Summary
Background
Long‐term glucocorticoids (HairGC) measured in scalp hair have been associated with body mass index (BMI), waist circumference (WC), and waist‐hip‐ratio (WHR) in several cross‐sectional studies. We aimed to investigate the magnitude, strength, and clinical relevance of these relations across all ages.
Methods
We performed a systematic review and meta‐analysis (PROSPERO registration CRD42020205187) searching for articles relating HairGC to measures of obesity. Main outcomes were bivariate correlation coefficients and unadjusted simple linear regression coefficients relating hair cortisol (HairF) and hair cortisone (HairE) to BMI, WC, and WHR.
Results
We included k = 146 cohorts (n = 34,342 individuals). HairGC were positively related to all anthropometric measurements. The strongest correlation and largest effect size were seen for HairE‐WC: pooled correlation 0.18 (95%CI 0.11–0.24; k = 7; n = 3,158; I 2 = 45.7%) and pooled regression coefficient 11.0 cm increase in WC per point increase in 10‐log‐transformed HairE (pg/mg) on liquid‐chromatography‐(tandem) mass spectrometry (LC–MS) (95%CI 10.1–11.9 cm; k = 6; n = 3,102). Pooled correlation for HairF‐BMI was 0.10 (95%CI 0.08–0.13; k = 122; n = 26,527; I 2 = 51.2%) and pooled regression coefficient 0.049 kg/m2 per point increase in 10‐log‐transformed HairF (pg/mg) on LC–MS (95%CI 0.045–0.054 kg/m2; k = 26; n = 11,635).
Discussion
There is a consistent positive association between HairGC and BMI, WC, and WHR, most prominently and clinically relevant for HairE‐WC. These findings overall suggest an altered setpoint of the hypothalamic–pituitary–adrenal axis with increasing central adiposity.
Keywords: adiposity, hair glucocorticoids, meta‐analysis, systematic review
1. BACKGROUND
The prevalence of obesity, defined in adults as a body mass index (BMI; weight in kg divided by height in meters squared) ≥ 30 kg/m2, has increased dramatically worldwide over the past decades. 1 An imbalance between energy intake and expenditure is regarded as the major cause of obesity. Numerous distinct characteristics and conditions can contribute to obesity within an individual. 2 One important contributing factor may be chronic exposure to the stress hormone cortisol, the major end‐product of the hypothalamic–pituitary–adrenal (HPA) axis. In healthy individuals, cortisol secretion and metabolism are closely linked and tightly regulated. Cortisol is converted by 11‐beta‐hydroxysteroid dehydrogenase type 2 (11β‐HSD‐2) to the biologically inactive cortisone in end‐organ tissues, but can be converted back to cortisol by 11‐beta‐hydroxysteroid dehydrogenase type 1 (11β‐HSD‐1) on tissue‐level. 3 Exposure to very high levels of endogenous or exogenous glucocorticoids (GC), such as in Cushing's syndrome, leads to a phenotype characterized by abdominal obesity and other features of the metabolic syndrome. 4 , 5 It is hypothesized that even a chronic mild increase of GC, that is, in the high‐physiological range, can contribute to overweight and obesity in the general population. 2 Despite many efforts over the last decades to explore this relation in different matrices such as blood, saliva and urine, conflicting results were found. 6 This may be due to cortisol's circadian rhythm, its pulsatile secretion, and the daily variation following changing circumstances such as acute stress. Hence, measurements that reflect a shorter term (minutes or hours for serum and saliva, days for urine) seem less suitable to investigate this association in the general population. 7
In the past decennium, a relatively novel technique has allowed researchers to study long‐term levels of GC by measuring cortisol and cortisone levels in scalp hair (HairF and HairE, respectively). Every centimeter of scalp hair is believed to represent the cumulative GC exposure of one month. 8 HairGC measurements are now considered an easily applicable, noninvasive and reproducible method for assessing long‐term GC exposure. 8 A systematic review and meta‐analysis by Stalder et al. that was conducted in September 2015 (when the number of studies that used HairGC started to increase rapidly) identified several possible influencers of HairF levels. The authors concluded that variation in HairF levels on study level could be related, among other factors, to differences in mean BMI of the study populations. 9 Gray et al. and Ling et al. also reported that BMI and BMI standard deviation score (SDS), that is, BMI z‐scores adjusted for age and sex that are most often used in pediatric studies, 10 were important determinants of HairF levels in children. 11 , 12 However, in the last years, many new large‐scale studies in various age categories have been published that have investigated the relation between HairGC and anthropometric features. Some of these studies showed a positive relation, 13 , 14 while other studies showed no relation between HairGC and anthropometric measurements. 15 , 16 It is unclear whether these conflicting results can be explained by differing population characteristics such as mean age, sex, and prevalence of obesity, use of corticosteroids, handling of outliers, or the various laboratory methods that were used.
Moreover, other anthropometric measurements than BMI are considered equally or even more relevant to cardiometabolic health, such as waist circumference (WC) and waist‐hip‐ratio (WHR), which both are markers of central adiposity. 17 These deserve specific attention as GC are known to particularly induce abdominal obesity. 18 Likewise, there are suggestions that hair cortisone might correlate stronger to obesity than hair cortisol itself. 19 However, a meta‐analysis that summarizes all evidence considering different anthropometric parameters in association with both HairF and HairE as well as relevant moderators of these relationships is missing.
Therefore, the aim of the current systematic review and meta‐analysis was to investigate the cross‐sectional relations between HairGC levels (HairF and HairE) and anthropometric measurements (BMI, BMI SDS, WC, and WHR) and to explore the possible influence of relevant characteristics of the population and laboratory methods.
2. METHODS
We performed this systematic review and meta‐analysis in concordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement and Meta‐analysis of Observational Studies in Epidemiology (MOOSE) checklist. 20 , 21 This systematic review was registered at the PROSPERO database (Registration number CRD42020205187, December 7, 2020). 22
2.1. Search strategy and selection criteria
A university health sciences librarian designed a comprehensive search to identify studies and conference abstracts concerning hair cortisol and/or hair cortisone and measurements of obesity. To avoid missing potentially relevant papers we designed a broad search strategy combining the elements “hair,” “cortisol/cortisone,” and “BMI/WC/WHR/anthropometrics”, including their synonyms without any restrictions other than “studies in humans”. The search was conducted in the following databases from inception up to November 16, 2020: Medline (Ovid), Embase, Cochrane, Web of Science, Scopus, Cinahl, PsycInfo, and Google Scholar. The complete search strategy is provided in the supporting information Appendix S1. Search results were exported to reference management software (EndNote version X9, Clarivate Analytics), and duplicates were removed prior to screening.
All identified studies were independently screened in two stages by two physicians (EV, OA, or MM) with a background in adult (EV and MM) and pediatric (OA) endocrinology. All studies that reported original HairGC data in humans were included in the title/abstract screening stage and were subsequently assessed full text. Disagreements were solved by discussion among the first authors (EV, OA, and MM), and the senior author (EvR) until consensus was reached. Additionally, reference lists of all included studies and relevant reviews were screened systematically for potentially relevant articles. 23 We included studies that reported cross‐sectional associations between HairGC and measurements of obesity. We excluded case reports, animal studies, review articles, non‐English or nonpeer reviewed studies, and studies in which hair sampling and weight measurements were not performed simultaneously (Figure 1). Pediatric studies that only included children younger than age 2 years were also excluded because BMI‐based definitions of obesity are not available for this age group. 10 We contacted all corresponding authors of articles that reported both HairGC and anthropometric data but did not report an association between these two outcomes to ask if they could provide us with an association measure. Of articles that also included patients with mental or physical diseases that are known to influence the relation between GCs and obesity, we only included the separate analyses of healthy controls if available. When data of the same participants were reported in several studies, we included the study that reported a bivariate association (correlation coefficient or unstandardized simple linear regression coefficient) between HairGC and measurements of obesity. If more than one article reported a bivariate association, we included the study with the largest sample size.
FIGURE 1.
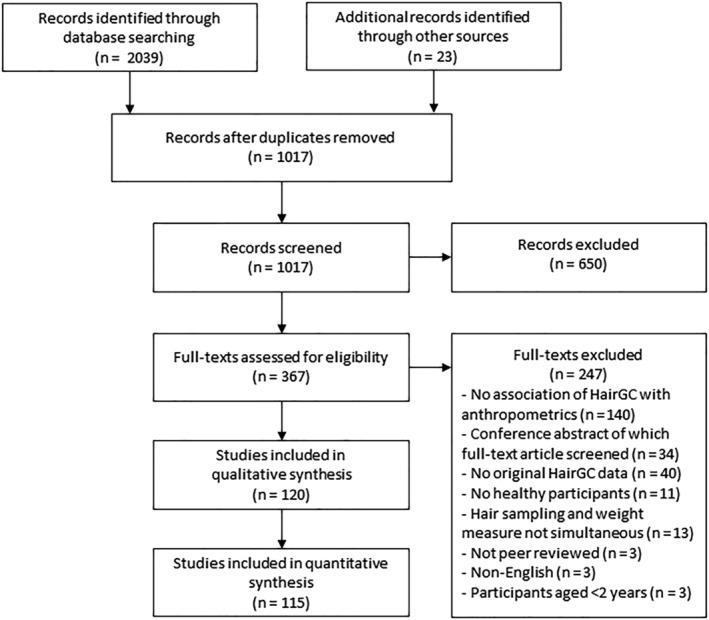
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram. HairGC, hair glucocorticoids
2.2. Data extraction
Descriptive, methodological, and outcome data were extracted from all included studies by two researchers independently (EV, OA, or MM) using a predesigned standardized data extraction sheet. Discrepancies were resolved by discussion among the first authors (EV, OA, and MM) and the senior author (EvR). The following descriptive data were extracted: study population characteristics (sample size and cohort characteristics: age, sex, prevalence of obesity, mean levels of HairF and HairE in pg/mg) and laboratory methods: liquid chromatography‐(tandem) mass spectrometry based measurements (LC–MS or LC–MS/MS, in this review further collectively abbreviated as LC–MS), enzyme‐linked immunosorbent assays (ELISA), or chemiluminescent immunoassays (CLIA). The reported outcomes of interest were any cross‐sectional associations between HairGC (HairF, HairE) and measurements of obesity, that is, BMI, BMI SDS, WC, and WHR. In studies presenting multiple data points of the same participants (e.g., before and after an intervention), only baseline associations were extracted. When insufficient data were reported for meta‐analysis, corresponding authors were contacted twice in a 2‐week time frame. In case of nonresponse, data were extracted from previous meta‐analyses where possible. 9 , 12
2.3. Risk of bias assessment
Risk of bias was assessed by two researchers independently (EV, OA, or MM) using the Quality In Prognostic Studies (QUIPS) tool. 24 In short, the QUIPS tool aids in the assessment of potential bias sources from the following study domains: study participation, study attrition, prognostic factor measurement, outcome measurement, confounding measurement, and statistical analysis. The subdomains on which risk of bias was assessed were the following: population selection criteria (QUIPS 1; study participation), the used laboratory methods (QUIPS 3; prognostic factor measurement), whether or not anthropometric measurements were objectively measured (QUIPS 4; outcome measurement), whether or not corticosteroid use was taken into account and whether any consideration was given to handling outliers in HairGC values (QUIPS 5; study confounding), and reporting of relevant statistics (QUIPS 6; statistical analysis and reporting). All subdomains were scored as ‘low’, ‘moderate’, or ‘high’ risk of bias on individual cohort level. We omitted the study attrition domain of the QUIPS tool (QUIPS 2) since it was not applicable to our cross‐sectional research question. Discrepancies between the researchers were solved by discussion among the first authors (EV, OA, and MM) and the senior author (EvR).
2.4. Qualitative synthesis
For the qualitative synthesis, we summarized all authors' conclusions regarding cross‐sectional associations between HairGC levels and obesity measurements, that is, correlation coefficients, regression coefficients, or comparison of HairGC levels and obesity measurements across categories.
2.5. Statistical analysis
All meta‐analyses were conducted in R version 3.6.3 with an α of 0.05. 25 For all descriptive data, medians and (interquartile) ranges were converted to means and standard deviations prior to analyses. 26 Furthermore, subgroup means from individual studies as well as the pooled means across all studies were pooled. 27 When not originally reported, standard errors were calculated based on reported confidence intervals or p‐values and degrees of freedom using the T‐distribution.
2.6. Meta‐analysis of correlation coefficients
For all studies reporting bivariate correlations (correlation coefficients), Fisher's r‐to‐z transformation was applied to transform individual correlations stratified on all combinations of HairGC (HairF and HairE) and obesity measurements (BMI, BMI SDS, WC, and WHR). As several studies reported correlations within distinct subgroups, we calculated the pooled correlation coefficients, 95% confidence intervals (CIs) and prediction intervals (PIs) using multilevel random effects models. 28 , 29 One study was excluded for all meta‐analyses, as the reported correlation coefficient for BMI versus HairF of the total cohort was 0.91. We assume this is a typographic error, as the authors state that they only found a statistically significant correlation in the highest tertile of the polygenic susceptibility score (which was reported to be 0.269, making a correlation of 0.91 for the total cohort impossible). 30 These authors did not respond to our contact attempts.
The I 2 statistic and Cochrane's Q test were used for the assessment of between‐study heterogeneity, with I 2 > 25% and p‐value for Cochrane's Q test <0.05 indicating heterogeneity. For all meta‐analyses with data from at least 10 cohorts, exploratory moderator analyses were performed using mixed‐effect models for categorical parameters (e.g., used laboratory method) and random effects models for continuous parameters (e.g., mean age of the study participants). Publication bias was assessed using contour‐enhanced funnel plots.
2.7. Meta‐analysis of unstandardized simple linear regression coefficients
For all studies reporting unstandardized simple linear regression coefficients between 10‐log transformed HairGC (HairF or HairE) in pg/mg as independent variable and untransformed obesity measurements (BMI, BMI SDS, WC, and WHR) as dependent variable, pooled regression coefficients and 95% CIs were calculated using the statistical approach described by Bini et al. and Becker & Wu. 31 , 32 In short, this approach allows pooling of linear regression coefficients using weighted least squares provided that the independent and dependent variables have been measured in the same manner across all studies. Therefore, we calculated pooled regression coefficients of 10‐log transformed HairGC on untransformed obesity measurements, stratified on laboratory method. Between‐study heterogeneity was assessed using the Qw‐statistic described by Bini et al. 31
3. RESULTS
The literature search identified 1017 unique citation titles of which a total of 120 studies 5 , 13 , 14 , 16 , 19 , 30 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 comprising 146 separate cohorts were included (Figure 1). This corresponds to a total of 34,342 included participants of which 15,698 (46%) were sampled from general population‐based studies (Table 1). The remaining 18,644 (54%) participants were sampled from studies where study inclusion was based on medical criteria (e.g., individuals with obesity), occupational characteristics (e.g., health‐care workers), or socio‐economic characteristics (e.g., children from low‐income parents). The majority of participants (24,004; 70%) were sampled from studies in adults (mean age ≥18 years). Most studies analyzed participants living in Germany (32/146 cohorts, 22%), The Netherlands (23/146 cohorts, 16%), and Canada (18/146 cohorts, 12%). For 70/146 cohorts (48%), correlation coefficients and/or regression coefficients that were not reported in original papers were obtained by contacting authors.
