Abstract
Laminin-332 pemphigoid is a rare and chronic autoimmune blistering disease which results in subepidermal blisters and erosive lesions predominantly localized to mucous membranes. As histologic inflammation is variable and non-complement-fixing IgG antibodies against laminin-332 are the predominant class of autoantibodies deposited at the epidermal basement membrane zone, we hypothesized that complement-independent pro-inflammatory and blistering pathways existed similarly to that previously shown in bullous pemphigoid. As autoantibodies to laminin α3 are most prevalent, we studied the major cellular response to blockade of laminin α3 using a well-characterized monoclonal antibody (P3H9-2). RNA-seq revealed upregulation of numerous desmosomal genes (DSG1, DSG3, DSC1, DSC3 and DSP) as well as KRT1 and KRT10. Additionally, P3H9-2-treated cells demonstrated downregulation of most hemidesmosomal genes. A pro-inflammatory response was not appreciated. Using pharmacological inhibitors, we identified both protein kinase C and NOTCH as key regulators of P3H9-2 induced differentiation. We lastly utilized 3D human skin equivalents to determine whether blockade of laminin α3 would lead to delayed blistering, consistent with keratinocyte differentiation. Significant blistering was noted after 72 h of treatment, with only minimal separation at 24 h. In summary, blockade of laminin α3 alters keratinocyte differentiation, representing a potential complement-independent mechanism of blistering.
Keywords: autoantibody, autoimmunity, epidermal differentiation, extracellular matrix, inflammation
1. BACKGROUND
Laminin-332 pemphigoid, a subset of mucous membrane pemphigoid (MMP), is a rare and chronic, autoimmune blistering disease which results in subepidermal blisters and erosive lesions predominantly localized to mucous membranes.1 The pathogenesis involves IgG autoantibodies against laminin-332 in the basement membrane zone (BMZ).1 While typically clinically indistinguishable from other forms of mucous membrane pemphigoid, laminin-332 pemphigoid appears to predispose to more aggressive laryngopharyngeal involvement.2–5 Complications from laminin-332 include scarring, which can lead to blindness or strictures along the oral-gastrointestinal and urogenital and tracts.3, 4
Laminin-332 is an extracellular glycoprotein and a vital component of the BMZ. It is a heterotrimer composed of the domains α3, β3 and γ2 with a large G domain at the base containing epidermal growth factor-based repeats.6, 7 While laminin α3 is the most frequently targeted domain in laminin-332 pemphigoid, patients often express varying amounts of β3 and γ2 antibodies.5, 8 The unique cross-shaped structure of laminin-332 and its various C-terminal domains allow numerous interactions with other structural proteins such as cell-surface receptors, α6β4 and α3β1 integrins, epidermal growth factor receptor and syndecan, as well as components of the basement membrane.7, 9 As such, laminin-332 plays an important role in maintaining the BMZ.9–22
Various models have shown the in vivo pathogenicity of anti-laminin-332 IgG by passive transfer using purified rabbit or human anti-laminin IgG.23–25 In neonatal mice, passive transfer experiments demonstrate the pathogenicity of laminin-332 antibodies in inducing blistering, despite a lack of inflammation or prominent mucosal involvement. This contrasts with many human histopathological findings which demonstrate extensive subepidermal separation accompanied by inflammatory infiltrates composed of lymphocytes, neutrophils, macrophages and eosinophils.25, 26 An adult model of laminin-332 pemphigoid using laminin α3 antibodies demonstrated a more characteristic inflammatory response, with characteristic clinical presentation, though this was Fc dependent.25 Yet previously, non-complement fixing, IgG4 antibodies against laminin-332 were identified as the predominant class of autoantibodies deposited at the epidermal BMZ.27 Thus, it is highly possible that complement-independent pro-inflammatory and blistering pathways exist in human disease, similarly to that shown in bullous pemphigoid (BP).28–32 As such, we hypothesized that laminin-332 pemphigoid-IgG would induce a similar pro-inflammatory response in keratinocytes, which could explain the histologic finding of acute inflammation and blistering, in the absence of complement-fixing antibodies.
