Summary
An impairment in next day driving performance has been reported for almost every drug currently United States Food and Drug Administration (FDA) approved for improvement of sleep in chronic and transient insomnia. Tasimelteon, a melatonin receptor agonist, demonstrated significant improvements in night‐time sleep, daytime naps, and sleep timing in non‐24‐hr sleep–wake disorder (Non‐24) by entraining these patients to a 24‐hr day as measured by melatonin and cortisol rhythms. Given this new mechanism of action of entraining the biological clock, we conducted a study to evaluate the potential effect tasimelteon may have on the ability to operate a motor vehicle. The study was conducted in 48 healthy adult subjects using a randomised, double‐blind, placebo and active (zopiclone 7.5 mg) controlled study with a 3‐period cross‐over design. Driving performance was assessed by measuring standard deviation of lateral position (SDLP) using the validated Cognitive Research Corporation Driving Simulator‐MiniSim. The difference in least square mean SDLP for tasimelteon was 1.22 cm reflecting a non‐significant increase in SDLP change from placebo (p = .1119). In contrast, treatment with the active control, zopiclone 7.5 mg, was associated with a meaningful and significant increase in SDLP, change from placebo for zopiclone was 4.14 cm (p < .0001). The lack of clinically meaningful and statistically significant finding with tasimelteon was further supported by the symmetry analysis, which showed the distribution of within‐subject differences between tasimelteon and placebo was symmetric about zero. At the FDA‐approved 20 mg dose to treat Non‐24, tasimelteon did not impair next‐day driving performance compared to placebo in adult healthy volunteers.
Keywords: circadian, melatonin agonist
1. INTRODUCTION
Next‐day residual effects resulting in daytime drowsiness and cognitive and psychomotor impairment after night‐time dosing is associated with most sleep aids. This highlights the need for the development of a new class of drug that improves sleep quality and quantity without unwanted residual effects. Tasimelteon is a melatonin receptor agonist approved by the United States Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) for the circadian rhythm sleep–wake disorder, non‐24‐hr sleep–wake disorder (Non‐24). The exact mechanism of action for tasimelteon is unknown, but it is believed to be mediated by the specific and high‐affinity binding of tasimelteon to the melatonin MT1 and MT2 receptors, which are thought to be involved in the control of circadian rhythms (Reppert & Weaver, 1995). The affinity of tasimelteon for the MT2 receptor is two‐ to four‐fold higher than its affinity for the MT1 receptor (Lavedan et al., 2015).
The aim of the present study was to examine the residual effects of tasimelteon 20 mg versus placebo on driving performance. Zopiclone was selected as the active control because of its well‐known next‐day residual effects, which are comparable to those observed with benzodiazepine hypnotics (Mets et al., 2011).
2. METHODS
This study was a double‐blind, double‐dummy, randomised, placebo‐controlled, three‐way crossover study in healthy adult subjects that evaluated the single‐dose effects of 20 mg tasimelteon, 7.5 mg zopiclone, and placebo. The study was conducted according to USA and international Good Clinical Practice standards, as well as all appropriate regulatory guidance (U.S. Food & Drug Administration, 2017). The protocol and all modifications and appropriate consent procedures were reviewed and approved by a properly constituted Institutional Review Board before study commencement. All study participants provided written informed consent prior to enrolment into the study. The study was conducted at Algorithme Pharma Inc.
