Abstract
Background
Obstructive sleep apnea (OSA) is a worldwide increasing syndrome, which, by promoting endothelial dysfunction, contributes to extend the cardiovascular risk. We evaluated the cardiovascular risk in a group of OSA patients.
Methods
A total of 185 OSA subjects (19 normal weight, 57 overweight, 109 obese), who entered the Ambulatory of Sleep Disorders of the Institute of Respiratory Diseases of the University of Bari, during 1 year, were enrolled in the study. We assessed anthropometric features, polysomnographic findings, cardiovascular risk factors, smoking habit, Pulmonary Function Test, Arterial Blood Gas Analysis, Epworth Questionnaire, and Charlson Co‐morbidities Index (CCI). Subjects were divided into three groups, according to their BMI: individuals with BMI ≥30 kg/m2 (Group 1 n = 109, mean age 61 ± 1; 74.3% men), individuals with BMI ranging from 25.0 to 29.9 kg/m2 defined as overweight subjects (Group 2 n = 57, mean age 58.8 ± 1.4; 77% men), and subjects with a BMI ranging from 18.5 to 24.9 kg/m2 defined as normal weight subjects (Group 3 n = 19, mean age 54.2 ± 2.3; 64,2% men).
Results
In the whole population, the percentage cardiovascular risk was weakly related with BMI (r = 0.33; P < .001), but not with AHI. The cardiovascular risk was strictly related to the obesity (P < .00002), while the Epworth Questionnaire score and the Charlson Co‐morbidity Index were respectively statistically higher in the group of obese individuals (P = .004, P = .0002) than in the other two sub‐groups. When AHI values were stratified in tertiles, the percentage cardiovascular risk did not vary with increasing AHI values (Figure 2).
Conclusions
Further studies are required to investigate the pivotal role of inflammation resulting from obesity, and underlying increased cardiovascular risk in OSA patients.
What is already known about this topic?
Obesity is a major risk factor for the development and progression of obstructive sleep apnea (OSA). Obesity may worsen OSA with increased fat deposition in tissues of the upper airway, and in the thorax, reducing chest compliance and functional capacity. The combination of both factors increase cardiovascular risk in these subjects. It is a matter of debate whether the severity of OSA per se can be considered as an independent factor of augmenting cardiovascular risk in obese patients.
What does this article add?
Although the sample size is small to make a fully adequate comparison among subgroups, results from the present study revealed the lack of a clear relationship between the severity of OSA and the extent of cardiovascular risk. Among our OSA population, we showed that the percentage of cardiovascular risk was related with Body Mass Index (BMI) but not with Apnea/hypopnea index (AHI). Therefore, the severity of OSA does not seem to increase, per se, the cardiovascular risk.
1. INTRODUCTION
The cardiovascular risk is defined by WHO/ISH by risk prediction charts, which indicate 10‐year risk of a fatal or nonfatal major cardiovascular event (myocardial infarction or stroke), according to age, sex, blood pressure, smoking status, total and HDL blood cholesterol, presence or absence of diabetes mellitus, and anti‐hypertensive treatment 1
Obstructive sleep apnea (OSA) is a worldwide increasing syndrome. Some risk factors determining the onset and progression of OSA, mainly those linked with body fat accumulation, also increase the cardiovascular risk. In particular, increased neck circumference and elevated Body Mass Index (BMI) are well‐known risk factors for cardiometabolic diseases, and are also considered traditional risk factors for OSA. These anatomical conditions are linked with recurrent obstruction of the pharyngeal airway during sleep, with resultant hypoxia and sleep fragmentation, leading to a persistent long‐term risk for several cardio‐pulmonary diseases, in subjects affected by OSA. 2 Chronic upper airways inflammation constantly promotes a systemic endothelial dysfunction 3 , 4 that worsens the cardio‐pulmonary activity, thus, affecting the left and the right heart performance, 5 increases risk of cardiovascular events, over time. 6
Obesity plays a well‐established pivotal role in the evolution of the cardiovascular risk in patients with OSA. 7 Indeed, in obese individuals, the eccentric ventricular hypertrophy and the diastolic heart failure are responsible for developing cardio‐myopathy, a well‐recognized complication in severe obese patients. 8 The augmented left ventricular filling pressure, transmitted into the pulmonary venous system, improves pulmonary venous pressures leading to increased pulmonary vascular resistance. 9 Obesity is also linked with elevated serum hs‐CRP levels in patients with sleep‐disordered breathing, 10 further contributing to chronic systemic inflammation.
