Abstract
Butane-oxidizing Arthrobacter (ATCC 27778) bacteria were shown to degrade low concentrations of methyl t-butyl ether (MTBE; range, 100 to 800 μg/liter) with an apparent half-saturation concentration (Ks) of 2.14 mg/liter and a maximum substrate utilization rate (kc) of 0.43 mg/mg of total suspended solids per day. Arthrobacter bacteria demonstrated MTBE degradation activity when grown on butane but not when grown on glucose, butanol, or tryptose phosphate broth. The presence of butane, tert-butyl alcohol, or acetylene had a negative impact on the MTBE degradation rate. Neither Methylosinus trichosporium OB3b nor Streptomyces griseus was able to cometabolize MTBE.
The prevalent use of methyl t-butyl ether (MTBE) for gasoline oxygenation has led to its introduction into groundwater from spills and leaky underground storage tanks. MTBE is poorly adsorbed, chemically and biologically stable, and very soluble in water, making it very mobile and persistent in the environment. The U.S. Environmental Protection Agency has recently proposed scaling back the use of MTBE in gasoline in light of the increasing frequency with which MTBE is found as a groundwater contaminant nationwide (16; http://www.epa.gov /swerust1/mtbe/browner.pdf). Concentrations of MTBE in groundwater have been reported to range from 0.5 μg/liter to >10 mg/liter. At least 20 states have established MTBE groundwater cleanup levels ranging from 20 to 400 μg/liter for groundwater for potable use (http://www.epa.gov/OUST/mtbe /sumtable.htm). The U.S. Environmental Protection Agency has established a health advisory level of 20 to 40 μg/liter for MTBE in drinking water (19).
Efforts to develop bioremediation processes to help combat MTBE contamination of groundwater have been hampered by the recalcitrance of MTBE. The highly branched nature of MTBE resists most bacterial enzymatic attacks. Only a few pure and mixed bacterial cultures that are able to biodegrade MTBE have been identified (4, 6, 7, 8, 17).
One potential MTBE biodegradation pathway involves the demethylation of MTBE to form tert-butyl alcohol (TBA) and formaldehyde (11, 17; K. L. Hurt, J. T. Wilson, and J. S. Cho, 5th Int. In Situ On-Site Bioremediation Symp., 1999), although the formation of tert-butyl formate from MTBE has also been observed (8). tert-Butyl formate is then hydrolyzed into TBA. In general, TBA is the first stable metabolite of MTBE, regardless of the type of bacterial cultures used. Hyman et al. (8) presented evidence that TBA is degraded by the same enzyme that degrades MTBE, although a soil microcosm was identified that was able to biodegrade TBA but not MTBE (M. J. Zenker, R. C. Borden, and M. A. Barlaz, 5th Int. In Situ On-Site Bioremediation Symp., 1999). TBA is biodegraded at a slower rate than MTBE (8, 17); thus, it tends to build up over time. If TBA is indeed degraded by the same enzyme that degrades MTBE, then the accumulation of TBA can have a detrimental effect on the MTBE biodegradation rate as a result of enzyme competition. Consequently, the effects of TBA on MTBE biodegradation need to be examined.
Most bacteria cannot utilize MTBE as a sole growth substrate. Consequently, many MTBE biodegradation studies have been conducted under cometabolic conditions, using growth substrates such as simple hydrocarbons (6, 8, 17). In addition, these studies have focused mainly on the degradation of moderate-to-high concentrations of MTBE (tens to hundreds of milligrams per liter), at which the observed biodegradation rates would likely be maximal. However, a large quantity of contaminated water contains MTBE at concentrations below 1 mg/liter. No work has been reported regarding the biodegradation of MTBE at low concentrations.
