Abstract
Understanding cellular electrical communications in both health and disease necessitates precise subcellular electrophysiological modulation. Nanomaterial-assisted photothermal stimulation was demonstrated to modulate cellular activity with high spatiotemporal resolution. Ideal candidates for such an application are expected to have high absorbance at the near-infrared window, high photothermal conversion efficiency, and straightforward scale-up of production to allow future translation. Here, we demonstrate two-dimensional Ti3C2Tx (MXene) as an outstanding candidate for remote, nongenetic, optical modulation of neuronal electrical activity with high spatiotemporal resolution. Ti3C2Tx’s photothermal response measured at the single-flake level resulted in local temperature rises of 2.31 ± 0.03 and 3.30 ± 0.02 K for 635 and 808 nm laser pulses (1 ms, 10 mW), respectively. Dorsal root ganglion (DRG) neurons incubated with Ti3C2Tx film (25 μg/cm2) or Ti3C2Tx flake dispersion (100 μg/mL) for 6 days did not show a detectable influence on cellular viability, indicating that Ti3C2Tx is noncytotoxic. DRG neurons were photothermally stimulated using Ti3C2Tx films and flakes with as low as tens of microjoules per pulse incident energy (635 nm, 2 μJ for film, 18 μJ for flake) with subcellular targeting resolution. Ti3C2Tx’s straightforward and large-scale synthesis allows translation of the reported photothermal stimulation approach in multiple scales, thus presenting a powerful tool for modulating electrophysiology from single-cell to additive manufacturing of engineered tissues.
Keywords: Ti3C2Tx MXene, optical, modulation, neurons, dorsal root ganglion
Introduction
A key challenge for studying complex neuronal functions is the ability to modulate neuronal activity with high spatiotemporal resolution.1,2 State-of-the-art microelectrode arrays (MEAs) can stimulate cells with high precision, but they lack cell-type specificity and require invasive implantation, which will result in tissue damage.3−5 Optogenetics is a major breakthrough that allows remote control without implanted bioelectronics both in vitro and in vivo, but its requirement for genetic modifications to drive expression of light-sensitive channels limits its clinical translatability.2,5,6 Alternatively, direct illumination of a neuron’s membrane with infrared (IR) laser pulses leads to membrane depolarization, thus resulting in an action potential without implantation or genetic modification.7,8 However, IR stimulation requires high incident energies per pulse to modulate the cellular activity, which can potentially damage the target cells and tissues.5,7,8
Nanomaterial-assisted photothermal stimulation provides a remote, nongenetic approach for stimulating cells and tissues with subcellular spatial (μm) and sub-millisecond temporal resolution.2,4 Recently, Au- (such as pristine and functionalized Au nanoparticles (AuNPs) and Au nanorods (AuNRs)),9,10 Si- (mesoporous Si particles, Si nanowires (SiNWs), and Au-decorated SiNWs),11−13 and C- (carbon nanotubes (CNTs), graphite particles, and nanowire-templated three-dimensional (3D) fuzzy graphene (NT-3DFG))5,9 based materials were demonstrated as promising candidates for photothermal stimulation without generating cellular stress. However, these materials face at least one of the following challenges: limited near-infrared (NIR) absorbance, low NIR photothermal conversion efficiency, and limited scalability of production.5,9−13
Transition metal carbides/nitrides (MXenes) have emerged as a rising class of two-dimensional (2D) nanomaterials where atomic layers of transition metals (M) sandwich 2D layer(s) of carbon or nitrogen atoms.14 Altering the atomic design of MXenes allows the synthesized nanomaterials to exhibit outstanding mechanical properties, high electrical conductivity, excellent electrochemical properties, and tunable optical responses.14 Therefore, MXenes have found applications across a wide variety of fields such as robotics, photonics, water desalination, and biomedical engineering.14−20 Ti3C2Tx21 have attracted significant attention in photothermal therapy (PTT) due to its high NIR absorbance, high photothermal conversion efficiency, and noncytotoxicity.19,21−23 Here we demonstrate that Ti3C2Tx is an excellent candidate for remote, nongenetic, photothermal stimulation of neuronal electrical activity with high spatiotemporal resolution. Moreover, the synthesis procedure of Ti3C2Tx allows large-scale production for establishing biointerfaces across multiple scales.24,25 Ti3C2Tx-based photothermal stimulation will provide a powerful toolset for understanding complex neuronal functions and enable optical-based therapeutics for neurological disorders.
Results and Discussion
2D Ti3C2Tx Is a Near-Infrared Absorber
Ti3C2Tx flakes were synthesized using a well-established protocol (see Materials and Methods),26 resulting in 2D flakes with a size of 9.72 ± 1.84 μm2 and thickness of 2.12 ± 0.41 nm based on atomic force microscopy (AFM) measurements, which suggests the presence of monolayer flakes (Figure 1A and Table S1).27 Ti3C2Tx flakes were confirmed via Raman spectroscopy (Figure 1B), where the spectra of individual flakes present four characteristic peaks (Figure 1B, red arrows). A few details can be gleaned from the spectra: the peaks at 147.4 and 200.3 cm–1 are attributed to the in-plane and out-of-plane stretching vibrations of Ti atoms, respectively, and the peaks at 640.4 and 723.1 cm–1 indicate in-plane and out-of-plane stretching vibrations of C atoms (Figure 1B and Table S2).28,29 The surface termination of the Ti3C2Tx flakes is dominated by −O groups, as indicated by the peak at 385.3 cm–1 (Figure 1B, gray region).28,29 Other terminations include −OH and −F groups, as a result of etching in an aqueous fluorine-containing solution (Figure 1B).
Figure 1.
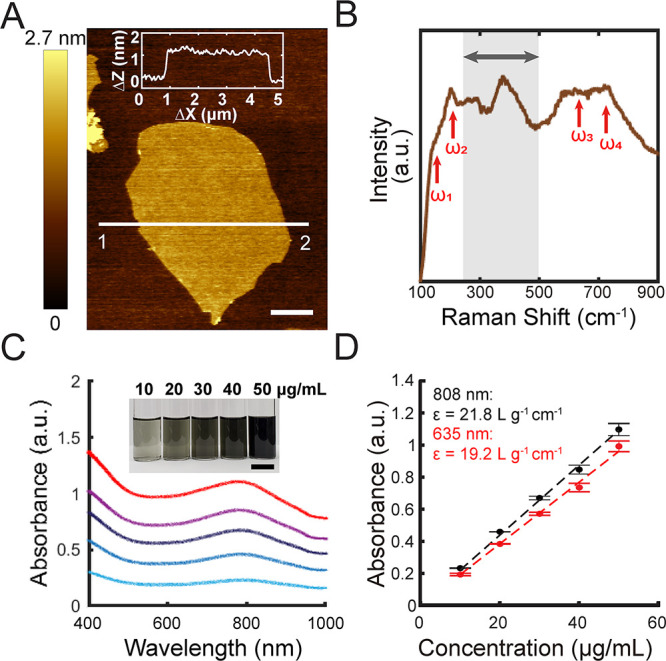
Structural and optical characterization of Ti3C2Tx. (A) Representative atomic force microscopy (AFM) image of a single-layer Ti3C2Tx flake dispersed on a Si/300 nm SiO2 substrate with an average thickness of 1.16 ± 0.11 nm. Inset: Thickness profile from point 1 to point 2. Scale bar is 1 μm. (B) Representative Raman spectrum of dispersed Ti3C2Tx flakes. Red arrows indicate the internal vibration modes of Ti3C2Tx, and the gray region indicates vibration modes of surface terminations, Tx. (C) Average vis–NIR absorption spectra of Ti3C2Tx suspensions of different concentrations (bottom to top: 10, 20, 30, 40, and 50 μg/mL) (n = 3). Inset: Optical image of Ti3C2Tx suspensions of different concentrations. Scale bar is 2 cm. (D) Absorbance as a function of Ti3C2Tx suspension concentration at 635 nm (red) and 808 nm (black), respectively. Data are presented as mean ± SD (n = 3).
