Abstract
Objective
To assess variations in nutritional interventions during chemoradiotherapy (CRT) among the Dutch Head and Neck Oncology centres (HNOCs).
Methods
An online questionnaire about nutritional interventions and dietetic practices was sent to 14 oncology dietitians of the HNOCs.
Results
The response rate was 93%. The number of scheduled dietetic consultations varied from two to seven during CRT. Most centres (77%) reported using a gastrostomy for tube feeding in the majority of patients. Gastrostomies were placed prophylactically upon indication (39%) or in all patients (15%), reactive (15%), or both (31%). For calculating energy requirements, 54% of the dietitians used the Food and Agriculture Organization/World Health Organization and United Nations University (FAO/WHO/UNU) formula and 77% uses 1.2–1.5 g/kg body weight for calculating protein requirements. Almost half of the centres (46%) reported to remove the gastrostomy between 8 and 12 weeks after CR. Most centres (92%) reported to end dietary treatment within 6 months after CRT.
Conclusion
This study shows substantial variation in dietetic practice, especially in the use of a gastrostomy for tube feeding, between the HNOCs. There is a need for concise dietetic guidelines.
Keywords: chemoradiotherapy, diet therapy, enteral nutrition, gastrostomy, head and neck neoplasms, nutritional intervention
1. INTRODUCTION
In patients with locally advanced head and neck squamous cell carcinoma (LAHNSCC) the standard treatment is primary or adjuvant radiotherapy with concurrent chemotherapy for 6 to 7 weeks (Pignon et al., 2009). Side effects of this chemoradiotherapy (CRT), for example, pain, dysphagia, mucositis, taste alterations, xerostomia, sticky saliva and nausea, impair oral nutritional intake (Bressan et al., 2016; Mulasi et al., 2020). As a consequence, these patients are at high risk of malnutrition (Bressan et al., 2016), which is characterised by unintended weight loss (Cederholm et al., 2019). Weight loss in patients with head and neck cancer (HNC) is associated with an increased rate of treatment interruption (Capuano et al., 2008; Paccagnella et al., 2010; Sealy et al., 2019), dose‐limiting toxicity (Wendrich et al., 2017), more severe radiation‐induced toxicity (Meyer et al., 2012), a lower quality of life (Capuano et al., 2010; Jager‐Wittenaar, Dijkstra, Vissink, van der Laan, et al., 2011; van den Berg et al., 2008) and a lower overall survival (Capuano et al., 2008; Datema et al., 2011; Langius et al., 2013). Intensive nutritional intervention has been shown to be beneficial in preventing weight loss and lowering CRT related toxicity (Isenring et al., 2004; Isenring et al., 2007; Valentini et al., 2012). Dietary treatment for malnourished patients also diminishes healthcare costs for HNC patients (Scholte, 2015). Therefore, dietary treatment is usually embedded in the HNC healthcare process from diagnosis until follow‐up.
In the Netherlands, HNC care is centralised in 14 head and neck oncology centres (HNOCs); 8 university hospitals; and 6 affiliated centres (van Overveld et al., 2017). Medical specialists of these centres involved in HNC care are united in the Dutch Head and Neck Cancer Society (NWHHT) (van Overveld et al., 2017). The members of the NWHHT, in consultation with members of the Allied Health Professionals for HNC (PWHHT), have developed the Dutch Head and Neck Cancer guidelines for standardisation and increasing quality of HNC care (Leemans et al., 2014). These guidelines do not provide guidance for the frequency of dietetic consultations during and after CRT. Also, the guidelines provide little information on the nutrition prescription (calculation of energy and protein needs) and nutritional interventions, such as tube feeding use, indications for gastrostomy placement and gastrostomy removal policy.
It is thereby unclear to what extent nutritional interventions vary between the HNOCs in the Netherlands. Therefore, the aim of this survey study is to evaluate current dietetic practice concerning dietary treatment, the dietetic care process, tube feeding and tube placement in patients with LAHNSCC treated with CRT at the HNOCs.
2. METHODS
In January 2019, an email with a link to an online questionnaire was sent to 14 oncology dietitians of all fourteen HNOCs in the Netherlands.
The questionnaire consisted of 18 questions concerning nutritional intervention during CRT for LAHNSCC patients (Data S1). The following topics were addressed: dietetic consultations during CRT; tube feeding use and route; calculation of energy and protein requirements; tube placement and removal policy and end of dietary treatment.
Respondents were asked to fill out the questionnaire within 3 weeks. After 3 weeks a reminder was sent to those who had not filled out the questionnaire. When information was unclear a request for further explanation was sent.
2.1. Ethical considerations
No ethical approval was needed for this survey on routine clinical practice and no patients were involved.
3. RESULTS
3.1. Dietetic consultations during treatment
Thirteen of the fourteen (93%) oncology dietitians completed the questionnaire. In all participating 13 centres, every LAHNSCC patient undergoing CRT was routinely referred to an oncology dietitian. In most centres (69%), dietetic consultations were scheduled weekly for all patients. Two centres (15%) reported scheduling between two and four dietetic consultations during the 7‐week treatment period and the remaining two centres (15%) determined the frequency of dietetic consultations depending on patients' needs and preferences. In all centres, all scheduled dietetic consultations were face‐to‐face contacts.
