Abstract
Background and aims
Diabetes mellitus (DM) is a risk factor for left ventricle (LV) diastolic dysfunction. Aim of this study was to investigate whether endothelial and/or autonomic dysfunction are associated with LV diastolic dysfunction in DM patients.
Methods
We studied 84 non‐insulin‐dependent type 2 DM (T2DM) patients with no heart disease by assessing: 1) LV diastolic function by echocardiography; 2) peripheral vasodilator function, by measuring flow‐mediated dilation (FMD) and nitrate‐mediate dilation (NMD); 3) heart rate variability (HRV) on 24‐h Holter electrocardiographic monitoring.
Results
Twenty‐five patients (29.8%) had normal LV diastolic function, while 47 (55.9%) and 12 (14.3%) showed a mild and moderate/severe diastolic dysfunction, respectively. FMD in these 3 groups was 5.25 ± 2.0, 4.95 ± 1.6 and 4.43 ± 1.8% (p = 0.42), whereas NMD was 10.8 ± 2.3, 8.98 ± 3.0 and 8.82 ± 3.2%, respectively (p = 0.02). HRV variables did not differ among groups. However, the triangular index tended to be lower in patients with moderate/severe diastolic dysfunction (p = 0.09) and a significant correlation was found between the E/e’ ratio and both the triangular index (r = −0.26; p = 0.022) and LF amplitude (r = −0.29; p = 0.011).
Conclusions
In T2DM patients an impairment of endothelium‐independent, but not endothelium‐dependent, dilatation seems associated with LV diastolic dysfunction. The possible role of cardiac autonomic dysfunction in diastolic dysfunction deserves investigation in larger populations of patients.
Keywords: cardiac autonomic dysfunction, endothelial dysfunction, left ventricle diastolic dysfunction, type 2 diabetes mellitus
1. INTRODUCTION
A subclinical myocardial disease in patients with type 2 diabetes mellitus (T2DM) may progress to heart failure (HF) even in the absence of myocardial ischaemia and hypertension. 1–3 In particular, T2DM is among the risk factors that may cause symptoms of HF in presence of preserved ejection fraction (HFpEF),4, 5 a syndrome characterized by impaired left ventricle (LV) diastolic relaxation and increased myocardial stiffness in presence of a normal LV contractility.6, 7 The mechanisms by which T2DM may result in HFpEF symptoms (mainly dyspnoea on effort), however, have not been fully elucidated. Endothelial dysfunction (ED) and autonomic neuropathy are frequent complications of T2DM, affecting up to one‐third of patients, and have both been associated with an increased risk of cardiovascular events. 8–11 Of note, both ED and cardiac autonomic dysfunction have recently been suggested to adversely affect LV diastolic function, resulting in altered LV filling patterns and symptoms of heart failure in presence of preserved LV systolic function.12, 13
ED might determine LV diastolic dysfunction as a result of repeated episodes of subendocardial ischaemia caused by coronary microvascular dysfunction consequent to the impaired endothelial NO production and resulting in abnormal cardiomyocyte relaxation. 14 Furthermore, since NO has also relaxing effects on myocardial cells, it has been hypothesised that an impaired NO production by the endocardial lining might also adversely affect relaxation of subendocardial myocytes, thus also favouring diastolic LV dysfunction. 15 Importantly, although with some differences, ED, when present, is usually diffused in the circulation; accordingly, several studies have shown that assessment of ED in peripheral is largely associated with coronary ED.16, 17
On the other hand, experimental studies have shown that increased adrenergic stimulation favours LV diastolic dysfunction thereby, likely increasing cardiomycyte stiffness and myocardial fibrosis18, 19 and a relation between sympatho‐vagal imbalance and LV diastolic dysfunction was reported in patients with HFpEF. 19
However, to the best of our knowledge, no previous study investigated the relation of LV diastolic function with cardiac autonomic function and endothelial function in patients with T2DM.
2. METHODS
We prospectively studied consecutive patients with T2DM, followed at the Diabetic Center of our University Hospital who fulfilled the following inclusion criteria: 1) no insulin therapy; 2) no evidence of cardiovascular disease, based on clinical history, physical examination, 12‐lead electrocardiogram, 2D‐Doppler echocardiography and carotid echo‐Doppler examination; 3) no significant systemic disease (e.g., liver and/or kidney failure, malignancies, chronic inflammatory disorders, neuromuscular disorders, psychiatric diseases).