In this work, we examined the abilities of several microorganisms to cometabolize MTBE at low concentrations (range, 100 to 800 μg/liter). It was determined that Arthrobacter bacteria readily degraded MTBE. The degradation kinetics were measured, and the effects of growth substrates and TBA buildup on bacterial cometabolism of MTBE were explored.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Nocardia (ENV425) and Arthrobacter (ATCC 27778) cultures were grown on basal salt medium (BSM) (17) with propane or lighter fluid butane (Colibri Premium Butane Fuel, 78% mixed butane), respectively, as the sole carbon and energy source. Both cultures were batch grown at room temperature with continuous shaking in 250-ml amber glass bottles sealed with screw caps fitted with Teflon-lined silicone septa (60 ml of growth medium). A 45-ml volume of propane or butane was added as an overpressure. Bottle headspaces were periodically purged with 200 ml of pure oxygen, and additional propane or butane was added. Methylosinus trichosporium OB3b PP358 (12) was maintained as a continuous culture in a chemostat as described by Aziz et al. (3). Streptomyces griseus (ATCC 13273) was maintained on a glucose-enriched medium, and its P-450 enzyme system was induced by soy flour as described by Sariaslani et al. (13).
Arthrobacter bacteria were also grown with n-butane (99% purity), 1-butanol (0.3%, vol/vol), glucose (2 g/liter), and a glucose-butane mixture (2 g/liter and 10% [vol/vol], respectively) and on tryptose phosphate broth (TPB; Difco). Alternatively, Arthrobacter bacteria were grown with lighter fluid butane in modified BSM (BSM-NO3) in which NH4Cl was replaced with an equal molar concentration of NaNO3. Unless otherwise noted, the Arthrobacter cultures reported herein were grown on lighter fluid butane in BSM.
All of the cells used in this experiment were harvested by centrifugation at 7,000 × g for 7 min, rinsed, and suspended in 3 ml of fresh medium.
Analytical techniques.
MTBE and TBA concentrations were measured using a gas chromatograph (GC). A 4-μl sample was injected into a Hewlett-Packard 5890A GC equipped with a 30-m Megabore DB-Wax column (J&W Scientific) with a 5-m guard column and a flame ionization detector. The injector and detector were maintained at 150 and 250°C, respectively. The initial column temperature was set at 40°C and maintained for 6 min after sample injection. The column temperature was then increased at a rate of 20°C/min to a final temperature of 150°C and maintained for 3 min. Hydrogen and air flow to the flame ionization detector was set at 35 and 400 ml/min, respectively. Helium was used as the carrier gas, and the column head pressure was set at 5 lb/in2. Nitrogen was used as makeup gas. The retention times of MTBE and TBA were 2.5 and 4.3 min, respectively. Concentrations were quantified against primary standard curves.
Cell mass was measured either gravimetrically or spectrophotometrically. Gravimetric analysis consisted of measurement of total suspended solids (TSS) as described in reference 2, using 47-mm-diameter Gelman type A/E filters. The spectrophotometric technique correlates the TSS concentration in a culture suspension with the A550 of the solution using a Perkin-Elmer Lambda 3B UV/VIS spectrophotometer. A calibration curve was developed, establishing the linear relationship between TSS and absorbance.
14C-radioactivity was measured on a Beckman LS 5000 TD liquid scintillation counter. Quench correction was done by the H number technique using the instrument's internal cesium-137 source. ScintiVerse II (Fisher Scientific) was used as the scintillation cocktail.
MTBE biodegradation assays and kinetic determination.
Using the headspace-free syringe method described by Aziz et al. (3), a harvested cell suspension was injected into a 60-cm3 disposable plastic syringe (Becton Dickinson) containing 40 ml of aerated growth medium, spiked with MTBE, and capped. At timed intervals, samples were collected by ejecting aliquots from the syringe through a disposable syringe filter (0.2-μm pore size; cellulose acetate) and directly into GC autosampler vials. A new syringe filter was used with each sample collected. Abiotic tests showed no loss of MTBE with time using the headspace-free syringe method. Inhibition assays with Arthrobacter bacteria were also conducted using the headspace-free syringe method described above. BSM was aerated and amended with butane, acetylene, or TBA prior to use. The dissolved oxygen concentration was measured at the end of every experiment to ensure that it did not fall below 3.5 mg/liter. Degradation and inhibition assays were conducted in duplicate.
Radiolabeled-MTBE assay.
