Abstract
People and wildlife are living in an increasingly urban world, replete with unprecedented human densities, sprawling built environments, and altered landscapes. Such anthropogenic pressures can affect multiple processes within an ecological community, from spatial patterns to interspecific interactions. We tested two competing hypotheses, human shields vs. human competitors, to characterize how humans affect the carnivore community using multispecies occupancy models. From 2017 to 2020, we conducted the first camera survey of city parks in Detroit, Michigan, and collected spatial occurrence data of the local native carnivore community. Our 12,106–trap night survey captured detection data for coyotes (Canis latrans), red foxes (Vulpes vulpes), raccoons (Procyon lotor), and striped skunks (Mephitis mephitis). Overall occupancy varied across species (Ψcoyote = 0.40, Ψraccoon = 0.54, Ψred fox = 0.19, Ψstriped skunk = 0.09). Contrary to expectations, humans did not significantly affect individual occupancy for these urban carnivores. However, co‐occurrence between coyote and skunk increased with human activity. The observed positive spatial association between an apex and subordinate pair supports the human shield hypothesis. Our findings demonstrate how urban carnivores can exploit spatial refugia and coexist with humans in the cityscape.
Keywords: camera survey, city, community structure, coyote, Detroit, distribution, human shield, occupancy, overlap
Introduction
Cities are highly heterogeneous landscapes of risk and reward, borne of unique interactions between anthropogenic and ecological processes (Alberti et al. 2003, Liu et al. 2007). As urbanization and land cover conversion rates continue to increase worldwide, cities have emerged as a new and unique habitat for wildlife. By 2050, over half of the global human population will live in a city while urban development is projected to grow by 120 million hectares globally by 2030 (Mcdonald et al. 2018, United Nations 2018). Cities can be a source or a sink for mammal species, a duality driven by both increases in availability of food sources and risks of mortality (Bateman and Fleming 2012, Lepczyk et al. 2017, Lamb et al. 2020). For example, cougars (Puma concolor) in an urban‐wildland system in Colorado successfully exploited anthropogenic food sources, yet faced a 6.5% increase in mortality risk in developed areas (Moss et al. 2016). Wildlife responses to the built environment are unsurprisingly driven by humans themselves and their induced modifications to landscapes through food provisioning, artificial habitat and light, and roads (Clucas and Marzluff 2011, Riley et al. 2014, Gaston et al. 2017).
Anthropogenic pressures affect wildlife communities from intraguild interactions down to behavioral shifts in individual species. Perturbations to higher trophic levels can have cascading impacts on ecosystem processes, which underscores the need to understand how carnivores respond to human activities (Terborgh and Estes 2010, Ripple et al. 2014). A study in the city of Chicago found that raccoons (Procyon lotor) comprised a larger relative proportion of the mesopredator community in urban compared to rural sites, irrespective of patch size (Prange and Gehrt 2004). Individual species’ responses to human activity are varied and depend on each species’ life history traits and behavioral tolerance of human encounters. Cottontail rabbits (Sylvilagus floridanus) in an urban area were more vigilant at sites where coyotes were absent, suggesting humans are a third “player” in a predator–prey–human system (Gallo et al. 2019). Evidently, urban systems produce a suite of complex and synergistic changes to ecological communities.
Despite evidence that human activity induces complex responses in urban wildlife, there is a dearth of studies that quantify these effects, particularly for terrestrial carnivores. A meta‐analysis of urban ecology studies found that only 10.2% of 244 studies quantified large mammal responses to urbanization and only 6% of all urbanization metrics employed in these studies explicitly considered humans (Moll et al. 2019). Worldwide population declines and range contraction in carnivores highlight the urgency to assess how spaces dominated by humans alter interactions within ecological communities (Ceballos and Ehrlich 2002, Ripple et al. 2014).
We leveraged a North American carnivore guild comprised of coyotes (Canis latrans), raccoons, red foxes (Vulpes vulpes), and striped skunks (Mephitis mephitis) to investigate how human activity influences spatial ecology within the community. We implemented the first camera survey of city parks in Detroit, Michigan from 2017 to 2020 to study the urban carnivores. By directly measuring human activity and not just proxies of human pressure such as housing density, we explicitly disentangled the effects of humans on wildlife from those related to the built environment.
In characterizing human effects, many relationships may occur in carnivore communities. We underscore two pervasive theoretical frameworks in the literature with distinct expectations for mammalian community response to fine‐scale human activity, but recognize these hypotheses are not mutually exclusive (Fig. 1). The human shield hypothesis (HSH) argues that humans differentially exert top‐down pressure on the apex (hereafter dominant) predator in the system, indirectly benefiting the subordinate competitors and facilitating greater spatial overlap between humans and subordinate species (Shannon et al. 2014, Moll et al. 2018). Anthropogenic pressure is known to mediate intraguild interactions (Berger 2007, Muhly et al. 2011, Gallo et al. 2019). For instance, red foxes have been shown to exploit highly developed core urban areas as spatial refugia to avoid their dominant coyote competitors (Moll et al. 2018). A contrasting approach frames humans as competitors (HCH), asserting that anthropogenic pressure affects multiple species irrespective of their trophic level or dominance hierarchy (Chapron and López‐Bao 2016, Farris et al. 2017). According to HCH, human presence is functionally similar to antagonism from another competitor in the guild, resulting in increased vigilance, competitive exclusion, and spatial avoidance across the entire community (Clinchy et al. 2016, Gallo et al. 2019, Suraci et al. 2019).
Fig. 1.