The presence of other cardiovascular risk factors was carefully assessed and drug therapy recorded in all patients. Hypertension was defined as blood pressure ≥140/90 mmHg or consumption of any antihypertensive drug. Hypercholesterolaemia was defined as total blood cholesterol >200 mg/dl, low‐density lipoprotein cholesterol >130 mg/dl, or use of lipid‐lowering drugs. Current smoking was defined as any cigarette smoked in the last 6 months.
The study complies with the Declaration of Helsinki and was approved by the local Institutional Review Board of our Institution. All patients were informed of the purpose and nature of the study and provided written, informed consent for participation.
2.1. Echocardiography
Mono‐bidimensional colour‐ and tissue‐Doppler echocardiography was performed in all patients using the ultrasound equipment Artida Toshiba (Toshiba Italy) and a 3.5‐MHz probe to measure the volumes and dimensions of heart chambers and assess LV systolic and diastolic function. Patients were positioned in the left lateral decubitus position and a 4‐chamber apical view was obtained. The sample volume of the pulsed‐wave Doppler was positioned immediately below the central point of contact of the two mitral leaflets in systole and the Doppler profile of the mitral flow was recorded. The E wave and A wave peaks were measured and the E/A wave ratio was calculated. Tissue‐Doppler imaging was then performed to measure the speed of displacement of the mitral annulus at the level of the lateral and septal wall. The echographic signal obtained at this level shows three different components: a peak systolic wave (S) and two waves of opposite polarity (e’ and A’) during the early and late phase of diastole, respectively. From pulsed‐wave Doppler and tissue‐Doppler images, we obtained the E/e’ ratio for both the lateral and septal wall; from the 2 measures the average E/e’ ratio was obtained, which was used in all subsequent analyses. The average E/e’ ratio, indeed, has been demonstrated to correlate significantly with left ventricular filling pressure and is therefore considered a valuable index of LV diastolic function. 15
A combination of E/A ratio (normal value ≥0.8), average E/e’ ratio (normal value <10), peak diastolic tricuspid velocity (normal value <2.8 m/s) and left atrial volume index (normal value < 34 ml/m2) was used to classify LV diastolic function in normal and as grade I (mild), grade II (moderate) or grade III (severe) diastolic dysfunction according to the criteria of the 2016 American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) guidelines. 16
Left ventricle ejection fraction (LVEF) was calculated according to the modified Simpson's rule (biplane methods of disks), following the current ASE/EACVI recommendations. 17
2.2. Peripheral arterial dilator function
All patients underwent assessment of systemic arterial dilator function, following a method described in detail elsewhere. 10 , 18–20 Vasodilator tests were performed by the same expert operator.
Endothelium‐dependent vasodilation was assessed by measuring flow mediated dilation (FMD). Briefly, subjects rested in a supine position for at least 10 min before testing. A 10‐MHz multifrequency linear array probe attached to a high‐resolution ultrasound machine was used to acquire images of the right brachial artery with a mechanical support maintaining the probe in a fixed position throughout the examination. Brachial artery diameter was measured throughout the test using a totally automated system that identifies the internal edges of the vessel and tracks the walls of the artery via differences in brightness intensity compared to the lumen of the vessel. The software provides a diameter measurement for every second. After obtaining basal images of the brachial artery, a forearm cuff, positioned 1 cm under the antecubital fossa, was inflated to 50 mm Hg above the systolic blood pressure and released after 5 min to elicit forearm reactive hyperaemia. FMD was calculated as the maximum percent change of the brachial artery diameter during hyperaemia compared to the basal diameter.
Endothelium‐independent dilator function was also assessed in our patients by measuring nitrate‐mediated dilation (NMD). To this aim, 10 min after full recovery from FMD assessment, brachial artery diameter was measured at baseline and for 15 min after administration of 25 μg of sublingual nitroglycerin, and NMD was expressed as the percent maximal increase of the artery diameter after nitroglycerin compared to baseline. 18–20
2.3. Cardiac autonomic function
Cardiac autonomic function was assessed by heart rate variability (HRV) on 24‐h electrocardiographic (ECG) Holter recordings, which were performed using 3‐channel digital recorders (FD5‐plus, Schiller Medilog). The bipolar chest leads CM5, CM1 and modified aVF were monitored and the data were analysed by an expert operator using the Medilog Darwin‐2 system (Schiller Medilog). HRV was assessed both in the time‐domain and frequency‐domain, as previously described. 21 , 22 Time domain HRV variables included the standard deviation of all RR intervals in the 24 h (SDNN), the mean of the standard deviations of RR intervals of all 5‐min segments in the 24 h (SDNNi) and the triangular index, obtained as the ratio between the total number of RR intervals in the 24 h and the number of RR intervals with the modal value. Frequency domain HRV was assessed by fast Fourier transform spectral analysis, obtaining the amplitudes of the RR interval changes in the ranges of very low‐frequency (VLF, 0.0033–0.04 Hz), low‐frequency (LF, 0.04–0.15 Hz) and high‐frequency (HF, 0.15–0.40 Hz). The LF/HF ratio was also calculated.
