Abstract
Synechocystis sp. strain PCC6308 cyanophycin synthetase was purified 72-fold in three steps by anion exchange chromatography on Q Sepharose, affinity chromatography on the triazine dye matrix Procion Blue HE-RD Sepharose, and gel filtration on Superdex 200 HR from recombinant cells of Escherichia coli. The native enzyme, which catalyzed the incorporation of arginine and aspartic acid into cyanophycin, has an apparent molecular mass of 240 ± 30 kDa and consists of identical subunits of 85 ± 5 kDa. The Km values for arginine (49 μM), aspartic acid (0.45 mM), and ATP (0.20 mM) indicated that the enzyme had a high affinity towards these substrates. During in vitro cyanophycin synthesis, 1.3 ± 0.1 mol of ATP per mol of incorporated amino acid was converted to ADP. The optima for the enzyme-catalyzed reactions were pH 8.2 and 50°C, respectively. Arginine methyl ester (99.5 and 97% inhibition), argininamide (99 and 96%), S-(2-aminoethyl) cysteine (43 and 42%), β-hydroxy aspartic acid (35 and 37%), aspartic acid β-methyl ester (38 and 40%), norvaline (0 and 3%), citrulline (9 and 7%), and asparagine (2 and 0%) exhibited an almost equal inhibitory effect on the incorporation of both arginine and aspartic acid, respectively, when these compounds were added to the complete reaction mixture. In contrast, the incorporation of arginine was diminished to a greater extent than that of aspartic acid, respectively, with canavanine (82 and 53%), lysine (36 and 19%), agmatine (33 and 25%), d-aspartic acid (37 and 30%), l-glutamic acid (13 and 5%), and ornithine (23 and 11%). On the other hand, canavanine (45% of maximum activity) and lysine (13%) stimulated the incorporation of aspartic acid, whereas aspartic acid β-methyl ester (53%) and asparagine (9%) stimulated the incorporation of arginine. [3H]lysine (15% of maximum activity) and [3H]canavanine (13%) were incorporated into the polymer, when they were either used instead of arginine or added to the complete reaction mixture, whereas l-glutamic acid was not incorporated. No effect on arginine incorporation was obtained by the addition of other amino acids (i.e., alanine, histidine, leucine, proline, tryptophan, and glycine). Various samples of chemically synthesized poly-α,β-d,l-aspartic acid served as primers for in vitro synthesis of cyanophycin, whereas poly-α-l-aspartic acid was almost inactive.
Cyanophycin (multi-l-arginyl-poly [l-aspartic acid]) is a protein-like cell inclusion that is unique to cyanobacteria. The polymer serves as a temporary nitrogen reserve, which accumulates during the transition from the exponential- to the stationary-growth phase and disappears when balanced growth resumes (9). The polymer contains aspartic acid and arginine in an equimolar ratio and consists of a polyaspartic acid backbone with arginine moieties linked to the β-carboxyl group of each aspartate by its α-amino group (17). At neutral pH and physiological ionic strength, cyanophycin is insoluble. The molecular weights of the polymer strands, as determined by polyacrylamide gel electrophoresis (PAGE), range from 25,000 to 100,000 (16), and the polymer exhibits a high polydispersity. Under optimal conditions, the unicellular Synechocystis sp. strain PCC6308 accumulated polyamide to a maximum of 16% (wt/wt) of its cell dry mass (2). Cyanophycin from this cyanobacterium can be chemically converted to a derivative with reduced arginine contents (W. Joentgen, T. Groth, A. Steinbüchel, T. Hai, and F. B. Oppermann, September 1998, German patent application DE19709024 A), which can be applied as a biodegradable substitute for polyacrylate in various technical processes (15).
Polymerization and degradation of cyanophycin are catalyzed by cyanophycin synthetase, which is encoded by cphA (18), and cyanophycinase, which is encoded by cphB (13), respectively. Cyanophycin synthetase has been purified from Anabaena variabilis (18) and from the thermophilic Synechococcus sp. strain MA19 (6). cphA was recently cloned from A. variabilis, Synechocystis sp. strain PCC6803, and Synechocystis sp. strain PCC6308 (1, 12, 18). Activity of cyanophycin synthetase is dependent on the presence of aspartic acid, arginine, ATP, Mg2+, and cyanophycin, which is used as a primer in the reaction mixture (1, 16–18). Recently, in vitro synthesis of cyanophycin was achieved by using chemically synthesized (β-Asp-Arg) as a primer instead of cyanophycin (4).
In this study we report on the purification of Synechocystis sp. strain PCC6308 cyanophycin synthetase from the soluble cell fraction of recombinant Escherichia coli and on the biochemical characterization of the enzyme.
