Abstract
Light‐induced melatonin suppression data from 29 peer‐reviewed publications was analysed by means of a machine‐learning approach to establish which light exposure characteristics (ie photopic illuminance, five α‐opic equivalent daylight illuminances [EDIs], duration and timing of the light exposure, and the dichotomous variables pharmacological pupil dilation and narrowband light source) are the main determinants of melatonin suppression. Melatonin suppression in the data set was dominated by four light exposure characteristics: (1) melanopic EDI, (2) light exposure duration, (3) pupil dilation and (4) S‐cone‐opic EDI. A logistic model was used to evaluate the influence of each of these parameters on the melatonin suppression response. The final logistic model was only based on the first three parameters, since melanopic EDI was the best single (photoreceptor) predictor that was only outperformed by S‐cone‐opic EDI for (photopic) illuminances below 21 lux. This confirms and extends findings on the importance of the metric melanopic EDI for predicting biological effects of light in integrative (human‐centric) lighting applications. The model provides initial and general guidance to lighting practitioners on how to combine spectrum, duration and amount of light exposure when controlling non‐visual responses to light, especially melatonin suppression. The model is a starting tool for developing hypotheses on photoreceptors’ contributions to light's non‐visual responses and helps identifying areas where more data are needed, like on the S‐cone contribution at low illuminances.
Keywords: guidance to lighting practitioners, humans, light exposure duration, machine learning, melatonin suppression, α‐opic EDI
1. INTRODUCTION
The interest in lighting solutions that integrate both visual and non‐visual responses is growing rapidly, and Human Centric Lighting 1 and Integrative Lighting (CIE 2 ) are commonly used terms to describe such lighting solutions. Despite this common interest and relevance, the scientific knowledge on how to design and quantify light conditions for their ability to elicit non‐image forming (NIF) responses is not complete yet. Some well‐known NIF responses are modulations of pupil size, 3 , 4 alertness, 5 temperature and heart rate, 6 , 7 phase shifting the circadian rhythm 8 and the suppression of nocturnal melatonin secretion. 7 , 9 , 10 NIF effects of light are strongly driven by a distinct class of photoreceptors: the melanopsin‐containing intrinsically photosensitive retinal ganglion cells (ipRGCs), which also receive inputs from rods and cones. 11 Traditionally, light measurements concentrate on the human visual system and the spectral sensitivity of the classical photoreceptors (rods and cones). The spectral luminous efficiency function for human photopic vision, V(λ), can be used to spectrally weight and quantify a light condition in terms of luminance or illuminance (CIE 18.2 in 1983 and CIE S 010/E:2004. Photometry—The CIE system of Physical Photometry 12 ). At low (il)luminance levels the V’(λ) function is used (see CIE S 010/E:2004 12 ) to describe human scotopic vision based on the spectral sensitivity of the rods in the human eye. Instead of weighting a spectrum with the spectral luminous efficiency functions, the spectrum can also be weighted with the spectral sensitivity functions of the five retinal photoreceptor classes that can contribute, via the ipRGCs, to the NIF effects of light mediated by photoreceptors in the human retina. 11 In December 2018, the international standard CIE S 026:2018 ‘CIE System for Metrology of Optical Radiation for ipRGC‐Influenced Responses to Light’ 13 was published. This standard defines five spectral weighting functions (eg action spectra), s α(λ), for the five (α‐opic) retinal photoreceptor classes: S‐cones, M‐cones, L‐cones, rods and melanopsin‐based photoreception of ipRGCs. These five spectral sensitivity functions are used to define light quantities and metrics that are SI compliant and compatible with earlier photometric standards. For each of the five (α‐opic) photoreceptors an α‐opic irradiance can be calculated by weighting the spectral irradiance of a given light source with an α‐opic spectral sensitivity function.
The five α‐opic irradiances are expressed in units on the energy scale (eg W/m2), the corresponding photometric quantities are the five α‐opic equivalent daylight illuminances (α‐opic EDIs), which are expressed in lux. 13 Each α‐opic EDI represents the (equivalent) illuminance of standard illuminant D65 14 that yields the same α‐opic irradiance as the test light.
The melanopic EDI (M) of a particular test light (condition) divided by its photopic illuminance (P) provides an M/P ratio that in CIE S 026:2018 is denoted as the melanopic daylight (D65) efficacy ratio (or melanopic DER). 15 This dimensionless ratio describes the melanopic efficacy of the test light, by definition it equals 1 when the spectrum of the test light conforms to D65.
Previous work has shown that the spectral sensitivity of the classical photoreceptors cannot accurately describe the overall light sensitivity of NIF responses. 7 , 11 , 16 , 17 , 18
In the present analysis, we adopt a machine‐learning algorithm to systematically analyse 29 studies on light‐induced melatonin suppression and explore which photoreceptors and which light exposure characteristics are the main determinants of melatonin suppression. The light exposure characteristics that were evaluated are: (photopic) illuminance, five α‐opic EDIs, duration and timing of the light exposure, pharmacological pupil dilation (y/n) and the nature of the light source (ie narrowband y/n). After establishing which characteristics of light exposure were significant predictors of melatonin suppression, we subsequently investigated how each of these individual predictors affect the logistic dose‐response relationship between the light stimulus and melatonin suppression.
2. METHODS
2.1. Studies selection
A literature search was executed to identify papers with data on the effect of light on human endogenous melatonin levels in saliva or blood (serum or plasma). The search used the electronic database PubMed with the following keywords: melatonin, light, circadian, human, irradiance, illuminance and considered studies published before March 2019. We only included studies with healthy adult participants. Studies had to provide a detailed description of the light characteristics: (1) the illuminance or irradiance, (2) the type of light source (narrowband or non‐narrowband), (3) for white light sources: (correlated) colour temperature (4) for narrowband light sources: spectral composition or spectral peak position and width of the peak, (5) the light exposure duration and (6) the start timing (external clock) of the light exposure. In addition, light exposure duration had to be at least 30 min and had to occur during the biological evening or night (ie the start of the light exposure had to be between 19:00 and 02:30 h). During this time window melatonin secretion usually starts in normally entrained people with no extreme chronotype under dim light (<8 lx) or no‐light conditions. The analysis only included studies that had a dim light control condition. In total, 29 studies were included. See Table 1.
TABLE 1.