2.4. Statistical analysis
Data are reported as mean and standard deviation for continuous variables and number and proportions for discrete variables. No variable showed a distribution different from the normal one according to Kolmogorov–Smirnov test. Thus, continuous variables were compared by the analysis of variance (ANOVA). Significant differences in outcome variables were adjusted for clinical/laboratory variables that showed significant or borderline (p ≤ 0.1) statistical differences among groups using a generalised linear model. Bonferroni correction was then applied for multiple between‐group comparisons. Categorical variables were compared by χ2 test. Since all patients with moderate/severe LV diastolic dysfunction had abnormally high E/e’ ratio, correlation analyses by Pearson's test were performed between the E/e’ ratio, as the most significant single variable for the presence of impaired diastolic function, and FMD, NMD and HRV parameters. A two‐tailed p value <0.05 was considered as statistically significant. The SPSS 21.0 statistical software (SPSS Italia Inc, Florence, Italy) was used for analyses.
3. RESULTS
3.1. General and echocardiographic findings
Overall, 84 patients were enrolled in the study. LV diastolic function was found to be normal in 25 patients (29.8%; Group 1), whereas some degree of diastolic dysfunction was found in 59 patients (70.2%). Specifically, 47 patients (55.9%) had mild diastolic dysfunction (grade I; Group 2), whereas 10 patients (11.9%) had moderate (grade II) and 2 patients (2.4%) severe (grade III) LV diastolic dysfunction. As only 2 patients had severe LV diastolic dysfunction, a single group of 12 patients with moderate or severe diastolic dysfunction was considered for analyses (Group 3).
The main demographic and clinical characteristics of the whole population and the 3 groups of patients are summarised in Table 1. As shown, patients of Group 3 were significantly older compared to patients of the other groups (p = 0.012). There were no significant differences among groups in other major clinical characteristics, except for a higher rate of dyslipidaemia in Group 3 patients (p = 0.043). Furthermore, glycated haemoglobin (HbA1c) serum levels also tended to be higher in patients with more severe forms of LV diastolic dysfunction (p = 0.096). The latter group of patients also showed a higher consumption of beta‐blockers (p = 0.041), angiotensin‐II receptor blockers (ARBs) (p = 0.025) and glinides (p = 0.007) compared to the other groups.
TABLE 1.
Main characteristics of the whole population and the three groups of patients
| Whole population (n = 84) | Group 1 (n = 25) | Group 2 (n = 47) | Group 3 (n = 12) | p | |
|---|---|---|---|---|---|
| Age (years) | 64.1 ± 10 | 59.4 ± 13 | 65.4 ± 8 | 68.7 ± 5 | 0.012 |
| Male (%) | 54 (64.3) | 17 (68.0) | 29 (61.7) | 8 (66.7) | 0.85 |
| BMI (Kg/m2) | 28.5 ± 4 | 29.1 ± 4 | 28.0 ± 4 | 29.1 ± 3 | 0.44 |
| Diabetes duration (years) | 13.6 ± 8 | 12.4 ± 7 | 13.8 ± 8 | 15.6 ± 6 | 0.50 |
| Hb1AC (%) | 7.16 ± 1.2 | 6.96 ± 1.1 | 7.10 ± 0.8 | 7.82 ± 2.2 | 0.096 |
| C‐reactive protein (mg/L) | 2.53 ± 4.5 | 2.42 ± 3.2 | 2.41 ± 5.3 | 3.22 ± 3.6 | 0.88 |
| CV risk factors | |||||
| Hypertension (%) | 52 (61.9) | 15 (60.0) | 27 (57.4) | 10 (83.3) | 0.25 |
| Hypercholesterolaemia (%) | 47 (56.0) | 10 (40.0) | 27 (57.4) | 10 (83.3) | 0.043 |
| Active smokers (%) | 16 (19.0) | 7 (28.0) | 8 (17.0) | 1 (8.3) | 0.31 |
