Abstract
Background
Chronic kidney disease (CKD) is often complicated by anaemia, which is associated with disease progression and increased hospital visits, decreased quality of life, and increased mortality.
Methods
A comprehensive literature search of English language peer‐reviewed articles in PubMed/MedLine published between 1998 and 2020 related to the treatment of anaemia of CKD was conducted. The United States Renal Database System and Dialysis Outcomes and Practice Patterns Study (DOPPS) data reports, the Centers for Disease Control and Prevention and the US Food and Drug Administration websites, and published congress abstracts in 2020 were surveyed for relevant information.
Results
Subgroups of patients with anaemia of CKD present a clinical challenge throughout the disease spectrum, including those with end‐stage kidney disease, advanced age or resistance to or ineligibility for current standards of care (ie, oral or intravenous iron supplementation, erythropoietin‐stimulating agents and red blood cell transfusions). In addition, those with an increased risk of adverse events because of comorbid conditions, such as cardiovascular diseases or diabetes, comprise special populations of patients with an unmet need for interventions to improve clinical outcomes. These comorbidities must be managed in parallel and may have a synergistic effect on overall disease severity.
Conclusions
Several therapies provide promising opportunities to address gaps with a standard of care, including hypoxia‐inducible factor prolyl hydroxylase inhibitors, which stimulate haematopoiesis through promoting modest increases in serum erythropoietin and improved iron homeostasis. The critical issues in the management of anaemia of CKD in these challenging phenotypes and the clinical utility of new therapeutic agents in development for the treatment of anaemia of CKD should be assessed and the information should be made available to healthcare providers.
Review criteria
A comprehensive literature search of English language peer‐reviewed articles in PubMed/MedLine published between 1998 and 2020 related to the treatment of anaemia of CKD was conducted. The United States Renal Database System and Dialysis Outcomes and Practice Patterns Study (DOPPS) data reports, the Centers for Disease Control and Prevention and the US Food and Drug Administration websites, and published congress abstracts in 2020 were surveyed for relevant information.
Message for the clinic
The management of chronic kidney disease (CKD) is often complicated by comorbid anaemia. Subgroups of patients with anaemia of CKD including those with end‐stage kidney disease, advanced age or resistance or hyporesponsiveness to erythropoietin‐stimulating agents present a clinical challenge throughout the disease spectrum. Multi‐modal treatment strategies and new therapeutic options that address current therapeutic challenges may be beneficial in the treatment of anaemia of CKD.
1. INTRODUCTION
Anaemia affects ~15.4% of patients with chronic kidney disease (CKD) in the US, an estimated 5.7 million people. 1 , 2 Anaemia prevalence increases with CKD stage, ranging from 8% at stage 1 to 53% at stage 5, 1 and with age, with 28% in patients aged 18‐63 years and 50% in patients aged 66‐85 years. 3 Race/ethnicity and sex also impact the prevalence of anaemia of CKD with increased risk in Black, Hispanic and female patients. 4
Compared with non‐anaemic counterparts, patients with anaemia of CKD have an increased risk of hospitalisations, 5 cardiovascular (CV) events, 6 heart failure, 7 progression to dialysis dependence 8 and all‐cause mortality. 9 Comorbid diabetes is linked to an increased prevalence of anaemia in CKD, 10 with greater frequency and higher severity 11 in later CKD stages. In a pooled analysis of diabetic patients with CKD, presence of anaemia was associated with an increase in cardiovascular disease (CVD) and all‐cause mortality risk compared with non‐anaemic counterparts. 12
Multi‐morbidity associated with anaemia of CKD adversely affects patient quality of life (QOL), with increased fatigue and decreased work productivity, 13 and leads to higher healthcare utilisation and costs. 3 , 14 Although the presence of anaemia may be indicative of sicker patients, a retrospective study found 38% higher adjusted mean total healthcare expense per patient per month in anaemic compared with non‐anaemic patients with CKD. 14 , 15 Management of anaemia of CKD may improve QOL 15 and lower costs by decreasing inpatient expenditures. 14
1.1. Pathophysiology of anaemia of chronic kidney disease
Anaemia of CKD is primarily a function of reduced erythropoietin (EPO) levels and impaired iron homeostasis 16 leading to decreased erythropoiesis (Figure 1). Insufficient EPO production following kidney damage occurs from functional deficiency in renal EPO‐producing cells 17 and desensitisation of hypoxia‐sensing mechanisms. 18 Iron is necessary for haemoglobin (Hb) synthesis, and iron availability is important for adequate tissue oxygenation. Iron deficiency in anaemia of CKD can stem from absolute iron deficiency, impaired dietary absorption or functional iron deficiency, in which systemic inflammation leads to insufficient iron release from internal stores resulting in iron‐deficient erythropoiesis. 16 The iron homeostasis regulator hepcidin is elevated in CKD because of inflammation 16 , 19 and decreased renal excretion 16 and is associated with decreased intestinal iron transport and increased iron sequestration. 19 Understanding the aetiology of anaemia of CKD and critical issues in the management of challenging patient phenotypes provide insights into treatment strategies for optimal patient care.
FIGURE 1.
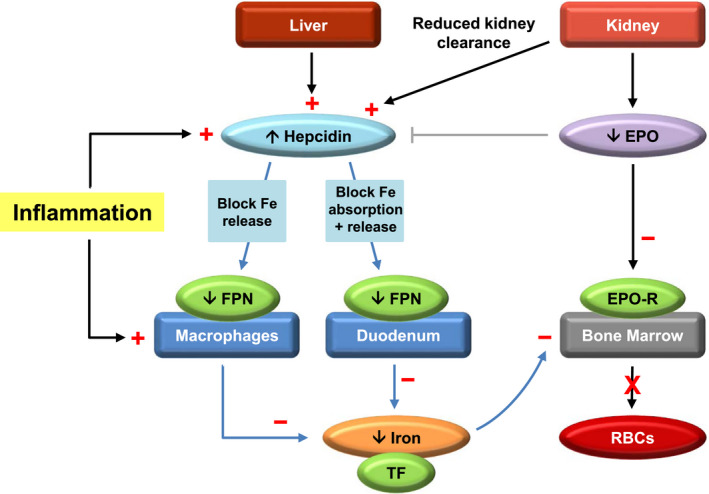
Pathophysiology of anaemia of CKD. Diagram shows aetiology of anaemia of CKD as a consequence of reduced EPO levels and impaired iron homeostasis. In CKD, inflammation and reduced renal clearance of hepcidin from kidney damage elevate hepcidin levels and leads to degradation of FPN. Decreased release of iron from internal stores (eg, dietary iron stored in the duodenum or iron from RBCs recycled by reticuloendothelial macrophages) restricts serum concentrations of TF‐bound iron available to meet the demands of haematopoiesis in the bone marrow. 16 , 19 Inflammation also enhances macrophage uptake of iron, further depleting serum iron levels. 160 Additionally, kidney damage in CKD results in decreased EPO production, leading to deficiency in erythrocyte levels, shortened RBC lifespan, and development of anaemia. 16 Black arrows represent physiological processes. Grey line indicates inhibition. Blue arrows show hepcidin‐mediated effects on iron metabolism. Positive (+) or negative (−) changes occurring with anaemia in CKD are in red. Abbreviations: CKD, chronic kidney disease; EPO, erythropoietin; EPO‐R, erythropoietin receptor; Fe, iron; FPN, ferroportin; RBC, red blood cell; TF, transferrin
2. METHODS
Information presented in this review was derived from a comprehensive literature search of English language peer‐reviewed articles in PubMed/MedLine database published from January 1998 to July 2020 related to the treatment of anaemia of CKD. Key search terms included anaemia, anaemia in/of chronic kidney disease, chronic kidney disease, CKD, dialysis, end‐stage kidney/renal disease, ESKD, ESRD, erythropoiesis, erythropoiesis‐stimulating agent, erythropoietin‐stimulating agent, ESA, functional iron deficiency, hepcidin, HIF‐PH, hyporesponse, hypoxia, hypoxia‐inducible factor prolyl hydroxylase, iron deficiency anaemia, etc. The United States Renal Database System (USRDS) and Dialysis Outcomes and Practice Patterns Study (DOPPS) data reports, the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines, verified websites from the Centers for Disease Control and Prevention and the US Food and Drug Administration, and published congress abstracts in 2020 were surveyed for relevant information. In addition, pharmaceutical product inserts, and official press releases related to clinical trial breaking news were included.
