Abstract
Background
This is the second update of previously published reviews in the Cochrane Library (2015, first update 2017). Interleukin‐5 (IL‐5) is the main cytokine involved in the proliferation, maturation, activation and survival of eosinophils, which cause airway inflammation and are a classic feature of asthma. Studies of monoclonal antibodies targeting IL‐5 or its receptor (IL‐5R) suggest they reduce asthma exacerbations, improve health‐related quality of life (HRQoL) and lung function in appropriately selected patients, justifying their inclusion in the latest guidelines.
Objectives
To compare the effects of therapies targeting IL‐5 signalling (anti‐IL‐5 or anti‐IL‐5Rα) with placebo on exacerbations, health‐related quality‐of‐life (HRQoL) measures and lung function in adults and children with chronic asthma, and specifically in those with eosinophilic asthma refractory to existing treatments.
Search methods
We searched CENTRAL, MEDLINE, Embase, and two trials registers, manufacturers' websites, and reference lists of included studies. The most recent search was 7 February 2022.
Selection criteria
We included randomised controlled trials comparing mepolizumab, reslizumab and benralizumab versus placebo in adults and children with asthma.
Data collection and analysis
Two review authors independently extracted data and analysed outcomes using a random‐effects model. We used standard methods expected by Cochrane.
Main results
Seventeen studies on about 7600 participants met the inclusion criteria. Six used mepolizumab, five used reslizumab, and six used benralizumab. One study using benralizumab was terminated early due to sponsor decision and contributed no data. The studies were predominantly on people with severe eosinophilic asthma, which was similarly but variably defined. One was in children aged 6 to 17 years; nine others included children over 12 years but did not report results by age group separately. We deemed the overall risk of bias to be low, with all studies contributing data of robust methodology. We considered the certainty of the evidence for all comparisons to be high overall using the GRADE scheme, except for intravenous (IV) mepolizumab and subcutaneous (SC) reslizumab because these are not currently licensed delivery routes.
The anti‐IL‐5 treatments assessed reduced rates of 'clinically significant' asthma exacerbation (defined by treatment with systemic corticosteroids for three days or more) by approximately half in participants with severe eosinophilic asthma on standard care (at least medium‐dose inhaled corticosteroids (ICS)) with poorly controlled disease (either two or more exacerbations in the preceding year or Asthma Control Questionnaire (ACQ) score of 1.5 or more), except for reslizumab SC. The rate ratios for these effects were 0.45 (95% confidence interval (CI) 0.36 to 0.55; high‐certainty evidence) for mepolizumab SC, 0.53 (95% CI 0.44 to 0.64; moderate‐certainty evidence) for mepolizumab IV, 0.43 (95% CI 0.33 to 0.55; high‐certainty evidence) for reslizumab IV, and 0.59 (95% CI 0.52 to 0.66; high‐certainty evidence) for benralizumab SC. Non‐eosinophilic participants treated with benralizumab also showed a significant reduction in exacerbation rates, an effect not seen with reslizumab IV, albeit in only one study. No data were available for non‐eosinophilic participants treated with mepolizumab.
There were improvements in validated HRQoL scores with all anti‐IL‐5 agents in severe eosinophilic asthma. This met the minimum clinically important difference (MCID) for the broader St. George's Respiratory Questionnaire (SGRQ; 4‐point change) for benralizumab only, but the improvement in the ACQ and Asthma Quality of Life Questionnaire (AQLQ), which focus on asthma symptoms, fell short of the MCID (0.5 point change for both ACQ and AQLQ) for all of the interventions. The evidence for an improvement in HRQoL scores in non‐eosinophilic participants treated with benralizumab and reslizumab was weak, but the tests for subgroup difference were negative.
All anti‐IL‐5 treatments produced small improvements in mean pre‐bronchodilator forced expiratory flow in one second (FEV1) of between 0.08 L and 0.15 L in eosinophilic participants, which may not be sufficient to be detected by patients.
There were no excess serious adverse events with any anti‐IL‐5 treatment; in fact, there was a reduction in such events with benralizumab, likely arising from fewer asthma‐related hospital admissions. There was no difference compared to placebo in adverse events leading to discontinuation with mepolizumab or reslizumab, but significantly more discontinued benralizumab than placebo, although the absolute numbers were small (42/2026 (2.1%) benralizumab versus 11/1227 (0.9%) placebo).
The implications for efficacy or adverse events are unclear.
Authors' conclusions
Overall this analysis supports the use of anti‐IL‐5 treatments as an adjunct to standard care in people with severe eosinophilic asthma and poor symptom control. These treatments roughly halve the rate of asthma exacerbations in this population. There is limited evidence for improved HRQoL scores and lung function, which may not meet clinically detectable levels. The studies did not report safety concerns for mepolizumab or reslizumab, or any excess serious adverse events with benralizumab, although there remains a question over adverse events significant enough to prompt discontinuation.
Further research is needed on biomarkers for assessing treatment response, optimal duration and long‐term effects of treatment, risk of relapse on withdrawal, non‐eosinophilic patients, children (particularly under 12 years), comparing anti‐IL‐5 treatments to each other and, in patients meeting relevant eligibility criteria, to other biological (monoclonal antibody) therapies. For benralizumab, future studies should closely monitor rates of adverse events prompting discontinuation.
Plain language summary
Mepolizumab, reslizumab or benralizumab for people already taking inhaled steroids and long‐acting beta 2‐agonists for their asthma
Key messages
• The asthma medicines mepolizumab, reslizumab and benralizumab reduced asthma attacks in selected individuals with severe asthma (those with high numbers of an inflammatory cell called an eosinophil in their blood).
• There were small improvements in quality of life questionnaire scores and breathing tests, but these may be too small to be detected by patients.
Review question
We considered in this review whether taking the medicines mepolizumab, reslizumab or benralizumab in addition to standard treatment (such as inhaled steroids and combination inhalers) are better than a placebo (a dummy medicine) for people with asthma.
Background
Asthma is an inflammatory lung condition characterised by the narrowing of the airways, breathlessness, a tight chest and reduced quality of life. It affects around 350 million people worldwide. Mepolizumab, reslizumab and benralizumab are 'anti‐IL‐5' treatments that may help to reduce asthma symptoms.
Study characteristics
Seventeen studies compared mepolizumab, reslizumab or benralizumab to a placebo in about 7600 people with asthma, most with severe disease. We summarised the results as they related to the occurrence of asthma attacks requiring additional treatment, quality of life, breathing tests, effects on a blood biomarker (the numbers of a type of inflammatory cell called eosinophils), and unwanted effects.
Key results
We found that participants with severe asthma, who had high numbers of eosinophils in their blood, benefited from taking mepolizumab, reslizumab or benralizumab through reduced asthma attacks. There were small improvements in quality of life and breathing tests, but these may be too small to be detected by patients. We agree with international guidelines that say that these medicines can be added to standard treatment for people with severe asthma. Further research is needed to clarify some aspects, such as how to assess treatment response and how long to give treatment for.
Quality of the evidence
The evidence included in this review is provided by very well‐designed studies. We are confident that participants in the studies were randomly placed into different treatment groups, that neither they nor the study team were aware of the treatment they were receiving, and that the small number who did not complete the study did not affect the findings.
This plain language summary is up to date as of 7 February 2022.
Summary of findings
Summary of findings 1. Mepolizumab subcutaneous (SC) compared to placebo for asthma.
| Mepolizumab (SC) compared to placebo for asthma | ||||||
| Patient or population: people with asthma Setting: community Intervention: mepolizumab (SC) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with mepolizumab (SC) | |||||
| Rate of clinically significant exacerbations requiring systemic corticosteroids Follow‐up: range 24‐52 weeks | The mean rate in the placebo group was 1.48 events per participant per yeara | The mean rate in the intervention group was 0.81 fewer events per participant per year (95% CI 0.66 fewer to 0.94 fewer) | Rate ratio 0.45 (0.36 to 0.55) | 936 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Rate of exacerbations requiring emergency department treatment or admission Follow‐up: range 24‐ 52 weeks | The mean rate in the placebo group was 0.15 events per patient per yearb | The mean rate in the intervention group was 0.10 fewer events per participant per year (95% CI 0.05 fewer to 0.12 fewer) | Rate ratio 0.36 (0.20 to 0.66) | 936 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Health‐related quality of life (ACQ) Scale from: 0‐6 (lower is better) Follow‐up: range 24‐52 weeks | The mean change in the placebo group ranged from −0.4 to −0.5 unitsc | The mean in the intervention group was 0.38 units fewer (0.50 fewer to 0.26 fewer) | ‐ | 1231 (3 RCTs) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Health‐related quality of life (SGRQ) Scale from: 0‐100 (lower is better) Follow‐up: range 24‐52 weeks | The mean change in the placebo group ranged from −7.9 to −9.0 unitsc | The mean change in the intervention group was 6.4 units fewer (8.9 fewer to 4.0 fewer) | ‐ | 1231 (3 RCTs) | ⊕⊕⊕⊕ High | A change of ≥ 4 points is considered the MCID |
| Pre‐bronchodilator FEV1 Follow‐up: range 24‐52 weeks | The mean change in the placebo group ranged from 0.086 L (± 0.031 L) to 0.120 L (0.047 to 0.192 L)c | The mean difference from placebo was a further 0.09 L (0.05 L to 0.14 L) | ‐ | 1231 (3 RCTs) | ⊕⊕⊕⊕ High | |
| Serious adverse events Follow‐up: range 24‐52 weeks | 95 per 1000 | 65 per 1000 (44 to 96) | Risk ratio 0.68 (0.46 to 1.01) | 1231 (3 RCTs) | ⊕⊕⊕⊕ High | |
| Clinically significant adverse events leading to discontinuation Follow‐up: range 24‐52 weeks | 15 per 1000 | 9 per 1000 (3 to 28) | Risk ratio 0.60 (0.19 to 1.85) | 1231 (3 RCTs) | ⊕⊕⊕⊕ High | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; CI: confidence interval; FEV1: forced expiratory volume in 1 second; MCID: minimum clinically important difference; SC: subcutaneous; SGRQ: St. George's Respiratory Questionnaire | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRounded mean of the rate in the placebo group of the three studies: 1.21 and 1.74. bRounded mean of the rate in the placebo group of the two studies: 0.10 and 0.20. cMean change in placebo taken from Chupp 2017 and Ortega 2014. Moore 2022 did not provide the mean change for each group.
Summary of findings 2. Mepolizumab intravenous (IV) compared to placebo for asthma.
| Mepolizumab (IV) compared to placebo for asthma | ||||||
| Patient or population: people with asthma Setting: community Intervention: mepolizumab (IV) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with mepolizumab (IV) | |||||
| Rate of clinically significant exacerbations requiring systemic corticosteroids Follow‐up: range 32‐52 weeks | The mean rate in the placebo group was 2.51 events per participant per yeara | The mean rate in the intervention groups was 1.18 fewer events per participant per year (1.41 fewer to 0.90 fewer) | Rate ratio 0.53 (0.44 to 0.64) | 751 (3 RCTs) | ⊕⊕⊕⊕ High | |
| Rate of exacerbations requiring emergency department treatment or admission Follow‐up: range 32‐52 weeks | The mean rate in the placebo group was 0.32 events per participant per yearb | The mean rate in the intervention groups was 0.15 fewer events per participant per year (0.22 fewer to 0.04 fewer) | Rate ratio 0.52 (0.31 to 0.87) | 690 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Health‐related quality of life (AQLQ) Scale from: 1‐7 (higher is better) Follow‐up: range 32‐52 weeks | The mean change in the placebo group ranged from 0.18 to 0.71 units | MD 0.21 higher (−0.06 lower to 0.47 higher) | ‐ | 369 (2 RCTs) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Health‐related quality of life (ACQ) Scale from: 0‐6 (lower is better) Follow‐up: range 32‐52 weeks | The mean change in the placebo group ranged from −0.59 to −0.50 units | MD −0.11 lower (−0.32 lower to 0.09 higher) | ‐ | 369 (2 RCTs) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Pre‐bronchodilator FEV1 (L) Follow‐up: range 32 weeks to 52 weeks | The mean change in the placebo group ranged from 0.060 L (± 0.038 L) to 0.086 L (± 0.031 L) | MD 0.08 L (0.02 L higher to 0.15 L higher) |
‐ | 690 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Serious adverse events Follow‐up: range 32‐52 weeks | 167 per 1000 | 99 per 1000 (62 to 157) | Risk ratio 0.59 (0.37 to 0.94) | 751 (3 RCTs) | ⊕⊕⊕⊕ High | |
| Clinically significant adverse events leading to discontinuation Follow‐up: range 32‐52 weeks | 26 per 1000 | 19 per 1000 (5 to 77) | Risk ratio 0.72 (0.18 to 2.92) | 751 (3 RCTs) | ⊕⊕⊕⊕ High | |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FEV 1: forced expiratory volume in 1 second; IV: intravenous; MCID: minimum clinically significant difference; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRounded mean of the rate in the placebo group of the three studies: 1.74, 2.40 and 3.4. bRounded mean of the rate in the placebo group of the two studies: 0.20 and 0.43.
Summary of findings 3. Reslizumab intravenous (IV) compared to placebo for asthma.
| Reslizumab (IV) compared to placebo for asthma | ||||||
| Patient or population: people with asthma Setting: community Intervention: reslizumab (IV) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with reslizumab (IV) | |||||
| Rate of clinically significant exacerbations requiring systemic corticosteroids Follow‐up: 52 weeks | The mean rate in the placebo group was 1.54 events per participant per year | The mean rate in the intervention groups was 0.93 fewer events per participant per year (1.09 fewer to 0.73 fewer) | Rate ratio 0.43 (0.33 to 0.55) | 953 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Rate of exacerbations requiring emergency department treatment or admission Follow‐up: 52 weeks | The mean rate in the placebo group was 0.12 events per participant per year | The mean rate in the intervention groups was 0.04 fewer events per participant per year (0.07 fewer to 0.02 more) | Rate ratio 0.67 (0.39 to 1.17) | 953 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Health‐related quality of life (AQLQ) Scale from: 1‐7 (higher is better) Follow‐up: range 16‐52 weeks | The mean change in the placebo group ranged from 0.779 to 0.89 units | MD 0.28 higher (0.17 higher to 0.39 higher) | ‐ | 1164 (3 RCTs) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Health‐related quality of life (ACQ) Scale from: 0 to 6 (lower is better) Follow‐up: range 16 weeks to 52 weeks | The mean change in the placebo group ranged from −0.368 to −0.80 units | MD ‐0.25 lower (−0.33 lower to −0.17 lower) | ‐ | 1652 (4 RCTs) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Pre‐bronchodilator FEV1 (L) Follow‐up: range 16‐52 weeks | The mean change in the placebo group ranged from 0.002 L (± 0.1216 L) to 0.215 (± 0.0484 L) | MD 0.11 L higher (0.07 L higher to 0.15 L higher) | ‐ | 1652 (4 RCTs) | ⊕⊕⊕⊕ High | |
| Serious adverse events Follow‐up: range 16‐52 weeks | 91 per 1000 | 72 per 1000 (51 to 102) | Risk ratio 0.79 (0.56 to 1.12) | 1656 (4 RCTs) | ⊕⊕⊕⊕ High | |
| Clinically significant adverse events leading to discontinuation Follow‐up: range 16‐52 weeks | 58 per 1000 | 38 per 1000 (25 to 59) | Risk ratio 0.66 (0.43 to 1.02) | 1659 (4 RCTs) | ⊕⊕⊕⊕ High | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FEV1: forced expiratory volume in 1 second; IV: intravenous MCID: minimum clinically significant difference; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 4. Reslizumab subcutaneous (SC) compared to placebo for asthma.
| Reslizumab (SC) compared with placebo for asthma | ||||||
|
Patient or population: people with asthma Settings: community Intervention: reslizumab (SC) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk for placebo | Risk with reslizumab (SC) | |||||
| Rate of clinically significant exacerbations requiring systemic corticosteroids Follow‐up: 52 weeks |
The mean rate in the placebo group was 0.79 events per participant per year | The mean rate in the intervention group was 0.2 fewer events per participant per year (0.38 fewer to 0.06 more) | Rate ratio 0.79 (0.56 to 1.11) |
464 (1 RCT) | ⊕⊕⊕⊕ High | |
| Rate of exacerbations requiring emergency department treatment or admission Follow‐up: 52 weeks | The mean rate in the placebo group was 0.05 events per participant per year | The mean rate in the intervention group was 0.003 fewer events per participant per year (0.03 fewer to 0.05 more) | Rate ratio 0.94 (0.43 to 2.05) | 464 (1 RCT) | ⊕⊕⊕⊕ High | |
| Health‐related quality of life (AQLQ) Scale from: 1‐7 (higher is better) Follow‐up: 52 weeks | The mean change in the placebo group was 1.06 | MD 0.08 higher (‐0.11 to 0.27) |
‐ | 464 (1 RCT) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Health‐related quality of life (ACQ) Scale from: 0 to 6 (lower is better) Follow up: 52 weeks | The mean change in the placebo group was ‐1.14 | MD ‐0.09 lower (‐0.27 to 0.09) |
‐ | 464 (1 RCT) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Health‐related quality of life (SGRQ) Scale from: 0 to 100 (lower is better) Follow up: 32 weeks | The mean change in the placebo group was ‐13.1 points | MD ‐3.3 lower (‐6.02 to ‐0.58 lower) | ‐ | 464 (1 RCT) | ⊕⊕⊕⊕ High | A change of ≥ 4 points is considered the MCID |
| Pre‐bronchodilator FEV1 (L) Follow‐up: 52 weeks | The mean change in the placebo group was 0.23 L | MD 0.14 higher (0.06 to 0.22) |
‐ | 46 4 (1 RCT) | ⊕⊕⊕⊕ High | |
| Serious adverse events Follow‐up: 52 weeks |
82 per 1000 | 80 per 1000 (41 to 151) | Risk ratio 0.97 (0.53 to 1.79) |
464 (1 RCT) | ⊕⊕⊕⊕ High | |
| Clinically significant adverse events leading to discontinuation Follow‐up: 52 weeks | 4 per 1000 | 19 per 1000 (2 to 166) | Risk ratio 4.87 (0.57 to 41.4) | 464 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FEV1: forced expiratory volume in 1 second; MCID: minimum clinically important difference; SC: subcutaneous; SGRQ: St. George's Respiratory Questionnaire | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded because of imprecision.
Summary of findings 5. Benralizumab subcutaneous (SC) compared to placebo for asthma.
| Benralizumab (SC) compared to placebo for asthma | ||||||
| Patient or population: people with asthma Setting: community Intervention: benralizumab (SC) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with benralizumab (SC) | |||||
| Rate of clinically significant exacerbations requiring systemic corticosteroids Follow‐up: range 24‐56 weeks | The mean rate in the placebo group was 1.2 events per participant per yeara | The mean rate in the intervention groups was 0.49 fewer events per participant per year (0.58 fewer to 0.41 fewer) | Rate ratio 0.59 (0.52 to 0.66) | 3112 (4 RCTs) |
⊕⊕⊕⊕ High | |
| Rate of exacerbations requiring emergency department treatment or admission Follow‐up: range 48‐56 weeks | The mean rate in the placebo group was 0.11 events per participant per yearb | The mean rate in the intervention groups was 0.04 fewer events per participant per year (0.06 fewer to 0.002 fewer) | Rate ratio 0.68 (0.47 to 0.98) | 1537 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | There is greater heterogeneity (I² = 43%) owing to inclusion of participants with less severe asthma in FitzGerald 2016 (a larger proportion who had only suffered 1 exacerbation the previous year, with correspondingly less potential for exacerbation) |
| Health‐related quality of life (AQLQ) Scale from: 1‐7 (higher is better) Follow‐up: range 48‐56 weeks | The mean change in the placebo group ranged from 0.98 to 1.31 units | MD 0.23 higher (0.11 higher to 0.35 higher)d | ‐ | 1541 (3 RCTs) | ⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Health‐related quality of life (ACQ) Scale from: 0‐6 (lower is better) Follow up: range 24‐56 weeks | The mean change in the placebo group ranged from −1.19 to −0.76 units | MD ‐0.26 lower (‐0.34 lower to ‐0.17 lower)e | ‐ | 2791 (4 RCTs) |
⊕⊕⊕⊕ High | A change of ≥ 0.5 points is considered the MCID |
| Pre‐bronchodilator FEV1 (L) Follow‐up: range 24‐56 weeks | The mean change in the placebo group ranged from ‐0.01 L to 0.239 L | MD 0.11 L higher (0.08 L higher to 0.15 L higher) | ‐ | 2786 (4 RCTs) |
⊕⊕⊕⊕ High | |
| Serious adverse events Follow‐up: range 24‐56 weeks | 130 per 1000 | 109 per 1000 (81 to 121) | Risk ratio 0.76 (0.62 to 0.93) | 3304 (5 RCTs) | ⊕⊕⊕⊕ High | |
| Clinically significant adverse events leading to discontinuation Follow‐up: range 48‐56 weeks | 9 per 1000 | 18 per 1000 (9 to 36 ) | Risk ratio 2.04 (1.03 to 4.03) |
3253 (4 RCTs) | ⊕⊕⊕⊕ High | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FEV1: forced expiratory volume in 1 second; IV: intravenous; MCID: minimum clinically significant difference; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRounded mean of the rate in the placebo group of the eosinophilic and non‐eosinophilic arms (as applicable) or the three studies: 1.33, 1.21, 0.68, 0.49, 0.93, 1.21. bRounded mean of the rate in the placebo group of the two studies: 0.18 and 0.04. cOne point deducted to reflect the level of heterogeneity on this outcome.
Background
This is the second update of previously published reviews in the Cochrane Library (2015, updated 2017), evaluating the effects of therapies targeting IL‐5 signalling (anti‐IL‐5 or anti‐IL‐5Rα) with placebo on asthma.
Description of the condition
Asthma is a chronic inflammatory condition affecting the airways in the lungs. It is characterised by symptoms of breathlessness, chest tightness, wheeze, and cough. These symptoms are due to variable airflow obstruction secondary to bronchial hyperresponsiveness and airway inflammation. These symptoms are variably and intermittently present in the natural course of the disease, with periods of acutely increased symptomatology called exacerbations.
An estimated 350 million people worldwide currently suffer from asthma, with numbers increasing (GBD Study 2017). Asthma causes a significant degree of morbidity and mortality: every year in the UK alone there are an estimated 2.7 million GP consultations, 121,000 hospital attendances, 93,900 admissions, and over 1000 deaths (Mukherjee 2016). The annual cost in the UK has been estimated at GBP 1.1 billion. Current treatments, such as inhaled corticosteroids (ICS) and bronchodilators are well established, yet despite these almost half of people living with asthma experience an exacerbation each year (Price 2014).
Asthma is now recognised as a heterogeneous disease comprised of a number of different clinical phenotypes and molecular endotypes, although the precise definition of these remains a work in progress (Wenzel 2012). Atopic or allergic asthma is generally considered the most common phenotype, representing roughly half of all people with asthma (Woodruff 2009). Atopic asthma is thought to be driven by an excess of 'type 2 inflammation': an elevated number of type 2 helper T (Th2) cells and the cytokines they secrete, interleukin 4 (IL‐4), IL‐5 and IL‐13. A separate pathophysiological mechanism, in which type 2 innate lymphoid cells (ILC2s) produce large amounts of IL‐5 and IL‐13 (and to a lesser degree, IL‐4) is hypothesised to be important in a subgroup of asthma sufferers with eosinophilia but no allergies (Brusselle 2013). This group is particularly important because they have severe asthma that is largely resistant to ICS and so have a high burden of disease.
The cytokines IL‐4, IL‐5 and IL‐13 produce many of the classic features of atopic asthma, for example, eosinophilia (IL‐5 controls the proliferation, survival and recruitment of eosinophils), raised immunoglobulin E (IgE) levels (the result of B cell class switching in response to IL‐4 and IL‐13), mucus hypersecretion and airway hyperresponsiveness, both a potential consequence of IL‐13 (Chung 2015). Treatments targeting these so called 'type 2 cytokines' have subsequently been developed and investigated for their potential in treating asthma.
Description of the intervention
One of the core pathological features of asthma is eosinophilic infiltration of the bronchial mucosa and airways (Kay 2015). Pro‐inflammatory mediators secreted by eosinophils cause damage to the epithelium, initiating vasodilatation, smooth muscle contraction and increased mucous secretion, which in turn is associated with increased airway hyperresponsiveness, asthma symptoms and airway narrowing (Liu 2013). Thus increased airway eosinophil counts, for example following reduction in the dose of maintenance ICS, cause increased symptoms and asthma exacerbations (Jatakanon 2000).
The proliferation, maturation, activation, recruitment and survival of eosinophils is under the control of IL‐5 (Lopez 1986), with the IL‐5 receptor being selectively expressed on eosinophils and basophils. Elevated levels of IL‐5 mRNA are seen in the bronchial biopsies of people with asthma and correlate with disease severity (Humbert 1997). IL‐5 signalling is therefore an attractive target in asthma, and has yielded three monoclonal antibodies: mepolizumab (trade name Nucala; GlaxoSmithKline), reslizumab (trade names Cinqair or Cinqaero; Teva) and benralizumab (trade name Fasenra; MedImmune/AstraZeneca). Mepolizumab and reslizumab both target IL‐5, whereas benralizumab binds the alpha chain of the IL‐5 receptor (IL‐5Rα) found on eosinophils and basophils.
How the intervention might work
Mepolizumab and reslizumab bind IL‐5 and interfere with its ligation to the IL‐5 receptor on eosinophils and basophils. Both reduce eosinophil counts in sputum, a sample from the lower airways, and blood in a dose‐dependent manner (Wang 2009). Benralizumab binds IL‐5Rα to inhibit its activation and induces eosinophil apoptosis by natural killer cells via antibody‐dependent cell‐mediated cytotoxicity (Kolbeck 2010). Benralizumab has also been shown to reduce sputum and blood eosinophil counts (Busse 2010).
All three have marketing licences for use in people with 'eosinophilic' asthma (variably defined) and it is logical that these drugs would be most effective in this subgroup of patients. Anti‐IL‐5 therapies might also theoretically be effective in patients with more relaxed definitions of eosinophilia, or in those defined as 'non‐eosinophilic' based on their blood eosinophil count but who may have an isolated elevation of eosinophils in the airways (i.e. sputum eosinophilia) or have an eosinophilia that has been suppressed by ICS treatment, or both.
Why it is important to do this review
As anti‐IL‐5 therapies become incorporated into national and international guidelines (e.g. the Global Initiative for Asthma (GINA)'s 2021 clinical consensus statement; GINA 2021), and clinical practice, it is important that the evidence is reviewed and made available in the Cochrane Library. The first Cochrane Review focused on mepolizumab, at the time the only anti‐IL‐5 agent licensed (Powell 2015). Reslizumab and benralizumab were subsequently approved by the US Food & Drug Administration and European Medicines Agency, so the scope of the updated review was broadened to consider all anti‐IL‐5 therapies (Farne 2017). They are compared to each other rather than pooled as there are potentially important differences in dose, route of administration (subcutaneous versus intravenous), and in the case of benralizumab, a significant difference in the mechanism of action that uniquely induces eosinophil and basophil apoptosis, which could improve efficacy, but equally increase the incidence of adverse events.
Objectives
To compare the effects of therapies targeting IL‐5 signalling (anti IL‐5 or anti‐IL‐5Rα) with placebo on exacerbations, health‐related quality‐of‐life (HRQoL) measures, and lung function in adults and children with chronic asthma, and specifically in those with eosinophilic asthma refractory to existing treatments.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) reported as full text, published as abstracts only, and unpublished data.
Types of participants
We included both adults and children with a diagnosis of asthma. We focused on collating data from people who had been reported as having eosinophilic asthma to analyse these individuals as a subgroup. We examined individual articles in order to determine how this group should be defined.
We excluded individuals with respiratory comorbidities such as cystic fibrosis, and current smokers. We included former smokers provided they had a smoking history of less than 10 pack years.
Types of interventions
We included studies that compared anti‐IL‐5 therapy with placebo, in addition to current standard care for asthma (ICS, with or without a second controller such as a long‐acting beta2 agonist (LABA)), provided the treatment period was 16 weeks or longer.
In the case of dose‐ranging studies, we included data only for participants on doses likely to be used clinically, specifically 75 mg intravenous (IV) or 100 mg subcutaneous (SC) injections of mepolizumab, 3 mg/kg IV or 110 mg reslizumab SC, and 20 to 30 mg benralizumab SC. These are the licensed doses except 75 mg IV mepolizumab (only licensed for SC administration), 110 mg reslizumab SC (only licensed for IV administration) and 20 mg SC benralizumab (30 mg is the licensed dose). However, for benralizumab, we included the 20 mg dose used in the three previous phase 2a dose‐ranging studies (Castro 2015a; Castro 2015b; Park 2016).
We planned to include the following co‐interventions provided they were not part of the randomised treatment: leukotriene antagonists (LTRA), inhaled bronchodilators (including LABA), inhaled and oral corticosteroids (ICS and OCS respectively), oral aminophyllines and macrolide antibiotics. We excluded studies that initiated a reduction in standard asthma management (e.g. oral corticosteroids) as part of the protocol, as we felt that they were too dissimilar for meaningful comparison.
Types of outcome measures
We referred to the joint American Thoracic Society (ATS) and European Respiratory Society (ERS) statement on standardising endpoints for asthma clinical studies to identify appropriate outcome measures (Reddel 2009). These recommend that clinical studies should assess outcomes relevant to both goals of asthma management: current control of asthma symptoms, and reduced risk of exacerbations and other adverse outcomes (e.g. accelerated lung function decline, treatment side effects). The joint ATS/ERS statement notes that exacerbation frequency, symptoms and lung function are often discordant, thus endpoints assessing each need to be considered.
Exacerbations are responsible for most of the morbidity, mortality and healthcare costs related to asthma, and therefore considered the primary outcome measure. The ATS/ERS statement defines severe exacerbations as including either use of systemic corticosteroids for at least three days, or emergency department treatment or admission requiring systemic corticosteroids (definitions in terms of changes from baseline in lung function, symptoms, or short‐acting β2 agonist use are not validated).
Lung function, specifically low pre‐bronchodilator forced expiratory flow in one second (FEV1), the most commonly reported lung function measure in clinical studies, is a strong independent predictor of asthma exacerbations (Osborne 2007), and is objective and reproducible. However, lung function and symptoms correlate poorly over time in individual patients, so it is recommended that both are monitored. There is no gold standard score for assessing asthma symptoms, with several validated and regularly used including the Asthma Control Questionnaire (ACQ) (Juniper 1999), Asthma Control Test (ACT) (Nathan 2004), Asthma Quality of Life Questionnaire (AQLQ; Juniper 1992), and the St George's Respiratory Questionnaire (SGRQ; Jones 1991). We considered any one of these an adequate measure of symptoms and health‐related quality of life (HRQoL).
Identifying potential patient safety issues are a priority in the evaluation of new drugs. We consider the decision to discontinue study medication because of an adverse event to be a useful clinical marker of severity with real‐world applicability, and have included this alongside serious adverse events, which would likely outweigh any potential benefits of the intervention.
Anti‐IL‐5 treatments should result in a reduction in eosinophils. Moreover, as discussed earlier, increased eosinophil counts are associated with symptoms and exacerbations (Jatakanon 2000). We have therefore included eosinophil counts in the peripheral blood as a secondary outcome, a measure that is readily available in hospitals and clinics.
Primary outcomes
Clinically significant asthma exacerbation, defined by treatment with a course (three days or more) of systemic corticosteroids (with or without hospital admission)
Secondary outcomes
Asthma exacerbation requiring emergency department treatment or admission
HRQoL (as measured by a validated questionnaire e.g. ACQ, AQLQ, SGRQ)
Measures of lung function (e.g. FEV1)
Serious adverse events
Clinically significant adverse events, defined as those prompting treatment discontinuation
Blood eosinophil counts
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from the following databases and trials registries:
Cochrane Airways Register, through the Cochrane Register of Studies (CRS) all years to 7 February 2022;
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 2) via the Cochrane Register of Studies, all years to 7 February 2022;
MEDLINE Ovid SP 1946 to 7 February 2022;
Embase Ovid SP 1974 to 7 February 2022;
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), all years to 7 February 2022;
World Health Organization International Clinical Trials Registry Platform (trialsearch.who.int/), all years to 7 February 2022.
Database search strategies are detailed in Appendix 1. The search strategies were developed and executed by the Cochrane Airways Information Specialist in collaboration with the review authors. We searched all databases from their inception to the present and imposed no restriction on language of publication. The search was first conducted in November 2013 and was updated in November 2014, March 2017, September 2020, March 2021 and February 2022.
Searching other resources
We checked the bibliographies of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information (clinical trials registers on the GlaxoSmithKline (manufacturer of mepolizumab) and AstraZeneca (benralizumab) websites; the Teva (reslizumab) website does not have a clinical trials register).
We searched for errata and retractions relevant to the included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) on 27 September 2021.
Data collection and analysis
Selection of studies
For this review update we used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments ‐ a service that matches records in the search results to records that have already been screened in Cochrane Crowd and have been labelled as 'RCT' or as 'Not an RCT'; the RCT classifier — a machine‐learning model that distinguishes RCTs from non‐RCTs (Marshall 2018); and if appropriate, Cochrane Crowd ‐ Cochrane's citizen science platform where 'the crowd' help to identify and describe health evidence (Noel‐Storr 2020).
Following this initial assessment, two review authors (HF, CP) independently screened titles and abstracts of all the potential studies identified in the search using Covidence software to select potentially relevant studies that met the inclusion criteria. The same two review authors (HF, CP) independently screened the full text of these studies to identify studies for inclusion and exclusion, and they recorded reasons for excluding the ineligible studies. Any disagreement was resolved through discussion or, if required, by consulting a third review author (SJM); however, this was not necessary. We identified and excluded duplicates and collated multiple reports of the same clinical study so that each clinical study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and a Characteristics of excluded studies table.
Data extraction and management
We used a data collection form to record study characteristics and outcome data that had been piloted on at least one study in the review. Two review authors (HF, AW for the 2017 review; EB, FY for the additional studies in the 2022 review) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any run‐in period, number of study centres and location, study setting, withdrawals and date of study
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria
Interventions: intervention, comparator, concomitant medications and excluded medications
Outcomes: primary and secondary outcomes specified and collected, and time points reported
Notes: funding for study and notable conflicts of interest of study authors
The same review authors independently extracted outcome data from included studies. We noted in the Characteristics of included studies if outcome data were not reported in a usable way. We planned to resolve disagreements by consensus or by involving a third author (SJM), but this was not necessary. We transferred the data into Review Manager 5 (RevMan 5; Review Manager 2020). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. The data extracted were additionally checked by the Cochrane Airways' statistician for the 2017 review; this role was fulfilled by one of the authors of the 2022 review (EB). Another review author (SJM) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (HF, AW for the 2017 review; EB, FY for the additional studies in the 2022 review) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve any disagreements by discussion or by involving another review author (SJM), but this was not necessary. We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We graded each potential source of bias as high, low or unclear, and provided a quotation from the study report together with a justification for this judgement in the description of the study under Included studies. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for an unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from that for a patient‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a study author, we noted this under the study description in Included studies .
