Abstract
Background
The enteric nervous system (ENS) is an extensive neural network embedded in the wall of the gastrointestinal tract that regulates digestive function and gastrointestinal homeostasis. The ENS consists of two main cell types; enteric neurons and enteric glial cells. In vitro techniques allow simplified investigation of ENS function, and different culture methods have been developed over the years helping to understand the role of ENS cells in health and disease.
Purpose
This review focuses on summarizing and comparing available culture protocols for the generation of primary ENS cells from adult mice, including dissection of intestinal segments, enzymatic digestions, surface coatings, and culture media. In addition, the potential of human ENS cultures is also discussed.
Keywords: adult mouse, enteric glial cells, enteric neurons, primary ENS culture, protocol
1. INTRODUCTION
Many aspects of gastrointestinal physiology are under the control of the enteric nervous system (ENS), 1 which is an extensive neural network embedded within the wall of the entire gastrointestinal tract and operates, to a large extent, independently from commands arriving from the central nervous system. 2 Enteric neurons and enteric glial cells (EGCs) form the core of the ENS and are assembled into two interconnected ganglionated plexus layers. 3 While the cell bodies of enteric neurons are mainly localized within the submucosal plexus (SMP), 4 and between the circular and longitudinal muscle layers, within the myenteric plexus (MP), 1 , 2 , 3 , 5 , 6 EGCs can also be found in the mucosa and muscle layers. Different enteric neuron subtypes can be classified according to their morphological, electrical, neurochemical, and molecular properties. 7 , 8 , 9 EGC subtypes can be classified based on their morphology and location. 8 , 10 , 11
Owing to its location close to the contractile gut musculature and intimate association with other intestinal cell types, such as epithelial, immune, and stromal cells, the development of adequate methodology to study the ENS has been a major challenge to the field. 12 , 13 , 14 The generation of in vitro techniques has allowed the acquisition, expansion, and manipulation of ENS cells for a wide variety of research questions. They include (but are not limited to) primary ENS cell cultures in (semi‐)monolayer, stem cell‐derived 3D neurospheres, induced ENS cells from stem cell origin, and established cell lines. Initially, the investigation of the ENS using in vitro systems was established mostly in guinea pig gut, 15 , 16 , 17 , 18 , 19 because of the extensive knowledge on anatomy, physiology, and chemical coding of guinea pig neurons. 9 , 20 However, the lack of genetically modified strains, as well as higher costs for housing and longer breeding times, made the ENS of mice the more commonly used exemplary. 21 Primary ENS cells in early culture systems were extracted from neonatal tissue, 18 , 19 , 22 but advancements in culture conditions, such as media and addition of growth factors have enabled the culture of adult primary ENS cells. To culture mature neuronal cells can still be challenging for different reasons: (1) they do not take part in cell division, so they can only be maintained for a limited amount of time; (2) the risk of overgrowth by other non‐ENS contaminants, for instance, fibroblasts and smooth muscle cells; (3) possible overgrowth of EGCs when the main interest is enteric neurons; and (4) risk of contamination by luminal contents containing bacteria and fungi. In this review, we summarize and compare currently available protocols for the isolation and culture of primary enteric neurons and EGCs obtained from the adult mouse intestine. We also discuss the methodology to generate human ENS cell cultures.
2. GENERATION OF MURINE PRIMARY ENS CULTURES
The generation of primary ENS cell cultures occurs in different steps. Below we discuss the different possibilities for isolation of the target tissue, the available approaches for the dissociation of the target cells, and possible culture maintenance techniques for murine primary ENS cultures. We will discuss culture methodology for ENS cultures focused on the isolation of neuronal cells (termed “ENS cultures”, as these cultures never consist of neurons solely) and for ENS cultures focused on the isolation of glial cells (termed “EGC cultures”, as they can be more purified).
2.1. Dissection and isolation
Depending on the research questions, different regions of the gut can be used (Figure 1A). In the small intestines of adult mice, for instance, the ENS meshwork is loosely arranged, with longer connecting nerve fiber strands resulting in a less compact structure.
FIGURE 1.
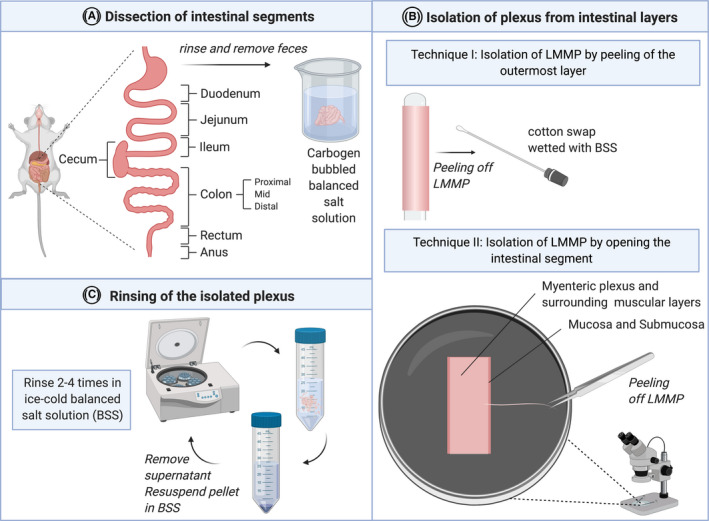
Schematic representation of the steps involved in the dissection of tissue to isolate ENS cells. A Tissue dissection from the adult mouse, highlighting the different segments of the intestines. Isolated segments are cleaned and kept viable in BSS. B Two different isolation techniques for the LMMPs. Technique I involves the isolation of LMMPs from unopened segments and technique II includes opening intestinal segments and isolating the LMMP from the underlying mucosa and submucosa. C Rinsing of isolated tissue pieces by centrifugation in ice‐cold BSS and resuspension of the pellet. Abbreviations: BSS = basal salt solution; LMMP = longitudinal muscle myenteric plexus. Created with BioRender.com.
In the colon, however, specifically in the proximal colon, ENS organization is more compact and dense within the MP, making the separation of the muscle layer from the underlying submucosal layer rather difficult, as observed in rat. 23
The organ of choice can be isolated from the animal by sterilizing and opening the abdominal wall, without injuring the underlying structures to expose the gastrointestinal tract, lifting the intestine with forceps, and cutting through the mesentery with scissors. After removing the intestine(s) from the animal, it is essential to keep the tissue viable by continuously maintaining adequate levels of ionic and osmotic balance. 24 This can be achieved by placing it immediately in a balanced basal salt solution (BSS) supplemented with carbohydrates (Figure 1A). Different basal salt solutions have been used including Hank's balanced salt solution (HBSS), 25 Krebs, 26 , 27 Krebs‐HBSS, 28 Krebs‐Ringer solution, 29 and DPBS, 30 , 31 generally containing the same essential constituents: calcium, magnesium, potassium, sodium, and phosphate. The function of BSS is fivefold: (I) the maintenance of intra‐ and extracellular osmotic balance during washing and dilution steps; (II) the preservation of intracellular water and ion concentrations essential for healthy cell physiology; (III) buffering the medium to maintain a physiological pH range; (IV) in case of supplementation with carbohydrates it provides a principle energy source; and (V) the provision of oxygen in case of bubbling with an O2/CO2 gas mixture. 24
Depending on the type of cell culture, different plexus layers may be prioritized. For example, for ENS cultures, composed of both neurons and EGCs, longitudinal muscle containing the myenteric plexus (LMMP) preparations are commonly used as the MP accommodates a larger number of neurons than the SMP. 25 , 26 , 27 , 29 , 32 However, it must be noted that SMP neurons and EGCs have different properties than MP neurons and EGCs. 33 , 34 , 35 Cultures consisting solely of MP neurons might thus not adequately represent the properties of SMP neurons, which has to be taken into consideration. For cultures focused on the isolation of EGCs, the submucosa and mucosa, as well as the LMMP, have been used as tissue sources, 30 , 31 indicating that either tissue source can be used depending on the research question.
Because the layers of the intestinal walls in rodents are very thin, soft, and delicate, the dissection and isolation of the ENS require careful tissue manipulation. Following the removal of debris and feces from the intestinal segments, the isolation of the plexus layers is usually performed by either of two techniques. Technique I includes cutting the intestine in small pieces and making a gentle incision along the line where the mesentery was attached after placing the segment on a small rod or syringe, followed by gentle stroking with a wetted cotton swap along the entire segment to peel away the LMMP (Figure 1B). Technique II involves longitudinal opening of the intestinal segment along the mesenteric line and then peeling off the LMMP (Figure 1B). In both techniques, it is crucial to cut along the mesenteric line, as the dissection of the MP is hindered here due to the presence of ducts and blood vessels. 36 It should be noted that technique II requires more time than technique I, but allows for a more precise separation of the muscularis externa from the lamina propria mucosa. The majority of the reviewed protocols utilized isolation technique I 26 , 27 , 28 , 29 compared to technique II. 25 , 31 Some modifications have been made on both techniques. For instance, Wahba et al. described a technique involving stretching and pinning of the unopened segment on a Sylgard plate instead of placing it on a rod, followed by scraping off the LMMP from the entire length of the intestinal segment with curved forceps. 29
In the protocol used by Verissimo et al. (2019) for EGC culture, technique II is adapted by only removing the mucosa to obtain ENS cells from the SMP and MP. 31 Wang et al. (2018), focusing on the isolation of EGCs from the mucosa, submucosa, and circular muscle, used technique I to separate the LMMP, but discarded it and used the remaining tissue containing the SMP and lamina propria for further processing. 30 Following the isolation of the plexus, dissociated cells are cleaned and separated from remaining debris by rinsing and centrifuging three to four times with BSS, a step shared by all analyzed protocols (Figure 1C).
