Abstract
In Hookworm infection, neutrophils have long had the image of the villain, being recruited to the site of larval migration because of damage but participating themselves in tissue injury. With recent developments in neutrophil biology, there is an increasing body of evidence for the role of neutrophils as effector cells in hookworm immunity. In particular, their ability to release extracellular traps, or neutrophil extracellular traps (NETs), confer neutrophils a larvicidal activity. Here, we review recent evidence in this nascent field and discuss the avenue for future research on NETs/hookworm interactions.
Keywords: helminth, hookworms, NETosis, neutrophils, neutrophil extracellular traps
1. INTRODUCTION
As the most abundant myeloid cell in the body, neutrophils play a crucial role in innate immune responses to infectious disease. Neutrophils account for more than half of circulating immune cells and largely outnumber other granulocytes such as eosinophils (2%–5%) and basophils (1%) in circulation. 1 These granulocytes display a diverse set of effector functions including phagocytosis and degranulation in response to small pathogens, and the release of DNA to form extracellular traps (ETs or neutrophil extracellular traps [NETs]) to fight larger pathogens such as fungal hyphae and multicellular parasites. 2
The process of NET formation, NETosis, was first described after phorbol 12‐myristate 13‐acetate (PMA) stimulation. 3 Brinkmann and colleagues then discovered the bactericidal activity of NETs, 2 heralding the intensive study of the NETosis mechanism and its role in pathogen control and disease development. The molecular pathways involved in NET formation still lack consensus, although chromatin decondensation is considered the endpoint. 4 This decondensation ultimately precipitates nuclear membrane rupture, whereupon the DNA mixes with cytoplasmic and granular proteins. 4 This “decorated” DNA is released into the environment, trapping pathogens and creating a micro‐environment rich in enzymes and other toxic molecules for pathogens, and simultaneously protecting neighboring tissue from immunopathology. DNA decondensation can either occur due to (i) the release of neutrophil elastase (NE) from granules, which then degrades histones in the nucleus; and/or (ii) the citrullination of histones by peptidyl arginine deiminase 4 (PAD4). In the first case, termed suicidal NETosis (with a loss of cell membrane), NADPH oxidase 2 (NOX2) leads to the formation of reactive oxygen species (ROS). H2O2, formed by superoxide dismutase (SOD) then becomes the substrate of myeloperoxidase (MPO), resulting in the release of NE. 4 In the second case, also termed, vital NETosis (without loss of membrane integrity), the mechanisms are less understood, but ROS are produced by mitochondria in a NOX‐independent manner. Of note, PAD4 can also be required for suicidal NETosis.
While neutrophils were not conventionally considered to be protective against helminth infection, here we review recent clinical and experimental evidence challenging this dogma, and suggest these neutrophils might have exerted a selective pressure on co‐evolved nematode parasites due to the larvicidal potential of NETs.
2. HOOKWORMS AND NEUTROPHILS: A CLINICAL PERSPECTIVE
Hookworms are clade V nematodes infecting a vast variety of mammals such as canids, felids and humans. 5 They are considered the most prevalent of the soil‐transmitted helminths (STH) and infect about 0.5 billion people worldwide principally in low‐ and middle‐income countries, where poverty and lack of adequate sanitation contribute to high infection rates. 6 Necator americanus, Ancylostoma ceylanicum and Ancylostoma duodenale are the three main species responsible for human infections. 6 Hookworms have a free‐living stage in humid soil, where eggs develop into infective larvae. Depending on the species, the infectious third‐stage larvae (L3) either are ingested or infect the host via skin penetration. From the skin, the larvae enter the blood circulation to reach the lungs. There, they further mature and are coughed up and swallowed, ultimately reaching the intestine. For all species, the adults reside in the intestine and reproduce, laying eggs that are passed on in the feces. Adult parasites can maintain themselves in their host for years, and individuals in endemic areas usually harbor increasing numbers of parasites with age.
To date, the hookworm research community is still unsure whether natural immunity is raised against hookworms, distinguishing these parasites from other STHs. This is attributed to the extensive immunomodulatory abilities of hookworms. 7 , 8 Researchers now propose that hookworms could be “Old Friends”: part of a natural human microbiome with a near‐symbiotic relationship. 9 Like other STH parasites, they are typically associated with raised levels of eosinophils in circulation and a modified Type 2 immune response. However, in‐depth characterization of the immune response triggered by hookworms is lacking, due in part to the absence of species that naturally infect rodents used in immunology research. Helminth infection is commonly associated with elevated granulocyte counts. Most reports focus on eosinophil levels, which can increase from 2% to 5% in a healthy individual to 40% in a helminth‐infected patient. 1 But are eosinophils the only granulocytes recruited in the context of helminth infection? Or are neutrophils also a common feature of hookworm infection?
To date, clinical evidence for neutrophil recruitment after hookworm infection is relatively poorly documented. Despite their role as “early responders,” they appear to have been overlooked because of their reputation as “professional phagocytes” unlikely to participate in the control of large pathogens.
Hookworms of cats and dogs can often cause zoonotic infections in human hosts, leading to a pathology called “cutaneous larvae migrans” in humans. As humans are not the natural host of these animal parasites, the hookworm larvae are unable to migrate from the skin where they entered the host. The impeded parasite causes local inflammation, where serpiginous tracks are often observed. Histological examination reveals eosinophil and neutrophil recruitment in close proximity to larvae. 10 , 11 , 12
While such reports suggest neutrophils might be recruited in the context of clinical hookworm infection, interestingly, a recent analysis of a cohort of 300 individuals living in a STH endemic area (Trichuris, hookworms and Ascaris) observed no increase in circulatory neutrophils due to these infections. 13 The authors further investigated whether the activation phenotype of the neutrophils might have been altered by infection, but once again no association with any of the STHs was found. 13 This absence of neutrophil response has previously been reported in smaller cohorts of hookworm‐infected individuals. For example, volunteers infected with N. americanus had increased levels of circulating eosinophils, but not neutrophils, 3 weeks post‐infection. 14
So why is there an apparent discrepancy between these reports? Could it be that only the skin/infective stages of the parasite are likely to cause neutrophil recruitment? The neutrophilia in cutaneous larvae migrans results from an acute encounter with the infective stage of hookworm. In contrast, in the STH studies, individuals had chronic, established infections with adults at the time of analysis. Hookworms, particularly the adult stage, have evolved a large arsenal of immunomodulation and evasion mechanisms, which we are just beginning to harness to treat systemic anti‐inflammatory diseases. 7 Where albendazole treatment reduces neutrophilia in cutaneous larvae migrans from zoonotic sources, 12 treatment of chronic infections results in an increase of circulatory neutrophils, 13 suggestive of active anti‐neutrophil modulation.
While we could not find clinical studies reporting neutrophil migration to the site of hookworm infection, there is indirect evidence that this recruitment might occur. Indeed, total products or secreted products of several STH have been shown to cause neutrophil chemotaxis. 15 , 16 One such protein is N. americanus Ancylostoma secreted protein 2 (Na‐ASP‐2), a protein involved in L3 invasion and specifically secreted at this infective stage. In an air‐pouch mouse model of localized in vivo inflammation (where the dermal skin is inflated with sterile air creating a bubble in which stimuli are introduced), recombinant Na‐ASP‐2 was shown to cause an influx of lymphocytes to the site of injection, predominantly neutrophils. 15 The crystal structure of Na‐ASP‐2 revealed a similar structure to CC‐chemokines, which are generally chemotactic for neutrophils and monocytes. Beyond hookworms, infective stages of similar nematodes induce neutrophil responses, for example, Strongyloides spp. Like hookworm, this Clade IV parasite enters its host by skin penetration. Rajamanickam et al. reported that Strongyloides stercoralis infected people from South India (presenting no other STH or filariae infection) had an increased number of circulating neutrophils. 17 Interestingly, the authors also found an increase in granular protein concentration, such as NE and MPO, in plasma suggesting that neutrophils were activated. Both neutrophils and their circulating proteins were reduced by anthelmintic treatment (ivermectin + albendazole, 6 months follow‐up), as with larvae migrans.