TABLE 1.
Overview of included cohorts
| Study | n | Age in years | BMI in kg/m2 Or BMI SDS M ± SD | % male | % obesity | HairF in pg/mg | HairE in pg/mg | HairGC analysis | Risk of bias a | Reported bivariate correlations | Reported regression coefficients |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M ± SD | M ± SD | M ± SD | |||||||||
| Adult cohorts | |||||||||||
| Abdulateef et al. (2019) | 65 | 33.1 ± 10.4 | 26.4 ± 5.7 | 9.8 | 28.1 | 17.2 | ELISA | 23311 | AC | AC | |
| Abell et al. (2016) | 3,634 | 69.8 ± 5.8 | 26.7 ± 4.5 | 68.4 | 19.8 | 12.6 ± 46.4 | LC–MS | 21121 | ACD | A | |
| Aguilo et al. (2018) | 53 | 56.7 ± 12.5 | 25.1 ± 3.9 | 30.2 | 9.4 | 14.0 ± 9.0 | ELISA | 23321 | A | A | |
| Berger et al. (2019)—Cohort WPHC | 207 | 40.3 ± 16.9 | 31.5 ± 7.2 | 44.4 | 14.2 ± 27.8 | ELISA | 23331 | A | |||
| Berger et al. (2019)—Cohort YPC | 122 | 19.4 ± 3.1 | 25.2 ± 6.9 | 43.4 | 7.8 ± 9.3 | ELISA | 23331 | A | |||
| Boesch et al. (2014) | 177 | 20.1 ± 1.1 | 23.6 ± 3.1 | 100 | 358.8 ± 159.1 | ELISA | 23131 | A | |||
| Bossé et al. (2018) | 598 | 64.9 ± 6.8 | 29.3 ± 6.5 | 80.6 | 36.9 | 11.9 ± 26.7 | CLIA | 32121 | AC | AC | |
| Brianda et al. (2020) | 134 | 24.6 ± 4.4 | 7.1 | 82.3 ± 94.3 | ELISA | 23311 | A | A | |||
| Castro‐Vale et al. (2020) | 128 | 49.1 ± 15.5 | 27.0 ± 3.9 | 68 | 4.7 ± 3.7 | LC–MS | 21331 | A | A | ||
| Cedillo et al. (2020) | 62 | 29.2 ± 7.5 b | 30.0 ± 7.7 b | 0 | 27.0 | 130.7 ± 124.5 b | ELISA | 23131 | A | ||
| Chan et al. (2014) | 57 | 44.5 ± 12.5 b , c | 27.6 ± 6.8 b , c | 45.6 | 33.3 | 98.8 ± 74.8 b , c | ELISA | 33121 | AC | ||
| Chen et al. (2013) | 53 | 40.7 ± 6.6 | 22.4 ± 2.9 | 98.11 | 18.9 ± 13.6 | LC–MS | 22331 | A | |||
| Chen et al. (2015)—female adults | 75 | 43.3 ± 8.9 | 30.2 ± 5.5 | 0 | 4.6 ± 3.4 | LC–MS | 21121 | AD | |||
| Chen et al. (2015)—male adults | 10 | 41.6 ± 9.2 | 29.4 ± 1.9 | 100 | 3.1 ± 1.5 | LC–MS | 21121 | AD | |||
| Davison et al. (2019) | 344 | 25.4 ± 1.5 b c | 23.7 ± 6.3 | 43 | 4.3 ± 4.9 | 6.3 ± 5.8 | LC–MS | 11121 | AE | ||
| Dettenborn et al. (2010)—employed | 28 | 32.6 ± 9.3 | 22.6 ± 3.8 | 42.9 | 7.1 ± 3.0 | CLIA | 22321 | A | |||
| Dettenborn et al. (2010)—unemployed | 31 | 36.7 ± 11.0 | 24.6 ± 6.3 | 3.2 | 10.2 ± 7.2 | CLIA | 22321 | A | |||
| Diebig et al. (2016) | 129 | 32.3 ± 12.1 | 24.4 ± 4.3 | 24 | 11.6 ± 13.2 | CLIA | 22331 | A | A | ||
| Dowlati et al. (2010)—controls | 87 | 65.7 ± 11.1 | 27.5 ± 4.9 | 80.5 | 185.3 ± 131.6 | ELISA | 33321 | A | |||
| Enge et al. (2020) | 470 | 38.6 ± 8.9 | 24.6 ± 4.9 | 34 | 8.3 | 6.1 ± 7.4 | LC–MS | 11311 | A | A | |
| Engert et al. (2018) | 332 | 40.7 ± 9.2 | 23.6 ± 3.3 | 40.7 | 1.6 ± 1.0 | 2.5 ± 0.7 | LC–MS | 11131 | AE | ||
| Etwel et al. (2014) | 39 | 23.8 ± 6.2 | 23.2 ± 4.8 | 0 | 257.2 ± 101.8 | ELISA | 23121 | A | |||
| Feeney et al. (2020) | 1876 | 66.4 ± 8.7 | 25.6 | 31.5 | 18.8 ± 48.1 | 12.4 ± 10.3 | LC–MS | 11111 | AE | AE | |
| Feller et al. (2014) | 654 | 65.8 ± 8.4 | 27.5 ± 4.4 | 46 | 35.1 ± 32.8 | CLIA | 12111 | ACD | |||
| Fischer et al. (2017) | 139 | 50.6 ± 14.6 | 27.5 ± 6.0 | 28 | 28 | ELISA | 13311 | A | |||
| Gao et al. (2014)—adult control | 23 | 41.5 ± 12.8 | 22.9 ± 2.7 | 61 | 0 | 4.3 ± 3.9 | LC–MS | 22321 | A | ||
| Gao et al. (2014)‐ adult earthquake survivor | 20 | 45.5 ± 14.2 | 23.4 ± 2.1 | 60 | 0 | 46.3 ± 48.4 | LC–MS | 22321 | A | ||
| Garcia‐Leon et al. (2018) | 62 | 33.0 ± 3.7 | 22.8 ± 2.9 | 0 | 127.9 ± 111.5 | ELISA | 13313 | A | |||
| Gidlow et al. (2016) | 132 | 41.4 ± 11.4 | 25.1 ± 4.8 | 28.9 | 10.8 ± 9.4 | ELISA | 13321 | A | A | ||
| Grass et al. (2015)—study I | 42 | 24.8 ± 5.7 | 21.3 ± 2.9 | 52.4 | 3.5 ± 2.3 | LC–MS | 11311 | A | |||
| Grass et al. (2015)—study II | 52 | 25.0 ± 4.9 | 22.8 ± 3.2 | 57.7 | 3.2 ± 3.8 | LC–MS | 11311 | A | |||
| Henley et al. (2014) | 109 | 29.8 | 16.2 | 592.2 ± 304.8 b | ELISA | 13123 | A | A | |||
| Hollenbach et al. (2018) | 59 | 36 ± 6 | 32.2 ± 9.8 | 3.4 | 27.8 ± 30.8 | ELISA | 13321 | A | |||
| Hunter et al. (2020) | 140 | 22.8 ± 6.0 | 27.2 ± 6.6 | 0 | 29 | 11.2 ± 23.5 | LC–MS | 21321 | A | A | |
| Jackson et al. (2017) | 2,527 | 67.9 ± 7.3 | 28.2 ± 5.2 | 41 | 30.5 | 30.5 ± 76.7 | LC–MS | 11131 | |||
| Janssens et al. (2017) | 111 | 43.4 ± 10.4 | 24.4 ± 3.8 | 60 | 10.8 | 14.9 ± 9.4 c | LC–MS | 21111 | AD | AD | |
| Kozik et al. (2015) | 66 | 71.9 ± 5.8 | 25.0 ± 4.0 | 33.3 | 25.8 ± 17.2 | ELISA | 13321 | AC | |||
| Kuehl et al. (2015) | 41 | 41.2 | 23.3 ± 3.6 b | 36.6 | 14.1 | 4.3 ± 4.2 b | 19.8 ± 21.5 b | CLIA | 22121 | ACEF | ACEG |
| Lanfear et al. (2020) | 41 | 68.1 ± 5.3 | 48 | 10.5 ± 13.6 | LC–MS | 11321 | D | ||||
| Larsen et al. (2016)—fathers | 231 | 40.3 ± 5.4 | 26.2 ± 3.7 | 100 | 177.4 ± 119.2 | ELISA | 23331 | A | A | ||
| Larsen et al. (2016)—mothers | 301 | 38.0 ± 4.3 | 26.6 ± 5.4 | 0 | 146.1 ± 102.3 | ELISA | 23331 | A | A | ||
| Lehrer et al. (2020) | 141 | 45.8 ± 15.2 | 32.6 | ELISA | 13122 | D | D | ||||
| Ling et al. (2020)—mothers | 35 | 29.7 ± 5.6 | 32.4 ± 7.0 | 0 | 58.1 | 7.0 ± 8.1 | ELISA | 23111 | A | A | |
| Manenschijn et al. (2013) | 283 | 74.8 ± 7.1 c | 27.4 ± 4.0 c | 33.9 | 23.2 ± 10.1 c | ELISA | 13121 | AC | |||
| Manenschijn, Koper et al. (2011) | 46 | ELISA | 23321 | CD | |||||||
| Mazgelyte et al. (2019) | 163 | 38.5 ± 9.3 | 26.6 ± 5.3 c | 100 | 237.8 ± 160.8 c | LC–MS | 21131 | AC | AC | ||
| McLennan et al. (2016) | 246 | 42.0 ± 11.2 | 26.4 ± 5.3 | 10.2 | 23.8 | 15.1 ± 14.6 | CLIA | 22311 | A | A | |
| Menning et al. (2015)—breast cancer no chemotherapy | 33 | 52.4 ± 7.3 | 24.0 ± 3.8 | 0 | 23.8 ± 16.6 | ELISA | 33331 | A | |||
| Menning et al. (2015)—controls | 38 | 50.1 ± 8.7 | 24.5 ± 3.5 | 0 | 27.0 ± 13.7 | ELISA | 23331 | A | |||
| Menning et al. (2015)—breast cancer chemotherapy | 32 | 50.2 ± 9.2 | 25.8 ± 4.5 | 0 | 33.4 ± 26.2 | ELISA | 33331 | A | |||
| Michaud et al. (2016) | 675 | 52.0 ± 15.2 | 28.2 ± 5.8 | 36.1 | 278.2 ± 553.8 | ELISA | 13331 | A | A | ||
| Mwanza et al. (2016) | 473 | 19.3 ± 1.4 | 31.4 ± 3.8 b | 61.3 | 7 | 11.4 ± 3.9 c | 35.0 ± 15.8 c | LC–MS | 12333 | ||
| Nery et al. (2018) | 16 | 37.5 ± 5.9 | 31.1 ± 6.1 | 0 | 50 | ELISA | 33331 | A | |||
| O'Brien et al. (2013) | 135 | 30.3 ± 12.8 | 35 | 14.5 ± 19.1 | ELISA | 23131 | D | ||||
| Olstad et al. (2016)—women | 70 | 43.4 ± 7.2 | 26.2 ± 6.0 | 0 | 18.6 | 123.7 ± 71.2 | ELISA | 23321 | A | A | |
| Ouellette et al. (2015)—high stress mothers | 30 | 38.2 ± 3.2 | 25.3 ± 6.0 | 0 | 244.6 ± 449.5 | ELISA | 23321 | A | |||
| Ouellette et al. (2015)—low stress mothers | 30 | 37.5 ± 5.2 | 29.9 ± 8.3 | 0 | 126.7 ± 165.4 | ELISA | 23321 | A | |||
| Pickett et al. (2020) | 91 | 24.6 ± 6.5 | 30.1 ± 7.7 | 0 | 42 | 68.0 ± 161.9 | ELISA | 13131 | AC | AC | |
| Pittner et al. (2020)—adults | 171 | 44.5 ± 14.8 | 25.8 ± 4.9 | 25.1 | 19.3 | 3.5 ± 5.7 | 8.6 ± 6.9 | LC–MS | 21321 | AE | AE |
| Pulopulos et al. (2014) | 54 | 64.8 ± 4.2 | 26.3 ± 3.5 b | 24.6 | 11.1 | 2.4 ± 2.2 b | LC–MS | 21111 | A | A | |
| Qi et al. (2014) | 39 | 30.2 ± 6.1 c | 21.5 ± 2.4 | 0 | 24.9 ± 20.0 c | LC–MS | 31311 | A | |||
| Radin et al. (2019) | 166 | 42.4 ± 5.1 | 25.5 ± 5.2 | 0 | 17.1 | 52.9 ± 24.3 b | ELISA | 23121 | ACD | ACD | |
| Saleem et al. (2013)—completers | 56 | 66 ± 11 | 27.3 ± 4.2 | 85.7 | 233.2 ± 173.0 | ELISA | 33111 | A | |||
| Saleem et al. (2013)—noncompleters | 43 | 61 ± 11 | 28.5 ± 5.0 | 70 | 153.5 ± 110.5 | ELISA | 33111 | A | |||
| Schalinski et al. (2015)—healthy controls | 12 | 31.9 ± 7.5 | 22.5 ± 4.1 | 0 | 9.1 | 12.6 ± 11.0 | CLIA | 12321 | A | A | |
| Schalinski et al. (2019)—healthy controls | 75 | 25.4 ± 6.7 | 23.4 ± 3.6 | 54.7 | 5.3 | 7.3 ± 5.4 b | CLIA | 22111 | A | A | |
| Serwinski et al. (2016) | 164 | 43.6 ± 9.8 | 24.1 ± 4.4 | 0 | 10.8 | 8.4 ± 6.3 | LC–MS | 21111 | A | A | |
| Skoluda et al. (2012)—controls | 70 | 36.6 ± 11.5 | 23.0 ± 2.5 | 17.1 | CLIA | 22321 | A | ||||
| Skoluda et al. (2012)—endurance athletes | 304 | 38.3 ± 11.6 | 22.7 ± 2.3 | 41.1 | CLIA | 22321 | A | ||||
| Smith, L. et al. (2019) | 3,741 | 68.4 ± 8.0 | 28.3 ± 5.3 | 33.6 | 26.2 ± 68.8 | LC–MS | 21131 | A | |||
| Stalder et al. (2010)—nonalcoholic controls | 20 | 43.7 ± 11.2 | 26.5 ± 3.6 | 80 | CLIA | 32331 | A | ||||
| Stalder et al. (2013) | 1,258 | 39.6 ± 7.3 c | 27.1 ± 3.5 c | 84.8 | 22.5 ± 11.7 c | 38.5 ± 16.3 c | LC–MS | 21111 | ACDEFG | ||
| Stalder et al. (2014)—caregivers | 20 | 71.2 ± 6.1 | 26.7 ± 3.8 | 5 | CLIA | 22311 | A | ||||
| Stalder et al. (2014)—controls | 20 | 72.2 ± 6.4 | 25.1 ± 3.9 | 15 | CLIA | 12311 | A | ||||