2.2. Cell dissociation
The dissociation of ENS cells for culturing can be achieved mechanically and/or enzymatically. Mechanical dissociation involves either cutting, sieving, or triturating the isolated tissue and can be much faster than enzymatic digestion. It is suitable for large amounts of soft tissue, but can lead to tissue damage and lower yield of viable cells. 37
Enzymatic digestion of cell‐cell adhesion components using proteases is the most common step for cell isolation from adjacent tissues. Proteases are distinctive in their molecular specificity, and different tissues therefore require different enzymatic activity depending on their matrix composition. 38 In addition, DNase can be used to digest the DNA that is released due to the digestion of connective tissue and smooth muscle cells, as it can form long strings that tie up cells. The most common enzymes used in the protocols analyzed in this review were collagenases, trypsin, DNase, or a combination thereof (Table 1). Only the study by Wang et al. made use of non‐enzymatic digestion (EDTA), which is also the only study using the submucosa, mucosa, and circular muscle instead of the LMMP.
TABLE 1.
Different enzymatic digestion conditions included in the reviewed protocols. Concentrations are shown as described in the protocols, temperatures are indicated in Celsius, incubation times are presented in minutes. Missing values indicated by (‐).
| Protocol source | Enzyme | Concentration | Serial/combinatory | Incubation time | Temperature |
|---|---|---|---|---|---|
| Zhang and Hu. 2013 25 |
Collagenase IV Trypsin |
1 mg/ml 0.05% |
Serial |
15 min 10 min |
37°C water bath shaker |
| Smith et al. 2013 27 |
Collagenase II Trypsin |
1.3 mg/ml 0.05% |
Serial |
60 min 7 min |
37°C water bath shaker |
| Lowette et al. 2014 28 |
Collagenase Protease Albumin |
14.67 mg/ml 10 mg/ml 5% in PBS |
Combinatory | 8 min | 37°C |
| Wahba et al. 2016 29 |
Collagenase IV Trypsin |
1 mg/ml 0.05% |
Serial |
15 min 10–15 min |
37°C water bath manual rotation |
| Brun and Akbarali. 2018 26 |
Collagenase I DNase I |
0.5 mg/ml 0.5 mg/ml |
Serial | 35 min | 37°C water bath shaker |
|
Verissimo et al. 2019 31 EGCs |
Collagenase II DNase I |
‐ | Combinatory | 60 min | 37°C |
|
Wang et al. 2018 30 EGCs |
Non‐enzymatic digestion | ‐ | ‐ | ‐ | ‐ |
Next to the choice of the particular enzyme(s) and their concentration, also the temperature and time of incubation are crucial determinants of successful enzymatic digestion. In order to allow the enzymes to reach their targets, the removal of fat tissue and unwanted muscle layers, and a sufficient level of mechanical dissociation prior to the digestion step greatly influence the efficacy of enzymatic dissociation of ENS cells. Together, these factors determine how well the tissue is dissociated and the subsequent cell viability. 39 After dissociation, enzymes should be removed from the samples before seeding with gentle centrifugation steps to ensure that no further damage is exerted on the cells. 37
One advantage of using enzymatic digestion for the isolation of ENS cells is the absence of collagen within the MP. The use of collagenase therefore allows almost complete digestion of surrounding muscle‐ and connective tissues while maintaining the structure of the ENS. 32 , 40 , 41 However, over‐digestion, thus destroying the extracellular matrix (ECM) and endogenous structure completely, will lead to lower viable cell yield.
Collagenase cleaves the peptide bonds between neutral amino acids and glycine, a sequence commonly found in collagen. 39 Different types of collagenase exist, each recommended for different types of tissue. Collagenase exists in crude and highly purified form, whereas the crude collagenase mixtures usually contain a mixture of collagenase, and other enzymes with tryptic activity, enabling the digestion of other ECM components. 39
Trypsin is a serine protease that cleaves peptide bonds at the C‐terminal end of positively charged side chains of lysine or arginine. It is innately found in the pancreas of most vertebrates and aids the cleavage of dietary proteins into peptides. 39 Trypsin has the strongest relative digestive power and is one of the most specific proteases known, 38 making it less effective for tissue dissociation due to its decreased selectivity for extracellular proteins. 38 The tryptic activity has to be neutralized by either serum or trypsin inhibitors to reduce residual activity after washing. 37 It is important to note that the use of trypsin can alter excitability of enteric neurons. 42 , 43 , 44 Neither of the protocols included in this review used trypsin alone for cell dissociation. It is mostly combined with collagenase to increase the specificity for ECM proteins, allowing the breakup of enteric ganglia and release of individual neurons. 29
Deoxyribonuclease I (DNase I) is an endonuclease that cleaves phosphodiester linkages in single‐ and double‐stranded DNA and has a less aggressive digestive capacity. It is often included in tissue dissociation mixtures to digest nucleic acids leaking into the dissociation medium, without damaging the intact cells in order to decrease viscosity and improve cell yield. 38 Caution is warranted when combined with other proteases, such as trypsin, which proteolytic activity inactivates other enzymes. Therefore, DNase I and trypsin should not be added together, but serially after washing. 37
Both serial and combinatory enzymatic digestion steps were used in the reviewed articles (Table 1), and the most common combination used was a type of collagenase together with trypsin, 25 , 27 , 29 followed by a collagenase and DNase I. 26 , 31 All enzymatic digestion steps were performed at 37°C.
2.3. Culture maintenance
It is essential for neuronal survival in vitro to provide a coating substrate to facilitate adhesion. 45 , 46 Prior investigations showed that ganglia in the adult (mammalian) MP are surrounded by an ECM arrangement of collagen IV, laminin, fibronectin, and proteoglycans. 47 , 48 It has been shown that poly‐D‐lysine coated coverslips can support the attachment of primary neurons and neural precursor cells, and specifically enteric neurons, when combined with fibronectin or laminin. 49 All reviewed studies reported to coat coverslips or wells themselves, although commercially available coated coverslips and culture plates are currently readily available. The most used coating substrates for enteric neuronal cultures include Matrigel, laminin, poly‐D‐lysine, and poly‐L‐lysine. However, the choice for the adequate coating substrate for ENS and EGC cultures also greatly depends on experimental setup and purpose. Coating is especially beneficial for downstream microscopic analysis, as cells less likely adhere to glass, the preferred material for most microscopic analyses, than to culture‐treated plastics. Care must be taken to ensure that the chosen coating does not interfere with downstream analyses, such as second‐harmonic generation imaging of cells grown on a collagen‐containing matrix, like Matrigel.
Poly‐D‐lysine and poly‐L‐lysine are chemically synthesized ECM molecules, and the most commonly used coating substrates to aid cell adhesion in pre‐treated tissue culture surfaces. The structure and molecular weight of Poly‐D‐lysine make it ideal for neuronal culture applications since this isoform is less readily digested by extracellular proteases. 50
Laminin is a glycoprotein component of the basement membrane which modulates cellular functions including attachment, spread, growth, and mobility by binding to itself and other matrix components. 51 Laminin is often used as a coating substrate in neuronal cultures since many neuronal cells express specific laminin‐binding proteins. 52 It is also part of the basement membrane and ECM surrounding the ENS, 48 as well as in the muscle layers of the adult mouse gut. 31 In enteric neuronal development, laminin also stimulates neurite outgrowth. 53
Fibronectin is an ECM glycoprotein produced by fibroblasts and is involved in cell migration and adhesion during ENS development. It is frequently used in vitro to enhance cell attachment and proliferation. Similar to laminin, fibronectin is part of the basement membrane in the MP. 48
Matrigel is a commercially available soluble basement membrane extract containing predominantly laminin, fibronectin, and proteoglycans. Its adherent matrices simulate the cells’ ECM environment more closely due to the mixture of several matrix components, 47 , 48 as is found in ECM in vivo. The usage of Matrigel in neuronal culture models is predominantly optimal for neural stem‐ or progenitor cells, 54 , 55 where it has been proven to enhance survival and differentiation of neural crest cells from human and mouse. 56 Zhang et al. 2013 also achieved the highest efficacy in attachment and growth of primary enteric neurons, and differentiating and neural stem/progenitor cells with Matrigel‐coated coverslips. 25 Similarly, Wahba et al. 2015 reached the highest cell density with Matrigel‐collagen coatings, compared to other single and double coating substrates. 29 The most commonly used double coatings are poly‐lysine and laminin, with poly‐D‐lysine being the prioritized isoform 27 , 28 , 30 , 31 over poly‐L‐lysine. 26 Matrigel coatings were applied in two ENS cultures. 25 , 29 Coating substrates in EGC cultures included poly‐D‐lysine‐laminin 30 and poly‐L‐lysine‐laminin. 31
Furthermore, the success of a cell culture experiment is greatly influenced by the choice of the cell culture medium , and the constituent cell types largely determine which medium is required. Basal culture media dispense a source of energy, maintain beneficial ionic strength, pH concentration, and take up debris and metabolites from cultured cells. 57 Essential components usually include inorganic salts, glucose or other carbohydrates, essential amino acids, vitamins (B complex), and phenol red as a pH indicator. 58 Besides these essential components, antibiotics and antimycotics are included to prevent microbial contamination. Despite the availability of specialized neuronal media (Neurobasal‐A, Gibco), standard DMEM medium supplemented with the F12 nutrient mix (Gibco) 25 , 28 , 29 , 30 , 31 and cell type‐specific supplements are most frequently used in murine primary ENS cultures.