MATERIALS AND METHODS
Organism, growth condition, harvesting of cells, and preparation of soluble cell fraction.
E. coli TOP 10 (Invitrogen, San Diego, Calif.) harboring plasmid pSK::cphAco (1) was grown at 37°C in Terrific broth medium (14). Growth was monitored by measuring the turbidity at 578 nm. When the turbidity reached 1.0, 1.0 mM isopropyl-β-d-thiogalactopyranoside was added to the culture. After entering the stationary-growth phase, the cells were harvested by centrifugation (15 min, 2,800 × g, 4°C), washed once with 50 mM Tris (pH 8.2) containing 20 mM KCl, 5 mM β-mercaptoethanol, and 1 mM EDTA, and resuspended with 2 ml of buffer per g of fresh cell mass. The cells were disintegrated by sonication for 1 min · ml−1 of cell suspension by using a Sonoplus GM200 sonifier (Bandelin Electronic, Berlin, Germany). The supernatant from high-speed centrifugation of the broken cells (1 h, 100,000 × g, 4°C) served as the soluble cell fraction.
Cyanophycin synthetase assay.
Unless otherwise stated, the enzyme activity was measured following the incorporation of [U-14C]l-arginine into insoluble cyanophycin by a modification of the radiometric procedure, which has been described recently (1). Fifty microliters of enzyme-containing sample was added to 71 μl of reaction mixture containing 88 mM Tris, 35 mM MgCl2, 35 mM KCl, 17.6 mM β-mercaptoethanol, 8.8 mM l-aspartic acid, 863 μM l-arginine, 17.6 μM [U-14C]l-arginine (specific activity: 10.3 GBq mmol−1; Amersham Pharmacia Biotech, Freiburg, Germany), and 1.53 mg of cyanophycin per ml of reaction mixture. After incubation of the mixture for 5 min at 28°C, the reaction was started by the addition of 4.0 μl of 94 mM ATP. In order to stop the reaction, 1 ml of ice-cold water was added. The precipitated cyanophycin was collected by centrifugation (15 min, 14,000 × g) and washed once with 50 mM Tris–2 mM EDTA (pH 8.2). After addition of 1 ml of 1.5 M HCl to dissolve the cyanophycin, one further centrifugation was performed to remove protein and other insoluble material. From the supernatant, 500 μl was removed and mixed with 5 ml of liquid scintillation counting cocktail hydroluma (J. T. Baker, Deventer, The Netherlands). Radioactivity was measured by using a model LS 6500 scintillation counter (Beckman Instruments GmBH, Munich, Germany).
Quantification of ADP in the reaction mixture was performed by a modification of a spectrophotometric procedure (7): 100 μl of the supernatant of the reaction mixture, which was obtained after a 5-min centrifugation at 14,000 × g, was added to 885 μl of assay mixture containing 50 mM Tris (pH 8.2), 20 mM KCl, 20 mM MgCl2, 1.1 mM phosphoenolpyruvate, 0.22 mM NADH, and 4.4 U of l-lactate dehydrogenase ml−1 (EC 1.1.1.27, from rabbit muscle; Sigma). The decrease in absorbance at 340 nm was monitored after addition of 10 μl of pyruvate kinase (400 U of pyruvate kinase ml−1, EC 2.7.1.40, from rabbit muscle; Sigma). After obtaining a constant level of absorbance, 5 μl of myokinase (800 U of myokinase ml−1, EC 2.7.4.3, from chicken muscle; Sigma) was added.
Purification of cyanophycin synthetase.
All steps were carried out at 7°C. A 50 mM Tris buffer (pH 8.2), containing 20 mM KCl, 5 mM β-mercaptoethanol, and 1 mM EDTA, was used throughout the purification procedure. The soluble cell fraction (approximately 5 g of protein) derived from about 30 g (wet weight) of cells was dialyzed against buffer and applied onto a column (2.6 by 10 cm; 53-ml bed volume [BV]) of Q Sepharose HiLoad (Amersham Pharmacia Biotech) equilibrated with buffer. After the column was washed with 2 BV of buffer, the protein was eluted with a KCl gradient (0 to 1,000 mM in 750 ml) at a constant flow rate of 2.5 ml · min−1. Fractions containing high enzyme activity were combined, dialyzed against buffer, and applied onto the triazine dye matrix Procion Blue HE-RD Sepharose CL-4B. Fractions containing high enzyme activity were combined, concentrated, and washed by ultrafiltration in a Diaflo chamber, using a YM30 membrane (both from Amicon Corp. Lexington, Ky.), and applied onto a column (2.6 by 60 cm; 330 ml BV) of Superdex 200 HR (Amersham Pharmacia Biotech) equilibrated with buffer, which was supplemented with 150 mM KCl. Protein was eluted with a constant flow rate of 1.0 ml · min−1. Fractions containing high enzyme activity were combined, concentrated, and washed by ultrafiltration. The enzyme preparation was stored at −20°C in the presence of 10% (vol/vol) dimethyl sulfoxide.