Overview of the different studies included in the analysis and their light exposure (LE) general characteristics
| Author(s) (year) | # Data points a | Light source | Narrow band | Light characteristics (CCT in K or peak wavelength in nm) | Pupil dilation | Start LE | LE duration(s) (min) |
|---|---|---|---|---|---|---|---|
|
Bojkowski et al., 1987 b [19] |
1 | Fluorescent | No | 5500 K | No | 00:30 | 30 |
|
Bojkowski et al., 1987 b [19] |
1 | Fluorescent | No | 5500 K | No | 01:30 | 30 |
|
Brainard et al., 1988 c [20] |
5 | tungsten, monochromatic filter | Yes | 509 nm | Yes | 02:00 | 60 |
|
Wright et al., 2000 d [21] |
3 | Fluorescent | No | 4000 K | No | 20:00 | 120, 180, 240 |
|
Zeitzer et al., 2000 e [22] |
21 | Fluorescent | No | 4000 K | No | 23:00 | 195 |
|
Brainard et al., 2001 b [9] |
68 | Xenon arc lamp & monochromator | Yes | 440, 460, 480, 505, 530, 555, 575, 600 | Yes | 02:00 | 90 |
|
Thapan et al., 2001 b [10] |
35 | Metal halide arc & monochromatic filter | Yes | 424, 456, 472, 496, 520, 548 | Yes | 23:30 | 30 |
|
Whitmore et al., 2001 b [23] |
2 | Fluorescent | No | 3500 K | No | 02:00 | 60 |
|
Whitmore et al., 2001 b [23] |
2 | Fluorescent + green filter | Yes | 530 | No | 02:00 | 60 |
|
Wright & Lack, 2001 b [24] |
8 | LED | Yes | 470, 497, 525, 595, 660 | No | 00:00 | 120 |
|
Wright & Czeisler, 2002 b [25] |
1 | LED | Yes | 497 | No | 00:00 | 120 |
|
Wright & Czeisler, 2002 b [25] |
1 | White LED | No | 4000 K | No | 00:00 | 120 |
|
Wright & Czeisler, 2002 b [25] |
1 | Fluorescent | No | 4000 K | No | 00:00 | 120 |
|
Gronfier et al., 2004 e [26] |
1 | Fluorescent | No | 4100 K | No | 1,1 h before habitual bedtime | 195 |
|
Wirz‐Justice et al., 2004 d [27] |
5 | Fluorescent | No | 4700 K | No | 21:00 | 30, 60, 90, 120, 180 |
|
Cajochen et al., 2005 d [6] |
6 | Xenon arc + interference filter | Yes | 460, 550 | No | 21:30 | 30, 60, 120 |
|
Herljevic et al., 2005 b [28] |
7 | Metal halide + monochromatic filter | Yes | 456, 548 | Yes | 23:30 | 30 |
|
Hanifin et al., 2006 b [29] |
3 | Xenon arc + monochromator | Yes | 460, 630, 700 | Yes | 02:00 | 90 |
|
Revell & Skene, 2007 b [30] |
3 | Ultra‐high‐pressure mercury | No | ~6600 K | Yes | 00:30 | 30 |
|
Revell & Skene, 2007 b [30] |
3 | Ultra‐high‐pressure mercury + interference filter | Yes | 479 | Yes | 00:30 | 30 |
| Brainard et al., 2008
b
[31] |
10 | LED | Yes | 420 nm, 460 nm | Yes | 02:00 | 90 |
|
Kozaki et al., 2008 d [32] |
3 | Fluorescent | No | 2300 K, 3000 K, 5000 K | No | 01:00 | 90 |
|
Gooley et al., 2010 e [8] |
8 | Xenon arc & monochromator | Yes | 460, 555 | Yes | 23:00 | 48.75, 146.25, 243.75, 341.25 f |
|
Revell et al., 2010 d [33] |
2 | Fluorescent | No | 4000 K, 17 000 K | Yes | 23:00 | 30 |
|
Revell et al., 2010 c [33] |
6 | Ultra‐high‐pressure mercury + interference filter | Yes | 437, 479, 532, 479 + 532, 437 + 470 | Yes | 23:00 | 30 |
|
Santhi et al., 2012 d [34] |
12 | Fluorescent yellow (TL16) | No | Yellow/blue‐depleted | No | 19:35 | 30, 60, 90, 120, 180, 240 |
|
Santhi et al., 2012 d [34] |
6 | Fluorescent (TLD 827) | No | 2700 K | No | 19:35 | 30, 60, 90, 120, 180, 240 |
|
Santhi et al., 2012 d [34] |
6 | Fluorescent (17000K) | No | 17 000 K | No | 19:35 | 30, 60, 90, 120, 180, 240 |
|
West et al., 2011 b [35] |
8 | LED | Yes | 469 | No | 02:00 | 90 |
|
West et al., 2011 b [35] |
1 | Fluorescent | No | 4000 K | No | 02:00 | 90 |
|
Chang et al., 2012 e [36] |
1 | Fluorescent | No | 4100 K | No | 0:30 | 120 |
|
Brainard et al., 2015 b [37] |
18 | Fluorescent | No | 4000 K, 17 000 K | No | 02:00 | 90 |
|
Gabel et al., 2017 d [38] |
2 | Fluorescent | No | 2800 K, 9000 K | No | 22:00 g | 180 |
|
Nowozin et al., 2017 b [18] |
18 | Fluorescent, white LED, halogen, high‐pressure sodium, Xe‐filled fluorescent, metal halide | No | 1500 K, 12 000 K | No | 22:00 | 30 |
|
Souman et al., 2018 d [5] |
5 | White LED (CRI 57) | No | 2609 K | No | 21:00 | 30, 60, 90, 120, 180 |
|
Souman et al., 2018 d [5] |
5 | White LED (CRI −22) | No | 2641 K | No | 21:00 | 30, 60, 90, 120, 180 |
|
Hanifin et al., 2019 e [39] |
2 | Fluorescent | No | 4000 K, 17 000 K | No | 22:45 | 195 |
|
Nagare et al., 2019 b [40] |
2 | White LED | No | 3000 K | No | 00:00 | 60 |
|
Nagare et al., 2019 b [40] |
2 | ‘cyan‐gap’ modified LED | No | cyan‐gap | No | 00:00 | 60 |
|
Nagare et al., 2019 b [40] |
32 | White LED | No | 2700 K, 6500 K | No | 23:00 | 30, 60, 120, 180 |
Number of data points that were extracted from the group mean melatonin (suppression) values as reported within a particular study for different light exposure durations and spectral compositions.
Study with dim light condition in which melatonin levels were >10 pg/ml at start time of light exposure. Light‐induced melatonin suppression corrected for the % change in melatonin in the dim light condition (see point 3 in the Section 2.2).
Study reporting % melatonin suppression relative to the values before lights on (see point 5 in Section 2.2).
Study reporting melatonin data as a function of time for both a dim light condition and the test light condition (see point 2 in Section 2.2).
Study reporting % suppression based on area under the curve (see point 4 in Section 2.2).
In Figure 2 the exposure duration points from this study were mapped to the closest light exposure bin: for instance the durations 48.75, 146.25, 243.75 and 341.25 min were mapped into the Figure 2 bins of 60, 180, ≥240 and ≥240 min, respectively.
When the light exposure started before the time point of the dim light melatonin onset (DLMO), it is assumed that the light exposure has only started at the DLMO, see Section 2.2.
2.2. Melatonin suppression calculations
The melatonin suppression data were analysed for six light exposure durations: 30, 60, 90, 120, 180 and 240 min and above. The following steps were followed to obtain percentage (%) of melatonin suppression for each of these time points (when sampled within a study) when melatonin suppression was not reported. If data had to be extracted from plots the WebPlotDigitizer was used.
-
1
In studies where the start time of the light exposure (LE) occurred before the time point of the dim light melatonin onset (DLMO, defined as the first melatonin sampling timepoint at which the salivary melatonin concentration approached 4 pg/ml), the light exposure prior to the DLMO was neglected in our analysis (since prior to DLMO, melatonin suppression cannot be accurately determined with current melatonin assays). Instead of using the actual start time of the light exposure, we assumed that the light exposure had only started at the DLMO.
-
2
In publications reporting melatonin data as a function of time for both a dim light condition and the test light condition(s), light‐induced melatonin suppression was calculated according to:
| (1) |
melatonin (tx ): melatonin concentration at the sampling time point tx .
-
3
In studies that had a dim light condition in which melatonin levels were above 10 pg/ml at the start time of the light exposure, light‐induced melatonin suppression of individuals was corrected for the % change in melatonin in the dim light condition during the same time interval (control‐adjusted change score as applied in refs 9, 37). This means that first, for each light condition, the per cent melatonin change score was determined according to:
| (2) |
∆ melatonin: melatonin change score (%) for a light condition at the sampling point tx ; melatonin (t 0): melatonin (baseline) concentration at the start of the light exposure t 0; melatonin (tx ): melatonin concentration at the sampling time point.
Next, the per cent control‐adjusted change scores were obtained by subtracting ∆ melatonin for the control (no‐light) condition from ∆ melatonin for the light condition.
-
4
For publications 8 , 22 , 36 , 39 where data on the area under the curve (ie the AUC from the start of the light exposure t 0, to the sampling time t x, for the melatonin concentration profile over time) was available and no data for steps 2–4 was available, light‐induced melatonin suppression was calculated according to:
| (3) |
The data points obtained by this function were allocated to the light exposure duration (∆t exposure) that corresponds to the midpoint of the AUC interval (ie ∆t exposure = 0.5 * (tx −t 0)).