The addition of serum to the basal culture media is used to provide signals for survival, growth, and differentiation. Generally fetal bovine serum (FBS), 26 , 27 , 28 , 29 , 30 , 31 in concentrations ranging from 1 to 10%, was used for ENS cultures. Only one protocol uses chick embryo extract (CEE) instead of FBS. 25 FBS is the most widely used form of serum due to low contents of complement factors and immunoglobulins. 59 However, serum‐free media and supplements allow culturing of neurons at low density, which enables the study of individual neurons and their projections. 60 Next to serum, certain growth factors, for example, nerve growth factor (NGF) and glial cell line‐derived neurotrophic factor (GDNF), and hormones are often added as well. These can be supplied individually or as part of commercially available supplement mixtures such as B27, N2, and G5. Despite the supplements, no major differences have been observed between the use of media for ENS cultures compared to EGC cultures. Table 2 gives a detailed overview of the culture protocols used in the reviewed studies, including culture type, isolation techniques, enzymatic digestion, coating substrates, and culture media.
TABLE 2.
Comparison of murine adult ENS primary culture protocols, with a detailed overview of mouse strains, age, dissection techniques, dissociation methods, coating agents, and culture medium specifications. Abbreviations: HBSS = Hank's balanced salt solution; FBS = fetal bovine serum.
| Source | Culture type | Mouse strain and age | Dissection technique, intestine segment, and BSS | Cell dissociation | Coating substrates and plating | Medium and maintenance |
|---|---|---|---|---|---|---|
|
Lowette et al. 2014 28 |
ENS culture |
Adult C57BL/N6 Mouse (8–9 weeks) |
Technique I Ileum Krebs‐HBSS |
Digestion solution: Collagenase (14.67 mg/ml), protease (10 mg/ml), albumin (5% in PBS). Incubation time: 8 min at 37°C. Stopped with Krebs, 10% FBS at RT. |
Poly‐D‐lysine‐laminin double coating: Poly‐D‐lysine hydrobromide (0.5 mg/ml in borate buffer). Laminin (20 µg/ml). |
Complete medium: DMEM‐F12 (1:1), 10% FBS, 1% glutamine, 0.5% pen/strep. Serum‐free medium: DMEM‐F12, with NGF (0.05%), N2 (0.2%), G5 (0.2%). Medium replaced by serum‐free medium after 24h. |
| Zhang and Hu. 2013 25 |
ENS culture |
Adult Mouse |
Technique II Complete small intestine HBSS |
Collagenase digestion medium: 1 mg/ml collagenase IV, 0.5 mM CaCl2, 10 mM HEPES in HBSS. Trypsin Digestion medium: 0.05% trypsin with 0.53 mM EDTA in HBSS. Digestion neutralizing medium: 500 U DNase I, 1 mg/ml BSA in DMEM/F12 medium. |
Matrigel coating: BD GF‐reduced Matrigel: 60% laminin, 30% collagen IV, 8% entactin, diluted 1:2000 in ice‐cold DMEM/F12 medium. Density of seeding: 1 × 105/cm2; with enteric neuronal culture medium. |
Enteric neuronal culture medium: DMEM/F12 medium (39.5 ml), chick embryo extract (7.5 ml), penicillin/streptomycin (100×, 0.5 ml), gentamicin (500×, 100 µl), amphotericin (100×, 0.5 ml), N2 (100×, 0.5 ml), B27 (50×, 1 ml), glutamine (100× 0.5 ml), FGF‐b (10 µg/ml, 50 µl), EGF (10 µg/ml, 100 µl), heparin (0.2%, 5 µl). Medium change every day. |
| Smith et al. 2013 27 | ENS culture | Adult Mouse |
Technique I Ileum Krebs solution |
Collagen digestion solution: 13 mg collagenase type II, 3 mg BSA in 10 ml carbogen‐bubbled Krebs. Incubation time: 60 min at 37°C. Trypsin digestion solution (0.05% trypsin): 5 ml of 0.05% trypsin solution. Incubation time: 7 min at 37°C in shaking water bath. Rinse medium: F12 medium with 10% FBS and antibiotic/antimycotic: to 500 ml bottle F12 media add 50 ml FBS and 5 ml antibiotic/antimycotic 100× liquid. |
Poly‐D‐lysine‐laminin double coating: 1 ml/25cm2 poly‐D‐lysine: 80 µl poly‐D‐lysine stock/CS. 5 µg/cm2 laminin: 200 µl stock/CS. |
Neuronal culture medium: Neurobasal‐A medium, B27, 2 mM L‐glutamine, 1% FBS, 10 ng/ml GDNF and antibiotic/antimycotic 100× liquid. Change half of medium every 2 days. |
|
Wahba et al. 2016 29 |
ENS culture |
CD−1 mouse (4–6 month) |
Technique Ib Complete small intestines Krebs‐Ringer Solution |
Collagenase digestion solution: 1 ml per mouse: 1 mg/ml collagenase IV, 0.5 mM CaCl2, 10 mM HPES in HBSS. Incubation time: 15 min at 37°C in water bath with manual constant rotation once every 5 min. Trypsin digestion solution: 0.05% trypsin, 0.54 mM EDTA in HBSS, 1 ml/mouse. Incubation time: 10 min in 37°C water bath with manual rotation and inverting of tube every 5 min once. |
5 different culture substrates tested: Poly‐D‐lysine (0.1 mg/ml, 50 µl, overnight at RT), Collagen (100 µg/ml, 50 µl, overnight at RT); Matrigel (MG) (1.8 mg/ml, 100 µl: 15 µl of MG in 85 µl culture media, 2h at 37°C); Poly‐D‐lysine /MG mix (Poly‐D‐lysine: 0.03 mg/ml 50 µl; MG: 0.5 mg/ml 15 µl in 85 µl media mix, 2h at 37°C); Collagen/MG mix: Collagen: 50 µl of 33µg/ml; MG: 0.5 mg/ml, 15 µl in 85 µl culture medium, 2h at 37°C). Wash once with pre‐warmed (37°C) HBSS. |
Complete medium: DMEM/F12, 2% (v/v) FBS, 100 U/ml pen, 100 µg/ml strep, 1 × B27, N2, 7.2 mg/L uridine triphosphate, 50 mg/L gentamycin. Medium change every day. |
|
Brun and Akbarali. 2018 26 |
ENS culture | Adult C57BL/6 J mice |
Technique I Ileum Krebs solution |
Neuronal digestion solution: 13 mg collagenase type II + 3 mg BSA in 10 ml RMPI medium 1640. Incubation time: 15 min at 37°C in water bath under shaking conditions. 0.05% Trypsin solution: 1 ml warmed 0.25% trypsin in 4 ml warmed RPMI medium 1640. Incubation time: 7 min at 37°C water bath shaking. Digestion stopped with Krebs solution. |
Poly‐D‐lysine‐laminin double coating: Dilute poly‐D‐lysine stock (0.01% in ddH2O) and take 150 µl stock/cm2. Incubate at RT for 30 min. Dilute laminin stock to 5 µg/ml with ddH2O, take 100 µl of laminin solution/cm2. Incubate at RT for 1h. |
Complete neuron medium: Neurobasal‐A medium/B27, 1 × L‐glutamine (2 mM), Pen/strep (100 U/ml and 100 µg/ml), sodium pyruvate (1 mM), 1%FBS, 10 ng/ml human recombinant GDNF. 150 µl of cell suspension in 1 ml complete medium. Medium change every 2 days. For electrophysiological studies: add 850 µl of cell/cm2 to the coated CS. |
| Verissimo et al. 2019 31 | EGC culture | Adult mouse |
Technique II Complete colon PBS with Pen/strep and fungizone |
Enzymatic dissociation: Collagenase II (Gibco), DNase I (Sigma chemical). 60 min at 37°C. Mechanical dissociation and centrifugation 2×. |
Poly‐L‐lysine‐laminin and Fibronectin single coating: Poly‐L‐lysine‐laminin substrate: 50 µg/ml in acid buffer (20 mM sodium acetate in 2 mM Calcium chloride), incubated with CS at least 12h at 37°C, 3× wash with PBS. Fibronectin substrate: 50 µg/ml in DMEM/F12, incubated with CS for 1h at 37°C, removed from wells and dried. Re‐plated after 3 days in culture to distribute cells homogenously. |
Medium: DMEM/F12, 2 mM glutamine, 3 mM sodium bicarbonate (NaHCO3), 0.5 mg/ml pen/step, 10%FBS, 2% chick embryo extract. |
| Wang et al. 2018 30 | EGC culture |
Adult C57BL/6 mice (8 weeks) |
Technique I, but isolation of mucosa, submucosa, and circular muscle Proximal small intestines DPBS w/o Ca2+ or Mg2+ |
Non‐enzymatic digestion: Sequential HEPES‐buffered EDTA incubations and gentle trituration. Isolation solution: EDTA/HEPES/DPBS dissociation solution (500 ml): sterile DPBS (w/o Ca2+ and Mg2+) to make final solution of 10 mM HEPES and 5 mM EDTA. Use 490 ml DPBS, 5 ml 1 m HEPES buffer, 5 ml 0.5 m EDTA stock. Cell recovery solution: commercially available. |
Poly‐D‐lysine ‐laminin double coating: 1 mg/ml poly‐D‐lysine stock diluted 1:10 in sterile tissue culture grade water. Final concentration: 100 µg/ml; 2 ml per well in 6‐well plates; Incubate 1 h. Dilute laminin (0.5 mg/ml stock) 1:50 in DPBS. final concentration: 10 µg/ml; 1 ml per 6‐well plate. Incubate 2h at 37°C, then remove laminin wash gently 3× with sterile DPBS. |
Glia cell resuspension media: 44.5 ml DMEM/F12, 5 ml FBS (10%), 500 µl Pen/Strep (100 U/ml Pen, 100 µg/ml Strep), 20 µl Gentamicin (20 µg/ml). Glial cell growth medium: 44 ml DMEM/F12, 5 ml FBS (10%), 500 µl Pen/strep (100 U/ml pen, 100 µg/ml strep), 20 µl Gentamicin (20 µg/ml), 50 µl GDNF (10 ng/ml), 500 µl L‐glutamine (2 mM). 200 µl cell suspension onto each CS. Complete medium change every day. |
2.4. Cell density, viability, and functionality
Although the protocols show similarities in the different steps of establishing culture systems (Table 2), the lack of representative outcome parameters regarding viability, functionality, and the presence of subtypes represents a significant limitation in our comparison.
