Summary
Despite the obesity epidemic, there are relatively few multidisciplinary obesity services in Australia, and only limited data on the effectiveness of these services. The aim of this study was to evaluate the effectiveness of a university hospital‐based weight management clinic—the ‘Healthy Weight Clinic’ in supporting patients to achieve clinically significant weight loss (≥5% reduction in body weight), weight maintenance, and changes in body composition. A retrospective review was conducted to determine weight and associated health outcomes in patients who attended an initial consultation in the first 2 years of the clinic—between March 2017 and March 2019. Follow up was at least 1 year for all patients. Patients who underwent bariatric surgery were excluded. Of 213 total patients, 172 patients attended more than one follow‐up consultation for lifestyle modification. Mean weight change and percentage total weight change at last follow‐up was −6.2 kg (SD 7.4) and − 6.0% (SD 6.9), respectively. For every additional clinic follow‐up, there was 21.4% increased odds of achieving clinically significant weight loss, and for every additional month of follow‐up, there was 10.1% increased odds of achieving clinically significant weight loss. Twenty percent of patients (34/172) maintained ≥5% of initial body weight loss for at least 1 year. Body composition measurements were also favourable, with significant changes in percentage skeletal muscle mass of +0.8% (SD 1.5) and in percentage fat mass by −1.4% (SD 3.2). Regular support in a structured holistic multidisciplinary obesity service enables patients to achieve clinically meaningful weight loss and improved skeletal muscle mass to body fat ratio, and maintain this loss for at least 1 year. Improved weight loss was associated with more patient visits and longer duration of attendance at the clinic.
Keywords: dietetics, endocrinology, exercise physiology, follow‐up, integrated care, interdisciplinary health team, obesity
What is already known
Multidisciplinary interventions can lead to more effective weight loss outcomes for patients.
There is considerable demand from both patients and other health professionals for specialist obesity services.
Publicly funded weight management services across Australia are limited in number and are under‐resourced.
What this study adds
This study reports on the real‐life outcomes of patients attending a privately funded Australian university hospital‐based weight management clinic—the ‘Healthy Weight Clinic’.
Through multidisciplinary care (endocrinology, dietetics and exercise physiology) patients were able to achieve clinically significant weight loss that is comparable to clinics in non‐Australian health systems.
Higher engagement and follow‐up with the clinic were associated with greater weight loss.
1. INTRODUCTION
Overweight or obesity was estimated to affect 67% of Australian adults in 2017–2018. 1 The adverse effects of overweight and obesity on risk of Type 2 diabetes, cardiovascular disease, certain cancers, all‐cause mortality, 2 , 3 and overall health and economic loss 4 , 5 , 6 are well recognized. The risk of these obesity‐related comorbidities and chronic diseases can be reduced, however, by weight loss of 5%–10%. 7 , 8 , 9 A systematic review with meta‐analysis of 34 randomized controlled trials of weight loss interventions for adults with obesity reported an 18% relative reduction in all‐cause mortality (relative risk 0.82, 95% CI: 0.71–0.95), with risk reduction also observed in 16 other RCTs for cardiovascular mortality and major adverse cardiac events. 10
Even when patients achieve clinically significant weight loss, long‐term weight loss maintenance is challenging. A meta‐analysis of 29 weight loss interventions in the United States, with at least a 2‐year follow‐up period, found that over a quarter of lost weight had been regained at 1 year and 80% of lost weight was regained by 5 years. 11 , 12 At the individual level, a multidisciplinary team approach with input from medical, dietetic, exercise, and behavioural experts is recommended for chronic disease management, weight loss, and long‐term weight maintenance. 7 , 8 , 12 , 13 , 14 , 15 , 16 , 17 The multidisciplinary approach allows each team member to deliver regular and ongoing care and support within their specific expertise. This can motivate patients to make small achievable and sustainable changes across lifestyle factors, while enabling better communication and coordination of a consistent patient‐centred care plan. 14
Compared to interventions that only address one behaviour, multicomponent interventions that target the combination of nutrition, physical activity, and behavioural modification have been found to promote greater weight loss. 7 , 18 In addition to their effectiveness, modelling has also shown that among women, multicomponent interventions are also more cost‐effective compared to routine primary care. 19 Multidisciplinary teams also have a central role in providing holistic care to support long‐term weight loss maintenance. 7 , 20
Australian clinical practice guidelines strongly endorse the multidisciplinary approach and estimated the strength of evidence to support this as Grade A. 8 However, it is recognized that there are inadequate obesity services in this country. A 2017 evaluation of multidisciplinary weight management services found only 16 public multidisciplinary weight management clinics in Australia. 21 Most of these facilities were found to be under‐resourced in infrastructure, staffing numbers, access to multidisciplinary specialists, as well as having poor public funding for adjunctive pharmacotherapy and bariatric surgery. Recognizing this health service gap, we established the Healthy Weight Clinic (HWC) at Macquarie University in 2017 to increase access to multidisciplinary services.
