Abstract
Plants are often exposed to multiple herbivores and densities of these attackers (or corresponding damage intensities) often fluctuate greatly in the field. Plant‐mediated interactions vary among herbivore species and with changing feeding intensity, but little is known about how herbivore identity and density interact to determine plant responses and herbivore fitness. Here, we investigated this question using Triadica sebifera (tallow) and two common and abundant specialist insect herbivores, Bikasha collaris (flea beetle) and Heterapoderopsis bicallosicollis (weevil). By manipulating densities of leaf‐feeding adults of these two herbivore species, we tested how variations in the intensity of leaf damage caused by flea beetle or weevil adults affected the performance of root‐feeding flea beetle larvae and evaluated the potential of induced tallow root traits to predict flea beetle larval performance. We found that weevil adults consistently decreased the survival of flea beetle larvae with increasing leaf damage intensities. In contrast, conspecific flea beetle adults increased their larval survival at low damage then decreased larval survival at high damage, resulting in a unimodal pattern. Chemical analyses showed that increasing leaf damage from weevil adults linearly decreased root carbohydrates and increased root tannin, whereas flea beetle adults had opposite effects as weevil adults at low damage and similar effects as them at high damage. Furthermore, across all feeding treatments, flea beetle larval survival correlated positively with concentrations of carbohydrates and negatively with concentration of tannin, suggesting that root primary and secondary metabolism might underlie the observed effects on flea beetle larvae. Our study demonstrates that herbivore identity and density interact to determine systemic plant responses and plant‐mediated effects on herbivores. In particular, effects are species‐specific at low densities, but converge at high densities. These findings emphasize the importance of considering herbivore identity and density simultaneously when investigating factors driving plant‐mediated interactions between herbivores, which advances our understanding of the structure and composition of herbivore communities and terrestrial food webs.
Keywords: above‐ and belowground herbivore interaction, conspecific and heterospecific herbivore interaction, density‐dependent effect, herbivore‐induced specific response, identity and density of herbivores, plant‐induced defense, plant‐herbivore interaction, resistance and susceptibility
INTRODUCTION
Upon herbivore attack, plants undergo substantial changes in primary and secondary metabolites (Johnson et al., 2016; Karban & Myers, 1989; Schwachtje & Baldwin, 2008). These induced responses can affect the performance of conspecific and heterospecific herbivores in both above‐ and belowground compartments and thereby drive herbivore community dynamics (Poelman & Dicke, 2014; Poelman & Kessler, 2016; Van Zandt & Agrawal, 2004). However, plant‐mediated interactions between herbivores remain difficult to predict, because the factors that determine these outcomes are not fully understood (Biere & Goverse, 2016; Haukioja, 1991; Nykänen & Koricheva, 2004).
A large body of evidence documents that herbivores elicit herbivore‐species‐specific plant responses (Agrawal, 2000; Ali & Agrawal, 2012; Karssemeijer et al., 2020; Kessler & Halitschke, 2007). Thus, herbivore identity is considered a key determinant of plant‐mediated interactions between herbivores (Kafle et al., 2017; Stout et al., 1997; Viswanathan et al., 2007). Some herbivores increase toxins and anti‐digestive compounds or decrease nutrients causing induced resistance to herbivores that arrive later (Denno et al., 2000; Soler et al., 2005), while others have opposite effects on these metabolites resulting in induced susceptibility to later attack (Robert et al., 2012a; Su et al., 2018). Such opposing effects are partially caused by highly specific herbivore‐associated cues (e.g., saliva, frass, and odor as well as feeding behavior and damage pattern) that are released and exert control during feeding (Basu et al., 2018; Erb et al., 2012a; Soler et al., 2013). Some of these cues are recognized specifically by plants and result in the elicitation of defense responses, while others can suppress plant defenses (Erb & Reymond, 2019; Kant et al., 2015).
A second factor that shapes plant‐mediated effects is herbivore density (Kaplan et al., 2008a; Masters, 1995). The initial herbivore‐species‐specific responses of plants are commonly assumed to be more intense at higher densities as specific herbivore‐derived cues are stronger (e.g., saliva; Karban & Baldwin, 1997, Agrawal & Karban, 2000, Eisenring et al., 2017). At the same time, high herbivore densities can result in resource overexploitation, thus reducing the fitness of later arrivers through a simple lack of food (Johnson & Amarasekare, 2015; Kaplan & Denno, 2007; Ohgushi & Sawada, 1998). Resource overexploitation can affect herbivores feeding on the same tissues, but also herbivores feeding on different tissues such as leaves versus roots. For instance, aboveground herbivory often strongly decreases plant root growth with increasing feeding intensity (Huang et al., 2012a; Hunt‐Joshi & Blossey, 2005; Masters, 1995; Wilson et al., 2021). The net effect of herbivore density is therefore likely a combination of increasing plant responses to the herbivore and increasing resource overexploitation (Robert et al., 2012b).
Following the concept that density‐dependent effects of herbivores partially reflect species‐specific plant induction patterns, we would expect that density‐dependent effects are at least partially species‐specific. For herbivores that trigger induced resistance, one would expect that with higher density, induced resistance and thus negative effects on other herbivores would increase, and that this pattern would be further accentuated by resource overexploitation. For herbivores that trigger induced susceptibility, one would expect a positive effect on other herbivores at lower densities when the remaining food for subsequent arrivers would be enough, and that this pattern would be attenuated, counteracted, or even reversed at higher densities due to increasing resource overexploitation. To date, plant‐mediated interactions between herbivores have predominantly been investigated either for one attacking herbivore species under single or multiple densities or for many attacking species under a given density (Huang et al., 2014; Kaplan et al., 2007; McKenzie et al., 2013; Soler et al., 2005). To the best of our knowledge, however, species‐specific density effects on plant‐mediated interactions between herbivores have not been investigated, thus limiting our understanding and prediction of this important aspect of plant‐herbivore community dynamics.
Here, we examined the species‐specific impacts of herbivore density using Triadica sebifera (Euphorbiaceae, tallow tree, hereafter “tallow”) and its associated herbivores Bikasha collaris (Coleoptera: Chrysomelidae, hereafter “flea beetle”) and Heterapoderopsis bicallosicollis (Coleoptera: Attelabidae, hereafter “weevil”). In our previous work, we found that flea beetle adults feeding on leaves improved root quality (reducing resistance and increasing nutrients) and facilitated conspecific larvae feeding on the roots (Huang et al., 2012b, 2013; Sun et al., 2019). In contrast, weevil adults feeding on leaves decreased root quality and inhibited flea beetle larvae feeding on the roots (Huang et al., 2014). We thus selected these two herbivore species in this study and performed a growth chamber experiment within which we manipulated the density of weevil adults and flea beetle adults to cause varying feeding intensities. By evaluating the performance of flea beetle larvae on these damaged plants, we tested whether the plant‐mediated effects of adult weevil and flea beetle attack on subsequent arriving flea beetle larvae depend on the densities of these two adult herbivores. Furthermore, by analyzing the changes in root quality (primary and secondary metabolites) and quantity (biomass), we examined whether these traits mediated observed indirect effects on flea beetle larvae.
MATERIAL AND METHODS
Study system
Tallow is a rapidly growing, subtropical tree, which is native to east and southeast Asia. It is widely distributed in central and southern China and is especially abundant in natural areas (e.g., forest) and disturbed habitats (e.g., agricultural fields, roadsides, gardens). At the field survey site, Hubei located in central China (31.58° N, 114.18° E), tallow is attacked by multiple herbivores (Appendix S1: Table S1) and feeding intensity on leaves varies drastically, ranging from 0% to 71.5% (Appendix S1: Figure S1a). Flea beetle and weevil adults were two of the most abundant herbivores (Appendix S1: Figure S1b) and the abundance of these two herbivores were strongly positively correlated with leaf damaged area (Appendix S1: Tables S1 and S2, Figure S1c,d). More details for the methods, statistical analyses and results about the field survey are available in Appendix S1. Previous host range tests indicated that the flea beetle and weevil are monophagous specialists that exclusively feed on tallow (Huang et al., 2011; Wang et al., 2009).