3.2. Tube feeding and feeding route
When asked what percentage of CRT patients required tube feeding, dietitians provided estimates ranging from 25% to 50% (n = 1), 50% to 75% (n = 7), and 75% to 100% (n = 5). In summary, all but one respondent (92%) estimated that more than half of all CRT patients required tube feeding at some point during CRT treatment. In most centres (77%), a gastrostomy was most frequently used (in 75% to 99% of patients) for the administration of tube feeding during CRT. In the remaining three centres (23%), a nasogastric tube was the preferred route (in 70% to 95% of their CRT patients). Four dietitians reported using a nasoduodenal or nasojejunal tube in a minority of patients (1% to 10%). Five centres (39%) reported placing a gastrostomy only prophylactically upon indication, thus in selected patients. Four centres (31%) reported placing gastrostomies both prophylactically upon indication or reactive. Two centres (15%) reported placing only reactive gastrostomies and two other centres (15%) placed prophylactic gastrostomies in all patients. Six out of the thirteen centres (46%) developed a centre‐specific protocol with indications for gastrostomy placement. Five other centres (38%) used selection criteria for gastrostomy placement as well, but these were not embedded in a protocol. Reported selection criteria for (prophylactic) gastrostomy placement include, among others: tumour location; tumour size; bilateral neck irradiation; malnutrition risk and pre‐treatment dysphagia. Detailed information on gastrostomy placement and selection criteria used can be found in Table 1.
TABLE 1.
Detailed information on gastrostomy placement and the presence of a gastrostomy placement protocol at the thirteen participating Dutch Head and Neck Oncology centres
| Respondent number | Gastrostomy placement | Selection criteria for gastrostomy placement | Protocol with indications |
|---|---|---|---|
| 1 | Reactive | Based on weight loss ≥10% and intake <50% | Yes |
| 2 | Prophylactic upon indication and reactive | Prophylactic based on criteria: very low BMI, large tumour, dysphagia. Reactive in case of severe complications during treatment and if nasogastric tube is not possible. Reactive often after CRT treatment | No |
| 3 | Prophylactic upon indication | If tumour is localised in oropharynx, oral cavity or nasopharynx. If tumour is localised elsewhere, it is based on insufficient intake and weight loss | Yes |
| 4 | Reactive | If nasogastric tube is not possible or not tolerated | No |
| 5 | Prophylactic upon indication and reactive | Prophylactic on indication in case of treatment with cisplatin, reactive if enteral nutrition is necessary (but then nasogastric tube is used instead of PEG/PRG) | No |
| 6 | Prophylactic upon indication and reactive | — | No |
| 7 | Prophylactic upon indication and reactive | No clear indicators, but at least 10% weight loss before treatment and dysphagia at baseline | No |
| 8 | Prophylactic (in all patients) | All patients receive a PEG/PRG tube prophylactic, unless it is not possible due to comorbidity. In that case, a nasogastric tube will be placed reactive | Yes |
| 9 | Prophylactic upon indication | If nutritional status is insufficient before start of CRT treatment | No |
| 10 | Prophylactic upon indication | In case of a primary tumour in oral cavity or oropharynx and/or bilateral neck irradiation | No |
| 11 | Prophylactic upon indication | If the physician expects that swallowing problems will be minimal (5% of the cases), a PEG or PRG tube is not placed prophylactic. In other cases, PEG or PRG tubes are placed before the treatment starts | Yes |
| 12 | Prophylactic (in all patients) | Prophylactic placement in almost every patient, except if there are contraindications or if the patients does not want a PEG or PRG tube. If the PEG tube is not placed prophylactic and tube feeding is needed in the last weeks of CRT, it will be provided via nasogastric tubes | Yes |
| 13 | Prophylactic upon indication | When at least one of the following applies: (1) T3/T4 tumour in oral cavity, oropharynx or hypopharynx; (2) nasopharyngeal tumour; (3) bilateral neck irradiation; (4) weight loss >5% in one month or >10% in three months; (5) low BMI (<18.5 or <20 when age >65 years); (6) dysphagia with insufficient intake | Yes |
3.3. Energy and protein requirements
For calculating resting energy expenditure (REE), seven dietitians (54%) reported using the equation of the Food and Agriculture Organization/World Health Organization and United Nations University (FAO/WHO/UNU, 1985), four dietitians (31%) reported using the Harris and Benedict equation (Roza & Shizgal, 1984), one respondent (8%) uses a fixed factor (30–35 kcal/kg) (Weir, 1949) and one respondent (8%) uses the mean of three different equations. None of the respondents measured REE using indirect calorimetry in routine care. In order to calculate total energy expenditure (TEE), all dietitians who use an REE prediction equation instead of a fixed factor, added a percentage between 30% and 50% for physical activity level, illness and thermic effect of food. Most dietitians (77%) reported using 1.2 to 1.5 g protein/kg body weight to calculate protein requirements during CRT treatment. Only one respondent (8%) uses more than 1.5 g protein/kg body weight and one respondent (8%) uses 1.0 to 1.2 g protein/kg body weight to calculate protein requirements. All but one dietitian (92%), reported using fat free mass or corrected body weight (e.g., body weight corresponding to a body mass index [BMI] of 27) instead of actual body weight for calculating protein requirements in overweight patients. For calculating energy requirements in overweight patients, the actual body weight is used in most institutions (69%).