2.1. Standard of care and its associated risks
Management of anaemia of CKD typically includes oral and intravenous (IV) iron, erythropoietin‐stimulating agents (ESAs) and red blood cell (RBC) transfusions 20 , 21 (Table 1). Iron supplementation is often necessary to treat iron deficiency, reduce anaemia‐related symptoms, allow dose sparing in patients on ESAs and avoid the need for blood transfusions. 22 Oral iron may be appropriate for non‐dialysis‐dependent (NDD) patients in earlier stages of the disease, as it is non‐invasive, inexpensive, and can be self‐administered. However, oral iron is poorly absorbed, and in patients requiring a high dose of iron to increase Hb levels, for example dialysis‐dependent CKD (DD‐CKD) and end‐stage kidney disease (ESKD), increased pill burden with oral iron is associated with gastrointestinal side effects and decreased regimen adherence. 23 Comparatively, IV iron is effective in raising Hb levels, and its use has increased over time, 23 perhaps because of greater iron requirements with ESA treatment 24 and ESA dose‐sparing practices. Concerns over IV iron include the potential for excessive accumulation, possible exacerbation of underlying systemic infection, and although uncommon, especially with high molecular weight iron dextran formulations, anaphylaxis. 20 , 25 For example, iron overload after IV iron therapy could lead to the generation of reactive oxygen species and further inflammation. 24 Additionally, observational studies have suggested an association between IV iron treatment and increased hospitalisation and mortality; a prospective cohort study in >30 000 haemodialysis (HD) patients found significantly higher mortality and hospitalisation risk in patients receiving ≥300 mg/month of IV iron for 4 months, 26 and a similar study in 58 000 HD patients showed a comparable trend. 27 Conversely, a large, randomised clinical trial showed significantly better outcomes in patients administered a high‐dose IV iron regimen proactively vs a low‐dose regimen reactively. In patients on HD, the risk of nonfatal CV events, any‐cause deaths, ESA doses and need for blood transfusions were lower in those patients proactively prescribed 400 mg/month (median monthly dose of 264 mg) when ferritin concentration was <700 μg/L and transferrin saturation (TSAT) <40% compared with patients treated reactively with 0‐400 mg/month of IV iron (median monthly dose of 145 mg) when ferritin concentration was <200 μg/L and TSAT < 20%. 28
TABLE 1.
| Advantage | Disadvantage | |
|---|---|---|
| Oral iron |
•Inexpensive •Easy to administer •Avoids an IV in patients not on dialysis 20 |
•Gastrointestinal side effects are common and may limit adherence 20 |
| IV iron |
•More efficacious than oral iron in raising Hb levels 30 , 31 |
•Increased frequency of serious AEs compared with oral iron, including CV events and infection 32 •Allergic and anaphylactoid reactions (rare) have been reported, particularly with iron dextran 33 •Care must be taken to avoid iron overload 24 ; high doses are associated with higher risks of mortality and hospitalisation 26 , 27 |
| ESA |
•When targeting Hb levels of 90‐110 g/L, decreases LVH and mortality and increases QOL 34 |
•Use of high doses is associated with higher rates of death, stroke, and other CV events 35 , 36 , 37 |
| Blood transfusion |
•Can be used if ESA treatment is not effective or if ESA risks are greater than benefits 20 |
•Risks include fever, allergic reactions, haemolytic reactions, transfusion‐related infections, and human leukocyte antigen sensitisation 20 |
Abbreviations: AEs, adverse events; CKD, chronic kidney disease; CV, cardiovascular; ESA, erythropoietin‐stimulating agent; Hb, haemoglobin; IV, intravenous; LVH, left ventricular hypertrophy; QOL, quality of life.
Erythropoietin‐stimulating agents have similar biological action as native EPO, 38 and both short‐acting (eg, epoetin alfa) and long‐acting (eg, darbepoetin alfa) drugs are used for the treatment of anaemia in DD and NDD patients. 39 A few clinical trials 35 , 36 , 40 , 41 , 42 have suggested there are risks associated with the use of higher ESA doses to target higher Hb levels. In the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial, a 34% increased risk of the composite endpoint (death, myocardial infarction [MI], hospitalisation for congestive heart failure [CHF] and stroke) was seen with epoetin alfa in patients randomised to the higher target Hb (135 g/L) compared with lower (113 g/L) level, with no incremental improvement in QOL. 36 Secondary analyses showed that high doses of epoetin alfa and associated hyporesponse were significantly associated with an increased risk of the composite endpoint, independent of Hb achieved. 43 Increased toxicity with high‐dose epoetin alfa was suggested as a contributing factor to these observations. 37 These trials along with observational data and meta‐analyses indicated that using higher ESA doses to achieve higher Hb targets may be detrimental and has led to recommendations by the US Food and Drug Administration and Kidney Disease: Improving Global Outcomes (KDIGO) against the use of ESAs to target Hb levels >110 g/L 44 and Hb > 115 g/L, respectively. 20
Blood transfusions may be necessary in patients with ESKD, ESA hyporesponders, and those precluded from ESA therapy because of, in some patients, recent stroke, pure red cell aplasia, 45 allergic reactions 46 or active cancer. 47 However, associated risks, although uncommon, include infection and severe hypersensitivity. 20 , 21 Additionally, alloimmunisation in patients receiving multiple blood transfusions and in multiparous women can result in haemolytic reactions 48 and may diminish chances for successful kidney transplantation. 47
2.2. Patients with challenging phenotypes
The treatment of anaemia of CKD in patients who are hyporesponsive to ESAs and in those with concurrent malignancies is particularly challenging with current standard of care; in addition, patients with coexisting CVD, secondary hyperparathyroidism (SHPT) and those who are older or undergoing dialysis will require special treatment considerations.
2.2.1. ESA hyporesponders
In ~5‐10% of patients with CKD, a suboptimal response or resistance to ESAs develops, where desired Hb concentrations cannot be reached despite increasingly higher doses of ESA. 49 The prevalence of ESA resistance may range from 10% to 20% in DD patients 50 , 51 and may in part be related to underlying disease severity. Absolute or functional iron deficiency is the most common cause of ESA resistance 49 and can arise in the presence or absence of anaemia. 52 Patients with absolute iron deficiency have hallmark low ferritin levels (indicator of low iron storage) while patients with functional iron deficiency have adequate internal iron stores but decreased capacity to release and utilise stored iron, as indicated by normal or high serum ferritin levels but low serum TSAT (ie, low circulating iron). However, ferritin is not a specific marker for iron storage, as it is an acute phase reactant affected by inflammation, infection and malignancy; thus high levels in CKD are not always indicative of adequate iron stores. 16 Functional iron deficiency is mediated through inflammatory elevation of hepcidin levels. 19 Renin‐angiotensin‐aldosterone system inhibitors (RAASi), angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers have been suggested to contribute to ESA hyporesponsiveness; discontinuation of these antihypertensives may be considered in some patients to regain ESA sensitivity, 49 but treatment interruption is not ideal since RAASi therapy provides cardiorenal benefits and is associated with improved survival. 53
There is no consensus on the exact parameters defining ESA hyporesponsiveness. Per KDIGO guidelines, ESA hyporesponse at the start of treatment is characterised by no increase from baseline in Hb concentration after the first month of ESA treatment on appropriate weight‐based dosing. 20 The inability to achieve or maintain Hb target levels by treatment with epoetin alfa >300 IU/kg/week subcutaneous or 450 IU/kg/week IV or darbepoetin alfa >1.5 microgram/kg/week has been considered inadequate ESA responsiveness. 21 Acute ESA hyporesponsiveness has been defined as a transient refractoriness (typically after an infectious episode) in Hb improvements with ESA dose escalation followed by response recovery within 4 months of treatment. 51 In chronic cases, ESA hyporesponsiveness can persist after 4 months of high‐dose treatment 51 with a decreased likelihood of recovery with higher transfusion and mortality rates than acute cases. 54 ESA‐resistant patients have higher rates of death, MI, CHF and stroke than responders. 35 , 37
Inflammation plays a key role in ESA resistance and is prevalent in patients with CKD. 55 Inflammation is associated with inhibition of erythropoiesis and increased levels of hepcidin and ferritin. 56 In HD patients, serum hepcidin levels were positively correlated with ESA resistance and inflammatory markers (ie, interleukin‐6 [IL‐6] and high‐sensitivity C‐reactive protein [CRP]) and negatively with Hb levels. 57 A higher risk of progression to ESKD and mortality has been shown for patients with higher CRP levels. 58 Inflammation was an independent risk factor for heart failure in patients with CKD. 7 As inflammation influences serum ferritin and transferrin, 56 other diagnostic tests may be needed to ascertain iron status in ESA hyporesponders such as content of reticulocyte haemoglobin, CRP, percentage of hypochromic erythrocytes and soluble transferrin receptor. 59
Addressing underlying inflammation, for example by improving general health and hygiene, optimising tunnelled catheter care to prevent infection, or by initiating the potentially less inflammatory modality of peritoneal dialysis vs HD, is key to effective treatment of anaemia of CKD 56 and may curb the development of ESA resistance. Nevertheless, inflammation is increased with catheter use even in the absence of infection, as evidenced by significantly decreased CRP levels in patients switched from a catheter to arteriovenous fistulas. 60 A systematic review found that patients with catheters had a higher risk of infection, CV events, hospitalisation and all‐cause mortality compared with those using fistulas or grafts for HD. 61
2.2.2. Patients with concurrent malignancy