-
5
In some publications, 20 , 33 light‐induced melatonin suppression was calculated according to Equation (2) with both ‘melatonin in dim(tx )’ replaced by ‘melatonin in dim(t 0)’ where t 0 refers to the melatonin values just before lights on.
-
6
When studies reported a zero (α‐opic equivalent daylight) illuminance, the (α‐opic equivalent daylight) illuminance was set to 10−6 lx for computational reasons.
The method used for each individual study is indicated in Table 1.
2.2.1. Quantification of light using five different photoreceptor sensitivity‐weighted inputs
In this paper, light doses are reported in terms of six different illuminances: the (photopic) illuminance and the L‐cone‐opic, M‐cone‐opic, S‐cone‐opic, melanopic and rhodopic equivalent daylight illuminance (EDI), all expressed in lx, as defined in CIE S 026:2018. These quantities are used to establish dose‐response relationships for light‐induced melatonin suppression. Here we preferred α‐opic EDIs over α‐opic irradiances because light practitioners typically use photometric units to specify their designs, installations and recommendations. To calculate the five α‐opic EDIs, we used a macro‐based Excel worksheet, called ‘Human Centric Lighting Toolkit’, which has been created by one of the co‐authors (Dieter Lang). This toolkit is non‐commercial and primarily intended to be used by scientists and lighting experts. It calculates melanopic and other α‐opic quantities for different light sources and intensities. A description of the basic functions of the melanopic toolkit used for the calculation scan be found in the Data S1.
The toolkit results are calculated based on the following documents and standards:
German standard DIN SPEC 5031–100:2015
Peer reviewed scientific paper ‘Measuring Light in the Melanopsin Age’ by Lucas et al. 11
Revised version of the ‘Irradiance Toolbox’, by Lucas et al. (2014).
The new SI‐compliant CIE metrics based on CIE S 026:2018 for user‐defined spectra, the ‘Human Centric Lighting Toolkit’ and the ‘CIE S 026 α‐opic Toolbox’ (available at: http://cie.co.at/news/launch‐cie‐s‐026‐toolbox‐and‐user‐guide) were cross‐checked and yield identical results.
Depending on the available information about the light sources that volunteers were exposed to, we conducted different calculation processes (see Data S1) to obtain the five α‐opic EDIs.
2.3. Dose‐response curves
The melatonin suppression data from all selected studies was plotted against the photopic illuminance and against each of the five α‐opic EDIs (see CIE 13 ). Earlier research has demonstrated that a four‐parameter logistic function as given in Equation (4) provides a good approximation of the sigmoidal dose‐response relationship between illuminance and melatonin suppression. 22 , 42
| (4) |
In this logistic model, the parameter a represents the lowest and c the highest value for the melatonin suppression response y (in %) at a given illuminance x (in lx). These lowest and highest values of y are asymptotically approached at very low or very high values of x, respectively. Parameter b represents the illuminance at which half of the full melatonin suppression response (ie ED50) occurs; d is the steepness of the curve at b. In our analysis, we adopted Equation (4) while constraining the parameters a and c to 0 (%) and 100 (%), respectively, that is, we assume that these values represent the minimum and maximum of the melatonin suppression response.
2.4. Data analysis
In order to identify the main predictors of the melatonin suppression response, the following independent variables were included in a random forest (RF) regression analysis: (1–5) the five α‐opic EDIs, as well as modulating cofactors like (6) light exposure start time, (7) exposure duration and (8) pharmacological pupil dilation (y/n). In the data set, pupil dilation (y/n) was heavily confounded with narrowband (y/n), rho = 0.83. To develop the RF model pupil dilation was used rather than narrowband, as pupil dilation was found to result in a slightly better fit quality as compared to narrowband in a pre‐analysis direct comparison. Ex‐post comparison of the optimal model with the one including narrowband instead of pupil dilation confirmed that decision.
Photopic illuminance was not included as a variable in the RF approach, since its V(λ) weighting is basically a linear combination of the spectral sensitivity functions of the M‐cone and the L‐cone. This means that in the RF approach the photopic illuminance does not add new information.
Random forest is a machine‐learning approach to solve classification and regression problems. It is an ensemble method based on large numbers (200+) of regression trees. 43 RF theory and procedures are extensively described in textbooks, for example, Hastie et al. 44 and various online resources. We employed the conditional RF algorithm by Strobl et al.. 45 The RF approach has no pre‐requirements for the data distribution, and inherently captures the non‐linear relationships and higher order interactions within the data. Furthermore, RF models employ ‘bagging’ (repeated random subsampling) and ensemble averaging, the latter acting protectively against overfitting the data (model variance). The quality of an RF model is determined by its prediction performance, respectively, the prediction error, which can be quantified as root mean square of error (RMSE). The optimal RF model is the model with the lowest RMSE and the lowest number of predictors. We also assessed RF model fits by using R 2 as a more traditional measure to select the best RF model (ie F‐statistics of hierarchically nested models). Finally, the RF predictors were integrated into a logistic non‐linear model to assess their impact on light‐induced melatonin suppression in more detail.
The randomness, which is an essential part of the RF model building process, produces slightly different outcomes for RMSA and R 2 when a model is built multiple times with the same data set and the same predictors. In order to account for that variability, we employed a 10‐fold cross validation (CV) process to attain an estimate of the prediction performance (as RMSE) for all 255 RF models, which resulted from fully crossing our set of eight predictor variables (255 unique combinations, 28−1 = 255). CV was conducted 56 times for each model, providing a distribution of RMSE for each model, which allowed an assessment of distinctness of competing models, that is, models with very close mean RMSE.
Mean RMSE values were employed for the stepwise forward selection of additional predictors to build a valid sequence of hierarchically nested RF models. Starting from a model with the single best predictor (lowest mean RMSE), subsequent predictors were added stepwise based on the capacity of the additional predictor to (maximally) reduce the mean RMSE of the model. This procedure was repeated to a point where any additional predictor would actually result in an RMSE increase. This point defined the preliminarily optimal model with the lowest mean RMSE and the least number of predictors. In a second step, the distinctness of adjacent mean RMSE values within the sequence of hierarchically nested models was evaluated by Welch's two samples t‐tests. If a model's mean RMSE was not significantly lower than the previous one, the chain of nested models would end with the previous model. To identify the model with the most substantial predictors, additionally mean R 2 of each model was derived by averaging 50 RF repetitions and compared between adjacent models in the nested chain by F‐statistics.
Lastly, the best RF model predictors were assessed within a logistic model that uses a single illuminance‐related variable x (Equation 5) to describe the melatonin suppression dose‐response relationship, that is, in this logistic model, x represents the best predictor out of the five illuminance‐related variables that were assessed in this study (ie five α‐opic EDIs). Hereto, additional predictors were sequentially introduced in Equation (4) as linear effects (Eb and Ed ) that interact with parameter b (midpoint x‐value of the function) and/or parameter d (slope in midpoint x‐value), see Equation (5).
| (5) |
For computational reasons the illuminances and α‐opic EDIs were multiplied by 106 (in order to move them out of the range between 0 and 1) before fitting the logistic dose‐response on the log10‐scale.
All calculations were performed with the R statistical computation environment v 4.0.3, 46 using the package party 47 for conditional (unbiased) random forest analysis and the basic R function nls for the non‐linear fitting procedure (NLS).
3. RESULTS
In Figure 1 the dose‐response curves for all five α‐opic EDIs (expressed in lx of D65) and photopic illuminance (expressed in lx) are shown for the different exposure durations. The upper row displays the overall data set in which all light exposure durations are included. Here, the largest adjusted R 2 values occurred when melatonin suppression was plotted against melanopic or rhodopic EDI. Apart for the 180‐min duration bin, all individual light exposure duration bins had the highest R 2 values for melanopic EDI. This might be due to the fact that for in the 180‐min exposure duration bin the melatonin suppression data set did not include any monochromatic light exposure and covered a relatively small range of spectral compositions across the illuminances range. This is illustrated in Figure 2, which displays both the photopic illuminance range and the range in melanopic EDI/photopic illuminance (ie the M/P ratio or melanopic DER) for each of the investigated exposure durations. Within the 30–120 min light duration bins, the gathered data covered a wide range of M/P ratios across the photopic illuminance.