3.4. Gastrostomy removal
Almost half of the centres (46%) reported that a gastrostomy is, on average, removed between 8 to 12 weeks after CRT (Figure 1). At all but two centres (85%), the dietitian and treating physician jointly decided when to remove the gastrostomy. Four dietitians (31%) mentioned that the patient is also involved in this decision making. Three centres (23%) developed a protocol for gastrostomy removal. These centres report that the gastrostomy will be removed when the patient has an adequate oral nutritional intake, a stable weight (or within acceptable range) and their gastrostomy has not been used for 2–6 weeks. One centre also added ‘safe swallowing function/no aspiration’ as a prerequisite for gastrostomy removal.
FIGURE 1.
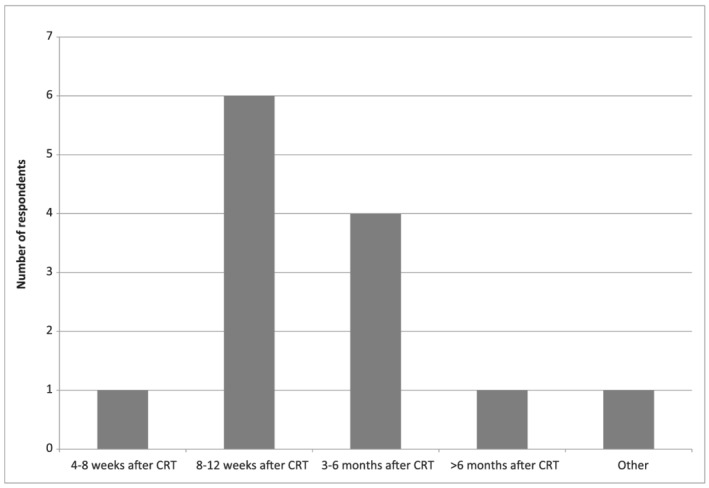
Estimated average time of gastrostomy removal after end of CRT treatment as reported by the 13 dietitians of the participating centres
3.5. End of dietary treatment
Most dietitians (76%) reported ending dietary treatment on average within 6 months after treatment. Two dietitians (15%) ended dietary treatment between 6 and 9 months and one dietitian (8%) ended dietary treatment, on average, more than 9 months after CRT treatment. However, several respondents denoted that there are considerable differences in the length of dietary treatment between patients, depending on patients' recovery after treatment, needs and nutritional intake. Reasons for ending dietary treatment varied per centre and included an adequate nutritional intake, weight stabilisation, reaching dietary treatment goals and removal of the gastrostomy. Two dietitians reported referring to a primary care dietitian if the patient is prolonged tube feeding dependent or if prolonged dietary treatment is indicated.
4. DISCUSSION
Results of this nationwide survey indicate that there is substantial variation in the number of scheduled dietetic consultations and tube placement (and removal) policy during CRT among the thirteen HNOCs participating in this study. Also, slight variations were reported in the calculation of energy and protein requirements and length of dietary treatment.
In all centres, all CRT patients are routinely referred to an oncology dietitian for face‐to‐face consultations, but the number of these consultations during CRT treatment varied between two and seven. Although the current Dutch Head and Neck cancer guidelines provide no information about the optimal frequency of dietetic consultations during CRT, in most centres (69%) they are scheduled weekly. This is in line with the Dutch Handbook ‘Nutrition in Cancer’ (Huitema & Jager‐Wittenaar, 2016) and guidelines from British Association of Head and Neck Oncologists (BAHNO) and Clinical Oncology Society of Australia (COSA) (Findlay et al., 2016; Talwar et al., 2016). Previous studies have shown that intensive, weekly nutritional intervention results in fewer treatment interruptions, less weight loss and milder symptoms of toxicity in HNC patients (Isenring et al., 2004; Paccagnella et al., 2010). These studies do not describe whether patients were compliant with the nutritional intervention. A more recent study showed that compliance with a dietary regimen with weekly nutritional counselling was low: as many as half of the patients missed more than 25% of scheduled appointments (Kabarriti et al., 2018). Future research should therefore gain insight into (non‐)compliance with weekly consultations and patients' needs and preferences considering the number and type of consultations.
Most dietitians were convinced that tube feeding is required for most patients during CRT treatment. Previous observational studies showed most LAHNSCC patients (68% to 81%) use tube feeding during CRT treatment (Karsten et al., 2019; van der Linden et al., 2017). In this survey, we did not verify the indications used for starting tube feeding. According to the Dutch malnutrition guideline, tube feeding in addition to oral intake is advised when 50% to 75% of calculated nutritional requirements are met, and full tube feeding is advised when less than 50% of requirements are met using only oral intake (Kruizenga et al., 2019). Tube feeding is commenced even earlier in this specific patient population in anticipation of side effects of treatment, usually occurring from the second week of treatment onward (Brown et al., 2017a).
