Summary
The extent to which behavioral weight management interventions affect health inequalities is uncertain, as is whether trials of these interventions directly consider inequalities. We conducted a systematic review, synthesizing evidence on how different aspects of inequality impact uptake, adherence, and effectiveness in trials of behavioral weight management interventions. We included (cluster‐) randomized controlled trials of primary care‐applicable behavioral weight management interventions in adults with overweight or obesity published prior to March 2020. Data about trial uptake, intervention adherence, attrition, and weight change by PROGRESS‐Plus criteria (place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status, social capital, plus other discriminating factors) were extracted. Data were synthesized narratively and summarized in harvest plots. We identified 91 behavioral weight loss interventions and 12 behavioral weight loss maintenance interventions. Fifty‐six of the 103 trials considered inequalities in relation to at least one of intervention or trial uptake (n = 15), intervention adherence (n = 15), trial attrition (n = 32), or weight outcome (n = 34). Most trials found no inequalities gradient. If a gradient was observed for trial uptake, intervention adherence, and trial attrition, those considered “more advantaged” did best. Alternative methods of data synthesis that enable data to be pooled and increase statistical power may enhance understanding of inequalities in behavioral weight management interventions.
Keywords: inequalities, interventions, obesity, weight management
Abbreviations
- BMI
body mass index
- OECD
Organization for Economic Collaboration and Development
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis
- RCT
randomized controlled trial
- SES
socioeconomic status
- USPSTF
United States Preventive Services Taskforce
- WLMs
behavioral weight loss maintenance interventions
- WLs
behavioral weight loss interventions
1. INTRODUCTION
Overweight and obesity are associated with an increased risk of several non‐communicable diseases including type 2 diabetes, cardiovascular disease, and some cancers (e.g., bowel and post‐menopausal breast). 1 , 2 Behavioral weight management interventions have been shown to be effective in promoting (behavioral weight loss interventions [WLs]) and maintaining (behavioral weight loss maintenance interventions [WLMs]) weight loss in those with overweight or obesity. 3 , 4 Interventions designed to support individuals to change their health behaviors, such as behavioral weight management interventions, typically require a high amount of personal agency (such as time, resource and education) in order to be effective. 5 , 6 In this way, they may benefit advantaged groups with higher personal agency more than those less advantaged. Consequently, behavioral weight management interventions may be inequitable and exacerbate health inequalities. 5 , 6 Health inequalities are systemic and avoidable differences in health outcomes between difference groups in a population. 7 Inequalities may arise at various stages of an intervention, such as uptake, adherence, or outcome and can occur across characteristics summarized by the PROGRESS‐Plus framework (place of residence, race/ethnicity, occupation, gender/sex, education, socioeconomic status [SES], social capital, plus other factors for which discrimination could occur such as age and sexual orientation). 8
Several previous systematic reviews have considered the relationship between characteristics where inequalities may occur and interventions for overweight or obesity. 9 , 10 , 11 , 12 , 13 , 14 The United States Preventive Services Taskforce (USPSTF) considered the overall effectiveness of behavioral and pharmacological interventions for overweight and obesity 3 and provided narrative comment about some aspects of inequality. The authors found that unless the intervention was targeted towards a specific ethnicity, ethnicity and SES were frequently not reported. Where these were reported, most participants were White and of mid‐to‐high SES.
The other systematic reviews we identified focused on a single characteristic where inequality might occur (one of race or ethnicity, gender, and SES). Seven reviews focused on race or ethnicity. 9 , 10 , 11 , 12 , 13 , 14 , 15 Two only included interventions that were targeted towards Latinos in America, 9 , 14 and one only included interventions that were tailored towards African American women. 10 Four systematic reviews included studies if they reported more than one race or ethnicity represented in their sample. Haughton et al. 13 found that only 2/60 WL studies conducted analysis of differential attendance by ethnicity and 8/60 conducted analysis of differential outcome by ethnicity. Across 71 trials of interventions that focused on using technology for weight loss, Rosenbaum et al. found there was low enrolment (trial uptake) of racial minorities. 12 Fitzgibbon et al. included all trials (n = 25) that reported including Black women (not only trials of interventions that were targeted towards Black women). 11 They found that Black women had lower weight loss and higher study attrition than other groups but no differences in intervention adherence. Tussing‐Humphreys et al. reviewed 17 studies of WLMs that included African American women 15 and found that African American women generally lost less weight during the weight loss phase and regained more weight during the maintenance phase when compared with Caucasian women.
We also identified systematic reviews that considered gender in behavioral weight management trials. 16 , 17 , 18 These reviews found that males are generally underrepresented in trials of WLs and WLMs 16 that male‐only interventions may effectively engage and assist men in achieving weight loss but, 17 in interventions that are delivered to males and females, achieved weight loss was similar. 18
Two systematic reviews synthesized data on inequalities by SES. Olstad et al., in a review of universal policies for obesity in adults and children, found no evidence to support the theory that interventions targeted towards individuals/households, such as behavioral weight management interventions, were more likely to be inequitable than population‐level interventions. 19 However, Olstad et al. noted that this may have been due to the few “agentic” (i.e., interventions requiring a high amount of personal agency in order to take effect) policies included in the review. In an earlier review, Hillier‐Brown et al. considered effectiveness of individual‐, community‐, and population‐level interventions at reducing socioeconomic inequalities in obesity. 20 The authors identified evidence from interventions targeting deprived groups, rather than those aimed at the population more generally. The authors only included studies reporting differential effects by SES and only looked at outcome measures rather than process measures (such as uptake and trial attrition).
The highlighted systematic reviews generally focused on a single PROGRESS‐Plus characteristic. It is useful to examine all of the PROGRESS‐Plus characteristics in a single review to gain a broader understanding of inequalities and identify any under‐researched characteristics. Furthermore, some of the previous systematic reviews restricted their inclusion criteria to specific races or ethnicities 9 , 14 or a specific subcategory of behavioral intervention. 12 Few reviews assessed if there were inequalities at trial stages other than weight outcome, especially in reviews that focused on SES. 19 , 20 It is important to understand at what stage inequalities occur in order to effectively address them.
Therefore, we aimed to:
Summarize the number and characteristics of trials of behavioral weight management interventions that report measures of inequalities as defined by the PROGRESS‐Plus criteria.
Identify, describe, and synthesize trial data on inequalities in the uptake of, adherence to, and effectiveness of behavioral weight management interventions.
Synthesize data on differential attrition in trials of behavioral weight management interventions.
2. METHODS
This review was completed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines and the PRISMA‐Equity extension. 21 , 22 Full details of the methods were published in the protocol, 23 and the review was registered on PROSPERO (CRD42020173242).
2.1. Eligibility criteria
Participants: adults aged 18 years and over with overweight or obesity (body mass index [BMI] ≥ 25 kg/m2 with no upper limit) who were deemed suitable (either by the applicable study team or healthcare practitioner) for WLs or WLMs. Participants may have additional risk factors such as hypertension, dyslipidemia, impaired glucose tolerance, or impaired fasting glucose. Studies were excluded if the population was not selected based on a weight‐related measure, included participants with BMI < 25 kg/m2 were selected based on having a chronic disease where weight loss is part of disease management or being pregnant, or if the intervention was targeted at parents to change behavior of children. Only studies conducted in member countries of the Organization for Economic Collaboration and Development (OECD) were eligible.
Interventions: behavioral weight management interventions with the primary aim of supporting weight loss or weight loss maintenance. Studies were included if they were conducted in, or were applicable to, primary care settings. Interventions may have been delivered alone or as part of a wider multicomponent intervention targeting diet and nutrition, physical activity, sedentary behavior, or a combination of these. Interventions may include but were not limited to, assessment with feedback, advice, provider training, goal‐setting, or exercise referral. Studies of pharmacological and surgical interventions were excluded unless the trial included behavioral only and control arms. Interventions were considered feasible for application to primary care if they were conducted in a healthcare setting or are widely available in the community at a national or regional level (such as commercial weight loss programs, text‐message based interventions); examples of settings that are not relevant to primary care include interventions delivered in inpatient settings or in residential care homes.
Comparators: wait‐list control, usual care, or minimal intervention (such as generic print or electronic materials).
Outcomes: studies must report a weight outcome (weight change in kg, ≥5% weight loss, or change in waist circumference) at either the 12‐ or 18‐month follow‐up.
Study designs: randomized or cluster‐randomized controlled trials (RCTs). Studies that were not available in English were excluded.
2.2. Search strategy and information sources
We adopted a two‐stage search strategy to identify relevant publications. The first stage involved identifying “parent” RCTs 24 (trials included in the USPSTF review 3 ). We then replicated the search used in the USPSTF review to identify relevant trials published since the USPSTF database search date (June 2017). The updated search was performed on March 5, 2020 in Medline, Cochrane CENTRAL, and PsycInfo (search strategy available in Supplementary File 1a). We used the USPSTF review due to the comprehensive search strategy used in the review that increased the likelihood of identifying relevant trails of behavioral weight management interventions for inclusion.
The second stage involved conducting a further Medline search to identify all further publications from each parent RCT.
2.3. Study selection
All behavioral weight management interventions included in the USPSTF report were included in the review. The titles and abstracts from the updated search were independently screened by two investigators (JMB and RAJ) using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Any discrepancies were resolved by consensus. Full texts of studies identified from the title and abstract screening as being potentially relevant were screened independently by two investigators (JMB and RAJ), and conflicts resolved through discussion. Trials already identified from the USPSTF report and included in our search results were de‐duplicated at this stage. Where necessary, conflicts were discussed with a third investigator (ALA) to reach consensus.
2.4. Data extraction
Data extraction was completed by one investigator (JMB) and checked by a second investigator (RAJ, JM, MDMcD, and RR). The data extraction form was developed using the Cochrane Public Health Group data extraction form, the Consolidated Standards of Reporting Trials 2010 statement, the Template for Intervention Description and Replication checklist, and the PROGRESS‐Plus criteria. 8 , 25 , 26 , 27
2.5. Outcomes
2.5.1. Trial and intervention uptake
Trial uptake was defined as participants accepting an invitation to participate in a trial of a behavioral weight management intervention. Therefore, we defined differential trial uptake as whether those who accepted invitation to participate differed from those who declined to take part by a measure of a PROGRESS‐Plus criterion.
Intervention uptake was defined as a participant attending or completing at least one session of the intervention. We defined differential intervention uptake as a statistically significant difference between participants attending at least one session of an intervention versus those who did not, by a measure of a PROGRESS‐Plus characteristic.
2.5.2. Intervention adherence
Adherence was defined as number of intervention sessions attended out of those offered or as engagement with any intervention component (such as completion of food diaries or number of times logged into a mobile application). Differential adherence was defined as a statistically significant difference in the number of sessions of an intervention attended or engagement with an intervention component by a measure of a PROGRESS‐Plus characteristic.
2.5.3. Trial attrition
Trial attrition was defined as those lost to follow‐up at the 12‐month follow‐up. Where attrition for the 12‐month time point was not reported, data reported up until 18 months of follow‐up were extracted instead. We considered differential trial attrition as a statistically significant difference in a measure of a PROGRESS‐Plus characteristic, between those who were and were not followed up in the intervention arm at this time point.
2.5.4. Weight outcome
Weight‐related outcomes (weight change in kilograms, >5% weight loss, or change in waist circumference in centimeters) at 12‐month follow‐up were extracted. If 12‐month follow‐up data were not reported, data for the closest time point after 12–18 months of follow‐up were extracted instead. Differential weight outcome was defined as a statistically significant difference by a measure of a PROGRESS‐Plus characteristic.
2.5.5. Categorization of more and less advantaged groups
We used the PROGRESS‐Plus framework, and previous inequality‐focused systematic reviews, to inform our categorization of which groups under each PROGRESS‐Plus criterion would be defined as “more advantaged” or “less advantaged.” 8 , 28 , 29 , 30 We considered “more advantaged” groups as follows: urban (place of residence, people living in urban areas often have more proximal access to healthcare and other amenities), White (race/ethnicity), employed full‐time (occupation), male (gender or sex), majority religion (religion), more education (Education), less deprived—for area based measures—or higher income level (SES), being married (social capital), and being older (PLUS). We categorized being older as “more advantaged” as evidence suggests older adults have fewer barriers to accessing primary care and are more likely to be offered weight management intervention in routine practice. 31 , 32 , 33 For other measures of the plus criterion, we considered those who spoke the predominant language, were born in the country where the trial was conducted, or were free from disability to be ‘more advantaged’.
