Abstract
Telemedicine gained interest in liver transplant patients but focused until now on the early post‐operative period. This prospective cohort study assessed feasibility, safety, and clinical beneficial effects of a telemedicine based remote monitoring program (TRMP) for the chronic follow‐up of adult liver transplant recipients. Between November 2017 and August 2019, a total of 87 of the 115 selected patients (76%) started the TRMP. Over the 2 years study period, none of the patients switched to standard follow‐up: 39/87 (45%) continued to do this autonomously and 48/87 (55%) stopped to report their data personally but communicated their lab values to the nurse. The other 28/115 (11%) patients who did not accept the TRMP continued the standard follow‐up. There was no difference in educational level between the three groups. Remote monitoring did not result in an increase in liver graft rejection and need of hospitalization. TRMP was associated with a higher number of tacrolimus level determinations and tacrolimus blood level concentrations could be kept lower. In conclusion, our results show that in patients with a stable clinical condition there is a high willingness to participate in TRMP and that this approach is safe. Remote monitoring allowed a stringent follow‐up of tacrolimus levels.
Keywords: kidney injury, liver transplant, remote monitoring, tacrolimus levels, telemedicine
1. INTRODUCTION
The outcome of liver transplantation has markedly improved over the last five decades in Europe, in part due to a better management of the immunosuppression in the chronic follow‐up of liver transplant recipients. 1 , 2 The aims of chronic follow‐up are (1) monitoring of immunosuppression, (2) assessment of disease recurrence, (3) management of renal dysfunction, (4) prevention and treatment of infection, and (5) prevention and treatment of diabetes, hypertension, cardiovascular disease, bone disease, and de novo tumors. 3 As the number of liver transplant recipient increases, follow‐up is becoming an increasing burden. Given that chronic follow‐up being typically concentrated at expert centers at a distance from many recipients, the logistic and socio‐economic burden on recipients may be considerable. 4
Over the past decade, the increasing availability and utilization of computers and smartphones have drastically changed delivery and communication in health care. 5 , 6 With its ability to eliminate geographic barriers, telemedicine is a rapidly expanding area of health care delivery. Telemedicine, a term coined in the 1970s, deviates from the traditional patient‐physician office visit and denotes “healing at a distance”. 5 , 6
Much of the published literature of telemedicine in the field of liver disease has described the use of video teleconferencing for the management of chronic hepatitis C patients. 6 , 7 , 8 , 9 Telemedicine has also gained traction in liver transplant patients but focused on the early post‐operative period. 10
In the chronic follow‐up of liver transplant recipients, telemedicine has the potential to decrease the number of outpatient visits while ensuring a continuous follow‐up of the immunosuppressive drugs which should be taken within a close therapeutic window to prevent graft rejection on the one hand and infections, diabetes mellitus, de novo tumors and especially nephrotoxicity on the other hand. 11 , 12
The present study assessed whether telemedicine based remote monitoring program (TRMP) reached the target population as well as the safety and potential clinical benefits of TRMP for the chronic follow‐up of liver transplant recipients.
2. METHODS
2.1. Study design and participants
This prospective cohort study was done at the University Hospitals Leuven, Belgium, who performs about 60–90 liver transplantations per year, and which currently has more than 900 liver transplant recipients in active chronic follow‐up. All patients aged 18 years or older with a liver transplantation ≥ 12 months ago who had a scheduled outpatient clinic visit between November 11, 2017 and August 21, 2019 were eligible for inclusion. Exclusion criteria were a combined transplant (e.g., kidney and liver), post‐transplant follow‐up partially performed in the referring hospital, the presence of medical conditions which required regular personal contact with the transplant team such as biliary strictures, severely decreased kidney function or kidney failure and other medical conditions which were relevant according to the investigator.
The study was undertaken in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the Ethics Committee Research UZ/KU Leuven (MP 011601).
2.2. Procedures
For the TRMP, the intention was to use Mynexuzhealth to communicate with the patients and Wintermute for the autonomously assessment of entered data ( = TRMP autonomously group). Mynexuzhealth is a secure platform for patients of the University Hospitals Leuven to access their lab results, overview of medical prescriptions and appointments via the website or mobile app (Figure 1). Wintermute is a Clinical Decision Support system that detects the entered values and automatically informs patients as well as the specialized nurse dedicated for TRMP whether the recorded parameters exceed predefined thresholds.
FIGURE 1.

Mynexuzhealth, a telemedicine based remote monitoring program (TRMP)
In order to assess whether the systems functioned well, patients were invited to use Mynexuzhealth and Wintermute 2 weeks prior to the next follow‐up visit to verify they understood everything. Patients received a username and a password for the TRMP system, or could log in with their electronic ID card.