| Stalder, Steudte et al. (2012)—study I | 155 | 24.1 ± 4.2 | 22.2 ± 3.4 | 26.5 | 3.9 | 17.7 ± 10.6 | CLIA | 22311 | A | ||
| Stalder, Steudte et al. (2012)—study II | 58 | 30.5 ± 12.1 | 24.0 ± 4.9 | 32.8 | 12.1 | 21.6 ± 16.0 | CLIA | 22311 | A | ||
| Staufenbiel et al. (2015) | 1,425 | 45.9 ± 13.8 | 28.2 | 3.6 ± 2.5 c | 11.1 ± 5.8 c | LC–MS | 11111 | ACEF | ACEG | ||
| Steudte et al. (2013)—nontraumatized controls | 28 | 37.6 ± 14.1 | 23.4 ± 3.05 | 10.7 | LC–MS | 11311 | A | ||||
| Steudte et al. (2013)—traumatized controls | 25 | 41.7 ± 12.3 | 23.8 ± 3.9 | 8 | LC–MS | 21311 | A | ||||
| Steudte, Kolassa et al. (2011) | 17 | 20.1 ± 5.7 | 21.4 ± 2.3 | 64.7 | CLIA | 22331 | A | ||||
| Steudte, Stalder et al. (2011) | 15 | 35.7 ± 9.3 | 22.9 ± 3.5 | 13.3 | CLIA | 12311 | A | ||||
| Steudte‐Schmiedgen et al. (2015)—nontraumatized soldiers | 129 | 26.2 ± 5.2 | 24.6 ± 2.7 | 100 | LC–MS | 21311 | A | ||||
| Steudte‐Schmiedgen et al. (2017) | 17 | 31.3 ± 9.4 b | 25.4 ± 5.0 | 11.8 | 14.1 ± 16.3 | 19.9 ± 11.3 | LC–MS | 21321 | AE | ||
| Suijker et al. (2018) | 15 | 45.2 ± 15.4 | 24.9 ± 4.7 | 43.8 | 12.5 | 21.7 ± 14.9 | ELISA | 31321 | A | A | |
| Van Aken et al. (2018) | 61 | 34.8 ± 6.7 b | 25.3 ± 4.6 b | 0 | 44.4 ± 36.2 b | ELISA | 23321 | A | |||
| Van den Heuvel, Stalder et al. (2020) | 216 | 43.8 ± 13.3 b | 31.6 ± 8.1 b | 0 | 53.7 | 6.3 ± 5.2 b , c | LC–MS | 21111 | ACD | ACD | |
| Van den Heuvel, Acker et al. (2020) | 164 | 46.5 ± 15.0 | 30.5 ± 7.3 c | 0 | 50.9 | 6.2 ± 6.4 | LC–MS | 11111 | ACD | ACD | |
| Van den Heuvel, Du Plessis et al. (2020) | 56 | 59.6 ± 8.7 | 29.5 ± 5.9 | 0 | 46.4 | 5.0 ± 4.5 b | 8.5 ± 6.2 | LC–MS | 31111 | ACDEFG | ACDEG |
| Van der Valk et al. (2020) | 51 | 40.7 ± 12.6 | 39.7 ± 5.6 | 27.5 | 100 | 5.8 ± 5.3 | 17.8 ± 13.8 | LC–MS | 31121 | ACEF | ACEG |
| Van Holland et al. (2012) | 27 | 46.2 ± 10.6 | 26 ± 4 | 81 | ELISA | 23331 | A | ||||
| Van Manen et al. (2019) | 32 | 47.8 ± 8.5 c | 27.8 ± 4.6 | 43.8 | 28.1 | 10.9 ± 11.7 | 23.9 ± 15.9 | LC–MS | 31121 | ACEF | ACEG |
| Walther et al. (2016) | 271 | 57.1 ± 10.7 | 25.4 ± 3.4 | 100 | 8.0 ± 6.3 | 24.6 ± 16.4 | LC–MS | 11333 | ADEG | ||
| Walton et al. (2013) | 10 | 28 ± 13 | 27.1 ± 3.6 | 30 | ELISA | 33311 | A | ||||
| Wang et al. (2019) | 68 | 32.5 ± 6.1 | 0 | 15 | 6.3 ± 6.5 c | LC–MS | 21323 | A | |||
| Wells et al. (2014) | 324 | 41.9 ± 15.8 | 27.0 ± 6.5 | 28.1 | 24.7 | 274.4 ± 222.0 | ELISA | 23311 | A | A | |
| Wester et al. (2014) | 47 | 45 ± 11.3 c | 23.4 | 100 | ELISA | 33123 | AC | ||||
| Wester et al. (2017) | 295 | 46.8 ± 11.7 c | 25.9 ± 4.3 | 25.4 | 19.32 | LC–MS | 11131 | AC | ACEG | ||
| Wester et al. (2017)—healthy controls | 174 | 36.3 ± 8.4 c | 26.8 ± 4.9 | 42.5 | ELISA | 23331 | A | ||||
| Wu et al. (2019) | 160 | 45.7 ± 9.8 | 26.6 ± 3.1 | 55.4 | 31 | 23.4 ± 30.5 | ELISA | 33121 | A | A | |
| Younge et al. (2015) | 151 | 41.3 ± 14.2 | 25.5 ± 4.9 | 37.1 | ELISA | 33111 | A | A | |||
| Zai et al. (2017) | 248 | 13333 | A | ||||||||
| Zekas et al. (2019) | 81 | 36.5 ± 6.2 | 100 | LC–MS | 21131 | C | |||||
| Pediatric cohorts | |||||||||||
| Bryson et al. (2020) | 297 | 3.1 ± 0.1 | 16.8 ± 1.8 | 39.4 | 22.9 | 8.5 ± 7.8 | ELISA | 23131 | A | A | |
| Chen et al. (2015)—female adolescents | 47 | 15.8 ± 3.1 | 24.3 ± 5.2 | 0 | 3.4 ± 1.9 | LC–MS | 21121 | AD | |||
| Chen et al. (2015)—male adolescents | 32 | 15.0 ± 2.1 | 21.7 ± 4.5 | 100 | 4.0 ± 2.5 | LC–MS | 21121 | AD | |||
| Condon et al. (2019) | 45 | 6.8 ± 2.1 | 0.7 ± 1.2 | 22.2 | 57.3 ± 112.7 | ELISA | 23131 | B | B | ||
| De Kruijff et al. (2020) | 278 | 10.8 ± 4.6 | −0.1 ± 1.0 | 51.1 | 0.8 | 3.1 ± 3.1 | LC–MS | 21311 | AB | AB | |
| Distel et al. (2019) | 52 | 8.4 ± 1.3 | 20.8 ± 4.4 | 39 | 29.3 | 20.6 ± 63.4 | ELISA | 23121 | A | A | |
| Evans et al. (2019) | 92 | 10.1 ± 0.3 | 17.3 ± 2.1 | 34.8 | 3.0 ± 4.5 | 10.1 ± 12.0 | LC–MS | 11121 | AE | A | |
| Föcker et al. (2016) | 20 | 17.3 ± 1.0 | −0.3 ± 1.1 | 0 | 12.6 ± 9.7 | CLIA | 12121 | B | |||
| Frisch et al. (2020) | 18 | 7.4 ± 1.0 | 15.8 ± 2.4 | 44 | 0 | 2.8 ± 2.4 | ELISA | 33321 | A | ||
| Gao et al. (2014)—young male control | 29 | 16.7 ± 0.6 | 21.4 ± 2.4 | 100 | 0 | 13.9 ± 10.9 | LC–MS | 22321 | A | ||
| Gao et al. (2014)—young male earthquake survivor | 20 | 16.8 ± 0.8 | 21.7 ± 2.4 | 100 | 0 | 25.3 ± 17.1 | LC–MS | 22321 | A | ||
| Genitsaridi et al. (2019) | 300 | 10.5 ± 2.6 | 25.7 ± 5.4 b | 25.3 | 46.7 | 8.9 ± 1.0 b | CLIA | 32131 | ACD | ||
| Gerber et al. (2017) | 318 | 7.3 ± 3.5 | 16.3 ± 2.2 | 46.9 | 8 | 12.2 ± 9.7 | CLIA | 12111 | AC | AC | |
| Golub et al. (2019) | 137 | 7.6 ± 0.6 | 16.1 ± 1.8 | 47.5 | 0 | ELISA | 13113 | A | |||
| Grunau et al. (2013)—full term | 42 | 7.8 ± 0.8 | 16.8 ± 3.2 | 35.7 | 416.2 ± 873.0 | ELISA | 33311 | A | |||
| Grunau et al. (2013)—pre‐term | 91 | 7.7 ± 0.3 | 15.7 ± 2.4 | 46.2 | 301.2 ± 560.8 | ELISA | 33311 | A | |||
| Hu et al. (2017) | 1,263 | 8.0 ± 0.8 | 47.3 | 11.8 ± 1.9 b | ELISA | 13123 | A | ||||
| Ilg et al. (2020) | 134 | 12.0 ± 4.0 | 18.6 ± 3.7 | 57 | 3.7 ± 2.3 | 13.4 ± 7.2 | LC–MS | 31323 | AE | ||
| Ince‐Askan et al. (2019) | 117 | 9.8 ± 2.4 b | 0.4 ± 1.1 b | 59.8 | 7.7 | 1.3 ± 1.0 b | 7.4 ± 3.5 b | LC–MS | 21131 | AE | ABCDEFG |
| Kamps et al. (2014) | 10 | 10.5 ± 1.3 | 0.1 ± 1.0 | 50 | 10 | 4.8 ± 4.0 | LC–MS | 11121 | AB | AB | |
| Larsen et al. (2016)—children | 363 | 5.4 ± 1.07 | 16.1 ± 1.2 | 55 | 146.5 ± 179.0 | ELISA | 23131 | A | BCD | ||
| Lehto et al. (2018) | 599 | 4.7 ± 0.9 | 15.9 ± 1.4 | 52 | 2.1 | 41 ± 77 | CLIA | 12131 | C | C | |
| Ling et al. (2020)—children | 35 | 4.7 ± 0.8 | 0.7 ± 1.0 | 51.4 | 20 | 32.0 ± 45.4 | ELISA | 23111 | B | AB | |
| Michels et al. (2017) | 81 | 12.7 ± 1.7 | −0.03 ± 0.9 | 53.6 | 0 | 25 ± 5 | LC–MS | 11121 | A | AB | |
| Murray et al. (2016) | 54 | 9.5 ± 0.3 | 17.6 ± 2.3 | 20.3 | 0 | 3.2 ± 2.9 b | ELISA | 33131 | A | A | |
| Olstad et al. (2016)—children | 30 | 14.3 ± 3.9 | 0.3 ± 1.1 | 56.7 | 10 | 96.6 ± 49.6 | ELISA | 23121 | B | B | |
| Ouellet‐Morin et al. (2016) | 34 | 17 | 26.5 | 33.0 ± 24.5 | ELISA | 13331 | A | A | |||
| Ouellette et al. (2015)—high stress daughters | 30 | 7.5 ± 0.7 | 15.3 ± 2.2 | 0 | 89.9 ± 235.1 | ELISA | 23321 | A | |||
| Ouellette et al. (2015)—low stress daughters | 30 | 7.7 ± 0.7 | 15.6 ± 2.8 | 0 | 104.4 ± 218.3 | ELISA | 23321 | A | |||
| Panter‐Brick et al. (2019) | 203 | 14.4 ± 1.7 | 0.0 ± 1.0 | 56.9 | 5.3 | 9.5 ± 10.0 | ELISA | 23121 | A | A | |
| Papafotiou et al. (2017)—normal weight | 25 | 7.8 ± 1.2 | −0.03 ± 0.6 | 0 | 1.2 ± 0.6 | LC–MS | 11121 | AB | A | ||
| Papafotiou et al. (2017)—obesity | 25 | 7.4 ± 1.3 | 2.9 ± 1.4 | 0 | 100 | 4.1 ± 5.0 | LC–MS | 31121 | A | AB | |
| Petimar et al. (2020)—mid‐childhood | 599 | 7.9 ± 0.8 | 0.3 ± 1.0 | 45.9 | 8.8 | 1.3 ± 1.5 c | LC–MS | 11121 | AC | ABC | |
| Pittner et al. (2020)—children | 61 | 12.4 ± 3.2 | 0.2 ± 1.0 | 42.6 | 3.3 | 1.6 ± 1.5 | 5.7 ± 2.9 | LC–MS | 21321 | BE | BE |
| Pyle Hennessey et al. (2020) | 100 | 5.8 ± 0.3 | 15.6 ± 1.7 | 48 | 3.1 | 6.8 ± 6.9 | ELISA | 23121 | A | A | |
| Schloss et al. (2018) | 75 | 4.6 ± 0.3 | 41.3 | ELISA | 23312 | A | |||||
| Slopen et al. (2018) | 344 | 2.1 ± 0.1 | 17.5 ± 1.7 | 43.2 | 11.1 | 19.0 ± 42.4 | ELISA | 13121 | A | A | |
| Smith, J. et al. (2019) | 114 | 8.5 ± 0.3 | 17.0 ± 2.6 | 42.1 | 4.2 ± 4.1 | ELISA | 23311 | AC | |||
| Sun et al. (2018) | 1,000 | 9.0 ± 0.9 | 18.6 ± 3.2 | 42.1 | 12.0 ± 2.0 c | ELISA | 13131 | A | A | ||
| Van Dammen et al. (2020) | 181 | 15.7 ± 2.0 | 20.3 ± 3.2 | 38.9 | 1.6 | 3.5 ± 2.1 | LC–MS | 12331 | AB | AB | |
| Vehmeijer et al. (2020) | 2042 | 6.1 ± 0.6 | 0.2 ± 0.9 | 47.5 | 3.6 | 1.9 ± 1.4 | 9.6 ± 7.4 | LC–MS | 11121 | AE | A |
| Vepsäläinen et al. (2021) | 565 | 4.8 ± 0.9 | 15.9 ± 1.5 | 37.9 | 2.1 | 40.9 ± 77.1 | CLIA | 12131 | A | A | |
| Wagner et al. (2019) | 434 | 12.0 | 38.5 | 9.9 | LC–MS | 11112 | B | ||||
| White et al. (2017) | 537 | 10.0 ± 3.1 b | 0.0 ± 0.8 | 49.3 | CLIA | 22322 | B | ||||
Note: Reported bivariate correlation/regression coefficient: A, HairF versus BMI; B, HairF versus BMI SDS; C, HairF versus WC; D, HairF versus WHR; E, HairE versus BMI; F, HairE versus WC; G, HairE versus WHR. Significant associations are represented in bold.
Abbreviations: CI, confidence interval; HairF, hair cortisol; HairE, hair cortisone; SDS, standard deviation score; WC, waist circumference; WHR, waist‐to‐hip ratio; LC–MS, liquid chromatography‐(tandem) mass spectrometry; ELISA, enzyme‐linked immunosorbent assay; CLIA, chemiluminescent immunoassay.