Thus, the complex interplays among OSA, insulin resistance, hypercholesterolemia, obesity, chronic systemic inflammation and elevated left ventricular filling is responsible for endothelial dysfunction, with Pulmonary Hypertension (PH) finally leading to increased cardiovascular risk. 11 , 12 , 13
However, besides the combined role of these factors, it is still under debate if the severity of OSA per se can be considered an independent factor in modulating the extent of the cardiovascular risk in obese patients. 14 A recent observational study has indicated that hypoxemia and cardio‐metabolic disorders in OSA may potentiate their unfavorable effects on cardiovascular diseases (CVD). Sleep fragmentation may indirectly predispose patients with OSA to an increased risk of CVD and, thus, cardio‐metabolic disorders and OSA synergistically should influence cardio‐metabolic risk patterns. 15 Although the cited study underscores the pathophysiological interactions between OSA and the relative excess risks of cardiovascular diseases, elements able to identify OSA as an independent, additional risk factor for CVD, are still lacking.
In the present study, we depicted the cardiovascular risk in OSA patients enrolled at the Sleep Ambulatory, and studied for anthropometric features, cardiovascular risk, and Polysomnographic findings, in order to establish, as primary outcome, the role of OSA to provoke increased cardiovascular risk, and, as second outcome, to evaluate whether the BMI could interact with OSA for increasing the health risk.
2. SUBJECTS AND METHODS
2.1. Patients
OSA patients entering, during the year 2016, the Ambulatory of Sleep Disorders of the Institute of Respiratory Diseases of the University of Bari were retrospectively evaluated. In enrolled subjects, we recorded anthropometric features, smoking habits, Pulmonary Function Test, polysomnographic findings, and data from Arterial Blood Gas Analysis. Subjects who showed signs of COPD at spirometry test, or were affected by others co‐morbidities, such as bronchial asthma or nasal polyps, were excluded from the study.
Daytime sleepiness was measured by the Epworth Questionnaire, and the death risk linked with comorbidities was assessed by the Charlson Co‐morbidities Index (CCI). The 10‐year risk of heart disease or stroke was calculated using the ASCVD algorithm. 16
We measured body weight (kg), height (m), and calculated BMI, ie, Quetelet's index as kilograms divided by meter squared (kg/m2). BMI ranging from 18.5 to 24.9 kg/m2 defined normal weight subjects. A BMI ranging from 25.0 to 29.9 kg/m2 defined overweight subjects, while a BMI ≥30 kg/m2 defined patients with obesity.
2.2. Pulmonary function test
Pulmonary function tests were performed by spirometry (PK Morgan Ltd; Gillingham, UK). The equipment was calibrated daily using a 3‐l syringe, and the analysis was performed in accordance to the guidelines of the American Thoracic Society. 17 The best of three reproducible values was expressed as a percentage of the predicted normal value.
2.3. Sleep study
In the sleep laboratory of the Institutes of Respiratory Diseases of the University of Bari, all patients were evaluated for sleep disordered breathing during one night. They were monitored continuously for about 8 hour using a portable cardiorespiratory monitoring (POLYMESAM®, MAP, Martinsried, Germany). The POLYMESAM® (PM) device consists of a recorder, to which multiple sensors are linked for the detection of the following signals: oxy‐hemoglobin saturation (by a finger sensor), heart rate (derived from three electrocardiogram (ECG) electrodes placed on the chest), snoring sound (by a microphone placed on the thyroid cartilage), body posture oro‐nasal airflow (by a threefold thermocouple sensor for both nostrils and mouth), and thoracic and abdominal movements (by stretch belts). Apnea was identified if the airflow was absent or nearly absent for at least 10 seconds. Hypopnea was considered when there was a decrease of airflow for at least 10 seconds in respirations, a 30‐percent reduction in ventilation, and a decrease in oxygen saturation.