Previous studies have demonstrated that blockade of integrin α3β1, a known binding partner of laminin α3, leads to terminal differentiation of keratinocytes.33 This differentiation could be decreased with growth of cells on purified laminin-332. Prior epitope mapping studies in patients with laminin-332 pemphigoid demonstrate that 65% of patients react with internal domains of laminin-332 ranging from the laminin-coiled coil domain to the G3 domain.25, 34 As the G1-3 domains interact with integrin α3β1 and α4β6,35, 36 we investigated the effect of blocking laminin α3 specifically in this region. P3H9-2 is a well-characterized monoclonal antibody that blocks laminin α3 from interacting with integrin α3β1.11, 35, 37–39
2. QUESTION ADDRESSED
Our goal was to assess whether antibody blockade of laminin α3 binding induces a pro-inflammatory transcriptional response and induces immune-independent blistering.
3. EXPERIMENTAL DESIGN
3.1. 2D cell culture
Primary adult human keratinocytes (PHKs) (Thermo Fisher Scientific) were cultured in EpiLife Medium with Human Keratinocyte Growth Supplement in a humidified atmosphere of 5% CO2 at 37°C. Fresh culture media were replaced with fresh media every 48 h. When confluency reached 80%–90%, cells were treated with anti-laminin α3 Clone P3H9-2 (R&D systems) 10 μg/ml, or Mouse IgG1 isotype control (ThermoFisher) for 24 h. The protein kinase C (PKC) inhibitor GO6983 (Tocris) 5 μM and the notch inhibitor tert-Butyl (S)-{(2S)-2-[2-(3,5-difluorophenyl)acetamido]propanamido}phenylacetate (DAPT) (Cayman Chemical) 10 μM were added to keratinocytes 2 h prior to treatment with P3H9-2. Finally, cells were washed with PBS twice and stored at −80°C until RNA extraction was performed.
3.2. 3D human skin equivalents
3D human skin equivalents (HSE) were initiated and propagated as described previously using primary neonatal human keratinocytes.40 Three independent cell lines derived from three donors were used for 3D HSEs. 3T3-J2 fibroblasts were used as previously described.40 After nine or eleven days of growth at the air-liquid interface, 3D cultures were treated with anti-laminin α3 Clone P3H9-2 at a concentration of 10 μg/ml or control vehicle for 72 or 24 h respectively. All cultures were harvested on day 12.
3.3. RNA-purification
Utilizing the miRNeasy Mini Kit, total RNA was extracted from PHKs using the manufacturer’s instructions (Qiagen Inc.). On-Column DNase I Digestion was used to prevent genomic DNA contamination.
3.4. Library preparation and sequencing
The quantity and quality of RNA were determined utilizing a Nanodrop 1000 spectrophotometer (Nanodrop), with RNA integrity number and concentration was confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). Isolated RNA samples were prepared for the development of transcriptome libraries. Poly (A) mRNA was extracted and enriched from total RNA utilizing oligo (dT)-attached magnetic beads according to the manufacturer’s (Illumina) instructions. Enriched and purified mRNA was fragmented into approximately 200 nt short mRNA. First-strand cDNA was synthesized using random hexamers as primers. Second-strand cDNA was constructed in a buffer containing dNTPs, DNA polymerase I and RNaseH. Appropriate fragments were isolated and enriched by PCR amplification. Lastly, the newly synthesized cDNA libraries of the samples were sequenced using an Illumina HiSeq sequencing platform.
3.5. Data processing and visualization
HISAT2 was used to map filtered clear reads to the reference genome. Mapped reads were converted to Fragments Per Kilobase of transcript per Million mapped reads (FPKM), and subsequently quantified with Cuffquant and Cuffnorm. DESeq was used to analyse differentially expressed genes. Screening criteria were established at fold change ≥2 and FDR <0.05. Gene Ontology (GO) was assessed using the topGO package, utilizing the gene ontology database (http://geneontology.org/). Heat maps were generated based on FPKM values using the heatmapper server (http://www.heatmapper.ca/).