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
We conducted the review according to this published protocol and have reported any deviations from it in Differences between protocol and review.
Measures of treatment effect
We analysed dichotomous data as rate ratios and risk ratios, and continuous data as mean differences or standardised mean differences, which are presented with 95% confidence intervals. We entered data presented on a scale with a consistent direction of effect.
We have undertaken meta‐analyses only where this was meaningful (i.e. if the treatments, participants and underlying clinical question were sufficiently similar for pooling to make sense).
Where multiple study arms were reported in a single study (Bjermer 2016; Castro 2014a; Park 2016; Pavord 2012a), we only included the arms with doses likely to be used clinically, that is, 75 mg IV or 100 mg mepolizumab SC, 3 mg/kg IV or 110 mg reslizumab SC, 20 to 30 mg SC benralizumab. We considered four‐weekly and eight‐weekly dosing schedules to be equally clinically valid and therefore pooled these data (Bleecker 2016; FitzGerald 2016). Mepolizumab and reslizumab can be administered by different routes (IV or SC); for the purpose of this review we considered these separately.
In future updates of this review, we will narratively describe skewed data reported as medians and interquartile ranges. Where multiple study arms are reported in a single study, we will include only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) are combined in the same meta‐analysis, we will halve the control (placebo) group to avoid double‐counting.
Unit of analysis issues
We did not identify any cross‐over studies or cluster‐randomised studies for inclusion in this version of the review. If cross‐over studies are identified in the future, we will seek data from a paired analysis from the study report or authors in order to appropriately include data in the review using the inverse variance method. If we identify cluster‐randomised studies in the future, then analyses will be at the level of the individual while allowing for the clustering in the data by using the intracluster correlation coefficient. If this is not reported in the study, then we will impute it from similar studies.
Dealing with missing data
We contacted study authors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when we identified a study only as an abstract). If this was not possible and we thought that the missing data would introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity between studies visually by inspection of the forest plots and using the Chi2 test (a P value less than 0.10 was considered significant due to the low power of the test). We also calculated the I² statistic (Higgins 2003); this describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). Values of I² statistic range from 0% to 100%, with 0% representing no heterogeneity and 100% representing considerable heterogeneity.
For this review, we defined heterogeneity as reported using the I² statistic as follows (Deeks 2022).
0% to 40%: heterogeneity might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
Assessment of reporting biases
If we are able to pool more than 10 studies for future versions, we will create and examine a funnel plot to explore possible small study biases and publication bias.
Data synthesis
In view of the considerable clinical heterogeneity between the included studies, we used a random‐effects model.
We combined data on outcomes at 6 months and 12 months. We also described data for other time points, where they were reported.
Subgroup analysis and investigation of heterogeneity
Provided we had included sufficient studies, we planned to carry out subgroup analyses according to:
eosinophilic individuals versus non‐eosinophilic individuals (as eosinophilia may be a prescribing requirement e.g. NICE 2017); and
age (e.g. 0 to 5 years, 6 to 16 years, 17 years and older).
Using the outcomes:
clinically significant asthma exacerbations;
HRQoL (as measured by a validated questionnaire); and
measures of lung function (e.g. FEV1).
We used the formal test for subgroup interactions in Review Manager 2020.
Sensitivity analysis
We planned to carry out the following sensitivity analyses if we included sufficient studies:
excluding studies with an overall high risk of bias;
excluding cross‐over studies and cluster‐randomised studies.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables using the following outcomes.
Rate of clinically significant exacerbations requiring systemic corticosteroids
Rate of exacerbations requiring emergency department treatment or admission
HRQoL (as measured by a validated questionnaire: e.g. ACQ, AQLA, SGRQ )
Measures of lung function (e.g. FEV1)
Serious adverse events
Clinically significant adverse events leading to discontinuation
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in chapter 8 (Higgins 2011) and Chapter 14 (Schünemann 2022) of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro GDT software. We have justified all decisions to downgrade or upgrade the certainty of the evidence using footnotes, and we have made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
We identified 1461 records (Figure 1) in updated database searches, after duplicates were removed and following initial assessment by Cochrane Screen4Me (Noel‐Storr 2020), for this review. The latest search was run on 7 February 2022.
1.
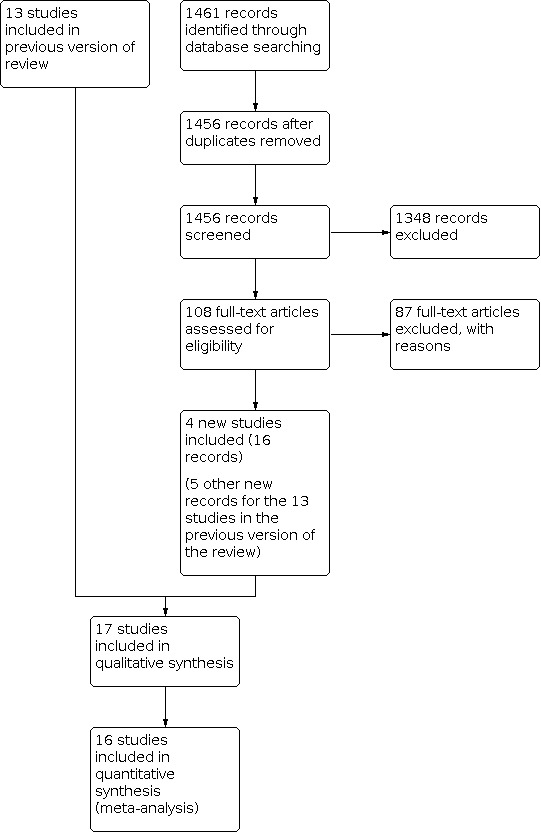
After removing additional duplicates, 1456 records remained.
Seventeen studies from 72 records met our inclusion criteria (see Characteristics of included studies).
There are no ongoing studies and no studies awaiting classification.
We excluded 289 records for various reasons (see Characteristics of excluded studies). In particular, we excluded records relating to the Bel 2014, Nair 2009 and Nair 2017a studies because their protocols included maintenance oral steroid dose reduction (as specified in Types of interventions).
Included studies
Table 6 compares the design, numbers, interventions and participant groups in the included studies. See Characteristics of included studies for details of each study.
1. Comparisons of study characteristics.
| Study (number of participants) | Design, follow‐up (weeks) | Baseline asthma severity | Baseline treatment | Intervention (route) | Primary and secondary outcomes |
|
Bernstein 2020 (468) |
RCT, double‐blind, placebo‐controlled, parallel‐group (52) | ACQ‐6 score ≥ 1.5; ≥ 2 exacerbations in the last year requiring systemic corticosteroids; blood eosinophils ≥ 300 cells/μL; and FEV1 bronchodilator reversibility of < 12% | Medium‐dose ICS for ≥ 3 months; + additional controller for ≥ 3 months; ± maintenance OCS | Reslizumab 110 mg (SC) or placebo every 4 weeks for 52 weeks (last dose at 48 weeks) |
|
|
Bjermer 2016 (315) |
RCT, double‐blind, placebo‐controlled, parallel‐group, fixed‐dosage, multicentre phase 3 (16) | Blood eosinophils ≥ 400 cells/μL during 2‐4‐week screening period; and ACQ‐7 score ≥ 1.5 | Medium‐dose ICS; maintenance OCS not allowed | Reslizumab 0.3 mg/kg or 3 mg/kg (IV) or placebo every 4 weeks for 4 doses |
|
|
Bleecker 2016 (1204) |
RCT double‐blind, parallel‐group, placebo‐controlled multicentre (52) | ≥ 2 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at enrolment; and FEV1 < 80% (if 12‐17 years old, < 90%) | Adults (> 18 years) high‐dose (≥ 500 μg/d FP or equivalent) ICS/LABA for ≥ 12 months Children (12‐17 years) at least medium‐dose (≥ 250 μg/day FP or equivalent) ICS/LABA |
Benralizumab 30 mg (SC) or placebo either every 4 weeks or every 4 weeks for the first 3 doses then every 8 weeks or placebo for 48 weeks |
|
|
Castro 2015a (489) and Castro 2015b (464) |
2 duplicate RCTs, double‐blind, placebo‐controlled, parallel‐group, multicentre, phase 3 (52) | Blood eosinophils ≥ 400 cells/μL during 2‐4‐week screening period; and ACQ‐7 score ≥ 1.5 | Medium‐dose ICS (i.e. ≥ 440 μg/day FP or equivalent daily); ± additional controller or maintenance OCS | Reslizumab 3 mg/kg (IV) or matching placebo every 4 weeks for 13 doses (last dose week 48) |
|
|
Castro 2014a (606) |
RCT double‐blind, placebo‐controlled, multicentre dose‐ranging (52) | 2‐6 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at least twice during screening; and morning pre‐bronchodilator FEV1 40%‐90% | Medium‐ to high‐dose ICS in combination with LABA for ≥ 12 months | Benralizumab 2 mg, 20 mg or 100 mg (SC) or placebo every 4 weeks for the first 3 doses, then every 8 weeks (total 7 doses) |
|
|
Chupp 2017 (551) |
RCT, double‐blind, placebo‐controlled (24) | Blood eosinophils ≥ 150 cells/μL at screening or ≥ 300 cells/μL in previous 12 months; and ≥ 2 exacerbations in previous 12 months; and FEV1 < 80% | High‐dose ICS for ≥ 12 months; + additional controller for ≥ 3 months; ± maintenance OCS | Mepolizumab 100 mg (SC) or placebo every 4 weeks for 24 weeks (last dose at 20 weeks) |
|
|
Corren 2016 (496) |
RCT double‐blind, placebo‐controlled, multicentre phase 3 (16) | ACQ‐7 score ≥ 1.5 (no selection based on blood eosinophils) | Medium‐dose ICS; maintenance OCS not allowed | Reslizumab 3 mg/kg (IV) or matching placebo every 4 weeks for 4 doses |
|
|
FitzGerald 2016 (1306) |
RCT, double‐blind, parallel‐group, placebo‐controlled multicentre (56) | ≥ 2 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at enrolment; and FEV1 < 80% | Medium‐ (≥ 250 μg/d FP or equivalent) to high‐dose (≥ 500 μg/d FP or equivalent) ICS/LABA for ≥ 12 months; high‐dose ICS/LABA for ≥ 3 months | Benralizumab 30 mg (SC) or placebo either every 4 weeks or every 4 weeks for the first 3 doses then every 8 weeks or placebo |
|
|
Haldar 2009 (61) |
RCT, double‐blind, placebo‐controlled, parallel‐group (50) | ≥ 3% sputum eosinophils; and ≥ 2 exacerbations in previous 12 months | High‐dose ICS | Mepolizumab 75 (IV) or matched placebo (150 mL of 0.9% saline) at monthly intervals for 1 year |
|
|
Harrison 2020 (656) |
RCT, double‐blind, placebo‐controlled, parallel‐group (24) | ≥ 2 exacerbations in the last year requiring systemic corticosteroids; FEV1 < 80%; blood eosinophils ≥ 300 cells/μL or ≥ 150 cells/μL and maintenance OCS or history of nasal polyps or ≥ 2 exacerbations in the last year or FVC < 65% or ≥ 18 years at asthma diagnosis; ACQ‐6 score ≥ 1.5; bronchodilator reversibility ≥ 12% or airway hyperresponsiveness or PEF variability ≥ 10% | Medium‐to‐high‐dose ICS + additional controller for ≥ 3 months; ± maintenance OCS | Benralizumab 30 mg (SC) or placebo every 8 weeks (first 3 doses every 4 weeks) for 24 weeks |
|
|
Jackson 2022 (290) |
RCT triple‐blind, placebo‐controlled | ≥ 2 exacerbations in the previous 12 months; peripheral blood eosinophils ≥ 150 cells/µL | For those aged 6‐11 years, treatment with at least medium‐ to high‐dose ICS For those ≥ 12 years of age, treatment with at least medium‐ to high‐dose ICS in combination with LABA |
Mepolizumab 40 mg (SC) for 6‐11 year‐olds, 100 mg (SC) for 12‐17 year‐olds every 4 weeks |
|
|
Moore 2022 (295) |
Randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre study (52) | Patients who had received continuous mepolizumab treatment for ≥ 3 years (initially as part of an RCT, Pavord 2012a, Ortega 2014, or Bel 2014) | Mepolizumab ≥ 6 months + controller medication ≥ 12 weeks | Mepolizumab 100 mg (SC) or placebo every 4 weeks for 52 weeks |
|
|
NCT01947946 (13) |
RCT double‐blind, parallel‐group, placebo‐controlled multicentre (48) | Uncontrolled asthma taking medium‐dose ICS plus LABA | Medium‐dose ICS (> 250 µg and ≤ 500 µg fluticasone dry powder formulation equivalents total daily dose) and LABA for at least 3 months prior to first visit | Benralizumab 30 mg (SC) or placebo either every 4 weeks or every 4 weeks for the first 3 doses then every 8 weeks or placebo | Asthma exacerbations over 48‐week treatment period |
|
Ortega 2014 (576) |
RCT, double‐blind, double‐dummy, phase 3 (32) | Blood eosinophils ≥ 150 cells/μL at screening or ≥ 300 cells/μL in previous 12 months; and ≥ 2 exacerbations in previous 12 months; and FEV1 < 80% | High‐dose ICS for ≥ 12 months; + additional controller for ≥ 3 months; ± maintenance OCS | Mepolizumab 75 mg (IV) or 100 mg (SC) or placebo every 4 weeks for 32 weeks |
|
|
Park 2016 (103) |
RCT double‐blind, placebo‐controlled, dose‐ranging multicentre (52) | 2‐6 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at least twice during screening; and morning pre‐bronchodilator FEV1 40%‐90% | Medium‐ to high‐dose ICS in combination with LABA for ≥ 12 months | Benralizumab 2 mg, 20 mg or 100 mg (SC) or placebo every 4 weeks for the first 3 doses, then every 8 weeks (total 7 doses) |
|
|
Pavord 2012a (621) |
Multicentre, double‐blind, placebo‐controlled (52) | ≥ 3% sputum eosinophils or blood eosinophil ≥ 300 cells/μL; and ≥ 2 exacerbations in previous 12 months | High‐dose ICS (i.e. ≥ 880 μg/d FP or equivalent daily); + additional controller; ± maintenance OCS | Mepolizumab 75 mg, 250 mg or 750 mg (IV) or placebo every 4 weeks for 13 doses |
|
| ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; ASUI: Asthma Symptom Utility Index; BDP: beclomethasone dipropionate; CASI: Composite Asthma Severity Index; CGI‐C: Clinician Global Impression of Change; ECP: eosinophil cationic protein; ED: emergency department; FEF25-75: forced expiratory flow at 25% to 75% of FVC; FeNO: exhaled fraction of nitric oxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; FP: fluticasone propionate; ICS: inhaled corticosteroid; IV: intravenous; LABA: long‐acting beta2 agonist; OCS: oral corticosteroid; PC20: histamine provocative concentration causing a 20% drop in FEV1; PEFR: peak expiratory flow rate; PGI‐C: Patient Global Impression of Change; PSIA: Predominant Symptom and Impairment Assessment; RCT: randomised controlled trial; SABA: short‐acting beta2‐agonists; SC: subcutaneous; SGRQ: St George's Respiratory Questionnaire; SNOT‐22: Sino‐Nasal Outcome Test‐22 | |||||
Mepolizumab
We included six studies that compared mepolizumab versus placebo, involving a total of 2294 participants distributed as follows: Chupp 2017 n = 551; Haldar 2009 n = 61; Jackson 2022 n = 290; Moore 2022 n = 195; Ortega 2014 n = 576; and Pavord 2012a n = 621.
Mepolizumab was administered intravenously (IV) in Haldar 2009 (at a dose of 750 mg) and Pavord 2012a (at doses of 75 mg, 250 mg and 750 mg), subcutaneously (SC) in Moore 2022 and Chupp 2017 (at a dose of 100 mg) and Jackson 2022 (at doses of 40 mg for 6 to 11 year‐olds and 100 mg for 12 to 17 year‐olds), and via both routes (75 mg IV or 100 mg SC) in Ortega 2014 over a range of treatment periods. For Pavord 2012a, we only included the arm dosed at 75 mg, as this is considered comparable to the 100 mg SC dose that is licensed (according to manufacturer's evidence submission to the UK's National Institute for Health and Care Excellence in November 2015 (NICE STA)).
The studies only included participants with eosinophilic asthma. Chupp 2017, Haldar 2009, Ortega 2014, and Pavord 2012a defined severe disease as requiring high‐dose ICS and a second controller medication plus a history of at least two exacerbations in the preceding 12 months. In addition Chupp 2017 and Ortega 2014 required that participants had impaired lung function despite treatment, with an FEV1 of less than 80%. Eosinophilia was defined as a blood eosinophil count of 150 cells or more per μL at screening or 300 cells or more per μL at some time during the previous year (Chupp 2017; Ortega 2014), or either a sputum eosinophil count of 3% or more (Haldar 2009) and/or a blood eosinophil count of 300 cells or more per μL (Pavord 2012a). The blood eosinophil thresholds used in Chupp 2017 and Ortega 2014 were identified as those that best predicted response to mepolizumab in a secondary analysis of previous studies (Ortega 2014; Pavord 2012a). Jackson 2022 used a minimum cut‐off of 150 cells or more per μL for inclusion in the study. Moore 2022 recruited severe eosinophilic patients who had earlier been enrolled in Pavord 2012a, Ortega 2014 or Bel 2014, which had the same blood eosinophil and disease severity criteria as Ortega 2014 but was excluded from the meta‐analysis as the intervention included steroid reduction. The intervention group for Moore 2022 continued mepolizumab, while the control group switched to placebo.
Reslizumab
We included five studies that compared reslizumab versus placebo, involving a total of 2232 participants distributed as follows: Bernstein 2020 n = 468, Bjermer 2016 n = 315, Castro 2015a n = 489; Castro 2015b n = 464; and Corren 2016 n = 496. Reslizumab was administered subcutaneously in Bernstein 2020 at a dose of 110 mg every 4 weeks. Reslizumab was administered intravenously in the remaining four studies over a range of treatment periods at a dose of 3 mg/kg, with an additional arm at a dose of 0.3 mg/kg in Bjermer 2016, which we did not include as it is 10 times lower than the licensed dose of 3 mg/kg.
All the participants had moderate to severe asthma, defined as requiring medium‐dose ICS. In addition, they had inadequate symptom control, with an ACQ of 1.5 or more. Castro 2015a and Castro 2015b furthermore required a history of at least one exacerbation in the preceding 12 months, and Bernstein 2020 required at least two exacerbations during the same time period. Three studies of reslizumab (Bjermer 2016; Castro 2015a; Castro 2015b), required participants to have a blood eosinophil count of 400 cells or more per μL, which has been shown to be predictive of a sputum eosinophil count of 3% or more in studies of participants with paired blood and sputum samples (Farooqui 2009; Van Veen 2009). Bernstein 2020 required a blood eosinophil count of 300 cells or more per μL at screening. Corren 2016 included participants with a range of eosinophil counts.
Benralizumab
We included six studies that compared benralizumab versus placebo, involving a total of 3888 participants distributed as follows: Bleecker 2016 n = 1204; Castro 2014a n = 606; FitzGerald 2016 n = 1306, Harrison 2020 n = 656, NCT01947946 n = 13 and Park 2016 n = 103. All studies administered benralizumab subcutaneously, with dosage varying from 2 mg to 100 mg every four or eight weeks over a range of treatment periods. We only included participants dosed with 20 mg or 30 mg benralizumab in the analysis, as 30 mg is the licensed dose and therefore we considered these the most clinically relevant. NCT01947946 was terminated due to sponsor decision after randomising 13 participants and contributes no data to the review.
The severity of asthma among participants varied from moderate to severe, defined as a requirement for maintenance therapy with medium‐ or high‐dose ICS plus LABA. Participants also had poor asthma control, determined by a history of at least two exacerbations in the previous 12 months and an ACQ of 1.5 or above in the studies contributing data. Harrison 2020 required a blood eosinophil count of 300 cells or more per μL at screening, but also accepted levels of 150 if other requirements were met. The remaining five studies included participants regardless of eosinophilia, but results were stratified by blood eosinophil count using a threshold of 300 cells or more per μL.
Excluded studies
We excluded 287 studies from the review: 115 (40%) because anti‐IL‐5 therapy had not been included in the study; 66 (23%) were an aggregation of studies; 37 (13%) were not randomised placebo‐controlled studies; 21 (7%) had a treatment period of less than 16 weeks; 20 (7%) were conducted on participants without a diagnosis of asthma; 15 (5%) were a post hoc analysis focusing on a specific subgroup (not specified in our protocol); 12 (4%) because the focus was on steroid reduction and 1 study (< 1%) was terminated early because of recruitment issues. (See Characteristics of excluded studies).
Risk of bias in included studies
Details of our risk of bias assessments are available in the Characteristics of included studies, and a summary of our assessment can be seen in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We deemed the majority of studies to be at low risk of bias for both random sequence generation and allocation concealment. Three studies (Bjermer 2016; Corren 2016; NCT01947946), presented no details on either random sequence generation or allocation concealment and were therefore judged as being unclear risk of bias, whereas a further three (Haldar 2009; Jackson 2022; Park 2016), presented no details on allocation concealment so we judged them as unclear risk of bias for that domain (Figure 3).
Blinding
We determined that all studies were at low risk of performance bias, and 11 were at low risk of detection bias; the risk of detection bias was unclear for six studies (Bernstein 2020; Bjermer 2016; Bleecker 2016; Moore 2022; NCT01947946; Park 2016Figure 3).
Incomplete outcome data
We considered 14 studies to be at low risk of attrition bias, with similar dropout rates in the different study arms ranging from 5% to 20% for the placebo and 2% to 23% for the treatment groups (Figure 3). We rated one study high risk due to substantial attrition (Moore 2022). We also deemed NCT01947946 to be high risk because no participants completed the study. There was insufficient information on a further study that has only been published in abstract form thus far, so we judged that study as unclear risk of bias (Jackson 2022).
Selective reporting
We considered the risk of reporting bias to be low in 15 studies (Figure 3), unclear in one study with insufficient data (Jackson 2022), and high in the terminated study (NCT01947946).
Other potential sources of bias
We did not note any other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Mepolizumab (SC) versus placebo
The data for this comparison come from four studies, with a combined total of 1521 participants with severe eosinophilic asthma (Chupp 2017; Jackson 2022; Moore 2022; Ortega 2014). Severe eosinophilia was defined as a blood eosinophil count of 300 cells or more per μL in the preceding 12 months or 150 cells or more per μL at screening, historically in the case of Moore 2022, which recruited participants already on mepolizumab from previous studies (Chupp 2017; Ortega 2014; Moore 2022). Jackson 2022 used a threshold of 150 eosinophils per μL. Our confidence in the results below is moderate to high, as the studies had a robust methodology. Chupp 2017 and Ortega 2014 were large studies, whilst the other two were more modest. Moore 2022 (n = 295) had substantial attrition from the blinded study arms (the study assessed cessation versus continuation of long‐term mepolizumab treatment, randomising participants on mepolizumab to placebo or continued mepolizumab; n = 84/151 in the placebo arm and n = 45/144 in the mepolizumab arm were switched to open‐label mepolizumab), but was otherwise methodologically sound. Jackson 2022 (n = 290) only had conference abstracts and a Clinicaltrials.gov registry entry available at the time of the search.
Primary outcomes
Clinically significant asthma exacerbation (as defined by treatment with a course of systemic corticosteroids, with or without hospital attendance or admission)
Mepolizumab SC resulted in a large reduction in clinically significant asthma exacerbations compared to placebo (rate ratio 0.45, 95% CI 0.36 to 0.55; 2 studies, 936 participants; high‐certainty evidence; Analysis 1.1). Jackson 2022 also examined this outcome but the information published to date does not include participant numbers for each arm and thus we omitted this study from the meta‐analysis. Like the other two studies, Jackson 2022 found a reduction in clinically significant exacerbations in the intervention group (rate ratio 0.73, 95% CI 0.56 to 0.95). Moore 2022 also examined clinically significant exacerbations but used a Cox proportional hazards model to estimate and compare the time to first exacerbation between the intervention and placebo groups. This study found that stopping mepolizumab SC treatment likely resulted in a shorter time to clinically significant exacerbations (hazard ratio 1.61, 95% CI 1.17 to 2.22).
1.1. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 1: Rate of exacerbations requiring systemic corticosteroids
Secondary outcomes
Exacerbations requiring emergency department treatment or admission
The rate of exacerbations requiring emergency department treatment or admission from the two studies (Chupp 2017; Ortega 2014), that contributed to this outcome was reduced in those receiving mepolizumab SC (rate ratio 0.36, 95% CI 0.20 to 0.66; 2 studies, 936 participants; high‐certainty evidence; Analysis 1.4); and the rate of exacerbations requiring admission in the same two studies was similarly reduced in mepolizumab SC participants versus those receiving placebo (rate ratio 0.31, 95% CI 0.13 to 0.73; 2 studies, 936 participants; high‐certainty evidence; Analysis 1.3). Moore 2022 found no evidence for an effect of mepolizumab SC on time to exacerbations requiring emergency department treatment or admission (hazard ratio 1.33, 95% CI 0.50 to 3.54; 295 participants).
1.4. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 4: Rate of exacerbations requiring ED treatment or admission
1.3. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 3: Rate of exacerbations requiring admission
HRQoL (as measured by a validated questionnaire e.g. ACQ, AQLQ, SGRQ)
Three studies (Chupp 2017; Moore 2022; Ortega 2014), contributed HRQoL data measured by the ACQ instrument, indicating a moderate effect in favour of mepolizumab SC versus placebo (mean difference (MD) −0.38, 95% CI −0.50 to −0.26; 1231 participants; moderate‐certainty evidence; Analysis 1.5), but this did not meet the minimum clinically important difference (MCID) of 0.5 points in the ACQ. However, mepolizumab SC reduced SGRQ scores by a clinically meaningful improvement in these studies (MD −6.37, 95% CI −8.76 to −3.98; I² = 36%; 3 studies, 1231 participants; high‐certainty evidence; Analysis 1.6); the MCID is −4 points for the SGRQ.
1.5. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 5: Health‐related quality of life (ACQ)
1.6. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 6: Health‐related quality of life (SGRQ)
The SGRQ is a 50‐item questionnaire with questions covering three domains: symptoms, activity, and impacts (psycho‐social). The ACQ has between five and seven items (there are three variations) focused on asthma symptoms and airflow limitation (the seven‐item ACQ includes short‐acting bronchodilator use for symptom relief and FEV1). The intervention may have had broader effects on activity and psycho‐social aspects that were not captured by the ACQ.
In a responder analysis, Chupp 2017 found 59% of participants treated with mepolizumab SC experienced an improvement greater than the MCID of 0.5 points in the ACQ, versus 42% of participants on placebo (P = 0.0014), and 73% had an improvement of greater than the MCID of 4 points in the SGRQ, versus 55% in the placebo arm (P < 0.0001). This is consistent with a post‐hoc responder analysis of the Ortega 2014 study, where 57% of the mepolizumab SC group achieved an improvement in the ACQ above the MCID compared to 45% of the placebo group (Llanos‐Ackert 2017).
Moore 2022 reported time to a 0.5‐point increase on the ACQ‐5 instrument, finding that stopping mepolizumab SC likely increased the time to a score increase compared to placebo (hazard ratio 1.52, 95% CI 1.13 to 2.04; 295 participants).
Measures of lung function (e.g. FEV1)
In a pooled analysis of three studies (Chupp 2017; Moore 2022; Ortega 2014), there was an increase of 90 mL in pre‐bronchodilator FEV1 in the mepolizumab SC group (MD 0.09, 95% CI 0.05 to 0.14; 3 studies, 1231 participants; high‐certainty evidence; Analysis 1.7). This is a relatively modest increase; although there is no universally accepted MCID for FEV1 in asthma; variability within a single testing session can be up to 0.12 L (data from a mixed pool of respiratory participants (Enright 2004)).
1.7. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 7: Pre‐bronchodilator FEV 1 (litres)
Serious adverse events
There was some slight evidence for fewer serious adverse events in participants receiving mepolizumab SC when the studies were pooled (risk ratio 0.68, 95% CI 0.46 to 1.01; 3 studies, 1231 participants; high‐certainty evidence; Analysis 1.8). This may be due to a reduction in asthma‐related serious adverse events (e.g. exacerbations requiring hospitalisation, which did decline), with Chupp 2017 reporting fewer serious adverse events with mepolizumab SC (15/273 (5%) on mepolizumab SC versus 22/278 (8%) on placebo) noting that 12 of these were asthma‐related: three (1%) with mepolizumab SC and nine (3%) with placebo. There were no reports of anaphylaxis after mepolizumab.
1.8. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 8: Serious adverse events
Clinically significant adverse events (defined as those prompting treatment discontinuation)
There was no evidence for a difference between the two groups with respect to adverse events prompting treatment discontinuation (rate ratio 0.60, 95% CI 0.19 to 1.85; 3 studies, 1231 participants; high‐certainty evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 9: Adverse events leading to discontinuation
Blood eosinophil counts
Insufficient data were available to analyse this outcome. However, Ortega 2014 reported a decrease in blood eosinophil counts by week 4, with a maximal drop of 86% by week 12 that was maintained during the study. Those participants who continued mepolizumab SC treatment in the Moore 2022 study had consistently lower eosinophil counts than those in the placebo group, a difference that persisted through the entire treatment period (stopping versus continuing mepolizumab: 6.19, 95% CI 4.89 to 7.83).
Mepolizumab (IV) versus placebo
The data for this comparison come from three studies (Haldar 2009; Ortega 2014; Pavord 2012a), with a combined total of 751 participants, all with severe eosinophilic asthma. There were no subgroups with non‐eosinophilic participants. Our confidence in the results is moderate, as IV delivery is not currently a licenced delivery route for mepolizumab, and although the results for exacerbations mirror those with mepolizumab SC, those for HRQoL measures do not.
Primary outcomes
Clinically significant asthma exacerbation (as defined by treatment with a course of systemic corticosteroids, with or without hospital attendance or admission)
All three studies reported the rate of clinically significant exacerbations, which were likely reduced in participants receiving mepolizumab IV compared to placebo (rate ratio 0.53, 95% CI 0.44 to 0.64; 3 studies, 751 participants; high‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 1: Rate of clinically significant exacerbations
Secondary outcomes
Exacerbations requiring emergency department treatment or admission
Two studies reported the rate of exacerbations requiring emergency department treatment or admission (Ortega 2014; Pavord 2012a). Mepolizumab IV probably reduces the rate of such exacerbations (rate ratio 0.52, 95% CI 0.31 to 0.87; I² = 5%; 2 studies, 690 participants; high‐certainty evidence; Analysis 2.2). For exacerbations requiring admission, there is little to no evidence for an effect of mepolizumab IV (rate ratio 0.61, 95% CI 0.33 to 1.13; 2 studies, 690 participants; Analysis 2.3).
2.2. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 2: Rate of exacerbations requiring emergency department treatment or admission
2.3. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 3: Rate of exacerbations requiring admission
These findings are consistent with results from the third, smaller study (Haldar 2009; 61 participants), which reported three admissions for asthma exacerbations in the mepolizumab IV group (n = 29) compared to 11 in the placebo group (n = 32; P = 0.07), albeit we have low confidence in this finding (risk ratio 0.82, 95% CI 0.61 to 1.09; 61 participants; Analysis 2.4).
2.4. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 4: People with one or more exacerbations
HRQoL (as measured by a validated questionnaire e.g. ACQ, AQLQ, SGRQ)
Two of the studies (Haldar 2009; Pavord 2012a), used the AQLQ and ACQ questionnaires, whereas the third used the SGRQ only (Ortega 2014). There is little to no evidence for a difference between mepolizumab IV and placebo for HRQoL when measured using the AQLQ instrument (MD 0.21, 95% CI −0.06 to 0.47; I² = 53%; 2 studies, 369 participants; high‐certainty evidence; Analysis 2.5). Similarly, there is little evidence for a difference between the two groups when measuring HRQoL using the ACQ in these studies (MD −0.11, 95% CI −0.32 to 0.09; 2 studies, 369 participants; high‐certainty evidence; Analysis 2.6). However, mepolizumab IV likely reduces SGRQ scores (MD −6.40, 95% CI −9.65 to −3.15; 1 study, 382 participants; Analysis 2.7). These results conflict with those for mepolizumab SC, but in cases with insufficient evidence to conclude an effect, the trend was in favour of mepolizumab and so it may be that the effect is relatively small and this outcome is therefore underpowered.
2.5. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 5: Health‐related quality of life (AQLQ)
2.6. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 6: Health‐related quality of life (ACQ)
2.7. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 7: Health‐related quality of life (SGRQ)
Measures of lung function (e.g. FEV1)
Two studies contributed lung function data (Ortega 2014; Pavord 2012a). Mepolizumab IV slightly increases pre‐bronchodilator FEV1 (litres) (MD 0.08 L, 95% CI 0.02 to 0.15; 2 studies, 690 participants; high‐certainty evidence; Analysis 2.8). This increase is comparable, but slightly smaller, than that for mepolizumab SC and, at an individual participant level, would be considered within the normal range of variability at a single session (Enright 2004).
2.8. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 8: Pre‐bronchodilator FEV 1 (litres)
Serious adverse events
Fewer serious adverse events occurred in the mepolizumab IV group (risk ratio 0.59, 95% CI 0.37 to 0.94; I² = 27%; 3 studies, 751 participants; high‐certainty evidence; Analysis 2.9). As with mepolizumab SC, this may be due to a reduction in asthma‐related serious adverse events but as the individual studies did not report a clear effect, there is no comment by the study authors.
2.9. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 9: Serious adverse events
Clinically significant adverse events (defined as those prompting treatment discontinuation)
All three studies reported this outcome, finding no evidence for a difference between mepolizumab IV and placebo (risk ratio 0.72, 95% CI 0.18 to 2.92; I² = 24%; 3 studies, 751 participants; high‐certainty evidence; Analysis 2.10).
2.10. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 10: Adverse events leading to discontinuation
Blood eosinophil counts
Only one small study (Haldar 2009), reported blood eosinophil counts. Mepolizumab IV likely reduces counts (MD −170.00, 95% CI −230.00 to −110.00; 1 study, 61 participants; Analysis 2.11). Ortega 2014 also reported a decrease in blood eosinophil counts by week 4, with a maximal drop of 83% by week 12 that was maintained during the study, but did not provide absolute counts that could be included.
2.11. Analysis.

Comparison 2: Mepolizumab (IV) versus placebo, Outcome 11: Blood eosinophil level (cells/μL)
Reslizumab (IV) versus placebo
The data for this comparison come from four studies (Bjermer 2016; Castro 2015a; Castro 2015b; Corren 2016), with a combined total of 1652 participants. One of these studies included participants with non‐eosinophilic asthma (Corren 2016). Our confidence in the results as applied to eosinophilic participants is high, as the studies were large and had a robust methodology. Where data were available for non‐eosinophilic participants, we have compared the effect estimate with that for eosinophilic participants using the test for subgroup difference.