Preparation of triazine dye matrix.
Procion Blue HE-RD (Imperial Chemical Industries Ltd., Berkshire, United Kingdom) was coupled to Sepharose CL-4B (Amercham Pharmacia Biotech) using the procedure of Atkinson et al. (3).
Electrophoresis.
Sodium dodecyl sulfate (SDS)-PAGE was performed in 11.5% (wt/vol) gels as described by Laemmli (8). Staining of proteins and cyanophycin was done with Serva Blue R. Protein concentration was determined by the procedure of Bradford (5).
Molecular mass determination.
The mass of the native purified enzyme was determined by gel filtration, as described previously (11).
RESULTS
Purification of cyanophycin synthetase.
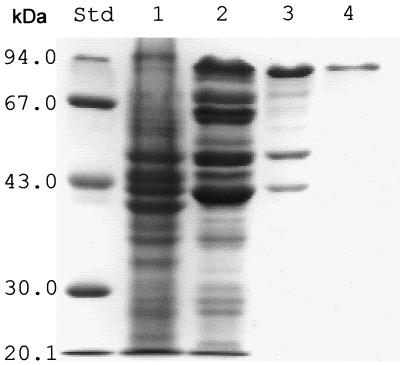
Cyanophycin synthetase was purified 72-fold to electrophoretic homogeneity with a recovery of 7.3% of the activity from the soluble cell extract of a recombinant E. coli strain during anion-exchange chromatography, affinity chromatography, and gel filtration chromatography (Table 1; Fig. 1). The cyanophycin synthetase content of the soluble cell proteins of the recombinant cells was calculated to 1.4% (wt/wt). SDS-PAGE of the final enzyme preparation resulted in one single protein band with an apparent molecular mass of 85 (±5.0) kDa (Fig. 1, lane 4), which is close to the theoretical mass of 95,085 Da that has been calculated from the cphA translational product (1). The molecular mass of the native enzyme was estimated as 240 (±30) kDa.
TABLE 1.
Purification of cyanophycin synthetase
| Purification step | Volume (ml) | Protein (mg) | Activity (U)a | Sp act (U · mg of protein−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Soluble cell fraction | 54 | 5,151 | 17,095 | 3.32 | 1 | 100 |
| Q Sepharose | 44 | 162.3 | 9,747 | 60 | 18 | 57 |
| Procion Blue | 9 | 17.46 | 2,653 | 152 | 46 | 15.5 |
| Superdex 200 | 6 | 5.28 | 1,257 | 238 | 72 | 7.3 |
Units are nanomoles of arginine incorporated into cyanophycin per minute.
FIG. 1.
SDS-PAGE of cyanophycin synthetase from recombinant E. coli at different steps of purification. Lane 1, soluble cell extract (75 μg protein); lane 2, after chromatography on Q Sepharose HiLoad (55 μg); lane 3, after affinity chromatography on Procion Blue HE-RD Sepharose (30 μg); lane 4, after gel filtration on Superdex 200 HR (13 μg). The sizes of molecular mass standard proteins are provided on the left. Protein bands were visualized by staining with Serva Blue R.
Dependency of purified cyanophycin synthetase on substrates, cosubstrate, and primer.