| CV therapy | |||||
| Antiaggregants (%) | 42 (50.0) | 8 (32.0) | 27 (57.4) | 7 (58.3) | 0.099 |
| Beta blockers (%) | 25 (29.8) | 8 (32.0) | 10 (21.3) | 7 (58.3) | 0.041 |
| Calcium antagonists (%) | 16 (19.0) | 5 (20.0) | 6 (12.8) | 5 (41.7) | 0.074 |
| ACE inhibitors (%) | 23 (27.4) | 3 (12.0) | 16 (34.0) | 4 (33.3) | 0.12 |
| ARBs (%) | 24 (28.6) | 8 (32.0) | 9 (19.1) | 7 (58.3) | 0.025 |
| Diuretics (%) | 18 (21.4) | 3 (12.0) | 11 (23.4) | 4 (33.3) | 0.29 |
| Statins (%) | 43 (51.2) | 10 (40.0) | 25 (53.2) | 8 (66.7) | 0.29 |
| Antidiabetic therapy | |||||
| Metformin (%) | 78 (92.9) | 23 (92.0) | 45 (95.7) | 10 (83.3) | 0.32 |
| Sulfonylureas (%) | 31 (36.9) | 10 (40.0) | 17 (36.2) | 4 (33.3) | 0.91 |
| Incretins (%) | 13 (15.5) | 5 (20.0) | 7 (14.9) | 1 (8.3) | 0.65 |
| DPP‐4 inhibitors (%) | 14 (16.7) | 1 (4.0) | 11 (23.4) | 2 (16.7) | 0.11 |
| Glinides (%) | 11 (13.1) | 2 (8.0) | 4 (8.5) | 5 (41.7) | 0.007 |
| GLP‐1 analogues (%) | 6 (7.1) | 2 (8.0) | 3 (6.4) | 1 (8.3) | 0.95 |
| SGLT‐2 inhibitors (%) | 2 (2.4) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 0.089 |
Note: Group 1 = patients with normal left ventricle diastolic function; Group 2 = patients with grade I (mild) left ventricle diastolic dysfunction; Group 3 = patients with grade II–III (moderate/severe) left ventricle diastolic dysfunction.
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin‐II receptor blockers; BMI, body mass index; CV, cardiovascular; DPP‐4, dipeptidyl peptidase‐4; GLP‐1, Glucagon‐like peptide‐1; HbA1c, glycated haemoglobin; SGLT‐2, sodium‐glucose co‐transporter‐2.
The main echocardiographic data of the 3 groups of patients are summarised in Table 2. As expected, the indices correlated to LV diastolic function differed significantly among groups. LVEF, however, was comparatively normal in the 3 groups (p = 0.27).
TABLE 2.
Main echocardiographic findings of the 3 groups of patients
| Group 1 (n = 25) | Group 2 (n = 47) | Group 3 (n = 12) | p | |
|---|---|---|---|---|
| E wave (cm/sec) | 73.4 ± 17.6 | 54.6 ± 8.5 | 82.3 ± 19.1 | <0.001 |
| A wave (cm/sec) | 75.3 ± 17.4 | 84.4 ± 12.3 | 91.9 ± 25.3 | 0.011 |
| E/A ratio | 0.98 ± 0.17 | 0.65 ± 0.08 | 0.95 ± 0.32 | <0.001 |
| E/e’ ratio | 7.12 ± 1.4 | 6.79 ± 1.2 | 11.83 ± 2.1 | <0.001 |
| DcT (msec) | 197.7 ± 41 | 200.8 ± 46 | 190.9 ± 39 | 0.78 |
| LAVi (mL/m2) | 24.4 ± 5.2 | 25.4 ± 4.4 | 36.2 ± 7.2 | <0.001 |
| Peak tricuspid vel. (m/s) | 2.2 ± 0.3 | 2.1 ± 0.3 | 2.6 ± 0.2 | <0.001 |
| LVEF (%) | 62.4 ± 3.1 | 61.7 ± 4.7 | 60.0 ± 4.0 | 0.27 |
Note: Group 1 = patients with normal left ventricle diastolic function; Group 2 = patients with grade I (mild) left ventricle diastolic dysfunction; Group 3 = patients with grade II–III (moderate/severe) left ventricle diastolic dysfunction.
Abbreviations: DcT, deceleration time; LAVi, left atrial volume index; LVEF, left ventricle ejection fraction; vel, velocity.
3.2. Peripheral arterial dilator function
The main findings concerning the assessment of arterial dilator function are summarised in Table 3, whereas individual data of both FMD and NMD are shown in Figure 1. The endothelium‐dependent peripheral vasodilator function did not show statistically significant differences between the 3 groups. FMD was, indeed, 5.25% ± 2.0% in patients of Group 1, 4.95% ± 1.6% in patients of Group 2 and 4.43 ± 1.8% in patients of Group 3 (p = 0.42).