Primary outcomes
Clinically significant asthma exacerbation (as defined by treatment with a course of systemic corticosteroids, with or without hospital attendance or admission)
Two studies reported this outcome (Castro 2015a; Castro 2015b), with the pooled data showing that reslizumab IV reduces clinically significant asthma exacerbations compared to placebo (rate ratio 0.43, 95% CI 0.33 to 0.55; 2 studies, 953 participants; high‐certainty evidence; Analysis 3.1). This analysis only included eosinophilic participants; there were no data for non‐eosinophilic participants.
3.1. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 1: Rate of exacerbations requiring systemic corticosteroids
Secondary outcomes
Exacerbations requiring emergency department treatment or admission
The same two studies included data on the exacerbations requiring emergency department treatment or admission, with no evidence for a difference between the treatment groups (rate ratio 0.67, 95% CI 0.39 to 1.17; 2 studies, 953 participants; high‐certainty evidence; Analysis 3.2). Again only eosinophilic participants were included.
3.2. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 2: Rate of exacerbations requiring emergency department treatment or admission
HRQoL (as measured by a validated questionnaire e.g. ACQ, AQLQ, SGRQ)
Three of the four studies used the AQLQ instrument (Bjermer 2016; Castro 2015a; Castro 2015b), and all four used ACQ. While reslizumab IV slightly improves HRQoL as measured by AQLQ scores, the MCID of 0.5 points or more is not reached (MD 0.28, 95% CI 0.17 to 0.39; 3 studies, 1164 participants; high‐certainty evidence; Analysis 3.3). This analysis only included eosinophilic participants; there were no data for non‐eosinophilic participants. Similarly, reslizumab IV slightly reduces ACQ scores, though falls short of the MCID of −0.5 points (MD −0.25, 95% CI −0.33 to −0.17; 4 studies, 1652 participants; high‐certainty evidence; Analysis 3.4). Only one study (Corren 2016), reported data from non‐eosinophilic participants, finding no evidence for a difference between reslizumab IV and placebo. Furthermore, there was no evidence for subgroup differences between eosinophilic and non‐eosinophilic participants (P = 0.19, I² = 41.1%).
3.3. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 3: Health‐related quality of life (AQLQ)
3.4. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 4: Health‐related quality of life (ACQ)
Measures of lung function (e.g. FEV1)
We noted a clear increase in pre‐bronchodilator FEV1 with reslizumab IV treatment, with data contributed by all four reslizumab IV studies (MD 0.11 L, 95% CI 0.07 to 0.15; I² = 21%; 4 studies, 1652 participants; high‐certainty evidence; Analysis 3.5). The absolute difference of 0.11 L is relatively modest, although there is no consensus around a MCID in FEV1 in asthma. For this outcome, data from non‐eosinophilic participants were available (again in only one study, Corren 2016), and for that subgroup we observed no evidence for a difference between reslizumab IV versus placebo. The formal test for subgroup differences yielded a P‐value of 0.13 (I² = 56.3%).
3.5. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 5: Pre‐bronchodilator FEV 1 (litres)
Serious adverse events
There is little to no effect of reslizumab IV on serious adverse events compared to placebo (risk ratio 0.79, 95% CI 0.56 to 1.12; I2 = 0%; 4 studies, 1656 participants; high‐certainty evidence; Analysis 3.6). We note that there was also no difference in the rate of hospitalisations due to asthma exacerbations between reslizumab IV and placebo (Analysis 3.2), which would have counted as serious adverse events. This may explain the difference in effect compared to mepolizumab SC.
3.6. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 6: Serious adverse events
Anaphylaxis was reported in three participants after receiving reslizumab (0.13%). One participant experienced breathlessness, wheezing and flushing shortly after the infusion, but did not have haemodynamic compromise (Corren 2016). All participants were treated for anaphylaxis with standard management at the study site and tested negative for antibodies to reslizumab.
Clinically significant adverse events (defined as those prompting treatment discontinuation)
There was a slight difference between reslizumab IV and placebo for this outcome, which was seen in all the reslizumab IV studies (risk ratio 0.66, 95% CI 0.43 to 1.02; 4 studies, 1659 participants; high‐certainty evidence; Analysis 3.7).
3.7. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 7: Adverse events leading to discontinuation
Blood eosinophil counts
The blood eosinophil counts were reduced in the reslizumab IV treatment group across all four studies (MD −476.83, 95% CI −499.32 to −454.34; I² = 15%; 4 studies, 1656 participants; Analysis 3.8). This analysis only included eosinophilic participants; note that a reduction in eosinophils amongst participants whose eosinophil counts are within the normal range to start with is not necessarily desirable or achievable.
3.8. Analysis.

Comparison 3: Reslizumab (IV) versus placebo, Outcome 8: Blood eosinophil level (cells/μL)
Reslizumab (SC) versus placebo
The data for this comparison come from a single study (Bernstein 2020), which randomised 468 patients. Many of the analyses for this study were stratified by blood eosinophil count, using 300 to less than 400 cells/μL and 400 cells or more per μL as the two analytical groups. However all participants had blood eosinophil counts of 300 or more cells/μL and participants who were truly non‐eosinophilic were excluded. The meta‐analyses for this comparison therefore use all participants. The additional results of the two subgroups (300 to < 400 cells/μL and ≥ 400 cells/μL) are discussed narratively, though we did not conduct a formal test of subgroup differences.
A further 177 participants were randomised as part of a second study reported in the same publication, but this study involved oral steroid dose reduction and was therefore excluded from this review.
Primary outcomes
Clinically significant asthma exacerbation (as defined by treatment with a course of systemic corticosteroids, with or without hospital attendance or admission)
There was no evidence for a difference in clinically significant asthma exacerbations between reslizumab SC and placebo (rate ratio 0.79, 95% CI 0.56 to 1.11; 464 participants; high‐certainty evidence; Analysis 4.1). In prespecified subgroup analyses, those with a blood eosinophil count of 400 cells or more per μL and treated with reslizumab SC experienced a lower rate of clinical asthma exacerbations (rate ratio 0.64, 95% CI 0.43 to 0.95; 373 participants), with no effect of treatment in a smaller group with more modest blood eosinophilia of 300 to less than 400 cells/μL (rate ratio 1.78, 95% CI 0.69 to 4.58; 90 participants). The threshold blood eosinophil count of 400 cells or more per μL is the same as that used in the studies of reslizumab IV, which did find reductions in clinically significant asthma exacerbations (Bjermer 2016; Castro 2015a; Castro 2015b; Corren 2016).
4.1. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 1: Rate of exacerbations requiring systemic corticosteroids
Secondary outcomes
Exacerbations requiring emergency department treatment or admission
There was no difference in the rate of clinical asthma exacerbations requiring emergency department treatment or hospitalisation between the two groups (rate ratio 0.94, 95% CI 0.43 to 2.05; 464 participants; high‐certainty evidence; Analysis 4.2). Subgroup analyses were not reported for this outcome.
4.2. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 2: Rate of exacerbations requiring emergency department treatment or admission
HRQoL (as measured by a validated questionnaire e.g. ACQ, AQLQ, SGRQ)
There was no observed effect of reslizumab SC on ACQ scores (MD −0.09, 95% CI −0.27 to 0.09; 464 participants; high‐certainty evidence; Analysis 4.4). Subgroup analysis confirmed no effect on either the higher blood eosinophil group with 400 cells or more per μL (MD −0.12, 95% CI −0.32 to 0.08; 373 participants) or the subgroup with 300 to less than 400 eosinophils/μL (MD 0.05, 95% CI −0.38 to 0.48; 90 participants). Similarly, there was no clear benefit of reslizumab SC on AQLQ scores in the overall population (MD 0.08, 95% CI −0.11 to 0.27; 464 participants; high‐certainty evidence) or in the subgroups with blood eosinophil counts of either 400 cells or more per μL (MD 0.08, 95% CI −0.14 to 0.30; 373 participants) or 300 to less than 400 cells/μL (MD 0.05, 95% CI −0.38 to 0.48; 90 participants; Analysis 4.3). Participants on reslizumab SC experienced a slight benefit in SGRQ score at week 32 (MD −3.30, 95% CI −6.02 to −0.58; 464 participants; high‐certainty evidence), but this was below the MCID and did not persist to week 52 (Analysis 4.5).
4.4. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 4: Health‐related quality of life (ACQ mean difference)
4.3. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 3: Health‐related quality of life (AQLQ mean difference)
4.5. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 5: Health‐related quality of life (SGRQ mean difference)
Measures of lung function (e.g. FEV1)
Pre‐bronchodilator FEV1 improved with reslizumab SC versus placebo (MD 0.14, 95% CI 0.06 to 0.22; 464 participants; high‐certainty evidence; Analysis 4.6). The same was seen in the subgroup with a blood eosinophil count 400 cells or more per μL (MD 0.15, 95% CI 0.05 to 0.25; 373 participants), though the 300 to less than 400 cells/μL subgroup had a weaker effect in the same direction (MD 0.13, 95% CI −0.05 to 0.31; 90 participants). The increases were modest for both groups but may have reached a clinically important difference, although this benchmark has not yet been established for FEV1 in asthma.
4.6. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 6: Pre‐bronchodilator FEV 1 (litres)
Serious adverse events
There was no difference in serious adverse events between the groups (risk ratio 0.97, 95% CI 0.53 to 1.79, high‐certainty evidence; Analysis 4.7).
4.7. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 7: Serious adverse events
Clinically significant adverse events (defined as those prompting treatment discontinuation)
There was no difference in adverse events prompting discontinuation (risk ratio 4.87, 95% CI 0.57 to 41.40; 468 participants; moderate‐certainty evidence; Analysis 4.8).
4.8. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 8: Adverse events leading to discontinuation
Blood eosinophil counts
Reslizumab SC treatment reduced blood eosinophil counts (MD −0.49, 95% CI −0.58 to −0.40; Analysis 4.9).
4.9. Analysis.

Comparison 4: Reslizumab (SC) versus placebo, Outcome 9: Blood eosinophil level (cells × 10⁹/litre)
Benralizumab (SC) versus placebo
The data for this comparison come from five studies (Bleecker 2016; Castro 2014a; FitzGerald 2016; Harrison 2020; Park 2016), with a combined total of 3304 participants. Four of the five studies included participants with an eosinophilic and non‐eosinophilic phenotype (Bleecker 2016; Castro 2014a; FitzGerald 2016; Park 2016), with more complete data presented for eosinophilic participants. In addition, two studies had additional treatment arms for four‐weekly and eight‐weekly dosing regimens (Bleecker 2016; FitzGerald 2016), shown separately in the meta‐analyses with the placebo group split across them (and adjusted accordingly). Our confidence in the results is high, as the studies were large and had a robust methodology. However, limited data were available on non‐eosinophilic subgroups, which were variably consistent with the findings in eosinophilic subgroups.
Primary outcomes
Clinically significant asthma exacerbation (as defined by treatment with a course of systemic corticosteroids, with or without hospital attendance or admission)
Benralizumab reduced clinically significant asthma exacerbations in all studies (rate ratio 0.59, 95% CI 0.52 to 0.66; I² = 21%; 4 studies, 3112 participants; high‐certainty evidence; Analysis 5.1). We observed this effect in both eosinophilic and non‐eosinophilic participants, with a stronger effect for the eosinophilic subgroup (eosinophilic: rate ratio 0.55, 95% CI 0.48 to 0.63; 4 studies, 2354 participants; versus non‐eosinophilic: rate ratio 0.69, 95% CI 0.56 to 0.85; 2 studies, 758 participants). However, there was no evidence for a subgroup difference (P = 0.08; I² = 68.4%).
5.1. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 1: Rate of exacerbations requiring systemic corticosteroids
Secondary outcomes
Exacerbations requiring emergency department treatment or admission
Two studies (Bleecker 2016; FitzGerald 2016), reported exacerbations requiring emergency department treatment or admission, finding that benralizumab likely decreased these exacerbations versus placebo (rate ratio 0.68, 95% CI 0.47 to 0.98; I² = 43%; 2 studies, 1537 participants; moderate‐certainty evidence; Analysis 5.2). This analysis only included eosinophilic participants; there were no data for non‐eosinophilic participants available. However, there was a considerable degree of heterogeneity (I² = 43%), despite Bleecker 2016 and FitzGerald 2016 having the same study design. Both studies noted heterogeneity in the exacerbation history of their participants, FitzGerald 2016 specifically commenting that participants recruited in Eastern Europe and South America had fewer exacerbations in the year before study entry than those recruited elsewhere. These would therefore have had less scope for a reduction in exacerbation rate. FitzGerald 2016 noted that participants who had had three or more exacerbations in the previous year saw the greatest effects of benralizumab treatment, at rates comparable to Bleecker 2016.
5.2. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 2: Rate of exacerbations requiring emergency department treatment or admission
HRQoL (as measured by a validated questionnaire e.g. ACQ, AQLQ, SGRQ)
Three studies included data on AQLQ scores (Bleecker 2016; Castro 2014a; FitzGerald 2016), four on ACQ scores (Bleecker 2016; Castro 2014a; FitzGerald 2016; Harrison 2020), and one on SGRQ scores (Harrison 2020). AQLQ mean difference was better in the benralizumab group (MD 0.23, 95% CI 0.11 to 0.35; 3 studies, 1541 participants; high‐certainty evidence; Analysis 5.3); for this particular outcome data were available only from eosinophilic participants. However, we observed a similar advantage in favour of benralizumab in combined eosinophilic and non‐eosinophilic participants when measuring HRQoL using the ACQ instrument (MD −0.26, 95% CI −0.34 to −0.17; I² = 37%; 4 studies, 2791 participants; high‐certainty evidence; Analysis 5.4). When analysing the non‐eosinophilic subgroup only, however, the evidence for this effect was weak (MD −0.14, 95% CI −0.30 to 0.02; 2 studies, 755 participants). There was also no strong evidence for subgroup differences (P = 0.09, I² = 64.3%). Neither the AQLQ and ACQ differences reached the MCID of 0.5 points or more. The one study that measured SGRQ scores found a strong improvement with benralizumab versus placebo among eosinophilic participants, exceeding the MCID of 4 points (MD −11.16, 95% CI −15.10 to −7.22; 1 study, 406 participants; Analysis 5.5).
5.3. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 3: Health‐related quality of life (AQLQ mean difference)
5.4. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 4: Health‐related quality of life (ACQ mean difference)
5.5. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 5: Health‐related quality of life (SGRQ mean difference)
One study (Harrison 2020), undertook a responder analysis, which found that 73% and 80% of participants receiving benralizumab experienced an improvement in excess of the MCID in the ACQ and SGRQ respectively, compared to 67% and 68% of participants on placebo (P = 0.019 and P = 0.001 respectively).
Measures of lung function (e.g. FEV1)
Pre‐bronchodilator FEV1 was superior in the benralizumab group (MD 0.11, 95% CI 0.08 to 0.15; I² = 35%; 4 studies, 2786 participants; high‐certainty evidence; Analysis 5.6; Bleecker 2016; Castro 2014a; FitzGerald 2016; Harrison 2020). However, only eosinophilic participants experienced this benefit: there was strong evidence for a difference between the two subgroups (P = 0.004; I² = 88.2%). The observed improvement of 0.11 L is relatively modest and of a similar magnitude to that seen with the mepolizumab and reslizumab interventions.
5.6. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 6: Pre‐bronchodilator FEV 1 (litres)
Serious adverse events
Serious adverse events were reduced in the benralizumab group across the five pooled studies (risk ratio 0.76, 95% CI 0.62 to 0.93; 5 studies, 3304 participants; high‐certainty evidence; Analysis 5.7). Two of the largest studies reported a reduced incidence of serious adverse events with benralizumab (FitzGerald 2016; Harrison 2020); the authors noted that the most commonly reported serious adverse event was worsening asthma, as did the authors of the other large study (Bleecker 2016). This was consistent with the effect of the treatment. There were no reports of anaphylaxis after benralizumab.
5.7. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 7: Serious adverse events
Clinically significant adverse events (defined as those prompting treatment discontinuation)
There were more clinically significant adverse events in the benralizumab group, pooled across all studies except Park 2016, which did not report this outcome (risk ratio 2.04, 95% CI 1.03 to 4.03; 4 studies, 3253 participants; high‐certainty evidence; Analysis 5.8), based on eosinophilic and non‐eosinophilic participants (including a subgroup of participants whose eosinophil status was not defined). The individual studies did not find evidence for an effect and thus there was no comment by the investigators. However, benralizumab has a different mechanism of action resulting in a larger reduction in eosinophils, which could result in an increase in adverse events. This is an area for further research.
5.8. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 8: Adverse events leading to discontinuation
Blood eosinophil levels (% change from baseline)
Although four studies included data on blood eosinophil levels, two provided this as a percentage change from baseline (Bleecker 2016; FitzGerald 2016), whilst the other two provided absolute counts (Castro 2014a; Park 2016). All found marked reductions in blood eosinophil levels with benralizumab. The pooled results of the studies showing this as a percentage change, which includes both eosinophilic and non‐eosinophilic participants, are shown in Analysis 5.9 (MD −104.74, 95% CI −116.12 to −93.35; I² = 36%; 2 studies, 2295 participants). In this analysis, there was strong evidence for subgroup differences (P = 0.002; I² = 90%). Castro 2014a reported mean values of 46 to 56 cells per μL in participants with 300 or more cells per μL at baseline, whereas Park 2016 saw reductions to around 0 cells per μL from a mean of 564 to 824 cells per μL (these data were shown graphically and we could not extract them for inclusion in the meta‐analysis).
5.9. Analysis.

Comparison 5: Benralizumab (SC) versus placebo, Outcome 9: Blood eosinophil level (% change from baseline)
Discussion
Summary of main results
Seventeen studies met the inclusion criteria for this systematic review (Bernstein 2020; Bjermer 2016; Bleecker 2016; Castro 2014a; Castro 2015a; Castro 2015b; Chupp 2017; Corren 2016; FitzGerald 2016; Haldar 2009; Harrison 2020; Jackson 2022; Moore 2022; NCT01947946; Ortega 2014; Park 2016; Pavord 2012a). Seven studies included adult participants only (Bernstein 2020; Castro 2014a; Corren 2016; Haldar 2009; Harrison 2020; Moore 2022; NCT01947946; Park 2016), one included six to 17 year‐olds only (Jackson 2022), and the remaining nine (Bernstein 2020; Bjermer 2016; Bleecker 2016; Castro 2015a; Castro 2015b; Chupp 2017; FitzGerald 2016; Ortega 2014; Pavord 2012a), recruited participants aged 12 years and over. Results in adolescents were not reported separately in the latter studies, and thus we could not perform a subgroup analysis on the paediatric population. However the results from the single study in children and adolescents, not yet published in full, are consistent with those in adults (Jackson 2022).
The results indicate that treatments targeting IL‐5 or the IL‐5 receptor reduce clinically significant asthma exacerbation rates by approximately half in participants with severe eosinophilic asthma already on standard care with a history of poor control ('clinically significant' exacerbations defined as episodes requiring at least three days' treatment with systemic corticosteroids; standard care defined as at least medium‐dose ICS; poor control defined as either two or more exacerbations in the preceding 12 months or an ACQ score of 1.5 or more). The effect size was comparable across the drugs and formulations, with the exception of reslizumab SC, which was relatively ineffective, although the study design and populations studied differed across studies and no head‐to‐head studies were performed. In addition, treatment with mepolizumab and benralizumab reduced rates of exacerbations requiring emergency department attendance or hospital admission. Non‐eosinophilic participants experienced a smaller reduction in asthma exacerbation rates when treated with benralizumab, but there was no evidence for a subgroup difference. No data were available for mepolizumab or reslizumab treatment in participants with non‐eosinophilic asthma; whether this finding will be replicated with mepolizumab and reslizumab is uncertain, as no studies to assess this are underway.
Mepolizumab SC, reslizumab and benralizumab all produced modest improvements in validated HRQoL scores (e.g. ACQ, AQLQ) in severe eosinophilic asthma. However, these did not exceed the MCID for ACQ and AQLQ. The effect size was largest with mepolizumab, although again, the study designs and populations enrolled differed with no head‐to‐head studies to assess this. Improvements in the SGRQ did reach the MCID but came from only four studies, in mepolizumab (Chupp 2017; Moore 2022; Ortega 2014), and benralizumab (Harrison 2020). This may be due to differences between the questionnaires: the SGRQ is a 50‐item questionnaire with three domains (symptoms, activity, and psychosocial impact); the ACQ is much shorter (five to seven items) and focuses on asthma symptoms and airflow limitation; however the AQLQ is more like the SGRQ, with 32 items in four domains (symptoms, activity, emotional function, environmental stimuli). It is therefore not entirely clear why there were differences between the SGRQ and the AQLQ in particular, although an analysis of the results by question domain might be illuminating in that regard. We found little to no evidence of improvement in ACQ and AQLQ scores in those treated with mepolizumab IV or ACQ scores in non‐eosinophilic participants treated with benralizumab (data were not available for mepolizumab or reslizumab).
All anti‐IL‐5 interventions produced an improvement in mean pre‐bronchodilator FEV1 of between 0.08 L and 0.15 L. There is no agreed definition of a MCID in FEV1 in asthma, but the reproducibility of FEV1 values in a single session in participants with a range of respiratory conditions is up to 0.12 L (Enright 2004), suggesting that the increase with anti‐IL‐5 drugs is modest and may not be noticed by participants.
Treatment with mepolizumab and reslizumab (both SC and IV) appeared to result in few adverse events, although there remain minor safety concerns over benralizumab. The pooled results of the benralizumab studies showed some evidence for a reduction in severe adverse events. This was most likely due to the reduction in asthma exacerbations requiring hospital admission that would be classed as severe adverse events. However, when considering adverse events prompting participants to discontinue the study drug, whilst mepolizumab and reslizumab treatment were no different to placebo, there were more discontinuations with benralizumab versus placebo, although the absolute numbers were small (42/2026 (2.1%) benralizumab versus 11/1227 (0.9%) placebo). A meta‐analysis of eight benralizumab placebo‐controlled randomised studies in asthma, including those excluded from this review because the treatment duration was less than 16 weeks or the protocol involved oral corticosteroid dose reduction, found that the excess non‐severe adverse events in those treated with benralizumab related to headache and pyrexia, with no excess of hypersensitivity, injection‐site reactions, nasopharyngitis, rhinitis, upper respiratory tract infection, influenza, cough, nausea, back pain or arthralgia (Liu 2019). This may be due to the different mechanism of action of benralizumab; further research is needed.
All anti‐IL‐5 treatments produced marked reductions in blood eosinophil levels. Benralizumab resulted in almost complete depletion of eosinophils from the peripheral circulation, in both eosinophilic and non‐eosinophilic participants, unlike mepolizumab and reslizumab where a few residual eosinophils remained. This is attributed to the ability of benralizumab to induce apoptosis of eosinophils. It is unclear whether this translates into greater clinical efficacy, a greater risk of adverse events, or both.
A single study (Bernstein 2020), assessed reslizumab SC, and found it did not reduce clinically significant asthma exacerbations in a population with blood eosinophil counts of 400 cells or more per μL. However, in a prespecified analysis, there was a reduction in exacerbation rates in a subgroup with blood eosinophil counts of 400 cells or more per μL, the same threshold as used in the reslizumab IV studies. Moreover, the study authors noted that the fixed SC dose used, 110 mg, was roughly equivalent to 1 mg/kg reslizumab IV for an average 70 kg man after accounting for bioavailability, compared to the licensed 3 mg/kg reslizumab IV dose. Thus the findings with reslizumab SC do not entirely contradict the results for the other formulations and drugs in this class.
Overall the evidence in this review supports the use of anti‐IL‐5 treatments as an adjunct to standard care (at least medium‐dose ICS) in people with severe eosinophilic asthma and a history of poor control (either two or more exacerbations in the preceding 12 months or an ACQ score of 1.5 or more).
Overall completeness and applicability of evidence
A reduction in asthma exacerbations is one of the primary goals of asthma management (GINA 2021). Asthma exacerbations are of major clinical significance as they are the primary cause of morbidity and mortality in asthma, and drive increased healthcare utilisation and cost (Zeiger 2016). This is particularly the case for those with severe asthma, who continue to suffer from frequent exacerbations despite existing treatment options and therefore have a high unmet need (Custovic 2013).
We found evidence of a reduction in the rate of clinically significant exacerbations in adults with severe eosinophilic asthma with poor control given anti‐IL‐5 treatment, with low heterogeneity between studies. Secondary outcomes included adverse event data showing that anti‐IL‐5 treatments are well tolerated. In addition, the few studies measuring the SGRQ, a broader HRQoL score, found significant improvements with anti‐IL‐5 interventions that exceeded the MCID. Statistically significant but more modest improvements, likely below levels that would be clinically detected by patients, were evident in the narrower AQLQ and ACQ scores, focusing on asthma symptoms, and lung function (FEV1). There were also large reductions in blood eosinophil levels but a relationship between these and symptoms is not established and thus this may also be of limited relevance to patients. The evidence on mepolizumab IV and reslizumab SC is of limited applicability as these drugs are not currently available in these formulations.
The included studies did not directly compare the different anti‐IL‐5 treatments, however the effect sizes versus placebo were similar. No head‐to‐head studies exist, although indirect treatment comparisons and network meta‐analyses have been published with differing results (Busse 2019a; Calzetta 2019; Casale 2019; Edris 2019; Edris 2021; He 2018; Iftikhar 2018; Ramonell 2020; Yan 2019), all limited by the differences in eligibility criteria and outcome measures across the studies. A single study comparing biologicals in asthma, omalizumab versus mepolizumab is ongoing (NCT03476109). Pragmatically, reslizumab is given by intravenous infusion necessitating a healthcare setting whereas mepolizumab and benralizumab are given subcutaneously, every four weeks and eight weeks respectively. Thus, there are practical advantages to mepolizumab and particularly benralizumab treatment.
Given the mechanism of action of anti‐IL‐5 agents, the studies were predominantly conducted in participants with severe eosinophilic asthma and poor control. It is therefore not possible to draw conclusions about those with milder or better‐controlled disease (e.g. ACQ less than 1.5 with no exacerbations) or non‐eosinophilic asthma. Eosinophilic and severe asthma were variably defined. Most studies considered blood eosinophil counts, although others used sputum eosinophil counts, which are not readily available in most hospitals or clinics (Haldar 2009; Pavord 2012a). The thresholds used to determine eosinophilia in blood counts varied, with the mepolizumab studies considering 150 cells or more per μL at screening or 300 cells or more per μL in the previous year (except Jackson 2022: 150 cells or more per μL), reslizumab studies using a cut‐off of 400 cells or more per μL (except Bernstein 2020's study of reslizumab SC: 300 cells or more per μL), and benralizumab studies 300 cells or more per μL (except Harrison 2020: 150 cells or more per μL). All the included studies defined severe asthma as a requirement to be on stable treatment with at least medium‐dose ICS, but most specified high‐dose ICS, often with additional controller medication(s). In addition, all studies restricted participants to those with uncontrolled asthma. This was either defined in terms of exacerbation history (usually at least two in the previous 12 months; e.g. the studies of mepolizumab), ACQ score (1.5 or more; e.g. the studies of reslizumab), or both (e.g. the studies of benralizumab). Given this heterogeneity, it is unclear exactly how best to select patients for anti‐IL‐5 treatment, although current evidence suggests that a measure of eosinophilia, treatment with at least medium‐dose ICS, and a history of poor control, defined as either two or more exacerbations in the last 12 months or an ACQ score of 1.5 or more, are necessary.
None of the included studies extended beyond a year, although several open‐label extension studies have since confirmed that the favourable safety profile of these drugs persists for at least between 1.5 and 3.5 years (Bourdin 2021; Busse 2019b; Fitzgerald 2019; Khatri 2019; Khurana 2019; Lugogo 2016; Murphy 2017). One study assessed the need for ongoing anti‐IL‐5 treatment, comparing cessation to continuation of long‐term mepolizumab (Moore 2022), finding that the clinical benefits are only sustained if treatment is continued.
The potential for anti‐IL‐5 treatments to enable a reduction in maintenance OCS in patients requiring this has been documented in several RCTs (Bel 2014; Bernstein 2020; Nair 2009; Nair 2017a). These studies incorporated differing OCS weaning regimens and we therefore considered them too heterogeneous for inclusion in the meta‐analysis, but have nonetheless consistently documented significant OCS‐sparing effects.
In summary, anti‐IL‐5 agents represent a new treatment option for severe eosinophilic asthma with poor control, a patient population with a high, unmet need.
Quality of the evidence
Using the GRADE system, we considered the certainty of the evidence for all comparisons to be high overall, except for mepolizumab IV and reslizumab SC, which are not currently licensed delivery routes for these drugs (we therefore regard this as indirect evidence). We are aware of the limitations in some studies and have detailed them in the Results, Figure 2 and Figure 3. We did not formally assess publication bias through the construction of a funnel plot due to the small number of included studies. However, our search strategy was thorough, including searching conference abstracts and ongoing studies, in order to identify unpublished studies.
Potential biases in the review process
This review and update was based on a published protocol (Powell 2013). We acknowledge the potential for publication bias in this review, as it is possible that we failed to identify unpublished studies that may have provided positive or negative outcomes, which in turn could have altered the treatment benefits. However, to the best of our knowledge, we identified a significant number of studies meeting our inclusion criteria through comprehensive and systematic database searches. We tried to address any study selection bias by having two review authors who independently evaluated all the identified studies. We also ensured that the assessment of each study was consistently in line with the inclusion criteria.
Agreements and disagreements with other studies or reviews
This review is the second update of a previous Cochrane Review, of which the first iteration focused solely on mepolizumab in asthma (Powell 2015, updated in Farne 2017). The previous version noted other reviews with similar findings (Cabon 2017; Li 2017; Liu 2013; Wang 2016; Yancey 2017a). Since then, several systematic reviews have assessed anti‐IL‐5 therapies versus placebo for treating asthma.
Agache 2020: a meta‐analysis of benralizumab, mepolizumab, reslizumab and also dupilumab and omalizumab to placebo, including studies incorporating OCS dose reduction
Calzetta 2019: a network meta‐analysis that compared mepolizumab, reslizumab and benralizumab (and other biologicals) to placebo, as well as each other
Edris 2019 a network meta‐analysis that compared mepolizumab, reslizumab and benralizumab (and other biologicals) to placebo, as well as each other
Edris 2021: a network meta‐analysis that compared mepolizumab, reslizumab and benralizumab (and other biologicals) to placebo, as well as each other
He 2018: a network meta‐analysis that compared mepolizumab, reslizumab and benralizumab to placebo, as well as each other
Henriksen 2018: a meta‐analysis and indirect comparison of mepolizumab and reslizumab (not benralizumab), including a study incorporating OCS dose reduction
Iftikhar 2018: a network meta‐analysis that compared mepolizumab, reslizumab and benralizumab (and other biologicals) to placebo, as well as each other
Ramonell 2020: a network meta‐analysis that compared benralizumab, mepolizumab, reslizumab and also dupilumab to placebo, as well as each other
Our findings are consistent with these reviews, despite the inclusion of additional recent studies (Bernstein 2020; Harrison 2020; Jackson 2022; Moore 2022). All the reviews highlight the need for further research in this area, particularly direct head‐to‐head comparisons of the different treatments.
Real‐world, observational studies of people with severe asthma receiving anti‐IL‐5 therapies have reported efficacy consistent with, and sometimes superior to, that seen in RCTs and in this review. The largest, an ongoing observational study of more than1800 adults with severe asthma in the USA, reported an annualised exacerbation rate of 0.62 per patient year in those receiving any biologic (including but not only anti‐IL‐5) compared to 0.88 in those on maintenance systemic corticosteroids and 0.91 in those not receiving biologics or maintenance systemic corticosteroids (Trevor 2021). In the 261 participants who started biologics during the study period, exacerbation rates fell by 43% in those treated with anti‐IgE compared to 62% in those starting another biological (breakdown by treatment not provided but likely to be overwhelmingly anti‐IL‐5/‐IL‐5Rα, rather than the more recently licensed anti‐IL‐4Rα drug dupilumab). Similar findings were reported for emergency department attendances and hospital admissions. Other outcomes (e.g. ACQ, lung function) were not analysed.
A systematic review and meta‐analysis of mepolizumab treatment for severe asthma identified 13 studies with 1457 participants and found improvements after 6 to 12 months' treatment in: exacerbations and hospital admissions, quantified in events per patient year; ACQ and ACT scores in excess of the MCID (1.03 and 6.52 points respectively); and 0.23 L in FEV1 (Li 2021). Real‐world reports of benralizumab are similarly consistent with the RCTs (e.g. Kavanagh 2021; Pelaia 2021).
Authors' conclusions
Implications for practice.
The studies that are currently available provide evidence to support the use of anti‐IL‐5 treatments in adults with severe eosinophilic asthma, which have been incorporated into national and international guidelines (e.g. Global Initiative for Asthma's 2021 clinical consensus statement, GINA 2021). These treatments appear to roughly halve the rate of asthma exacerbations in this patient population, for whom exacerbations are particularly troublesome (Custovic 2013). Importantly, no concerns about safety were reported for mepolizumab or reslizumab, and there were no excess serious adverse events with benralizumab, although there was a small but significant incidence of adverse events prompting discontinuation of benralizumab. There is limited evidence for improvement in health‐related quality‐of‐life scores and lung function, which may not meet clinically detectable levels.
Whilst the majority of studies included children over the age of 12 years, these did not provide sufficient data to reach a conclusion about efficacy and safety in this population. The initial findings of Jackson 2022, a study in those aged 6 to 17 years, only available in abstract form, suggest similar efficacy to adults but the full results are eagerly awaited.
Implications for research.
Further research is needed to identify biomarkers for assessing treatment response, what the optimal duration of treatment is, the long‐term effects of treatment and risk of relapse on withdrawal, the impact of eosinophil‐depleting treatment on parasitic or helminth infections, and to clarify how best to define the people who will benefit from this treatment, considering the availability of tests (e.g. sputum cell differentials) and thresholds (for blood eosinophil counts). Research is also needed in people with non‐eosinophilic asthma and younger age groups, both under 12 years, in whom there has been only one incompletely published study, and 12 to 18 years old, for whom data have not been reported separately.
With regard to benralizumab in particular, future studies and observational studies should closely monitor the incidence of adverse events leading to discontinuation.
There will be some people who are eligible for more than one anti‐IL‐5 agent and potentially also treatment with anti‐immunoglobulin E (omalizumab) and the newer treatments, anti‐IL‐4 receptor α subunit (blocking IL‐4 and IL‐13 signalling; dupilumab), and anti‐thymic stromal lymphopoietin (TSLP; tezepelumab). At present there are no direct comparisons from head‐to‐head studies, leaving the clinician faced with such patients in an evidence‐free quandary. The variability in enrolment criteria present an obstacle to conducting a network meta‐analysis. High‐quality, head‐to‐head studies are required; one is indeed underway comparing omalizumab and mepolizumab (NCT03476109), more will be needed.