FIGURE 1.
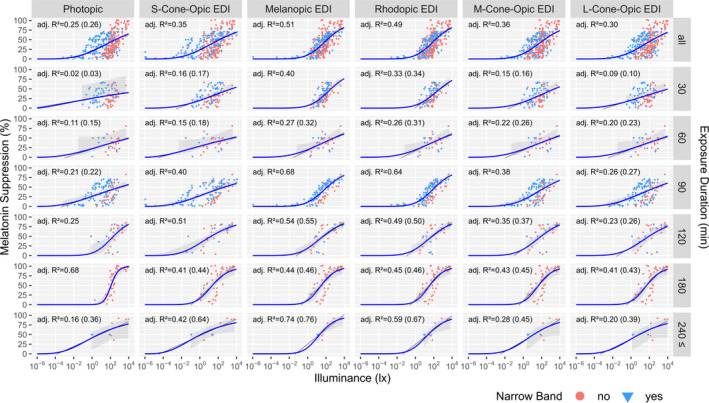
Dose‐response relationship for % melatonin suppression as a function of both photopic illuminance and each of the five α‐opic EDIs for all light exposure durations (top row, N = 326 data points). In the lower rows, the data are split up in bins with different light exposure durations, thus representing % melatonin suppression as determined after 30, 60, 90, 120, 180 and ≥240 min of light exposure. Data points located between the designated bin limits were assigned to the closest bin, as well as all points beyond 240 min were assigned to that bin. The coloured symbols indicate whether the light is narrowband (light blue triangles) or not (red circles). The 95% confidence bands (grey areas) for the logistic fits (blue) were derived using a higher order Taylor expansion and Monte Carlo simulation method, which is only applicable (and shown) for the range of actual data points. Adjusted R 2 values for the logistic fits are provided for each panel. If a linear fit had the same or higher adj. R 2, it is indicated in brackets and the linear fit line is drawn (dark grey)
FIGURE 2.
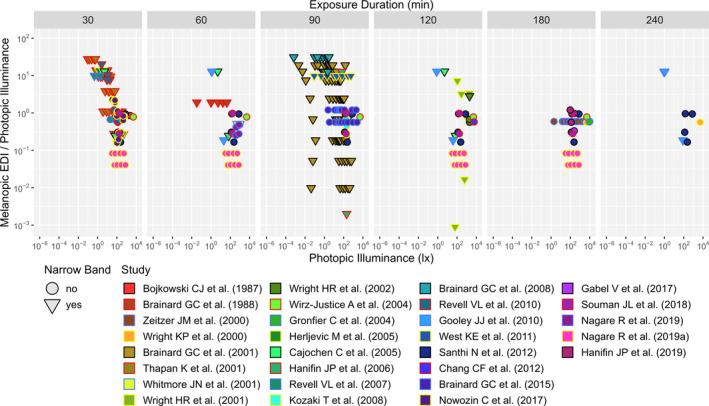
Distribution of photopic illuminance and M/P ratio (ie melanopic DER) for each of the 7 light exposure duration bins. Data points located between the designated bin limits were assigned to the closest bin, as well as all points beyond 240 min were assigned to that bin. Triangles denote narrowband and circles non‐narrowband light conditions. One data point is not displayed due to its position far outside the figure range. It belongs to a narrow band light source in the 90 min bin from the Hanifin et al. 29 study: photopic illuminance = 100.49 and M/P = 10−6.45
Figure 3 shows the loess‐smoothed R 2 values for a single‐predictor logistic dose‐response model that is either based on one of the five α‐opic EDIs or the photopic illuminance while restricting the data analysis to various upper thresholds in (photopic) illuminance, where the x‐axis in Figure 3 represents the various upper threshold values. This allowed to asses the role of the different α‐opic EDIs as a function of illuminance levels. At illuminances below 21 lx, S‐cone‐opic EDI was the best single predictor of the melatonin suppression response. For illuminances between 22 and 30 lx, melanopic EDI and S‐cone‐opic EDI were equally good predictors. For photopic illuminances above 30 lx, the melanopic EDI became a better predictor than any of the other α‐opic EDIs. The same procedure was performed in order to asses the role of the different α‐opic EDIs as a function of light exposure duration. When tested for various upper thresholds in exposure duration melanopic EDI was confirmed to be the best single predictor (see Figure S1).
FIGURE 3.
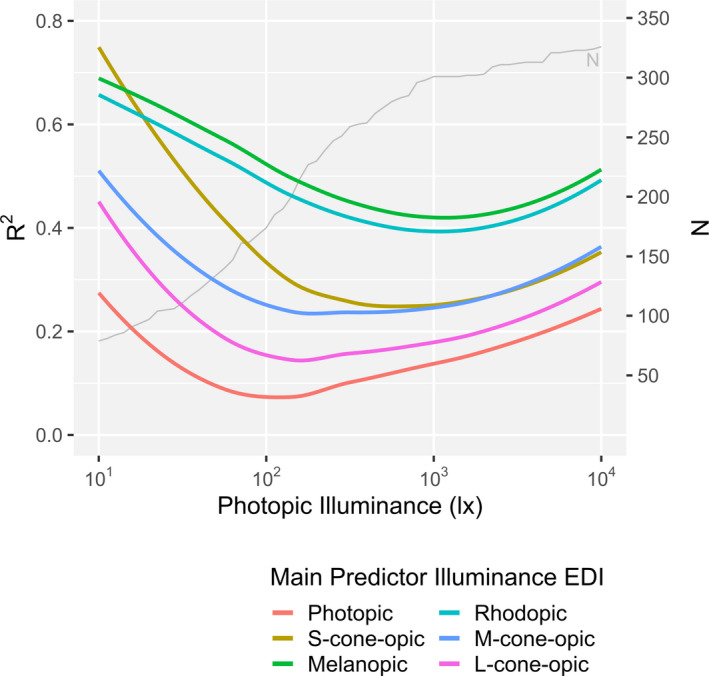
Smoothed R 2 value sequence for single‐predictor logistic dose‐response models (Equation 5) for each α‐opic illuminance. The sequence starts with a data set for which the maximum photopic illuminance is 10 lx (N = 79) and continues including successively more data points with a maximum photopic illuminance that is denoted as cut‐off on the x‐axis, until the whole data set (N = 326, photopic illuminance = 10 000 lx) is included in the model. The lines represent a loess‐fit of the actually calculated values. The S‐cone EDI outperforms melanopic EDI only at photopic illuminances below approximately 21 lux
In the RF analysis, melanopic EDI was the best single predictor for light‐induced melatonin suppression, see Table 2, which corroborated our results as presented in Figures 1 and 3. The rows in Table 2 show the hierarchical sequence of RF models with 1 (top row) to 6 (bottom row) predictors. As indicated by lowest RMSE value of 15.19% the best RF model included 4 predictors. These were, in order of importance: melanopic EDI, light exposure duration, pupil dilation (y/n) and S‐cone‐opic EDI.
TABLE 2.