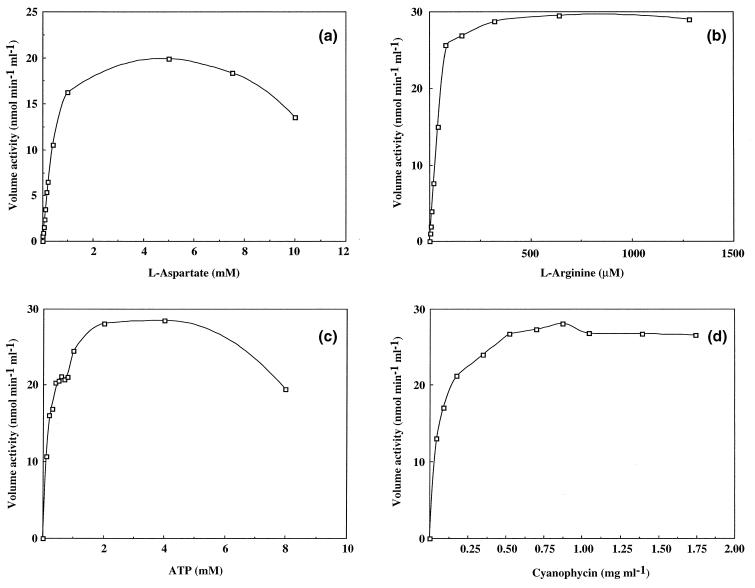
Time courses of the incorporation of the amino acid substrates exhibited a clear dependency on the presence of both amino acids (data not shown). The incorporation of only one amino acid substrate in the absence of the other was very low (2.4% in the absence of arginine and 1.2% in the absence of aspartic acid), as shown in Table 2. The dependency of the enzyme activity on the concentration of the amino acid substrates, ATP, and the primer cyanophycin was determined (Fig. 2), and the corresponding Km values, calculated from the Hanes equation, were 450, 49, and 200 μM and 35 μg ml−1 for l-aspartic acid, l-arginine, ATP, and cyanophycin, respectively. Depending on these Km values, the conditions of the cyanophycin synthetase assay, which was described recently (1), were modified. After increasing the concentration of aspartic acid and arginine approximately 50-fold, the activity of cyanophycin synthetase increased about 30-fold.
TABLE 2.
Incorporation of different radiolabeled amino acids into cyanophycin using purified cyanophycin synthetasea
| Condition | Radioactive compoundb | Enzyme activityc (% of complete reaction) |
|---|---|---|
| Complete | l-[U-14C]arginine | 100 |
| Without l-aspartic acid | l-[U-14C]arginine | 1.2 |
| Without l-arginine | l-[U-14C]aspartic acid | 2.4 |
| Plus canavanine | l-[U-3H]canavanine | 12 |
| l-Canavanine instead of l-arginine | l-[U-3H]canavanine | 13 |
| l-Lysine instead of l-arginine | l-[4,5-3H]lysine | 15 |
| l-Glutamic acid instead of l-arginine | l-[U-14C]glutamic acid | 0.1 |
| l-Glutamic acid instead of l-aspartic acid | l-[U-14C]glutamic acid | 0.3 |
Data represent the means of four determinations.
Final concentrations of the radioactive substrates were as follows: l-[U-14C]arginine, 0.5 mM; l-[U-14C]aspartic acid, 5 mM; l-[U-3H]canavanine, 1 mM; l-[4,5-3H]lysine, 1 mM; l-[U-14C]glutamic acid, 0.5 mM (when added instead of l-arginine) or 5 mM (when added instead of l-aspartic acid).
The activity corresponding to 100% was determined as the incorporation of 109 nmol of arginine min−1 ml−1.
FIG. 2.
The activity of purified cyanophycin synthetase as a function of the concentration of (a) l-aspartic acid, (b) l-arginine, (c) ATP, and (d) cyanophycin.
Previous investigations of Ziegler revealed that, during enzyme-catalyzed cyanophycin synthesis in complete reaction mixture, ATP was converted to ADP. The reaction mixtures obtained after 1 h of incubation of the purified enzyme under optimized conditions were used to determine the ratio of ADP formed per amino acid incorporated (Table 3) and were calculated as 1.3 ADP per amino acid in the presence of both amino acids, ATP, Mg2+, and cyanophycin. Formation of AMP was not detected. No ADP was detectable after incubation in the absence of either enzyme, ATP, or Mg2+. Residual ADP formation in the presence of only one amino acid substrate and in the absence of cyanophycin correlated to a corresponding small amount of incorporated amino acid.
TABLE 3.
Formation of ADP during cyanophycin synthetase-catalyzed incorporation of amino acid substratea
| Condition | ADPb (mM) | Argininec incorporated (mM) | Aspartic acidc incorporated (mM) |
|---|---|---|---|
| Complete | 0.56 | 0.216 | 0.202 |
| Without aspartic acid | 0.04 | 0.003 | N.D.d |
| Without arginine | 0.07 | N.D. | 0.005 |
| Without ATP | <0.02 | <0.0005 | N.D. |
| Without cyanophycin | 0.05 | 0.003 | N.D. |
| Without enzyme | <0.02 | <0.0005 | N.D. |
| Without MgCl2 | <0.02 | <0.0005 | N.D. |
Data represent the mean of four determinations.
The ADP content in the reaction mixture and the amount of amino acids incorporated into cyanophycin were determined after 1 h of incubation at 28°C, as described in Materials and Methods.
The incorporation of arginine and aspartic acid was determined in separate runs.
N.D., not determined.
Optimum pH and temperature and stability at elevated temperature.