This study aimed to evaluate the outcomes of the HWC in supporting clinically significant weight loss and weight maintenance, and body composition changes. The findings contribute data for the effectiveness of a multidisciplinary weight management approach in an Australian academic private health care facility.
2. METHODS
2.1. Study design
This study was part of the Macquarie Health institutional audit and quality review initiative. Approval was granted by the Institutional Human Research Ethics Committee and Clinical Innovation and Audit Committee (CIAC MQCIAC2019002). This was a retrospective chart review of data collected from all patients with BMI ≥25 kg/m2 who attended at least one consultation at the HWC in the 2‐year period March 2017 to March 2019. Patient follow‐up data were collected until July 2020. Data were collected for outcomes from the lifestyle modification cohort of the clinic provided by the endocrinologist, dietitian, and exercise physiologist, including use of adjunctive pharmacotherapies. Patients who underwent bariatric surgery were excluded.
2.2. Study setting and multidisciplinary team consultations
The HWC at Macquarie University, Australia, is a privately funded teaching hospital‐affiliated specialist weight management clinic. The clinic uses a multidisciplinary model, engaging an interdisciplinary health care team of endocrinologists, dietitians, exercise physiologists, and bariatric surgical specialists to target physiology and behaviour change regarding diet and physical activity (Table S1). Operating out of one clinic allows for multi‐specialist collaboration and communication, with the aim of delivering a consistent evidence‐based medicine treatment plan and patient‐centred care.
There are no specific inclusion or exclusion criteria for patients to engage with the HWC services, other than having a BMI ≥25 kg/m2. The typical pathway of care within the HWC and the specific strategies applied by each of the multidisciplinary care members are outlined in Figure S1. All patients consulted with the endocrinologist for medical assessment and management of metabolic and obesity‐related comorbidities. Appropriate referrals were made by the endocrinologist to additional medical specialities (most commonly to sleep and respiratory medicine, cardiology, rheumatology, and bariatric surgery). Adjunctive pharmacotherapies for weight loss were prescribed to patients as required.
The Accredited Practising Dietitian and Accredited Exercise Physiologist, respectively, conducted comprehensive dietary and physical activity assessments with patients, including determining the socioecological factors influencing food and beverage intake, and physical activity. The dietitian also assessed the presence of disordered eating patterns and the patient's relationship with food according to the nutrition care process to facilitate delivery of medical nutrition therapy. The exercise physiologist conducted postural, mobility, and balance assessments to identify movement limitations or specific musculoskeletal issues. Team care members developed a consistent individualized intervention plan in negotiation with the patient. This plan encompassed education and behaviour counselling. This was achieved through motivational interviewing, SMART goal setting, and self‐monitoring using digital technologies such as nutrition apps and a wearable activity monitor. These technologies were used to encourage behaviour change with diet and nutrition and physical activity habits, build self‐efficacy, and facilitate adherence to diet and activity recommendations. 22
Patients were typically reviewed on a four to six weekly basis with at least one of the HWC team. At these reviews, progress with SMART (specific, measurable, achievable, realistic, and time‐bound) goals and anthropometry measures were evaluated. Feedback was provided and adjustments to goals or new goals were developed, with engagement of further behavioural change techniques as necessary.
2.3. Data collection
Anthropometric measures (weight, waist circumference, and body composition) were collected as part of routine measurements taken by the dietitian, exercise physiologist, or endocrinologist at each consultation and recorded in the electronic medical record. Height was measured at baseline using a stadiometer calibrated at installation. Weight was measured with minimum clothing (including emptied pockets and without shoes) on bariatric digital weighing scales. Waist circumference was measured in centimetres at the umbilicus using a flexible plastic tape in the standing position.
Patient body composition data were determined from bioimpedance spectroscopy analysis using the Impedimed SOZO device (Impedimed) to measure fat mass and skeletal muscle mass. Routine body composition measurements began in February 2018 upon availability of the SOZO machine in the clinic. Laboratory tests (fasting blood glucose, HbA1c, insulin resistance, lipid profile total cholesterol, HDL and LDL cholesterol, triglycerides) were also collected as part of routine medical care. Pre‐diabetes was defined as an HbA1c of 5.7%–6.4%, and Type 2 diabetes HbA1c ≥6.5% or a 75‐g oral glucose tolerance test consistent with diabetes, as per the American Diabetes Association guidelines. 23 Data on disordered eating patterns were determined by the dietitian and extracted from the consultation notes and/or transcribed consultation summary letters.
2.4. Statistical analysis
Descriptive statistics were used to calculate patient baseline characteristics as well as changes in BMI classes. Independent t tests for continuous variables and chi‐square tests for categorical variables were used to assess any differences between patients who only attended the initial consultation compared to those who reattended the clinic. Paired t tests were used to compare weight and body composition changes by patients and assess differences between time points. Multiple regression was carried out to assess the relationship between weight loss and the number of patient visits to the clinic and length of clinic follow‐up and binary logistic regression analysis was used to determine the odds of achieving clinically significant weight loss. All statistical analyses were conducting using SPSS version 28 (IBM Corp.).