Arthrobacter bacteria were tested with radiolabeled MTBE to confirm the mineralization of MTBE. The assay was conducted using the headspace-free syringe method. The syringe was filled with 55 ml of aerated BSM, 0.9 μCi of uniformly labeled [14C]MTBE (10.1 mCi/mmol; lot no. 3048-175B; Dupont New England Nuclear Products) in 12 μl of ethanol, and unlabeled MTBE. Harvested Arthrobacter bacteria were then added to the syringe. The resulting biomass concentration was 1,100 mg of TSS/liter, and the total initial MTBE concentration was 670 μg/liter. At timed intervals, aliquots were collected from the syringe and analyzed for MTBE and TBA concentrations using GC and total radioactivity and 14CO2 production using a liquid scintillation counter. 14CO2 was separated by trapping the volatile components in solvents and base as previously described (9).
Transformation capacity.
A cell suspension was injected into a 250-ml amber glass bottle containing 200 ml of BSM and 2.15 μmol of MTBE to provide a TSS concentration of 1,100 mg/liter. The bottle was capped with a screw top Mininert valve. Aliquots of 2 to 3 ml were withdrawn from the bottle at timed intervals and syringe filtered directly into GC autosampler vials for MTBE and TBA measurement. When the MTBE concentration had decreased by 90% of the initial concentration, the experiment was paused to remove any remaining TBA. The cell suspension was centrifuged, rinsed with BSM, and resuspended with 10 ml of BSM. The suspended cells were injected into a new amber glass bottle containing enough BSM and MTBE to bring the total liquid volume to 80 to 90% of the volume of the cell suspension before centrifugation, and the experiment was continued. The process was repeated until the degradation rate fell to approximately 10% of that at the beginning of the experiment. Progressively smaller amber glass bottles were used as the total volume of the cell suspension decreased, such that the headspace volume was never greater than 25% of the total volume of the bottle. The biomass concentration was determined for each dosing period by measuring the optical density at 550 nm. The MTBE concentrations for each dosing ranged from 950 to 4,100 μg/liter.
RESULTS AND DISCUSSION
Bacterial strain selection.
Three bacterial strains were tested for the ability to biodegrade MTBE at low concentrations. The methanotroph M. trichosporium OB3b PP358 was selected because of its production of a nonspecific soluble methane monooxygenase that is capable of degrading various chlorinated solvents (3, 5, 15). S. griseus, which has long been exploited for its ability to degrade a diverse array of structurally complex xenobiotics (13, 14, 18), was studied in order to evaluate a P-450 enzyme system for the biodegradation of MTBE (17). The last organism tested was Arthrobacter sp. strain ATCC 27778. This strain was isolated from a natural- and domestic- gas-contaminated site and has been shown to utilize butane and butanol as growth substrates (10). Because most of the identified MTBE-cometabolizing bacteria utilize alkane as a feed substrate (6, 8, 17), we hypothesized that a culture that had been previously exposed to a mixture of hydrocarbons, including branched butane, would have the ability to produce the necessary enzymes for MTBE degradation. The MTBE degradation activity of the test strains was compared to that of ENV425, a Nocardia strain that utilizes propane as an energy and carbon source and is a known MTBE degrader (17).
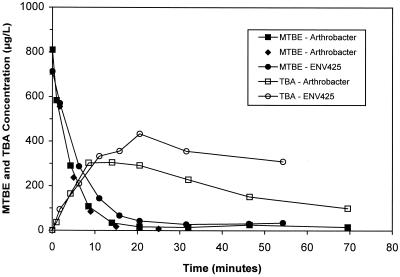
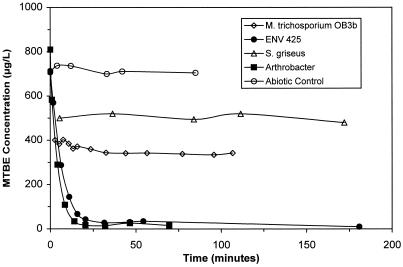
Arthrobacter bacteria incubated in MTBE at 600 μg/liter were found to biodegrade >90% of the MTBE present within 30 min. Simultaneous production of TBA, the primary metabolite of MTBE, was also observed. Mass balances of MTBE and TBA showed that TBA accumulated at a rate slower than that at which MTBE was being biodegraded. This observation suggested that a portion of the TBA being produced was simultaneously being degraded along with MTBE. Comparing Arthrobacter to ENV425, the former accumulated less TBA during MTBE degradation (Fig. 1), indicating a more rapid initial rate of TBA degradation by Arthrobacter. Neither M. trichosporium OB3b nor S. griseus was able to oxidize MTBE to any appreciable extent within the 2-h time frame of the experiment (Fig. 2).
