A comprehensive understanding of the life‐history strategies and habitat use of species is essential for developing accurate ecological models and effective management and conservation strategies. For example, omitting critical habitats of endangered species when estimating their abundance and when designing conservation plans can severely limit our understanding of population dynamics and lead to poor management outcomes. Here we describe important new observations of the distribution and habitat use of Longfin Smelt (Spirinchus thaleichthys) in the San Francisco Estuary, California, USA. Longfin Smelt are planktivorous forage fish found in estuarine and coastal waters from San Francisco Bay, California to the Aleutian Islands, Alaska (Garwood 2017). This species was once a dominant forage fish in the estuary, even supporting a small commercial fishery prior to the 1970s (Skinner 1962, Moyle 2002); however, this genetically distinct population has collapsed to approximately 1% of its historic (pre‐1980) abundance, and details regarding its life history and drivers of population dynamics remain uncertain (Nobriga and Rosenfield 2016).
Longfin Smelt are thought to live for 1–2 yrs in the coastal Pacific Ocean before returning to tidal freshwater habitats of the Sacramento–San Joaquin Delta (“upper estuary”), where they are believed to spawn (Moyle 2002, Rosenfield and Baxter 2007; Fig. 1a). Though the San Francisco Estuary is a well‐studied system, long‐term fishery surveys were designed for other species (e.g., striped bass); thus population models for Longfin Smelt have depended largely on data from the upper estuary and open‐water bay habitats. Targeted studies are needed to assess whether existing data sets and resultant models accurately describe Longfin Smelt distributions, behaviors, and population dynamics. For example, a recent study observed high densities of Longfin Smelt larvae in previously unsampled wetland habitats in the upper estuary (Grimaldo et al. 2017). Similarly, it has been hypothesized that Longfin Smelt may also inhabit shallow tidal wetlands of the many smaller watersheds throughout San Francisco Bay and San Pablo Bay for spawning, rearing, and feeding (Fig. 1b).
Figure 1.
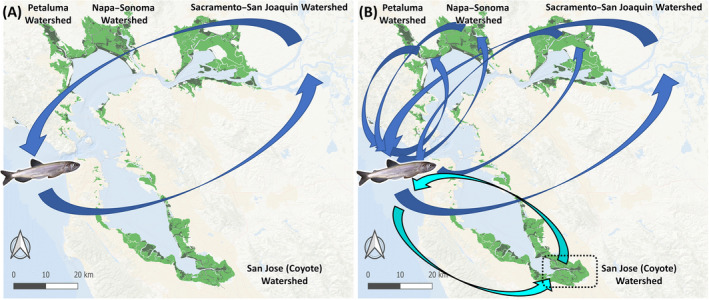
Life history of the Longfin Smelt. (A) Classic life‐history model showing a 2‐yr semi‐anadromous life history, with adults migrating upstream to spawn in the Sacramento–San Joaquin Watershed and subsequent downstream dispersal of larvae and juveniles to bay and coastal habitats. (B) Alternative life‐history model indicating spawning and hatching in a variety of smaller watersheds throughout the San Francisco Estuary (SFE). Our observations in the Coyote Creek Watershed in the southern SFE (dashed box) confirm that reproductive adult Longfin Smelt visit this watershed annually and successfully spawn in years of high precipitation. This life‐history strategy was likely more common and successful prior to extensive degradation of brackish wetland habitats and damming and diversion of lesser watersheds throughout the estuary. Green shaded areas represent historic (light) and existing (dark) brackish wetlands (San Francisco Estuary Institute and Aquatic Science Center [SFEI ASC] 2017).