The activity of cyanophycin synthetase was measured at different pH values in the range of 6.2 to 9.7. Highest activity was obtained by using Tris buffer adjusted to pH 8.2, which is in accordance with previously published results for cyanophycin synthetase from Anabaena cylindrica (16).
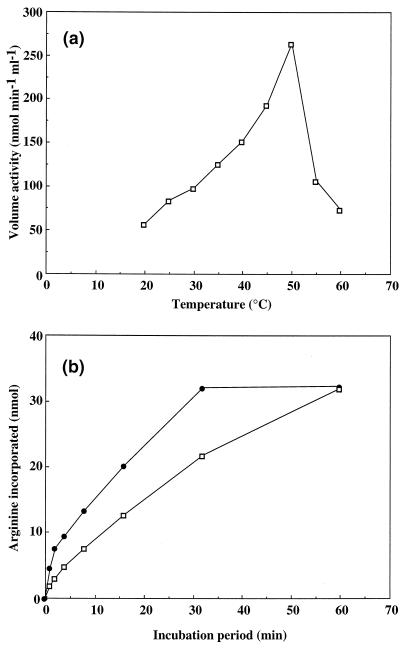
The effect of the assay temperature on the activity of cyanophycin synthetase was studied by incubating the reaction mixture at different temperatures. Maximum activity of cyanophycin synthetase was obtained at 50°C (Fig. 3a). Prolonged incubation at that temperature revealed that cyanophycin synthetase became inactive after 30 min, whereas the enzyme was still active when it was incubated at 28°C for 30 min (Fig. 3b).
FIG. 3.
The influence of the incubation temperature on the cyanophycin synthetase-catalyzed incorporation of arginine. (a) Cyanophycin synthetase activity as a function of the incubation temperature. The enzyme activity was determined for 4 min after start of the reaction at the indicated temperature. (b) Time courses of the incorporation of arginine into cyanophycin at 28°C (▫) and 50°C (●). The incorporated amount of arginine was determined at the specified time points in 125 μl of complete reaction mixture, as described in Materials and Methods. Purified cyanophycin synthetase was used for these experiments.
Substrate specificity.
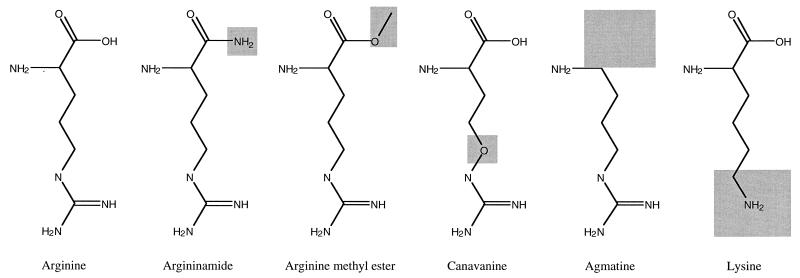
Based on the finding that incorporation of arginine and aspartic acid occurred at a high rate only in the presence of both amino acids (Table 2), we performed a screening procedure for compounds that (i) affected the activity of cyanophycin synthetase or (ii) were incorporated instead of or in addition to arginine or aspartic acid into the polymer (Fig. 4). The structure formulas of arginine-analogous compounds are shown in Fig. 5.
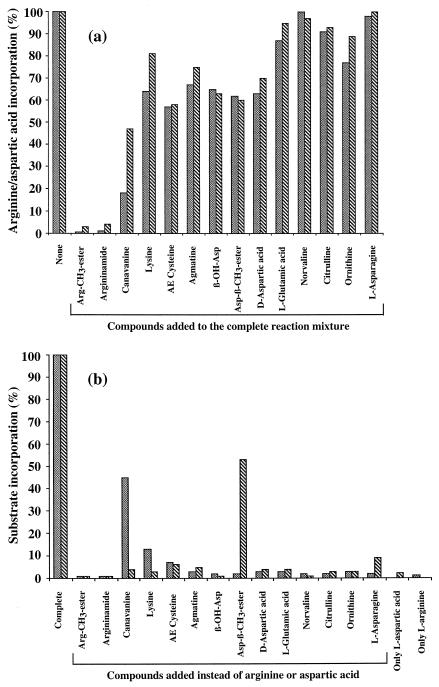
FIG. 4.
