Abstract
In this study, different injection solutions containing opioid and nonopioid compounds used for patient‐controlled analgesia in hospice and palliative care were evaluated in terms of analyte stability. Investigated injection solutions contained different combinations of morphine, hydromorphone, metamizole and esketamine. For the practical implementation, samples from infusion pumps were daily drawn over a period of 7 days at 22 and 37°C. Quantitative measurements were performed on a high‐performance liquid chromatography system with ultraviolet detection applying a validated analytical method. All compounds apart from morphine showed no evident changes in concentration. However, a significant loss of morphine was observed for injection mixtures containing both morphine and metamizole at 37°C. After 7 days, only 72% of the initially measured morphine concentration was measured in the binary and 77% in the ternary mixture. Furthermore, an additional compound was detected that could represent the morphine‐metamizole‐adduct, “metamorphine”. Based on these results, a significantly reduced morphine concentration must be expected after only 3 days if an injection solution mixture containing both morphine and metamizole is administered to a patient at 37°C. Since the analgesic effects of morphine–metamizole adducts have not yet been thoroughly investigated, further clinical studies are necessary before accurate conclusions can be drawn in this regard.
Keywords: analgesic injection mixture, hospice and palliative care, morphine, patient‐controlled analgesia, stability evaluation
1. INTRODUCTION
The aim of analgesia is to relieve or prevent suffering from pain, which is a receptor‐mediated response that is transmitted along nerve fibres and perceived in the brain. Pain can be averted by inhibiting certain receptors, blocking the transmission of neurotransmitters or altering the perception within the brain (Cox, 2009). For this purpose, mainly analgesics are used, which are generally classified into two main groups, opioid and nonopioid compounds (Curtis et al., 2019; Raja et al., 2018). Both classes of analgesics exhibit several adverse side effects. Therefore, more than 20 years ago, multimodal pain management was introduced to improve analgesia and to reduce the incidence of negative events (Breivik et al., 2008; Jahr et al., 2011). Multimodal or balanced pain management refers to a technique in which analgesics with different mechanisms are simultaneously administered to a patient. The rationale behind this concept is to lower drug doses and therefore reduce unwanted side effects, yet achieve sufficient analgesia owing to additive or synergistic effects between different analgesics (Greene, 2002; Kehlet & Dahl, 1993). Consequently, it is an opioid‐sparing means of analgesia, while at the same time providing superior pain control (Rowbotham et al., 2008).
Many patients have considerable difficulty in taking oral medications. Nevertheless, to ensure the best possible balanced pain management and to avoid the use of multiple infusion needles, different drugs are often combined and injected as a mixture (Graham & Clark, 2005; María et al., 2018; Mukoreka & Sisay, 2015). In this context, infusion pumps (also called syringe pumps or drivers) are used to deliver medications by intravenous, arterial or subcutaneous routes (Harrs et al., 2013). One major benefit of this is that drug administrations are much more convenient for both the patient and the medical practitioner (McPherson, 2019). Furthermore, the use of patient‐controlled analgesia (PCA) even allows the patient to determine the frequency of medication administration, which usually results in lower opioid doses, improved mobility, minimal sedation and higher patient satisfaction (Urden et al., 2019).
However, it is also well known that combining two or more substances can lead to physical as well as chemical changes in the initial compounds of a mixture (Connors et al., 1986; Gikic et al., 2000; O'Connell & Haile, 2011). As a result, combinations of different analgesics may also have an effect on the chemical stability of single chemicals, which has already been investigated in numerous publications (Chen et al., 2014, 2015, 2018; Fang et al., 2016, 2017; Gu et al., 2015; Lee et al., 2020; Selbach et al., 2011). When injection mixtures are administered over several days or weeks, compounds can degrade and/or react with other substances, especially when exposed to moisture, light, ambient air or elevated temperatures. As a result, it is possible that the efficacy of the drug may also be reduced or altered (Aschenbrenner & Venable, 2009). Therefore, stabilities of applied analgesics in single‐injection solutions and injection mixtures must be thoroughly investigated to minimize the risk of adverse effects and to ensure their consistent analgesic effect in the period of administration to the patient (Akash & Rehman, 2020).
In hospice and palliative care, different analgesic injection solution mixtures containing morphine or hydromorphone in different combinations with esketamine and metamizole are used in PCA to achieve improved pain management. The Tyrolean Oncology Working Group (Tiroler Arbeitskreis für Onkologie), in cooperation with the Tyrolean Hospice Association (Tiroler Hospiz Gemeinschaft), has released a recommendation brochure on the topic of palliative care. It was reported, for example, that morphine can be mixed with metamizole or esketamine for use in PCA infusion pumps (Lukas et al., 2016). However, it was not stated over what period of time and under which storage conditions these mixtures are supposed to be stable. As previously mentioned, storage conditions can have a huge impact on analyte stabilities and should therefore be taken very seriously. Several research articles have already examined the chemical stabilities of morphine (Ping et al., 2019; Vermeire, 1999), hydromorphone (Hildebrand et al., 2001; Nassr et al., 2003), metamizole (Xiang et al., 2007) and esketamine (Ancedy et al., 2021; Donnelly, 2013; Huvelle et al., 2016) in saline solution. However, it is hardly known whether these analgesics are also stable as mixtures. Furthermore, the majority of reports were mainly concerned with stabilities under normal storage conditions. For example, Donnelly has shown that a mixture of morphine and esketamine was stable (analyte concentration changes ≤ 10%) for 91 days at 5 and 23°C (Donnelly, 2009). A study by Schmid et al. indicated that morphine–esketamine mixtures did not significantly change, regarding their analyte concentrations, for at least 4 days at room temperature and at a pH value between 5.5 and 7.5 (exact temperature and stability limits were not given in this study; Schmid et al., 2002). Ensom et al. demonstrated that mixtures containing hydromorphone and esketamine were stable (analyte concentration changes ≤ 10%) for at least 7 days at 25°C (Ensom et al., 2009). Müller evaluated the stability of preparations containing morphine and metamizole. In her work she demonstrated that mixtures were stable (analyte concentration changes ≤ 10%) for 21 days in the refrigerator (Müller, 2009). Despite their importance for use in hospice and palliative care, analyte stabilities have hardly been evaluated in PCA infusion pumps at 37°C. In many cases, however, pumps and their medication cassette reservoirs are carried on or in close proximity to the body and may therefore be exposed to elevated temperatures, like 37°C. Furthermore, sunlight and radiators can also increase the temperature of the injection solution. Only Müller reported that morphine might react in the presence of metamizole at 37°C to a morphine–metamizole adduct (“metamorphine”; Müller, 2009). Accordingly, this study aimed to investigate the stability of different injection solution mixtures containing morphine or hydromorphone in combination with metamizole and esketamine in PCA infusion pumps at ambient (22°C) as well as body temperature (37°C). In this context, emphasis was laid on the ternary injection mixture containing morphine, metamizole and esketamine, which has not yet been studied.