Flea beetle adults feed on leaves causing small holes and oviposit on the soil surface at the base of tallow, and larvae feed on roots forming elongate tunnels (Huang et al., 2011). In Hubei province in central China, there are typically five generations per year with adults present beginning in late April until November when they start to overwinter in the rhizosphere (Huang et al., 2011). Under laboratory conditions, flea beetle adults can live more than 200 days on tallow and begin to oviposit about 17 days after eclosion (Wheeler et al., 2017). Adults oviposit in clutches every 3 days with about 24 eggs per clutch (Wheeler et al., 2017). The duration of the life cycle is about 33–36 days from egg to adult (egg, 8–9 days; larva, 17–18 days; pupa, 8–9 days; Huang et al., 2011).
Weevil adults feed on leaves making large scars. Female adults roll leaves for oviposition, and eggs, larvae and pupae develop inside the rolled leaves (Wang et al., 2009). In Hubei, there are six or seven generations per year with adults present beginning in May until October when they start to overwinter in the litter around tallow (Wang et al., 2009). Under laboratory conditions, weevil adults can live more than 80 days on tallow and begin to oviposit about 17 days after eclosion (Steininger et al., 2013). Adults oviposit 1–4 eggs per rolled leaf (Steininger et al., 2013). The duration of the life cycle is about 12–16 days from egg to adult (egg, 4–5 days; larva, 5–7 days; pupa, 3–4 days; Wang et al., 2009).
Species‐specific impacts of herbivore density on herbivore interactions
We collected seeds from 20 trees at the field survey site that were separated by at least 50 m in November 2018. We randomly selected 100 seeds from each maternal tree, completely mixed them and soaked them in water with laundry detergent (10 g/L) for 2 days to remove waxy coats. Then, we placed them in moist sand at 4°C for 3 months to break dormancy (Huang et al., 2010). We sowed seeds in 50‐cell seeding trays filled with seedling substrate (Klasmann‐Deilmann GmbH, Geeste, Germany) in a growth chamber (14 h light/10 h dark with 24°/18°C temperature, relative humidity 50%–70%) at Wuhan Botanical Garden, Chinese Academy of Sciences, Hubei, China (30.61° N, 114.54° E). Four weeks later, we individually transplanted similar‐sized plants with four or five leaves into pots (14 cm in height, 12 cm in diameter) filled with a homogenized 50:50 mixture of seedling substrate and sand. Plants were watered every 2 days and were rearranged every week to eliminate possible impacts of environmental heterogeneity within the growth chamber.
We collected flea beetle adults and weevil rolled leaves at the field survey site in June 2019. Flea beetle adults were reared on 3‐month‐old tallow plants in a nylon mesh cage (1 × 1 × 1 m, 0.8 mm mesh sieve) in growth chamber with same conditions as already described. To obtain flea beetle larvae, naturally mated flea beetle adults were transferred to Petri dishes (10 cm in diameter, one pair per Petri dish). Each Petri dish contained a tallow leaf as food (Hubei population) and corrugated moist filter paper as oviposition substrate. We checked the leaves and oviposition substrates every 3 days and placed eggs into a new Petri dish with moist filter paper under constant darkness. Field collected flea beetle adults and laboratory reared newly hatched flea beetle larvae were used for the subsequent experiments. To ensure that we could obtain sufficient newly hatched flea beetle larvae simultaneously, we conducted egg collections on 300 pairs of mating adults. Weevil‐rolled leaves were put on wet hand towels enclosed within a nylon mesh cage (0.7 × 0.7 × 0.7 m, 0.8 mm mesh sieve). Newly emerged weevil adults were used for the subsequent experiments. To obtain sufficient newly hatched weevil adults, we collected more than 3000 rolled leaves in the field.
To examine herbivore identity and density dependent effects on plant induced resistance and susceptibility to flea beetle larvae, we conducted a bioassay by exposing flea beetle larvae to the roots of tallow seedlings that experienced prior leaf damage by different densities of weevil or flea beetle adults. Three weeks after transplantation, we selected plants with 10 fully expanded leaves, enclosed them individually in nylon mesh cages (14 cm in diameter, 40 cm in height, and 0.8 mm mesh sieve), and placed them in the growth chamber under the conditions described above. During the experiment, plants were watered as needed and were rearranged every week. Subsequently, we randomly assigned them to one of five densities of weevil adults, or one of five densities of flea beetle adults. Because per capita leaf removal is higher for weevils than flea beetles, we released 0, 2, 4, 6, or 8 newly emerged weevil adults or 0, 4, 10, 20, or 32 field collected flea beetle adults into each cage. There were 30 replicates for each combination (herbivory identity × herbivory density, 300 pots). Herbivores were allowed to feed on tallow for 1 week. To prevent oviposition by flea beetle adults into the soil, the mesh cage of each pot was sealed by string tied to the base of plant stem (below all leaves). We also sealed other pots to eliminate the possible impact of string.
One week after herbivore feeding, we removed all adults for both herbivores and assessed the leaf damaged area for each plant. The amount of damage was determined by visual estimate for each leaf by a 5% interval category, then averaging the visual estimates for all leaves per plant. Leaf damage at the highest density for both herbivores was around 70%, which was consistent with leaf damage levels observed in our field survey (Appendix S1: Figure S1a). Then, we punched a hole (1 cm in diameter, 2 cm in depth) in the soil at the base of each plant, transferred 10 newly hatched flea beetle larvae into the hole and covered them with moist soil. We recorded the number of emerging flea beetle adults and removed them every day. This process lasted 32 days, which was enough for flea beetle development from larva to adult.
Species‐specific impacts of herbivore density on plant traits
To evaluate changes in root traits that influence flea beetle larvae, we measured root biomass and root primary and secondary metabolites of tallow seedlings that experienced prior leaf damage by different densities of weevil or flea beetle adults. We used the same procedures as described above with a different set of 300 plants (i.e., seedling transplantation, cage installation, adult herbivore addition and removal, leaf damage measurement). We cleaned roots with tap water then weighed, flash froze in liquid nitrogen, finely ground, and stored them at −80°C. Our previous studies indicated that primary metabolites and tannin play a critical role in mediating above‐ and belowground herbivore interactions on tallow (Huang et al., 2013, 2014). We determined glucose, fructose, and starch using their corresponding assay kits (Beijing Solarbio Science & Technology, Beijing, China). Protein was extracted by a plant protein extraction kit (Beijing Solarbio Science & Technology) and quantified by a protein assay kit (Takara Biomedical Technology, Beijing, China). All procedures followed the manufacturers' instructions. Tannin was measured using a radial diffusion assay (Hagerman, 1987) with a tannic acid standard (Sigma‐Aldrich, St. Louis, Missouri, USA). All chemical concentrations were expressed as mg/g fresh mass.
Statistical analyses
To examine the species‐specific impacts of herbivore density on larval flea beetle performance (larval survival) and plant root traits (biomass and primary and secondary metabolites), we conducted a series of regression analyses. As the percentage of leaf‐damaged areas caused by adult weevils and adult flea beetles were strongly positively correlated with their corresponding densities in the herbivore performance experiments and plant trait analyses (Appendix S1: Figure S2a–d), we therefore used the percentage of leaf‐damaged area instead of herbivore density as the explanatory variable. The response variable of larval flea beetle survival (binomial data) was analyzed using a generalized linear model (GLM) with a binominal distribution and a logit link function, and the response variables of plant root traits (continuous data) were analyzed using linear models (LMs). Because the effects of leaf damage on plant‐mediated herbivore interactions could be nonlinear (e.g., U‐ or hump‐shaped), we first fit two models for each response variable, one with the percentage of leaf damaged area entered as a linear term only and the other with both linear and quadratic damage terms. Then, we selected the model with the best fit based on the lowest Akaike information criterion (AIC; Burnham & Anderson, 2002). Significant effects of the explanatory variables were assessed with z tests in GLMs and t tests in LMs. Goodness of fit of models were reported as [1 – (residual deviance/null deviance)] in GLMs (Zuur et al., 2009) and the coefficient of determination was reported as R 2 in LMs. The plant root data were log e ‐transformed where necessary to improve the fit of model residuals. Adult weevil and adult flea beetle herbivory treatments were analyzed separately.