Risk of bias: 1, low risk of bias; 2, moderate risk of bias; 3, high risk of bias. Each number represents the assessed QUIPS domains. The following definitions were used for low, moderate of high risk of bias: QUIPS 1 (study participation), 1: population‐based sampling, 2: population selection on nonmedical or social conditions, 3: population selection on medical conditions not evidently related to disturbances of the HPA‐axis; QUIPS 2 (study attrition), not applicable and therefore not scored; QUIPS 3 (prognostic factor measurement), 1: HairGC analysis using LC–MS, 2: HairGC analysis using CLIA, 3: HairGC analysis using ELISA; QUIPS 4 (outcome measurement), 1: anthropometric measurements objectively measured, 2: anthropometric measurements self‐reported; QUIPS 5 (study confounding), 1: both outliers and corticosteroid use taken into account, 2: only outliers or only corticosteroid use taken into account, 3: outliers and corticosteroid use both not taken into account; QUIPS 6 (statistical analysis and reporting), 1: relevant statistics fully reported, 2: relevant statistics partly reported, 3: relevant statistics not reported.
Pooled subgroup means.
Means calculated from either median and interquartile range, or from median and range.
3.1. Description of study characteristics
The weighted mean age of cohorts involving adults (available for n = 23,467) was 53.3 ± 18.4 years and weighted mean BMI (n = 19,653) was 27.0 ± 5.4 kg/m2. For studies involving children, weighted mean age (n = 9904) was 7.8 ± 3.3 years and weighted mean BMI SDS (n = 4108) was 0.2 ± 1.0. Forty‐three of the 146 cohorts (29%) included children (mean age <18 years). The majority of the cohorts had a population that was predominantly female (104 cohorts had >50% females), although the proportion of females within all included subjects was 44%.
Of the 43 pediatric cohorts, two specifically included only children with obesity, 63 , 99 whereas the other 41 cohorts either had no criteria regarding weight status or included only children with normal weight. In adults, 2 of the 103 cohorts exclusively included adults with obesity (BMI ≥ 30 kg/m2), 131 , 141 whereas the other 101 cohorts either had no criteria regarding weight status or included only adults with normal weight or overweight. In 12 of the 103 adult cohorts (12%), the mean BMI of the included population was 30 kg/m2 or higher. Details on the mean BMI of the studies can be found in Table 1.
BMI was the most commonly reported obesity measurement in 138/146 cohorts (95%), followed by WC in 30/146 cohorts (21%), WHR in 20/146 cohorts (14%), and BMI SDS in 16/43 pediatric cohorts (37%).
For 145 cohorts (99%) the used laboratory method was reported, which were ELISA (63/145 cohorts, 43%), LC–MS or LC–MS/MS (56/145 cohorts, 39%), or CLIA (26/145 cohorts, 18%). In all cohorts HairF was reported, whereas HairE was additionally reported in 19/146 cohorts (13%).
Mean crude HairGC concentrations across the studies varied widely with reported means ranging from 1.2–592.2 pg/mg for HairF and 2.45–38.48 pg/mg for HairE. Mean HairF concentrations were higher in studies that used an ELISA (weighted mean 95.6 ± 236.4 pg/mg) compared with studies that used CLIA (24.0 ± 45.1 pg/mg) or LC–MS (mean 13.4 ± 13.4 pg/mg and mean 12.2 ± 39.5 pg/mg in a sensitivity analysis without Mazgelyte et al., 86 which was a significant outlier in mean HairF level). All HairE analyses except for one 78 were performed using LC–MS. In the studies that reported both HairE and HairF concentrations, HairE levels in most cases were higher than HairF levels (Table 1).
3.2. Risk of bias
Risks of bias assessments on cohort level are presented in Table 1. With respect to the selection of the population domain (QUIPS 1), 25 (17%) cohorts had a high, 75 (52%) medium, and 46 (31%) low risk of bias. Regarding the prognostic factor (HairGC) measurement domain (QUIPS 3), 65 (45%) cohorts had a high, 31 (21%) medium, and 50 (34%) low risk of bias. For the outcome measurement domain (QUIPS 4), 75 (51%) cohorts had a moderate and 71 (49%) a low risk of bias. In the domain of accounting for possible confounders (QUIPS 5), 37 (25%) cohorts had a high, 64 (44%) medium, and 45 (31%) low risk of bias. With regard to the statistical domain (QUIPS 6), 10 (7%) cohorts had a high, 4 (3%) medium, and 132 (89%) low risk of bias.
3.3. Qualitative synthesis
An overview of all outcomes reporting any relation between HairGC and obesity measurements is shown in the supporting information Table S1.
3.4. Quantitative synthesis
3.4.1. Meta‐analysis of correlation coefficients
In total, 140/146 cohorts (96%) from 115 unique studies were included in the meta‐analyses of correlations, comprising data of 28,830 participants. The pooled correlation coefficients ranged from 0.10–0.18 (all p < 0.0001). The strongest pooled correlation was found for HairE versus WC (pooled r = 0.18; Table 2 and supporting information Figures S1–S6). Meta‐regressions and subgroup analyses were possible for the associations between HairF versus BMI, BMI SDS, WC, and WHR and HairE versus BMI. In subgroup analyses, neither applied laboratory methods nor population‐based sampling moderated the correlations between HairGC and obesity measurements (all p‐values >0.05, Table 3). Subgroup analyses on all QUIPS domains showed no moderation by risk of bias categories except for QUIPS domain 4 (assessment of outcome, that is, self‐reported BMI vs. measured): studies with self‐reported BMI showed stronger correlations with HairF than studies with measured BMI (pooled r of 0.15 vs. 0.07, respectively; Q = 14.34, p < 0.0001).
TABLE 2.
Pooled correlation coefficients
| k cohorts | n participants | Pooled r | 95% CI | 95% PI | P‐value | Between‐study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| I 2 (%) | Q | P‐value | |||||||
| HairF versus BMI | 122 | 26,527 | 0.10 | 0.08; 0.13 | −0.04; 0.24 | <0.0001 | 51.2 | 221.4 | <0.0001 |
| HairF versus BMI SDS | 11 | 1,247 | 0.12 | 0.06; 0.18 | 0.06; 0.18 | <0.0001 | 0.0 | 11.8 | 0.30 |
| HairF versus WC | 24 | 11,006 | 0.11 | 0.07; 0.15 | −0.03; 0.26 | <0.0001 | 68.3 | 59.7 | <0.0001 |
| HairF versus WHR | 16 | 6,786 | 0.11 | 0.07; 0.15 | 0.03; 0.19 | <0.0001 | 28.4 | 22.3 | 0.10 |
| HairE versus BMI | 16 | 8,210 | 0.11 | 0.07; 0.15 | 0.00; 0.21 | <0.0001 | 52.7 | 31.0 | 0.01 |
| HairE versus WC | 7 | 3,158 | 0.18 | 0.11; 0.24 | 0.06; 0.29 | <0.0001 | 45.7 | 9.6 | 0.14 |
| HairE versus WHR | 2 | 1,314 | NA a | NA | NA | NA | NA | NA | NA |
Abbreviations: CI, confidence interval; HairF, hair cortisol; HairE, hair cortisone; NA, not applicable; PI, prediction interval; SDS, standard deviation score; WC, waist circumference; WHR, waist‐to‐hip ratio.
Meta‐analysis not performed due to small number of cohorts.
TABLE 3.
Results of subgroup analyses in the meta‐analyses of correlation coefficients
| Moderator | k cohorts | I 2 (%) | Pooled r | 95% CI | Q between | P‐value | |
|---|---|---|---|---|---|---|---|
| HairF versus BMI | QUIPS 1: Study participation (population‐based sampling) | 0.34 | 0.55 | ||||
| Yes | 34 | 51 | 0.10 | 0.07; 0.13 | |||
| No | 88 | 51 | 0.11 | 0.08; 0.14 | |||
| QUIPS 3: Prognostic factor measurement (HairGC analysis method) | 0.05 | 0.98 | |||||
| LC–MS | 47 | 51 | 0.10 | 0.07; 0.14 | |||
| ELISA | 52 | 35 | 0.10 | 0.07; 0.14 | |||
| CLIA | 21 | 66 | 0.11 | 0.05; 0.17 | |||
| QUIPS 4: Outcome (anthropometric) measurement | 14.34 | <0.001 | |||||
| Self‐reported | 67 | 22 | 0.15 | 0.12; 0.18 | |||
| Objectively measured | 55 | 62 | 0.07 | 0.04; 0.10 | |||
| QUIPS 5: Study confounding | 2.74 | 0.43 | |||||
| CS use and outliers handled | 39 | 62 | 0.13 | 0.09; 0.17 | |||
| Only outliers handled | 22 | 29 | 0.09 | 0.05; 0.13 | |||
| Only CS use handled | 33 | 30 | 0.10 | 0.06; 0.15 | |||
| Neither handled | 28 | 51 | 0.08 | 0.03; 0.12 | |||
| QUIPS 6: Statistical analysis (Relevant statistics fully reported) | 0.01 | 0.93 | |||||
| Yes | 118 | 50 | 0.10 | 0.08; 0.13 | |||
| No | 4 | 65 | 0.10 | −0.04; 0.23 | |||
| HairF versus BMI SDS | QUIPS 1: Study participation (population‐based sampling) | 0.12 | 0.73 | ||||
| Yes | 4 | 0 | 0.14 | 0.01; 0.27 | |||
| No | 7 | 0 | 0.12 | 0.05; 0.18 | |||
| QUIPS 3: Prognostic factor measurement (HairGC analysis method) | 0.63 | 0.73 | |||||
| LC–MS | 6 | 70.7 | 0.06 | −0.13; 0.25 | |||
| ELISA | 3 | 0 | 0.07 | −0.13; 0.26 | |||
| CLIA | 2 | 0 | 0.13 | 0.04; 0.21 | |||
| QUIPS 4: Outcome (anthropometric) measurement | 2.11 | 0.15 | |||||
| Self‐reported | 4 | 0 | 0.14 | 0.08; 0.20 | |||
| Objectively measured | 7 | 32.1 | −0.01 | −0.19; 0.18 | |||
| QUIPS 5: Study confounding | 0.86 | 0.83 | |||||
| Both handled | 2 | 0 | 0.13 | 0.03; 0.24 | |||
| Only outliers handled | 2 | 0 | 0.13 | 0.04; 0.21 | |||
| Only CS use handled | 5 | 60.8 | −0.01 | −0.31; 0.28 | |||
| Neither handled | 2 | 0 | 0.13 | 0.00; 0.26 | |||
| HairF versus WC | QUIPS 1: Study participation (population‐based sampling) | 3.95 | 0.05 | ||||
| Yes | 9 | 65 | 0.07 | 0.02; 0.13 | |||
| No | 15 | 60 | 0.15 | 0.09; 0.20 | |||
| QUIPS 3: Prognostic factor measurement (HairGC analysis method) | 0.17 | 0.92 | |||||
| LC–MS | 12 | 78 | 0.11 | 0.05; 0.18 | |||
| ELISA | 7 | 4 | 0.10 | 0.02; 0.17 | |||
| CLIA | 5 | 77 | 0.11 | 0.02; 0.21 | |||
| QUIPS 4: Outcome (anthropometric) measurement | 0.67 | 0.41 | |||||
| Self‐reported | 3 | 40 | 0.18 | 0.01; 0.35 | |||
| Objectively measured | 21 | 71 | 0.11 | 0.06; 0.15 | |||
| QUIPS 5: Study confounding | 5.90 | 0.12 | |||||
| Both handled | 9 | 68 | 0.08 | 0.02; 0.15 | |||
| Only outliers handled | 3 | 0 | 0.16 | 0.13; 0.19 | |||
| Only CS use handled | 7 | 33 | 0.13 | 0.05; 0.21 | |||
| Neither handled | 5 | 77 | 0.10 | −0.03; 0.23 | |||
| HairF versus WHR | QUIPS 1: Study participation (population‐based sampling) | 0.56 | 0.46 | ||||
| Yes | 4 | 57 | 0.15 | 0.03; 0.26 | |||
| No | 12 | 36 | 0.10 | 0.04; 0.15 | |||
| QUIPS 3: Prognostic factor measurement (HairGC analysis method) | 0.34 | 0.56 | |||||
| LC–MS | 11 | 33 | 0.10 | 0.05; 0.15 | |||
| ELISA | 4 | 76 | 0.16 | −0.03; 0.34 | |||
| QUIPS 4: Outcome (anthropometric) measurement | 5.79 | 0.02 | |||||
| Self‐reported | 2 | 0 | 0.36 | 0.16; 0.53 | |||
| Objectively measured | 14 | 36 | 0.10 | 0.06; 0.14 | |||
| QUIPS 5: Study confounding | 2.85 | 0.24 | |||||
| Both handled | 6 | 53 | 0.09 | 0.02; 0.15 | |||
| Only outliers handled | 5 | 0 | 0.13 | 0.10; 0.16 | |||
| Only CS use handled | 4 | 57 | 0.23 | 0.06; 0.38 | |||
| Neither handled | 1 | NA | NA | NA | |||
| HairE versus BMI | QUIPS 1: Study participation (population‐based sampling) | 0.02 | 0.89 | ||||
| Yes | 6 | 40 | 0.11 | 0.07; 0.15 | |||
| No | 9 | 46 | 0.12 | 0.02; 0.21 | |||
| QUIPS 4: Outcome (anthropometric) measurement | 0.24 | 0.62 | |||||
| Self‐reported | 3 | 78 | 0.22 | −0.20; 0.57 | |||
| Objectively measured | 12 | 59 | 0.12 | 0.07; 0.16 | |||
| QUIPS 5: Study confounding | 8.08 | 0.04 | |||||
| Both handled | 4 | 55 | 0.16 | 0.11; 0.21 | |||
| Only outliers handled | 4 | 0 | 0.07 | 0.04; 0.11 | |||
| Only CS use handled | 5 | 0 | 0.09 | −0.02; 0.20 | |||
| Neither handled | 2 | 61 | 0.05 | −0.12; 0.21 | |||
Note: Subgroup analyses were only performed when data of at least 2 cohorts were available within a subgroup and 10 cohorts across all subgroups. Bold text indicates statistically significant effect (P‐value < 0.05).
Abbreviations: CI, confidence interval; HairF, hair cortisol; SDS, standard deviation score; WC, waist circumference; WHR, waist‐to‐hip ratio; LC–MS, liquid chromatography‐(tandem) mass spectrometry; ELISA, enzyme‐linked immunosorbent assay; CLIA, chemiluminescent immunoassay.