What's new
| Date | Event | Description |
|---|---|---|
| 7 February 2022 | New search has been performed | New literature search run |
| 7 February 2022 | New citation required but conclusions have not changed | Three new studies involving ~1300 people added to the review. |
History
Protocol first published: Issue 11, 2013 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 29 March 2017 | New citation required and conclusions have changed | Scope broadened to encompass all Anti IL 5 therapies (reslizumab and benralizumab), rather than mepolizumab alone Review substantively redrafted Inclusion criteria applied more strictly resulting in exclusion of five (out of eight) mepolizumab studies Search updated leading to the inclusion of 10 new studies (one mepolizumab, four reslizumab and five benralizumab) Groups on doses of the trial medications that are not clinically relevant (e.g. 10 times higher or lower) have been excluded from the analysis Outcomes revised to focus on validated symptom scores, only a pre‐bronchodilator measure of lung function, subgroups for eosinophilia or otherwise New author team |
| 29 March 2017 | New search has been performed | New literature search run |
Acknowledgements
We would particularly like to acknowledge the excellent support and assistance from Emma Dennett, Liz Stovold and Emma Jackson of the Cochrane Airways Review Group, together with the greatly appreciated guidance from Chris Cates (Cochrane Airways Review Group Co‐ordinating Editor). We thank Emma Dennett for screening an update search in February 2022 and for assistance in updating the search section and PRISMA diagram.
The information provided by Prof. Peter Barnes regarding a study included in the previous version of this review (Leckie 2000), and the support provided by Birgit Smith, Jane Dennis, Laura Sansum and Anette Blümle with translation on non‐English study reports is also very much appreciated.
The Background and Methods section of this review are based on a standard template used by Cochrane Airways.
We gratefully acknowledge the significant contribution to the previous versions of this review of Lynne Bax (Farne 2017), and Kerry Dwan and Nicola Walters (Powell 2015).
The authors and Cochrane Airways’ Editorial Team are grateful to the following peer and consumer reviewers for their time and comments: Francesco Menzella (Italy), Tim Donovan (UK), Adil Adatia (Canada) and Marwah Anas El‐Wegoud (Egypt).
This project was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Database search strategies
| Database/search platform/date of last search | Search strategy |
| Airways Register (via Cochrane Register of Studies) Date of most recent search: 7 February 2022 | #1 AST:MISC1 #2 MeSH DESCRIPTOR Asthma Explode All #3 asthma*:ti,ab #4 #1 or #2 or #3 #5 MeSH DESCRIPTOR Antibodies, Monoclonal #6 MeSH DESCRIPTOR Antibodies, Monoclonal, Humanized #7 human* NEAR2 monoclonal* NEAR2 antibod* #8 mepolizumab #9 SB24056 or SB‐24056 #10 Bosatria or Nucala #11 benralizumab* #12 MEDI‐563 #13 reslizumab* #14 Cinquil or Cinqair #15 CEP‐38072 #16 "anti‐interleukin 5" #17 "anti‐IL5" #18 "anti‐IL‐5" #19 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 #4 AND #19 |
| CENTRAL (via Cochrane Register of Studies) Date of most recent search: 7 February 2022 | #1 AST:MISC1 #2 MeSH DESCRIPTOR Asthma Explode All #3 asthma*:ti,ab #4 #1 or #2 or #3 #5 MeSH DESCRIPTOR Antibodies, Monoclonal #6 MeSH DESCRIPTOR Antibodies, Monoclonal, Humanized #7 human* NEAR2 monoclonal* NEAR2 antibod* #8 mepolizumab #9 SB24056 or SB‐24056 #10 Bosatria or Nucala #11 benralizumab* #12 MEDI‐563 #13 reslizumab* #14 Cinquil or Cinqair #15 CEP‐38072 #16 "anti‐interleukin 5" #17 "anti‐IL5" #18 "anti‐IL‐5" #19 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 #4 AND #19 |
| MEDLINE (Ovid) ALL Date of most recent search: 7 February 2022 | 1 exp asthma/ 2 asthma*.tw. 3 1 or 2 4 Antibodies, Monoclonal, Humanized/tu [Therapeutic Use] 5 mepolizumab.tw. 6 (Bosatria or Nucala).tw. 7 benralizumab.tw. 8 MEDI‐563.tw. 9 reslizumab.tw. 10 (Cinquil or Cinqair).tw. 11 CEP‐38072.tw. 12 "anti‐interleukin 5".tw. 13 "anti‐IL5".tw. 14 "anti‐IL‐5".tw. 15 or/4‐14 16 3 and 15 17 (controlled clinical trial or randomized controlled trial).pt. 18 (randomized or randomised).ab,ti. 19 placebo.ab,ti. 20 dt.fs. 21 randomly.ab,ti. 22 trial.ab,ti. 23 groups.ab,ti. 24 or/17‐23 25 Animals/ 26 Humans/ 27 25 not (25 and 26) 28 24 not 27 29 16 and 28 |
| Embase (Ovid) Date of most recent search: 7 February 2022 | 1 exp asthma/ 2 asthma$.tw. 3 1 or 2 4 mepolizumab/ 5 mepolizumab.tw. 6 (Bosatria or Nucala).tw. 7 benralizumab.tw. 8 MEDI‐563.tw. 9 benralizumab/ 10 reslizumab/ 11 (Cinquil or Cinqair).tw. 12 CEP‐38072.tw. 13 "anti‐interleukin 5".tw. 14 "anti‐IL5".tw. 15 "anti‐IL‐5".tw. 16 or/4‐15 17 Randomized Controlled Trial/ 18 randomization/ 19 controlled clinical trial/ 20 Double Blind Procedure/ 21 Single Blind Procedure/ 22 Crossover Procedure/ 23 (clinica$ adj3 trial$).tw. 24 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 25 exp Placebo/ 26 placebo$.ti,ab. 27 random$.ti,ab. 28 ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 29 (crossover$ or cross‐over$).ti,ab. 30 or/17‐29 31 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 32 human/ or normal human/ or human cell/ 33 31 and 32 34 31 not 33 35 30 not 34 36 16 and 35 |
| ClinicalTrials.gov Date of most recent search: 7 February 2022 | Study type: interventional
Condition: asthma Intervention: mepolizumab or reslizumab or benralizumab |
| WHO trials portal Date of most recent search: 7 February 2022 | Condition: asthma Intervention: mepolizumab or reslizumab or benralizumab |
Data and analyses
Comparison 1. Mepolizumab (SC) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Rate of exacerbations requiring systemic corticosteroids | 2 | 936 | Rate Ratio (IV, Random, 95% CI) | 0.45 [0.36, 0.55] |
| 1.1.1 Eosinophilic | 2 | 936 | Rate Ratio (IV, Random, 95% CI) | 0.45 [0.36, 0.55] |
| 1.2 At least one clinically significant exacerbation | 1 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.93] |
| 1.2.1 Eosinophilic | 1 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.93] |
| 1.3 Rate of exacerbations requiring admission | 2 | 936 | Rate Ratio (IV, Random, 95% CI) | 0.31 [0.13, 0.73] |
| 1.3.1 Eosinophilic | 2 | 936 | Rate Ratio (IV, Random, 95% CI) | 0.31 [0.13, 0.73] |
| 1.4 Rate of exacerbations requiring ED treatment or admission | 2 | 936 | Rate Ratio (IV, Random, 95% CI) | 0.36 [0.20, 0.66] |
| 1.4.1 Eosinophilic | 2 | 936 | Rate Ratio (IV, Random, 95% CI) | 0.36 [0.20, 0.66] |
| 1.5 Health‐related quality of life (ACQ) | 3 | 1231 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.50, ‐0.26] |
| 1.5.1 Eosinophilic | 3 | 1231 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.50, ‐0.26] |
| 1.6 Health‐related quality of life (SGRQ) | 3 | 1231 | Mean Difference (IV, Random, 95% CI) | ‐6.37 [‐8.76, ‐3.98] |
| 1.6.1 Eosinophilic | 3 | 1231 | Mean Difference (IV, Random, 95% CI) | ‐6.37 [‐8.76, ‐3.98] |
| 1.7 Pre‐bronchodilator FEV 1 (litres) | 3 | 1231 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.05, 0.14] |
| 1.7.1 Eosinophilic | 3 | 1231 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.05, 0.14] |
| 1.8 Serious adverse events | 3 | 1231 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.01] |
| 1.8.1 Eosinophilic | 3 | 1231 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.01] |
| 1.9 Adverse events leading to discontinuation | 3 | 1231 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.85] |
| 1.9.1 Eosinophilic | 3 | 1231 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.85] |
1.2. Analysis.

Comparison 1: Mepolizumab (SC) versus placebo, Outcome 2: At least one clinically significant exacerbation
Comparison 2. Mepolizumab (IV) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Rate of clinically significant exacerbations | 3 | 751 | Rate Ratio (IV, Random, 95% CI) | 0.53 [0.44, 0.64] |
| 2.1.1 Eosinophilic | 3 | 751 | Rate Ratio (IV, Random, 95% CI) | 0.53 [0.44, 0.64] |
| 2.2 Rate of exacerbations requiring emergency department treatment or admission | 2 | 690 | Rate Ratio (IV, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 2.2.1 Eosinophilic | 2 | 690 | Rate Ratio (IV, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 2.3 Rate of exacerbations requiring admission | 2 | 690 | Rate Ratio (IV, Random, 95% CI) | 0.61 [0.33, 1.13] |
| 2.3.1 Eosinophilic | 2 | 690 | Rate Ratio (IV, Random, 95% CI) | 0.61 [0.33, 1.13] |
| 2.4 People with one or more exacerbations | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4.1 Eosinophilic | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5 Health‐related quality of life (AQLQ) | 2 | 369 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.06, 0.47] |
| 2.5.1 Eosinophilic | 2 | 369 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.06, 0.47] |
| 2.6 Health‐related quality of life (ACQ) | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.32, 0.09] |
| 2.6.1 Eosinophilic | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.32, 0.09] |
| 2.7 Health‐related quality of life (SGRQ) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.7.1 Eosinophilic | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.8 Pre‐bronchodilator FEV 1 (litres) | 2 | 690 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.02, 0.15] |
| 2.8.1 Eosinophilic | 2 | 690 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.02, 0.15] |
| 2.9 Serious adverse events | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.94] |
| 2.9.1 Eosinophilic | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.94] |
| 2.10 Adverse events leading to discontinuation | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.92] |
| 2.10.1 Eosinophilic | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.92] |
| 2.11 Blood eosinophil level (cells/μL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.11.1 Eosinophilic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Reslizumab (IV) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Rate of exacerbations requiring systemic corticosteroids | 2 | 953 | Rate Ratio (IV, Fixed, 95% CI) | 0.43 [0.33, 0.55] |
| 3.1.1 Eosinophilic | 2 | 953 | Rate Ratio (IV, Fixed, 95% CI) | 0.43 [0.33, 0.55] |
| 3.2 Rate of exacerbations requiring emergency department treatment or admission | 2 | 953 | Rate Ratio (IV, Fixed, 95% CI) | 0.67 [0.39, 1.17] |
| 3.2.1 Eosinophilic | 2 | 953 | Rate Ratio (IV, Fixed, 95% CI) | 0.67 [0.39, 1.17] |
| 3.3 Health‐related quality of life (AQLQ) | 3 | 1164 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.17, 0.39] |
| 3.3.1 Eosinophilic | 3 | 1164 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.17, 0.39] |
| 3.4 Health‐related quality of life (ACQ) | 4 | 1652 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| 3.4.1 Eosinophilic | 4 | 1260 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.36, ‐0.19] |
| 3.4.2 Non‐eosinophilic | 1 | 392 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.33, 0.09] |
| 3.5 Pre‐bronchodilator FEV 1 (litres) | 4 | 1652 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.15] |
| 3.5.1 Eosinophilic | 4 | 1260 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.08, 0.16] |
| 3.5.2 Non‐eosinophilic | 1 | 392 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.07, 0.14] |
| 3.6 Serious adverse events | 4 | 1656 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.12] |
| 3.6.1 Eosinophilic | 3 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.22] |
| 3.6.2 Eosinophil status unknown | 1 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.34, 2.88] |
| 3.7 Adverse events leading to discontinuation | 4 | 1659 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 1.02] |
| 3.7.1 Eosinophilic | 3 | 1163 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.37, 1.20] |
| 3.7.2 Eosinophil status unknown | 1 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.23] |
| 3.8 Blood eosinophil level (cells/μL) | 4 | 1656 | Mean Difference (IV, Fixed, 95% CI) | ‐476.83 [‐499.32, ‐454.34] |
| 3.8.1 Eosinophilic | 4 | 1656 | Mean Difference (IV, Fixed, 95% CI) | ‐476.83 [‐499.32, ‐454.34] |
Comparison 4. Reslizumab (SC) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Rate of exacerbations requiring systemic corticosteroids | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 Eosinophilic | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Rate of exacerbations requiring emergency department treatment or admission | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.1 Eosinophilic | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3 Health‐related quality of life (AQLQ mean difference) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3.1 Eosinophilic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4 Health‐related quality of life (ACQ mean difference) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4.1 Eosinophilic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.5 Health‐related quality of life (SGRQ mean difference) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.5.1 Eosinophilic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.6 Pre‐bronchodilator FEV 1 (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.6.1 Eosinophilic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.7.1 Eosinophilic | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.8 Adverse events leading to discontinuation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.8.1 Eosinophilic | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.9 Blood eosinophil level (cells × 10⁹/litre) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9.1 Eosinophilic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Benralizumab (SC) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Rate of exacerbations requiring systemic corticosteroids | 4 | 3112 | Rate Ratio (IV, Fixed, 95% CI) | 0.59 [0.52, 0.66] |
| 5.1.1 Eosinophilic | 4 | 2354 | Rate Ratio (IV, Fixed, 95% CI) | 0.55 [0.48, 0.63] |
| 5.1.2 Non‐eosinophilic | 2 | 758 | Rate Ratio (IV, Fixed, 95% CI) | 0.69 [0.56, 0.85] |
| 5.2 Rate of exacerbations requiring emergency department treatment or admission | 2 | 1537 | Rate Ratio (IV, Fixed, 95% CI) | 0.68 [0.47, 0.98] |
| 5.2.1 Eosinophilic | 2 | 1537 | Rate Ratio (IV, Fixed, 95% CI) | 0.68 [0.47, 0.98] |
| 5.3 Health‐related quality of life (AQLQ mean difference) | 3 | 1541 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 5.3.1 Eosinophilic | 3 | 1541 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 5.4 Health‐related quality of life (ACQ mean difference) | 4 | 2791 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.34, ‐0.17] |
| 5.4.1 Eosinophilic | 4 | 2036 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.40, ‐0.20] |
| 5.4.2 Non‐eosinophilic | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.30, 0.02] |
| 5.5 Health‐related quality of life (SGRQ mean difference) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.5.1 Eosinophilic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6 Pre‐bronchodilator FEV 1 (litres) | 4 | 2786 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.08, 0.15] |
| 5.6.1 Eosinophilic | 4 | 2048 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.11, 0.19] |
| 5.6.2 Non‐eosinophilic | 2 | 738 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.03, 0.10] |
| 5.7 Serious adverse events | 5 | 3304 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.62, 0.93] |
| 5.7.1 Eosinophilic | 3 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.56, 0.93] |
| 5.7.2 Non‐eosinophilic | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.57, 1.27] |
| 5.7.3 Eosinophil status unknown | 2 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.37, 1.51] |
| 5.8 Adverse events leading to discontinuation | 4 | 3253 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.03, 4.03] |
| 5.8.1 Eosinophilic | 3 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.89, 5.73] |
| 5.8.2 Non‐eosinophilic | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.54, 6.05] |
| 5.8.3 Eosinophil status unknown | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.31, 10.69] |
| 5.9 Blood eosinophil level (% change from baseline) | 2 | 2295 | Mean Difference (IV, Fixed, 95% CI) | ‐104.74 [‐116.12, ‐93.35] |
| 5.9.1 Eosinophilic | 2 | 1537 | Mean Difference (IV, Fixed, 95% CI) | ‐101.74 [‐113.27, ‐90.21] |
| 5.9.2 Non‐eosinophilic | 2 | 758 | Mean Difference (IV, Fixed, 95% CI) | ‐216.81 [‐287.35, ‐146.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bernstein 2020.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 468 participants with unstable asthma
|
|
| Interventions | Reslizumab will be administered SC in a dose of 110 mg every 4 weeks vs placebo | |
| Outcomes | The primary objective of this study is to determine the effect of reslizumab (110 mg) administered SC every 4 weeks on clinical asthma exacerbations in adults and adolescents with asthma and elevated blood eosinophils who are inadequately controlled on standard care asthma therapy Primary outcomes
Secondary outcomes
|
|
| Notes | Estimated study completion date: January 2018 Responsible party: Teva Branded Pharmaceutical Products, R&D Inc. International multicentre study with 201 centres. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as: "Participants were assigned using an interactive web response system (IWRS)." |
| Allocation concealment (selection bias) | Low risk | Reported as: "Patients and investigators were masked to treatment assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as: "Patients and investigators were masked to treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information on outcome assessor blindness |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was < 10% in both groups. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported, with additional outcomes presented in supplement |
Bjermer 2016.
| Study characteristics | ||
| Methods | Parallel, double‐blind RCT with a 16‐week treatment phase | |
| Participants | 315 participants (42 male) with moderate‐severe asthma, with airway reversibility, blood eosinophilia, ACQ score of at least 1.5, and taking ICS
|
|
| Interventions | IV infusion of reslizumab 0.3 mg/kg, reslizumab 3.0 mg/kg, or placebo once every 4 weeks (total of 4 doses) | |
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | 68 locations across 13 countries Funded by Teva Branded Pharmaceutical Products R&D, Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Allocation concealment (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated, no clarification available from study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly more withdrawals in placebo group (20/105, 19%) than treatment arms (12‐17%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Bleecker 2016.
| Study characteristics | ||
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study run over 48 weeks | |
| Participants | 1204 participants with symptomatic asthma were randomised to 1 of 3 groups (benralizumab 30 mg 4 weeks, benralizumab 30 mg 8 weeks, or placebo)
|
|
| Interventions | Benralizumab SC 30 mg/mL every 4 weeks or every 8 weeks vs placebo | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Multi‐centre study in 374 centres from 17 countries Funded by AstraZeneca and Kyowa Hakko Kirin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was assigned a unique enrolment number and randomisation code by an interactive web‐based voice response system |
| Allocation concealment (selection bias) | Low risk | The identity of the treatment allocation was not made available to the participants, investigators involved in participant treatment or clinical assessment, or study funder |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (participant, caregiver and investigator) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated, no clarification available from study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rates were relatively low (10.1%‐12.8%) |
| Selective reporting (reporting bias) | Low risk | Unless otherwise specified, all results were presented for participants with baseline blood eosinophilia |
Castro 2014a.
| Study characteristics | ||
| Methods | Double‐blind, dose‐ranging RCT | |
| Participants | 606 participants with uncontrolled asthma randomised and 535 completed
|
|
| Interventions | 6 arms: benralizumab 2 mg or benralizumab 20 mg or benralizumab 100 mg or placebo delivered by 2 SC injections every 4 weeks for the first 3 doses (weeks 1, 4, and 8), then every 8 weeks (weeks 16, 24, 32, and 40) | |
| Outcomes | Primary outcomes
Secondary outcomes in eosinophilic individuals
|
|
| Notes | 52‐week multi‐national study with sites in 10 countries. The study protocol was developed by MedImmune and the corresponding author. The investigators collected and had full access to all study data, which were analysed by the funding source. The analysis was done solely by MedImmune; however, study authors helped determine which analyses were done and could request further ad‐hoc analyses. The report was written by the study authors with a medical writer funded by the funding source. The corresponding author had final responsibility for decision to submit for publication. Funding: MedImmune |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive web/voice‐response system for random assignment |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by the vendor systems and no study personnel or site had access to the system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, treating physicians, study investigators, and study statisticians were masked to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were even across groups. |
| Selective reporting (reporting bias) | Low risk | Results for most but not all listed primary and secondary outcomes were reported (e.g. symptoms score, AQLQ – shown in supplementary material in graphs only) |
Castro 2015a.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 489 participants with moderate‐severe asthma (medium dose of ICS, inadequate control ACQ ≥ 1.5, and at least 1 exacerbation in the past 12 months)
|
|
| Interventions | IV infusion of reslizumab 3 mg/kg or matching placebo every 4 weeks (13 doses with last dose in week 48) | |
| Outcomes | Primary outcomes (per protocol)
Secondary outcomes (per protocol)
|
|
| Notes | 128 clinical research centres. The research was funded by Teva Branded Pharmaceutical Products R&D. Teva employees were involved in the study design, data collection and analysis, and in the writing of this manuscript. All study authors had full access to all study data and had final responsibility for the decision to submit for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with use of interactive response technology with computerised central randomisation. |
| Allocation concealment (selection bias) | Low risk | The funder’s clinical personnel involved in the study were also masked to the study drug identity until the database was locked for analysis and the treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators remained masked to treatment assignment during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and investigators remained masked to treatment assignment during the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low and even across the groups (11%‐14%) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome measures were reported. |
Castro 2015b.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 464 participants with moderate‐severe asthma (medium dose of ICS, inadequate control ACQ ≥ 1.5 and at least 1 exacerbation in the past 12 months)
|
|
| Interventions | IV infusion of reslizumab 3 mg/kg or matching placebo every 4 weeks (13 doses with last dose in week 48) | |
| Outcomes | Primary outcomes (per protocol)
Secondary outcomes (per protocol)
|
|
| Notes | Funding: Teva Branded Pharmaceutical Products R&D. Teva employees were involved in the study design, data collection and analysis, and in the writing of this manuscript. All study authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with use of interactive response technology with computerised central randomisation. |
| Allocation concealment (selection bias) | Low risk | The funder’s clinical personnel involved in the study were also masked to the study drug identity until the database was locked for analysis and the treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low and even across the groups (11%‐14%) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome measures were reported |
Chupp 2017.
| Study characteristics | ||
| Methods | Multicentre, placebo‐controlled, double‐blind, parallel‐group study | |
| Participants | 551 participants with severe eosinophilic asthma Male (%): mepolizumab 125 (46); placebo, 101 (36)
|
|
| Interventions | Mepolizumab 100 mg SC every 4 weeks for a period of 24 weeks (total of 6 doses) along with their respective standard care treatment, versus placebo (0.9% sodium chloride) SC every 4 weeks for a period of 24 weeks (total of 6 doses) along with their respective standard care treatment | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using an interactive voice‐response system and a centralised, computer‐generated, permuted‐block design of block size six |
| Allocation concealment (selection bias) | Low risk | Participants, investigators, other site staff, and the entire study team including those assessing outcomes data were masked to treatment assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the treatment arm 5 participants were withdrawn from the study: 2 withdrew consent, 2 experienced an adverse event and 1 was lost to follow‐up. In the placebo arm 14 participants were withdrawn from study: 6 withdrew consent, 2 experienced an adverse event, 2 withdrew due to poor efficacy, 2 were lost to follow‐up and 2 were withdrawn on a physician's decision. |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
Corren 2016.
| Study characteristics | ||
| Methods | Parallel, double‐blind | |
| Participants | 496 participants with moderate‐severe asthma (based on at least medium‐dose ICS, inadequate control ACQ ≥ 1.5)
|
|
| Interventions | Reslizumab IV 3.0 mg/kg or placebo once every 4 weeks (total of 4 doses) | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | 66 study locations across the USA Funding: Teva Branded Pharmaceutical Products R&D, Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Allocation concealment (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts comparable in each group (16/98, 16%, placebo vs 58/398, 15%, reslizumab) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported with numbers, except blood eosinophil counts only shown as a chart |
FitzGerald 2016.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants | 1306 participants with moderate‐severe (medium‐high‐dose ICS + LABA, ≥ 2 asthma exacerbations last 12 months, FEV1 < 80% predicted), ACQ‐6 ≥ 1.5 at enrolment
|
|
| Interventions | 56 weeks (final follow‐up at 60 weeks). Benralizumab SC 30 mg every 4 weeks for 56 weeks or every 4 weeks for 3 doses then 8 weeks thereafter for 56 weeks | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: AstraZeneca and Kyowa Hakko Kirin. 303 clinical research centres in 11 countries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment groups using an interactive web‐based voice‐response system. Randomisation was stratified by ICS dosage at enrolment (high or medium), geographic region, age group (adult or adolescent), and peripheral blood eosinophil count at enrolment (< 300 cells/μL or ≥ 300 cells/μL) |
| Allocation concealment (selection bias) | Low risk | The study investigator assigned randomisation codes sequentially in each stratum as participants became eligible for randomisation, until each stratum was full |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To preserve blinding, participants and study centre staff were masked to treatment allocation, placebo solution was visually matched with benralizumab solution, and both placebo and benralizumab were provided in accessorised (needle guards and finger phalanges), prefilled syringes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low: placebo 11.1% (49/440); benralizumab 30 mg every 4 weeks 9.6% (41/425); benralizumab 30 mg every eight weeks 13.4% (59/441) |
| Selective reporting (reporting bias) | Low risk | Results for all listed primary and secondary outcomes were reported. |
Haldar 2009.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 61 participants had refractory eosinophilic asthma and a history of recurrent severe exacerbations.
|
|
| Interventions | Mepolizumab IV (750 mg) vs matched placebo (150 mL of 0.9% saline) at monthly intervals for 1 year | |
| Outcomes | Reported as: "[P]rimary outcome measure was the number of severe exacerbations/participant during the 50‐week treatment phase. Secondary outcomes included a change in asthma symptoms, scores on the Asthma Quality of Life Questionnaire (AQLQ, in which scores range from 1 to 7, with lower values indicating more severe impairment and a change of 0.5 unit considered to be clinically important), forced expiratory volume in 1 second (FEV1) after use of a bronchodilator, airway hyperresponsiveness, and eosinophil counts in the blood and sputum." | |
| Notes | Single‐centre study conducted at Institute for Lung Health, Leicester, UK Supported by GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as: "Stratified randomisation with use of the minimisation method, which was performed by an independent clinician. Participants were randomly assigned with the use of the minimisation method to receive 12 infusions of either 750 mg of mepolizumab delivered intravenously or matched placebo (150 mL of 0.9% saline) at monthly intervals between visits 3 and 14. The criteria used for minimisation were the frequency of exacerbations in the previous 12 months, the baseline eosinophil count in the sputum and the number of participants taking oral corticosteroids." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported as: "A total of 61 of the 63 participants ( one required and operation and one withdrew consent) who were screened started treatment and constituted the modified intention‐to‐treat population. Thirty‐two participants were randomly assigned to receive placebo. Overall, 94.9% of treatment visits were completed. Participants who withdrew completed a mean of 4.6 treatment visits (38.3%)." |
| Selective reporting (reporting bias) | Low risk | No apparent indication of reporting bias |
Harrison 2020.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled, phase 3b study | |
| Participants | 656 participants aged 18‐75 with severe eosinophilic asthma
|
|
| Interventions | Benralizumab SC every 8 weeks at 30 mg (1st 3 doses given 4 weeks apart) for 24 weeks vs matched placebo. Patients were randomised 2:1 intervention to placebo | |
| Outcomes | Primary
Secondary
|
|
| Notes | Study conducted from July 2017‐September 2019 at 221 research centres across Europe and North America. Supported by AstraZeneca. NCT03170271 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a web‐based system. |
| Allocation concealment (selection bias) | Low risk | Treatment group assignment as well as kit allocation was performed by a web‐based system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported as: "...eosinophil, basophil, and monocyte counts were removed from any central laboratory reports sent to investigative sites to prevent unintentional unblinding of treatment allocation post‐dose.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92.3% of participants completed intervention, and 95.2% of participants completed placebo |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
Jackson 2022.
| Study characteristics | ||
| Methods | Randomised, parallel‐group, placebo‐controlled study | |
| Participants | 290 total participants (age 6‐17 years) Main inclusion criteria:
|
|
| Interventions | Mepolizumab administered SC every 4 weeks, 40 mg for 6‐11 year‐olds and 100 mg for 12‐17 year‐olds for 52 weeks | |
| Outcomes | Primary
Secondary
|
|
| Notes | Conference abstract. NCT03292588 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation is random |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ClinicalTrials.gov reports triple masking (participant, care provider, investigator) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ClinicalTrials.gov reports triple masking (participant, care provider, investigator) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not provided |
| Selective reporting (reporting bias) | Unclear risk | Challenging to assess reporting bias from a short abstract |
Moore 2022.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 195 adults with severe eosinophilic asthma who had previous long‐term treatment with mepolizumab
|
|
| Interventions | Mepolizumab SC 100 mg every 4 weeks for 52 weeks vs placebo (but can switch to open‐label mepolizumab after a severe exacerbation). Placebo is discontinuation from previous mepolizumab treatment | |
| Outcomes | Primary
Secondary
|
|
| Notes | Study conducted from January 2016‐July 2019 at 75 centres in 14 countries Supported by GlaxoSmithKline. NCT02555371 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an interactive response technology system. |
| Allocation concealment (selection bias) | Low risk | Reported as: "[Treatement] will be administered by a designated blinded member of the site staff. Once prepared, mepolizumab and placebo will be identical in appearance." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as: "The blinding of all those involved in the evaluation of the study treatment (e.g. physician/nurse as well as the subject) shall be maintained at all times." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as: "The blinding of all those involved in the evaluation of the study treatment (e.g. physician/nurse as well as the subject) shall be maintained at all times." Biomarker results will be masked to outcome assessors to protect unblinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 41% of the placebo group and 67% of the intervention group finished the randomised study. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
NCT01947946.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled, phase 3 efficacy and safety study | |
| Participants | 13 participants with uncontrolled asthma taking medium‐dose ICS plus LABA
|
|
| Interventions | Fixed 30 mg dose of benralizumab every 4 weeks or fixed 30 mg dose of benralizumab, every 4 weeks for the first 3 doses and then every 8 weeks thereafter versus placebo | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Study terminated due to sponsor decision after recruitment of 13 participants. No participant completed the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as double‐blind, but blinding of outcome assessment not specifically described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study terminated due to decision of sponsor after recruitment of 13 participants. No reason given for decision to terminate |
| Selective reporting (reporting bias) | High risk | Study terminated due to decision of sponsor after recruitment of 13 participants. No reason given for decision to terminate. Original secondary outcomes listed removed from study registration. Outcomes could not be incorporated into meta‐analysis |
Ortega 2014.
| Study characteristics | ||
| Methods | Randomised, double‐blind, double‐dummy, phase 3 study | |
| Participants | 576 participants with recurrent asthma exacerbations and evidence of eosinophilic inflammation despite high doses of inhaled glucocorticoids to 1 of 3 study groups
|
|
| Interventions | Mepolizumab in a 75 mg IV dose vs mepolizumab in a 100 mg SC dose vs placebo every 4 weeks for 32 weeks | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | 32‐week treatment intervention, with 1‐6 week run‐in and 8‐week follow‐up. Conducted in Baltimore, Middlesex, Ghent, Vancouver, Parma, Marseille and Paris Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated permuted block schedule |
| Allocation concealment (selection bias) | Low risk | Treatment allocations will be concealed via the RandAll system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Mepolizumab and placebo were identical in appearance and were administered by a staff member who was unaware of the study group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study drugs were prepared by staff members who were aware of the study group assignments but were not involved in study assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% (placebo), 8% (IV), 5% (SC) did not complete the study |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
Park 2016.