For Wintermute, the transplant physicians entered a diary request thereby indicating the optimal lab values for the specific patient. Accordingly, every 4 months the patients were asked to visit their general practitioner for blood tests and subsequently enter data on immunosuppressive drug levels, serum creatinine, glomerular filtration rate (GFR), alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), alkaline phosphatase (ALP), gamma‐glutamyl transferase (GGT), and bilirubin level in the diary. In the case of an abnormality (red flag), the specialized nurse discussed the results with the transplant physician. In case immunosuppressive medication dose adjustments were needed, patients were informed via the mailing system in Mynexuzhealth. After dose adjustments, patients were asked to contact their general practitioner again for a new blood test within 14 days to check if immunosuppressive drug levels were within the required range.
Participants in the TRMP group were instructed to plan one routine outpatient visit per year. Patients who did not accept the TRMP continued their routine follow‐up visits in our hospital every 4 months according to the local protocol ( = standard follow‐up group). 13 Patients in the standard follow‐up group gave consent for data collection with respect to the current study and were subsequently used as controls.
In case patients within the TRMP were not willing anymore to personally report their data in the system, they were switched to the standard follow‐up group or they continued within TRMP but communicated their lab values to the specialized nurse. The nurse subsequently discussed the results with the transplant physician and an advice was reported by Mynexuzhealth or e‐mail ( = TRMP not autonomously group).
2.3. Baseline
Baseline was defined as the time patients were asked to be followed up by means of TRMP. Demographic information (age, sex, race), educational level, active alcohol abuse (yes vs. no), active smoking (yes vs. no), body mass index (BMI) ≥ 25 kg/m2 (yes vs. no), year from liver transplantation to inclusion, primary disease leading to liver transplantation, immunosuppressive therapy, possible nephrotoxic drug use (none, one, two, or more), creatinine level, GFR categories (≥ 90 (G1), 60 – 89 (G2), 45 – 59 (G3a), 30 – 44 (G3b) ml/min/1.73m2), glucose level, LDL cholesterol level, history of arterial hypertension (yes vs. no), diabetes mellitus (yes vs. no), cardiovascular disease (yes vs. no), and non‐liver malignancy at baseline were collected for all patients. Educational level was ranked as low (schooling 9 years), moderate (schooling 9–12 years), or high (schooling 12 years). 14 More than two drinks for females and three drinks for males per day represented alcohol abuse. 15 According to international practice guideline, primary diseases leading to liver transplantation were categorized in cirrhosis, cancers, cholestatic diseases, acute hepatic failure, metabolic diseases, and other. 3 Patient's medication list at baseline was assessed for the most common possible nephrotoxic drugs as previously outlined by Patel and Sapra. 16
2.4. Aims of the study
We primarily assessed whether liver transplant recipients were reached by the TRMP. It was determined how many patients initiated telemedicine as well as how many patients continued to use telemedicine with Wintermute ( = TRMP autonomously group) or without Wintermute ( = TRMP not autonomously group). This study also determined how many patients preferred the standard follow‐up and the reason why. In the TRMP autonomously group, the percentage of required data entered in Mynexuzhealth was assessed. In both the TRMP autonomously group and TRMP not autonomously group, a survey further assessed satisfaction using a 1 – 5 scale on the following five aspects of telemedicine: (1) overall I am satisfied with Mynexuzhealth, (2) I could rely on timely assistance through Mynexuzhealth, (3) it was simple to use this system, (4) completion of the diary was promptly, and (5) I trust that follow‐up through telemedicine was of a high standard as in‐person outpatient visits. Patients were also asked to provide suggestions to improve the TRMP.