Two-dimensional Ti3C2Tx flakes exhibit surface plasmon (SP) resonance where the interband transition to the vacant energy state of the functional groups results in an enhanced absorption in the NIR window.30,31 Similar optical absorption properties were observed in our Ti3C2Tx suspensions (Figure 1C), where the suspensions have an absorption peak at ca. 785 nm due to the localized surface plasmon resonance (LSPR) effect (Figure 1C).30,32,33 The extinction coefficients at 635 and 808 nm were calculated as 19.2 and 21.8 L g–1 cm–1, respectively (Figure 1D), which is in good agreement with published data (25.2 L g–1 cm–1 at 808 nm).19 Compared with NIR-active absorbers such as AuNRs,34 and additional absorbers such as SiNWs or NT-3DFG,5,35 the position of the transverse surface plasmon peak in Ti3C2Tx does not depend on the lateral flake size due to the consistency of interband transition and can be tuned by altering the surface chemistry of the material via the synthesis protocol.36,37 The NIR window is crucial for biomedical applications, especially for in vivo studies, since the radiation in this window has deeper penetration depths in tissue due to the relatively low absorption by water and hemoglobin.38 Therefore, Ti3C2Tx emerges as a promising candidate for NIR photothermal modulation.
Single-Flake Ti3C2Tx Is Photothermally Active
Ti3C2Tx exhibits high photothermal conversion efficiency and has been used in PTT for tumor ablation and enhanced water evaporation.19,22 However, the photothermal performance of Ti3C2Tx has only been evaluated at the macroscale using suspensions.19,22,23 Such measurements do not represent the photothermal effect at a single-flake level due to the interference from the solvent. Precise evaluation of the photothermal effect of Ti3C2Tx flakes is necessary prior to stimulating cells and tissues with subcellular precision. Here, we have characterized the photothermal performance of single Ti3C2Tx flakes using a micropipette-based technique (Figure 2A, Figure S1) (see Materials and Methods).5
Figure 2.
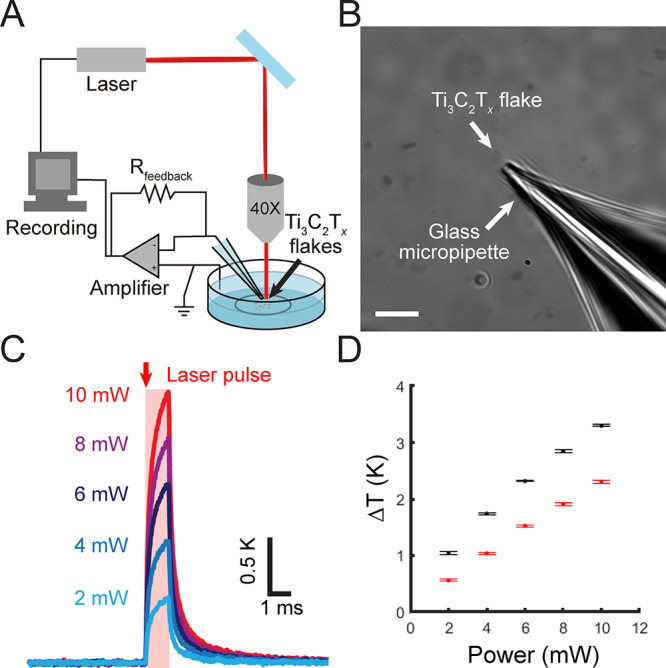
Single Ti3C2Tx flake is photothermally active. (A) Schematic of the micropipette-based temperature measurement technique to evaluate the photothermal response of isolated Ti3C2Tx flakes. (B) Optical image of an isolated Ti3C2Tx flake with a glass micropipette adjacent to the flake. Scale bar is 10 μm. (C) Average temperature change as a function of time for an isolated Ti3C2Tx flake (shown in B) under 635 nm laser illumination with a pulse width of 1 ms and different incident laser powers. Data are presented as the average of 10 individual pulses. (D) Maximum temperature change as a function of incident laser power measured for two isolated representative Ti3C2Tx flakes under 635 nm (red) and 808 nm (black) laser pulses with a pulse width of 1 ms. Data are presented as mean ± SD for 10 individual laser pulses.
The photothermal performance of Ti3C2Tx was characterized with both 635 and 808 nm lasers (Figure 2 and Figure S2). For a representative isolated flake (Figure 2B), the local temperature change increased from 0.68 ± 0.02 to 2.31 ± 0.03 K with an increase in the incident laser power from 2 to 10 mW, respectively (635 nm laser, 1 ms pulse, ca. 20 μm spot size) (Figure 2C, Figure S2, and Table S3). Illuminating isolated Ti3C2Tx flakes with a NIR laser (808 nm, 10 mW, 1 ms pulse, ca. 20 μm spot size) resulted in a local temperature change of 3.30 ± 0.02 K (Figure 2D, Figure S2, and Table S4). The greater photothermal performance at 808 nm is attributed to Ti3C2Tx’s increased absorption in the NIR window. Our results present that the energy density required by isolated Ti3C2Tx flakes to elicit a similar rise in local temperature is at least 1 order of magnitude lower than for AuNRs39 and AuNPs,10 2 orders of magnitude lower than Si-based materials (SiNWs and Au-decorated SiNWs),12,13 and comparable to recently developed C-based materials (NT-3DFG) (Table S7).5 Thus, Ti3C2Tx is an outstanding photothermally active material at the single-flake level.
To illustrate the ease of implementing Ti3C2Tx in photothermal modulation of electrophysiology, we have also evaluated the photothermal response of Ti3C2Tx as a film. We prepared Ti3C2Tx films by drop-casting known concentrations of Ti3C2Tx suspensions onto glass coverslips (Figure S3; see Materials and Methods). The top-view scanning electron microscopy (SEM) images of a 25 μg/cm2 Ti3C2Tx film exhibited a compact morphology, and the cross-section SEM image revealed that the film thickness is 90 ± 35 nm (n = 3 samples, 5 images per sample, 3 measurements per image) (Figure S3). For a representative spot on 25 μg/cm2 Ti3C2Tx, the local temperature change was 10.66 ± 0.05 K with the incident laser power of 10 mW (635 nm laser, 1 ms pulse, ca. 20 μm spot size). The measured temperature change is ca. 5-fold higher compared to individual Ti3C2Tx flakes, which is attributed to the accumulating photothermal effect of multiple flakes (Figure S4 and Table S5). Thus, both the photothermally active Ti3C2Tx films and flakes can potentially allow photothermal modulation of cellular activity with lower incident energies.
Ti3C2Tx Flakes Adhere to the Cell Membrane
Photothermal stimulation of cells leverages on inducing an instantaneous change in the cell membrane capacitance.9 This requires the photothermally active agent to be in close proximity of the plasma membrane.9 We investigated the interaction between Ti3C2Tx flakes and dorsal root ganglion (DRG) neurons with two types of interfaces: (1) DRG neurons directly cultured on Ti3C2Tx films and (2) DRG neurons incubated with dispersed Ti3C2Tx flakes. A 25 μg/cm2 Ti3C2Tx film was selected due to its high coverage as well as its semitransparent nature, which allowed transmitted light imaging of the DRG cells using a custom-built microscope (Figure S5). Incubating Ti3C2Tx films in physiological conditions did not result in a change of structure and surface terminations, proved by Raman spectra and SEM images before and after incubation. (Figure S6).