2.1. Subjects
A total of 48 subjects (20 females and 28 males) were enrolled in this study. Subjects were male and female aged 21–55 years, with a body mass index (BMI) of >18 and <30 kg/m2 (BMI = weight (kg)/[height (m)]2), and possessing a valid driver’s license for ≥3 years with reported annual mileage ≥3000 km. Subjects’ eligibility to participate in the study were assessed at the screening visit during the pre‐randomisation phase. Subjects were excluded if they had a current complaint or diagnosis of insomnia or any other sleep disorder. Additionally, subjects were excluded if they used tobacco products 3 months prior; napped habitually more than three times per week; had a history (within the past 6 months) of alcohol or drug dependence or present evidence of such abuse; or excessive caffeine consumption (>3 caffeinated beverages/day). During the screening visit, subjects were given instructions on sleep hygiene and asked to follow these instructions throughout the study. To control for the potential differences in prior sleep–wake history among subjects, they were required to sleep between 7.0 and 8.5 hr per night and have a habitual bedtime between 22:00 and 24:00 hours. This was verified using a post‐sleep questionnaire, which subjects were required to complete 1 hr after awakening until the next study visit. Subjects that met all inclusion‐exclusion criteria were able to continue to the Randomisation Phase. During this phase, the use of concomitant medication was prohibited except for oral contraceptives and acetaminophen. Additionally, smoking was prohibited while participating in this study, alcohol was prohibited from 72‐hr prior to each dose administration, and xanthine (e.g. caffeine) containing beverages or food was prohibited from 36‐hr prior to each study dosing until the end of the test day.
2.2. Study design and procedures
The Randomisation Phase comprised of three experimental sessions, each separated by a 6–9‐day washout period. Subjects received a single dose of one of the following treatments in a random sequence order during each of the three treatment periods: 20 mg tasimelteon + zopiclone placebo, 7.5 mg zopiclone (over encapsulated) + tasimelteon placebo, or zopiclone placebo + tasimelteon placebo. Subjects’ target bedtime at the study site was based on habitual bedtime (−30 min) to control for circadian phase. Study assessments were set relative to the actual dosing time of each individual subject. Subjects were dosed ~30 min before their target bedtime and were given an 8‐hr opportunity to sleep at the study site. Simulated driving was assessed using the Cognitive Research Corporation’s Driving Simulator (CRCDS Mini‐Sim; Figure 1a). The study employed the CRCDS Country Vigilance‐Divided Attention (CVDA) driving scenario, a 62.1 mile (100 km), monotonous, two‐lane highway driving task that includes a secondary visual vigilance task (divided attention) (Simen et al., 2015). The driving simulator has been shown to be sensitive to alcohol (Kay et al., 2013) and the next‐day residual effects of zopiclone (Simen et al., 2015). Subjects started the driving session ~1‐hr after awakening to avoid potential impairments in cognition and sensory‐motor performance due to sleep inertia.
FIGURE 1.
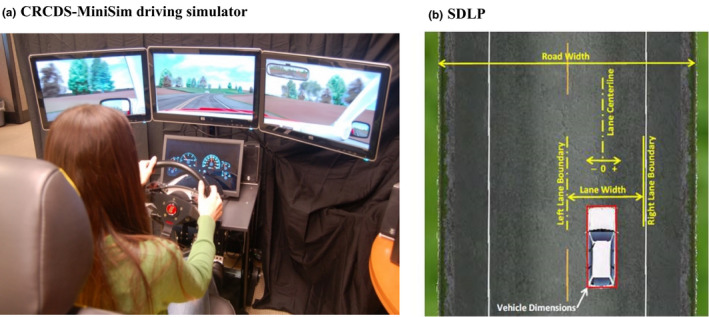
Simulated driving performance. (a) Subjects performed the Country Vigilance Divided Attention driving scenario on the Cognitive Research Corporation’s Driving Simulator (CRCDS)‐MiniSim driving simulator. (b) The standard deviation of lateral position (SDLP in cm) is an index of road tracking error or “weaving.” SDLP score difference between placebo and 0.05% blood alcohol concentration [BAC] for the CRCDS has been found to be 4.4 cm using this model (data on file at Cognitive Research Corporation)
Subjective sleepiness was measured with the Karolinska Sleepiness Scale (KSS), which was administered ~9‐hr post‐dose; just before driving. A self‐perceived safety to drive questionnaire was completed by the subjects before the driving session, after they completed the KSS. Subjects were instructed to operate the driving simulator for ~1‐hr at a speed of 88.5 km/hr (55 mph) while maintaining lane position. After the driving session, subjects completed a visual analogue scale (VAS) assessing self‐perceived motivation and driving performance.