| Study characteristics | ||
| Methods | Parallel | |
| Participants | 103: 38 male (age 53.2, 55.6, 51.4, 50.8) Moderate/severe (based on ICS dose (medium/high), exacerbation history, and ACQ ≥ 1.5 on at least 2 occasions) participants also had to demonstrate post‐bronchodilator FEV1 reversibility ≥ 12% and ≥ 200 mL, or a positive response to methacholine challenge (PC20 ≤ 8 mg/mL)
|
|
| Interventions | SC doses given at weeks 1, 4, 8, 16, 24, 32, 40. Benralizumab 2 mg, 20 mg or 100 mg SC | |
| Outcomes | Primary outcomes
Secondary outcomes
Exploratory endpoints included blood eosinophil counts. |
|
| Notes | 32 sites in South Korea and Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eosinophilic participants were randomised using a central, interactive web‐response system |
| Allocation concealment (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study medication was administered … in a blinded fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated, no clarification available from study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates relatively high but even across groups (19.2% for placebo vs 16.0%‐23.1% for treatment groups) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Pavord 2012a.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, placebo‐controlled study | |
| Participants | 621 participants with severe asthma despite receiving high doses of standard asthma medications
|
|
| Interventions | 13 total IV infusions of mepolizumab (750 mg), mepolizumab (250 mg), mepolizumab (75 mg) or placebo given every 4 weeks | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | 52‐week study conducted at 81 centres in 13 countries (Argentina, Australia, Canada, Chile, France, Germany, South Korea, Poland, Romania, Russia, Ukraine, the UK and the USA) Supported by GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone‐based system and computer‐generated, randomly permuted block schedule stratified by whether treatment with OCS was required |
| Allocation concealment (selection bias) | Low risk | Mepolizumab and placebo were prepared by unmasked site staff who were not involved in study assessments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Mepolizumab and placebo were prepared by unmasked site staff who were not involved in study assessments. Both treatments were identical in appearance and were given to participants by a masked member of the site staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data analysts were masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for with information on reasons for having withdrawn. Some participants not included in results due to ‘poor efficacy’ |
| Selective reporting (reporting bias) | Low risk | No apparent indication of reporting bias |
ACQ: Asthma Control Questionnaire; ALT: alanine aminotransferase; Alk Phos: alkaline phosphatase; AQLQ: Asthma Quality of Life Questionnaire; AST: aspartate aminotransferase; ASUI: Asthma Symptom Utility Index; ECP: eosinophil cationic protein; ED: emergency department; EQ‐5D‐5L: Euroquol 5‐dimension, 5‐level healthcare instrument; FEF: forced expiratory flow; FeNO: exhaled fraction of nitric oxide; FEV1: Forced expiratory volume in 1 second; FP: fluticasone propionate; FVC: forced vital capacity; HRQoL: health‐related quality of life; ICS: inhaled corticosteroid; ICU: intensive care unit; IL: interleukin; IQR: interquartile range; IV: intravenous; LABA: long‐acting beta2‐agonist; OCS: oral corticosteroids; PC20: histamine provocative concentration causing a 20% drop in FEV1;PEFR: peak expiratory flow rate; RCT: randomised controlled trial; SC: subcutaneous; SD: standard deviation; SGRQ: St. George's Respiratory Questionnaire; ULN: Upper Limit of Normal; VC: vital capacity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albers 2016 | Post‐hoc analysis of observational study |
| Albers 2020 | Data pooled from 2 studies, also post hoc analysis looking at participants grouped by prior omalizumab use, which is not a subgroup prespecified in our protocol |
| Alvarez‐Cuesta 1994 | Intervention used in study (cat extract immunotherapy) is not anti‐IL‐5 therapy |
| Armentia 1992 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Austin 2016 | Aggregation of 2 clinical trials |
| Austin 2016a | Not an RCT (analysis of the characteristics of patients that entered 2 trials of mepolizumab) |
| Ayres 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Bardford 2018 | Post hoc analysis of mepolizumab study (MUSCA, Chupp 2017), looking at participants grouped by prior omalizumab use, which is not a subgroup prespecified in our protocol. |
| Bel 2014 | Focus of trial is on steroid reduction and therefore does not meet our predefined inclusion criteria |
| Berger 2003 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Bernstein 2020a | Not asthma (wrong disease group) |
| Bjermer 2017 | Only pooled data (from > 1 RCT) presented |
| Blanken 2012 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Blanken 2013 | Intervention used in study (pavilizumab) is not anti‐IL‐5 therapy |
| Bleecker 2018a | Data pooled from 2 studies |
| Bleecker 2018b | Only pooled data (from > 1 RCT) presented |
| Boulet 1997 | Intervention used in study (anti‐IgE antibody e25) is not anti‐IL‐5 therapy |
| Bourdin 2018 | Post hoc analysis of reslizumab study looking at patients grouped by presence or absence of allergen‐specific IgE, which is not a subgroup prespecified in our protocol. |
| Bourdin 2020 | This is not an RCT (it is a type of meta‐analysis of other RCTs) |
| Bousquet 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Bousquet 2011 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Brightling 2014 | Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy |
| Brown 2007 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Brusselle 2016 | Aggregation of 2 clinical trials |
| Brusselle 2017 | Data pooled from 2 studies, also post hoc analysis looking at participants grouped by baseline step 4/5 therapy, which is not a subgroup prespecified in our protocol. |
| Bryant 1975a | Not a RCT |
| Bryant 1975b | Not a RCT |
| Buhl 2000a | Intervention used in study (rhumab‐25) is not anti‐IL‐5 therapy |
| Buhl 2000b | Intervention used in study (rhumab‐25) is not anti‐IL‐5 therapy |
| Buhl 2002 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Bush 1985 | Intervention used in study (soybean oil) is not anti‐IL‐5 therapy |
| Busse 2001 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Busse 2008 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Busse 2015 | Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy |
| Buttner 2003 | Treatment < 16 weeks |
| Caffarelli 2000 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Canvin 2016 | Aggregation of 2 clinical trials |
| Carr 2017 | Data pooled from 2 studies |
| Carr 2019 | Only pooled data (from > 1 RCT) presented |
| Castro 2011 | < 16 weeks in length |
| Castro 2014 | Intervention used in study (dupilumab) is not anti‐IL‐5 therapy |
| Castro 2018 | Data pooled from 2 studies, also post hoc analysis looking at participants grouped by whether screening and baseline eosinophils > 400 or not, which is not a subgroup prespecified in our protocol |
| Chandra 1989 | Intervention used in study (various foods) is not anti‐IL‐5 therapy |
| Chanez 2017 | Only pooled data (from > 1 RCT) presented |
| Chanez 2017a | Only pooled data (from > 1 RCT) presented |
| Chanez 2018 | Open‐label extension not an RCT |
| Chanez 2019 | Post hoc analysis of reslizumab study comparing participants who didn’t have a blood eosinophil response to reslizumab to those who did, which is not a subgroup prespecified in our analysis |
| Chauhan 2017 | Only pooled data (from > 1 RCT) presented |
| Chervinsky 2003 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Chipps 2017 | Pooled data only (from SIROCCO (Bleecker 2016) and CALIMA FitzGerald 2016) studies; data are already in the analysis) |
| Chipps 2018 | Only pooled data (from > 1 RCT) presented |
| Chipps 2019 | Only pooled data (from > 1 RCT) presented |
| Chipps 2020 | Only pooled data (from > 1 RCT) presented |
| Clavel 1998 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Corren 2003 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Corren 2010 | Intervention used in study (il‐4r alpha antagonist) is not anti‐IL‐5 therapy |
| Cullell‐Young 2002 | Not a RCT |
| Dasgupta 2016 | Participants did not have a diagnosis of asthma (COPD patients) |
| De Boever 2014 | Intervention used in study (anti‐IL‐13 mab) is not anti‐IL‐5 therapy |
| Djukanovic 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| DuBuske 2018 | Data pooled from 2 studies |
| Ebner 1989 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Eckman 2010 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| El‐Nawawy 2000 | Not a RCT |
| EUCTR2012‐004385‐17‐BE | The study participants did not have asthma |
| EUCTR2015‐001152‐29‐BE | Not an RCT and endpoints are not applicable as this is a long‐term access programme |
| EUCTR2016‐001831‐10‐NL | No placebo arm/single treatment arm and treatment duration < 16 weeks |
| EUCTR2016‐002405‐19‐DE | Participants do not have a diagnosis of asthma, no placebo arm, treatment duration < 16 weeks |
| EUCTR2017‐003958‐16‐NL | Treatment duration < 16 weeks (12 weeks) |
| Fahy 1997 | Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy |
| Fahy 1999 | Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy |
| Ferguson 2016a | Benralizumab for mild to moderate, persistent asthma: the BISE phase III study ‐ duration < 16 weeks (12 weeks) |
| Ferguson 2016b | Treatment duration < 16 weeks in length |
| Ferguson 2017 | Study duration < 16 weeks (12 weeks) |
| Finn 2003 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| FitzGerald 2017a | Post hoc analysis of CALIMA (FitzGerald 2016), benralizumab study looking at the subset of patients from Japan, which is not a subgroup prespecified in our protocol. |
| FitzGerald 2017b | Only pooled data (from > 1 RCT) presented |
| FitzGerald 2018 | Only pooled data (from > 1 RCT) presented |
| Fitzgerald 2019 | 2‐year safety study including 1 year in RCT (3 studies pooled) and 1 year open‐label extension |
| Flood‐Page 2003 | Treatment < 16 weeks |
| Flood‐Page 2007 | Treatment < 16 weeks |
| Frew 1998 | Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy |
| Garcia 2013 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Garin 2019 | Only pooled data (from > 1 RCT) presented |
| Gauvreau 2011 | Intervention used in study ( anti‐IL‐13) is not anti‐IL‐5 therapy |
| Gauvreau 2014a | Intervention used in study (quilizumab) is not anti‐IL‐5 therapy |
| Gauvreau 2014b | Intervention used in study (anti‐tslp) is not anti‐IL‐5 therapy |
| Gauvreau 2014c | Intervention used in study (OX40L antagonist) is not anti‐IL‐5 therapy |
| Gauvreau 2015a | Intervention used in study (ligelizumab) is not anti‐IL‐5 therapy |
| Gauvreau 2015b | Intervention used in study (ligelizumab) is not anti‐IL‐5 therapy |
| Gevaert 2013 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Gibson 2021 | Wrong study design |
| Goldman 2017 | Only pooled data (from > 1 RCT) presented |
| Gopalan 2017a | Data pooled from 2 studies |
| Gopalan 2017b | Data pooled from 2 studies |
| Gordon 1972 | Intervention used in study is not anti‐IL‐5 therapy |
| Greenberg 1991 | Participants do not have a diagnosis of asthma |
| Gunsoy 2016 | Not a randomised, placebo‐controlled trial |
| Han 2009 | Intervention used in study (jade screen powder) is not anti‐IL‐5 therapy |
| Hanania 2011 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Hanania 2013 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Hanania 2014 | Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy |
| Hanania 2015 | Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy |
| Harris 2016 | Intervention used in study (quilizumab) is not anti‐IL‐5 therapy |
| Hendeles 2015 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Hickey 2019 | Only pooled data (from > 1 RCT) presented |
| Hill 1982 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Hodsman 2013 | Intervention used in study ( anti‐IL‐13) is not anti‐IL‐5 therapy |
| Holgate 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Hoshino 2012 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Howarth 2020 | Study focused on nasal polyps rather than asthma |
| Humbert 2005 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Humbert 2008 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Humbert 2009 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Humbert 2017 | Post hoc analysis looking at participants in a reslizumab trial (BREATH) grouped by omalizumab eligibility, which is not a subgroup prespecified in our protocol |
| Humbert 2019 | Data pooled from 2 mepolizumab studies (MENSA, MUSCA), post hoc analysis looking at participants grouped by omalizumab eligibility, which is not a subgroup prespecified in our protocol |
| Iino 2019 | Study in eosinophilic otitis media not asthma |
| Jackson 2019 | Pooled data only from trials that are already included (Bleecker 2016; FitzGerald 2016) |
| Jacobs 2018 | Pooled data only from trials that are already included (Castro 2015a; Castro 2015b) |
| Jacquemin 1995 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Jimenez 2019 | Aggregation of 5 studies |
| Jutel 2005 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Kang 1988 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Katial 2019 | Subgroup analysis by subgroups not relevant to our protocol |
| Katial 2020 | Post hoc analysis of benralizumab study looking at patients grouped by high or low FeNO, which is not a subgroup prespecified in our protocol |
| Kim 2020 | Pooled data only: mepolizumab IV (Pavord 2012a), and mepolizumab SC (Ortega 2014) |
| Kips 2003 | Treatment < 16 weeks |
| Kon 2001 | Intervention used in study (anti‐cd4) is not anti‐IL‐5 therapy |
| Kopp 2009 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Kopp 2013 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Korenblat 2018 | Only pooled data (from > 1 RCT) presented |
| Kreindler 2019 | Pooled data only from trials that are already included (Bleecker 2016; FitzGerald 2016) |
| Kuang 2018 | Not asthma (hypereosinophilic syndrome) |
| Kuang 2019 | Not asthma (hypereosinophilic syndrome) |
| Kulus 2010 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Lam 2018 | Not a RCT (cost‐effectiveness analysis incorporating multiple inputs, including but not only data from 2 RCTs) |
| Lanier 2003 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Lanier 2009 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Laviolette 2013 | Treatment < 16 weeks |
| Leckie 2000 | Treatment < 16 weeks |
| Leynadier 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Li 2016 | Review article, not a RCT |
| Lizaso 2008 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Lugogo 2016 | Not a randomised, placebo‐controlled trial |
| Lugogo 2020 | The intervention includes a reduction in standard asthma management (OCS maintenance), and as per our protocol, we have excluded such studies as the heterogeneity in study design makes them too dissimilar to compare |
| Manning 2018 | Only pooled data (from > 1 RCT) presented |
| Maselli 2017 | Trial involves OCS dose reduction (1 of our exclusion criteria) |
| Maspero 2016 | Combined secondary analysis of 2 trials |
| Maspero 2018 | Pooled data from 2 trials (Bleecker 2016; FitzGerald 2016) |
| Maspero 2019 | Only pooled data (from > 1 RCT) presented |
| Massanari 2009 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Massanari 2010 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Mathur 2019 | Pooled data from 2 trials (Bleecker 2016; FitzGerald 2016) |
| Mathur 2020 | Only pooled data (from > 1 RCT) presented |
| Maunoury 2018 | Not a RCT |
| Menzies‐Gow 2019a | Trial involves OCS dose reduction (one of our exclusion criteria) |
| Menzies‐Gow 2019b | Trial involves OCS dose reduction (one of our exclusion criteria) |
| Metzger 1998 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Milgrom 1999 | Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy |
| Milgrom 2001 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Modlin 1977 | Participants do not have diagnosis of asthma |
| Moran 2020 | Not an RCT (observational study of patients with asthma and COPD) |
| Moss 1987 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Murphy 2018 | Only pooled data (from > 1 RCT) presented |
| Nair 2009 | Focus of trial is on steroid reduction and therefore does not meet our predefined inclusion criteria |
| Nair 2016 | All participants do not have a diagnosis of asthma |
| Nair 2017a | Trial involves OCS dose reduction (1 of our exclusion criteria) |
| Nair 2017b | Substudy of a trial involving OCS dose reduction (one of our exclusion criteria) |
| Nair 2017c | Trial involves OCS dose reduction (one of our exclusion criteria) |
| Nair 2020 | Only pooled data (from > 1 RCT) presented |
| NCT00783289 | Treatment duration < 16 weeks |
| NCT00802438 | Not RCT |
| NCT01290887 | Study does not include a placebo arm |
| NCT01366521 | Phase 2 study comparing three doses of mepolizumab. This trial does not have a placebo arm. |
| NCT01471327 | Focus of study was on tolerability, pharmacokinetics and pharmacodynamics of single dose SB‐240563 administered IV to Japanese healthy male participants. People with asthma were not included in the study. |
| NCT01520051 | Duration < 16 weeks, additional intervention (experimental rhinovirus infection) |
| NCT01691859 | This study does not include a placebo group. Multi‐centre, open‐label, long‐term safety study with total sample receiving 100 mg mepolizumab administered SC (no control group) |
| NCT01842607 | This study does not include a placebo group. Multi‐centre, open‐label, long‐term safety study with total sample receiving 100 mg mepolizumab administered SC (no control group) |
| NCT02020889 | The study participants did not have asthma |
| NCT02075255 | Focus of trial is on oral steroid reduction |
| NCT02135692 | This study does not include a placebo group. Multi‐centre, open‐label, long‐term study of SC administered mepolizumab 100 mg in addition to standard care, in participants with severe eosinophilic asthma |
| NCT02258542 | Not a RCT (an extension study with no placebo arm) |
| NCT02293265 | Aim of study is to provide a "reliable description of the severe asthma patient landscape with respect to the potential eligibility for treatment with mepolizumab, omalizumab, and reslizumab". No pharmaceutical intervention in study |
| NCT02377427 | Treatment period < 16 weeks |
| NCT02417961 | Not a RCT |
| NCT02501629 | Focus of trial is on oral steroid reduction |
| NCT02559791 | Not placebo‐controlled ‐ single treatment arm only |
| NCT02594332 | Study terminated (recruitment problems) |
| NCT02654145 | Not placebo‐controlled. Single treatment arm only |
| NCT02808819 | Not an RCT |
| NCT02814643 | Treatment duration < 16 weeks |
| NCT02821416 | Duration < 16 weeks, additional intervention (allergen challenge) |
| NCT02869438 | Treatment duration < 16 weeks |
| NCT02937168 | Treatment duration < 16 weeks |
| NCT02968914 | Not a placebo‐controlled trial |
| NCT03014674 | Not a placebo‐controlled trial and treatment duration < 16 weeks |
| NCT03021304 | No placebo arm/single treatment arm, treatment duration < 16 weeks |
| NCT03476109 | No placebo comparator |
| NCT04617171 | Treatment of participants with acute asthma exacerbation, not chronic asthma |
| NCT04710134 | Ineligble study design |
| Nelsen 2019 | Pooled data from 2 trials (Chupp 2017; Ortega 2014), and a 3rd observational study |
| Newbold 2016 | Not an RCT |
| Newbold 2017 | Pooled data from 2 trials (Bleecker 2016; FitzGerald 2016) |
| Niven 2008 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Noga 2003 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Noga 2008 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Noonan 2013 | Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy |
| Nowak 2015 | Treatment < 16 weeks |
| O'Quinn 2019 | Only pooled data (from > 1 RCT) presented |
| Oba 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Oh 2013 | Intervention used in study (anti‐IL‐9) is not anti‐IL‐5 therapy |
| Ohashi 1997 | Participants do not have a diagnosis of asthma |
| Ohman 1984 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Ohta 2009 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Ohta 2017 | Post hoc analysis of CALIMA (FitzGerald 2016), benralizumab study looking at the subset of patients from Japan, which is not a subgroup prespecified in our protocol |
| Ong 2005 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Ortega 2018a | Only pooled data (from > 1 RCT) presented |
| Ortega 2018b | Only pooled data (from > 1 RCT) presented |
| Panettieri 2017 | Post hoc analysis of reslizumab study looking at participants who had an FEV1 < 50%, which is not a subgroup prespecified in our analysis |
| Park 1998 | Not an RCT |
| Park 2018 | Post hoc analysis of SIROCCO (Bleecker 2016), benralizumab study looking at the subset of patients from Korea, which is not a subgroup prespecified in our protocol |
| Park 2019 | Post hoc analysis of SIROCCO (Bleecker 2016) benralizumab study looking at the subset of patients from Korea, which is not a subgroup prespecified in our protocol |
| Parker 2010 | Intervention used in study (anti‐IL‐9) is not anti‐IL‐5 therapy |
| Pauli 1984 | Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy |
| Pavord 2012 | Posthoc analysis of Pavord 2012a and Ortega 2014 stratified by prior use of anti‐IgE therapy |
| Pelaia 2016 | Study is not an RCT |
| Pham 2016 | An analysis of sera collected from asthma patients enrolled in 2 clinical studies |
| Piper 2012 | Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy |
| Piper 2013 | Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy |
| Pouliquen 2015 | Study has no placebo arm or clinical endpoints |
| Pouliquen 2016 | Aggregation of 2 clinical trials |
| Prazma 2016 | Study is not a randomised, placebo controlled trial |
| Prieto 2006 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Pui 2010 | Intervention used in study (air/diesel exhaust +/‐ antioxidant) is not anti‐IL‐5 therapy |
| Ranade 2015 | Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy |
| Rose 2009 | Intervention used in study (pneumococcal vaccine) is not anti‐IL‐5 therapy |
| Roskos 2018 | Pooled data from 2 trials (Bleecker 2016; FitzGerald 2016) |
| Roufosse 2020 | Not asthma (hypereosinophilic syndrome) |
| Sakamoto 1984 | Not an RCT |
| Scheerens 2011 | Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy |
| Scheerens 2012 | Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy |
| Scheerens 2014 | Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy |
| Shaw 2019 | Trial involves oral corticosteroid dose reduction (one of our exclusion criteria). |
| Shrimanker 2018 | Only pooled data (from > 1 RCT) presented |
| Shrimanker 2019 | Only pooled data (from > 1 RCT) presented |
| Siergiejko 2011 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Silk 1998 | Intervention used in study (pneumococcal vaccine) is not anti‐IL‐5 therapy |
| Silkoff 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Simoes 2007 | Intervention used in study (pavilizumab) is not anti‐IL‐5 therapy |
| Singh 2010 | Intervention used in study (anti‐IL‐13) is not anti‐IL‐5 therapy |
| Slavin 2009 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Soler 2001 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Sorkness 2013 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Steinfeld 2020 | Not asthma (hypereosinophilic syndrome) |
| Sthoeger 2007 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Sugaya 1994 | Intervention used in study (influenza vaccine) is not anti‐IL‐5 therapy |
| Swanson 2014 | Intervention used in study (dupilumab) is not anti‐IL‐5 therapy |
| Szymaniak 1998 | Not an RCT |
| Tanaka 1993 | Intervention used in study (influenza vaccine) is not anti‐IL‐5 therapy |
| Terr 1969 | Study predates monoclonal treatments |
| Vanlandingham 2019 | Only pooled data (from > 1 RCT) presented |
| Van Rensen 2009 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Vignola 2004 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Virchow 2016 | Aggregation of 2 clinical trials |
| Virchow 2017 | Only pooled data (from > 1 RCT) presented |
| Virchow 2019 | Only pooled data (from > 1 RCT) presented |
| Virchow 2020 | Only pooled data (from > 1 RCT) presented |
| Virchow 2020a | Only pooled data (from >1 RCT) presented |
| Wang 2015 | Pharmacometrics assessment of phase IIb data to characterise the exposure‐response relationship with benralizumab in adults with asthma |
| Wang 2017 | Only pooled data (from > 1 RCT) presented |
| Wark 2003 | Intervention used in study (itraconazole) is not anti‐IL‐5 therapy |
| Wechsler 2017 | Pooled data from 2 trials (Castro 2015a; Castro 2015b) |
| Wechsler 2017a | Only pooled data (from > 1 RCT) presented |
| Wechsler 2019 | Only pooled data (from > 1 RCT) presented |
| Wechsler 2020 | Post hoc analysis of reslizumab studies looking at participants who had ≥ 2 or ≥ 3 exacerbations, which is not a subgroup prespecified in our analysis |
| Weinstein 2016 | Combined secondary analysis of 2 trials |
| Wenzel 2009 | Intervention used in study (golimumab) is not anti‐IL‐5 therapy |
| Wenzel 2013 | Interventionused in study (dupilumab) is not anti‐IL‐5 therapy |
| Wenzel 2013a | Intervention used in study (dupilumab) is not anti‐IL‐5 therapy |
| Wenzel 2014 | Intervention used in study (dupilumab) is not anti‐IL‐5 therapy |
| Xu 2017 | Pooled data from 2 trials (Bleecker 2016; FitzGerald 2016) |
| Xu 2018 | Pooled data from 2 trials (Bleecker 2016; FitzGerald 2016) |
| Yan 2015 | Participants do not have a diagnosis of asthma |
| Yancey 2017 | Analysis of subgroup not relevant to our protocol (participants with ≥ 3 exacerbations and eosinophils ≥ 300/μL) |
| Yancey 2020 | Analysis of subgroup not relevant to our protocol |
| Zangrilli 2019 | Pooled data from 2 trials (Bleecker 2016; FitzGerald 2016) |
| Zetterstrom 1972 | Participants do not all have diagnosis of asthma |
| Zhu 2013 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
| Zielen 2013 | Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
COPD: chronic obstructive pulmonary disease; FeNO: exhaled fraction of nitric oxide; IV: intravenous; OCS: oral corticosteroid; RCT: randomised controlled trial; SC: subcutaneous
Differences between protocol and review
We initially planned to use a fixed‐effect model for meta‐analysis, but we agreed with a peer reviewer who suggested that a random‐effects model was more appropriate in view of the substantial clinical heterogeneity between the studies.
The scope was broadened to encompass all anti‐IL‐5 therapies, that is, including reslizumab and benralizumab in addition to mepolizumab. Since the protocol was written, reslizumab and benralizumab have also been licensed. These agents are all designed for the same patients and are therefore comparable.
Data from study arms on doses not deemed clinically relevant (e.g. 10 times more or less than the dose that has marketing approval) were excluded. Similarly, studies where an additional intervention was the withdrawal of systemic corticosteroid were also excluded.
Outcomes were revised to focus on validated symptom scores (i.e. excluding non‐validated scores, as these cannot be readily compared across studies) and only a pre‐bronchodilator measure of lung function (as per American Thoracic Society/European Respiratory Society guidelines on standardising endpoints for clinical asthma studies). Subgroups were set as eosinophilic or otherwise, as these agents are primarily designed for eosinophilic asthma.
The original protocol stated that included studies should be a minimum of 16 weeks in duration; we have clarified that there should be a minimum of 16 weeks' treatment.
Congenital heart disease had been listed as an exclusion criteria previously but this was removed as there was no reason why these conditions in particular should be excluded.
The number of studies identified was insufficient to conduct subgroup analyses or formally assess for reporting bias.
Contributions of authors
SM, HF and CP contributed to the rewriting of the Background and Methods sections. HF and CP independently selected studies for the review, HF, AW, EB and FY extracted the data, and EB entered the data into the Review Manager 2020 file with cross‐checking by Christopher Cates, the Cochrane Airways Group statistician. HF, SM, EB and AW wrote the Results section, and HF, CP and SM co‐authored the Discussion and Conclusions.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor) edited the review and advised on methodology.
Sally Spencer (Co‐ordinating Editor): signed off the review prior to copyediting.
Alexander Mathiousdakis (Contact Editor): edited the review; advised on methodology, interpretation and content, assisted with sign‐off of the review.
Emma Dennett (Deputy Co‐ordinating Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review. Emma also assisted the authors in screening a pre‐publication search update in February 2022.
Emma Jackson (Managing Editor): co‐ordinated peer review; edited the reference and other sections of the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Data were checked by Emma Banchoff who was invited to join the author team after making a significant contribution to the review.
Sources of support
Internal sources
-
All, Other
The authors declare that no such funding was received for this systematic review
External sources
-
Cochrane Airways, UK
This project was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group
Declarations of interest
HF: none known
AW: none known
CP: none known
FY: none known
SM: none known
EB: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bernstein 2020 {published data only}
- Bernstein JA, Virchow JC, Murphy K, Maspero JF, Jacobs J, Adir Y, et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid-dependent asthma: results from two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respiratory Medicine 2020;8:461–74. [DOI] [PubMed] [Google Scholar]
- NCT02452190. Study of reslizumab in patients with uncontrolled asthma and elevated blood eosinophils. clinicaltrials.gov/show/NCT02452190 (first received 13 May 2015).
- NCT02501629. An efficacy and safety study of reslizumab subcutaneous in patients with oral corticosteroid dependent asthma and elevated blood eosinophils. www.clinicaltrials.gov/ct2/show/NCT02501629 (first received 17 July 2015).
Bjermer 2016 {published data only}
- Bjermer L, Lemiere C, Maspero J, Ciesielska M, O'Brien C, Zangrilli J. A randomized phase 3 study of the efficacy and safety of reslizumab in subjects with asthma with elevated eosinophils. European Respiratory Journal 2014;44(Suppl 58):P299. [CENTRAL: 1053372] [EMBASE: 71849984] [Google Scholar]
- Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 2016;150(4):789-98. [CENTRAL: 1139859] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Maspero J, Bjermer L, Lemiere C, Ciesielska M, O'Brien C, Zangrilli J. A randomized phase 3 study assessing patient reported outcomes and safety of reslizumab in patients with asthma with elevated eosinophils. Annals of Allergy, Asthma and Immunology 2014;113(5 Suppl 1):A21. [CENTRAL: 1020022] [EMBASE: 71679175] [Google Scholar]
- NCT01270464. A study to evaluate the efficacy and safety of reslizumab (0.3 or 3.0 mg/kg) as treatment for patients (12-75 years of age) with eosinophilic asthma. clinicaltrials.gov/ct2/show/NCT01270464 (first received 29 December 2010).
Bleecker 2016 {published data only}
- Bleecker E, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Benralizumab provides significant improvements for patients with severe, uncontrolled asthma: SIROCCO Phase III results. European Respiratory Journal 2016;48:OA4832. [Google Scholar]
- Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta 2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016;388(10056):2115–27. [DOI] [PubMed] [Google Scholar]
- NCT01928771. Efficacy and safety study of benralizumab added to high-dose inhaled corticosteroid plus LABA in patients with uncontrolled asthma. clinicaltrials.gov/show/NCT01928771 (first received 16 August 2013).
Castro 2014a {published data only}
- Castro M, Gossage DL, Ward CK, Wu Y, Khatri DB, Molfino NA, et al. Benralizumab reduces exacerbations and improves lung function in adults with uncontrolled eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2014;189:B101. [CENTRAL: 1131488] [EMBASE: 72043211] [Google Scholar]
- Castro M, Wenzel S, Kolbeck R, Khatry D, Christine W, Wu Y, et al. A phase 2 study of benralizumab on exacerbations, lung function, and asthma control in adults with uncontrolled eosinophilic asthma. European Respiratory Journal 2014;44 Suppl 58:2909. [CENTRAL: 1053384] [Google Scholar]
- Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respiratory Medicine 2014;2(11):879-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Eck S, Castro M, Sinibaldi D, White W, Folliot K, Gossage D, et al. Benralizumab effect on blood basophil counts in adults with uncontrolled asthma. European Respiratory Journal 2014;44 Suppl 58:297. [CENTRAL: 1053403] [EMBASE: 71849982] [Google Scholar]
- NCT01238861. Study to evaluate the efficacy and safety of MEDI-563 in adults with uncontrolled asthma. clinicaltrials.gov/ct2/show/NCT01238861 (first received 9 November 2010).
- Wang B, Yan L, Hutmacher M, White WI, Ward CK, Nielsen J, et al. Exposure-response analysis for determination of benralizumab optimal dosing regimen in adults with asthma. American Journal of Respiratory and Critical Care Medicine 2014;189:A1324. [CENTRAL: 1035648] [EMBASE: 72043774] [Google Scholar]
Castro 2015a {published data only}
- Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulmonary Pharmacology and Therapeutics 2017;43:39-45. [DOI] [PubMed] [Google Scholar]
- Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(5):355-66. [DOI] [PubMed] [Google Scholar]
- Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(4):e15. [DOI: 10.1016/S2213-2600(15)00042-9] [EMBASE: 2015833476] [DOI] [PubMed] [Google Scholar]
- NCT01287039. A study to evaluate the efficacy and safety of reslizumab (3.0 mg/kg) in the reduction of clinical asthma exacerbations in patients (12-75 years of age) with eosinophilic asthma. clinicaltrials.gov/ct2/show/NCT01287039 (first received 28 January 2011).
Castro 2015b {published data only}
- Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(5):355-66. [DOI] [PubMed] [Google Scholar]
- Castro M, Zangrilli J, Wechsler ME. Corrections to Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(4):e15. [EMBASE: 2015833476] [DOI] [PubMed] [Google Scholar]
- NCT01285323. A study to evaluate the efficacy and safety of reslizumab in patients with eosinophilic asthma. clinicaltrials.gov/ct2/show/NCT01285323 (first received 25 January 2011).
Chupp 2017 {published data only}
- Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respiratory Medicine 2017;5(5):390-400. [DOI] [PubMed] [Google Scholar]
- NCT02281318. A randomised, double-blind, placebo-controlled, parallel-group, multi-centre 24-week study to evaluate the efficacy and safety of mepolizumab adjunctive therapy in subjects with severe eosinophilic asthma on markers of asthma control. clinicaltrials.gov/show/NCT02281318 (first received 30 October 2014).
Corren 2016 {published data only}
- Corren J, Weinstein S, Janka L, O'Brien C, Zangrilli J. A randomized phase 3 study of reslizumab efficacy in relation to blood eosinophil levels in patients with moderate to severe asthma. European Respiratory Journal 2014;44 Suppl 58:4673. [CENTRAL: 1053393] [EMBASE: 71849958] [Google Scholar]
- Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 2016;150(4):799-810. [CENTRAL: 1139856] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01508936. A 16-week, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of reslizumab (3.0 mg/kg) treatment in patients with moderate to severe asthma. clinicaltrials.gov/show/NCT01508936 (first received 3 January 2012).
FitzGerald 2016 {published data only}
- FitzGerald JM, Bleecker E, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab reduces exacerbations in severe, uncontrolled asthma: results of the phase III CALIMA trial [OA1969]. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016.
- FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388(10056):2128-41. [DOI] [PubMed] [Google Scholar]
- NCT01914757. A multicentre, randomized, double-blind, parallel group, placebo controlled, phase 3 study to evaluate the efficacy and safety of benralizumab in asthmatic adults and adolescents inadequately controlled on inhaled corticosteroid plus long-acting beta 2agonist (CALIMA). clinicaltrials.gov/show/NCT01914757 (first received 31 July 2013).
Haldar 2009 {published data only}
- EUCTR2005-001932-61-GB. Mepolizumab and exacerbation frequency in refractory eosinophilic asthma. A randomised, double blind, placebo controlled, parallel group trial. clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2005-001932-61-GB (first received 16 November 2005).
- Gupta S, Halder P, Hargadon B, Sousa A, Marshall RP, Wardlaw AJ, et al. Assessment of changes in airways dimensions with mepolizumab treatment in refractory eosinophilic asthma. In: American Thoracic Society International Conference; 2009 May 15-20 San Diego. 2009:A3641. 4900100000024535
- Haldar P, Brightling C, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab (anti-IL5) and exacerbation frequency in refractory eosinophilic asthma. American Journal of Critical Care and Respiratory Medicine 2009;179:A3638. 4900100000024597 [Google Scholar]
- Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. New England Journal of Medicine 2009;360(10):973-84. 4900100000023473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar P, Brightling CE, Singapuri A, Hargadon B, Gupta S, Monteiro W, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. Journal of Allergy and Clinical Immunology 2014;133(3):921-3. [EMBASE: 2014156329] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shrimanker R, Hargaden B, Bradding P, Wardlaw AJ, Brightling CE, Green R, et al. Characterisation of exacerbations of severe eosinophilic asthma (SEA) on mepolizumab compared to placebo. European Respiratory Journal 2017;50(61):PA3971. [Google Scholar]
- Shrimanker R, Keene O, Bratton DJ, Yancey S, Heaney L, Pavord ID. Characterisation of exacerbations of severe eosinophilic asthma on mepolizumab compared to placebo. American Journal of Respiratory and Critical Care Medicine 199;9:A2671. [Google Scholar]
Harrison 2020 {published data only}
- Canonica GW, Harrison TW, Chanez P, Menzella F, Louis R. Benralizumab efficacy for severe, eosinophilic asthma with a diagnosis of nasal polyposis: results from the phase IIIb ANDHI trial. European Journal of Allergy and Clinical Immunology 2020;75(109):114. [Google Scholar]
- Harrison TW, Chanez P, Menzella F, Canonica GW, Louis R, Cosio BG, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respiratory Medicine 2021;9(3):260-74. [DOI] [PubMed] [Google Scholar]
- Kreindler J, Chanez P, Bourdin A, Burden A, Gil EG. Comprehensive response to benralizumab by patients with nasal polyposis and severe, eosinophilic asthma. Annals of Allergy, Asthma and Immunology 2020;125(5):S29. [Google Scholar]
- NCT03170271. A study of the safety and effectiveness of benralizumab to treat patients with severe uncontrolled asthma (ANDHI) [A multicenter, randomized, double-blind, parallel group, placebo controlled, phase 3b study to evaluate the safety and efficacy of benralizumab 30 mg SC in patients with severe asthma uncontrolled on standard of care treatment]. www.clinicaltrials.gov/ct2/show/NCT03170271 (first received 31 May 2017).
Jackson 2022 {published data only}
- Altman M, Bacharier L, Villarreal M, Gill M, Liu A, Gruchalla R, et al. Distinct airway inflammatory pathways associated with asthma exacerbations are modulated by mepolizumab therapy in children. In: Journal of Allergy and Clinical Immunology. Vol. 149 Suppl 2. 2022:AB146.
- Jackson D, Bacharier L, Gergen P, Gagalis L, Villarreal M, Gill M, et al. Phenotype-directed therapy with mepolizumab for urban children with exacerbation-prone asthma. Journal of Allergy and Clinical Immunology 2022;149(2):AB146. [Google Scholar]
- NCT03292588. A trial of mepolizumab adjunctive therapy for the prevention of asthma exacerbations in urban children (MUPPITS-2). https://clinicaltrials.gov/ct2/show/NCT03292588 (first received 29 March 2022).
Moore 2022 {published data only}
- Bel EH, Moore WC, Kornmann O, Poirier C, Kaneko N, Smith SG, et al. Continued long-term mepolizumab in severe eosinophilic asthma protects from asthma worsening versus stopping mepolizumab: COMET trial. European Respiratory Journal 2020;56:5280. [Google Scholar]
- Liu M, Bel E, Kornmann O, Humbert M, Kaneko N, Martin N, et al. Clinician/patient perception: asthma severity decreases and response increases with continuing versus stopping long-term mepolizumab (COMET). Annals of Allergy, Asthma & Immunology 2020;125(5):S30. [Google Scholar]
- Liu M, Bel E, Kornmann O, Humbert M, Kaneko N, Martin N, et al. Clinician/patient perception: asthma severity decreases and response increases with continuing versus stopping long-term mepolizumab (COMET). Annals of Asthma, Allergy and Immunology 2020;201:A4211. [Google Scholar]
- Moore WC, Kornmann O, Humbert M, Poirier C, Bel EH, Kaneko N, et al. Outcomes following continuation or stopping long-term mepolizumab treatment in patients with severe eosinophilic asthma: the randomized COMET trial. ATS Journals 2020;201(1):Meeting Abstracts A421. [Google Scholar]
- Moore WC, Kornmann O, Humbert M, Poirier C, Bel EH, Kaneko N, et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). European Respiratory Journal 2022;59:2100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02555371. Cessation versus continuation of long-term mepolizumab in severe eosinophilic asthma patients. clinicaltrials.gov/show/NCT02555371 (first received 17 September 2015).