Cultured DRG neurons adhered well to the Ti3C2Tx film without any delamination (Figure 3A and B). The Ti3C2Tx film was observed only below the cultured cells, and no Ti3C2Tx flakes were observed inside the cell membrane of the DRG neurons (Figure 3A and B). In the case of dispersed Ti3C2Tx flakes on DRG neurons, the flakes were randomly distributed throughout the cell culture and adhered to the cell membrane without any observed internalization after a 24 h incubation period (Figure 3C,D and Figure S7). This close interaction between Ti3C2Tx flakes and DRG neurons establishes a promising interface to allow an efficient photothermal stimulation.9
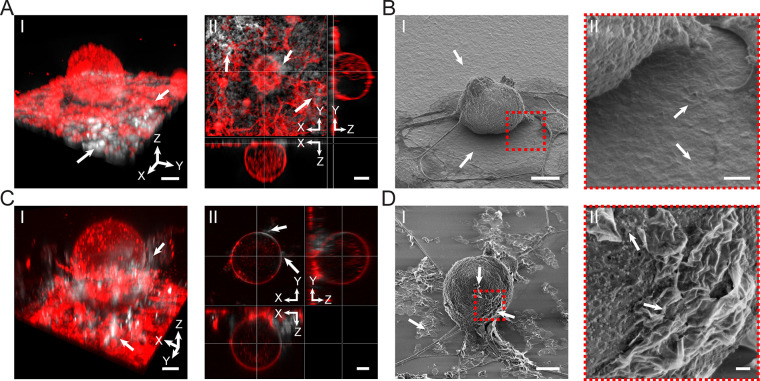
Figure 3.
Ti3C2Tx interfaces with DRG neurons. (A) Interface between Ti3C2Tx films and DRG neurons. (I) Representative 3D reconstructed confocal laser scanning fluorescence image of DRG neurons labeled with plasma membrane stain (red color, CellMask plasma membrane dye) seeded on a 25 μg/cm2 Ti3C2Tx film (white color). The white arrows denote Ti3C2Tx flakes. Scale bar is 10 μm. (II) Orthogonal sections of the cell in I. Scale bar is 10 μm. (B) Representative SEM image of DRG neurons seeded on a Ti3C2Tx film. Scale bar is 10 μm. (II) Expanded view of the marked red dashed box in I. Scale bar is 1 μm. (C) Interface between dispersed Ti3C2Tx flakes and DRG neurons. (I) Representative 3D reconstructed confocal laser scanning fluorescence image of DRG neurons labeled with plasma membrane stain (red color, CellMask plasma membrane dye) incubated for 24 h with a dispersion of 100 μg/mL Ti3C2Tx flakes (white color). The white arrows denote Ti3C2Tx flakes attached to the neuron. Scale bar is 10 μm. (II) Orthogonal sections of the cell in I. Scale bar is 10 μm. (D) Representative SEM image of a DRG neuron incubated with 100 μg/mL Ti3C2Tx flakes. White arrows denote Ti3C2Tx flakes interfaced with the neuron and dispersed on the substrate. Scale bar is 10 μm. (II) Expanded view of the marked red dashed box in I. Scale bar is 1 μm.
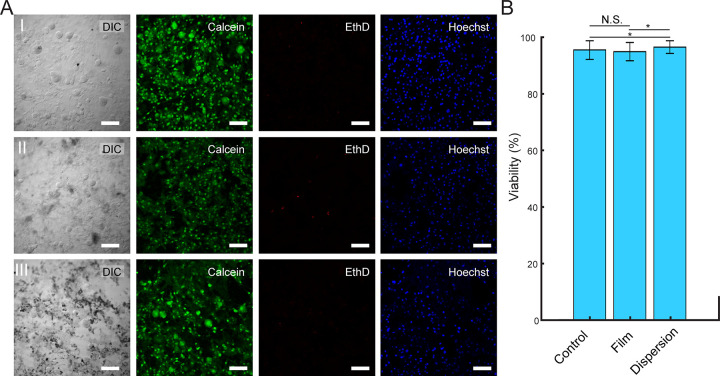
Prior to employing Ti3C2Tx films and flakes for photothermal stimulation, it is necessary to evaluate their cytotoxicity. To this end, we investigated the cytotoxicity by incubating DRG neurons for up to 6 days with Ti3C2Tx films (25 μg/cm2) and Ti3C2Tx flakes (100 μg/mL). The calculated viabilities were 95.4 ± 3.4%, 94.8 ± 3.2%, and 96.5 ± 2.2% for control samples, Ti3C2Tx films, and dispersed Ti3C2Tx flakes, respectively (Figure 4). The results indicate that Ti3C2Tx (as films or flakes) has no detectable influence on neuronal viability (Figure 4).
Figure 4.
Ti3C2Tx films and dispersions do not have detectable influence on DRG neuron viability. (A) Live/Dead assay performed on DRG neurons incubated (I) without Ti3C2Tx, (II) with 25 μg/cm2 Ti3C2Tx film, and (III) with a 100 μg/mL dispersion of Ti3C2Tx flakes for 6 days. Green (Calcien AM), red (ethidium homodimer-1), and blue (Hoechst) denote live cells, dead cells, and cell nuclei, respectively. Scale bars are 100 μm. (B) Viability (%) of DRG neurons incubated without Ti3C2Tx (control), on a 25 μg/cm2 Ti3C2Tx film (film), and with a 100 μg/mL dispersion of Ti3C2Tx flakes (dispersion). Data are presented as mean ± SD (n = 5 dishes per condition, 10 images per dish). N.S. denotes no significant difference. The asterisks (*) denote statistically significant difference with p < 0.05 (one-way ANOVA and post hoc Tukey).
Ti3C2Tx Film Allows Photothermal Stimulation of the DRG Network
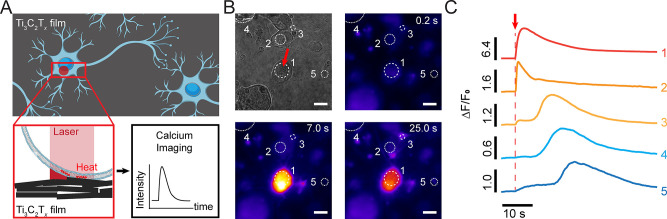
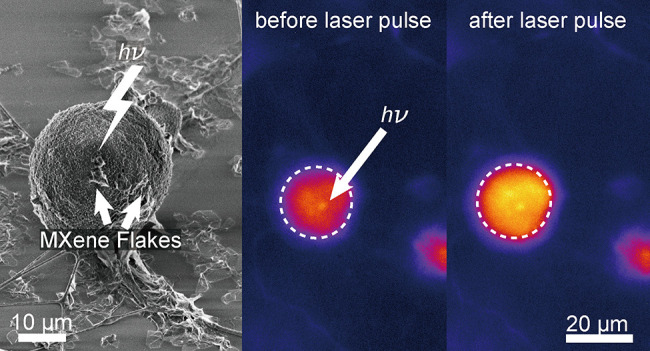
To demonstrate the photothermal neuronal modulation capabilities of the Ti3C2Tx film, DRG neurons were cultured on a Ti3C2Tx film, and neuronal electrical activity during stimulation was evaluated using Ca2+ imaging (Figure 5A and Figure S8). Ca2+ is a ubiquitous secondary messenger that plays a key role in signal transduction in excitable cells. Therefore, monitoring changes in Ca2+ intracellular concentration during an action potential can be used as an indicator to study cellular electrical activity.5,40 To evaluate the effect of photothermal stimulation on the Ca2+ dynamics, DRG neurons were labeled with a Ca2+ indicator, CalBryte 520 AM (see Materials and Methods). Ca2+ influx was observed after applying a single 1 ms, 10 mW laser pulse (635 nm, 10 μJ (ca. 3.2 J/cm2) per pulse) to the DRG neuron–film interface (Figure 5B, Movie S1). The intracellular Ca2+ transient of the laser-targeted neuron (Figure 5B, region of interest 1 (ROI 1)) demonstrated a rapid rise and slow decay, which is in agreement with the reported Ca2+ transients for DRG neurons (Figure 5C).5,35 The Ti3C2Tx film-based optical modulation of DRG neurons is highly reproducible (Figure S8, Table S6, and Movie S2). Photothermal stimulation with high-energy laser pulses can potentially lead to cellular phototoxicity due to the high local temperature change at the cell–material interface.5 Therefore, it is necessary to minimize the local temperature change required for safe photothermal stimulation by optimizing the overall energy of the incident laser pulse.5 We observed that laser energies as low as 2 μJ (ca. 0.6 J/cm2) incident at the DRG–Ti3C2Tx interface can successfully stimulate DRG neurons (Table S6). Stimulation of the targeted DRG neuron resulted in delayed Ca2+ transients in neighboring cells (Figure 5B and C, ROI 2–5, and Movie S1). The propagation of the Ca2+ wave in the network is attributed to the presence of gap junctions and extracellular diffusion of agonists between the cells.41−43 The exact communication mechanisms between the cells need further investigations.
