Abstract
Background
Oesophagogastric anastomosis is mainly complicated by its tediousness. We hope to modify an oesophagogastric anastomotic technique that simplifies anastomosis.
Methods
We conducted a retrospective analysis of 57 cases executed using reverse‐puncture anastomotic (RPA) technique and 64 cases of manual purse anastomosis (MPA) technique for robot‐assisted minimally invasive oesophagectomy (RAMIE). Baseline characteristics and perioperative outcomes were analysed.
Results
There were no significant differences between the 2 groups with regards to demographic data and clinical features. All patients had R0 resection. Relative to MPA, RPA group experienced significantly shorter operation times (232.5 ± 33.84 min vs. 262.3 ± 83.94 min, p = 0.038).RPA group patients had shorter anastomotic times relative to MPA group patients (10.5 ± 3.4 min vs. 18.3 ± 4.1 min, p = 0.014). No adverse events were observed.
Conclusions
Reverse‐puncture anastomosis is safe, feasible in RAMIE. This approach has the potential to efficiently shorten the anastomotic time and ensure safe operation.
Keywords: 3D, oesophagectomy, minimal invasive surgery, oesophagus
1. INTRODUCTION
Oesophageal cancer (EC) is the sixth most common cause of cancer‐associated deaths worldwide. And its global incidence has increased significantly in the past 4 decades. 1 , 2 In China, EC is a burden and a strain on the health care system. 3 Radical oesophagectomy is the cornerstone of multimodal treatment aimed at curing EC. 4 Ivor Lewis 5 and McKeown 6 oesophagectomy are the main minimally invasive oesophagectomy (MIE) methods. 7
Transthoracic oesophagectomy is associated with a considerable degree of morbidity 8 and oesophagogastric anastomosis leakage is a significant source of perioperative morbidity and occasional oesophagectomy mortality. 9 Various techniques are currently used for oesophagogastric reconstruction but their effects on postoperative anastomotic leakage and morbidity have not been examined. 10 Cervical anastomosis is easier to execute, and convenient for treating anastomotic leakage in case of postoperative complication. Intrathoracic anastomosis after MIE is associated with a lower rate of anastomotic leakage and better functional outcomes. 11
Multiple factors may cause anastomotic complications, including surgical technique. The most commonly used anastomotic methods following oesophagectomy are hand‐sewn (HS), circular‐stapled and semi‐mechanical linear‐stapled (LS) 12 techniques. Here, we find that no anastomotic technique is superior in terms of limiting post‐operative complications or shortening anastomotic time. 13 However, with advancements in surgical instrumentation, robot‐assisted minimally invasive oesophagectomy (RAMIE) has become increasingly popular. RAMIE has the potential to avoid some of the pitfalls of conventional TAMIE, especially lymph node dissection of recurrent laryngeal nerve. Robotic surgery offers better dexterity and lower surgeon fatigue. This approach aims to reduce surgical trauma and postoperative complications, thereby improving postoperative outcomes. 14 Thus, we hope to develop an ideal anastomotic technique that saves time, whilst ensuring operation safety.
Here, we describe reverse‐puncture anastomotic (RPA), an improved RAMIE‐based gastroesophageal anastomotic technique that aims to simplify the complex steps of manual purse. We offer suggestions on treatment options for surgeons regarding oesophagogastric reconstruction approaches using a gastric tube during oesophagectomy.
2. MATERIALS AND METHODS
2.1. Patients
We retrospectively analysed clinical data on 121 patients treated in Affiliated Jinling Hospital, Medical School of Nanjing University between January 2017 and August 2019. Of these, 57 underwent RPA, whilst 64 underwent Manual‐Puncture Anastomotic (MPA) technique for oesophagectomy. The diagnoses and clinical information were collected by consulting medical records of the patients. All those patients routinely underwent endoscopic ultrasound examination, chest computed tomography (CT).The demographic and clinical characteristics were composed of age, sex, body mass index (BMI) and basic illness (including hypertension, diabetes and chronic obstructive pulmonary disease [COPD]). Tumour associated data, in terms of tumour histology, size and location were also collected. The surgical related data included total‐operative time, anastomotic time, intraoperative bleeding, lymph‐nodes removed, postoperative hospital stay, intraoperative adverse events and complications. The decision of the site of the anastomosis depended on the tumour resection margin. Passage was re‐established by a gastric conduit. Anastomotic leakages were detected by endoscopy. Diagnostic criteria of anastomotic leakage accordingly to the ECCG. Ethical approval for the study was granted by our institutional review board and written informed consent was obtained from each participant.