TABLE 3.
Main findings of arterial dilator function tests
| Group 1 (n = 25) | Group 2 (n = 47) | Group 3 (n = 12) | p | |
|---|---|---|---|---|
| FMD assessment | ||||
| Basal artery diameter (mm) | 3.98 ± 0.70 | 4.09 ± 0.64 | 4.19 ± 0.61 | 0.62 |
| Peak artery diameter (mm) | 4.19 ± 0.75 | 4.29 ± 0.68 | 4.38 ± 0.61 | 0.71 |
| Artery diameter increase (mm) | 0.21 ± 0.09 | 0.20 ± 0.07 | 0.19 ± 0.07 | 0.64 |
| FMD (%) | 5.25 ± 2.0 | 4.95 ± 1.6 | 4.43 ± 1.8 | 0.42 |
| NMD assessment | ||||
| Basal artery diameter (mm) | 4.00 ± 0.71 | 4.11 ± 0.66 | 4.16 ± 0.61 | 0.75 |
| Peak artery diameter (mm) | 4.41 ± 0.77 | 4.45 ± 0.68 | 4.57 ± 0.70 | 0.81 |
| Artery diameter increase (mm) | 0.43 ± 0.11 | 0.36 ± 0.13 | 0.37 ± 0.15 | 0.27 |
| NMD (%) | 10.8 ± 2.3 | 8.98 ± 3.0 | 8.82 ± 3.2 | 0.027 |
Note: Group 1 = patients with normal left ventricle diastolic function; Group 2 = patients with grade I (mild) left ventricle diastolic dysfunction; Group 3 = patients with grade II–III (moderate/severe) left ventricle diastolic dysfunction.
Abbreviations: FMD, flow‐mediated dilation; NMD, nitrate‐mediate dilation.
FIGURE 1.
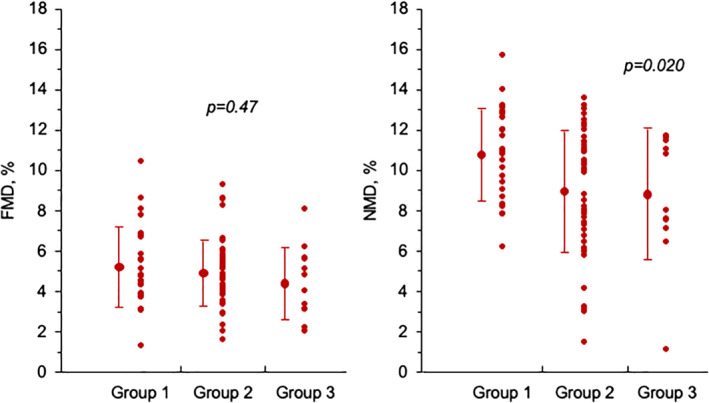
Individual data, with average and standard deviation, of flow‐mediated dilation (FMD) and nitrate mediated dilation (NMD) in the 3 groups of patients. Group 1 = patients with normal left ventricle diastolic function; Group 2 = patients with grade I (mild) left ventricle diastolic dysfunction; Group 3 = patients with grade II–III (moderate/severe) left ventricle diastolic dysfunction
In contrast, we found that endothelium‐independent vasodilator function was lower in our patients of Group 2 and Group 3 as compared to those of Group 1. NMD was, indeed, 10.8% ± 2.3%, 8.98 ± 3.0 and 8.82 ± 3.2 in the three groups, respectively (p = 0.02 after adjustment for confounding clinical/laboratory variables). However, no significant correlation was found between the E/e’ ratio and both FMD (r = −0.10; p = 0.36) and NMD (r = −0.13; p = 0.25).
3.3. Cardiac autonomic function
Twenty‐four‐hour Holter ECG monitoring was available for analyses in 77 patients (92%); 5 patients, indeed, refused to undergo the test, whereas 2 recordings were unreliable due to technical issues.
No significant differences were observed between the 3 groups of patients in HRV variables, both in the time‐domain and frequency‐domain (Table 4). However, the triangular index showed a trend toward lower values in patients of Group 3 (p = 0.09). Furthermore, a significant, although mild, correlation was found between the E/e’ ratio and both the triangular index in the time domain (r = −0.26; p = 0.022) and LF amplitude in the frequency domain (r = −0.29; p = 0.011) (Figure 2).
TABLE 4.