In most of the responding centres (77%), a gastrostomy is the preferred route for the administration of tube feeding, although the optimal route for tube feeding administration has not yet been established. A nasogastric tube has the advantage of its relatively low costs and easy placement procedure in an outpatient setting (Corry et al., 2009). However, in contrast to gastrostomies, nasogastric tubes dislodge more often and patients find them more inconvenient (Corry et al., 2009). A gastrostomy is preferred when tube feeding is expected to be necessary for at least four weeks (Arends et al., 2016; Willemsen et al., 2019).
Insertion of a prophylactic gastrostomy in all patients has been subject of debate (Chen et al., 2010). In the Netherlands, there is currently a shift from prophylactic gastrostomy in all CRT patients towards prophylactic gastrostomy in selected patients or reactive gastrostomy placement, which is illustrated by the results of this survey: most centres that placed a gastrostomy did so upon indication only. In two centres, however, all patients treated with CRT received a prophylactic gastrostomy. This is in contrast with the Dutch Head and Neck Cancer guideline, that states that a gastrostomy should be placed only upon indication and therefore not in all CRT patients (Langius et al., 2013). Although evidence is low, we support the recommendation to place a prophylactic gastrostomy only in selected patients because 9% to 47% of prophylactic gastrostomies are never used during CRT (Madhoun et al., 2011; van der Linden et al., 2017), and complication rates are high (Grant et al., 2009; Strijbos et al., 2018). Moreover, prophylactic gastrostomy insertion in all CRT patients might increase long‐term dysphagia and tube feeding dependency due to atrophy of the swallowing muscles in the prolonged absence of oral intake (Oozeer et al., 2011; Williams et al., 2012).
To better predict which patients would benefit from a prophylactic gastrostomy, we recently developed and internally validated a prediction model for tube feeding dependency for at least four weeks during CRT which can be used as a tool to support personalised decision making on prophylactic gastrostomy insertion (Willemsen et al., 2019).
There is no consensus on when to remove a gastrostomy. Most centres reported removing the gastrostomy, on average, between 8 and 12 weeks after CRT. It is essential to stimulate oral intake during and after CRT, to closely monitor tube use and to remove the gastrostomy as soon as possible after CRT treatment to prevent long‐term dysphagia (Berthiller et al., 2016; Brown et al., 2017b). Three centres have already formulated indications on when to remove the gastrostomy. Future studies should focus on the optimal timing of gastrostomy removal and criteria for gastrostomy removal, as information in literature is lacking. It should also be noted that in 70% of the centres the patient was not mentioned as being involved in this gastrostomy removal decision making, suggesting that there is ample opportunity to increase the use of shared‐decision making.
Several methods were used to calculate energy requirements of patients. This is no surprise, because for calculating a patients' individual energy requirement, various prediction equations for REE can be used, for example Harris and Benedict, the FAO/WHO/UNU and Schofield formula (FAO/WHO/UNU, 1985; Roza & Shizgal, 1984; Schofield, 1985; Weijs et al., 2008). The Dutch Head and Neck cancer guidelines provide no information on which formula is best to use in HNC patients. The FAO/WHO/UNU formula seems to perform best in patients with a BMI < 30 and the Harris and Benedict in patients with a BMI > 30 (Huitema & Jager‐Wittenaar, 2016; Kruizenga et al., 2016). An earlier study showed that the Harris and Benedict underestimates REE in a CRT population with a BMI < 25 (Garcia‐Peris et al., 2005). Therefore, the FAO/WHO/UNU (for BMI < 30) or the Harris and Benedict equation (for BMI > 30) seem to be the best prediction equations for calculating REE, until a population specific formula for calculating REE in HNC patients has been developed. All respondents reported calculating TEE by multiplying REE with 1.3–1.5 (physical activity level and illness rate), which is in line with general guidelines for cancer patients (Beijer et al., 2016; Kruizenga et al., 2019).
Some variations in calculating protein requirements were observed. Although most dietitians (77%) use 1.2 to 1.5 g protein/kg bodyweight, which is also used for malnourished patients (Deutz et al., 2014), the optimal protein requirement for cancer patients has not yet been determined (Arends et al., 2016). Recommendations vary between 1.0 and 2.0 g protein/kg bodyweight per day depending on disease stage, type of treatment and complications (Arends et al., 2016; DDO Group, 2012). There is some evidence that protein requirements can be even higher as 1.7 g/kg bodyweight in patients receiving combination therapy (Jager‐Wittenaar, Dijkstra, Vissink, Langendijk, et al., 2011).
Although most dietitians (76%) participating in this survey reported ending dietary treatment shortly (0 to 6 months) after CRT, it is known that late toxicity rates of CRT are considerable. For instance, van den Berg reported that as few as 15.6% of HNC patients were able to eat without restrictions 44 months after treatment and the majority of patients reported to still experiencing a dry mouth and sticky saliva at their late morbidity clinic (van den Berg et al., 2014). Patients with these late toxicities may benefit from long term dietary treatment.
Results of this survey provide a nice overview of dietetic care for HNC in the Netherlands, although it has some limitations. For answering some survey questions, we relied on the judgement of the respondent and we could not verify answers with objective data. Since all are experienced HNC dietitians, we think this would not highly affect our results. However, the number of years of experience in the field of HNC might differ between respondents, but this was not asked in our survey. In the Netherlands, there is no national specialisation or training to be a HNC dietitian, which might explain some variation in care between dietitians and centres.