Because a significant quadratic term is not a rigorous test of a U‐ or hump‐shaped relationship (Simonsohn, 2018), we thus conducted additional two‐line tests for models with a significant quadratic term to validate the existence of such relationships (Harman et al., 2020). In this method, we estimated two regression lines, which were interrupted at the break point estimated using the Robin Hood algorithm. U‐ or hump‐shaped relationships could be determined if both regression lines have significant slopes but opposite signs. Larval survival was analyzed based on a binominal distribution with a logit link function. The plant root traits were analyzed based on a Gaussian distribution and transformed as above in their quadratic models.
To investigate whether changes in root quality and quantity might be the causal mechanism underlying plant‐mediated herbivore interactions, we conducted simple linear regression analyses across all feeding treatments to test the dependence of larval flea beetle survival on root biomass, protein, glucose, fructose, starch, or tannin. It should be noted that although plant induced responses are specific to species of attacking herbivore, performance of responding herbivores is mainly dependent on the changes in plant traits (e.g., primary and secondary metabolites). Thus, data from adult weevil and adult flea beetle herbivory treatments were analyzed together.
All analyses were performed in R (version 3.6.3, R Development Core Team, 2020). The package STATS was used to fit the GLMs and LMs. For the two‐line test, we used the online R code provided by Simonsohn (2018).
RESULTS
Species‐specific impacts of density on herbivore interactions
The best model fit for adult weevil herbivory and larval flea beetle survival was a linear regression (Appendix S1: Table S3). Adult weevil leaf damage decreased larval flea beetle survival (Figure 1a). However, the best model fit for adult flea beetle herbivory and larval flea beetle survival included both linear and quadratic terms (Appendix S1: Table S3). At low damage levels, adult flea beetle herbivory increased larval survival, with the highest survival around 18% of leaf damaged area. However, this positive effect weakened with increasing leaf damage, and beyond around 38%, adult flea beetle herbivory decreased larval survival (Figure 1b). The two lines analysis further showed clear evidence that there was a positive relationship between adult flea beetle herbivory and larval survival at low damage levels (averaged leaf damaged area <18%: slope = 4.89, p < 0.001) and a negative relationship at high damage levels (averaged leaf damaged area >18%: slope = −5.82, p < 0.001), validating the presence of the hump‐shaped relationship (Appendix S1: Table S4). Thus, the amount of herbivore damage modulated plant‐mediated effects in a species‐specific manner.
FIGURE 1.
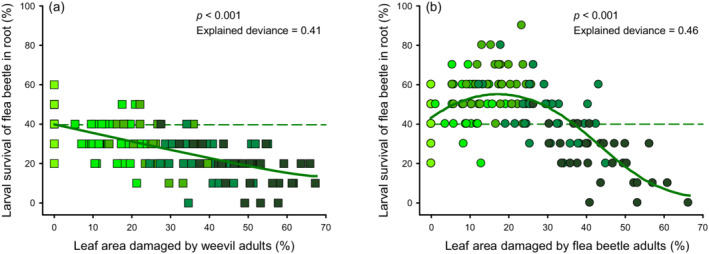
Density‐dependent effects mediate species‐specific induced resistance and susceptibility differently. Relationships between larval flea beetle survival (Bikasha collaris) and the percentage of leaf damaged area caused by (a) adult weevils (Heterapoderopsis bicallosicollis, squares) or (b) adult flea beetles (B. collaris, circles). The best fits included a linear term for the percentage of leaf damaged area of adult weevil herbivory (a), and linear and quadratic terms for the percentage of leaf damaged area of adult flea beetle herbivory (b). Percentage of leaf damaged area = 0 indicates healthy plants. The p values and explained deviances are given. Data points represent individual replicates (n = 30). Colors from light to dark in green indicate increasing herbivore density (adult weevil density: 0, 2, 4, 6, or 8 per plant; adult flea beetle density: 0, 4, 10, 20 or 32 per plant). The dotted lines indicate larval flea beetle survival on the healthy plants
Species‐specific impacts of density on plant traits
Consistent with their impact on larval flea beetle survival, the severity of leaf attack by adult weevils had a linear effect on most root metabolites (Appendix S1: Table S5). Concentrations of all root carbohydrates (glucose, fructose, and starch) significantly decreased with increasing leaf damage (Figure 2a–c). Conversely, root tannin increased linearly with increasing leaf damage (Figure 2d). For plants attacked by adult flea beetles, unimodal patterns were observed on most root metabolites (Appendix S1: Table S5), with higher root carbohydrates and lower tannin at low damage levels, and lower root carbohydrates and higher tannin at high damage levels (Figure 2e–h). The two‐line analysis further validated the presence of the hump‐shaped relationship for root carbohydrates and U‐shaped relationship for tannin (Appendix S1: Table S4). Root protein and root biomass were not significantly affected by adult weevil or adult flea beetle attack (Appendix S1: Figure S3).
FIGURE 2.
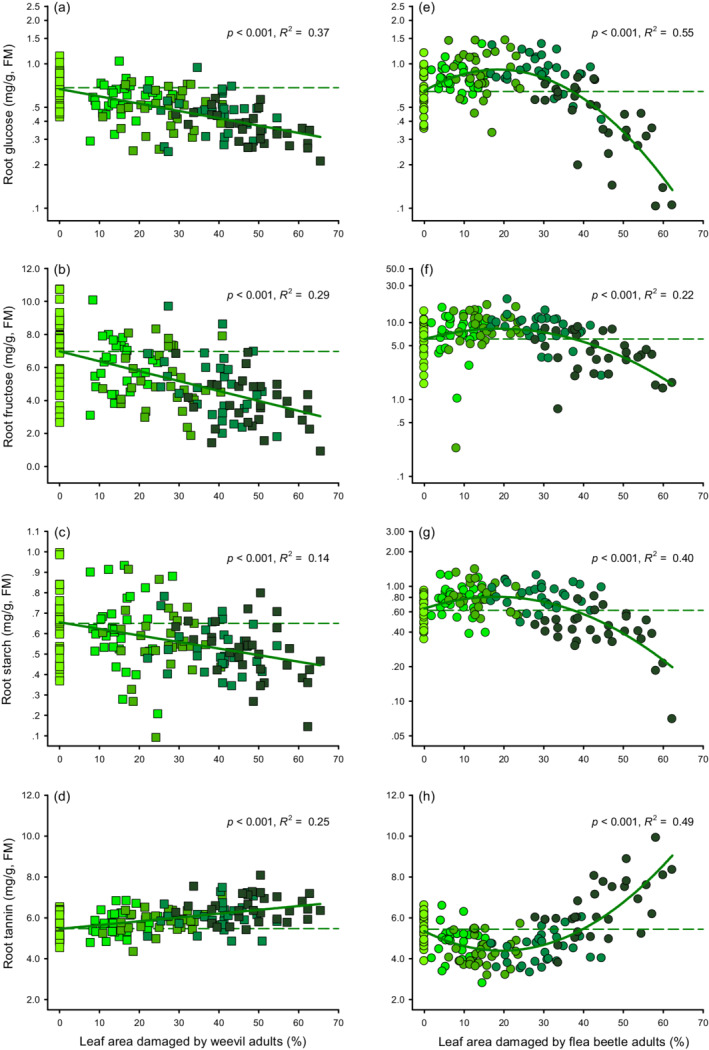
Density‐dependent effects mediate species‐specific plant induced responses differently. Relationships between root glucose, fructose, starch, and tannin and the percentage of leaf damaged area caused by (a–d) adult weevils (Heterapoderopsis bicallosicollis, squares) or (e–h) adult flea beetles (Bikasha collaris, circles). The best fits included a linear term for the percentage of leaf damaged area of adult weevil herbivory (a–d), and linear and quadratic terms for the percentage of leaf damaged area of adult flea beetle herbivory (e–h). Concentration of glucose (a) in adult weevil herbivory treatment and concentrations of glucose (e), fructose (f), and starch (g) in adult flea beetle herbivory treatments were log e ‐transformed. Percentage of leaf damaged area = 0 indicates healthy plants. The p values and R 2 are given. Data points represent individual replicates (n = 30). Colors from light to dark in green indicate increasing herbivore density (adult weevil density: 0, 2, 4, 6, or 8 per plant; adult flea beetle density: 0, 4, 10, 20, or 32 per plant). The dotted lines indicate the concentrations of plant metabolites of the healthy plants. Note the log e scale of metabolites (a, e, f, and g) on the y‐axis. FM, fresh mass
In addition, larval flea beetle survival for an experimental treatment increased with root glucose (Figure 3a), fructose (Figure 3b), and starch (Figure 3c) concentrations of the treatment but it decreased with root tannin (Figure 3d). Larval survival of an experimental treatment did not depend on root protein (p = 0.080) or mass (p = 0.157). Thus, the strength of herbivore damage modulated systemic changes in plant primary metabolism and defense in a species‐specific manner, and these induction patterns in turn affected herbivore fitness.