2.2. Inclusion criteria
(1) Patients were preoperatively diagnosed with distal esophagus and oesophagogastric junction squamous cell carcinoma of esophagus. (2) No enlargement of cervical lymph nodes based on preoperative CT. (3) Head CT, bone scan and PET‐CT suggest that there is no distant metastasis of cancer, poor cardiac, lung function or severe arrhythmia.
2.3. Exclusion criteria
(1) Patients with severe comorbidities, such as impaired cardiac, kidney, liver and/or lung function. (2) Patients with severe chest adhesions, history of right chest trauma, surgery and tuberculosis. (3) The tumour involved a large proportion of the stomach and the patient required total or subtotal gastrectomy and oesophagojejunostomy. (4) Patients refused to undergo this modified surgical approach.
2.4. Surgical procedure
2.4.1. Preoperative preparation
Preoperative preparation was same as for conventional surgery. Intubation can be done via double‐lumen endotracheal intubation or single lumen with a bronchial occluder. After intubation, intravenous‐inhalation combined anaesthesia was given and an artificial pneumothorax applied on the lung, on the side of the operation to ensure its complete collapse.
2.5. Abdominal operation
The patient was in a supine position. About 12 mm trocar under the umbilicus was placed into an robotic observation hole. An incision with a length of about 1 cm was taken from the left upper part of the umbilicus, the right upper part of the umbilicus and 2 cm below the bilateral ribs. Artificial pneumoperitoneum was performed, and the lens was placed under the umbilicus for exploration. The exploration results are as shown above. Lift the colon, cut the gastrocolic ligament, free the great curvature of the stomach to the secluded entrance and keep the right blood vessel of the gastric omentum. Free the left side of the greater curvature of stomach to below the gastric ligament. Cut off the short gastric artery, free the bottom of the stomach to the cardia, turn up the stomach, expose the left gastric artery, separate the left gastric artery, clean the surrounding lymph nodes by skeletal ligation and ligate the left gastric artery at the distal end and proximal end, respectively. Cut off the left artery from the middle with ultrasonic knife, and cut off the hepatogastric ligament and its lymph nodes from the right side of the cardia down to the toughness of the hepatoduodenum, enlarge the muscle hole, remove the device, and take a 5‐cm incision along the midline under the xiphoid process.
2.6. Thoracic operation
With help of the robot, we tried to free the thoracic oesophagus and execute lymph node dissections. For this, the patient is usually in the left lateral position, slightly leaning forward if necessary to expose the oesophagus (Figure 1). Four ports were then positioned as shown in Figure 1. A 12‐mm camera port was then inserted in the sixth intercostal space (ICS) at the anterior axillary line. Two 8‐mm ports were inserted in the ninth ICS at the posterior axillary line and in the fourth ICS at the anterior axillary line, respectively, for the first and second working ports. Finally, a 12‐mm assistant port was created in the seventh ICS of anterior axillary line. This port allows the assistant to pull the lung and expose the oesophagus better. Thus, the oesophagus can be successfully separated using the two robotic arms. The oesophagus was mobilised en bloc from the thoracic inlet to the gastroesophageal junction. Next, the corresponding lymph node parts were dissected and the conduit pulled up through the hiatus. MPA and RPA were used for RAMIE.
FIGURE 1.