Overall substantial variation was found in nutritional interventions during CRT in the Dutch centres. Previously, van Overveld et al. assessed variation in quality of HNC care in the Netherlands (van Overveld et al., 2018). They demonstrated variation was associated with patient characteristics (tumour stage, tumour subsite and performance status) and hospital characteristics (volume of HNC care). Variation in nutritional interventions during CRT is not likely to be influenced by patient characteristics as all CRT patients have advanced disease and a sufficient performance status is usually a prerequisite for CRT treatment. Although we did not assess differences in hospital volume of HNC, this is likely to vary between university hospitals and affiliated centres. This might influence the available dietetic full‐time equivalents (FTE's) and thereby the number of scheduled consultations during CRT and length of dietary treatment. Hospital dietetic services in the Netherlands are paid from a fixed hospital budget. This is in contrast to medical specialists who receive budget for every new HNC patient by opening a diagnose treatment combination (DTC) (Hasaart, 2011). From this case‐based budget all hospital services from first consultation until the completion of treatment should be paid, but strangely allied health services do not receive any payment from this DTC. By increasing hospital volume of HNC, the frequency of dietetic contacts and duration of follow up will be lowered as it does not fit available hospital dietetic FTE's. To be able to offer high‐quality dietetic care in the hospital, payment of hospital dietetic services need to be changed.
For all of the topics assessed in this survey current literature provides some guidance, as discussed above, which can be used in clinical practice. Although available evidence and level of evidence varies, we should be able to develop concise dietetic guidelines for HNC, as has already been done by the British Association of Head and Neck Oncologists (BAHNO) and Clinical Oncology Society of Australia (COSA) (Findlay et al., 2016; Talwar et al., 2016). These guidelines provide guidance on dietetic intervention and frequency of contact and also for prophylactic gastrostomy placement. To create support for and commissioning of these dietetic guidelines in the Netherlands it should be integrated in the Dutch Head and Neck Cancer guidelines which are currently updated. We therefore advise the NWHHT and PWHHT to combine their knowledge and develop multidisciplinary head and neck cancer guidelines, not focusing solely on medical treatment but on multidisciplinary care, including allied health care as has been done by the British Association of Head and Neck Oncologists.
In conclusion, this study shows considerable variation in dietetic practice between the Dutch Head and Neck Oncology centres. To reduce variation between centres and dietitians, we advise to reconsider the current fixed budget for dietetic services and develop a national training or specialisation to become a HNC. Most importantly, we should develop and implement multidisciplinary HNC guidelines based on the available literature, which provide guidance on dietetic care throughout the whole HNC care process including frequency of contact, nutrition prescription and tube placement.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
FUNDING INFORMATION
The authors did not receive any funding for this project.
Supporting information
Data S1. Appendix S1. Questionnaire on dietetic consultations; tube feeding use; tube feeding route; calculation of energy and protein requirements; tube placement and removal policy and end of dietary treatment in patients with LAHNSCC treated with chemoradiotherapy at the Head and Neck Oncology Centres.
ACKNOWLEDGEMENTS
The authors would like to thank the dietitians of the Dutch Head and Neck Oncology centres for completing the questionnaire.
Kok, A. , van der Lugt, C. , Leermakers‐Vermeer, M. J. , de Roos, N. M. , Speksnijder, C. M. , & de Bree, R. (2022). Nutritional interventions in patients with head and neck cancer undergoing chemoradiotherapy: Current practice at the Dutch Head and Neck Oncology centres. European Journal of Cancer Care, 31(1), e13518. 10.1111/ecc.13518
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Arends, J. , Bachmann, P. , Baracos, V. , Barthelemy, N. , Bertz, H. , Bozzetti, F. , & Preiser, J. C. (2016). ESPEN guidelines on nutrition in cancer patients. Clinical Nutrition, 36(1), 11–48. 10.1016/j.clnu.2016.07.015 [DOI] [PubMed] [Google Scholar]
- Beijer, S. D. P. , Doornink, N. , ten Have, H. , van Lieshout, R. , & Vogel, J. (2016). Voedingsbehoefte en voedingsadvies. In Vogel J. (Ed.), Handboek voeding bij kanker (second ed.) (pp. 64–80). De Tijdstroom. [Google Scholar]
- Berthiller, J. , Straif, K. , Agudo, A. , Ahrens, W. , Bezerra Dos Santos, A. , Boccia, S. , & Lee, Y. C. (2016). Low frequency of cigarette smoking and the risk of head and neck cancer in the INHANCE consortium pooled analysis. International Journal of Epidemiology, 45(3), 835–845. 10.1093/ije/dyv146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan, V. , Stevanin, S. , Bianchi, M. , Aleo, G. , Bagnasco, A. , & Sasso, L. (2016). The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: A systematic review. Cancer Treatment Reviews, 45, 105–119. 10.1016/j.ctrv.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Brown, T. , Banks, M. , Hughes, B. G. M. , Lin, C. , Kenny, L. M. , & Bauer, J. D. (2017a). Impact of early prophylactic feeding on long term tube dependency outcomes in patients with head and neck cancer. Oral Oncology, 72, 17–25. 10.1016/j.oraloncology.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Brown, T. E. , Banks, M. D. , Hughes, B. G. M. , Lin, C. Y. , Kenny, L. M. , & Bauer, J. D. (2017b). Randomised controlled trial of early prophylactic feeding vs standard care in patients with head and neck cancer. British Journal of Cancer, 117(1), 15–24. 10.1038/bjc.2017.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano, G. , Gentile, P. C. , Bianciardi, F. , Tosti, M. , Palladino, A. , & Di, P. M. (2010). Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Supportive Care in Cancer, 18(4), 433–437. 10.1007/s00520-009-0681-8 [DOI] [PubMed] [Google Scholar]