2. METHODS
2.1. Reagents, materials and instrumentation
Methanol (MeOH), HPLC‐grade (Chromasolv), was purchased from Sigma Aldrich (St Louis, USA), an affiliate of Merck (Darmstadt, Germany). Trifluoroacetic acid (TFA, ≥99.9%) was obtained from Carl Roth (Karlsruhe, Germany). Water was purified with a Millipore Milli‐Q® water purification system (Bedford, MA, USA). The internal standard potassium sorbate (>99.0%) was purchased from Merck. The opioid‐based medications, Vendal® containing the chemical morphine (40,000 mg L−1) and Hydal® containing hydromorphone (20,000 mg L−1), were purchased from Gerot Lannach (Lannach, Austria) and Mundipharma GmbH (Frankfurt am Main, Germany), respectively. The drug Ketanest® S containing esketamine (25,000 mg L−1) as the active ingredient was obtained from Pfizer (New York, USA). Metamizole (500,000 mg L−1), containing medications, including Metamizol Kalceks and Novalgin®, was purchased from Fresenius Kabi (Bad Homburg vor der Höhe, Germany) and Sanofi (Paris, France), respectively. The analgesic standard stock solution, used for validation, was prepared by mixing certain volumes of Vendal®, Hydal®, Metamizol Kalceks and Ketanest® S in a volumetric flask and making up to the mark with water, and contained the following analyte concentrations: 1000 mg L−1 morphine, 500 mg L−1 hydromorphone, 1,500 mg L−1 metamizole and 500 mg L−1 esketamine. The internal standard stock solutions contained different concentrations of potassium sorbate (1,000; 10,000 and 200,000 mg L−1) dissolved in water. All stock solutions were stored at 4°C in the dark.
Preparation of sample solutions was performed with Terumo® syringes (5 ml) with Luer Lock from Terumo Europe (Leuven, Belgium) by the injection of certain drug and saline solution volumes into CADD™ medication cassette reservoirs (100 ml) from Smiths Medical ASD Inc. (Minneapolis, MN, USA). Samples were drawn from CADD‐Prizm® VIP ambulatory infusion pumps of model 6100 from Smiths Medical ASD Inc., which were located in a temperature controlled room (22°C) or heat chamber (37°C; U 40 from Memmert GmbH + Co. KG, Schwabach, Germany). Samples were stored in a Froster Labo 730 from the company Kirsch (Willstätt‐Sand, Germany).
Quantitative measurements were performed on a 1100 Series HPLC Value System from Agilent (Santa Clara, CA, USA) equipped with a 1100 Series diode array detector. Separation of analytes was executed using a Kinetex 2.6u PFP 100A, 150 × 4.6 mm analytical column from Phenomenex (Torrance, CA, USA). The mobile phase consisted of 0.1 vol% TFA in water (eluent A) and MeOH (eluent B). A gradient program was applied using following settings (min/vol% of eluent B): 0/20, 10/85, 11/20, 16/20. The injection volume was set to 5 μl, the mobile phase flow‐rate was 0.6 ml min−1, the injection temperature was 4°C and the temperature of the column oven was set to 40°C. Detection of the analytes was performed at 210 nm. Qualitative analyses were additionally performed on a LC–MS Acquity Arc System from Waters Corporation (Milford, MA, USA) equipped with an Acquity QDa detector and a 2998 photodiode array detector (split ratio 1:9). Separations were carried out on an Excel 3 C18‐PFP, 150 × 4.6 mm analytical column from Advanced Chromatography Technologies Ltd (Aberdeen, Scotland). Separation was accomplished with a mobile phase consisting of 0.1 vol% FA in water (eluent C) and MeOH (eluent B). A gradient program was applied (min/vol% of eluent B): 0/20, 20/80, 20.1/20, 25/20. For enhancing ionization inside the ESI source, a mixture of 70 vol% ACN in water and 0.1 vol% FA was used as the mobile phase from the split to the QDa detector. All flow rates were set to 0.5 ml min−1, the injection volume was 5 μl and the column temperature was set to 40°C. Detection was performed in ESI+ using a full scan between 50 and 1,000 m/z with a sampling frequency of 8 Hz and a cone voltage of 15 V. The mass accuracy was automatically calibrated using an internal calibration oil prior to measurements.
2.2. Validation of the quantitative LC–UV method
Validation was accomplished according to the guidelines for quality assurance in forensic–toxicological analyses of the Society of Toxicological and Forensic Chemistry and included the linearity of calibration, processed sample stability and freeze–thaw stability (Peters et al., 2009). Detailed validation results can be found in the Supplementary Information.
A calibration model was established by spiking and diluting the analgesic standard stock solution with internal standard (1,000 mg L−1) and water. Three independent and equal dilution series were prepared with analyte concentration levels ranging from 25 to 450 mg L−1 and a consistent internal standard concentration of 200 mg L−1. Every concentration level was measured three times to obtain a total of nine data points per level.
Processed sample stability at 4°C was tested by measuring the internal standard potassium sorbate at 200 mg L−1 and analyte concentrations at maximum and minimum calibration level every hour for a total of 24 h. For the implementation, the analgesic standard stock solution was spiked with internal standard and diluted with water to obtain analyte concentrations at maximum and minimum calibration levels. The two obtained samples were subsequently pooled into six aliquots each and measured successively.
Three freeze–thaw cycles were performed to guarantee analyte stabilities after freezing and thawing samples three times. Therefore, three aliquots (1 ml) of analgesic standard stock solution were pipetted into three separate amber glass vials and placed into a freezer at −28°C for 24 h. On the following day, samples were thawed at ambient temperature for 3 hours. Subsequently, 100 μl of each sample was transferred into a separate vial and measured with HPLC–UV three times each. The previous described freeze–thaw procedure was replicated two more times.