2.4. Statistics
Data are presented as mean ± standard error (SEM) or as percentages. One‐way analysis of variance (ANOVA) assessed inter‐group differences. Differences between two groups were tested by Student's t test for unpaired data. The Chi‐square test was used to compare proportions. The Pearson correlation coefficient was used for correlations. Results were considered significant at the 5% critical level (P < .05). Statistical analyses were performed with NCSS10 Statistical Software (NCSS, LLC. Kaysville, Utah, USA).
2.5. Study approval
Since the study was retrospective, the patients did not sign any informed consent, while the Local Ethic Committee approved the study number 1089, July the31st 2009, with note protocol no. 24961/DS 03/26/2009, and approved the study number protocol no. 44696/DS 06‐03‐2009 no. 527/DG/2004 modified by the protocol no. 828/DG/2007.
3. RESULTS
A total of 185 OSA subjects (19 normal weight, 57 overweight, 109, obese) entered the study. Anthropometric data and sleep study from normal weight, overweight, and obese subjects are depicted in Table 1.
TABLE 1.
Anthropometric data, smoking habit, sleep Questionnaire, Charlson co‐morbidities Index score, from normal weight, overweight, and obese subjects, entering the study
| Normal weight | Overweight | Obese | P | |
|---|---|---|---|---|
| n. | 19 | 57 | 109 | |
| Men | 12 | 44 | 81 | |
| Age (y) | 54.2 ± 2.3 | 58.8 ± 1.4 | 61 ± 1.0* | .02 |
| BMI (kg/m2) | 23.1 ± 1.0 | 27.6 ± 0.6* | 35.9 ± 0.4* , ° | .0000 |
| Smokers (%) | 15.8% | 15.8% | 74.3% | <.05 |
| Systemic Hypertension (%) | 31.6% | 35% | 59.6% | <.05 |
| Type 2 Diabetes (%) | 5.3% | 3.5% | 27.5% | <.05 |
| Total cholesterol (mg/dL) | 200.6 ± 3.0 | 202.4 ± 1.7 | 218.1 ± 1.2* , ° | .00001 |
| HDL cholesterol (mg/dL) | 61.3 ± 1.8 | 60.0 ± 1.0 | 53.9 ± 0.8* , ° | .0001 |
| Apnea Hypopnea Index (AHI) | 34.8 ± 3.1 | 35.5 ± 1.8 | 43.3 ± 1.3* , ° | .0006 |
| Cardiovascular risk (%) | 10.2 ± 3.1 | 13.7 ± 1.8 | 22.3 ± 1.3* , ° | .00002 |
| Epworth score | 6.8 ± 1.1 | 6.4 ± 0.7 | 8.9 ± 0.5 ° | .004 |
| Charlson Co‐morbidities Index | 1.8 ± 0.4 | 1.8 ± 0.2 | 2.7 ± 0.1* , ° | .0002 |
P < 05 vs normal weight.
P < .05 vs overweight.
The prevalence of smokers was higher in the obese subset. Obese subjects also showed higher rates of systemic hypertension and type 2 diabetes, higher serum concentration of total cholesterol, and lower concentration of HDL cholesterol, as compared with lean and overweight subjects.
The cardiovascular risk and the Charlson Co‐morbidities Index were significantly higher in obese, than in normal weight and overweight subjects. A similar trend was also evident for the Epworth score.
The Apnea Hypopnea Index (AHI) was higher in obese than in lean and overweight subjects. Overall, a direct relationship was evident between AHI and BMI (r = 0.23, P = .001, Figure 1). The percentage cardiovascular risk was weakly related with BMI (r = 0.33; P < .001), but not with AHI.
FIGURE 1.