NCT01947946 {published data only}
- NCT01947946. Efficacy and safety study of benralizumab added to medium-dose inhaled corticosteroid plus LABA in patients with uncontrolled asthma. clinicaltrials.gov/show/NCT01947946 (first received 11 September 2013).
Ortega 2014 {published data only}
- Albers FC, Lugogo N, Gilson MJ, Price R, Yancey SW. Long-term safety and efficacy of mepolizumab in patients with severe eosinophilic asthma. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl 1):AB14. [CENTRAL: 1135293] [EMBASE: 72196801] [Google Scholar]
- Albers FC, Price R, Ortega H, Yancey SW, Nelsen LM. Effect of mepolizumab in severe eosinophilic asthma patients in relation to their baseline ACQ-5 and SGRQ scores. European Journal of Allergy and Clinical Immunology 2016;71:257-8. [Google Scholar]
- Albers FC, Price RG, Smith SG, Yancey SW. Mepolizumab efficacy in patients with severe eosinophilic asthma receiving different controller therapies. Journal of Allergy and Clinical Immunology 2017;140(5):1464-6. [DOI] [PubMed] [Google Scholar]
- Basu A, Dalal A, Canonica GW, Forshag M, Yancey SW, Nagar S, et al. Economic analysis of the phase III MENSA study evaluating mepolizumab for severe asthma with eosinophilic phenotype. Expert Review of Pharmacoeconomics and Outcomes Research 2017;17(2):121-31. [DOI] [PubMed] [Google Scholar]
- Forshag M, Dalal AA, Ortega H, Yancey S, Gunsoy NB, Canonica G. Healthcare resource use associated with exacerbations in patients with severe eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2015;191:A4174. [CENTRAL: 1107017] [EMBASE: 72052049] [Google Scholar]
- Jackson D, Price RG, Yancey SW, Buhl R, Wenzel S. Characterising individual response to mepolizumab treatment. European Respiratory Journal 2020;56:S64. [Google Scholar]
- Magnan AA, Bourdin A, Prazma CM, Albers FC, Price RG, Yancey SW, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. European Journal of Allergy and Clinical Immunology 2016;71(9):1335-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01691521. MEA115588 A randomised, double-blind, double-dummy, placebo-controlled, parallel-group, multi-centre study of the efficacy and safety of mepolizumab adjunctive therapy in subjects with severe uncontrolled refractory asthma. clinicaltrials.gov/ct2/show/NCT01691521 (first received 20 September 2012). 4900126000001339
- Ortega H, Liu M, Pavord I, Brusselle G, FitzGerald JM, Chetta A, et al. Reduction in exacerbations with mepolizumab in severe eosinophilic asthma: MENSA study. European Respiratory Journal 2014;44 Suppl 58:2906. [CENTRAL: 1053453] [Google Scholar]
- Ortega H, Mayer B, Yancey S, Katial R. Response to treatment with mepolizumab in elderly patients. American Journal of Respiratory and Critical Care Medicine 2015;191:A4177. [CENTRAL: 1107016] [EMBASE: 72052052] [Google Scholar]
- Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. New England Journal of Medicine 2014;371(13):1198-207. [EMBASE: 25199059] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respiratory Medicine 2016;4(7):549–56. [CENTRAL: 1161094] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shimoda T, Odajima H, Okamasa A, Kawase M, Komatsubara M, Mayer B, et al. Efficacy and safety of mepolizumab in Japanese patients with severe eosinophilic asthma. Allergology International 2017;66(3):445-51. [DOI] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Adachi M, Kim M-K, Imai N, Nakanishi T, Ohta K, Tohda Y, et al. A phase 2a study of benralizumab for patients with eosinophilic asthma in South Korea and Japan. American Journal of Respiratory and Critical Care Medicine 2015;191(Meeting Abstracts):A2495. [CENTRAL: 1101040] [EMBASE: 72050330] [Google Scholar]
- NCT01412736. A phase IIa study of KHK4563 (4563-003) [A phase IIa, double-blind, placebo-controlled dose-ranging study to evaluate the efficacy and safety of KHK4563 in adults with uncontrolled, suspected eosinophilic asthma]. clinicaltrials.gov/show/NCT01412736 (first received 7 August 2011).
- Park HS, Kim MK, Imai N, Nakanishi T, Adachi M, Ohta K, et al. A phase 2a study of benralizumab for patients with eosinophilic asthma in South Korea and Japan. International Archives of Allergy and Immunology 2016;169(3):135-45. [CENTRAL: 1152856] [EMBASE: 20160326586] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pavord 2012a {published data only}
- NCT01000506. Dose ranging efficacy and safety with mepolizumab in severe asthma (DREAM) [A multicenter, randomized, double-blind, placebo-controlled, parallel group, dose ranging study to determine the effect of mepolizumab on exacerbation rates in subjects with severe uncontrolled refractory asthma]. clinicaltrials.gov/ct2/show/NCT01000506 (accessed 16 January 2015). 4900126000001337
- Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. European Respiratory Journal 2014;44(1):239-41. [DOI] [PubMed] [Google Scholar]
- Ortega H, Li H, Suruki R, Albers F, Gordon D, Yancey S. Cluster analysis and characterization of response to mepolizumab: a step closer to personalized medicine for patients with severe asthma. Annals of the American Thoracic Society 2014;11(7):1011-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ortega HG, Chupp G, Bardin P, Bourdin A, Hartley B, Humbert M. The role of mepolizumab in atopic and non-atopic patients with refractory eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2013;187:A3861. [CENTRAL: 1129378] [EMBASE: 71983360] [Google Scholar]
- Pavord I, Korn S, Howarth P, Bleecker E, Buhl R, Keene O, et al. Mepolizumab (anti-IL-5) reduces exacerbations in patients with refractory eosinophilic asthma. European Respiratory Journal 2012;40 Suppl 56:36s [349]. 4900100000068022 [Google Scholar]
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380(9842):651-9. 4900100000063432 [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albers 2016 {published data only}
- Albers F, Cockle S, Gunsoy N, Shin JY, Nelsen L, Muellerova H. Eligibility for mepolizumab, omalizumab and reslizumab in the EU population: The IDEAL study [PA4216]. In: European Respiratory Society 26thAnnual Congress; 2016 Sep 3-7; London. 2016.
Albers 2020 {published data only}
- Albers FC, Hozawa S, Bratton DJ, Yancey SW, Prazma CM, Mumbert M, et al. Mepolizumab treatment in patients with severe eosinophilic asthma and prior omalizumab use. Allergy 2020;75(4):942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez‐Cuesta 1994 {published data only}
- Alvarez-Cuesta E, Cuesta-Herranz J, Puyana-Ruiz J, Cuesta-Herranz C, Blanco-Quiros A. Monoclonal antibody-standardized cat extract immunotherapy: risk-benefit effects from a double-blind placebo study. Journal of Allergy and Clinical Immunology 1994;93(3):556-66. 4900100000004347 [DOI] [PubMed] [Google Scholar]
Armentia 1992 {published data only}
- Armentia A, Arranz M, Martin JM, la Fuente R, Sanchez P, Barber D, et al. Evaluation of immune complexes after immunotherapy with wheat flour in bakers' asthma. Annals of Allergy 1992;69(5):441-4. 4900100000003916 [PubMed] [Google Scholar]
Austin 2016 {published data only}
- Austin D, Pouliquen I, Keene O, Yancey S. Blood eosinophil dose response to oral corticosteroids in a population of patients with severe asthma [PA1110]. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016.
Austin 2016a {published data only}
- Austin D, Pouliquen I, Keene O, Yancey S. Blood eosinophil dose response to oral corticosteroids in a population of patients with severe asthma. European Respiratory Journal 2016;48:PA1110. [Google Scholar]
Ayres 2004 {published data only}
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy 2004;59(7):701-8. 4900100000017042 [DOI] [PubMed] [Google Scholar]
Bardford 2018 {published data only}
- Bardford E, Nelsen L, Bratton D, Albers F, Taille C, Magnan A. Efficacy of mepolizumab in patients with severe eosinophilic asthma who had previously received omalizumab treatment. Pneumologie 2018;72:S17. [Google Scholar]
Bel 2014 {published data only}
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene O, Yancey SW, et al. Oral corticosteroid-sparing effect of mepolizumab in severe eosinophilic asthma: The SIRIUS study. European Respiratory Journal 2014;44 Suppl 58:2907. [CENTRAL: 1053369] [Google Scholar]
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. New England Journal of Medicine 2014;371(13):1189-97. [CENTRAL: 1013105] [EMBASE: 25199060] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01691508. Mepolizumab steroid-sparing study in subjects with severe refractory asthma [MEA115575: a randomised, double-blind, placebo-controlled, parallel-group, multicenter study of mepolizumab adjunctive therapy to reduce steroid use in subjects with severe refractory asthma]. clinicaltrials.gov/ct2/show/NCT01691508 (first received 20 September 2012). 4900126000001341
- Sehmi R, Smith SG, Kjarsgaard M, Radford K, Boulet LP, Lemiere C, et al. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone-dependent severe asthma. Clinical and Experimental Allergy 2015;46(6):793-802. [CENTRAL: 1129187] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berger 2003 {published data only}
- Berger W, Gupta N, McAlary M, Fowler-Taylor A, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Annals of Allergy, Asthma and Immunology 2003;91(2):182-8. 4900102000000524 [4900102000000524] [DOI] [PubMed] [Google Scholar]
Bernstein 2020a {published data only}
- Bernstein JA, Singh U, Rao MB, Berendts K, Zhang X, Mutasim D. Benralizumab for chronic spontaneous urticaria. New England Journal of Medicine 2020;383(14):1389-91. [DOI] [PubMed] [Google Scholar]
Bjermer 2017 {published data only}
- Bjermer L, Garin M, Sun SX. Clinically meaningful improvements with reslizumab in patient-reported outcomes and lung function in a sub-population defined by the EU indication with >=3 exacerbations. European Respiratory Journal 2017;50:PA4687. [Google Scholar]
Blanken 2012 {published data only}
- Blanken M, Rovers M, Sanders E, Bont L. Ethical considerations and rationale of the MAKI trial: a multicenter double-blind randomized placebo-controlled trial into the preventive effect of palivizumab on recurrent wheezing associated with respiratory syncytial virus infection in children with a gestational age of 33-35 weeks. Contemporary Clinical Trials 2012;33(6):1287-92. 4900100000067009 [DOI] [PubMed] [Google Scholar]
Blanken 2013 {published data only}
- Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. New England Journal of Medicine 2013;368(19):1791-9. 4900100000077900 [4900100000077900] [DOI] [PubMed] [Google Scholar]
Bleecker 2018a {published data only}
- Bleecker E, McDonald M, Garin MC. Decreases in blood eosinophil levels correlate with lung function improvement with reslizumab treatment. American Journal of Respiratory and Critical Care Medicine 2018;197:A1362. [Google Scholar]
Bleecker 2018b {published data only}
- Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. European Respiratory Journal 2018;52(4):1800936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulet 1997 {published data only}
- Boulet LP, Chapman KR, Cote J, Kalra S, Bhagat R, Swystun VA, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1835-40. 4900100000005712 [DOI] [PubMed] [Google Scholar]
Bourdin 2018 {published data only}
- Bourdin A, McDonald M, Vanlandingham R. Reslizumab is effective in asthma patients with or without allergen specific IgE. European Journal of Allergy and Clinical Immunology 2018;73(105):202-3. [Google Scholar]
Bourdin 2020 {published data only}
- Bourdin A, Husereau D, Molinari N, Golam S, Siddiqui MK, Lindner L, et al. Matching-adjusted comparison of oral corticosteroid reduction in asthma: systematic review of biologics. Clinical and Experimental Allergy 2020;50(4):442-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bousquet 2004 {published data only}
- Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest 2004;125(4):1378-86. 4900100000016325 [DOI] [PubMed] [Google Scholar]
Bousquet 2011 {published data only}
- Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy 2011;66(5):671-8. 4900100000026344 [DOI] [PubMed] [Google Scholar]
Brightling 2014 {published data only}
- Brightling CE, She D, Ranade K, Piper E. Efficacy and safety of tralokinumab, an anti-il-13 monoclonal antibody, in a phase 2b study of uncontrolled severe asthma. American Journal of Respiratory and Critical Care Medicine 2014;189:A6670. [CENTRAL: 1035526] [EMBASE: 72047302] [Google Scholar]
Brown 2007 {published data only}
- Brown R, Turk F, Dale P, Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy 2007;62(2):149-53. 4900100000019948 [4900100000019948] [DOI] [PubMed] [Google Scholar]
Brusselle 2016 {published data only}
- Brusselle G, McElhattan J, Canvin J, Buhl R. Reslizumab (RES) in asthma patients (pts) with severe eosinophilic asthma stratified by GINA asthma steps 4 and 5: analysis of two phase 3, placebo (PBO)-controlled trials [PA4107]. In: European Respiratory Society 26thAnnual Congress; 2016 Sep 3-7; London. 2016.
Brusselle 2017 {published data only}
- Brusselle G, Canvin J, Weiss S, Sun SX, Buhl R. Stratification of eosinophilic asthma patients treated with reslizumab and GINA Step 4 or 5 therapy. ERJ Open Research 2017;3:00004-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryant 1975a {published data only}
- Bryant DH, Burns MW, Lazarus L. The correlation between skin tests, bronchial provocation tests and the serum level of IgE specific for common allergens in patients with asthma. Clinical Allergy 1975;5(2):145-57. 4900100000023985 [DOI] [PubMed] [Google Scholar]
Bryant 1975b {published data only}
- Bryant DH, Burns MW, Lazarus L. Identification of IgG antibody as a carrier of reaginic activity in asthmatic patients. Journal of Allergy and Clinical Immunology 1975;56(6):417-28. 4900100000024002 [DOI] [PubMed] [Google Scholar]
Buhl 2000a {published data only}
- Buhl R, Kunkel G, Soler M, Bensch G, Wolfe J, Noga O, et al. RhuMAb-25 improves asthma-specific quality of life in patients with allergic asthma. European Respiratory Journal 2000;16 Suppl 31:465s. 4900100000013847 [Google Scholar]
Buhl 2000b {published data only}
- Buhl R, Soler M, Fox H, Ashby M, McAlary M, Cooper J, et al. Recombinant humanized monoclonal antibody (rhuMAb) E25 in the prevention of serious asthma exacerbations. European Respiratory Journal 2000;16 Suppl 31:277s. 4900100000013848 [Google Scholar]
Buhl 2002 {published data only}
- Buhl R, Soler M, Matz J, Townley R, O'Brien J, Noga O, et al. Omalizumab provides long-term control in patients with moderate-to- severe allergic asthma. European Respiratory Journal 2002;20(1):73-8. 4900100000012867 [DOI] [PubMed] [Google Scholar]
Bush 1985 {published data only}
- Bush RK, Taylor SL, Nordlee JA, Busse WW. Soybean oil is not allergenic to soybean-sensitive individuals. Journal of Allergy and Clinical Immunology 1985;76(2 Pt 1):242-5. 4900100000001757 [DOI] [PubMed] [Google Scholar]
Busse 2001 {published data only}
- Busse WW, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Della Cioppa G, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. Journal of Allergy and Clinical Immunology 2001;108(2):184-90. 4900100000010636 [4900100000010636] [DOI] [PubMed] [Google Scholar]
Busse 2008 {published data only}
- Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, et al. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine 2008;178(10):1002-8. 4900100000022833 [DOI] [PubMed] [Google Scholar]
Busse 2015 {published data only}
- Busse WW, Wang M, Gibson J, Gottlow M, Braddock M, Colice G. TROPOS: designing a clinical trial to evaluate the oral corticosteroid-sparing effect of a biologic in severe asthma. Clinical Investigation 2015;5(8):723-30. [CENTRAL: 1096145] [EMBASE: 2015401568] [Google Scholar]
Buttner 2003 {published data only}
- Buttner C, Lun A, Splettstoesser T, Kunkel G, Renz H. Monoclonal anti-interleukin-5 treatment suppresses eosinophil but not T-cell functions. European Respiratory Journal 2003;21(5):799-803. 4900100000015258 [DOI] [PubMed] [Google Scholar]
- Buttner C, Splettstosser T, Lun A, Renz H, Kunkel G. The influence of anti-IL-5 anti-bodies on leucocyte differentiation and function in patients with asthmatic. Pneumologie 2001;55(SH1):S69. [Google Scholar]
Caffarelli 2000 {published data only}
- Caffarelli C, Sensi LG, Marcucci F, Cavagni G. Preseasonal local allergoid immunotherapy to grass pollen in children: a double-blind, placebo-controlled, randomized trial. Allergy 2000;55(12):1142-7. 4900100000009985 [DOI] [PubMed] [Google Scholar]
Canvin 2016 {published data only}
- Canvin J, Noble R, Djukanovic R, Curran M, Weiss S. Early identification of responders to reslizumab at 16 weeks using an algorithm derived from the pivotal clinical studies of severe eosinophilic asthma (SEA) patients [OA2998]. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016.
Carr 2017 {published data only}
- Carr W, McDonald M, Meizlik P. Reslizumab improves spirometric lung age in patients with severe eosinophilic asthma. Annals of Allergy, Asthma and Immunology 2017;119(5):S57. [DOI] [PubMed] [Google Scholar]
Carr 2019 {published data only}
- Carr WW, McDonald M, Meizlik P. Effect of intravenously administered reslizumab on spirometric lung age in patients with moderate-to-severe eosinophilic asthma. Allergy and Asthma Proceedings 2019;40(4):240-9. [DOI] [PubMed] [Google Scholar]
Castro 2011 {published data only}
- Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. American Journal of Respiratory and Critical Care Medicine 2011;184(10):1125-32. 4900100000056192 [DOI] [PubMed] [Google Scholar]
- Castro M, Mathur S, Hargreave F, Xie F, Young J, Wilkins HJ, et al. Reslizumab in the treatment of poorly controlled asthma in patients with eosinophilic airway inflammation. Annals of Allergy, Asthma and Immunology 2010;105(5 Suppl):A43. 4900100000054093 [Google Scholar]
- Mathur S, Castro M, Hargreave F, Xie F, Wilkins HJ, Henkel T, et al. Efficacy of reslizumab in patients with poorly controlled eosinophilic asthma: subgroup analysis of patients with nasal polyps. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB84. 4900100000026800 [Google Scholar]
- NCT00587288. Efficacy and safety study of reslizumab to treat poorly controlled asthma [An efficacy and safety study of reslizumab in the treatment of poorly controlled asthma in subjects with eosinophilic airway inflammation]. clinicaltrials.gov/show/NCT00587288 (first received 7 January 2008).
Castro 2014 {published data only}
- Castro M, Teper A, Wang L, Pirozzi G, Radin A, Graham N, et al. Responder analysis for FEV1 improvement with dupilumab in patients with persistent asthma and elevated eosinophil levels. American Journal of Respiratory and Critical Care Medicine 2014;189:A1321. [CENTRAL: 1131453] [EMBASE: 72043771] [Google Scholar]
Castro 2018 {published data only}
- Castro M, Adir Y, Chanez P, Mcdonald M, Hickey L, Garin M. Consistent efficacy across endpoints with reslizumab despite short term variability in blood eosinophil levels. European Respiratory Journal 2018;52:PA1043. [Google Scholar]
Chandra 1989 {published data only}
- Chandra RK, Singh G, Shridhara B. Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Annals of Allergy 1989;63(2):102-6. 4900100000002896 [PubMed] [Google Scholar]
Chanez 2017 {published data only}
- Chanez P, Garin M, McDonald M, Humbert M. Reslizumab reduces severe exacerbations associated with emergency department visit or hospitalization and improves measures of lung function in patients on maintenance oral corticosteroids (OCS). European Respiratory Journal 2017;50:PA4691. [Google Scholar]
Chanez 2017a {published data only}
- Chanez P, McDonald M, Garin M, Murphy K. Early decreases in blood eosinophil levels with reslizumab. American Journal of Respiratory and Critical Care Medicine 2017;195:A3190. [DOI] [PubMed] [Google Scholar]
Chanez 2018 {published data only}
- Chanez P, Adir Y, Castro M, Pahus L, Mcdonald M, Hickey L, et al. Intravenous (IV) reslizumab improves asthma control, symptoms, and quality of life in patients with historically elevated blood eosinophils. European Respiratory Journal 2018;52:PA600. [Google Scholar]
Chanez 2019 {published data only}
- Chanez P, Hickey L, Garin M, Castro M. Evaluation of eosinophilic asthma patients without blood eosinophil (EOS) response to intravenous (IV) reslizumab in a post-hoc analysis of 52-week placebo-controlled phase 3 studies. Journal of Allergy and Clinical Immunology 2019;143(2):AB97. [Google Scholar]
Chauhan 2017 {published data only}
- Chauhan A, Garin M, Sun S. Efficacy of reslizumab in adults with severe eosinophilic asthma with >=3 exacerbations in the previous year: analyses at weeks 16 and 52 of two placebo-controlled phase 3 trials. European Respiratory Journal 2017;50:PA2626. [Google Scholar]
Chervinsky 2003 {published data only}
- Chervinsky P, Casale T, Townley R, Tripathy I, Hedgecock S, Fowler-Taylor A, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Annals of Allergy, Asthma and Immunology 2003;91(2):160-7. 4900100000015346 [4900100000015346] [DOI] [PubMed] [Google Scholar]
Chipps 2017 {published data only}
- Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Efficacy of benralizumab for patients with severe, uncontrolled atopic asthma by serum immunoglobulin E concentrations. Pneumologie 2018;72:S14. [DOI] [PubMed] [Google Scholar]
Chipps 2018 {published data only}
- Chipps BE, Hirsch I, Trudo F, Alacqua M, Zangrilli JG. Demographics, clinical characteristics, and response to benralizumab treatment for patients with severe, eosinophilic asthma and fixed airflow obstruction. American Journal of Respiratory and Critical Care Medicine 2018;197:A2489. [Google Scholar]
Chipps 2019 {published data only}
- Chipps BE, Hirsch I, Trudo F, Alacqua M, Zangrilli JG. Benralizumab efficacy for patients with fixed airflow obstruction and severe, uncontrolled eosinophilic asthma. Annals of Allergy and Asthma Immunology 2020;124(1):79-86. [DOI] [PubMed] [Google Scholar]
Chipps 2020 {published data only}
- Chipps B, Corren J, Israel E, Barker P, Kreindler J, Newbold P. Influence of key clinical baseline characteristics on benralizumab response for patients with severe, uncontrolled asthma and moderate blood eosinophilia. Journal of Allergy and Clinical Immunology 2020;145(2):AB22. [Google Scholar]
Clavel 1998 {published data only}
- Clavel R, Bousquet J, Andre C. Clinical efficacy of sublingual-swallow immunotherapy: a double-blind, placebo-controlled trial of a standardized five-grass-pollen extract in rhinitis. Allergy 1998;53(5):493-8. 4900100000023536 [DOI] [PubMed] [Google Scholar]
Corren 2003 {published data only}
- Corren J, Casale T, Deniz Y, Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. Journal of Allergy and Clinical Immunology 2003;111(1):87-90. 4900100000015187 [DOI] [PubMed] [Google Scholar]
Corren 2010 {published data only}
- Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(8):788-96. 4900100000024877 [DOI] [PubMed] [Google Scholar]
Cullell‐Young 2002 {published data only}
- Cullell-Young M, Bayes M, Leeson PA. Omalizumab: treatment of allergic rhinitis, treatment of asthma. Drugs of the Future 2002;27(6):537-45. 4900100000050549 [Google Scholar]
Dasgupta 2016 {published data only}
- Dasgupta A, Kjarsgaard M, Capaldi D, Radford K, Aleman F, Parraga G, et al. Mepolizumab in COPD with eosinophilic bronchitis: a randomized clinical trial [PA305]. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016.
De Boever 2014 {published data only}
- De Boever EH, Ashman C, Cahn AP, Locantore NW, Overend P, Pouliquen IJ, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. Journal of Allergy and Clinical Immunology 2014;133(4):989-96. [CENTRAL: 984687] [EMBASE: 2014224342] [PMID: ] [DOI] [PubMed] [Google Scholar]
Djukanovic 2004 {published data only}
- Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. American Journal of Respiratory and Critical Care Medicine 2004;170(6):583-93. 4900100000017162 [DOI] [PubMed] [Google Scholar]
DuBuske 2018 {published data only}
- DuBuske L, Newbold P, Wu Y, Trudo F, Gopalan G. Seasonal variability of exacerbations in patients with severe, uncontrolled eosinophilic asthma and clinical benefits of benralizumab: pooled analysis of the SIROCCO and CALIMA trials. Journal of Allergy and Clinical Immunology 2018;141(2):AB12. [DOI] [PubMed] [Google Scholar]
Ebner 1989 {published data only}
- Ebner H, Neuchrist C, Havelec L, Kraft D. Comparative studies of the effectiveness of specific immunotherapy in house dust mite allergy [Vergleichende untersuchungen zur wirksamkeit einer spezifischen immuntherapie bei hausstaubmilben-allergie]. Wiener Klinische Wochenschrift 1989;101(15):504-11. 4900100000002906 [PubMed] [Google Scholar]
Eckman 2010 {published data only}
- Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. Journal of Allergy and Clinical Immunology 2010;125(4):889-95.e7. 4900100000024734 [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Nawawy 2000 {published data only}
- El-Nawawy AA, Massoud MN, El-Nazzar SY, Ramy BB. Pulmonary tuberculosis as a cause of recurrent wheezy chest: the value of serological diagnosis using IgG antibodies to mycobacterium 38 kDa antigen. Journal of Tropical Pediatrics 2000;46(1):53-4. 4900100000008353 [DOI] [PubMed] [Google Scholar]
EUCTR2012‐004385‐17‐BE {published data only}
- 2012-004385-17. Study of mepolizumab versus placebo in addition to standard of care for the treatment of eosinophilic granulomatosis with polyangiitis [A double-blind, randomised, placebo-controlled study to investigate the efficacy and safety of mepolizumab in the treatment of eosinophilic granulomatosis with polyangiitis in subjects receiving standard of care therapy]. www.clinicaltrialsregister.eu/ctr-search/ (first received 2 November 2013).
EUCTR2015‐001152‐29‐BE {published data only}
- 2015-001152-29. A long-term access programme for asthmatic subjects who participated in a GSK-sponsored clinical study with mepolizumab. www.clinicaltrialsregister.eu/ctr-search/search?query=A+long-term+access+programme+for+asthmatic+subjects+who+participated+in+a+GSK-sponsored+clinical+study+with+mepolizumab (first received 10 July 2015).
EUCTR2016‐001831‐10‐NL {published data only}
- 2016-001831-10. A real-world use study of safety syringe for the administration of mepolizumab in severe asthma. clinicaltrialsregister.eu/ctr-search/search?query=A+Real-World+Use+Study+of+Safety+Syringe+for+the+Administration+of+Mepolizumab+in+Severe+Asthma (first received 14 February 2017).
EUCTR2016‐002405‐19‐DE {published data only}
- 2016-002405-19. A phase 3 pharmacokinetic study in healthy volunteers of mepolizumab as liquid formulation versus powder for solution formulation. clinicaltrialsregister.eu/ctr-search/ (first received 5 December 2017).
EUCTR2017‐003958‐16‐NL {published data only}
- 2017-003958-16-NL. Effect of reslizumab on small airways in asthma. RESSAPEA. www.clinicaltrialsregister.eu/ctr-search/search?query=RESSAPEA (first received 10 August 2018).
Fahy 1997 {published data only}
- Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1828-34. 4900100000005711 [DOI] [PubMed] [Google Scholar]
Fahy 1999 {published data only}
- Fahy JV, Cockcroft DW, Boulet LP, Wong HH, Deschesnes F, Davis EE, et al. Effect of aerosolized anti-IgE (E25) on airways responses to inhaled allergen in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 1999;160(3):1023-7. 4900100000006519 [4900100000006519] [DOI] [PubMed] [Google Scholar]
Ferguson 2016a {published data only}
- Ferguson G, FitzGerald JM, Bleecker E, Laviolette M, Bernstein D, LaForce C, et al. Benralizumab for mild to moderate, persistent asthma: The BISE phase III study. European Respiratory Journal 2016;48:OA1791. [Google Scholar]
Ferguson 2016b {published data only}
- Ferguson G, FitzGerald JM, Bleecker E, Laviolette M, Bernstein D, LaForce C, et al. Benralizumab for mild to moderate, persistent asthma: The BISE phase III study. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. Vol. 48. 2016:Suppl 60.