The primary endpoint, standard deviation of lateral position (SDLP, a measure of lane weaving; Figure 1b), was analysed using a mixed model with fixed effects for sequence (six possible treatment orders), period (three experimental sessions) and treatment (three study drugs). An unstructured covariance structure and Kenward‐Roger degrees of freedom were used. In the event an unstructured covariance structure failed to converge, a variance components covariance structure was assumed.
Secondary endpoints were evaluated similarly. Lane exceedance was log‐transformed (1n[x + 1]) prior to analyses. Pair‐wise comparisons for self‐assessed readiness to drive (“Right now do you feel safe to drive?”) were analysed using the McNemar test. Furthermore, pairwise within participant differences in SDLP between drug and placebo were tested for symmetry about zero (Laska et al., 2012) using the maximally selected McNemar test.
Tasimelteon was to be considered non‐inferior to placebo if the upper 95% confidence limit in the difference in SDLP between tasimelteon and placebo did not exceed 4.4 cm (equal to the previously found difference between placebo and 0.05% blood alcohol concentration [BAC] for the CRCDS). Formal statistical tests (where performed) were two‐sided and tested at the α = 0.05 significance level with no adjustments for multiple comparisons.
3. RESULTS
3.1. Primary endpoint: SDLP
Following treatment with tasimelteon 20 mg, the upper limit of the 95% confidence interval (CI) for the change in SDLP between tasimelteon and placebo (2.74 cm) did not exceed the pre‐established non‐inferiority criterion of 4.4 cm (p = .1119; Table 1). There was a non‐significant increase in SDLP for tasimelteon compared to placebo (1.22 cm, p = .1119). Furthermore, symmetry analysis showed that the distribution of within‐subject differences between tasimelteon and placebo was symmetric about zero (maximum McNemar Statistic <7.043), with three subjects showing an increase >4.4 cm and seven subjects showing an improvement of equal magnitude. The distribution of within‐subject difference scores from placebo for tasimelteon and zopiclone are shown in Figure 2.
TABLE 1.
Standard deviation of lateral position (SDLP in cm)
|
20 mg Tasimelteon (N = 48) |
7.5 mg Zopiclone (N = 46) |
Placebo (N = 46) |
|
|---|---|---|---|
| Mean (SD) | 30.82 (6.394) | 32.96 (7.286) | 29.59 (5.104) |
| LS means a | 30.84 | 33.75 | 29.61 |
| Median | 29.58 | 31.85 | 28.91 |
| Min, max | 22.2, 51.1 | 23.9, 68.9 | 22.5, 53.2 |
| p value for period a | .8852 | ||
| p value for sequence a | .0186 | ||
| 20 mg Tasimelteon versus placebo | 7.5 mg Zopiclone versus placebo | 20 mg Tasimelteon versus 7.5 mg zopiclone | |
| Difference in LS means a | 1.22 | 4.14 | −2.92 |
| 95% CI a | (−0.29, 2.74) | (2.60, 5.68) | (−4.43, −1.41) |
| p value a | .1119 | <.0001 | .0002 |
| Non‐inferiority b | Non‐inferior/2.74<4.4 | n/a | n/a |
CI, confidence interval; LS, least square; N, number of subjects; n/a, not applicable; SD, standard deviation.
Mixed‐effects model with fixed effects for sequence, period, and treatment, with repeated observations for subjects for each of the driving time points, an unstructured covariance structure, and Kenward‐Roger degrees of freedom. Estimated differences are first treatment label listed minus second treatment label. p value tests null hypothesis that difference in LS means = 0 versus alternative hypothesis that difference in LS means ≠ 0.
Non‐inferiority is concluded if the upper bound of the 95% CI is less than the non‐inferiority margin, which is 4.4.
FIGURE 2.