In meta‐regressions, we found that studies that included larger proportions of males showed stronger correlations between HairF and WC (estimated slope 0.0022 per percentage point increase in proportion of males, 95% CI 0.0010 to 0.0033, p = 0.0002) and HairF and WHR (estimated slope 0.0011 per percentage point increase in proportion of males, 95% CI 0.0001 to 0.0021, p = 0.02; Table 4 and supporting information Figures S7 and S8). Furthermore, studies including more participants with obesity showed weaker correlations between HairF and BMI (estimated slope −0.0029 per percentage point increase in proportion of participants with obesity, 95% CI −0.0049 to −0.0010, p = 0.0028), and studies with higher BMI SDS showed weaker correlations between HairF and BMI SDS (Table 4 and supporting information Figure S9). Mean age and mean HairF concentration of the study population did not moderate the correlations between HairGC and obesity measurements (all p‐values >0.05, Table 4). In contrast, higher mean HairE was associated with stronger positive correlations (estimated slope 0.0046 per point increase in mean HairE on study level, 95% CI 0.0025–0.0068, p < 0.0001). Visual inspection of the funnel plots showed no evidence for publication bias; that is, no systematic trends were found between standard error (as proxy for study sample size) and magnitude and direction of the reported correlation coefficients (supporting information Figures S10–S15).
TABLE 4.
Results of meta‐regressions in the meta‐analyses of correlation coefficients
| Moderator | k cohorts | % Between‐study heterogeneity explained | Estimate (slope) | 95% CI | Q m | P‐value | |
|---|---|---|---|---|---|---|---|
| HairF versus BMI | Mean age | 120 | 0.3 | 0.0006 | −0.0005; 0.0017 | 1.32 | 0.25 |
| Mean BMI | 113 | 0.7 | 0.0003 | −0.0050; 0.0057 | 0.01 | 0.90 | |
| Adults only | 84 | 0.7 | −0.0082 | −0.0180; 0.0016 | 2.70 | 0.10 | |
| Mean HairF | 115 | 0.002 | 0.0000 | −0.0002; 0.0003 | 0.10 | 0.76 | |
| LC–MS | 44 | 2.1 | 0.0008 | −0.0042; 0.0057 | 0.09 | 0.76 | |
| CLIA | 23 | 7.4 | −0.0025 | −0.0092; 0.0041 | 0.55 | 0.46 | |
| ELISA | 47 | 0.03 | 0.0000 | −0.0003; 0.0003 | 0.02 | 0.88 | |
| % obesity | 57 | 11.9 | −0.0029 | −0.0049; −0.0010 | 8.95 | 0.0028 | |
| % males | 122 | 2.5 | 0.0003 | −0.0006; 0.0011 | 0.38 | 0.54 | |
| HairF versus BMI SDS | Mean age | 11 | 11.0 | 0.0127 | −0.0091; 0.0344 | 1.30 | 0.25 |
| % males | 10 | 18.6 | 0.0037 | −0.0012; 0.0087 | 2.18 | 0.14 | |
| Mean BMI SDS | 10 | 86.4 | −0.2108 | −0.3408; −0.0807 | 10.09 | 0.0015 | |
| Mean HairF | 10 | 1.03 | −0.0006 | −0.0040; 0.0028 | 0.12 | 0.73 | |
| HairF versus WC | Mean age | 23 | 21.9 | 0.0011 | −0.0007; 0.0028 | 1.46 | 0.23 |
| Mean BMI | 20 | 9.3 | 0.0013 | −0.0081; 0.0106 | 0.07 | 0.79 | |
| Adults only | 17 | 18.3 | −0.0080 | −0.0267; 0.0108 | 0.69 | 0.41 | |
| Mean HairF | 21 | 0.002 | 0.0003 | −0.0006; 0.0012 | 0.46 | 0.50 | |
| % obesity | 16 | 0.03 | −0.0002 | −0.0030; 0.0027 | 0.02 | 0.89 | |
| % males | 23 | 39.5 | 0.0022 | 0.0010; 0.0033 | 14.29 | 0.0002 | |
| HairF versus WHR | Mean age | 15 | 10.7 | 0.0024 | −0.0006; 0.0055 | 2.10 | 0.12 |
| Mean BMI | 12 | 13.3 | −0.0120 | −0.0315; 0.0074 | 1.47 | 0.23 | |
| Adults only | 10 | 54.4 | −0.0170 | −0.0377; 0.0037 | 2.59 | 0.11 | |
| Mean HairF | 14 | 4.0 | 0.0014 | −0.0020; 0.0047 | 0.65 | 0.42 | |
| LC–MS | 11 | 25.2 | 0.0056 | −0.0013; 0.0126 | 2.53 | 0.11 | |
| % males | 15 | 28.7 | 0.0011 | 0.0001; 0.0021 | 5.07 | 0.02 | |
| HairE versus BMI | Mean age | 15 | 27.9 | 0.0016 | −0.0004; 0.0035 | 2.56 | 0.11 |
| Mean BMI | 12 | 47.2 | 0.0096 | −0.0006; 0.0197 | 3.41 | 0.07 | |
| Mean HairE | 13 | 65.2 | 0.0046 | 0.0025; 0.0068 | 17.96 | <.0001 | |
| % males | 15 | 12.2 | 0.0010 | −0.0010; 0.0030 | 0.99 | 0.32 |
Note: Meta‐regressions were only performed when data of at least 10 cohorts were available. Bold text indicates statistically significant effect (P‐value < 0.05).
Abbreviations: CI, confidence interval; Qm, Cochrane's Q for the moderator; HairF, hair cortisol; SDS, standard deviation score; WC, waist circumference; WHR, waist‐to‐hip ratio; LC–MS, liquid chromatography‐(tandem) mass spectrometry; ELISA, enzyme‐linked immunosorbent assay; CLIA, chemiluminescent immunoassay; NA, not available or not applicable.
3.4.2. Meta‐analysis of regression coefficients
The pooled regression coefficients stratified on analysis method are presented in Table 5. The pooled regression coefficient for 10‐log transformed HairF as independent variable on BMI as dependent variable measured for LC–MS‐based measurements was based on the largest number of cohorts (k = 26 cohorts comprising 11,635 individuals). The pooled regression coefficient for LC–MS‐based measurements was 0.049 kg/m2 (95% CI 0.045–0.054; Table 5). This indicates that for LC–MS‐based measurements, 1 point increase in 10‐log HairF was associated with 0.049 kg/m2 higher BMI. One point increase in 10‐log HairE was associated with 1.15 kg/m2 higher BMI (95% CI 0.987–1.310 kg/m2). The highest pooled regression coefficient was found for HairE on dependent variable WC, where 1 point increase in 10‐log HairE was associated with 11.0 cm larger WC (95% CI 10.1–11.9 cm) on LC–MS. There was no significant between‐study heterogeneity (all p‐values >0.05, Table 5).
TABLE 5.
Pooled regression coefficients
| k cohorts | n participants | Analysis method | Pooled beta | 95% CI | Between‐study heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Q w | P‐value | ||||||
| HairF independent—BMI dependent | 8 | 1,984 | CLIA | 0.02 | 0.016; 0.03 | 0.26 | >0.05 |
| 26 | 11,635 | LC–MS | 0.05 | 0.045; 0.054 | 0.50 | >0.05 | |
| HairF independent—BMI SDS dependent | ‐ | ‐ | CLIA | ‐ | ‐ | ||
| 6 | 998 | LC–MS | 0.20 | 0.14; 0.27 | 0.11 | >0.05 | |
| HairF independent—WC dependent | 4 | 1,556 | CLIA | 0.02 | 0.02; 0.03 | 0.13 | >0.05 |
| 10 | 4,259 | LC–MS | 1.26 | 1.08; 1.44 | 0.15 | >0.05 | |
| HairF independent—WHR dependent | ‐ | ‐ | CLIA | ‐ | ‐ | ||
| 5 | 1,805 | LC–MS | −0.01 | −0.01; −0.00 | 0.00 | >0.05 | |
| HairE independent—BMI dependent | CLIA | ‐ | ‐ | ||||
| 9 | 5,266 | LC–MS | 1.15 | 0.98; 1.31 | 0.08 | >0.05 | |
| HairE independent—WC dependent | CLIA | ‐ | ‐ | ||||
| 6 | 3,102 | LC–MS | 11.0 | 10.1; 11.9 | 0.05 | >0.05 | |
Note: ‐, meta‐analysis not performed due to insufficient number of cohorts. Bold text indicates statistically significant effect (P‐value < 0.05).
Abbreviations: CI, confidence interval; HairF, hair cortisol; HairE, hair cortisone; SDS, standard deviation score; WC, waist circumference; WHR, waist‐to‐hip ratio; LC–MS, liquid chromatography‐(tandem) mass spectrometry; CLIA, chemiluminescent immunoassay.
4. DISCUSSION
In the current systematic review including 34,342 unique subjects, HairGC levels showed a significant positive relation with anthropometric measurements. In the meta‐analyses, pooled correlation coefficients ranged between 0.10 for hair cortisol versus BMI and 0.18 for hair cortisone versus WC. The largest effect size was found for the relation between hair cortisone and WC: one point increase in 10‐log‐transformed hair cortisone concentration (e.g., an increase from 1 pg/mg to 10 pg/mg) on LC–MS‐based assays was associated with 11 cm larger WC. For the outcome BMI, an increase of 1.15 kg/m2 per one point increase in 10‐log transformed hair cortisone on LC–MS‐based assays was found. Moderator analysis in the meta‐analyses of correlation coefficients showed that a higher percentage of male participants was associated with stronger correlations in the relations between hair cortisol versus WC and hair cortisol versus WHR. A higher percentage of participants with obesity of the included cohorts was associated with less strong correlations in the relation hair cortisol versus BMI. Interestingly, no evidence was found for a moderating influence on study level of other important covariates that are known to influence either HairGC or obesity measurements in individual persons, namely age, laboratory methods, and handling of outliers and exogenous corticosteroid use.
In the largest of our meta‐analyses, for HairF versus BMI (n = 26,527 participants), we confirmed the modest positive relations in exploratory analyses of Stalder et al. and Ling et al. between HairF and BMI/BMI SDS. 9 , 12 Evidently, there is a relation between measures of obesity and long‐term glucocorticoid levels, a relation that has been controversial for measurement of GC levels in other matrices that reflect shorter time periods. 6 As GC are known to contribute to central adiposity, for example, in Cushing's syndrome, it might be possible that in the study of a gradually developing disease such as obesity, long‐term GC measurements offer a different and perhaps more appropriate perspective to the role of the HPA‐axis.
The current study indicates that this relation is strongest (i.e., the highest correlation coefficient and the largest effect size) for cortisone, the inactive form of cortisol, and WC. Although the pooled correlation coefficients and pooled regression coefficients for the most frequently studied outcome HairF versus BMI were statistically significant (pooled correlation coefficient 0.10, pooled regression coefficient 0.049 kg/m2 increase in BMI per 1 point increase in 10‐log transformed HairF on LC–MS), the small effect size here seems to have less clinical relevance compared with the large effect size we found for the relation HairE versus WC. We believe that the consistency of our findings across all studied outcomes is indicative of an altered setpoint of the HPA‐axis in obesity. This may induce or aggravate obesity, although causality cannot be proven by our study because of its limitation to cross‐sectional associations. Yet, the fact that HairGC apparently relate strongly to measures of abdominal obesity matches the paradigm that chronic exposure to higher levels of GCs specifically induce abdominal obesity. 18 Importantly, specifically abdominal obesity increases mortality, for example, by compromising cardiometabolic health and increasing the risk of many chronic diseases. 147
Previous meta‐analyses already demonstrated an overall relation between HairF and BMI. However, this was investigated in smaller groups that also included individuals with psychosocial or biological factors affecting the HPA‐axis such as post‐traumatic stress disorder, 9 or limited to children only. 12 Therefore, another important aim of our study was to identify moderators and subgroups within this relation on study level. This could improve the eventual applicability of HairGC measurements in the context of weight variability and additionally increase our understanding of the underlying biological mechanisms.
Strikingly, the pooled correlations between parameters of obesity and cortisone, the inactive form of cortisol, tended to be stronger than the relations with cortisol itself. The equilibrium between cortisol and cortisone is controlled by the enzymes 11β‐hydroxysteroid dehydrogenase types 1 and 2 both in the circulation (which is mostly determined by hepatic enzyme activity) as well as at tissue level, differing per tissue type. 148 With regard to scalp hair, it has been suggested that human hair follicles display a functional equivalent of the HPA‐axis and can synthesize cortisol, 149 although this finding has until now not been confirmed by others. However, there are currently no reports regarding balance between cortisol and cortisone at the shaft level. Therefore, it is believed that at least HairF represents cumulative circulating levels of cortisol, 150 which presumably also holds true for HairE and cortisone. Perhaps this more stable circulating “reservoir” of inactive cortisol can be seen as a better indicator of chronic hypercortisolism related to adiposity, considering the stronger relations that we found for HairE. Moreover, this matches previous findings that HairE has a better diagnostic efficacy than HairF in the diagnostic screening for endogenous hypercortisolism. 4
Furthermore, in contrast to Ling et al., 12 our meta‐analyses did not indicate that LC–MS‐based cortisol measurements had a stronger relation to obesity than ELISA or CLIA‐based measurements. In principle, the LC–MS‐based method has a higher specificity than the ELISA method because it mostly lacks the interference from other steroid compounds. 151 The finding that LC–MS‐based studies did not show a higher correlation for cortisol and obesity measurements than ELISA‐based studies could also point towards an actual biological effect that in obesity, there is a more general activation of the HPA‐axis. This general activation could lead to increased levels of other steroid hormones such as cortisone, which could potentially reduce issues associated with cross‐reactivity in this context.
The percentage of males included was a significant influencer of the relation between WC and HairF, with a similar trend for WHR and HairF, but not for HairF and BMI. For both WC and WHR, cut‐off values are sex‐specific, with males generally having a larger WC and WHR than females. This might contribute to the stronger associations between HairGC and anthropometric measurements in studies that contain more males. Unfortunately, lack of raw data hampered stratification for sex.
We also observed that studies that had a high percentage of participants with obesity found less strong associations between HairF and BMI. Although HairGC levels may explain less of the weight variability in cohorts with individuals with obesity compared with cohorts that include wider weight ranges, it has clearly been established that individuals with obesity in general have higher HairGC than individuals without obesity, 14 , 141 , 152 an observation that is confirmed by our current analyses. It might be possible that within individuals with obesity, HairGC relate more to metabolic health than to anthropometrics per se. Another explanation could be the presence of a certain “tipping point,” perhaps the development of hepatic steatosis, that may interfere with cortisol‐metabolizing enzymes, leading to or maintaining the state of hypercortisolism.
In contrast to our expectations, we found that studies using self‐reported BMI reported stronger correlations to HairGC levels than studies using objective anthropometric features (r = 0.15 and r = 0.07, respectively, for HairF‐BMI). One possible explanation for this finding could include higher perceived weight stigma in individuals with obesity. Weight stigma is associated with adverse psychological consequences, such as anxiety, lower self‐esteem, poor quality of life, as well as with higher HairF levels. 153 When perceived weight stigma would cause individuals with obesity to overestimate their own weight, this could result in stronger correlations between BMI and HairGC levels, although this is highly speculative. Other possible areas of bias, for example, the selection of participants (whether or not the participant selection was population‐based or based on medical, occupational or socio‐economic characteristics), the consideration of possible confounders (outliers of HairGC measurements and corticosteroid use), and the statistical reporting all did not affect the outcomes.