Effects produced by the addition or substitution of analogous compounds structurally related to aspartic acid and arginine on the incorporation of both amino acids. (a) Aspartic acid (▧) and arginine (░⃞) incorporation in the presence of a 5 mM concentration of the analogous compounds, which were added to the complete reaction mixture containing 5 mM l-[U-14C]aspartic acid and 0.5 mM l-[2,3,4,5-3H]arginine, as described in Materials and Methods. (b) Effect of the analogous compounds on the incorporation of arginine and aspartic acid when they were added in place of aspartic acid (▧) at a final concentration of 5 mM or in place of arginine (░⃞) at a final concentration of 0.5 mM. The activity corresponding to 100% was determined as the incorporation of 10 nmol of aspartic acid · min−1 · ml−1 and 11 nmol of arginine · min−1 · ml−1, respectively, as described in Materials and Methods using purified cyanophycin synthetase. Values were calculated on the basis of four runs (run standard deviation, ca. 10%).
FIG. 5.
Structural formulas of arginine and analogous compounds tested as substrates for cyanophycin synthetase. Changes of the structures with respect to the natural substrate arginine are shaded.
In the first series of experiments (Fig. 4a), a third additional compound was added to the complete reaction mixture, and the incorporations of 14C-labeled aspartic acid and 3H-labeled arginine were measured simultaneously. The 14 compounds, which exhibited more or less severe inhibitory effects on cyanophycin synthetase activity, are depicted in Fig. 4a. On the basis of the occurrence of different effects on the incorporation of arginine and aspartic acid, the inhibitory compounds can be divided into two groups: (i) the majority of the compounds (i.e., arginine methyl ester, argininamide, S-(2-aminoethyl) cysteine, β-hydroxy aspartic acid, aspartic acid β-methyl ester, norvaline, citrulline, and asparagine) exhibited an almost equal effect on the incorporation of both substrates, as shown in Fig. 4a; (ii) six compounds (i.e., canavanine, lysine, agmatine, d-aspartic acid, l-glutamic acid, and ornithine) showed a remarkably stronger negative effect on the incorporation of arginine than on aspartic acid. This discrepancy may indicate that these compounds had been incorporated along with arginine into the polymer. No effect on the incorporation rates was obtained from proteinogenic amino acids other than arginine and aspartic acid (i.e., alanine, histidine, leucine, proline, tryptophan, and glycine [data not shown]).
In a second series of experiments as part of the screening, all compounds were added in place of either arginine or aspartic acid to the reaction mixture (Fig. 4b). Canavanine or lysine led to a remarkable increase in the incorporation of [14C]aspartic acid when it was added instead of arginine to the reaction mixture, compared to the level of incorporation in an assay performed with only aspartic acid. This clearly indicated again that canavanine and lysine could be incorporated into the polymer instead of arginine. On the other hand, aspartic acid β-methyl ester and asparagine were found to stimulate the incorporation of [3H]arginine, which can be explained by the incorporation of either of these compounds into the polymer in place of aspartic acid.
Of the compounds mentioned above that exhibited different effects in either series of experiments, canavanine, lysine, and l-glutamic acid were directly tested for their incorporation into cyanophycin by using their radioisomers (Table 2). For canavanine and lysine, incorporation into cyanophycin could be confirmed by these experiments. However, no hint was obtained from the radiometric experiments for the incorporation of l-glutamic acid. Incorporation of l-glutamic acid was found to be negligible in vitro when the purified cphA translational product was used (Table 2), although it had been found previously that l-glutamic acid instead of arginine was incorporated into cyanophycin in vitro when crude cell extracts from Synechocystis sp. strain PCC6308 were used (1) and although it was reported in the literature that l-glutamic acid replaced arginine as a main constituent of cyanophycin in nitrogen-limited cells of this bacterium (10).
Primer specificity.
In the absence of a primer, the activity of the purified enzyme was almost negligible (Table 4). Various aspartic acid-containing polymers were tested for their ability to act as a primer for cyanophycin synthetase in place of cyanophycin. Although only a very low level of incorporation of arginine into poly-α-l-aspartic acid homopolymers was found, significant incorporation of arginine to poly-α,β-d,l-aspartic polymers from different commercial sources occurred (Table 4).
TABLE 4.
Cyanophycin synthetase-catalyzed incorporation of arginine into aspartic acid containing polymersa
| Polymer added as primerb | Incorporation of argininec (% of maximum value)d |
|---|---|
| Cyanophycin | 100 |
| Without primer | 0.2 |
| Poly-α,β-dl-aspartic acid (from Bayer AG, Leverkusen, Germany; lot, LP OC 618; color, yellowish) | 97 |
| Poly-α,β-dl-aspartic acid (Bayer; lot, LP OC 617; color, beige) | 25 |
| Poly-α,β-dl-aspartic acid (Sigma; lot, 64H5510; color, white) | 49 |
| Poly-α-l-aspartic acid (Sigma) | 3.4 |
| Poly-α-l-aspartic acid, pentamer (Sigma) | 0.5 |
Data represent the mean of four determinations.