Statistics for distinctness within the hierarchical sequence of nested random forest regression models as evaluated by decrease of RMSE and increase of R 2
| Model | RMSE statistics a | R 2 statistics b | ||||||
|---|---|---|---|---|---|---|---|---|
| RMSE % (SD) | t | df | p | R 2 | adj. R 2 | F | p | |
| Melanopic | 18.03 (0.16) | 0.63 | 0.63 | |||||
| Melanopic + Duration | 16.09 (0.12) | 65.94 | 73.28 | <.0001 | 0.70 | 0.70 | 75.35 | <.0001 |
| Melanopic + Duration + Pupil Dilation | 15.41 (0.15) | 23.66 | 76.89 | <.0001 | 0.728 | 0.725 | 34.54 | <.0001 |
| Melanopic + Duration + Pupil Dilation + S‐cone | 15.19 (0.19) | 6.91 | 78.92 | <.0001 | 0.744 | 0.741 | 20.25 | <.0001 |
| Melanopic + Duration + Pupil Dilation + S‐cone + L‐cone | 15.21 (0.16) | −1.07 | 82.00 | .29 | 0.747 | 0.743 | 3.84 | .10 |
| Melanopic + Duration + Pupil Dilation + S‐cone + L‐cone + M‐cone | 15.27 (0.15) | −1.55 | 80.90 | .13 | 0.751 | 0.746 | 5.20 | .05 |
56 repetitions of 10‐fold CV, Welch Two Sample t‐test.
Mean R 2 of 50 RF repetitions, F‐test df = (1324).
Although S‐cone‐opic EDI was identified by RF as valuable additional predictor resulting in a decrease of RMSE of 0.22% and a statistically significant rise of R 2 of 0.016, that incremental change appears to be very small and of debatable practical relevance. This result can be explained by the observed slightly better performance of S‐cone‐opic EDI in the illuminance range below 21 photopic lux and is discussed further down. In view of the exiguity of the effect and the methodical difficulties arising from integrating more than one illuminance‐related contribution into the logistic function model, the authors deemed it justifiable to drop S‐cone‐opic EDI from further consideration in the translation of the RF model to the logistic function model, which was performed by applying an NLS fitting procedure.
Melatonin suppression is primarily regarded as a function of the one chosen α‐opic predictor melanopic EDI, which is assigned to the place of x in Equation (5). The influence of exposure duration and pupil dilation was further evaluated in terms of a linear combination of their individual and combined effects Eb and Ed (on the midpoint b, and on the slope d, respectively, see Equation 5). All nine possible combinations of exposure duration and pupil dilation acting alone or together as Eb and/or Ed in the logistic model were realized and the model with exposure duration and pupil dilation (y/n) acting together as Ed , see Equation (6), provided the best fit while comprising only significant coefficients, see Table 3 and Equation (7). RMSE of that model was 15.56% and adjusted R 2 was 0.63, which signified an improvement over the model with melanopic EDI as single predictor (RMSE = 17.90%; adj. R 2 = 0.51 vs. 0.63: F (1,323) = 52.15, p < .0001).
| (6) |
where: suppressionmelatonin = melatonin suppression (%); EDImelanopic = melanopic EDI (lx); b = illuminance at which half of the full melatonin suppression response (ie ED50) occurs under the hypothetical condition that exposure duration were 0 and pupils were undilated; ∆t exposure = exposure duration (min); βe = regression weight of exposure duration; dilpupil = pupil dilation applied: 0 = no, 1 = yes; βp = regression weight of pupil dilation; d = steepness of the curve at b
| (7) |
TABLE 3.
Final logistic model (Equation 7) parameter estimates and statistics
| Parameter | Estimate | SE | t | p a | CI95% |
|---|---|---|---|---|---|
| b | 9.002 | 0.121 | 74.415 | <.0001 | 8.7654 to 9.2396 |
| d | 7.496 | 0.505 | 14.858 | <.0001 | 6.5075 to 8.4853 |
| β e | −0.008 | 0.001 | −8.710 | <.0001 | −0.0093 to −0.0059 |
| β p | −0.462 | 0.095 | −4.848 | <.0001 | −0.649 to −0.2753 |
df = 322.
Figure 4A,B show melatonin suppression for cases without pharmacological pupil dilation as predicted by the RF model and the final logistic model (Equation 7 with parameter values as given in Table 3, which are based on the assumption of 100% melatonin suppression at infinite illuminance, as opposed to scaling melatonin suppression as a % of the maximum measured response, see discussion section), respectively, for cases without pharmacological pupil dilation. While the RF model yielded a close representation of the data, it makes no valid assumptions regarding the physiological processes underlying the dose‐response relationship. On the other hand, the logistic model of Equation (4) has been shown to accurately describe many physiological dose‐response relationships. 22 , 42 As such, the logistic model of Equation (7) was further applied to predict the % melatonin suppression as a function of the melanopic EDI and the light exposure duration (without pharmacological pupil dilation). The result is shown in Figure 5. Different melatonin suppression levels (coloured lines) are depicted as a function of melanopic EDI and light exposure duration. The figure adopts a shading to indicate the light thresholds as proposed in a recent preprint with recommendations for healthy daytime (>250 lx melanopic EDI), evening (<10 lx melanopic EDI) and night time (<1 lx melanopic EDI) indoor light exposures 48 as reference for typical lighting scenarios. Figure 5 also shows that 50% melatonin suppression can be achieved with illuminances as low as 10 lx melanopic EDI provided that exposure is of sufficient duration.
FIGURE 4.
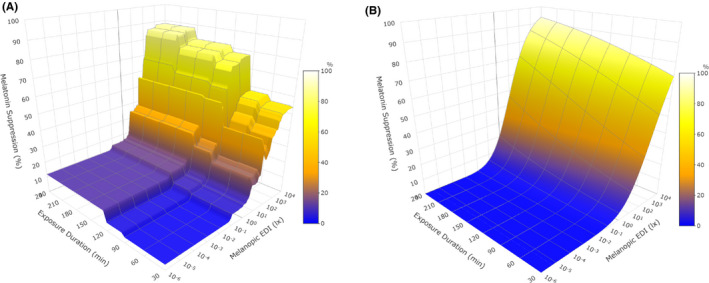
Surface plots of model predictions for melatonin suppression by melanopic EDI (10−6 lx – 104 lx), exposure duration (30–240 min) and pupil dilation constrained to ‘undilated’. (A) Random forest model: RMSE = 15.41%, adj. R 2 = 0.73. (B) Logistic dose‐response model (Equation 7): RMSE = 13.30%, adj. R 2 = 0.63
FIGURE 5.
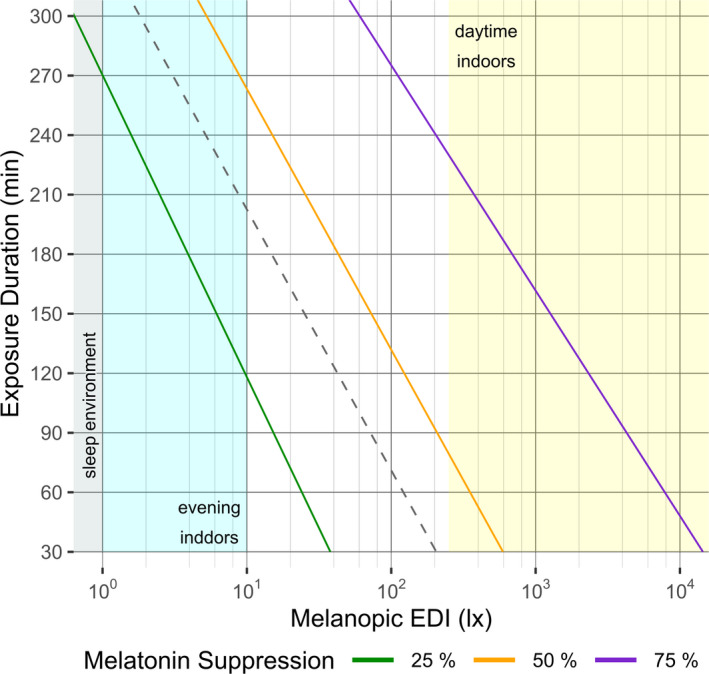
Melatonin suppression for different combinations of exposure duration and melanopic EDI as predicted by the logistic dose‐response model (Equation 7) for an undilated pupils scenario. The coloured lines represent the profiles of three levels of melatonin suppression. The coloured areas indicate ranges of melanopic EDI that are recommended for practicular application contexts: ideal sleep environment (grey area), sleep supportive evening setting at home (cyan area) and daytime indoor environment (yellow area). The dashed grey line indicates the profile of 50% melatonin suppression under a dilated pupils condition, it is shifted to the left as compared to the undilated pupils scenario, which signifies a higher sensitivity.