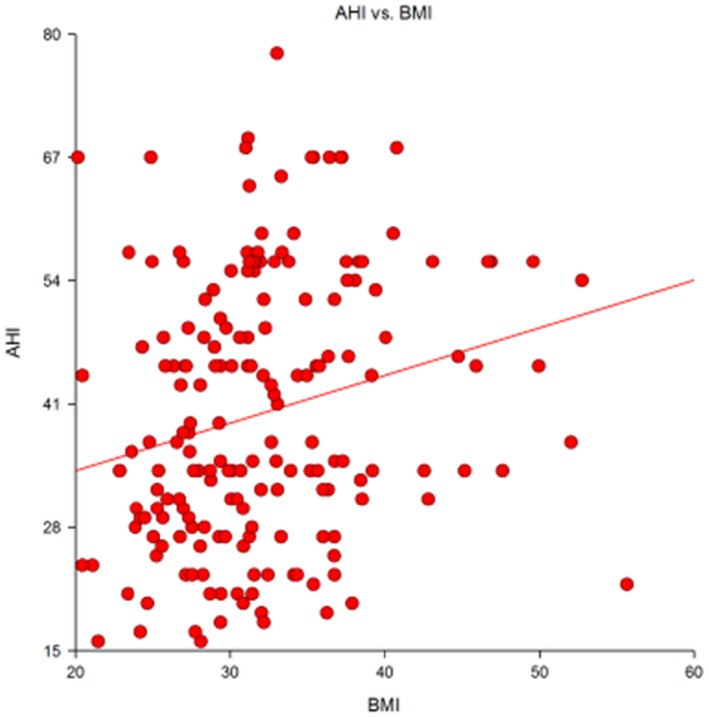
Direct correlation (r = 0.23, P = .001) between the Apnea Hypopnea Index (AHI) and the Body Mass Index (BMI) in a group of 185 patients with OSA
When AHI values were stratified in tertiles, the percentage cardiovascular risk did not vary with increasing AHI values (P = NS, ANOVA) (Figure 2).
FIGURE 2.
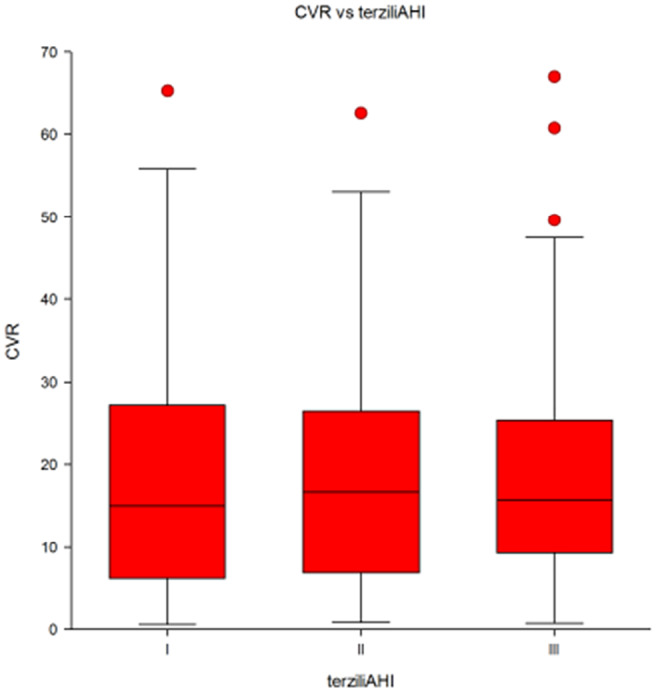
Average percentage cardiovascular risk (CVR) in a group of 185 patients with OSA selected according to tertiles of Apnea Hypopnea Index (AHI). I, 1st tertile; II, 2nd tertile; III, 3rd tertile of AHI
4. DISCUSSION
In this study, we found, in the whole OSA population, that the percentage of cardiovascular risk was related with BMI but not with AHI, indicating the lack of an independent role of the severity of OSA in modulating the cardiovascular risk at individual level.
In fact, as expected, AHI values were higher in obese than in lean and overweight subjects, and a direct relationship was evident between AHI and BMI. However, when AHI values were stratified in tertiles, the percentage cardiovascular risk did not vary with increasing AHI values.
Thus, the severity of OSA does not seem to increase, per se, the cardiovascular risk. Conversely, while the coexistence of a severe degree of OSA with a higher BMI might be likely responsible for the worsening of the prognosis.