Figure 5.
DRG electrical activity can be modulated by the Ti3C2Tx film. (A) Schematic representing a photothermal stimulation event at the interface between DRG neurons and the Ti3C2Tx film. (B) Bright field and time series fluorescence images of a representative DRG neuron interfaced with a Ti3C2Tx film and labeled with a Ca2+ indicator (CalBryte 520 AM). A 635 nm laser pulse of 10 mW power and 1 ms pulse duration was applied at t = 5.6 s. Red arrow indicates the laser target spot. The white circles denote the ROIs for fluorescence intensity analysis. Scale bars are 20 μm. (C) Normalized Ca2+ fluorescence intensity as a function of time for the ROIs marked in B. Red arrow denotes the starting point of the applied laser pulse (t = 5.6 s).
As reported previously, photothermal stimulation with Si-, Au-, and C- based materials follows the opto-capacitive mechanism.5,9,35,44 The opto-capacitive mechanism dictates that the light-induced temperature gradient (dT/dt) close to the cell membrane will result in a change in the membrane capacitance, followed by the generation of a transient capacitive current, Ic.9 Thus, targeting the laser pulse away from the cell–film interface will not generate sufficient Ic to elicit an action potential. To illustrate this point, illuminating the Ti3C2Tx film at a point away from a DRG neuron with the same laser pulse conditions did not result in any change in Ca2+ fluorescence intensity (Figure S9). This corroborates that the photothermal stimulation with the Ti3C2Tx film requires a close interface between the film and the target DRG neurons.
DRG–Ti3C2Tx Flake Interface Allows High-Resolution Neuronal Modulation
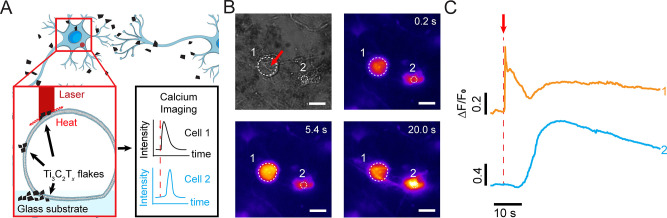
To achieve optical stimulation with subcellular resolution without coating the entire substrate with Ti3C2Tx, isolated Ti3C2Tx flakes were interfaced with DRG neurons (Figure 6A). A single laser pulse (635 nm, 18 mW, and 1 ms pulse, 18 μJ (ca. 5.7 J/cm2) per pulse) at the DRG neuron and Ti3C2Tx flake interface resulted in an elicit action potential as can be seen in the apparent intracellular Ca2+ transient (Figure 6B and C, Movie S3, and Table S6). We note that the energy needed per pulse to stimulate a DRG neuron is higher for dispersed flakes as compared to films due to the higher flake density of the Ti3C2Tx film, which results in a higher local temperature change (Figure S4 and Table S5). Targeting the laser at a point on the DRG neuron but not on the Ti3C2Tx flake did not result in any Ca2+ transients (off-Ti3C2Tx, Figure S10). Additionally, targeting the laser at a point away from the DRG neurons did not result in any Ca2+ transients as well (off-cell, Figure S10). These results indicate that isolated Ti3C2Tx flakes allow effective optical modulation with subcellular spatial resolution.
Figure 6.
Optical modulation of DRG activity with dispersed Ti3C2Tx flakes. (A) Schematic of DRG neuron network and dispersed Ti3C2Tx flake interface. (B) Bright field image and time series fluorescence images of a representative DRG neuron labeled with a Ca2+ indicator (CalBryte 520 AM). A 635 nm laser pulse of 18 mW power and 1 ms pulse duration was applied at t = 5.0 s. Red arrow indicates the laser target spot. The white circles denote the ROIs for fluorescence intensity analysis. Scale bars are 20 μm. (C) Normalized Ca2+ fluorescence intensity as a function of time for the ROIs marked in B. Red arrow denotes the starting point of the applied laser pulse (t = 5.0 s).
Conclusions
In this work, we demonstrate that 2D Ti3C2Tx (MXene) can be used for remote and nongenetic modulation of neuronal electrical activity with subcellular resolution. We have measured the Ti3C2Tx photothermal performance at the single-flake level and observed a local temperature change of 2.31 ± 0.03 and 3.30 ± 0.02 K under 635 and 808 nm pulses (10 mW and 1 ms), respectively. Ti3C2Tx flakes interfaced with DRG neurons do not alter cellular viability and are not internalized; instead they adhere closely to the cell membrane. Both Ti3C2Tx films and flakes enabled photothermal stimulation of DRG neurons with microjoule-scale energies per laser pulse (2 μJ (ca. 0.6 J/cm2) for film and 18 μJ (ca. 5.7 J/cm2) for flakes). The effective energy densities for photothermal stimulation with Ti3C2Tx are at least 1 order of magnitude lower than those for existing Au-9,10 and Si-11−13 based photothermal agents and comparable to C-based materials (NT-3DFG) (Table S8).5 Compared with a photoelectrically active p–i–n Si membrane and SiNWs,12,45 Ti3C2Tx necessities similar incident energy densities to stimulate neurons. Besides the low incident energy densities, Ti3C2Tx will allow translation of the reported photothermal stimulation approach in future biomedical engineering applications and interventions with the ease of straightforward and large-scale synthesis.24 Furthermore, this technique can be potentially combined with electrical stimulation and employed in tissue engineering for building the prototype of practical therapeutics.46−48
Materials and Methods
Synthesis of Ti3C2Tx
Ti3C2Tx synthesis follows a previously published protocol.26 TiC (99.5%, ∼325 mesh, Alfa Aesar, catalog no. 40178-30), Ti (99.5%, ∼325 mesh, Alfa Aesar, catalog no. 42624-22), and Al (99.5%, ∼325 mesh, Alfa Aesar, catalog no. 11067-30) powders in a 2:1:1 mass ratio were mixed with zirconia balls at a 2:1 zirconia-to-powder ratio in a ball mill at 70 rpm for 18 h. The mixed powder was placed in an alumina crucible, covered by graphite foil, and placed into a tube furnace. The tube furnace was purged with a 100 sccm Ar flow for 30 min at room temperature followed by heating to 1380 °C for 2 h at a heating/cooling rate of 3 °C/min. The Ti3AlC2 block was milled to form a Ti3AlC2 powder using a TiN-coated milling bit. One gram of Ti3AlC2 powder was then slowly mixed with 10 mL of 9 M HCl (Fisher Scientific, catalog no. 320331) and stirred for 4 h at room temperature. The mixture was vacuum filtered with a 5 μm filter membrane, and the residual powder was washed with DI-H2O until the pH of the filtrate was ca. 6. The washed Ti3AlC2 powder was dried in a vacuum oven for 6 h at 80 °C and was collected after sieving through a 450-mesh (32 μm) particle sieve. A 6 mL amount of DI-H2O, 12 mL of 12 M HCl, and 2 mL of 50 wt % HF (Acros Organics, catalog no. AC223330250) were added in a 60 mL high-density polyethylene bottle. A 1 g portion of Ti3AlC2 power was added to the prepared solution and mixed at 400 rpm for 24 h at 35 °C.
