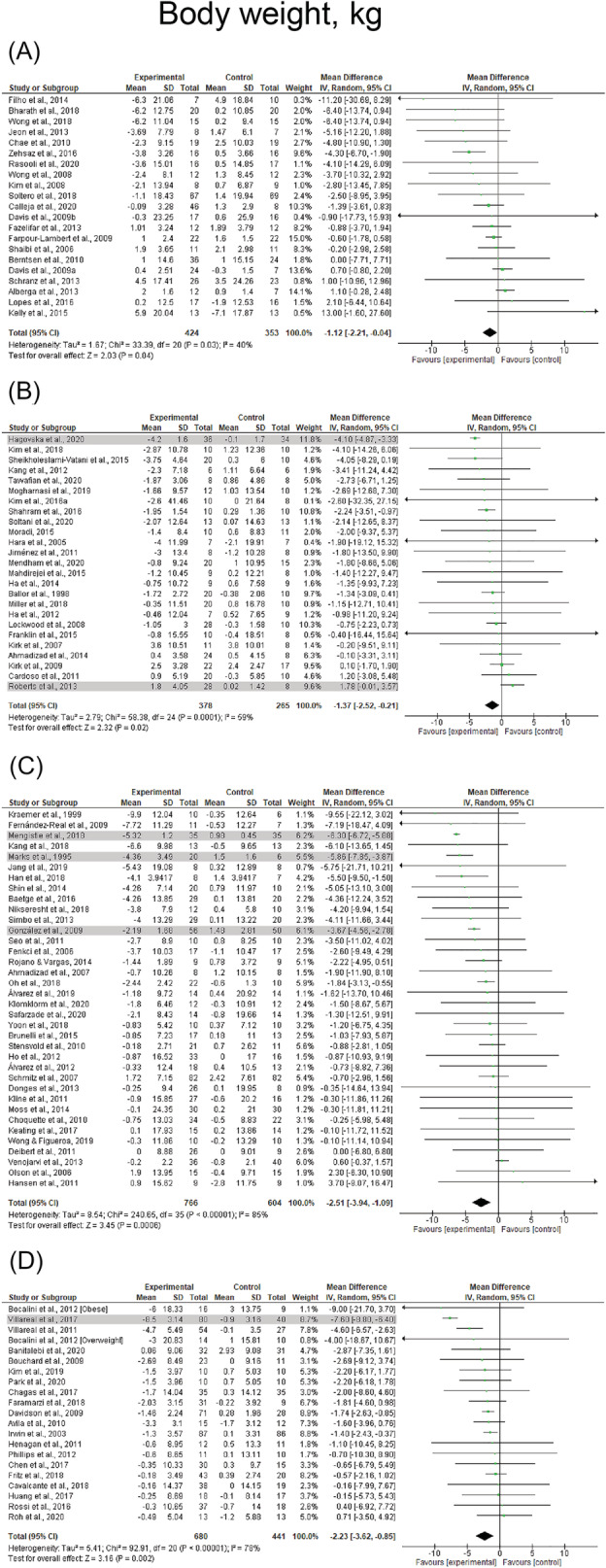
Mean difference effects of resistance‐based exercise compared with control on body weight in children/adolescents (A), young adults (B), middle‐aged adults (C), and older adults with overweight/obesity (D). Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis; studies deemed outliers are highlighted in gray
FIGURE 7.
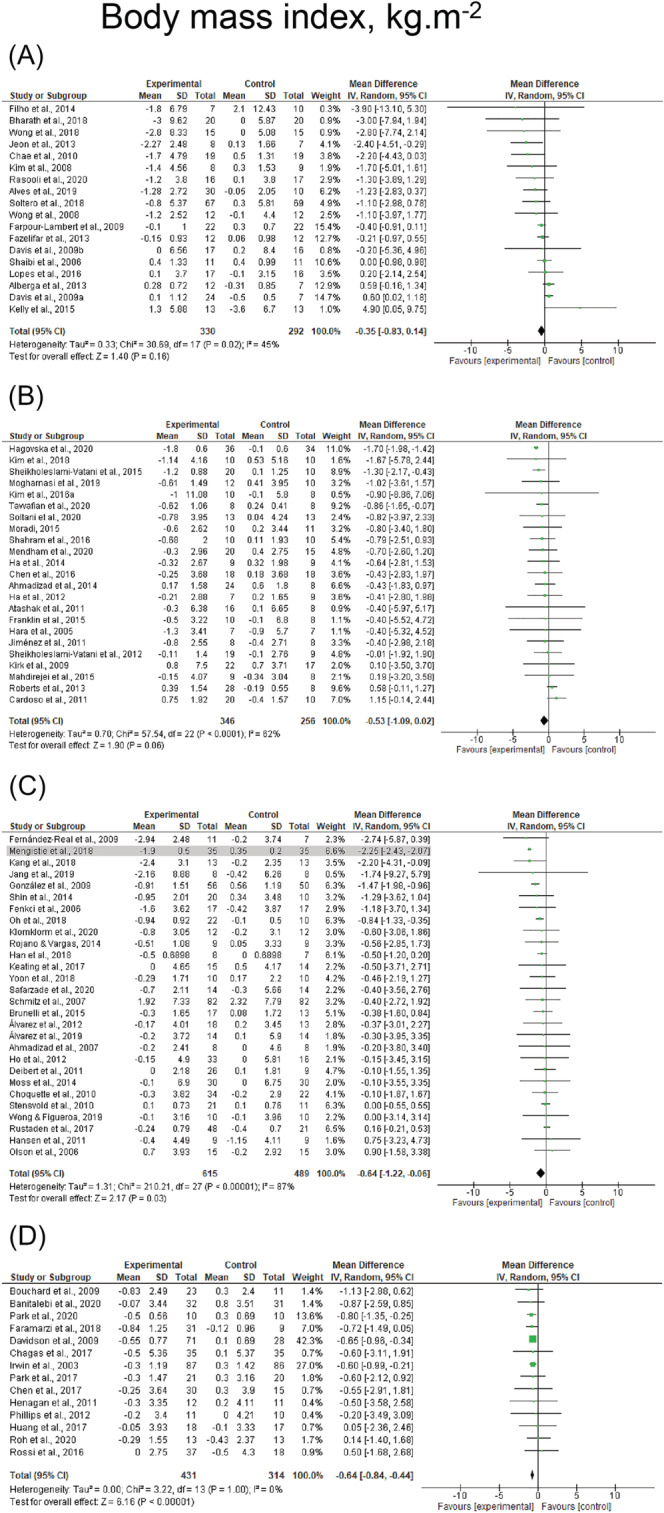
Mean difference effects of resistance‐based exercise compared with control on body mass index in children/adolescents (A), young adults (B), middle‐aged adults (C), and older adults with overweight/obesity (D). Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis; studies deemed outliers are highlighted in gray
4. DISCUSSION
In the present systematic review and meta‐analysis, we examined the effects of resistance‐based exercise programs compared with groups without intervention in individuals with overweight/obesity across the lifespan. The main findings of this study are (1) supervised resistance‐based exercise programs significantly reduces body fat percentage and whole‐body fat mass in participants with overweight and obesity regardless of age and sex, with supervised resistance‐based exercise programs combined with a caloric restriction being the most effective intervention; (2) regional adiposity measures were significantly reduced following resistance‐based exercise programs, with greater effects observed in middle‐aged and older adults as well as following combined resistance and aerobic exercise; (3) supervised resistance training alone is the most effective intervention for increasing lean mass, whereas lean mass was preserved in interventions undertaking a caloric restriction component that included resistance exercise; and (4) body weight and BMI were significantly reduced by supervised resistance‐based exercise programs in all age categories except children/adolescents, with greater effects when undertaking resistance training + caloric restriction or combined resistance and aerobic exercise + caloric restriction. Therefore, resistance‐based training is an effective option within multicomponent therapy programs for targeting fat and weight loss while maintaining lean mass in individuals with overweight/obesity. These results are clinically relevant and can be immediately used to improve current practice by expanding the exercise modalities within multicomponent therapy programs targeting obesity.