Similarly, the veterinary‐relevant hematophagous parasite Haemonchus contortus causes neutrophil recruitment to the sheep abomasum for the first 7 days of infection. 18 Interestingly, the differential neutrophil response to STH observed between stages might not only occur due to immune evasion but also be intrinsic to the parasite antigen. Indeed, H. contortus antigens from L3 but not from the adult stage were shown to induce IL‐4 release from neutrophils in vitro. 19
3. HOOKWORMS AND NEUTROPHILS: WHAT LABORATORY MODELS CAN TELL US
Neutrophil recruitment to STH could thus be associated with the infective stage, however, the literature is too limited to reach any significant conclusion. Nevertheless, laboratory investigations have uncovered more detailed associations between neutrophils and hookworm.
Nippostrongylus brasiliensis is a rodent nematode model with a life cycle that closely resembles that of the hookworm N. americanus. This nematode has been shown to cause neutrophil recruitment early on after infection in both the skin and the lungs. 20 , 21 , 22 , 23 , 24 In laboratory mice, secondary infection confers sterilizing immunity unlike in natural infection settings. In this context, neutrophils have also been shown to be recruited in granuloma‐like formations around the larvae of both N. brasiliensis and the strictly intestinal parasite Heligmosmoides polygyrus. 25 , 26 , 27 , 28 As with clinical studies, injection of Strongyloides spp. using a diffusion chamber model in mice caused neutrophil accumulation 1 day post‐infection. 29
More nuanced results were observed for hookworm canine laboratory infections. Ancylostoma caninum, but not Ancylostoma brasiliense, was shown to cause an increase in circulating neutrophils between the early days of infection to the patent phase. 30 These results point out that not all hookworms are made equal regarding neutrophil recruitment and activation, and it would certainly be interesting to characterize these differences. Altogether, such studies illustrate that the infective stages of STH parasites often cause neutrophil recruitment to the site of infection.
4. NEUTROPHILS AND HOOKWORM'S EXCRETORY/SECRETORY PRODUCTS: A SELECTIVE PRESSURE?
Given the long evolutionary history hookworms share with their specific host, parasitic helminths are experts in modulating the immune system to prevent their expulsion. In the last 30 years, research has turned its attention towards studying how the excretory/secretory (ES) products of hookworms contribute to immunomodulation, with proteins, lipids, microvesicles, miRNA, and various metabolites found at this host–parasite interface. 7 , 31 To date, only proteins have been well‐studied, and these are the subject of a recent and extensive review. 7 Hookworms express a plethora of putative or fully demonstrated neutrophil evasion mechanisms. Here, we discuss key examples (Table 1).
TABLE 1.
Immunomodulatory hookworm excretory/secretory proteins display documented or putative neutrophil‐specific functions
| Neutrophil‐related functions | Other immunomodulatory functions | |||
|---|---|---|---|---|
| L3 hookworm immuno‐evasion | ||||
|
DNase II N.br, N.am, A.ce |
✓ | Degrades DNA backbone of NETs 20 | ? | Could degrade ETs from other cells e.g. monocytes |
|
ASP−2 A.ce, N.am |
✓ | Chemoattractant for neutrophils in vitro and in vivo 15 | ✓ | Chemoattractant for monocytes induces antibody responses |
|
KI−1 A.ce, A.ca |
✓ |
Inhibits neutrophil elastase (low expression in L3) 51 |
✓ | Inhibits trypsin and other elastases |
|
HpARI* H.po |
✓ | Inhibits release of IL−33 alarmin 84 | ||
|
HpBARI* H.po |
✓ | Binds and blocks ST2 (IL−33 receptor) 85 | ||
|
MIF* A.ce |
? | Binds the pro‐inflammatory MIF receptor (CD74) which increases MPO expression | ? | Binds the pro‐inflammatory MIF receptor (CD74) on monocytes 86 |
|
MTP−2 A.ce |
✓ | Induces TNFα and IFNγ release from macrophages 87 | ||
|
PAF inhibitor N.br |
✓ | Inhibits PAF, a chemoattractant of eosinophils and neutrophils 35 | ||
| Adult hookworm immuno‐evasion | ||||
|
TIL−1 A.ce, A.du |
✓ | Inhibits neutrophil elastase 52 | ||
|
APs A.ca |
✓ | Various anti‐coagulant peptides 88 | ||
|
NIF, Gp55 (H.co) A.ca, A.ce |
✓ | Blocks neutrophil migration via CD11b/CD18 integrin 37 , 40 | ||
|
Calreticulin(‐like) N.am |
? | Prevents complement‐mediated neutrophil activation 89 | ✓ | Prevents C1q deposition (from L4 to adult stage) |
|
SODs N.am, A.ce, N.br |
? | Protects against oxidation 90 , 91 , 92 | ||
|
PRXs A.ce |
? | Protects against oxidation 93 | ||
|
TMP−1 A.ca |
✓ | De‐activates DCs, induces T regulatory cells, and inhibits matrix metalloproteases 94 , 95 | ||
|
TMP−2 A.ca |
✓ | Matrix metalloprotease inhibitor 94 | ||
|
Acetylcholinesterase N.am |
? | Could prevent the release of neutrophil chemotactic factors from epithelial cells 96 | ||
✓, demonstrated function;?, putative or predicted function; *, also expressed in adults.
The third‐stage larvae (L3) and adult stages of hookworms and related STH express a number of excretory/secretory (ES) proteins, 7 some with known or putative anti‐neutrophil activity.
Of the 8 characterized proteins secreted by hookworm infective larvae or their laboratory model counterparts, 5 have confirmed or putative activity associated with neutrophils. Two are related to chemotaxis (ASP‐2 as attractant 15 and PAF inhibitor as blocker 35 ). The three others could impair NETs formation (DNase‐II 20 , KI‐1 51 and MIF 7 , 86 ). Two ES not associated with neutrophils have been discovered in Heligmosmoides polygyrus and inhibit IL‐33/ST2 pathway (HpARI and HpBARI 84 , 85 ). Finally, MTP‐2 is an astacin‐like metalloprotease that enhances the expression of TNFα and IFNγ in classically activated (LPS‐stimulated) macrophages. 87
In the adult stage, more proteins with immunomodulatory properties have been characterized. Of 12 notable proteins we could find described in the literature, 7 had potential anti‐neutrophil activity. NIF, 37 and its homologue gp55 40 in H. contortus, block neutrophil chemotaxis. Acetylcholinesterase could also decrease neutrophil recruitment indirectly by blocking epithelial cells chemokine secretions. 96 Once again, several ES protein activities were consistent with anti‐NET activity (TIL‐1, MIF, SODs, PRXs). Similar to KI‐1, TIL‐1 has been shown to inhibit NE. 52 Both SODs 90 , 91 , 92 and PRXs 93 could affect NETosis by decreasing oxidative stress. Finally, a calreticulin‐like protein, identified in Necator americanus could contribute to complement evasion, 89 and thus indirectly decrease neutrophil trapping and NETosis. Non‐neutrophil‐related proteins include metalloprotease inhibitors (TMP‐1 and 2), which have been shown to affect dendritic cell polarization and inhibit host matrix metalloproteases (MMP)‐2, −7, and −13. 94 , 95 APs have been shown to have anticoagulant activity. 88
Checkmark indicates function described in vivo or in vitro, interrogation mark indicates putative function from known activity of protein or predicted function of sequence.
Abbreviations: A.ca, A. caninum; A.ce, A. ceylanicum; Ac‐TMP‐2, tissue inhibitor of metalloprotease 2; A.du, A. duodenale; APs, anticoagulant proteins; ASP‐2, ancylostoma‐secreted proteins; H.co, H. contortus; KI‐1, Kunitz‐type inhibitor 1 from Ancylostoma ceylanicum; MIF homologue of macrophage migration inhibitory factor; MTP‐2, metalloprotease 2; N.am, N. americanus; N.br, N. brasiliensis; TIL‐1 trypsin‐inhibtor like serine protease inhibitor.
The color is to help the reader assess if the evasion products has been linked to neutrophil quickly. Dark blue is used for when Neutro association is demonstrated, light blue, when it is hypothetical and grey for a mechanism unrelated to neutrophil.