4. RESULTS
4.1. Keratinocyte treatment with P3H9-2 results in transcriptional changes compatible with differentiation
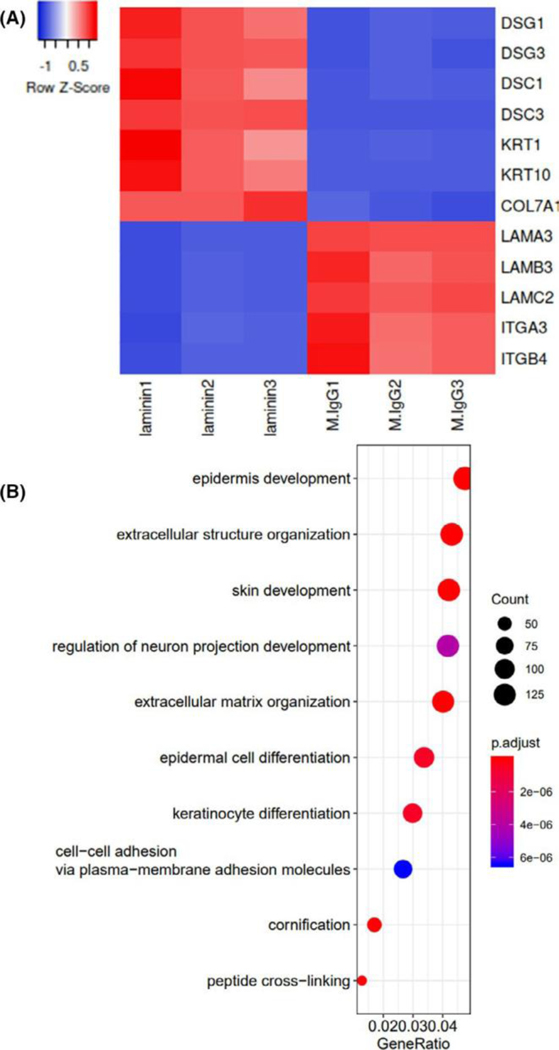
To determine the major cellular response to blockade of laminin α3, we performed RNA-seq on cultured PHKs. RNA-seq revealed upregulation of numerous desmosomal genes (DSG1, DSG3, DSC1, DSC3, DSP) as well as KRT1 and KRT10. Additionally, P3H9-2-treated cells demonstrated downregulation of fibronectin, as well most laminins and integrins except LAMB4 and ITGB8, which were overall expressed to a much lower level than other laminins and integrins respectively. COL17A1 expression was notably unaffected (log2FC = 0.02, P-adj = 0.65). Notable differentially expressed structural genes are shown in a heat map in Figure 1A. A pro-inflammatory transcriptome was not appreciated, with significant downregulation of all detected CXCL chemokines, as well as IL1B, IL1A, IL23A and IL36B. Interestingly MMP9 was significantly downregulated as well. A complete list of differentially expressed genes is provided in Table S1.
FIGURE 1.
Blockade of laminin alpha-3 alters the keratinocyte transcriptome. Primary human keratinocytes were incubated with laminin alpha-3 blocking antibody (clone: P3H9-2) or Isotype control (n=3/condition). RNA-seq was subsequently performed. Fold change ≥2 and FDR <0.05 were set as screening criteria. A) Select differentially expressed epithelial genes are shown as a heat map, revealing upregulation of desmosomal genes and downregulation of hemidesmosomal genes. B) Gene ontology for biologic processes of all significantly differentially expressed genes reveals marked enrichment of epidermal genes
We next performed GO analysis to provide an unbiased enrichment of gene sets, which demonstrated profound enrichment of genes involved in epidermal development and extracellular structures as expected (Figure 1B, Figure S1). KEGG pathway annotation demonstrated enrichment of signal transduction molecules (Figure S2).
