Abstract
The COVID-19 pandemic presented challenges to the healthcare system while catalyzing the adoption of virtual care. The need for remote assessment and real-time monitoring of physiological vital signs has driven towards a need for virtual care solutions. This paper presents the outcome of a multidisciplinary collaboration to ensure clinical usability of a remote contactless sensing technology, VitalSeer, and to help close gaps between emerging technologies and clinical practice. The paper describes the user-centric data-driven clinical approach to address the needs as identified by clinical experts through the iterative and agile development cycle. It highlights findings from preliminary studies to validate proof-of-concept VitalSeer’s adoptability, accessibility and usability. The studies on volunteers demonstrated the accuracy of VitalSeer’s heart rate model at a low MAE of 0.74 (bpm) and a RMSE of 1.2 bpm, below the threshold of clinical grade contact-based sensors. The paper concludes with a discussion on the technology implications in emergency medicine and community care.
Introduction
COVID-19 has propelled the health system to change from predominantly in-person services to virtual care, where healthcare providers use technology, like video-calls, to examine and communicate with the patients. Virtual care has been used for decades; however, its adoption rate has only significantly increased since the beginning of the COVID-19 pandemic1. Virtual platforms with common and affordable information technologies such as computers, smart phones and tablets are becoming well accepted by physicians and patients, and can be helpful during emergencies and outbreaks2–4. Such remote healthcare delivery approach can also support social distancing when accessing needed medical consultations, and reduce unnecessary emergency department visits5–7.
Knowing patients’ vital signs in the course of virtual visits is critical to assess the severity of illnesses and determine whether their conditions warrant in person visits8. Yet, vital sign measurement has not been integrated into the most current routine virtual care workflows. This can limit the scope of virtual care and, ultimately, the quality of care delivered9. For example, in COVID-19 patients with silent hypoxia10, their oxygen saturation (SpO2) can be dangerously low and require hospitalization, yet they may not experience dyspnea as a subjective symptom, or exhibit obvious shortness of breath on video11. Furthermore, a recent study found that SpO2 and Respiratory Rate (RR) at the time of presentation to the hospital are predictive of the risk of patient mortality12. Therefore, during virtual care encounters with patients, clinical questioning coupled with heart rate (HR), RR, and SpO2 assessment are critical to accurately evaluate their need for in person assessment or hospitalization.
While patients can acquire home-based devices and wearables to assess their vital signs, only a small portion may have the means and self-management knowledge to use the devices. To optimize equity to access, accurate and economical methods to assess vital signs remotely need to be developed for patients who have no or limited access to home-based devices. Contactless sensing systems to measure vital signs is a promising approach13,14 that might be more convenient to deploy and use compared to existing wired or wireless alternatives.
Proposed Contactless Sensing Solution: This paper presents the employment of the user-centric data-driven clinical approach for the development, from ideation to pre-clinical proof-of-concept , of VitalSeer, a contactless sensing system solution. In virtual care, “contactless sensing” refers to processes whereby physiological signals are obtained from contactless hardware (for example: microphone, video cameras, etc.) and processed to extract vital signs or other biofeedback information. VitalSeer is based on remote photoplethysmography (rPPG), following similar fundamental principles as the contact-based PPG used in pulse oximeters. However, instead of using diodes and photosensors in an enclosed device, rPPG is obtained using ambient light and regular, external color cameras as illustrated in Figure 1. VitalSeer software aims to conduct remote monitoring of critical vital signs including HR, RR and SpO2.
To support patient self-monitoring and enable decision support for clinicians, the developed software needs to be clinically relevant, adoptable, accessible, easy-to-use and low-cost. Such a system would be important in the context of delivering virtual care, and particularly in the context of the current COVID-19 pandemic. The iterative, clinically driven, user-centric development process taken to build the solution was grounded in a close collaboration between multidisciplinary teams to achieve usability and clinical pertinence, helping to close the gap between rapidly emerging technologies and clinical practice. As a demonstration of this work, the development, testing and findings reported in this paper are based on VitalSeer’s HR model.