2.4.1. ENS cultures
Zhang and Hu (2013) proposed a standardized protocol for the isolation of primary ENS cells, with the extension of generating neurospheres starting from the same protocol. Briefly, the intestines of 8 week‐old mice were isolated by technique II described above (Figure 1B), and enzymatic digestion was performed with collagenase, followed by trypsin. After 1–2 days in culture, the cells attached to the substrate and developed longer processes. Morphological changes could be observed daily, and the attempts to form plexuses over a layer of glial cells, resembling ganglionic structures, were seen after 5–7 days in vitro. Staining with the neuronal marker βIII tubulin validated the presence of enteric neurons in these cultures. However, no data on the density, count, or functionality of cultured cells were shown in this study, nor in other studies using the same protocol. 61 , 62 Brun and Akbarali (2018) developed a protocol for the isolation of ENS cultures from the ileum of adult mice, providing two variations depending on the subsequent use of the cell culture: (1) a gentler digestion method involving only collagenase II is proposed for suspension cultures, which were used to assess the phenotype of neurons after stimulation; (2) extensive digestion for electrophysiological studies, involving serial treatment with collagenase II and trypsin. However, no results regarding cell viability, density, count, and functionality were reported for this protocol. 63 , 64
The protocols by Smith et al. and Wahba et al. both assessed functionality of the cultured ENS cells by calcium imaging after one week in vitro, confirming functionally viable cells in culture, 29 identifying two electrophysiological distinct neuronal subtypes with a current clamp; synaptic (S‐) and after‐hyperpolarization (AH‐)neurons. 27 Both protocols are based on dissection technique I and performed serial digestion with collagenase and trypsin, while Wahba et al. used DMEM/F12 and Smith et al used Neurobasal‐A medium supplemented with B27. Moreover, Wahba et al. tested a variety of coating substrates (Table 2). Immunofluorescence staining after 5–7 days in culture for DAPI, βIII tubulin, neuronal subtype markers, and the glial cell marker GFAP confirmed the presence of distinctive neurochemical subtypes (nNOS, VIP, and ChAT) in culture established by Wahba et al., and the presence of EGCs and enteric neurons in both studies. In the study by Wahba et al., the cellular density was found to be the highest when using collagen‐Matrigel double coatings, whereas Smith et al. kept the cell density low to avoid contamination, reaching 10%–40% confluence after one day in culture with Poly‐D‐lysine‐aminin coatings.
The cell culture approach developed by Smith et al. has been adopted in several other studies, investigating viability, and functionality of enteric neurons in response to fungal extracts 65 and morphine, 66 as well as in studies using optogenetics analysis. 67 Furthermore, the same protocol has been used to study EGCs, 68 to generate other protocols for EGC cultures 30 and was used together with intestinal stem cells and epithelial cells in co‐culture models. 69
The protocol for primary ENS cultures used by Lowette et al. (2014) to investigate the role of corticosterone in the ENS, was similar to the study from Smith et al. in most steps, except for the enzymatic digestion, in which a combinatory digestion mixture of collagenase and proteases was used (Table 1). However, no data regarding viability, heterogeneity, or density of ENS cells in culture was described and can therefore not be compared. 28
2.4.2. EGC cultures
The protocols for EGCs cultures showed similarities by using DMEM/F12 culture media and serial enzymatic digestion with collagenase and DNase. The protocol by Wang et al. (2018), however, applies an entirely different approach by isolating the EGCs from the submucosa and lamina propria, instead of extracting them from the LMMP, followed by enzyme‐free digestion using EDTA incubation. 30 Flow cytometry confirmed the presence of more than 95% EGCs after three days in culture. Yields of about 40,000–100,000 cells morphologically consistent with EGCs in vivo were confirmed after 1–3 days in culture on poly‐D‐lysine‐laminin substrate and expressed the main glial cell markers such as S100β, GFAP, and Sox10. Verissimo et al. (2019) established a protocol for the isolation of primary EGCs from both the SMP and MP to investigate the effects of laminin and environmental cues on the differentiation of EGCs into neurons. 31 Immunofluorescence staining for glial‐ (GFAP and Sox10) and neuron‐specific markers (βIII‐tubulin) was employed to investigate the proportions of cells expressing one or both markers. The authors showed increased numbers of cells expressing both glial markers on the control substrate fibronectin and decreasing numbers of cells expressing both glial and neuronal markers on laminin substrate after seven days in vitro. Cell viability and activity were not assessed, and cultures were maintained for 21 days. Although comparing the ENS culture protocols in terms of outcomes remains challenging, murine primary ENS cultures have been used for many different research applications (a non‐exhaustive list of experimental methods used in ENS cultures is shown in Table 3).
TABLE 3.
Key applications of murine ENS cultures.
| ENS culture type | Application |
|---|---|
| ENS culture | Phenotypic assessment (immunofluorescence) 25 , 26 , 27 , 29 , 61 , 62 , 63 , 64 , 65 , 66 , 69 |
| Assessment of gene expression (RNA) 63 | |
| Assessment of protein levels/alterations 61 | |
| Cytokine measurements 69 | |
| Cytotoxicity 62 | |
| Viability 65 | |
| Activity (electrophysiology) 26 , 27 , 66 , 67 | |
| Activity (calcium imaging) 28 , 65 , 67 | |
| Optogenetics 67 | |
| Co‐culture 69 | |
| EGC culture | Phenotypic assessment (immunofluorescence) 30 , 31 , 68 , 112 |
| Assessment of gene expression (RNA) 31 | |
| Assessment of protein levels 68 | |
| Cytotoxicity 112 | |
| Viability 112 | |
| Activity (calcium imaging) 30 |
3. GENERATION OF HUMAN ENS CULTURES
All protocols for the isolation and culture of human primary ENS cells to date focus on the isolation of EGCs 70 , 71 , 72 , 73 (Table 4). Cells are generally isolated from the MP of small intestine, 70 , 71 , 72 , 73 , 74 but SMP 73 and also colon have been used as well. 73 , 74 Technique II is used for tissue dissection 32 , 70 , 71 , 72 , 73 , 74 as the size of the tissue renders the use of technique I inadequate. The human ENS is bigger and more complex, therefore, requiring longer digestion times for cell dissociation, which has been tested in direct comparison with murine and rat ENS. 74 Most protocols use a combination of protease and collagenase 71 , 72 , 73 , 74 for tissue dissociation, while Grubišić et al. have successfully used a serial digestion with liberase (a blend of collagenase and protease) and DNase. The primary human EGCs are either grown in a culture dish without coating, 70 , 71 , 72 or on glass slides coated with gelatin, 74 or double coated with laminin and poly‐D‐lysine. 73 In all protocols, the human EGCs are maintained on DMEM‐F12 medium with 10% fetal calf serum (FCS) 71 , 72 , 74 or FBS 70 , 73 and antibiotic/antimycotic, without additional (glia‐specific) supplements. Instead, for purifying the culture of human primary EGC, magnetic beads linked to specific targets, such as Thy‐1.1 71 , 72 or D7‐Fib 70 , 73 are often used to eliminate fibroblasts from the culture. With this method, Liñán‐Rico et al. and Grubišić et al. reached a cell enrichment of 10,000–20,000 fold. Soret et al. took a different approach and switched the medium after 48h to a DMEM‐based medium (not DMEM‐F12) without an additional purification step. They report a purity of around 80% EGCs and 20% other cell types, determined by immunohistochemistry.