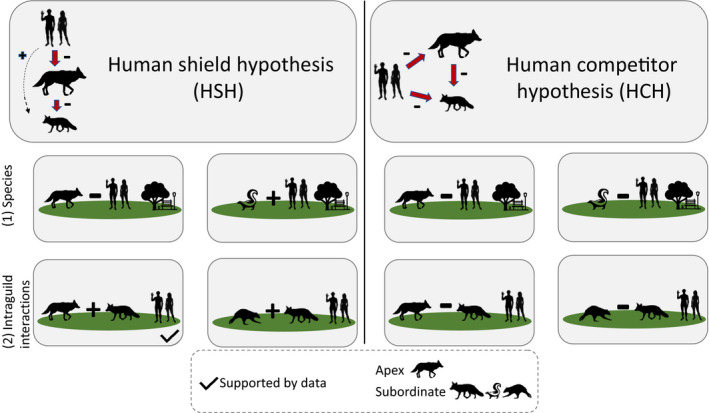
Conceptual framework for the effects of humans on (1) individual carnivore species and (2) pairwise intraguild interactions under two hypotheses: humans as shields (HSH) and humans as competitors (HCH). HSH1: Humans reduce dominant carnivore occupancy and increase subordinate carnivore occupancy. HSH2: Increased conditional occupancy for dominant–subordinate and subordinate–subordinate species pairs. HCH1: Humans reduce occupancy for both dominant and subordinate carnivore species. HCH2: Reduced conditional occupancy for dominant–subordinate and subordinate–subordinate species pairs.
Here, we addressed the following questions to test whether effects from anthropogenic pressures on urban carnivores align with expectations of the HSH or HCH: (1) how do humans influence space use of individual carnivore species? (2) How are pairwise interactions affected by human activity within a competing carnivore guild?
With HSH, subordinate carnivores will exploit the spatial refugia created by human top‐down pressures on dominant predators and spatially overlap with humans at the park scale (Geffroy et al. 2015, Moll et al. 2018). We categorized raccoon, red fox, and skunk as subordinate based on body size, trophic level, and known antagonistic interaction with coyotes, which we categorized as dominant (Fedriani et al. 2000). First, for individual species, we expected human activity to reduce dominant carnivore occupancy and concurrently increase subordinate carnivore occupancy (Berger 2007, Muhly et al. 2011). Second, for pairwise interactions, we expected human activity to increase dominant–subordinate and subordinate–subordinate carnivore species pair conditional occupancy (Smith et al. 2018).
Conversely, with HCH, humans can function like a superior competitor, reduce the niche space, and thus spatially displace carnivores regardless of whether they are dominant or subordinate (Everatt et al. 2019). First, for individual species we expected human activity to reduce both dominant and subordinate carnivore occupancy (George and Crooks 2006). Second, for pairwise interactions we expect human activity to reduce both subordinate–dominant and subordinate–subordinate carnivore conditional occupancy (Magle et al. 2014).
Materials and Methods
Study area
We surveyed the carnivore community at 24 urban parks throughout Detroit, a ˜370‐km² city in southeastern Michigan, USA, using remotely triggered cameras (Fig. 2). Parks sampled as part of our study represent 51% of the total area of the green space in city parks (City of Detroit 2015). The Detroit River runs along the city’s southern boundary and the Rouge River runs through the southwestern districts including an automotive manufacturing plant. Over the last 70 yr, Detroit has experienced a substantial population decline, dropping from 1.8 million residents at the height of its industrial era in 1950 to approximately 673,000 residents in 2016 (U.S. Census Bureau 2016). Current human population density is 1,819/km compared to 2,374/km found in the smaller mid‐western city of Milwaukee, Wisconsin. The number of empty lots peaked at roughly 120,000 vacant lots (33% of total parcels) and 48,000 abandoned buildings (13% of parcels) in 2010 (Detroit Residential Parcel Survey 2010, Raleigh and Galster 2015). Over time, many empty lots have progressed through early successional stages and have even developed enough vegetative cover to support small mammal populations, a key prey source for urban carnivores (Bateman and Fleming 2012).
Fig. 2.
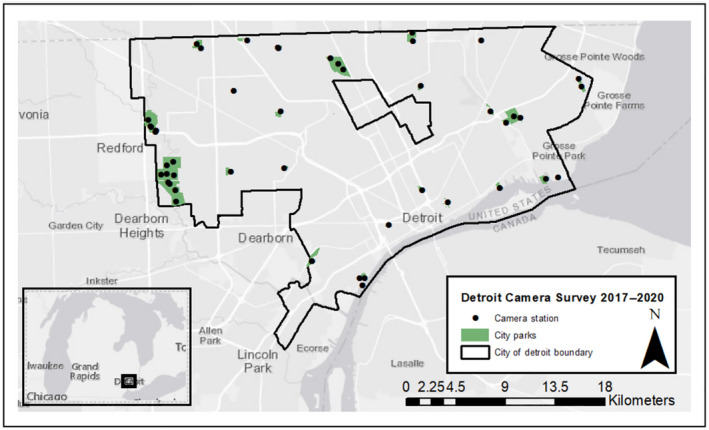
Study area—City of Detroit, Michigan. Shaded green polygons represent the 24 city parks in the Detroit Metro Parks system included in the analysis, and black dots denote camera stations in the study.
Camera survey
We conducted a 3‐yr, noninvasive survey by installing motion‐triggered trail cameras (Reconyx© PC 850, 850C, 900, 900C) throughout city parks during the fall–winter season in Detroit (November 2017–March 2018, November 2018–February 2019, November 2019–March 2020). We deployed 39 stations across 23 city parks in 2017, 41 stations across 24 parks in 2018, and 36 stations at 23 parks in 2019. The same parks were sampled across 2017–2020, with the exception of 2018, where a new site was added. We selected parks to ensure representation of ecological and anthropogenic features such as park size, vegetation cover, distance to water, trails, and built infrastructure such as visitor pavilions and playgrounds. Site selection for camera placement within parks was based on animal sign such as tracks, scat, or natural trails to improve detection of carnivores and their prey. Unbaited cameras were placed approximately 0.5 m from the ground on trees >10 cm in diameter, following standard protocol for mesocarnivore camera trap studies (Cove et al. 2012). Camera settings were set to high sensitivity, with three images captured per trigger at 1‐s intervals, and 15‐s delay between triggers. For parks with >1 camera station, the average distance between cameras was 1,416 m, and the average distance between parks was 3,200 m.