Less advantaged groups were defined as follows: rural (place of residence), ethnicity other than white (race/ethnicity), not employed full‐time (occupation), female (gender or sex), minority religion (religion), less education (education), more deprived—for area‐based measures—or lower income level (SES), not married (social capital), and being younger (PLUS). For other measures of the plus criterion, we considered those who did not speak the predominant language or were not born in the country where the trial was conducted, or who had a disability, to be “less advantaged.”
2.6. Quality assessment
Firstly, we extracted the categorization of quality assessment (good, fair, or poor) given to the studies included in the USPSTF report. Next, we replicated the quality assessment process from the USPSTF report for the additional studies identified as meeting eligibility criteria. 34 Studies were graded as “good” if follow‐up was ≥80%, valid measurement instruments used, interventions clearly outlined, and confounders were appropriately controlled for in analysis. A study was rated as “fair” if some minor limitations occurred. For example, there may be minor differences in follow‐up, follow‐up <80%, or not all potential confounders accounted for. A “poor” rating was given to a study if major limitations existed, such as unreliable weight measurement methods (e.g., invalidated scales), inadequately conducted randomization, or important confounders given little or no consideration.
2.6.1. Deviations from original protocol
We originally planned to use Cochrane's Risk of Bias (RoB) 2 tool to conduct risk of bias assessment. 23 Instead, we aligned our methods for quality assessment with those used in the USPSTF report, 3 as the USPSTF method incorporates risk of bias assessment into its quality assessment of included trials.
2.7. Data synthesis
Due to heterogeneity in intervention types and measures of the PROGRESS‐Plus criteria, such as country‐specific measures of SES or ethnicity, it was deemed not possible to conduct a meta‐analysis. Therefore, we conducted a narrative review with the addition of harvest plots. Harvest plots were originally proposed by Ogilvie et al., as a method of synthesizing evidence of differential effectiveness of public health interventions where a meta‐analysis may not be appropriate. 35 In the harvest plots, bar height equates to the sample size of the study, with the smallest bars representing studies with 0–200 participants, and the tallest bars studies with 801+ participants. A study was categorized as favoring a particular group if a statistically significant difference was observed. The harvest plots were produced using Microsoft PowerPoint (version 2016, Microsoft Corporation).
3. RESULTS
The PRISMA Flow Diagram (Figure 1) shows the number of papers identified in each stage of the review. 21 A total of 103 studies met the inclusion criteria, 89 of which were extracted from the USPSTF report and a further 14 identified through the replicated search strategy. As publication families of each included trial were identified, information was extracted from 266 publications across the 103 studies.
FIGURE 1.
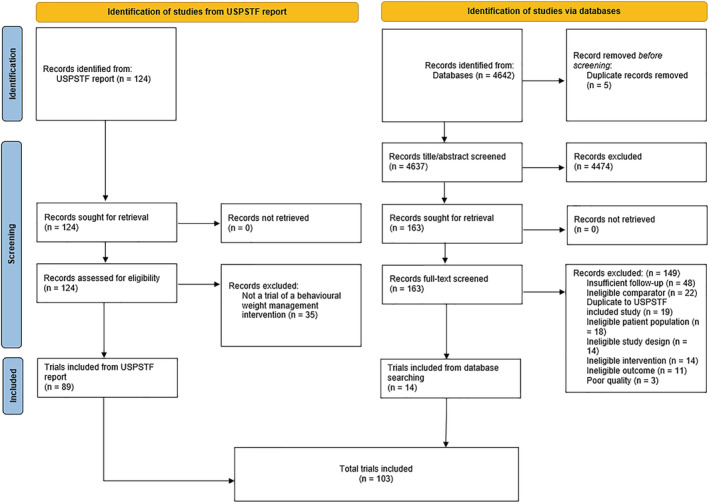
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) flow diagram for the inclusion of studies
3.1. Study characteristics
Of the 103 included studies (Tables 1 and S1), 90 were trials of WLs, 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 and 13 were of WLMs. 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 Across the studies, there were a total of 36,805 participants, with the sample size of each study ranging from 30 44 to 2,161 participants. 93 Sixty‐six percent of participants were female. Most studies were from the United States (n = 57).
TABLE 1.
Characteristics of included trials
| Number of trials (%) | Citations | |
|---|---|---|
| Intervention type | ||
| Weight loss | 90 (87.4) | 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 |
| Weight loss maintenance | 13 (12.6) | 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 |
| Database searching vs. identification from USPSTF report | ||
| Studies identified from database searching | 14 (13.6) | 84 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 135 , 136 , 137 , 138 |
| Studies identified from USPSTF report | 89 (86.4) | 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 |
| Trial location | ||
| United States | 57 (55.3) | 36 , 37 , 42 , 43 , 44 , 45 , 46 , 47 , 52 , 55 , 56 , 57 , 63 , 64 , 65 , 66 , 68 , 69 , 71 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 83 , 84 , 85 , 87 , 90 , 92 , 93 , 94 , 96 , 98 , 101 , 102 , 104 , 105 , 107 , 110 , 111 , 112 , 113 , 115 , 117 , 119 , 123 , 127 , 129 , 130 , 131 , 133 , 134 |
| United Kingdom | 16 (15.5) | 38 , 39 , 40 , 41 , 48 , 49 , 53 , 61 , 67 , 95 , 100 , 114 , 124 , 132 , 135 , 138 |
| Australia | 5 (4.9) | 59 , 116 , 118 , 125 , 128 |
| Finland | 5 (4.9) | 86 , 103 , 108 , 122 , 126 |
| Japan | 4 (3.9) | 60 , 91 , 99 , 137 |
| Germany | 3 (2.9) | 54 , 97 , 136 |
| The Netherlands | 3 (2.9) | 58 , 82 , 109 |
| Spain | 3 (2.9) | 106 , 120 , 121 |
| Canada | 2 (1.9) | 70 , 89 |
| Sweden | 2 (1.9) | 50 , 88 |
| Norway | 1 (1.0) | 62 |
| Portugal | 1 (1.0) | 72 |
| Multiple countries | 1 (1.0) | 51 |
| Sample size at baseline | ||
| 0–200 | 41 (39.8) | 36 , 44 , 45 , 46 , 48 , 50 , 54 , 57 , 58 , 59 , 64 , 65 , 67 , 71 , 76 , 80 , 81 , 86 , 88 , 90 , 91 , 97 , 99 , 101 , 102 , 103 , 104 , 107 , 110 , 112 , 113 , 119 , 120 , 121 , 124 , 128 , 129 , 130 , 132 , 136 , 137 |
| 201–400 | 32 (31.1) | 39 , 42 , 43 , 47 , 52 , 55 , 56 , 60 , 61 , 62 , 63 , 68 , 69 , 72 , 75 , 77 , 83 , 84 , 87 , 92 , 98 , 111 , 114 , 115 , 116 , 117 , 118 , 125 , 126 , 133 , 134 , 138 |
| 401–600 | 15 (14.6) | 37 , 41 , 66 , 70 , 73 , 78 , 79 , 82 , 85 , 94 , 108 , 122 , 123 , 131 , 135 |
| 601–800 | 5 (4.9) | 49 , 51 , 53 , 96 , 105 |
| >800 | 10 (9.7) | 38 , 40 , 74 , 89 , 93 , 95 , 100 , 106 , 109 , 127 |
| Proportion female at baseline | ||
| 0% (all male) | 6 (5.8) | 49 , 59 , 66 , 103 , 109 , 128 |
| 1–49% | 11 (10.7) | 39 , 48 , 54 , 56 , 58 , 73 , 74 , 91 , 97 , 119 , 133 |
| 50–99% | 70 (68.0) | 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 46 , 51 , 52 , 53 , 55 , 60 , 61 , 62 , 63 , 64 , 65 , 67 , 69 , 70 , 71 , 75 , 76 , 77 , 78 , 79 , 81 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 92 , 93 , 95 , 96 , 98 , 99 , 100 , 106 , 107 , 108 , 111 , 112 , 113 , 114 , 115 , 116 , 118 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 130 , 131 , 132 , 134 , 135 , 136 , 137 , 138 |
| 100% (all female) | 16 (15.5) | 45 , 47 , 50 , 57 , 68 , 72 , 80 , 82 , 94 , 101 , 102 , 104 , 105 , 110 , 117 , 129 |
| Mean age (years) at baseline a | ||
| 18–40 | 11 (10.7) | 50 , 52 , 59 , 68 , 72 , 75 , 76 , 85 , 86 , 101 , 117 |
| 41–54 | 63 (61.2) | 37 , 38 , 42 , 43 , 45 , 46 , 47 , 49 , 51 , 53 , 55 , 56 , 57 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 69 , 70 , 71 , 73 , 74 , 79 , 81 , 83 , 84 , 87 , 88 , 89 , 93 , 95 , 96 , 97 , 98 , 100 , 102 , 103 , 104 , 107 , 109 , 111 , 112 , 114 , 115 , 116 , 118 , 119 , 121 , 122 , 123 , 124 , 126 , 128 , 129 , 130 , 131 , 132 , 134 , 135 , 136 , 138 |
| ≥55 | 28 (27.2) | 36 , 39 , 40 , 41 , 44 , 48 , 54 , 58 , 67 , 77 , 78 , 80 , 82 , 90 , 91 , 92 , 94 , 99 , 105 , 106 , 108 , 110 , 113 , 120 , 125 , 127 , 133 , 137 |
| Number of PROGRESS‐Plus measures reported at baseline | ||
| 0 | 0 (0.0) | |
| 1 | 0 (0.0) | |
| 2 | 20 | 44 , 46 , 58 , 60 , 70 , 88 , 97 , 99 , 103 , 106 , 108 , 112 , 121 , 129 , 130 , 131 , 134 , 136 , 137 |
| 3 | 16 | 36 , 43 , 51 , 54 , 85 , 87 , 90 , 91 , 95 , 96 , 100 , 105 , 107 , 113 , 122 , 132 |
| 4+ | 67 | 37 , 38 , 39 , 40 , 41 , 42 , 45 , 47 , 48 , 49 , 50 , 52 , 53 , 55 , 56 , 57 , 59 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 86 , 89 , 92 , 93 , 94 , 98 , 101 , 102 , 104 , 109 , 110 , 111 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 123 , 124 , 126 , 127 , 128 , 133 , 135 , 138 |
| Number of PROGRESS‐Plus measures differential trial uptake considered by | ||
| 0 | 89 (86.4) | 36 , 37 , 39 , 41 , 43 , 44 , 46 , 47 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 67 , 69 , 72 , 73 , 75 , 76 , 77 , 78 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 129 , 130 , 131 , 132 , 135 , 136 , 137 , 138 |
| 1 | 6 (5.8) | 48 , 58 , 68 , 70 , 71 , 119 |
| 2 | 4 (3.9) | 42 , 45 , 66 , 114 |
| 3 | 3 (2.9) | 40 , 74 , 143 |
| 4+ | 1 (1.0) | 79 |
| Number of PROGRESS‐Plus measures differential intervention uptake considered by | ||
| 0 | 98 (95.1) | 36 , 38 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 |
| 1 | 3 (2.9) | 40 , 52 , 68 |
| 2 | 0 (0.0) | |
| 3 | 0 (0.0) | |
| 4+ | 1 (1.0) | 37 |
| Number of PROGRESS‐Plus measures differential adherence considered by | ||
| 0 | 88 (85.4) | 36 , 39 , 41 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 53 , 54 , 56 , 57 , 58 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 69 , 72 , 74 , 75 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 115 , 116 , 117 , 118 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 |
| 1 | 7 (6.8) | 40 , 52 , 55 , 68 , 70 , 73 , 76 |
| 2 | 1 (1.0) | 59 |
| 3 | 2 (1.9) | 71 , 114 |
| 4+ | 5 (4.9) | 37 , 38 , 42 , 119 , 127 |
| Number of PROGRESS‐Plus measures differential trial attrition considered by | ||
| 0 | 71 (68.9) | 37 , 39 , 40 , 43 , 46 , 48 , 49 , 55 , 56 , 58 , 60 , 64 , 73 , 74 , 75 , 76 , 78 , 80 , 81 , 82 , 83 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 123 , 124 , 125 , 126 , 129 , 130 , 131 , 132 , 133 , 134 |
| 1 | 6 (5.8) | 51 , 52 , 59 , 63 , 67 , 69 |
| 2 | 7 (6.8) | 44 , 50 , 62 , 65 , 66 , 70 , 117 |
| 3 | 7 (6.8) | 36 , 41 , 54 , 57 , 71 , 72 , 114 |
| 4+ | 12 (11.7) | 38 , 42 , 45 , 47 , 53 , 61 , 68 , 77 , 79 , 116 , 127 , 135 |
| Number of PROGRESS‐Plus measures differential weight outcome considered by | ||
| 0 | 72 (69.9) | 36 , 37 , 41 , 45 , 47 , 48 , 50 , 51 , 54 , 55 , 57 , 58 , 60 , 62 , 63 , 64 , 65 , 66 , 67 , 70 , 71 , 72 , 76 , 77 , 80 , 81 , 82 , 83 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 129 , 130 , 131 , 132 , 133 , 134 , 136 , 137 , 138 |
| 1 | 10 (9.7) | 40 , 46 , 52 , 53 , 56 , 59 , 61 , 69 , 116 , 118 |
| 2 | 8 (7.8) | 44 , 73 , 78 , 79 , 93 , 115 , 117 , 135 |
| 3 | 7 (6.8) | 38 , 39 , 43 , 74 , 114 , 126 , 128 , 143 |
| 4+ | 6 (5.8) | 42 , 49 , 68 , 75 , 84 , 127 |
Abbreviations: PROGRESS‐Plus, place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status, social capital, plus other discriminating factors; USPSTF, United States Preventive Services Taskforce.