- Capuano, G. , Grosso, A. , Gentile, P. C. , Battista, M. , Bianciardi, F. , Di Palma, A. , & Di Palma, M. (2008). Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head & Neck, 30(4), 503–508. 10.1002/hed.20737 [DOI] [PubMed] [Google Scholar]
- Cederholm, T. , Jensen, G. L. , Correia, M. , Gonzalez, M. C. , Fukushima, R. , Higashiguchi, T. , & Compher, C. (2019). GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Journal of Cachexia, Sarcopenia and Muscle, 10(1), 207–217. 10.1002/jcsm.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, A. M. , Li, B. Q. , Lau, D. H. , Farwell, D. G. , Luu, Q. , Stuart, K. , & Vijayakumar, S. (2010). Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. International Journal of Radiation Oncology, Biology, Physics, 78(4), 1026–1032. 10.1016/j.ijrobp.2009.09.036 [DOI] [PubMed] [Google Scholar]
- Corry, J. , Poon, W. , McPhee, N. , Milner, A. D. , Cruickshank, D. , Porceddu, S. V. , & Peters, L. J. (2009). Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo)radiation. Head & Neck, 31(7), 867–876. 10.1002/hed.21044 [DOI] [PubMed] [Google Scholar]
- Datema, F. R. , Ferrier, M. B. , & Baatenburg de Jong, R. J. (2011). Impact of severe malnutrition on short‐term mortality and overall survival in head and neck cancer. Oral Oncology, 47(9), 910–914. 10.1016/j.oraloncology.2011.06.510 [DOI] [PubMed] [Google Scholar]
- Deutz, N. E. , Bauer, J. M. , Barazzoni, R. , Biolo, G. , Boirie, Y. , Bosy‐Westphal, A. , & Calder, P. C. (2014). Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clinical Nutrition, 33(6), 929–936. 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch Dietitians in Oncology Group . (2012). General and tumor specific nutrition and dietary intervention guidelines.
- FAO/WHO/UNU . (1985). Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organization Technical Report Series, 724, 1–206. [PubMed] [Google Scholar]
- Findlay, M. , Bauer, J , Brown, T , & Head and Neck Guideline Steering Committee . (2016). COSA: Head and neck cancer nutrition guidelines/nutrition implementation—Radiotherapy and chemotherapy/what are the effective methods of implementation to ensure positive outcomes. In: Evidence‐based practice guidelines for the nutritional management of adult patients with head and neck cancer.
- Garcia‐Peris, P. , Lozano, M. A. , Velasco, C. , de La, C. C. , Iriondo, T. , Breton, I. , & Navarro, C. (2005). Prospective study of resting energy expenditure changes in head and neck cancer patients treated with chemoradiotherapy measured by indirect calorimetry. Nutrition, 21(11–12), 1107–1112. 10.1016/j.nut.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Grant, D. G. , Bradley, P. T. , Pothier, D. D. , Bailey, D. , Caldera, S. , Baldwin, D. L. , & Birchall, M. A. (2009). Complications following gastrostomy tube insertion in patients with head and neck cancer: A prospective multi‐institution study, systematic review and meta‐analysis. Clinical Otolaryngology, 34(2), 103–112. 10.1111/j.1749-4486.2009.01889.x [DOI] [PubMed] [Google Scholar]
- Hasaart, F. (2011). Incentives in the diagnosis treatment combination payment system for specialist medical care. (PhD). Maastricht University. (ISBN 978‐94‐6159‐089‐3) [Google Scholar]
- Huitema, S. , & Jager‐Wittenaar, H. (2016). Handboek voeding bij kanker. In Vogel J. (Ed.), Handboek voeding bij kanker (2nd ed.) (pp. 286–314). De Tijdstroom. [Google Scholar]
- Isenring, E. A. , Bauer, J. D. , & Capra, S. (2007). Nutrition support using the American dietetic association medical nutrition therapy protocol for radiation oncology patients improves dietary intake compared with standard practice. Journal of the American Dietetic Association, 107(3), 404–412. 10.1016/j.jada.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Isenring, E. A. , Capra, S. , & Bauer, J. D. (2004). Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. British Journal of Cancer, 91(3), 447–452. 10.1038/sj.bjc.6601962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager‐Wittenaar, H. , Dijkstra, P. U. , Vissink, A. , Langendijk, J. A. , van der Laan, B. F. , Pruim, J. , & Roodenburg, J. L. (2011). Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head & Neck, 33(6), 863–870. 10.1002/hed.21546 [DOI] [PubMed] [Google Scholar]
- Jager‐Wittenaar, H. , Dijkstra, P. U. , Vissink, A. , van der Laan, B. F. , van Oort, R. P. , & Roodenburg, J. L. (2011). Malnutrition and quality of life in patients treated for oral or oropharyngeal cancer. Head & Neck, 33(4), 490–496. 10.1002/hed.21473 [DOI] [PubMed] [Google Scholar]
- Kabarriti, R. , Bontempo, A. , Romano, M. , McGovern, K. P. , Asaro, A. , Viswanathan, S. , & Garg, M. K. (2018). The impact of dietary regimen compliance on outcomes for HNSCC patients treated with radiation therapy. Support Care Cancer, 26(9), 3307–3313. 10.1007/s00520-018-4198-x [DOI] [PubMed] [Google Scholar]
- Karsten, R. T. , Stuiver, M. M. , van der Molen, L. , Navran, A. , de Boer, J. P. , Hilgers, F. J. M. , & Smeele, L. E. (2019). From reactive to proactive tube feeding during chemoradiotherapy for head and neck cancer: A clinical prediction model‐based approach. Oral Oncology, 88, 172–179. 10.1016/j.oraloncology.2018.11.031 [DOI] [PubMed] [Google Scholar]
- Kruizenga, H. , Beijer, S. , Huisman‐de Waal, G. , Jonkers‐Schuitema, C. , Klos, M. , Remijnse‐Meester, W. , Thijs, A. , Tieland. M. , Vasse, E. , & Witteman, B. (2019). Richtlijn ondervoeding. Stuurgroep ondervoeding.