After camera retrieval, at least two members of the Applied Wildlife Ecology Lab at the University of Michigan classified images to species and confirmed accuracy. Any unresolved photos were classified as “unknown” and removed from the analysis. We implemented a 30‐min quiet period to account for pseudoreplication and improve independence for analysis, given that some animals tend to remain in front of the camera and trigger it multiple times. Domestic dogs and cats were excluded from the analysis, as we could not differentiate between feral animals who form part of the local carnivore community and those that were temporarily roaming from their owners. Focal species in this study are relatively common in the eastern United States and comprise a guild that is hierarchically structured, ideal for investigating the effects of human presence on the space use of a carnivore community in urban environments.
Modeling carnivore occupancy
Camera trap data were formatted for occupancy analysis by generating weekly detection histories (i.e., presence “1” or absence “0” of each species at each camera location) using the R package camtrapR (Niedballa et al. 2016). We used a Bayesian multispecies occupancy modeling approach and fit a series of candidate models to test hypotheses about anthropogenic effects on individual carnivore species and intraguild interactions (Rota et al. 2016). The latent occupancy state (Ψ) was modeled as a multivariate Bernoulli random variable (MVB), where Zi = {Zi 1, Zi 2, Zi 3, Zi 4} represents the four‐dimensional vector of binary detection data for the four focal carnivores (1) (Dai et al. 2013). Each occupancy state represents possible scenarios; that is, Ψ1111 denotes all four species are present, Ψ0000 denotes all carnivores are absent.
| (1) |
Interaction parameters were included in the model to calculate individual occupancy estimates as well as conditional probabilities for each species. Conditional probabilities reflect occupancy estimates given the presence of a competitor (e.g., occupancy estimates of red fox, given the presence of coyote). We modeled natural parameters (f 1, f 2, f 3, f 4, f 12, f 13, f 14, f 23, f 24, f 34) as linear functions to obtain the probability of each community state, where each subscript denotes one of the four species: 1 = coyote, 2 = raccoon, 3 = red fox, 4 = skunk, following derivations of Rota et al. (2016). The number of gray fox (Urocyon cinereoargenteus) detections was insufficient for inclusion in the occupancy models and were excluded from the analysis (n = 11 detections) (Mackenzie et al. 2002).
The detection process was modeled as a function of covariates including: park area (km2) (AREA), number of trap nights (TN), camera type (CAM), and NDVI as a measure of vegetation density (VEG); occupancy was modeled as a function of human detections per trap night (HUM), distance to school (km) (DSCH), year (YR), AREA, and NDVI. Top models were selected with an information‐theoretic approach using Akaike’s information criterion (AIC) to identify the model with the lowest ΔAIC and greatest weight (w) (Mackenzie and Bailey 2004). Models were run using the unmarked package in Program R; model fit was assessed using residual sum of squares (RSS) with the parametric bootstrap function ‘parboot’ (Fiske and Chandler 2011, R Development Core Team 2017). We assessed the effects of human activity on carnivore occupancy using the HUM covariate coefficient estimate (βHUM) from the model with the lowest ΔAIC. A negative βHUM signifies that humans decreased individual species or conditional pairwise occupancy, and a positive βHUM indicated that human activity increased these occupancy estimates. We calculated the 95% confidence interval for each βHUM to determine whether it was a strong predictor of occupancy and concluded that intervals overlapping zero were poor predictors and thus did not affect occupancy.
Results
Our 12,106–trap night survey yielded detections of coyotes (n = 220), raccoons (n = 1,496), red foxes (n = 88), and striped skunks (n = 38) in a highly urbanized landscape from 2017–2020. The proportion of sites occupied (uncorrected for imperfect detection) varied by species (Table 1). We recorded 1,103 human detections at 24 parks, resulting in a naïve occupancy estimate of 0.96 based on the proportion of occasions where humans were present across our study period.
Table 1.
Summary of 2017–2020 Detroit Metro Parks camera survey including total and average number of trap nights, camera stations, parks, detections for all species, and number and proportion of parks occupied by each species (i.e., naïve occupancy).
| Detroit camera survey 2017–2020 | Total (no.) | No. detections | No. parks occupied (proportion) |
|---|---|---|---|
| Trap nights | 12,106 | ||
| Average trap nights per camera | 96 | ||
| No. camera stations | 49 | ||
| Parks | 24 | ||
| Species | |||
| Human | 1,103 | 23 (0.96) | |
| Coyote | 220 | 16 (0.66) | |
| Red Fox | 88 | 7 (0.29) | |
| Gray fox | 11 | 2 (0.08) | |
| Raccoon | 1,496 | 19 (0.79) | |
| Skunk | 38 | 6 (0.25) | |
| Domestic dog | 600 | 24 (1.0) | |
| Domestic cat | 439 | 23 (0.96) | |
Species response to human activity
Contrary to expectations of both HCH and HSH, human activity did not significantly alter single‐species occupancy estimates for any of the four focal carnivore species (Fig. 3A). Human βHUM coefficients derived from the top‐performing model for individual species occupancy were coyote (βHUM = −0.13, 95% confidence interval [CI]: −11.6, 11.3), raccoon (βHUM = −1.3, CI: −3.9, 1.3), red fox (βHUM = 1.5, CI: −1.5, 4.5), and skunk (βHUM = −2.9, CI: −14.8, 8.9). Other environmental variables were strong predictors of individual‐species occupancy. For example, DSCH increased occupancy for raccoons (βDSCH = 0.001, CI: 0.0008, 0.0012). VEG and AREA were negative predictors for red foxes, but they were positive and negative predictors for coyotes, respectively (Table 2).