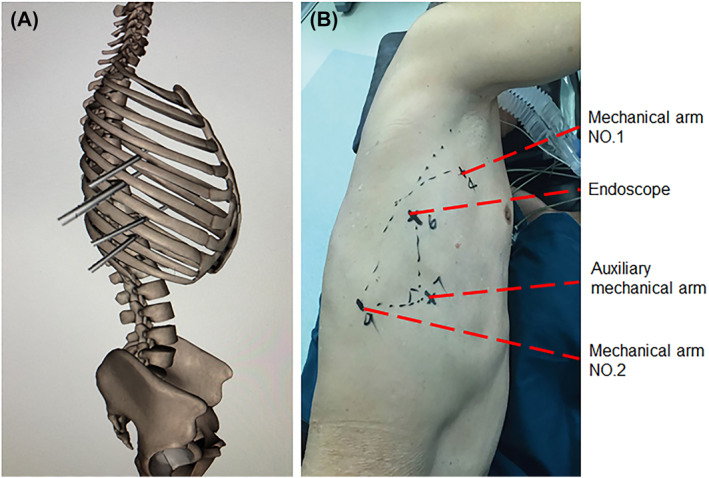
Port positions for minimally invasive oesophagectomy
2.7. RPA technique
Firstly, a knot was made through the small hole at the tip of the puncture head. This knot should not be too tight, as this would make it difficult to pull out the circular stapler. The length of the oesophageal hemi‐transection should be kept at between 2 and 3 cm. Next, we inserted a 60 mm × 38 mm circular stapler into the oesophagus (Figure 2A). We then used an endoscopic stapler to close the oesophageal stump (Figure 2B). Next, we slowly, vertically pulled out the circular stapler which was tied with a knot (Figure 2C) with the tubular stomach pulled into the chest through the enlarged hiatus. After that, the circular staple was used to pierce the centre rod of the completed tubular stomach and docked with an anvil (Figure 2D). An endoscopic stapler was then used to close the incision on the tubular‐stomach and purse‐string suture used to reinforce the anastomotic stoma. Finally, integrity of the anastomosis was examined (Figure 3).
FIGURE 2.
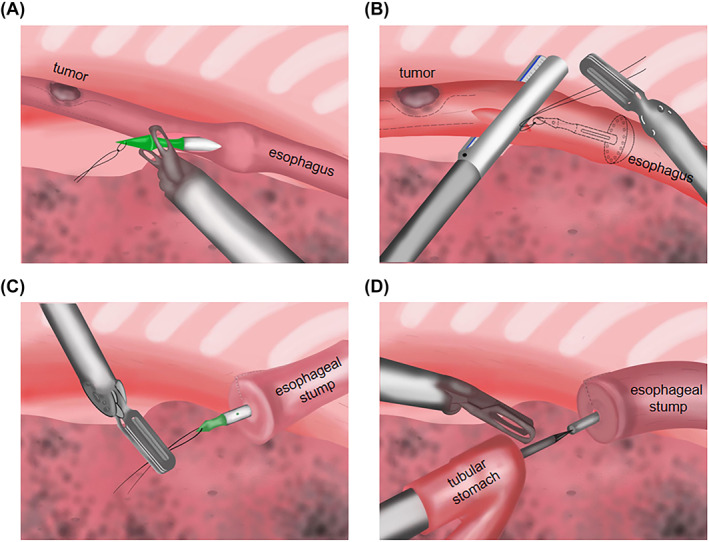
A) Circular stapler inserted into the oesophagus. (B) Use an endoscopic stapler to close an oesophageal stump. (C) Slowly, vertically pull out the circular stapler that was tied with a knot. (D) Let the circular staple pierce the centre rod of the completed tubular stomach and docked with an anvil
FIGURE 3.
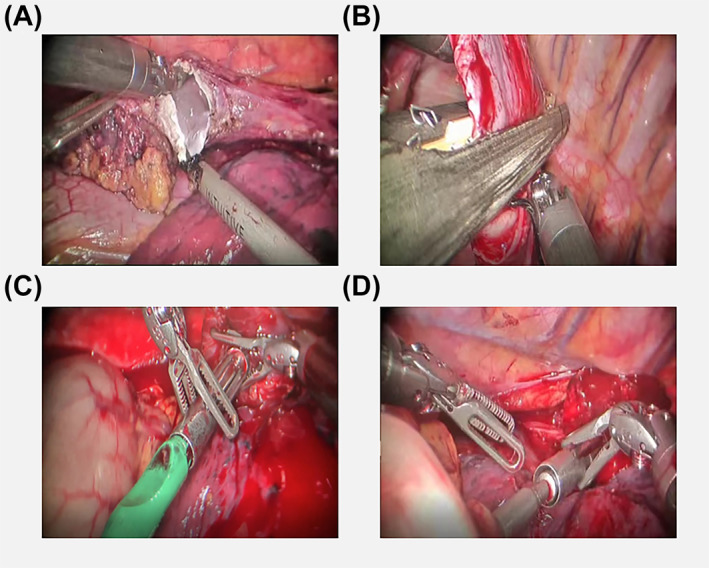
Pictures of the operation
2.8. MPA technique
The first three stitches of purse string in turn were at 3, 12 and 9 O’clock positions of the oesophageal wall. Especially the final stitch was used to assist the oesophagus to rotate 90° clockwise, which facilitate placement. Then we rotated the oesophagus 90° counterclockwise to facilitate placement of the fourth stitch at the 3 o’clock position. After that, a transverse incision was made in the anterior oesophageal wall, 2–3 cm distal to the purse‐string suture. The incision was held open at the 3 and 9 o’clock positions, straighten the oesophagus inferiorly and posteriorly and the anvil of a 25‐mm circular stapler was inserted. The purse string is tied tight and knotted with a knot pusher. After the first knot was finished, one end of the string was re‐circled through back wall of oesophagus and tied to complete the second knot. The distal oesophagus was transected 0.5 cm from the purse string. The gastric conduit was pulled into the thoracic cavity, and the circular stapler was inserted through the main port to construct an oesophagogastric end‐to‐side anastomosis. At last, the gastric conduit was stapled with a linear stapler.