Polymers were added in a final concentration of 0.87 mg · ml−1 to the reaction mixture.
The amount of incorporated arginine was measured in the reaction mixture after 10 h of incubation at 28°C.
The maximum value for each primer corresponding to 100% was determined as 430 nmol of arginine ml−1.
DISCUSSION
The availability of a reliable method for the determination of cyanophycin synthetase activity, which was published previously (1), greatly facilitated the purification and characterization of the Synechocystis sp. strain PCC6308 cyanophycin synthetase and made the first kinetic data about the reaction catalyzed by this cyanophycin synthetase available. The low Km values, which were determined for both amino acid substrates and for ATP, indicated the high affinity of this enzyme for these reactants, in particular, arginine. The polymerization of arginine and aspartic acid was almost completely dependent on the presence of both of these amino acids; synthesis of polyaspartic acid did not occur. These results distinguish the cyanophycin synthetase of Synechocystis sp. strain PCC6308 from the enzymes of A. variabilis (18) and A. cylindrica (16), which exhibited significant residual activity in the presence of only one of these amino acids. The release of 2.6 ADP per incorporated pair of amino acids corresponds well with the activation of two carboxylic groups, which is needed for the formation of the two peptide bonds per cyanophycin building block.
In vitro synthesis of cyanophycin occurred at a significant rate only in the presence of cyanophycin; only negligible amounts of perceptible material were formed during long incubation times (≥1 h) in the absence of cyanophycin. Since, on the other hand, fast-growing recombinant cells of E. coli were able to synthesize cyanophycin de novo in large quantities (1), the question arose about the nature of the primer for cyanophycin synthesis in both E. coli and the cyanobacteria. Our findings that poly-α,β-d,l-aspartic acid polymers with a heterogeneous structure can serve as primers in vitro are puzzling. Since these polymers were chemically synthesized by simple thermocondensation of aspartic acid leading to variable amounts of unknown byproducts and structures inside the polymer matrix, the nature of the priming motif in these artificial polymers is still unknown. However, this study clearly indicates that other related molecules can prime the reaction catalyzed by the cyanophycin synthetase. In vivo, related molecules such as, for example, precursors of the peptidoglycan or even proteins or peptides occurring in E. coli may substitute for the cyanophycin that is lacking at the beginning of synthesis. Another solution of this “chicken and egg” problem may be the fact that the cyanophycin synthesis is not completely abolished in the absence of cyanophycin or that during the long period of in vivo synthesis sufficient cyanophycin primer molecules to start the strand elongation are synthesized.
Purified cyanophycin synthetase did not accept l-glutamic acid as substrate. This is in contrast to the incorporation of l-glutamic acid into Synechocystis sp. strain PCC6308 cyanophycin, which was found to occur either in vivo in nitrogen-limited cells (10) or in vitro in cell extracts of this bacterium (1). It is interesting that l-glutamic acid is introduced into the polymer in vivo in place of arginine and is not introduced in place of aspartic acid (10); therefore, it is hardly conceivable that activation of these two completely different amino acids occurs at the same catalytic site. Because of the relatively small size of cyanophycin synthetase with respect to the complexity of the catalyzed reaction and in accordance with the primary structure of the enzyme, the presence of one additional catalytic site for l-glutamic acid is not very likely as well. Therefore, another yet unknown enzyme component is most probably responsible for the in vivo incorporation of l-glutamic acid into cyanophycin.
It is also interesting that l-lysine and canavanine were found to be incorporated instead of arginine into the polymer. l-Lysine has been previously reported as a minor constituent of cyanophycin from recombinant cells of E. coli harboring A. variabilis cphA (18). Since lysine shows some similarity to arginine with respect to length and net charge, a cyanophycin synthetase-catalyzed incorporation of this amino acid is comprehensible. Argininamide and arginine methyl ester were found to inhibit the incorporation of both arginine and aspartic acid drastically, which indicates that these two compounds are not accepted by this cynophycin synthetase as substrates instead of arginine.
In conclusion, this study clearly demonstrated the suitability of cyanophycin synthetase for the biosynthesis of novel biopolymers. Because of the flexibility of cyanophycin synthetase with respect to the chemical structure of the primer required for initiation of the polymerization reaction, polyamides with other constituents than aspartic acid and arginine may be synthesized as well as novel block copolymers.
ACKNOWLEDGMENT
This study was supported by a fellowship to Elsayed Aboulmagd provided from the government of the Arabic Republic of Egypt.