Our findings that resistance training alone and combined resistance and aerobic exercise can significantly reduce fat mass were in agreement with previous systematic reviews and meta‐analyses. 5 , 7 , 140 However, among the interventions investigated in this study, resistance‐based exercise programs combined with caloric restriction were the most effective for reducing body fat percentage and whole‐body fat mass in participants who are overweight or obese. Interestingly, the results achieved by either resistance training alone + caloric restriction or combined resistance and aerobic exercise + caloric restriction were similar and comparable to changes observed in adults with overweight/obesity undertaking aerobic exercise alone plus caloric restriction when compared with no intervention control groups. 77 , 78 , 131 , 141 In the studies from Marks et al., 77 Kraemer et al., 78 Villareal et al., 131 and Yoshimura et al., 141 for example, the aerobic exercise program combined with caloric restriction resulted in an average fat mass reduction of ~5 kg following 12 to 26 weeks of intervention in adults with overweight/obesity. In addition, the effects derived from the resistance‐based exercise programs combined with caloric restriction in our study were observed in 12 to 48 weeks, without an apparent effect for intervention duration. Apart from contributing to successful weight loss in individuals with obesity, the ~5 kg reduction in fat mass observed following resistance‐based exercise programs combined with a caloric restriction compared with groups without intervention is critical for cardiometabolic health. 142 , 143 As previously reported, 142 , 143 both body fat percentage and fat distribution are associated with an increased risk for hypertension and cardiovascular disease. Therefore, our results expand current recommendations for individuals with overweight/obesity, 2 , 3 indicating that resistance training could be used as a sole exercise intervention within a multicomponent therapy program for individuals undergoing caloric restriction interventions, potentially reducing the risk for cardiovascular disease in this population.
Beyond the clinical relevance of whole‐body fat mass, both visceral and subcutaneous fat mass depots are also associated with cardiometabolic health and systemic inflammation in individuals with obesity. Both VAT and SAT were significantly reduced following resistance‐based exercise programs in the present study, with somewhat greater effects observed when undertaking combined resistance and aerobic exercise. The reduction of 0.7 SMD observed following combined resistance and aerobic exercise in VAT is larger to those reported in previous meta‐analyses, 8 , 9 , 10 , 11 , 12 although the effects are still considered small‐to‐moderate. In the study of Maillard et al., 11 for example, high‐intensity interval training was associated with a reduction in VAT of ~0.2 SMD in adults with overweight/obesity. Likewise, general aerobic exercise promoted a reduction in VAT of ~0.3 SMD, as observed in the study of Ismail et al. 8 A potential explanation for the different findings reported previously, 8 , 9 , 10 , 11 , 12 and this study could be related to the additional effect derived from combined resistance and aerobic exercise, resulting in a higher effect on VAT and SAT. Therefore, even without a dietary intervention, combined resistance and aerobic exercise can significantly reduce abdominal fat with greater effects than interventions comprising only aerobic exercise modalities, 8 , 11 previously deemed the most effective modality for reducing overall abdominal fat. Moreover, our findings are that middle‐aged and older adults benefit the most from exercise on VAT and SAT outcomes. These age groups are the most affected by cardiovascular risk factors, 144 and therefore, our findings are of particular interest. In previous studies, 145 , 146 , 147 increased visceral and subcutaneous fat was associated with ~20% to 80% increased risk of incident hypertension, hypertriglyceridemia, and metabolic syndrome in middle‐aged and older adults. Furthermore, the benefits observed in VAT and SAT could reduce the progression of metabolic syndrome, attenuating the chronic side effects from comorbidities in these age groups. 148
Although greater effects were observed when undertaking resistance training or combined resistance and aerobic exercise, as previously reported, 5 , 7 the result that resistance training can at least help preserve lean mass while undergoing caloric restriction is meaningful for this population. In the systematic review of Weinheimer et al., 149 the authors reported that ~70% of studies only undertaking caloric restriction present reductions ≥1.5 kg of lean mass in middle‐aged and older adults. Similarly, Garrow and Summerbell 150 predicted that ~20% to 30% of weight loss following caloric restriction could be unrelated to fat mass in adults. Substantial reductions of 2–3 kg are also observed in lean mass following aerobic exercise alone plus caloric restriction. 78 , 131 Additionally, our results are in agreement with a previous meta‐analysis, 151 demonstrating that resistance training is associated with an increase of ~0.8 kg in lean mass compared with caloric restriction interventions in older adults with obesity, although they were not compared with caloric restriction only programs in the present study. These results are of great importance as resistance training can reduce the risk of sarcopenia and frailty as well as improve physical function and quality of life in this population. 152 , 153 Moreover, the clinical implications of lean mass have become clearer with advances in the investigation of myokines. 154 Several myokines, including myostatin, 155 interleukin 6 (IL‐6), 156 and brain‐derived neurotrophic factor (BDNF), 157 are produced, expressed, and released by muscle contraction and may account for protection against proinflammatory adipokines under conditions of obesity. 154 Therefore, maintenance or accrual of lean mass, only achieved with resistance exercise in this population, is of clinical importance as it can potentially improve resting energy expenditure and accrue benefits for weight loss 158 as well as promote reductions in chronic inflammation. 154
A substantial reduction in body weight was observed following either resistance training or combined resistance and aerobic exercise with caloric restriction when compared with no intervention control groups. This result is of importance for clinical practice as resistance exercise can be used regardless of an aerobic exercise component when combined with caloric restriction and still lead to a reduction of ~5.5 kg in body weight compared with no intervention control groups. In addition, this substantial change may be explained by reductions in fat rather than lean mass given the anabolic effect from resistance training which attenuated a reduction in lean mass during weight loss. The magnitude of weight loss we observed with resistance‐based programs + caloric restriction is similar to previous studies examining aerobic training only + caloric restriction. 77 , 78 , 131 , 141 Therefore, our results support the utilization of resistance training + caloric restriction as part of multicomponent therapy programs for adults with overweight or obesity to reduce body weight and BMI.
The strength of the present review are as follows: (1) inclusion of 116 studies with ~4000 participants who are overweight or obese; (2) a broad eligibility criteria and control of different definitions and cut‐off points for individuals with overweight or obesity; (3) inclusion of published and unpublished studies written in three different languages; (4) a conservative approach of assuming a correlation of 0.5 for studies not reporting sufficient data for meta‐analysis; and (5) a range of subgroup analyses based on population characteristics and exercise modalities. However, the present study also has limitations. First, most studies included were of high risk of bias because of concerns regarding the randomization process, measurement of outcomes, and selection of reported results, and this may affect the precision and magnitude of effects of resistance‐based exercise interventions. Second, most data were pooled from different methods of body composition assessment such as dual energy X‐ray absorptiometry, bioelectrical impedance, and anthropometry (i.e., skinfolds), and this may increase the heterogeneity across studies. Third, age groups were categorized based on the average age, and this may not fully represent the sample of each study included. Fourth, we did not include comparisons between resistance‐based exercise programs and dietary interventions only. This may be considered a limitation to estimate the direct contribution of resistance exercise or caloric restriction to weight loss and lean mass accruing. Additional research is required to evaluate the individual impact of exercise or caloric restriction on body composition in individuals with overweight/obesity.