TABLE 4.
Comparison of human adult ENS primary culture protocols, with a detailed overview of donor material, dissection techniques, dissociation methods, coating agents, and culture medium specifications. Abbreviations: FCS = fetal calf serum; BSA = bovine serum albumin; HBSS = Hank's balanced salt solution; FBS = fetal bovine serum.
| Source | Culture type | Donors | Dissection technique, intestine segment, and BSS | Cell dissociation | Coating substrates and plating | Medium and maintenance |
|---|---|---|---|---|---|---|
| Cirillo et al. 2011 72 and Turco et al. 2014 71 | EGC culture | Colorectal cancer patients |
Technique II Small intestine Krebs |
Digestion solution: Protease (1 mg/ml), collagenase (1.25 mg/ml). Incubation time: 30 min at 37°C. |
No coating. |
Medium: DMEM‐F12, 10% heat‐inactivated FCS, 1% antibiotic‐antimycotic solution. Grow for 3–4 weeks and use Thy−1.1 antibody‐coated magnetic beads while passaging to eliminate fibroblasts and smooth muscle cells. Perform purification twice. |
| Soret et al. 2013 74 | EGC culture | Patients with colorectal cancer, polyps, Hirschprung, fistula, sigmoiditis, volvulus, or pancreatic adenoma |
Technique II Jejunum, ileum, colon (normal segments of resection margin) Krebs |
Digestion solution: 250 µl protease (type I from bovine pancreas; stock solution: 5 g/l), 250 µl collagenase (Clostridium histolyticum; stock solution:20 g/L), and 400 µl BSA (stock solution: 50 g/L) in 5 ml of complete medium. Dissociated in a GentelMACS dissociator procedure A−01: 25s at cycles of 400 rpm clockwise and 300 rpm counter clockwise, once before and once after incubation in digestion solution. Incubation time: 45 min at 37°C on a rocker. |
Gelatin coating: gelatin type A from Porcine skin 90–110; 0.5% in PBS. |
Complete medium: DMEM/F12 supplemented with 10% heat‐inactivated FCS, 100 IU/ml penicillin, 100 µg/ml streptomycin, 1.1 µg/ml amphotericin B, 20 µg/ml gentamicin, 6 mM glutamine, 2.1 g/l NaHCO3. After 48h half the medium is replaced with complete DMEM medium: DMEM 4.5 g/L glucose, 10% FCS, 2 mM glutamine, 50 IU/ml penicillin, 50 µg/ml streptomycin. |
| Liñán‐Rico et al. 2016 73 | EGC culture | Patients with polyps undergoing colectomy or patients undergoing Roux‐en‐Y‐ bypass surgery |
Technique II Sigmoid colon or jejunum Krebs |
Digestion solution: Protease (1 mg/ml), collagenase (1 mg/ml) in HBSS. Incubation time: 60 min at 37°C. Spin down and resuspend in HBSS (once) and then in DMEM‐F12 0.1% BSA and DNase (50 µg/ml). Collect ganglia with micropipette. |
Laminin/Poly‐D‐Lysine coating: 20 mg/ml on 50 mm bottom glass #0 culture dishes. |
Medium: DMEM‐F12 (1:1), 10% FBS, penicillin (100 U/ml), streptomycin (100 µg/ml), amphotericin B (0.25 µg/ml). Grow for 3–4 weeks and use magnetic microbeads linked to D7‐Fib while passaging to eliminate fibroblasts. Perform purification twice. |
| Grubišić et al. 2020 70 | EGC culture | Patients with Crohn's disease |
Technique II Ileum Krebs |
Digestion solution: Liberase (0.125 mg/ml), Amphotericin B (0.5µg/ml) in DMEM‐F12. Incubation time: 60 min at 37°C. Spin down and resuspend in DMEM‐F12 0.1% BSA and DNase (50 µg/ml). Collect ganglia with micropipette. |
No coating. |
Medium: DMEM‐F12 (1:1), 10% FBS, penicillin (100 U/ml), streptomycin (100 µg/ml), amphotericin B (0.25 µg/ml). Grow for 3–4 weeks and use magnetic microbeads linked to D7‐Fib while passaging to eliminate fibroblasts. Perform purification twice. |
In addition to direct isolation of ENS cells from human tissue, methods have been developed to differentiate human pluripotent stem cells into ENS cells. These cells are self‐renewing and can be used for many applications. Several protocols have been established to generate neural crest‐derived cells from pluripotent stem cells 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 and further differentiate them to produce enteric neural lineages. 83 , 84 , 85 , 86
Generally, neural crest cell differentiation protocols depend on the manipulation of the BMP, WNT, FGF, NOTCH, TGFβ, and EGF pathways. In 2009, Chambers et al. formulated dual SMAD inhibition in pluripotent stem cells to produce neural crest cells in 11 days. 87 This protocol has since been further optimized by the addition of the WNT activator CHIR99021 to the differentiation recipe, enhancing the efficiency of differentiation to neural crest cells. 82 , 88 Later, Fattahi et al. used retinoic acid (RA) between days 6 and 11 to promote the vagal fate of the neural crest cells which then expressed HOXB2‐B5, PAX3, EDNRB, and RET. 85 After an intermediate step of suspension culture for 4 days in FGF2 and CHIR99021, further differentiating vagal neural crest cells using ascorbic acid and GDNF yielded TuJ+ neurons expressing a spectrum of markers including 5‐HT, GABA, and nNOS. The presence of glial cells in these cultures was not reported. In 2019, Barber et al. optimized the differentiation protocol further by replacing the undefined serum in culture media, and by the use of BMP4 in the first 2 days to promote neural crest specification. 83 With this culture method, not only TuJ+ neurons were evident, but also GFAP+ and SOX10+ glial cells. The parallel inhibition of NOTCH‐signaling skews the differentiation toward neuronal lineages. 84
On the other hand, a combination of EGF and FGF2 was used to induce the formation, and maintenance of neural crest cells that can be directed to their vagal fate using RA. 77 , 78 , 89 Coculturing these neural crest cells with pluripotent stem cell‐derived intestinal organoids or gut explants, or implanting them into aneural gut tissue promotes their differentiation into enteric neurons and glia. 77 , 89 , 90
4. DISCUSSION
This review provides an overview and comparison of different protocols to isolate and culture primary ENS cells from adult mice. Overall, the reviewed articles described similar approaches to obtain primary cultures from the ENS. However, because different outcome parameters were used, a direct comparison between protocols was difficult.
Different sections of the mouse intestine are used for primary ENS cultures, with a preference for the small intestines, because of differences in muscle layer thickness, plexus density, 27 and total number of ENS cells. 91 It is important that dissection of the intestinal segments is done rapidly, as lengthy dissection times may reduce cell viability, due to degradation of the tissue in the presence of microbes and pancreatic enzymes, as the tissue is no longer protected against autodigestion by a functional intestinal barrier. 92 , 93 In general, two techniques are being used for the isolation of ENS cells. Technique I, the extraction of the LMMP from the unopened intestines (on a rod), is a faster procedure than technique II, extracting the LMMP after opening the intestines in a Sylgard dish, and technique I is therefore recommended.
We found significant variation in the steps involving cell dissociation. Both a combination of digestive enzymes, serial enzymatic digestions, and enzyme‐free cell dissociation are being applied. Combinations of different enzymes allow for almost complete dissociation of the tissue, while preventing over‐digestion caused by long incubation times or high enzyme concentrations. Depending on the specific gut region that is targeted, incubation times have to be adapted. 32 Also, adult tissue appears to be more challenging to dissociate due to increased complexity of the connective tissue and ECM with age. 37 The use of collagenase for primary ENS cells is ideal due to the lack of internal collagen within the MP, allowing almost complete digestion of surrounding muscle‐ and connective tissue while keeping ENS structures mostly intact. 32 , 40 , 41 Trypsin, the enzyme used in all reviewed protocols, has a very high digestive capacity, 38 making incubation times of no longer than 10 minutes optimal.
