Abstract
Regulatory T cells (Tregs) are a subset of T cells responsible for the regulation of immune responses, thereby maintaining immune homeostasis and providing immune tolerance to both self and non-self-antigens. An increasing number of studies revealed Treg numbers and functions in a variety of autoimmune diseases. Treg deficiency can cause the development of several autoimmune skin diseases including vitiligo, alopecia areata, pemphigoid and pemphigus, psoriasis, and systemic sclerosis. Many clinical trials have been performed for autoimmune conditions using polyclonal Tregs, but efficiency can be significantly improved using antigen-specific Tregs engineered using T cell receptor (TCR) or chimeric antigen receptor (CAR) constructs. In this review, we systematically reviewed altered frequencies, impaired functions, and phenotypic features of Tregs in autoimmune skin conditions. We also summarized new advances in TCR and CAR based antigen-specific Tregs tested both in animal models and in clinics. The advantages and limitations of each approach were carefully discussed emphasizing possible clinical relevance to patients with autoimmune skin diseases. Moreover, we have reviewed potential approaches for engineering antigen-specific Tregs, and strategies for overcoming possible hurdles in clinical applications. Thereby, antigen-specific Tregs can be infused using autologous adoptive cell transfer to restore Treg numbers and to provide local immune tolerance for autoimmune skin disorders.
Keywords: Antigen-specific, Autoimmune skin diseases, Chimeric antigen receptor, Regulatory T cells, T cell receptor
1. The role of regulatory T cells (Tregs) in preventing autoimmunity
Tregs are a subpopulation of T cells, which play a key role in the regulation of the immune response. By controlling the immune response to self and foreign antigens, Tregs can prevent the development of autoimmune disorders [1]. Tregs are generated from immature CD4 single positive cells receiving T cell receptor (TCR) signals of intermediate strength to escape clonal deletion in the thymus and are committed to differentiate into thymic or natural Tregs (tTregs or nTregs) [2]. Alternatively, naïve CD4+ T cells can differentiate into inducible Tregs (iTregs) when exposed to transforming growth factor β (TGF-β) and interleukin (IL-2) in the periphery. This classical subset of iTregs stably expresses the transcription factor forkhead box protein 3 (FOXP3). Type 1 T regulatory cells (Tr1) is another most extensively studied subset of iTregs. Tr1 cells are induced by interleukin (IL-10) and are FoxP3-negative. These two distinct populations of iTregs produce IL-10 and TGF-β to elicit their suppressive activities [3,4].
iTregs maintain peripheral tolerance, modulate effector T cell responses in several autoimmune diseases and prevent allograft rejection [5]. The possibility of generating in vitro expanded antigen-specific iTregs has encouraged their clinical use in autoimmunity and Graft vs. Host Disease (GvHD) [6]. Circulating Tregs are considered heterogeneous, mainly due to their plasticity, and acquite features specific to the type of immune response that they control. Under inflammatory conditions and in autoimmune diseases, Tregs can lose FOXP3 expression and convert into effector type producing Th1, Th2, or Th17 cytokines, which impairs immune homeostasis and contributes to the progression and pathogenesis of the disease [5,7]. Therefore, highly stable FOXP3 expression in vitro is associated with a low risk for interleukin (IL-17) production in vivo under inflammatory conditions [7]. The suppression mechanisms of Tregs can be direct, by cell-to-cell contact, or indirect, through a bystander effect [8,9]. Examples of bystander mechanisms include the secretion of cytokines such as IL-10, TGF-β and IL-35 and the production of granzyme and perforin, enzymes leading to apoptosis in target cells [10]. Moreover, high expression of CD25 receptors enables Tregs to take up more IL-2 and “starve” the surrounding cells of this cytokine [11]. In the absence of Tregs, as in patients with FOXP3 mutations, autoreactive T cells can persistently attack healthy cells [8,12]. Limited availability of Tregs was observed in several autoimmune diseases such as rheumatoid arthritis (RA), alopecia areata, multiple necrosis, and vitiligo [13].
2. Overview of autoimmune skin conditions and principle immune mechanisms
Autoimmune disease is a condition arising from an abnormal immune response to self-antigens that can affect any part of the body, including the skin [14]. Autoimmune skin disorders include alopecia areata, pemphigoid and pemphigus, vitiligo, psoriasis, and systemic sclerosis (Fig. 1) [15]. Upon progression, autoimmune skin disorders severely affect the quality of life and can become life-threatening [16–18]. Both genetic and environmental factors might be involved in their etiology [19,20]. Greater knowledge of the genetic underpinnings will allow early recognition of persons predisposed to these diseases, whereas identification of environmental triggers and possible autoantigens can uncover opportunities for preventing autoimmunity [21].
Fig. 1.