2.9. Statistical analysis
This is a safety and feasibility study of a modified surgical technique. Categorical data are expressed as values and percentage. Continuous data are reported as mean ± SD. Data were prospectively recorded on Microsoft Office Excel 2003. Data were analysed using SPSS (IBM). Student’s t‐test or Wilcoxon rank‐sum test was used to compare continuous variables between groups. Chi‐squared test or Fisher’s exact test was used to compare categorical data. A 2‐sided p < 0.05 indicates statistical significance.
3. RESULTS
3.1. Patient characteristics and anastomotic techniques
We retrospectively analysed clinical data on 121 patients with a mean age of 65 years (range 45–77 years) treated at our hospital between January 2017 and August 2019. A total of 57 patients underwent RPA and 64 with traditional technique for RAMIE. Demographic and clinical features are shown in Table 1. No significant differences were observed between the RPA and MPA‐treated patients with regards to age, sex, body mass index, basic illness and neoadjuvant therapy.
TABLE 1.
Demographic and clinical characteristics
| RPA n (%) (N = 57) | MPA n (%) (N = 64) | p‐value | |
|---|---|---|---|
| Age, years | |||
| Median (range) | 64.7 (45–79) | 65.6 (50–77) | 0.737 |
| ≥60 | 41 (71.9) | 47 (73.4) | 0.933 |
| <60 | 16 (28.1) | 17 (26.6) | |
| Gender | |||
| Male | 47 (82.5) | 52 (81.3) | 0.678 |
| Female | 10 (17.5) | 12 (18.7) | |
| BMI, kg/m2 | |||
| ≤25 | 21 (36.8) | 25 (39.1) | 0.944 |
| >25 | 36 (63.2) | 39 (60.9) | |
| Basic illness | |||
| Hypertension | 15 (26.3) | 21 (32.8) | 0.350 |
| Diabetes | 4(7.0) | 5(7.8) | |
| COPD | 3(5.3) | 5(7.8) | |
| Neoadjuvant therapy | |||
| Radio‐therapy only | 2(3.5) | 3(4.7) | 0.287 |
| Chemo‐therapy only | 0(0.0) | 1(1.6) | |
| Chemo‐radio therapy | 1(1.8) | 1(1 .6) |
Abbreviations: BMI, Body mass index; COPD, chronic obstructive pulmonary disease; MPA, manual purse anastomosis; RPA, reverse‐puncture anastomosis.
3.2. Cancer outcome
Detailed results on tumour associated data are listed in Table 2. All patients had R0 resection, including the distal oesophagus and cardia, Most of the cardia were adenocarcinomas. Thus, there were no statistically significant differences between the groups with regards to tumour histology (p = 0.837), size (p = 0.313), location (p = 0.681) and TNM classification (p = 0.749)
TABLE 2.
Tumour associated data
| RPA n (%) (N = 57) | MPA n (%) (N = 64) | p‐value | ||
|---|---|---|---|---|
| Tumour histology | ||||
| Squamous‐cell carcinoma | 30 (52.6) | 37 (57.8) | 0.837 | |
| Adenocarcinoma | 26 (45.6) | 26 (40.6) | ||
| Small‐cell carcinoma | 1 (1.8) | 1 (1.6) | ||
| Tumour size | ||||
| T1 | 18 (31.6) | 19 (29.7) | 0.313 | |
| T2 | 25 (43.9) | 12 (18.7) | ||
| T3 | 13 (22.8) | 29 (45.3) | ||
| T4 | 1 (1.7) | 4 (6.3) | ||
| Tumour location | ||||
| Distal oesophagus | 35 (61.4) | 33 (51.6) | 0.681 | |
| Cardia | 22 (38.6) | 31 (48.4) | ||
| TNM classification | ||||
| I | 16 (28.1) | 26 (40.6) | 0.749 | |
| II | 15 (26.3) | 13 (20.3) | ||
| III | 25 (43.9) | 24 (37.5) | ||
| IV | 1 (1.7) | 1 (1.6) | ||
Abbreviations: MPA, manual purse anastomosis; RPA, reverse‐puncture anastomosis.