3. RESULTS
3.1. Baseline characteristics of HWC patients
A total of 239 patients attended the HWC in the 2‐year study period (March 2017–March 2019). Twenty‐six patients underwent bariatric surgery and were excluded from the study. The study cohort thus included 213 individuals who received lifestyle modification (with or without adjunctive pharmacotherapy).
Demographic data for age, initial weight, and BMI are shown in Table 1. During the study and follow‐up period (up until June 2020), 172/213 (80.8%) patients attended the HWC more than once but 41 (19.2%) patients only attended the initial consultation. There were no significant differences in baseline characteristics between these two cohorts. The mean follow‐up period for those who reattended the clinic was 10.8 months (range: 1–39 months; SD 9.6). Of the 172 reattending patients, 140 (81.4%) utilized adjunct pharmacotherapies, (metformin, liraglutide, dulaglutide) as shown in Table 1. Data were collected prior to the availability of other glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) therapies in the Australia (e.g., semaglutide and exenatide).
TABLE 1.
Baseline characteristics of all patients, those who only attended the initial consultation and those who reattended the Healthy Weight Clinic
| Characteristics | All patients (n = 213) | Patients who only attended initial consultation (n = 41) | Patients who reattended the clinic (n = 172) |
|---|---|---|---|
| Gender | |||
| Female | 143 (67.1%) | 29 (70.7%) | 114 (66.3%) |
| Male | 70 (32.9%) | 12 (29.3%) | 58 (33.7%) |
| Mean age (years) | 51.3 (SD 15.0) | 51 (SD 15.1) | 51.8 (SD 14.9) |
| Anthropometric data | |||
| Mean weight (kg) | 104.9 (SD 20.4) | 103.9 (SD 20.4) | 105.1 (SD 20.5) |
| Mean BMI (kg/m2) |
37.3 (SD 6.8) Male: 37.2 (SD 5.8) Female: 37.3 (SD 7.3) |
37.3 (SD 7.7) Male: 34.9 (SD 5.7) Female: 38.3 (SD 8.2) |
37.2 (SD 6.6) Male: 37.7 (SD 5.7) Female: 37.3 (SD 7.3) |
| Mean excess weight (amount of weight over BMI 25 kg/m2) (kg) | 34.3 (SD 18.5) | 33.7 (SD 19.9) | 34.4 (SD 18.2) |
| Mean waist circumference (cm) |
115.5 (SD 15.5) Male: 125.0 (SD 15.8) Female: 110.9 (SD 13.1) |
114.7 (SD 12.3) Male: 117.9 (SD 8.6) Female: 113.3 (SD 13.7) |
115.7 (SD 16.2) Male: 126.5 (SD 16.6) Female: 110.9 (SD 13.1) |
| Smoking status | |||
| Never | 146 (68.5%) | 28 (68.3%) | 118 (68.6%) |
| Quit ≥10 years | 19 (8.9%) | 1 (2.4%) | 18 (10.5%) |
| Quit <10 years | 16 (7.5%) | 1 (2.4%) | 15 (8.7%) |
| Current | 10 (4.7%) | 3 (7.3%) | 7 (4.1%) |
| Unknown | 22 (10.3%) | 8 (19.5%) | 14 (8.1%) |
| Medical history | |||
| Pre‐diabetes | 44 (20.7%) | 3 (7.3%) | 41 (23.8%) |
| Insulin resistance | 44 (20.7%) | 6 (14.6%) | 38 (22.1%) |
| Type 2 diabetes | 27 (12.7%) | 3 (7.3%) | 24 (14.0%) |
| Dyslipidaemia | 83 (39.0%) | 12 (29.3%) | 71 (41.3%) |
| Hypertension | 72 (33.8%) | 14 (34.1%) | 58 (33.7%) |
| Joint and mobility pain | 75 (35.2%) | 12 (29.3%) | 63 (36.6%) |
| Chronic back pain | 36 (16.9%) | 6 (14.6%) | 30 (17.4%) |