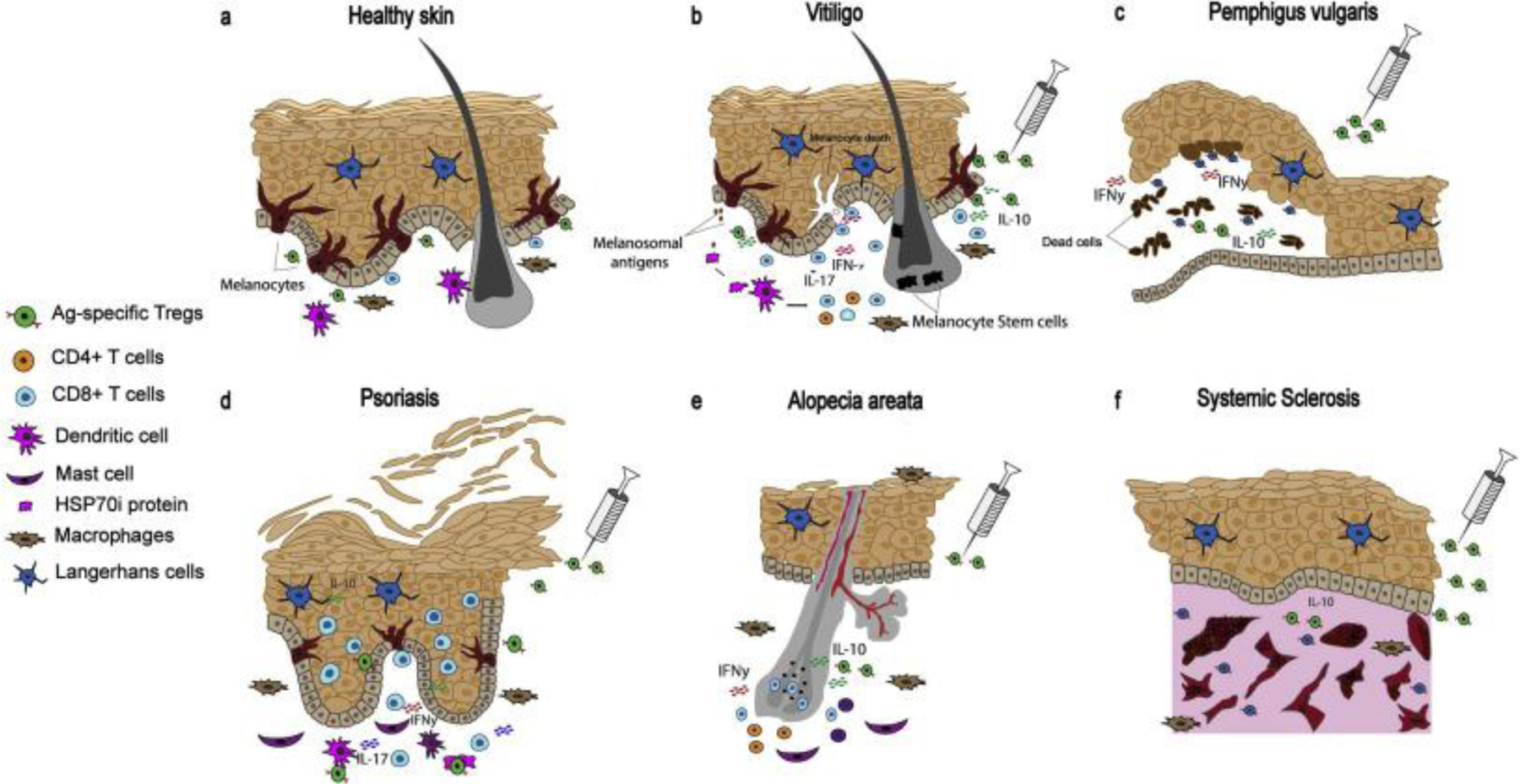
Autoimmune etiology of autoimmune skin diseases. (a) Illustration of immune surveillance in healthy skin. Adoptive transfer of antigen-specific Tregs to control (b) autoimmune depigmentation in vitiligo, (c) autoimmune blistering of the skin in pemphigus vulgaris, (d) autoimmune psoriatic patches in psoriasis, (e) autoimmune hair loss in alopecia areata, (f) autoimmune fibrosis in systemic sclerosis.
2.1. Vitiligo
The above conditions affect the skin and can all potentially benefit from a greater Treg activity on site. Here we provide a comparison to benefits achievable for progressive depigmentation in vitiligo. Vitiligo is a skin condition caused by the loss of melanocytes [22]. Vitiligo has been identified as a T cell mediated autoimmune disease (Fig. 1b) [23]. Several studies support an increased abundance of CD8+ T cells reactive to tyrosinase, melanoma antigen recognized by T cells (MART-1) and other melanosomal proteins, as shown by HLA multimer staining of circulating lymphocytes from patients [24]. Another name for MART-1 is MelanA; in contrast to TYRP1, MART-1 and other melanosomal proteins induce cytotoxic T-cells, but not humoral responses [25]. These melanosomal proteins might thus be considered autoantigens [23,26]. Indeed, cytotoxic responses to these and other melanocyte antigens can eliminate melanocytes and cause depigmentation [23]. Recent studies suggest the role of exosomes derived under pathological conditions can impact Tregs and Th17 cells [27] and CD8+ balance [28], suggesting a contribution for exosomes in the underlying mechanism of melanocyte destruction in vitiligo [29]. In vitiligo, inducible heat shock protein 70 (HSP70i) plays a central role in the autoimmune response by activating dendritic cells (DCs). These activated DCs further initiate autoimmune reactivity towards melanocytes [30,31].