Methods
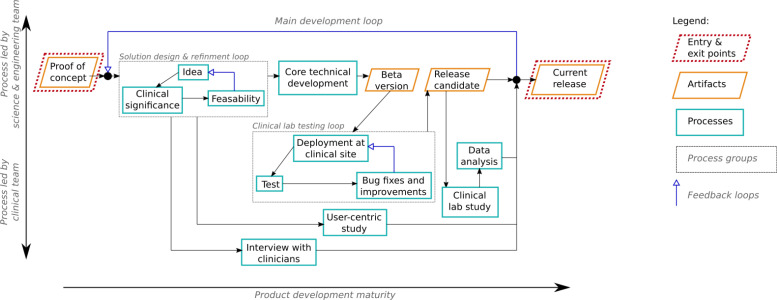
Overview: An iterative technology design and development approach was undertaken for the development of the VitalSeer contactless sensing solution. An intertwined approach ensures that the technology being developed is well aligned with the clinical context. The approach comprised of three main steps: 1) healthcare provider interviews, solution requirements definition and design, 2) solution development and testing, and technology benchmarking, and technology validation through clinical research studies. Each of these steps is described in more detail below. Feedback loops allow for iterative development, which in turn allows for the gradual refinement of the quality of the solution. Figure 2 illustrates this development process. The diagram provides a schematic view of the key processes led by both the clinical team (toward the left-hand side of the diagram) and/or the science and engineering team (toward the right-hand side). The overall methodology consists of a main development loop leading from a proof-of-concept implementation to an updated release version of the solution. Two sub-loops (steps 1 and 2 above) are also included to represent shorter-term, fast-paced iterations. To support the development, two rounds of in-lab studies were conducted. These two rounds are designated as “VitalSeer 1.0” and “VitalSeer 1.2” clinical research study through the rest of this paper. After each study, the results were fed back to the design stage to inform the development of the next version.
Figure 2.
VitalSeer development. The three feedback loops highlight the iterative development process: solution design & refinement, clinical lab testing and main development.
Healthcare provider interviews, solution requirements definition and design: Exploratory interviews with healthcare providers were conducted to better understand current practices, utility, and challenges with vital sign measurement in virtual care. The interview guide is available upon request to the corresponding author. Healthcare providers with experience providing virtual care (defined as providing care, using technology and tools remotely, or at a distance from the patient) in 2020 or before were recruited. Interviews were between 30 and 60 minutes and conducted over videoconferencing. The protocol of questions used to facilitate the interviews aimed to collect insights and challenges in providing virtual and COVID-related care, and the potential impacts, opportunities, and clinical applications of contactless sensing. Meanwhile, the multidisciplinary team initiated the solution design by constructing use cases that outlined the detailed workflows, scenarios, and conditions under which the proposed contactless sensing technology would operate. The use cases were refined with feedback from both the clinical and technology teams and the feedback collected through the interviews, with particular attention paid to the user experience. Next, the use cases were translated into a technology development document that defined and presented the specific requirements, features and modules of the solution. Table 1 in the Results section documents the outcome of this process.
Table 1.
Summary of clinical requirements linked to corresponding technology features/components
| Objective and requirement | Performance indicator | VitalSeer feature/component implementation |
|---|---|---|
| Clinical adoptability by both healthcare professionals and patients |
|
|
| Clinical accessibility for the users |
|
|
| User-friendliness/ ease of use and functional usability |
|
|
Solution development, testing, and technology benchmarking: Guided by the results in Table 1, the core technical development commenced which allowed for features and components of the solution to be implemented within the framework architecture. As beta software versions were iteratively released by the technical team, the clinical and technology teams conducted testing (see clinical lab testing loop in Figure 2). These tests systematically identified issues/bugs, test systemperformance, stability, accuracy, robustness and usability prior to the release candidate version for clinical evaluation with study participants. At the end of each testing loop, the technology and clinical teams jointly reviewed and interpreted the Masimo Root reference sensor and VitalSeer testing results to inform areas for refinement. Additional recommendations, observations, and clinical/user-centric insights into the data values and trends contributed to continual improvements to VitalSeer. These improvements are related to the graphical user interface (GUI), data acquisition process, system configuration and setup, calibration of key parameters and test conditions including, but not limited to, lighting configuration, subject’s motion and skin tone.