4.1. Hookworm ES products block neutrophil recruitment
Several studies report that hookworm ES reduces or abolishes neutrophil responses and immunopathology. For example, whole ES isolated from N. brasiliensis abrogates Lipopolysaccharides (LPS)‐induced lung neutrophilia. 32 , 33 Similarly, H. polygyrus also reduces the level of neutrophil‐specific chemokines in mice in a model of contact hypersensitivity. 34
While hookworm ES proteins have now been studied for several decades, only a few functions are fully characterized. A platelet activating factor (PAF) hydrolase (Table 1) was identified in adult stages of N. brasiliensis and found to inhibit PAF, a potent chemoattractant of eosinophils and neutrophils. 35 Another notable hookworm ES product is neutrophil inhibitory factor (NIF), identified while studying A. caninum extracts. 36 This glycoprotein binds to the Mac‐1 integrin (CD11b/CD18) on leukocytes, preventing the adhesion and transmigration of neutrophils. By transfecting the NIF gene into human endothelial cell cultures and murine lungs, it was demonstrated that this molecule effectively blocks the recruitment and migration of neutrophils both in vitro and in vivo. 37 , 38 NIF has since been shown to be produced by a wide variety of hookworms and related helminths. Notably, however, despite being predicted in its genome, no protein has been found in N. americanus. 8 , 39 In H. contortus, gp55, a NIF homologue, was further shown to reduce neutrophil effector functions by blocking H2O2 release by binding to Cd11b/Cd18. 40
As neutrophils are typically implicated in tissue damage, 21 , 24 it has long been assumed that the anti‐neutrophil activity of ES was to dampen immunopathology that would ultimately damage host and helminth alike. More recent advances in our understanding of neutrophil biology 41 and functions have pressed the research community to focus on neutrophils as also being effector cells against hookworms. Interestingly, hookworms do also express secreted molecules that could potentially block neutrophil effector functions.
4.2. Hookworm ES products may block neutrophil effector functions
Most of the evasion molecules that could inhibit neutrophil effector functions have been studied before neutrophils were considered as potential anti‐helminth effector cells. As such, the function of some ES products discussed in this section is putative or predicted (Table 1).
Indirect evidence that neutrophils might contribute to parasite control comes from approaches where hookworm ES have been blocked. For example, Ali and collaborators demonstrate that vaccination with NIF reduces A. ceylanicum fecundity in hamsters, as measured by a reduction in eggs per gram of feces. 36 This mimics clinical observations from Papua New Guinea that N. americanus‐infected patients with IgE responses to ES products have lower levels of egg production. 42 , 43
Both N. americanus and the ruminant nematode H. contortus secrete a calreticulin‐like molecule (NaCalr). NaCalr was shown in vitro to block C1q‐induced haemolysis via the complement pathway and therefore has a putative role in preventing opsonization and activation of leukocytes such as neutrophils. 44 , 45 Like for NIF, immunization with NaCalr conferred some protection against challenge infection, with a reduction of 43%–49% worms in the lungs of mice. 46
NETosis has recently been shown to be triggered in response to various nematodes. 47 We reported that extracellular traps were released early on after both natural skin penetration and intradermal infection with N. brasiliensis. 20 Previously, in mice infected with N. brasiliensis no NETs were found surrounding lung L3, but NETs were reported in the skin around dead L3. 22 , 24 This apparent discrepancy can be explained by an active anti‐NETosis evasion mechanism (Figure 1). Indeed, we demonstrated that while NETs could not be observed at late time points around living larvae both in vivo and in vitro, they were present around dead larvae. Using live imaging, we proved that in all cases NETs were released, but that they were quickly destroyed in the presence of ES products expressed by living parasites. We further identified a DNase‐II highly conserved in Clade V nematodes (including N. americanus, A. ceylanicum), which is secreted and able to degrade NETs in vivo and in vitro. 20
FIGURE 1.
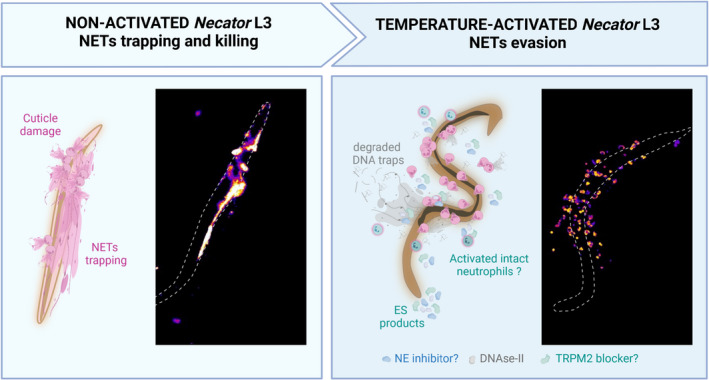
Hookworms actively evade NETosis. The non‐activated infectious larvae of hookworm are trapped by neutrophil extracellular traps (NETs) released by neutrophils isolated from human blood. During the transition to parasitism, heat‐activation causes hookworms to secrete anti‐NETs evasion molecules. Three mechanisms of evasion are illustrated: (i) a DNase‐II capable of degrading NETs to evade trapping and cuticle damage, demonstrated in Necator brasiliensis and N. americanus and (ii) a Kunitz‐type Inhibitor (Ace‐KI1) identified in Ancylostoma ceylanicum is proposed to block the formation of NETs by inhibiting NE activity, (iii) an unidentified blocker of TRMP‐2 inhibits oxidative stress‐induced NETs formation. This mechanism has been demonstrated in the cestode Mesocestoides corti. Fluorescent images were obtained by co‐culture of circulatory human neutrophils with N. americanus L3 for 3 h. Activated larvae were placed at 37°C for one night before co‐culture to stimulate ES release. NETs are stained using sytox green and are represented with the LUT fire in Fiji. The figure has been made using Biorender
Neutrophil elastase, a key molecule in NETs formation, has been shown in vitro to be directly toxic to the flukes Fasciola hepatica and Schistosomes by damaging their tegument. 48 , 49 NE has also been suggested to damage the much harder nematode cuticle of Trichinella. 50 Adult worms of A. ceylanicum and A. caninum have been shown to produce a Kunitz‐type inhibitor‐1 (AceKI‐1 or AceK1), which is a tight‐binding inhibitor of trypsin, chymotrypsin, pancreatic elastase, and NE. 51 Ancylostoma duodenale also expresses a serine protease inhibitor with two trypsin inhibitor‐like domains (AduTIL‐1). 52 This likely reflects the reproductive niche of hookworms in the digestive tract, but might also be an evasion strategy from neutrophil attack (Figure 1). Unfortunately, the impact of NE inhibition via AceKI1 on NETosis has not been studied so far.
Hookworms express several antioxidant enzymes, such as SODs, which could interfere with the process of NET formation (Figure 1). Interestingly, extracts of the tapeworm Mesocestoides corti were shown to block the formation of stress‐induced NETs. 53 In this study, parasite products were co‐cultured with neutrophils in the presence of H2O2, and a reduction in NETs was observed. The authors then further determined that ES products block the Transient Receptor Potential Cation Channel Subfamily M Member 2 (TRPM2) as well as downstream autophagy pathways. While the authors did not define which specific parasite molecule(s) is (are) responsible for this activity, other studies have investigated the role of cestode ES in protecting against H2O2‐induced cell death. One such study treated Echinococcus granulosus with H2O2 to establish a list of parasite proteins secreted in response to reactive oxygen species. 54 At the top of this list are gluthatione‐S‐transferases, a well‐characterized family of enzymes involved in detoxification. In hookworms, three GSTs have been identified, with GST‐1 being a lead human vaccine candidate due to its heme detoxification function. It might thus be interesting to investigate the role of GSTs in NETosis evasion.
In this section, we have shown that hookworms have evolved a considerable defense arsenal against neutrophil activity, both blocking recruitment as well as neutrophil effector functions. We therefore argue that this reflects a selective pressure exerted by neutrophils on parasite fitness. In the next section, we discuss the proven and potential larvicidal activity of neutrophils.