Secondary, we assessed the safety of TRMP at the end of study follow‐up. End of study follow‐up was at August 21, 2020, that is, 1 year after the last inclusion. Safety assessment consisted of the following: the number of outpatient visits, emergency visits, non‐elective hospitalizations, number of tacrolimus (TAC) level determinations, number of TAC levels outside target level, TAC concentration, liver graft rejection (yes vs. no), re‐transplantation (yes vs. no), acute kidney injury (yes vs. no), chronic kidney disease progression (yes vs. no), last creatinine level, disease recurrence (yes vs. no), de novo malignancy development (yes vs. no), de novo cardiovascular disease (yes vs. no), community acquired infections (yes vs. no), mortality (yes vs. no). Liver graft rejection was documented based on liver biopsy or suspected on the presence of liver test abnormalities which improved after increase of the immunosuppressive drugs. In line with the recommendations of Kidney Disease Improving Global Outcomes (KDIGO) guidelines, acute kidney injury was defined as an increase in serum creatinine by ≥ .3 mg/dl within 48 h, increase in serum creatinine to ≥ 1.5 times baseline within 7 days or urine volume ≤ .5 ml/kg/h for 6 h. 17 Chronic kidney disease progression was defined as a decline in GFR category (≥ 90 (G1), 60–89 (G2), 45–59 (G3a), 30–44 (G3b), 15–29 (G4), or < 15 (G5) ml/min/1.73m2) or a sustained decline in GFR of more than 5 ml/min/1.73 m2/year. 18 Cardiovascular disease included clinical documentation (acute myocardial infarction, acute coronary syndrome, coronary revascularization, and other arterial revascularization procedures, stroke and transient ischemic attack, aortic aneurysm, and peripheral artery disease) or documentation on imaging (significant plaque on coronary angiography or carotid ultrasound). 19 Community‐acquired infections were defined as infections that were contracted outside of a health care facility or were diagnosed within 48 h of admission. 20
2.5. Statistical analysis
TRMP group consisted of those patients who personally entered data in the diary with Wintermute ( = TRMP autonomously group) and those patients without Wintermute ( = TRMP not autonomously group). Patients in the standard follow‐up group were used as controls.
Categorical data were presented as n (%) and differences between the TRMP autonomously group, TRMP not autonomously group and standard follow‐up group were analyzed using chi‐squared test or Fisher's exact test. Continuous data were expressed as median ± interquartile range (IQR) and comparison of continuous variables between the three groups was done with the Kruskal‐Wallis H test. We used the McNemar's test and Wilcoxon signed‐rank test to compare data within TRMP group at different time points (e.g., when comparing 12 months prior and after TRMP use). Multivariate analyses were not performed due to expected data sparseness.
Statistical analysis was performed with the SPSS software version 25 (IBM Corp ©, Armonk, NY, USA). The level of statistical significance was set at P < .050 in two‐tailed tests.
3. RESULTS
A total of 256 post‐liver transplantation outpatients were evaluated to be monitored with the use of TRMP of whom 55% (n = 141) did not meet the in‐ and exclusion criteria, and 11% (n = 28) were not interested, providing reasons such as “the preference of having physical contact with the physician” (n = 14), “no/limited experience with information technology (IT)” (n = 10), “inability to understand the Dutch language” (n = 2) and “lack of a computer” (n = 2) (Figure 2). The 28 patients who fulfilled the inclusion criteria but did not accept the TRMP were subsequently categorized as “the standard follow‐up group”.
FIGURE 2.
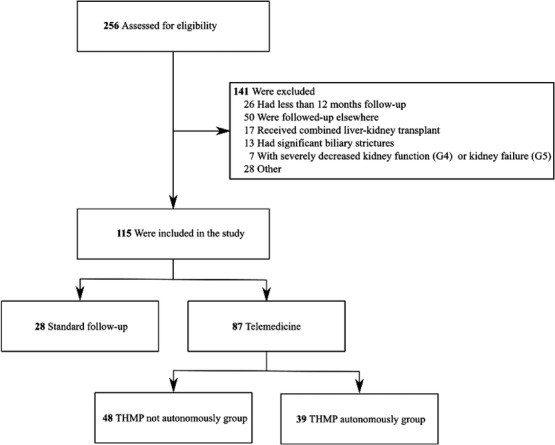
Flowchart of the study. TRMP: telemedicine based remote monitoring program
A total of 87 (75.7%) out of 115 eligible patients initiated TRMP. Furthermore, 39 (44.8%) out of 87 patients in the TRMP group continued to use the Wintermute system ( = TRMP autonomously group). None of the patients in the TRMP group switched to the standard follow‐up group. During study follow‐up, 48 (55.2%) out of 87 patients in the TRMP group were at a certain moment not willing to personally report their data in the system anymore but communicated their lab values to the specialized nurse ( = TRMP not autonomously group).
Patients in the TRMP autonomously group personally entered 1526 (90.9%) out of the 1679 required data. They reported a median satisfactory score of 4/5 on all five aspects of telemedicine: overall satisfaction, timely assistance, ease to use, promptly completion of the diary and high standard of follow‐up through telemedicine. The same satisfactory score of 4/5 on all aspects of telemedicine was reported in the TRMP not autonomously group. Both the TRMP autonomously group as the TRMP not autonomously group were asked to provide suggestions to improve the TRMP. Some of the provided recommendations were as follows: “could the laboratory results not be implemented automatically in mynexuzhealth”, “the general practitioner did not request the trough level of the immunosuppression”, “too many actions to access mynexuzhealth” and “confusion regarding SGPT/SGOT versus ALAT/ASAT”.