The percentage of immunosuppressive Tregs in vitiligo skin is greatly reduced compared to healthy skin, suggesting that autoreactive cytotoxic T cells remain uninhibited, contributing to depigmentation in vitiligo [32]. Increased Treg infiltration was directly associated with repigmentation in a spontaneous mouse model of the disease, which is suggestive of a role for Tregs in preventing autoimmunity towards melanocytes [33]. Indeed, reduced numbers of skin Tregs with impaired function have been described for vitiligo patients [32,34–36]. Contested by some, decreased numbers of circulating Tregs have been reported in vitiligo patients as well [35], particularly in patients with active disease [34,35,37]. Reduced Treg numbers were found in unaffected and perilesional skin, but this population may ultimately repopulate lesional skin of vitiligo patients, and restore immune homeostasis [32,38–41].
TGF-β can polarize naïve T cells to a Tregs phenotype, and induce FOXP3 expression in iTregs [42], while also supporting Treg function [43]. Importantly, the levels of TGF-β are reduced in serum and skin of vitiligo patients, potentially affecting Treg suppressive activity [44,45]. TGF-β titers, as well as IL-10 levels, are selectively decreased in active, but not stable vitiligo [46,47]. In vitiligo, reduced Treg infiltration to the skin has been explained by the limited expression of CCL22 in vitiligo patient skin, which otherwise favors Treg trafficking to a CCL2-rich environment [32]. A broad knowledge that exists about target antigens selectively expressed by melanocytes targeted in vitiligo can open the door to antigen-specific Treg therapy for this disease.
2.2. Pemphigus and pemphigoid
Autoimmune bullous diseases are classified as pemphigus and pemphigoid [48]. In pemphigus, autoantibodies target desmosomal proteins, leading to disruption of epidermal adhesion and subsequent intraepidermal blistering. In pemphigoid, autoantibodies target structural proteins of the dermo-epidermal junction, causing tense, subepidermal blistering. The most common types of pemphigus and pemphigoid diseases are pemphigus vulgaris (PV), which is mediated by circulating autoantibodies directed to desmoglein 3 (Dsg3) on the keratinocyte cell surface, and bullous pemphigoid (BP) caused by autoantibodies to the structural protein BP180 at the epidermal basement membrane zone (Fig. 1c) [49].
The contribution of Tregs to BP remains controversial. Recent studies showed a significant reduction in circulating Treg frequency [50,51] and decreased numbers of FOXP3+ and IL-10+ cells were observed in BP skin, while no differences were found in TGF-β cells when compared to psoriasis, atopic dermatitis and control skin. The inflammatory milieu may dictate decreased homing of Tregs in BP-affected skin as an unintended consequence, rather than a cause of disease [52]. Circulating CD4+CD25high Tregs express a normal level of cutaneous lymphocyte antigen (CLA), and their inhibitory capacity was not affected in BP [53]. In fact, Treg-depletion in the DEREG mouse model of BP induced excessive inflammation and blistering, increased neutrophil infiltrates and expression of Th1 and Th2 cytokines and chemokines, suggesting a role of Tregs in BP pathogenesis [54].
In PV patients, a greatly reduced number of Tregs are found [55]. Expression of FOXP3 is downregulated among circulating patient Tregs of PV patients, suggesting reduced suppressive function [56]. Despite abundant infiltration of Tregs into PV skin [52], the link between Treg dysfunction and the progression of PV has become more evident from studies employing the Treg-depleted PV mouse model.
Increasing or decreasing the number of Tregs, by adoptive transfer or antibody-mediated depletion, impacts autoreactive antibody production and disease development in PV mice [57,58]. Meanwhile, Dsg3-specific Tregs can be developed from Dsg3−/− mice and adoptively transferred into Dsg3-sufficient mice to effectively suppress anti-Dsg3 antibody production in the PV mouse model [57]. Type 1 regulatory cells (Tr1 cells) are responsible for maintaining immune tolerance against self and non-self-antigens [59]. Dsg3-reactive Tr1 cells that inhibit proliferation of Dsg3-responsive autoreactive Th cells via secretion of IL-10 and TGF-β are more frequent in peripheral blood of healthy donors expressing PV-predisposing HLA than in actual PV patients [60]. Thus, autoantigen-specific Tregs can serve to suppress Dsg3-specific immune responses and associated disease in PV.