In patients with cancer, CKD can be a risk factor for malignancy, likely because of heightened inflammation and oxidative stress, or may arise consequently from cancer cell toxins or myelosuppressive and nephrotoxic anticancer therapies. 62 , 63 Chronic kidney disease in cancer patients can be exacerbated by anaemia stemming from chemotherapy, radiation therapy, inflammation, and repeated blood sampling. 64 Anaemia is common in cancer patients and is associated with lower treatment response rates and increased mortality. 64 As the standard of care for cancer therapy‐related anaemia, ESAs may reduce the need for blood transfusions. 64 However, anaemia correction with ESAs to higher Hb targets in cancer patients receiving chemotherapy has been linked to the progression of malignancy and adverse CV events such as thrombosis, hypertension, and stroke, although the risk is not uniform across cancer types. 64 While no conclusive association has been shown between low‐dose ESA use and cancer risk, 64 current guidelines recommend their use with caution and only with Hb levels <100 g/L in patients with NDD‐ or DD‐CKD with an active or history of malignancy. 20 , 64 However, except for some patients with myelodysplastic syndromes, ESAs are not recommended in cancer patients with non‐chemotherapy‐associated anaemia. 65 For these patients, RBC transfusion may be the only option, bringing with it concerns of allosensitisation and jeopardised future kidney transplantation. 20 , 64
2.2.3. Patients with cardiovascular disease
Chronic kidney disease and CVD are linked pathophysiological states that can exacerbate each other. Patients with comorbidities have a mortality rate at least twice as high as patients with CKD alone, 66 and most of these deaths are caused by CV complications, 67 especially among patients on dialysis. 40 Either CKD or CVD can lead to the development of anaemia, which further triggers reciprocal disease progression and is more common in patients with more severe CKD and CVD. 68 Anaemia in CKD is associated with left ventricular hypertrophy 69 and CHF, 68 stemming from tissue hypoxia and peripheral vasodilation and subsequent compensatory increases in cardiac output that place a high work burden on the heart and may result in resistance to CHF therapy and poorer clinical outcomes and survival. 68 This complex triad of anaemia, CKD, and CVD, referred to as the cardiorenal anaemia syndrome (CRAS), 68 is particularly challenging clinically because of increased morbidity and mortality, heightened systemic inflammation and iron imbalance and the need for multidisciplinary treatment approaches. Mortality risk was significantly higher in CRAS patients compared with heart failure (HF) patients with either comorbid anaemia or CKD, 70 and risks of CV complications and progression to renal replacement therapy (RRT) were higher in hospitalised HF patients with CRAS compared with HF patients with CKD without anaemia. 71 Pro‐inflammatory markers are elevated in anaemic HF patients 72 and may cause progression of kidney damage 73 and cardiac remodelling. 73 As discussed, CKD alone is an inflammatory state that when combined with inflammation of CVD and anaemia further contributes to ESA hyporesponsiveness. Although IV iron therapy is beneficial, ESA use is not recommended in patients with anaemia and HF because of the lack of therapeutic benefit on CV outcomes. 74 A systematic review and meta‐analysis of nine randomised clinical trials found ESA administration after acute MI and percutaneous coronary intervention to be relatively “safe,” and to improve short‐term (≤6 months) cardiac function, 75 but no reduction in incidence of major adverse cardiovascular events (MACE), including recurrent MI and stroke, was shown long term with epoetin beta treatment. 76 In the Trial to Reduce Cardiovascular Events With Aranesp® Therapy (TREAT), patients with anaemia of NDD‐CKD and comorbid diabetes treated with darbepoetin alfa did not achieve reduction in the composite outcome of either death or a cardiovascular event but required fewer cardiac revascularisation procedures compared with placebo. 42 Clinical guidelines with an integrated treatment approach to address these obstacles are needed to improve outcomes in patients with CRAS.
2.2.4. Patients on dialysis
In 2017 in the US, 746 557 individuals needed RRT to survive, of whom 70% received dialysis and 30% received a transplant. 77 Of 124 500 patients with incident ESKD in 2017, ~97% were initiated on dialysis and 3% started with a transplant. 77 Anaemia is prevalent in patients undergoing dialysis, up to 89% in the US. 78 Chronic inflammation is common in dialysis patients 79 and, along with lack of adequate dietary iron and functional iron deficiency, 16 can lead to declining erythropoiesis over time and worsening anaemia. 80 Retention of blood in the dialyzer adds to blood loss. Although blood loss varies by dialyzer used, estimates indicate >508 mg of total iron may be lost annually from HD. 81 Anaemia in DD‐CKD patients is associated with increased hospitalisation rates, greater mortality, and higher economic burden. 82 Poor QOL is a concern in DD‐ESKD patients and is associated with worse survival. 83
Treatment with epoetin alfa 84 and darbepoetin alfa 85 in DD‐CKD patients without CVD resulted in maintenance of target Hb levels, a reduced need for blood transfusions, and improvements in energy and fatigue; however, similar to NDD‐CKD patients with anaemia, ESA doses to target normal Hb levels are associated with dose‐dependent adverse effects, including higher mortality rates. 86 In the US, ~80% of HD patients were given ESA therapy in 2016, 87 and ~10‐20% of patients on dialysis are estimated to be hyporesponsive to ESAs. 50 , 51 , 54 Parenteral IV iron therapy can overcome functional iron deficiency in patients on dialysis with limited treatment‐related adverse events and is convenient to administer by infusion during dialysis sessions. However, the optimal IV iron dose to use with various ESA doses is difficult to ascertain and complicated by poor diagnostic measures of iron status. Care must be taken to minimise potential risk of iron overload, especially in patients who are iron‐resistant because of underlying inflammation and elevated levels of hepcidin. 20 , 88
2.2.5. Patients with secondary hyperparathyroidism
Secondary hyperparathyroidism, a common complication of CKD, is marked by excessive secretion of parathyroid hormone (PTH), an important regulator of serum calcium levels, in response to hyperphosphatemia from decreased kidney function and hypocalcaemia from impaired bone and mineral metabolism. 89 Parathyroid hormone is implicated in the development of renal anaemia and ESA resistance. 90 It impedes erythropoiesis by inhibiting EPO synthesis 91 and shortens the survival of RBCs. 91 Lower levels of PTH appear to improve the responsiveness to ESA treatment, as patients with relative hypoparathyroidism appeared to respond better to ESA treatment than those with stable PTH. 92 Accordingly, patients who received parathyroidectomy had reduced requirement for exogenous EPO. 91
Treatment of SHPT may improve anaemia and associated morbidity and mortality 91 through coordinated intervention with a low‐phosphorus diet and medication with phosphate binders, vitamin D analogs, and calcimimetics. 93 However, cooperative therapy is difficult to manage in patients with a constellation of CKD, SHPT, and anaemia, and lack of conclusive efficacy or potential risks may offset possible benefits. 93 Although vitamin D derivatives have been shown to suppress PTH secretion, their effect on controlling bone pain and reducing mortality are contradictory. 93 A recent randomised clinical trial in anaemic CKD patients on HD confirmed that vitamin D is dispensable for anaemia management since its administration did not improve EPO levels, 91 despite some studies suggesting that vitamin D may enhance erythropoiesis outside of its effect on PTH. 94 The most recent KDIGO guidelines do not recommend vitamin D supplementation in most adult patients with NDD‐CKD in stages 3a–5, but reserve it for patients in stages 4‐5 CKD with severe and progressive hyperparathyroidism. 95 Treatment of SHPT with calcimimetics has resulted in higher levels of Hb, lower doses of ESAs, 91 and decreased CV hospitalisation, but its use has been limited by high rates of gastrointestinal effects, hypocalcaemia, over‐suppression of PTH and non‐adherence to oral calcimimetics. 96 Multifactorial treatment considerations and risk‐benefit assessments are necessary for optimal management of comorbidities in patients with CKD, SHPT and anaemia.
2.2.6. Elderly patients
The prevalence of anaemia of CKD in older patients (≥66 years) is ~50% compared with 28% in younger patients (18‐63 years) and increases with age and CKD stage. 3 Older patients often have malnutrition and/or inflammatory conditions with reduced erythropoiesis and increased hepcidin compared with younger patients, 97 which contributes to insufficient Hb. Although Hb levels decrease with age and optimal Hb targets in older patients may be lower than those in younger patients, higher Hb targets in older patients with more rigorous treatment of anaemia may not improve outcomes. In patients prescribed ESAs or iron, a significant association of high Hb levels and improved QOL was seen in patients <65 years but not in those ≥65 years, although QOL parameters are different in younger vs older patients. 98 Dose escalation with ESAs and IV iron in elderly patients is more closely associated with adverse outcomes such as CV effects, hospitalisation and mortality 97 ; increased age is associated with ESA resistance, 99 and a significantly higher weekly ESA dose per kilogram body weight is required to achieve the same target Hb levels in patients ≥65 years compared with those <65 years. 97
Stage 3‐5 NDD‐CKD patients aged 66‐85 years with anaemia were shown to have an increased prevalence of CV conditions compared with corresponding age‐matched, non‐anaemic patients (arteriosclerotic heart disease [52.2% vs 36.4%], CHF [40.5% vs 20.1%] and dysrhythmia [43.8% vs 28.0%]) and compared with younger (18‐63 years) counterparts (arteriosclerotic heart disease [52.2% vs 23.6%], CHF [40.5% vs 21.4%], and dysrhythmia [43.8% vs 19.5%]). 3 Similarly, there may be an increased risk of mortality in elderly HD patients with low Hb levels. Comorbidities in elderly patients with CKD can lead to reduced daily activity, which is associated with increased mortality risk. 100 Following hospital discharge, anaemia and CKD are significantly associated with 1‐year mortality rates in older patients, representing post‐treatment vulnerability in this population without proper follow‐up. 100 Given the frequency of anaemia of CKD and associated comorbidities in the elderly, screening such patients for these disorders and mobility limitations may decrease healthcare utilisation and improve survival, particularly if accompanied by effective treatment intervention.