3.1. Baseline characteristics
Compared to the other groups, patients in TRMP autonomously group were younger (median age 59.1 years), the median age was 67.2 years in the TRMP not autonomously group (P = .016) and 66.0 years in the standard follow‐up group (P = .002). Median driving distance for one outpatient visit in University Hospitals Leuven was 77.5 miles for the TRMP autonomously group, this was 68.2 miles in the TRMP not autonomously group (P = .359) and 44.2 for standard follow‐up group (P = .006). Arterial hypertension was known among 41.0% patients in the TRMP autonomously group, and this number was 66.7% in the TRMP not autonomously group (P = .017) and 42.9% in the standard follow up‐group (P = .881). Other baseline characteristics were comparable between the three groups, including the educational level (Table 1).
TABLE 1.
Baseline characteristics (n = 115)
| Characteristics |
TRMP autonomously (n = 39) |
TRMP not autonomously (n = 48) |
Standard follow‐up (n = 28) |
P value |
|---|---|---|---|---|
| Median age at inclusion, years | 59.1 ± 12.17 | 67.2 ± 17.20 | 66.0 ± 11.77 | .006 |
| Male sex, n | 24 (61.5) | 29 (60.4) | 14 (50.0) | .592 |
| Caucasian race, n | 39 (100.0) | 48 (100.0) | 28 (100.0) | … |
| Distance to hospital ( miles) | 77.5 ± 93.0 | 68.2 ± 47.8 | 44.2 ± 33.3 | .006 |
| Educational level, n (%) | ||||
| Low | 11 (28.2) | 11 (25.0) | 10 (38.5) | .335 |
| Moderate | 15 (38.5) | 20 (45.5) | 13 (50.0) | |
| High | 13 (33.3) | 13 (29.5) | 3 (11.5) | |
| Active alcohol abuse, n (%) | 0 (.0) | 1 (2.1) | 2 (7.1) | .254 |
| Active smoking, n (%) | 1 (2.6) | 1 (2.1) | 2 (7.1) | .557 |
| BMI ≥ 25 kg/m2 | 18 (50.0) | 33 (68.8) | 21 (75.0) | .081 |
| Median years from LT to inclusion | 6.4 ± 8.42 | 7.4 ± 12.92 | 7.0 ± 9.28 | .740 |
| Primary indication for LT, n (%) | .167 | |||
| Acute hepatic failure | 5 (12.8) | 2 (4.2) | 0 (.0) | |
| Cirrhosis | 17 (43.6) | 36 (75.0) | 20 (71.4) | |
| HCC | 2 (5.1) | 1 (2.1) | 1 (3.6) | |
| Cholestatic diseases | 6 (15.4) | 4 (8.3) | 2 (7.1) | |
| Metabolic diseases | 4 (10.3) | 1 (2.1) | 2 (7.1) | |
| Others | 5 (12.8) | 4 (8.3) | 3 (10.7) | |
| Etiologies leading to LT, n (%) | .339 | |||
| Alcohol | 8 (20.5) | 14 (29.2) | 9 (32.1) | |
| NASH | 4 (10.3) | 10 (20.8) | 4 (14.3) | |
| HCV | 4 (10.3) | 3 (6.3) | 5 (17.9) | |
| Others | 23 (59.0) | 21 (43.8) | 10 (35.7) | |
| Immunosuppressive therapy, n (%) | .368 | |||
| TAC | 16 (41.0) | 21 (43.8) | 10 (35.7) | |
| TAC+MMF | 10 (25.6) | 9 (18.8) | 12 (42.9) | |
| TAC+EVR | 7 (17.9) | 7 (14.6) | 2 (7.1) | |
| Others | 6 (15.4) | 11 (22.9) | 4 (14.3) | |
| Possible nephrotoxic drug use, n (%) | .205 | |||
| None | 16 (41.0) | 10 (20.8) | 10 (35.7) | |
| One drug | 14 (35.9) | 18 (37.5) | 11 (39.3) | |
| Two or more drugs | 9 (23.1) | 20 (41.7) | 7 (25.0) | |
| Median creatinine level (mg/dl) | 1.0 ± .35 | 1.1 ± .36 | 1.0 ± .38 | .729 |
| GFR categories (ml/min/1.73m2), n (%) | .465 | |||
| G1 ≥ 90 | 9 (23.1) | 8 (16.7) | 3 (10.7) | |
| G2 60 – 89 | 18 (46.2) | 19 (39.6) | 16 (57.1) | |
| G3a 45 – 59 | 9 (23.1) | 12 (25.0) | 4 (14.3) | |
| G3b 30 – 44 | 3 (7.7) | 9 (18.8) | 5 (17.9) | |
| Median glucose level (mg/dl) | 97.0 ± 17.25 | 101.0 ± 35.00 | 104.0 ± 31.25 | .115 |
| Median LDL cholesterol level (mg/dl) | 88.0 ± 39.50 | 91.0 ± 36.50 | 95.5 ± 29.25 | .506 |
| Arterial hypertension , n (%) | 16 (41.0) | 32 (66.7) | 12 (42.9) | .031 |
| Diabetes mellitus, n (%) | 9 (23.1) | 20 (41.7) | 10 (35.7) | .185 |
| Cardiovascular disease, n (%) | 6 (15.4) | 8 (16.7) | 3 (10.7) | .845 |
| Non‐liver malignancy, n (%) | 3 (7.7) | 5 (10.4) | 3 (10.7) | .851 |
Data are n (%) or median ± interquartile range, IQR.