REFERENCES
- 1.Aboulmagd E, Oppermann-Sanio F B, Steinbüchel A. Molecular characterization of the cyanophycin synthetase from Synechocystissp. strain PCC6308. Arch Microbiol. 2000;174:297–306. doi: 10.1007/s002030000206. [DOI] [PubMed] [Google Scholar]
- 2.Allen M M. Cyanobacterial cell inclusions. Annu Rev Microbiol. 1984;38:1–25. doi: 10.1146/annurev.mi.38.100184.000245. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson T, Hammond P M, Hartwell R D, Hughes P, Scaven M D, Sherwood R F, Small A P, Bruton C J, Harvey M J, Lowe C R. Triazine-dye affinity chromatography. Biochem Soc Trans. 1981;9:290–293. doi: 10.1042/bst0090290. [DOI] [PubMed] [Google Scholar]
- 4.Berg H, Ziegler K, Piotukh K, Baier K, Lockau W, Volker-Engert R. Biosynthesis of the cyanobacterial reserve polymer multi-l-arginyl-poly-l-aspartic acid (cyanophycin). Mechanism of the cyanophycin synthetase reaction studied with synthetic primers. Eur J Biochem. 2000;267:5561–5570. doi: 10.1046/j.1432-1327.2000.01622.x. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Hai T, Oppermann-Sanio F B, Steinbüchel A. Purification and characterization of cyanophycin and cyanophycin synthetase from the thermophilic Synechococcussp. MA19. FEMS Microbiol Lett. 1999;181:229–236. doi: 10.1111/j.1574-6968.1999.tb08849.x. [DOI] [PubMed] [Google Scholar]
- 7.Jaworek D, Gruber W, Bergmeyer H U. Adenosin-5′-diphosphat und Adenosin-5′-monophosphat. In: Bergmeyer H U, editor. Methoden der enzymatischen Analyse Verlag Chemie. Germany: Weinheim; 1974. pp. 2051–2055. [Google Scholar]
- 8.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Mackerras A H, De Chazal N M, Smith G D. Transient accumulations of cyanophycin in Anabaena cylindrica and Synechocystis6308. J Gen Microbiol. 1990;136:2057–2065. [Google Scholar]
- 10.Merritt M V, Sid S S, Mesh L, Allen M M. Variations in the amino acid composition of cyanophycin in the cyanobacterium Synechocystissp. PCC6308 as a function of growth conditions. Arch Microbiol. 1994;162:158–166. doi: 10.1007/BF00314469. [DOI] [PubMed] [Google Scholar]
- 11.Oppermann F B, Schmidt B, Steinbüchel A. Purification and characterization of acetoin:2,6-dichlorophenolindophenol oxidoreductase, dihydrolipoamide dehydrogenase, and dihydrolipoamide acetyltransferase of the Pelobacter carbinolicusacetoin dehydrogenase enzyme system. J Bacteriol. 1991;173:757–767. doi: 10.1128/jb.173.2.757-767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oppermann-Sanio F B, Hai T, Aboulmagd E, Hezayen F F, Jossek S, Steinbüchel A. Biochemistry of microbial polyamide metabolism. In: Steinbüchel A, editor. Biochemical principles and mechanisms of biosynthesis and biodegradation of polymers. Weinheim, Germany: Wiley-VCH; 1999. pp. 185–193. [Google Scholar]
- 13.Richter R, Hejazi M, Kraft R, Ziegler K, Lockau W. Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-l-arginyl-poly-l-aspartic acid (cyanophycin). Molecular cloning of the gene of Synechocystis sp. PCC 6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur J Biochem. 1999;263:163–169. doi: 10.1046/j.1432-1327.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 15.Schwamborn M. Chemical synthesis of polyaspartates: a biodegradable alternative to currently used polycarboxylate homo- and copolymers. Polym Degrad Stab. 1998;59:39–45. [Google Scholar]
- 16.Simon R D. The biosynthesis of multi-l-arginyl-poly(l-aspartic acid) in the filamentous cyanobacterium Anabaena cylindrica. Biochim Biophys Acta. 1976;422:407–418. doi: 10.1016/0005-2744(76)90151-0. [DOI] [PubMed] [Google Scholar]
- 17.Simon R D, Weathers P. Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in cyanobacteria. Biochim Biophys Acta. 1976;420:165–176. doi: 10.1016/0005-2795(76)90355-x. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler K, Diener A, Herpin C, Richter R, Deutzmann R, Lockau W. Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-l-arginyl poly-l-aspartate (cyanophycin) Eur J Biochem. 1998;254:154–159. doi: 10.1046/j.1432-1327.1998.2540154.x. [DOI] [PubMed] [Google Scholar]