4.2. Blockade of laminin α3 results in delayed blistering in 3D culture
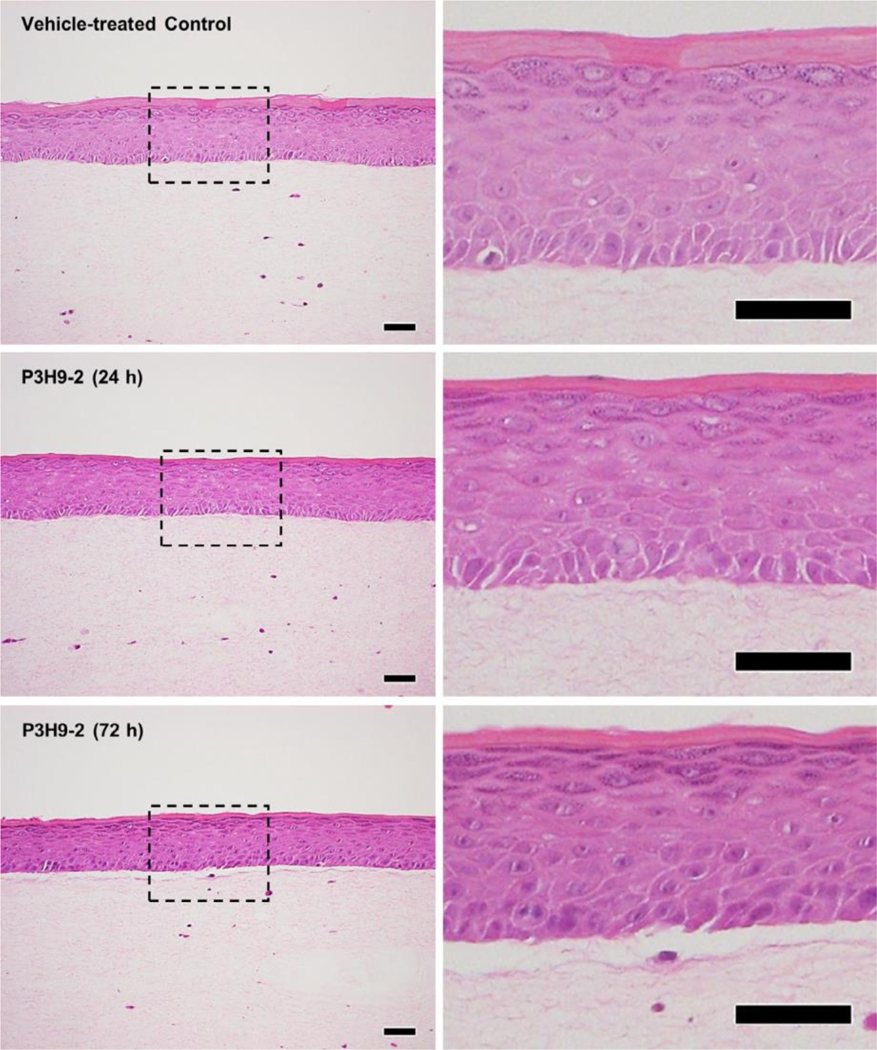
In light of the significant downregulation of hemidesmosomal genes, we next hypothesized that these transcriptional effects may alone be capable of inducing physiologic blistering, rather than by physical blockade of laminin-α3/integrin interactions. Traditional cryosection models do not utilize active keratinocyte signalling and thus have failed to demonstrate consistent complement-independent blistering pathways with autoantibodies against laminin-332. We thus utilized 3D HSEs. While blistering was not appreciated at 24 h, blistering was noted at 72 h, thus suggesting that steric hindrance of laminin-integrin alone did not cause the blistering, but rather alterations in keratinocyte interaction at the BMZ (Figure 2).
FIGURE 2.