FIG. 1.
MTBE degradation and TBA production by Arthrobacter and ENV425.
FIG. 2.
Cometabolism of MTBE by Arthrobacter, ENV425, M. trichosporium OB3b, and S. griseus.
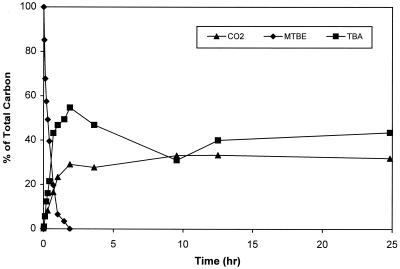
MTBE mineralization by Arthrobacter was confirmed using uniformly labeled [14C]MTBE in a degradation assay (Fig. 3). The formation of 14CO2 from [14C]MTBE degradation was measured and determined to closely follow the demethylation of MTBE to form TBA. At 42 min, approximately 16% of the radiolabeled carbon was captured as 14CO2. This corresponded to 80% MTBE degradation, assuming that CO2 was formed solely from the demethylation of MTBE. GC measurement of the sample confirmed that 80% of the initial MTBE was degraded.
FIG. 3.
Formation of [14C]TBA and 14CO2 from degradation of uniformly radiolabeled MTBE by Arthrobacter.
MTBE was completely degraded within 2 h, with 30% of the radioactivity being captured as 14CO2 and the remaining 70% being recovered as dissolved radiolabeled products. Of these dissolved products, 79% (55% of the original 14C) was identified as TBA, and the remaining 21% was unidentified radioactive products. TBA continued to be degraded even after all of the MTBE had been degraded. Analysis of the biomass present at the end of the experiment showed no accumulation of radiolabeled products within the cells. Mass balance closure on 14C was >97%.
In addition to degradation of MTBE, Arthrobacter bacteria were tested for the ability to degrade TBA alone. Butane-grown cultures were able to biodegrade TBA at 0.07 nmol/mg of TSS/h.
MTBE degradation kinetics.
The Monod kinetic parameters KS (apparent half-saturation coefficient) and kc (maximum specific rate of substrate utilization) for MTBE degradation by Arthrobacter and ENV425 were determined for cultures grown with nitrate or ammonia as the nitrogen source (Table 1). For each bacterial strain tested, five or six degradation assays were performed with a range of initial MTBE concentrations (100 to 22,000 μg/liter). The data from these degradation assays were then simultaneously fitted to the Monod equation to obtain a unique set of Monod parameters (3). The calculated pseudo-first-order rate constant (k1) values based on the measured Monod parameters were closely bracketed by the k1 measured from independent degradation assays (Table 1).
TABLE 1.
Monod kinetic parameters
| Strain | Nitrogen source | kca (mg/mg of TSS/day) | KSa (mg/liter) | Calculated k1b (liters/mg of TSS/day) | Observed k1c (liters/mg of TSS/day); no. of expts |
|---|---|---|---|---|---|
| ATCC 27778 | NH4+ | 0.43 (0.37–0.50) | 2.14 (1.50–3.00) | 0.20 | 0.12–0.30; 7 |
| ATCC 27778 | NO3− | 0.18 (0.17–0.19) | 1.61 (1.25–1.85) | 0.12 | 0.08–0.12; 3 |
| ENV425 | NH4+ | 0.20 (0.11–0.36) | 1.17 (0.10–5.45) | 0.17 | 0.08–0.22; 6 |
Values in parentheses are the lower and upper bounds of the 95% confidence interval.
Calculated k1 = kc/KS.
Observed k1 measured from degradation assays that were independent of kinetic-parameter assays used to calculate kc and KS.