- Kruizenga, H. M. , Hofsteenge, G. H. , & Weijs, P. J. (2016). Predicting resting energy expenditure in underweight, normal weight, overweight, and obese adult hospital patients. Nutrition & Metabolism (London), 13, 85. 10.1186/s12986-016-0145-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langius, J. A. , Bakker, S. , Rietveld, D. H. , Kruizenga, H. M. , Langendijk, J. A. , Weijs, P. J. , & Leemans, C. R. (2013). Critical weight loss is a major prognostic indicator for disease‐specific survival in patients with head and neck cancer receiving radiotherapy. British Journal of Cancer, 109(5), 1093–1099. 10.1038/bjc.2013.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans, C. R. , Langendijk, J. A. , De Boer, J. P. , Terhaard, C. H. J. , Roodenburg, J. L. N. , Klomp, F. W. J. , & Werker, P. M. N. (2014). Richtlijn hoofd‐halstumoren. 228.
- Madhoun, M. F. , Blankenship, M. M. , Blankenship, D. M. , Krempl, G. A. , & Tierney, W. M. (2011). Prophylactic PEG placement in head and neck cancer: How many feeding tubes are unused (and unnecessary)? World Journal of Gastroenterology, 17(8), 1004–1008. 10.3748/wjg.v17.i8.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, F. , Fortin, A. , Wang, C. S. , Liu, G. , & Bairati, I. (2012). Predictors of severe acute and late toxicities in patients with localized head‐and‐neck cancer treated with radiation therapy. International Journal of Radiation Oncology, Biology, Physics, 82(4), 1454–1462. 10.1016/j.ijrobp.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Mulasi, U. , Vock, D. M. , Jager‐Wittenaar, H. , Teigen, L. , Kuchnia, A. J. , Jha, G. , & Earthman, C. P. (2020). Nutrition status and health‐related quality of life among outpatients with advanced head and neck cancer. Nutrition in Clinical Practice, 35, 1129–1137. 10.1002/ncp.10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oozeer, N. B. , Corsar, K. , Glore, R. J. , Penney, S. , Patterson, J. , & Paleri, V. (2011). The impact of enteral feeding route on patient‐reported long term swallowing outcome after chemoradiation for head and neck cancer. Oral Oncology, 47(10), 980–983. 10.1016/j.oraloncology.2011.07.011 [DOI] [PubMed] [Google Scholar]
- Paccagnella, A. , Morello, M. , Da Mosto, M. C. , Baruffi, C. , Marcon, M. L. , Gava, A. , & Marchiori, C. (2010). Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer, 18(7), 837–845. 10.1007/s00520-009-0717-0 [DOI] [PubMed] [Google Scholar]
- Pignon, J. P. , le Maitre, A. , Maillard, E. , & Bourhis, J. (2009). Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): An update on 93 randomised trials and 17,346 patients. Radiotherapy and Oncology, 92(1), 4–14. 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- Roza, A. M. , & Shizgal, H. M. (1984). The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. The American Journal of Clinical Nutrition, 40(1), 168–182. 10.1093/ajcn/40.1.168 [DOI] [PubMed] [Google Scholar]
- Schofield, W. N. (1985). Predicting basal metabolic rate, new standards and review of previous work. Human Nutrition. Clinical Nutrition, 39(Suppl 1), 5–41. [PubMed] [Google Scholar]
- Scholte, R. L. M. (2015). De waarde van diëtetiek bij ondervoede patiënten in het ziekenhuis. SEO Economisch Onderzoek. [Google Scholar]
- Sealy, M. J. , Dechaphunkul, T. , van der Schans, C. P. , Krijnen, W. P. , Roodenburg, J. L. N. , Walker, J. , & Baracos, V. E. (2019). Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clinical Nutrition, 39, 501–509. 10.1016/j.clnu.2019.02.029 [DOI] [PubMed] [Google Scholar]
- Strijbos, D. , Keszthelyi, D. , Bogie, R. M. M. , Gilissen, L. P. L. , Lacko, M. , Hoeijmakers, J. G. J. , & Masclee, A. A. M. (2018). A systematic review and meta‐analysis on outcomes and complications of percutaneous endoscopic versus radiologic gastrostomy for enteral feeding. Journal of Clinical Gastroenterology, 52(9), 753–764. 10.1097/MCG.0000000000001082 [DOI] [PubMed] [Google Scholar]