The mixture was centrifuged in a 175 mL centrifuge tube at 3500 rpm for 5 min, followed by decantation and redispersing the sediment with DI-H2O. This process was repeated until the supernatant reached a pH of ca. 6 to obtain a multilayer Ti3C2Tx sediment. The sediment was dispersed into 50 mL of a 0.5 M LiCl solution (99.3% Chem-Impex Int. catalog no. 30595) and stirred at 400 rpm for 4 h at room temperature. The sediment was washed with DI-H2O and centrifuged at 3500 rpm for 5 min. The supernatant was decanted, and the sediment was redispersed in DI-H2O and centrifuged at 3500 rpm for 1 h. This step was repeated three more times. Finally, the Ti3C2Tx suspension was centrifuged at 3500 rpm for 20 min and the supernatant was collected.
Atomic Force Microscopy (AFM)
AFM was performed using a MultiMode 8-HR (Bruker) in tapping mode. A 0.5 mg/mL Ti3C2Tx suspension was spin-coated at 2000 rpm for 2 min on an O2-plasma-treated Si/300 nm SiO2 substrate, followed by spinning at 5000 rpm for 15 s to dry the sample. A Si tip (Budget Sensors, USA, Tap300Al-G; f0 = 300 kHz, k = 40 N/m) was used to characterize the samples. Scan speed was 0.8 Hz.
Raman Spectroscopy
Raman spectra of isolated Ti3C2Tx flakes were acquired using NT-MDT Spectra (NT-MDT Spectrum Instruments) with a 532 nm excitation laser using a 100×/0.7 NA objective. To prepare the sample, 20 μL of a 10 μg/mL Ti3C2Tx suspension was drop-casted on a Si/600 nm SiO2 substrate coated with 100 nm Au. The spectra were acquired with a 0.5 neutral density filter and a 30 s acquisition time. The measured laser power through the objective was 1.95 mW (PM100D power meter equipped with a S121C detector, Thorlabs). The spectra were acquired from 10 randomly distributed points across 3 independent samples.
Visible–Near Infrared (Vis-NIR) Absorption Spectroscopy
Vis–NIR absorption spectroscopy was characterized using an ASEQ LR1-T broad range photospectrometer (ASEQ Instruments). Vis–NIR absorption spectra were acquired from five different concentrations of Ti3C2Tx suspensions (10, 20, 30, 40, and 50 μg/mL). Three independent spectra were measured for each concentration, and the results are presented as the mean of these three independent measurements. The extinction coefficient was determined following the Beer–Lambert law:49
where A is the absorbance, ε is the extinction coefficient, L is the length of the cuvette (1 cm), and c is the concentration of Ti3C2TX suspensions. Linear curve fitting was performed using MATLAB (MathWorks) to calculate the extinction coefficients.
Scanning Electron Microscopy (SEM)
SEM imaging was performed using a FEI Quanta 600 field emission gun SEM. Images were acquired with an acceleration voltage of 2–20 kV and 5 mm working distance. No additional conductive coating was applied to any of the samples for SEM imaging.
Ti3C2Tx Film Preparation
Ti3C2Tx films of different densities were prepared on 0.9 cm × 0.9 cm microscope cover glass (Fisher Scientific, catalog no. 12-541A). The substrates were cleaned in an ultrasonic bath (Crest Ultrasonics, model no. CP230D) in acetone for 5 min, followed by isopropyl alcohol washing and N2 blow drying. The surfaces were coated with poly-l-lysine (PLL) (Sigma-Aldrich, catalog no. P8920). Ti3C2Tx suspensions (50 μL) of different concentrations were drop-casted and air-dried at room temperature to obtain 15, 25, 35, 45, and 55 μg/cm2 Ti3C2Tx films.
Photothermal Characterization
Photothermal characterization of isolated Ti3C2Tx flakes was performed using a previously described micropipette technique with a custom-built microscope.5 635 or 808 nm laser (NaKu Technology) pulses were delivered using a custom-built microscope with a 40×/0.80 NA water immersion objective (Nikon). The duration of the laser pulses was controlled using a transistor–transistor logic (TTL) signal. Prior to the photothermal characterization, the output power of the laser through the objective was calibrated under continuous wave operation using a photodiode power meter (PM100D with an S121C detector, Thorlabs).
Voltage-clamp measurement was performed using a patch-clamp amplifier (A-M Systems model 2400) controlled by WinWCP software (open-source). Micropipettes were pulled from glass capillaries with a 1.5 mm outer diameter and 0.86 mm inner diameter (Sutter Instruments, catalog no. BF150-86-10) using a micropipette puller (P-97, Sutter Instruments) for a final resistance of ca. 1–2 MΩ in 1× phosphate buffered saline (1× PBS) solution (Corning, catalog no. 21-040-CV). For photothermal characterization, Ti3C2Tx flakes were dispersed on a PLL-modified glass-bottom dish (Matsunami, catalog no. D35-14-1.5-U). Briefly, the dish was treated under UV-Ozoneat 25 °C for 10 min followed by addition of 100 μL of 0.02% (w/v) PLL for 5 min. The PLL solution was aspirated, and the dish was washed three times with DI-H2O and N2 blow-dried. Twenty microliters of a 10 μg/mL Ti3C2Tx suspension was drop-casted on the surface-treated dish and air-dried at room temperature. During the photothermal characterization, the dish was filled with 4 mL of 1× PBS solution and a Ag/AgCl wire was placed in the chamber. The temperature of the solution was continuously measured using a digital thermometer (Signstek, 6802-II). The isolated Ti3C2Tx flake was selected through the microscope, and the glass micropipette tip was controlled by a manipulator to get approximately 1–2 μm from the selected flake to record the local temperature change. The tip was located at the edge of the flake to avoid the light reflected by the pipette walls. The current through the micropipette and the resistance of the micropipette were continuously recorded in voltage-clamp mode with holding potential Vp of 75 mV. One millisecond laser pulses with power ranging from 2 to 10 mW were applied. The photothermal current (ΔIthermal(t)) can be expressed using the following equation:5
| 1 |
where ΔIthermal(t), R0, R(t), and I0 are the photothermal current, the micropipette resistance at room temperature, the micropipette resistance during laser illumination, and the dark-state current, respectively. To calibrate the resistance of the micropipette as a function of temperature, 4 mL of 1× PBS solution was heated to ca. 50 °C and added into a Petri dish. A thermocouple was placed near the micropipette’s tip to measure the temperature. To establish a calibration curve, the micropipette’s resistance was recorded until a bath temperature of 25 °C was reached (Figure S1). The dependence between the micropipette’s resistance and temperature follows the Arrhenius law:50
| 2 |
where R(t), R0, A, T, and C are the measured micropipette resistance, the room-temperature micropipette resistance, the calibration curve slope, the temperature, and the calibration curve intercept, respectively. Photothermal characterization of 7 and 10 independent Ti3C2Tx flakes was performed with 635 and 808 nm lasers, respectively. Photothermal characterization of Ti3C2Tx films (25 μg/cm2) was performed under 635 nm laser illumination at 10 randomly selected spots across two representative film samples.
Dorsal Root Ganglion (DRG) Cell Culture
DRG neurons were obtained from adult male Sprague–Dawley rats (Envigo) and were harvested as described previously.5,42 Rats with weights between 240 and 400 g were pair-housed with a 12:12 light:dark cycle with food and water ad libitum, at the University of Pittsburgh. The experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and followed NIH guidelines for laboratory animal use. Prior to harvesting DRG neurons, rats were anesthetized with an intraperitoneal injection of a mixture containing 55 mg/kg ketamine (Henry Schein, catalog no. 056344), 5.5 mg/kg xylazine (Henry Schein, catalog no. 033197), and 1.1 mg/kg aceprozamine (Henry Schein, catalog no. 003845). Rats were subsequently perfused with 1× PBS, and DRG neurons were collected, enzymatically treated, and mechanically dissociated to prepare cell seeding suspensions.