Efforts have been made to induce tolerance in PV by administering Dsg3 amino acids 186–204, which specifically bind PV-associated HLA-DR alleles, but this did not affect autoantibody titers against Dsg3 [57], possibly due to an absence of co-stimulation required to generate antigen-specific Tregs in the trial.
2.3. Psoriasis
Psoriasis is a chronic inflammatory skin disease mediated by both innate and adaptive immune responses. The disease is characterized by incomplete, defective basal keratinocyte differentiation induced by an inflammatory cascade in the dermis involving dendritic cells, macrophages, mast cells, and T cells (Fig. 1d) [61]. Parallels have been drawn between the etiopathomechanism of psoriasis and vitiligo [62–64], which has contributed to a rich history of therapeutics repurposed from psoriasis to vitiligo, including light therapies [65–67]. However, the skin microenvironment in active disease is quite different in either case. Nevertheless, both psoriasis and vitiligo can benefit from immune tempering measures, and functional skin Tregs can be of therapeutic benefit in either case. Several autoantigens derived from keratinocytes have been identified to explain the dominant T cell component observed in psoriatic skin. This includes LL37 cathelicidin/nucleic acid complexes, a newly recognized group of lipid antigens [68]. These may trigger initial activation of T cells, particularly IL-17-producing Th17 cells, T helper (Th)1 and Th22 cells [68].
A Treg-related autoimmune etiology of psoriasis has been reported [69]. Despite contradictory data concerning the frequency of Tregs in peripheral blood of psoriasis patients, a profound difference in Treg functional activity has been observed. Sugiyama H. et al. showed that circulating CD4+CD25high cytotoxic T-lymphocyte antigen 4-positive (CTLA-4+) FOXP3high Tregs of patients with chronic plaque type psoriasis display an anergic phenotype and exhibit decreased potential to suppress T cell proliferation in vitro [70–72]. In patients with moderate and severe psoriasis, tempered suppressive activity was accompanied by increased type 1 cytokines, STAT3 phosphorylation and proinflammatory cytokines (IL-6, IL-21 and IL-23) [73]. A pathogenic switch of patient Tregs into IL-17-producing RORγt+ Th17 was observed, with CD4+FOXP3+/−IL-17+ cells infiltrating the lesional dermis of patients with severe disease [74]. Interestingly, the proportion of Treg among CD4+ T cells was elevated in non-lesional skin of patients with chronic plaque psoriasis [75], whereas decreased Treg infiltration was associated with acute but not chronic skin lesions, suggesting that a transient deficiency in Tregs supports the initiation of new lesions to exacerbate psoriasis [71].
A therapeutic role for Tregs in restoring self-tolerance has been demonstrated in mouse models of psoriasis. Tregs suppressed infiltration of lesional skin by GM-CSF+ T cells, resulting in disease regression, while Treg depletion exacerbated imiquimod-induced disease [76]. Such depletion of FoxP3+ Tregs allowed for type I IFN production by mononuclear phagocytes and accumulation of CD8+ T cells [77]. In human TNF-α-induced psoriatic arthritis, Tregs suppressed macrophage infiltration and the development of psoriasis [78]. In the PL/J murine model, Treg activity reduced psoriatic skin inflammation, and CD18 expression proved essential for the Treg-DC crosstalk that induces proliferation and TGF-β-dependent function of alloantigen-specific Tregs [79]. In conclusion, there is a definitive and unique role for Tregs in psoriasis pathogenesis, and great potential for Treg-based therapeutics in this keratinocyte-directed disease.
2.4. Alopecia areata
Alopecia areata (AA) is one of the most common autoimmune diseases that affects hair follicles. The autoimmunity is site-specific and is mediated by CD8+ T cells activated by aberrant epithelial MHC-I and MHC-II expression in the bulb of the hair follicle (Fig. 1e) [80,81]. The striking similarities between the etiology of vitiligo and AA further extend to the antigens targeted in either disease, as both melanocyte and keratinocyte-derived antigens in hair follicles may be targeted in alopecia [82]. Potential AA-associated antigens include keratinocyte-derived trichohyalin 1 (TCHH-1), trichohyalin 2 (TCHH-2) and melanocyte-derived tyrosinase (TYR), tyrosinase-related protein (TYRP2) [80,82].
Aberrant Treg function has been linked to AA pathology. Decreased frequencies of Tregs have been observed in the skin, in draining lymph nodes and also in the spleen of C3H/HeJ mice with chronic and transplanted AA. Low infiltration of Tregs in the AA lesions in mice was accompanied by low CD40 expression [83]. Transfer of Tregs obtained from skin draining lymph nodes of mice with a normal pelage to AA-affected mice prevented systemic AA development and site-specific hair loss in the latter [84].