In conclusion, this study provides evidence that resistance‐based exercise programs are effective and should be considered as part of a multicomponent therapy program when caloric restriction is utilized in adults with overweight or obesity. Considering the similar effect on fat and weight loss and unique effect on lean mass, resistance training rather than aerobic exercise alone should be considered within any multicomponent fat loss prescription for individuals with overweight/obesity. These results expand current guidelines to improve existing exercise clinical practice 1 , 2 , 3 with the potential to counteract cardiometabolic complications associated with increased fat mass and body weight while avoiding loss of muscle mass.
CONFLICT OF INTERESTS
No conflict of interest statement' in the first proofs.
AUTHOR CONTRIBUTIONS
Pedro Lopez had full access to all of the data in the study and takes responsibility for the for the integrity of the data and the accuracy of the data analysis. Conception and design: Pedro Lopez and Anderson Rech. Acquisition, analysis, or interpretation of data: Pedro Lopez, Elisa R. Nonemacher, Victória M. Wendt, Renata N. Bassanesi, Douglas J. P. Turella, and Anderson Rech. Drafting of the manuscript: Pedro Lopez, Dennis R. Taaffe, Daniel. A. Galvão, Robert U. Newton, Elisa R. Nonemacher, Victória M. Wendt, Renata N. Bassanesi, Douglas J. P. Turella, Anderson Rech. Critical revision of the manuscript for important intellectual content: Pedro Lopez, Dennis R. Taaffe, Daniel. A. Galvão, Robert U. Newton, Elisa R. Nonemacher, Victória M. Wendt, Renata N. Bassanesi, Douglas J. P. Turella, Anderson Rech. Statistical analysis: Pedro Lopez.
Supporting information
Table S1. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in children/adolescents who are overweight or obese.
Table S2. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in young adults who are overweight or obese.
Table S3. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in middle‐aged adults who are overweight or obese.
Table S4. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in older adults who are overweight or obese.
Table S5. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass, body weight and body mass index in children/adolescents who are overweight or obese.
Table S6. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass and body weight and body mass index in young adults who are overweight or obese.
Table S7. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass, body weight and body mass index in middle‐aged adults who are overweight or obese.
Table S8. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass, body weight and body mass index in older adults who are overweight or obese.
Figure S1. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), combined resistance and aerobic exercise (C), combined resistance and aerobic exercise + caloric restriction (D) and combined resistance and aerobic exercise + healthy diet (E) compared with control on body fat percentage in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S2. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), resistance training + protein supplementation (D), combined resistance and aerobic exercise (E) and combined resistance and aerobic exercise + caloric restriction (F) compared with control on whole‐body fat mass in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S3. Mean difference effects of resistance‐based exercise programs compared with control on trunk fat mass (A), visceral adipose tissue (B) and subcutaneous adipose tissue (C) in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. COMB, Combined resistance and aerobic exercise; RET, resistance training; I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S4. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), combined resistance and aerobic exercise (D) and combined resistance and aerobic exercise + caloric restriction (E) compared with control on whole‐body lean mass in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S5. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), combined resistance and aerobic exercise (D), combined resistance and aerobic exercise + caloric restriction (E) and combined resistance and aerobic exercise + healthy diet (F) compared with control on body weight in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S6. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), resistance training + protein supplementation (D), combined resistance and aerobic exercise (E) and combined resistance and aerobic exercise + caloric restriction (F) compared with control on body mass index in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
ACKNOWLEDGMENTS
Pedro Lopez is supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence (CRE) in Prostate Cancer Survivorship Scholarship. Daniel A. Galvão and Robert U. Newton are funded by a NHMRC CRE in Prostate Cancer Survivorship. The results of the study are presented clearly, honestly, without fabrication, falsification, or inappropriate data manipulation. No financial support was received to conduct the present study or for the preparation or publication of this manuscript. Sponsors were not involved in the study design, analysis or interpretation of data, manuscript writing, and decision to submit the manuscript for publication. Open access publishing facilitated by Edith Cowan University, as part of the Wiley ‐ Edith Cowan University agreement via the Council of Australian University Librarians.
Lopez P, Taaffe DR, Galvão DA, et al. Resistance training effectiveness on body composition and body weight outcomes in individuals with overweight and obesity across the lifespan: A systematic review and meta‐analysis. Obesity Reviews. 2022;23(5):e13428. doi: 10.1111/obr.13428
Funding information NHMRC CRE
REFERENCES
- 1. Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947‐1956. doi: 10.1016/s0140-6736(16)00271-3 [DOI] [PubMed] [Google Scholar]
- 2. Semlitsch T, Stigler FL, Jeitler K, Horvath K, Siebenhofer A. Management of overweight and obesity in primary care—a systematic overview of international evidence‐based guidelines. Obes Rev. 2019;20(9):1218‐1230. doi: 10.1111/obr.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459‐471. doi: 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 4. Oppert JM, Bellicha A, van Baak MA, et al. Exercise training in the management of overweight and obesity in adults: synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes Rev. 2021;22(Suppl 4):e13273. doi: 10.1111/obr.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morze J, Rücker G, Danielewicz A, et al. Impact of different training modalities on anthropometric outcomes in patients with obesity: a systematic review and network meta‐analysis. Obes Rev. 2021;22(7):e13218 doi: 10.1111/obr.13218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta‐analysis. Obes Rev. 2021;22(2):e13137. doi: 10.1111/obr.13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellicha A, van Baak MA, Battista F, et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: an overview of 12 systematic reviews and 149 studies. Obes Rev. 2021;22(Suppl 4):e13256. doi: 10.1111/obr.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta‐analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13(1):68‐91. doi: 10.1111/j.1467-789X.2011.00931.x [DOI] [PubMed] [Google Scholar]
- 9. Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta‐analysis. PLoS ONE. 2013;8(2):e56415. doi: 10.1371/journal.pone.0056415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. González‐Ruiz K, Ramírez‐Vélez R, Correa‐Bautista JE, Peterson MD, García‐Hermoso A. The effects of exercise on abdominal fat and liver enzymes in pediatric obesity: a systematic review and meta‐analysis. Child Obes. 2017;13(4):272‐282. doi: 10.1089/chi.2017.0027 [DOI] [PubMed] [Google Scholar]
- 11. Maillard F, Pereira B, Boisseau N. Effect of high‐intensity interval training on total, abdominal and visceral fat mass: a meta‐analysis. Sports Med. 2018;48(2):269‐288. doi: 10.1007/s40279-017-0807-y [DOI] [PubMed] [Google Scholar]
- 12. Khalafi M, Malandish A, Rosenkranz SK, Ravasi AA. Effect of resistance training with and without caloric restriction on visceral fat: a systemic review and meta‐analysis. Obes Rev. 2021;22(9):e13275. doi: 10.1111/obr.13275 [DOI] [PubMed] [Google Scholar]
- 13. Furlan AD, Pennick V, Bombardier C, van Tulder M, Editorial Board CBRG. Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34(18):1929‐1941. doi: 10.1097/BRS.0b013e3181b1c99f [DOI] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. Mapping of reporting guidance for systematic reviews and meta‐analyses generated a comprehensive item bank for future reporting guidelines. J Clin Epidemiol. 2020;118:60‐68. doi: 10.1016/j.jclinepi.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 16. Lefebvre C, Manheimer E, Glanville J, Higgins J & Green S Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 (updated March 2011). The Cochrane Collaboration, 2011. Available from Www Cochrane‐Handbook Org 2011.