DRG neurons were then seeded on uncoated and Ti3C2Tx film coated glass-bottom dishes. Briefly, dishes were treated with UV-Ozone at 25 °C for 10 min and sterilized with 70% ethanol (Pharmco-Aaper, catalog no. 111000200) and UV illumination for 1 h. The dishes were then rinsed three times with sterile DI-H2O, treated with 300 μL of 50 μg/mL poly-d-lysine (PDL) (Sigma-Aldrich, catalog no. P6407) at room temperature for 1 h, rinsed three times with sterile DI-H2O, and dried for 30 min in a cell culture hood under sterile conditions. The PDL-coated dishes were incubated with 250 μL of 20 μg/mL Laminin (Corning, catalog no. 354232) at 37 °C with 5% CO2 for 1 h and subsequently rinsed three times with sterile 1× PBS. DRG neurons were seeded on the PDL-laminin-treated dishes and incubated at 37 °C with 5% CO2 for 3 h prior to adding 4 mL of media. The media was composed of Dulbecco’s modified Eagle’s medium (Corning, catalog no. 15-013-CM) + 1% Glutamax (ThermoFisher, catalog no. 35050-061) + 1% penicillin/streptomycin (ThermoFisher, catalog no. 15140122) + 10% fetal bovine serum (Invitrogen, catalog no. 10082147). The DRG neurons were incubated at 37 °C with 5% CO2 for 24–48 h until use.
Interfacing DRG Neurons with Ti3C2Tx
To investigate the DRG-Ti3C2Tx interfaces, DRG neurons were interfaced with Ti3C2Tx films and flakes. DRG–film interfaces followed the sample preparation protocol described above. To interface DRG neurons and dispersed Ti3C2Tx flakes, a Ti3C2Tx suspension was sterilized under UV illumination for 1 h and added to the DRG neuron culture with a final concentration of 100 μg/mL. The dishes with dispersed flakes were incubated at 37 °C with 5% CO2 for 24 h. CellMask orange plasma membrane stain (ThermoFisher, catalog no. C10045) was used to stain the cell membrane. The cell cultures were incubated with dye (5 μg/mL) for 15 min at 37 °C with 5% CO2. Tyrode’s buffer solution (Sigma, catalog no. T2145) with a pH of ca. 7.4 supplemented with 1 g/L NaHCO3 (Sigma, catalog no. S576) was constantly perfused with carbogen gas containing 5% CO2 and 95% O2 (Airgas) for 20 min. The samples were gently washed three times with carbogenated Tyrode’s solution. The DRG neuron and Ti3C2Tx interface was imaged using z-stack live-cell imaging with differential interference contrast (DIC) and scanning laser confocal fluorescence imaging with a 40×/0.80 NA water immersion objective. The 3D reconstructions and orthogonal sections were generated using Imaris 3/4D Image Visualization and Analysis software (Oxford Instruments).
The samples were then fixed using 4% paraformaldehyde (Electron Microscopy Sciences, catalog no. 15710) in 1× PBS for 20 min on a plate shaker at room temperature. The samples were rinsed three times with 1× PBS, followed by washing three times with DI-H2O. DI-H2O was serially replaced with 100% ethanol (25%, 50%, 75%, 90%, and 100%). During each exchange, the samples were incubated at room temperature for 10 min. The 100% ethanol was serially exchanged with hexamethyldisilazane (HMDS) (Sigma-Aldrich, catalog no. 440191) (50% and 100%) and incubated at room temperature for 20 min during each exchange. HMDS was then aspirated, and the samples were dried in a clean hood at room temperature. Fixed and dried samples were imaged using SEM without any additional surface coatings with an acceleration voltage and working distance of 2 kV and 5 mm, respectively.
Viability Assay
Viability analysis was performed on DRG neurons seeded on glass-bottom dishes (control), seeded on 25 μg/cm2 Ti3C2Tx films (film), and incubated with a 100 μg/mL dispersion of Ti3C2Tx flakes (dispersion). The samples were prepared following the above sample preparation protocols. For all samples, the seeding density of DRG neurons was 4500 cells/dish. To maintain neuronal phenotypes, culture media was supplemented with 10 nM/mL 2.5S NGF (Sigma-Aldrich, catalog no. 01-125). To minimize the proliferation of glial cells, mitotic inhibitors uridine (Sigma-Aldrich, catalog no. 3750) and deoxyuridine (Sigma-Aldrich, catalog no. D5412) were added to the culture media at a final concentration of 40 μM. The samples were maintained in culture at 37 °C with 5% CO2 for 6 days before performing the viability assay. For the assay, the samples were labeled with a Live/Dead assay kit (ThermoFisher, catalog no. L3224). The live cells were labeled with 2 μM calcein acetoxymethyl (Calcein AM); the dead cells were labeled with 4 μM ethidium homodimer-1 (EthD-1); and the nuclei were labeled with 1 μM Hoechst 33342 (ThermoFisher, catalog no. 62249). After adding the dyes, the samples were incubated for 15 min at 37 °C with 5% CO2. The samples were then washed three times with warm carbogenated Tyrode’s solution. DIC and scanning laser confocal fluorescence imaging were performed using an upright confocal microscope and a 20×/0.50 NA water immersion objective (Nikon). Viability quantification was performed for 5 dishes per condition and 10 images per dish. The viability was calculated as
Ca2+ Imaging of Photothermal Stimulation
To Ca2+ image the neurons during photothermal stimulation, DRG neurons interfaced with Ti3C2Tx films and dispersed Ti3C2Tx flakes were labeled with a Ca2+ indicator dye by incubating the samples with 5 μM CalBryte-520 (AAT Bioquest, catalog no. 20650) for 60 min at 37 °C followed by 30 min at room temperature in the dark. The samples were then washed three times with carbogenated Tyrode’s solution and were loaded on the stage of the custom-built microscope. A continuous flow of fresh carbogenated Tyrode’s solution at 22 °C was maintained through the sample during the photothermal stimulation experiment.
Bright field illumination was performed using a white LED (6 W LED dual gooseneck illuminator, AMScope, product no. SKU: LED-6W), while the fluorescence imaging was performed using a 473 nm laser (UltraLasers, product no. CST-L-473-50-OEM) and a green fluorescent protein (GFP) filter (Thorlabs, catalog no. MF525-39). The Ca2+ fluorescence and bright field images were acquired using a monochromatic complementary metal oxide semiconductor camera (Basler acA3088-57 μm, Basler) using Pylon Viewer (Basler). Bright field images were acquired before laser illumination, and the sequence of Ca2+ fluorescence images was acquired at five frames per second during laser illumination. The laser pulses from a 635 nm laser were controlled by TTL signal and applied on the flake–cell interface through a 40×/0.80 NA water immersion objective. ImageJ (Java-based open-source image processing software, NIH) and MATLAB were used for fluorescence intensity analysis. Data are presented as (F – F0)/F0vs time, where F0 is the average intensity of the first five seconds before applying the laser pulse, and F is the time-dependent fluorescence intensity. Photothermal stimulation of the film– and dispersed flake–cell interface was performed on 13 and 3 DRG neuron networks, respectively.
Acknowledgments
T.C.-K. acknowledges funding support from the National Science Foundation [Award No. CBET1552833], the Defense Advanced Research Projects Agency [Award No. AWD00001593 (416052-5)], and the National Institutes of Health [Award No. R21EB029164]. Y.G acknowledges funding support from the National Science Foundation [Award No. DMR-1740795]. F.V. acknowledges funding support from the National Institutes of Health [Award No. K12HD073945]. We also acknowledge support from the Department of Materials Science and Engineering Materials Characterization Facility supported by Grant MCF-677785.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.1c04431.