2.3. Infusion pump installation, sampling and sample preparation
All five investigated injection solutions applied for hospice and palliative care were prepared at the Innsbruck University Hospital, Pharmacy Department. For their preparation, specific drug volumes and certain amounts of saline solution (0.9 wt%) were directly injected into medication cassettes and thoroughly mixed. Subsequently, each medication cassette was attached to an infusion pump. Every injection solution was separately prepared according to this procedure. Drug and saline volumes as well as resulting analyte concentrations for all injection solutions are given in Table 1. Six independent infusion pumps, containing the same type of injection solution at a time, were installed at the Hospital Pharmacy. Three pumps were stored at 22°C and three at 37°C. Samples with a total volume of 1.0 ml were drawn once (day 0–3) or twice a day (day 4–7) over a period of 7 days. Samples were stored in Eppendorf tubes® (2 ml) in a freezer at −28°C until analysis.
TABLE 1.
The drug and saline solution volumes (V) used for the preparation of analgesic injection solutions and resulting analyte concentrations (c)
| Injection solution | V (opioid drug)/ml | V (nonopioid drugs)/ml | V (saline solution, 0.9 wt%)/ml | c (opioid)/mg L−1 | c (nonopioids)/mg L−1 |
|---|---|---|---|---|---|
| Morphine | 25 | — | 75 | 10,000 | — |
| Hydromorphone | 20 | — | 80 | 4,000 | ‐ |
| Hydromorphone/esketamine | 20 | 20 | 60 | 4,000 | 5,000 |
| Morphine/metamizole | 25 | 50 | 25 | 10,000 | 250,000 |
| Morphine/metamizole/esketamine | 25 | 50/20 | 5 | 10,000 | 250,000/5000 |
Prior to HPLC measurements, samples were thawed at ambient temperature for 3 h, spiked with internal standard (1:1 vol%) and subsequently diluted with water to obtain final analyte concentrations ranging from 80 to 200 mg L−1. Samples containing one or two of the components morphine, hydromorphone and esketamine were spiked with internal standard (10,000 mg L−1) and subsequently diluted with water to obtain a total dilution of 1:25 vol%. For the practical implementation, 100 μl of thawed sample was combined with 100 μl of internal standard (10,000 mg L−1). Subsequently, 40 μl of this mixture was diluted with 960 μl of water and an aliquot measured via HPLC–UV. A second independent sample preparation had to be performed if metamizole was present in the sample. Owing to its very high concentration, a more concentrated internal standard (200,000 mg L−1) and a higher dilution had to be used. For the implementation, 100 μl of the metamizole‐containing sample was combined with 100 μl of the concentrated internal standard. Subsequently, 10 μl of this mixture was diluted with 4,990 μl of water to obtain a total dilution of 1:500 vol%. In order to assess the accuracy, reliability and repeatability of the obtained data, sample preparation and HPLC–UV measurements were all carried out in triplicate.
2.4. Data evaluation, the creation of time series and stability assessment
For data evaluation, initial analyte concentrations at day zero were compared with concentrations obtained from measurements on consecutive days. Therefore, in the first step, analyte peak areas were divided by the peak area of the internal standard. In our case, the internal standard (potassium sorbate) was primarily used to account for concentration changes during sample preparation (Moldoveanu & David, 2015). The obtained peak area ratios were subsequently compared with the average peak area ratio at day zero (which served as reference value and was therefore set as 100%) to obtain “relative analyte concentrations”. Then the mean relative analyte concentration and corresponding standard deviation (SD) values were calculated for each sampling time and analyte. These values were subsequently used for graphical data representation. In addition, deviations from the reference value (100%) were calculated by subtracting the reference value from relative analyte concentrations.
The first sample was directly drawn after setting up the infusion pumps at day zero. From day zero to day three, sampling was performed once a day at 15:00. Starting with day four, samples were drawn twice a day at 7:30 and 15:00. For the graphical representation, calculated relative analyte concentrations and corresponding SD values were plotted against storage time. In this context, the first sampling at 15:00 at day zero was set as the starting point.
Injection solutions were considered to be stable if relative analyte concentrations were maintained in an interval of 90–110% compared with the initially measured value on day zero. In other words, a decrease or increase >10% from the initial concentration was considered significant. This specific interval was applied to better compare this work with related studies where similar stability limits were applied (Donnelly, 2009; Ensom et al., 2009; Müller, 2009). A downward or upward trend in concentration was considered to exist when there was a linear correlation observed between relative analyte concentration and storage time (slope test, P > 0.05).
3. RESULTS
The study investigated the chemical stabilities of morphine and hydromorphone with and without the addition of metamizole and esketamine in injection solutions at 22 and 37°C. The two storage temperatures were chosen because infusion pumps are usually exposed to temperatures within this range. Detailed stability results are given in the Supplementary Information.
3.1. LC–UV method validation
For the evaluation of calibration measurements, analyte peak areas were divided by the area of the internal standard. Subsequently, peak area ratios were plotted against analyte concentrations. The obtained straight lines showed linear behaviours with correlation coefficients ranging from 0.9996 to 0.9999. The accuracy and precision of the calibration model were validated and gave bias values between −3.9 and 1.6% and a maximum relative standard deviation (RSD) value of 0.74%.
Evaluation of the processed sample stability was performed by setting the analyte and internal standard peak areas obtained from the first measurement as 100% (initial values). All peak areas obtained from consecutive measurements were subsequently compared with these initial values. Analyte and internal standard concentrations after 24 h at 4°C in the autosampler deviated at most between 0.2 and 3.1% from the initial concentration, which indicated sufficient stability within the measurement time.
For measurement evaluation of freeze–thaw experiments, analyte peak areas were divided by the area of the internal standard. Subsequently, peak area ratios obtained from measurements before freezing were set as 100%. All peak area ratios obtained after one, two and three freeze–thaw cycles were compared with the mean value before freezing. Analyte concentrations did not significantly change when performing a total of three freeze–thaw cycles with maximum deviations from the value before freezing of 1.2–3.7%.
3.2. Single analgesic injection solutions
Relative analyte concentration changes of single injection solutions containing morphine or hydromorphone at 22 and 37°C are displayed in Figure 1(a–d). Morphine concentrations deviated from the initial concentration by a maximum of −3.5% (22°C) and 3.2% (37°C), with the highest SD values being 3.6% (22°C) and 1.7% (37°C). Hydromorphone concentrations deviated by a maximum of 4.6% (22°C) and 3.9% (37°C) with maximum SD values of 2.2% (22°C) and 1.7% (37°C). The obtained results showed that concentration changes for both opioids were clearly within the defined interval of ±10%. Therefore, it can be said that morphine as well as hydromorphone single injection solutions stored at ambient as well as body temperature are stable for use in PCA within at least 7 days.