2.2.7. Novel challenges from coronavirus disease 2019
The recent coronavirus disease 2019 (COVID‐19) pandemic has shown a disproportionately higher disease burden and severity and poorer outcomes for patients with CKD and ESKD with or without other comorbidities. 101 , 102 Health records and epidemiological studies have shown stage 4‐5 CKD, ESKD with dialysis, and kidney transplants to be high‐risk for severe complications and hospitalisations 103 ; the prevalence of kidney disease among hospitalised patients with COVID‐19 was found to be as high as 13%‐30% in some cohorts. 103 , 104 Patients with ESKD had a 37% higher risk of in‐hospital death compared with non‐ESKD counterparts with similar baseline demographics and comorbidities. 101 While the exact mechanism of this increased risk of adverse outcomes in patients with preexisting kidney disease is yet to be fully elucidated, it is believed that the heightened inflammation and immune system dysregulation, as well as the activation of the complement system, are believed to contribute to the pathogenesis. 101 , 105
In addition to its prevalence in patients with CKD, anaemia with dysregulated iron metabolism has been observed in patients with COVID‐19 and is associated with systemic inflammation and is often severe. 106 During COVID‐19 infection, an immune response driven by a cytokine storm may induce a hyperinflammatory state. 101 , 106 Among 11 265 hospitalised patients with COVID‐19, haemoglobin levels decreased and ferritin levels increased with rising inflammation, suggesting that inflammation‐induced, hepcidin‐mediated iron restriction in erythropoiesis may be responsible for the occurrence and exacerbation of anaemia in these patients. 106 Although ESAs have been used to treat anaemia of CKD with or without COVID‐19, 107 their use in patients with CKD and COVID‐19 may have reduced efficacy due to inflammation‐induced resistance. 106 Furthermore, it has been suggested that ESA use may be dangerous in these patients because severe COVID‐19 is characterised by venous and arterial blood clots, and ESAs tend to induce a prothrombotic state. 106 In patients on dialysis and maintenance ESA therapy, some nephrologists have recommended continuing outpatient ESA regimens in the inpatient setting but targeting lower Hb levels of 8‐9 g/dL, with no dose escalation in order to mitigate the risk of thrombosis. 106 Although several putative benefits of recombinant erythropoietin have been proposed, 108 in patients with COVID‐19 and anaemia with or without kidney disease, avoidance of ESA use has been suggested since the risks outweigh the benefits, even if blood transfusions may be necessary. 106 , 107
2.3. New therapeutic options in development
2.3.1. Hypoxia‐inducible factor prolyl hydroxylase inhibition
Recognition of tissue hypoxia occurs by the hypoxia‐inducible factor (HIF) system. In normoxia, the oxygen‐sensing subunit of HIF (HIF‐α) rapidly undergoes proteasomal degradation after hydroxylation by prolyl hydroxylases (PH), but under hypoxic conditions, HIF‐α is stabilised, resulting in transcription of a number of genes that support erythropoiesis, including EPO, EPO receptors and genes involved in iron metabolism 109 (Figure 2). A new class of orally administered drugs aimed at transiently stabilising HIF/HIF‐α levels represents a possible treatment strategy for anaemia of CKD. These agents inhibit the activity of hypoxia‐inducible factor prolyl hydroxylase (HIF‐PH), leading to stabilisation of intact HIF‐α. Inhibitors of HIF‐PH induce EPO synthesis at significantly lower levels than the supraphysiologic levels observed with ESA therapy. 110
FIGURE 2.
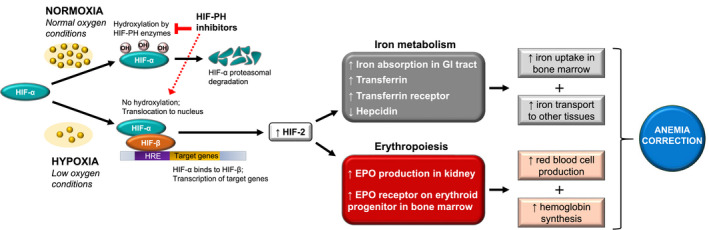
Schematic representation of HIF‐PH inhibitor effects on anaemia. Under normal oxygen conditions, HIF‐α is hydroxylated by HIF‐PH enzymes and degraded by the proteasome. In hypoxia, HIF‐α is not degraded and translocates to the nucleus where it combines with HIF‐β and other coactivators to bind to the HRE and initiate transcription of target genes for iron metabolism and erythropoiesis. 11 HIF‐PH inhibitors promote activation of the hypoxia‐inducible pathway (dashed red arrow) by blocking the activity of HIF‐PH enzymes responsible for HIF protein degradation. As a result, HIF proteins accumulate and act as transcriptional regulators of target genes whose expression has important consequences for functional iron deficiency anaemia in CKD: increased endogenous EPO expression by kidney cells and upregulation of EPO‐R on the surface of bone marrow erythroid precursor cells leads to erythropoiesis, rise in Hb levels, 11 and subsequent increases in iron demands 11 , 109 ; HIF proteins boost iron availability for EPO‐induced RBC production by reducing levels of hepcidin, increasing iron absorption in the gastrointestinal tract, and mobilising iron transport to bone marrow and other tissues via upregulation of TF, TF receptor, and ceruloplasmin. 109 Anaemia correction with HIF‐PH inhibitors may increase tissue oxygenation, leading to improvement in anaemia‐related symptoms, such as fatigue and reduced physical and mental ability, and may potentially reduce the risk of adverse events associated with comorbidities. 11 Abbreviations: CKD, chronic kidney disease; EPO, erythropoietin; EPO‐R, erythropoietin receptor; GI, gastrointestinal; Hb, haemoglobin; HIF, hypoxia‐inducible factor; HIF‐PH, hypoxia‐inducible factor prolyl hydroxylase; HRE, hypoxia response element; OH, hydroxyl group; RBC, red blood cell; TF, transferrin
Although no HIF‐PH inhibitors have been approved in the US at the time of publication of this review, roxadustat, the first‐in‐class orally administered HIF‐PH inhibitor, is in late‐stage development for the treatment of anaemia of CKD and is currently approved for the treatment of anaemia in patients with NDD‐ and DD‐CKD in China and Japan, and the HIF‐PH inhibitors vadadustat and daprodustat are approved for marketing in patients with DD‐ and NDD‐CKD in Japan (Table 2). Roxadustat was noninferior to epoetin alfa in patients with DD‐CKD 111 and superior to placebo in those with NDD‐CKD 112 in raising Hb levels with similar frequency of adverse events, except hyperkalaemia, which was reported more often in the roxadustat treatment groups. Phase 3 studies in Japan suggested that roxadustat was noninferior to ESA therapy and effective at maintaining Hb levels within a target range in ESA‐naïve patients undergoing HD 113 or peritoneal dialysis. 114
TABLE 2.
Hypoxia‐inducible factor prolyl hydroxylase inhibitors
| Drug name | Study design | Dosing frequency in clinical trials | Outcomes from clinical trials | Status a | |
|---|---|---|---|---|---|
| DD‐CKD | NDD‐CKD | ||||
| Daprodustat (GSK‐1278863) |
Phase 2 RCTs |
Phase 2 RCTs |
QD 121 , 122 , 123 |
↑ Hb ↓ Hepcidin ↓ Ferritin ↑ TIBC |
Approved in patients with anaemia of DD‐CKD and NDD‐CKD in Japan 124 |
|
Phase 3 RCTs |
Phase 3 RCTs |
||||
| Desidustat (ZYAN1) |
Phase 2 RCTs CTRI/2017/05/008534 126 |
3x/week 126 |
↑ Hb ↓ Hepcidin ↑ TIBC |
Phase 3 clinical trials are ongoing 127 |
|
|
Phase 3 RCTs |
Phase 3 RCTs |
||||
| Enarodustat (JTZ‐951) |
Phase 2 RCTs JapicCTI‐152892 128 |
Phase 2 RCTs JapicCTI‐152881 129 |
QD 128 , 129 |
↑ Hb ↓ Hepcidin ↓ Ferritin ↑ TIBC ↓ TSAT |
NDA filed in Japan in November 2019 in patients with anaemia of DD‐CKD and NDD‐CKD 130 |
|
Phase 3 RCTs JapicCTI‐173700 130 JapicCTI‐173701 130 JapicCTI‐173702 130 JapicCTI‐183938 130 |
Phase 3 RCTs JapicCTI‐173699 130 JapicCTI‐183870 130 |
||||
| Molidustat (BAY‐853934) |
Phase 2 RCTs NCT02064426 (OLE) 132 |
Phase 2 RCTs NCT02055482 (OLE) 132 |
QD 131 |
↑ Hb ↓ Hepcidin ↓ Ferritin ↑ or stable TIBC ↓ or stable TSAT |
Phase 3 clinical trials are ongoing 133 |
|
Phase 3 RCTs |
Phase 3 RCTs |
||||
| Roxadustat (FG‐4592) |
Phase 2 RCTs |
Phase 2 RCTs |
3x/week 111 , 112 , 113 , 114 , 139 , 140 |
↑ Hb ↓ Hepcidin ↓ Ferritin (variable in ESKD patients) ↑ Transferrin ↑ TIBC ↓ or stable TSAT |
Approved in patients with anaemia of DD‐CKD and NDD‐CKD in China and Japan 141 , 142 NDA filed in December 2019 in Canada, Mexico, Taiwan, Philippines, and Singapore in patients with anaemia of DD‐CKD and NDD‐CKD 141 NDA Filed in US in Feb 2020 in patients with anaemia of DD‐CKD and NDD‐CKD 141 |
|
Phase 3 RCTs |
Phase 3 RCTs |
||||
| Vadadustat (AKB‐6548) |
Phase 2 RCTs |
Phase 2 RCTs |
QD 150 , 152 , 153 |
↑ Hb ↓ Hepcidin ↓ Ferritin ↑ TIBC |
Approved in patients with anaemia of DD‐CKD and NDD‐CKD in Japan 142 |
|
Phase 3 RCTs 154 |
Phase 3 RCTs 142 |
||||
Abbreviations: DD‐CKD, dialysis‐dependent chronic kidney disease; ESKD, end‐stage kidney disease; Hb, haemoglobin; NDA, new drug application; NDD‐CKD, non‐dialysis‐dependent chronic kidney disease; OLE, open‐label extension; QD, once daily; RCT, randomised clinical trial; TIBC, total iron‐binding capacity; TSAT, transferrin saturation.