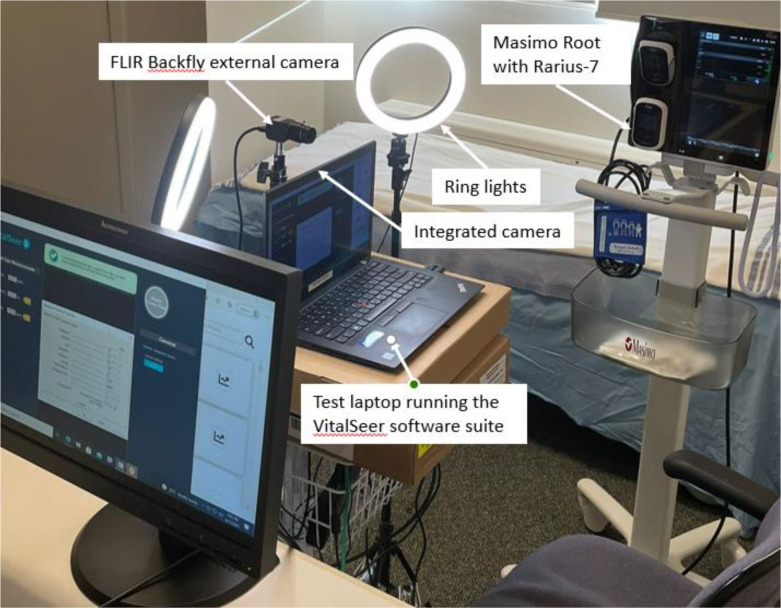
In consultation with Biomedical technology experts at Vancouver General Hospital (VGH) in Vancouver, BC, the FDA-approved Masimo Root patient monitoring and connectivity platform with Radius-7 wearable patient monitor, used by VGH for continuous patient monitoring, was identified as the reliable ground truth equipment. Due diligence on the Masimo Root system was performed by testing and verification of equipment specifications, including performance, usage, safety, vital sign data acquisition, instrumentation maintenance, sterilization/sanitation, and calibration. The clinical research team was trained on the optimal use of the Masimo systemthrough iterative dialogues and an in-depth product education session with Masimo product and technical experts. This became the benchmark against which the veracity of datasets of HR, RR and SpO2 vital signs acquired by VitalSeer were compared and verified. The test setup, shown in Figure 3, was composed of the Masimo Root equipment as the ground truth sensor, and VitalSeer installed on a Lenovo T470s laptop (Windows 10), connected to Ethernet for optimal bandwidth and with laptop webcam and FLIR cameras as input imaging devices.
Figure 3.
Test and validation setup in a simulated clinical setting, composed of a laptop running VitalSeer application with integrated and FLIR cameras and the Masimo Root reference equipment
Technology validation through clinical research studies: Following the clinical lab testing loops, candidate versions of the VitalSeer software were released and used in a clinical research study, for which details are provided below. The study was approved by UBC clinical research ethics board and received institutional approval by Vancouver Coastal Health. All protocols were designed to follow the relevant COVID-related research restrictions.
Prospective participants were recruited through a provincial online research platform (https://www.reachbc.ca/). Interested volunteers who were 19 years old or older and able to attend a one-hour test session in the research lab located in Vancouver were eligible to participate. Volunteers were excluded if they were unable to read and understand English, were unable to display a clean face (i.e. no facial hair; no facial tattoos; no makeup; no glasses) during the session, were in exacerbation (i.e. experiencing worsening symptoms of an existing illness) or displaying abnormal physiological parameters, or if they were unable to sit still for two minutes. All participants were also required to pass a COVID screening questionnaire upon their arrival to the research lab.
During each test session, the physiological parameters (HR for VitalSeer 1.0; HR, RR and SpO2 for VitalSeer 1.2) were taken by the contactless sensing system via a 2-minute video recording of the face of the seated participant, along with the same measurements from the medical grade Masimo Root contact-type system. For consistency purposes, 3 to 5 2-minute recordings were taken per participant. At the end of the test session, each participant was invited to complete a usability feedback survey about their overall VitalSeer experience.
The raw input data (VitalSeer video footage and the Masimo Root log) was collected through the VitalSeer software. This allowed for reprocessing the data at any selected time with different parameters and algorithms, using the same processing pipeline as the live data collection. All electronic data was saved on a password protected, encrypted and firewall protected network server shared drive on the test laptop.
Meta-information associated with the recording sessions (e.g. participant ID, hardware information, lighting conditions) were stored within a data repository, REDCap (Research Electronic Data Capture), which allows the re-use of any recorded data offline and grouping data according to certain conditions. All other study participant data (ex. demographics) was de-identified and stored in REDCap. A secure data sharing process using MS OneDrive was established for teams to share and access data extracts from VitalSeer, Masimo, and the REDCap repository.