3.3. Surgery‐related information
There were no intraoperative complications or conversions to open surgery during surgery. Perioperative period related information is shown in Table 3. Median operation time was 252 min (range 144–378 min). Relative to the MPA group, the RPA group experienced a significantly shorter operative time (232.5 ± 33.84 min vs. 262.3 ± 83.94 min, p = 0.038). RPA group had shorter anastomotic time relative to MPA group (10.5 ± 3.4 min vs. 18.3 ± 4.1 min p = 0.014). Intraoperative blood in the RPA group was lower than in the MPA group (297.60 ± 89.748 min vs. 204.729 ± 52.220 min, p = 0.029). The removed lymph nodes were similar between the 2 groups (25.252 ± 8.381 min vs. 22.351 ± 7.609 min, p = 0.805). Anastomotic leakage were similar between the 2 groups (3.1% vs. 5.3%, p = 0.294). Detailed complications, including pulmonary embolism, acute respiratory distress syndrome, pneumonia, wound infection, pneumothorax, postoperative urinary retention chylothorax and vocal cord paralysis, atrial fibrillation, were similar between the 2 groups. Postoperative hospital stay was shorter in the RPA group relative to the MPA group (9.486 ± 1.968 min vs. 17.76 ± 2.297 min, p = 0.031).
TABLE 3.
Perioperative period related information
| Characteristics a | RPA n (%) (N = 57) | MPA n (%) (N = 64) | p‐value |
|---|---|---|---|
| Total operative time, min | 232.5 ± 33.84 | 262.3 ± 83.94 | 0.038 |
| Anastomotic time, min | 10.5 ± 3.4 | 18.3 ± 4.1 | 0.014 |
| Intraoperative bleeding, ml | 204.729 ± 52.220 | 297.60 ± 89.748 | 0.029 |
| Lymph‐nodes removed, pcs | 22.351 ± 7.609 | 25.252 ± 8.381 | 0.805 |
| Postoperative hospital – stay, day | 9.486 ± 1.968 | 17.76 ± 5.297 | 0.031 |
| Major complications | |||
| Anastomotic leakage | 2 (3.1) | 3 (5.3) | 0.294 |
| Pulmonary embolism | 0 (0.0) | 1 (1.8) | |
| ARDS | 1 (1.6) | 2 (3.5) | |
| Minor complications pneumonia | |||
| Pneumonia | 1 (1.6) | 2 (3.5) | 0.261 |
| Wound infection | 0 (0.0) | 1 (1.8) | |
| Pneumothorax | 1 (1.6) | 0 (0.0) | |
| Postoperative urinary retention | 1 (1.6) | 0 (0.0) | |
| Chylothorax | 0 (0.0) | 0 (0.0) | |
| Vocal cord paralysis | 0 (0.0) | 0 (0.0) | |
| Atrial fibrillation | 1 (1.6) | 2 (3.5) |
Abbreviations: ARDS, acute respiratory distress syndrome; MPA, manual purse anastomosis; RPA, reverse‐puncture anastomosis.
Categoric data are expressed as number (%) and continuous data as mean ± SD or median (interquartile range).