Regarding cellular adhesion, combinations of different coating substrates can enhance the complexity of the adherence matrix in in vitro systems, resulting in increased cell adhesion and survival. Wahba et al. (2015) investigated the differences of coating substrates on cell yield and density of cultures using poly‐D‐lysine, collagen, and Matrigel as single coating substrates, and poly‐D‐lysine‐Matrigel and collagen‐Matrigel double coating. 29 Using DAPI to label individual cells, the authors observed the highest cell densities with a collagen‐Matrigel double coating. With respect to cell attachment, similar results were obtained for poly‐D‐lysine and Matrigel as single coatings. Adding poly‐D‐lysine or collagen to Matrigel coatings further improved cell attachement. 29
Coating substrates may also influence cell differentiation in culture systems, more specifically, different compositions of the neural ECM can modulate the differentiation of ENS progenitors. 47 A similar setup was used in the study by Verissimo et al. (2019). By using primary EGC cultures from adult murine intestines, the authors investigated the effects of laminin and other environmental cues on the neurogenic potential of EGCs. The study suggests that laminin possibly simulates the endogenous ECM microenvironment, resulting in significant inhibition of neuronal trans‐differentiation of EGCs in adult mice, compared to fibronectin‐coated plates. 31 This emphasizes the importance of selecting an appropriate coating substrate for different adult ENS culture types. However, it is still not fully elucidated how EGCs activate their neurogenic potential in vitro and in vivo. Understanding the potential ability of these cells to give rise to neurons is challenging but would provide beneficial advancements on the role of ENS cells in regeneration and aging. As is the case for coating substrates, medium composition also influences (trans)differentiation and proliferation. The addition of specific growth factors, such as GDNF, can favor survival and proliferation of ENS (precursor) cells. 94
One of the limitations of the current review was the lack of available data on the assessment of important cellular outcome parameters, such as cell viability and differentiation. The primary focus of the studies included was to characterize morphological and neurochemically defined subtypes of ENS cells in vitro by immunofluorescence assays, as well as imaging and electrophysiological studies to assess the functional properties of ENS populations. In order to assess and compare isolation protocols, it is important to validate if morphology, gene expression, and activity of ENS cells in vitro represent their characteristics in vivo. Potential markers to assess EGC cultures are S100β, Sox10, and GFAP, as they represent the main EGC phenotype in vivo. 10 For ENS cultures, the presence and percentage of βIII‐tubulin+ cells in the culture should be reported, ideally with other neuronal subtype markers such as nNOS and ChAT. Depending on the research question, other validation methods can be added. This will improve the comparison between ENS culture protocols and the translatability of in vitro models. For example, Schneider et al. have validated purinergic receptor expression in their EGC cultures, similar to in vivo expression, 95 and the protocols by Smith et al. and Wahba et al. have validated neuronal functionality in their ENS cultures. 27 , 29
Applications for primary ENS cultures include assessment of ENS response to different signals, such as GLP‐1, 96 HIV protein, 97 and gastrin, 68 and studying effects of drugs and toxic agents on cell viability, proliferation, differentiation, and disease development. 66 Co‐culture models with ENS cells and epithelium are also used to assess possible interactions and cell‐cell communication. 69 , 98 However, it is difficult to study the effects of one specific cell type in these primary cultures, as they are generally a mix of different cell types including enteric neurons and glia. Of note, even though neuronal markers, like βIII tubulin, are expressed after several days of culture, it is possible that these enteric neurons are derived from enteric glia cells or enteric glia‐like precursor cells that are present in adult mice 99 , 100 , 101 , 102 instead of being isolated enteric neurons.
Although this review focuses on primary ENS cultures from adult mice, other available and robust methods for culturing ENS cells from distinct developmental stages should not be overlooked. A vast amount of primary ENS culture protocols that are currently published have focused on embryonic‐ or postnatal animals as a tissue source to obtain ENS cells. 103 , 104 , 105
Although they are less favorable to study the physiology of mature enteric neurons and glia as they are stem cell‐derived cultures, the generation of 3D neurospheres is another possibility to study the ENS in vitro. They are mainly used to assess proliferation, multipotency, and stemness 99 , 101 , 106 or are grown to serve as a source of donor cells in cell transplantation studies. 107 , 108 Recently, progress has been made in the generation of ENS cultures, thereby generating a possibility to study human ENS cells, enabling genetic editing and regenerative medicine purposes. 109 In addition, intestinal organoids with a functional ENS can be generated to study cell‐cell communication in a complex and more holistic approach. 89 , 110 , 111 Nonetheless, primary murine ENS cultures are still a valuable tool to understand fundamental aspects of ENS cell biology.
The use of primary ENS cultures from mice as a model to investigate the role of the ENS in health and disease has been instrumental to advance our current understanding of ENS physiology and disease. Despite their limitations, primary cultures represent the physiological state of the cells better when compared to cell lines (generally derived from cancer cells), especially in the gut. ENS cultures allow studies on the molecular pathways that drive the physiological and pathological properties of these cells, which can be difficult to observe in vivo. Although the absence of microenvironmental cues acting in vivo significantly reduces the physiological relevance of in vitro studies, the improved accessibility to manipulate different components of the ENS outside the organism represents an important advantage for the understanding of specific questions related to ENS cell biology.
AUTHOR CONTRIBUTIONS
VM and WB were involved in manuscript concept. TTK was involved in initial manuscript draft and figure design. SS was involved in writing and shaping the manuscript. MI contributed to the section about human ENS cultures. VM, WB, and ACBF were involved in critical manuscript review. All authors approved the final version of the submitted review.
Schonkeren SL, Küthe TT, Idris M, Bon‐Frauches AC, Boesmans W, Melotte V. The gut brain in a dish: Murine primary enteric nervous system cell cultures. Neurogastroenterology & Motility.2022;34:e14215. 10.1111/nmo.14215
Funding information
This research was funded by The Netherlands Organisation for Scientific Research (NWO) Veni grant, grant number 016.186.124, VIDI grant, grant number 016.196.367, and HESTIA grant, grant number 1154.18.045, as well as by a grant from the Research Foundation Flanders (FWO), grant number G036320N
Contributor Information
Simone L. Schonkeren, Email: veerle.melotte@maastrichtuniversity.nl.
Tara T. Küthe, Email: veerle.melotte@maastrichtuniversity.nl.
REFERENCES
- 1. Vergnolle N, Cirillo C. Neurons and glia in the enteric nervous system and epithelial barrier function. Physiology. 2018;33(4):269‐280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286‐294 [DOI] [PubMed] [Google Scholar]
- 3. Hansen MB. The enteric nervous system I: Organisation and classification. Pharmacol Toxicol. 2003;92(3):105‐113 [DOI] [PubMed] [Google Scholar]
- 4. Furness JB. The enteric nervous system. Wiley Online Library. 2006. [Google Scholar]
- 5. Furness JB. Structure of the enteric nervous system. The enteric nervous system. Oxford, UK: Blackwell Publishing; 2006:3‐28 [Google Scholar]
- 6. Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17(6):338‐351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morarach K, Mikhailova A, Knoflach V, et al. Diversification of molecularly defined myenteric neuron classes revealed by single cell RNA‐sequencing. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeisel A, Hochgerner H, Lonnerberg P, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174(4):999–1014. e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81(1–3):87‐96 [DOI] [PubMed] [Google Scholar]
- 10. Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63(2):229‐241 [DOI] [PubMed] [Google Scholar]
- 11. Drokhlyansky E, Smillie CS, Van Wittenberghe N, et al. The Human and Mouse Enteric Nervous System at Single‐Cell Resolution. Cell. 2020;182(6):1606‐1622. e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boesmans W, Hao MM, Vanden Berghe P. Optogenetic and chemogenetic techniques for neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2018;15(1):21‐38 [DOI] [PubMed] [Google Scholar]
- 13. Chow A K, Gulbransen B D. Potential roles of enteric glia in bridging neuroimmune communication in the gut. American Journal of Physiology‐Gastrointestinal and Liver Physiology. 2017;312(2):G145–G152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schemann M, Camilleri M. Functions and Imaging of Mast Cell and Neural Axis of the Gut. Gastroenterology. 2013;144(4):698‐704.e694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanani M, Xia Y, Wood JD. Myenteric ganglia from the adult guinea‐pig small‐intestine in tissue‐culture. Neurogastroenterol Motil. 1994;6(2):103‐118 [DOI] [PubMed] [Google Scholar]