From October through April, which encompasses the spawning season, and in all years from 2011 to 2019, we observed persistent and occasionally dense aggregations of adult Longfin Smelt in marshes and sloughs of the Coyote Creek watershed in the southernmost part of San Francisco Bay (Figs. 1b, 2a,b). Many of the adults were in late‐stage spawning condition and expressed eggs and milt upon capture (Fig. 2c). Postlarval recruits (Fig. 2d) were also observed in April–May of 2017 and 2019, with each of these years characterized by anomalously high precipitation and freshwater outflow (and persistent low‐salinity spawning and rearing habitat). Thus, the potential for spawning was apparent in all years, whereas recruitment success appeared to be limited by freshwater outflow, as has been described for Longfin Smelt in the upper estuary (Kimmerer 2002, Nobriga and Rosenfield 2016). The highest catches of recruits and adults were often within shallow recently restored tidal marshes and adjacent sloughs (Fig. 2a), suggesting that (1) previous surveys have likely omitted substantial fractions of the San Francisco Estuary Longfin Smelt population and (2) that tidal marsh restoration may benefit all life stages of this threatened species.
Figure 2.
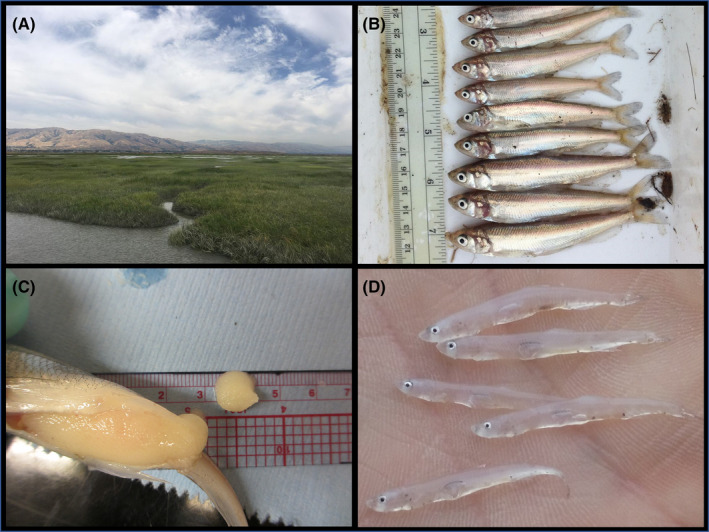
(A) Example of a restored salt marsh in South San Francisco Bay where relatively high densities of (B) adult Longfin Smelt were often observed during the spawning season. Many of the adults captured were expressing (C) milt or eggs (shown), indicative of spawning. In 2017 and 2019, (D) postlarval Longfin Smelt recruits were also observed in restored marshes and adjacent sloughs, indicating successful spawning and rearing in Lower South San Francisco Bay during years of high precipitation and freshwater outflow.
Surveys were conducted monthly from 2011 to 2019 at 2–3 m depth in marsh and slough habitats of the lower Coyote Creek watershed, including two restored tidal marshes and two open‐water bay stations. The Coyote Creek watershed of South San Francisco Bay drains much of Silicon Valley and feeds directly into the Alviso Marsh Complex—a macrotidal Mediterranean‐type estuary with a 4‐m tidal range that is composed of several major tidal sloughs (Upper and Lower Coyote Creek, Alviso Slough, and Artesian Slough) networked throughout historically expansive salt marsh habitats. Sampling was conducted by towing an otter trawl with a 4.3 × 1.5 m opening and 0.6‐cm cod end mesh into the current at 3 km/h for 5–10 min and counting the number of fish in each tow.
High abundances of Longfin Smelt were unexpected in these habitats, let alone evidence for successful spawning and recruitment. Most of the historic wetland habitats in the region were dredged, diked, and converted to solar evaporation ponds for salt production in the mid‐1900s (Nichols et al. 1986) and little freshwater runoff reaches the estuary because of numerous upstream dams and diversions that capture and redirect most instream flows for human use (Grossinger et al. 2007). Alviso Marsh also receives over 100 million gallons per day of low‐salinity, warm, and nutrient‐rich wastewater effluent directly into its sloughs (San Jose–Santa Clara Regional Wastewater Facility [SJSCRWF] 2018). Historically, there has been little interest in studying ecological communities in this highly degraded ecosystem. However, recent commitments to restore 15,000 acres of wetland habitat in the region (Valoppi 2018) have provided the motivation to establish baselines and document changes in aquatic communities in response to restoration efforts over the past decade. Surprisingly, our observations suggest that Longfin Smelt are relatively abundant and can successfully spawn and rear in wetlands of South San Francisco Bay, and that restoration of marsh habitats and increases in freshwater outflow to these smaller watersheds could benefit this threatened species.