Movies S1: Ca2+ influx achieved by DRG–Ti3C2Tx film interfaces (MP4)
Movie S2: Ca2+ influx achieved by DRG–Ti3C2Tx film interfaces (MP4)
Movie S3: Ca2+ influx achieved by DRG–Ti3C2Tx flake interface (MP4)
Figures of micropipette calibration, photothermal response of Ti3C2Tx flakes under varying laser wavelengths, characterization of Ti3C2Tx films, photothermal response of Ti3C2Tx films, photothermal stimulation microscope setup, stability evaluation of Ti3C2Tx film in physiological conditions, orthogonal sections of DRG–Ti3C2Tx flake interface at different z-axis positions, Ca2+ influx achieved by DRG–Ti3C2Tx film interface, off-cell stimulation of DRG–Ti3C2Tx film interface, off-cell and off-Ti3C2Tx stimulation of DRG–Ti3C2Tx flake interface; tables of AFM results, Raman spectra results, photothermal response of Ti3C2Tx flakes (635 and 808 nm illumination), photothermal response of Ti3C2Tx films (635 nm illumination), laser conditions on DRG–Ti3C2Tx film interface and DRG–Ti3C2Tx flake interface to stimulate DRG neurons, comparison of temperature rise of reported photothermal agents, comparison of DRG neuron stimulation energy densities of reported photothermal agents (PDF)
Author Contributions
T.C.-K. designed the research; Y.W., R.G., J.E.H., A.G., and D.A.P. performed the experiments; all authors analyzed the data and discussed the results; and Y.W., R.G., and T.C.-K. wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Pisanello F.; Sileo L.; De Vittorio M. Micro- and Nanotechnologies for Optical Neural Interfaces. Front. Neurosci. 2016, 10, 70. 10.3389/fnins.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. F.; Tian B. Nongenetic Optical Methods for Measuring and Modulating Neuronal Response. ACS Nano 2018, 12 (5), 4086–4095. 10.1021/acsnano.8b02758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musk E. An Integrated Brain-Machine Interface Platform with Thousands of Channels. Journal of Medical Internet Research 2019, 21 (10), e16194 10.2196/16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivnay J.; Wang H.; Fenno L.; Deisseroth K.; Malliaras G. G. Next-Generation Probes, Particles, and Proteins for Neural Interfacing. Science Advances 2017, 3 (6), e1601649 10.1126/sciadv.1601649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S. K.; Garg R.; Scopelliti M. G.; Pinto B. I.; Hartung J. E.; Kim S.; Murphey C. G.; Johnson N.; San Roman D.; Bezanilla F. Remote Nongenetic Optical Modulation of Neuronal Activity Using Fuzzy Graphene. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (24), 13339–13349. 10.1073/pnas.1919921117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nat. Methods 2011, 8 (1), 26–29. 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. G.; Homma K.; Villarreal S.; Richter C.-P.; Bezanilla F. Infrared Light Excites Cells by Changing Their Electrical Capacitance. Nat. Commun. 2012, 3 (1), 1–11. 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. D.; Kao C.; Jansen E. D.; Konrad P. E.; Mahadevan-Jansen A. Application of Infrared Light for in Vivo Neural Stimulation. J. Biomed. Opt. 2005, 10 (6), 064003 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- Carvalho-de-Souza J. L.; Pinto B. I.; Pepperberg D. R.; Bezanilla F. Optocapacitive Generation of Action Potentials by Microsecond Laser Pulses of Nanojoule Energy. Biophys. J. 2018, 114 (2), 283–288. 10.1016/j.bpj.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-de-Souza J. L.; Treger J. S.; Dang B.; Kent S. B.; Pepperberg D. R.; Bezanilla F. Photosensitivity of Neurons Enabled by Cell-Targeted Gold Nanoparticles. Neuron 2015, 86 (1), 207–217. 10.1016/j.neuron.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Carvalho-de-Souza J. L.; Wong R. C.; Luo Z.; Isheim D.; Zuo X.; Nicholls A. W.; Jung I. W.; Yue J.; Liu D.-J. Heterogeneous Silicon Mesostructures for Lipid-Supported Bioelectric Interfaces. Nat. Mater. 2016, 15 (9), 1023–1030. 10.1038/nmat4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Li X.; Liu B.; Yi J.; Fang Y.; Shi F.; Gao X.; Sudzilovsky E.; Parameswaran R.; Koehler K. Rational Design of Silicon Structures for Optically Controlled Multiscale Biointerfaces. Nature Biomedical Engineering 2018, 2 (7), 508–521. 10.1038/s41551-018-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y.; Jiang Y.; Acaron Ledesma H.; Yi J.; Gao X.; Weiss D. E.; Shi F.; Tian B. Texturing Silicon Nanowires for Highly Localized Optical Modulation of Cellular Dynamics. Nano Lett. 2018, 18 (7), 4487–4492. 10.1021/acs.nanolett.8b01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VahidMohammadi A.; Rosen J.; Gogotsi Y.. The World of Two-Dimensional Carbides and Nitrides (MXenes). Science 2021, 372 ( (6547), ), eabf1581. 10.1126/science.abf1581. [DOI] [PubMed] [Google Scholar]
- Rasool K.; Pandey R. P.; Rasheed P. A.; Buczek S.; Gogotsi Y.; Mahmoud K. A. Water Treatment and Environmental Remediation Applications of Two-Dimensional Metal Carbides (MXenes). Mater. Today 2019, 30, 80–102. 10.1016/j.mattod.2019.05.017. [DOI] [Google Scholar]
- Driscoll N.; Richardson A. G.; Maleski K.; Anasori B.; Adewole O.; Lelyukh P.; Escobedo L.; Cullen D. K.; Lucas T. H.; Gogotsi Y. Two-Dimensional Ti3C2 MXene for High-Resolution Neural Interfaces. ACS Nano 2018, 12 (10), 10419–10429. 10.1021/acsnano.8b06014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G.; Ciou J.-H.; Liu Y.; Jiang Y.; Lee P. S. Leaf-Inspired Multiresponsive MXene-Based Actuator for Programmable Smart Devices. Science Advances 2019, 5 (7), eaaw7956 10.1126/sciadv.aaw7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhon Y. I.; Koo J.; Anasori B.; Seo M.; Lee J. H.; Gogotsi Y.; Jhon Y. M. Metallic MXene Saturable Absorber for Femtosecond Mode-Locked Lasers. Adv. Mater. 2017, 29 (40), 1702496. 10.1002/adma.201702496. [DOI] [PubMed] [Google Scholar]
- Lin H.; Wang X.; Yu L.; Chen Y.; Shi J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17 (1), 384–391. 10.1021/acs.nanolett.6b04339. [DOI] [PubMed] [Google Scholar]
- Dai C.; Lin H.; Xu G.; Liu Z.; Wu R.; Chen Y. Biocompatible 2D Titanium Carbide (MXenes) Composite Nanosheets for pH-Responsive MRI-Guided Tumor Hyperthermia. Chem. Mater. 2017, 29 (20), 8637–8652. 10.1021/acs.chemmater.7b02441. [DOI] [Google Scholar]
- Naguib M.; Kurtoglu M.; Presser V.; Lu J.; Niu J.; Heon M.; Hultman L.; Gogotsi Y.; Barsoum M. W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23 (37), 4248–4253. 10.1002/adma.201102306. [DOI] [PubMed] [Google Scholar]
- Li R.; Zhang L.; Shi L.; Wang P. MXene Ti3C2: An Effective 2D Light-To-Heat Conversion Material. ACS Nano 2017, 11 (4), 3752–3759. 10.1021/acsnano.6b08415. [DOI] [PubMed] [Google Scholar]
- Han X.; Huang J.; Lin H.; Wang Z.; Li P.; Chen Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthcare Mater. 2018, 7 (9), 1701394. 10.1002/adhm.201701394. [DOI] [PubMed] [Google Scholar]