Blocking laminin alpha-3 results in delayed blistering at 72 h. Ex vivo 3D human skin equivalents were treated with laminin alpha-3 blocking antibody (clone: P3H9-2) or vehicle control (n=3/condition). Cultures were harvested for histology at 24 and 72 h, revealing blistering at 72 h, but not 24. Scale bars =50 µm
4.3. PKC and NOTCH regulate laminin α3 maintenance of basal keratinocytes
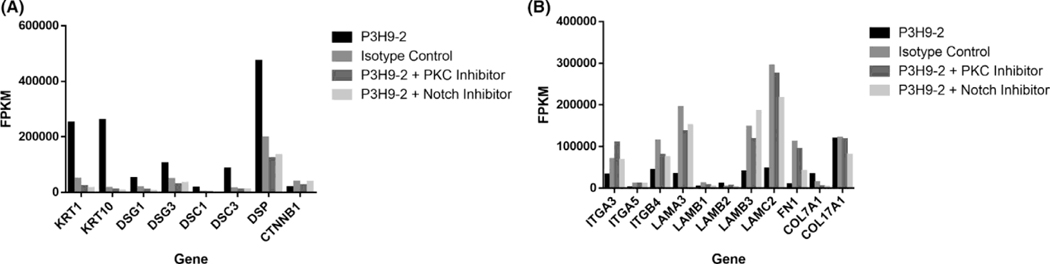
Given the profound effect of PKC on regulation of keratinocyte differentiation,41–43 we next investigated whether cell signalling resulting from laminin α3 blockade was ultimately regulated by PKC. PHKs were pretreated with the PKC inhibitor GO6983 followed by the addition of P3H9-2 mAb. Overwhelmingly, PKC inhibition prevented P3H9-2-mediated keratinocyte transcriptional changes, preventing upregulation of KRT1, KRT10, and desmosomal genes, as well as the downregulation of hemidesmosomal genes. Notably, inhibition of PKC had no effect on COL17a1 expression, consistent with our initial observation that blockade of laminin α3 did not affect COL17a1 expression (Figure 3A).
FIGURE 3.
Inhibition of PKC and NOTCH mitigates laminin alpha-3 transcriptional changes to key desmosomal and hemidesmosomal genes. Primary human keratinocytes were incubated with laminin alpha-3 blocking antibody (clone: P3H9-2) or isotype control in the following pretreatment with the PKC inhibitor GO6983 and the NOTCH inhibitor DAPT at a concentration of 5μM and 10μM respectively (n=3/condition). RNA-seq was subsequently performed. Expression of select A) desmosomal and B) hemidesmosomal genes is shown for each condition expressed as normalized fragments per kilobase million (FPKM), revealing PKC and NOTCH-dependent inhibition of P3H9-2-mediated keratinocyte differentiation
Lastly, given the transcriptional upregulation of NOTCH1 and NOTCH3 we observed in our samples, the required role of PKC in NOTCH activation,44 and the involvement of NOTCH signalling on keratinocyte differentiation,45, 46 we explored this mechanism further. We utilized DAPT, a γ-secretase inhibitor that indirectly inhibits NOTCH. Like PKC blockade, inhibition of notch signalling prevented P3H9-2 induced keratinocyte differentiation (Figure 3B).
5. CONCLUSIONS AND PERSPECTIVES
The aim of this study was to test the hypothesis that autoantibodies to laminin-332 trigger a pro-inflammatory response in keratinocytes and induces immune-independent blistering. While we did not identify a pro-inflammatory transcriptome, we noted significant keratinocyte differentiation, with downregulation of most hemidesmosomal genes. This antibody-mediated transcriptional change could be reversed with both a PKC and NOTCH inhibitor. Functionally, this was sufficient to induce blistering in an ex-vivo 3D culture model at 72 h, but not 24 h, suggesting that integrin-mediated signalling rather than steric hindrance of laminin α3/integrin was sufficient to induce blistering. As laminin-332 globular domains 1–3 are known to interact with integrin α3β1 as well as α6β4, we cannot determine whether monoclonal antibody treatment resulted in blockade of α3β1, which has been previously described, but also α6β4.35, 47
Recently, an inducible knockout of laminin α3 in basal keratinocytes has been shown to induce blistering in vivo.48 This loss of laminin α3 resulted in significant alterations in cytokeratin, cell stress and cornified envelope expression. This is in line with our findings of blockade induced differentiation and blistering. Notably, this model utilized a knockout towards the N-terminus of laminin α3, presumably affecting laminin binding to a larger number of protein-binding partners than just our monoclonal antibody approach.