As expected, given the large number of included studies, we observed a relatively high between‐study heterogeneity in our meta‐analyses of correlation coefficients, up to an I 2 of 68% for HairF versus WC. Although some of our studied moderators could explain part of this heterogeneity, the majority is still unexplained. Hence, there may be a role for other factors that are known to influence HairGC levels and/or obesity that we did not account for in the current report. For example, a recent meta‐analysis demonstrated that adversity also relates to long‐term GC levels, although this relation is complex and depends on the type and timing of adversity and on the studied population. 154 Adversity and stressful conditions can have similar complex relations to obesity. 155 We did not include these factors as possible moderators in our analyses due to a lack of universally accepted definitions that we could apply to all studies. However, we do not suspect a major influence of stressful conditions on our results as sensitivity analyses focusing on population‐based cohorts were comparable with the analyses based on all data.
A major strength of the current study was our comprehensive search in which we included all studies that reported any association between measures of adiposity and HairGC levels, including studies that did not primarily aim to investigate these associations. To minimize the risk of publication bias due to incomplete reporting of results based on statistical significance, we contacted corresponding authors of all included studies for additional information. In addition, we contacted all corresponding authors of studies that reported anthropometric measurements and HairGC but not an association. This yielded additional information for 70 cohorts (48%). This limits the risk of publication bias, which was also confirmed by our funnel plots (supporting information Figures S10–S15). Moreover, an important addition of our work compared with the two systematic reviews and meta‐analyses that have already been published on this topic was that we studied both the active form cortisol and the inactive form cortisone, their relations to different measures of adiposity, and also investigated effect sizes complementary to correlations. This has yielded the valuable conclusion that both the strongest correlation as well as the strongest, clinically relevant effect size are actually seen for HairE versus WC, instead of the most commonly studied association HairF versus BMI. Another strength of our study is that we focused on studies that did not include participants with severe diseases affecting GC levels, which have therefore not disturbed our findings.
A limitation of our study was that we obtained data that are related to full cohorts instead of individual person‐data. This restricts our conclusions to comparisons across cohorts instead of across individuals. However, by pooling regression coefficients, we could provide an effect size that is applicable on individual level. Other limitations relate to the lack of standardization of HairGC analysis methods and the usefulness of HairGC itself, as there are still numerous issues unsolved. For example, the ubiquitously reported growth speed of scalp hair, 1 cm per month, may vary considerably by ethnicity and season. 8 Other issues represent the high prevalence of overall CS use (which may influence basal cortisol levels and were found to be used by 11% of the Dutch population, a number that may be even higher in other countries 140 , 156 ), hair characteristics such as color, treatment and washing frequency, 157 and the unresolved issue of how to handle HairGC outliers. 158 , 159 These characteristics were often not reported in the included studies, which prevented comparison across studies. Then again, the results of our analyses in the subgroup of studies that accounted for outliers and corticosteroid use, the two issues that are most likely related to obesity, did not differ significantly from the results in the subgroup of studies that did not account for outliers, corticosteroid use, or neither. It should however be noted that we only assessed whether studies handled outliers at all and that the exact manner of handling outliers in (psycho)endocrine research is still a separate topic of discussion. 159 Lastly, this review only included cross‐sectional associations while any conclusion on the prognostic or predictive value of HairGC for future obesity should come from studies investigating longitudinal relations, which have however until now only been performed scarcely. 15 , 134
Altogether, we confirmed a consistent positive association between anthropometric measurements and hair glucocorticoids. This relation was most often studied for hair cortisol and BMI but showed the strongest correlation and largest effect size for hair cortisone and WC. These relations were not influenced by mean age, mean BMI, or mean HairGC levels nor by the used laboratory methods of the studies. However, the percentage of males, the percentage of participants with obesity, and objective measurement of weight instead of self‐reported weight represented important features to take into account when assessing hair glucocorticoids in cohorts. Although causality is not yet proven, our results suggest that higher long‐term glucocorticoid levels measured in scalp hair, especially cortisone, may contribute to or reflect the state of specifically central adiposity. Future longitudinal studies should investigate whether higher hair glucocorticoid levels can have clinical relevance in predicting the development or deterioration of obesity. Our results emphasize the importance of accounting for BMI and/or WC or WHR when interpreting hair glucocorticoid levels in individuals or on a group level.
FUNDING INFORMATION
OA, BvdV, EvdA, and EvR are supported by the Elisabeth Foundation, a nonprofit organization supporting academic obesity research. EvR is supported by the Netherlands Organization of Scientific Research NWO, ZonMW Vidi Grant/Award Number: 91716453.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest for all authors.
AUTHOR CONTRIBUTIONS
EvdV, OA, and MM: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, and writing‐original draft. AA: data curation, formal analysis, investigation, visualization, and writing‐review and editing. VW, AI, EvdA, YdR, and BvdV: formal analysis, investigation, methodology, supervision, validation, and writing‐review and editing. TS: data curation, formal analysis, investigation, supervision, validation, and writing‐review and editing. SH: conceptualization, formal analysis, investigation, methodology, supervision, validation, visualization, writing‐review and editing. EvR: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing‐review and editing.
Supporting information
Appendix S1. Search strategy.
Table S1. Qualitative synthesis.
Figure S1. Forest plot for the meta‐analysis of correlation coefficients between HairF and BMI.
Figure S2. Forest plot for the meta‐analysis of correlation coefficients between HairF and BMI SDS.
Figure S3. Forest plot for the meta‐analysis of correlation coefficients between HairF and WC.
Figure S4. Forest plot for the meta‐analysis of correlation coefficients between HairF and WHR.
Figure S5. Forest plot for the meta‐analysis of correlation coefficients between HairE and BMI.
Figure S6. Forest plot for the meta‐analysis of correlation coefficients between HairE and WC.
Figure S7. Bubble plot for the meta‐regression on proportion of males in the meta‐analysis of correlation coefficients between HairF and WC.
Figure S8. Bubble plot for the meta‐regression on proportion of males in the meta‐analysis of correlations between HairF and WHR.
Figure S9. Bubble plot for the meta‐regression on proportion of individuals with obesity in the meta‐analysis of correlations between HairF and BMI.
Figure S10. Funnel plot for the meta‐analysis of correlation coefficients between HairF and BMI.
Figure S11. Funnel plot for the meta‐analysis of correlation coefficients between HairF and BMI SDS.
Figure S12. Funnel plot for the meta‐analysis of correlation coefficients between HairF and WC.
Figure S13. Funnel plot for the meta‐analysis of correlation coefficients between HairF and WHR.
Figure S14. Funnel plot for the meta‐analysis of correlation coefficients between HairE and BMI.
Figure S15. Funnel plot for the meta‐analysis of correlation coefficients between HairE and WC.
ACKNOWLEDGMENTS
The authors wish to thank Wichor Bramer, Maarten Engel, and Sabrina Gunput from the Erasmus MC Medical Library for developing and updating the search strategies. We also wish to thank all authors that were contacted who provided us with additional information.
van der Valk E, Abawi O, Mohseni M, et al. Cross‐sectional relation of long‐term glucocorticoids in hair with anthropometric measurements and their possible determinants: A systematic review and meta‐analysis. Obesity Reviews. 2022;23(3):e13376. doi: 10.1111/obr.13376
Eline van der Valk, Ozair Abawi, and Mostafa Mohseni shared first authorship.
Funding information Elisabeth Foundation, a non‐profit organization supporting academic obesity research; Netherlands Organization of Scientific Research NWO; ZonMW Vidi, Grant/Award Number: 91716453
REFERENCES
- 1. World Health Organisation . Obesity and Overweight Fact Sheet. 2016. [Google Scholar]
- 2. Van der Valk ES, Van den Akker ELT, Savas M, et al. A comprehensive diagnostic approach to detect underlying causes of obesity in adults. Obes Rev. 2019;20(6):795‐804. 10.1111/obr.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomlinson JW, Stewart PM. Cortisol metabolism and the role of 11beta‐hydroxysteroid dehydrogenase. Best Pract Res Clin Endocrinol Metab. 2001;15(1):61‐78. [DOI] [PubMed] [Google Scholar]
- 4. Savas M, Wester VL, de Rijke YB, et al. Hair glucocorticoids as biomarker for endogenous Cushing's syndrome: Validation in two independent cohorts. Neuroendocrinology. 2019;109(2):171‐178. 10.1159/000498886 [DOI] [PubMed] [Google Scholar]
- 5. Wester VL, Reincke M, Koper JW, et al. Scalp hair cortisol for diagnosis of Cushing's syndrome. Eur J Endocrinol. 2017;176(6):695‐703. [DOI] [PubMed] [Google Scholar]
- 6. Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic‐pituitary‐adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology. 2015;62:301‐318. [DOI] [PubMed] [Google Scholar]
- 7. van der Valk ES, Savas M, van Rossum EFC. Stress and obesity: Are there more susceptible individuals? Curr Obes Rep. 2018;7(2):193‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greff MJE, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, van Uum SHM. Hair cortisol analysis: An update on methodological considerations and clinical applications. Clin Biochem. 2019;63:1‐9. [DOI] [PubMed] [Google Scholar]
- 9. Stalder T, Steudte‐Schmiedgen S, Alexander N, et al. Stress‐related and basic determinants of hair cortisol in humans: A meta‐analysis. Psychoneuroendocrinology. 2017;77:261‐274. [DOI] [PubMed] [Google Scholar]
- 10. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284‐294. [DOI] [PubMed] [Google Scholar]
- 11. Gray NA, Dhana A, van der Vyver L, van Wyk J, Khumalo NP, Stein DJ. Determinants of hair cortisol concentration in children: A systematic review. Psychoneuroendocrinology. 2018;87:204‐214. [DOI] [PubMed] [Google Scholar]
- 12. Ling J, Kao TA, Robbins LB. Body mass index, waist circumference and body fat are positively correlated with hair cortisol in children: A systematic review and meta‐analysis. Obes Rev. 2020;21(10):e13050. [DOI] [PubMed] [Google Scholar]
- 13. Abell JG, Stalder T, Ferrie JE, et al. Assessing cortisol from hair samples in a large observational cohort: The Whitehall II study. Psychoneuroendocrinology. 2016;73:148‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson SE, Kirschbaum C, Steptoe A. Hair cortisol and adiposity in a population‐based sample of 2,527 men and women aged 54 to 87 years. Obesity. 2017;25(3):539‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petimar J, Rifas‐Shiman SL, Hivert MF, Fleisch AF, Tiemeier H, Oken E. Childhood hair cortisol concentration and early teen cardiometabolic outcomes. Pediatr Obes. 2019;15(3):e12592. 10.1111/ijpo.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vepsäläinen H, Hautaniemi H, Sääksjärvi K, et al. Do stressed children have a lot on their plates? A cross‐sectional study of long‐term stress and diet among Finnish preschoolers. Appetite. 2021;157:104993. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. Geneva: World Health Organization; 8–11 DECEMBER 2008; 2008. [Google Scholar]
- 18. Fardet L, Feve B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74(15):1731‐1745. [DOI] [PubMed] [Google Scholar]
- 19. Stalder T, Kirschbaum C, Alexander N, et al. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2573‐2580. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: A proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 22. Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst Rev. 2012;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bramer WM. Reference checking for systematic reviews using endnote. J Med Libr Assoc. 2018;106(4):542‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427‐437. [DOI] [PubMed] [Google Scholar]
- 25. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: https://www.R-project.org/; 2020. [Google Scholar]
- 26. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.1 2020; www.training.cochrane.org/handbook. Accessed October 22, 2020. [Google Scholar]
- 28. Viechtbauer W. Conducting Meta‐Analyses in R With the Metafor Package. 2010; https://cris.maastrichtuniversity.nl/en/publications/conducting-meta-analyses-in-r-with-the-metafor-package. Accessed 18–03‐2021, 2021. [Google Scholar]
- 29. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐analysis. John Wiley & Sons; 2011. [Google Scholar]
- 30. Sun Y, Fang J, Wan Y, Hu J, Xu Y, Tao F. Polygenic differential susceptibility to cumulative stress exposure and childhood obesity. Int J Obes. 2018;42(6):1177‐1184. [DOI] [PubMed] [Google Scholar]
- 31. Bini LM, Coelho AS, Diniz‐Filho JA. Is the relationship between population density and body size consistent across independent studies? A meta‐analytical approach. Braz J Biol. 2001;61(1):1‐6. 10.1590/S0034-71082001000100002 [DOI] [PubMed] [Google Scholar]
- 32. Becker BJ, Wu M‐J. The synthesis of regression slopes in meta‐analysis. Stat Sci. 2007;22(3):414‐429. 10.1214/07-STS243 [DOI] [Google Scholar]
- 33. Abdulateef DS, Mahwi TO. Assessment of hair cortisol in euthyroid, hypothyroid, and subclinical hypothyroid subjects. Endocrine. 2019;63(1):131‐139. [DOI] [PubMed] [Google Scholar]
- 34. Aguiló S, García E, Arza A, Garzón‐Rey JM, Aguiló J. Evaluation of chronic stress indicators in geriatric and oncologic caregivers: A cross‐sectional study. Stress. 2018;21(1):36‐42. [DOI] [PubMed] [Google Scholar]
- 35. Berger M, Taylor S, Harriss L, et al. Hair cortisol, allostatic load, and depressive symptoms in Australian Aboriginal and Torres Strait Islander people. Stress. 2019;22(3):312‐320. [DOI] [PubMed] [Google Scholar]
- 36. Boesch M, Sefidan S, Annen H, et al. Hair cortisol concentration is unaffected by basic military training, but related to sociodemographic and environmental factors. Stress. 2014;18(1):35‐41. 10.3109/10253890.2014.974028 [DOI] [PubMed] [Google Scholar]