Status at the time of manuscript publication; none of these agents has been approved in the US.
In DD‐CKD patients with anaemia, preliminary data from several recent global phase 3 trials, including the US‐based SIERRAS, 143 the European PYRENEES, 145 and the worldwide HIMALAYAS trials 146 have shown the noninferiority of roxadustat to epoetin alfa in increasing Hb levels. Significant improvements from baseline Hb levels and reduction in IV iron use were seen in patients with DD‐CKD with roxadustat vs epoetin alfa in the worldwide ROCKIES 144 trial. In addition, roxadustat was associated with a reduction in hepcidin levels from baseline compared with little change after epoetin alfa in DD‐CKD 111 and vs placebo in NDD‐CKD patients. 112 Apparent inflammation based on high CRP levels did not affect the Hb response with roxadustat; in a sub‐analysis of patients with elevated CRP levels, roxadustat treatment led to greater increases in Hb compared with epoetin alfa, indicating that roxadustat may benefit patients with inflammation and anaemia of CKD. 111 In NDD‐CKD patients with anaemia, significant increases from baseline in Hb levels were observed with roxadustat treatment vs placebo in preliminary data from several phase 3 trials, including the ALPS, ANDES, and OLYMPUS trials. 145 , 147 , 148
An additional effect of roxadustat is the improvement in lipids; a decrease in total and low‐density lipoprotein (LDL) cholesterol and triglycerides, and improvement in LDL to high‐density lipoprotein ratio has been shown. 111 In an open‐label, randomised, non‐comparative clinical trial in ESA‐naïve Japanese patients with DD‐CKD, daprodustat treatment provided an increase in mean Hb from baseline after 4 weeks and attainment of target mean Hb after 8 weeks, which was maintained for 24 weeks. No treatment‐related serious adverse events were reported. 121 In Japanese patients with NDD‐CKD, preliminary results showed noninferiority of daprodustat to ESA in achieving target Hb levels and no clinical differences in adverse events of special interest, including ocular, cardiovascular, and cancer‐related adverse events. 122 Similar efficacy results were reported in phase 3, randomised, active‐controlled studies with vadadustat in Japanese patients with DD‐ 152 and NDD‐CKD 153 ; however, in preliminary safety results from patients with NDD‐CKD, vadadustat did not meet the primary safety endpoint of noninferiority vs darbepoetin alfa with time to first occurrence of MACE. 155
Phase 2b trials of molidustat, 131 , 132 , 156 enarodustat 128 , 129 and desidustat 126 have reported similar results. Notably, all HIF‐PH inhibitors have hepcidin‐lowering effects, which may reduce IV iron requirements and increase dietary iron absorption and iron release from internal stores. 111 , 112 , 118 , 126 , 128 , 129 , 131 , 149 , 150 , 151 , 156
Associated with the stimulation of numerous genes related to the cellular response to hypoxia, HIF‐PH inhibitors may also have non‐haematologic effects such as angiogenesis, tumour growth, and fibrosis. Although such responses have not been observed in clinical trials, ongoing studies will further evaluate the long‐term safety and efficacy of these agents. 110
2.3.2. Hepcidin antagonism
Early clinical studies investigating the ability of hepcidin antagonists (ie, PRS 080, L‐oligonucleotide Lexaptepid Pegol [NOX‐H94] and the humanised monoclonal antibody [mAb] LY2787106) to improve functional iron deficiency in patients with anaemia of CKD by increasing iron availability through binding and inhibition of hepcidin have shown promising preliminary results. The development of these particular agents has been discontinued, and the clinical utility of anti‐hepcidin‐based approaches remains to be evaluated. 157 , 158
Other strategies to modify hepcidin levels currently in preclinical development include decreasing hepcidin production via ligand sequestration or small molecule inhibition of the signalling pathway involved in hepcidin transcription (ie, bone morphogenetic pathway); downregulation of the inflammatory pathway by neutralising IL‐6 with mAbs or small interfering RNA‐mediated targeting of hepcidin or hepcidin regulator mRNA; neutralisation of hepcidin with specific mAbs; interference of hepcidin‐ferroportin interaction without blocking iron export using either small molecule inhibitors or anti‐ferroportin antibodies; and inhibition of ferroportin receptor endocytosis or increased production of ferroportin receptor. 159
3. CONCLUSIONS
For a substantial subset of patients with CKD, anaemia management remains suboptimal and challenging. In particular, for patients who are hyporesponsive to ESAs or have other common comorbid conditions such as diabetes, CVD, functional iron deficiency, and heightened inflammatory status, current standard of care may be inadequate. Newer treatment options, such as HIF‐PH inhibitors, that lower hepcidin levels and provide more physiologic levels of EPO and maintain higher Hb levels without major adverse outcomes may be beneficial.
DISCLOSURES
David S. Goldfarb, MD: Consultant, Alnylam, AstraZeneca, Retrophin, Synlogic; Research, Akebia, Dicerna; Owner, Dr Arnie's, Inc Sheena Pramod, MD: has nothing to disclose. The authors have not received any funding or honoraria for their work.
AUTHOR CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript and take responsibility for the integrity of the work as a whole. All authors and medical writers from inScience Communications wrote the first draft of the manuscript. All authors participated in subsequent drafts, approved the submission of the manuscript, and are fully accountable for all aspects of the work.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Rohan Keshwara, PhD, and Meri D. Pozo, PhD, CMPP, of inScience Communications, Springer Healthcare (Philadelphia, PA), provided medical writing support. Funding for editorial support for this manuscript was provided by AstraZeneca.
Pramod S, Goldfarb DS. Challenging patient phenotypes in the management of anaemia of chronic kidney disease. Int J Clin Pract. 2021;75:e14681. 10.1111/ijcp.14681
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study. All presented data are from published studies.
REFERENCES
- 1. Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Chronic Kidney Disease in the United States, 2019. https://www.cdc.gov/kidneydisease/publications‐resources/2019‐national‐facts.html. Accessed May 7, 2020
- 3. St Peter WL, Guo H, Kabadi S, et al. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non‐dialysis‐dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol. 2018;19(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20(9):1501‐1510. [DOI] [PubMed] [Google Scholar]
- 5. Portoles J, Gorriz JL, Rubio E, et al. The development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney disease. BMC Nephrol. 2013;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang JM, Chen SC, Huang JC, Su HM, Chen HC. Anemia and left ventricular hypertrophy with renal function decline and cardiovascular events in chronic kidney disease. Am J Med Sci. 2014;347(3):183‐189. [DOI] [PubMed] [Google Scholar]
- 7. He J, Shlipak M, Anderson A, et al. Risk factors for heart failure in patients with chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) study. J Am Heart Assoc. 2017;6(5):5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nohara N, Io H, Matsumoto M, et al. Predictive factors associated with increased progression to dialysis in early chronic kidney disease (stage 1–3) patients. Clin Exp Nephrol. 2016;20(5):740‐747. [DOI] [PubMed] [Google Scholar]
- 9. Sato Y, Fujimoto S, Konta T, et al. Anemia as a risk factor for all‐cause mortality: obscure synergic effect of chronic kidney disease. Clin Exp Nephrol. 2018;22(2):388‐394. [DOI] [PubMed] [Google Scholar]
- 10. O'Mara NB. Anemia in patients with chronic kidney disease. Diabetes Spectrum. 2008;21(1):12‐19. [Google Scholar]
- 11. Fishbane S, Spinowitz B. Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018. Am J Kidney Dis. 2018;71(3):423‐435. [DOI] [PubMed] [Google Scholar]
- 12. Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all‐cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005;16(11):3403‐3410. [DOI] [PubMed] [Google Scholar]
- 13. Covic A, Jackson J, Hadfield A, Pike J, Siriopol D. Real‐world impact of cardiovascular disease and anemia on quality of life and productivity in patients with non‐dialysis‐dependent chronic kidney disease. Adv Ther. 2017;34(7):1662‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wish J, Schulman K, Law A, Nassar G. Healthcare expenditure and resource utilization in patients with anaemia and chronic kidney disease: a retrospective claims database analysis. Kidney Blood Press Res. 2009;32(2):110‐118. [DOI] [PubMed] [Google Scholar]
- 15. Pergola PE, Pecoits‐Filho R, Winkelmayer WC, et al. Economic burden and health‐related quality of life associated with current treatments for anaemia in patients with CKD not on dialysis: a systematic review. Pharmacoecon Open. 2019;3(4):463‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23(10):1631‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Souma T, Suzuki N, Yamamoto M. Renal erythropoietin‐producing cells in health and disease. Front Physiol. 2015;6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiang CK, Tanaka T, Inagi R, Fujita T, Nangaku M. Indoxyl sulfate, a representative uremic toxin, suppresses erythropoietin production in a HIF‐dependent manner. Lab Invest. 2011;91(11):1564‐1571. [DOI] [PubMed] [Google Scholar]
- 19. Ganz T, Nemeth E. Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol. 2016;36(2):87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kidney Disease Improving Global Outcomes . KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279‐335. [Google Scholar]
- 21. Mikhail A, Brown C, Williams JA, et al. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017;18(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kidney Disease Improving Global Outcomes . Chapter 2: use of iron to treat anemia in CKD. Kidney Int Suppl. 2012;2(4):292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gardiner R, Roshan D, Brennan A, Connolly D, Murray S, Reddan D. Trends in the treatment of chronic kidney disease‐associated anaemia in a cohort of haemodialysis patients: the Irish experience. Ir J Med Sci. 2019;188(1):223‐230. [DOI] [PubMed] [Google Scholar]
- 24. Vaziri ND. Safety issues in iron treatment in CKD. Semin Nephrol. 2016;36(2):112‐118. [DOI] [PubMed] [Google Scholar]
- 25. Del Vecchio L, Longhi S, Locatelli F. Safety concerns about intravenous iron therapy in patients with chronic kidney disease. Clin Kidney J. 2016;9(2):260‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailie GR, Larkina M, Goodkin DA, et al. Data from the dialysis outcomes and practice patterns study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015;87(1):162‐168. [DOI] [PubMed] [Google Scholar]
- 27. Kalantar‐Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG. Time‐dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16(10):3070‐3080. [DOI] [PubMed] [Google Scholar]
- 28. Macdougall IC, White C, Anker SD, et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N Engl J Med. 2019;380(5):447‐458. [DOI] [PubMed] [Google Scholar]
- 29. National Clinical Guideline Center Anaemia management in chronic kidney disease: clinical guideline. NICE Guideline, 2015: 8. https://www.ncbi.nlm.nih.gov/books/NBK299242/pdf/Bookshelf_NBK299242.pdf. Accessed March 18, 2020.