Results
Healthcare provider interview findings: Five physicians – 2 family physicians and 3 emergency physicians – participated in interviews. Two practiced in rural/remote locations, and 3 practiced in urban areas. The main gaps identified with remote vital sign measurement in virtual care included patient inaccessibility to available and affordable equipment; inability to collect objective and reliable measures of vital signs remotely; and inconsistent methods to follow patients over time. The potential benefits and opportunities of a contactless sensing technology, like VitalSeer, were described as increased engagement and motivation for patients to monitor their own health; improved patient confidence in accessing virtual services with more complete, accurate and objective information; increased physician confidence in decision making; and delivery of effective virtual care to the patients in their own homes. The participating physicians also highlighted the importance of establishing usability, reliability, affordability, trustworthiness, privacy, and data integration with other digital patient information platforms as required to establish VitalSeer as an innovative and scalable technology for virtual care for both patients and clinicians.
Responding to the findings from the interviews, and in conjunction with expert feedback from the clinical team, two use cases for the VitalSeer technology were proposed and refined: (1) short-term scenario: a stand-alone downloadable application and (2) long-term scenario: integration into existing virtual care platform(s).
VitalSeer design, development and testing: A set of clinical and user-centric objectives and requirements, based on the healthcare provider interview findings, were defined to drive the development of VitalSeer. Table 1 outlines the objectives and requirements, their associated performance indicators and the VitalSeer solution features/components.
The final proposed solution is composed of three main components:
VitalSeer Library: a portable Application Programming Interface (APIs) to interface with the input devices (e.g. cameras) and the backend server, and perform image processing and feature extraction;
VitalSeer Application: user-facing application (currently PC based, Linux or Windows) that guides users through the vital sign estimation sessions and displays near real-time vital signs measures;
VitalSeer Backend: the remote server that converts a time series of processed video features into vital signs by hosting the vital sign assessment models.
Responding to the defined use cases, VitalSeer can be launched either from a web browser (in a workflow that is compatible with virtual care) or through a downloadable desktop application. It includes different configurations to estimate different vital signs, each including the required hardware to be activated (e.g. camera, microphone) and the required processing pipeline, as well as information on how to display the results..
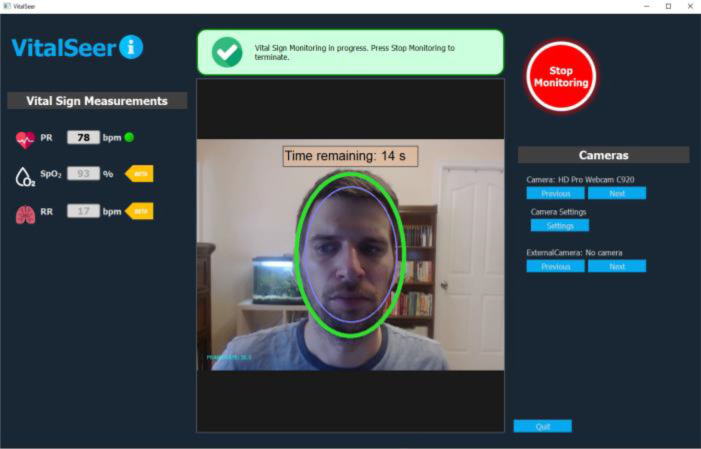
Figure 4 shows the VitalSeer application being used to display a subject’s vital sign estimations computed using only video recording of his face in near real-time. As examples of user-friendliness, the green oval is displayed to help the user adjust distance from the camera and thus guide the user for valid vitals assessment. The message box, above the video, is included in order to display the status of the acquisition and the presence of any issues. The panel on the left displays the three vitals, HR, RR and SpO2 (in this paper the evaluation focusses on HR only).
Figure 4.
VitalSeer GUI used for display of real-time video-based vital sign estimations.
In addition to the feature implementation presented in Table 1, the proposed software architecture is extensible to support additional operating systems by integrating the required APIs to access the input devices. Multiple level devices such as integrated laptop cameras, external webcams as well as industrial grade cameras (e.g. FLIR BlackFly) were interfaced and tested on various platforms such as desktop computers and laptops of different specifications.