FIGURE 1.
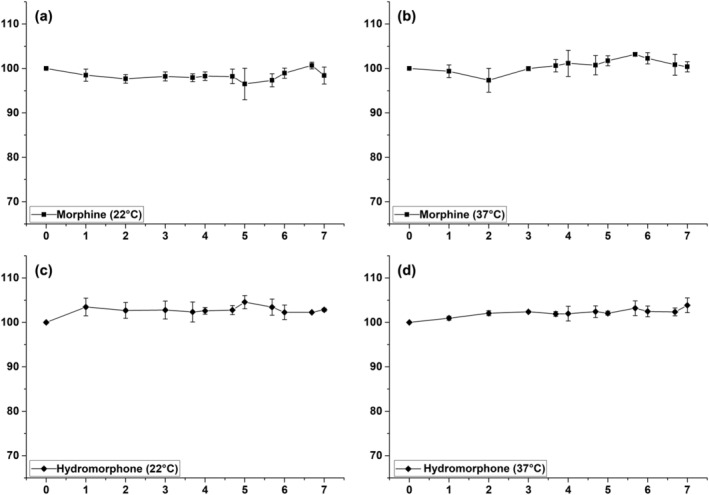
Stability evaluation of single injection solutions, including morphine at 22°C (a) and 37°C (b) and hydromorphone at 22°C (c) and 37°C (d), within a storage period of 7 days. Relative analyte concentration in percent plotted against storage time in days with corresponding SD values as error bars
3.3. Binary injection solution mixtures
Stability evaluation for the binary injection solution containing hydromorphone and esketamine stored at 22 and 37°C is displayed in Figure 2(a and b). Hydromorphone concentrations deviated by a maximum of 8.6% (22°C) and 2.4% (37°C) from the initial concentration with maximum SD values of 5.5% (22°C) and 2.4% (37°C). Esketamine concentrations showed a maximum deviation of 8.8% (22°C) and −2.6% (37°C) with maximum SD values of 5.0% (22°C) and 2.4% (37°C). Both analgesics exhibited maximum concentration changes within the defined interval of ±10%. Therefore, binary injection solution mixtures containing hydromorphone and esketamine stored at both ambient and body temperature are stable for at least 7 days when used in PCA.
FIGURE 2.
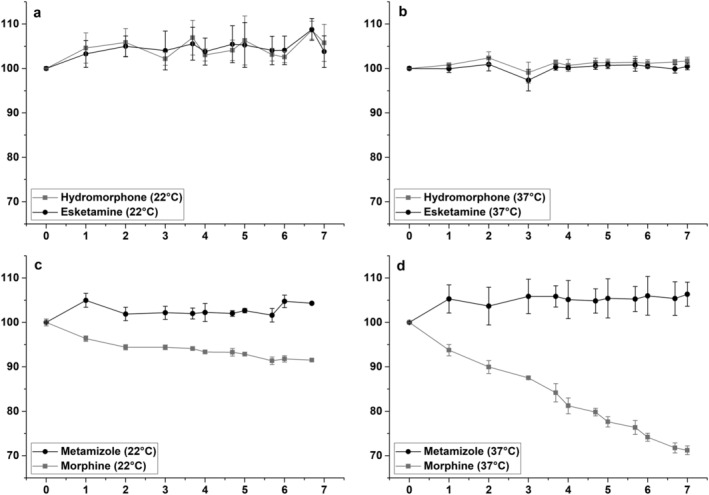
Stability evaluation of binary injection mixtures, including hydromorphone and esketamine at 22°C (a) and 37°C (b) as well as metamizole and morphine at 22°C (c) and 37°C (d), within a storage period of 7 days. Relative analyte concentration in percent plotted against storage time in days with corresponding SD values as error bars
A graphical representation of the binary injection mixture containing morphine and metamizole is given in Figure 2(c) and (d). Metamizole concentrations deviated by a maximum of 5.0% (22°C) and 6.0% (37°C) from the initial concentration with maximum SD values of 2.0% (22°C) as well as 4.4% (37°C). Morphine concentration showed a maximum deviation of −8.6% (22°C) and −28.2% (37°C) with maximum SD values of 0.9% (22°C) and 2.1% (37°C). The obtained results show that at a storage temperature of 22°C, metamizole and morphine concentration changes stay within the defined interval of ±10%. Also, at 37°C metamizole concentrations did not significantly change; however, morphine concentrations rapidly decreased with increasing storage time. After 7 days, only 71.8% of the initially measured morphine concentration was detected. Linear regression analysis showed that there was a linear correlation between morphine concentration and storage time at 37°C, indicating that morphine stability is dependent on the duration of storage. In summary, the binary mixture containing morphine and metamizole is stable during its use in PCA at 22°C for at least 7 days. However, a significantly reduced morphine concentration must be expected when PCA pumps are stored at 37°C during patient administration after only 3 days.
3.4. Ternary injection solution mixtures
The results for stability evaluation of the ternary injection mixture containing morphine, esketamine and metamizole at 22°C and 37°C are displayed in Figure 3. Esketamine concentrations deviated by a maximum of 1.3% (22°C) and −3.7% (37°C) from the initial concentration with maximum SD values of 4.7% (22°C) and 1.8% (37°C). Metamizole concentration showed a maximum deviation of 5.9% (22°C) and 2.6% (37°C) with maximum SD values of 1.8% (22°C) and 1.9% (37°C). Morphine concentrations deviated by a maximum of −6.5% (22°C) and −23.4% (37°C) with maximum SD values of 4.2% (22°C) and 1.8% (37°C). The obtained results at a storage temperature of 22°C showed that esketamine, metamizole and morphine concentrations remained within the defined interval of ±10%. Similar results were obtained for esketamine and metamizole concentrations at 37°C. On the other hand, morphine concentrations constantly decreased at 37°C with increasing storage time, so that by day seven only 76.6% of the initially measured morphine concentration could be detected. Furthermore, a linear correlation between morphine concentration and storage time was observed, indicating that morphine stability is dependent on the duration of storage. In conclusion, it can be said that the ternary mixture containing esketamine, metamizole and morphine is stable during its use in PCA at 22°C for at least 7 days. However, a significantly reduced morphine concentration must be expected after only 3 days if administration is conducted with PCA infusion pumps at 37°C.