- 30. Van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S; United States Iron Sucrose Clinical Trials G . A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis‐dependent CKD. Kidney Int. 2005;68(6):2846‐2856. [DOI] [PubMed] [Google Scholar]
- 31. Macdougall IC, Bock AH, Carrera F, et al. FIND‐CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dialysis Transplant. 2014;29(11):2075‐2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agarwal R, Kusek JW, Pappas MK. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015;88(4):905‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang C, Graham DJ, Kane RC, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314(19):2062‐2068. [DOI] [PubMed] [Google Scholar]
- 34. Kaplan J. Roxadustat and anemia of chronic kidney disease. N Engl J Med. 2019;381(11):1070‐1072. [DOI] [PubMed] [Google Scholar]
- 35. Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363(12):1146‐1155. [DOI] [PubMed] [Google Scholar]
- 36. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085‐2098. [DOI] [PubMed] [Google Scholar]
- 37. Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin‐alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. AMGEN . Epogen [package insert]. AMGEN, Inc. 2012. [Google Scholar]
- 39. Sakaguchi Y, Hamano T, Wada A, Masakane I. Types of erythropoietin‐stimulating agents and mortality among patients undergoing hemodialysis. J Am Soc Nephrol. 2019;30(6):1037‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584‐590. [DOI] [PubMed] [Google Scholar]
- 41. Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071‐2084. [DOI] [PubMed] [Google Scholar]
- 42. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019‐2032. [DOI] [PubMed] [Google Scholar]
- 43. McCullough PA, Barnhart HX, Inrig JK, et al. Cardiovascular toxicity of epoetin‐alfa in patients with chronic kidney disease. Am J Nephrol. 2013;37(6):549‐558. [DOI] [PubMed] [Google Scholar]
- 44. FDA Drug Safety Communication . Modified dosing recommendations to improve the safe use of Erythropoiesis‐Stimulating Agents (ESAs) in chronic kidney disease. 2011. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐drug‐safety‐communication‐modified‐dosing‐recommendations‐improve‐safe‐use‐erythropoiesis. Accessed March 18, 2020.
- 45. Casadevall N, Nataf J, Viron B, et al. Pure red‐cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346(7):469‐475. [DOI] [PubMed] [Google Scholar]
- 46. Weber G, Gross J, Kromminga A, Loew HH, Eckardt KU. Allergic skin and systemic reactions in a patient with pure red cell aplasia and anti‐erythropoietin antibodies challenged with different epoetins. J Am Soc Nephrol. 2002;13(9):2381‐2383. [DOI] [PubMed] [Google Scholar]
- 47. Kidney Disease Improving Global Outcomes . Chapter 4: red cell transfusion to treat anemia in CKD. Kidney Int Suppl. 2012;2(4):311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elshimy A, Fouad T, Hasan A. Screening and identification of red blood cell alloantibodies among hemodialysis patients in National Institute of Urology and Nephrology. J Med Sci Res. 2018;1(4):261‐265. [Google Scholar]
- 49. Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis‐stimulating agent hyporesponsiveness. Nephrology. 2007;12(4):321‐330. [DOI] [PubMed] [Google Scholar]
- 50. Luo J, Jensen DE, Maroni BJ, Brunelli SM. Spectrum and burden of erythropoiesis‐stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68(5):763‐771. [DOI] [PubMed] [Google Scholar]
- 51. Gilbertson DT, Peng Y, Arneson TJ, Dunning S, Collins AJ. Comparison of methodologies to define hemodialysis patients hyporesponsive to epoetin and impact on counts and characteristics. BMC Nephrol. 2013;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soppi ET. Iron deficiency without anemia – a clinical challenge. Clin Case Rep. 2018;6(6):1082‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Evans M, Palaka E, Furuland H, et al. The value of maintaining normokalaemia and enabling RAASi therapy in chronic kidney disease. BMC Nephrol. 2019;20(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sibbel SP, Koro CE, Brunelli SM, Cobitz AR. Characterization of chronic and acute ESA hyporesponse: a retrospective cohort study of hemodialysis patients. BMC Nephrol. 2015;16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kaysen GA. Biochemistry and biomarkers of inflamed patients: why look, what to assess. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S56‐S63. [DOI] [PubMed] [Google Scholar]
- 56. Ueda N, Takasawa K. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. 2018;10(9):1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. El Sewefy DA, Farweez BA, Behairy MA, Yassin NR. Impact of serum hepcidin and inflammatory markers on resistance to erythropoiesis‐stimulating therapy in haemodialysis patients. Int Urol Nephrol. 2019;51(2):325‐334. [DOI] [PubMed] [Google Scholar]
- 58. McCausland FR, Claggett B, Burdmann EA, et al. C‐reactive protein and risk of ESRD: results from the trial to reduce cardiovascular events with aranesp therapy (TREAT). Am J Kidney Dis. 2016;68(6):873‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goldstein SL, Ikizler TA, Zappitelli M, Silverstein DM, Ayus JC. Non‐infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int. 2009;76(10):1063‐1069. [DOI] [PubMed] [Google Scholar]
- 61. Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24(3):465‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Malyszko J, Tesarova P, Capasso G, Capasso A. The link between kidney disease and cancer: complications and treatment. Lancet. 2020;396(10246):277‐287. [DOI] [PubMed] [Google Scholar]
- 63. Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol. 2010;23(3):253‐262. [PMC free article] [PubMed] [Google Scholar]
- 64. Thavarajah S, Choi MJ. The use of erythropoiesis‐stimulating agents in patients with CKD and cancer: a clinical approach. Am J Kidney Dis. 2019;74(5):667‐674. [DOI] [PubMed] [Google Scholar]
- 65. Bohlius J, Bohlke K, Castelli R, et al. Management of cancer‐associated anemia with erythropoiesis‐stimulating agents: ASCO/ASH clinical practice guideline update. Blood Adv. 2019;3(8):1197‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. United States Renal Data System . 2018 USRDS Annual Data Report. Volume 1: CKD in the United States – Chapter 3: Morbidity and Mortality in Patients with CKD. 2018. https://www.usrds.org/annual‐data‐report/previous‐adrs/. Accessed March 18, 2020.