Related data were obtained in humans. Low levels of CD4+FOXP3+ and CD25+FOXP3+ Tregs were found in the scalp lesions of patients with AA [85]. Compared to healthy controls, among CD4+CD25+FOXP3+, CD39-expressing Tregs were also reduced in the hair follicles of patients with active AA. However, no significant reduction in the percentage of circulating CD25+FOXP3+ Tregs or their memory subset CD25+FOXP3+CD45RO+ were found in AA patients with active disease [86]. At the same time, subcutaneous injections of low-doses of IL-2 to AA patients, resistant to conventional treatments, induced the recruitment of CD4+CD25+FOXP3+ Tregs into lesional skin and successful hair regrowth [87].
Single-nucleotide polymorphisms have been reported in chromosome regions encoding CD25, the ikaros family member Eos (IKZF4), CTLA-4, and FOXP3 in AA patients [88]. Data related to Treg-related cytokine abundance in AA are conflicting, with a decrease in serum TGF-β levels reported by some [89], and an increase by others when compared to healthy controls [90]. Surprisingly, no other cytokine differences (including IL-10) have been detected or reported in AA [89], but the impaired suppressive activity of circulating CD4+CD25+T CD127low/− Tregs of AA patients was found in vitro [91]. Despite the fact that the mechanisms that render Treg dysfunction in AA patients and their recruitment defects to a site of AA are currently unknown, the data account for the crucial role of Tregs in the pathogenesis of AA and suggest the potential of their therapeutic utilization.
2.5. Systemic sclerosis
Systemic sclerosis (SSc) is a complex systemic autoimmune disease characterized by thickened and sclerotic skin, which also affects organs such as the lungs, the gastrointestinal tract, the heart and the kidneys [92]. SSc is the most severe connective tissue disease, associated with high mortality. The pathology of SSc involves three pathways, namely: (1) vasculopathy, (2) fibrosis caused by fibroblast dysfunction generating excessive accumulation of matrix components, and (3) aberrant immune activation leading to the production of autoantibodies and cell-mediated autoimmunity (Fig. 1f) [93].
Treg cells from SSc patients can inhibit T-cell proliferation and IL-2, IL-4, IL-5, IL-10, IFN-γ and TNF-α production, thus suggesting the presence of functional Tregs in SSc [94]. However, decreased frequencies of Tregs are found in peripheral blood of untreated SSc patients and SSc lesions [94–102]. The frequency of circulating Tregs is inversely correlated with disease activity, age of onset, staging and complexity in SSc [103–105]. The reduced abundance of circulating Tregs was associated with a marked increase in Th17 cells [106], and not surprisingly, decreased IL-10 and TGF-β serum levels. Comparable findings were reported for SSc lesional skin [102], though a reduction in Tregs was not accompanied by increased cutaneous Th17 [107]. Instead, in diffuse SSC, IL-4 and IL-13 producing Tregs are significantly increased to drive the differentiation and proliferation of fibroblasts and excess fibrosis [108].
Despite limited knowledge of the mechanism by which Tregs participate in tissue fibrosis and SSc, Treg augmentation could be a viable therapy for the disease. For example, in a clinical trial of chronic GvHD-cases that closely resemble that of scleroderma, patients were treated with low-dose IL-2. This treatment can boost Tregs, and it led to a decrease in skin fibrosis in some patients [109]. In SSc patients undergoing autologous hematopoietic stem cell transplantation, improved Treg function correlated with reduced skin fibrosis [110]. Also, in a bleomycin-induced pulmonary fibrosis mouse model, adoptive transfer of Tregs significantly reduced pulmonary fibrosis [111]. However, only late depletion of Tregs led to increased fibrosis [112], suggesting that Tregs therapy might be effective only in the late stages of SSc.
Some opposing data have been recently published. Though TGF-β is clearly involved in Treg suppressive functions, it is also strongly implicated with SSc pathogenesis, and targeting TGF-β-regulated gene expression with neutralizing antibody improved SSc clinical symptoms [113]. It is thus possible that TGF-β-regulated gene expression primarily mediates clinical manifestations of SSc. Further investigation of the therapeutic potential of Treg in SSc is needed. Treg activity may be enhanced by antigen specificity, potentially targeting overexpressed matrix components found in (proximity to) hyperactivated fibroblasts.
3. Treg-based methods tested to suppress autoimmune skin conditions
Therapeutics to enhance Tregs function offer a potential tool for the treatment of autoimmune diseases, and can serve to replace the chronic use of immunosuppressants that can be associated with undesirable side effects. Clinical trials have been initiated using polyclonal Tregs for autoimmune skin diseases; a phase I non-randomized, open-label clinical study was conducted for active cutaneous lupus. Patients received a single dose of 108 autologous, polyclonal Tregs (NCT02428309), but the study was terminated due to limited recruitment feasibility. In an ongoing Treg-based, phase I clinical trial for PV and pemphigus foliaceus, patients have been administered a single dose of 1–2.5×108 autologous Tregs, and the results of this study are pending (NCT03239470). Numerous preclinical studies addressing the treatment of autoimmune skin disorders hail the benefits of using antigen-specific Tregs expressing either a transgenic TCR or a CAR, and await translation to a clinical trial using genetically modified Tregs [6,114–118].