Main heart rate variability data in the 3 groups of patients
| Group 1 (n = 22) | Group 2 (n = 44) | Group 3 (n = 11) | p | |
|---|---|---|---|---|
| Time domain | ||||
| SDANN (msec) | 106.4 ± 31.1 | 98.3 ± 32.7 | 107.7 ± 32.3 | 0.30 |
| SDNNi (msec) | 43.9 ± 14.7 | 39.2 ± 17.9 | 41.4 ± 16.9 | 0.19 |
| VFC | 32.9 ± 9.5 | 29.8 ± 12.2 | 25.5 ± 14.1 | 0.09 |
| pNN50 (%) | 4.5 ± 4.3 | 4.8 ± 7.9 | 3.3 ± 3.1 | 0.73 |
| Frequency‐domain | ||||
| VLF (msec) | 29.6 ± 9.0 | 27.8 ± 12.2 | 27.2 ± 12.2 | 0.47 |
| LF (msec) | 18.3 ± 7.8 | 16.7 ± 9.1 | 13.7 ± 5.8 | 0.21 |
| HF (msec) | 10.8 ± 5.3 | 10.2 ± 6.6 | 9.2 ± 3.6 | 0.65 |
| LF/HF ratio | 1.90 ± 0.7 | 1.82 ± 0.6 | 1.57 ± 0.6 | 0.26 |
Note: Group 1 = patients with normal left ventricular diastolic function; Group 2 = patients with grade I (mild) left ventricular diastolic dysfunction; Group 3 = patients with grade II–III (moderate/severe) left ventricular diastolic dysfunction. See “Methods” for definition of heart rate variability parameters.
FIGURE 2.
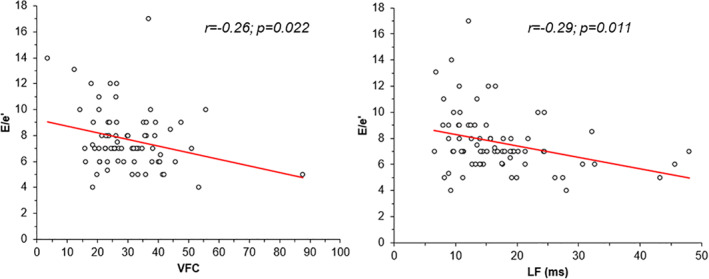
Correlation between the E/e’ ratio and the heart rate variability (HRV) variables triangular index and low‐frequency amplitude
4. DISCUSSION
In this study we aimed at assessing whether endothelial dysfunction and/or cardiac autonomic dysfunction may play a pathophysiologic role in LV diastolic dysfunction of T2DM patients. Our results show that, in non‐insulin‐dependent T2DM, LV diastolic dysfunction is not associated with systemic endothelial dysfunction; however, a lower endothelium‐independent vasodilation was observed in patients with impaired as compared to those with normal diastolic function. On the other hand, no clear association also emerged between cardiac autonomic dysfunction, as assessed by HRV, and diastolic dysfunction.
T2DM is associated with a sizeable increase of the risk of cardiovascular and, in particular, coronary events. Coronary involvement may concern both large epicardial arteries and coronary microcirculation. 20 However, several data suggest that T2DM may also cause direct abnormalities of cardiomyocyte metabolism and function, resulting in a ‘diabetic cardiomyopathy’. 3 Moreover, T2DM has also consistently been found to be a significant risk factor for the development of the clinical syndrome of HFpEF,4, 5 which is typically characterized by impaired LV relaxation during diastole. Indeed, diastolic dysfunction has been found in up to 75% of T2DM patients, with symptoms of HFpEF reported in about 30%–40% of patients. 4
The pathophysiologic mechanisms of diastolic dysfunction in T2DM, however, remain poorly known. Both ED and cardiac autonomic dysfunction are frequent complications of T2DM and both have pathophysiologic consequences that might favour LV diastolic dysfunction. 21–25
An abnormal function of vascular endothelial cells in T2DM is likely to be associated with an abnormal function also of endocardial cells, which usually contribute to myocardial relaxation through subendocardial relase of NO,12, 13 thus resulting in LV diastolic dysfunction.13, 23 Furthermore, vascular endothelial dysfunction may favour increased arterial stiffening, as assessed by arterial elastance, as well as increased systemic vascular resistance, thus favouring an increase in blood pressure, which poses increased workload to the myocardium, resulting in LV hypertrophy, a frequent finding and cause of diastolic dysfunction in HFpEF patients.12, 23, 24, 25
Our study, however, failed to show a correlation between diastolic dysfunction and endothelium‐dependent systemic vascular dilator function, as FMD showed no significant difference between patients with or without diastolic dysfunction or between patients with mild compared to patients with moderate/severe diastolic dysfunction.