The effect of pharmacological pupil dilation on the effective dose for 50% melatonin suppression (ED50) is shown by the dotted line, which is shifted to lower melanopic EDIs as compared to the ED50 for undilated pupils (ie without pharmacological pupil dilation). Our model predicts that pharmacological pupil dilation reduces the various ED thresholds (ED10, ED50 and ED75, all expressed in lx melanopic EDI) to 34.5% of the ED threshold without pharmacological pupil dilation. In the Data S2, a tool is provided to predict % melatonin suppression from melanopic EDI, pupil dilation y/n and exposure duration (or vice versa, except for pupil dilation y/n).
For a 90‐min exposure duration our model predicts ED50 values of about 208 lx melanopic EDI and 72 lx melanopic EDI for undilated and dilated pupils, respectively. Phillips et al., 49 report, under undilated pupils conditions, an overall ED50 of 12.7 lx melanopic EDI 50 , this value is based on the AUC interval from baseline DLMO, about 2 h before habitual bedtime, to the final melatonin assay 1 h post‐habitual bedtime. This implies a total exposure duration of 3 h, which we allocate to an exposure duration at its midpoint: 90 min (see also point 4 in the sub‐section Section 2.2). For the same 90‐min exposure duration Prayag et al., 7 under dilated pupil conditions, reported an EC70 value of ~60 ‘melanopic lx’, which corresponds to a melanopic EDI of ~54 lx. 15 This EC70 value gives the light dose at which 70% of the maximum response occurs. Since the maximum response in Prayag et al. was about 70% (control‐adjusted) melatonin suppression, their EC70 corresponds to 70 * 70 = 49% melatonin suppression. Therefore, their EC70 value can be compared to our ED50 values and those of Phillips et al. 49 (ie the latter two ED50 values are assuming that 100% suppression at infinite illuminance is achievable).
4. DISCUSSION
The melatonin suppression response depends on the level of light exposure and this dose‐response relationship can be accurately described by means of a four‐parameter logistic model. 22 , 42 Recent analyses indicate that melanopsin weighted quantities such as the melanopic EDI can be used to describe the overall light sensitivity of melatonin suppression across a range of studies. 16 , 50 Although some first evaluations on the influence of pupil dilation and light exposure duration on melatonin suppression are becoming available, 16 the impact of different factors such as the timing and duration of light exposure along with pupil dilation has not yet been investigated systematically. In the present analysis, we thoroughly explored to what extent melatonin suppression can be predicted based on the following light exposure characteristics: (photopic) illuminance, α‐opic EDIs (quantifying the activation of each of the five α‐opic photoreceptors), exposure duration, pharmacological pupil dilation (y/n), exposure timing.
Our analysis followed a three‐step approach. Firstly, we adopted an approach where a single, illuminance‐based parameter was used to describe light dose within a regular logistic model that has been reported to accurately describe many physiological dose‐response relationships. Secondly, we adopted a machine‐learning approach that is independent of data structure (ie a RF) and allows to identify which light exposure characteristics are the main predictors of the melatonin suppression response. Lastly, we adopted a smoother approach in which the significant predictors from the RF approach were further assessed and integrated in a final model that is based on a modified version of the most successful logistic model from the first step. 22 , 42
4.1. Model outputs: α‐opic photoreceptors
The results from the regular logistic model approach as displayed in Figures 1 and 3 showed that melatonin suppression in general does not exhibit a clear sigmoidal dose‐response relationship with photopic illuminance apart from the 180 min bin, that did not contain any narrowband light condition and had a spectral variation (as judged from the melanopic EDI/illuminance ratio) that was quite limited as compared to the other bins. As such this bin is more easily dominated by photopic illuminance as compared to the other light exposure duration bins. The clearest sigmoidal dose‐response relationship occurs when light dose was quantified in terms of melanopic EDI. The RF approach confirmed that melanopic EDI is the most important predictor to describe melatonin suppression. In view of the key role that melanopsin plays in non‐image forming responses, 4 , 16 , 17 and in particular, the melatonin suppression response, 7 , 9 , 10 its predictive value comes as no surprise. The best fitting RF model described melatonin suppression by means of four significant predictors: melanopic EDI, light exposure duration, pupil dilation (y/n) and S‐cone‐opic EDI. Earlier work already discussed and explored that a potential interaction between melanopsin‐ and S‐cone‐photoreception could influence melatonin suppression under certain conditions. 7 , 16 , 51 Spitschan et al., 52 showed that at illuminances around 170 lx, a change in S‐cone activation of about 2 orders of magnitude has no effects on the melatonin concentrations across a 2 h light exposure duration. Although a potential role of saturation effects cannot be fully excluded at illuminances of 170 lux, 49 this result might also be interpreted as support for the idea that the S‐cone role in melatonin suppression might be illuminance dependent. Moreover, our analysis suggests that the S‐cone contribution to melatonin suppression might depend on light intensity but not so much on light exposure duration. Only for (photopic) illuminances below 21 lux the logistic fit based on S‐cone EDI outperformed the melanopic EDI‐based logistic model (see Figure 3). When exploring light exposure duration, the melanopic EDI‐based logistic model was the best logistic model across all light exposure durations (see Figure S1). Further research is warranted to evaluate the S‐cone‐opic and melanopic influence on melatonin suppression for different light intensities, especially for low (photopic) illuminances as well as for light exposure durations below 30 min (ie including flashes and intermittent light exposures). Melanopsin‐based photoreception is implicated in retinal adaptation of the human primary cone visual pathway, 53 and this might account for an S‐cone role that is illuminance and time‐of‐day dependent. Suggesting a substantial contribution from the photopic visual system in mesopic illuminance conditions (0.05–50 lux), which are prevalent in night‐time outdoor and street lighting scenarios.
4.2. Model outputs: exposure duration and pupil dilation
The final logistic model was based on the most successful single‐predictor logistic model (Equation 5, with x = melanopic EDI) and described melatonin suppression across the full data set in terms of three predictors; melanopic EDI, exposure duration and pupil dilation, see Equation (7). Because of the significance of light exposure duration as predictor for melatonin suppression, we also explored the combined effect of the different α‐opic EDIs and light exposure duration (ie using α‐opic EDI * light exposure duration as an additional predictor) within a separate RF analysis. However, such combined variables did not outperform the single variables as predictors, and therefore, such combinations were not further considered.
Our predictions (Figure 5) differed from those reported by Phillips et al. 49 but are in agreement with the results of Prayag et al. 7 The RF model yields a generalized prediction that is based on many studies with different experimental contexts and not necessarily provides a good description of every individual experimental context. We find an about 10‐fold difference in ED50 value for melatonin suppression between the shortest and longest light exposure duration, which is in good agreement with Brown. 16 In our analysis, we pooled existing published data and created a single response curve from it. This neglects the variability between different experimental contexts and designs. The earlier studies have explored individual dose‐response curves for very similar or identical experimental designs. However, the differences between the current and earlier findings are well within the range of inter‐individual differences in light sensitivity of melatonin suppression between the most sensitive and least sensitive individuals ED50 is reported to vary from about 350 to 6 lux (ie from about 181 to 3.1 lx melanopic EDI), respectively. 49 , 50
It is worth noticing the complexity involved when comparing % melatonin suppression data between studies, as % suppression very likely not only depends on the light exposure, but also on the method used to assess melatonin suppression, for example, plasma versus saliva assessments 54 , 55 and when the % suppression is based on when using AUC: which light exposure duration is assumed to apply for the AUC interval.