- 67. Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504‐2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Silverberg DS, Wexler D, Iaina A, Steinbruch S, Wollman Y, Schwartz D. Anemia, chronic renal disease and congestive heart failure–the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. 2006;38(2):295‐310. [DOI] [PubMed] [Google Scholar]
- 69. Weiner DE, Tighiouart H, Vlagopoulos PT, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16(6):1803‐1810. [DOI] [PubMed] [Google Scholar]
- 70. Lu KJ, Kearney LG, Hare DL, et al. Cardiorenal anemia syndrome as a prognosticator for death in heart failure. Am J Cardiol. 2013;111(8):1187‐1191. [DOI] [PubMed] [Google Scholar]
- 71. Kim CJ, Choi IJ, Park HJ, et al. Impact of cardiorenal anemia syndrome on short‐ and long‐term clinical outcomes in patients hospitalized with heart failure. Cardiorenal Med. 2016;6(4):269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Anand IS. Heart failure and anemia: mechanisms and pathophysiology. Heart Fail Rev. 2008;13(4):379‐386. [DOI] [PubMed] [Google Scholar]
- 73. Colombo PC, Ganda A, Lin J, et al. Inflammatory activation: cardiac, renal, and cardio‐renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17(2):177‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137‐e161. [DOI] [PubMed] [Google Scholar]
- 75. Li J, Xu H, Gao Q, Wen Y. Effect of erythropoiesis‐stimulating agents in acute ST‐segment elevation myocardial infarction: a systematic review. Eur J Clin Pharmacol. 2012;68(5):469‐477. [DOI] [PubMed] [Google Scholar]
- 76. Steppich B, Groha P, Ibrahim T, et al. Effect of Erythropoietin in patients with acute myocardial infarction: five‐year results of the REVIVAL‐3 trial. BMC Cardiovasc Disord. 2017;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. United States Renal Data System . 2019 USRDS annual data report. volume 2: ESRD in the United States reference Table D.1: treatment modalities. 2019. https://www.usrds.org/annual‐data‐report/previous‐adrs/. Accessed August 10, 2020.
- 78. Arbor Research Collaborative for Health . Erythropoiesis Stimulating Agent (ESA) use, last 3 months. The DOPPS Practice Monitor. 2020. https://www.dopps.org/dpm/DPMSlideBrowser.aspx?type=Topic&id=1&graphid=78. Accessed August 5, 2020
- 79. Pisoni RL, Bragg‐Gresham JL, Young EW, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(1):94‐111. [DOI] [PubMed] [Google Scholar]
- 80. de Francisco AL, Stenvinkel P, Vaulont S. Inflammation and its impact on anaemia in chronic kidney disease: from haemoglobin variability to hyporesponsiveness. NDT Plus. 2009;2(Suppl_1):i18‐i26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tsukamoto T, Matsubara T, Akashi Y, Kondo M, Yanagita M. Annual iron loss associated with hemodialysis. Am J Nephrol. 2016;43(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 82. Collins AJ, Li S, St Peter W, et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol. 2001;12(11):2465‐2473. [DOI] [PubMed] [Google Scholar]
- 83. Mapes DL, Lopes AA, Satayathum S, et al. Health‐related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int. 2003;64(1):339‐349. [DOI] [PubMed] [Google Scholar]
- 84. Foley RN, Curtis BM, Parfrey PS. Erythropoietin therapy, hemoglobin targets, and quality of life in healthy hemodialysis patients: a randomized trial. Clin J Am Soc Nephrol. 2009;4(4):726‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sinha SD, Bandi VK, Bheemareddy BR, et al. Efficacy, tolerability and safety of darbepoetin alfa injection for the treatment of anemia associated with chronic kidney disease (CKD) undergoing dialysis: a randomized, phase‐III trial. BMC Nephrol. 2019;20(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Koulouridis I, Alfayez M, Trikalinos TA, Balk EM, Jaber BL. Dose of erythropoiesis‐stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am J Kidney Dis. 2013;61(1):44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. United States Renal Data System . 2018 USRDS annual data report. Volume 2: ESRD in the United States – chapter 2: clinical indicators and preventative care. 2018. https://www.usrds.org/annual‐data‐report/previous‐adrs/. Accessed March 18, 2020.
- 88. Rostoker G, Vaziri ND, Fishbane S. Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs. 2016;76(7):741‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Saliba W, El‐Haddad B. Secondary hyperparathyroidism: pathophysiology and treatment. J Am Board Fam Med. 2009;22(5):574‐581. [DOI] [PubMed] [Google Scholar]
- 90. Kalantar‐Zadeh K, Lee GH, Miller JE, et al. Predictors of hyporesponsiveness to erythropoiesis‐stimulating agents in hemodialysis patients. Am J Kidney Dis. 2009;53(5):823‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tanaka M, Komaba H, Fukagawa M. Emerging association between parathyroid hormone and anemia in hemodialysis patients. Ther Apher Dial. 2018;22(3):242‐245. [DOI] [PubMed] [Google Scholar]
- 92. Al Saran K, Sabry A, Hassan AH. Effect of relative hypoparathyroidism on the responsiveness to recombinant human erythropoietin in chronic hemodialysis patients: a single Saudi center experience. Saudi J Kidney Dis Transpl. 2013;24(4):825‐831. [DOI] [PubMed] [Google Scholar]
- 93. Yuen NK, Ananthakrishnan S, Campbell MJ. Hyperparathyroidism of renal disease. Perm J. 2016;20(3):15‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Miskulin DC, Majchrzak K, Tighiouart H, et al. Ergocalciferol supplementation in hemodialysis patients with vitamin D deficiency: a randomized clinical trial. J Am Soc Nephrol. 2016;27(6):1801‐1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kidney Disease: Improving Global Outcomes . KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD‐MBD). Kidney Int Suppl. 2017;7(1):1‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bover J, Urena P, Ruiz‐Garcia C, et al. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11(1):161‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kuragano T, Mizusaki K, Kimura T, Nakanishi T. Anemia management considering the pathophysiology of elderly chronic kidney disease patients. Contrib Nephrol. 2019;198:135‐143. [DOI] [PubMed] [Google Scholar]
- 98. de Goeij MC, Meuleman Y, van Dijk S, et al. Haemoglobin levels and health‐related quality of life in young and elderly patients on specialized predialysis care. Nephrol Dial Transplant. 2014;29(7):1391‐1398. [DOI] [PubMed] [Google Scholar]
- 99. Rossert J, Gassmann‐Mayer C, Frei D, McClellan W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transplant. 2007;22(3):794‐800. [DOI] [PubMed] [Google Scholar]
- 100. Lattanzio F, Corsonello A, Montesanto A, et al. Disentangling the impact of chronic kidney disease, anemia, and mobility limitation on mortality in older patients discharged from hospital. J Gerontol A Biol Sci Med Sci. 2015;70(9):1120‐1127. [DOI] [PubMed] [Google Scholar]
- 101. Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end‐stage kidney disease hospitalized with COVID‐19. Kidney Int. 2020;98:1530‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID‐19) infection. Int Urol Nephrol. 2020;52:1193‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Oetjens MT, Luo JZ, Chang A, et al. Electronic health record analysis identifies kidney disease as the leading risk factor for hospitalization in confirmed COVID‐19 patients. PLoS One. 2020;15:e0242182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97:829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID‐19: friend and foe?. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fishbane S, Hirsch JS. Erythropoiesis‐stimulating agent treatment in patients With COVID‐19. Am J Kidney Dis. 2020;76:303‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Leventhal J, Angeletti A, Cravedi P. EPO in patients with COVID‐19: more than an Erythropoietic hormone. Am J Kidney Dis. 2020;76:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ehrenreich H, Weissenborn K, Begemann M, Busch M, Vieta E, Miskowiak KW. Erythropoietin as candidate for supportive treatment of severe COVID‐19. Mol Med. 2020;26:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gupta N, Wish JB. Hypoxia‐inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69(6):815‐826. [DOI] [PubMed] [Google Scholar]
- 110. Haase VH. HIF‐prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int. 2017;21(Suppl 1):S110‐S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen N, Hao C, Liu BC, et al. Roxadustat treatment for anemia in patients undergoing long‐term dialysis. N Engl J Med. 2019;381(11):1011‐1022. [DOI] [PubMed] [Google Scholar]
- 112. Chen N, Hao C, Peng X, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381(11):1001‐1010. [DOI] [PubMed] [Google Scholar]
- 113. Akizawa T, Ueno M, Shiga T, Reusch M. Oral roxadustat three times weekly in ESA‐naive and ESA‐converted patients with anemia of chronic kidney disease on hemodialysis: results from two phase 3 studies. Ther Apher Dial. 2020;24:628‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open‐label study. Ther Apher Dial. 2020;24(2):115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Akizawa T, Tsubakihara Y, Nangaku M, et al. Effects of daprodustat, a novel hypoxia‐inducible factor prolyl hydroxylase inhibitor on anemia management in japanese hemodialysis subjects. Am J Nephrol. 2017;45(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 116. Holdstock L, Meadowcroft AM, Maier R, et al. Four‐week studies of oral hypoxia‐inducible factor‐prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27(4):1234‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cizman B, Sykes AP, Paul G, Zeig S, Cobitz AR. An exploratory study of daprodustat in erythropoietin‐hyporesponsive subjects. Kidney Int Rep. 2018;3(4):841‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Meadowcroft AM, Cizman B, Holdstock L, et al. Daprodustat for anemia: a 24‐week, open‐label, randomized controlled trial in participants on hemodialysis. Clin Kidney J. 2019;12(1):139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Brigandi RA, Johnson B, Oei C, et al. A novel hypoxia‐inducible factor‐prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: a 28‐day, phase 2a randomized trial. Am J Kidney Dis. 2016;67(6):861‐871. [DOI] [PubMed] [Google Scholar]
- 120. Holdstock L, Cizman B, Meadowcroft AM, et al. Daprodustat for anemia: a 24‐week, open‐label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J. 2019;12(1):129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tsubakihara Y, Akizawa T, Nangaku M, et al. A 24‐week anemia correction study of daprodustat in Japanese dialysis patients. Ther Apher Dial. 2020;24(2):108‐114. [DOI] [PubMed] [Google Scholar]
- 122. Kimura T, Nangaku M, Hamano T, et al. Efficacy and safety of daprodustat compared with epoetin beta pegol in Japanese non‐dialysis patients with anemia of chronic kidney disease: a 52‐week, open‐label, randomized controlled phase 3 trial [late‐breaking clinical trial]. Proceedings of the ERA‐EDTA 2019 congress; June 13–16, 2019; Budapest, Hungary.