On the other hand, we surprisingly found an impaired endothelium‐independent peripheral dilator function in patients with any evidence of LV diastolic dysfunction compared to those with normal LV diastolic function, as indicated by lower NMD values, even after adjustment for significant clinical variables and drugs. The reasons for this finding are not immediately clear, but it is possible that an impairment of a maximal dilator response to direct stimulation of vascular smooth muscle cells may better reflect an increase in systemic vascular resistance and LV afterload which translate into LV overload and impairment of cardiomyocyte relaxation. 25
Cardiac autonomic dysfunction in T2DM patients usually initially results in sympatho‐vagal imbalance, with a predominance of sympathetic activity over an impaired vagal function.26, 27 This may cause alterations in cardiomyocyte metabolism, resulting in an impairment of cardiomyocyte relaxation. Also, sympathetic predominance may determine coronary microvascular dysfunction, with a consequent impairment of myocardial perfusion, which has also been suggested to contribute to abnormal LV diastolic relaxation. 28
In our study, we also failed to find a clear relation between LV diastolic dysfunction and cardiac autonomic dysfunction in T2DM. HRV parameters, indeed, did not differ significantly between patients with normal LV diastolic function and those with mild or moderate diastolic dysfunction.
However, the triangular index tended to be lower, although just above statistical significance, in patients with moderate/severe LV diastolic dysfunction, as compared to the other 2 groups. Furthermore, the same index and LF amplitude showed a significant, although slight, correlation with E/e’ ratio, which is considered the single most valuable echocardiographic index of increased LV diastolic pressure and, therefore, diastolic dysfunction. Thus, these results suggest that further studies are desirable to better assess, in larger populations, whether some relation may actually exist between cardiac sympatho/vagal imbalance and LV diastolic function.
It is worth noting that our data suggest some relation between a more difficult glycaemic control and LV diastolic dysfunction. Patients with moderate/severe diastolic dysfunction, indeed, showed the highest values of HbA1c, although just above statistical significance, and the highest consumption of glinide, as compared to the other 2 groups. Among other clinical/laboratory variables, on the other hand, only age and a history of hypercholesterolaemia were associated with LV dysfunction.
Some limitations of our study should be acknowledged. First, the number of patients included was relatively small and therefore minor true differences among groups in FMD and HRV might not have been detected as significant; furthermore, our population mainly included patients with a mild form of LV diastolic dysfunction, as only 2 and 10 patients, respectively, had a severe (III grade) or moderate (II grade) form of diastolic dysfunction. Therefore, further studies, in larger groups of patients, are needed to establish whether endothelial dysfunction and/or cardiac autonomic dysfunction present a different prevalence in patients with more severe forms of LV diastolic dysfunction. Second, we did not perform any measurement of shear stimulus during FMD assessment; therefore, we cannot exclude that different entities of shear stress were achieved in the 3 groups and influenced in some way the results, despite the same method of forearm ischaemia was carefully applied in all patients. Thus, our data need to be confirmed in studies also assessing a measure of shear stimulus. Finally, a confirmation of the independent association of NMD with LV diastolic dysfunction should also be obtained in further studies, as the limited number of patients included in our study might have not allowed appropriate correction for potentially confounding variables.
5. CONCLUSIONS
Our data in T2DM patients failed to demonstrate a relation between LV diastolic dysfunction and endothelial dysfunction, whereas they showed a significant relationship between peripheral endothelium‐independent arterial dilator function and LV diastolic dysfunction. The tendency to lower values of the triangular index and a significant inverse correlation between some HRV parameters and the echocardiographic E/e’ ratio, on the other hand, suggest that an impairment of cardiac autonomic function might contribute to LV diastolic dysfunction, although this should be clarified in larger studies.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest to disclose.
ETHICS STATEMENT
The study complies with the Declaration of Helsinki and was approved by our Institutional Review Board. All patients were informed of the purpose and nature of the study and provided written, informed consent for participation.
AUTHOR CONTRIBUTIONS
Gaetano Antonio Lanza, Saverio Tremamunno and Antonio De Vita designed the study, analysed the data, and drafted the manuscript; Veronica Melita, Gessica Ingrasciotta, Eleonora Ruscio, Monica Filice, Linda Tartaglione, Gaetano Emanuele Rizzo, Mauro Di Leo and Tamara Felici selected and enrolled the patients and performed the exams; Saverio Tremamunno, Antonio De Vita, Angelo Villano, Antonio Bisignani and Salvatore Emanuele Ravenna collected data and handled the database; Dario Pitocco and Gaetano Antonio Lanza critically reviewed the manuscript. All authors have read and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/dmrr.3484.