- 17. Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41(2):323‐339. doi: 10.1177/0145445516673998 [DOI] [PubMed] [Google Scholar]
- 18. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1(3):98‐101. [Google Scholar]
- 22. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991‐996. doi: 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 23. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455‐463. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 24. Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38(7):1208‐1215. doi: 10.1249/01.mss.0000227304.88406.0f [DOI] [PubMed] [Google Scholar]
- 25. Kim HJ, Lee S, Kim TW, et al. Effects of exercise‐induced weight loss on acylated and unacylated ghrelin in overweight children. Clin Endocrinol (Oxf). 2008;68(3):416‐422. doi: 10.1111/j.1365-2265.2007.03058.x [DOI] [PubMed] [Google Scholar]
- 26. Wong PC, Chia MY, Tsou IY, et al. Effects of a 12‐week exercise training programme on aerobic fitness, body composition, blood lipids and C‐reactive protein in adolescents with obesity. Ann Acad Med Singapore. 2008;37(4):286‐293. [PubMed] [Google Scholar]
- 27. Davis JN, Tung A, Chak SS, et al. Aerobic and strength training reduces adiposity in overweight Latina adolescents. Med Sci Sports Exerc. 2009;41(7):1494‐1503. doi: 10.1249/MSS.0b013e31819b6aea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis JN, Kelly LA, Lane CJ, et al. Randomized control trial to improve adiposity and insulin resistance in overweight Latino adolescents. Obesity (Silver Spring). 2009;17(8):1542‐1548. doi: 10.1038/oby.2009.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farpour‐Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre‐pubertal obese children. J Am Coll Cardiol. 2009;54(25):2396‐2406. doi: 10.1016/j.jacc.2009.08.030 [DOI] [PubMed] [Google Scholar]
- 30. Berntsen S, Mowinckel P, Carlsen KH, et al. Obese children playing towards an active lifestyle. Int J Pediatr Obes. 2010;5(1):64‐71. doi: 10.3109/17477160902957166 [DOI] [PubMed] [Google Scholar]
- 31. Chae HW, Kwon YN, Rhie YJ, et al. Effects of a structured exercise program on insulin resistance, inflammatory markers and physical fitness in obese Korean children. J Pediatr Endocrinol Metab. 2010;23(10):1065‐1072. doi: 10.1515/jpem.2010.168 [DOI] [PubMed] [Google Scholar]
- 32. Davis JN, Gyllenhammer LE, Vanni AA, et al. Startup circuit training program reduces metabolic risk in Latino adolescents. Med Sci Sports Exerc. 2011;43(11):2195‐2203. doi: 10.1249/MSS.0b013e31821f5d4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alberga AS, Farnesi BC, Lafleche A, Legault L, Komorowski J. The effects of resistance exercise training on body composition and strength in obese prepubertal children. Phys Sportsmed. 2013;41(3):103‐109. doi: 10.3810/psm.2013.09.2028 [DOI] [PubMed] [Google Scholar]
- 34. Fazelifar S, Ebrahim K, Sarkisian V. Effect of concurrent training and detraining on anti‐inflammatory biomarker and physical fitness levels in obese children. Revista Brasileira de Medicina Do Esporte. 2013;19(5):349‐354. [Google Scholar]
- 35. Jeon JY, Han J, Kim HJ, Park MS, Seo DY, Kwak YS. The combined effects of physical exercise training and detraining on adiponectin in overweight and obese children. Integr Med Res. 2013;2(4):145‐150. doi: 10.1016/j.imr.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schranz N, Tomkinson G, Parletta N, Petkov J, Olds T. Can resistance training change the strength, body composition and self‐concept of overweight and obese adolescent males? A randomised controlled trial. Br J Sports Med. 2014;48(20):1482‐1488. doi: 10.1136/bjsports-2013-092209 [DOI] [PubMed] [Google Scholar]
- 37. Albuquerque Filho N, Reboucas GM, Matos VAF, de Mello Salgueiro CC, Knackfuss MI, de Medeiros HJ. Effect of concurrent training on body composition and lipid profile in overweight adolescents. J Exer Physiol Online. 2014;17(6):33‐44. [Google Scholar]
- 38. Kelly LA, Loza A, Lin X, et al. The effect of a home‐based strength training program on type 2 diabetes risk in obese Latino boys. J Pediatr Endocrinol Metab. 2015;28(3–4):315‐322. doi: 10.1515/jpem-2014-0470 [DOI] [PubMed] [Google Scholar]
- 39. Lopes WA, Leite N, da Silva LR, et al. Effects of 12 weeks of combined training without caloric restriction on inflammatory markers in overweight girls. J Sports Sci. 2016;34(20):1902‐1912. doi: 10.1080/02640414.2016.1142107 [DOI] [PubMed] [Google Scholar]
- 40. Zehsaz F, Farhangi N, Ghahramani M. The response of circulating omentin‐1 concentration to 16‐week exercise training in male children with obesity. Phys Sportsmed. 2016;44(4):355‐361. doi: 10.1080/00913847.2016.1248223 [DOI] [PubMed] [Google Scholar]
- 41. Bharath LP, Choi WW, Cho JM, et al. Combined resistance and aerobic exercise training reduces insulin resistance and central adiposity in adolescent girls who are obese: randomized clinical trial. Eur J Appl Physiol. 2018;118(8):1653‐1660. doi: 10.1007/s00421-018-3898-8 [DOI] [PubMed] [Google Scholar]
- 42. Soltero EG, Olson ML, Williams AN, et al. Effects of a community‐based diabetes prevention program for Latino youth with obesity: a randomized controlled trial. Obesity (Silver Spring). 2018;26(12):1856‐1865. doi: 10.1002/oby.22300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong A, Sanchez‐Gonzalez MA, Son WM, Kwak YS, Park SY. The effects of a 12‐week combined exercise training program on arterial stiffness, vasoactive substances, inflammatory markers, metabolic profile, and body composition in obese adolescent girls. Pediatr Exerc Sci. 2018;30(4):480‐486. doi: 10.1123/pes.2017-0198 [DOI] [PubMed] [Google Scholar]
- 44. Alves ASR, Venancio TL, Honorio SAA, Martins J, Manuel C. Multicomponent training with different frequencies on body composition and physical fitness in obese children. An Acad Bras Cienc. 2019;91(4):1‐10. [DOI] [PubMed] [Google Scholar]
- 45. Calleja M, Caetano Feitoza N, Falk B, et al. Increased dairy product consumption as part of a diet and exercise weight management program improves body composition in adolescent females with overweight and obesity—a randomized controlled trial. Pediatr Obes. 2020;15(12):e12690. doi: 10.1111/ijpo.12690 [DOI] [PubMed] [Google Scholar]
- 46. Rasooli SA, Fathi R, Golzar FA, Baghersalimi M. The effect of circuit resistance training on plasma levels of amino acids, alpha‐hydroxybutyrate, mannose, and urinary levels of glycine conjugated adducts in obese adolescent boys. Appl Physiol Nutr Metab. 2021;46(6):561‐570. doi: 10.1139/apnm-2020-0171 [DOI] [PubMed] [Google Scholar]
- 47. Ballor DL, Katch VL, Becque MD, Marks CR. Resistance weight training during caloric restriction enhances lean body weight maintenance. Am J Clin Nutr. 1988;47(1):19‐25. doi: 10.1093/ajcn/47.1.19 [DOI] [PubMed] [Google Scholar]
- 48. Hara T, Fujiwara H, Nakao H, Mimura T, Yoshikawa T, Fujimoto S. Body composition is related to increase in plasma adiponectin levels rather than training in young obese men. Eur J Appl Physiol. 2005;94(5–6):520‐526. doi: 10.1007/s00421-005-1374-8 [DOI] [PubMed] [Google Scholar]
- 49. Kirk EP, Washburn RA, Bailey BW, LeCheminant JD, Donnelly JE. Six months of supervised high‐intensity low‐volume resistance training improves strength independent of changes in muscle mass in young overweight men. J Strength Cond Res. 2007;21(1):151‐156. doi: 10.1519/00124278-200702000-00027 [DOI] [PubMed] [Google Scholar]