OSA consists of repeated episodes of extrathoracic upper airway obstruction associated with intra‐arterial hypoxemia and hypercapnia. 18 As a chronic condition, OSA is also responsible for endothelial dysfunction, with early atherosclerosis plaque evidence and rapid increase of cardiovascular risk. 6 Even very recently, several studies have pointed out the role of the degree of OSA as factor likely responsible for increasing cardiovascular risk. 19 On the other hand, by the contrast, recent evidences have derived by the results of an observational 1 year study did not establish any correlation between severe obstructive sleep apnea syndrome and increased cardiovascular risk, as well as augmented cardiovascular co‐morbidities. 20 This debate has involved, over time, several investigators, who hypothesized both possible scenarios. Thus, it remains under discussion the primary role of OSA for developing increased cardiovascular risk in these subjects. 21
A validate assessment established that the metabolic syndrome commonly coexists with OSA, and obesity is perhaps the strongest predictor of OSA with 40‐60% of obese subjects suffering from sleep disorders breathing. 22 The burden of obesity is worrisome; the obesity prevalence is globally increasing, affecting 10% to 25% of the world population and is associated with a wide range of abnormalities, including elevated systemic as well as elevated pulmonary artery pressures, 23 both sustained by systemic inflammation. 7 , 10 Furthermore, obesity is characterized by increased production and secretion of a wide range of inflammatory molecules, including TNF‐alpha and interleukin‐6, which have both, local effects on adipose tissue physiology, and systemic effects on other organs, such as the cardiovascular system. 12 , 24 Thus, contributing to activate various inflammatory cells, such as lymphocytes and monocytes, leading to over‐expression of pro‐inflammatory mediators, that may establish irreversible endothelial dysfunction. The latter observation has been recently demonstrated in a rabbit model. 25
In two recent studies, 3 , 26 endothelial peripheral dysfunction in OSA patients has been significantly found in mild OSA and in minimally symptomatic OSA. The endothelial dysfunction decreased by 3 months of C‐PAP treatment. Indeed, in our study, we demonstrated that moderate or severe OSA patients show a progressive brachial endothelial dysfunction, established by flow‐mediated dilation (FMD) assay. The most evident finding was the significant reversibility of FMD in the group of patients treated with C‐PAP therapy, for at least 3 months. 3
In addition, OSA‐associated chronic intermittent hypoxia (CIH), in obesity, may complicate adipose tissue hypoxia with over‐production of adipose tissue inflammation, by increasing macrophage infiltration and activating redox‐sensitive transcription factors, as well as ER stress signaling proteins. 27 In a very recent study, the effect of OSA and C‐PAP on changes in adipose tissue inflammatory/hypoxia markers has been evaluated, which was assessed by testing mRNA levels of genes related to macrophage infiltration, hypoxia, and ER stress. 28 In this investigation, authors performed abdominal subcutaneous adipose tissue biopsies from OSA and non‐OSA obese (BMI > 35) individuals, at baseline and after 24 weeks (T1) of weight‐loss intervention plus continuous positive airway pressure (C‐PAP), or weight‐loss intervention alone, respectively. In obese individuals with OSA, the reduction of CIH, by a correct C‐PAP therapy, associated with a weight‐loss intervention strategy, decreased mRNA expression of markers related to tissue hypoxia, reduced plasma levels of some pro‐inflammatory cytokines and growth factors, ER stress, inflammation, and, finally, macrophage infiltration, 28
The endothelial, as well as, the adipose tissue dysfunction, with the consequent increased cardiovascular risk, are an early phenomenon in OSA patients, and, according to our results, show‐up more severe in subjects with a BMI higher than 30, while did not appear statistically significant as compared with the degree of OSA. Thus, the current study demonstrates that newly diagnosed subjects with OSA are already at risk for cardiovascular events, and patients with higher levels of BMI, as well as with higher levels of AHI, experience also the presence of more elevated number of co‐morbidities, as indicated by the results of Charlson Co‐morbidities Index (Table 1). In order to avoid this condition, OSA should be diagnosed as soon as possible in patients with elevated BMI, who likely improve their endothelial dysfunction, and subsequently their cardiovascular risk with C‐PAP therapy. This treatment has been shown to significantly decrease the cardiovascular risk and the development of several co‐morbidities, often responsible for fatal events. 3 , 25 , 26
This study has several limitations. First, the sample size is small to make a fully adequate comparison among subgroups. Although results from this study revealed the lack of a clear relationship between the severity of OSA and the extent of cardiovascular risk, further studies are needed to confirm our results, in particular in overweight and normal weight subjects. Second, the newly diagnosed population of OSA individuals was evaluated retrospectively, and it is unknown whether these subjects had a secondary benefit or not by using C‐PAP, in terms of cardiovascular risk, as compared with them who refused or were intolerant. Third, the rate of smokers was higher in obese patients, as compared with the other two subgroups of patients. This unbalance might play a significant role in increasing the cardiovascular risk. However, most of enrolled subjects were past‐smokers or very mild smokers who never acted as habitual smokers, because of their increasing overweight.