- 123. Akizawa T, Nangaku M, Yonekawa T, et al. Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double‐blind, phase 3 trial [abstract SaO036]. Nephrol Dial Transplant. 2019;34:i350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Daprodustat – GlaxoSmithKline . AdisInsight drugs [Internet document; Updated July 13, 2020]. https://adisinsight.springer.com/drugs/800030089. Accessed August 10, 2020.
- 125. Akizawa T, Nangaku M, Yonekawa T, et al. Efficacy and safety of daprodustat compared with darbepoetin alfa in japanese hemodialysis patients with anemia: a randomized, double‐blind, phase 3 trial. Clin J Am Soc Nephrol. 2020;15(8):1155‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Parmar DV, Kansagra KA, Patel JC, et al. Outcomes of desidustat treatment in people with anemia and chronic kidney disease: a phase 2 study. Am J Nephrol. 2019;49(6):470‐478. [DOI] [PubMed] [Google Scholar]
- 127. Desidustat – Zydus Cadila . AdisInsight drugs [Internet document; Updated July 28, 2020]. https://adisinsight.springer.com/drugs/800041749. Accessed August 10, 2020.
- 128. Akizawa T, Nangaku M, Yamaguchi T, et al. Enarodustat, conversion and maintenance therapy for anemia in hemodialysis patients: a randomized, placebo‐controlled phase 2b trial followed by long‐term trial. Nephron. 2019;143(2):77‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Akizawa T, Nangaku M, Yamaguchi T, et al. A placebo‐controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long‐term trial. Am J Nephrol. 2019;49(2):165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Enarodustat – Japan Tobacco AdisInsight drugs [Internet document; Updated December 30, 2019]. https://adisinsight.springer.com/drugs/800035317. Accessed August 10, 2020.
- 131. Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14(1):28‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Akizawa T, Macdougall IC, Berns JS, et al. Long‐term efficacy and safety of molidustat for anemia in chronic kidney disease: DIALOGUE extension studies. Am J Nephrol. 2019;49(4):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Molidustat – Bayer HealthCare Pharmaceuticals . AdisInsight drugs [Internet document; Updated April 11, 2020]. https://adisinsight.springer.com/drugs/800033038. Accessed August 10, 2020.
- 134. Chen N, Qian J, Chen J, et al. Phase 2 studies of oral hypoxia‐inducible factor prolyl hydroxylase inhibitor FG‐4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32(8):1373‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Provenzano R, Besarab A, Wright S, et al. Roxadustat (FG‐4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6‐ to 19‐week, open‐label, active‐comparator, dose‐ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67(6):912‐924. [DOI] [PubMed] [Google Scholar]
- 136. Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG‐4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27(4):1225‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Provenzano R, Besarab A, Sun CH, et al. Oral hypoxia‐inducible factor prolyl hydroxylase inhibitor roxadustat (FG‐4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol. 2016;11(6):982‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Besarab A, Provenzano R, Hertel J, et al. Randomized placebo‐controlled dose‐ranging and pharmacodynamics study of roxadustat (FG‐4592) to treat anemia in nondialysis‐dependent chronic kidney disease (NDD‐CKD) patients. Nephrol Dial Transplant. 2015;30(10):1665‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Akizawa T, et al. Phase 3, randomized, double‐blind, active‐comparator (Darbepoetin Alfa) conversion study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. Paper presented at: American Society of Nephrology Kidney Week. 2018. [DOI] [PMC free article] [PubMed]
- 140. T Akizawa, et al. Phase 3, multicenter, randomized, open‐label, non‐comparative study of intermittent oral roxadustat in ESA‐naive CKD patients not on dialysis in japan. Paper presented at: American Society of Nephrology (ASN) Kidney Week. 2019.
- 141. Roxadustat – FibroGen . AdisInsight drugs [Internet document; Updated August 06, 2020]. https://adisinsight.springer.com/drugs/800023523. Accessed August 10, 2020.
- 142. Astellas‐Fibrogen . Astellas receives approval of EVRENZO® (roxadustat) in Japan for the treatment of anemia of chronic kidney disease in adult patients not on dialysis. 2020. https://www.astellas.com/system/files/news/2020‐11/20201127_en_1.pdf. Accessed December 7, 2020.
- 143. Charytan C, Manllo‐Karim R, Martin E, Steer D, Bernardo M, Dua S. SIERRAS: a phase 3, open‐label, randomized, active‐controlled study of the efficacy and safety of roxadustat in the maintenance treatment of anemia in subjects with ESRD on stable dialysis [SA‐P0227]. J Am Soc Nephrol. 2019;30:822. [Google Scholar]
- 144. Fishbane S, Pollack C, El‐Shawahawy M, Escudero E, Rastogi A. ROCKIES: an international, phase 3, randomized, open‐label, active‐controlled study of roxadustat for anemia in dialysis‐dependent CKD patients [TH‐OR022]. J Am Soc Nephrol. 2019;30:6. [Google Scholar]
- 145. Esposito C, Czicky B, Tataradze A, Reusch M, Han C, Sulowicz W. Two phase 3, multicenter, randomized studies of intermittent oral roxadustat in anemic CKD patients on (PYRENEES) and not on (ALPS) dialysis [SA‐P0225]. J Am Soc Nephrol. 2019;30:822. [Google Scholar]
- 146. Provenzano R, Evgeny S, Liubov E, Kordeyeva S. HIMALAYAS: a phase 3, randomized, open‐label, active‐controlled study of the efficacy and safety of roxadustat in the treatment of anemia in incident‐dialysis patients [TH‐OR021]. J Am Soc Nephrol. 2019;30:5. [Google Scholar]
- 147. Coyne D, Rodger S, Shin S, Kim S. ANDES: a phase 3, randomized, double‐blind, placebo controlled study of the efficacy and safety of roxadustat for the treatment of anemia in CKD patients not on dialysis [SA‐P0228]. J Am Soc Nephrol. 2019;30:822. [Google Scholar]
- 148. Fishbane S, El‐Shawahawy M, Pecoits‐Filho R, van Bui P. OLYMPUS: a phase 3, randomized, double‐blind, placebo‐controlled, international study of roxadustat efficacy in patients with non‐dialysis‐dependent (NDD) CKD and anemia [TH‐OR023]. J Am Soc Nephrol. 2019;30:5. [Google Scholar]
- 149. Haase VH, Chertow GM, Block GA, et al. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis‐stimulating agents. Nephrol Dial Transplant. 2019;34(1):90‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis‐dependent chronic kidney disease. Kidney Int. 2016;90(5):1115‐1122. [DOI] [PubMed] [Google Scholar]
- 151. Martin ER, Smith MT, Maroni BJ, Zuraw QC, deGoma EM. Clinical trial of vadadustat in patients with anemia secondary to stage 3 or 4 chronic kidney disease. Am J Nephrol. 2017;45(5):380‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nangaku N, Kondo K, Ueta K, et al. Randomized, double‐blinded, active‐controlled (darbepoetin alfa), phase 3 study of vadadustat in CKD patients with anemia on hemodialysis in Japan [abstract TH‐OR024]. J Am Soc Nephrol. 2019;30:6. [Google Scholar]
- 153. Nangaku N, Kondo K, Kokado Y, et al. Randomized, open‐label, active‐controlled (darbepoetin alfa), phase 3 study of vadadustat for treating anemia in non‐dialysis‐dependent CKD patients in Japan [abstract SA‐PO229]. J Am Soc Nephrol. 2019;30:823. [Google Scholar]
- 154. Vadadustat – Akebia Therapeutics . AdisInsight drugs [Internet document; Updated July 16, 2020]. https://adisinsight.springer.com/drugs/800031427. Accessed August 10, 2020.
- 155. Akebia Therapeutics . Akebia Therapeutics Announces Top‐Line Results from its PRO2TECT Global Phase 3 Program of Vadadustat for Treatment of Anemia Due to Chronic Kidney Disease in Adult Patients Not on Dialysis. 2020. https://ir.akebia.com/node/11301/pdf. Accessed September 29, 2020.
- 156. Akizawa T, Macdougall IC, Berns JS, et al. Iron regulation by molidustat, a daily oral hypoxia‐inducible factor prolyl hydroxylase inhibitor, in patients with chronic kidney disease. Nephron. 2019;143(4):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Poli M, Asperti M, Ruzzenenti P, Regoni M, Arosio P. Hepcidin antagonists for potential treatments of disorders with hepcidin excess. Front Pharmacol. 2014;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Renders L, Budde K, Rosenberger C, et al. First‐in‐human Phase I studies of PRS‐080#22, a hepcidin antagonist, in healthy volunteers and patients with chronic kidney disease undergoing hemodialysis. PLoS One. 2019;14(3):e0212023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fung E, Nemeth E. Manipulation of the hepcidin pathway for therapeutic purposes. Haematologica. 2013;98(11):1667‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Soares MP, Hamza I. Macrophages and iron metabolism. Immunity. 2016;44(3):492‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study. All presented data are from published studies.