VitalSeer validation through clinical research studies – product validation findings and participant feedback: A total of 35 healthy volunteers were enrolled into the study. Of the 35, 22 participated in the VitalSeer 1.0 clinical study sessions, and 9 of the 22 returned to participate in VitalSeer 1.2 study sessions as well. In addition, 13 new volunteers were also recruited to participate in VitalSeer 1.2 study sessions.
Data used for the vital sign estimate analysis came from different sources. A data processing step took place prior to the analysis step, which included the proper synchronization of the time reference of each device, e.g. sampling between the camera and Masimo Root. This was realized by compensating for the observed offset. The offset information was also used to ensure the clocks on all devices were synchronized in subsequent beta testing loops and clinic research experiments. For each 2-minute recording, 10-second running windows with 2 second overlaps were used to compute the heart rate estimations. To evaluate the accuracy of the HR model, a linear interpolation of all Masimo Root pulse rates within the corresponding VitalSeer analysis windows was performed. The error was calculated as the difference between the interpolated Masimo Root readings and the Vital Seer estimations.
There was a total of 165 recordings collected from the 44 test sessions across the VitalSeer 1.0 and 1.2 studies. Considering factors such as the stability in the pulse rate readings from the reference Masimo Root device, as well as the unexpected excessive subject motion in the acquired data, a total of 17757 pulse rate estimations (approximately 110 pulse rate estimations per 2-minute recording) was confirmed by the data processing step. VitalSeer also provides a confidence level on the HR estimation. This confidence level decreases as the signal-to-noise ratio (SNR) decreases. Signal quality can be impacted by various factors such as low lighting conditions, poor camera quality or unexpected motions of the face. When the confidence level is below a certain threshold, the HR estimation is filtered out. Fine tuning this threshold is essential to balance usability and accuracy of the vital sign estimation; in this study, this threshold was set to 90%, see details in15. Accuracy metrics were only computed for values which meet this confidence threshold. Of the 17757 pulse rate estimations performed, 86.1% of the HR estimations (15285) were above the confidence threshold.
The first row in Table 2 presents the model accuracy results and the prediction error as a function of the Masimo HR values. The results showed that the computed bias was negligible, Mean Absolute Error (MAE) was below 1 beat per minute, and the Root Mean Square Error (RMSE) of 1.2 beats per minute was below the claimed RMSE of FDA-approved clinical grade contact-based sensors utilized for heart rate measurements (on the order of 3 beats per minute, under ideal conditions)16.
Table 2.
VitalSeer 1.0 and 1.2 clinical studies – HR data analysis summary.
| Studies | Readings with confidence level >90% | Mean Error (bpm) | RMSE (bpm) | MAE (bpm) | % of values within 2 bpm | % of values within 5 bpm | Outlier ratio (error >10 bpm) % |
|---|---|---|---|---|---|---|---|
| Aggregated VitalSeer 1.0 and 1.2 studies with integrated webcam | 86.1% (n=15285 predictions) | -0.07 | 1.20 | 0.74 | 93.1 | 99.3 | 0.05 |
| Pilot with FLIR Blackfly camera (concurrent to VitalSeer 1.2 study) | 97.2% (n=9843) | -0.06 | 1.09 | 0.68 | 94.6 | 99.3 | 0.01 |
It was observed that most low confidence recordings were observed with participants who had darker skin tones (IV and above on the Fitzpatrick scale). We hypothesized that this might be related to camera settings, and a pilot run including the same subjects that was conducted using an industrial grade camera (i.e. FLIR Blackfly) to evaluate if the use of higher quality video capture devices could improve the quality of the signal. This pilot included 92 2-minutes videos that were recorded concurrently to those used in the main study, so subjects, lighting and reference pulse rate were identical. The results of the pilot (n=10127 pulse rate estimations) are presented in the second data row of Table 2. Using an industrial grade camera significantly increases the signal to noise ratio, leading to less readings rejected due to low confidence levels. However, the accuracy of the HR measurements of the FLIR camera versus the laptop integrated camera well confirmed the proposed low-cost consumer-grade based solution. Of note, VitalSeer using the FLIR Blackfly video data yielded accurate HR estimations for subjects with darker skin.