Mean age not available for Perri et al. 130
Thirty‐six of the included studies explicitly stated that the intervention targeted a specific group that covered at least one of the PROGRESS‐Plus criteria. 37 , 39 , 42 , 43 , 45 , 47 , 49 , 50 , 57 , 59 , 62 , 63 , 65 , 66 , 68 , 69 , 72 , 75 , 76 , 78 , 80 , 81 , 82 , 85 , 94 , 102 , 103 , 104 , 105 , 109 , 110 , 113 , 117 , 119 , 124 , 129 Specifically, 10 studies targeted a specific race or ethnic group (such as Black African‐American or Hispanic), 43 , 47 , 57 , 63 , 65 , 69 , 76 , 102 , 113 , 124 three targeted specific occupations, 103 , 109 , 119 22 studies targeted based on gender or sex (six interventions were targeted at men 49 , 59 , 66 , 103 , 109 , 128 and 16 at women 45 , 47 , 50 , 57 , 68 , 72 , 80 , 82 , 94 , 101 , 102 , 104 , 105 , 110 , 117 , 129 ), eight targeted low‐income groups, 37 , 42 , 57 , 65 , 68 , 69 , 81 , 102 and five targeted particular age groups. 39 , 75 , 78 , 85 , 129 Participants belonging to certain health groups were also targeted in eight of the studies identified, 39 , 45 , 50 , 62 , 68 , 80 , 105 , 110 such as postpartum women or those at elevated risk of breast cancer.
3.2. Quality assessment
As shown in Figure 1, we scored three studies as “poor” quality; these were excluded from our synthesis. 139 , 140 , 141 Of the 14 studies identified in our updated search, 11 were scored “fair” quality, and three were scored “good” quality. In total, 74 studies were of “fair” quality, and 29 of “good” quality (Table S1).
3.3. Findings
At baseline, all 103 trials reported age and almost all (n = 101) reported participant gender or sex (Figure 2). The next most commonly reported baseline measures were race/ethnicity (n = 67), education (n = 57), SES (n = 40), social capital (predominantly marital status, n = 33), and occupation (n = 31). Nine trials reported measures (other than age) that meet the definition of “plus” according to the PROGRESS‐Plus characteristics—the most common measures meeting this criterion was health literacy and language at home. The least commonly reported measures at baseline were place of residence (n = 1) and religion (n = 1). Sexual orientation was not reported in any of the included studies. Fifty‐six of the 103 trials considered inequalities in intervention or trial uptake (n = 15), intervention adherence (n = 15), trial attrition (n = 32), or weight outcome (n = 34) in relation to at least one PROGRESS‐Plus criteria.
FIGURE 2.
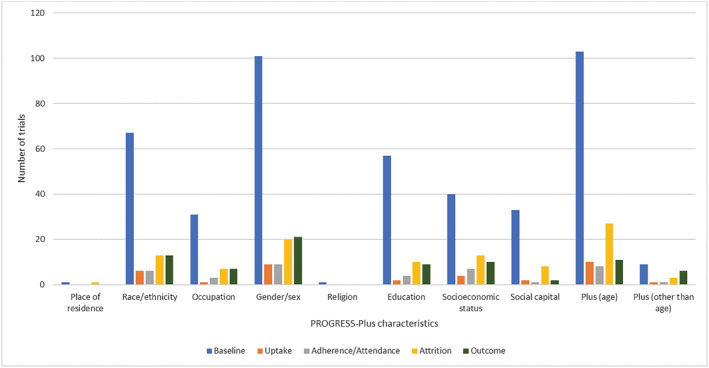
Number of trials reporting PROGRESS‐Plus (place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status, social capital, plus other discriminating factors) criteria at each trial stage
3.3.1. Inequalities and uptake
Trial uptake
Twenty‐nine analyses (WLs = 28, WLMs = 1) across 15 trials (Figure 3) examined inequalities in trial uptake. 38 , 40 , 42 , 45 , 48 , 58 , 66 , 68 , 70 , 71 , 74 , 79 , 114 , 119 , 127
FIGURE 3.
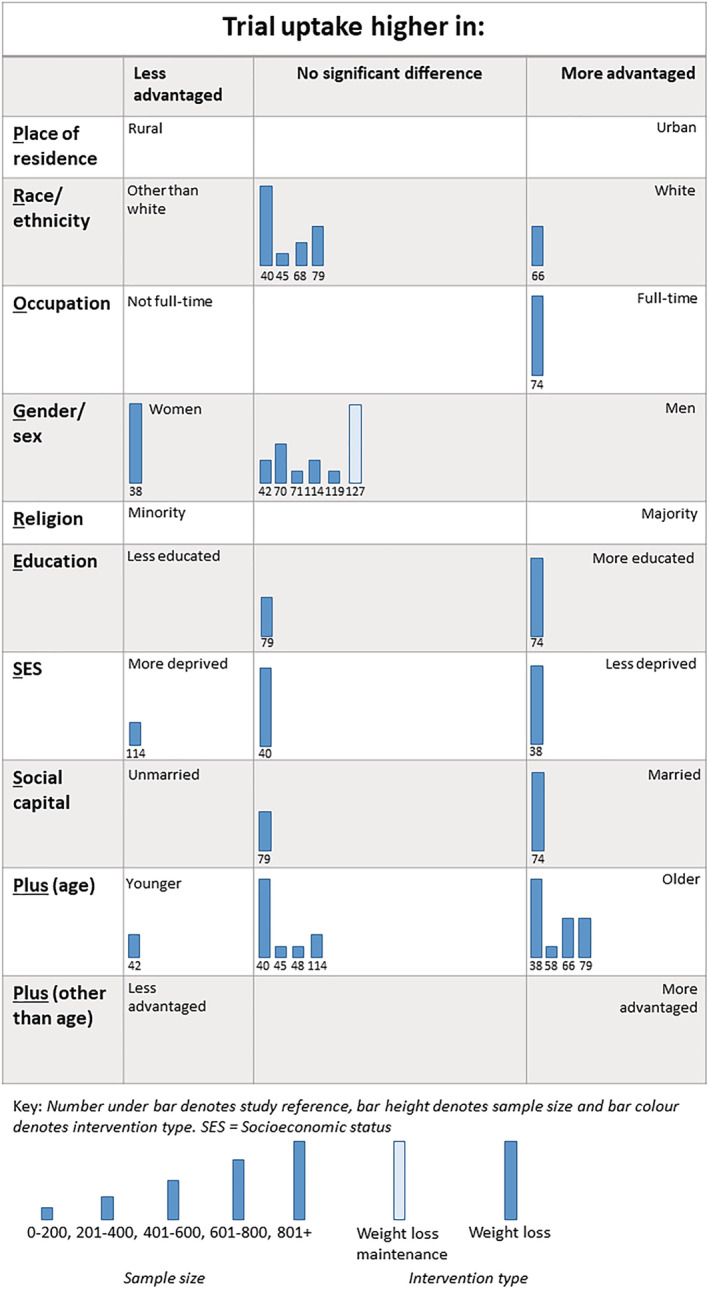
Harvest plot of inequalities in trial uptake
In the 28 analyses across 14 trials of WLs, 16 analyses found no evidence that trial uptake favored more or less advantaged. Three analyses found that trial uptake was highest in “less advantaged” groups and nine analyses found trial uptake was highest in “more advantaged” groups.
One study (one analysis) considered if differential trial uptake occurred in WLMs trials. This analysis found no evidence that trial uptake favored more or less advantaged.
Intervention uptake
Seven analyses across four trials (all WLs) considered whether there were inequalities in intervention uptake (Figure 4). 37 , 40 , 66 , 73 One study considered inequalities by race or ethnicity, two studies considered inequalities by gender, one by SES, one by social capital (marital status), one by age, and one by protocol language (English versus Spanish). One analysis found that intervention uptake favored “less advantaged,” 40 three analyses found no gradient, 37 two found intervention uptake favored “more advantaged,” 66 while one analysis was unclear in whether it favored a particular group or not. 73
FIGURE 4.
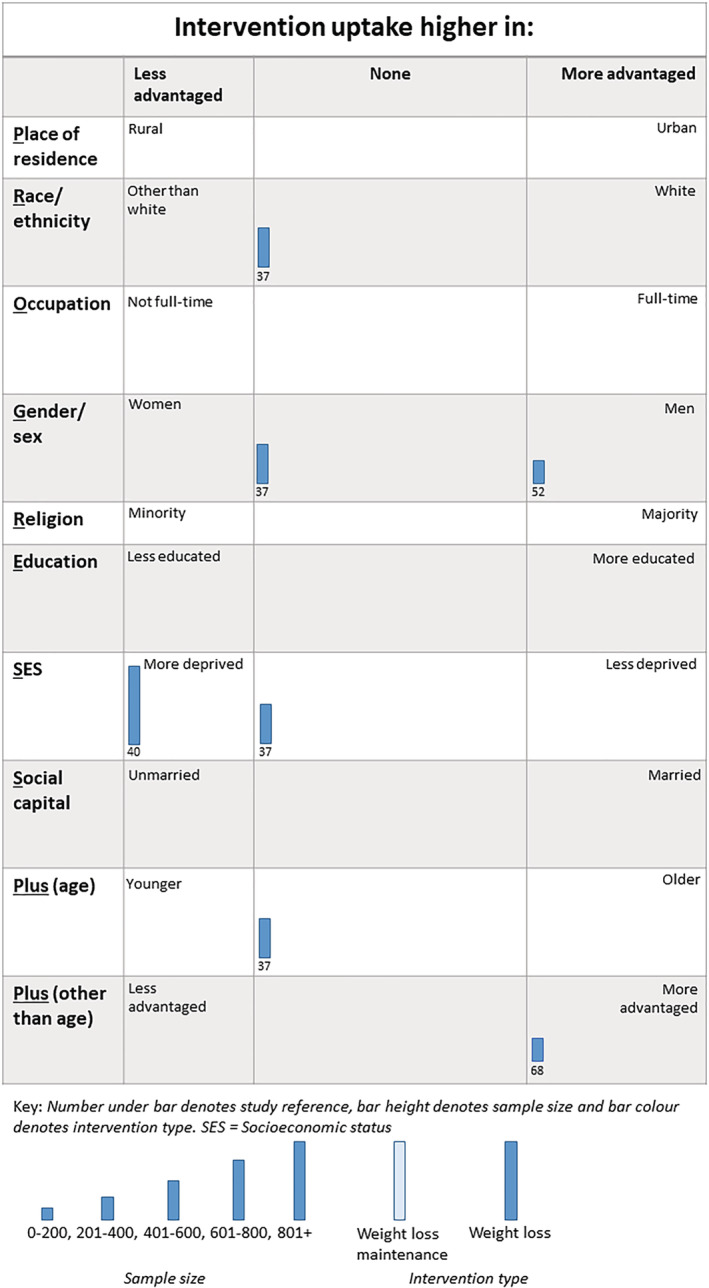
Harvest plot of inequalities in intervention uptake
3.3.2. Inequalities and intervention adherence
Thirty‐nine analyses (WLs = 34, WLMs = 5) from 15 trials (Figure 5) examined inequalities in intervention adherence.