| Osteoarthritis | 27 (12.7%) | 4 (9.8%) | 23 (13.4%) |
| Obstructive sleep apnoea | 50 (23.5%) | 11 (26.8%) | 39 (22.7%) |
| Depression | 39 (18.3%) | 7 (17.1%) | 32 (18.6%) |
| Non‐alcoholic fatty liver disease (NAFLD) | 37 (17.4%) | 3 (7.3%) | 34 (19.8%) |
| Ischaemic heart disease | 9 (4.2%) | 2 (4.9%) | 7 (4.1%) |
| Polycystic ovarian syndrome | 31 (14.6%) | 3 (7.3%) | 28 (16.3%) |
| Eating pattern | |||
| Emotional/stress eating | 57 (26.8%) | 14 (34.1%) | 43 (25%) |
| Boredom/mindless eating | 15 (7.0%) | 2 (4.9%) | 13 (7.6%) |
| Meal skipping (no binge style habits) | 23 (10.8%) | 2 (4.9%) | 21 (12.2%) |
| Binge eating (triggered by meal skipping) | 18 (8.5%) | 3 (7.3%) | 15 (8.7%) |
| Binge eating (emotional or stress induced) | 19 (8.9%) | 6 (14.6%) | 13 (7.6%) |
| Laboratory tests | |||
| Mean HbA1c (% and mmol/mol) |
5.80% (SD 1.1) 40 mmol/mol (SD −11.5) |
5.80 (SD 1.4) 40 mmol/mol (SD −8.19) |
5.80% (SD 1.1) 40 mmol/mol (SD −11.5) |
| Mean total cholesterol (mmol/L) | 5.11 (SD 1.17) | 5.50 (SD 1.0) | 5.06 (SD 1.2) |
| Mean high‐density lipoprotein (mmol/L) | 1.29 (SD 0.35) | 1.29 (SD 0.35) | 1.29 (SD 0.4) |
| Mean low‐density lipoprotein (mmol/L) | 3.11 (SD 1.09) | 2.79 (SD 1.0) | 3.17 (SD 1.1) |
| Mean triglycerides (mmol/L) | 1.50 (SD 0.71) | 1.51 (SD 0.64) | 1.50 (SD 0.7) |
| Pharmacotherapy (including in combination) | |||
| Metformin | 154 (72.3%) | 24 (58.5%) | 130 (75.6%) |
| Liraglutide | 69 (32.4%) | 11 (26.8%) | 58 (33.7%) |
| Dulaglutide | 7 (3.3%) | 0 (0%) | 7 (4.1%) |
| Duromine | 26 (12.2%) | 3 (7.3%) | 23 (13.4%) |
| Orlistat | 10 (4.7%) | 0 (0%) | 10 (5.8%) |
| Contrave | 2 (0.9%) | 0 (0%) | 2 (1.2%) |
| Length of clinic follow‐up | |||
| Did not return | 41 (19.2%) | 41 (100%) | 0 (0%) |
| 1–2 months | 42 (19.7%) | N/A | 42 (24.4%) |
| 3–5 months | 30 (14.1%) | N/A | 30 (17.4%) |
| 6–8 months | 18 (8.5%) | N/A | 18 (10.5%) |
| 9–11 months | 21 (9.9%) | N/A | 21 (12.2%) |
| 12–18 months | 20 (9.4%) | N/A | 20 (11.6%) |
| 19–24 months | 22 (10.3%) | N/A | 22 (12.8%) |
| 25–36 months | 18 (8.5%) | N/A | 18 (10.5%) |
| >36 months | 1 (0.5%) | N/A | 1 (0.6%) |
| Number of patient visits to clinic | |||
| Only initial appointment | 41 (19.2%) | 41 (100%) | 0 (0%) |
| 1–4 follow‐up visits | 47 (22.1%) | N/A | 47 (27.3%) |
| 5–9 follow‐up visits | 66 (31.9%) | N/A | 66 (38.4%) |
| 10–14 follow‐up visits | 33 (15.5%) | N/A | 33 (19.2%) |
| 15–19 follow‐up visits | 17 (8.0%) | N/A | 17 (9.9%) |
| More than 20 follow‐up visits | 9 (4.2%) | N/A | 9 (5.2%) |
3.2. Weight and body composition outcomes
Patients who reattended the clinic at least once achieved a mean weight change of −6.2 kg (SD 7.4) at last follow‐up, with a mean change in percentage body weight of −6.0% (SD 6.9) (Table 2). Consistent and significant weight loss from baseline was achieved between 3 to 24 months. Significant changes in weight were only observed between baseline and each time period and not between time points (p > .05).
TABLE 2.