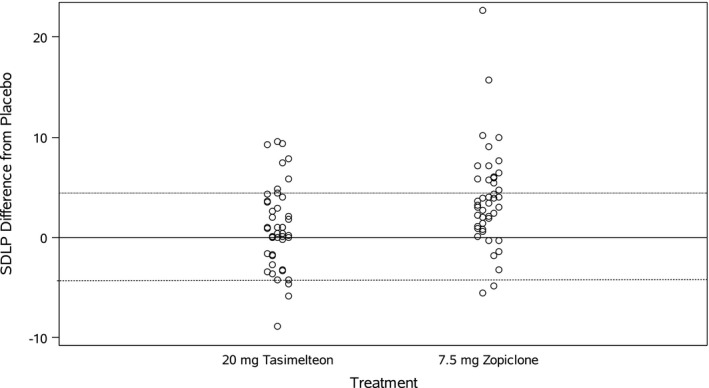
Within‐subject difference scores in standard deviation of lateral position (SDLP) by treatment. Distribution of within‐subject difference scores for tasimelteon and zopiclone. Dotted lines indicate thresholds for impairment (>4.4 cm) and improvement (<−4.4 cm). Three individuals treated with tasimelteon exceeded the 4.4 cm SDLP threshold (<−4.4 cm), whereas seven subjects showed a decrease in SDLP of this magnitude (>4.4 cm) after treatment with tasimelteon
The increase in SDLP was significantly greater (2.92 cm, p = .0002; Table 1) for zopiclone compared to tasimelteon. Furthermore, three subjects taking tasimelteon compared to 16 subjects taking zopiclone exceeded the 4.4 cm SDLP threshold.
3.2. Model sensitivity
The sensitivity of the driving simulator was established by the significantly worse performance (p < .0001; Table 1) on zopiclone 7.5 mg compared to placebo on SDLP. Symmetry analysis comparing pairwise within‐subject differences in SDLP between zopiclone and placebo showed that the differences between zopiclone and placebo are not symmetric about zero (the McNemar value exceeded the critical value of 7.048).
3.3. Driving secondary endpoint: lane exceedance
The number of lane exceedances is an indication of lane position control (the driver’s ability to stay within his/her lane), measured by the number of times the front, left, or right tire of the vehicle crosses over the right or left lane boundary. While the number of lane exceedances was not higher for tasimelteon compared to placebo (p = .3085), there was significantly more lane exceedances after zopiclone treatment compared to placebo (p < .0001; Table 2). The difference between tasimelteon and zopiclone with respect to number of lane exceedances was also significant (p < .0001).
TABLE 2.
Statistical analysis of lane exceedance (count) – log(x + 1) value (intent‐to‐treat population)
|
20 mg Tasimelteon (N = 48) |
7.5 mg Zopiclone (N = 46) |
Placebo (N = 46) |
|
|---|---|---|---|
| Mean (SD) | 2.60 (1.245) | 3.11 (0.949) | 2.42 (1.072) |
| Median | 2.63 | 3.07 | 2.52 |
| Min, max | 0.0, 5.2 | 1.1, 5.9 | 0.0, 5.4 |
| LS means a | 2.57 | 3.24 | 2.44 |
| p value for period a | .7952 | ||
| p value for sequence a | .0620 | ||
| 20 mg Tasimelteon versus placebo | 7.5 mg Zopiclone versus placebo | 20 mg Tasimelteon versus 7.5 mg zopiclone | |
| Difference in LS means a | 0.13 | 0.80 | −0.66 |
| 95% CI a | (−0.12, 0.39) | (0.53, 1.06) | (−0.92, −0.41) |
| p value a | .3085 | <.0001 | <.0001 |
CI, confidence interval; LS, least square; N, number of subjects; SD, standard deviation.
Mixed‐effects model with fixed effects for sequence, period, and treatment, with repeated observations for subjects for each of the driving time points, an unstructured covariance structure, and Kenward‐Roger degrees of freedom. Estimated differences are first treatment label listed minus second treatment label.
The severity of lane exceedance is assessed by related measures, including lane exceedance maximum (the maximum lateral deviation that the vehicle travelled from the lane centre) and duration of exceedance (the amount of time that it took for the driver to make corrections to bring the vehicle back into the lane of travel). For these measures, performance was significantly worse for zopiclone compared to placebo. By contrast, no significant increases were seen for tasimelteon compared to placebo.
3.4. Other secondary endpoints
Prior to beginning the drive, subjects were asked to self‐report their sleepiness and indicate if they felt “safe to drive.” There was no significant increase in subjective sleepiness following dosing with either tasimelteon (p = .1446) or zopiclone (p = .8268). Similarly, about the same number of subjects indicated that they felt safe to drive following treatment with tasimelteon (44/46 subjects [95.7%]) and zopiclone (43/46 subjects [93.5%]).