During the post-testing interviews with the participants on the various aspects of the experience and the technology, over 75% of participants agreed (37%) or strongly agreed (42%) it was easy to meet the requirement of having a clean face (i.e. no makeup, no facial hair, etc.) in order to have vital signs estimated by the technology; 95% of participants in VitalSeer 1.0 study, who took part in an average of 3.32 2-minute recordings per study session, reported it was comfortable to sit steady for about two minutes. However, in VitalSeer 1.2 study, where, on average, participants were asked to sit for 4.18 recordings per study sessions, 78% reported it was comfortable to sit steady for two minutes at a time.
At the end of each test session, participants in each study were asked if they felt the contactless sensing technology would be valuable for their own health management and care. Almost all, 93%, agreed or strongly agreed contactless sensing would be useful for self-monitoring. All also agreed or strongly agreed that they would be willing to share their vital sign readings with their healthcare provider online.
A majority, 86%, felt this method of vital signs measurement would be helpful for health care providers to monitor their patients remotely.
Discussion
In this work, a clinical research study framework and infrastructure was designed, built and tested, for the development of an innovative technology solution, between a multidisciplinary team with clinical and technical backgrounds. This partnership and the corresponding iterative development methodology fostered the lockstep development of a contactless sensing platform whose features and components were shaped to meet clinical and user-centric objectives, and to address the needs of virtual care. This collaboration needs to be grounded in a solid framework to guide the development of digital health applications that consider continuous and rigorous clinical evaluation and include end-users in the development loop17.
From the clinical adoptability perspective, analyses of study recordings above the 90% confidence threshold (i.e. with high SNR) suggest the VitalSeer HR model attains the clinical adoptability performance goal of continuous, high-accuracy vital sign estimations by producing results with negligible computed bias, low MAE and an RMSE that is favorable compared to FDA-approved clinical grade contact-based sensors.
From the clinical accessibility perspective, accessibility of VitalSeer was addressed by the design and implementation of a hardware and operating system agnostic architecture. Through the testing, it was shown that the system could accommodate consumer-grade hardware while maintaining prediction accuracy. Hardware agnosticism is also further supported by the fact that model evaluation metrics were comparable between the consumer-grade (e.g. laptop built-in cameras) and industrial-grade cameras. With a relatively high confidence threshold of 90% utilized in this analysis with the consumer-grade hardware, 14% of predictions were rejected in the analysis, which could be considered significant. However, in practice most rejected values are dispersed in time, so this rejection rate often translates into less frequent updates of the computed value (e.g. one update every 1-4s instead of every 1s), which might be suitable in many cases. Also, the confidence threshold is a design parameter, and it is possible to deduce the rejection rate to about 5% at the cost of a slightly increase accuracy15 to better fit a specific application. Future work should consider the integration and validation of the technology into and with other platforms such as mobile and tablet devices, while expanding the model capabilities to accommodate more variable environmental (in-the-wild) conditions. Such an endeavor will also allow the idea of operating system agnosticism to be evaluated.
From the ease-of-use perspective, the application user interface was developed and iterated over time with continual feedback from clinicians and other end-users. The resulting user interface is now a version that, among other features, provides real-time guidance and feedback (e.g. displays alerts/messages) about the position of the face and illumination management, reporting and automatic recovery when signal and vitals acquisition was challenging. Future work should continue to seek end-user feedback on operating VitalSeer in various environments so as to continuously adapt and improve the ease-of-use and usability of the application. This type of future endeavor should constitute studies that are inclusive to ensure VitalSeer’s ability to meet a wide range of needs.
From the affordability perspective, VitalSeer currently leverages generally available and relatively low-cost technologies to test and validate the viability of conducting virtual vital sign measurement. With further development and refinement, VitalSeer can operate under lower-bandwidth conditions, with less computing resource requirements and lower-resolution video cameras, thereby further reducing overall cost and increasing overall affordability for users of VitalSeer.
Limitations: First, the study was conducted under controlled lighting conditions to compute vital signs. An extensive validation would be needed to confirm that different lighting situations will still result in accurate data acquisition.
Second, most of study participants had relatively light skin tones. Based on the standardized Fitzpatrick skin tone scale18 from grades I to VI (the higher grade, the darker), they fall within grades I to III. Preliminary results from two subjects in the cohort suggest that the extraction of viable physiological signals is more challenging with regards to darker skin tones, which is consistent with reports in the literature19, not only for rPPG but also for regular contact PPG20, and thus, merits further investigation and work. Our findings must be validated on darker skin tones since less light gets reflected by darker skin tones and this may impact vital signs computations.