While pupil dilation was shown to be a significant determinant of melatonin suppression, pharmacologically dilated pupils are not a natural condition. Pupils will change and adapt to the environmental light levels. Nonetheless, a relevant observation was that the midpoint (ie ED50) of the melatonin suppression dose‐response relationship as predicted by the logistic model was affected by both pharmacological pupil dilation and the light exposure duration while the slope was not significantly affected by these two variables.
4.3. Limitations
To our knowledge, this is the first time that a machine‐learning approach has been used to evaluate which light exposure characteristics are the most important determinants of melatonin suppression. Within the data range used to build the model, the RF model predictions are expected to be more reliable as compared to the logistic model predictions. However, an RF model lacks any layer of abstraction for predictions outside its original data range. In order to assess the biological effects of the predictors across a wider data range, a logistic model was built based on the most important predictors of the RF model. The main difference between both models can be observed in Figure 4A,B (constrained to ‘undilated’ pupils, for comparison to ‘dilated’ pupils see Figure S2). As compared to the logistic model, the RF model gives a more accurate description of data within the data range that the model is based on. However, the RF model shows a large variability, which is most likely due to the different experimental contexts that were combined within the present analysis. It merits to be noted that the RF model does not take into consideration the dependency of the data points that are derived from the same study. The discrete jumps between the different light exposure durations in Figure 4A are artefacts of the RF analysis due to the discrete steps in exposure duration within the data set. On the other hand, the logistic model makes assumptions about some properties of the underlying physiology of the melatonin suppression response, predicting more gradual changes in melatonin suppression when changing exposure duration or melanopic EDI. For some of the light exposure durations, data availability was quite limited, and more research is needed to further improve the logistic model and its reliability.
Finally, we note that most melatonin suppression studies have used constant light exposures, while recent evidence suggests that temporal variations in illuminance (such as the 10 min breaks with bright or dim light during medium intensity nocturnal light exposure as described by Lee et al. 56 ) can also influence melatonin suppression. Also, certain dynamics have not been considered when pooling all data together. For instance, it is known that prior light history plays a role; the sensitivity of the circadian system to evening light is reported to be lower when the preceding light exposure is increased. 57 , 58 , 59 , 60 For short light exposure durations the differences between saliva and plasma derived % melatonin suppression (which we ignored in the present analysis) are expected to become more important and pronounced. 54 , 55 , 61 For long light exposure durations, the melatonin suppression and phase shifting effects of light can occur intertwined. Moreover, an assessment of melatonin suppression beyond the Syn‐off point 62 might no longer be useful or meaningful. And lastly, it is important to note that the present model allows for a 100% melatonin suppression (at infinite illuminance), independently of light exposure duration and light source. There is some indication for substantial melatonin suppression at short light exposure durations of ~20 min. 63 , 64 , 65 However, achieving 100% melatonin suppression might require a light condition that is extremely bright, and potentially unpleasant or even harmful to the eyes. For these reasons such extreme brightness may not be accessible experimentally. When comparing melatonin suppression data across studies it is important to keep this assumption (ie 100% melatonin suppression at infinite illuminance) in mind. Some studies report percentage suppression relative to the maximum suppression response observed within the experimental data set and not relative to the maximum suppression possible (see also our earlier comparison of our ED50 model predictions with the EC70 data from Prayag et al. 7 at the end of the Section 3.
4.4. Outlook and further research
In this paper, we present a simple mathematical model that can be used to assess the biological functionality of different light sources based on melatonin suppression data collected before March 2019. The predictions of this model should be tested against independent data sets as soon as these become available. However, the current model is a tool and useful starting point for the development of new hypotheses and to identify gaps in data availability. For instance, there is limited data available for longer light exposure durations (above 180 min), especially to assess the influence of spectral composition for these durations. Although the discovery of the ipRGCs has resulted in a large number of studies that explored different spectral compositions of light, the currently available data has a bias towards lower illuminances for narrowband sources, and to higher illuminances for non‐narrowband sources. Expansion of the available data range will enable for a better understanding of the influence of an individual photoreceptor on a particular NIF response. The strength and limitation of the current model with respect to its ability to describe the current melatonin suppression data set is shown in the supplementary data as a 3D plot (see supplementary data VideoS1). Despite the clear limitations, the model provides lighting practitioners with some initial and general guidance on what light levels, spectral compositions and durations to choose when attempting to control melatonin suppression and related non‐visual responses to light within integrative lighting solutions.
Supporting information
Data S1
Data S2
Video S1
ACKNOWLEDGEMENT
We would like to thank Drs. Domien Beersma, Ruta Lasauskaite, Jakob Löffler, Renske Lok, Philipp Novotny, Karin Smolders, Herbert Plischke and Katharina Wulff for their contributions to the dose‐response relationship work of the project Accelerate SSL Innovation for Europe (FP7‐ICT‐2013‐11‐619249).
Giménez MC, Stefani O, Cajochen C, Lang D, Deuring G, Schlangen LJM. Predicting melatonin suppression by light in humans: Unifying photoreceptor‐based equivalent daylight illuminances, spectral composition, timing and duration of light exposure. J Pineal Res. 2022;72:e12786. doi: 10.1111/jpi.12786
Giménez and Stefani should be considered joint first author.
Schlangen and Deuring should be considered joint senior author.
Funding information
This study is (partially) supported by funds from LightingEurope (Rue Belliard 205, B‐1040 Brussels, Belgium) and The European Union and the nationals contributing in the context of the ECSEL Joint Undertaking programme (2021‐2024) under the grant #101007319.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Houser KW, Esposito T. Human‐centric lighting: foundational considerations and a five‐step design process. Front Neurol. 2021;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CIE . CIE S 017/E:2020 ILV: International Lighting Vocabulary. 2nd ed. International Standard. 2020. https://cie.co.at/publications/ilv‐international‐lighting‐vocabulary‐2nd‐edition‐0 [Google Scholar]
- 3. Gooley JJ, Mien IH, St. Hilaire MA, et al. Melanopsin and rod‐cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012;32:14242‐14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spitschan M, Jain S, Brainard DH, Aguirre GK. Opponent melanopsin and S‐cone signals in the human pupillary light response. Proc Natl Acad Sci USA. 2014;2014:15568‐15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Souman JL, Tinga AM, te Pas SF, van Ee R, Vlaskamp BNS. Acute alerting effects of light: a systematic literature review. Behav Brain Res. 2018;337:228‐239. [DOI] [PubMed] [Google Scholar]
- 6. Cajochen C, Münch M, Szymon K, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311‐1316. [DOI] [PubMed] [Google Scholar]
- 7. Prayag AS, Najjar RP, Gronfier C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J Pineal Res. 2019;66:1‐8. [DOI] [PubMed] [Google Scholar]
- 8. Gooley JJ, Rajaratnam SM, Brainard GC, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405‐6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non‐rod, non‐cone photoreceptor system in humans. J Physiol. 2001;535:261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CIE . ISO 23539:2005(E)/CIE S 010/E:2004. Photometry ‐ The CIE System of Physical Photometry. International Standard; 2004. [Google Scholar]