Ferguson 2017 {published data only}
- Ferguson G, FitzGerald JM, Bleecker E, Laviolette M, Bernstein D, LaForce C, et al. Benralizumab for patients with mild to moderate, persistent asthma (BISE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respiratory Medicine 2017;5(7):568-76. [DOI] [PubMed] [Google Scholar]
Finn 2003 {published data only}
- Finn A, Gross G, Van Bavel J, Lee T, Windom H, Everhard F, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. Journal of Allergy and Clinical Immunology 2003;111(2):278-84. 4900100000013800 [DOI] [PubMed] [Google Scholar]
FitzGerald 2017a {published data only}
- FitzGerald JM, Ohta K, Adachi M, Tohda Y, Kamei T, Kato M, et al. Benralizumab reduces exacerbations in Japanese patients with severe, uncontrolled asthma: subgroup analysis of the CALIMA Trial. Journal of Allergy and Clinical Immunology 2017;139(2):AB10. [Google Scholar]
FitzGerald 2017b {published data only}
- FitzGerald JM, Bleecker ER, Menzies-Gow A, Zangrilli JG, Metcalfe P, Hirsch I, et al. Characterizing responders to benralizumab for severe asthma: pooled analysis of the SIROCCO and CALIMA studies. European Respiratory Journal 2017;50:OA2902. [DOI] [PubMed] [Google Scholar]
FitzGerald 2018 {published data only}
- FitzGerald JM, Bleecker ER, Menzies Gow A, Zangrilli JG, Hirsch I, Metcalfe P, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respiratory Medicine 2018;6(1):51-64. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2019 {published data only}
- Fitzgerald JM, Bleecker ER, Bourdin A, Busse WW, Ferguson GT, Brooks L, et al. Two-year integrated efficacy and safety analysis of benralizumab SIROCCO, CALIMA, ZONDA, and BORA trials in severe asthma. American Journal of Respiratory and Critical Care Medicine 2019;199(1):A2676. [Google Scholar]
Flood‐Page 2003 {published data only}
- Flood-Page P, Menzies-Gow A, Wangoo A, Barnes NC, Barkans J, Phipps S, et al. Intravenous administration of an anti-IL-5 monoclonal antibody to mild atopic asthmatics reduces the expression of tenascin, procollagen 111 and lumican in the bronchial mucosal reticular basement membrane: evidence for a role for eosinophils in airways remodelling. QJM : Monthly Journal of the Association of Physicians 2003;96(11):860. [CENTRAL: 493553] [4900100000017354] [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. American Journal of Respiratory and Critical Care Medicine 2003;167(2):199-204. 4900100000013790 [4900100000013790] [DOI] [PubMed] [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Phipps S, Compton C, Walls C, Barnes NC, et al. Reduction of tissue eosinophils in mild atopic asthmatics by an anti-IL-5 monoclonal antibody (mepolizumab) is associated with inhibition of tenascin deposition with the bronchial epithelial basement. American Journal of Respiratory and Critical Care Medicine 2002;165 Suppl 8:B42. 4900100000013133 [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. Journal of Clinical Investigation 2003;112(7):1029-36. 4900100000015627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AB, Flood-Page PT, Menzies-Gow AN, Robinson DS. Effect of anti-Il-5 (mezolizumab) on airway eosinophils in asthmatics. Allergy and Clinical Immunology International 2004;Suppl 1:298-301. 4900100000051661 [4900100000051661] [Google Scholar]
- Menzies-Gow AN, Flood-Page PT, Compton C, Walls C, Sehmi R, Robinson DS, et al. A double-blind placebo-controlled, parallel group study to assess the effect of mepolizumab (humanised monoclonal anti-il-5-antibody) on bone marrow and peripheral blood eosinophils and eosinophil progenitors in atopic asthmatics. American Journal of Respiratory and Critical Care Medicine 2002;165 Suppl 8:B50. 4900100000013192 [Google Scholar]
- Menzies-Gow AN, Flood-Page PT, Sehmi R, Burman J, Hamid Q, Robinson DS, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. Journal of Allergy and Clinical Immunology 2003;111(4):714-9. 4900100000015225 [4900100000015225] [DOI] [PubMed] [Google Scholar]
- Phipps S, Flood-Page PT, Menzies-Gow AN, Wangoo A, Barnes N, Barkans J, et al. Anti-IL-5 (mepolizumab) reduces the expression of tenascin procollagen III and lumican in the reticular basement membrane of human atopic asthmatics. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl):S278. 4900100000018828 [Google Scholar]
Flood‐Page 2007 {published data only}
- Flood-Page PT, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. American Journal of Respiratory and Critical Care Medicine 2007;176(11):1062-71. 4900100000021401 [DOI] [PubMed] [Google Scholar]
Frew 1998 {published data only}
- Frew AJ. Effects of anti-IgE in asthmatic subjects. Thorax 1998;53 Suppl 2:S52-7. 4900100000010183 [4900100000010183] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2013 {published data only}
- Garcia G, Magnan A, Chiron R, Contin-Bordes C, Berger P, Taillé C, et al. A proof-of-concept, randomized, controlled trial of omalizumab in patients with severe, difficult-to-control, nonatopic asthma. Chest 2013;144(2):411-9. [CENTRAL: 872766] [EMBASE: 2013508319] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garin 2019 {published data only}
- Garin M, Hickey L, Mustafa SS. A high proportion of exacerbation-prone patients with uncontrolled eosinophilic asthma receiving IV reslizumab experienced zero asthma exacerbations through 52 weeks (wks) of treatment. Journal of Allergy and Clinical Immunology 2019;143(2):AB98. [Google Scholar]
Gauvreau 2011 {published data only}
- Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. American Journal of Respiratory and Critical Care Medicine 2011;183(8):1007-14. 4900100000026369 [DOI] [PubMed] [Google Scholar]
Gauvreau 2014a {published data only}
- Gauvreau GM, Harris JM, Boulet LP, Scheerens H, Fitzgerald JM, Putnam WS, et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Science Translational Medicine 2014;6(243):243ra85. [CENTRAL: 994063] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gauvreau 2014b {published data only}
- Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. New England Journal of Medicine 2014;370(22):2102-10. [CENTRAL: 991614] [4900126000014666] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gauvreau 2014c {published data only}
- Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Mayers I, Carlsten C, et al. OX40L blockade and allergen-induced airway responses in subjects with mild asthma. Clinical and Experimental Allergy 2014;44(1):29-37. [CENTRAL: 961627] [EMBASE: 2013812052] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gauvreau 2015a {published data only}
- Gauvreau G, Boulet L-P, Leigh R, Cockcroft DW, Davis BE, Mayers I, et al. A predictive model for determining the efficacy of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting the allergen-induced early asthmatic response. American Journal of Respiratory and Critical Care Medicine 2015;191(Meeting Abstracts):A2488. [CENTRAL: 1101075] [EMBASE: 72050323] [Google Scholar]
Gauvreau 2015b {published data only}
- Gauvreau GM, Boulet LP, Leigh R, Cockcroft DW, Davis BE, Mayers I, et al. QGE031 (ligelizumab) is more effective than omalizumab and placebo in inhibiting allergen-induced early asthmatic response: results from a predictive modeling study. European Respiratory Journal 2015;46 Suppl 59:PA5091. [CENTRAL: 1135083] [EMBASE: 72107321] [Google Scholar]
Gevaert 2013 {published data only}
- Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. Journal of Allergy and Clinical Immunology 2013;131(1):110-6.e1. 4900100000072110 [DOI] [PubMed] [Google Scholar]
Gibson 2021 {published data only}
- Gibson PG, Prazma CM, Chupp GL, Bradford ES, Forshag M, Mallett SA, et al. Mepolizumab improves clinical outcomes in patients with severe asthma and comorbid conditions. Respiratory Research 2021;22(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman 2017 {published data only}
- Goldman M, Hirsch I, Zangrilli J, Newbold P, Xu X. The association between blood eosinophil count and benralizumab efficacy for adult patients with severe, uncontrolled asthma. Current Medical Research Opinion 2017;33(9):1605-13. [DOI] [PubMed] [Google Scholar]
Gopalan 2017a {published data only}
- Gopalan G, Xu X, O'Quinn S, Hirsch I. Asthma symptom improvements with benralizumab are associated with improvements in activity functions and quality of life for patients with severe, uncontrolled asthma: results of pooled phase III benralizumab studies. Thorax 2017;72(3):P68. [Google Scholar]
Gopalan 2017b {published data only}
- Gopalan G, O'Quinn S, Xu X, Hirsch I. Patient-reported activity impairment, stress, and tiredness improvement in patients with severe, uncontrolled asthma with eosinophilic inflammation: pooled results from two phase III trials of benralizumab. Respirology 2017;22(3):4–87. [Google Scholar]
Gordon 1972 {published data only}
- Gordon VH, Caplinger KJ, Meade JHJ, Thompson C. Correlation of type specific fluorescent antibodies to ragweed with symptomatology: double-blind study. Annals of Allergy 1972;30(9):507-17. 4900100000000354 [PubMed] [Google Scholar]
Greenberg 1991 {published data only}
- Greenberg RN, Wilson KM, Kunz AY, Wedel NI, Gorelick KJ. Randomized, double-blind phase II study of anti-endotoxin antibody (E5) as adjuvant therapy in humans with serious gram-negative infections. Progress in Clinical and Biological Research 1991;367:179-86. 4900100000003509 [PubMed] [Google Scholar]
Gunsoy 2016 {published data only}
- Gunsoy N, Cockle S, Nelsen L, Albers F, Doyle S. Association between EQ-5D and changes in asthma symptoms, severity, and Qol in patients with severe eosinophilic asthma. Value in Health 2016;19(7):A558. [Google Scholar]
Han 2009 {published data only}
- Han JQ, Zhu YX. Efficacy and regulation of humoral immunity of jade screen powder as an adjunct therapy in children with asthma. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2009;11(7):587-8. 4900100000024253 [PubMed] [Google Scholar]
Hanania 2011 {published data only}
- Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Annals of Internal Medicine 2011;154(9):573-82. 4900100000026408 [DOI] [PubMed] [Google Scholar]
Hanania 2013 {published data only}
- Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. American Journal of Respiratory and Critical Care Medicine 2013;187(8):804-11. 4900100000077549 [4900100000077549] [DOI] [PubMed] [Google Scholar]
Hanania 2014 {published data only}
- Hanania NA, Noonan MJ, Corren J, Korenblat P, Zheng Y, Putnam W, et al. Efficacy and safety of lebrikizumab in severe uncontrolled asthma: results from the LUTE and VERSE phase II randomized, double-blind, placebo-controlled trials. Journal of Allergy and Clinical Immunology 2014;133(2 Suppl):AB402. [CENTRAL: 985853] [EMBASE: 71351759] [Google Scholar]
Hanania 2015 {published data only}
- Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax 2015;70(8):748-56. [CENTRAL: 1072983] [EMBASE: 2015350098] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2016 {published data only}
- Harris JM, Maciuca R, Bradley MS, Cabanski CR, Scheerens H, Lim J, et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respiratory Research 2016;17(1):29. [CENTRAL: 1139820] [EMBASE: 20160229671] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendeles 2015 {published data only}
- Hendeles L, Khan YR, Shuster JJ, Chesrown SE, Abu-Hasan M. Omalizumab therapy for asthma patients with poor adherence to inhaled corticosteroid therapy. Annals of Allergy, Asthma and Immunology 2015;114(1):58-62.e2. [CENTRAL: 1038271] [EMBASE: 2014983010] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hickey 2019 {published data only}
- Hickey L, Carr W, Garin M. Clinically meaningful improvements in asthma control questionnaire (ACQ) scores occur early, while asthma-related quality of life (AQLQ) scores continue to improve over 52 weeks, among patients with eosinophilic asthma receiving IV reslizumab. Journal of Allergy and Clinical Immunology 2019;143(2):AB96. [Google Scholar]
Hill 1982 {published data only}
- Hill DJ, Smart IJ, Hosking CS. Specific cellular and humoral immunity in children with grass pollen asthma. Clinical Allergy 1982;12(1):83-9. 4900100000001231 [DOI] [PubMed] [Google Scholar]
Hodsman 2013 {published data only}
- Hodsman P, Ashman C, Cahn A, De Boever E, Locantore N, Serone A, et al. A phase 1, randomized, placebo-controlled, dose-escalation study of an anti-IL-13 monoclonal antibody in healthy subjects and mild asthmatics. British Journal of Clinical Pharmacology 2013;75(1):118-28. 4900100000070660 [DOI] [PMC free article] [PubMed] [Google Scholar]
Holgate 2004 {published data only}
- Holgate ST, Chuchalin AG, Hebert J, Lotvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clinical and Experimental Allergy 2004;34(4):632-8. 4900100000016275 [DOI] [PubMed] [Google Scholar]
Hoshino 2012 {published data only}
- Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration 2012;83(6):520-8. 4900100000062323 [DOI] [PubMed] [Google Scholar]
Howarth 2020 {published data only}
- Howarth P, Chupp G, Nelsen LM, Bradford ES, Bratton DJ, Smith SG, et al. Severe eosinophilic asthma with nasal polyposis: a phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. Journal of Allergy and Cinical Immunology 2020;145(6):1713-15. [DOI] [PubMed] [Google Scholar]
Humbert 2005 {published data only}
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60(3):309-16. 4900100000018078 [DOI] [PubMed] [Google Scholar]
Humbert 2008 {published data only}
- Humbert M, Berger W, Rapatz G, Turk F. Add-on omalizumab improves day-to-day symptoms in inadequately controlled severe persistent allergic asthma. Allergy 2008;63(5):592-6. 4900100000021856 [4900100000021856] [DOI] [PubMed] [Google Scholar]
Humbert 2009 {published data only}
- Humbert M, Boulet LP, Niven RM, Panahloo Z, Blogg M, Ayre G. Omalizumab therapy: patients who achieve greatest benefit for their asthma experience greatest benefit for rhinitis. Allergy 2009;64(1):81-4. 4900100000023513 [DOI] [PubMed] [Google Scholar]
Humbert 2017 {published data only}
- Humbert M, Castro M, McDonald M, Germinaro M. Efficacy of reslizumab in asthma patients eligible for omalizumab treatment. American Journal of Respiratory and Critical Care Medicine 2017;195:A4688. [Google Scholar]
Humbert 2019 {published data only}
- Humbert M, Albers FC, Bratton DJ, Yancey SW, Liu MC, Hozawaf S, et al. Effect of mepolizumab in severe eosinophilic asthma according to omalizumab eligibility. Respiratory Medicine 2019;154:69-75. [DOI] [PubMed] [Google Scholar]
Iino 2019 {published data only}
- Iino Y, Takahashi E, Ida S, Kikuchi S. Clinical efficacy of anti-IL-5 monoclonal antibody mepolizumab in the treatment of eosinophilic otitis media. Auris Nasus Larynx 2019;46(2):196-203. [DOI] [PubMed] [Google Scholar]
Jackson 2019 {published data only}
- Jackson DJ, Humbert M, Hirsch I, Newbold P, Garcia Gil E. Association of baseline blood eosinophil counts and serum IgE concentrations on exacerbations and benralizumab efficacy for patients with severe, uncontrolled asthma. In: British Thoracic Society Winter Meeting; 2019 Dec 4-6; London. Vol. 74. 2019:A34-5.
Jacobs 2018 {published data only}
- Jacobs J, Hickey L, Vanlandingham R. Improvements with reslizumab treatment in patient-reported sleep quality in patients with inadequately controlled eosinophilic asthma [Annals of Allergy, Asthma and Immunology]. 2018 121;5:S43-4. [Google Scholar]
Jacquemin 1995 {published data only}
- Jacquemin MG, Saint-Remy JM. Specific down-regulation of anti-allergen IgE and IgG antibodies in humans associated with injections of allergen-specific antibody complexes. Therapeutic Immunology 1995;2(1):41-52. 4900100000004951 [PubMed] [Google Scholar]
Jimenez 2019 {published data only}
- Jimenez J, Malone D. The cost-effectiveness of Il-5 inhibitors for persons with eosinophilic asthma. In: ISPOR Conference; 2019 May 18-12; New Orleans. Vol. 22. 2019:S354.
Jutel 2005 {published data only}
- Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. Journal of Allergy and Clinical Immunology 2005;116(3):608-13. 4900100000019019 [DOI] [PubMed] [Google Scholar]
Kang 1988 {published data only}
- Kang BC, Johnson J, Morgan C, Chang JL. The role of immunotherapy in cockroach asthma. Journal of Asthma 1988;25(4):205-18. 4900100000008263 [DOI] [PubMed] [Google Scholar]
Katial 2019 {published data only}
- Katial R, Siddiqui S, Barker P, Kwiatek J. Clinical efficacy characterization of benralizumab for patients with nasal polyposis and severe, uncontrolled eosinophilic asthma. Annals of Allergy, Asthma and Immunology 2019;123(5):S26. [Google Scholar]
Katial 2020 {published data only}
- Katial R, Kreindler J, Hirsch I, Newbold P, Gil EG. Blood eosinophil count and fractional exhaled nitric oxide as a combined predictor of treatment response to benralizumab for patients with severe, eosinophilic asthma. Chest 2020;158(4):A2660-1. [Google Scholar]
Kim 2020 {published data only}
- Kim MK, Park HS, Park CS, Min SJ, Albers FC, Yancey SW, et al. Efficacy and safety of mepolizumab in Korean patients with severe eosinophilic asthma from the DREAM and MENSA studies. Korean Journal of Internal Medicine 2020;36(2):362-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kips 2003 {published data only}
- Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstiens HA, Postma DS, et al. Results of a phase I trial with SCH55700, a humanized anti-IL-5 antibody, in severe persistent asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A505. [CENTRAL: 429039] [DOI] [PubMed] [Google Scholar]
- Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstjens HA, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. American Journal of Respiratory and Critical Care Medicine 2003;167(12):1655-9. 4900100000015121 [DOI] [PubMed] [Google Scholar]
Kon 2001 {published data only}
- Kon OM, Sihra BS, Loh LC, Barkans J, Compton CH, Barnes NC, et al. The effects of an anti-CD4 monoclonal antibody, keliximab, on peripheral blood CD4+ T-cells in asthma. European Respiratory Journal 2001;18(1):45-52. 4900100000011111 [4900100000011111] [DOI] [PubMed] [Google Scholar]
Kopp 2009 {published data only}
- Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann KC, Sieder C, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clinical and Experimental Allergy 2009;39(2):271-9. 4900100000023284 [DOI] [PubMed] [Google Scholar]
Kopp 2013 {published data only}
- Kopp MV, Hamelmann E, Bendiks M, Zielen S, Kamin W, Bergmann KC, et al. Transient impact of omalizumab in pollen allergic patients undergoing specific immunotherapy. Pediatric Allergy and Immunology 2013;24(5):427-33. [CENTRAL: 870929] [EMBASE: 2013483684] [PMID: ] [DOI] [PubMed] [Google Scholar]
Korenblat 2018 {published data only}
- Korenblat PE, McDonald M, Garin M. Improvements in individual asthma control questionnaire (ACQ-5) questions with reslizumab in patients with inadequately controlled asthma and elevated blood eosinophils: pooled analysis of two phase 3 trials. In: AAAAI and WAO Joint Congress; 2018 March 2-5; Orlando. Vol. 141. 2018:AB17.
Kreindler 2019 {published data only}
- Kreindler J, Katial R, Barker P, Newbold P. Benralizumab treatment is not associated with oral corticosteroid-like increases in weight and blood pressure. Annals of Allergy, Asthma and Immunology 2019;123(5):S39. [Google Scholar]
Kuang 2018 {published data only}
- Kuang FL, Alao H, Kumar S, Powers A, Quezado M, Wang Z, et al. Benralizumab (anti-IL=Ra) depletes gut tissue eosinophilia and improves symptoms in hypereosinophilic syndrome with gastrointestinal involvement. Journal of Allergy and Clinical Immunology 2018;141:AB196. [Google Scholar]
Kuang 2019 {published data only}
- Kuang FL, Legrand F, Makiya M, Ware J, Wetzler L, Brown T, et al. Benralizumab for PDGFRA-negative hypereosinophilic syndrome. New England Journal of Medicine 2019;380(14):1336-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulus 2010 {published data only}
- Kulus M, Hebert J, Garcia E, Fowler Taylor A, Fernandez Vidaurre C, Blogg M. Omalizumab in children with inadequately controlled severe allergic (IgE-mediated) asthma. Current Medical Research and Opinion 2010;26(6):1285-93. 4900100000025421 [DOI] [PubMed] [Google Scholar]
Lam 2018 {published data only}
- Lam J, Hay JW, Salcedo J, Kenyon NJ. An evaluation of the cost-effectiveness of reslizumab in poorly controlled eosinophilic asthma. Value in Health 2018;21(S235):PRS29. [Google Scholar]
Lanier 2003 {published data only}
- Lanier BQ, Corren J, Lumry W, Liu J, Fowler-Taylor A, Gupta N. Omalizumab is effective in the long-term control of severe allergic asthma. Annals of Allergy, Asthma and Immunology 2003;91(2):154-9. [CENTRAL: 440250] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lanier 2009 {published data only}
- Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. Journal of Allergy and Clinical Immunology 2009;124(6):1210-6. [CENTRAL: 731495] [EMBASE: 2009623072] [PMID: ] [DOI] [PubMed] [Google Scholar]
Laviolette 2013 {published data only}
- Gossage DL, Laviolette M, Gauvreau GM, Leigh R, Kolbeck R, Wu Y. Depletion of airway eosinophils by benralizumab an anti-IL5 receptor alpha monoclonal antibody. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A3961. [CENTRAL: 834337] [EMBASE: 71988597] [Google Scholar]
- Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. Journal of Allergy and Clinical Immunology 2013;132(5):1086-96.e5. [CENTRAL: 874819] [EMBASE: 2013693094] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00659659. A study to evaluate the safety, tolerability and effects of MEDI-563 in adults with asthma. clinicaltrials.gov/ct2/show/NCT00659659 (first received 11 April 2008).
Leckie 2000 {published data only}
- Leckie MJ, Ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000;356(9248):2144-8. 4900100000010079 [DOI] [PubMed] [Google Scholar]
Leynadier 2004 {published data only}
- Leynadier F, Doudou O, Gaouar H, Le Gros V, Bourdeix I, Guyomarch-Cocco L, et al. Effect of omalizumab in health care workers with occupational latex allergy. Journal of Allergy and Clinical Immunology 2004;113(2):360-1. 4900100000016361 [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li J, Lin C, Du J, Xiao B, Du C, Sun J, et al. The efficacy and safety of reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: a systematic review and meta-analysis. Journal of Asthma 2016;54(3):300-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lizaso 2008 {published data only}
- Lizaso MT, Tabar AI, Garcia BE, Gomez B, Algorta J, Asturias JA, et al. Double-blind, placebo-controlled alternaria alternata immunotherapy: in vivo and in vitro parameters. Pediatric Allergy and Immunology 2008;19(1):76-81. 4900100000021781 [DOI] [PubMed] [Google Scholar]
Lugogo 2016 {published data only}
- Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clinical Therapeutics 2016;38(9):2058-2070.e1. [DOI: 10.1016/j.clinthera.2016.07.010] [DOI] [PubMed] [Google Scholar]
Lugogo 2020 {published data only}
- Lugogo N, Kreindler J, Katial R, Barker P, Gil EG. Benralizumab reduces exacerbations for patients with OCS-dependent severe asthma regardless of early improvement in FEV1. Chest 2020;158(4):A44-6. [Google Scholar]
Manning 2018 {published data only}
- Manning ME, Garin M, Jiang B, Sun SX. Impact of reslizumab on duration of exacerbations in patients with severe eosinophilic asthma. Journal of Allergy and Clinical Immunology 2018;141(2):AB17. [Google Scholar]
Maselli 2017 {published data only}
- Maselli DJ, Peters JI. In severe asthma, benralizumab reduced daily oral glucocorticoid dose and asthma exacerbations at 6 months. ACP Journal Club 2017;167(8):1-1. [DOI] [PubMed] [Google Scholar]
Maspero 2016 {published data only}
- Maspero J, Jacobs J, Garin M. Improvements in asthma quality of life questionnaire (AQLQ) domains with reslizumab in patients with inadequately controlled asthma and elevated blood eosinophils. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl 1):AB15. [CENTRAL: 1135292] [EMBASE: 72196802] [Google Scholar]
Maspero 2018 {published data only}
- Maspero J, Harrison T, Werkstrom V, Wu Y, Gopalan G, Zangrilli J. Clinical efficacy of benralizumab in patients with severe, uncontrolled eosinophilic asthma and nasal polyposis: pooled analysis of the SIROCCO and CALIMA trials. Journal of Allergy and Clinical Immunology 2018;141(2):AB12. [Google Scholar]
Maspero 2019 {published data only}
- Maspero JF, Hickey L, Garin MC. Multiple components contribute to greater improvements in quality of life with iv reslizumab in the most eosinophilic asthma patients compared with the overall patient population. American Journal of Respiratory and Critical Care Medicine 2019;199:A7085. [Google Scholar]
Massanari 2009 {published data only}
- Massanari M, Kianifard F, Zeldin RK, Geba GP. Efficacy of omalizumab in cat-allergic patients with moderate-to-severe persistent asthma. Allergy and Asthma Proceedings 2009;30(5):534-9. [CENTRAL: 728392] [PMID: ] [DOI] [PubMed] [Google Scholar]
Massanari 2010 {published data only}
- Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. Journal of Allergy and Clinical Immunology 2010;125(2):383-9. 4900100000024484 [DOI] [PubMed] [Google Scholar]
Mathur 2019 {published data only}
- Mathur SK, Modena BD, Coumou H, Kamboj AP, Barker P, Kreindler J, et al. Change in post-bronchodilator FEV 1 for patients with severe asthma with eosinophilic features following benralizumab therapy. In: European Academy of Allergy and Clinical Immunology Congress; 2019 June 1-5; Lisbon. 2019.
Mathur 2020 {published data only}
- Mathur SK, Modena BD, Coumou H, Barker P, Kreindler JL, Zangrilli JG. Postbronchodilator lung function improvements with benralizumab for patients with severe asthma. Allergy 2020;75(6):1507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maunoury 2018 {published data only}
- Maunoury F, Pribil C, Aubier M, Nachbaur G, Doyle S. Cost-effectiveness of mepolizumab in severe eosinophilic asthma patients in France. Value in Health 2018;21 Suppl 3:S413. [Google Scholar]
Menzies‐Gow 2019a {published data only}
- Menzies-Gow A, Corren J, Bel E, Maspero J, Heaney L, Gurnell M, et al. Oral corticosteroid tapering during benralizumab treatment of severe, uncontrolled eosinophilic asthma: PONENTE phase IIIb clinical trial. American Journal of Respiratory and Critical Care Medicine 2019;199:A1320. [Google Scholar]
Menzies‐Gow 2019b {published data only}
- Menzies-Gow A, Corren J, Bel EH, Maspero J, Heaney LG, Gurnell M, et al. Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial. ERJ Open Research 2019;5(3):00009-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metzger 1998 {published data only}
- Metzger WJ, Fick RB, Bush RK, Busse W, Casale T, Chodosh S. Corticosteroid (CS) withdrawal in a study of recombinant humanized monoclonal antibody to IgE (rhu MAbE25) [Abstract]. Journal of Allergy and Clinical Immunology 1998;101(1 Suppl):S231. 4900100000023644 [Google Scholar]
Milgrom 1999 {published data only}
- Milgrom H, Fick RB, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. RhuMAb-E25 Study Group. New England Journal of Medicine 1999;341(26):1966-73. 4900100000007996 [DOI] [PubMed] [Google Scholar]
Milgrom 2001 {published data only}
- Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001;108(2):E36. 4900100000010744 [DOI] [PubMed] [Google Scholar]
Modlin 1977 {published data only}
- Modlin JF, Smith DH, Harding L. Clinical trials of bivalent A/New Jersey/76-A/Victoria/75 influenza vaccines in high-risk children. Journal of Infectious Diseases 1977;136 Suppl:S626-31. 4900100000000785 [DOI] [PubMed] [Google Scholar]
Moran 2020 {published data only}
- Moran AM, Ramakrishnan S, Borg CA, Connolly CM, Couillard S, Mwasuku CM, et al. Blood eosinophil depletion with mepolizumab, benralizumab, and prednisolone in eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2020;202(9):1314-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moss 1987 {published data only}
- Moss RB, Hsu YP, Kwasnicki JM, Sullivan MM, Reid MJ. Isotypic and antigenic restriction of the blocking antibody response to ryegrass pollen: correlation of rye group I antigen-specific IgG1 with clinical response. Journal of Allergy and Clinical Immunology 1987;79(2):387-98. 4900100000002212 [DOI] [PubMed] [Google Scholar]
Murphy 2018 {published data only}
- Murphy KR, McDonald M, Garin MC. Improvements in exacerbation rate and lung function with weight-based intravenous reslizumab dosing in patients with baseline high body weight. American Journal of Respiratory and Critical Care Medicine 2018;197:A1356. [Google Scholar]
Nair 2009 {published data only}
- Ayars AG, Altman LC, Potter-Perigo S, Radford K, Wight TN, Nair P. Sputum hyaluronan and versican in severe eosinophilic asthma. International Archives of Allergy and Immunology 2013;161(1):65-73. 4900100000077338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayars AG, Altman LC, Potter-Perigo S, Wight TN, Nair P. Sputum hyaluronan as a biomarker of airway remodeling in severe asthma. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB8. 4900100000027030 [Google Scholar]
- Nair P, Kjarsgaard M, Armstrong S, Efthimiadis A, O'Byrne PM, Hargreave FE. Nitric oxide in exhaled breath is poorly correlated to sputum eosinophils in patients with prednisone-dependent asthma. Journal of Allergy and Clinical Immunology 2010;126(2):404-6. [CENTRAL: 759797] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nair P, Pizzichini M, Kjarsgaard M, Inman M, Efthimiadis A, Pizzichini E, et al. Prednisone sparing effect of mepolizumab on eosinophilic bronchitis with or without asthma a randomized placebo controlled trial. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. 2008:A568[#509]. 4900100000023006
- Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. New England Journal of Medicine 2009;360(10):985-93. 4900100000023472 [DOI] [PubMed] [Google Scholar]
- NCT00292877. The prednisone-sparing effect of anti-IL-5 antibody (SB-240563). clinicaltrials.gov/ct2/show/NCT00292877 (first received 15 February 2006). 4900100000020456
Nair 2016 {published data only}
- Nair PK, Dasgupta A, Kjarsgaard M, Capaldi D, Radford K, Aleman FP, et al. Mepolizumab in COPD with eosinophilic bronchitis: a randomized clinical trial. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl 1):AB392. [CENTRAL: 1135274] [EMBASE: 72197694] [Google Scholar]
Nair 2017a {published data only}
- Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. New England Journal of Medicine 2017;376(25):2448-58. [DOI] [PubMed] [Google Scholar]
Nair 2017b {published data only}
- Nair P, Mukherjee M, Fang Lim H, Huang C, Radford K, Boulet L-P, et al. Benralizumab attenuates airway eosinophilopoietic processes in prednisone-dependent asthma. European Respiratory Journal 2017;50(61):OA3217. [Google Scholar]
Nair 2017c {published data only}
- Nair P, Wenzel SE, Rabe K-F, Bourdin A, Lugogo N, Kuna P, et al. Benralizumab significantly reduced oral corticosteroid dosages and asthma exacerbation rates for patients with severe, uncontrolled asthma: results of the ZONDA phase III trial. American Journal of Respiratory and Critical Care Medicine 2017;195:A4678. [Google Scholar]
Nair 2020 {published data only}
- Nair P, Bardin P, Humbert M, Murphy KR, Hickey L, Garin M, et al. Efficacy of intravenous reslizumab in oral corticosteroid-dependent asthma. Journal of Allergy and Clinical Immunology Practice 2020;8(2):555-64. [DOI] [PubMed] [Google Scholar]
NCT00783289 {published data only}
- NCT00783289. A phase 2a study to evaluate the safety and tolerability of MEDI-563 in adults with asthma (MEDI-563). clinicaltrials.gov/show/NCT00783289 (first received 30 October 2008).
NCT00802438 {published data only}
- NCT00802438. Eosinophilic airway inflammation and mepolizumab. clinicaltrials.gov/ct2/show/NCT00802438 (first received 4 December 2008). 4900126000001344
NCT01290887 {published data only}
- NCT01290887. Open-label extension study to evaluate the long-term safety and efficacy of reslizumab (3.0 mg/kg) as treatment for patients (12 through 75 years of age) with eosinophilic asthma. clinicaltrials.gov/show/NCT01290887 (first received 4 February 2011).
NCT01366521 {published data only}
- NCT01366521. Dose ranging pharmacokinetics and pharmacodynamics study with mepolizumab in asthma patients with elevated eosinophils. clinicaltrials.gov/ct2/show/NCT01366521 (first received 12 May 2011). 4900126000001335
NCT01471327 {published data only}
- NCT01471327. Japanese phase 1 study of mepolizumab. clinicaltrials.gov/ct2/show/NCT01471327 (first received 10 November 2011). 4900126000001343
NCT01520051 {published data only}
- NCT01520051. Mepolizumab treatment for rhinovirus-induced asthma exacerbations (MATERIAL) [The efficacy of mepolizumab treatment on rhinovirus induced asthma exacerbations]. clinicaltrials.gov/show/NCT01520051 (first received 25 January 2012). 4900126000001342
NCT01691859 {published data only}
- NCT01691859. MEA112997 open-label long term extension safety study of mepolizumab in asthmatic subjects. clinicaltrials.gov/ct2/show/NCT01691859 (first received 13 September 2012). 4900126000001338
NCT01842607 {published data only}
- NCT01842607. A study to determine long-term safety of mepolizumab in asthmatic subjects. clinicaltrials.gov/ct2/show/NCT01842607 (first received 25 April 2013). 4900126000001336
NCT02020889 {published data only}
- NCT02020889. A study to investigate mepolizumab in the treatment of eosinophilic granulomatosis with polyangiitis. clinicaltrials.gov/ct2/show/NCT02020889 (first received 19 December 2013).
NCT02075255 {published data only}
- NCT02075255. Efficacy and safety study of benralizumab to reduce OCS use in patients with uncontrolled asthma on high dose inhaled corticosteroid plus LABA and chronic OCS therapy. clinicaltrials.gov/show/NCT02075255 (first received 14 February 2014).
NCT02135692 {published data only}
- NCT02135692. A Phase 3a, repeat dose, open-label, long-term safety study of mepolizumab in asthmatic subjects. clinicaltrials.gov/show/NCT02135692 (first received 8 May 2014).
NCT02258542 {published data only}
- NCT02258542. A safety extension study to evaluate the safety and tolerability of benralizumab (MEDI-563) in asthmatic adults and adolescents on inhaled corticosteroid plus LABA (BORA). clinicaltrials.gov/show/NCT02258542 (first received 14 September 2014).
NCT02293265 {published data only}
- NCT02293265. Cross-sectional study for identification and description of severe asthma patients. clinicaltrials.gov/show/NCT02293265 (first received 27 October 2014).
NCT02377427 {published data only}
- NCT02377427. Pharmacokinetics and pharmacodynamics of mepolizumab administered subcutaneously in children. clinicaltrials.gov/ct2/show/NCT02377427 (first received 26 February 2015).
NCT02417961 {published data only}
- NCT02417961. Study to assess functionality, reliability, and performance of a pre-filled syringe with benralizumab administered at home. clinicaltrials.gov/show/NCT02417961 (first received 12 March 2015).
NCT02501629 {published data only}
- NCT02501629. An efficacy and safety study of reslizumab subcutaneous in patients with oral corticosteroid dependent asthma and elevated blood eosinophils. clinicaltrials.gov/show/NCT02501629 (first received 14 July 2015).
NCT02559791 {published data only}
- NCT02559791. Anti-interleukin-5 (IL5) monoclonal antibody (MAb) in prednisone-dependent eosinophilic asthma. clinicaltrials.gov/ct2/show/NCT02559791 (first received 22 September 2015).
NCT02594332 {published data only}
- NCT02594332. Effects of mepolizumab compared to placebo on airway physiology in patients with eosinophilic asthma: MEMORY study (MEMORY). clinicaltrials.gov/show/NCT02594332 (first received 31 August 2015).
NCT02654145 {published data only}
- NCT02654145. Omalizumab to mepolizumab switch study in severe eosinophilic asthma patients. clinicaltrials.gov/ct2/show/NCT02654145 (first received 11 January 2016).
NCT02808819 {published data only}
- NCT02808819. A safety extension study with benralizumab for asthmatic adults on inhaled corticosteroid plus long-acting beta 2 agonist (MELTEMI). clinicaltrials.gov/show/NCT02808819 (first received 7 June 2016).
NCT02814643 {published data only}
- NCT02814643. Study to evaluate the potential effect of benralizumab on the humoral immune response to the seasonal influenza vaccination in adolescent and young adult patients with severe asthma (ALIZE). clinicaltrials.gov/show/NCT02814643 (first received 15 June 2016).
NCT02821416 {published data only}
- NCT02821416. Study to evaluate the effect of benralizumab on allergen-induced inflammation in mild, atopic asthmatics (ARIA). clinicaltrials.gov/show/NCT02821416 (first received 17 June 2016).
NCT02869438 {published data only}
- NCT02869438. A study to evaluate the onset of effect and time course of change in lung function with benralizumab in severe, uncontrolled asthma patients with eosinophilic inflammation. clinicaltrials.gov/ct2/show/NCT02869438 (first received 16 August 2016).
NCT02937168 {published data only}
- NCT02937168. An imaging study using PET/CT to characterize the effect of intravenous reslizumab on airway inflammation. clinicaltrials.gov/show/NCT02937168 (first received 14 October 2016).
NCT02968914 {published data only}
- NCT02968914. Pharmacokinetic comparability of benralizumab using accessorized pre-filled syringe or autoinjector in healthy volunteers. clinicaltrials.gov/show/NCT02968914 (first received 28 October 2016).
NCT03014674 {published data only}
- NCT03014674. A study to compare the pharmacokinetics of mepolizumab as a liquid drug in a safety syringe or an autoinjector versus lyophilised drug. clinicaltrials.gov/show/NCT03014674 (first received 15 December 2016).
NCT03021304 {published data only}
- NCT03021304. Study of mepolizumab safety syringe in asthmatics. clinicaltrials.gov/ct2/show/NCT03021304 (first received 12 January 2017).
NCT03476109 {published data only}
- NCT03476109. Study of magnitude and prediction of response to omalizumab and mepolizumab in adult severe asthma (PREDICTUMAB) [Predictive factors and magnitude of response to omalizumab and mepolizumab in allergic and eosinophilic severe asthma: a pragmatic multicenter trial in Belgium]. clinicaltrials.gov/ct2/show/NCT03476109 (first received 23 March 2018).
NCT04617171 {published data only}
- NCT04617171. Benralizumab initiated during severe asthma attack [Randomized double blind placebo controlled trial of benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, initiated during hospitalization for severe asthma attack in reducing severe exacerbations: phase 2B study]. clinicaltrials.gov/ct2/show/ (first received 5 November 2020).
NCT04710134 {published data only}
- NCT04710134. Efficacy of reslizumab dose escalation in patients with severe asthma. https://ClinicalTrials.gov/show/NCT04710134 ( first received 14 January 2022).
Nelsen 2019 {published data only}
- Nelsen LM, Cockle SM, Gunsoy NB, Jones P, Albers FC, Bradford ES, et al. Impact of exacerbations on St George's Respiratory Questionnaire score in patients with severe asthma: post hoc analyses of two clinical trials and an observational study. Journal of Asthma 2019;9:1-11. [DOI] [PubMed] [Google Scholar]
Newbold 2016 {published data only}
- Newbold P, Liu H, Pham T-H, Damera G, Sridhar S. Modulation of inflammation by benralizumab in eosinophilic airway disease. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016:[PA4902].
Newbold 2017 {published data only}
- Newbold P, Hirsch I, Trudo F, Goldman M. Effects of immunoglobin e concentration, eosinophil concentration, and atopy status on benralizumab efficacy in asthma. Annals of Allergy, Asthma and Immunology 2017;119(5):S56. [DOI] [PubMed] [Google Scholar]
Niven 2008 {published data only}
- Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respiratory Medicine 2008;102(10):1371-8. 4900100000023545 [DOI] [PubMed] [Google Scholar]
Noga 2003 {published data only}
- Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. International Archives of Allergy and Immunology 2003;131(1):46-52. 4900100000015254 [4900100000015254] [DOI] [PubMed] [Google Scholar]
Noga 2008 {published data only}
- Noga O, Hanf G, Kunkel G, Kleine-Tebbe J. Basophil histamine release decreases during omalizumab therapy in allergic asthmatics. International Archives of Allergy and Immunology 2008;146(1):66-70. [CENTRAL: 628876] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noonan 2013 {published data only}
- Noonan M, Korenblat P, Mosesova S, Scheerens H, Arron JR, Zheng Y, et al. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. Journal of Allergy and Clinical Immunology 2013;132(3):567-74.e12. [CENTRAL: 872124] [EMBASE: 2013545353] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nowak 2015 {published data only}
- Molfino NA, Nowak R, Silverman RA, Rowe BH, Smithline H, Khan F. Reduction in the number and severity of exacerbations following acute severe asthma: results of a placebo-controlled, randomized clinical trial with benralizumab. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2753. [CENTRAL: 834405] [EMBASE: 71987342] [Google Scholar]
- NCT00768079. A phase 2 study to evaluate the safety and efficacy of intravenously administered MEDI-563 (MEDI-563). clinicaltrials.gov/ct2/show/NCT00768079 (first received 3 October 2008).