- 16. Vanden Berghe P, Tack J, Andrioli A, Missiaen L, Janssens J. Receptor‐induced Ca2+ signaling in cultured myenteric neurons. American Journal of Physiology‐Gastrointestinal and Liver Physiology. 2000;278(6):G905‐G914 [DOI] [PubMed] [Google Scholar]
- 17. Jessen KR, Saffrey MJ, Burnstock G. The enteric nervous system in tissue culture. I. Cell types and their interactions in explants of the myenteric and submucous plexuses from guinea pig, rabbit and rat. Brain Res. 1983;262(1):17‐35 [DOI] [PubMed] [Google Scholar]
- 18. Zhou X, Galligan JJ. Non‐additive interaction between nicotinic cholinergic and P2X purine receptors in guinea‐pig enteric neurons in culture. The Journal of physiology. 1998;513(3):685‐697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mulholland MW, Romanchuk G, Lally K, Simeone DM. Nerve growth factor promotes neurite outgrowth in guinea pig myenteric plexus ganglia. Am J Physiol. 1994;267(4):G716‐722 [DOI] [PubMed] [Google Scholar]
- 20. Brookes SJ. Classes of enteric nerve cells in the guinea‐pig small intestine. Anat Rec. 2001;262(1):58‐70 [DOI] [PubMed] [Google Scholar]
- 21. Freshney RI. Culture of animal cells: A manual of basic technique and specialized applications, 6th edn. Glasgow: Wiley‐Blackwell John Wiley & Sons; 2015. [Google Scholar]
- 22. Korman LY, Nylen ES, Finan TM, Linnoila RI, Becker KL. Primary culture of the enteric nervous system from neonatal hamster intestine: Selection of vasoactive intestinal polypeptide‐containing neurons. Gastroenterology. 1988;95(4):1003‐1010 [DOI] [PubMed] [Google Scholar]
- 23. Schäfer K‐H, Saffrey MJ, Burnstock G, Mestres‐Ventura P. A new method for the isolation of myenteric plexus from the newborn rat gastrointestinal tract. Brain Res Protoc. 1997;1(2):109‐113 [DOI] [PubMed] [Google Scholar]
- 24. Defined FRI, Media and Supplements. In. Culture of animal cells: a manual of basic technique and specialized applications, 6th edn. Glasgow: Wiley‐Blackwell John Wiley & Sons; 2015:99‐114 [Google Scholar]
- 25. Zhang Y, Hu W. Mouse enteric neuronal cell culture. Methods Mol Biol. 2013;1078:55‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brun P, Akbarali HI. Culture of neurons and smooth muscle cells from the myenteric plexus of adult mice. Methods Mol Biol. 2018;1727:119‐125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith TH, Ngwainmbi J, Grider JR, Dewey WL, Akbarali HI. An in‐vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. JoVE. 2013;78:e50688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lowette K, Tack J, Vanden Berghe P. Role of corticosterone in the murine enteric nervous system during fasting. American Journal of Physiology‐Gastrointestinal and Liver Physiology. 2014;307(9):G905‐G913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wahba G, Hebert A‐E, Grynspan D, Staines W, Schock S. A rapid and efficient method for dissociated cultures of mouse myenteric neurons. J Neurosci Methods. 2016;261:110‐116 [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, Ocadiz‐Ruiz R, Sundaresan S, et al. Isolation of enteric glial cells from the submucosa and lamina propria of the adult mouse. JoVE. 2018;138:e57629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veríssimo CP, Carvalho JDS, Silva FJMd, Campanati L, Moura‐Neto V, Coelho‐Aguiar JDM. Laminin and environmental cues act in the inhibition of the neuronal differentiation of enteric glia in vitro. Frontiers in Neuroscience. 2019;13(914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grundmann D, Klotz M, Rabe H, Glanemann M, Schäfer K‐H. Isolation of high‐purity myenteric plexus from adult human and mouse gastrointestinal tract. Sci Rep. 2015;5:9226‐9226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med. 2009;13(7):1193‐1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao M, Gershon MD. Enteric nervous system development: what could possibly go wrong? Nat Rev Neurosci. 2018;19(9):552‐565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fung C, Vanden Berghe P. Functional circuits and signal processing in the enteric nervous system. Cell Mol Life Sci. 2020;77(22):4505‐4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Osorio N, Delmas P. Patch clamp recording from enteric neurons in situ. Nat Protoc. 2011;6(1):15‐27 [DOI] [PubMed] [Google Scholar]
- 37. Freshney RI. Primary Culture. In: Culture of animal cells: a manual of basic technique and specialized applications. 6 ed. Glasgow: Wiley‐Blackwell John Wiley and Sons. 2015:773 [Google Scholar]
- 38. Corporation WB. In: Santangelo C, ed. Worthington Biochemical Corporation Tissue Dissociation Guide, 13th edn. Santangelo: Worthington Biochemical Corporation; 2011. Accessed 20–05‐2020 [Google Scholar]
- 39. Mótyán JA, Tóth F, Tőzsér J. Research applications of proteolytic enzymes in molecular biology. Biomolecules. 2013;3(4):923‐942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gershon MD. Functional Anatomy of the Enteric Nervous System. In: Holschneider AM, Puri P, eds. Hirschsprung's Disease and Allied Disorders. Springer: Berlin, Heidelberg; 2008:21‐49 [Google Scholar]
- 41. Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4(2):195‐204 [DOI] [PubMed] [Google Scholar]
- 42. Reed DE, Barajas‐Lopez C, Cottrell G, et al. Mast cell tryptase and proteinase‐activated receptor 2 induce hyperexcitability of guinea‐pig submucosal neurons. The Journal of Physiology. 2003;547(2):531‐542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Linden DR, Manning BP, Bunnett NW, Mawe GM. Agonists of proteinase‐activated receptor 2 excite guinea pig ileal myenteric neurons. Eur J Pharmacol. 2001;431(3):311‐314 [DOI] [PubMed] [Google Scholar]
- 44. Kugler EM, Mazzuoli G, Demir IE, Ceyhan GO, Zeller F, Schemann M. Activity of protease‐activated receptors in primary cultured human myenteric neurons. Front Neurosci. 2012;6:133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Voloboueva L, Sun X, Ouyang YB, Giffard RG. Cell Culture: Primary Neural Cells.; 2017.In: Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier [Google Scholar]
- 46. Scholz WK. Cell Adhesion and Growth on Coated or Modified Glass or Plastic Surfaces. Technical Bulletin Thermo Fisher Scientific. 2010;13(1–2) [Google Scholar]
- 47. Raghavan S, Gilmont RR, Bitar KN. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials. 2013;34(28):6649‐6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bannerman PGC, Mirsky R, Jessen KR, Timpl R, Duance VC. Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and guinea pig. J Neurocytol. 1986;15(6):733‐743 [DOI] [PubMed] [Google Scholar]
- 49. Gordon J, Amini S, White MK. General overview of neuronal cell culture. Methods in molecular biology (Clifton, NJ). 2013;1078:1‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Freshney RI. Culture Vessels and Substrates. In: Culture of animal cells: a manual of basic technique and specialized applications, 6th edn. Glasgow: Wiley‐Blackwell John Wiley & Sons. 2015:89‐98 [Google Scholar]
- 51. Colognato H, Yurchenco PD. Form and function: The laminin family of heterotrimers. Dev Dyn. 2000;218(2):213‐234. [DOI] [PubMed] [Google Scholar]
- 52. Dertinger S K W, Jiang X, Li Z, Murthy V N, Whitesides G M. Gradients of substrate‐bound laminin orient axonal specification of neurons. Proceedings of the National Academy of Sciences. 2002;99(20):12542–12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rauch U, Schäfer KH. The extracellular matrix and its role in cell migration and development of the enteric nervous system. Eur J Pediatr Surg. 2003;13(3):158‐162 [DOI] [PubMed] [Google Scholar]
- 54. Qian L, Saltzman WM. Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials. 2004;25(7):1331‐1337 [DOI] [PubMed] [Google Scholar]
- 55. Lee S‐W, Lee HJ, Hwang HS, Ko K, Han DW, Ko K. Optimization of Matrigel‐based culture for expansion of neural stem cells. Animal Cells and Systems. 2015;19(3):175‐180 [Google Scholar]
- 56. Ramos‐Hryb AB, Da‐Costa MC, Trentin AG, Calloni GW. Matrigel supports neural, melanocytic and chondrogenic differentiation of trunk neural crest cells. Int J Dev Biol. 2013;57(11–12):885‐890 [DOI] [PubMed] [Google Scholar]
- 57. Springer‐Protocols . In: Kasper C, Charwat V, Lavrentieva A, eds. Cell Culture Technology, 1st edn. Cham, Switzerland: Springer International Publishing; 2018. Accessed 2020 [Google Scholar]
- 58. Springer‐Protocols . Cell Culture Media. In: Kasper C, Charwat V, Lavrentieva A, ed. Cell Culture Technology. 1 ed. Cham, Switzerland: Springer International Publishing; 2018:168 [Google Scholar]
- 59. van der Valk J, Bieback K, Buta C, et al. Fetal bovine serum (FBS): past–present–future. ALTEX. 2018;35(1):99‐118 [DOI] [PubMed] [Google Scholar]
- 60. Thermofisher . Neurobiology Protocol Handbook. In. Vol. Carlsbad. CA: Life Technologies; 2020:2012 [Google Scholar]