As an alternative to autologous Treg transfer, therapeutic agents can be used to support Treg function. Drug-based treatment is usually considered more cost-effective and may come at a lower risk for adverse events compared to adoptive transfer of live cells. A limited set of clinical trials of this kind is being conducted. Recent studies report that low-dose IL-2 has been associated with the promotion and expansion of Tregs in patients with autoimmune conditions [119,120]. This includes a large-scale, ongoing clinical trial to test low-dose IL-2 for the treatment of multiple autoimmune disorders, including skin conditions such as psoriasis, systemic sclerosis and Systemic Lupus Erythematosus (SLE) (NCT01988506) [120,121]. A recent review also discussed the potential of using low-dose IL-2 for RA [122]. In animal studies, rapamycin promoted Treg development via protein kinase B with subsequent mTOR inhibition [123], and provided benefit by reducing the effector T cell: Treg ratio [124–127]. However, in clinical trials of rapamycin, no significant benefit has been reported to date [124–127].
Supporting Treg skin homing can also be considered for the treatment of autoimmune skin diseases. By cutaneous CCL22 overexpression in a mouse model of vitiligo, Treg numbers were restored and continued treatment was able to suppress vitiligo development [128]. Delivery of CCL22 might be achieved by local needle-free jet injection of DNA to attract Treg to the injection site [129]. The same approach can support anti-tumor responses to melanoma, by redirecting Tregs away from the tumor and towards the skin [130].
Supporting microbial diversity or supplying particular microbes can also serve as preventive or therapeutic measures to drive Treg activity and alleviating autoimmune responses of the skin. This approach is gaining increased attention [131]. In one study, oral administration of Bifidobacterium infantis was associated with increased IL-10 secretion and FOXP3 expression in patient blood [132], and in an increase in Tregs in a mouse model of S. typhimurium disease [133]. We found that neomycin treatment can alter the microbiome of vitiligo-prone mice to significantly delay depigmentation and promote the infiltration of Tregs to the skin [134].
4. Why include antigen specificity?
In early-phase clinical trials, polyclonal Tregs have been used to prevent autoimmunity after allogeneic hematopoietic stem cell transplantation (HSCT) and in patients with type-1 diabetes, demonstrated treatment safety and efficacy [135]. However, the inadvertent suppression of immune responses to infection or malignancies can form an important consideration when applying polyclonal Tregs [136]. Moreover, generating polyclonal Tregs in numbers sufficient for clinical use can be challenging. These factors might be overcome by including antigen specificity to adoptively transferred cells. Animal studies performed for diabetes, central nervous system demyelinating autoimmune diseases, and allograft transplantation demonstrated that antigen-specific Tregs can provide superior protection from autoimmunity over polyclonal Tregs [116,137–144]. Thus, using antigen-specific Tregs might overcome the two primary challenges with polyclonal Treg therapy, namely production of potent Tregs with relevant specificity to provide superior protection from autoimmune activity.
5. TCRs and CARs: A functional comparison
Antigen-specific Tregs can be generated using TCR or CAR constructs. Engineered Tregs with specific TCR can be generated at high efficiency using retroviral or lentiviral constructs, but the use of TCR-Tregs is limited by its MHC-restriction requirement, which impacts the number of patients that will benefit from this therapy [145]. On the other hand, TCR-Tregs can respond to antigen regardless of the intended cellular expression site of the molecule. Tregs transduced with genes encoding a CAR will overcome this MHC-dependence. The latter constructs are instead composed of a single-chain variable fragment, scFv - the binding portion of a monoclonal antibody, followed by an extracellular hinge region, a transmembrane region, and intracellular TCR signaling domains. In CAR-T cell therapy, optimizing and selecting the correct CAR affinity and intracellular signaling domains is crucial for the resulting therapeutic activity and cellular persistence of the resulting T cells [146]. Since CARs are constructed using antibody variable regions, they hold a higher affinity to their cognate antigen compared to TCRs [147]. A limitation to the use of CAR Tregs is that a suitable antigen must be identified that is readily accessible by antibodies, generally on the surface of the targeted cell [148]. Moreover, high-affinity CAR stimulation, at least in CD8 T cells, can ultimately result in reduced activity and loss of specificity of host T cells [115]. Therefore, the optimal affinity range for the domains responsible for the specificity of the CARs, and the benefit of high affinity interaction form an area of active investigation.