5. NEUTROPHIL‐MEDIATED KILLING OF HOOKWORMS
We have established in the previous sections that neutrophil recruitment is a feature of hookworm infections and that hookworms have evolved various strategies of evading the neutrophil attack. This suggests that hookworm fitness can be affected by neutrophils. But can these granulocytes kill a helminth, and by which mechanism(s), given the large size of hookworms?
Interestingly, the toxic activity of neutrophils was assessed against various nematodes in vitro in the early days of immunoparasitology. 50 However, knowledge of neutrophil effector functions was incomplete at the time, and their role in anti‐helminth immunity has only been studied in detail more recently. Two types of neutrophil‐mediated killing have emerged from recent studies: (i) direct killing, associated with neutrophil trapping, NETosis and granule toxicity and (ii) indirect killing, associated with enhanced type 2 immunity.
5.1. Direct toxicity of neutrophil to hookworm parasites: netosis killing
The web‐like DNA that forms NETs is decorated with antimicrobial molecules such as histones, MPO, and NE, which have been demonstrated to kill bacterial and fungal pathogens. 41 Here, we discuss the pathways invoking NETosis, and the mechanisms by which NETs reduce parasite infectivity and viability (Figure 2).
FIGURE 2.
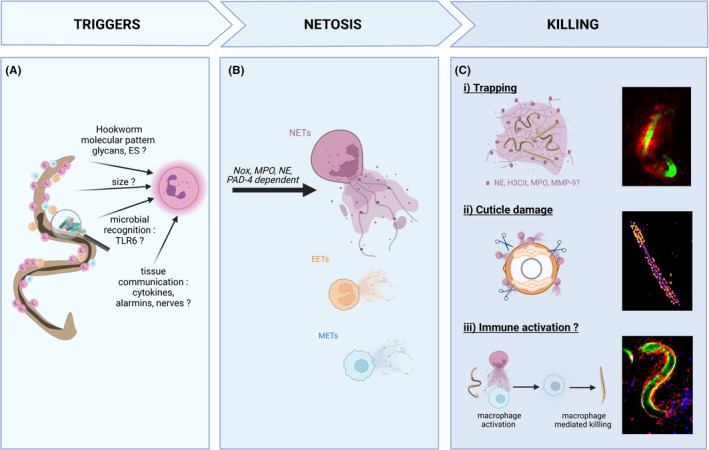
Neutrophil extracellular traps kill larvae via a variety of mechanisms. Neutrophil extracellular traps (NETs) are induced in response to hookworm and related helminth larvae. (A) The mechanism of NETs induction is not yet characterized. The current hypothesis include parasite‐specific products such as glycans and excretory/secretory (ES) products, the multicellular size of larvae, recognition of microbiome/soil‐derived microbial signatures via toll‐like receptors (TLR), or indirect activation from as‐yet‐unknown immune or non‐immune cells. (B) Following hookworm detection, NETosis induction requires NADPH oxidase (Nox), myeloperoxidase (MPO), neutrophil elastase (NE), and peptidylarginine deiminase 4 (PAD4). While neutrophils have been the primary study of hookworm‐induced NETosis, emerging evidence suggests other cells may form extracellular traps such as eosinophils (EETs) and monocytes/macrophages (METs). (C) In the absence of immuno‐evasion, NETs can participate in larval killing by direct or indirect mechanisms not mutually exclusive: (i) Larvae are mechanically trapped by NETs. L3 are potentially exposed to a high concentration of “decorating” enzymes (NE, citrullinated histone H3 (H3Cit), MPO, and matrix metalloprotease 9 [MMP‐9]) or killed by other immune cells recruited to the traps and (ii) the cuticle of larvae is damaged by neutrophil enzymes such as NE, causing increased permeability to sytox green. (iii) NETs directly activate other immune cells, such as macrophage to potentiate their larvicidal activity. Immunofluorescence microscopy of Necator brasiliensis L3: (i) intravital imaging of larvae in the skin (CFSE stained, green) trapped by NETs stained with the DNA binding dye sytox blue (red). (ii) Larvae killed by NETs in vitro and stained with sytox green for 3 h. The damaged cuticle lets the otherwise impermeant dye through to stain the internal structures of the worm. Fluorescence intensity represented with the fire LUT in Fiji. (iii) Intravital imaging of neutrophils (Ly6G‐PE red) adhered to larvae (CFSE‐stained, green) surrounded by monocytes (Ly6C‐BV421, blue) 6 h post intradermal inoculation. For more details about the methodology, please see. 20 The figure has been made using Biorender
5.1.1. Neutrophil extracellular traps larvicidal activity
Neutrophils that are recruited towards larvae or their products have been shown to bind and trap larvae 20 which can be enhanced by antibody‐dependent binding as shown during secondary infection with H. polygyrus. 55 To bypass macrophage‐mediated killing of the parasite, mice were challenged with L3 only 4 days after primary infection and passively immunized with serum from primed mice to artificially increase antibody titers. Following this transfer, worm burdens were significantly reduced. They further demonstrated in vitro that “altered” neutrophils from immunized mice swarmed and killed the larvae – although this now four‐decade‐old paper did not further describe this alteration, it was perhaps an early report of trained immunity. Similarly, in mice vaccinated with A. caninum, inoculation of L3 intraperitoneally caused neutrophils to bind to the larvae in an antibody‐dependent manner. The authors then conducted SEM on the larvae and observed damage to the cuticle, including what they described as a swelling, focal collapse of cuticle, and deformation. 56
We have recently observed similar damage to the cuticle of N. brasiliensis exposed in vitro to neutrophils, as shown by increased permeability of the L3 cuticle to the impermeant DNA binding dye Sytox‐Green. 20 In this study, we further characterized the neutrophil toxicity to the larvae resulting from the release of NETs, formed around larvae after skin penetration or intradermal injection. Neutrophil depletion or NETosis blockade (PAD‐4‐KO, NE inhibitor, and DNAse treatment) all increased parasite survival, measured by the number of adults in the intestine. We also confirmed that N. americanus‐induced NETosis in vitro. As mentioned previously, N. brasiliensis and N. americanus secrete a DNAse‐II that cleaves extracellular traps in response to this attack. In a co‐culture assay of L3 with neutrophils, neutralizing the DNAse‐II evasion activity with anti‐serum increased the percentage of dead larvae, proving that NETs can directly impair larval survival. Altogether, this illustrates that NETs can have larvicidal activity against hookworms and that those parasites have evolved an evasion strategy against this attack (Figure 2).
NETosis appears to be initiated against various helminths, both STH and vector‐borne. Notably it has been observed in vitro against Strongyloides stercolaris, 57 H. contortus, 58 , 59 Strongyloides ratti, 60 the ruminant parasite Ostertagia ostertagi, 61 as well as several filariae. 47 What is most intriguing is that this response is not just limited to parasite nematodes; even if quite artificial, the free‐living clade V nematode Caenorhabditis elegans is also able to trigger NETs in vitro, proving the triggers of NETosis are well‐conserved amongst nematodes. 61
To date, no consensus has emerged regarding the importance of NETs in STH larvicidal activity. Key differences between studies are as follows: (i) So far, immuno‐evasion has only been observed in hookworm, with the live larvae of other species becoming durably entrapped in NETs 20 , 57 , 59 ; (ii) Cuticle damage was only reported in N. americanus, N. brasiliensis and A. caninum 20 , 56 ; (iii) While neutrophil‐induced killing was found in all models, NETs degradation was not always sufficient to reverse killing. 57 , 58 , 62 Whether this means that NETs can damage some helminths and not others is not clear. Indeed, some of these results might be inherent to the assay, and not reflective of NETs larvicidal potential in vivo. For example, in the S. stercolaris in vitro system, incubating the larvae with a mouse or human neutrophils gave diverging results. 57 In both cases, NETs were released and contributed to larval trapping. However, with human cells, DNase‐I treatment reduced the killing of the larvae from 90% to 20%, while with murine neutrophils no reduction in the killing was observed. Further work, using in vivo studies, is required to confirm the larvicidal activity of NETs in other STH.