Fig. 3.
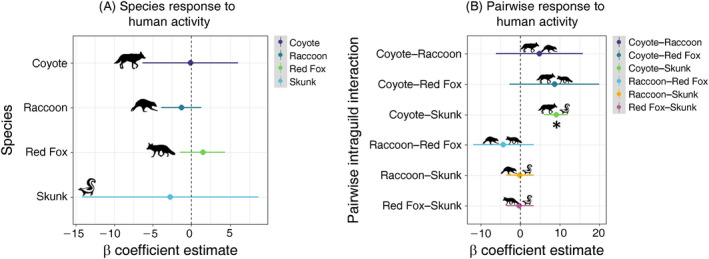
Human effects on (A) individual carnivore species and (B) pairwise intraguild interactions using β coefficient estimates for the human trap night (HUM) covariate and 95% confidence intervals shown from top model. Asterisks denote significant effect with interval not overlapping 0.
Table 2.
Summary of candidate multispecies occupancy models with covariates including year (YR), park area (km2) (AREA), human detections per trap night (HUM), distance to school (km) (DSCH), and NDVI (VEG) as a measure of vegetation density.
| Model | Equation | K | AIC | ΔAIC | w | RSS P‐value |
|---|---|---|---|---|---|---|
| 3 |
ρCoy (VEG); ΨCoy (AREA + VEG + HUM), ρRac (.); ΨRac (DSCH + HUM), ρFox (VEG); ΨFox (AREA + VEG + HUM), ρSku (.); ΨSku (HUM), ρCoy‐Rac (.); ΨCoy‐Rac (AREA + HUM), ρCoy‐Fox (.); ΨCoy‐Fox (AREA + VEG + HUM), ρCoy‐Sku (.); ΨCoy‐Sku (AREA + HUM), ρRac‐Fox (.); ΨRac‐Fox (VEG + HUM), ρRac‐Sku (.); ΨRac‐Sku (HUM), ρFox‐Sku (.); ΨFox‐Sku (HUM) |
36 | 3,356.85 | 0.00 | 0.74 | <0.01 |
| 1 |
ρCoy (.); ΨCoy (AREA + VEG), ρRac (.); ΨRac (DSCH + HUM), ρFox (.); ΨFox (AREA + VEG), ρSku (.); ΨSku (.), ρCoy‐Rac (.); ΨCoy‐Rac (AREA + HUM), ρCoy‐Fox (.); ΨCoy‐Fox (AREA + VEG), ρCoy‐Sku (.); ΨCoy‐Sku (AREA), ρRac‐Fox (.); ΨRac‐Fox (VEG + HUM), ρRac‐Sku (.); ΨRac‐Sku (DSCH), ρFox‐Sku (.); ΨFox‐Sku (DSCH) |
29 | 3,359.52 | 2.67 | 0.19 | <0.01 |
| 2 |
ρCoy (NDVI); ΨCoy (AREA + VEG), ρRac (.); ΨRac (DSCH), ρFox (NDVI); ΨFox (AREA + VEG), ρSku (.); ΨSku (.), ρCoy‐Rac(.); ΨCoy‐Rac (AREA), ρCoy‐Fox(.); ΨCoy‐Fox (AREA + VEG), ρCoy‐Sku(.); ΨCoy‐Sku (AREA), ρRac‐Fox(.); ΨRac‐Fox (VEG), ρRac‐Sku(.); ΨRac‐Sku (.), ρFox‐Sku(.); ΨFox‐Sku (.) |
26 | 3,363.63 | 6.78 | 0.026 | <0.01 |
Dot models (.) denote null (i.e., constant) detection () or occupancy (ψ). Coy, coyote; Rac, raccoon; Sku, skunk. HUM = #human detections/trap night; DSCH = distance to nearest school (km). Akaike’s information criterion (AIC), delta AIC, AIC model weight (w), and model goodness‐of‐fit residual sum of squares (RSS P value) are listed.
Pairwise intraguild interaction response to human activity
Human activity altered how urban carnivores use space with respect to their intraguild competitors. The inclusion of HUM as a covariate for all individual and pairwise interaction occupancy estimates greatly improved model inference, demonstrating that fine‐scale measures of human activity are informative in explaining carnivore occupancy. Human coefficient estimates for coyote–raccoon (βHUM = 4.8, CI: −6.2, 15.8), coyote–red fox(βHUM = 8.7, CI: −2.6, 20.0), raccoon–red fox (βHUM = −3.9, CI: −11.3, 3.5), raccoon–skunk (βHUM = −0.12, CI: −0.4, 0.2), and fox–skunk (βHUM = −0.2, CI: −1.0, 0.6), overlapped zero, signaling that humans were weak predictors of conditional occupancy for these species pairs. However, human activity significantly increased the likelihood that skunks would occupy an area where coyotes were present (βHUM = 9.0, CI: 8.7, 9.2), in support of the human shield hypothesis with intraguild interactions between dominant and subordinate species pair (HSH, Fig. 3B).
Discussion
Our study provides necessary insights into how human activity can affect space use in the carnivore community in urban parks. Common proxies for human activity derived from landscape‐level metrics of urbanization such as housing density, percent impervious surfaces, or road density may not capture the resolution necessary to determine fine‐scale consequences for wildlife (Tablado and Jenni 2017). The inclusion of indices of direct human activity is therefore needed in future urban ecology studies to disentangle the effects of humans from the built environment (Nickel et al. 2020). Further, the design of parks and urban green spaces should include considerations of how human presence shapes animal communities to balance the needs of people and wildlife adequately.