There were two VAS items administered post‐drive; one addressed the subject’s motivation to drive, and the other addressed the subject’s perception of how well they had driven. Subjects who had taken tasimelteon reported being significantly less motivated to perform at the best of their ability compared to placebo (p = .0468). In contrast, after treatment with zopiclone there was no significant reduction in motivation to perform well (p = .3665). Subjects rated their driving performance as not statistically different from placebo after treatment with tasimelteon (p = .2538). In contrast, following dosing with zopiclone subjects reported that their driving performance was significantly worse than following placebo (p = .0338).
4. DISCUSSION
The present study demonstrates that on a validated driving performance measure (SDLP) with known sensitivity for next‐day residual effects of sedatives, there were not statistically significant or clinically relevant effects on subjects’ performance after treatment with tasimelteon 20 mg. Specifically, the upper limit of the 95% CI for tasimelteon (2.74 cm) did not exceed the pre‐established non‐inferiority criterion of 4.4 cm (p < .0001). Furthermore, compared to placebo there was no significant increase in SDLP after tasimelteon treatment. Analysis of the distribution of changes in SDLP scores by symmetry analysis confirmed the lack of driving impairment after treatment with tasimelteon. The sensitivity of the study was established using zopiclone 7.5 mg, which demonstrated impairment of next‐day performance compared to placebo on measures considered sensitive to sedation. Furthermore, the effects observed for placebo and zopiclone in the present study were consistent with those previously reported for the CRCDS‐MiniSim driving simulator (Simen et al., 2015). The present study used zopiclone 7.5 mg, which is within the typical therapeutic range of dosages for zopiclone of 5–7.5 mg daily for younger adults (Cimolai, 2007).
It is worrisome that despite the decline in their driving performance after dosing with zopiclone, 93.5% of subjects judged themselves as being safe to drive prior to the drive, suggesting that subjects lacked awareness of their driving impairment before driving. Subjects were able to recognise their driving impairment at the completion of their drive, as subjects dosed with zopiclone reported that their driving performance was significantly worse compared to placebo (p = .0338). Interestingly, subjects who had taken tasimelteon reported being less motivated to perform at the best of their ability compared to placebo (p = .0468). This could be interpreted as an isolated finding as no other results correlate or it could be that the subjects on tasimelteon felt calmer and more confident in their driving abilities, thus did not need to be as motivated to perform well. To capture this confidence in driving, more recent studies have instituted a measure of perceived “effort” by asking “How much effort did you have to put forward to drive”, which may have better clarified this question.
In contrast to the lack of impairment by tasimelteon seen in the present study, ramelteon, another melatonin agonist, was found to significantly impair driving performance, as well as cognitive functioning, memory, and psychomotor performance the morning after bedtime administration (Mets et al., 2011; Staner et al., 2005). Ramelteon is noted to have a long‐acting active metabolite (M‐II) which may contribute to the finding of impaired next‐day driving performance.
The present study included healthy volunteers as the objective of the study was to understand whether tasimelteon can affect driving performance when taken the night before driving. Performing this study in healthy volunteers allows us to understand the specific effects of the drugs in the absence of sleep disturbances. Therefore, subjects with insomnia and other sleep disorders were excluded, and all subjects were educated on sleep hygiene.
In the present study, objective measures of sleep were not collected because tasimelteon was not expected to impair sleep and the study participants did not have any sleep disturbances. While the benefit of collecting objective sleep measures would be limited, the lack of this information prevented us from evaluating any potential association between sleep architecture with driving performance.
In summary, analysis of the next‐morning residual effect 9‐hr after bedtime administration of 20 mg tasimelteon on measures of simulated driving performance in healthy adults demonstrated no clinically meaningful effect on driving performance relative to placebo.