Third, in VitalSeer versions tested, the participants needed to sit motionless in front of the camera for two minutes for the vital signs to be computed and tracked across time. The VitalSeer sensing session had to be restarted if there was an object obscuring the patient’s face (e.g. a hand moving in front of the face), or if there was a significant movement within the region of interest such as a sneeze or a cough.
Fourth, our subjects were healthy volunteers with no severe illnesses during measurements. The platform will need to be tested with subjects experiencing a larger range of HR, RR, and SpO2 illnesses for broader use in all clinical contexts and illness severity.
Another limitation of the study relates to conducting in-person clinical research studies in the midst of the COVID-19 crisis, which limited recruitment and access to clinical settings to carry out the test sessions.
Conclusion
Empowering and bringing the technology to users has drawn increasing attention in the past decades, as this approach has been demonstrated to be critical for success of an innovation. The close clinical and technical teamwork on VitalSeer, presented in this paper, promotes excellence in health technology innovation by applying best practices in clinical research in the context of innovative medical device by clinical end-users and virtual care solution development by technology experts. The clinical insights collected from healthcare professionals and user-centric data effectively inform the VitalSeer research team in the development of the platform from a conceptual idea to its laboratory creation as a pre-clinical proof-of-concept. Next steps will include further usability studies to further investigate and validate the technology readiness for formal clinical trial to test its effectiveness in real world setting. Incorporating the experiences and insights of the end users, both healthcare providers and patients, will continue to be vital to inform the development of VitalSeer, ultimately to achieve successful implementation in virtual care settings to transform healthcare.
Our study also demonstrates the importance of the multidisciplinary nature of the collaboration to create this dynamic environment for science, engineering, medical, research and technology teams to iteratively exchange and realize innovative ideas in a rapid and disciplined fashion. The ongoing knowledge translation and dissemination activities will further contribute to socializing the technology with healthcare providers, patients and policy-makers for solution deployment, scale up, and sustainability.
Figures & Table
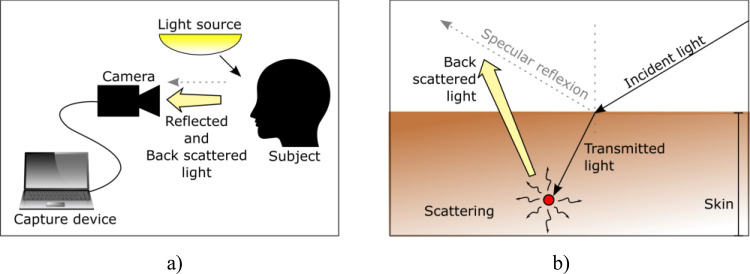
Figure 1.
Illustration of the rPPG principle. a) A capture device equipped with a camera is used to capture light that is back scattered from a subject’s exposed skin subjected to an external light source, b) Details of the light scattering and reflection processes.
References
- 1.Whitelaw S, Mamas MA, Topol E, Van Spall HGC. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit Heal [Internet] 2020;2(8):e435–40. doi: 10.1016/S2589-7500(20)30142-4. . Available from: http://dx.doi.org/10.1016/S2589-7500(20)30142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jnr BA. Use of Telemedicine and Virtual Care for Remote Treatment in Response to COVID-19 Pandemic. J Med Syst [Internet] 2020;44(7):1. doi: 10.1007/s10916-020-01596-5. Jun 15 [cited 2021 Aug 12].9. Available from: https://link.springer.com/article/10.1007/s10916-020-01596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serper M, Cubell AW, Deleener ME, Casher TK, Rosenberg DJ, Whitebloom D, et al. Telemedicine in Liver Disease and Beyond: Can the COVID-19 Crisis Lead to Action? Spec Artic | Hepatol. 2020;72(2) doi: 10.1002/hep.31276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai Y, Wang Y, Zhang M, Gittell JH, Jiang S, Chen B, et al. From Isolation to Coordination: How Can Telemedicine Help Combat the COVID-19 Outbreak? [cited 2021 Aug 12]; Available from: https://doi.org/10.1101/2020.02.20.20025957.