4. DISCUSSION
RAMIE has the potential to avoid some of the pitfalls of conventional thoracoscopic‐assisted minimally invasive oesophagectomy (TAMIE), especially lymph node dissection. Robotic surgery offers better dexterity and lower surgeon fatigue. With a surgeon‐controlled stable 3D camera, an ergonomic operating position, and EndoWristed instruments with 7 degrees of freedom, this system is better suited to train beginners. 15 , 16 We find that with regards to cancer outcomes, there were no significant differences in terms of clear resection margins, tumour histology, size and location, when using robotic versus thoracoscopic procedures. Stapled intrathoracic anastomosis is mainly used when the tumour is located at the distal oesophagus and oesophagogastric junction and includes transthoracic or transoral placement of the anvil and introduction of the circular stapler through a small thoracotomy. 17 The OrVil technique is too expensive to be popular, whilst transoral insertion of the anvil raises the chance of thoracic infection and throat injury. 18 In our group, we usually use MPA for oesophagogastric anastomosis during intrathoracic anastomosis because it does not require specialised devices. However, the technique is tedious and difficult to master. RPA omits the manual purse anastomosis (MPA) step. Anastomotic time (from oesophageal wall incision to oesophageal transection) was 10.5 ± 3.4 min, which compares well with the 10–18 min reported before, 19 indicating that RPT saves time whilst ensuring operation safety. Here, we find the total operative time in RPA and MPA was 232.5 ± 33.8 and 262.3 ± 83.94 min, respectively, which compares well with the 5.03 ± 1.04 h reported before. 14 We shortened the total operative time through increased experience and standardisation at each step. The number of lymph node dissection in RPA and MPA group was 25.252 ± 8.381 and 22.351 ± 7.609 pcs, respectively. Lymph node dissection is critical for patient survival after a radical oesophagectomy, 20 which is prone to a high metastatic rate. However, lymph node dissection is technically challenging due to a narrow operative space. 21 The feasibility and safety of the robot‐assisted lymphadenectomy were demonstrated in a previous study. 22 One of main postoperative complications of EC resection is anastomotic leakage, whose incidence using various anastomosis approaches in the minimally invasive Ivor‐Lewis oesophagectomy ranges from 0% to 10%, without statistically significant differences 17 , 18 , 23 Here, the anastomotic leakage rate was 3.1% in the RPA group, which is consistent with past studies. According to the doctor’s personal experience and intraoperative situation. Relative to the RPA group, the MPA group will observe for a few more days in hospital. So, the postoperative hospital stay was shorter in the RPA group relative to the MPA group (p = 0.031), Additionally, a fixed configuration for a conventional circular stapler and puncture head can be used to lower hospital costs.
Our approach has the following characteristics: (1) Anvil placement is simple as the MPA step is omitted. (2) It can efficiently remove large tumours that cannot be pulled out and then anastomosed at the neck. (3) A knot is made through the small hole at the tip of the puncture head. The knot should be tied to the side to avoid forming an angle that would make it hard to pull out. (4) This method is modified circular‐stapled end‐to‐side (CEEA) anastomosis. Past studies show that CEEA effectively reduces leakage rate following esophagogastrostomy. 24
This study had some constraints. It was conducted in a nonrandomised, retrospective manner. Additionally, the technical procedure used depended on surgeon and patient preferences. The small sample size in our study could result in selection bias. This is still an early experience for RAILE.
In conclusion, our data show that this technique is feasible and reliable, and offer suggestions on treatment options for surgeons performing oesophagogastric reconstruction using a gastric tube during oesophagectomy.
CONFLICT OF INTEREST
The authors acknowledge that they have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Hao Peng makes substantial contributions to conception and design. Hao Peng, Yi Yang Liu, Rong Chun Wang and Hao Chen contribute to acquisition of data. Hao Peng, Yi Yang Liu, Maimaitijiang Aimudula and Xiaolong Liu contribute to analysis and interpretation of data. All authors participate in drafting the article or revising it critically for important intellectual content. All authors give final approval of the version to be published.
ACKNOWLEDGEMENT
None.
Peng H, Liu YY, Aimudula M, et al. A safe and effective anastomotic technique for robot‐assisted minimally invasive oesophagectomy: reverse‐puncture anastomosis. Int J Med Robot. 2022;18(1):e2336. 10.1002/rcs.2336
Hao Peng and Yi Yang Liu contributed equally to this work.
Contributor Information
Haizhu Song, Email: njyijun@163.com.