A potential explanation as to why some helminth larvae are killed by NETs and not others may lie in a difference in neutrophil activation, rather than in the worms themselves. Indeed, different stimuli have been shown to cause changes in the “NETome,” that is,the molecules decorating the released DNA in NETs. 63 , 64 This raises the question of whether there are helminth‐specific NET decorations. The protein composition of helminth‐induced NETs has not been studied to date, and the only information known is that MPO, NE and histones are present. 20 , 59 , 61 However, Ehrens et al. 60 report the release of MMP‐9 in the supernatant of S. ratti stimulated neutrophils but did not confirm its association to the released DNA. Given that NE is suggested to damage Trichinella sp. 50 , it would be an interesting avenue of future research to determine whether NE or other NET‐associated proteins are responsible for NETs‐induced damage to the hookworm cuticle.
5.1.2. Neutrophil extracellular traps induction
Neutrophil extracellular traps can be invoked via several different pathways and signaling events, as discussed above. 65 , 66 In all reported models of STH‐induced NETosis, neutrophil elastase blockade was found to prevent NET formation. 20 , 59 , 61 Blockade of MPO and NOX activity was also reported to block NETosis in vitro. 59 , 61 Interestingly, NETosis in response to Dirofilariae immitis (a Clade III nematode) was shown to be independent of NOX activity, 62 suggesting that several types of NETs could be induced by different helminths. In NOX‐independent NETosis, PADs were shown to be required to decondensate DNA by hypercitrullination. 67 PAD4 was shown to be required for NET killing in vivo after N. brasiliensis infection, and NETs were heavily decorated with citrullinated Histone 3, 20 though unfortunately, we did not investigate the role of NOX.
Interestingly, NOX‐independent NETosis has been reported to be a much faster event than NOX‐dependent NETosis. Rapid induction of NETosis was reported in vitro after stimulation with S. stercolaris and H. contortus, respectively, 57 , 59 which might suggest NOX‐independent NETosis does occur after STH stimulation. Notably, both species induced NETs more rapidly than with highly potent artificial stimuli PMA. However, with both N. brasiliensis and O. ostertagi, NETs induction has been shown to be slower than with PMA. 20 , 61 This slow NETosis has been shown to be associated with some neutrophils remaining NETosis‐free several hours after culture within Candida albicans and Group B streptococcus, 66 while PMA induced NETosis in virtually all neutrophils. Similar to those slow inducers, a large majority of neutrophils free from NETosis were reported for both N. brasiliensis and S. ratti. 20 , 60 Why such a low number of neutrophils enter NETosis in response to helminth infection is an interesting question, currently without an answer. It may be that a specific polarization of neutrophil is required (as described in Chen, 2016) or that neutrophil requires a signal above a threshold to undergo NETosis, that PMA and other “potent inducers” reliably cross, while helminth does not, maybe to prevent “NETosis storms.”
Further work is required to understand the mechanisms of NETs formation against helminths and to decipher if all NET induction mechanisms impair parasite viability.
5.2. Indirect toxicity of neutrophils: a role for netosis in type 2 protective immunity?
The role of neutrophils in type 2 immune responses, particularly in helminth infection, is increasingly appreciated. 68 Here, we focus on the indirect role of NETs in driving type 2 protective immunity to hookworm.
5.2.1. Priming of type 2 immunity
As with many infections or injuries, neutrophils are quickly recruited to, and swarm, hookworm larvae after their entry into the host. 20 Neutrophils and inflammatory monocytes have been shown to be the main cells taking up fluorescently labelled N. brasiliensis L3 antigens in the skin. 22 Given that NETs have been shown to interact with dendritic cells (DCs) and alter their polarization. 69 , 70 Pellefigues and collaborators explored the role of NETs in the priming of type 2 inducing DCs after dead L3 injection. The IFN‐I signature of DCs (required for Th2 induction in helminth models) was not found to be dependent on NETosis, as this signature was not abrogated by exogenous DNase treatment or neutrophil depletion. 22 This however does not exclude the role of NETs in shaping type 2 responses entirely. Indeed, in type 2 respiratory virus model, NETs have been recently shown to sustain the intensity and priming of the type 2 response by controlling recruitment of monocyte‐derived DCs, 71 proving that NETs can be involved in Type 2 immune priming/tuning. Further studies of NETosis in the initiation of Type 2 immunity are thus needed.
5.2.2. Activation of effector cells
IL‐4 activated macrophages are a hallmark of anti‐helminth immunity, and many forms of neutrophil‐macrophage communication have been reported. 72 In Strongyloides infection, NETs were shown to be required for killing larvae but could do so only in the presence of macrophages. 57 Similarly, in an N. brasiliensis re‐infection model, primed macrophages from neutrophil‐depleted mice, transferred to naïve animals, could no longer protect against infection, 27 suggesting an important role for neutrophils in priming macrophage larvicidal activity. Nippostrongylus brasiliensis or LPS‐treated neutrophils had a distinct transcriptome from one another, including prototypical M2 markers (il‐13, chi3l3, retnla, arg‐1). Whether this transcriptomic profile is associated with NETosis has not been investigated in this study. 27
Recent research, outside the field of helminthology, has begun to explore the potential for NETs to contribute to macrophage activation. Notably, NETs have been shown to activate macrophages via NLRP3 leading to increased activation of CD86+ macrophages and increased expression of IL‐1b, IL‐6 and TNFa (Qiongyi et al. Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult‐onset Still's disease, PMID: 30616678). Furthermore, IL‐8 has been shown to cause the release of NETs and further activate macrophage activation and release of IL‐8 in the context of atherosclerosis (An, 2019). Future research could thus investigate how NETs or their clearance changes macrophage polarization and function in the context of hookworm infection.
6. HOOKWORMS AND NETOSIS: OUTSTANDING QUESTIONS REMAIN
While NETosis is an increasingly documented response against STH infection, there remain several key questions surrounding the mechanisms and requirements of this effector response. First, what are the molecular triggers of helminth‐induced NETosis? Second, what other cells contribute to killing during NETosis, and could eosinophil and monocyte ETs contribute to helminth control?
6.1. Triggers for nets
Size has been proposed as a trigger for NETosis. Indeed, Bransk and collaborators proposed that neutrophils selectively release NETs in response to fungal hyphae or bacterial aggregates, but not small yeasts or non‐aggregated bacteria. 73 The absence of phagosomes that form when neutrophils encounter large pathogens allows for NE to be slowly released into the cytosol. This in turn promotes the decondensation of chromatin, leading to the release of NETs. These molecular events could certainly occur when a neutrophil encounters a helminth, although it is yet to be explored. However, in this size‐dependent model, NETosis is slow and can take up to 4 hours. 73 Hookworms and helminths on the other hand have been shown to trigger NETs within 30 minutes to 1 hour post‐encounter. Thus, it is likely that other triggers are involved in the induction of NETosis. 26 , 57 , 59 , 61
In filarial parasites, it is well documented that NET formation is driven by the presence of the bacterial symbiont Wolbachia. 47 Indeed, treatment of Brugia malayi with doxycycline prior to infection abolishes NETosis. This raises the possibility that it is not hookworms per se that are recognized by the immune system, but associated microbial pathogens. While hookworms do not have known endosymbionts, the infective larvae are likely to be covered with soil‐derived bacteria. It stands to reason that host immunity could evolve responses to this hookworm‐associated microbial signature.