Contrary to our expectations, humans did not influence single‐species occupancy, a result that supports neither HSH or HCH. Our hypothetical framework hinged on either a positive or negative effect of humans on urban carnivore space use. However, our observed lack of effect for individual species may reflect an alternate hypothesis, wherein humans do not directly influence space use at a fine scale, likely as a result of heightened behavioral plasticity in urban adapters (Lowry et al. 2013). Key resources such as den sites and prey availability may be intrinsically concentrated in urban green spaces, given that they are essentially habitat fragments embedded in an urban matrix, thus funneling carnivores into parks regardless of human activity (Marzluff 2005). Our results are consistent with a growing body of evidence that underscores the importance of urban greenspaces to ensure long‐term persistence carnivore community (Gallo et al. 2017).
We found that the influence of human activity altered intraguild interactions for only one species pair. Spatial interaction between coyotes and skunks increased significantly with human activity, indicating that humans effectively shielded skunks from coyotes, consistent with HSH. As the smallest carnivore in our study, skunks face antagonism from coyotes ranging from interference competition to direct killing (Fisher and Stankowich 2018). To date, there has been little evidence of skunks spatially avoiding coyotes; thus we present a novel example of how skunk spatial ecology is mediated by human activity (Prange and Gehrt 2007, Ritchie and Johnson 2009). The long‐term effects of such increased spatial overlap are unknown between this species pair. If humans continue to shield skunks from dominant competitors, the result is a net positive for skunks, as coexistence is facilitated. Alternatively, if the interaction strength between humans and coyotes weakens, yet skunks continue to use humans as a shield, they could potentially be lured into more frequent antagonistic interactions with coyotes and face increased mortality risk, akin to an ecological trap (Robertson and Hutto 2006, Bateman and Fleming 2012). Carnivores may leverage the temporal niche dimension to avoid competitive exclusion, though our study did not explore this aspect of urban carnivore ecology and represents a fruitful future direction.
If humans function as shields and facilitate spatial overlap in the carnivore community, then urban areas could serve as key refugia in cities and increase co‐existence in an otherwise highly competitive guild. Paradoxically, the human shield hypothesis indicates that urbanization does not inherently result in biodiversity loss at the patch scale, given that subordinate species can exploit refugia (Moll et al. 2018, Lewis et al. 2019). However, biodiversity loss due to urbanization at both the landscape and global scale remains a concern for conservation efforts (Mcdonald et al. 2013, Mcintyre 2014, Lewis et al. 2015).
In addition to wild carnivores, domestic dogs and cats were commonly detected and often were without human company. Human affiliates such as dogs and cats are widely recognized as having significant detrimental effects on wildlife (Lenth et al. 2008, Vanak and Gompper 2009, Loss et al. 2013). Despite this, the distinction between free‐roaming, feral, or simply temporarily off leash remains unresolved in our study system. As a result, we were unable to determine whether these human affiliates were true long‐term members of the local carnivore assembly, and thus they were excluded from the analysis. Eeven leashed dogs sometimes harass and injure wildlife including some of the focal species in this study (Hughes and Macdonald 2013). However, how these antagonistic interactions determine the composition, structure, and distribution of carnivore communities in urban spaces is not well understood. Because dogs are one of the most widely distributed terrestrial carnivores, filling this knowledge gap should be a key consideration for future studies to inform natural resource managers seeking to mitigate dogs’ effects on wildlife (Gehrt et al. 2010).
Our study provides insight into the intraguild interactions of an urban carnivore community in a midsized city, the population of which has declined over the last 70 yr. Homogenization patterns expected in urban systems do not necessarily scale down to smaller cities (Collins et al. 2002). Moreover, the historical trajectory and relationship between population decline, housing vacancy, and vegetation varies by city (Schwarz et al. 2018). Notably, this emigration of people from the city of Detroit is complex and tied to various historical socioeconomic biases (Xie et al. 2018). Further, we recognize that how people are distributed in the city and who has access to green spaces is not equitable and is a consequence of discriminatory housing and city planning policies that impact a myriad of ecological processes (Watkins and Gerrish 2018, Schell et al. 2020). This economic and racial segregation of neighborhoods introduces a bias to our understanding of human–wildlife interactions in cities (Alberti et al. 2020). The location, maintenance level, and surrounding characteristics of parks are also unequally distributed in cities; this inequity can potentially attract or deter carnivore species and confound the interpretation of our results (Elliott et al. 2019, Huang et al. 2020). Further studies are needed to disentangle urban carnivore coexistence patterns and resource availability from socioeconomic factors driven by racial disparities and environmental injustices (Wilson et al. 2008).
Finally, our study could inform how natural resource managers and city planners approach urban design and offer opportunities for collaboration with ecologists. Given that urban carnivores seek spatial refuge from human activity hotspots, future park designs could incorporate wildlife zones where the use of walking trails diverts human foot traffic around rather than through important habitat (Hess et al. 2014). Urban planners are thus tasked with promoting access to natural areas for the public, while still conserving habitat for wildlife. City parks are an important resource for urbanites and provide recreational, cultural, psychological, and physiological benefits to visitors (Soga and Gaston 2020). Therefore, finding a balance between the well‐being of people and wildlife is a fundamental challenge of the 21st century (Chawla 2015, Rigolon 2016, Liu et al. 2017).