FIGURE 5.
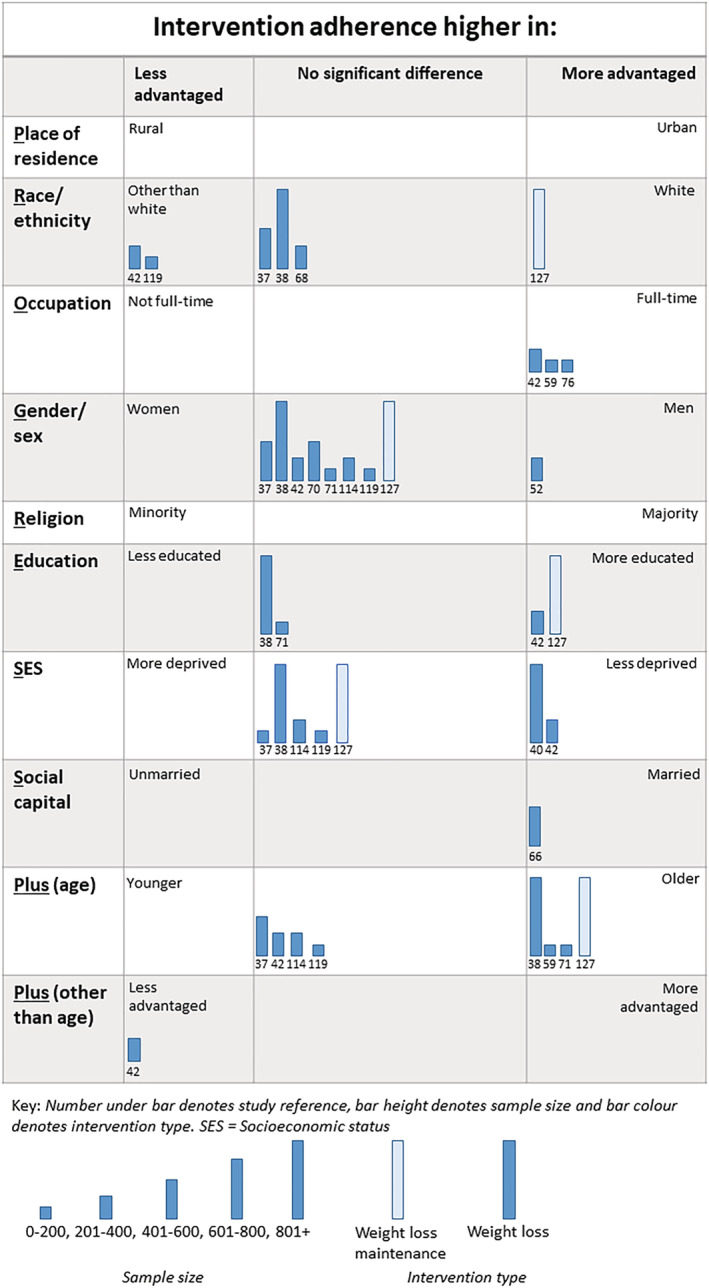
Harvest plot of inequalities in adherence to behavioral weight management interventions
We found 34 analyses across 14 trials of WLs that examined inequalities in intervention adherence. 37 , 38 , 40 , 42 , 52 , 55 , 59 , 68 , 70 , 71 , 73 , 76 , 114 , 119 Twenty of the 34 analyses found no gradient. Eleven analyses found that intervention adherence favored more advantaged groups (i.e., that intervention adherence was highest in these groups) and three found that intervention adherence was highest in the less advantaged groups. Intervention adherence was higher in those who had a full‐time occupation versus not full‐time (3/3 analyses) and also appeared to be higher in older participants (3/7 analyses).
Five analyses, from one trial, explored inequalities in adherence to WLMs. 127 Three out of the five analyses favored the more advantaged groups (1/1 analysis of ethnicity, 1/1 analysis of education, and 1/1 analysis of age). The remaining two analyses found that intervention adherence did not favor either less or more advantaged groups.
3.3.3. Inequalities and trial attrition
In total, 93 (WLs = 78, WLMs = 15) analyses across 32 trials considered inequalities in trial attrition (Figure 6).
FIGURE 6.
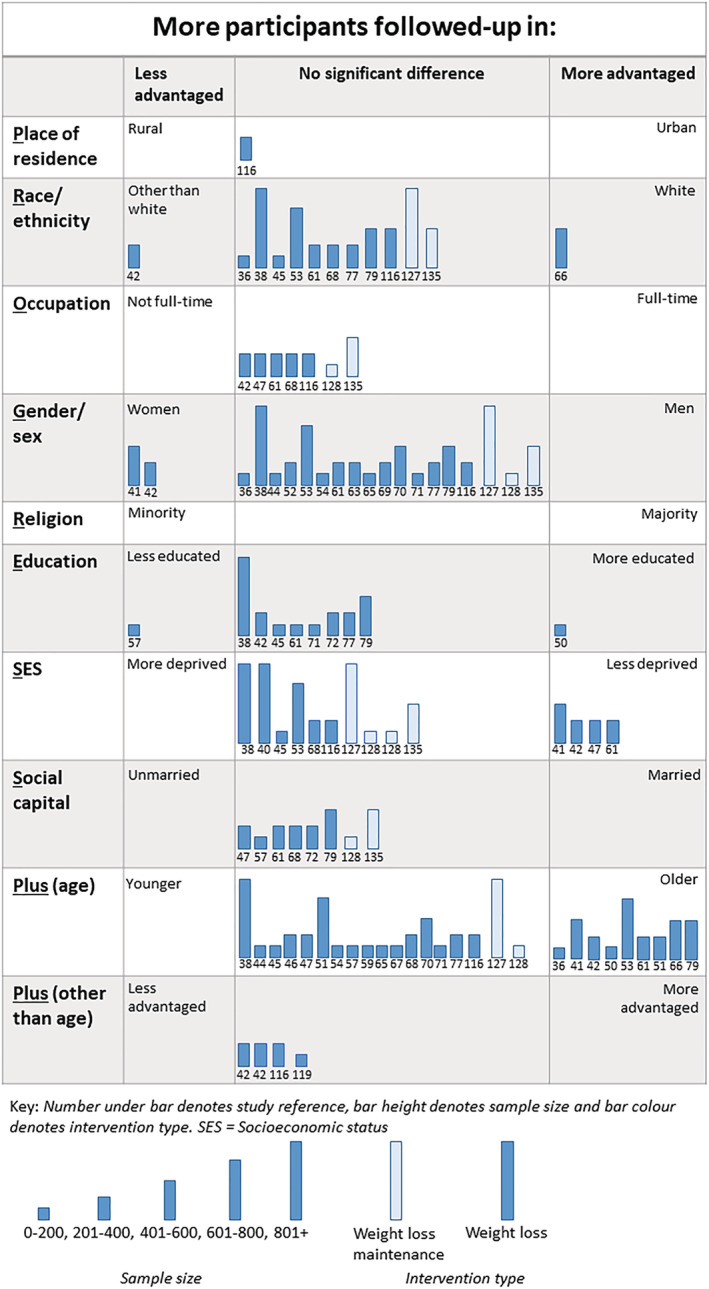
Harvest plot of inequalities in trial attrition
Seventy‐eight analyses from 29 trials assessed inequalities in trial attrition in WLs. 36 , 38 , 41 , 42 , 44 , 45 , 47 , 50 , 51 , 52 , 53 , 54 , 57 , 59 , 61 , 62 , 63 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 77 , 79 , 114 , 116 The majority of these analyses (n = 59) found that trial attrition favored no particular group; four analyses found that trial attrition was lower in less advantaged groups, and in 15 analyses, trial attrition was lower in more advantaged groups. Most of the analyses favoring “more advantaged” were of age, followed by SES (i.e., those who were older or of a less deprived SES were less likely to be lost to follow‐up). There was little evidence to suggest inequalities in trial attrition by other PROGRESS‐Plus criteria in trial attrition.
All analyses (15 across three trials) considering if there were inequalities in trial attrition in WLMs found that trial attrition did not favor any particular group. 127 , 128 , 135
3.3.4. Inequalities and weight outcome
We identified 79 analyses (WLs = 64, WLMs = 15) across 34 trials that considered inequalities in weight outcome (Figure 7). The results of four of these analyses (three for gender or sex 60 , 64 , 70 and one for occupation 126 ) were unclear and are consequently omitted from the Harvest plot.
FIGURE 7.
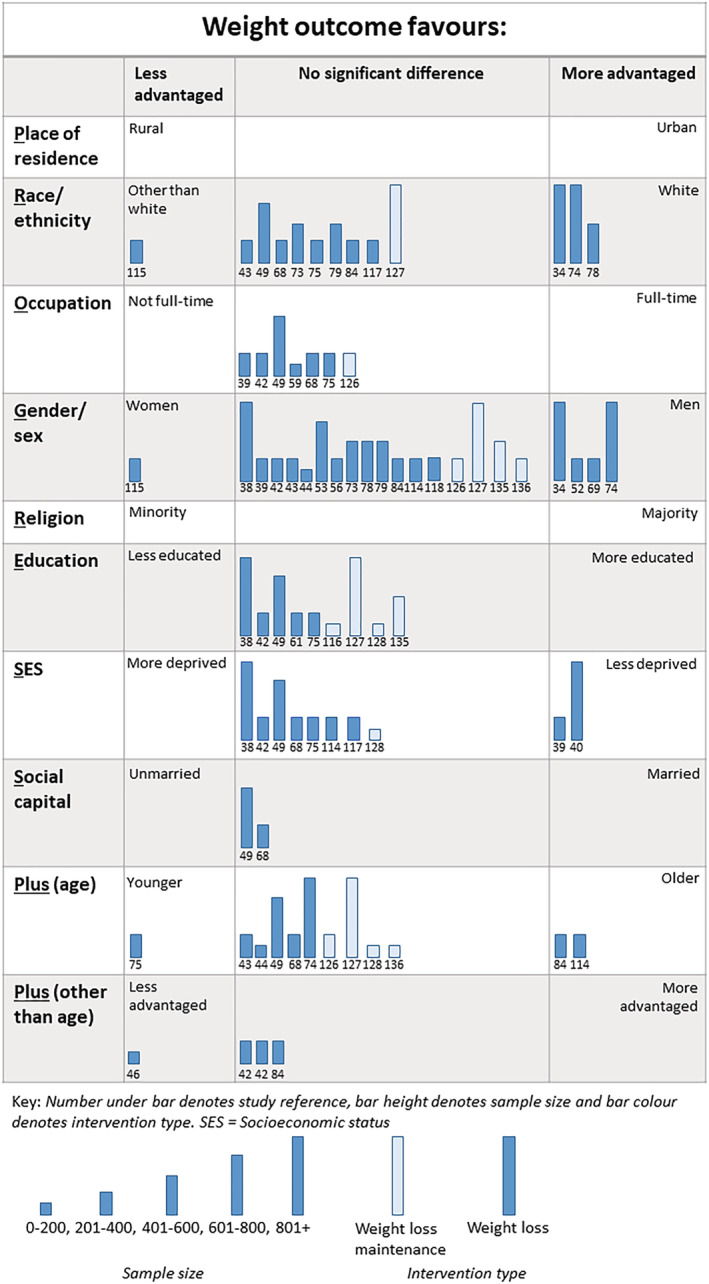
Harvest plot of inequalities in weight outcome
Sixty‐five analyses in 30 trials of WLs considered inequalities in weight loss. 36 , 37 , 38 , 39 , 40 , 42 , 43 , 44 , 46 , 49 , 52 , 53 , 56 , 59 , 60 , 64 , 68 , 69 , 70 , 73 , 74 , 75 , 78 , 79 , 84 , 93 , 114 , 115 , 117 , 118 Four analyses found that less advantaged groups lost more weight, 11 that more advantaged groups lost more weight, and the majority (n = 50) found that weight loss favored neither less nor more advantaged groups. For gender or sex, men lost more weight than women in three out of 17 analyses conducted, whereas women lost more weight in one of the 17 analyses. For SES, two of nine analyses favored those in less advantaged groups.