While in BP complement-independent mechanisms of blistering and inflammatory response have been well described,31, 49 it has not been studied in laminin-332 pemphigoid. Previous studies have provided conflicting evidence regarding complement-independent blistering mechanisms. We have recently studied the subunit-dependent responses of keratinocytes treated with IgG from a series of patients with antibodies against only the laminin α3,β3, or γ2.50 Here, we identified a pro-inflammatory effect, particularly in patients with antibodies against laminin β3. Likewise, keratinocyte-treated antibodies from a small cohort of patients with antibodies against laminin α3 did not demonstrate significant differentiation markers. Given laminin α3’s interaction with numerous binding partners, it is likely that these patients did not express antibodies targeting the same globular domain epitope as in the present study utilizing a monoclonal antibody. As such, this phenomenon appears epitope dependent, whereby blockade of the G2 domain contributes towards keratinocyte differentiation, which in itself can induce differentiation but not upregulation of inflammatory markers. This represents a potential mechanism that may account for complement-independent blistering in laminin-332 pemphigoid.
Several study limitations must be noted. As DAPT is a y-secretase inhibitor, NOTCH is only indirectly inhibited. Other potential targets include amyloid precursor protein, E-cadherin and ErbB4. Notably, P3H9-2 did not affect gene expression of APP, CDH1 or ERBB4 genes, though non-transcriptional alternative regulation of these signalling molecules cannot be ruled out. Similarly, while we demonstrated that PKC and NOTCH prevented downregulation of most hemidesmosomal genes in 2D culture, we did not test this in 3D culture, given the effect of these pharmacologics on keratinocyte differentiation, which would presumably affect stratification of 3D HSEs. Lastly, while it is known that a majority of patients have autoantibodies targeting the globular domain of laminin α3, the functional ability of patient autoantibodies to block laminin α3 and integrin α3β1 interactions is unclear.
In conclusion, we have characterized the keratinocyte transcriptome as a response to blockade of laminin α3. While we did not identify a pro-inflammatory transcriptional effect, we identified a complement-independent mechanism of blistering through direct blockade of laminin α3 leading to cell differentiation. Further characterization of patient samples targeting different epitopes of laminin α3 will provide more insight into direct pathologic effects on keratinocytes.
Supplementary Material
ACKNOWLEDGEMENTS
BEPW is supported by grant K01AR072773 awarded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). A portion of this work was performed with the Northwestern University Skin Biology and Diseases Resource-based Center funded by grant P30AR075049 funded by NIAMS.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
All data sets have been included in the supplementary tables.