While hookworm NETosis induction could be bacterial dependent, it is however independent of TLR‐2 and TLR‐4, as NETs were still observed around dead N. brasiliensis L3 in TLR‐2‐KO and TLR‐4‐KO. 22 Similar results were obtained with TLR‐4 inhibitor treatment in O. ostertagi assays, 61 proving that TLR‐2 and TLR‐4 ligands are not required for hookworm‐induced NETosis. While specific helminth PAMPs have still not been characterized, several hookworm molecules could be triggering NETosis. The glycan‐rich cuticle of hookworms could be recognized by neutrophils, as lipophosphoglycans of Trypanasoma spp. have been shown to induce NETs. 74 Another potential PAMP could be aspartic protease‐1 (APR‐1), the lead vaccine candidate against hookworms, as fungal aspartic proteases of C. albicans were shown to trigger NETosis. 75
Finally, NETosis could happen in response to environmental cues such as danger signals or cytokine alarmins. Indeed, cytokine (IL‐8) and chemokine binding (to IFN‐R and CXCR1) has been shown to be capable of triggering NETs formation. 76 To date, such a role for the cytokine milieu has not been addressed in STH. Instead, a recent study reports that IL‐4, a hallmark of anti‐helminth response, limits NETs formation. 77
6.2. Extracellular traps from other cells
Since the discovery of NETs, DNA traps have been shown to be a general feature of leukocytes, including eosinophils (EETs), macrophage (METs), mast cells, and basophils (BETs). 78 , 79 Literature is even more limited for these cells than neutrophils, but a few reports are pointing towards their involvement in hookworm infection.
As mentioned previously, Strongyloides infection has been shown to trigger egress of both neutrophils and eosinophils to the site of infection. 60 Interestingly, Ehrens and collaborators, reported recently that S. ratti infection triggers both NETosis and EETosis with larvicidal activity. EETosis was also demonstrated against filarial parasites Litomosoides sigmodontis and D. immitis, suggesting a conserved mechanism of helminth defense. 80
Strongyloides larvae were shown to cause the release of METs from human cells in vitro. 57 The subsequent killing of larvae then required a combination of neutrophils and macrophages, and was reversed by DNase treatment. Interestingly, the authors report that in co‐cultures of mouse neutrophils, macrophages and larvae, NETs but not METs are released. The killing was then still present but not reversed by DNAse treatment. 57 This suggests that METs might be involved in Strongyloides killing but that redundant mechanisms exist.
Basophils are known to be important for early priming of the immune response against N. brasiliensis, notably releasing IL‐4 and priming macrophages in secondary responses. 81 , 82 Nothing is yet known regarding their DNA release during helminth infection, but it would be interesting to investigate the contribution of BETs to anti‐helminth immunity.
7. CONCLUSION
Hookworms appear to have evolved complex mmune‐evasion mechanisms specifically tailored towards neutrophil effector functions, with many mechanisms still remaining to be elucidated. It also appears that neutrophils, and in particular their NETs, can have larvicidal activity directed against at least the infective stage of hookworm. Thus, we argue that neutrophils are an important player in anti‐hookworm immunity and that their role in clinical infection should be explored further.
Clinical investigations of the immune response to hookworm infections are scarce, as local tissues are hard to access in humans. Quite recently, controlled challenge infections with hookworms have been designed to test the efficacy of hookworm vaccine candidates during clinical trials. 83 This approach has created an interesting opportunity for researchers to study the immune response to hookworm infections in otherwise hookworm‐naïve individuals, notably the early events after infection, which may be associated with neutrophil responses and potential larvicidal activity.
Attempts to develop anti‐hookworm vaccines have highlighted the need to exploit early immune responses to the infective stage, rather than established adult worms. Given neutrophils role as first responders, deciphering the neutrophil/hookworm interplay might pave the way towards new vaccine targets and uncover neutrophil evasion mechanisms that could be harnessed to combat neutrophil‐mediated diseases.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pim.12911.
ACKNOWLEDGEMENTS
We thank Adam A. T. Smith, & Sonja I. Repetti for critically reviewing the work and providing feedback. Open Access Funding provided by Universitat Basel.
Doolan R, Bouchery T. Hookworm infections: Reappraising the evidence for a role of neutrophils in light of NETosis. Parasite Immunol. 2022;44:e12911. doi: 10.1111/pim.12911
Funding information
This work was supported by a career development grant SNF‐PRIMA PR00P3_193084 covering the salary of TB and RD.
Contributor Information
Rory Doolan, Email: rory.doolan@swisstph.ch.
Tiffany Bouchery, Email: tiffany.bouchery@swisstph.ch.
REFERENCES
- 1. Reimert CM, Fitzsimmons CM, Joseph S, et al. Eosinophil activity in Schistosoma mansoni infections in vivo and in vitro in relation to plasma cytokine profile pre‐ and posttreatment with praziquantel. Clin Vaccine Immunol. 2006;13(5):584‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532‐1535. [DOI] [PubMed] [Google Scholar]
- 3. Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12‐myristate 13‐acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59(2):229‐240. [DOI] [PubMed] [Google Scholar]
- 4. Boeltz S, Amini P, Anders HJ, et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26(3):395‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6(3):177‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:16088. [DOI] [PubMed] [Google Scholar]
- 7. Abuzeid AMI, Zhou X, Huang Y, Li G. Twenty‐five‐year research progress in hookworm excretory/secretory products. Parasit Vectors. 2020;13(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang YT, Gao X, Rosa BA, et al. Genome of the human hookworm Necator americanus . Nat Genet. 2014;46(3):261‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorimer J. Hookworms make us human: the microbiome, eco‐immunology, and a probiotic turn in western health care. Med Anthropol Q. 2019;33(1):60‐79. [DOI] [PubMed] [Google Scholar]
- 10. Balfour E, Zalka A, Lazova R. Cutaneous larva migrans with parts of the larva in the epidermis. Cutis. 2002;69(5):368‐370. [PubMed] [Google Scholar]
- 11. Damante JH, Chinellato LE, Oliveira FT, Soares CT, Fleury RN. Larva migrans in the oral mucosa: report of two cases. Braz Dent J. 2011;22(2):166‐170. [DOI] [PubMed] [Google Scholar]
- 12. Maxfield L, Crane JS. Cutaneous Larva Migrans. StatPearls; 2021. [PubMed] [Google Scholar]
- 13. de Ruiter K, Tahapary DL, Sartono E, et al. The effect of helminths on granulocyte activation: a cluster‐randomized placebo‐controlled trial in Indonesia. J Infect Dis. 2019;219(9):1474‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White CJ, Maxwell CJ, Gallin JI. Changes in the structural and functional properties of human eosinophils during experimental hookworm infection. J Infect Dis. 1986;154(5):778‐783. [DOI] [PubMed] [Google Scholar]
- 15. Bower MA, Constant SL, Mendez S. Necator americanus: the Na‐ASP‐2 protein secreted by the infective larvae induces neutrophil recruitment in vivo and in vitro. Exp Parasitol. 2008;118(4):569‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Connell AE, Redding KM, Hess JA, Lok JB, Nolan TJ, Abraham D. Soluble extract from the nematode Strongyloides stercoralis induces CXCR2 dependent/IL‐17 independent neutrophil recruitment. Microbes Infect. 2011;13(6):536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajamanickam A, Munisankar S, Bhootra Y, Dolla CK, Nutman TB, Babu S. Elevated systemic levels of eosinophil, neutrophil, and mast cell granular proteins in strongyloides stercoralis infection that diminish following treatment. Front Immunol. 2018;9:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bowdridge SA, Zajac AM, St NDR. Croix sheep produce a rapid and greater cellular immune response contributing to reduced establishment of Haemonchus contortus. Vet Parasitol. 2015;208(3–4):204‐210. [DOI] [PubMed] [Google Scholar]