FIGURE 3.
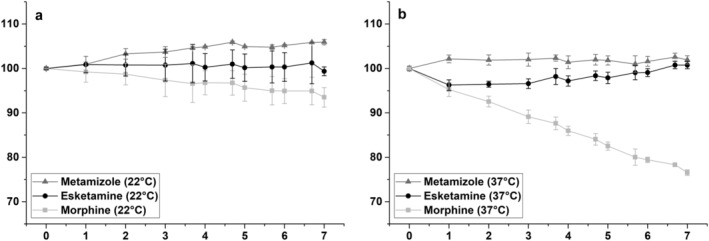
Stability evaluation of the ternary injection mixture, including morphine, metamizole and esketamine at 22°C (a) and 37°C (b), within a storage period of 7 days. Relative analyte concentration in percent plotted against storage time in days with corresponding SD values as error bars
3.5. Morphine–metamizole adduct
Ternary and binary injection mixtures containing metamizole and morphine stored at 37°C exhibited an additional peak in the chromatogram starting with day one. Its peak area steadily increased with increasing storage time. The unknown substance was detected between metamizole and the internal standard with a retention time of 8.2 min. Further qualitative analyses on a LC–MS system were performed in order to determine its origin. The results of the ternary injection mixture after 7 days of storage in positive ion mode showed that the unknown peak mainly consisted of the m/z signals 515, 258, 218, 537 and 553. The m/z signals 515, 258, 537 and 553 probably belong to the morphine–metamizole adduct “metamorphine”, which can be formed by the reaction of 4‐methylaminoantipyrine, formaldehyde and morphine (Müller, 2009). The degradation of metamizole and the associated decrease in metamizole concentration was probably not measured owing to the large excess of metamizole in the injection solutions (metamizole was present in a 25‐fold excess compared with morphine). However, further MS–MS analyses would have to be carried out in order to prove these assumptions and be able to make more precise statements about the structure and origin of the resulting morphine–metamizole adduct.
4. DISCUSSION
To our knowledge, this is the first scientific study evaluating the stability of a ternary injection mixture containing morphine, metamizole and esketamine at 22 and 37°C. Furthermore, PCA infusion pumps were used as a storage and sampling device for all of the investigated injection solutions. Therefore, the influence of the medication cassette reservoir and infusion pump on analyte stabilities was also taken into account.
4.1. Storage temperature and resulting measures
The results demonstrated that the storage temperature had a huge influence on the stability of injection solutions containing both metamizole and morphine as active analgesic ingredients. When PCA infusion pumps were stored at 22°C, no significant changes in morphine concentrations were observed over a period of 7 days, whereas morphine concentrations linearly decreased at 37°C with a significant loss in morphine concentration after only 3 days of storage. Temperatures of 37°C can occur, for example, when PCA pumps are directly located on or in close proximity to the body, near radiators or in direct sunlight. These circumstances should be avoided, as they cause these injection solutions to decompose more quickly and the formation of metamizole–morphine adducts is possible. Therefore, it is of utmost importance to inform medical practitioners and subsequently patients about the instability of morphine–metamizole mixtures at elevated temperatures, like 37°C. If they are aware of the enormous impact of storage conditions, measures can be taken to prevent the solutions from heating up during administration. As we now know, the solution should be stable at 22°C over a period of at least 7 days. Therefore, there should be no concerns if these mixtures are used correctly under the right storage conditions in PCA.
4.2. Comparison of binary and ternary mixture
Only minor differences regarding the decrease of morphine concentration were found in the binary compared to the ternary injection mixture. After 7 days, 72% of the initially measured morphine concentration was detected in the binary and 77% in the ternary injection mixture. This indicates that the addition of esketamine to the injection solution only has a minor influence on the stability of morphine and metamizole. The concentration of esketamine was not significantly affected by the other two active pharmaceutical ingredients, demonstrating its compatibility with morphine and metamizole.
4.3. Consequences for the patient
This study, however, cannot estimate the impact of reduced morphine concentrations on the patient during medical treatment, nor is it possible to predict the effects of emerging adducts like “metamorphine”. Nevertheless, it is very likely that the formed adducts are potentially analgesic, as no morphine–metamizole mixture has yet been reported to show a time‐dependent loss of analgesic effects (Trittler et al., 2011). As there are no conclusive studies on this subject in the literature, we must wait for further clinical studies to be carried out in order to be able to make definite statements in this regard.
4.4. Juridical perspective
From a juridical point of view, every medication has to be validated for a therapeutic use and approved by a ruling authority of a government (Development & Approval Process (Drugs), 2019). If certain drugs are combined and used as a mixture, it is basically necessary to get approval before its application (FDA, 2019). However, the “off‐label” use of medications is very common and legal, even if it is often done without adequate supporting data. When there is a high need and no other options are available, it can allow medical practitioners to adopt new practices based on emerging evidence, which can be a huge advantage. However, this can also have negative consequences, especially when there are no studies available concerning drug safety and efficacy (Stafford, 2008).
Injection solution mixtures containing opioid and nonopioid active ingredients used in PCA for hospice and palliative care are not approved medicinal products, but patient‐specific extemporaneous preparations. In many hospitals, such mixtures are still prepared under uncontrolled conditions on wards and in outpatient clinics. In June 2016 the Council of Europe published the article “Resolution CM/Res(2016)2 on good reconstitution practices in health care establishments for medicinal products for parenteral use” (Committee of Ministers, 2016) with the aim of preventing harm to patient health from incorrect reconstitution and providing guidance to the healthcare establishment in deciding where reconstitution should take place: in a pharmacy or in a clinical facility. This decision should be based on a risk assessment and support professionals in healthcare establishments, such as pharmacists, nurses etc., in planning and implementing reconstitutions. It is the responsibility of the management to ensure that systems are in place for safe reconstitution. Risks associated with complex reconstitution procedures, like the preparation of analgesic mixtures investigated in this study, include, for example, microbiological contamination, incorrect composition or risks related to the pharmacological activity. Therefore, the preparation of injection solutions containing opioid and nonopioid compounds applied in PCA should take place in an environmentally controlled area in the pharmacy or under the full responsibility of a pharmacist. In Austria, for example, magisterial preparations in pharmacies should be prepared in accordance with the provisions of the European Pharmacopoeia (European Pharmacopoeia, 2019) as defined in Section 1 of the Pharmacopoeia Act. If the Pharmacopoeia does not contain any regulations on the preparation, they shall be prepared in accordance with the state of the art (Gesamte Rechtsvorschrift für Apothekenbetriebsordnung 2005, Fassung vom 02.09.2021, 2019). For hospital pharmacies operating under a GMP certificate, manufacturing specifications corresponding to the respective state of the art must be available for each manufactured medication, each concentration and each batch size (Gesamte Rechtsvorschrift für Arzneimittelbetriebsordnung 2009, Fassung vom 02.09.2021, 2019).