- 37. Bossé S, Stalder T, D'Antono B. Childhood trauma, perceived stress, and hair cortisol in adults with and without cardiovascular disease. Psychosom Med. 2018;80(4):393‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brianda ME, Roskam I, Mikolajczak M. Hair cortisol concentration as a biomarker of parental burnout. Psychoneuroendocrinology. 2020;117:104681. [DOI] [PubMed] [Google Scholar]
- 39. Bryson HE, Mensah F, Goldfeld S, Price AMH. Using hair cortisol to examine the role of stress in children's health inequalities at 3 years. Acad Pediatr. 2019;20(2):193‐202. 10.1016/j.acap.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 40. Castro‐Vale I, van Rossum EFC, Staufenbiel SM, Severo M, Mota‐Cardoso R, Carvalho D. Hair cortisol as a marker of intergenerational heritage of war? A study of veterans and their offspring. Psychiatry Investig. 2020;17(10):976‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cedillo YE, Lomax RO, Fernandez JR, Moellering DR. Physiological significance of discrimination on stress markers, obesity, and LDL oxidation among a European American and African American cohort of females. Int J Behav Med. 2020;27(2):213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan J, Sauvé B, Tokmakejian S, Koren G, van Uum S. Measurement of cortisol and testosterone in hair of obese and non‐obese human subjects. Exp Clin Endocrinol Diabetes. 2014;122(6):356‐362. [DOI] [PubMed] [Google Scholar]
- 43. Chen Z, Li J, Zhang J, et al. Simultaneous determination of hair cortisol, cortisone and DHEAS with liquid chromatography‐electrospray ionization‐tandem mass spectrometry in negative mode. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;929:187‐194. [DOI] [PubMed] [Google Scholar]
- 44. Chen X, Gelaye B, Velez JC, et al. Caregivers' hair cortisol: A possible biomarker of chronic stress is associated with obesity measures among children with disabilities. BMC Pediatr. 2015;15(15):9. 10.1186/s12887-015-0322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Condon EM, Holland ML, Slade A, Redeker NS, Mayes LC, Sadler LS. Associations between maternal caregiving and child indicators of toxic stress among multiethnic urban families. J Pediatr Health Care. 2019;33(4):425‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davison B, Singh GR, McFarlane J. Hair cortisol and cortisone as markers of stress in Indigenous and non‐Indigenous young adults. Stress. 2019;22(2):210‐220. [DOI] [PubMed] [Google Scholar]
- 47. de Kruijff I, Noppe G, Kieviet N, et al. LC‐MS/MS‐based reference intervals for hair cortisol in healthy children. Psychoneuroendocrinology. 2020;112:104539. [DOI] [PubMed] [Google Scholar]
- 48. Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long‐term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404‐1409. [DOI] [PubMed] [Google Scholar]
- 49. Diebig M, Bormann KC, Rowold J. A double‐edged sword: Relationship between full‐range leadership behaviors and followers' hair cortisol level. Leadersh Q. 2016;27(4):684‐696. [Google Scholar]
- 50. Distel LML, Egbert AH, Bohnert AM, Santiago CD. Chronic stress and food insecurity: examining key environmental family factors related to body mass index among low‐income Mexican‐origin youth. Fam Community Health. 2019;42(3):213‐220. 10.1097/FCH.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 51. Dowlati Y, Herrmann N, Swardfager W, et al. Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatr Dis Treat. 2010;6:393‐400. [PMC free article] [PubMed] [Google Scholar]
- 52. Enge S, Fleischhauer M, Hadj‐Abo A, et al. Comparison of hair cortisol concentrations between self‐ and professionally‐collected hair samples and the role of five‐factor personality traits as potential moderators. Psychoneuroendocrinology. 2020;122:104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Engert V, Kok BE, Puhlmann LMC, et al. Exploring the multidimensional complex systems structure of the stress response and its relation to health and sleep outcomes. Brain Behav Immun. 2018;73:390‐402. [DOI] [PubMed] [Google Scholar]
- 54. Etwel F, Russell E, Rieder MJ, van Uum SH, Koren G. Hair cortisol as a biomarker of stress in the 2011 Libyan war. Clin Invest Med. 2014;37(6):E403‐E408. [DOI] [PubMed] [Google Scholar]
- 55. Evans BE, Beijers R, Hagquist C, de Weerth C. Childhood urbanicity and hair steroid hormone levels in ten‐year‐old children. Psychoneuroendocrinology. 2019;102:53‐57. [DOI] [PubMed] [Google Scholar]
- 56. Feeney JC, O'Halloran AM, Kenny RA. The association between hair cortisol, hair cortisone, and cognitive function in a population‐based cohort of older adults: Results from the Irish longitudinal study on ageing. J Gerontol A Biol Sci Med Sci. 2020;75(2):257‐265. 10.1093/gerona/gly258 [DOI] [PubMed] [Google Scholar]
- 57. Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology. 2014;39:132‐140. 10.1016/j.psyneuen.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 58. Fischer S, Duncko R, Hatch SL, et al. Sociodemographic, lifestyle, and psychosocial determinants of hair cortisol in a South London community sample. Psychoneuroendocrinology. 2017;76:144‐153. [DOI] [PubMed] [Google Scholar]
- 59. Föcker M, Stalder T, Kirschbaum C, et al. Hair cortisol concentrations in adolescent girls with anorexia nervosa are lower compared to healthy and psychiatric controls. Eur Eat Disord Rev. 2016;24(6):531‐535. 10.1002/erv.2466 [DOI] [PubMed] [Google Scholar]
- 60. Frisch N, Eichler A, Plank AC, Golub Y, Moll GH, Kratz O. Exploring reference values for hair cortisol: Hair weight versus hair protein. Ther Drug Monit. 2020;42(9):902‐908. 10.1097/FTD.0000000000000782 [DOI] [PubMed] [Google Scholar]
- 61. Gao W, Zhong P, Xie Q, et al. Temporal features of elevated hair cortisol among earthquake survivors. Psychophysiology. 2014;51(4):319‐326. [DOI] [PubMed] [Google Scholar]
- 62. Garcia‐Leon MA, Peralta‐Ramirez MI, Arco‐Garcia L, et al. Hair cortisol concentrations in a Spanish sample of healthy adults. PLoS ONE. 2018;13(9):e0204807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Genitsaridi SM, Karampatsou S, Papageorgiou I, et al. Hair cortisol concentrations in overweight and obese children and adolescents. Horm Res Paediatr. 2019;1‐8. 10.1159/000504913 [DOI] [PubMed] [Google Scholar]
- 64. Gerber M, Endes K, Brand S, et al. In 6‐ to 8‐year‐old children, hair cortisol is associated with body mass index and somatic complaints, but not with stress, health‐related quality of life, blood pressure, retinal vessel diameters, and cardiorespiratory fitness. Psychoneuroendocrinology. 2017;76:1‐10. [DOI] [PubMed] [Google Scholar]
- 65. Gidlow CJ, Randall J, Gillman J, Silk S, Jones MV. Hair cortisol and self‐reported stress in healthy, working adults. Psychoneuroendocrinology. 2016;63:163‐169. [DOI] [PubMed] [Google Scholar]
- 66. Golub Y, Kuitunen‐Paul S, Panaseth K, et al. Salivary and hair cortisol as biomarkers of emotional and behavioral symptoms in 6–9year old children. Physiol Behav. 2019;209:112584. 10.1016/j.physbeh.2019.112584 [DOI] [PubMed] [Google Scholar]
- 67. Grass J, Kirschbaum C, Miller R, Gao W, Steudte‐Schmiedgen S, Stalder T. Sweat‐inducing physiological challenges do not result in acute changes in hair cortisol concentrations. Psychoneuroendocrinology. 2015;53:108‐116. [DOI] [PubMed] [Google Scholar]
- 68. Grunau RE, Cepeda IL, Chau CM, et al. Neonatal pain‐related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PLoS ONE. 2013;8(9):e73926. 10.1371/journal.pone.0073926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Henley P, Lowthers M, Koren G, et al. Cultural and socio‐economic conditions as factors contributing to chronic stress in sub‐saharan African communities. Can J Physiol Pharmacol. 2014;92(9):725‐732. [DOI] [PubMed] [Google Scholar]
- 70. Hollenbach JP, Gherlone N, Simoneau T, Sylvester F, Cloutier MM. Caregiver's hair cortisol is a potential biomarker of a child's asthma. Am J Respir Crit Care Med. 2018;197:A2016. [Google Scholar]
- 71. Hu JJ, Duan XN, Fang J, et al. Association between hair cortisol concentration and overweight and obesity in 6‐9 years old childhood. Chung Hua Yu Fang I Hsueh Tsa Chih. 2017;51(12):1065‐1068. [DOI] [PubMed] [Google Scholar]
- 72. Hunter SK, Hoffman MC, McCarthy L, et al. Black American maternal prenatal choline, offspring gestational age at birth, and developmental predisposition to mental illness. Schizophr Bull. 2020;47(4):896‐905. 10.1093/schbul/sbaa171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ilg L, Kirschbaum C, Li SC, et al. No association of antenatal synthetic glucocorticoid exposure and hair steroid levels in children and adolescents. J Clin Endocrinol Metab. 2020;105(3):E575‐E582. [DOI] [PubMed] [Google Scholar]
- 74. Ince‐Askan H, van den Akker ELT, de Rijke YB, van Rossum EFC, Hazes JMW, Dolhain R. Associations between antenatal prednisone exposure and long‐term cortisol and cortisone concentrations in children born to women with rheumatoid arthritis: results from a nationwide prospective cohort study. RMD Open. 2019;5(1):e000852. 10.1136/rmdopen-2018-000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Janssens H, Clays E, Fiers T, Verstraete AG, de Bacquer D, Braeckman L. Hair cortisol in relation to job stress and depressive symptoms. Occup Med (Lond). 2017;67(2):114‐120. [DOI] [PubMed] [Google Scholar]
- 76. Kamps AW, Molenmaker M, Kemperman R, van der Veen BS, Bocca G, Veeger NJ. Children with asthma have significantly lower long‐term cortisol levels in their scalp hair than healthy children. Acta Paediatr. 2014;103(9):957‐961. 10.1111/apa.12685 [DOI] [PubMed] [Google Scholar]
- 77. Kozik P, Hoppmann CA, Gerstorf D. Future time perspective: Opportunities and limitations are differentially associated with subjective well‐being and hair cortisol concentration. Gerontology. 2015;61(2):166‐174. [DOI] [PubMed] [Google Scholar]
- 78. Kuehl LK, Hinkelmann K, Muhtz C, et al. Hair cortisol and cortisol awakening response are associated with criteria of the metabolic syndrome in opposite directions. Psychoneuroendocrinology. 2015;51:365‐370. [DOI] [PubMed] [Google Scholar]
- 79. Lanfear JH, Voegel CD, Binz TM, Paul RA. Hair cortisol measurement in older adults: Influence of demographic and physiological factors and correlation with perceived stress. Steroids. 2020;163:108712. [DOI] [PubMed] [Google Scholar]
- 80. Larsen SC, Fahrenkrug J, Olsen NJ, Heitmann BL. Association between hair cortisol concentration and adiposity measures among children and parents from the “healthy start” study. PLoS ONE. 2016;11(9):e0163639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lehrer HM, Goosby BJ, Dubois SK, Laudenslager ML, Steinhardt MA. Race moderates the association of perceived everyday discrimination and hair cortisol concentration. Stress. 2020;23(5):539‐537. 10.1080/10253890.2019.1710487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lehto E, Ray C, Vepsäläinen H, et al. Increased health and wellbeing in preschools (DAGIS) study—Differences in children's energy balance‐related behaviors (EBRBs) and in long‐term stress by parental educational level. Int J Environ Res Public Health. 2018;15(10):2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ling J, Xu D, Robbins LB, Kao TSA. Obesity and hair cortisol: Relationships varied between low‐income preschoolers and mothers. Matern Child Health J. 2020;24(12):1495‐1504. [DOI] [PubMed] [Google Scholar]
- 84. Manenschijn L, Koper JW, Lamberts SWJ, van Rossum EFC. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10):1032‐1036. 10.1016/j.steroids.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 85. Manenschijn L, Schaap L, van Schoor NM, et al. High long‐term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013;98(5):2078‐2083. 10.1210/jc.2012-3663 [DOI] [PubMed] [Google Scholar]
- 86. Mazgelytė E, Karčiauskaitė D, Linkevičiūtė A, et al. Association of hair cortisol concentration with prevalence of major cardiovascular risk factors and Allostatic load. Med Sci Monit. 2019;25:3573‐3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McLennan SN, Ihle A, Steudte‐Schmiedgen S. Hair cortisol and cognitive performance in working age adults. Psychoneuroendocrinology. 2016;67:100‐103. 10.1016/j.psyneuen.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 88. Menning S, de Ruiter MB, Veltman DJ, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—The role of fatigue. Neuroimage: Clinical. 2015;7:547‐554. 10.1016/j.nicl.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Michaud DS, Feder K, Keith SE, et al. Self‐reported and measured stress related responses associated with exposure to wind turbine noise. J Acoust Soc am. 2016;139(3):1467‐1479. [DOI] [PubMed] [Google Scholar]
- 90. Michels N, van de Wiele T, de Henauw S. Chronic psychosocial stress and gut health in children: Associations with calprotectin and fecal short‐chain fatty acids. Psychosom Med. 2017;79(8):927‐935. [DOI] [PubMed] [Google Scholar]
- 91. Murray CR, Simmons JG, Allen NB, et al. Associations between dehydroepiandrosterone (DHEA) levels, pituitary volume, and social anxiety in children. Psychoneuroendocrinology. 2016;64:31‐39. [DOI] [PubMed] [Google Scholar]
- 92. Mwanza C, Chen Z, Zhang Q, Chen S, Wang W, Deng H. Simultaneous HPLC‐APCI‐MS/MS quantification of endogenous cannabinoids and glucocorticoids in hair. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1028:1‐10. [DOI] [PubMed] [Google Scholar]
- 93. Nery SF, Paiva SPC, Vieira EL, et al. Mindfulness‐based program for stress reduction in infertile women: Randomized controlled trial. Stress Health. 2018;35(1):49‐58. 10.1002/smi.2839 [DOI] [PubMed] [Google Scholar]
- 94. O'Brien KM, Tronick EZ, Moore CL. Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress Health. 2013;29(4):337‐344. [DOI] [PubMed] [Google Scholar]
- 95. Olstad DL, Ball K, Wright C, Abbott G, Brown E, Turner AI. Hair cortisol levels, perceived stress and body mass index in women and children living in socioeconomically disadvantaged neighborhoods: The READI study. Stress. 2016;19(2):158‐167. [DOI] [PubMed] [Google Scholar]
- 96. Ouellet‐Morin I, Laurin M, Robitaille MP, et al. Validation of an adapted procedure to collect hair for cortisol determination in adolescents. Psychoneuroendocrinology. 2016;70:58‐62. [DOI] [PubMed] [Google Scholar]
- 97. Ouellette SJ, Russell E, Kryski KR, et al. Hair cortisol concentrations in higher‐ and lower‐stress mother‐daughter dyads: A pilot study of associations and moderators. Dev Psychobiol. 2015;57(5):519‐534. [DOI] [PubMed] [Google Scholar]
- 98. Panter‐Brick C, Wiley K, Sancilio A, Dajani R, Hadfield K. C‐reactive protein, Epstein‐Barr virus, and cortisol trajectories in refugee and non‐refugee youth: Links with stress, mental health, and cognitive function during a randomized controlled trial. Brain Behav Immun. 2019;87:207‐217. 10.1016/j.bbi.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Papafotiou C, Christaki E, van den Akker ELT, et al. Hair cortisol concentrations exhibit a positive association with salivary cortisol profiles and are increased in obese prepubertal girls. Stress. 2017;20(2):217‐222. [DOI] [PubMed] [Google Scholar]