ACKNOWLEDGMENTS
We do not have acknowledgement to include.
Open Access Funding provided by Universita Cattolica del Sacro Cuore within the CRUI–CARE Agreement.
Tremamunno S, De Vita A, Villano A, et al. Relation of endothelial and cardiac autonomic function with left ventricle diastolic function in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2022;38(2):e3484. 10.1002/dmrr.3484
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Seferović PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853‐872. [DOI] [PubMed] [Google Scholar]
- 2. Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124:121‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602 [DOI] [PubMed] [Google Scholar]
- 4. McHugh K, DeVore AD, Wu J, et al. Heart failure with preserved ejection fraction and diabetes: JACC State‐of‐the‐Art review. J Am Coll Cardiol. 2019;73:602‐611. [DOI] [PubMed] [Google Scholar]
- 5. Chirinos JA, Bhattacharya P, Kumar A, et al. Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8:e011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phan TT, Abozguia K, Nallur Shivu G, et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. [DOI] [PubMed] [Google Scholar]
- 7. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507‐515. [DOI] [PubMed] [Google Scholar]
- 8. Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta‐analysis. Diabetes Care. 2003;6:1895–1901. [DOI] [PubMed] [Google Scholar]
- 9. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. [DOI] [PubMed] [Google Scholar]
- 10. Villano A, Mencarelli E, Melita V, et al. Endothelial dysfunction and cardiovascular outcome in asymptomatic patients with type 2 diabetes: a pilot study. Diabetes Metab Res Rev. 2020;36:e3215. [DOI] [PubMed] [Google Scholar]
- 11. Cosentino F, Lüscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S54‐S61. [PubMed] [Google Scholar]
- 12. Kishimoto S, Kajikawa M, Maruhashi T, et al. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2017;231:181–187. [DOI] [PubMed] [Google Scholar]
- 13. Somsen GA, Dubois EA, Brandsma K, et al. Cardiac sympathetic neuronal function in left ventricular volume and pressure overload. Cardiovasc Res. 1996;31:132–138. [PubMed] [Google Scholar]
- 14. Crea F, Bairey Merz CN, Beltrame JF, et al. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38:473‐477. [DOI] [PubMed] [Google Scholar]
- 15. Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235‐1241. [DOI] [PubMed] [Google Scholar]
- 16. Teragawa H, Ueda K, Matsuda K, et al. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol. 2005;28:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grimm D, Elsner D, Schunkert H, et al. Development of heart failure following isoproterenol administration in the rat: role of the renin‐angiotensin system. Cardiovasc Res. 1998;37:91‐100. [DOI] [PubMed] [Google Scholar]
- 18. Verloop WL, Beeftink MM, Santema BT, et al. A systematic review concerning the relation between the sympathetic nervous system and heart failure with preserved left ventricular ejection fraction. PLoS One. 2015;10:e0117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silberman GA, Fan TH, Liu H, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park JH, Marwick TH. Use and limitations of E/e’ to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound. 2011;19:169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277‐314. [DOI] [PubMed] [Google Scholar]
- 22. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1‐39.e14. [DOI] [PubMed] [Google Scholar]
- 23. Faulx MD, Wright AT, Hoit BD. Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am Heart J. 2003;145:943‐951. [DOI] [PubMed] [Google Scholar]
- 24. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2‐H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nerla R, Tarzia P, Sestito A, et al. Effect of bariatric surgery on peripheral flow‐mediated dilation and coronary microvascular function. Nutr Metabol Cardiovasc Dis. 2012;22:626‐634. [DOI] [PubMed] [Google Scholar]
- 26. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European society of cardiology and the North American Society of pacing and electrophysiology. Eur Heart J. 1996;17:354‐381. [PubMed] [Google Scholar]
- 27. Lanza GA, Ruscio E, Ingrasciotta G, et al. Relation of vascular dilator function and cardiac autonomic function with coronary angiography findings in patients with non‐ST segment elevation acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. Published online April 22, 2020. 10.1177/2048872620918714 [DOI] [PubMed] [Google Scholar]
- 28. Tousoulis D, Papageorgiou N, Androulakis E, et al. Diabetes mellitus‐associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013;62:667‐676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