- 13. CIE . CIE S 026:2018 CIE System for Metrology of Optical Radiation for ipRGC‐Influenced Responses to Light. International Standard; 2018. [Google Scholar]
- 14. CIE . ISO 11664‐2:2007(E)/CIE S 014‐2/E:2006. Colorimetry — Part 2: CIE Standard Illuminants for Colorimetry. International Standard; 2006. [Google Scholar]
- 15. Schlangen LJM, Price LLA. The lighting environment, its metrology, and non‐visual responses. Front Neurol. 2021;12:624861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown TM. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. J Pineal Res. 2020;69:1‐14. [DOI] [PubMed] [Google Scholar]
- 17. Hommes V, Giménez MC. A revision of existing Karolinska Sleepiness Scale responses to light: a melanopic perspective. Chronobiol Int. 2015;32:750‐756. [DOI] [PubMed] [Google Scholar]
- 18. Nowozin C, Wahnschaffe A, Rodenbeck A, et al. Applying melanopic lux to measure biological light effects on melatonin suppression and subjective sleepiness. Curr Alzheimer Res. 2017;14:1042‐1052. [DOI] [PubMed] [Google Scholar]
- 19. Bojkowski CJ, Aldhous ME, English J, Franey C, Poulton AL, Skene DJ, Arendt J. Suppression of nocturnal plasma melatonin and 6‐sulphatoxymelatonin by bright and dim light in man. Horm Metab Res. 1987;19:437‐440. [DOI] [PubMed] [Google Scholar]
- 20. Brainard GC, Lewy AJ, Menaker M, et al. Dose‐response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Res. 1988;454:212‐218. [DOI] [PubMed] [Google Scholar]
- 21. Wright KP, Myers BL, Plenzler SC, Drake CL, Badia P. Acute effects of bright light and caffeine on nighttime melatonin and temperature levels in women taking and not taking oral contraceptives. Brain Res. 2000;873:310‐317. [DOI] [PubMed] [Google Scholar]
- 22. Zeitzer JM, Dijk D‐J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitmore JN, French J, Fischer JR. Psychophysiological effects of a brief nocturnal light exposure. J Hum Ergol (Tokyo). 2001;30:267‐272. [PubMed] [Google Scholar]
- 24. Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18:801‐808. [DOI] [PubMed] [Google Scholar]
- 25. Wright KP, Czeisler CA. Absence of circadian phase resetting in response to bright light behind the knee. Science. 2002;297:571. [DOI] [PubMed] [Google Scholar]
- 26. Gronfier C, Wright KP, Kronauer RE, Jewett ME, Czeisler CA Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174‐E181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wirz‐Justice A, Kräuchi K, Cajochen C, Danilenko KV, Renz C, Weber JM. Evening melatonin and bright light administration induce additive phase shifts in dim light melatonin onset. J Pineal Res. 2004;36:192‐194. [DOI] [PubMed] [Google Scholar]
- 28. Herljevic M, Middleton B, Thapan K, Skene DJ. Light‐induced melatonin suppression: age‐related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237‐242. [DOI] [PubMed] [Google Scholar]
- 29. Hanifin JP, Stewart KT, Smith P, Tanner R, Rollag M, Brainard GC. High‐intensity red light suppresses melatonin. Chronobiol Int. 2006;23:251‐268. [DOI] [PubMed] [Google Scholar]
- 30. Revell VL, Skene DJ. Light‐induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiol Int. 2007;24:1125‐1137. [DOI] [PubMed] [Google Scholar]
- 31. Brainard GC. Sensitivity of the human circadian system to short‐wavelength (420‐nm) light. J Biol Rhythms. 2008;23:379‐386. [DOI] [PubMed] [Google Scholar]
- 32. Kozaki T, Koga S, Toda N, Noguchi H, Yasukouchi A. Effects of short wavelength control in polychromatic light sources on nocturnal melatonin secretion. Neurosci Lett. 2008;439:256‐259. [DOI] [PubMed] [Google Scholar]
- 33. Revell VL, Barrett DCG, Schlangen LJM, Skene DJ. Predicting human nocturnal nonvisual responses to monochromatic and polychromatic light with a melanopsin photosensitivity function. Chronobiol Int. 2010;27:1762‐1777. [DOI] [PubMed] [Google Scholar]
- 34. Santhi N, Thorne HC, van der Veen DR, et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012;53:47‐59. [DOI] [PubMed] [Google Scholar]
- 35. West KE, Jablonski MR, Warfield B, et al. Blue light from light‐emitting diodes elicits a dose‐dependent suppression of melatonin in humans. J Appl Physiol (1985). 2011;1985(110):619‐626. [DOI] [PubMed] [Google Scholar]
- 36. Chang A‐M‐M, Santhi N, St Hilaire M, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103‐3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brainard GC, Hanifin JP, Warfield B, et al. Short‐wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res. 2015;58:352‐361. [DOI] [PubMed] [Google Scholar]
- 38. Gabel V, Reichert CF, Maire M, et al. Differential impact in young and older individuals of blue‐enriched white light on circadian physiology and alertness during sustained wakefulness. Sci Rep. 2017;7:7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanifin JP, Lockley SW, Cecil K, et al. Randomized trial of polychromatic blue‐enriched light for circadian phase shifting, melatonin suppression, and alerting responses. Physiol Behav. 2019;198:57‐66. [DOI] [PubMed] [Google Scholar]
- 40. Nagare R, Plitnick B, Figueiro MG. Effect of exposure duration and light spectra on nighttime melatonin suppression in adolescents and adults. Light Res Technol. 2019;2001(51):530‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagare R, Rea MS, Plitnick B, Figueiro MG. Effect of white light devoid of ‘Cyan’ spectrum radiation on nighttime melatonin suppression over a 1‐h exposure duration. J Biol Rhythms. 2019;34:195‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeitzer JM, Khalsa SBS, Boivin DB, Duffy JF, Shanahan TL, Kronauer RE, Czeisler CA. Temporal dynamics of late‐night photic stimulation of the human circadian timing system. Am J Physiol Regul Integr Comp Physiol 2005;289:R839‐R844. [DOI] [PubMed] [Google Scholar]
- 43. Breiman L. Random forests. Mach Learn. 2001;45:5‐32. [Google Scholar]
- 44. Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer; 2009. [Google Scholar]
- 45. Strobl C, Boulesteix A‐L, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 47. Strobl C, Boulesteix A‐L, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brown T, Brainard G, Cajochen C, et al. Recommendations for Healthy Daytime, Evening, and Night‐Time Indoor Light Exposure. (2020) doi: 10.20944/preprints202012.0037.v1 [DOI]
- 49. Phillips AJK, Vidafar P, Burns AC, et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci USA. 2019;116:12019‐12024. doi: 10.1073/pnas.1901824116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cain SW, McGlashan EM, Vidafar P, et al. Evening home lighting adversely impacts the circadian system and sleep. Sci Rep. 2020;10:19110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown TM, Thapan K, Arendt J, Revell VL, Skene DJ. S‐cone contribution to the acute melatonin suppression response in humans. J Pineal Res. 2021;71:e12719. doi: 10.1111/jpi.12719 [DOI] [PubMed] [Google Scholar]
- 52. Spitschan M, Lazar R, Yetik E, Cajochen C. No evidence for an S cone contribution to acute neuroendocrine and alerting responses to light. Curr Biol. 2019;29(24):R1297‐R1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long‐term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12:191‐198. [DOI] [PubMed] [Google Scholar]
- 54. Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457‐466. [DOI] [PubMed] [Google Scholar]
- 55. McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Melatonin Rhythm in Human Plasma Saliva. J Pineal Res. 1987;4:177‐183. [DOI] [PubMed] [Google Scholar]
- 56. Lee S‐I, Kinoshita S, Noguchi A, et al. Melatonin suppression during a simulated night shift in medium intensity light is increased by 10‐minute breaks in dim light and decreased by 10‐minute breaks in bright light. Chronobiol Int. 2020;37:897‐909. [DOI] [PubMed] [Google Scholar]
- 57. Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 2006;21:394‐404. [DOI] [PubMed] [Google Scholar]
- 59. Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610‐3614. [DOI] [PubMed] [Google Scholar]
- 60. te Kulve M, Schlangen LJM, van Marken Lichtenbelt WD. Early evening light mitigates sleep compromising physiological and alerting responses to subsequent late evening light. Sci Rep. 2019;9:16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin supression by light is intensity dependent. J Pineal Res. 1989;6:149‐156. [DOI] [PubMed] [Google Scholar]
- 62. Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227‐236. [DOI] [PubMed] [Google Scholar]
- 63. Rahman SA, Wright KP, Lockley SW, Czeisler CA, Gronfier C. Characterizing the temporal dynamics of melatonin and cortisol changes in response to nocturnal light exposure. Sci Rep. 2019;9:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6‐11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data S2
Video S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