6. Currently available TCRs and CARs of potential use for autoimmune skin diseases
Current cancer immunotherapies can involve using TCR and CAR-transduced T cells, with CD19-reactive CAR T cells now approved for the treatment of B cell lymphoma [149]. The outcomes suggest that similar approaches could hold promise for the treatment of autoimmune diseases [150,151]. Ex vivo-expanded autologous polyclonal CD4+CD25high CD127low/− regulatory T cells have been in clinical trials for Type 1 Diabetes and GvHD [152]. These Tregs were sorted based on surface markers to purify Tregs from peripheral blood, and sorted Tregs were cultured with clinical-grade activation beads in the presence of IL-2 [153]. Prior to infusion, FOXP3 expression and viability were established [152,154]. The use of CAR constructs to generate alloantigen-specific Tregs has shown promise in preclinical studies for organ transplantation, to prevent GvHD [6]. In one example, Tregs expressing an HLA-A2–specific CAR (A2-CAR) maintained a stable phenotype, with superior suppressive activity to polyclonal Tregs in vitro and prevention of xenogeneic GvHD caused by HLA-A2+ T cells in mice [6]. Another recent study reports the use of CAR Tregs targeting ganglioside D3 (GD3) antigen in a vitiligo prone mouse model. The results show enhanced protection from depigmentation when compared to untransduced Tregs [155]. The scheme of adoptive Treg therapy is shown in Fig. 2.
Fig. 2.
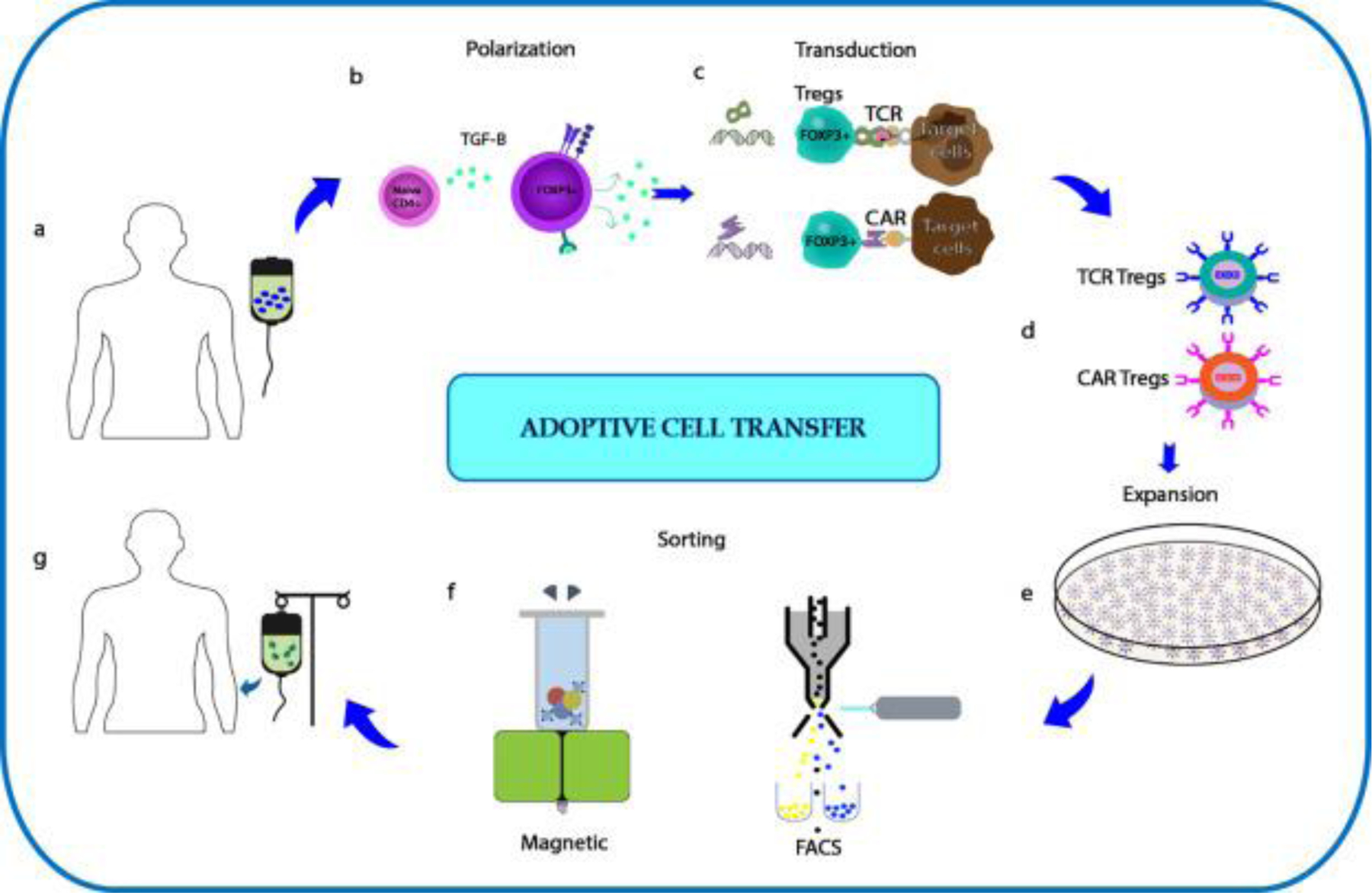
Scheme for Adoptive Treg transfer. (a) Leukapheresis of lymphocytes from peripheral blood of the patient. (b) Polarization of CD4+ T cells in to FOXP3+ Tregs in the presence of TGF-β. (c) Viral transduction of FOXP3+ Tregs with either TCR or CAR construct. (d) Tregs transduced with TCR and CAR construct. (e) Ex vivo expansion of Tregs to generate sufficient numbers for adoptive Treg transfer. (f) Sorting for Tregs using either magnetic or FACS based methods. (g) Infusion of either TCR or CAR Tregs into patients.