- 19. Middleton D, Garza JJ, Greiner SP, Bowdridge SA. Neutrophils rapidly produce Th2 cytokines in response to larval but not adult helminth antigen. Parasite Immunol. 2020;42(1):e12679. [DOI] [PubMed] [Google Scholar]
- 20. Bouchery T, Moyat M, Sotillo J, et al. Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe. 2020;27(2):277‐89. e6. [DOI] [PubMed] [Google Scholar]
- 21. Chen F, Liu Z, Wu W, et al. An essential role for TH2‐type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18(2):260‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pellefigues C, Tang SC, Schmidt A, et al. Toll‐like receptor 4, but not neutrophil extracellular traps, promote IFN type I expression to enhance Th2 responses to Nippostrongylus brasiliensis . Front Immunol. 2017;8:1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pesce JT, Liu Z, Hamed H, et al. Neutrophils clear bacteria associated with parasitic nematodes augmenting the development of an effective Th2‐type response. J Immunol. 2008;180(1):464‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sutherland TE, Logan N, Ruckerl D, et al. Chitinase‐like proteins promote IL‐17‐mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol. 2014;15(12):1116‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anthony RM, Urban JF Jr, Alem F, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12(8):955‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouchery T, Kyle R, Camberis M, et al. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat Commun. 2015;6:6970. [DOI] [PubMed] [Google Scholar]
- 27. Chen F, Wu W, Millman A, et al. Neutrophils prime a long‐lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014;15(10):938‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morimoto M, Morimoto M, Whitmire J, et al. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol. 2004;172(4):2424‐2430. [DOI] [PubMed] [Google Scholar]
- 29. Abraham D, Rotman HL, Haberstroh HF, et al. Strongyloides stercoralis: protective immunity to third‐stage larvae inBALB/cByJ mice. Exp Parasitol. 1995;80(2):297‐307. [DOI] [PubMed] [Google Scholar]
- 30. Dias SR, Cunha DE, da Silva SM, Dos Santos HA, Fujiwara RT, Rabelo EM. Evaluation of parasitological and immunological aspects of acute infection by Ancylostoma caninum and Ancylostoma braziliense in mixed‐breed dogs. Parasitol Res. 2013;112(6):2151‐2157. [DOI] [PubMed] [Google Scholar]
- 31. Yeshi K, Creek DJ, Anderson D, et al. Metabolomes and lipidomes of the infective stages of the gastrointestinal nematodes, Nippostrongylus brasiliensis and Trichuris muris . Metabolites. 2020;10(11):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keir PA, Brown DM, Clouter‐Baker A, Harcus YM, Proudfoot L. Inhibition of neutrophil recruitment by ES of Nippostrongylus brasiliensis . Parasite Immunol. 2004;26(3):137‐139. [DOI] [PubMed] [Google Scholar]
- 33. Zhao M, Brown DM, Maccallum J, Proudfoot L. Effect of Nippostrongylus brasiliensis L3 ES on inflammatory mediator gene transcription in lipopolysaccharide lung inflammation. Parasite Immunol. 2009;31(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 34. Filbey KJ, Mehta PH, Meijlink KJ, Pellefigues C, Schmidt AJ, Le Gros G. The gastrointestinal helminth heligmosomoides bakeri suppresses inflammation in a model of contact hypersensitivity. Front Immunol. 2020;11:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blackburn CC, Selkirk ME. Inactivation of platelet‐activating factor by a putative acetylhydrolase from the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunology. 1992;75(1):41‐46. [PMC free article] [PubMed] [Google Scholar]
- 36. Ali F, Brown A, Stanssens P, Timothy LM, Soule HR, Pritchard DI. Vaccination with neutrophil inhibitory factor reduces the fecundity of the hookworm Ancylostoma ceylanicum . Parasite Immunol. 2001;23(5):237‐249. [DOI] [PubMed] [Google Scholar]
- 37. Lo SK, Rahman A, Xu N, et al. Neutrophil inhibitory factor abrogates neutrophil adhesion by blockade of CD11a and CD11b beta(2) integrins. Mol Pharmacol. 1999;56(5):926‐932. [DOI] [PubMed] [Google Scholar]
- 38. Zhou MY, Lo SK, Bergenfeldt M, et al. In vivo expression of neutrophil inhibitory factor via gene transfer prevents lipopolysaccharide‐induced lung neutrophil infiltration and injury by a beta2 integrin‐dependent mechanism. J Clin Invest. 1998;101(11):2427‐2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Logan J, Pearson MS, Manda SS, et al. Comprehensive analysis of the secreted proteome of adult Necator americanus hookworms. PLoS Negl Trop Dis. 2020;14(5):e0008237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anbu KA, Joshi P. Identification of a 55 kDa Haemonchus contortus excretory/secretory glycoprotein as a neutrophil inhibitory factor. Parasite Immunol. 2008;30(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 41. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134‐147. [DOI] [PubMed] [Google Scholar]
- 42. Pritchard DI, Quinnell RJ, Walsh EA. Immunity in humans to Necator americanus: IgE, parasite weight and fecundity. Parasite Immunol. 1995;17(2):71‐75. [DOI] [PubMed] [Google Scholar]
- 43. Quinnell RJ, Slater AF, Tighe P, Walsh EA, Keymer AE, Pritchard DI. Reinfection with hookworm after chemotherapy in Papua New Guinea. Parasitology. 1993;106(Pt 4):379‐385. [DOI] [PubMed] [Google Scholar]
- 44. Kasper G, Brown A, Eberl M, et al. A calreticulin‐like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins. Parasite Immunol. 2001;23(3):141‐152. [DOI] [PubMed] [Google Scholar]
- 45. Suchitra S, Joshi P. Characterization of Haemonchus contortus calreticulin suggests its role in feeding and immune evasion by the parasite. Biochim Et Biophys Acta Gen Subj. 2005;1722(3):293‐303. [DOI] [PubMed] [Google Scholar]
- 46. Winter JA, Davies OR, Brown AP, Garnett MC, Stolnik S, Pritchard DI. The assessment of hookworm calreticulin as a potential vaccine for necatoriasis. Parasite Immunol. 2005;27(4):139‐146. [DOI] [PubMed] [Google Scholar]
- 47. Diaz‐Godinez C, Carrero JC. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci Rep. 2019;39(1):BSR20180916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freudenstein‐Dan A, Gold D, Fishelson Z. Killing of schistosomes by elastase and hydrogen peroxide: implications for leukocyte‐mediated schistosome killing. J Parasitol. 2003;89(6):1129‐1135. [DOI] [PubMed] [Google Scholar]
- 49. Serradell MC, Guasconi L, Masih DT. Involvement of a mitochondrial pathway and key role of hydrogen peroxide during eosinophil apoptosis induced by excretory‐secretory products from Fasciola hepatica . Mol Biochem Parasitol. 2009;163(2):95‐106. [DOI] [PubMed] [Google Scholar]
- 50. Bass DA, Szejda P. Eosinophils versus neutrophils in host defense. J Clin Invest. 1979;64(5):1415‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Milstone AM, Harrison LM, Bungiro RD, Kuzmic P, Cappello M. A broad spectrum Kunitz type serine protease inhibitor secreted by the hookworm Ancylostoma ceylanicum . J Biol Chem. 2000;275(38):29391‐29399. [DOI] [PubMed] [Google Scholar]
- 52. Jin X, Deng L, Li H, et al. Identification and characterization of a serine protease inhibitor with two trypsin inhibitor‐like domains from the human hookworm Ancylostoma duodenale . Parasitol Res. 2011;108(2):287‐295. [DOI] [PubMed] [Google Scholar]
- 53. Chauhan A, Sharma A, Tripathi JK, et al. Helminth derived factors inhibit neutrophil extracellular trap formation and inflammation in bacterial peritonitis. Sci Rep. 2021;11(1):12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cancela M, Paes JA, Moura H, Barr JR, Zaha A, Ferreira HB. Unraveling oxidative stress response in the cestode parasite Echinococcus granulosus . Sci Rep. 2019;9(1):15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Penttila IA, Ey PL, Jenkin CR. Infection of mice with Nematospiroides dubius: demonstration of neutrophil‐mediated immunity in vivo in the presence of antibodies. Immunology. 1984;53(1):147‐154. [PMC free article] [PubMed] [Google Scholar]
- 56. Shuhua X, Hotez PJ, Binggui S, et al. Electron and light microscopy of neutrophil responses in mice vaccinated and challenged with third‐stage infective hookworm (Ancylostoma caninum) larvae. Parasitol Int. 2001;50(4):241‐248. [DOI] [PubMed] [Google Scholar]