Abbreviations: TRMP, telemedicine based remote monitoring program; BMI, body mass index; NASH, non‐alcoholic steatohepatitis; HCV, hepatitis C virus; TAC, tacrolimus; EVR, everolimus; MMF, mycophenolate mofetil; GFR, glomerular filtration rate.
3.2. Safety of telemedicine based remote monitoring program
Table 2 shows the outcomes at end of study follow‐up for the three groups. Median follow‐up was 2.0 years in TRMP autonomously group, this was 2.1 years in TRMP not autonomously group (P = .636) and 2.4 years in standard follow‐up group (P = .038). When compared to TRMP autonomously group (median outpatients visits: 2.0), there was a similar number of 2.0 outpatient visits in TRMP not autonomously group (P = .265) while this was significantly higher in the standard follow‐up group (P < .001) with a median of 5.0 outpatient visits. In the TRMP autonomously group a median number of 8.0 TAC levels were determined during study follow‐up, this was 7.0 in TRMP not autonomously group (P = .117) and 6.0 in standard follow‐up group (P = .001). Occurrence of acute kidney injury was 0% in TRMP autonomously group, this was 6.3% in TRMP not autonomously group (P = .412) and 14.3% in standard follow‐up group (P = .027).
TABLE 2.
Outcomes at end of study follow‐up (n = 115)
| Outcome |
TRMP autonomously (n = 39) |
TRMP not autonomously (n = 48) |
Standard follow‐up (n = 28) |
P value |
|---|---|---|---|---|
| Median follow‐up, years | 2.0 ± .93 | 2.1 ± .89 | 2.4 ± .44 | .034 |
| Median outpatient visits | 2.0 ± 1.00 | 2.0 ± 3.00 | 5.0 ± 2.00 | <.001 |
| Median emergency visits | .0 ± .00 | .0 ± .00 | .0 ± .00 | .595 |
| Median hospitalizations | .0 ± .00 | .0 ± .00 | .0 ± .00 | .451 |
| Median number of TAC level determinations | 8.0 ± 6.00 | 7.0 ± 7.00 | 6.0 ± 3.00 | .013 |
| Median TAC levels outside target level | 1.0 ± 2.25 | 1.0 ± 2.50 | 1.0 ± 2.00 | .819 |
| Median TAC concentration | 4.7 ± 1.72 | 4.6 ± 1.74 | 4.4 ± 2.76 | .707 |
| Liver graft rejection | 1 (2.6) | 0 (.0) | 1 (3.6) | .337 |
| Re‐transplantation | 0 (.0) | 0 (.0) | 0 (.0) | … |
| Acute kidney injury | 0 (.0) | 3 (6.3) | 4 (14.3) | .048 |
| Chronic kidney disease progression | 1 (2.6) | 3 (6.3) | 2 (7.1) | .658 |
| Median last creatinine level (mg/dl) | 1.0 ± .29 | 1.1 ± .32 | 1.0 ± .29 | .524 |
| Disease recurrence | 0 (.0) | 1 (2.1) | 1 (3.6) | .714 |
| De novo malignancy development | 1 (2.6) | 0 (.0) | 1 (3.6) | .337 |
| De novo cardiovascular disease | 2 (5.1) | 0 (.0) | 2 (7.1) | .162 |
| Community acquired infections | 2 (5.1) | 4 (8.3) | 6 (21.4) | .106 |
| Mortality | 0 (.0) | 1 (2.1) | 0 (.0) | 1.000 |
Data are n (%) or median ± interquartile range, IQR.