FIGURE 3.
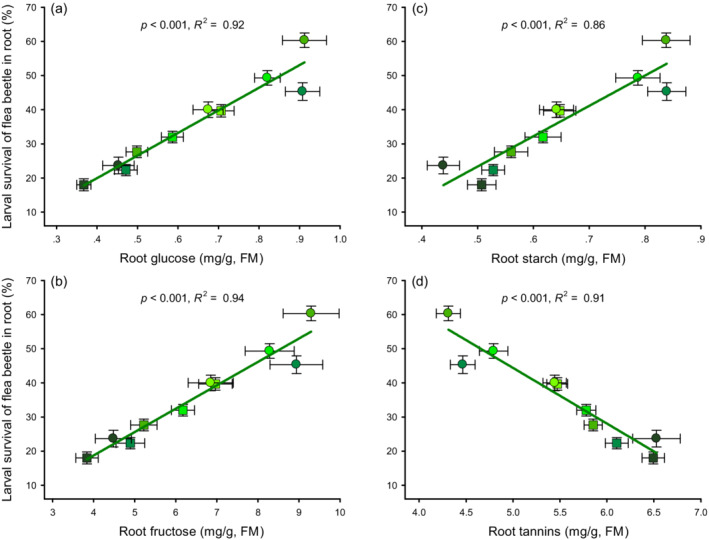
Herbivore survival is strongly associated with root primary and secondary metabolites. Relationships between larval flea beetle survival (Bikasha collaris) and tallow root (a) glucose, (b) fructose, (c) starch, and (d) tannin induced by adult weevil (Heterapoderopsis bicallosicollis, squares) or adult flea beetle (B. collaris, circles) herbivory within an herbivore × density treatment. Values are means ± SE. The p values and R 2 are given. Colors from light to dark indicate increasing herbivory density (adult weevil density: 0, 2, 4, 6, or 8 per plant; adult flea beetle density: 0, 4, 10, 20, or 32 per plant). FM, fresh mass
DISCUSSION
Herbivore identity and density are known to be two major drivers of plant‐mediated interactions among herbivores (Erwin et al., 2013; Underwood, 2000), but their combined effects are poorly understood. This study demonstrates the effect of herbivore density (or damage intensity) on fitness of subsequent herbivores depends on initial herbivore identity and is likely mediated by changes in plant primary metabolism and defense. Therefore, these results help us better understand complex plant‐mediated interactions among herbivores in the field.
We found that adult weevil herbivory decreases larval flea beetle survival in a density‐dependent manner, which is consistent with our prediction that high levels of herbivory intensity would consistently increase the initial induced resistance. Similar results have been also found in many studies that examined effects of induced responses to several different levels of initial attacker density or damage on subsequent herbivores (He et al., 2018; Karban & Baldwin, 1997; Simelane, 2006; Wei et al., 2016). In contrast to adult weevil herbivory, we found that herbivory by adult flea beetles facilitates larval survival at lower feeding intensity, an effect that is reversed at higher feeding intensity. Such shifts have also been observed in other studies (Hausmann & Miller, 1989; Meiners et al., 2005; Pettersson et al., 1998). For example, Pineda et al. (2017) found that Pieris brassicae caterpillars preferred the wild crucifer Brassica nigra infested by Brevicoryne brassicae aphids at low or medium densities over uninfested plants, but preferred uninfested plants to those at high infestation density. Likewise, Goodsman et al. (2015) found that attack by Dendroctonus rufipennis beetles increased after moderate Choristoneura biennis caterpillar infestation but decreased after severe C. biennis outbreaks at the landscape scale. By incorporating two types of herbivores that induced distinct responses in the same plant, we demonstrate, to the best of our knowledge for the first time, that plant‐mediated effects on herbivores are species‐specific at low initial herbivore densities, but converge at high herbivore densities, which is likely to be a common phenomenon among herbivores feeding on the same host plant.
As food resources of herbivores, the amount of available resource can influence the performance of herbivores (Robert et al., 2012b; Walker et al., 2008). However, in the current study, root biomass was not strongly affected by increasing adult flea beetle or weevil feeding intensities, which suggested that observed plant‐mediated density‐dependent interactions between herbivores did not reflect lack of food resources, and thus the potential effect of resource overexploitation in this system was excluded. A similar result was also found in the mustard Brassica nigra on which feeding by aboveground herbivore Pieris brassicae did not affect root biomass but significantly decreased survival and growth of the belowground herbivore Delia radicum (Soler et al., 2007).
In addition, plant quality (e.g., primary and secondary metabolites) also plays a critical role in determining the performance of herbivores (Mithöfer & Boland, 2012; Wan et al., 2019). In this study, adult weevil herbivory consistently reduced three carbohydrates and increased tannin with increasing feeding intensity. In contrast, with adult flea beetle feeding, increased the three carbohydrates and reduced tannin only occurred as feeding intensity was initially increasing, before it attenuated and finally reversed as feeding intensity increased. These findings coupled with the fact that flea beetle larval survival was positively correlated with each carbohydrate and negatively correlated with tannin across all feeding treatments suggested that changes in plant quality might underlie observed plant‐mediated density‐dependent interactions between herbivores (He et al., 2021; Robert et al., 2012b; Soler et al., 2005).
The observed systemic effects on carbohydrates and tannin are likely the combined result of herbivore‐species‐specific plant responses and increasing leaf damage. At low damage, adult weevil and flea beetle infestations caused opposing responses in each of these two types of metabolites, which is consistent with our previous finding about tannin at a comparatively lower intensity of damage (~10%) caused by adult weevils and adult flea beetles separately (Huang et al., 2014). Sarmento et al. (2011) also found similar divergence in proteinase inhibitors, an inducible defense compound, in tomato (Solanum lycopersicum) that were separately infested by two spider mite congeners, Tetranychus evansi and Tetranychus urticae at a single density. Such variation in plant metabolites induced by different herbivore species belonging to the same feeding guild is likely the result of herbivore‐specific cues (Basu et al., 2018; Schuman & Baldwin, 2016). For example, in maize Zea mays, attack by the cotton leafworm Spodoptera littoralis induced the emission of volatiles while the fall armyworm Spodoptera frugiperda suppressed such emission, and similar impacts were further confirmed when their oral secretions were released on artificial damaged leaves (De Lange et al., 2020). We thus hypothesize that adult flea beetles produce specific cues that boost root carbohydrates and suppress tannin, while adult weevils produce cues that have the opposite effect. More work is required to understand the identities and contributions of herbivore‐specific cues of flea beetle adults and weevil adults to damage‐induced responses in tallow.
Interestingly, systemic plant responses in terms of carbohydrates and tannin of the two herbivores converged at high feeding intensities, suggesting that consistently intensified initial herbivore‐induced plant response under high feeding intensity might be only applicable to herbivores that trigger initial induced resistance but not to herbivores that trigger induced susceptibility. In fact, in addition to herbivore‐species‐specific plant responses, damage itself could also induce changes in plant primary and secondary metabolism. On the one hand, removal of plant tissues will decrease photosynthetic activity and nutrient uptake, which, in turn, decreases the plant's nutritional status (Nabity et al., 2009; Zangerl et al., 2002). On the other hand, plants can perceive the elicitors from their own damaged cells and activate general defensive responses (Erb & Reymond, 2019). Both of them have been well demonstrated in many studies using mechanical damage (Erb et al., 2012b; Machado et al., 2017; Mithöfer et al., 2005). For example, in wild‐type tobacco, simulated herbivore feeding by pattern wheels on leaves significantly decreased contents of sugar and starch, but increased content of nicotine in leaves (Machado et al., 2013). Contrary to the induced defenses elicited by herbivore‐species‐specific cues, plant‐induced responses triggered by damage are often assumed to be difficult for herbivores to overcome (Duran‐Flores & Heil, 2014, 2016; Heil, 2009, 2012), and its intensity increases with the amount of damage (Canham et al., 1999; Heil et al., 2001, 2012). Hence, the observed induced responses in plant metabolism and plant‐mediated interactions between herbivores at high damage are probably attributed to the joint effect of increasing herbivore‐induced responses and increasing damage‐induced nutrient limitation and defensive responses.