Acknowledgments
First, we recognize implementing our field research with camera traps was conducted on lands originally belonging to the People of the Three Fires. Our work is not human‐subjects research requiring IRB review, though we remain grateful to authorities granting permission for our research and their efforts to manage coupled human–natural ecosystems. Our sincere thanks to members past and present of the Applied Wildlife Ecology (AWE) Lab at the University of Michigan, specifically K. Mills, R. Malhotra, S. Lima, S. Bower, and G. Gadsden, who contributed to the data collection, image sorting, ArcGIS expertise, and logistical support of this project. We thank our partners at the Detroit Zoological Society for their financial support and the City of Detroit for collaboration, permits, and access to the parks in our study. We thank all of our volunteers for their assistance with camera checks as well as the Michigan ZoomIN online community for their contribution to image classification.
Gámez, S. , and Harris N. C.. 2021. Living in the concrete jungle: carnivore spatial ecology in urban parks. Ecological Applications 31(6):e02393. 10.1002/eap.2393
Corresponding Editor: Aaron J. Wirsing.
Footnotes
Open Research
Data (Gámez and Harris 2021) are available from the Dryad Digital Repository:2
Literature Cited
- Alberti, M. , et al. 2020. The complexity of urban eco‐evolutionary dynamics. BioScience 70:772–793. [Google Scholar]
- Alberti, M. , Marzluff J. M., Shulenberger E., Bradley G., Ryan C., and Zumbrunnen C.. 2003. Integrating humans into ecology: opportunities and challenges for studying urban ecosystems. BioScience 53:1169–1179. [Google Scholar]
- Bateman, P. W. , and Fleming P. A.. 2012. Big city life: carnivores in urban environments. Journal of Zoology 287:1–23. [Google Scholar]
- Berger, J. 2007. Fear, human shields and the redistribution of prey and predators in protected areas. Biology Letters 3:620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos, G. , et al. 2002. Mammal population losses and the extinction crisis. Science 296:904. [DOI] [PubMed] [Google Scholar]
- Chapron, G. , and López‐Bao J. V.. 2016. Coexistence with large carnivores informed by community ecology. Trends in Ecology & Evolution 31:578–580. [DOI] [PubMed] [Google Scholar]
- Chawla, L. 2015. Benefits of nature contact for children. Journal of Planning Literature 30:433–452. [Google Scholar]
- City of Detroit . 2015. Detroit open data portal. https://data.detroitmi.gov/
- Clinchy, M. , Zanette L. Y., Roberts D., Suraci J. P., Buesching C. D., Newman C., and Macdonald D. W.. 2016. Fear of the human “super predator” far exceeds the fear of large carnivores in a model mesocarnivore. Behavioral Ecology 27:1826–1832. [Google Scholar]
- Clucas, B. , and Marzluff, J. , et al. 2011. Coupled relationships between humans and other organisms in urban areas. Pages 140–144 in Niemela J., Breuste J., Elmqvist T., Guntenspergen G, James P., and McIntyre N., editors. Urban ecology: patterns, processes, and applications. Oxford University Press, Oxford, UK. [Google Scholar]
- Collins, M. , Vázquez D., and Sanders N.. 2002. Species‐area curves, homogenization and the loss of global diversity. Evolutionary Ecology Research 4:457–464. [Google Scholar]
- Cove, M. V. , Jones B. M., Bossert A. J., Clever D. R., Dunwoody R. K., White B. C., and Jackson V. L.. 2012. Use of camera traps to examine the mesopredator release hypothesis in a fragmented midwestern landscape. American Midland Naturalist 168:456–465. [Google Scholar]
- Dai, B. , Ding S., and Wahba G.. 2013. Multivariate Bernoulli distribution. Bernoulli 19:1465–1483. [Google Scholar]
- Detroit Residential Parcel Survey . 2010. Citywide report for vacant and occupied housing. http://www.detroitparcelsurvey.org/
- Elliott, J. R. , Korver–Glenn E., and Bolger D.. 2019. The successive nature of city parks: making and remaking unequal access over time. City & Community 18:109–127. [Google Scholar]
- Everatt, K. T. , Moore J. F., and Kerley G. I. H.. 2019. Africa’s apex predator, the lion, is limited by interference and exploitative competition with humans. Global Ecology and Conservation 20:e00758. [Google Scholar]
- Farris, Z. J. , Gerber B. D., Valenta K., Rafaliarison R., Razafimahaimodison J. C., Larney E., Rajaonarivelo T., Randriana Z., Wright P. C., and Chapman C. A.. 2017. Threats to a rainforest carnivore community: A multi‐year assessment of occupancy and co‐occurrence in Madagascar. Biological Conservation 210:116–124. [Google Scholar]
- Fedriani, J. M. , Fuller T. K., Sauvajot R. M., and York E. C.. 2000. Competition and intraguild predation among three sympatric carnivores. Oecologia 125:258–270. [DOI] [PubMed] [Google Scholar]
- Fisher, K. A. , and Stankowich T.. 2018. Antipredator strategies of striped skunks in response to cues of aerial and terrestrial predators. Animal Behaviour 143:25–34. [Google Scholar]
- Fiske, I. , and Chandler R.. 2011. unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software 1:2011. [Google Scholar]
- Gallo, T. , Fidino M., Lehrer E. W., and Magle S. B.. 2017. Mammal diversity and metacommunity dynamics in urban green spaces: implications for urban wildlife conservation. Ecological Applications 27:2330–2341. [DOI] [PubMed] [Google Scholar]
- Gallo, T. , Fidino M., Lehrer E. W., and Magle S.. 2019. Urbanization alters predator‐avoidance behaviours. Journal of Animal Ecology 88:793–803. [DOI] [PubMed] [Google Scholar]