- Talwar, B. , Donnelly, R. , Skelly, R. , & Donaldson, M. (2016). Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. The Journal of Laryngology and Otology, 130(S2), S32–S40. 10.1017/S0022215116000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini, V. , Marazzi, F. , Bossola, M. , Micciche, F. , Nardone, L. , Balducci, M. , & Martorana, G. E. (2012). Nutritional counselling and oral nutritional supplements in head and neck cancer patients undergoing chemoradiotherapy. Journal of Human Nutrition and Dietetics, 25(3), 201–208. 10.1111/j.1365-277X.2011.01220.x [DOI] [PubMed] [Google Scholar]
- van den Berg, M. G. , Rasmussen‐Conrad, E. L. , van Nispen, L. , van Binsbergen, J. J. , & Merkx, M. A. (2008). A prospective study on malnutrition and quality of life in patients with head and neck cancer. Oral Oncology, 44(9), 830–837. 10.1016/j.oraloncology.2007.11.002 [DOI] [PubMed] [Google Scholar]
- van den Berg, M. G. , Rutten, H. , Rasmussen‐Conrad, E. L. , Knuijt, S. , Takes, R. P. , van Herpen, C. M. , & Merkx, M. A. (2014). Nutritional status, food intake, and dysphagia in long‐term survivors with head and neck cancer treated with chemoradiotherapy: A cross‐sectional study. Head & Neck, 36(1), 60–65. 10.1002/hed.23265 [DOI] [PubMed] [Google Scholar]
- van der Linden, N. C. , Kok, A. , Leermakers‐Vermeer, M. J. , de Roos, N. M. , de Bree, R. , van Cruijsen, H. , & Terhaard, C. H. (2017). Indicators for enteral nutrition use and prophylactic percutaneous endoscopic gastrostomy placement in patients with head and neck cancer undergoing chemoradiotherapy. Nutrition in Clinical Practice, 32(2), 225–232. 10.1177/0884533616682684 [DOI] [PubMed] [Google Scholar]
- van Overveld, L. F. , Braspenning, J. C. , & Hermens, R. P. (2017). Quality indicators of integrated care for patients with head and neck cancer. Clinical Otolaryngology, 42(2), 322–329. 10.1111/coa.12724 [DOI] [PubMed] [Google Scholar]
- van Overveld, L. F. J. , Takes, R. P. , Braspenning, J. C. C. , Baatenburg de Jong, R. J. , de Boer, J. P. , Brouns, J. J. A. , & Hermens, R. (2018). Variation in integrated head and neck cancer care: Impact of patient and hospital characteristics. Journal of the National Comprehensive Cancer Network, 16(12), 1491–1498. 10.6004/jnccn.2018.7061 [DOI] [PubMed] [Google Scholar]
- Weijs, P. J. , Kruizenga, H. M. , van Dijk, A. E. , van der Meij, B. S. , Langius, J. A. , & Knol, D. (2008). Validation of predictive equations for resting energy expenditure in adult outpatients and inpatients. Clinical Nutrition, 27(1), 150–157. 10.1016/j.clnu.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Weir, J. B. (1949). New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of Physiology, 109(1–2), 1–9. 10.1113/jphysiol.1949.sp004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendrich, A. W. , Swartz, J. E. , Bril, S. I. , Wegner, I. , de Graeff, A. , Smid, E. J. , & Pothen, A. J. (2017). Low skeletal muscle mass is a predictive factor for chemotherapy dose‐limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncology, 71, 26–33. 10.1016/j.oraloncology.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Willemsen, A. C. H. , Kok, A. , van Kuijk, S. M. J. , Baijens, L. W. J. , de Bree, R. , Devriese, L. A. , Hoebers, F. J. P. , Lalisang, R. I. , Schols, A. M. W. J. , Terhaard, C. H. J. , & Hoeben, A. (2019). Prediction model for tube feeding dependency during chemoradiotherapy for at least four weeks in head and neck cancer patients: A tool for prophylactic gastrostomy decision making. Clinical Nutrition, 39, 2600–2608. 10.1016/j.clnu.2019.11.033 [DOI] [PubMed] [Google Scholar]
- Williams, G. F. , Teo, M. T. , Sen, M. , Dyker, K. E. , Coyle, C. , & Prestwich, R. J. (2012). Enteral feeding outcomes after chemoradiotherapy for oropharynx cancer: A role for a prophylactic gastrostomy? Oral Oncology, 48(5), 434–440. 10.1016/j.oraloncology.2011.11.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Appendix S1. Questionnaire on dietetic consultations; tube feeding use; tube feeding route; calculation of energy and protein requirements; tube placement and removal policy and end of dietary treatment in patients with LAHNSCC treated with chemoradiotherapy at the Head and Neck Oncology Centres.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