- 5.Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet [Internet] 2020 Feb;395(10223):514–23. doi: 10.1016/S0140-6736(20)30154-9. . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarvis CI, Van Zandvoort K, Gimma A, Prem K, Klepac P, Rubin GJ, et al. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med [Internet] 2020 Dec 7;18(1):124. doi: 10.1186/s12916-020-01597-8. . Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morawska L, Milton DK. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin Infect Dis [Internet]. 2020 Jul 6;6(939). Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa939/5867798. [DOI] [PMC free article] [PubMed]
- 8.Rohmetra H, Raghunath N, Narang · Pratik, Chamola V, Guizani M, Naga ·, et al. AI-enabled remote monitoring of vital signs for COVID-19: methods, prospects and challenges. [cited 2021 Aug 12]; Available from: https://doi.org/10.1007/s00607-021-00937-7.
- 9.Pham C, Poorzargar K, Nagappa M, Saripella A, Parotto M, Englesakis M, et al. 2021. Effectiveness of consumer-grade contactless vital signs monitors: a systematic review and meta-analysis. J Clin Monit Comput [Internet]. Jul 9 [cited 2021 Aug 15]; Available from: https://doi.org/10.1007/s10877-021-00734-9.
- 10.Herrmann J, Mori V, Bates JHT, Suki B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun [Internet]. 2020 Dec 28;11(1):4883. Available from: http://www.nature.com/articles/s41467-020-18672-6. [DOI] [PMC free article] [PubMed]
- 11.Tobin MJ, Laghi F, Jubran A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am J Respir Crit Care Med [Internet] 2020 Aug 1;202(3):356–60. doi: 10.1164/rccm.202006-2157CP. . Available from: https://www.atsjournals.org/doi/10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee NA, Jensen PN, Harris AW, Nguyen DD, Huang HD, Cheng RK, et al. Admission respiratory status predicts mortality in COVID‐19. Influenza Other Respi Viruses [Internet]. 2021 May 24;irv.12869. Available from: https://onlinelibrary.wiley.com/doi/10.1111/irv.12869. [DOI] [PMC free article] [PubMed]
- 13.Negishi T, Abe S, Matsui T, Liu H, Kurosawa M, Kirimoto T, et al. Contactless Vital Signs Measurement System Using RGB-Thermal Image Sensors and Its Clinical Screening Test on Patients with Seasonal Influenza. Sensors [Internet]. 2020;20. Available from: www.mdpi.com/journal/sensors. [DOI] [PMC free article] [PubMed]
- 14.Sun G, Nakayama Y, Dagdanpurev S, Abe S, Nishimura H, Kirimoto T, et al. Remote sensing of multiple vital signs using a CMOS camera-equipped infrared thermography system and its clinical application in rapidly screening patients with suspected infectious diseases. Int J Infect Dis [Internet]. 2017 Feb;55:113-7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1201971217300103. [DOI] [PMC free article] [PubMed]
- 15.Rivest-Hénault D, Pagiatakis C., Bernhardt R., Vaughan T., Falardeau B., Smith M.S.D., Jiang D. 2021. , Quasi real-time contactless physiological sensing using consumer-grade cameras. In: Proceedings of 2021 IEEE International Conference on Healthcare Informatics (ICHI), In Press.
- 16.Corporation M. Masimo RD SETTM Series [Internet]. 2018. Available from: https://techdocs.masimo.com/globalassets/techdocs/pdf/lab-10131a-eifu.pdf.
- 17.Duffy A, Christie G MS. Examining Challenges to the Incorporation of End Users in the Design of Digital Health Interventions: Protocol for a Systematic Review. JMIR Res Protoc. 2021;10(7):e28083. doi: 10.2196/28083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware OR, Dawson JE, Shinohara MM TS. 105(2):77–80. Racial limitations of fitzpatrick skin type. Cutis. [PubMed] [Google Scholar]
- 19.Nowara EM, McDuff D VA. A Meta-Analysis of the Impact of Skin Tone and Gender on Non-Contact Photoplethysmography Measurements. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops. 2020. p. 284-5.
- 20.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. N Engl J Med [Internet] 2020 Dec 17;383(25):2477–8. doi: 10.1056/NEJMc2029240. . Available from: http://www.nejm.org/doi/10.1056/NEJMc2029240. [DOI] [PMC free article] [PubMed] [Google Scholar]