- Gámez, S. , and Harris N. C.. 2021. Living in the concrete jungle: carnivore spatial ecology in urban parks. Dryad, data set. 10.5061/dryad.59zw3r263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston, K. J. , Davies T. W., Nedelec S. L., and Holt L. A.. 2017. Impacts of artificial light at night on biological timings. Annual Review of Ecology, Evolution, and Systematics 48:49–68. [Google Scholar]
- Geffroy, B. , Samia D. S. M., Bessa E., and Blumstein D. T.. 2015. How nature‐based tourism might increase prey vulnerability to predators. Trends in Ecology & Evolution 30:755–765. [DOI] [PubMed] [Google Scholar]
- Gehrt S. D., et al. editors. 2010. Urban carnivores: ecology, conflict, and conservation. The Johns Hopkins University Press, Baltimore, Maryland, USA. [Google Scholar]
- George, S. L. , and Crooks K. R.. 2006. Recreation and large mammal activity in an urban nature reserve. Biological Conservation 133:107–117. [Google Scholar]
- Hess, G. R. , et al. 2014. Integrating wildlife conservation into urban planning. Pages 239–278 in Mccleery R. A., Moorman C. E., and Peterson M. N., editors. Urban wildlife conservation: theory and practice. Springer, Boston, Massachusetts, USA. [Google Scholar]
- Huang, J.‐H. , Hipp J. A., Marquet O., Alberico C., Fry D., Mazak E., Lovasi G. S., Robinson W. R., and Floyd M. F.. 2020. Neighborhood characteristics associated with park use and park‐based physical activity among children in low‐income diverse neighborhoods in New York City. Preventive Medicine 131:105948. [DOI] [PubMed] [Google Scholar]
- Hughes, J. , and Macdonald D. W.. 2013. A review of the interactions between free‐roaming domestic dogs and wildlife. Biological Conservation 157:341–351. [Google Scholar]
- Lamb, C. T. , Ford A. T., McLellan B. N., Proctor M. F., Mowat G., Ciarniello L., Nielsen S. E., and Boutin S.. 2020. The ecology of human–carnivore coexistence. Proceedings of the National Academy of Sciences of the United States of America 117:17876–17883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, B. E. , Knight R. L., and Brennan M. E.. 2008. The effects of dogs on wildlife communities. Natural Areas Journal 28:218–227. [Google Scholar]
- Lepczyk, C. A. , Aronson M. F. J., Evans K. L., Goddard M. A., Lerman S. B., and MacIvor J. S.. 2017. Biodiversity in the city: fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience 67:799–807. [Google Scholar]
- Lewis, A. , Bouman M., Winter A., Hasle E., Stotz D., Johnston M., Klinger K., Rosenthal A., and Czarnecki C.. 2019. Does nature need cities? Pollinators reveal a role for cities in wildlife conservation. Frontiers in Ecology and Evolution 7:220. [Google Scholar]
- Lewis, J. S. , Bailey L. L., VandeWoude S., and Crooks K. R.. 2015. Interspecific interactions between wild felids vary across scales and levels of urbanization. Ecology and Evolution 5:5946–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Li F., Li J., and Zhang Y.. 2017. The relationships between urban parks, residents' physical activity, and mental health benefits: A case study from Beijing, China. Journal of Environmental Management 190:223–230. [DOI] [PubMed] [Google Scholar]
- Liu, J. , et al. 2007. Complexity of coupled human and natural systems. Science 317:1513–1516. [DOI] [PubMed] [Google Scholar]
- Loss, S. R. , Will T., and Marra P. P.. 2013. The impact of free‐ranging domestic cats on wildlife of the United States. Nature Communications 4:1396. [DOI] [PubMed] [Google Scholar]
- Lowry, H. , Lill A., and Wong B. B. M.. 2013. Behavioural responses of wildlife to urban environments. Biological Reviews 88:537–549. [DOI] [PubMed] [Google Scholar]
- MacKenzie, D. I. , and Bailey L. L.. 2004. Assessing the fit of site‐occupancy models. Journal of Agricultural, Biological, and Environmental Statistics 9:300–318. [Google Scholar]
- MacKenzie, D. I. , Nichols J. D., Lachman G. B., Droege S., Andrew Royle J., and Langtimm C. A.. 2002. Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. [Google Scholar]
- Magle, S. B. , Simoni L. S., Lehrer E. W., and Brown J. S.. 2014. Urban predator–prey association: coyote and deer distributions in the Chicago metropolitan area. Urban Ecosystems 17:875–891. [Google Scholar]
- Marzluff, J. M. 2005. Island biogeography for an urbanizing world: how extinction and colonization may determine biological diversity in human‐dominated landscapes. Urban Ecosystems 8:157–177. [Google Scholar]
- Mcdonald, R. I. , et al. 2013. Urbanization and global trends in biodiversity and ecosystem services. Pages 31–52 in Elmqvist T., Fragkias M., Goodness J., Güneralp B., Marcotullio P. J., Mcdonald R. I., Parnell S., Schewenius M., Sendstad M., Seto K. C., and Wilkinson C., editors. Urbanization, biodiversity and ecosystem services: challenges and opportunities: a global assessment. Springer, Dordrecht, Netherlands. [Google Scholar]
- McDonald, R. I. , Güneralp B., Huang C.‐W., Seto K. C., and You M.. 2018. Conservation priorities to protect vertebrate endemics from global urban expansion. Biological Conservation 224:290–299. [Google Scholar]
- Mcintyre, N. E. 2014. Wildlife responses to urbanization: patterns of diversity and community structure in built environments. Pages 103–115 in Mccleery R. A., Moorman C. E., and Peterson M. N., editors. Urban wildlife conservation: theory and practice. Springer, Boston, Massachusetts, USA. [Google Scholar]
- Moll, R. J. , Cepek J. D., Lorch P. D., Dennis P. M., Robison T., Millspaugh J. J., and Montgomery R. A.. 2018. Humans and urban development mediate the sympatry of competing carnivores. Urban Ecosystems 21:765–778. [Google Scholar]