REFERENCES
- 1.Egan CA, Lazarova Z, Darling TN, Yee C, Yancey KB. Anti-epiligrin cicatricial pemphigoid: clinical findings, immunopathogenesis, and significant associations. Medicine. 2003;82(3):177–186. [DOI] [PubMed] [Google Scholar]
- 2.Lazarova Z, Hsu R, Yee C, Yancey KB. Antiepiligrin cicatricial pemphigoid represents an autoimmune response to subunits present in laminin 5 (alpha3beta3gamma2). Br J Dermatol. 1998;139(5):791–797. [DOI] [PubMed] [Google Scholar]
- 3.Chiorean R, Danescu S, Virtic O, et al. Molecular diagnosis of antilaminin 332 (epiligrin) mucous membrane pemphigoid. Orphanet J Rare Dis. 2018;13(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol. 2018;54(1):26–51. [DOI] [PubMed] [Google Scholar]
- 5.Amber KT, Bloom R, Hertl M. A systematic review with pooled analysis of clinical presentation and immunodiagnostic testing in mucous membrane pemphigoid: association of anti-laminin-332 IgG with oropharyngeal involvement and the usefulness of ELISA. J Eur Acad Dermatol Venereol. 2016;30(1):72–77. [DOI] [PubMed] [Google Scholar]
- 6.Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19(4):309–317. [DOI] [PubMed] [Google Scholar]
- 7.Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7(5):370–380. [DOI] [PubMed] [Google Scholar]
- 8.Goletz S, Probst C, Komorowski L, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol. 2019;180(1):149–156. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto O, Bachy S, Odenthal U, et al. Normal human keratinocytes bind to the alpha3LG4/5 domain of unprocessed laminin-5 through the receptor syndecan-1. J Biol Chem. 2003;278(45):44168–44177. [DOI] [PubMed] [Google Scholar]
- 10.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. [DOI] [PubMed] [Google Scholar]
- 11.Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65(4):599–610. [DOI] [PubMed] [Google Scholar]
- 12.Nievers MG, Schaapveld RQ, Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999;18(1):5–17. [DOI] [PubMed] [Google Scholar]
- 13.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16(7):376–383. [DOI] [PubMed] [Google Scholar]
- 14.Geerts D, Fontao L, Nievers MG, et al. Binding of integrin alpha-6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J Cell Biol. 1999;147(2):417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nievers MG, Kuikman I, Geerts D, Leigh IM, Sonnenberg A. Formation of hemidesmosome-like structures in the absence of ligand binding by the (alpha)6(beta)4 integrin requires binding of HD1/plectin to the cytoplasmic domain of the (beta)4 integrin subunit. J Cell Sci. 2000;113(Pt 6):963–973. [DOI] [PubMed] [Google Scholar]
- 16.Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149(4):969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterk LM, Geuijen CA, van den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115(Pt 6):1161–1173. [DOI] [PubMed] [Google Scholar]
- 18.Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145(6):1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with lami nin 5 to promote stable epithelial-stromal attachment. J Cell Biol. 1996;132(6):1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen BP, Ren XD, Schwartz MA, Carter WG. Ligation of integrin alpha 3beta 1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J Biol Chem. 2001;276(47):43860–43870. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen BP, Gil SG, Carter WG. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J Biol Chem. 2000;275(41):31896–31907. [DOI] [PubMed] [Google Scholar]
- 23.Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest. 1996;98(7):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarova Z, Hsu R, Yee C, Yancey KB. Human anti-laminin 5 autoantibodies induce subepidermal blisters in an experimental human skin graft model. J Invest Dermatol. 2000;114(1):178–184. [DOI] [PubMed] [Google Scholar]
- 25.Heppe EN, Tofern S, Schulze FS, et al. Experimental laminin 332 mucous membrane pemphigoid critically involves C5aR1 and reflects clinical and immunopathological characteristics of the human disease. J Invest Dermatol. 2017;137(8):1709–1718. [DOI] [PubMed] [Google Scholar]
- 26.Rose C, Schmidt E, Kerstan A, et al. Histopathology of antilaminin 5 mucous membrane pemphigoid. J Am Acad Dermatol. 2009;61(3):433–440. [DOI] [PubMed] [Google Scholar]
- 27.Hsu R, Lazarova Z, Yee C, Yancey KB. Noncomplement fixing, IgG4 autoantibodies predominate in patients with anti-epiligrin cicatricial pemphigoid. J Invest Dermatol. 1997;109(4):557–561. [DOI] [PubMed] [Google Scholar]