5. CONCLUSION
Scientific studies evaluating the stability of certain drug mixtures can detect composition changes at an early stage and thereby limit or prevent further off‐label administrations, which is a protective measure for both medical practitioners and their patients. Consequently, this study is an important contribution to the already existing state of knowledge.
AUTHOR CONTRIBUTIONS
The study was supervised by M. Rainer, H. Oberacher and G. K Bonn and was largely designed by H. Oberacher, M. Rainer, M. Harder, U. EI Horvath, A. Schlager, S. Kerndler, F. Schüllner and M. Jeske. The preparation of analgesic injection solutions and installation of PCA pumps was mainly performed by U. EI Horvath and A. Schlager in association to the team of the aseptic production. U. EI Horvath, S. Kerndler and F. Schüllner carried out sample collection. Sample preparation, sample measurements as well as data evaluation was conducted by M. Harder and A. Fiegl‐Lechner. Interpretation of the obtained data was mainly performed by H. Oberacher, M. Rainer and M. Harder. The manuscript was drafted by M. Harder. All authors critically reviewed and revised the final manuscript and have approved it for publication in the Journal of Biomedical Chromatography.
DECLARATION OF CONFLICTING INTEREST
There are no conflicts to declare.
Supporting information
Table S1. Concentration levels (c1–c6) for analyte calibrations.
Table S2. Results obtained from analyte calibrations.
Table S3. Results obtained from processed sample stability at calibration maximum at 4°C.
Table S4. Results obtained from processed sample stability at calibration minimum at 4°C.
Table S5. Results obtained from freeze–thaw stability.
Table S6. Results for individual analgesic injection solution containing hydromorphone at storage temperatures of 22 and 37°C.
Table S7. Results for individual analgesic injection solution containing morphine at storage temperatures of 22 and 37°C.
Table S8. Results for binary analgesic injection mixture containing hydromorphone and esketamine at a storage temperature of 22°C.
Table S9. Results for binary analgesic injection mixture containing hydromorphone and esketamine at a storage temperature of 37°C.
Table S10. Results for binary analgesic injection mixture containing morphine and metamizole at a storage temperature of 22°C.
Table S11. Results for binary analgesic injection mixture containing morphine and metamizole at a storage temperature of 37°C.
Table S12. Results for ternary analgesic injection mixture containing morphine, esketamine and metamizole at a storage temperature of 22°C.
Table S13. Results for ternary analgesic injection mixture containing morphine, esketamine and metamizole at a storage temperature of 37°C.
Table S14. Assigned m/z signals obtained from MS analysis in positive ion mode of the ternary injection mixture after 7 days of storage.
Figure S1. Chromatogram obtained from HPLC–UV measurement at calibration maximum (c6), including signals for morphine (1), hydromorphone (2), metamizole (3), potassium sorbate (4) and esketamine (5).
ACKNOWLEDGEMENTS
We would like to thank the team of the aseptic production (Innsbruck University Hospital, Pharmacy Department) for their support.
Harder, M. , Fiegl‐Lechner, A. , Oberacher, H. , Horvath, U. E. I. , Schlager, A. , Jeske, M. , Kerndler, S. , Schüllner, F. , Bonn, G. K. , & Rainer, M. (2022). Stability evaluation of morphine, hydromorphone, metamizole and esketamine containing analgesic mixtures applied for patient‐controlled analgesia in hospice and palliative care. Biomedical Chromatography, 36(4), e5340. 10.1002/bmc.5340
DATA AVAILABILITY STATEMENT
More comprehensive data can be obtained from the Supplementary Information.
REFERENCES
- Akash, M. S. H. , & Rehman, K. (2020). Drug stability and chemical kinetics (1st ed.). Springer. 10.1007/978-981-15-6426-0 [DOI] [Google Scholar]
- Ancedy, D. , Sebti, M. , Postaire, M. , Vidal, F. , Cisternino, S. , & Schlatter, J. (2021). Stability of 10‐mg/ml and 50‐mg/ml ketamine oral solutions. American Journal of Health‐System Pharmacy: AJHP: Official Journal of the American Society of Health‐System Pharmacists, 78(9), 825–831. 10.1093/ajhp/zxab066 [DOI] [PubMed] [Google Scholar]
- Aschenbrenner, D. S. , & Venable, S. J. (2009). Study guide to accompany drug therapy in nursing (3rd ed.). Lippincott Williams & Wilkins. [Google Scholar]
- Breivik, H. , Campbell, W. , Nicholas, M. , & Rice, A. S. C. (2008). Clinical pain management (first paperback edition). CRC Press. 10.1201/b13562 [DOI] [Google Scholar]
- Chen, F. , Fang, B. , Li, P. , Zhu, X. , & Zhou, B. (2014). Physico‐chemical stability of butorphanol‐tramadol and butorphanol‐fentanyl patient‐controlled analgesia infusion solutions over 168 hours. Die Pharmazie—an International Journal of Pharmaceutical Sciences, 69(8), 585–588. 10.1691/ph.2014.4507 [DOI] [PubMed] [Google Scholar]
- Chen, F.‐C. , Shi, X.‐Y. , Li, P. , Yang, J.‐G. , & Zhou, B.‐H. (2015). Stability of butorphanol–tropisetron mixtures in 0.9% sodium chloride injection for patient‐controlled analgesia use. Medicine, 94(6), e432. 10.1097/MD.0000000000000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P. , Chen, F. , & Zhou, B.‐H. (2018). Compatibility and stability of dezocine and tropisetron in 0.9% sodium chloride injection for patient‐controlled analgesia administration. Medicine, 97(50), e13698. 10.1097/MD.0000000000013698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee of Ministers . (2016). Resolution CM/Res(2016)2 on good reconstitution practices in health care establishments for medicinal products for parenteral use. https://www.edqm.eu/sites/default/files/resolution_cm_res_2016_2_good_reconstitution_practices_in_health_care_establishments_for_medicinal_products_for_parenteral_use_pdf