- 50. Lockwood CM, Moon JR, Tobkin SE, et al. Minimal nutrition intervention with high‐protein/low‐carbohydrate and low‐fat, nutrient‐dense food supplement improves body composition and exercise benefits in overweight adults: a randomized controlled trial. Nutr Metab (Lond). 2008;5:11. doi: 10.1186/1743-7075-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kirk EP, Donnelly JE, Smith BK, et al. Minimal resistance training improves daily energy expenditure and fat oxidation. Med Sci Sports Exerc. 2009;41(5):1122‐1129. doi: 10.1249/MSS.0b013e318193c64e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith AE, Lockwood CM, Moon JR, et al. Physiological effects of caffeine, epigallocatechin‐3‐gallate, and exercise in overweight and obese women. Appl Physiol Nutr Metab. 2010;35(5):607‐616. doi: 10.1139/h10-056 [DOI] [PubMed] [Google Scholar]
- 53. Atashak S, Peeri M, Jafari A, Azarbayjani MA. Effects of ginger supplementation and resistance training on lipid profiles and body composition in obese men. J Med Plant Res. 2011;5(16):3827‐3832. [Google Scholar]
- 54. Cardoso GA. Efeito do consumo de chá verde aliado ou não ao treinamento de força sobre a composição corporal e taxa metabólica de repouso em mulheres com sobrepeso ou obesas. Universidade de São Paulo; 2011. [Google Scholar]
- 55. Jiménez ÓH, Ramírez‐Vélez R. El entrenamiento con pesas mejora la sensibilidad a la insulina y los niveles plasmáticos de lípidos, sin alterar la composición corporal en sujetos con sobrepeso y obesidad. Endocrinol Nutr. 2011;58(4):169‐174. [DOI] [PubMed] [Google Scholar]
- 56. Ha CH, So WY. Effects of combined exercise training on body composition and metabolic syndrome factors. Iran J Public Health. 2012;41(8):20‐26. [PMC free article] [PubMed] [Google Scholar]
- 57. Kang H‐J, Lee YS, Park D‐S, Kang D‐H. Effects of 12‐week circuit weight training and aerobic exercise on body composition, physical fitness, and pulse wave velocity in obese collegiate women. Soft Computing. 2012;16(3):403‐410. [Google Scholar]
- 58. Sheikholeslami Vatani D, Ahmadi Kani Golzar F. Changes in antioxidant status and cardiovascular risk factors of overweight young men after six weeks supplementation of whey protein isolate and resistance training. Appetite. 2012;59(3):673‐678. doi: 10.1016/j.appet.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 59. Roberts CK, Croymans DM, Aziz N, Butch AW, Lee CC. Resistance training increases SHBG in overweight/obese, young men. Metabolism. 2013;62(5):725‐733. doi: 10.1016/j.metabol.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ahmadizad S, Ghorbani S, Ghasemikaram M, Bahmanzadeh M. Effects of short‐term nonperiodized, linear periodized and daily undulating periodized resistance training on plasma adiponectin, leptin and insulin resistance. Clin Biochem. 2014;47(6):417‐422. doi: 10.1016/j.clinbiochem.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 61. Croymans DM, Krell SL, Oh CS, et al. Effects of resistance training on central blood pressure in obese young men. J Hum Hypertens. 2014;28(3):157‐164. doi: 10.1038/jhh.2013.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ha CH, Swearingin B, Jeon YK, Lee M. Effects of combined exercise on HOMA‐IR, HOMA β‐cell and atherogenic index in Korean obese female. Sport Sci Health. 2015;11(1):49‐55. [Google Scholar]
- 63. Franklin NC, Robinson AT, Bian JT, et al. Circuit resistance training attenuates acute exertion‐induced reductions in arterial function but not inflammation in obese women. Metab Syndr Relat Disord. 2015;13(5):227‐234. doi: 10.1089/met.2014.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mahdirejei TA, Razi M, Barari A, et al. A comparative study of the effects of endurance and resistance exercise training on PON1 and lipid profile levels in obese men. Sport Sci Health. 2015;11(3):263‐270. [Google Scholar]
- 65. Moradi F. Changes of serum adiponectin and testosterone concentrations following twelve weeks resistance training in obese young men. Asian J Sports Med. 2015;6(4):e23808. doi: 10.5812/asjsm.23808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sheikholeslami‐Vatani D, Siahkouhian M, Hakimi M, Ali‐Mohammadi M. The effect of concurrent training order on hormonal responses and body composition in obese men. Sci Sport. 2015;30(6):335‐341. [Google Scholar]
- 67. Chen CK, Ismail NS, Al‐Safi AA. Effects of brisk walking and resistance training on cardiorespiratory fitness, body composition, and lipid profiles among overweight and obese individuals. J Phys Edu Sport. 2016;16(3):957‐963. [Google Scholar]
- 68. Kim HJ, Lee HJ, So B, Son JS, Yoon D, Song W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study. Physiol Res. 2016;65(2):271‐279. doi: 10.33549/physiolres.932997 [DOI] [PubMed] [Google Scholar]
- 69. Shahram S, Elham Y, Abdolali B. The effect of endurance and resistance training on inflammatory cytokines in sedentary young women. Acta Med Mediter. 2016;32:999‐1002. [Google Scholar]
- 70. Kim JW, Ko YC, Seo TB, Kim YP. Effect of circuit training on body composition, physical fitness, and metabolic syndrome risk factors in obese female college students. J Exerc Rehabil. 2018;14(3):460‐465. doi: 10.12965/jer.1836194.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miller T, Mull S, Aragon AA, Krieger J, Schoenfeld BJ. Resistance training combined with diet decreases body fat while preserving lean mass independent of resting metabolic rate: a randomized trial. Int J Sport Nutr Exerc Metab. 2018;28(1):46‐54. doi: 10.1123/ijsnem.2017-0221 [DOI] [PubMed] [Google Scholar]
- 72. Mogharnasi M, TaheriChadorneshin H, Abbasi‐Deloei N. Effect of exercise training type on plasma levels of vaspin, nesfatin‐1, and high‐sensitivity C‐reactive protein in overweight and obese women. Obes Med. 2019;13:34‐38. [Google Scholar]
- 73. Hagovska M, Švihra J, Buková A, Dračková D, Horbacz A, Nagyová I. Effect of an exercise programme for reducing abdominal fat on overactive bladder symptoms in young overweight women. Int Urogynecol J. 2020;31(5):895‐902. doi: 10.1007/s00192-019-04157-8 [DOI] [PubMed] [Google Scholar]
- 74. Mendham AE, Larsen S, George C, et al. Exercise training results in depot‐specific adaptations to adipose tissue mitochondrial function. Sci Rep. 2020;10(1):3785. doi: 10.1038/s41598-020-60286-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Soltani N, Marandi SM, Kazemi M, Esmaeil N. Combined all‐extremity high‐intensity interval training regulates immunometabolic responses through toll‐like receptor 4 adaptors and A20 downregulation in obese young females. Obes Facts. 2020;13(3):415‐431. doi: 10.1159/000509132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tavvafian N, Darabi H, Ahani A, et al. Effects of glycyrrhizic acid supplementation during nonlinear resistance training on inflammatory markers and muscular damage indices in overweight young men. Obes Med. 2020;17:100178. [Google Scholar]
- 77. Marks BL, Ward A, Morris DH, Castellani J, Rippe JM. Fat‐free mass is maintained in women following a moderate diet and exercise program. Med Sci Sports Exerc. 1995;27(9):1243‐1251. [PubMed] [Google Scholar]
- 78. Kraemer WJ, Volek JS, Clark KL, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc. 1999;31(9):1320‐1329. doi: 10.1097/00005768-199909000-00014 [DOI] [PubMed] [Google Scholar]
- 79. Fenkci S, Sarsan A, Rota S, Ardic F. Effects of resistance or aerobic exercises on metabolic parameters in obese women who are not on a diet. Adv Ther. 2006;23(3):404‐413. doi: 10.1007/bf02850161 [DOI] [PubMed] [Google Scholar]