The KS for Arthrobacter grown on butane with ammonia as its nitrogen source is 2.14 mg/liter. This value is much lower than those reported for propane-grown Xanthobacter (KS = 210 mg/liter) or propane-grown Mycobacterium vaccae (KS = 82 mg/liter) (8). The calculated k1 for propane-oxidizing Xanthobacter (8) is 0.015 liters/mg of TSS/day, compared to 0.20 liters/mg of TSS/day for Arthrobacter. These Ks and k1 values indicate that Arthrobacter has a greater affinity for MTBE and can degrade MTBE faster than Xanthobacter under low-concentration conditions. However, the higher Xanthobacter kc value (51 nmol/min/mg of protein), compared to that of Arthrobacter (6.78 nmol/min/mg of protein, assuming that the total protein is 50% of the biomass), makes Xanthobacter better suited for bioremediation of MTBE contamination at high concentrations. In addition to Arthrobacter, ENV425 also had a low KS value (KS = 1.17 mg/liter). Both ENV425 and Arthrobacter were able to biodegrade MTBE at 650 μg/liter at about the same rate (k1 = 0.17 and 0.20 liters/mg of TSS/day, respectively).
Factors affecting MTBE degradation.
The growth substrates played an important role in the ability of Arthrobacter bacteria to biodegrade MTBE. Arthrobacter bacteria were grown in batches on butane, 1-butanol, glucose, and glucose plus butane and in TPB. Cells that were grown with butanol, glucose, or TPB had no detectable MTBE degradation activity (Table 2). Slight MTBE degradation activity was observed in cultures that were fed a combination of butane and glucose (k1 = 0.01 liters/mg-day), while cultures given only butane had the highest rate constant (k1=0.20 liters/mg-day). The type of butane used as a growth substrate did not have any apparent effect on the k1 values (Table 2). Arthrobacter bacteria grown on either n-butane (99.0% purity) or lighter fluid butane were able to degrade MTBE at the same rate (k1 = 0.20 liters/mg-day), with no initial lag period (data not shown).
TABLE 2.
Effects of growth substrate on MTBE degradation by Arthrobacter bacteria
| Substrate | k1 (liters/mg of TSS/day) |
|---|---|
| Glucose | NDAa |
| Glucose + butaneb | 0.01 |
| Butaneb | 0.20 |
| Butaneb + NO3c | 0.12 |
| n-Butane | 0.20 |
| 1-Butanol | NDA |
| TPB | NDA |
NDA, no degradation activity observed.
butane = lighter fluid butane.
NaNO3 was used as inorganic nitrogen in the growth medium instead of ammonia.
The presence of butane affected the rate of MTBE biodegradation. Butane-grown Arthrobacter incubated with an initial MTBE concentration of 450 μg/liter and an initial butane concentration of 2.5 mg/liter was only able to degrade 15% of the MTBE present after 20 min of incubation (data not shown). Control samples not incubated with butane degraded 95% of the MTBE within the same time period. Exposure to acetylene had an adverse impact on the ability of Arthrobacter bacteria to biodegrade both MTBE and TBA. TBA production from MTBE degradation by Arthrobacter in the presence of acetylene was suppressed by 86% compared to the control. In addition, Arthrobacter biodegradation of TBA alone was also inhibited in the presence of acetylene.
Acetylene's and butane's inhibitory effects on the ability of Arthrobacter bacteria to degrade MTBE, plus the fact that only butane-grown cells were able to degrade MTBE, suggest that a butane-oxidizing enzyme is responsible for MTBE degradation by Arthrobacter bacteria. The role of a butane-oxidizing enzyme in MTBE biodegradation is further emphasized by the inability of Arthrobacter bacteria grown on butanol to degrade MTBE. Hyman et al. (8) observed similar phenomena in that propanol-grown Xanthobacter and M. vaccae were not able to degrade MTBE, although the same strains grown on propane effectively degrade MTBE. Since bacterial cultures typically oxidize alkanes to their corresponding alcohols as the initial step in alkane degradation, it is likely that a butanol-grown cell would have all of the same enzymes as a butane-grown cell except for the enzyme which initiates the butane oxidation step.
It has been suggested that TBA and MTBE are degraded by the same enzyme (8). Consequently, the effect of TBA on the MTBE biodegradation rate is of interest. MTBE degradation assays were conducted with either MTBE alone (control) or a mixture of MTBE and TBA (Table 3). In the presence of equal molar concentrations of TBA and MTBE, the rate of MTBE biodegradation by Arthrobacter bacteria was 86% of that of the control. The inhibitory effect of TBA increased with increasing TBA concentrations. The MTBE degradation rate of cultures incubated with a high concentration of TBA was 14% of that of the control. The apparent half-saturation coefficient for TBA, KS,TBA, was calculated as 1.1 to 2.3 mg/liter (Table 3).