Weight and body composition changes for patients who reattended the clinic (n = 172)
| Outcome | Time point | ||||||
|---|---|---|---|---|---|---|---|
| At last follow up | Baseline to 3 months | 3–6 months | 6–9 months | 9–12 months | 12–24 months | 24–36 months | |
| Weight outcomes | |||||||
| n | 172 | 147 | 111 | 91 | 72 | 30 | 3 |
| Mean weight change, kg (SD) | −6.2 (7.4)* | −4.6 (4.5)* | −1.4 (2.9)* | −1.2 (2.7)* | −0.8 (1.9)* | −0.8 (5.6) | −3.5 (15.2) |
| Mean BMI change, kg/m2 (SD) | −2.3 (2.7)* | −1.7 (1.6)* | −0.5 (1.1)* | −0.5 (1.0)* | −0.3 (0.7)* | −0.3 (2.0) | −1.2 (5.8) |
| Mean percentage weight change, % (SD) | −6.0 (6.9)* | −4.3 (4.0) | −1.3 (2.9)* | −1.3 (2.8) | −0.9 (2.1) | −0.9 (5.5) | −4.2 (19.4) |
| Waist circumference outcome | |||||||
| n | 56 | 53 | 45 | 34 | 23 | 5 | 1 |
| Mean waist circumference change, cm (SD) | −6.9 (7.7)* | −3.9 (5.0)* | −2.2 (3.6)* | −1.3 (2.6)* | −1.2 (2.4)* | −5.1 (4.7) | −1.3 (N/A) |
| Body composition outcomes | |||||||
| n | 59 | 59 | 55 | 43 | 38 | 8 | 0 |
| Mean SMM change, kg (SD) | −0.6 (1.2)* | −0.4 (1.0)* | −0.1 (0.9) | −0.1 (0.9) | −0.2 (0.9) | −0.6 (1.0) | N/A |
| Mean percentage SMM change, % (SD) | 0.8 (1.5)* | 0.5 (1.1)* | 0.4 (1.1)* | 0.2 (1.1) | 0.2 (1.0) | −0.1 (1.1) | N/A |
| Mean FM change, kg (SD) | −3.7 (5.4)* | −2.5 (3.6)* | −1.5 (3.0)* | −0.7 (2.7) | −0.8 (2.2)* | −0.9 (4.0) | N/A |
| Mean percentage FM change, % (SD) | −1.4 (3.2)* | −0.9 (2.6)* | −0.8 (2.4)* | −0.2 (2.5) | −0.4 (2.3) | −0.5 (3.2) | N/A |
Note: *p value <.05 against baseline.
Abbreviations: FM, fat mass; N/A, not applicable; SMM, skeletal muscle mass.
Weight loss of ≥5% of initial body weight was achieved by 51.2% of patients (n = 88/172) and 29.1% (n = 50/172) achieved between 5% and 9.9% weight loss (Table S2). At last follow‐up, seven patients (4.1%) achieved a healthy weight range BMI, and a shift in BMI classes by at least one classification group was achieved by a third of the patient cohort (31.9%; n = 55/172) (see Table S3 and Figure S2).
Consistent significant reductions in waist circumferences were observed against baseline up until 9–12 months, with a waist circumference change at last follow‐up of −6.9 cm (SD 7.7). In those patients who had body composition measurements taken (n = 59), patients had a significant fat mass reduction of 3.7 kg (SD 5.4) at last follow‐up, equivalent to a −1.4% (SD 3.2) reduction in percentage fat mass as a proportion of body weight (Table 2). While absolute muscle mass had decreased by 0.6 kg (SD 1.2), overall proportion of skeletal muscle mass to body weight increased by 0.8% (SD 1.5) muscle mass.
3.3. Weight maintenance
Weight maintenance in the cohort occurred between 15 and 33 months (Figure S3). Further ongoing weight loss out to 36 months is shown but sample size at that time point was small (n = 3) due to the defined study period. Thirty‐four patients (19.8%) lost ≥5% of initial body weight and maintained it for at least 1 year.
3.4. Relationship between engagement with clinic and weight outcomes
Figure 1 is a graphical representation of the percentage total body weight change according to number of follow‐up visits to the clinic. Patients who engaged in 5–9 follow‐up visits with the clinic, achieved a mean weight loss of 5.7%, and a mean weight loss of 10.0% was achieved when patients attended between 10 and 14 follow‐up visits.
FIGURE 1.
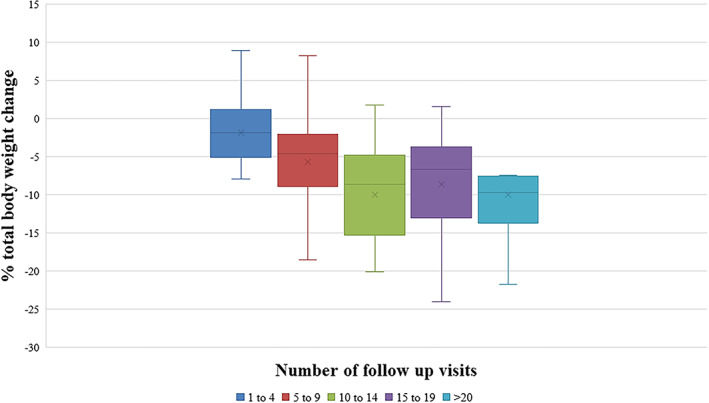
Percentage total body weight change according to the number of follow‐up visits with the clinic
From multiple regression analyses both the number of patient follow‐up visits with HWC clinicians, and the length of clinic follow‐up, significantly predicted percentage weight loss achieved by patients in our cohort (F(7163) = 6.26, p < .001, R 2 = .21). Age, gender, baseline weight, whether patients had a disordered eating pattern, and use of pharmacotherapies were not significantly associated with percentage total weight loss (p > .05).