- Shuck C. E.; Sarycheva A.; Anayee M.; Levitt A.; Zhu Y.; Uzun S.; Balitskiy V.; Zahorodna V.; Gogotsi O.; Gogotsi Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater. 2020, 22 (3), 1901241. 10.1002/adem.201901241. [DOI] [Google Scholar]
- Naguib M.; Unocic R. R.; Armstrong B. L.; Nanda J. Large-Scale Delamination of Multi-Layers Transition Metal Carbides and Carbonitrides “MXenes. Dalton Transactions 2015, 44 (20), 9353–9358. 10.1039/C5DT01247C. [DOI] [PubMed] [Google Scholar]
- Mathis T. S.; Maleski K.; Goad A.; Sarycheva A.; Anayee M.; Foucher A. C.; Hantanasirisakul K.; Shuck C. E.; Stach E. A.; Gogotsi Y. Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ACS Nano 2021, 15 (4), 6420–6429. 10.1021/acsnano.0c08357. [DOI] [PubMed] [Google Scholar]
- Lipatov A.; Alhabeb M.; Lukatskaya M. R.; Boson A.; Gogotsi Y.; Sinitskii A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Advanced Electronic Materials 2016, 2 (12), 1600255. 10.1002/aelm.201600255. [DOI] [Google Scholar]
- Hu T.; Wang J.; Zhang H.; Li Z.; Hu M.; Wang X. Vibrational Properties of Ti3C2 and Ti3C2T2 (T= O, F, OH) Monosheets by First-Principles Calculations: A Comparative Study. Phys. Chem. Chem. Phys. 2015, 17 (15), 9997–10003. 10.1039/C4CP05666C. [DOI] [PubMed] [Google Scholar]
- Sarycheva A.; Gogotsi Y. Raman Spectroscopy Analysis of the Structure and Surface Chemistry of Ti3C2Tx MXene. Chem. Mater. 2020, 32 (8), 3480–3488. 10.1021/acs.chemmater.0c00359. [DOI] [Google Scholar]
- Sarycheva A.; Makaryan T.; Maleski K.; Satheeshkumar E.; Melikyan A.; Minassian H.; Yoshimura M.; Gogotsi Y. Two-Dimensional Titanium Carbide (MXene) as Surface-Enhanced Raman Scattering Substrate. J. Phys. Chem. C 2017, 121 (36), 19983–19988. 10.1021/acs.jpcc.7b08180. [DOI] [Google Scholar]
- Dillon A. D.; Ghidiu M. J.; Krick A. L.; Griggs J.; May S. J.; Gogotsi Y.; Barsoum M. W.; Fafarman A. T. Highly Conductive Optical Quality Solution-Processed Films of 2D Titanium Carbide. Adv. Funct. Mater. 2016, 26 (23), 4162–4168. 10.1002/adfm.201600357. [DOI] [Google Scholar]
- Jiang X.; Kuklin A. V.; Baev A.; Ge Y.; Ågren H.; Zhang H.; Prasad P. N. Two-Dimensional MXenes: From Morphological to Optical, Electric, and Magnetic Properties and Applications. Phys. Rep. 2020, 848, 1–58. 10.1016/j.physrep.2019.12.006. [DOI] [Google Scholar]
- El-Demellawi J. K.; Lopatin S.; Yin J.; Mohammed O. F.; Alshareef H. N. Tunable Multipolar Surface Plasmons in 2D Ti3C2Tx MXene Flakes. ACS Nano 2018, 12 (8), 8485–8493. 10.1021/acsnano.8b04029. [DOI] [PubMed] [Google Scholar]
- Eom K.; Im C.; Hwang S.; Eom S.; Kim T.-S.; Jeong H. S.; Kim K. H.; Byun K. M.; Jun S. B.; Kim S. J. Synergistic Combination of Near-Infrared Irradiation and Targeted Gold Nanoheaters for Enhanced Photothermal Neural Stimulation. Biomed. Opt. Express 2016, 7 (4), 1614–1625. 10.1364/BOE.7.001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Parameswaran R.; Li X.; Carvalho-de-Souza J. L.; Gao X.; Meng L.; Bezanilla F.; Shepherd G. M.; Tian B. Nongenetic Optical Neuromodulation with Silicon-Based Materials. Nat. Protoc. 2019, 14 (5), 1339. 10.1038/s41596-019-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleski K.; Shuck C. E.; Fafarman A. T.; Gogotsi Y. The Broad Chromatic Range of Two-Dimensional Transition Metal Carbides. Adv. Opt. Mater. 2021, 9 (4), 2001563. 10.1002/adom.202001563. [DOI] [Google Scholar]
- Anayee M.; Kurra N.; Alhabeb M.; Seredych M.; Hedhili M. N.; Emwas A.-H.; Alshareef H. N.; Anasori B.; Gogotsi Y. Role of Acid Mixtures Etching on the Surface Chemistry and Sodium Ion Storage in Ti3C2Tx MXene. Chem. Commun. 2020, 56 (45), 6090–6093. 10.1039/D0CC01042A. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Mancini M. C.; Nie S. Second Window for in Vivo Imaging. Nat. Nanotechnol. 2009, 4 (11), 710–711. 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J.; Needham K.; Brown W. G.; Nayagam B. A.; McArthur S. L.; Yu A.; Stoddart P. R. Gold-Nanorod-Assisted Near-Infrared Stimulation of Primary Auditory Neurons. Adv. Healthcare Mater. 2014, 3 (11), 1862–1868. 10.1002/adhm.201400027. [DOI] [PubMed] [Google Scholar]
- Grienberger C.; Konnerth A. Imaging Calcium in Neurons. Neuron 2012, 73 (5), 862–885. 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Giacobassi M. J.; Leavitt L. S.; Raghuraman S.; Alluri R.; Chase K.; Finol-Urdaneta R. K.; Terlau H.; Teichert R. W.; Olivera B. M. An Integrative Approach to the Facile Functional Classification of Dorsal Root Ganglion Neuronal Subclasses. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (10), 5494–5501. 10.1073/pnas.1911382117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. G.; Zhang X.; Gold M. S. Intracellular Calcium Regulation Among Subpopulations of Rat Dorsal Root Ganglion Neurons. J. Physiol. 2006, 577 (1), 169–190. 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E.; Giaume C. Astrocyte Calcium Waves: What They Are and What They Do. Glia 2006, 54 (7), 716–725. 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaksin M.; Shapira E.; Kimmel E.; Shoham S. Thermal Transients Excite Neurons through Universal Intramembrane Mechanoelectrical Effects. Phys. Rev. X 2018, 8 (1), 011043 10.1103/PhysRevX.8.011043. [DOI] [Google Scholar]
- Parameswaran R.; Carvalho-de-Souza J. L.; Jiang Y.; Burke M. J.; Zimmerman J. F.; Koehler K.; Phillips A. W.; Yi J.; Adams E. J.; Bezanilla F. Photoelectrochemical Modulation of Neuronal Activity with Free-Standing Coaxial Silicon Nanowires. Nat. Nanotechnol. 2018, 13 (3), 260–266. 10.1038/s41565-017-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Hudson A.; Shiwarski D.; Tashman J.; Hinton T.; Yerneni S.; Bliley J.; Campbell P.; Feinberg A. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365 (6452), 482–487. 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- Wang K.; Frewin C. L.; Esrafilzadeh D.; Yu C.; Wang C.; Pancrazio J. J.; Romero-Ortega M.; Jalili R.; Wallace G. High-Performance Graphene-Fiber-Based Neural Recording Microelectrodes. Adv. Mater. 2019, 31 (15), 1805867. 10.1002/adma.201805867. [DOI] [PubMed] [Google Scholar]
- Quigley A. F.; Razal J. M.; Thompson B. C.; Moulton S. E.; Kita M.; Kennedy E. L.; Clark G. M.; Wallace G. G.; Kapsa R. M. A Conducting-Polymer Platform with Biodegradable Fibers for Stimulation and Guidance of Axonal Growth. Adv. Mater. 2009, 21 (43), 4393–4397. 10.1002/adma.200901165. [DOI] [PubMed] [Google Scholar]
- Swinehart D. F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39 (7), 333. 10.1021/ed039p333. [DOI] [Google Scholar]
- Yao J.; Liu B.; Qin F. Rapid Temperature Jump by Infrared Diode Laser Irradiation for Patch-Clamp Studies. Biophys. J. 2009, 96 (9), 3611–3619. 10.1016/j.bpj.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.