- 57. Bonne‐Annee S, Kerepesi LA, Hess JA, et al. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis . Microbes Infect. 2014;16(6):502‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garza JJ, Greiner SP, Bowdridge SA. Ovine vital neutrophil extracellular traps bind and impair Haemonchus contortus L3 in a breed‐dependent manner. Parasite Immunol. 2018;40(9):e12572. [DOI] [PubMed] [Google Scholar]
- 59. Munoz‐Caro T, Rubio RM, Silva LM, et al. Leucocyte‐derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasit Vectors. 2015;8:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ehrens A, Rudiger N, Heepmann L, et al. Eosinophils and neutrophils eliminate migrating strongyloides ratti larvae at the site of infection in the context of extracellular DNA trap formation. Front Immunol. 2021;12:715766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mendez J, Sun D, Tuo W, Xiao Z. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi . Sci Rep. 2018;8(1):17598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Munoz‐Caro T, Conejeros I, Zhou E, et al. Dirofilaria immitis microfilariae and third‐stage larvae induce canine NETosis resulting in different types of neutrophil extracellular traps. Front Immunol. 2018;9:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chapman EA, Lyon M, Simpson D, et al. Caught in a trap? proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front Immunol. 2019;10:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petretto A, Bruschi M, Pratesi F, et al. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS One. 2019;14(7):e0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Bont CM, Koopman WJH, Boelens WC, Pruijn GJM. Stimulus‐dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim Biophys Acta Mol Cell Res. 2018;1865(11):1621‐1629. [DOI] [PubMed] [Google Scholar]
- 66. Kenny EF, Herzig A, Kruger R, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6:e24437. doi: 10.7554/eLife.24437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase‐independent NETosis induced by calcium influx. Proc Natl Acad Sci USA. 2015;112(9):2817‐2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. El‐Naccache DW, Chen F, Chen N, Gause WC. The NET effect of neutrophils during helminth infection. Cell Host Microbe. 2020;27(2):165‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Minns D, Smith KJ, Findlay EG. Orchestration of adaptive T cell responses by neutrophil granule contents. Mediators Inflamm. 2019;2019:8968943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sangaletti S, Tripodo C, Chiodoni C, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120(15):3007‐3018. [DOI] [PubMed] [Google Scholar]
- 71. Toussaint M, Jackson DJ, Swieboda D, et al. Host DNA released by NETosis promotes rhinovirus‐induced type‐2 allergic asthma exacerbation. Nat Med. 2017;23(6):681‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bouchery T, Harris N. Neutrophil‐macrophage cooperation and its impact on tissue repair. Immunol Cell Biol. 2019;97(3):289‐298. doi: 10.1111/imcb.12241 [DOI] [PubMed] [Google Scholar]
- 73. Branzk N, Lubojemska A, Hardison SE, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15(11):1017‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang K, Jiang N, Sang X, Feng Y, Chen R, Chen Q. Trypanosoma brucei lipophosphoglycan induces the formation of neutrophil extracellular traps and reactive oxygen species burst via toll‐like receptor 2, toll‐like receptor 4, and c‐Jun N‐terminal kinase activation. Front Microbiol. 2021;12:713531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zawrotniak M, Bochenska O, Karkowska‐Kuleta J, et al. Aspartic proteases and major cell wall components in Candida albicans trigger the release of neutrophil extracellular traps. Front Cell Infect Microbiol. 2017;7:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Teijeira A, Garasa S, Gato M, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52(5):856‐871. e8. [DOI] [PubMed] [Google Scholar]
- 77. Impellizzieri D, Ridder F, Raeber ME, et al. IL‐4 receptor engagement in human neutrophils impairs their migration and extracellular trap formation. J Allergy Clin Immunol. 2019;144(1):267‐279. e4. [DOI] [PubMed] [Google Scholar]
- 78. Daniel C, Leppkes M, Munoz LE, Schley G, Schett G, Herrmann M. Extracellular DNA traps in inflammation, injury and healing. Nat Rev Nephrol. 2019;15(9):559‐575. [DOI] [PubMed] [Google Scholar]
- 79. Williams TL, Rada B, Tandon E, Gestal MC. NETs and EETs, a whole web of mess. Microorganisms. 2020;8(12):1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ehrens A, Lenz B, Neumann AL, et al. Microfilariae trigger eosinophil extracellular DNA traps in a dectin‐1‐dependent manner. Cell Rep. 2021;34(2):108621. [DOI] [PubMed] [Google Scholar]
- 81. Min B, Prout M, Hu‐Li J, et al. Basophils produce IL‐4 and accumulate in tissues after infection with a Th2‐inducing parasite. J Exp Med. 2004;200(4):507‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Obata‐Ninomiya K, Ishiwata K, Tsutsui H, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med. 2013;210(12):2583‐2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Diemert D, Campbell D, Brelsford J, et al. Controlled human hookworm infection: accelerating human hookworm vaccine development. Open forum. Infect Dis. 2018;5(5):ofy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Osbourn M, Soares DC, Vacca F, et al. HpARI protein secreted by a helminth parasite suppresses interleukin‐33. Immunity. 2017;47(4):739‐51 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vacca F, Chauche C, Jamwal A, et al. A helminth‐derived suppressor of ST2 blocks allergic responses. Elife. 2020;9:e54017. doi: 10.7554/eLife.54017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cho Y, Jones BF, Vermeire JJ, et al. Structural and functional characterization of a secreted hookworm macrophage migration inhibitory factor (MIF) that interacts with the human MIF receptor CD74. Journal of Biological Chemistry. 2007;282(32):23447–23456. doi: 10.1074/jbc.m702950200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Baska P, Wisniewski M, Krzyzowska M, et al. Molecular cloning and characterisation of in vitro immune response against astacin‐like metalloprotease Ace‐MTP‐2 from Ancylostoma ceylanicum . Exp Parasitol. 2013;133(4):472‐482. [DOI] [PubMed] [Google Scholar]
- 88. Mieszczanek J, Harrison LM, Vlasuk GP, Cappello M. Anticoagulant peptides from Ancylostoma caninum are immunologically distinct and localize to separate structures within the adult hookworm. Mol Biochem Parasitol. 2004;133(2):319‐323. [DOI] [PubMed] [Google Scholar]
- 89. Loukas A, Brown AP, Pritchard DI. Na‐ctl‐2, a cDNA encoding a C‐type lectin expressed exclusively in adult Necator americanus hookworms. DNA Seq. 2002;13(1):61‐65. [DOI] [PubMed] [Google Scholar]
- 90. Batra S, Singh SP, Gupta S, Katiyar JC, Srivastava VM. Reactive oxygen intermediates metabolizing enzymes in Ancylostoma ceylanicum and Nippostrongylus brasiliensis . Free Radic Biol Med. 1990;8(3):271‐274. [DOI] [PubMed] [Google Scholar]
- 91. Taiwo FA, Brophy PM, Pritchard DI, Brown A, Wardlaw A, Patterson LH. Cu/Zn superoxide dismutase in excretory‐secretory products of the human hookworm Necator americanus. An electron paramagnetic spectrometry study. Eur J Biochem. 1999;264(2):434‐438. [DOI] [PubMed] [Google Scholar]
- 92. Brophy PM, Patterson LH, Pritchard DL. Secretory nematode SOD–offensive or defensive? Int J Parasitol. 1995;25(7):865‐866. [DOI] [PubMed] [Google Scholar]
- 93. Nguyen JB, Pool CD, Wong CY, et al. Peroxiredoxin‐1 from the human hookworm Ancylostoma ceylanicum forms a stable oxidized decamer and is covalently inhibited by conoidin A. Chem Biol. 2013;20(8):991‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhan B, Gupta R, Wong SP, et al. Molecular cloning and characterization of Ac‐TMP‐2, a tissue inhibitor of metalloproteinase secreted by adult Ancylostoma caninum . Mol Biochem Parasitol. 2008;162(2):142‐148. [DOI] [PubMed] [Google Scholar]
- 95. Cuellar C, Wu W, Mendez S. The hookworm tissue inhibitor of metalloproteases (Ac‐TMP‐1) modifies dendritic cell function and induces generation of CD4 and CD8 suppressor T cells. PLoS Negl Trop Dis. 2009;3(5):e439. doi: 10.1371/journal.pntd.0000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pritchard DI, Leggett KV, Rogan MT, McKean PG, Brown A. Necator americanus secretory acetylcholinesterase and its purification from excretory‐secretory products by affinity chromatography. Parasite Immunol. 1991;13(2):187‐199. [DOI] [PubMed] [Google Scholar]