For every additional clinical encounter and extra month of follow up, there was further significant percentage weight loss achieved: 0.24% (SD 0.21, p = .02) for each additional clinic visit and 0.21% (SD 0.30, p < .001) for each additional month. Moreover, for every additional follow‐up visit with the clinic, there was 21.4% greater odds of being a patient who would achieve clinically significant (≥5%) weight loss (OR = 1.21, 95% CI: 1.12–1.31, p < .001). Attending each additional three follow‐up appointments was associated with a 78.9% increase in the odds of achieving clinically significant weight loss. Similarly, for each additional month of follow up, there was 10.1% greater odds of achieving ≥5% weight loss (OR = 1.10, 95% CI: 1.06–1.15, p < .001), meaning that with every additional 6 months of follow‐up with the clinic, there is a 78.1% increase in the likelihood of achieving clinically significant weight loss.
4. DISCUSSION
This study showed that lifestyle modification, with adjunctive pharmacotherapy when required, at an Australian multidisciplinary weight management clinic resulted in half of patients achieving clinically significant weight loss as well as beneficial changes in body composition that were maintained, for overall health benefits. More patient visits and longer duration of attendance at the clinic were also associated with greater odds of achieving clinically significant weight loss.
The 6.2 kg mean weight loss and 6.0% reduction in percentage body weight reported here are comparable to other international multidisciplinary weight interventions that employed endocrinologists, dietitians, and exercise physiologists along with adjunctive pharmacotherapy. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 These studies reported weight change ranging from −3.5 to −9.5 kg at last follow‐up, and 24% to 51% of participants achieving clinically significant weight loss. Our results are also comparable to other Australian weight management clinics, such as the publicly funded Canberra Obesity Management Services that demonstrated a mean weight change of −6.2 kg with −5% change in mean percentage body weight. 33 Clinically significant weight loss (≥5% of initial body weight) was achieved by a higher proportion of our patients (51.2%) compared to the Canberra clinic (36%).
Our data reinforce the importance of regular and ongoing care in helping patients to achieve meaningful weight loss and can be used to advocate for lifestyle‐based weight management programs delivered in multidisciplinary clinics, with at least three follow‐up visits over a minimum 6‐month period to increase the odds of achieving clinically significant weight loss. These findings are consistent with other behavioural weight loss programs that indicate that greater adherence to attendance is associated with greater weight loss. 34 , 35 Similarly, long‐term weight maintenance could be attributed to expertise from the multidisciplinary HWC team who support and facilitate patient adherence through dietary, physical activity, psychological, behavioural and medical strategies necessary for long‐term weight loss success. 12 , 20 , 36 In particular, this can include building habits of maintaining regularity of eating patterns, low‐caloric low‐fat diets, high levels of physical activity, and self‐monitoring of weight, 36 aspects also addressed by the National Obesity Strategy for all Australians. 37
According to systematic reviews, use of adjunctive pharmacotherapies in additional to behaviour‐based interventions have also been found to support greater weight loss, less weight regain, 38 , 39 and in promoting weight loss maintenance, 40 compared to behavioural interventions alone. Interestingly, pharmacotherapy use was not a significant predictor for weight loss among our patient population. In part, this may have been due to the small proportion (18.6%) who did not utilize pharmacotherapies, but were in an action stage of change.
4.1. Strengths and limitations
This study provides a comprehensive description of real‐life clinical data linked to a well‐defined program structure. This study has been able to monitor weight changes longitudinally to examine trends over time, with successful outcomes for most patients in our data to date. Future studies can examine patient weight loss and maintenance for longer follow‐up periods, such as to 5 years, to support the benefit of privately funded multidisciplinary weight clinics such as this one. Evidence of benefit may help to counter efforts to reduce financial support for clinics like ours, which can be compared unfavourably by finance analysts to services such as those involving procedural specialists. It has been noted that interventions achieving long‐term weight management can also reduce health care use for up to 5 years after the intervention. 41 Attrition rates are common in the clinical setting, likely exacerbated by patient expectations and access issues to clinical services, which has also been previously documented by the Canberra Obesity Management Services. 33
With the recent release of Australia's National Obesity Strategy 2022–2032, 37 the positive outcomes for patient care from our clinic could be used to support the strategy of developing and improving access to integrated models of care and referral pathways in order to prevent and manage unhealthy weight gain and obesity. Our data may be informative for health policy advisors and obesity lobby groups who can use clinical evidence to inform funding for program development.
5. CONCLUSIONS
We have demonstrated that clinically significant weight and body composition (fat mass and muscle mass) improvements are achievable through a multidisciplinary holistic clinic. The likelihood of patients achieving these benefits is associated with number of clinic visits and the number of months that patients continue to regularly attend the clinic. This can be valuable information to discuss with patients, encouraging them to persist with attendance and remain compliant with recommendations in order to achieve the set goals.