TABLE 3.
Effects of various TBA concentrations on degradation of MTBE by Arthrobacter bacteria
| Initial concn of test compound (mg/liter)
|
Relative activitya (%) | KS,TBAb (mg/liter) | |
|---|---|---|---|
| MTBE | TBA | ||
| 0.53 | 0 | 100 | |
| 0.60 | 0.53 | 86 | 2.3 |
| 0.58 | 6.45 | 14 | 1.1 |
The MTBE degradation rate of each culture degrading a mixture of MTBE and TBA was compared to the rate of MTBE degradation by Arthrobacter bacteria degrading MTBE only.
KS,TBA, apparent half-saturation coefficient for TBA.
MTBE transformation capacity.
The transformation capacity, Tc, defined as the maximum mass of substrate that can be transformed per unit mass of cells (1), was measured for butane-grown Arthrobacter bacteria. Transformation capacity was determined by consecutively dosing a culture with MTBE until the MTBE degradation rate decreased to approximately 10% of the initial value. In order to minimize the inhibition effect of TBA on MTBE degradation, cells were centrifuged and resuspended in fresh medium before each additional dosing of MTBE. This process prevented excessive accumulation of TBA in the culture medium but did not prevent simultaneous degradation of MTBE and TBA. Mass balance calculations from GC measurements showed that approximately 35% of the TBA formed from MTBE was thus degraded. The Tc for butane-grown Arthrobacter was determined to be 20 μg of MTBE/mg of biomass (Fig. 4). This value is relatively small compared to the cometabolism of other chemicals. For example, the Tc of OB3b cometabolizing TCE ranged from 26 to 108 μg/mg of TSS (5). Since TBA was removed from the culture before each MTBE dosage, the bacterial cells were not being significantly inhibited by a buildup of TBA. Because the enzyme was not being inhibited, then the decrease in the MTBE degradation rate with time is due to either the consumption of reducing power within the cells or the production of toxic oxidation products from MTBE degradation. Although TBA was periodically removed from the cell culture, some of the TBA was still degraded. Consequently, it is possible that a metabolite further down in the degradation chain is toxic and thus partly caused the observed decrease in the MTBE degradation rate with time. Alternatively, a low Tc value can be indicative of the fact that Arthrobacter lacks the ability to store excess reducing power under the conditions under which it was grown. Additional research into the effect of adding reducing power to the culture medium during degradation assays would help clarify this matter.
FIG. 4.
Transformation capacity of Arthrobacter degrading MTBE. Cells were centrifuged and resuspended in fresh medium before each addition of MTBE.
The widespread occurrence of MTBE in groundwater has generated a demand for the development of effective remediation technologies. Both Arthrobacter and ENV425 appear to be viable candidates for use in bioreactor systems to treat MTBE-contaminated water. Their higher affinity for MTBE enables both bacterial strains to effectively treat low-level MTBE contamination. Additional research is needed to optimize the growth conditions of these organisms so as to obtain the best MTBE remediation rates. In addition, bioreactors using Arthrobacter to treat MTBE-contaminated water must be designed so as to minimize competitive inhibition effects among MTBE, TBA, and butane.
ACKNOWLEDGMENTS
This work was supported by a grant from the Gulf Coast Hazardous Substance Research Center.
We thank Robert J. Steffan (Envirogen, Inc.) for his generous donation of the ENV425 bacterial culture used in this research.