- Connors, K. A. , Amidon, G. L. , & Stella, V. J. (1986). Chemical stability of pharmaceuticals: A handbook of pharmacists. Wiley. [Google Scholar]
- Cox, F. (2009). Perioperative pain management. Wiley‐Blackwell. http://site.ebrary.com/lib/alltitles/docDetail.action?docID=10345950 [Google Scholar]
- Curtis, K. , Ramsden, C. , Shaban, R. Z. , Fry, M. , & Considine, J. (2019). Emergency and trauma Care for Nurses and Paramedics (3rd ed.). Elsevier. [Google Scholar]
- Donnelly, R. F. (2009). Physical compatibility and chemical stability of ketamine‐morphine mixtures in polypropylene syringes. The Canadian Journal of Hospital Pharmacy, 62(1), 28–33. 10.4212/cjhp.v62i1.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly, R. F. (2013). Stability of diluted ketamine packaged in glass vials. The Canadian Journal of Hospital Pharmacy, 66(3), 198. 10.4212/cjhp.v66i3.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensom, M. H. H. , Decarie, D. , Leung, K. , & Montgomery, C. (2009). Stability of hydromorphone–ketamine solutions in glass bottles, plastic syringes, and IV bags for pediatric use. The Canadian Journal of Hospital Pharmacy, 62(2), 112–118. 10.4212/cjhp.v62i2.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Pharmacopoeia . (2019). European Pharmacopoeia 9.0–9.8 (9th German ed.). Deutscher Apotheker. [Google Scholar]
- Fang, B. , Wang, L. , Gu, J. , Chen, F. , & Shi, X.‐Y. (2016). Physicochemical stability of ternary admixtures of butorphanol, ketamine, and droperidol in polyolefin bags for patient‐controlled analgesia use. Drug Design, Development and Therapy, 10, 3873–3878. 10.2147/DDDT.S123411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, B.‐X. , Wang, L.‐H. , Liu, H.‐M. , Chen, F.‐C. , & Liu, J. (2017). Stability study of dezocine in 0.9% sodium chloride solutions for patient‐controlled analgesia administration. Medicine, 96(35), e7979. 10.1097/MD.0000000000007979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . (2019). Development & Approval Process (Drugs). https://www.fda.gov/drugs/development-approval-process-drugs
- FDA . (2019). Postmarketing Safety Reporting for Combination Products Guidance for Industry and FDA Staff. Final Guidance. https://www.fda.gov/media/111788/download
- Gesamte Rechtsvorschrift für Apothekenbetriebsordnung 2005, Fassung vom 02.09.2021 . (2019). https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&&Gesetzesnummer=20005989
- Gesamte Rechtsvorschrift für Arzneimittelbetriebsordnung 2009, Fassung vom 02.09.2021 . (2019). https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&&Gesetzesnummer=20003947
- Gikic, M. , Di Paolo, E. R. , Pannatier, A. , & Cotting, J. (2000). Evaluation of physicochemical incompatibilities during parenteral drug administration in a paediatric intensive care unit. Pharmacy World & Science: PWS, 22(3), 88–91. 10.1023/a:1008780126781 [DOI] [PubMed] [Google Scholar]
- Graham, F. , & Clark, D. (2005). The syringe driver and the subcutaneous route in palliative care: The inventor, the history and the implications. Journal of Pain and Symptom Management, 29(1), 32–40. 10.1016/j.jpainsymman.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Greene, S. A. (2002). Veterinary anesthesia and pain management secrets. The Secrets Series. Hanley & Belfus. http://www.sciencedirect.com/science/book/9781560534426 [Google Scholar]
- Gu, J. , Qin, W. , Chen, F. , & Xia, Z. (2015). Long‐term stability of tramadol and ketamine solutions for patient‐controlled analgesia delivery. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 21, 2528–2534. 10.12659/MSM.894066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrs, P. , Nagy, S. , & Vardaxis, N. (2013). Mosby's dictionary of medicine, nursing and health professions (third Australian & New Zealand ed.). Mosby Australia. [Google Scholar]
- Hildebrand, K. R. , Elsberry, D. E. , & Anderson, V. C. (2001). Stability and compatibility of hydromorphone hydrochloride in an implantable infusion system. Journal of Pain and Symptom Management, 22(6), 1042–1047. 10.1016/S0885-3924(01)00364-5 [DOI] [PubMed] [Google Scholar]
- Huvelle, S. , Godet, M. , Hecq, J.‐D. , Gillet, P. , Bihin, B. , Jamart, J. , & Galanti, L. (2016). Long‐term stability of ketamine hydrochloride 50 mg/ml injection in 3 ml syringes. Annales Pharmaceutiques Françaises, 74(4), 283–287. 10.1016/j.pharma.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Jahr, J. S. , Watkins‐Pitchford, J. M. , & Sinatra, R. S. (Eds.) (2011). The essence of analgesia and analgesics. Cambridge University Press. 10.1017/CBO9780511841378 [DOI] [Google Scholar]
- Kehlet, H. , & Dahl, J. B. (1993). The value of "multimodal" or "balanced analgesia" in postoperative pain treatment. Anesthesia and Analgesia, 77(5), 1048–1056. 10.1213/00000539-199311000-00030 [DOI] [PubMed] [Google Scholar]
- Lee, C. H. , Kim, A. R. , Lee, M. K. , Oh, J. S. , Lee, D. K. , & Choi, S. S. (2020). Intravenous patient‐controlled analgesia: In vitro stability profiles of mixtures containing fentanyl, hydromorphone, oxycodone, nefopam, ondansetron, and ramosetron. Journal of Analytical Science and Technology, 11(1), 1–8. 10.1186/s40543-020-00230-w [DOI] [Google Scholar]
- Lukas, P. , Gastl, G. , Wöll, E. , Eisterer, W. , Stauder, R. , Gunsilius, E. , Gattringer, K. , & Kastner, S. (2016). Palliative Care: Empfehlungen für die Betreuung schwerkranker Menschen am Lebensende in Tirol (2nd ed.). https://www.hospiz-tirol.at/wp-content/uploads/2016/03/Palliativ-Care_2016.pdf
- María, E. B. , Fuensanta, S. R. , & Catalina, B. O. (2018). Determination of compatibility and stability of haloperidol and morphine mixtures used in palliative care. Brazilian Journal of Pharmaceutical Sciences, 54(2). 10.1590/s2175-97902018000217352 [DOI] [Google Scholar]