Finally, the major limitation of the study was the lack of data about the description of major lung co‐morbidities associated with OSA, such as COPD. However, results from the respiratory function tests demonstrated that all subsets of patients have a comparable, and not significant reduction of the respiratory indices.
In conclusion, although the coexistence of OSA and excessive fat accumulation cooperate in determining the global health risk in obese subjects, the extent of the cardiovascular risk, in these subjects, seems independent from the severity of OSA. Further studies are needed in order to better explore the role of common pathogenic pathways, as systemic inflammation, but also to possibly identity differently modulable primary and secondary prevention strategies to ameliorate the quality of life and the prognosis of these complex patients.
5. INFORMED CONSENT
Informed consent was obtained from all individuals participants included in the study.
CONFLICT OF INTEREST
The authors declare that they gave no conflict of interest.
AUTHORS’ CONTRIBUTION
Pierluigi Carratù conceived the study, made data interpretation, and wrote the manuscript. Agostino Di Ciaula edited the manuscript, made statistical analysis, and approved the final revision of the manuscript. Silvano Dragonieri designed the study, approved the final form of the manuscript. Teresa Ranieri collected data, approved the final form of the manuscript. Marco Matteo Ciccone participated to the study and approved the final revision of the manuscript. Piero Portincasa participated to the study design, edited the manuscript, approved the final form of the manuscript. Onofrio Resta participated to the study, approved the final revision of the manuscript.
ETHICAL APPROVAL
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee with the 1964 Helsinki declaration, and its later amendments or comparable ethical standards.
Carratù P, Di Ciaula A, Dragonieri S, et al. Relationships between Obstructive Sleep Apnea Syndrome and cardiovascular risk in a naïve population of southern Italy. Int J Clin Pract. 2021;75:e14952. doi: 10.1111/ijcp.14952
Funding information
Open Access Funding provided by Universita degli Studi di Bari Aldo Moro within the CRUI‐CARE Agreement.
WOA Institution: Universita degli Studi di Bari Aldo Moro
Blended DEAL: CARE
Pierluigi Carratù and Agostino Di Ciaula share equal contribution.