The joint effect of herbivore identity and damage intensity on plant induced responses may be an important mechanism for the maintenance of a diversity of conspecific and heterospecific herbivores in above‐ and belowground compartments (Figure 4). In previous studies at low density of flea beetle larvae, we found that larval flea beetle herbivory induced leaf volatiles attractive to conspecific adults and increased their feeding, while induced leaf volatiles repellent to heterospecific leaf‐feeding herbivores, including adult weevils, which in turn decreased their occurrence, damage, and performance (Figure 4a; Huang et al., 2012b, Huang et al., 2014, Li et al., 2016, Sun et al., 2019). In such conditions, without the mediating effects of adult flea beetles at high density we observed in this study, increased adult flea beetle damage at higher densities would continually increase food quality for larval flea beetles and then promote their survival, which, in turn, would intensify negative effects on adult weevils via increased repellent volatiles. As a result, heterospecific aboveground herbivores might be excluded from the tallow system by the flea beetle and herbivore diversity on tallow would decrease (Figure 4b). However, because of mediating effects of adult flea beetles at high density, increased adult flea beetles can inhibit larval flea beetle survival, which, in turn, might weaken the negative impact on adult weevils through decreasing repellent volatile production, and allow adult weevils to persist on tallow (Figure 4c). Thus, such mediating effects of damage intensity shifting from induced susceptibility to induced resistance for conspecific belowground herbivores may be very important for heterospecific aboveground herbivores, in particular for specialists, such as weevils in this study, because they cannot survive on other host plants (Wang et al., 2009). Future chemical analyses and manipulative field studies would be needed to fully understand such patterns.
FIGURE 4.
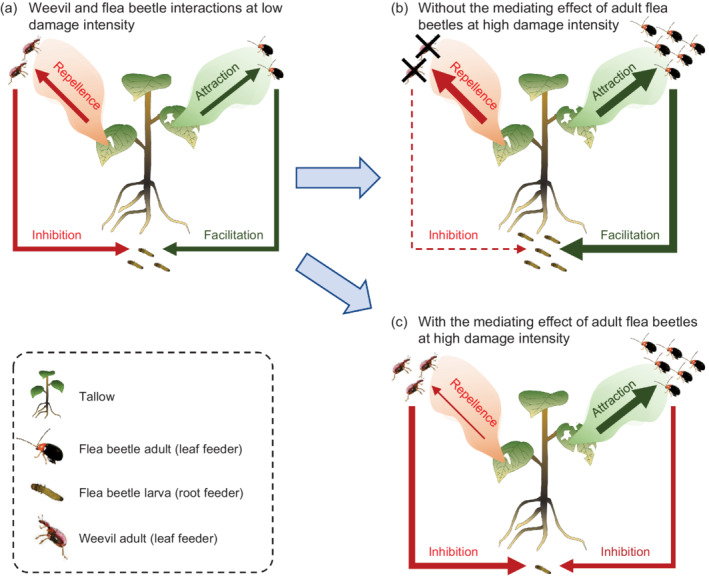
Conceptual models depicting how species‐specific induced responses and ecological consequences depend on damage intensity in system of tallow (Triadica sebifera) that is attacked by aboveground flea beetle adults (Bikasha collaris) and weevil adults (Heterapoderopsis bicallosicollis) as well by belowground flea beetle larvae. (a) At low damage intensities of aboveground herbivores, flea beetle adults and larvae reciprocally facilitate each other's performances via increasing attractive volatiles and root quality (Huang et al., 2012b; Sun et al., 2019), while weevil adults and flea beetle larvae inhibit each other via increasing repellent volatiles and decreasing root quality (Huang et al., 2014; Li et al., 2016; Sun et al., 2019). Mediating effect indicates that the impact of adult flea beetles on their larvae shifts from positive to negative with increasing damage intensity, as shown in this study. (b) Without the mediating effect of adult flea beetles at high damage intensity, increased densities of adult flea beetles would continually promote conspecific larval survival through increasing root quality, which in turn would intensify the negative effects on adult weevils via increased repellent volatiles and finally might exclude weevils from the tallow system. (c) With the mediating effect of adult flea beetles at high damage intensity, increased adult flea beetles would inhibit their larval survival through decreasing root quality, which in turn would weaken negative effects on adult weevils via decreasing repellent volatiles and finally might maintain weevil on the tallow system. Red lines indicate repellence or inhibition; green lines indicate attraction or facilitation
In summary, this study shows that herbivore identity and density interact to determine systemic plant responses and plant‐mediated interactions between herbivores. While herbivore‐species‐specific effects on plant responses and subsequent herbivores dominate at low densities, they converge at high herbivore densities. This study highlights the importance of considering herbivore identity and density simultaneously when identifying factors influencing induced plant responses to herbivory and plant‐mediated effects. Furthermore, a large body of studies show that induced plant responses are often systemic (Bezemer & van Dam, 2005; Gutbrodt et al., 2011; Kaplan et al., 2008b). Our findings, therefore, are probably able to be applied broadly, including to interactions among spatially separated herbivores (between leaf and root herbivores) and herbivores feeding on same compartment (between leaf herbivores and between root herbivores). Currently, it is well known that herbivore‐induced changes in the plant's traits can affect species richness and abundance of herbivore communities via both bottom‐up and top‐down effects (Ohgushi, 2005; Turlings & Erb, 2018). Understanding the combined effects of herbivore identity and density will help us further understand the mechanisms that shape the organization and diversity of herbivore communities including conspecific and heterospecific herbivores.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Jinlong Wan and Wei Huang conceived the project and designed the research; Jinlong Wan, Jiahui Yi, Zhibin Tao, Zhikun Ren, Evans O. Otieno and Baoliang Tian carried out field survey, herbivore performance experiments and chemical analyses; Jinlong Wan, Evan Siemann, Wei Huang, and Matthias Erb analyzed and interpreted the data; Wei Huang, Jinlong Wan, Jianqing Ding, Evan Siemann,ss and Matthias Erb wrote the initial version of the manuscript, and all authors contributed to its revision and gave final approval for publication.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This work was funded by the National Natural Science Foundation of China (31822007 and 32071660 to W. Huang, 32001239 to J. Wan), the Natural Science Foundation of Hubei Province (2020CFA064 to W. Huang) and Application Foundation Frontier Project of Wuhan (2019020701011495 to W. Huang). We are grateful to Susu Dai, Jialiang Zhang, Minyan He, Jie Ren, Wenchao Qin, and Qingqing Dong for their assistance in field and growth‐chamber works. We also thank Subject Matter Editor James T. Cronin and two anonymous referees for their constructive comments and suggestions on statistical analysis and conceptual model.