- McPherson, M. L. M. (2019). Demystifying opioid conversion calculations: A guide for effective dosing (second ed.). American Society of Health‐System Pharmacists. [Google Scholar]
- Moldoveanu, S. , & David, V. (2015). Modern sample preparation for chromatography. Elsevier. [Google Scholar]
- Mukoreka, J. , & Sisay, I. (2015). Safe practice in syringe pump management. Nursing Times, 111(14), 19–21. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26182586/ [PubMed] [Google Scholar]
- Müller, S. (2009). Stabilität von binären Arzneistoffkombinationen in Schmerzmittelreservoiren zur kontinuierlichen parenteralen Applikation (Dissertation). Albert‐Ludwigs‐Universität, Freiburg im Breisgau. file:///C:/Users/csaq1535/AppData/Local/Temp/Dissertation_Mueller_Simone_Nov_2009‐1.pdf
- Nassr, S. , Dubuc, M. C. , Lavoie, P. , & Brazier, J. L. (2003). HPLC–DAD methods for studying the stability of solutions containing hydromorphone, ketorolac, haloperidol, midazolam, famotidine, metoclopramide, dimenhydrinate, and scopolamine. Journal of Liquid Chromatography & Related Technologies, 26(17), 2909–2929. 10.1081/JLC-120025053 [DOI] [Google Scholar]
- O'Connell, J. P. , & Haile, J. M. (2011). Thermodynamics: Fundamentals for applications (first paperback ed.). Cambridge University Press. http://www.loc.gov/catdir/description/cam051/2004057542.html [Google Scholar]
- Peters, F. T. , Hartung, M. , Herbold, M. , Schmitt, G. , Daldrup, T. , Mußhoff, F. , & Skopp, G. (2009). Requirements for the validation of analytical methods: Version 1. https://www.gtfch.org/cms/images/stories/files/appendix_b_gtfch_2020090601.pdf
- Ping, L. , Juan, H. , Li, R.‐L. , Feng, Z. Y. , Zhou, X.‐J. , Liu, M. F. , Jing, B. , & Jiang, L. (2019). Stability of hydromorphone hydrochloride and morphine under different clinical infusion conditions. Indian Journal of Pharmaceutical Sciences, 81(6), 1140–1146. 10.36468/pharmaceutical-sciences.615 [DOI] [Google Scholar]
- Raja, S. , Liu, S. S. , Fishman, S. , & Cohen, S. P. (2018). Essentials of pain medicine (fourth ed.). Elseiver. [Google Scholar]
- Rowbotham, D. J. , Macintyre, P. E. , & Walker, S. M. (2008). Clinical pain management: Volume 1: Acute pain (2nd ed.). Hodder Arnold. http://site.ebrary.com/lib/alltitles/docDetail.action?docID=10388187 [Google Scholar]
- Schmid, R. , Koren, G. , Klein, J. , & Katz, J. (2002). The stability of a ketamine–morphine solution. Anesthesia and Analgesia, 94(4), 898–900. 10.1097/00000539-200204000-00023 [DOI] [PubMed] [Google Scholar]
- Selbach, S. , Diederich, W. E. , Fett, S. , Fründ, D. , Koch, T. , & Eberhart, L. H. J. (2011). Stability‐indicating HPLC assays for the determination of piritramide and droperidol in PCA solution. Journal of Clinical Pharmacy and Therapeutics, 36(2), 161–165. 10.1111/j.1365-2710.2010.01169.x [DOI] [PubMed] [Google Scholar]
- Stafford, R. S. (2008). Regulating off‐label drug use—Rethinking the role of the FDA. New England Journal of Medicine, 358(14), 1427–1429. 10.1056/NEJMp0802107 [DOI] [PubMed] [Google Scholar]
- Trittler, R. , Feuerstein, T. J. , & Heydenreich, M. (2011, May 13). Metamorphin im Schmerzmittelcocktail Pharmakologisch relevant? [Poster.] Universitätsklinikum Freiburg: Berlin. https://www.adka.de/index.php?eID=dumpFile&t=f&f=910&token=f9975a8e4c59741bd688962fb0b44f94bd771435
- Urden, L. D. , Stacy, K. M. , & Lough, M. E. (2019). Priorities in critical care nursing. Elsevier Health Sciences. [Google Scholar]
- Vermeire, A. (1999). Stability and compatibility of morphine. International Journal of Pharmaceutics, 187(1), 17–51. 10.1016/S0378-5173(99)00181-7 [DOI] [PubMed] [Google Scholar]
- Xiang, Q. , Niu, G. , Wu, X. , & Chen, G. (2007). Stability and determination of metamizole sodium by capillary electrophoresis analysis combined with infra‐red spectroscopy. Chemical Research in Chinese Universities, 23(6), 654–658. 10.1016/S1005-9040(07)60142-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Concentration levels (c1–c6) for analyte calibrations.
Table S2. Results obtained from analyte calibrations.
Table S3. Results obtained from processed sample stability at calibration maximum at 4°C.
Table S4. Results obtained from processed sample stability at calibration minimum at 4°C.
Table S5. Results obtained from freeze–thaw stability.
Table S6. Results for individual analgesic injection solution containing hydromorphone at storage temperatures of 22 and 37°C.
Table S7. Results for individual analgesic injection solution containing morphine at storage temperatures of 22 and 37°C.
Table S8. Results for binary analgesic injection mixture containing hydromorphone and esketamine at a storage temperature of 22°C.
Table S9. Results for binary analgesic injection mixture containing hydromorphone and esketamine at a storage temperature of 37°C.
Table S10. Results for binary analgesic injection mixture containing morphine and metamizole at a storage temperature of 22°C.
Table S11. Results for binary analgesic injection mixture containing morphine and metamizole at a storage temperature of 37°C.
Table S12. Results for ternary analgesic injection mixture containing morphine, esketamine and metamizole at a storage temperature of 22°C.
Table S13. Results for ternary analgesic injection mixture containing morphine, esketamine and metamizole at a storage temperature of 37°C.
Table S14. Assigned m/z signals obtained from MS analysis in positive ion mode of the ternary injection mixture after 7 days of storage.
Figure S1. Chromatogram obtained from HPLC–UV measurement at calibration maximum (c6), including signals for morphine (1), hydromorphone (2), metamizole (3), potassium sorbate (4) and esketamine (5).
Data Availability Statement
More comprehensive data can be obtained from the Supplementary Information.