We identified 15 analyses of inequalities in weight loss maintenance across five WLMs. 126 , 127 , 128 , 135 , 136 None of the analyses found the inequalities in the weight outcome of WLMs (i.e., there was no significant difference observed in weight loss maintenance by any measure of inequality).
4. DISCUSSION
This comprehensive systematic review found that most trials of behavioral weight management interventions do not examine whether differential trial/intervention uptake, intervention adherence, trial attrition, or outcome occurs in different social groups. In those that did examine differences, most found no gradient (e.g., intervention uptake or trial attrition was not higher in either more or less advantaged groups). Where a gradient was observed, it mostly favored those who were “more advantaged.” This was not the case for weight outcomes, for which a similar number of trials favored “less advantaged” groups as those favoring “more advantaged.” Our findings suggest that inequalities may occur in intervention/trial uptake, intervention adherence, and trial attrition, although behavioral weight management interventions may be equitable for those who reach the 12‐month follow‐up.
In this review, we examined two types of behavioral weight management interventions: interventions targeting weight loss (WLs) and interventions targeting weight loss maintenance (WLMs). There were differences in inequalities observed between the two intervention types; evidence of inequalities in WLMs was only present in intervention adherence and not in trial or intervention uptake, whereas there was some evidence of inequalities at all stages in WLs. There may be several underpinning reasons for this. First, we identified fewer trials of WLMs than of WLs (13 vs. 90), meaning that there were fewer data available for WLMs. Second, it is possible that inequalities in behavioral weight management interventions are being generated in the interventions targeting weight loss, and those who are less successful in a weight loss intervention may be less likely to be invited to take part in a further weight loss maintenance trial.
We found some evidence to suggest, when taking into account the age of people invited to take part in a weight management trial, a significantly higher proportion of older people took up the offer in four of the 10 studies we identified that examined this. This is supported by survey data showing that older people report better access to primary care142 and by evidence from a UK‐based population‐based cohort study which observed that weight management interventions were more often accessed by older participants. 33 The study authors also noted that weight management interventions were more often accessed by women and those in deprivation. 33 We found that, overall, two thirds of total participants across the trials included in our review were female. This is similar to the findings of previous systematic review which focused on the issue of male inclusion in RCTs in WLs, which found that only 27% of participants were male. 16 When accounting for the total number of men and women invited to take part in the trial, we did not find evidence to suggest there were inequalities in those who were likely to accept invitation to the trial or to the intervention arm. It has previously been observed that the proportion of male participants in studies of commercial weight management programs is higher when all eligible in the population are invited than when patients are invited opportunistically, suggesting the inequality in male participation can be reduced by inviting more men to take part.143
When compared with the wider RCT literature, trials of behavioral weight management interventions are atypical in that recruitment favors women and older participants. Outside of trials of behavioral weight management interventions, for example, in RCTs used for cardiovascular guidelines or drug and vaccine research, recruitment tends to favor men and younger participants. 144 , 145 , 146 Therefore, this may suggest that a nuanced perspective of inequalities should be taken when addressing behavioral weight management interventions, as some groups that are typically underresearched—women and older people—are the most researched. Hence, there is less research in groups that would typically be considered as advantaged in other health and wider societal domains.
Although there is some evidence in our systematic review to suggest that trial uptake is higher in less socioeconomically advantaged groups, we found that intervention adherence, trial attrition, and weight outcome favored those who were less deprived. This supports findings from trials of other behavioral interventions (such as those targeting smoking cessation) where attrition is higher or intervention/adherence is lower in those who are more deprived. 147 , 148 , 149 , 150 Future studies should consider how participants from more deprived backgrounds can remain engaged in both the intervention and the trial itself, to ensure that the benefit of the intervention can be received more equally across different socioeconomic groups. For example, incentives may have an important role in improving participation in trials of health interventions, particularly in groups that are typically underrepresented. However, due to a lack of relevant data reported in the included studies, we have been unable to examine this.
Our findings of inequalities in behavioral weight management interventions by race or ethnicity are broadly similar to previously conducted systematic reviews we identified. We found that few studies reported if there was differential adherence or outcome by race or ethnicity, supporting the findings of Tussing‐Humphreys et al. and Haughton et al. 13 , 15 We did not have sufficient evidence to support Tussing‐Humphreys et al. and Kong et al. findings that WL and WLM led to less weight loss and more weight regain in African American women though. 10 , 15 This may have been due to the different inclusion criteria used across the reviews, leading to variation in the studies included (17 studies in the Tussing‐Humphreys review, 103 in this review).
There are several factors by which discrimination or differential health outcomes may occur that were either not captured at all or were only partially captured, in this review. Some factors, such as sexual orientation, were not measured in any of the 103 trials included in our review. This is despite there being known inequalities in weight by sexual orientation. For example, women who identify as lesbian are more likely to have overweight or obesity than women who identify as heterosexual. 151 , 152 The National Health Service in England has highlighted the need for further research to gain a better contextual understanding of weight issues in this group. 153 Other factors that discrimination or differential health outcomes can occur by, such as gender and social capital, were only captured in a limited way. For example, gender was predominantly recorded in trials as either male or female, which does not reflect the full spectrum of gender. Similarly, despite its broad definition, social capital was only captured in trials as marital status, meaning that the full nature of people's personal support networks was not captured. Additionally, depending on the categorization of marital status, this measure may not reflect contemporary attitudes towards relationships and marriage. Similarly, our consideration of several PROGRESS‐Plus characteristics, such as place of residence (urban vs. rural), was binary, which loses detail in the complexity of people's circumstances and living arrangements. Future trials should consider broader categorizations of factors, such as of gender, to ensure demographic information fully represents how participants wish to identify.
Due to heterogeneity in intervention types and measures of the PROGRESS‐Plus criteria, such as country‐specific measures of SES or ethnicity, we deemed it was not appropriate to conduct a meta‐analysis. We suggest that future research should identify if a common range of measures covering the PROGRESS‐Plus criteria could be reported across trials of behavioral interventions, as well as identifying key stages of a trial (such as trial uptake, intervention adherence and outcome) for which differences in these measures should be reported. While reporting common measures across key stages of a trial will not overcome the issue that most trials may not have sufficient statistical power to identify if inequalities are present, more consistent reporting would facilitate future meta‐analyses that could address inequalities‐focused research questions.
4.1. Strengths and limitations
To our knowledge, this systematic review is the most up‐to‐date and comprehensive review investigating the association between indicators of inequality and behavioral weight management interventions published to date. In particular, it is the first to investigate the impact of inequalities at several stages of an intervention (such as trial or intervention uptake and follow‐up). Utilizing the PROGRESS‐Plus criteria ensured a comprehensive examination of inequality beyond the individual measures (such as SES 20 or gender 16 ) that previous reviews have focused on. In using “publication families,” 24 we endeavored to capture all papers published from each included trial. Furthermore, we also contacted authors of trials included in this review to request any missing relevant data, aiding the completeness of our data collection.
Despite the usefulness of harvest plots in graphically synthesizing information across studies that cannot be meta‐analyzed, they are unable to overcome the limitation of low statistical power of the individual studies in detecting differential effects of interventions. This is pertinent when considering the impact inequalities have on trial/intervention uptake, adherence, attrition, and effectiveness, as studies are generally only sufficiently powered to detect a significant difference in weight outcomes between the intervention and control groups. This is likely attributable, in part, to analyses of inequalities rarely being part of the main analysis plan and such analyses often being performed post hoc. A large number of our included studies had a relatively small sample size (e.g., 41 studies had 0–200 participants). This may explain why a large number of studies included in our harvest plots found no inequality gradient for any of the outcomes we studied. It may be that an inequality gradient is present in some of these studies, but there was insufficient statistical power to detect it. Future research should consider alternative methods of data synthesis, such as individual participant data meta‐analysis, when addressing inequality‐focused research questions of intervention studies. In individual participant data meta‐analyses, different measures of an inequality criterion (such as SES) can be harmonized and pooled, providing greater statistical power to detect significant differences in uptake, adherence, attrition, and intervention effectiveness.
A further limitation of our review is that, although we took a comprehensive approach to considering various indicators of inequality and their interaction with behavioral weight management interventions, we did not consider weight status (e.g., higher BMI category vs. lower BMI category) as a factor where differential uptake, adherence, attrition, or effectiveness may occur. Higher weight status is associated with increased weight stigma, which is linked to worsened mental and physical health, and healthcare avoidance. 154 , 155 , 156 Therefore, inequalities in behavioral weight management interventions may also exist in this group. Furthermore, we only set out to include studies from high‐income (OECD) countries, meaning that the results cannot be extrapolated to low‐ and middle‐income countries. Similarly, by using a minimum BMI cut‐off of 25 kg/m2, we may have excluded a number of studies conducted across Asia‐Pacific countries that typically use lower BMI cut‐offs for overweight (23–24.9 kg/m2) and obesity (≥25 kg/m2). 157 Finally, we were unable to conduct any meaningful analysis of intersectionality—for which two or more characteristics where inequalities may occur interact and produce inequality that is distinct and specific from the inequalities arising from individual characteristics. 158 Given RCTs are typically only sufficiently powered to detect an interaction between outcome and intervention arm, they are not designed in a way that facilitates consideration of intersectionality. It is, however, an important issue for future research to address intersectionality in terms of differential intervention outcomes, as well as building on recent research that explored intersectional differences in prevalence of obesity. 159 , 160 , 161
5. CONCLUSION
We found that most trials of behavioral weight management interventions did not consider whether inequalities in trial or intervention uptake, adherence, trial attrition, or weight outcomes occurred by a measure of the PROGRESS‐Plus criteria. This is likely to have been because analyses of inequalities in trials are often post hoc and commonly are not included in the main analysis plan of a trial, as RCTs are generally only sufficiently powered to detect an interaction between trial arm and the primary outcome. In studies that did conduct such analyses, most found that no inequalities gradient was present. In the studies that did find a gradient, they mostly found that the intervention favored those who were “more advantaged” for uptake, adherence, and trial attrition. However, this was not the case for weight outcomes at 12‐month follow‐up, where there was a more equal balance between trials favoring more and less advantaged groups. These findings may suggest that behavioral weight management interventions are equitable for those who reach the 12‐month follow‐up. Future research should include standard measures of the PROGRESS‐Plus criteria and consider alternative methods of data synthesis, such as meta‐analysis of individual participant data, when addressing inequality‐focused questions in trials of interventions. This would help to overcome limitations such as insufficient statistical power, in order to detect potential differences by measures of inequalities.
CONFLICT OF INTEREST
ALA is principal investigator on two publicly funded (NIHR, MRC) trials where the intervention is provided by WW (formerly Weight Watchers) at no cost. SJG is principal investigator on a publicly funded (NIHR) trial in which the intervention is provided by WW (formerly Weight Watchers) at no cost. MPK has undertaken consultancy for Slimming World, and led the obesity and weight management guidelines development for NICE from 2005 until 2014.
Supporting information
Table S1: Detailed Study Characteristics
ACKNOWLEDGMENTS
JMB, RAJ, SJG, and ALA are supported by the Medical Research Council (MRC) (Grant MC_UU_00006/6). MDMcD is supported by an Australian Government Research Training Program Scholarship.