CONFLICTS OF INTEREST
Catherine M. Dean declares that she is a member of Macquarie University Council, which is an unpaid role for the governing body of the University. The other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Juliana Chen, Joanna Jaques, and Veronica Preda contributed to study design and application. Data extraction was done by Zoe Rock, Juliana Chen, and Joanna Jaques. Harpreet Kaur and Juliana Chen analysed the data. Juliana Chen interpreted the results and drafted the first version of the manuscript. Zoe Rock, Joanna Jaques, Catherine M. Dean, Reginald V. Lord, and Veronica Preda contributed to interpretation of the results and revising the manuscript. All authors read and approved the final manuscript submitted.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
The authors acknowledge the assistance of Kyrollos Louka in data extraction and Professor Benoit Liquet‐Weiland for his helpful statistical guidance.
Open access publishing facilitated by Macquarie University, as part of the Wiley‐Macquarie University agreement via the Council of Australian University Librarians.
Chen J, Kaur H, Jaques J, et al. Association of clinically significant weight loss with number of patient visits and months of attendance at an Australian multidisciplinary weight management clinic. Clinical Obesity. 2022;12(3):e12520. doi: 10.1111/cob.12520
REFERENCES
- 1. Australian Bureau of Statistics . National Health Survey: First Results, 2017–18. cat. no. 4364.0.55.001. https://www.abs.gov.au/statistics/health/health-conditions-and-risks/national-health-survey-first-results/latest-release.
- 2. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Global BMI Mortality Collaboration , Di Angelantonio E, Bhupathiraju S, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776‐786. doi: 10.1016/s0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Australian Institute of Health and Welfare . Australian burden of disease study: impact and causes of illness and death in Australia 2015. Accessed April 25, 2020. https://www.aihw.gov.au/getmedia/c076f42f-61ea-4348-9c0a-d996353e838f/aihw-bod-22.pdf.aspx?inline=true.
- 5. Crosland P, Ananthapavan J, Davison J, Lambert M, Carter R. The economic cost of preventable disease in Australia: a systematic review of estimates and methods. Aust N Z J Public Health. 2019;43(5):484‐495. doi: 10.1111/1753-6405.12925 [DOI] [PubMed] [Google Scholar]
- 6. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378(9793):815‐825. doi: 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 7. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25_suppl 2):S102‐S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Health and Medical Research Council . Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Accessed April 18, 2020. https://www.nhmrc.gov.au/file/4916/download?token=5RaPGL3n.
- 9. National Institute for Health and Care Excellence . Weight management: lifestyle services for overweight or obese adults. Accessed April 18, 2020. https://www.nice.org.uk/guidance/ph53/resources/weight-management-lifestyle-services-for-overweight-or-obese-adults-pdf-1996416726469.
- 10. Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta‐analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson JW, Konz EC, Frederich RC, Wood CL. Long‐term weight‐loss maintenance: a meta‐analysis of US studies. Am J Clin Nutr. 2001;74(5):579‐584. doi: 10.1093/ajcn/74.5.579 [DOI] [PubMed] [Google Scholar]
- 12. Hall KD, Kahan S. Maintenance of lost weight and long‐term management of obesity. Med Clin North Am. 2018;102(1):183‐197. doi: 10.1016/j.mcna.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zwar N, Hasan I, Hermiz O, Vagholkar S, Comino E, Harris M. Multidisciplinary care plans and diabetes‐‐benefits for patients with poor glycaemic control. Aust Fam Physician. 2008;37(11):960‐962. [PubMed] [Google Scholar]
- 14. Foster D, Sanchez‐Collins S, Cheskin LJ. Multidisciplinary team‐based obesity treatment in patients with diabetes: current practices and the state of the science. Diabetes Spectrum. 2017;30(4):244. doi: 10.2337/ds17-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGill M, Felton A‐M. New global recommendations: a multidisciplinary approach to improving outcomes in diabetes. Prim Care Diabetes. 2007;1(1):49‐55. doi: 10.1016/j.pcd.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 16. Ara S. A literature review of cardiovascular disease management programs in managed care populations. J Managed Care Pharm. 2004;10(4):326‐344. doi: 10.18553/jmcp.2004.10.4.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Department of Health and Ageing . Review of cardiovascular disease programs: final report. London, UK: Ernst & Young. Accessed April 22, 2020. https://www1.health.gov.au/internet/main/publishing.nsf/Content/BF19A2491D59835ECA257BF0001ED86C/$File/review.pdf.