Abbreviation: TAC: tacrolimus.
Over the follow‐up period, emergency visits, hospitalizations, liver graft rejection, re‐transplantation, chronic kidney disease progression, last creatinine level, disease recurrence, de novo malignancy development, de novo cardiovascular disease development, community acquired infections, and mortality did not differ significantly between the three study groups.
3.3. Outcomes within 12 months prior versus after the use of TRMP
A total of 69 out of 87 patients in the TRMP group started using telemedicine two or more years after liver transplantation. These patients were subsequently included in the subgroup analysis; Table 3 illustrates the disease outcomes within 12 months prior versus after the use of telemedicine for the TRMP autonomously and not autonomously group separately.
TABLE 3.
Outcomes within 12 months prior versus after the use of TRMP (n = 69)
| Outcome |
TRMP autonomously (n = 31) |
TRMP not autonomously (n = 38) |
||||
|---|---|---|---|---|---|---|
| Pre | Post | P value | Pre | Post | P value | |
| Median outpatient visits | 2.0 ± 1.00 | 1.0 ± .00 | < .001 | 2.0 ± 1.00 | 1.0 ± 1.00 | < .001 |
| Median emergency visits | .0 ± .00 | .0 ± .00 | 1.000 | .0 ± .00 | .0 ± .00 | .414 |
| Median hospitalizations | .0 ± .00 | .0 ± .00 | 1.000 | .0 ± .00 | .0 ± .00 | .518 |
| Median number of TAC level determinations | 3.0 ± 3.00 | 4.5 ± 3.75 | < .001 | 2.0 ± 1.00 | 4.0 ± 2.75 | .003 |
| Median TAC levels outside target level | 1.0 ± 1.00 | 1.0 ± 2.00 | .524 | 1.0 ± 1.00 | 1.0 ± 1.00 | .171 |
| Median TAC concentration | 5.1 ± 2.16 | 4.7 ± 2.23 | .038 | 5.4 ±2.5 | 4.4 ± 2.01 | .002 |
| Liver graft rejection | 2 (6.5) | 1 (3.2) | 1.000 | 2 (5.7) | 0 (.0) | … |
| Acute kidney injury | 0 (.0) | 0 (.0) | … | 5 (13.9) | 0 (.0) | … |
| Chronic kidney disease progression | 0 (.0) | 0 (.0) | … | 1 (2.8) | 0 (.0) | … |
Data are n (%) or median ± interquartile range, IQR.
Abbreviation: TAC: tacrolimus.
In both patient groups who used the remote monitoring program the number of outpatients visit dropped (P < .001 in both TRMP autonomously and not autonomously group), more TAC level determinations were performed (respectively, P < .001 and P = .003) and TAC blood level concentrations could be kept lower (respectively, P = .038 and P = .002) without an effect on the rejection rate in the sub‐analysis of pre‐ and post‐intervention
4. DISCUSSION
Among our patients with a history of liver transplantation more than 1 year ago and who had a stable medical condition, we found a high interest rate (76%) in remote monitoring for their post‐transplant follow‐up. Over the 2 years study period, none of the patients in TRMP group switched to standard follow‐up and almost half of the patients in TRMP group continued to do this autonomously with Wintermute, a Clinical Decision system which only reacts in case laboratory tests were outside predetermined ranges. The other participants in TRMP group stopped to use Wintermute but further participated in the remote monitoring program by communicating their lab values to the nurse. Patients who preferred the traditional follow‐up lived closer to the transplant unit and this could play a role in their decision.
Overall willingness of liver transplant patients to use telemedicine has previously been reported between 56% and 93%. 21 , 22 , 23 , 24 , 25 The wide range can be explained by different features of the adopted telemedicine system, the extent of data collection and patient characteristics. 24
Barriers to the widespread adoption of telemedicine include technology use among older adults and costs of implementation. 22 , 26 Only 14% of our study population did indicate no/limited experience with information technology (IT), with only two individuals not possessing a computer/smartphone. Interestingly, there was no difference in educational level between the groups and the high participation rate in follow‐up with TRMP demonstrates the feasibility of this remote monitoring system also in patients with older age.
In addition to the feasibility, the use of TRMP was safe. There was no difference in the number of emergency visits, hospitalizations, liver graft rejection, re‐transplantation, chronic kidney disease progression, community acquired infections, or mortality between the three study groups.