- 100. Petimar J, Rifas‐Shiman SL, Hivert MF, Fleisch AF, Tiemeier H, Oken E. Prenatal and childhood predictors of hair cortisol concentration in mid‐childhood and early adolescence. PLoS ONE. 2020;15(2):e0228769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pickett S, McCoy TP, Odetola L. The influence of chronic stress and emotions on eating behavior patterns and weight among young African American women. West J Nurs Res. 2020:193945919897541;(11):894‐902. 10.1177/0193945919897541 [DOI] [PubMed] [Google Scholar]
- 102. Pittner K, Buisman RSM, van den Berg LJM, et al. Not the root of the problem‐hair cortisol and cortisone do not mediate the effect of child maltreatment on body mass index. Front Psych. 2020;11:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pulopulos MM, Hidalgo V, Almela M, Puig‐Perez S, Villada C, Salvador A. Hair cortisol and cognitive performance in healthy older people. Psychoneuroendocrinology. 2014;44:100‐111. [DOI] [PubMed] [Google Scholar]
- 104. Pyle Hennessey EM, Kepinska O, Haft SL, et al. Hair cortisol and dehydroepiandrosterone concentrations: Associations with executive function in early childhood. Biol Psychol. 2020;155:107946. 10.1016/j.biopsycho.2020.107946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qi X, Zhang J, Liu Y, Ji S, Chen Z, Sluiter JK. Relationship between effort–reward imbalance and hair cortisol concentration in female kindergarten teachers. J Psychosom Res. 2014;76(4):329‐332. 10.1016/j.jpsychores.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 106. Radin RM, Mason AE, Laudenslager ML, Epel ES. Maternal caregivers have confluence of altered cortisol, high reward‐driven eating, and worse metabolic health. PLoS ONE. 2019;14(5):e0216541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Saleem M, Herrmann N, Swardfager W, et al. Higher cortisol predicts less improvement in verbal memory performance after cardiac rehabilitation in patients with coronary artery disease. Cardiovasc Psychiatry Neurol. 2013;2013:1‐8. 10.1155/2013/340342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schalinski I, Elbert T, Steudte‐Schmiedgen S, Kirschbaum C. the cortisol paradox of trauma‐related disorders: Lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS ONE. 2015;10(8):e0136921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schalinski I, Teicher MH, Rockstroh B. Early neglect is a key determinant of adult hair cortisol concentration and is associated with increased vulnerability to trauma in a transdiagnostic sample. Psychoneuroendocrinology. 2019;108:35‐42. [DOI] [PubMed] [Google Scholar]
- 110. Schloß S, Ruhl I, Müller V, et al. Low hair cortisol concentration and emerging attention‐deficit/hyperactivity symptoms in preschool age. Dev Psychobiol. 2018;60(6):722‐729. [DOI] [PubMed] [Google Scholar]
- 111. Serwinski B, Salavecz G, Kirschbaum C, Steptoe A. Associations between hair cortisol concentration, income, income dynamics and status incongruity in healthy middle‐aged women. Psychoneuroendocrinology. 2016;67:182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Skoluda N, Dettenborn L, Stalder T. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology. 2012;37(5):611‐617. 10.1016/j.psyneuen.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 113. Slopen N, Roberts AL, LeWinn KZ, et al. Maternal experiences of trauma and hair cortisol in early childhood in a prospective cohort. Psychoneuroendocrinology. 2018;98:168‐176. 10.1016/j.psyneuen.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 114. Smith JD, Johnson KA, Whittle S, Allen NB, Simmons JG. Measurement of cortisol, dehydroepiandrosterone, and testosterone in the hair of children: Preliminary results and promising indications. Dev Psychobiol. 2019;61(6):962‐970. [DOI] [PubMed] [Google Scholar]
- 115. Smith L, Firth J, Grabovac I, et al. The association of grip strength with depressive symptoms and cortisol in hair: A cross‐sectional study of older adults. Scand J Med Sci Sports. 2019;29(10):1604‐1609. [DOI] [PubMed] [Google Scholar]
- 116. Stalder T, Tietze A, Steudte S, Alexander N, Dettenborn L, Kirschbaum C. Elevated hair cortisol levels in chronically stressed dementia caregivers. Psychoneuroendocrinology. 2014;47:26‐30. [DOI] [PubMed] [Google Scholar]
- 117. Stalder T, Kirschbaum C, Heinze K, et al. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol‐dependent individuals. Biol Psychol. 2010;85(3):357‐360. [DOI] [PubMed] [Google Scholar]
- 118. Stalder T, Steudte S, Alexander N, et al. Cortisol in hair, body mass index and stress‐related measures. Biol Psychol. 2012;90(3):218‐223. [DOI] [PubMed] [Google Scholar]
- 119. Staufenbiel SM, Koenders MA, Giltay EJ, Elzinga BM. Recent negative life events increase hair cortisol concentrations in patients with bipolar disorder. Stress. 2014;17(6):451‐459. 10.3109/10253890.2014.968549 [DOI] [PubMed] [Google Scholar]
- 120. Staufenbiel SM, Penninx B, Rijke YB. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology. 2015;60:182–194. 10.1016/j.psyneuen.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 121. Steudte S, Kirschbaum C, Gao W, et al. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013;74(9):639‐646. [DOI] [PubMed] [Google Scholar]
- 122. Steudte S, Kolassa IT, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology. 2011;36(8):1193‐1200. [DOI] [PubMed] [Google Scholar]
- 123. Steudte S, Stalder T, Dettenborn L, et al. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res. 2011;186(2–3):310‐314. [DOI] [PubMed] [Google Scholar]
- 124. Steudte‐Schmiedgen S, Wichmann S, Stalder T, et al. Hair cortisol concentrations and cortisol stress reactivity in generalized anxiety disorder, major depression and their comorbidity. J Psychiatr Res. 2017;84:184‐190. [DOI] [PubMed] [Google Scholar]
- 125. Suijker I, Savas M, van Rossum EFC, Langendonk JG. Hair cortisol is elevated in patients with erythropoietic protoporphyria and correlates with body mass index and quality of life. Br J Dermatol. 2018;178(5):1209‐1210. [DOI] [PubMed] [Google Scholar]
- 126. van Aken M, Oosterman J, van Rijn T, et al. Hair cortisol and the relationship with chronic pain and quality of life in endometriosis patients. Psychoneuroendocrinology. 2018;89:216‐222. [DOI] [PubMed] [Google Scholar]
- 127. van Dammen L, de Rooij SR, Behnsen PM, Huizink AC. Sex‐specific associations between person and environment‐related childhood adverse events and levels of cortisol and DHEA in adolescence. PLoS ONE. 2020;15(6):e023371. 10.1371/journal.pone.0233718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. van den Heuvel LL, Acker D, du Plessis S, et al. Hair cortisol as a biomarker of stress and resilience in South African mixed ancestry females. Psychoneuroendocrinology. 2020;113:104543. [DOI] [PubMed] [Google Scholar]
- 129. van den Heuvel LL, du Plessis S, Stalder T, et al. Hair glucocorticoid levels in Parkinson's disease. Psychoneuroendocrinology. 2020;117:104704. [DOI] [PubMed] [Google Scholar]
- 130. van den Heuvel LL, Stalder T, du Plessis S, Suliman S, Kirschbaum C, Seedat S. Hair cortisol levels in posttraumatic stress disorder and metabolic syndrome. Stress. 2020;1‐36(5):577‐589. 10.1080/10253890.2020.1724949 [DOI] [PubMed] [Google Scholar]
- 131. van der Valk ES, van der Voorn B, Iyer AM, et al. In adults with obesity, copeptin is linked with BMI but is not associated with long‐term exposure to cortisol and cortisone. Eur J Endocrinol. 2020;183(6):669‐676. [DOI] [PubMed] [Google Scholar]
- 132. van Holland BJ, Frings‐Dresen MH, Sluiter JK. Measuring short‐term and long‐term physiological stress effects by cortisol reactivity in saliva and hair. Int Arch Occup Environ Health. 2012;85(8):849‐852. 10.1007/s00420-011-0727-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. van Manen MJG, Wester VL, van Rossum EFC, et al. Scalp hair cortisol and testosterone levels in patients with sarcoidosis. PLoS ONE. 2019;14(6):e021576. 10.1371/journal.pone.0215763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vehmeijer FOL, Santos S, Gaillard R, et al. Associations of hair cortisol concentrations with general and organ fat measures in childhood. J Clin Endocrinol Metab. 2020;106(2):e551‐e561. 10.1210/clinem/dgaa785 [DOI] [PubMed] [Google Scholar]
- 135. Wagner M, Kratzsch J, Vogel M, et al. Hair cortisol concentration in healthy children and adolescents is related to puberty, age, gender, and body mass index. Horm Res Paediatr. 2019;92(4):1‐8. 10.1159/000504914 [DOI] [PubMed] [Google Scholar]
- 136. Walther A, Ehlert U. Hair, nail or still saliva? Cortisol measurement in peripheral body substrates and its association with sex steroids and body composition in a sample of middle‐aged and older men. Psychoneuroendocrinology. 2016;71S:74. 10.1016/j.psyneuen.2016.07.191 [DOI] [Google Scholar]
- 137. Walton DM, Macdermid JC, Russell E, Koren G, van Uum S. Hair‐normalized cortisol waking response as a novel biomarker of hypothalamic‐pituitary‐adrenal axis activity following acute trauma: A proof‐of‐concept study with pilot results. Pain Res Treat. 2013;2013:876871. 10.1155/2013/876871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang C, Dai J, Li J. Mediating effects of hair cortisol on the mutual association of job burnout and insomnia: A retrospective exploratory study. J Psychiatr Res. 2019;117:62‐67. [DOI] [PubMed] [Google Scholar]
- 139. Wells S, Tremblay PF, Flynn A, et al. Associations of hair cortisol concentration with self‐reported measures of stress and mental health‐related factors in a pooled database of diverse community samples. Stress. 2014;17(4):334‐342. [DOI] [PubMed] [Google Scholar]
- 140. Wester VL, Noppe G, Savas M, van den Akker ELT, de Rijke YB, van Rossum EFC. Hair analysis reveals subtle HPA axis suppression associated with use of local corticosteroids: The Lifelines cohort study. Psychoneuroendocrinology. 2017;80:1‐6. [DOI] [PubMed] [Google Scholar]
- 141. Wester VL, Staufenbiel SM, Veldhorst MAB, et al. Long‐term cortisol levels measured in scalp hair of obese patients. Obesity. 2014;22(9):1956‐1958. [DOI] [PubMed] [Google Scholar]
- 142. White LO, Ising M, von Klitzing K, et al. Reduced hair cortisol after maltreatment mediates externalizing symptoms in middle childhood and adolescence. J Child Psychol Psychiatry. 2017;58(9):998‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wu YK, Berry DC, Schwartz TA. Weight stigma and acculturation in relation to hair cortisol among Asian Americans with overweight and obesity: A cross‐sectional study. Health Psychol Open. 2019;6(1):2055102919829275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Younge JO, Wester VL, van Rossum EF, et al. Cortisol levels in scalp hair of patients with structural heart disease. Int J Cardiol. 2015;184:71‐78. 10.1016/j.ijcard.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 145. Zai C, George J, Irwin D, et al. Stress response genes and hair cortisol levels in first nation communities. Eur Neuropsychopharmacol. 2017;27:S320. [Google Scholar]
- 146. Žekas V, Matuzevičiene R, Karčiauskaite D, et al. Chronic and oxidative stress association with total count of endothelial microvesicles in healthy young male plasma. Adv Clin Exp Med. 2019;28(5):683‐692. 10.17219/acem/94144 [DOI] [PubMed] [Google Scholar]
- 147. Jayedi A, Soltani S, Zargar MS, Khan TA, Shab‐Bidar S. Central fatness and risk of all cause mortality: Systematic review and dose‐response meta‐analysis of 72 prospective cohort studies. BMJ. 2020;370:m3324. 10.1136/bmj.m3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Anderson AJ, Andrew R, Homer NZM, et al. Effects of obesity and insulin on tissue‐specific recycling between cortisol and cortisone in men. J Clin Endocrinol Metab. 2020;106(3):e1206–e1220. 10.1210/clinem/dgaa896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic‐pituitary‐adrenal axis and synthesize cortisol. FASEB j. 2005;19(10):1332‐1334. [DOI] [PubMed] [Google Scholar]
- 150. Short SJ, Stalder T, Marceau K, et al. Correspondence between hair cortisol concentrations and 30‐day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016;71:12‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab. 2013;98(10):3971‐3973. [DOI] [PubMed] [Google Scholar]
- 152. Veldhorst MAB, Noppe G, Jongejan MHTM, et al. Increased scalp hair cortisol concentrations in obese children. J Clin Endocrinol Metab. 2014;99(1):285‐290. [DOI] [PubMed] [Google Scholar]
- 153. Jackson SE, Kirschbaum C, Steptoe A. Perceived weight discrimination and chronic biochemical stress: A population‐based study using cortisol in scalp hair. Obesity. 2016;24(12):2515‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Khoury JE, Bosquet Enlow M, Plamondon A, Lyons‐Ruth K. The association between adversity and hair cortisol levels in humans: A meta‐analysis. Psychoneuroendocrinology. 2019;103:104‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tomiyama AJ. Stress and obesity. Annu Rev Psychol. 2019;70(1):703‐718. [DOI] [PubMed] [Google Scholar]
- 156. Savas M, Muka T, Wester VL, et al. Associations between systemic and local corticosteroid use with metabolic syndrome and body mass index. J Clin Endocrinol Metab. 2017;102(10):3765‐3774. [DOI] [PubMed] [Google Scholar]
- 157. Staufenbiel SM, Penninx BW, de Rijke YB, van den Akker EL, van Rossum EF. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology. 2015;60:182‐194. 10.1016/j.psyneuen.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 158. Marceau K, Wang W, Robertson O, Shirtcliff EA. A systematic review of hair cortisol during pregnancy: Reference ranges and methodological considerations. Psychoneuroendocrinology. 2020;122:104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Herbers J, Miller R, Walther A, et al. How to deal with non‐detectable and outlying values in biomarker research: Best practices and recommendations for univariate imputation approaches. Compr Psychoneuroendocrinol. 2021;100052. 10.1016/j.cpnec.2021.100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.
Table S1. Qualitative synthesis.
Figure S1. Forest plot for the meta‐analysis of correlation coefficients between HairF and BMI.
Figure S2. Forest plot for the meta‐analysis of correlation coefficients between HairF and BMI SDS.
Figure S3. Forest plot for the meta‐analysis of correlation coefficients between HairF and WC.
Figure S4. Forest plot for the meta‐analysis of correlation coefficients between HairF and WHR.
Figure S5. Forest plot for the meta‐analysis of correlation coefficients between HairE and BMI.
Figure S6. Forest plot for the meta‐analysis of correlation coefficients between HairE and WC.
Figure S7. Bubble plot for the meta‐regression on proportion of males in the meta‐analysis of correlation coefficients between HairF and WC.
Figure S8. Bubble plot for the meta‐regression on proportion of males in the meta‐analysis of correlations between HairF and WHR.
Figure S9. Bubble plot for the meta‐regression on proportion of individuals with obesity in the meta‐analysis of correlations between HairF and BMI.
Figure S10. Funnel plot for the meta‐analysis of correlation coefficients between HairF and BMI.
Figure S11. Funnel plot for the meta‐analysis of correlation coefficients between HairF and BMI SDS.
Figure S12. Funnel plot for the meta‐analysis of correlation coefficients between HairF and WC.
Figure S13. Funnel plot for the meta‐analysis of correlation coefficients between HairF and WHR.
Figure S14. Funnel plot for the meta‐analysis of correlation coefficients between HairE and BMI.
Figure S15. Funnel plot for the meta‐analysis of correlation coefficients between HairE and WC.