Birch JM, Jones RA, Mueller J, et al. A systematic review of inequalities in the uptake of, adherence to, and effectiveness of behavioral weight management interventions in adults. Obesity Reviews. 2022;23(6):e13438. doi: 10.1111/obr.13438
Funding information Medical Research Council, Grant/Award Number: MC_UU_00006/6; Australian Government Research Training Program Scholarship
REFERENCES
- 1. Lauby‐Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794‐798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Afshin A, Alexander LT, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1659‐1724. doi: 10.1016/s0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O'Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity‐related morbidity and mortality in adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;320(11):1172‐1191. doi: 10.1001/jama.2018.7777 [DOI] [PubMed] [Google Scholar]
- 4. Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta‐analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams J, Mytton O, White M, Monsivais P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. 2016;13(4):e1001990. doi: 10.1371/journal.pmed.1001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Backholer K, Beauchamp A, Ball K, et al. A framework for evaluating the impact of obesity prevention strategies on socioeconomic inequalities in weight. Am J Public Health. 2014;104(10):e43‐e50. doi: 10.2105/AJPH.2014.302066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCartney G, Popham F, McMaster R, Cumbers A. Defining health and health inequalities. Public Health. 2019;172:22‐30. doi: 10.1016/j.puhe.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Neill J, Tabish H, Welch V, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56‐64. doi: 10.1016/j.jclinepi.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 9. Perez LG, Arredondo EM, Elder JP, Barquera S, Nagle B, Holub CK. Evidence‐based obesity treatment interventions for Latino adults in the U.S.: a systematic review. Am J Prev Med. 2013;44(5):550‐560. doi: 10.1016/j.amepre.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kong A, Tussing‐Humphreys LM, Odoms‐Young AM, Stolley MR, Fitzgibbon ML. Systematic review of behavioural interventions with culturally adapted strategies to improve diet and weight outcomes in African American women. Obes Rev. 2014;15(Suppl 4(0 4)):62‐92. doi: 10.1111/obr.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitzgibbon ML, Tussing‐Humphreys LM, Porter JS, Martin IK, Odoms‐Young A, Sharp LK. Weight loss and African‐American women: a systematic review of the behavioural weight loss intervention literature. Obes Rev. 2012;13(3):193‐213. doi: 10.1111/j.1467-789X.2011.00945.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenbaum DL, Piers AD, Schumacher LM, Kase CA, Butryn ML. Racial and ethnic minority enrollment in randomized clinical trials of behavioural weight loss utilizing technology: a systematic review. Obes Rev. 2017;18(7):808‐817. doi: 10.1111/obr.12545 [DOI] [PubMed] [Google Scholar]
- 13. Haughton CF, Silfee VJ, Wang ML, et al. Racial/ethnic representation in lifestyle weight loss intervention studies in the United States: a systematic review. Prev Med Rep. 2018;9:131‐137. doi: 10.1016/j.pmedr.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morrill KE, Lopez‐Pentecost M, Molina L, et al. Weight Loss Interventions for Hispanic Women in the United States: A Systematic Review. J Environ Public Health. 2021;2021:8714873. doi: 10.1155/2021/8714873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tussing‐Humphreys LM, Fitzgibbon ML, Kong A, Odoms‐Young A. Weight loss maintenance in African American women: a systematic review of the behavioral lifestyle intervention literature. J Obesity. 2013;2013:437369‐437369. doi: 10.1155/2013/437369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity. 2012;20(6):1234‐1239. doi: 10.1038/oby.2011.140 [DOI] [PubMed] [Google Scholar]
- 17. Young MD, Morgan PJ, Plotnikoff RC, Callister R, Collins CE. Effectiveness of male‐only weight loss and weight loss maintenance interventions: a systematic review with meta‐analysis. Obes Rev. 2012;13(5):393‐408. doi: 10.1111/j.1467-789X.2011.00967.x [DOI] [PubMed] [Google Scholar]
- 18. Williams RL, Wood LG, Collins CE, Callister R. Effectiveness of weight loss interventions—Is there a difference between men and women: a systematic review. Obes Rev. 2015;16(2):171‐186. doi: 10.1111/obr.12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olstad DL, Teychenne M, Minaker LM, et al. Can policy ameliorate socioeconomic inequities in obesity and obesity‐related behaviours? A systematic review of the impact of universal policies on adults and children. Obes Rev. 2016;17(12):1198‐1217. doi: 10.1111/obr.12457 [DOI] [PubMed] [Google Scholar]
- 20. Hillier‐Brown FC, Bambra CL, Cairns JM, Kasim A, Moore HJ, Summerbell CD. A systematic review of the effectiveness of individual, community and societal‐level interventions at reducing socio‐economic inequalities in obesity among adults. Int J Obesity (2005). 2014;38(12):1483‐1490. doi: 10.1038/ijo.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Welch V, Petticrew M, Tugwell P, et al. PRISMA‐Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012;9(10):e1001333. doi: 10.1371/journal.pmed.1001333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Birch JM, Griffin SJ, Kelly MP, Ahern AL. Inequalities in the uptake of, adherence to and effectiveness of behavioural weight management interventions: systematic review protocol. BMJ Open. 2020;10(11):e039518. doi: 10.1136/bmjopen-2020-039518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orkin AM, McArthur A, Venugopal J, et al. Defining and measuring health equity in research on task shifting in high‐income countries: a systematic review. SSM Popul Health. 2019;7:100366‐100366. doi: 10.1016/j.ssmph.2019.100366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schulz KF, Altman DG, Moher D. CONSORT statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010/03/24 2010. 2010;8(1):18. doi: 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ: British Med J. 2014;348(mar07 3):g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 27. Cochrane Public Health Group . Cochrane public health group data extraction and assessment template. 2011.
- 28. Attwood S, van Sluijs E, Sutton S. Exploring equity in primary‐care‐based physical activity interventions using PROGRESS‐Plus: a systematic review and evidence synthesis. Int J Behav Nutr Phys Act. 2016;13(1):60. doi: 10.1186/s12966-016-0384-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crowther M, Avenell A, MacLennan G, Mowatt G. A further use for the Harvest plot: a novel method for the presentation of data synthesis. Res Synth Methods. 2011;2(2):79‐83. doi: 10.1002/jrsm.37 [DOI] [PubMed] [Google Scholar]
- 30. Nanninga S, Lehne G, Ratz T, Bolte G. Impact of public smoking bans on social inequalities in children's exposure to tobacco smoke at home: an equity‐focused systematic review. Nicotine Tob Res. 2018;21(11):1462‐1472. doi: 10.1093/ntr/nty139 [DOI] [PubMed] [Google Scholar]
- 31. Corscadden L, Levesque JF, Lewis V, Strumpf E, Breton M, Russell G. Factors associated with multiple barriers to access to primary care: an international analysis. Int J Equity Health. 2018;17(1):28. doi: 10.1186/s12939-018-0740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weaver RG, Manns BJ, Tonelli M, et al. Access to primary care and other health care use among western Canadians with chronic conditions: a population‐based survey. CMAJ Open. 2014;2(1):E27‐E34. doi: 10.9778/cmajo.20130045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Booth HP, Prevost AT, Gulliford MC. Access to weight reduction interventions for overweight and obese patients in UK primary care: population‐based cohort study. BMJ Open. 2015;5(1):e006642. doi: 10.1136/bmjopen-2014-006642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. U.S. Preventive Services Task Force . Procedure manual. 2020. Accessed 09/08/2021. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/inline-files/procedure-manual-2020_3.pdf
- 35. Ogilvie D, Fayter D, Petticrew M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol. 2008;8(1):8. doi: 10.1186/1471-2288-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the diabetes prevention program into the community. The DEPLOY pilot study. Am J Prev Med Oct. 2008;35(4):357‐363. doi: 10.1016/j.amepre.2008.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ackermann RT, Liss DT, Finch EA, et al. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015;105(11):2328‐2334. doi: 10.2105/ajph.2015.302641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahern AL, Wheeler GM, Aveyard P, et al. Extended and standard duration weight‐loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. Lancet (London, England). 2017;389(10085):2214–2225. 10.1016/S0140-6736(17)30647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderson AS, Craigie AM, Caswell S, et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ. 2014;348:g1823. doi: 10.1136/bmj.g1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aveyard P, Lewis A, Tearne S, et al. Screening and brief intervention for obesity in primary care: a parallel, two‐arm, randomised trial. The Lancet. 2016;388(10059):2492‐2500. doi: 10.1016/S0140-6736(16)31893-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beeken RJ, Leurent B, Vickerstaff V, et al. A brief intervention for weight control based on habit‐formation theory delivered through primary care: results from a randomised controlled trial. Int J Obesity (2005). 2017;41(2):246‐254. doi: 10.1038/ijo.2016.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172(7):565‐574. doi: 10.1001/archinternmed.2012.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christian JG, Byers TE, Christian KK, et al. A computer support program that helps clinicians provide patients with metabolic syndrome tailored counseling to promote weight loss. J am Diet Assoc. 2011;111(1):75‐83. doi: 10.1016/j.jada.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 44. Cohen MD, D'Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Fam Med. 1991;23(1):25‐28. [PubMed] [Google Scholar]
- 45. Demark‐Wahnefried W, Jones LW, Snyder DC, et al. Daughters and mothers against breast cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120(16):2522‐2534. doi: 10.1002/cncr.28761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischer HH, Fischer IP, Pereira RI, et al. Text message support for weight loss in patients with prediabetes: a randomized clinical trial. Diabetes Care. 2016;39(8):1364‐1370. doi: 10.2337/dc15-2137 [DOI] [PubMed] [Google Scholar]
- 47. Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity reduction black intervention trial (ORBIT): 18‐month results. Obesity (Silver Spring). 2010;18(12):2317‐2325. doi: 10.1038/oby.2010.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Greaves C, Gillison F, Stathi A, et al. Waste the waist: a pilot randomised controlled trial of a primary care based intervention to support lifestyle change in people with high cardiovascular risk. Int J Behav Nutr Phys Act. 2015;12(1):1. doi: 10.1186/s12966-014-0159-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunt K, Wyke S, Gray CM, et al. A gender‐sensitised weight loss and healthy living programme for overweight and obese men delivered by Scottish Premier League football clubs (FFIT): a pragmatic randomised controlled trial. The Lancet. 2014;383(9924):1211‐1221. doi: 10.1016/S0140-6736(13)62420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huseinovic E, Bertz F, Leu Agelii M, Hellebö Johansson E, Winkvist A, Brekke HK. Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr. 2016;104(2):362‐370. doi: 10.3945/ajcn.116.135673 [DOI] [PubMed] [Google Scholar]
- 51. Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378(9801):1485‐1492. doi: 10.1016/s0140-6736(11)61344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jeffery RW, Wing RR, Thorson C, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61(6):1038‐1045. doi: 10.1037//0022-006x.61.6.1038 [DOI] [PubMed] [Google Scholar]
- 53. Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: lighten up randomised controlled trial. BMJ. 2011;343:d6500. doi: 10.1136/bmj.d6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kulzer B, Hermanns N, Gorges D, Schwarz P, Haak T. Prevention of diabetes self‐management program (PREDIAS): effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care. 2009;32(7):1143‐1146. doi: 10.2337/dc08-2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kumanyika SK, Fassbender JE, Sarwer DB, et al. One‐year results of the Think Health! study of weight management in primary care practices. Obesity (Silver Spring). 2012;20(6):1249‐1257. doi: 10.1038/oby.2011.329 [DOI] [PubMed] [Google Scholar]