- 28.Van den Bergh F, Eliason SL, Burmeister BT, Giudice GJ. Collagen XVII (BP180) modulates keratinocyte expression of the proinflammatory chemokine, IL-8. Exp Dermatol. 2012;21(8):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt E, Reimer S, Kruse N, Bröcker EB, Zillikens D. The IL-8 release from cultured human keratinocytes, mediated by antibodies to bullous pemphigoid autoantigen 180, is inhibited by dapsone. Clin Exp Immunol. 2001;124(1):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messingham KN, Srikantha R, DeGueme AM, Fairley JA. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187(1):553–560. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt E, Reimer S, Jainta S, et al. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol. 2000;115(5):842–848. [DOI] [PubMed] [Google Scholar]
- 32.Amber KT, Chernyavsky A, Agnoletti AF, Cozzani E, Grando SA. Mechanisms of pathogenic effects of eosinophil cationic protein and eosinophil-derived neurotoxin on human keratinocytes. Exp Dermatol. 2018;27(12):1322–1327. [DOI] [PubMed] [Google Scholar]
- 33.Symington BE, Carter WG. Modulation of epidermal differentiation by epiligrin and integrin alpha 3 beta 1. J Cell Sci. 1995;108(Pt 2):831–838. [DOI] [PubMed] [Google Scholar]
- 34.Lazarova Z, Yee C, Lazar J, Yancey KB. IgG autoantibodies in patients with anti-epiligrin cicatricial pemphigoid recognize the G domain of the laminin 5 alpha-subunit. Clinical Immunol. 2001;101(1):100–105. [DOI] [PubMed] [Google Scholar]
- 35.Mizushima H, Takamura H, Miyagi Y, et al. Identification of integrin-dependent and -independent cell adhesion domains in COOH-terminal globular region of laminin-5 alpha 3 chain. Cell Growth Differ. 1997;8(9):979–987. [PubMed] [Google Scholar]
- 36.Rousselle P, Beck K. Laminin 332 processing impacts cellular behavior. Cell Adh Migr. 2013;7(1):122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wayner EA, Gil SG, Murphy GF, Wilke MS, Carter WG. Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J Cell Biol. 1993;121(5):1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzales M, Haan K, Baker SE, et al. A cell signal pathway involving laminin-5, alpha3beta1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol Biol Cell. 1999;10(2):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987;105(4):1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnette C, Koetsier JL, Hoover P, Getsios S, Green KJ. In vitro model of the epidermis: connecting protein function to 3D structure. Methods Enzymol. 2016;569:287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denning MF. Epidermal keratinocytes: regulation of multiple cell phenotypes by multiple protein kinase C isoforms. Int J Biochem Cell Biol. 2004;36(7):1141–1146. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Murata S, Yaoita H, Nakagawa H. An antibody to BP 180 kDa antigen is able to induce an increase of intracellular Ca2+ concentration in DJM-1 (human squamous cell carcinoma) cells. Autoimmunity. 2002;35(4):271–276. [DOI] [PubMed] [Google Scholar]
- 43.Lee YS, Yuspa SH, Dlugosz AA. Differentiation of cultured human epidermal keratinocytes at high cell densities is mediated by endogenous activation of the protein kinase C signaling pathway. J Invest Dermatol. 1998;111(5):762–766. [DOI] [PubMed] [Google Scholar]
- 44.Steinbuck MP, Winandy S. A review of notch processing with new insights into ligand-independent notch signaling in T-cells. Front Immunol. 2018;9:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangarajan A, Talora C, Okuyama R, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20(13):3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dlugosz AA, Yuspa SH. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol. 1993;120(1):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JM, Park WH, Min BM. The PPFLMLLKGSTR motif in globular domain 3 of the human laminin-5 alpha3 chain is crucial for integrin alpha3beta1 binding and cell adhesion. Exp Cell Res. 2005;304(1):317–327. [DOI] [PubMed] [Google Scholar]
- 48.Tayem R, Niemann C, Pesch M, et al. Laminin 332 is indispensable for homeostatic epidermal differentiation programs. J Invest Dermatol. 2021;141(11):2602–2610.e3. [DOI] [PubMed] [Google Scholar]
- 49.Natsuga K, Nishie W, Shinkuma S, et al. Antibodies to pathogenic epitopes on type XVII collagen cause skin fragility in a complement-dependent and -independent manner. J Immunol. 2012;188(11):5792–5799. [DOI] [PubMed] [Google Scholar]
- 50.Bao L, Li J, Solimani F, et al. Subunit-specific reactivity of autoantibodies against laminin-332 reveals direct inflammatory mechanisms [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sets have been included in the supplementary tables.