REFERENCES
- 1.Alvarez-Cohen L, McCarty P L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991;57:228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Public Health Association. Total suspended solids dried at 103–105°C. In: Greenberg A E, Trussel R R, Clesceri L S, editors. Standard methods for the examination of water and wastewater. 16th ed. Washington, D.C.: American Public Health Association; 1985. pp. 96–97. [Google Scholar]
- 3.Aziz C E, Georgiou G, Speitel G E., Jr Cometabolism of chlorinated solvents and binary chlorinated solvent mixtures using M. trichosporium OB3b PP358. Biotechnol Bioeng. 1999;65:100–107. doi: 10.1002/(sici)1097-0290(19991005)65:1<100::aid-bit12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Cowan R M, Park K. Hazardous & Industrial Waste. Proceedings of the Mid-Atlantic Industrial Waste Conference. Lancaster, Pa: Technomic Publishing Co.; 1996. Biodegradation of the gasoline oxygenates MTBE, ETBE, TAME, TBA, and TAA by aerobic mixed cultures; pp. 523–530. [Google Scholar]
- 5.Fitch M W. Trichloroethylene degradation by Methylosinus trichosporium OB3.b in a hollow fiber membrane reactor. Ph.D. thesis. Austin: University of Texas; 1996. [Google Scholar]
- 6.Garnier P M, Auria R, Augur C, Revah S. Cometabolism biodegradation of methyl t-butyl ether by Pseudomonas aeruginosa grown on pentane. Appl Microbiol Biotechnol. 1999;51:498–503. doi: 10.1007/s002530051423. [DOI] [PubMed] [Google Scholar]
- 7.Hanson J R, Ackerman C E, Scow K M. Biodegradation of methyl tert-butyl ether by a bacterial pure culture. Appl Environ Microbiol. 1999;65:4788–4792. doi: 10.1128/aem.65.11.4788-4792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman M, Won P K, Williamson K, O'Reilly M. Cometabolism of MTBE by alkane-utilizing microorganisms. In: Wickramanayake G B, Hinchee R E, editors. Natural attenuation: chlorinated and recalcitrant compounds. Columbus, Ohio: Battelle Press; 1998. pp. 321–326. [Google Scholar]
- 9.Marinucci A C, Bartha R. Apparatus for monitoring the mineralization of volatile 14C-labeled compounds. Appl Environ Microbiol. 1979;38:1020–1022. doi: 10.1128/aem.38.5.1020-1022.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLee A G, Kormendy A C, Wayman M. Isolation and characterization of n-butane-utilizing microorganisms. Can J Microbiol. 1972;18:1191–1195. doi: 10.1139/m72-186. [DOI] [PubMed] [Google Scholar]
- 11.Mo K, Lora C O, Wanken A E, Javanmardian M, Yang X, Kulpa C F. Biodegradation of methyl t-butyl ether by pure bacterial cultures. Appl Microbiol Biotechnol. 1997;47:69–72. doi: 10.1007/s002530050890. [DOI] [PubMed] [Google Scholar]
- 12.Phelps P, Agarwal S, Speitel G E, Jr, Georgiou G. Methylosinus trichosporium OB3b mutants having constitutive expression of soluble methane monooxygenase in the presence and absence of high levels of copper. Appl Environ Microbiol. 1992;58:3701–3708. doi: 10.1128/aem.58.11.3701-3708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sariaslani F S, Kunz D A. Induction of Cytochrome P-450 in Streptomyces griseus by soybean flour. Biochem Biophys Res Commun. 1986;141:405–410. doi: 10.1016/s0006-291x(86)80187-5. [DOI] [PubMed] [Google Scholar]
- 14.Sariaslani F S, Trower M K, Buchholz S E. Xenobiotic transformations by Streptomyces griseus. Dev Ind Microbiol. 1989;30:161–171. [Google Scholar]
- 15.Speitel G E, Jr, Thompson R C, Weissman D. Biodegradation kinetics of Methylosinus trichosporium OB3b at low concentrations of chloroform in the presence and absence of enzyme competition by methane. Water Res. 1993;27:15–24. [Google Scholar]
- 16.Squillace P J, Zogorski J S, Wilber W G, Price C V. Preliminary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993–1994. Environ Sci Technol. 1996;30:1721–1730. [Google Scholar]
- 17.Steffan R J, McClay K, Vainberg S, Condee C W, Zhang D. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl Environ Microbiol. 1997;63:4216–4222. doi: 10.1128/aem.63.11.4216-4222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trower M K, Sariaslani F S, O'Keefe D P. Purification and characterization of a soybean flour-induced cytochrome P-450 from Streptomyces griseus. J Bacteriol. 1989;171:1781–1787. doi: 10.1128/jb.171.4.1781-1787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Environmental Protection Agency. Drinking water advisory: consumer acceptability advice and health effects analysis on methyl tertiary-butyl ether (MTBE). EPA 822-F-97–008. U.S. Washington, D.C.: Environmental Protection Agency; 1997. [Google Scholar]