Taking into account the small sample size of the current study, TRMP had also clinical beneficial effects. It allowed a more stringent follow‐up of the level of immunosuppression. The closer follow‐up of the tacrolimus level resulted in lower TAC concentrations without more rejections. In the short‐term follow‐up, there was a lower rate of acute kidney injury in the TRMP group. It would be worth to investigate whether TRMP could reduce immunosuppression‐related side effects at the long‐term.
Telemedicine also improves access to expert centers, certainly in situations such as the COVID‐19 pandemic. The current study demonstrated that TRMP after liver transplantation resulted in a declining need of outpatient visits. This is also an important benefit of telemedicine since the survival of patients after liver transplantation has improved significantly over the last decades and the increase in liver transplant patients in chronic follow‐up results more and more in difficulties with regard to logistics. 27 Finally, most post‐liver transplant patients have an active professional or social life and the current telemedicine system allowed them not to give up unnecessary time in traveling and waiting at the outpatient clinic.
In the liver transplant setting, the use of telemedicine is limited to a handful of data suggesting that telemedicine could improve hospital readmissions 90 days after liver transplantation. 10 , 22 By contrast, telemedicine in heart failure is extensively studied with randomized controlled trials and meta‐analyses suggesting even reductions in morbidity and mortality. 28 , 29
The efficacy of complex interventions such as telemedicine depends on the context in which they are applied and can be grouped into two modalities: synchronous and asynchronous telemedicine. 30 Teleconsultation is an example of interactive telemedicine which provides synchronous real‐time interactions between health care provider and patient. For our study, we chose an asynchronous telemonitoring system (i.e., Mynexuzhealth) allowing patients to enter data which were then later analyzed by Wintermute or one specialized nurse dedicated for the TRMP of liver transplant recipients.
This study had limitations. The trial has a small sample size and should be interpreted accordingly. The approach was not validated in an independent group of patients and was only assessed in our own liver unit. The follow‐up of these patients was only 2 years and the drop out may increase later on. We found a more stringent control of immunosuppression but were not able to demonstrate the influence on the long‐term outcome of these patients. Although we collected data regarding the number of outpatient visits, we did not systematically collect information on the number of phone calls or time required to answer patients’ mails, and therefore cannot thoroughly comment on differences in the amount of time required by the staff to monitor patients within each group. Finally, this study showed a high satisfactory score among patients in the TRMP group but did not assess satisfactory among all providers.
In conclusion, our results show a high participation rate in TRMP for post‐transplant follow‐up in patients in a stable clinical condition, reduced outpatient visits and ensured a more stringent follow‐up of tacrolimus levels between outpatient visits. In an era of rising availability of computers and smartphones, telemedicine could be a valuable tool for reorganizing liver transplant care towards more personalized and value‐based healthcare.
AUTHOR CONTRIBUTIONS
Özgür Muhammet Koc, Marleen Pierco, Kathleen Remans, Thijs Van den Hende, Jef Verbeek, Hannah Van Malenstein, Schalk Van der Merwe, Geert Robaeys, Diethard Monbaliu, Jacques Pirenne, Bart Van den Bosch, Fabienne Dobbels, and Frederik Nevens contributed to the conception and design of the study. Frederik Nevens supervised Özgür Muhammet Koc to collect data. Following statistical analysis of data, Özgür Muhammet Koc drafted the first version of the paper, the co‐authors critically revised the article and approved the final version to be submitted, including the authorship list.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
We would like to acknowledge Lena Smets for the help of verifying the data, Bart Decuypere, and Egon Nijns for the IT support.
Koc ÖM, Pierco M, Remans K, et al. Telemedicine based remote monitoring after liver transplantation: feasible in a select group and a more stringent control of immunosuppression. Clin Transplant. 2022;36:e14494. 10.1111/ctr.14494
DATA AVAILABILITY STATEMENT
Proposals should be directed to the corresponding author; to gain access, data requestors will need to sign a data access agreement. Only individual participant data that underlie the results reported in this article, after de‐identification, will be shared.