CONFLICT OF INTEREST
RT is an employee of Vanda and a stockholder. MF is an employee of Vanda and a stockholder. GB is an employee of Vanda and a stockholder. CP is an employee of Vanda and a stockholder. GGK provided advice for the present study, and his institution, Cognitive Research Corporation, received payment from Vanda Pharmaceuticals for equipment rental (driving simulators) and other contract research organization support services. Independent of the current study, GGK has served as a consultant for Avanir, Dart, Pfizer, and Sprout; Cognitive Research Corporation has received grants and consultancy payments from Avanir, Dart, Pfizer, and Sprout. GGK is the publisher of CogScreen® for which he receives licensing fees. CX is an employee of Vanda and a stockholder. MHP is Chief Executive Officer of Vanda.
AUTHOR CONTRIBUTIONS
This study was designed by RT, GGK, GB, CP, and MHP. RT and MF acquired the data. GGK helped analysed the data. GGK, GB, CP, and MHP interpreted the results of the study. RT and MF drafted the manuscript. GGK, GB, CP, and MHP reviewed the manuscript.
ACKNOWLEDGEMENT
The study team is grateful to the individuals who participated in this clinical study. This work was funded by Vanda Pharmaceuticals. This study was not submitted to a clinical trial registry.
Torres, R. , Fisher, M. , Birznieks, G. , Polymeropoulos, C. , Kay, G. G. , Xiao, C. , & Polymeropoulos, M. H. (2022). Simulated driving performance in healthy adults after night‐time administration of 20 mg tasimelteon. Journal of Sleep Research, 31, e13430. 10.1111/jsr.13430
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Cimolai, N. (2007). Zopiclone: Is it a pharmacologic agent for abuse? Canadian Family Physician, 53(12), 2124–2129. [PMC free article] [PubMed] [Google Scholar]
- Kay, G. , Ahmad, O. , Brown, T. , & Veit, A. (2013). Comparison of the MiniSim and STISIM driving simulators for the detection of impairment: An alcohol validation study (pp. 191–197). 6th International Driving Symposium on Human Factors in Driver Assessment, Training, and Vehicle Design at: Bolton Landing, NY, USA. Retrieved from: 10.17077/drivingassessment.1487 [DOI] [Google Scholar]
- Laska, E. , Meisner, M. , & Wanderling, J. (2012). A maximally selected test of symmetry about zero. Statistics in Medicine, 31(26), 3178–3191. 10.1002/sim.5384 [DOI] [PubMed] [Google Scholar]
- Lavedan, C. , Forsberg, M. , & Gentile, A. J. (2015). Tasimelteon: A selective and unique receptor binding profile. Neuropharmacology, 91, 142–147. 10.1016/j.neuropharm.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Mets, M. A. J. , de Vries, J. M. , de Senerpont Domis, L. M. , Volkerts, E. R. , Olivier, B. , & Verster, J. C. (2011). Next‐day effects of Ramelteon (8 mg), Zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep, 34(10), 1327–1334. 10.5665/SLEEP.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert, S. M. , & Weaver, D. R. (1995). Melatonin madness. Cell, 83(7), 1059–1062. 10.1016/0092-8674(95)90131-0 [DOI] [PubMed] [Google Scholar]
- Simen, A. A. , Gargano, C. , Cha, J.‐H. , Drexel, M. , Bautmans, A. N. , Heirman, I. , Laethem, T. , Hochadel, T. , Gheyle, L. , Bleys, K. , Beals, C. , Stoch, A. , Kay, G. G. , & Struyk, A. (2015). A randomized, crossover, placebo‐controlled clinical trial to assess the sensitivity of the CRCDS Mini‐Sim to the next‐day residual effects of zopiclone. Therapeutic Advances in Drug Safety, 6(3), 86–97. 10.1177/2042098615579314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staner, L. , Ertlé, S. , Boeijinga, P. , Rinaudo, G. , Arnal, M. A. , Muzet, A. , & Luthringer, R. (2005). Next‐day residual effects of hypnotics in DSM‐IV primary insomnia: A driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology (Berlin), 181(4), 790–798. 10.1007/s00213-005-0082-8 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research (2017). Evaluating drug effects on the ability to operate a motor vehicle guidance for industry. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