How important are brackish wetland habitats to Longfin Smelt? To answer this, future studies should build upon these observations, expanding the geographic scope of sampling to encompass other brackish marshes and sloughs, and assessing the relative importance of these habitats to the adult population. Estuary‐wide sampling of brackish wetland habitats should be conducted during spawning and recruitment periods and in years with different precipitation, temperature, and other climate parameters. Multiple gear types should be deployed to quantify both larval and adult abundances, and methods should match or be calibrated against long‐term monitoring programs so that relative contributions can be directly compared among habitat types and regions. Future modeling efforts could assess sensitivity and elasticity of population dynamics to production and survival of young‐of‐the‐year age classes in different regions and habitats of the estuary.
Can tidal marsh and watershed restoration benefit Longfin Smelt? To address this, it is important to quantify the conservation value of tidal marsh habitats to Longfin Smelt. Comparative studies of abundance, feeding rates, growth rates, diets, and mortality rates of Longfin Smelt in marshes versus open‐water habitats would be valuable. Trace‐element chemistry of otoliths (fish ear bones) could be used to quantify the relative contributions of different regions and habitat types to adult Longfin Smelt populations (Hobbs et al. 2007). Isotopes of Sr and O in otoliths can be used to reconstruct time‐resolved histories of salinity, providing key information about habitat requirements and ontogenetic movement patterns and behaviors (Hobbs et al. 2010). Additional chemical tools (e.g., δ34S and Mn) could prove useful for reconstructing the use of wetland versus open‐water habitats by Longfin Smelt.
Longfin Smelt are at record low abundance and likely no longer serve their historic ecological function in the San Francisco Estuary. The population was listed as “threatened” in 2009 under the California Endangered Species Act (CESA) and designated as “warranted but precluded” for listing in 2012 under the federal Endangered Species Act (ESA). For 7 years, the species has remained precluded from ESA protections despite rapid and continuous declines in abundance. In the face of numerous ecological impacts, a warming climate (Cloern et al. 2011, Jeffries et al. 2016), and precluded protection, the future of longfin smelt remains bleak in the estuary (Hobbs et al. 2017).
For nine consecutive years, however, we have observed previously undescribed aggregations of Longfin Smelt that were attempting to spawn in restored and under‐explored tidal wetlands of South San Francisco Bay. Furthermore, we observed successful recruitment in years of high freshwater outflow. These observations may help explain the positive recruitment–freshwater outflow relationship and long‐term decline of Longfin Smelt (Kimmerer 2002, Kimmerer et al. 2009, Nobriga and Rosenfield 2016). For example, expansion of shallow low‐salinity spawning and rearing habitats throughout the entire estuary in wet years may explain in part the positive relationship between interannual variation in freshwater outflow and Longfin Smelt recruitment. Similarly, the historic degradation of lesser watersheds and brackish wetlands throughout the San Francisco Estuary may have contributed to the long‐term decline of this species. Thus, the observations reported herein could transform our fundamental understanding of the habitat needs of Longfin Smelt and the interventions needed to conserve and restore this distinct and imperiled population.
Lewis, L. S. , Willmes M., Barros A., Crain P. K., and Hobbs J. A.. 2020. Newly discovered spawning and recruitment of threatened Longfin Smelt in restored and underexplored tidal wetlands. Ecology 101(1):e02868. 10.1002/ecy.2868
Corresponding Editor: John Pastor.