- 56. Ma J, Yank V, Xiao L, et al. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113‐121. doi: 10.1001/2013.jamainternmed.987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low‐income minority women. Obesity (Silver Spring, Md). 2008;16(11):2462‐2467. doi: 10.1038/oby.2008.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mensink M, Blaak EE, Corpeleijn E, Saris WH, de Bruin TW, Feskens EJ. Lifestyle intervention according to general recommendations improves glucose tolerance. Obes Res. 2003;11(12):1588‐1596. doi: 10.1038/oby.2003.211 [DOI] [PubMed] [Google Scholar]
- 59. Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12‐month outcomes and process evaluation of the SHED‐IT RCT: an internet‐based weight loss program targeting men. Obesity (Silver Spring). 2011;19(1):142‐151. doi: 10.1038/oby.2010.119 [DOI] [PubMed] [Google Scholar]
- 60. Nakade M, Aiba N, Suda N, et al. Behavioral change during weight loss program and one‐year follow‐up: Saku Control Obesity Program (SCOP) in Japan. Asia Pac J Clin Nutr. 2012;21(1):22‐34. [PubMed] [Google Scholar]
- 61. Nanchahal K, Power T, Holdsworth E, et al. A pragmatic randomised controlled trial in primary care of the Camden Weight Loss (CAMWEL) programme. BMJ Open. 2012;2(3):e000793. doi: 10.1136/bmjopen-2011-000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nilsen V, Bakke PS, Gallefoss F. Effects of lifestyle intervention in persons at risk for type 2 diabetes mellitus ‐ results from a randomised, controlled trial. BMC Public Health. 2011;11(1):893. doi: 10.1186/1471-2458-11-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ockene IS, Tellez TL, Rosal MC, et al. Outcomes of a Latino community‐based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102(2):336‐342. doi: 10.2105/AJPH.2011.300357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pacanowski CR, Levitsky DA. Frequent self‐weighing and visual feedback for weight loss in overweight adults. J Obes. 2015;2015:763680. doi: 10.1155/2015/763680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health. 2010;100(Suppl 1):S232‐S239. doi: 10.2105/ajph.2009.170910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Patrick K, Calfas KJ, Norman GJ, et al. Outcomes of a 12‐month web‐based intervention for overweight and obese men. Ann Behav Med. 2011;42(3):391‐401. doi: 10.1007/s12160-011-9296-7 [DOI] [PubMed] [Google Scholar]
- 67. Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009;9(1):342. doi: 10.1186/1471-2458-9-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Phelan S, Hagobian T, Brannen A, et al. Effect of an internet‐based program on weight loss for low‐income postpartum women: a randomized clinical trial. JAMA. 2017;317(23):2381‐2391. doi: 10.1001/jama.2017.7119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosas LG, Thiyagarajan S, Goldstein BA, et al. The effectiveness of two community‐based weight loss strategies among obese, low‐income US Latinos. J Acad Nutr Diet Apr. 2015;115(4):537, e2‐550. doi: 10.1016/j.jand.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ross R, Lam M, Blair SN, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Intern Med. 2012;172(5):414‐424. doi: 10.1001/archinternmed.2011.1972 [DOI] [PubMed] [Google Scholar]
- 71. Shapiro JR, Koro T, Doran N, et al. Text4Diet: a randomized controlled study using text messaging for weight loss behaviors. Prev Med. 2012;55(5):412‐417. doi: 10.1016/j.ypmed.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 72. Silva MN, Vieira PN, Coutinho SR, et al. Using self‐determination theory to promote physical activity and weight control: a randomized controlled trial in women. J Behav Med. 2010;33(2):110‐122. doi: 10.1007/s10865-009-9239-y [DOI] [PubMed] [Google Scholar]
- 73. Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the trials of hypertension prevention. Arch Intern Med Apr. 1993;153(7):849‐858. doi: 10.1001/archinte.1993.00410070039006 [DOI] [PubMed] [Google Scholar]
- 74. Stevens VJ, Obarzanek E, Cook NR, et al. Long‐term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med. 2001;134(1):1‐11. doi: 10.7326/0003-4819-134-1-200101020-00007 [DOI] [PubMed] [Google Scholar]
- 75. Svetkey LP, Batch BC, Lin P‐H, et al. Cell phone intervention for you (CITY): a randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring, Md). 2015;23(11):2133‐2141. doi: 10.1002/oby.21226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Narayan KM, Hoskin M, Kozak D, et al. Randomized clinical trial of lifestyle interventions in Pima Indians: a pilot study. Diabet Med. 1998;15(1):66‐72. doi:10.1002/(sici)1096‐9136(199801)15:1<66::Aid‐dia515>3.0.Co;2‐a [DOI] [PubMed] [Google Scholar]
- 77. Thomas JG, Raynor HA, Bond DS, et al. Weight loss in Weight Watchers Online with and without an activity tracking device compared to control: a randomized trial. Obesity (Silver Spring). 2017;25(6):1014‐1021. doi: 10.1002/oby.21846 [DOI] [PubMed] [Google Scholar]
- 78. Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. Jama. 1998;279(11):839‐846. doi: 10.1001/jama.279.11.839 [DOI] [PubMed] [Google Scholar]
- 79. Wylie‐Rosett J, Swencionis C, Ginsberg M, et al. Computerized weight loss intervention optimizes staff time: the clinical and cost results of a controlled clinical trial conducted in a managed care setting. J Am Diet Assoc. 2001;101(10):1155‐1162. doi: 10.1016/s0002-8223(01)00284-x [DOI] [PubMed] [Google Scholar]
- 80. Cadmus‐Bertram L, Nelson SH, Hartman S, Patterson RE, Parker BA, Pierce JP. Randomized trial of a phone‐ and web‐based weight loss program for women at elevated breast cancer risk: the HELP study. J Behav Med Aug. 2016;39(4):551‐559. doi: 10.1007/s10865-016-9735-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chirinos DA, Goldberg RB, Llabre MM, et al. Lifestyle modification and weight reduction among low‐income patients with the metabolic syndrome: the CHARMS randomized controlled trial. J Behav Med. 2016;39(3):483‐492. doi: 10.1007/s10865-016-9721-2 [DOI] [PubMed] [Google Scholar]
- 82. de Vos BC, Runhaar J, Bierma‐Zeinstra SM. Effectiveness of a tailor‐made weight loss intervention in primary care. Eur J Nutr. 2014;53(1):95‐104. doi: 10.1007/s00394-013-0505-y [DOI] [PubMed] [Google Scholar]
- 83. Eaton CB, Hartman SJ, Perzanowski E, et al. A randomized clinical trial of a tailored lifestyle intervention for obese, sedentary, primary care patients. Ann Fam Med. 2016;14(4):311‐319. doi: 10.1370/afm.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Godino JG, Golaszewski NM, Norman GJ, et al. Text messaging and brief phone calls for weight loss in overweight and obese English‐ and Spanish‐speaking adults: a 1‐year, parallel‐group, randomized controlled trial. PLoS Med. 2019;16(9):e1002917. doi: 10.1371/journal.pmed.1002917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Godino JG, Merchant G, Norman GJ, et al. Using social and mobile tools for weight loss in overweight and obese young adults (Project SMART): a 2 year, parallel‐group, randomised, controlled trial. Lancet Diabetes Endo. 2016;4(9):747‐755. doi: 10.1016/S2213-8587(16)30105-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1‐year effectiveness study. Public Health Nutr. 2009;12(12):2382‐2391. doi: 10.1017/s1368980009005230 [DOI] [PubMed] [Google Scholar]
- 87. Jakicic JM, Otto AD, Lang W, et al. The effect of physical activity on 18‐month weight change in overweight adults. Obesity (Silver Spring, Md). 2011;19(1):100‐109. doi: 10.1038/oby.2010.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jansson SP, Engfeldt P, Magnuson A, Pt GL, Liljegren G. Interventions for lifestyle changes to promote weight reduction, a randomized controlled trial in primary health care. BMC Res Notes. 2013;6(1):213. doi: 10.1186/1756-0500-6-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jenkins DJA, Boucher BA, Ashbury FD, et al. Effect of current dietary recommendations on weight loss and cardiovascular risk factors. J Am Coll Cardiol. 2017;69(9):1103‐1112. doi: 10.1016/j.jacc.2016.10.089 [DOI] [PubMed] [Google Scholar]
- 90. Jones DW, Miller ME, Wofford MR, et al. The effect of weight loss intervention on antihypertensive medication requirements in the hypertension optimal treatment (HOT) study. Am J Hypertens. 1999;12(12 Pt 1–2):1175‐1180. doi: 10.1016/s0895-7061(99)00123-5 [DOI] [PubMed] [Google Scholar]
- 91. Kanke S, Kawai T, Takasawa N, Mashiyama Y, Ishii A, Kassai R. Interventions for body weight reduction in obese patients during short consultations: an open‐label randomized controlled trial in the Japanese primary care setting. Asia Pac Fam Med. 2015;14(1):5‐5. doi: 10.1186/s12930-015-0022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Katula JA, Vitolins MZ, Rosenberger EL, et al. One‐year results of a community‐based translation of the diabetes prevention program: healthy‐living partnerships to prevent diabetes (HELP PD). Project Diabetes Care. 2011;34(7):1451‐1457. doi: 10.2337/dc10-2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kuller LH, Pettee Gabriel KK, Kinzel LS, et al. The women on the move through activity and nutrition (WOMAN) study: final 48‐month results. Obesity (Silver Spring). 2012;20(3):636‐643. doi: 10.1038/oby.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Little P, Stuart B, Hobbs FR, et al. An internet‐based intervention with brief nurse support to manage obesity in primary care (POWeR+): a pragmatic, parallel‐group, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(10):821‐828. doi: 10.1016/s2213-8587(16)30099-7 [DOI] [PubMed] [Google Scholar]
- 96. Logue E, Sutton K, Jarjoura D, Smucker W, Baughman K, Capers C. Transtheoretical model‐chronic disease care for obesity in primary care: a randomized trial. Obes Res. 2005;13(5):917‐927. doi: 10.1038/oby.2005.106 [DOI] [PubMed] [Google Scholar]
- 97. Luley C, Blaik A, Götz A, et al. Weight loss by telemonitoring of nutrition and physical activity in patients with metabolic syndrome for 1 year. J Am Coll Nutr. 2014;33(5):363‐374. doi: 10.1080/07315724.2013.875437 [DOI] [PubMed] [Google Scholar]
- 98. Marrero DG, Palmer KNB, Phillips EO, Miller‐Kovach K, Foster GD, Saha CK. Comparison of commercial and self‐initiated weight loss programs in people with prediabetes: a randomized control trial. Am J Public Health. 2016;106(5):949‐956. doi: 10.2105/AJPH.2015.303035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mitsui T, Shimaoka K, Tsuzuku S, Kajioka T, Sakakibara H. Gentle exercise of 40 minutes with dietary counseling is effective in treating metabolic syndrome. Tohoku J Exp Med. 2008;215(4):355‐361. doi: 10.1620/tjem.215.355 [DOI] [PubMed] [Google Scholar]
- 100. Moore H, Summerbell C, Greenwood D, et al. Improving management of obesity in primary care: cluster randomised trial. BMJ. 2003;327(7423):1085‐1080. doi: 10.1136/bmj.327.7423.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nicklas JM, Zera CA, England LJ, et al. A web‐based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2014;124(3):563‐570. doi: 10.1097/aog.0000000000000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. O'Brien MJ, Perez A, Scanlan AB, et al. PREVENT‐DM comparative effectiveness trial of lifestyle intervention and metformin. Am J Prev Med. 2017;52(6):788‐797. doi: 10.1016/j.amepre.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Puhkala J, Kukkonen‐Harjula K, Mansikkamäki K, et al. Lifestyle counseling to reduce body weight and cardiometabolic risk factors among truck and bus drivers‐‐a randomized controlled trial. Scand J Work Environ Health. 2015;41(1):54‐64. doi: 10.5271/sjweh.3463 [DOI] [PubMed] [Google Scholar]
- 104. Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity (Silver Spring). 2007;15(4):939‐949. doi: 10.1038/oby.2007.614 [DOI] [PubMed] [Google Scholar]
- 105. Rock CL, Flatt SW, Byers TE, et al. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33(28):3169‐3176. doi: 10.1200/JCO.2015.61.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rodriguez‐Cristobal JJ, Alonso‐Villaverde C, Panisello JM, et al. Effectiveness of a motivational intervention on overweight/obese patients in the primary healthcare: a cluster randomized trial. BMC Fam Pract. 2017;18(1):74. doi: 10.1186/s12875-017-0644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity (Silver Spring). 2010;18(8):1614‐1618. doi: 10.1038/oby.2009.457 [DOI] [PubMed] [Google Scholar]
- 108. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343‐1350. doi: 10.1056/nejm200105033441801 [DOI] [PubMed] [Google Scholar]
- 109. van Wier MF, Dekkers JC, Hendriksen IJ, et al. Effectiveness of phone and e‐mail lifestyle counseling for long term weight control among overweight employees. J Occup Environ Med. 2011;53(6):680‐686. doi: 10.1097/JOM.0b013e31821f2bbb [DOI] [PubMed] [Google Scholar]
- 110. von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2012;125(3):699‐704. doi: 10.1016/j.ygyno.2012.03.042 [DOI] [PubMed] [Google Scholar]