- 80. Olson TP, Dengel DR, Leon AS, Schmitz KH. Moderate resistance training and vascular health in overweight women. Med Sci Sports Exerc. 2006;38(9):1558‐1564. doi: 10.1249/01.mss.0000227540.58916.0e [DOI] [PubMed] [Google Scholar]
- 81. Ahmadizad S, Haghighi AH, Hamedinia MR. Effects of resistance versus endurance training on serum adiponectin and insulin resistance index. Eur J Endocrinol. 2007;157(5):625‐631. doi: 10.1530/eje-07-0223 [DOI] [PubMed] [Google Scholar]
- 82. Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ, Warren M, Jensen MD. Strength training and adiposity in premenopausal women: strong, healthy, and empowered study. Am J Clin Nutr. 2007;86(3):566‐572. doi: 10.1093/ajcn/86.3.566 [DOI] [PubMed] [Google Scholar]
- 83. Fernández‐Real JM, Izquierdo M, Moreno‐Navarrete JM, et al. Circulating soluble transferrin receptor concentration decreases after exercise‐induced improvement of insulin sensitivity in obese individuals. Int J Obes (Lond). 2009;33(7):768‐774. doi: 10.1038/ijo.2009.99 [DOI] [PubMed] [Google Scholar]
- 84. González FG, García JCF, Rubio AB, et al. Mejora a corto plazo del peso y la calidad de vida de mujeres obesas posmenopáusicas mediante un programa ambulatorio de ejercicio físico. Med Clin. 2009;133(14):533‐538. [DOI] [PubMed] [Google Scholar]
- 85. Choquette S, Riesco É, Cormier É, Dion T, Aubertin‐Leheudre M, Dionne IJ. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6‐month double‐blind controlled trial. Br J Nutr. 2011;105(8):1199‐1209. doi: 10.1017/s0007114510004897 [DOI] [PubMed] [Google Scholar]
- 86. Stensvold D, Tjønna AE, Skaug EA, et al. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol (1985). 2010;108(4):804‐810. doi: 10.1152/japplphysiol.00996.2009 [DOI] [PubMed] [Google Scholar]
- 87. Deibert P, Solleder F, König D, et al. Soy protein based supplementation supports metabolic effects of resistance training in previously untrained middle aged males. Aging Male. 2011;14(4):273‐279. doi: 10.3109/13685538.2011.565091 [DOI] [PubMed] [Google Scholar]
- 88. Hansen E, Landstad BJ, Brønn R, Gundersen KT, Svebak S. Exercise‐induced changes in body fat, upper leg skeletal muscle area, BMI and body weight in overweight people with risk of developing Type 2 diabetes. Acta Kinesiologiae Universitatis Tartuensis. 2011;17:66‐79. [Google Scholar]
- 89. Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631‐1640. doi: 10.5665/sleep.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Seo DI, So WY, Ha S, et al. Effects of 12 weeks of combined exercise training on visfatin and metabolic syndrome factors in obese middle‐aged women. J Sports Sci Med. 2011;10(1):222‐226. [PMC free article] [PubMed] [Google Scholar]
- 91. Álvarez C, Ramírez R, Flores M, Zúñiga C, Celis‐Morales CA. Efectos del ejercicio físico de alta intensidad y sobrecarga en parámetros de salud metabólica en mujeres sedentarias, pre‐diabéticas con sobrepeso u obesidad. Rev Med Chil. 2012;140(10):1289‐1296. [DOI] [PubMed] [Google Scholar]
- 92. Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. doi: 10.1186/1471-2458-12-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Donges CE, Duffield R, Guelfi KJ, Smith GC, Adams DR, Edge JA. Comparative effects of single‐mode vs. duration‐matched concurrent exercise training on body composition, low‐grade inflammation, and glucose regulation in sedentary, overweight, middle‐aged men. Appl Physiol Nutr Metab. 2013;38(7):779‐788. doi: 10.1139/apnm-2012-0443 [DOI] [PubMed] [Google Scholar]
- 94. Simbo SY. Effects of Exercise and Diet‐induced Weight Loss in Overweight/Obese Women on Characterization of Serum/White Blood Cells, microRNAs and Cytokine Gene Transcription. 2013.
- 95. Venojärvi M, Wasenius N, Manderoos S, et al. Nordic walking decreased circulating chemerin and leptin concentrations in middle‐aged men with impaired glucose regulation. Ann Med. 2013;45(2):162‐170. doi: 10.3109/07853890.2012.727020 [DOI] [PubMed] [Google Scholar]
- 96. Moss J, Tew GA, Copeland RJ, et al. Effects of a pragmatic lifestyle intervention for reducing body mass in obese adults with obstructive sleep apnoea: a randomised controlled trial. Biomed Res Int. 2014;2014:102164. doi: 10.1155/2014/102164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rojano D, Vargas G. Efectos de una dieta hipocalórica y de un programa de ejercicio físico de corta duración en el perfil lipídico y en la composición corporal de mujeres menopáusicas con sobrepeso. Revista Andaluza de Medicina del Deporte. 2014;7(3):95‐100. [Google Scholar]
- 98. Shin JS, Huh YS. Effect of intake of gardenia fruits and combined exercise of middle‐aged obese women on hormones regulating energy metabolism. J Exerc Nutr Biochem. 2014;18(1):41‐49. doi: 10.5717/jenb.2014.18.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brunelli DT, Chacon‐Mikahil MP, Gáspari AF, et al. Combined training reduces subclinical inflammation in obese middle‐age men. Med Sci Sports Exerc. 2015;47(10):2207‐2215. doi: 10.1249/mss.0000000000000658 [DOI] [PubMed] [Google Scholar]
- 100. Baetge C, Earnest CP, Lockard B, et al. Efficacy of a randomized trial examining commercial weight loss programs and exercise on metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab. 2017;42(2):216‐227. doi: 10.1139/apnm-2016-0456 [DOI] [PubMed] [Google Scholar]
- 101. Keating SE, Hackett DA, Parker HM, et al. Effect of resistance training on liver fat and visceral adiposity in adults with obesity: a randomized controlled trial. Hepatol Res. 2017;47(7):622‐631. doi: 10.1111/hepr.12781 [DOI] [PubMed] [Google Scholar]
- 102. Rustaden AM, Haakstad LAH, Paulsen G, Bø K. Effects of BodyPump and resistance training with and without a personal trainer on muscle strength and body composition in overweight and obese women—a randomised controlled trial. Obes Res Clin Pract. 2017;11(6):728‐739. doi: 10.1016/j.orcp.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 103. Han CJ, Korde LA, Reding S, et al. Investigation of a lifestyle intervention in women at high risk of breast cancer. West J Nurs Res. 2018;40(7):976‐996. doi: 10.1177/0193945917697227 [DOI] [PubMed] [Google Scholar]
- 104. Kang SJ, Kim JH, Gang Z, et al. Effects of 12‐week circuit exercise program on obesity index, appetite regulating hormones, and insulin resistance in middle‐aged obese females. J Phys Ther Sci. 2018;30(1):169‐173. doi: 10.1589/jpts.30.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mengistie AB, Reddy R, Babu MS. The effects of workout‐based combination of aerobic and resistance exercise training in obese adults of Northwest Ethiopia. Int J Sport Exerc. 2013;3(1):96‐116. [Google Scholar]
- 106. Nikseresht M. Comparison of serum cytokine levels in men who are obese or men who are lean: effects of nonlinear periodized resistance training and obesity. J Strength Cond Res. 2018;32(6):1787‐1795. doi: 10.1519/jsc.0000000000002039 [DOI] [PubMed] [Google Scholar]