Jun Yi, Email: songhaizhu@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon a reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Thrumurthy SG, Chaudry MA, Thrumurthy S, Mughal M. Oesophageal cancer: risks, prevention, and diagnosis. BMJ. 2019;366:l4373. [DOI] [PubMed] [Google Scholar]
- 3. Shen X, Chen T, Shi X, et al. Modified reverse‐puncture anastomotic technique vs. traditional technique for total minimally invasive Ivor‐Lewis esophagectomy. World J Surg Oncol. 2020;18(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383‐2396. [DOI] [PubMed] [Google Scholar]
- 5. Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18‐31. [DOI] [PubMed] [Google Scholar]
- 6. McKeown KC. Total three‐stage oesophagectomy for cancer of the oesophagus. Br J Surg. 1976;63(4):259‐262. [DOI] [PubMed] [Google Scholar]
- 7. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700‐b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gutschow CA, Staiger RD. Comment on: Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy.Ann Surg. 2019;270(6):e123‐e124. [DOI] [PubMed] [Google Scholar]
- 9. Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg. 2004;16(2):124‐132. [DOI] [PubMed] [Google Scholar]
- 10. Schroder W, Raptis DA, Schmidt HM, et al. Anastomotic techniques and associated morbidity in total minimally invasive transthoracic esophagectomy: results from the EsoBenchmark database. Ann Surg. 2019;270(5):820‐826. [DOI] [PubMed] [Google Scholar]
- 11. van Workum F, Berkelmans GH, Klarenbeek BR, Nieuwenhuijzen G, Luyer M, Rosman C. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta‐analysis. J Thorac Dis. 2017;9(Suppl 8):S826‐S833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kukar M, Ben‐David K, Peng JS, et al. Minimally invasive Ivor Lewis esophagectomy with linear stapled anastomosis associated with low leak and stricture rates. J Gastrointest Surg. 2020;24(8):1729‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rostas JW, Graffree BD, Scoggins CR, McMasters KM, Martin R. Long‐term outcomes after hand‐sewn versus circular‐stapled (25 and 29 mm) anastomotic technique after esophagogastrectomy for esophageal cancer. J Surg Oncol. 2018;117(3):469‐472. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Han Y, Gan Q, et al. Early outcomes of robot‐assisted versus thoracoscopic‐assisted Ivor Lewis esophagectomy for esophageal cancer: a propensity score‐matched study. Ann Surg Oncol. 2019;26(5):1284‐1291. [DOI] [PubMed] [Google Scholar]
- 15. Spinoglio G, Summa M, Priora F, Quarati R, Testa S. Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum. 2008;51(11):1627‐1632. [DOI] [PubMed] [Google Scholar]
- 16. Baik SH, Lee WJ, Rha KH, et al. Robotic total mesorectal excision for rectal cancer using four robotic arms. Surg Endosc. 2008;22(3):792‐797. [DOI] [PubMed] [Google Scholar]
- 17. Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc. 2012;26(7):1795‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhan B, Chen J, Du S, Xiong Y, Liu J. Using the hand‐sewn purse‐string stapled anastomotic technique for minimally invasive Ivor Lewis esophagectomy. Thorac Cardiovasc Surg. 2019;67(7):578‐584. [DOI] [PubMed] [Google Scholar]
- 19. Zhang RQ, Xia WL, Kang NN, Ge W, Chen AG, Zhu KC. Purse‐string stapled anastomotic technique for minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2012;94(6):2133‐2135. [DOI] [PubMed] [Google Scholar]
- 20. Tsurumaru M, Kajiyama Y, Udagawa H, Akiyama H. Outcomes of extended lymph node dissection for squamous cell carcinoma of the thoracic esophagus. Ann Thorac Cardiovasc Surg. 2001;7(6):325‐329. [PubMed] [Google Scholar]
- 21. Yang Y, Zhang X, Li B, et al. Robot‐assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma: protocol for a multicenter prospective randomized controlled trial (RAMIE trial, robot‐assisted minimally invasive esophagectomy). BMC Cancer. 2019;19(1):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim DJ, Park SY, Lee S, Kim HI, Hyung WJ. Feasibility of a robot‐assisted thoracoscopic lymphadenectomy along the recurrent laryngeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc. 2014;28(6):1866‐1873. [DOI] [PubMed] [Google Scholar]
- 23. Xiao P, Zhuang X, Shen Y, et al. Reverse‐puncture anastomotic technique for minimally invasive Ivor‐Lewis esophagectomy. Ann Thorac Surg. 2015;100(6):2372‐2375. [DOI] [PubMed] [Google Scholar]
- 24. Zhang H, Wang Z, Zheng Y, et al. Robotic side‐to‐side and end‐to‐side stapled esophagogastric anastomosis of Ivor Lewis esophagectomy for cancer. World J Surg. 2019;43(12):3074‐3082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon a reasonable request.