- 111. Wadden TA, Volger S, Sarwer DB, et al. A Two‐Year Randomized Trial of Obesity Treatment in Primary Care Practice. N Engl J Med. 2011;365(21):1969‐1979. doi: 10.1056/NEJMoa1109220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21(3):350‐359. doi: 10.2337/diacare.21.3.350 [DOI] [PubMed] [Google Scholar]
- 113. Yeh MC, Heo M, Suchday S, et al. Translation of the diabetes prevention program for diabetes risk reduction in Chinese immigrants in New York City. Diabetic Med J British Diabetic Assoc. 2016;33(4):547‐551. doi: 10.1111/dme.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Astbury NM, Aveyard P, Nickless A, et al. Doctor referral of overweight people to low energy total diet replacement treatment (DROPLET): pragmatic randomised controlled trial. BMJ (Clin Res Ed). 2018;362:k3760. doi: 10.1136/bmj.k3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bennett GG, Steinberg D, Askew S, et al. Effectiveness of an app and provider counseling for obesity treatment in primary care. Am J Prev Med. 2018;55(6):777‐786. doi: 10.1016/j.amepre.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fjeldsoe BS, Goode AD, Phongsavan P, et al. Get healthy, stay healthy: evaluation of the maintenance of lifestyle changes six months after an extended contact intervention. JMIR Mhealth Uhealth. 2019;7(3):e11070. doi: 10.2196/11070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Haire‐Joshu D, Schwarz CD, Steger‐May K, et al. A randomized trial of weight change in a national home visiting program. Am J Prev Med. 2018;54(3):341‐351. doi: 10.1016/j.amepre.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tapsell LC, Lonergan M, Batterham MJ, et al. Effect of interdisciplinary care on weight loss: a randomised controlled trial. BMJ Open. 2017;7(7):e014533. doi: 10.1136/bmjopen-2016-014533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Viglione C, Bouwman D, Rahman N, et al. A technology‐assisted health coaching intervention vs. enhanced usual care for primary care‐based obesity treatment: a randomized controlled trial. BMC Obes. 2019;6(1):4. doi: 10.1186/s40608-018-0226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fernández‐Ruiz VE, Armero‐Barranco D, Paniagua‐Urbano JA, Sole‐Agusti M, Ruiz‐Sánchez A, Gómez‐Marín J. Short‐medium‐long‐term efficacy of interdisciplinary intervention against overweight and obesity: Randomized controlled clinical trial. Int J Nurs Pract. 2018;24(6):e12690. doi: 10.1111/ijn.12690 [DOI] [PubMed] [Google Scholar]
- 121. Tárraga Marcos ML, Panisello Royo JM, Carbayo Herencia JA, Rosich Domenech N, Alins Presas J, Tárraga López PJ. Effect on the lipid parameters of an intervention to reduce weight in overweight and obese patients. Clin Investig Arterioscler. 2017;29(3):103‐110. Efecto sobre los parámetros lipídicos de una intervención para reducir peso en pacientes con sobrepeso y obesidad. doi: 10.1016/j.arteri.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 122. Teeriniemi AM, Salonurmi T, Jokelainen T, et al. A randomized clinical trial of the effectiveness of a web‐based health behaviour change support system and group lifestyle counselling on body weight loss in overweight and obese subjects: 2‐year outcomes. J Intern Med. 2018;284(5):534‐545. doi: 10.1111/joim.12802 [DOI] [PubMed] [Google Scholar]
- 123. Appel LJ, Clark JM, Yeh H‐C, et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959‐1968. doi: 10.1056/NEJMoa1108660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bhopal RS, Douglas A, Wallia S, et al. Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: a family‐cluster randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(3):218‐227. doi: 10.1016/s2213-8587(13)70204-3 [DOI] [PubMed] [Google Scholar]
- 125. Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23(6):1241‐1249. doi: 10.1097/01.hjh.0000170388.61579.4f [DOI] [PubMed] [Google Scholar]
- 126. Pekkarinen T, Kaukua J, Mustajoki P. Long‐term weight maintenance after a 17‐week weight loss intervention with or without a one‐year maintenance program: a randomized controlled trial. Journal of Obesity. 2015;2015:651460. doi: 10.1155/2015/651460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. Jama. 2008;299(10):1139‐1148. doi: 10.1001/jama.299.10.1139 [DOI] [PubMed] [Google Scholar]
- 128. Young MD, Callister R, Collins CE, Plotnikoff RC, Aguiar EJ, Morgan PJ. Efficacy of a gender‐tailored intervention to prevent weight regain in men over 3 years: a weight loss maintenance RCT. Obesity (Silver Spring). 2017;25(1):56‐65. doi: 10.1002/oby.21696 [DOI] [PubMed] [Google Scholar]
- 129. Cussler EC, Teixeira PJ, Going SB, et al. Maintenance of weight loss in overweight middle‐aged women through the Internet. Obesity (Silver Spring). 2008;16(5):1052‐1060. doi: 10.1038/oby.2008.19 [DOI] [PubMed] [Google Scholar]
- 130. Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long‐term management of obesity. J Consult Clin Psychol. 1988;56(4):529‐534. doi: 10.1037//0022-006x.56.4.529 [DOI] [PubMed] [Google Scholar]
- 131. Sherwood NE, Crain AL, Martinson BC, et al. Enhancing long‐term weight loss maintenance: 2 year results from the keep it off randomized controlled trial. Prev Med. 2013;56(3–4):171‐177. doi: 10.1016/j.ypmed.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Simpson SA, McNamara R, Shaw C, et al. A feasibility randomised controlled trial of a motivational interviewing‐based intervention for weight loss maintenance in adults. Health Technol Assess. 2015;19(50) v‐vi, xix‐xxv:1‐378. doi: 10.3310/hta19500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Voils CI, Olsen MK, Gierisch JM, et al. Maintenance of weight loss after initiation of nutrition training: a randomized trial. Ann Intern Med. 2017;166(7):463‐471. doi: 10.7326/m16-2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self‐regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563‐1571. doi: 10.1056/NEJMoa061883 [DOI] [PubMed] [Google Scholar]
- 135. Daley A, Jolly K, Madigan C, et al. A brief behavioural intervention to promote regular self‐weighing to prevent weight regain after weight loss: a RCT. Southampton (UK): NIHR J Library. 2019;7(7):1‐66. doi: 10.3310/phr07070 [DOI] [PubMed] [Google Scholar]
- 136. Mai K, Brachs M, Leupelt V, et al. Effects of a combined dietary, exercise and behavioral intervention and sympathetic system on body weight maintenance after intended weight loss: Results of a randomized controlled trial. Metabolism. 2018;83:60‐67. doi: 10.1016/j.metabol.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 137. Nakata Y, Sasai H, Tsujimoto T, Hashimoto K, Kobayashi H. Web‐based intervention to promote weight‐loss maintenance using an activity monitor: a randomized controlled trial. Prev Med Rep. 2019;14:100839. doi: 10.1016/j.pmedr.2019.100839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sniehotta FF, Evans EH, Sainsbury K, et al. Behavioural intervention for weight loss maintenance versus standard weight advice in adults with obesity: A randomised controlled trial in the UK (NULevel Trial). PLoS Med. 2019;16(5):e1002793. doi: 10.1371/journal.pmed.1002793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kinsey AW, Gowey MA, Tan F, et al. Similar weight loss and maintenance in African American and White women in the improving weight loss (ImWeL) trial. Ethn Health. 2021;26(2):251‐263. doi: 10.1080/13557858.2018.1493435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sellman D, Schroder R, Deering D, Elmslie J, Foulds J, Frampton C. Psychosocial enhancement of the green prescription for obesity recovery: a randomised controlled trial. N Z Med J. 2017;130(1450):44‐54. [PubMed] [Google Scholar]
- 141. Tanaka NI, Murakami H, Aiba N, Morita A, Watanabe S, Miyachi M. Effects of 1‐year weight loss intervention on abdominal skeletal muscle mass in Japanese overweight men and women. Asia Pac J Clin Nutr. 2019;28(1):72‐78. doi: 10.6133/apjcn.201903_28(1).0011 [DOI] [PubMed] [Google Scholar]
- 142. Fisher R & Fraser C. Who gets in? What does the 2020 GP patient survey tell us about access to general practice? The Health Foundation. Accessed 11/10/21,
- 143. Ahern AL, Aveyard P, Boyland EJ, Halford JC, Jebb SA. Inequalities in the uptake of weight management interventions in a pragmatic trial: an observational study in primary care. Br J Gen Pract. 2016;66(645):e258‐e263. doi: 10.3399/bjgp16X684337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. 2014;174(11):1868‐1870. doi: 10.1001/jamainternmed.2014.4758 [DOI] [PubMed] [Google Scholar]
- 145. Tinetti ME. The gap between clinical trials and the real world: Extrapolating treatment effects from younger to older adults. JAMA Intern Med. 2014;174(3):397‐398. doi: 10.1001/jamainternmed.2013.13283 [DOI] [PubMed] [Google Scholar]
- 146. Helfand BKI, Webb M, Gartaganis SL, Fuller L, Kwon C‐S, Inouye SK. The exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019—missing the target. JAMA Intern Med. 2020;180(11):1546‐1549. doi: 10.1001/jamainternmed.2020.5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wiseman KP, Coa KI, Prutzman YM. Predictors of Retention in an adult text messaging smoking cessation intervention program: cohort study. JMIR Mhealth Uhealth. 2019;7(8):e13712. doi: 10.2196/13712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Metse AP, Hizam NAN, Wiggers J, Wye P, Bowman JA. Factors associated with retention in a smoking cessation trial for persons with a mental illness: a descriptive study. BMC Med Res Methodol. 2018;18(1):177. doi: 10.1186/s12874-018-0640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ligthart KAM, Buitendijk L, Koes BW, van Middelkoop M. The association between ethnicity, socioeconomic status and compliance to pediatric weight‐management interventions—a systematic review. Obes Res Clin Pract. 2017;11(5, Supplement 1):1‐51. doi: 10.1016/j.orcp.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 150. O'Mahen HA, Woodford J, McGinley J, et al. Internet‐based behavioral activation—treatment for postnatal depression (Netmums): a randomized controlled trial. J Affect Disord. 2013;150(3):814‐822. doi: 10.1016/j.jad.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 151. Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16(10):1828. doi: 10.3390/ijerph16101828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Semlyen J, Curtis TJ, Varney J. Sexual orientation identity in relation to unhealthy body mass index: individual participant data meta‐analysis of 93 429 individuals from 12 UK health surveys. J Public Health. 2019;42(1):98‐106. doi: 10.1093/pubmed/fdy224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. NHS England . Menu of evidence‐based interventions and approaches for addressing and reducing health inequalities: tier 2 weight management services. https://www.england.nhs.uk/ltphimenu/prevention/tier-2-weight-management-services/ (Accessed 11/08/2021)
- 154. Alimoradi Z, Golboni F, Griffiths MD, Broström A, Lin C‐Y, Pakpour AH. Weight‐related stigma and psychological distress: a systematic review and meta‐analysis. Clin Nutr. 2020;39(7):2001‐2013. doi: 10.1016/j.clnu.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 155. Tomiyama AJ, Carr D, Granberg EM, et al. How and why weight stigma drives the obesity ‘epidemic’ and harms health. BMC Med. 2018;16(1):123. doi: 10.1186/s12916-018-1116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Puhl RM, Lessard LM, Himmelstein MS, Foster GD. The roles of experienced and internalized weight stigma in healthcare experiences: perspectives of adults engaged in weight management across six countries. PLOS ONE. 2021;16(6):e0251566. doi: 10.1371/journal.pone.0251566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Pan WH, Yeh WT. How to define obesity? Evidence‐based multiple action points for public awareness, screening, and treatment: an extension of Asian‐Pacific recommendations. Asia Pac J Clin Nutr. 2008;17(3):370‐374. [PubMed] [Google Scholar]
- 158. Council of Europe . Intersectionality and multiple discrimination. Accessed 12/01/2022, https://www.coe.int/en/web/gender-matters/intersectionality-and-multiple-discrimination
- 159. Abshire DA, Wippold GM, Wilson DK, Pinto BM, Probst JC, Hardin JW. Rurality, gender, and obesity: an intersectionality perspective on rural men's health. Am J Public Health. 2021;111(10):1761‐1763. doi: 10.2105/ajph.2021.306482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Makowski AC, Kim TJ, Luck‐Sikorski C, von dem Knesebeck O. Social deprivation, gender and obesity: multiple stigma? Results of a population survey from Germany. BMJ Open. 2019;9(4):e023389. doi: 10.1136/bmjopen-2018-023389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Polos J, Koning S, McDade T. Do intersecting identities structure social contexts to influence life course health? The case of school peer economic disadvantage and obesity. Soc Sci Med. 2021;289:114424. doi: 10.1016/j.socscimed.2021.114424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Detailed Study Characteristics