- 107. Oh M, Kim S, An KY, et al. Effects of alternate day calorie restriction and exercise on cardio‐metabolic risk factors in overweight and obese adults: an exploratory randomized controlled study. BMC Public Health. 2018;18(1):1124. doi: 10.1186/s12889-018-6009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cho AR, Moon JY, Kim S, et al. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism. 2019;93:52‐60. doi: 10.1016/j.metabol.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 109. Yoon JR, Ha GC, Ko KJ, Kang SJ. Effects of exercise type on estrogen, tumor markers, immune function, antioxidant function, and physical fitness in postmenopausal obese women. J Exerc Rehabil. 2018;14(6):1032‐1040. doi: 10.12965/jer.1836446.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Álvarez C, Ramírez‐Campillo R, Lucia A, Ramírez‐Vélez R, Izquierdo M. Concurrent exercise training on hyperglycemia and comorbidities associated: non‐responders using clinical cutoff points. Scand J Med Sci Sports. 2019;29(7):952‐967. doi: 10.1111/sms.13413 [DOI] [PubMed] [Google Scholar]
- 111. Jang SH, Paik IY, Ryu JH, Lee TH, Kim DE. Effects of aerobic and resistance exercises on circulating apelin‐12 and apelin‐36 concentrations in obese middle‐aged women: a randomized controlled trial. BMC Womens Health. 2019;19(1):23 doi: 10.1186/s12905-019-0722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wong A, Figueroa A. The effects of low intensity resistance exercise on cardiac autonomic function and muscle strength in obese postmenopausal women. J Aging Phys Act. 2019;27(4):855‐860. doi: 10.1123/japa.2018-0418 [DOI] [PubMed] [Google Scholar]
- 113. Klomklorm A, Ruangthai R, Vaithanomsat P, Sukatta U, Phoemsapthawee J. Concurrent training and Eri silkworm pupae ingestion improve resting and exercise fat oxidation and energy expenditure in obese adults. J Exerc Rehabil. 2020;16(5):467‐479. doi: 10.12965/jer.2040682.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Safarzade A, Alizadeh H, Bastani Z. The effects of circuit resistance training on plasma progranulin level, insulin resistance and body composition in obese men. Horm Mol Biol Clin Invest. 2020;41(2):20190050. doi: 10.1515/hmbci-2019-0050 [DOI] [PubMed] [Google Scholar]
- 115. Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra‐abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323‐330. doi: 10.1001/jama.289.3.323 [DOI] [PubMed] [Google Scholar]
- 116. Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise‐induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silver Spring). 2006;14(11):1921‐1930. doi: 10.1038/oby.2006.224 [DOI] [PubMed] [Google Scholar]
- 117. Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16(1):66‐72. doi: 10.1097/gme.0b013e31817dacf7 [DOI] [PubMed] [Google Scholar]
- 118. Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169(2):122‐131. doi: 10.1001/archinternmed.2008.558 [DOI] [PubMed] [Google Scholar]
- 119. Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol. 2010;109(3):517‐525. doi: 10.1007/s00421-010-1387-9 [DOI] [PubMed] [Google Scholar]
- 120. Henagan TM, Phillips MD, Cheek DJ, Kirk KM, Barbee JJ, Stewart LK. The melanocortin 3 receptor: a novel mediator of exercise‐induced inflammation reduction in postmenopausal women? J Aging Res. 2011;2011:512593. doi: 10.4061/2011/512593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218‐1229. doi: 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bocalini DS, Lima LS, de Andrade S, et al. Effects of circuit‐based exercise programs on the body composition of elderly obese women. Clin Interv Aging. 2012;7:551‐556. doi: 10.2147/cia.S33893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44(11):2099‐2110. doi: 10.1249/MSS.0b013e3182644984 [DOI] [PubMed] [Google Scholar]
- 124. Kim H, Kim M, Kojima N, et al. Exercise and nutritional supplementation on community‐dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc. 2016;17(11):1011‐1019. doi: 10.1016/j.jamda.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 125. Rossi FE, Fortaleza AC, Neves LM, et al. Combined training (aerobic plus strength) potentiates a reduction in body fat but demonstrates no difference on the lipid profile in postmenopausal women when compared with aerobic training with a similar training load. J Strength Cond Res. 2016;30(1):226‐234. doi: 10.1519/jsc.0000000000001020 [DOI] [PubMed] [Google Scholar]
- 126. Chagas EFB, Bonfim MR, Turi BC, Brondino NCM, Monteiro HL. Effect of moderate‐intensity exercise on inflammatory markers among postmenopausal women. J Phys Act Health. 2017;14(6):479‐485. doi: 10.1123/jpah.2016-0319 [DOI] [PubMed] [Google Scholar]
- 127. Chen HT, Chung YC, Chen YJ, Ho SY, Wu HJ. Effects of different types of exercise on body composition, muscle strength, and IGF‐1 in the elderly with sarcopenic obesity. J Am Geriatr Soc. 2017;65(4):827‐832. doi: 10.1111/jgs.14722 [DOI] [PubMed] [Google Scholar]
- 128. Huang SW, Ku JW, Lin LF, Liao CD, Chou LC, Liou TH. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: a pilot randomized controlled trial. Eur J Phys Rehabil Med. 2017;53(4):556‐563. doi: 10.23736/s1973-9087.17.04443-4 [DOI] [PubMed] [Google Scholar]
- 129. Liao CD, Tsauo JY, Lin LF, et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: a CONSORT‐compliant prospective randomized controlled trial. Medicine (Baltimore). 2017;96(23):e7115. doi: 10.1097/md.0000000000007115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Park J, Park H. Effects of 6 months of aerobic and resistance exercise training on carotid artery intima media thickness in overweight and obese older women. Geriatr Gerontol Int. 2017;17(12):2304‐2310. doi: 10.1111/ggi.12972 [DOI] [PubMed] [Google Scholar]
- 131. Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376(20):1943‐1955. doi: 10.1056/NEJMoa1616338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cavalcante EF, Ribeiro AS, Nascimento MA, et al. Effects of different resistance training frequencies on fat in overweight/obese older women. Int J Sports Med. 2018;39(7):527‐534. doi: 10.1055/a-0599-6555 [DOI] [PubMed] [Google Scholar]
- 133. Faramarzi M, Bagheri L, Banitalebi E. Effect of sequence order of combined strength and endurance training on new adiposity indices in overweight elderly women. Isokinetics Exer Sci. 2018;26(2):105‐113. [Google Scholar]
- 134. Fritz NB, Juesas Á, Gargallo P, et al. Positive effects of a short‐term intense elastic resistance training program on body composition and physical functioning in overweight older women. Biol Res Nurs. 2018;20(3):321‐334. doi: 10.1177/1099800418757676 [DOI] [PubMed] [Google Scholar]
- 135. Kim SW, Jung WS, Park W, Park HY. Twelve weeks of combined resistance and aerobic exercise improves cardiometabolic biomarkers and enhances red blood cell hemorheological function in obese older men: a randomized controlled trial. Int J Environ Res Public Health. 2019;16(24):5020. doi: 10.3390/ijerph16245020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Banitalebi E, Faramarzi M, Ghahfarokhi MM, SavariNikoo F, Soltani N, Bahramzadeh A. Osteosarcopenic obesity markers following elastic band resistance training: a randomized controlled trial. Exp Gerontol. 2020;135:110884. doi: 10.1016/j.exger.2020.110884 [DOI] [PubMed] [Google Scholar]
- 137. Park W, Jung WS, Hong K, Kim YY, Kim SW, Park HY. Effects of moderate combined resistance‐ and aerobic‐exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int J Environ Res Public Health. 2020;17(19):7233. doi: 10.3390/ijerph17197233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ribeiro AS, Schoenfeld BJ, Dos Santos L, et al. Resistance training improves a cellular health parameter in obese older women: a randomized controlled trial. J Strength Cond Res. 2020;34(10):2996‐3002. doi: 10.1519/jsc.0000000000002773 [DOI] [PubMed] [Google Scholar]