- Nowak RM, Parker JM, Silverman RA, Rowe BH, Smithline H, Khan F, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor alpha monoclonal antibody, after acute asthma. American Journal of Emergency Medicine 2015;33(1):14-20. [CENTRAL: 1038284] [EMBASE: 2014976517] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Quinn 2019 {published data only}
- O’Quinn S, Xiao Xu X, Hirsch I. Daily patient-reported health status assessment improvements with benralizumab for patients with severe, uncontrolled eosinophilic asthma. Journal of Asthma and Allergy 2019;12:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oba 2004 {published data only}
- Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. Journal of Allergy and Clinical Immunology 2004;114(2):265-9. 4900100000016686 [DOI] [PubMed] [Google Scholar]
Oh 2013 {published data only}
- Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respiratory Research 2013;14:93. [CENTRAL: 871533] [EMBASE: 2013599844] [4900100000090024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohashi 1997 {published data only}
- Ohashi Y, Nakai Y, Tanaka A, Kakinoki Y, Ohno Y, Masamoto T, et al. Serum levels of specific IgE, soluble interleukin-2 receptor, and soluble intercellular adhesion molecule-1 in seasonal allergic rhinitis. Annals of Allergy, Asthma and Immunology 1997;79(3):213-20. 4900100000005807 [DOI] [PubMed] [Google Scholar]
Ohman 1984 {published data only}
- Ohman JL, Findlay SR, Leitermann KM. Immunotherapy in cat-induced asthma. Double-blind trial with evaluation of in vivo and in vitro responses. Journal of Allergy and Clinical Immunology 1984;74(3 Pt 1):230-9. 4900100000007076 [DOI] [PubMed] [Google Scholar]
Ohta 2009 {published data only}
- Ohta K, Miyamoto T, Amagasaki T, Yamamoto M, 1304 Study Group. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology 2009;14(8):1156-65. 4900100000024432 [DOI] [PubMed] [Google Scholar]
Ohta 2017 {published data only}
- Ohta K, Adachi M, Tohda Y, Kamei T, Kato M, Mark Fitzgerald J, et al. Efficacy and safety of benralizumab in Japanese patients with severe, uncontrolled eosinophilic asthma. Allergology International 2018;67(2):266‐72. [DOI] [PubMed] [Google Scholar]
Ong 2005 {published data only}
- Ong YE, Menzies-Gow A, Barkans J, Benyahia F, Ou TT, Ying S, et al. Anti-IgE (omalizumab) inhibits late-phase reactions and inflammatory cells after repeat skin allergen challenge. Journal of Allergy and Clinical Immunology 2005;116(3):558-64. 4900100000019018 [DOI] [PubMed] [Google Scholar]
Ortega 2018a {published data only}
- Ortega H, Albers F, Llanos JP, Bradford E, Price G, Pouliquen I, Castro M. Impact of weight on the efficacy of mepolizumab in patients with severe eosinophilic asthma. Pneumologie 2018;72:S01. [DOI: 10.1055/s-0037-1619160] [DOI] [Google Scholar]
Ortega 2018b {published data only}
- Ortega H, Menzies-Gow A, Llanos-Ackert J-P, Forshag MS, Albers FC, Gunsoy N, et al. Improvement of lung function measured by AM PEF in patients with severe eosinophilic asthma treated with mepolizumab: a combined analysis of the MENSA and MUSCA studies. Journal of Allergy and Clinical Immunology 2018;141(2):AB16. [Google Scholar]
Panettieri 2017 {published data only}
- Panettieri R, McDonald M, Germinaro M. Efficacy of reslizumab in eosinophilic asthma patients with low lung function. Annals of Allergy, Asthma and Immunology 2017;119(5):S54. [Google Scholar]
Park 1998 {published data only}
- Park CS, Choi YS, Ki SY, Moon SH, Jeong SW, Uh ST, et al. Granulocyte macrophage colony-stimulating factor is the main cytokine enhancing survival of eosinophils in asthmatic airways. European Respiratory Journal 1998;12(4):872-8. [CENTRAL: 156846] [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2018 {published data only}
- Park H-S, Lee SH, Werkstrom V, Wu Y, Zangrilli J, Gopalan G. Benralizumab reduces exacerbations and improves lung function in patients from republic of Korea with severe, uncontrolled asthma: subgroup analysis of the SIROCCO Trial. Journal of Allergy and Clinical Immunology 2018;141(2):AB14. [Google Scholar]
Park 2019 {published data only}
- Park HS, Lee SH, Lee SY, Kim MK, Lee BJ, Werkstrom V, et al. Efficacy and safety of benralizumab for Korean patients with severe, uncontrolled eosinophilic asthma. Allergy, Asthma and Immunology Research 2019;11(4):508-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2010 {published data only}
- Parker J, Brazinsky S, Miller DS, Nayak A, Korenblat PE, Sari S, et al. Randomized, double-blind, placebo-controlled, multicenter phase 2A study to evaluate the effect of a humanized interleukin-9 monoclonal antibody (MEDI-528) on exercise-induced bronchospasm. American Journal of Respiratory and Critical Care Medicine 2010;181:A5394. 4900100000025282 [Google Scholar]
Pauli 1984 {published data only}
- Pauli G, Bessot JC, Bigot H, Delaume G, Hordle DA, Hirth C, et al. Clinical and immunologic evaluation of tyrosine-adsorbed Dermatophagoides pteronyssinus extract: a double-blind placebo-controlled trial. Journal of Allergy and Clinical Immunology 1984;74(4 Pt 1):524-35. 4900100000007226 [DOI] [PubMed] [Google Scholar]
Pavord 2012 {published data only}
- Albers FC, Price RG, Yancey SW, Bradford E. Efficacy of mepolizumab in reducing exacerbations in patients with severe eosinophilic asthma who would be eligible for omalizumab treatment. In: 35th Annual Congress of the European Academy of Allergy and Clinical Immunology; 2016 11-15 June; Vienna. Vol. 71. 2016:66-7.
Pelaia 2016 {published data only}
- Pelaia G, Vatrella A, Busceti MT, Gallelli L, Preiano M, Lombardo N, et al. Role of biologics in severe eosinophilic asthma - focus on reslizumab. Therapeutics and Clinical Risk Management 2016;12:1075-82. [EMBASE: 20160514755] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pham 2016 {published data only}
- Pham T-H, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respiratory Medicine 2016;111:21-9. [CENTRAL: 1139843] [EMBASE: 20160044298] [DOI] [PubMed] [Google Scholar]
Piper 2012 {published data only}
- Piper E, She D, Molfino NA. Subgroup analysis of a phase 2A randomized, double-blind, placebo-controlled study of tralokinumab, an anti-IL-13 monoclonal antibody, in moderate to severe asthma. American Journal of Respiratory and Critical Care Medicine 2012;185:A2759. [CENTRAL: 1107530] [EMBASE: 71987348] [Google Scholar]
Piper 2013 {published data only}
- Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. European Respiratory Journal 2013;41(2):330-8. 4900100000073595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Pouliquen 2015 {published data only}
- Pouliquen IJ, Kornmann O, Barton SV, Price JA, Ortega HG. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizumab. International Journal of Clinical Pharmacology and Therapeutics 2015;53(12):1015-27. [CENTRAL: 1096155] [EMBASE: 20160138058] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pouliquen 2016 {published data only}
- Pouliquen I, Austin D, Gunsoy N, Yancey S. A weight-based exacerbation dose-response analysis of mepolizumab in severe asthma with eosinophilic phenotype [PA4106]. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016.
Prazma 2016 {published data only}
- Prazma CM, Bel EH, Barnes NC, Price R, Albers FC, Yancey SW. Steroid sparing response with mepolizumab: durability of steroid reduction in severe asthma. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl 1):AB16. [CENTRAL: 1135291] [EMBASE: 72196807] [Google Scholar]
Prieto 2006 {published data only}
- Prieto L, Gutierrez V, Colas C, Tabar A, Perez-Frances C, Bruno L, et al. Effect of omalizumab on adenosine 5'-monophosphate responsiveness in subjects with allergic asthma. International Archives of Allergy and Immunology 2006;139(2):122-31. 4900100000019265 [DOI] [PubMed] [Google Scholar]
Pui 2010 {published data only}
- Pui M, Lay JC, Alexis NE, Carlsten C. Flow cytometry to identify leukocyte sub-populations in blood and induced sputum in asthmatic and healthy volunteers exposed to diesel exhaust. Allergy, Asthma, and Clinical Immunology 2010;6 Suppl 3:P7. 4900100000054152 [Google Scholar]
Ranade 2015 {published data only}
- Ranade K, Manetz S, Liang M, Lee R, Kuziora M, She D, et al. Effect of tralokinumab on serum periostin and IgE levels in uncontrolled severe asthma. European Respiratory Journal 2015;46 Suppl 59:OA1770. [CENTRAL: 1135122] [EMBASE: 72106787] [Google Scholar]
Rose 2009 {published data only}
- Rose MA, Gruendler M, Schubert R, Kitz R, Schulze J, Zielen S. Safety and immunogenicity of sequential pneumococcal immunization in preschool asthmatics. Vaccine 2009;27(38):5259-64. 4900100000024282 [DOI] [PubMed] [Google Scholar]
Roskos 2018 {published data only}
- Roskos L, Wang B, Yan L, Yu B, Barker P, Goldman M. Longitudinal modeling of prebronchodilator FEV1 response to benralizumab for patients with severe asthma. Pneumologie 2018;72(S 01):S14-15. [Google Scholar]
Roufosse 2020 {published data only}
- Roufosse F, Kahn J-E, Gleich GJ, Rothenberg ME, Wardlaw AJ, Yun Kirby S, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. Hemasphere 2020;4:67-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakamoto 1984 {published data only}
- Sakamoto Y, Nakagawa T, Ito K, Miyamoto T. Solid-phase radioimmunoassay for the measurement of IgG antibodies specific for the house dust mite, Dermatophagoides farinae. Annals of Allergy 1984;52(4):303-8. 4900100000008622 [PubMed] [Google Scholar]
Scheerens 2011 {published data only}
- Scheerens H, Arron JR, Su Z, Zheng Y, Putnam W, Erickson RW, et al. Predictive and pharmacodynamic biomarkers of interleukin-13 blockade: effect of lebrikizumab on late phase asthmatic response to allergen challenge. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB164. 4900100000026957 [Google Scholar]
Scheerens 2012 {published data only}
- Scheerens H, Arron J, Choy D, Mosesova S, Lal P, Matthews J. Lebrikizumab reduces serum periostin in asthma patients with elevated baseline periostin. European Respiratory Journal 2012;40 Suppl 56:387s [P2167]. [CENTRAL: 839426] [EMBASE: 71926597] [Google Scholar]
Scheerens 2014 {published data only}
- Scheerens H, Arron JR, Zheng Y, Putnam WS, Erickson RW, Choy DF, et al. The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clinical and Experimental Allergy 2014;44(1):38-46. [CENTRAL: 961626] [EMBASE: 2013812053] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2019 {published data only}
- Shaw D, Menzies-Gow A, Bourdin A, Barker P, Garcia GE. Reduced long-term cumulative OCS exposure for benralizumab-treated patients with severe asthma. Thorax 2019;74(2):A136-7. [Google Scholar]
Shrimanker 2018 {published data only}
- Shrimanker R, Pavord ID, Price R, Bradford ES, Keene ON, Yancey S. Fractional exhaled nitric oxide and the peripheral blood eosinophil count as biomarkers of the response to mepolizumab in patients with severe eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2018;197:A5964. [Google Scholar]
Shrimanker 2019 {published data only}
- Shrimanker R, Price R, Wenzel SE, Yancey S, Pavord I. Fractional exhaled nitric oxide and peripheral blood eosinophil count prognostic and predictive biomarkers in severe eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2019;199:A1296. [DOI] [PubMed] [Google Scholar]
Siergiejko 2011 {published data only}
- Siergiejko Z, Swiebocka E, Smith N, Peckitt C, Leo J, Peachey G, et al. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Current Medical Research and Opinion 2011;27(11):2223-8. 4900100000056233 [DOI] [PubMed] [Google Scholar]
Silk 1998 {published data only}
- Silk H, Zora J, Goldstein J, Tinkelman D, Schiffman G. Response to pneumococcal immunization in children with and without recurrent infections. Journal of Asthma 1998;35(1):101-12. 4900100000008189 [DOI] [PubMed] [Google Scholar]
Silkoff 2004 {published data only}
- Silkoff PE, Romero FA, Gupta N, Townley RG, Milgrom H. Exhaled nitric oxide in children with asthma receiving Xolair (omalizumab), a monoclonal anti-immunoglobulin E antibody. Pediatrics 2004;113(4):e308-12. 4900100000016337 [DOI] [PubMed] [Google Scholar]
Simoes 2007 {published data only}
- Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. Journal of Pediatrics 2007;151(1):34-42, 42.e1. 4900100000023930 [DOI] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh D, Kane B, Molfino NA, Faggioni R, Roskos L, Woodcock A. A phase 1 study evaluating the pharmacokinetics, safety and tolerability of repeat dosing with a human IL-13 antibody (CAT-354) in subjects with asthma. BMC Pulmonary Medicine 2010;10:3. 4900100000024732 [DOI] [PMC free article] [PubMed] [Google Scholar]
Slavin 2009 {published data only}
- Slavin RG, Ferioli C, Tannenbaum SJ, Martin C, Blogg M, Lowe PJ. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. Journal of Allergy and Clinical Immunology 2009;123(1):107-113.e3. 4900100000022846 [4900100000022846] [DOI] [PubMed] [Google Scholar]
Soler 2001 {published data only}
- Soler M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. European Respiratory Journal 2001;18(2):254-61. 4900100000011115 [DOI] [PubMed] [Google Scholar]
Sorkness 2013 {published data only}
- Sorkness CA, Wildfire JJ, Calatroni A, Mitchell HE, Busse WW, O'Connor GT, et al. Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. Journal of Allergy and Clinical Immunology in Practice 2013;1(2):163-71. [CENTRAL: 991371] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinfeld 2020 {published data only}
- Steinfeld J, Rofousse F, Kahn J, Gleich G, Rothenberg M, Wardlaw A, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine 2020;201:A4212. [Google Scholar]
Sthoeger 2007 {published data only}
- Sthoeger ZM, Eliraz A, Asher I, Berkman N, Elbirt D. The beneficial effects of Xolair (Omalizumab) as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available treatment (GINA 2002 step IV - the Israeli arm of the INNOVATE study). Israel Medical Association Journal 2007;9(6):472-5. 4900100000021263 [PubMed] [Google Scholar]
Sugaya 1994 {published data only}
- Sugaya N, Nerome K, Ishida M, Matsumoto M, Mitamura K, Nirasawa M. Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well-matched type B. JAMA 1994;272(14):1122-6. 4900100000004523 [PubMed] [Google Scholar]
Swanson 2014 {published data only}
- Swanson BN, Wang L, Ming J, Hamilton JD, Teper A, Dicioccio T, et al. Exhaled nitric oxide (FENO) and t-helper 2 cell biomarkers: can they predict treatment response to dupilumab, an il-4ra antibody, in an eosinophilic asthma population? Journal of Allergy and Clinical Immunology 2014;133(2 Suppl):AB85. [CENTRAL: 985863] [EMBASE: 71351035] [Google Scholar]
Szymaniak 1998 {published data only}
- Szymaniak L. An attempt to block histamine release from basophils granulocytes with antibodies obtained as a result of long-term immunization [Proba blokowania uwalniania histaminy z granulocytow zasadochlonnych przeciwcialami uzyskanymi w wyniku dlugotrwalej immunizacji bakteriami]. Annales Academiae Medicae Stetinensis 1998;44:45-64. 4900100000006192 [PubMed] [Google Scholar]
Tanaka 1993 {published data only}
- Tanaka Y, Ueda K, Miyazaki C, Nakayama M, Kusuhara K, Okada K, et al. Trivalent cold recombinant influenza live vaccine in institutionalized children with bronchial asthma and patients with psychomotor retardation. Pediatric Infectious Disease Journal 1993;12(7):600-5. 4900100000004155 [DOI] [PubMed] [Google Scholar]
Terr 1969 {published data only}
- Terr AI. Immunologic bases for injection therapy of allergic diseases. Medical Clinics of North America 1969;53(6):1257-64. 4900100000000151 [PubMed] [Google Scholar]
Vanlandingham 2019 {published data only}
- Vanlandingham RG, Gubbi A, Corren J. Variability in absolute blood eosinophil (EOS) counts over 52 weeks in patients with inadequately controlled eosinophilic asthma receiving placebo in two duplicate clinical trials. Journal of Allergy and Clinical Immunology 2019;143(2):AB98. [Google Scholar]
Van Rensen 2009 {published data only}
- Van Rensen EL, Evertse CE, Van Schadewijk WA, Van Wijngaarden S, Ayre G, Mauad T, et al. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti-IgE treatment. Allergy 2009;64(1):72-80. 4900100000023512 [DOI] [PubMed] [Google Scholar]
Vignola 2004 {published data only}
- Vignola AM, Humbert M, Bousquet J, Boulet LP, Hedgecock S, Blogg M, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 2004;59(7):709-17. 4900100000017155 [DOI] [PubMed] [Google Scholar]
Virchow 2016 {published data only}
- Virchow JC, Zangrilli J, Weiss S, Korn S. Reslizumab (RES) in patients (pts) with inadequately controlled asthma and elevated blood eosinophils (EOS): analysis of two phase 3, placebo-controlled trials [OA1797]. In: European Respiratory Society 26th Annual Congress; 2016 Sep 3-7; London. 2016.
Virchow 2017 {published data only}
- Virchow JC, Garin M, McDonald M, Buhl R. Clinically meaningful FEV1 response with reslizumab achieved early and sustained over 52 weeks. European Respiratory Journal 2017;50:PA4690. [Google Scholar]
Virchow 2019 {published data only}
- Virchow JC, Hickey L, Garin M. High peripheral blood eosinophil (EOS) levels are associated with low FEV1 reversibility (REV) in patients with severe eosinophilic asthma. Journal of Allergy and Clinical Immunology 2019;143(2):AB5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virchow 2020 {published data only}
- Virchow JC, Hickey L, Du E, Garin M. In patients with severe asthma with eosinophilia in reslizumab clinical trials, high peripheral blood eosinophil levels are associated with low FEV1 reversibility. Allergy and Asthma Clinical Immunology 2020;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virchow 2020a {published data only}
- Virchow JC, McDonald M, Garin M, Korn S. Reslizumab as add-on therapy in patients with refractory asthma. BMJ Open Respiratory Research 2020;7:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang B, Yan L, Hutmacher M, Roskos L. Pharmacometrics enabled rational determination of optimal dosing regimen for benralizumab pivotal studies in adults and adolescents with asthma. Clinical Pharmacology and Therapeutics 2015;97:S78. [CENTRAL: 1066945] [EMBASE: 71771470] [Google Scholar]
Wang 2017 {published data only}
- Wang B, Yan L, Yu B, Barker P, Goldman M, Roskos L. Longitudinal modeling of prebronchodilator FEV1 response to benralizumab for patients with severe asthma. European Respiratory Journal 2017;50:PA3968. [Google Scholar]
Wark 2003 {published data only}
- Wark PA, Hensley MJ, Saltos N, Boyle MJ, Toneguzzi RC, Epid GD, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2003;111(5):952-7. 4900100000015247 [DOI] [PubMed] [Google Scholar]
Wechsler 2017 {published data only}
- Wechsler M, Garin M, McDonald M, Korn S. Reslizumab for uncontrolled eosinophilic asthma in patients who experienced a single exacerbation in the previous year: sub-analysis of two phase 3 trials. European Respiratory Journal 2017;50:PA3960. [Google Scholar]
Wechsler 2017a {published data only}
- Wechsler ME, Korn S, Sun SX, Garin M. Impact of reslizumab on healthcare resource utilization in adult patients with severe eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2017;195:A2639. [Google Scholar]
Wechsler 2019 {published data only}
- Wechsler M, Hickey L, Garin MC, Chauhan A. Response rate improvement and systemic corticosteroid burden reduction with intravenous (IV) reslizumab in a subgroup of asthma patients with GINA 4/5 severity and >=2 clinical asthma exacerbations (CAEs) in the prior 12 months. American Journal of Respiratory and Critical Care Medicine 2019;199:A1271. [Google Scholar]
Wechsler 2020 {published data only}
- Wechsler ME, Hickey L, Garin M, Chauhan A. Efficacy of reslizumab treatment in exacerbation-prone patients with severe eosinophilic asthma. Journal of Allergy and Clinical Immunology Practice 2020;8(10):3434-42. [DOI] [PubMed] [Google Scholar]
Weinstein 2016 {published data only}
- Weinstein SF, Germinaro M, Bardin P, Korn S, Bateman ED. Efficacy of reslizumab with asthma, chronic sinusitis with nasal polyps and elevated blood eosinophils. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl 1):AB86. [CENTRAL: 1135290] [EMBASE: 72197039] [Google Scholar]
Wenzel 2009 {published data only}
- Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. American Journal of Respiratory and Critical Care Medicine 2009;179(7):549-58. 4900100000023441 [4900100000023441] [DOI] [PubMed] [Google Scholar]
Wenzel 2013 {published data only}
- Wenzel SE, Pirozzi G, Wang L, Kirkesseli S, Rocklin R, Radin A, et al. Efficacy and safety of SAR231893/REGN668 in patients with moderate-to-severe, persistent asthma and elevated eosinophil levels. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A6068. [CENTRAL: 870803] [EMBASE: 71984701] [Google Scholar]
Wenzel 2013a {published data only}
- Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. New England Journal of Medicine 2013;368(26):2455-66. 4900100000076900 [DOI] [PubMed] [Google Scholar]
Wenzel 2014 {published data only}
- Wenzel SE, Teper A, Wang L, Pirozzi G, Radin A, Graham N, et al. ACQ5 improvement with dupilumab in patients with persistent asthma and elevated eosinophil levels: responder analysis from a 12-week proof-of-concept placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A1323. [CENTRAL: 1131452] [EMBASE: 72043773] [Google Scholar]
Xu 2017 {published data only}
- Xu X, O'Quinn S, Hirsch I, Gopalan G. Asthma symptom improvements with benralizumab are associated with improvements in activity functions and quality of life for patients with severe, uncontrolled asthma: results of pooled phase iii benralizumab studies. American Journal of Respiratory and Critical Care Medicine 2017;195:A4682. [Google Scholar]
Xu 2018 {published data only}
- Xu X, O'Quinn S, Hirsch I, Gopalan G. Asthma symptom improvements with benralizumab are associated with improvements in activity function and quality of life for patients with severe, uncontrolled asthma. Pneumologie 2018;72(S 01):S15-16. [Google Scholar]
Yan 2015 {published data only}
- Yan L, Roskos L, Ward CK, She D, Merwe R, Wang B. Pharmacokinetics and pharmacodynamics of benralizumab in subjects with moderate-to-severe chronic obstructive pulmonary disease. Clinical Pharmacology and Therapeutics 2015;97:S95. [CENTRAL: 1066944] [EMBASE: 71771521] [Google Scholar]
Yancey 2017 {published data only}
- Yancey S, Albers FC, Bradford ES, Keene ON, Price R. Mepolizumab in patients ≥3 exacerbations and eosinophil count ≥300 cells/μL. European Respiratory Journal 2017;50:PA3958. [Google Scholar]
Yancey 2020 {published data only}
- Yancey S, Corren J, Price R, Castro M. Exacerbation reduction in patients based upon baseline eosinophil counts and FEV1 reversibility. Journal of Allergy and Clinical Immunology 2020;145(2):AB26. [Google Scholar]
Zangrilli 2019 {published data only}
- Zangrilli JG, Wenzel S, Brusselle G, Hirsch I, Rastogi. Benralizumab efficacy in patients with uncontrolled eosinophilic asthma by age at diagnosis. Pneumologie 2019;73:S 01. [DOI: 10.1055/s-0039-1678224] [DOI] [Google Scholar]
Zetterstrom 1972 {published data only}
- Zetterstrom O, Fagerberg E, Wide L. An investigation of pollen extracts from different deciduous trees in patients with springtime allergy in Sweden. Acta Allergologica 1972;27(1):15-21. 4900100000008122 [DOI] [PubMed] [Google Scholar]
Zhu 2013 {published data only}
- Zhu R, Zheng Y, Putnam WS, Visich J, Eisner MD, Matthews JG, et al. Population-based efficacy modelling of omalizumab in patients with severe allergic asthma inadequately controlled with standard therapy. AAPS Journal 2013;15(2):559-70. 4900126000000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
Zielen 2013 {published data only}
- Zielen S, Lieb A, De La Motte S, Wagner F, Monchy J, Fuhr R, et al. Omalizumab protects against allergen-induced bronchoconstriction in allergic (immunoglobulin E-mediated) asthma. International Archives of Allergy and Immunology 2013;160(1):102-10. 4900100000072111 [DOI] [PubMed] [Google Scholar]
Additional references
Agache 2020
- Agache I, Beltran J, Akdis C, Akdis M, Canelo-Aybar C, Canonica GW, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy 2020;75(5):1023-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bourdin 2021
- Bourdin A, Shaw D, Menzies-Gow A, FitzGerald JM, Bleecker ER, Busse WW, et al. Two-year integrated steroid-sparing analysis and safety of benralizumab for severe asthma. Journal of Asthma 2021;58(4):514-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brusselle 2013
- Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nature Medicine 2013;19(8):977-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Busse 2010
- Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. Journal of Allergy and Clinical Immunology 2010;125(6):1237-1244.e2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Busse 2019a
- Busse W, Chupp G, Nagase H, Albers FC, Doyle S, Shen Q, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. Journal of Allergy and Clinical Immunology 2019;143(1):190-200. [PMID: ] [DOI] [PubMed] [Google Scholar]
Busse 2019b
- Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Sproule S, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respiratory Medicine 2019;7(1):46-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cabon 2017
- Cabon Y, Molinari N, Marin G, Vachier I, Gamez AS, Chanez P, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clinical and Experimental Allergy 2017;47(1):129-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Calzetta 2019
- Calzetta L, Matera MG, Rogliani P. Monoclonal antibodies in severe asthma: is it worth it? Expert Opinion on Drug Metabolism and Toxicology 2019;15(6):517-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Casale 2019
- Casale TB, Pacou M, Mesana L, Farge G, Sun SX, Castro M. Reslizumab compared with benralizumab in patients with eosinophilic asthma: a systematic literature review and network meta-analysis. Journal of Allergy and Clinical Immunology 2019;7(1):122-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chung 2015
- Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet 2015;386(9998):1086-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed prior to 7 June 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Custovic 2013
- Custovic A, Johnston SL, Pavord I, Gaga M, Fabbri L, Bel EH, et al. EAACI position statement on asthma exacerbations and severe asthma. Allergy 2013;68(12):1520-31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Edris 2019
- Edris A, De Feyter S, Maes T, Joos G, Lahousse L. Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis. Respiratory Research 2019;20(1):179. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edris 2021
- Edris A, Lahousse L. Monoclonal antibodies in type 2 asthma: an updated network meta-analysis. Minerva Medica 2021;112(5):573-81. [DOI: 10.23736/S0026-4806.21.07623-0] [DOI] [PubMed] [Google Scholar]
Enright 2004
- Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. American Journal of Respiratory and Critical Care Medicine 2004;169(2):235-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farooqui 2009
- Farooqui N, Khan BQ, Wan JY, Lieberman P. Blood eosinophils as markers of inflammation in asthma. Annals of Allergy, Asthma, and Immunology 2009;103(5):A56. [Google Scholar]
GBD Study 2017
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respiratory Medicine 2017;5(9):691-706. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2021
- Global Initiative for Asthma (GINA). Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021. www.ginasthma.org 2021.
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed before 8 June 2022. Hamilton (ON): McMaster university (developed by Evidence Prime). Available at gradepro.org.
He 2018
- He LL, Zhang L, Jiang L, Xu F, Fei DS. Efficacy and safety of anti-interleukin-5 therapy in patients with asthma: a pairwise and Bayesian network meta-analysis. International Immunopharmacology 2018;64:223-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Henriksen 2018
- Henriksen DP, Bodtger U, Sidenius K, Maltbaek N, Pedersen L, Madsen H, et al. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma - a systematic review and meta-analysis. European Clinical Respiratory Journal 2018;5(1):1536097. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Humbert 1997
- Humbert M, Corrigan CJ, Kimmitt P, Till SJ, Kay AB, Durham SR. Relationship between IL-4 and IL-5 mRNA expression and disease severity in atopic asthma. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):704-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Iftikhar 2018
- Iftikhar IH, Schimmel M, Bender W, Swenson C, Amrol D. Comparative efficacy of anti IL-4, IL-5 and IL-13 drugs for treatment of eosinophilic asthma: a network meta-analysis. Lung 2018;196(5):517-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jatakanon 2000
- Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. American Journal of Respiratory and Critical Care Medicine 2000;161(1):64-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jones 1991
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respiratory Medicine 1991;85:25-31. [DOI] [PubMed] [Google Scholar]
Juniper 1992
- Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992;47(2):76-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Juniper 1999
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. European Respiratory Journal 1999;14(4):902-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kavanagh 2021
- Kavanagh JE, Hearn AP, Dhariwal J, d'Ancona G, Douiri A, Roxas C, et al. Real-2orld 3ffectiveness of benralizumab in severe eosinophilic asthma. Chest 2021;159(2):496-506. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kay 2015
- Kay AB. The early history of the eosinophil. Clinical and Experimental Allergy 2015;45(3):575-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Khatri 2019
- Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. Journal of Allergy and Clinical Immunology 2019;143(5):1742-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Khurana 2019
- Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: The COSMEX Study. Clinical Therapeutics 2019;41(10):2041-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kolbeck 2010
- Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. Journal of Allergy and Clinical Immunology 2010;125(6):1344-1353.e2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2017
- Li J, Wang F, Lin C, Du J, Xiao B, Du C, et al. The efficacy and safety of reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: a systematic review and meta-analysis. Journal of Asthma 2017;54(3):300-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2021
- Li H, Zhang Q, Wang J, Gao S, Li C, Wang J, et al. Real-world effectiveness of mepolizumab in severe eosinophilic asthma: a systematic review and meta-analysis. Clinical Therapeutics 2021;43(6):192-e208. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2013
- Liu Y, Zhang S, Li DW, Jiang S-J. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials. PLoS One 2013;8(3):e59872. [DOI: 10.1371/journal.pone.0059872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2019
- Liu W, Ma X, Zhou W. Adverse events of benralizumab in moderate to severe eosinophilic asthma: a meta-analysis. Medicine 2019;98(22):e15868. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Llanos‐Ackert 2017
- Llanos-Ackert JP, Price RG, Forshag M, Yancey S, Liu MC. Asthma control in patients with severe eosinophilic asthma treated with mepolizumab. American Journal of Respiratory and Critical Care Medicine 2017;195:A3192. [Google Scholar]
Lopez 1986
- Lopez AF, Begley CG, Williamson DJ, Warren DJ, Vadas MA, Sanderson CJ. Murine eosinophil differentiation factor. An eosinophil-specific colony-stimulating factor with activity for human cells. Journal of Experimental Medicine 1986;163(5):1085-99. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomised controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukherjee 2016
- Mukherjee M, Stoddart A, Gupta RP, Nwaru BI, Farr A, Heaven M, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Medicine 2016;14(1):113. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2017
- Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. Journal of Allergy and Clinical Immunology 2017;5:1572-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nathan 2004
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. Journal of Allergy and Clinical Immunology 2004;113(1):59-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2017
- National Institute for Health and Care Excellence (NICE). Mepolizumab for treating severe refractory eosinophilic asthma. www.nice.org.uk/guidance/ta431 (accessed prior to 20 July 2017).
NICE STA
- National Institue for Health and Care Excellence, single technology appraisal, mepolizumab for treating severe eosinophilic asthma [ID798]. https://www.nice.org.uk/guidance/ta671/documents/committee-papers-4 (accessed 15 June 2022).
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Affengruber L, Gartlehner G. Citation screening using crowdsourcing and machine learning produced accurate results: evaluation of Cochrane's modified Screen4Me service. Journal of Clinical Epidemiology 2020;130:23-31. [DOI] [PubMed] [Google Scholar]
Osborne 2007
- Osborne ML, Pedula KL, O'Hollaren M, Ettinger KM, Stibolt T, Buist AS, et al. Assessing future need for acute care in adult asthmatics: the Profile of Asthma Risk Study: a prospective health maintenance organization-based study. Chest 2007;132(4):1151-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pelaia 2021
- Pelaia C, Crimi C, Benfante A, Caiaffa MF, Calabrese C, Carpagnano GE, et al. Therapeutic effects of benralizumab assessed in patients with severe eosinophilic asthma: real-life evaluation correlated with allergic and non-allergic phenotype expression. Journal of Asthma and Allergy 2021;14:163-73. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2014
- Price D, Fletcher M, Van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Primary Care Respiratory Medicine 2014;24:14009. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramonell 2020
- Ramonell RP, Iftikhar IH. Effect of anti-IL5, anti-IL5R, anti-IL13 therapy on asthma exacerbations: a network meta-analysis. Lung 2020;198(1):95-103. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddel 2009
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. American Journal of Respiratory and Critical Care Medicine 2009;180(1):59-99. [DOI: 10.1164/rccm.200801-060ST] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Trevor 2021
- Trevor J, Lugogo N, Carr W, Moore WC, Soong W, Panettieri RA Jr, et al. Severe asthma exacerbations in the United States: incidence, characteristics, predictors, and effects of biologic treatments. Annals of Allergy, Asthma and Immunology 2021;127(5):579-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Veen 2009
- Van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. Journal of Allergy and Clinical Immunology 2009;124(3):615-7, 617.e1-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2009
- Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clinical and Experimental Allergy 2009;39(6):798-806. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016
- Wang FP, Liu T, Lan Z, Li SY, Mao H. Efficacy and safety of anti-interleukin-5 therapy in patients with asthma: a systematic review and meta-analysis. PloS One 2016;11(11):e0166833. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenzel 2012
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nature Medicine 2012;18(5):716-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Woodruff 2009
- Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major sub phenotypes of asthma. American Journal of Respiratory and Critical Care Medicine 2009;180(5):388-95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2019
- Yan K, Balijepalli C, Sharma R, Barakat S, Sun SX, Falcao S, et al. Reslizumab and mepolizumab for moderate-to-severe poorly controlled asthma: an indirect comparison meta-analysis. Immunotherapy 2019;11(17):1491-505. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yancey 2017a
- Yancey SW, Ortega HG, Keene ON, Mayer B, Gunsoy NB, Brightling CE, et al. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. Journal of Allergy and Clinical Immunology 2017;139(4):1167-75.e2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zeiger 2016
- Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. Journal of Allergy and Clinical Immunology 2016;4(1):120-9.e3. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Farne 2017
- Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD010834. [DOI: 10.1002/14651858.CD010834.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 2013
- Powell C, Milan SJ, Dwan K, Walters N. Mepolizumab versus placebo for asthma. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD010834. [DOI: 10.1002/14651858.CD010834] [DOI] [PubMed] [Google Scholar]
Powell 2015
- Powell C, Milan SJ, Dwan K, Bax L, Walters N. Mepolizumab versus placebo for asthma. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD010834. [DOI: 10.1002/14651858.CD010834.pub2] [DOI] [PubMed] [Google Scholar]