Literature Cited
- Cloern, J. E. , et al. 2011. Projected evolution of California's San Francisco Bay‐Delta‐River System in a century of climate change. PLoS ONE 6:e24465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood, R. S. 2017. Historic and contemporary distribution of longfin smelt (Spirinchus thaleichthys) along the California coast. California Fish and Game 103:96–117. [Google Scholar]
- Grimaldo, L. , Feyrer F., Burns J., and Maniscalco D.. 2017. Sampling uncharted waters: examining rearing habitat of larval Longfin Smelt (Spirinchus thaleichthys) in the Upper San Francisco Estuary. Estuaries and Coasts 40:1771–1784. [Google Scholar]
- Grossinger, R. M. , Striplen C. J., Askevold R. A., Brewster E., and Beller E. E.. 2007. Historical landscape ecology of an urbanized California valley: wetlands and woodlands in the Santa Clara Valley. Landscape Ecology 22:103–120. [Google Scholar]
- Hobbs, J. A. , Bennett W. A., Burton J., and Gras M.. 2007. Classification of larval and adult delta smelt to nursery areas by use of trace elemental fingerprinting. Transactions of the American Fisheries Society 136:518–527. [Google Scholar]
- Hobbs, J. A. , Lewis L. S., Ikemiyagi N., Sommer T., and Baxter R. D.. 2010. The use of otolith strontium isotopes (87Sr/86Sr) to identify nursery habitat for a threatened estuarine fish. Environmental Biology of Fishes 89:557–569. [Google Scholar]
- Hobbs, J. A. , Peter M. B., Fangue N., and Connon R. E.. 2017. Is extinction inevitable for delta smelt and longfin smelt? An opinion and recommendations for recovery. San Francisco Estuary and Watershed Science 15:1–19. [Google Scholar]
- Jeffries, K. M. , Connon R. E., Davis B. E., Komoroske L. M., Britton M. T., Sommer T., Todgham A. E., and Fangue N. A.. 2016. Effects of high temperatures on threatened estuarine fishes during periods of extreme drought. Journal of Experimental Biology 219:1705–1716. [DOI] [PubMed] [Google Scholar]
- Kimmerer, W. J. 2002. Effects of freshwater flow on abundance of estuarine organisms: physical effects or trophic linkages? Marine Ecology Progress Series 243:39–55. [Google Scholar]
- Kimmerer, W. J. , Gross E. S., and MacWilliams M. L.. 2009. Is the response of estuarine nekton to freshwater flow in the San Francisco Estuary explained by variation in habitat volume? Estuaries and Coasts 32:375–389. [Google Scholar]
- Moyle, P. B. 2002. Inland fishes of California. University of California Press, Berkeley, California, USA. [Google Scholar]
- Nichols, F. H. , Cloern J. E., Luoma S. N., and Peterson D. H.. 1986. The modification of an estuary. Science 231:567–573. [DOI] [PubMed] [Google Scholar]
- Nobriga, M. L. , and Rosenfield J. A.. 2016. Population dynamics of an estuarine forage fish: disaggregating forces driving long‐term decline of Longfin Smelt in California's San Francisco Estuary. Transactions of the American Fisheries Society 145:44–58. [Google Scholar]
- Rosenfield, J. A. , and Baxter R. D.. 2007. Population dynamics and distribution patterns of longfin smelt in the San Francisco estuary. Transactions of the American Fisheries Society 136:1577–1592. [Google Scholar]
- SFEI ASC (San Francisco Estuary Institute and Aquatic Science Center). 2017. Bay Area aquatic resource inventory (BAARI) Version 2.1 GIS Data. http://www.sfei.org/data/baari-version-21-gis-data
- SJSCRWF (San Jose–Santa Clara Regional Wastewater Facility). 2018. Annual self monitoring report. http://www.sanjoseca.gov/ArchiveCenter/ViewFile/Item/3507
- Skinner, J. E. 1962. An historical review of the fish and wildlife resources of the San Francisco Bay Area. Department of Fish and Game Water Projects Branch Report No. 1.
- Valoppi, L. 2018. Phase 1 studies summary of major findings of the South Bay Salt Pond Restoration Project, South San Francisco Bay, California: U.S. Geological Survey Open‐File Report 2018‐1039. 10.3133/ofr20181039 [DOI]