Wan, Jinlong , Yi Jiahui, Tao Zhibin, Ren Zhikun, Otieno Evans O., Tian Baoliang, Ding Jianqing, Siemann Evan, Erb Matthias, and Huang Wei. 2022. “Species‐Specific Plant‐Mediated Effects between Herbivores Converge at High Damage Intensity.” Ecology 103(5): e3647. 10.1002/ecy.3647
Handling Editor: James T. Cronin
Funding information Application Foundation Frontier Project of Wuhan, Grant/Award Number: 2019020701011495; National Natural Science Foundation of China, Grant/Award Numbers: 31822007, 32001239, 32071660; Natural Science Foundation of Hubei Province, Grant/Award Number: 2020CFA064
DATA AVAILABILITY STATEMENT
Data (Wan et al., 2021) are available in Dryad at https://doi.org/10.5061/dryad.cvdncjt5h
REFERENCES
- Agrawal, A. A. 2000. “Specificity of Induced Resistance in Wild Radish: Causes and Consequences for Two Specialist and Two Generalist Caterpillars.” Oikos 89: 493–500. [Google Scholar]
- Agrawal, A. A. , and Karban R.. 2000. “Specificity of Constitutive and Induced Resistance: Pigment Glands Influence Mites and Caterpillars on Cotton Plants.” Entomologia Experimentalis et Applicata 96: 39–49. [Google Scholar]
- Ali, J. G. , and Agrawal A. A.. 2012. “Specialist Versus Generalist Insect Herbivores and Plant Defense.” Trends in Plant Science 17: 293–302. [DOI] [PubMed] [Google Scholar]
- Basu, S. , Varsani S., and Louis J.. 2018. “Altering Plant Defenses: Herbivore‐Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores.” Molecular Plant‐Microbe Interactions 31: 13–21. [DOI] [PubMed] [Google Scholar]
- Bezemer, T. M. , and van Dam N. M.. 2005. “Linking Aboveground and Belowground Interactions Via Induced Plant Defenses.” Trends in Ecology & Evolution 20: 617–24. [DOI] [PubMed] [Google Scholar]
- Biere, A. , and Goverse A.. 2016. “Plant‐Mediated Systemic Interactions between Pathogens, Parasitic Nematodes, and Herbivores Above‐ and Belowground.” Annual Review of Phytopathology 54: 499–527. [DOI] [PubMed] [Google Scholar]
- Burnham, K. P. , and Anderson D. R.. 2002. Model Selection and Multimodel Inference: A Practical Information‐Theoretic Approach, Second ed. New York: Springer‐Verlag. [Google Scholar]
- Canham, C. D. , Kobe R. K., Latty E. F., and Chazdon R. L.. 1999. “Interspecific and Intraspecific Variation in Tree Seedling Survival: Effects of Allocation to Roots Versus Carbohydrate Reserves.” Oecologia 121: 1–11. [DOI] [PubMed] [Google Scholar]
- De Lange, E. S. , Laplanche D., Guo H., Xu W., Vlimant M., Erb M., Ton J., and Turlings T. C. J.. 2020. “ Spodoptera Frugiperda Caterpillars Suppress Herbivore‐Induced Volatile Emissions in Maize.” Journal of Chemical Ecology 46: 344–60. [DOI] [PubMed] [Google Scholar]
- Denno, R. F. , Peterson M. A., Gratton C., Cheng J., Langellotto G. A., Huberty A. F., and Finke D. L.. 2000. “Feeding‐Induced Changes in Plant Quality Mediate Interspecific Competition between Sap‐Feeding Herbivores.” Ecology 81: 1814–27. [Google Scholar]
- Duran‐Flores, D. , and Heil M.. 2014. “Damaged‐Self Recognition in Common Bean (Phaseolus Vulgaris) Shows Taxonomic Specificity and Triggers Signaling Via Reactive Oxygen Species (ROS).” Frontiers in Plant Science 5: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran‐Flores, D. , and Heil M.. 2016. “Sources of Specificity in Plant Damaged‐Self Recognition.” Current Opinion in Plant Biology 32: 77–87. [DOI] [PubMed] [Google Scholar]
- Eisenring, M. , Meissle M., Hagenbucher S., Naranjo S. E., Wettstein F., and Romeis J.. 2017. “Cotton Defense Induction Patterns under Spatially, Temporally and Quantitatively Varying Herbivory Levels.” Frontiers in Plant Science 8: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, M. , Meldau S., and Howe G. A.. 2012a. “Role of Phytohormones in Insect‐Specific Plant Reactions.” Trends in Plant Science 17: 250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, M. , Glauser G., and Robert C. A. M.. 2012b. “Induced Immunity against Belowground Insect Herbivores‐ Activation of Defenses in the Absence of a Jasmonate Burst.” Journal of Chemical Ecology 38: 629–40. [DOI] [PubMed] [Google Scholar]
- Erb, M. , and Reymond P.. 2019. “Molecular Interactions Between Plants and Insect Herbivores.” Annual Review of Plant Biology 70: 527–57. [DOI] [PubMed] [Google Scholar]
- Erwin, A. C. , Geber M. A., and Agrawal A. A.. 2013. “Specific Impacts of Two Root Herbivores and Soil Nutrients on Plant Performance and Insect–Insect Interactions.” Oikos 122: 1746–56. [Google Scholar]
- Goodsman, D. W. , Goodsman J. S., McKenney D. W., Lieffers V. J., and Erbilgin N.. 2015. “Too Much of a Good Thing: Landscape‐Scale Facilitation Eventually Turns into Competition between a Lepidopteran Defoliator and a Bark Beetle.” Landscape Ecology 30: 301–12. [Google Scholar]
- Gutbrodt, B. , Mody K., Wittwer R., and Dorn S.. 2011. “Within‐Plant Distribution of Induced Resistance in Apple Seedlings: Rapid Acropetal and Delayed Basipetal Responses.” Planta 233: 1199–207. [DOI] [PubMed] [Google Scholar]
- Hagerman, A. E. 1987. “Radial Diffusion Method for Determining Tannin in Plant Extracts.” Journal of Chemical Ecology 13: 437–49. [DOI] [PubMed] [Google Scholar]
- Harman, R. R. , Goddard J., Shivaji R., and Cronin J. T.. 2020. “Frequency of Occurrence and Population‐Dynamic Consequences of Different Forms of Density‐Dependent Emigration.” American Naturalist 195: 851–67. [DOI] [PubMed] [Google Scholar]
- Haukioja, E. 1991. “Induction of Defenses in Trees.” Annual Review of Entomology 36: 25–42. [Google Scholar]
- Hausmann, S. M. , and Miller J. R.. 1989. “Ovipositional Preference and Larval Survival of the Onion Maggot (Diptera: Anthomyiidae) as Influenced by Previous Maggot Feeding.” Journal of Economic Entomology 82: 426–9. [Google Scholar]
- He, M. , Chen J., Ding J., and Lu X.. 2018. “Differing Interactions between an Introduced Beetle and a Resident Root Nematode Mediated by an Invasive Plant and its Native Congener.” Plant Ecology 219: 803–12. [Google Scholar]
- He, M. , Zhang J., Siemann E., Yi J., Qin W., Sun X., Ding J., and Huang W.. 2021. “Herbivory of a Biocontrol Agent on a Native Plant Causes an Indirect Trait‐Mediated Non‐target Effect on a Native Insect.” Journal of Ecology 109: 2692–704. [Google Scholar]
- Heil, M. 2009. “Damaged‐Self Recognition in Plant Herbivore Defence.” Trends in Plant Science 14: 356–63. [DOI] [PubMed] [Google Scholar]
- Heil, M. 2012. “Damaged‐Self Recognition as a General Strategy for Injury Detection.” Plant Signaling & Behavior 7: 576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil, M. , Ibarra‐Laclette E., Adame‐Álvarez R. M., Martínez O., Ramirez‐Chávez E., Molina‐Torres J., and Herrera‐Estrella L.. 2012. “How Plants Sense Wounds: Damaged‐Self Recognition Is Based on Plant‐Derived Elicitors and Induces Octadecanoid Signaling.” PLoS One 7: e30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil, M. , Koch T., Hilpert A., Fiala B., Boland W., and Linsenmair K. E.. 2001. “Extrafloral Nectar Production of the Ant‐Associated Plant, Macaranga Tanarius, Is an Induced, Indirect, Defensive Response Elicited by Jasmonic Acid.” Proceedings of the National Academy of Sciences USA 98: 1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. , Carrillo J., Ding J., and Siemann E.. 2012a. “Interactive Effects of Herbivory and Competition Intensity Determine Invasive Plant Performance.” Oecologia 170: 373–82. [DOI] [PubMed] [Google Scholar]