- 139. Roh HT, Cho SY, So WY. A cross‐sectional study evaluating the effects of resistance exercise on inflammation and neurotrophic factors in elderly women with obesity. J Clin Med. 2020;9(3):842‐852. doi: 10.3390/jcm9030842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wewege MA, Desai I, Honey C, et al. The effect of resistance training in healthy adults on body fat percentage, fat mass and visceral fat: a systematic review and meta‐analysis. Sports Med. 2021;1‐14. doi: 10.1007/s40279-021-01562-2 [DOI] [PubMed] [Google Scholar]
- 141. Yoshimura E, Kumahara H, Tobina T, et al. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes. 2014;2014:197216. doi: 10.1155/2014/197216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ye S, Zhu C, Wei C, et al. Associations of body composition with blood pressure and hypertension. Obesity (Silver Spring). 2018;26(10):1644‐1650. doi: 10.1002/oby.22291 [DOI] [PubMed] [Google Scholar]
- 143. Chen GC, Arthur R, Iyengar NM, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40(34):2849‐2855. doi: 10.1093/eurheartj/ehz391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow‐up study of 14 786 middle‐aged men and women in Finland. Circulation. 1999;99(9):1165‐1172. doi: 10.1161/01.cir.99.9.1165 [DOI] [PubMed] [Google Scholar]
- 145. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39‐48. doi: 10.1161/circulationaha.106.675355 [DOI] [PubMed] [Google Scholar]
- 146. Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring). 2013;21(9):E439‐E447. doi: 10.1002/oby.20135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of changes in abdominal fat quantity and quality with incident cardiovascular disease risk factors. J Am Coll Cardiol. 2016;68(14):1509‐1521. doi: 10.1016/j.jacc.2016.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Morales‐Palomo F, Moreno‐Cabañas A, Ramirez‐Jimenez M, et al. Exercise reduces medication for metabolic syndrome management: a 5‐year follow‐up study. Med Sci Sports Exerc. 2021;53(7):1319‐1325. doi: 10.1249/mss.0000000000002591 [DOI] [PubMed] [Google Scholar]
- 149. Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat‐free mass in middle‐aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68(7):375‐388. doi: 10.1111/j.1753-4887.2010.00298.x [DOI] [PubMed] [Google Scholar]
- 150. Garrow JS, Summerbell CD. Meta‐analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr. 1995;49(1):1‐10. [PubMed] [Google Scholar]
- 151. Sardeli AV, Komatsu TR, Mori MA, Gáspari AF, Chacon‐Mikahil MPT. Resistance training prevents muscle loss induced by caloric restriction in obese elderly individuals: a systematic review and meta‐analysis. Nutrients. 2018;10(4):423‐432. doi: 10.3390/nu10040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Talar K, Hernández‐Belmonte A, Vetrovsky T, Steffl M, Kałamacka E, Courel‐Ibáñez J. Benefits of resistance training in early and late stages of frailty and sarcopenia: a systematic review and meta‐analysis of randomized controlled studies. J Clin Med. 2021;10(8):1630‐1663. doi: 10.3390/jcm10081630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lopez P, Pinto RS, Radaelli R, et al. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin Exp Res. 2018;30(8):889‐899. doi: 10.1007/s40520-017-0863-z [DOI] [PubMed] [Google Scholar]
- 154. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457‐465. doi: 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 155. Feldman BJ, Streeper RS, Farese RV Jr, Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci USA. 2006;103(42):15675‐15680. doi: 10.1073/pnas.0607501103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol Rev. 2008;88(4):1379‐1406. doi: 10.1152/physrev.90100.2007 [DOI] [PubMed] [Google Scholar]
- 157. Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Role of exercise‐induced brain‐derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94(12):1153‐1160. doi: 10.1113/expphysiol.2009.048561 [DOI] [PubMed] [Google Scholar]
- 158. Hunter GR, Fisher G, Neumeier WH, Carter SJ, Plaisance EP. Exercise training and energy expenditure following weight loss. Med Sci Sports Exerc. 2015;47(9):1950‐1957. doi: 10.1249/mss.0000000000000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in children/adolescents who are overweight or obese.
Table S2. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in young adults who are overweight or obese.
Table S3. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in middle‐aged adults who are overweight or obese.
Table S4. Characteristics of randomised controlled trials examining resistance‐based exercise programs on body composition and body weight measures in older adults who are overweight or obese.
Table S5. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass, body weight and body mass index in children/adolescents who are overweight or obese.
Table S6. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass and body weight and body mass index in young adults who are overweight or obese.
Table S7. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass, body weight and body mass index in middle‐aged adults who are overweight or obese.
Table S8. Individual risk of bias assessment for examining body fat percentage, fat mass, lean mass, body weight and body mass index in older adults who are overweight or obese.
Figure S1. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), combined resistance and aerobic exercise (C), combined resistance and aerobic exercise + caloric restriction (D) and combined resistance and aerobic exercise + healthy diet (E) compared with control on body fat percentage in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S2. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), resistance training + protein supplementation (D), combined resistance and aerobic exercise (E) and combined resistance and aerobic exercise + caloric restriction (F) compared with control on whole‐body fat mass in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S3. Mean difference effects of resistance‐based exercise programs compared with control on trunk fat mass (A), visceral adipose tissue (B) and subcutaneous adipose tissue (C) in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. COMB, Combined resistance and aerobic exercise; RET, resistance training; I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S4. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), combined resistance and aerobic exercise (D) and combined resistance and aerobic exercise + caloric restriction (E) compared with control on whole‐body lean mass in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S5. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), combined resistance and aerobic exercise (D), combined resistance and aerobic exercise + caloric restriction (E) and combined resistance and aerobic exercise + healthy diet (F) compared with control on body weight in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.
Figure S6. Mean difference effects of resistance training only (A), resistance training + caloric restriction (B), resistance training + low‐sugar diet (C), resistance training + protein supplementation (D), combined resistance and aerobic exercise (E) and combined resistance and aerobic exercise + caloric restriction (F) compared with control on body mass index in individuals with overweight/obesity. Overall subgroup analyses conducted with a random‐effects model. I 2 represents the heterogeneity test; diamonds represent pooled estimates of random‐effect meta‐analysis.