REFERENCES
- 1. European guidelines on CVD prevention in clinical practice 2016. Eur J Prev Cardiol. 2016;23(11):NP1‐NP96. [DOI] [PubMed] [Google Scholar]
- 2. Rana D, Torrilus C, Ahmad W, Okam NA, Fatima T, Jahan N. Obstructive sleep apnea and cardiovascular morbidities: a review article. Cureus. 2020;12(9):e10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kohler M, Craig S, Pepperell JC, et al. CPAP improves endothelial function in patients with minimally symptomatic OSA: results from a subset study of the MOSAIC trial. Chest. 2013;144(3):896‐902. [DOI] [PubMed] [Google Scholar]
- 4. Carratù P, Carratù L, Resta O. What is more affected in patients with obstructive sleep apnea: the right or the left heart? Pol Arch Med Wewn. 2016;126(4):217‐218. [DOI] [PubMed] [Google Scholar]
- 5. Archontogeorgis K, Voulgaris A, Nena E, et al. Cardiovascular risk assessment in a cohort of newly diagnosed patients with obstructive sleep apnea syndrome. Cardiol Res Practice. 2018;2018:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carneiro G, Zanella MT. Obesity metabolic and hormonal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events. Metabolism. 2018;pii:S0026–0495(18)30074‐X. [DOI] [PubMed] [Google Scholar]
- 7. Carratù P, Ventura VA, Maniscalco M, et al. Echocardiographic findings and plasma endothelin‐1 levels in obese patients with and without obstructive sleep apnea. Sleep Breath. 2016;20(2):613‐619. [DOI] [PubMed] [Google Scholar]
- 8. Wong CY, O’Moore‐Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110(19):3081‐3087. [DOI] [PubMed] [Google Scholar]
- 9. Segers VF, Brutsaert DL, De Keulenaer GW. Pulmonary hypertension and right heart failure in heart failure with preserved left ventricular ejection fraction: pathophysiology and natural history. Curr Opin Cardiol. 2012;27(3):273‐280. [DOI] [PubMed] [Google Scholar]
- 10. Sharma SK, Mishra HK, Sharma H, et al. Obesity, and not obstructive sleep apnea, is responsible for increased serum hs‐CRP levels in patients with sleep‐disordered breathing in Delhi. Sleep Med. 2008;9(2):149‐156. [DOI] [PubMed] [Google Scholar]
- 11. Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33(2):318‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reardon CA, Lingaraju A, Schoenfelt KQ, et al. Obesity and insulin resistance promote atherosclerosis through an IFNγ‐regulated macrophage protein network. Cell Rep. 2018;23(10):3021‐3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol. 2010;106(2):284‐286. [DOI] [PubMed] [Google Scholar]
- 14. Xie J‐Y, Liu W‐X, Ji L, et al. Relationship between inflammatory factors and arrhythmia and heart rate variability in OSAS patients. Eur Rev Med Pharmacol Sci. 2020;24(4):2037‐2053. [DOI] [PubMed] [Google Scholar]
- 15. Zhao X, Li X, Xu H, et al. Shan Kai Yin (2019) Relationships between cardiometabolic disorders and obstructive sleep apnea: Implications for cardiovascular disease risk. J Clin Hypertens (Greenwich). 2019;21(2):280‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goff DC, Lloyd‐Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49‐73. [DOI] [PubMed] [Google Scholar]
- 17. American Thoracic Society . Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107‐1136. [DOI] [PubMed] [Google Scholar]
- 18. Patil SP, Schneider H, Schwartz AR, et al. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kendzerska T, Leung RS, Atzema CL, et al. Cardiovascular consequences of obstructive sleep apnea in women: a historical cohort study. Sleep Med. 2020;68:71‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papanikolaou J, Ntalapascha M, Makris D, et al. Diastolic dysfunction in men with severe obstructive sleep apnea syndrome but without cardiovascular or oxidative stress‐related comorbidities. Ther Adv Respir Dis. 2019;13:175346661988007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berger G, Berger R, Oksenberg A. Progression of snoring and obstructive sleep apnoea: the role of increasing weight and time. Eur Respir J. 2009;33(2):338‐345. [DOI] [PubMed] [Google Scholar]
- 22. Punjabi N, Sorkin J, Katzel L, et al. Sleep‐disordered breathing and insulin resistance in middle‐aged and overweight men. Am J Respir Crit Care Med. 2002;165:677‐682. [DOI] [PubMed] [Google Scholar]
- 23. Friedman SE, Andrus BW. Obesity and pulmonary hypertension: a review of pathophysiologic mechanisms. J Obes. 2012;50(5):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4‐12. [PubMed] [Google Scholar]
- 25. Alarcon G, Roco J, Medina M, Medina A, Peral M, Jerez S. High fat diet‐induced metabolically obese and normal weight rabbit model shows early vascular dysfunction: mechanisms involved. Int J Obes (Lond). 2018;42(9):1535‐1543. [DOI] [PubMed] [Google Scholar]
- 26. Ciccone MM, Favale S, Scicchitano P, et al. Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients. Int J Cardiol. 2012;158(3):383‐386. [DOI] [PubMed] [Google Scholar]
- 27. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perrini S, Cignarelli A, Quaranta VN, et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. Insight. 2017;2(17):e94379. [DOI] [PMC free article] [PubMed] [Google Scholar]