REFERENCES
- 1. Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57(3):675–688. [DOI] [PubMed] [Google Scholar]
- 2. Adam R, Karam V, Cailliez V, et al. Annual Report of the European Liver Transplant Registry (ELTR) ‐ 50‐year evolution of liver transplantation. Transpl Int. 2018;31(12):1293–1317. [DOI] [PubMed] [Google Scholar]
- 3. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–485. [DOI] [PubMed] [Google Scholar]
- 4. Neuberger J. Follow‐up of liver transplant recipients. Best Pract Res Clin Gastroenterol. 2020;46‐47:101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryu S, Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth 2009 (Global Observatory for eHealth Series, Volume 2). [Google Scholar]
- 6. Piao C, Terrault NA, Sarkar S. Telemedicine: an evolving field in hepatology. Hepatol Commun. 2019;3(5):716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serper M, Volk ML. Current and future applications of telemedicine to optimize the delivery of care in chronic liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the. Am Gastroenterol Assoc. 2018;16(2):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beste LA, Glorioso TJ, Ho PM, et al. Telemedicine specialty support promotes hepatitis c treatment by primary care providers in the department of veterans affairs. Am J Med. 2017;130(4):432–438. [DOI] [PubMed] [Google Scholar]
- 9. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ertel AE, Kaiser TE, Abbott DE, Shah SA. Use of video‐based education and tele‐health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160(4):869–876. [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez‐Perálvarez M, Germani G, Darius T, et al. Tacrolimus exposure after liver transplantation in randomized controlled trials: too much for too long. Am J Transplant. 2013;13(5):1371–1372. [DOI] [PubMed] [Google Scholar]
- 12. Park ES, Peccoud MR, Wicks KA, et al. Use of an automated clinical management system improves outpatient immunosuppressive care following liver transplantation. J Am Med Inform Assoc. 2010;17(4):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nevens F, Pirenne J. Renal disease in the allograft recipient. Best Pract Res Clin Gastroenterol. 2020;46‐47:101690. [DOI] [PubMed] [Google Scholar]
- 14. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497–1504. [DOI] [PubMed] [Google Scholar]
- 15. EASL clinical practice guidelines: management of alcohol‐related liver disease. J Hepatol. 2018;69(1):154–181. [DOI] [PubMed] [Google Scholar]
- 16. Patel JB, SA. Nephrotoxic Medications. [Updated 2020 Jun 3]. StatPearls [Internet. Treasure Island (FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK553144/. Available from. [Google Scholar]
- 17. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. [DOI] [PubMed] [Google Scholar]
- 18. Stevens PE. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. [DOI] [PubMed] [Google Scholar]
- 19. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Todorovic Markovic M, Pedersen C, Gottfredsson M, Todorovic Mitic M, Gaini S. Focus of infection and microbiological etiology in community‐acquired infections in hospitalized adult patients in the Faroe Islands. BMC Infect Dis. 2019;19(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine D, Torabi J, Choinski K, Rocca JP, Graham JA. Transplant surgery enters a new era: increasing immunosuppressive medication adherence through mobile apps and smart watches. Am J Surg. 2019;218(1):18–20. [DOI] [PubMed] [Google Scholar]
- 22. Lee TC, Kaiser TE, Alloway R, Woodle ES, Edwards MJ, Shah SA. Telemedicine based remote home monitoring after liver transplantation: results of a randomized prospective trial. Ann Surg. 2019;270(3):564–572. [DOI] [PubMed] [Google Scholar]
- 23. Santonicola A, Zingone F, Camera S, Siniscalchi M, Ciacci C. Telemedicine in the COVID‐19 era for liver transplant recipients: an Italian lockdown area experience. Clin Res Hepatol Gastroenterol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vanhoof JMM, Vandenberghe B, Geerts D, et al. Technology experience of solid organ transplant patients and their overall willingness to use interactive health technology. J Nurs Scholarsh. 2018;50(2):151–162. [DOI] [PubMed] [Google Scholar]
- 25. Ertel AE, Kaiser T, Shah SA. Using telehealth to enable patient‐centered care for liver transplantation. JAMA Surg. 2015;150(7):674–675. [DOI] [PubMed] [Google Scholar]
- 26. Gajarawala SN, Pelkowski JN. Telehealth benefits and barriers. J Nurse Pract. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol. 2019;70(4):745–758. [DOI] [PubMed] [Google Scholar]
- 28. Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet. 2011;378(9792):731–739. [DOI] [PubMed] [Google Scholar]
- 29. Stotts MJ, Grischkan JA, Khungar V. Improving cirrhosis care: the potential for telemedicine and mobile health technologies. World J Gastroenterol. 2019;25(29):3849–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Minary L, Trompette J, Kivits J, Cambon L, Tarquinio C, Alla F. Which design to evaluate complex interventions? Toward a methodological framework through a systematic review. BMC Med Res Methodol. 2019;19(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Proposals should be directed to the corresponding author; to gain access, data requestors will need to sign a data access agreement. Only individual participant data that underlie the results reported in this article, after de‐identification, will be shared.