- Huang, W. , Carrillo J., Ding J., and Siemann E.. 2012b. “Invader Partitions Ecological and Evolutionary Responses to Above‐ and Belowground Herbivory.” Ecology 93: 2343–52. [DOI] [PubMed] [Google Scholar]
- Huang, W. , Siemann E., Wheeler G. S., Zou J., Carrillo J., and Ding J.. 2010. “Resource Allocation to Defence and Growth Are Driven by Different Responses to Generalist and Specialist Herbivory in an Invasive Plant.” Journal of Ecology 98: 1157–67. [Google Scholar]
- Huang, W. , Siemann E., Xiao L., Yang X., and Ding J.. 2014. “Species‐Specific Defence Responses Facilitate Conspecifics and Inhibit Heterospecifics in Above–Belowground Herbivore Interactions.” Nature Communications 5: 4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. , Siemann E., Yang X., Wheeler G. S., and Ding J.. 2013. “Facilitation and Inhibition: Changes in Plant Nitrogen and Secondary Metabolites Mediate Interactions between Above‐ground and Below‐ground Herbivores.” Proceedings of the Royal Society B 280: 20131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. , Wheeler G. S., Purcell M. F., and Ding J.. 2011. “The Host Range and Impact of Bikasha Collaris (Coleoptera: Chrysomelidae), a Promising Candidate Agent for Biological Control of Chinese Tallow, Triadica Sebifera (Euphorbiaceae) in the United States.” Biological Control 56: 230–8. [Google Scholar]
- Hunt‐Joshi, T. R. , and Blossey B.. 2005. “Interactions of Root and Leaf Herbivores on Purple Loosestrife (Lythrum Salicaria).” Oecologia 142: 554–63. [DOI] [PubMed] [Google Scholar]
- Johnson, C. A. , and Amarasekare P.. 2015. “A Metric for Quantifying the Oscillatory Tendency of Consumer‐Resource Interactions.” American Naturalist 185: 87–99. [DOI] [PubMed] [Google Scholar]
- Johnson, S. N. , Erb M., and Hartley S. E.. 2016. “Roots under Attack: Contrasting Plant Responses to below‐ and Aboveground Insect Herbivory.” New Phytologist 210: 413–8. [DOI] [PubMed] [Google Scholar]
- Kafle, D. , Hänel A., Lortzing T., Steppuhn A., and Wurst S.. 2017. “Sequential Above‐ and Belowground Herbivory Modifies Plant Responses Depending on Herbivore Identity.” BMC Ecology 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant, M. R. , Jonckheere W., Knegt B., Lemos F., Liu J., Schimmel B. C. J., Villarroel C. A., et al. 2015. “Mechanisms and Ecological Consequences of Plant Defence Induction and Suppression in Herbivore Communities.” Annals of Botany 115: 1015–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, I. , and Denno R. F.. 2007. “Interspecific Interactions in Phytophagous Insects Revisited: A Quantitative Assessment of Competition Theory.” Ecology Letters 10: 977–94. [DOI] [PubMed] [Google Scholar]
- Kaplan, I. , Halitschke R., Kessler A., Rehill B. J., Sardanelli S., and Denno R. F.. 2008a. “Physiological Integration of Roots and Shoots in Plant Defense Strategies Links Above‐ and Belowground Herbivory.” Ecology Letters 11: 841–51. [DOI] [PubMed] [Google Scholar]
- Kaplan, I. , Halitschke R., Kessler A., Sardanelli S., and Denno R. F.. 2008b. “Effects of Plant Vascular Architecture on Aboveground–Belowground‐Induced Responses to Foliar and Root Herbivores on Nicotiana Tabacum .” Journal of Chemical Ecology 34: 1349–59. [DOI] [PubMed] [Google Scholar]
- Kaplan, I. , Lynch M. E., Dively G. P., and Denno R. F.. 2007. “Leafhopper‐Induced Plant Resistance Enhances Predation Risk in a Phytophagous Beetle.” Oecologia 152: 665–75. [DOI] [PubMed] [Google Scholar]
- Karban, R. , and Baldwin I. T.. 1997. Induced Responses to Herbivory. Chicago, IL: Chicago University Press. [Google Scholar]
- Karban, R. , and Myers J. H.. 1989. “Induced Plant Responses to Herbivory.” Annual Review of Ecology and Systematics 20: 331–48. [Google Scholar]
- Karssemeijer, P. N. , Reichelt M., Gershenzon J., van Loon J., and Dicke M.. 2020. “Foliar Herbivory by Caterpillars and Aphids Differentially Affects Phytohormonal Signalling in Roots and Plant Defence to a Root Herbivore.” Plant, Cell & Environment 43: 775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, A. , and Halitschke R.. 2007. “Specificity and Complexity: The Impact of Herbivore‐Induced Plant Responses on Arthropod Community Structure.” Current Opinion in Plant Biology 10: 409–14. [DOI] [PubMed] [Google Scholar]
- Li, X. , Guo W., Siemann E., Wen Y., Huang W., and Ding J.. 2016. “Plant Genotypes Affect Aboveground and Belowground Herbivore Interactions by Changing Chemical Defense.” Oecologia 182: 1107–15. [DOI] [PubMed] [Google Scholar]
- Machado, R. A. R. , Baldwin I. T., and Erb M.. 2017. “Herbivory‐Induced Jasmonates Constrain Plant Sugar Accumulation and Growth by Antagonizing Gibberellin Signaling and Not by Promoting Secondary Metabolite Production.” New Phytologist 215: 803–12. [DOI] [PubMed] [Google Scholar]
- Machado, R. A. R. , Ferrieri A. P., Robert C. A. M., Glauser G., Kallenbach M., Baldwin I. T., and Erb M.. 2013. “Leaf‐Herbivore Attack Reduces Carbon Reserves and Regrowth from the Roots Via Jasmonate and Auxin Signaling.” New Phytologist 200: 1234–46. [DOI] [PubMed] [Google Scholar]
- Masters, G. J. 1995. “The Effect of Herbivore Density on Host Plant Mediated Interactions Between Two Insects.” Ecological Research 10: 125–33. [Google Scholar]
- McKenzie, S. W. , Vanbergen A. J., Hails R. S., Jones T. H., and Johnson S. N.. 2013. “Reciprocal Feeding Facilitation Between Above‐ and Below‐ground Herbivores.” Biology Letters 9: 20130341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners, T. , Hacker N. K., Anderson P., and Hilker M.. 2005. “Response of the Elm Leaf Beetle to Host Plants Induced by Oviposition and Feeding: The Infestation Rate Matters.” Entomologia Experimentalis et Applicata 115: 171–7. [Google Scholar]
- Mithöfer, A. , and Boland W.. 2012. “Plant Defense against Herbivores: Chemical Aspects.” Annual Review of Plant Biology 63: 431–50. [DOI] [PubMed] [Google Scholar]
- Mithöfer, A. , Wanner G., and Boland W.. 2005. “Effects of Feeding Spodoptera Littoralis on Lima Bean Leaves. II. Continuous Mechanical Wounding Resembling Insect Feeding Is Sufficient to Elicit Herbivory‐Related Volatile Emission.” Plant Physiology 137: 1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabity, P. D. , Zavala J. A., and DeLucia E. H.. 2009. “Indirect Suppression of Photosynthesis on Individual Leaves by Arthropod Herbivory.” Annals of Botany 103: 655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykänen, H. , and Koricheva J.. 2004. “Damage‐Induced Changes in Woody Plants and their Effects on Insect Herbivore Performance: A Meta‐Analysis.” Oikos 104: 247–68. [Google Scholar]
- Ohgushi, T. 2005. “Indirect Interaction Webs: Herbivore‐Induced Effects through Trait Change in Plants.” Annual Review of Ecology, Evolution, and Systematics 36: 81–105. [Google Scholar]
- Ohgushi, T. , and Sawada H.. 1998. “What Changed the Demography of an Introduced Population of an Herbivorous Lady Beetle?” Journal of Animal Ecology 67: 679–88. [Google Scholar]