- Moll, R. J. , Cepek J. D., Lorch P. D., Dennis P. M., Tans E., Robison T., Millspaugh J. J., and Montgomery R. A.. 2019. What does urbanization actually mean? A framework for urban metrics in wildlife research. Journal of Applied Ecology 56:1289–1300. [Google Scholar]
- Moss, W. E. , Alldredge M. W., and Pauli J. N.. 2016. Quantifying risk and resource use for a large carnivore in an expanding urban–wildland interface. Journal of Applied Ecology 53:371–378. [Google Scholar]
- Muhly, T. B. , Semeniuk C., Massolo A., Hickman L., and Musiani M.. 2011. Human activity helps prey win the predator–prey space race. PLoS One 6:e17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel, B. A. , Suraci J. P., Allen M. L., and Wilmers C. C.. 2020. Human presence and human footprint have non‐equivalent effects on wildlife spatiotemporal habitat use. Biological Conservation 241:108383. [Google Scholar]
- Niedballa, J. , Sollmann R., Courtiol A., and Wilting A.. 2016. camtrapR: an R package for efficient camera trap data management. Methods in Ecology and Evolution 7:1457–1462. [Google Scholar]
- Prange, S. , and Gehrt S. D.. 2004. Changes in mesopredator‐community structure in response to urbanization. Canadian Journal of Zoology 82:1804–1817. [Google Scholar]
- Prange, S. , and Gehrt S. D.. 2007. Response of skunks to a simulated increase in coyote activity. Journal of Mammalogy 88:1040–1049. [Google Scholar]
- Raleigh, E. , and Galster G.. 2015. Neighborhood disinvestment, abandonment, and crime dynamics. Journal of Urban Affairs 37:367–396. [Google Scholar]
- R Development Core Team . 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R‐project.org/ [Google Scholar]
- Rigolon, A. 2016. A complex landscape of inequity in access to urban parks: A literature review. Landscape and Urban Planning 153:160–169. [Google Scholar]
- Riley, S. P. D. , et al. 2014. Wildlife friendly roads: the impacts of roads on wildlife in urban areas and potential remedies. Pages 323–360 in Mccleery R. A., Moorman C. E., and Peterson M. N., editors. Urban wildlife conservation: theory and practice. Springer, Boston, Massachusetts, USA. [Google Scholar]
- Ripple, W. J. , et al. 2014. Status and ecological effects of the world’s largest carnivores. Science 343:1241484. [DOI] [PubMed] [Google Scholar]
- Ritchie, E. G. , and Johnson C. N.. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters 12:982–998. [DOI] [PubMed] [Google Scholar]
- Robertson, B. A. , and Hutto R. L.. 2006. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87:1075–1085. [DOI] [PubMed] [Google Scholar]
- Rota, C. T. , Ferreira M. A. R., Kays R. W., Forrester T. D., Kalies E. L., McShea W. J., Parsons A. W., and Millspaugh J. J.. 2016. A multispecies occupancy model for two or more interacting species. Methods in Ecology and Evolution 7:1164–1173. [Google Scholar]
- Schell, C. J. , Dyson K., Fuentes T. L., Des Roches S., Harris N. C., Miller D. S., Woelfle‐Erskine C. A., and Lambert M. R.. 2020. The ecological and evolutionary consequences of systemic racism in urban environments. Science 369:eaay4497. [DOI] [PubMed] [Google Scholar]
- Schwarz, K. , Berland A., and Herrmann D. L.. 2018. Green, but not just? Rethinking environmental justice indicators in shrinking cities. Sustainable Cities and Society 41:816–821. [Google Scholar]
- Shannon, G. , Cordes L. S., Hardy A. R., Angeloni L. M., and Crooks K. R.. 2014. Behavioral responses associated with a human‐mediated predator shelter. PLoS One 9:e94630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. A. , Thomas A. C., Levi T., Wang Y., and Wilmers C. C.. 2018. Human activity reduces niche partitioning among three widespread mesocarnivores. Oikos 127:890–901. [Google Scholar]
- Soga, M. , and Gaston K. J.. 2020. The ecology of human–nature interactions. Proceedings of the Royal Society B 287:20191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraci, J. P. , Clinchy M., Zanette L. Y., and Wilmers C. C.. 2019. Fear of humans as apex predators has landscape‐scale impacts from mountain lions to mice. Ecology Letters 22:1578–1586. [DOI] [PubMed] [Google Scholar]
- Tablado, Z. , and Jenni L.. 2017. Determinants of uncertainty in wildlife responses to human disturbance. Biological Reviews 92:216–233. [DOI] [PubMed] [Google Scholar]
- Terborgh J. E., and Estes J. A., editors. 2010. Trophic cascades: predators, prey, and the changing dynamics of nature. Island Press, Washington, D.C., USA. [Google Scholar]
- United Nations . 2018. World urbanization prospects: the 2019 revision. https://www.un.org/en/events/citiesday/assets/pdf/the_worlds_cities_in_2018_data_booklet.pdf
- U.S. Census Bureau . 2016. Decennial census of population and housing.
- Vanak, A. T. , and Gompper M. E.. 2009. Dogs Canis familiaris as carnivores: their role and function in intraguild competition. Mammal Review 39:265–283. [Google Scholar]
- Watkins, S. L. , and Gerrish E. d.. 2018. The relationship between urban forests and race: A meta‐analysis. Journal of Environmental Management 209:152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. , Hutson M., and Mujahid M.. 2008. How planning and zoning contribute to inequitable development, neighborhood health, and environmental injustice. Environmental Justice 1:211–216. [Google Scholar]
- Xie, Y. , Gong H., Lan H., and Zeng S.. 2018. Examining shrinking city of Detroit in the context of socio‐spatial inequalities. Landscape and Urban Planning 177:350–361. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data (Gámez and Harris 2021) are available from the Dryad Digital Repository:2