- 18. Kirk SFL, Penney TL, McHugh TL, Sharma AM. Effective weight management practice: a review of the lifestyle intervention evidence. Int J Obes. 2011;36:178. doi: 10.1038/ijo.2011.80 [DOI] [PubMed] [Google Scholar]
- 19. Roux L, Kuntz KM, Donaldson C, Goldie SJ. Economic evaluation of weight loss interventions in overweight and obese women. Obesity. 2006;14(6):1093‐1106. doi: 10.1038/oby.2006.125 [DOI] [PubMed] [Google Scholar]
- 20. Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle GR. Long‐term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37‐46. doi: 10.2147/DMSO.S89836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atlantis E, Kormas N, Samaras K, et al. Clinical obesity services in public hospitals in Australia: a position statement based on expert consensus. Clin Obes. 2018;8(3):203‐210. doi: 10.1111/cob.12249 [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Gemming L, Hanning R, Allman‐Farinelli M. Smartphone apps and the nutrition care process: current perspectives and future considerations. Patient Educ Couns. 2018;101(4):750‐757. doi: 10.1016/j.pec.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 23. American DA. 2. Classification and diagnosis of diabetes: standards of medical Care in diabetes—2021. Diabetes Care. 2021;44(suppl_1):S15‐S33. doi: 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 24. Mottalib A, Tomah S, Hafida S, et al. Intensive multidisciplinary weight management in patients with type 1 diabetes and obesity: a one‐year retrospective matched cohort study. Diabetes Obes Metab. 2019;21(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 25. Venchiarutti RL, Byth K, Marks JL, Chand A, Blumenthal CS. Comparing the effectiveness of general dietary advice versus a very low energy diet in an obese outpatient population in Australia. Eat Weight Disord. 2017;11:1‐9. [DOI] [PubMed] [Google Scholar]
- 26. Jennings A, Hughes CA, Kumaravel B, et al. Evaluation of a multidisciplinary tier 3 weight management service for adults with morbid obesity, or obesity and comorbidities, based in primary care. Clin Obes. 2014;4(5):254‐266. doi: 10.1111/cob.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamga‐Ngande CN, Carpentier AC, Nadeau‐Marcotte F, et al. Effectiveness of a multidisciplinary program for management of obesity: the Unite d'Enseignement, de Traitement et de Recherche sur l'Obesite (UETRO) database study. Research support, non‐U.S. Gov't. Metab Synd Rel Disorders. 2009;7(4):297‐304. [DOI] [PubMed] [Google Scholar]
- 28. Steele T, Narayanan RP, James M, James J, Mazey N, Wilding JPH. Evaluation of Aintree LOSS, a community‐based, multidisciplinary weight management service: outcomes and predictors of engagement. Clin Obes. 2017;7(6):368‐376. [DOI] [PubMed] [Google Scholar]
- 29. Srivastava G, Buffington C. Early weight loss outcomes from a newly established hospital‐affiliated specialized obesity care delivery model in Central Florida. Int J Obes. 2019;43(1):132‐138. doi: 10.1038/s41366-018-0092-3 [DOI] [PubMed] [Google Scholar]
- 30. Wharton S, VanderLelie S, Sharma AM, Sharma S, Kuk JL. Feasibility of an interdisciplinary program for obesity management in Canada. Research support, non‐U.S. Gov't. Can Fam Phys. 2012;58(1):e32‐e38. [PMC free article] [PubMed] [Google Scholar]
- 31. Kuk JL, Wharton S. Differences in weight change trajectory patterns in a publicly funded adult weight management centre. Obes Sci Pract. 2016;2(2):215‐223. doi: 10.1002/osp4.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, Liraglutide, or both combined. N Engl J Med. 2021;384(18):1719‐1730. doi: 10.1056/NEJMoa2028198 [DOI] [PubMed] [Google Scholar]
- 33. Brightman L, Huang H‐CC, Dugdale P. Determining patient attendance, access to interventions and clinical outcomes in a publicly funded obesity programme: results from the Canberra obesity management service. Clin Obes. 2019;9(4):e12325. doi: 10.1111/cob.12325 [DOI] [PubMed] [Google Scholar]
- 34. Acharya SD, Elci OU, Sereika SM, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009;3:151‐160. doi: 10.2147/ppa.s5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piernas C, MacLean F, Aveyard P, et al. Greater attendance at a community weight loss programme over the first 12 weeks predicts weight loss at 2 years. Obes Facts. 2020;13(4):349‐360. doi: 10.1159/000509131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wing RR, Phelan S. Long‐term weight loss maintenance. Am J Clin Nutr. 2005;82(1):222S‐225S. doi: 10.1093/ajcn/82.1.222S [DOI] [PubMed] [Google Scholar]
- 37. Commonwealth of Australia . National Obesity Strategy 2022–2032. Accessed March 15, 2022. https://apo.org.au/sites/default/files/resource-files/2022-03/apo-nid316810.pdf.
- 38. LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O'Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity‐related morbidity and mortality in adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;320(11):1172‐1191. doi: 10.1001/jama.2018.7777 [DOI] [PubMed] [Google Scholar]
- 39. Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24(9):1073‐1079. doi: 10.1007/s11606-009-1042-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs. 2009;24(1):58‐80. doi: 10.1097/01.jcn.0000317471.58048.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krishnaswami A, Sidney S, Sorel M, Smith W, Ashok R. Temporal changes in health care utilization among participants of a medically supervised weight management program. Perm J. 2019;23:18‐134. doi: 10.7812/TPP/18-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