- Pettersson, J. , Karunaratne S., Ahmed E., and Kumar V.. 1998. “The Cowpea Aphid, Aphis Craccivora, Host Plant Odours and Pheromones.” Entomologia Experimentalis et Applicata 88: 177–84. [Google Scholar]
- Pineda, A. , Soler R., Pastor V., Li Y., and Dicke M.. 2017. “Plant‐Mediated Species Networks: The Modulating Role of Herbivore Density.” Ecological Entomology 42: 449–57. [Google Scholar]
- Poelman, E. H. , and Dicke M.. 2014. “Plant‐Mediated Interactions Among Insects Within a Community Ecological Perspective.” Annual Plant Reviews 47: 309–38. [Google Scholar]
- Poelman, E. H. , and Kessler A.. 2016. “Keystone Herbivores and the Evolution of Plant Defenses.” Trends in Plant Science 21: 477–85. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . 2020. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Robert, C. A. M. , Erb M., Duployer M., Zwahlen C., Doyen G. R., and Turlings T. C. J.. 2012a. Herbivore‐Induced Plant Volatiles Mediate Host Selection by a Root Herbivore. New Phytologist 194:1061–1069. [DOI] [PubMed] [Google Scholar]
- Robert, C. A. M. , Erb M., Hibbard B. E., French B. W., Zwahlen C., and Turlings T. C. J.. 2012b. A Specialist Root Herbivore Reduces Plant Resistance and Uses an Induced Plant Volatile to Aggregate in a Density‐Dependent Manner. Functional Ecology 26:1429–1440. [Google Scholar]
- Sarmento, R. A. , Lemos F., Bleeker P. M., Schuurink R. C., Pallini A., Oliveira M. G. A., Lima E. R., Kant M., Sabelis M. W., and Janssen A.. 2011. “A Herbivore that Manipulates Plant Defence.” Ecology Letters 14: 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman, M. C. , and Baldwin I. T.. 2016. “The Layers of Plant Responses to Insect Herbivores.” Annual Review of Entomology 61: 373–94. [DOI] [PubMed] [Google Scholar]
- Schwachtje, J. , and Baldwin I. T.. 2008. “Why Does Herbivore Attack Reconfigure Primary Metabolism?” Plant Physiology 146: 845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simelane, D. O. 2006. “Effect of Herbivory by Teleonemia Scrupulosa on the Performance of Longitarsus Bethae on their Shared Host, Lantana Camara .” Biological Control 39: 385–91. [Google Scholar]
- Simonsohn, U. 2018. “Two Lines: A Valid Alternative to the Invalid Testing of U‐Shaped Relationships with Quadratic Regressions.” Advances in Methods and Practices in Psychological Science 1: 538–55. [Google Scholar]
- Soler, R. , Bezemer T. M., Cortesero A. M., Van der Putten W. H., Vet L. E. M., and Harvey J. A.. 2007. “Impact of Foliar Herbivory on the Development of a Root‐Feeding Insect and its Parasitoid.” Oecologia 152: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler, R. , Bezemer T. M., van der Putten W. H., Vet L. E. M., and Harvey J. A.. 2005. “Root Herbivore Effects on Above‐ground Herbivore, Parasitoid and Hyperparasitoid Performance Via Changes in Plant Quality.” Journal of Animal Ecology 74: 1121–30. [Google Scholar]
- Soler, R. , Erb M., and Kaplan I.. 2013. “Long Distance Root‐Shoot Signalling in Plant‐Insect Community Interactions.” Trends in Plant Science 18: 149–56. [DOI] [PubMed] [Google Scholar]
- Steininger, M. S. , Wright S. A., Ding J., and Wheeler G. S.. 2013. “Biology and Host Range of Heterapoderopsis Bicallosicollis: A Potential Biological Control Agent for Chinese Tallow Triadica Sebifera .” Biocontrol Science and Technology 23: 816–28. [Google Scholar]
- Stout, M. J. , Workman K. V., Bostock R. M., and Duffey S. S.. 1997. “Specificity of Induced Resistance in the Tomato, Lycopersicon Esculentum .” Oecologia 113: 74–81. [DOI] [PubMed] [Google Scholar]
- Su, Q. , Chen G., Mescher M. C., Peng Z., Xie W., Wang S., Wu Q., et al. 2018. “Whitefly Aggregation on Tomato Is Mediated by Feeding‐Induced Changes in Plant Metabolites that Influence the Behaviour and Performance of Conspecifics.” Functional Ecology 32: 1180–93. [Google Scholar]
- Sun, X. , Siemann E., Liu Z., Wang Q., Wang D., Huang W., Zhang C., and Ding J.. 2019. “Root‐Feeding Larvae Increase their Performance by Inducing Leaf Volatiles that Attract Above‐ground Conspecific Adults.” Journal of Ecology 107: 2713–23. [Google Scholar]
- Turlings, T. C. J. , and Erb M.. 2018. “Tritrophic Interactions Mediated by Herbivore‐Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential.” Annual Review of Entomology 63: 433–52. [DOI] [PubMed] [Google Scholar]
- Underwood, N. 2000. “Density Dependence in Induced Plant Resistance to Herbivore Damage: Threshold, Strength and Genetic Variation.” Oikos 89: 295–300. [Google Scholar]
- Van Zandt, P. A. , and Agrawal A. A.. 2004. “Community‐Wide Impacts of Herbivore‐Induced Plant Responses in Milkweed (Asclepias Syriaca).” Ecology 85: 2616–29. [Google Scholar]
- Viswanathan, D. V. , Lifchits O. A., and Thaler J. S.. 2007. “Consequences of Sequential Attack for Resistance to Herbivores when Plants Have Specific Induced Responses.” Oikos 116: 1389–99. [Google Scholar]
- Walker, M. , Hartley S. E., and Jones T. H.. 2008. “The Relative Importance of Resources and Natural Enemies in Determining Herbivore Abundance: Thistles, Tephritids and Parasitoids.” Journal of Animal Ecology 77: 1063–71. [DOI] [PubMed] [Google Scholar]
- Wan, J. , Huang B., Yu H., and Peng S.. 2019. “Reassociation of an Invasive Plant with its Specialist Herbivore Provides a Test of the Shifting Defence Hypothesis.” Journal of Ecology 107: 361–71. [Google Scholar]
- Wan, J. , Yi J., Tao Z., Ren Z., Otieno E. O., Tian B., Ding J., Siemann E., Erb M. and Huang W.. 2021. “Species Specific Plant‐Mediated Effects between Herbivores Converge at High Damage Intensity.” Dryad, data set. 10.5061/dryad.cvdncjt5h. [DOI] [PMC free article] [PubMed]
- Wang, Y. , Ding J., Wheeler G. S., Purcell M. F., and Zhang G.. 2009. “ Heterapoderopsis Bicallosicollis (Coleoptera: Attelabidae): A Potential Biological Control Agent for Triadica Sebifera .” Environmental Entomology 38: 1135–44. [DOI] [PubMed] [Google Scholar]
- Wei, H. , He M., Lu X., and Ding J.. 2016. “Differences in Interactions of Aboveground and Belowground Herbivores on the Invasive Plant Alternanthera Philoxeroides and Native Host A. Sessilis .” Biological Invasions 18: 3437–47. [Google Scholar]
- Wheeler, G. S. , Steininger M. S., and Wright S.. 2017. “Quarantine Host Range of Bikasha Collaris, a Potential Biological Control Agent of Chinese Tallow Tree (Triadica Sebifera) in North America.” Entomologia Experimentalis et Applicata 163: 184–96. [Google Scholar]
- Wilson, C. H. , Vendramini J. M., Sollenberger L. E., and Flory S. L.. 2021. “Root Production in a Subtropical Pasture Is Mediated by Cultivar Identity and Defoliation Severity.” Tropical Grasslands‐Forrajes Tropicales 9: 144–58. [Google Scholar]
- Zangerl, A. R. , Hamilton J. G., Miller T. J., Crofts A. R., Oxborough K., Berenbaum M. R., and de Lucia E. H.. 2002. “Impact of Folivory on Photosynthesis Is Greater than the Sum of its Holes.” Proceedings of the National Academy of Sciences USA 99: 1088–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur, A. F. , Ieno E. N., Walker N. J., Saveliev A. A., and Smith G. M.. 2009. Mixed Effects Models and Extensions in Ecology with R. New York: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data (Wan et al., 2021) are available in Dryad at https://doi.org/10.5061/dryad.cvdncjt5h