- 61. Sampath C, Srinivasan S, Freeman ML, Gangula PR. Inhibition of GSK‐3β restores delayed gastric emptying in obesity‐induced diabetic female mice. American Journal of Physiology‐Gastrointestinal and Liver Physiology. 2020;319(4):G481‐G493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ye L, Li G, Goebel A, et al. Caspase‐11–mediated enteric neuronal pyroptosis underlies Western diet–induced colonic dysmotility. J Clin Investig. 2020;130(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brun P, Zamuner A, Peretti A, et al. 3D Synthetic peptide‐based architectures for the engineering of the enteric nervous system. Sci Rep. 2019;9(1):1‐12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brun P, Scarpa M, Marchiori C, et al. Herpes simplex virus type 1 engages toll like receptor 2 to recruit macrophages during infection of enteric neurons. Front Microbiol. 2018;9:2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brand B, Stoye NM, Guilherme MDS, et al. Identification of Patulin from Penicillium coprobium as a Toxin for Enteric Neurons. Molecules. 2019;24(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith TH, Ngwainmbi J, Hashimoto A, Dewey WL, Akbarali HI. Morphine dependence in single enteric neurons from the mouse colon requires deletion of β‐arrestin2. Physiol Rep. 2014;2(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hibberd TJ, Feng J, Luo J, et al. Optogenetic Induction of Colonic Motility in Mice. Gastroenterology. 2018;155(2):514‐528.e516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sundaresan S, Meininger CA, Kang AJ, et al. Gastrin Induces Nuclear Export and Proteasome Degradation of Menin in Enteric Glial Cells. Gastroenterology. 2017;153(6):1555‐1567.e1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Puzan M, Hosic S, Ghio C, Koppes A. Enteric Nervous System Regulation of Intestinal Stem Cell Differentiation and Epithelial Monolayer Function. Sci Rep. 2018;8(1):6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grubišić V, McClain JL, Fried DE, et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 2020;32(10): 108100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Turco F, Sarnelli G, Cirillo C, et al. Enteroglial‐derived S100B protein integrates bacteria‐induced Toll‐like receptor signalling in human enteric glial cells. Gut. 2014;63(1):105‐115 [DOI] [PubMed] [Google Scholar]
- 72. Cirillo C, Sarnelli G, Turco F, et al. Proinflammatory stimuli activates human‐derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil. 2011;23(9):e372‐e382 [DOI] [PubMed] [Google Scholar]
- 73. Liñán‐Rico A, Turco F, Ochoa‐Cortes F, et al. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm Bowel Dis. 2016;22(8):1812‐1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soret R, Coquenlorge S, Cossais F, Meurette G, Rolli‐Derkinderen M, Neunlist M. Characterization of human, mouse, and rat cultures of enteric glial cells and their effect on intestinal epithelial cells. Neurogastroenterol Motil. 2013;25(11):e755‐e764 [DOI] [PubMed] [Google Scholar]
- 75. Lee G, Chambers SM, Tomishima MJ, Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5(4):688 [DOI] [PubMed] [Google Scholar]
- 76. Zeltner N, Lafaille FG, Fattahi F, Studer L. Feeder‐free derivation of neural crest progenitor cells from human pluripotent stem cells. JoVE (Journal of Visualized Experiments). 2014;87:e51609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li W, Huang L, Zeng J, et al. Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells. Mol Psychiatry. 2018;23(3):499‐508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bajpai R, Chen DA, Rada‐Iglesias A, et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463(7283):958‐962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kerosuo L, Nie S, Bajpai R, Bronner ME. Crestospheres: long‐term maintenance of multipotent, premigratory neural crest stem cells. Stem cell reports. 2015;5(4):499‐507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu Q, Spusta SC, Mi R, et al. Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl Med. 2012;1(4):266‐278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hackland JO, Frith TJ, Thompson O, et al. Top‐down inhibition of BMP signaling enables robust induction of hPSCs into neural crest in fully defined, xeno‐free conditions. Stem cell reports. 2017;9(4):1043‐1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci. 2011;108:19240‐19245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barber K, Studer L, Fattahi F. Derivation of enteric neuron lineages from human pluripotent stem cells. Nat Protoc. 2019;14(4):1261‐1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Frith TJ, Gogolou A, Hackland JO, et al. Retinoic Acid Accelerates the Specification of Enteric Neural Progenitors from In‐Vitro‐Derived Neural Crest. Stem cell reports. 2020;15(3):557‐565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fattahi F, Steinbeck JA, Kriks S, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531(7592):105‐109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lai FP‐L, Lau S‐T, Wong JK‐L, et al. Correction of Hirschsprung‐associated mutations in human induced pluripotent stem cells via clustered regularly interspaced short palindromic repeats/Cas9, restores neural crest cell function. Gastroenterology. 2017;153(1):139–153. e138 [DOI] [PubMed] [Google Scholar]
- 87. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275‐280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mica Y, Lee G, Chambers SM, Tomishima MJ, Studer L. Modeling neural crest induction, melanocyte specification, and disease‐related pigmentation defects in hESCs and patient‐specific iPSCs. Cell Rep. 2013;3(4):1140‐1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Workman MJ, Mahe MM, Trisno S, et al. Engineered human pluripotent‐stem‐cell‐derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23(1):49‐59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schlieve CR, Fowler KL, Thornton M, et al. Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue‐engineered small intestine. Stem Cell Reports. 2017;9(3):883‐896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130(10):2187‐2198 [DOI] [PubMed] [Google Scholar]
- 92. Schmid‐Schönbein GW. Inflammation and the autodigestion hypothesis. Microcirculation. 2009;16(4):289‐306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Altshuler AE, Kistler EB, Schmid‐Schönbein GW. Autodigestion: Proteolytic Degradation and Multiple Organ Failure in Shock. Shock. 2016;45(5):483‐489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitorsin vitro. Dev Biol. 1998;200(1):116‐129 [DOI] [PubMed] [Google Scholar]
- 95. Schneider R, Leven P, Glowka T, et al. A novel P2X2‐dependent purinergic mechanism of enteric gliosis in intestinal inflammation. EMBO Mol Med. 2021;13(1):e12724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Grasset E, Puel A, Charpentier J, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP‐1 resistance through an enteric NO‐dependent and gut‐brain axis mechanism. Cell Metab. 2017;25(5):1075‐1090.e1075 [DOI] [PubMed] [Google Scholar]
- 97. Ngwainmbi J, De DD, Smith TH, et al. Effects of HIV‐1 tat on enteric neuropathogenesis. The Journal of Neuroscience. 2014;34(43):14243‐14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Walsh KT, Zemper AE. The enteric nervous system for epithelial researchers: Basic anatomy, techniques, and interactions with the epithelium. Cellular and Molecular Gastroenterology and Hepatology. 2019;8(3):369‐378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kulkarni S, Micci M‐A, Leser J, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci. 2017;114:E3709‐E3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Suárez‐Rodríguez R, Belkind‐Gerson J. Cultured nestin–positive cells from postnatal mouse small bowel differentiate ex vivo into neurons, glia, and smooth muscle. Stem Cells. 2004;22(7):1373‐1385 [DOI] [PubMed] [Google Scholar]
- 101. Joseph NM, He S, Quintana E, Kim Y‐G, Núñez G, Morrison SJ. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Investig. 2011;121(9):3398‐3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121(9):3412‐3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130(25):6387 [DOI] [PubMed] [Google Scholar]
- 104. Hegewald C, Alt R, Hetz S, et al. Reduced oxygen stress promotes propagation of murine postnatal enteric neural progenitors in vitro. Neurogastroenterol Motil. 2011;23(10):e412‐e424 [DOI] [PubMed] [Google Scholar]
- 105. Dettmann HM, Zhang Y, Wronna N, et al. Isolation, expansion and transplantation of postnatal murine progenitor cells of the enteric nervous system. PLoS One. 2014;9(5):e97792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lindley RM, Hawcutt DB, Connell MG, Edgar DH, Kenny SE. Properties of secondary and tertiary human enteric nervous system neurospheres. J Pediatr Surg. 2009;44(6):1249‐1256 [DOI] [PubMed] [Google Scholar]
- 107. Lindley RM, Hawcutt DB, Connell MG, et al. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology. 2008;135(1):205‐216.e206 [DOI] [PubMed] [Google Scholar]
- 108. Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56(4):489‐496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chng SH, Pachnis V. Enteric Nervous System: lessons from neurogenesis for reverse engineering and disease modelling and treatment. Curr Opin Pharmacol. 2020;50:100‐106 [DOI] [PubMed] [Google Scholar]
- 110. Mahe MM. Engineering a second brain in a dish. Brain Res. 2018;1693:165‐168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chukwurah E, Osmundsen A, Davis SW, Lizarraga SB. All Together Now: Modeling the Interaction of Neural With Non‐neural Systems Using Organoid Models. Frontiers in Neuroscience. 2019;13(582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang X‐K, Liu X‐M, Hu Q, et al. Dexmedetomidine rescues enteric glial cells from oxidative stress‐induced intestinal ischemia‐reperfusion injury through promoting mitochondrial translocation of telomerase reverse transcriptase. SSRN Electronic Journal. 2019; 10.2139/ssrn.3397181 [DOI] [Google Scholar]