In another example, a chimeric autoantibody receptor (CAAR) specific for pathogenic antibody-producing B cells has been developed for the treatment of PV [156]. This CAAR was generated by fusing an autoantigen (Dsg3) to a CD137/CD3ζ signaling domain. Preclinical studies in a mouse model of PV showed that these Dsg3-CAAR T cells were functional against human anti-Dsg3 B cells and prevented blistering without measurable off-target toxicity. Thus, CAAR T-reg therapy might hold potential for deleterious PV by eliminating self-reactive B cells, and opens new avenues for the use of CAR Tregs in other autoimmune skin conditions [156,157].
7. Opportunities for antigen-specificity in vitiligo
Melanocytes express several currently known melanosomal proteins such as TRP-1 and TRP-2 [158]. Tyrosinase meanwhile, is a target antigen that has been targeted by TCR transgenic Tregs in a mouse model of melanoma. Brusko et al. tested human tyrosinase specific TCR (TyrTCR) transduced antigen-specific Tregs on a tumor model, and results showed the effective suppression of antigen-specific T cells to facilitate tumor growth [159]. Therefore, these TyrTCR Tregs might likewise suppress effector T cells in vitiligo and serve as a potential treatment for the disease. The concept is then, that tyrosinase can serve as a target for antigen-specific Tregs to suppress autoreactive T cells in vitiligo without eliciting non-specific immunosuppression [160]. Especially with the local deficiency of peripheral Tregs in vitiligo in mind, adoptive transfer of antigen-specific Tregs responsive to melanocytes might prevent general immunosuppression and bring superior specificity to the treatment.
8. Setbacks: how to generate Tregs in bulk and maintain a Treg profile
Adoptive Treg transfer has been administered in clinics for GvHD, transplantation, and autoimmune diseases [152,161]. However, ex vivo expansion and maintenance can become laborious and require manual handling, posing a risk for contamination. Isolation of Tregs for a clinical purpose can be performed using the “CliniMACS Prodigy®” fully automated system that meets GMP standards [162]. Peripheral blood can serve as a source of Tregs, with purification performed by magnetic enrichment in the sterile CliniMACS system. The isolation protocol includes the depletion of CD8+ and CD19+ lymphocytes followed by enrichment of CD25+ cells with a purity of FOXP3+ estimated at >90% and with yield up to 2.12 × 109 cells [162]. Besides, flow cytometry-based sorting was also frequently used in clinical trials based on CD4 and CD25, and lack of CD127 surface marker expression to isolate Tregs of high purity (>99%) [152,163,164]. Among sorted Tregs, the CD4+CD25+CD127lowCD45RA+ Tregs subset is most stable for FOXP3 expression, demonstrating resistance to Th17 effector conversion [165].
However, Tregs make up only ~3% of total T cells in peripheral blood, creating a challenge to generating sufficient numbers for infusion. Protocols for the expansion of Tregs are critical to achieving large numbers for clinical administration [166–168]. Polyclonal Tregs were 300-fold expanded in 5 weeks using activator beads and high dose IL-2 [169]. Some suggest adding rapamycin during expansion [164,170]. This immunosuppressant inhibits the mechanistic target of rapamycin (mTOR) protein kinase, which can inhibit effector T cell proliferation and favors FOXP3 upregulation [171], conferring higher stability and suppressive ability to the expanded Tregs [170]. Following a Treg expansion protocol using TGF-β together with rapamycin and IL-2, the expanded Tregs demonstrated higher suppression compared to the use of rapamycin alone [172]. Others developed a protocol to generate antigen-specific Tregs from co-culture with activated allogeneic B-cells or donor-derived DCs in the presence of IL-2 in organ transplantations. These antigen-specific Tregs also showed superior suppressive ability in vitro and in vivo compared to polyclonally expanded Tregs [153].
9. A reality check- Will antigen specific Tregs become available for autoimmune diseases?
In contrast to conventional immunosuppressive drugs, biologics, alkylating agents, and antimetabolites, Tregs can provide greater specificity with complex therapeutic benefits, and restore immune tolerance in various autoimmune diseases [173]. The application of polyclonal Tregs for autoimmune diseases has already have been attempted in clinical trials [152]. Overall, this and other phase I clinical studies provided answers to the isolation and expansion that surrounded Treg immunotherapy [152,163,174,175]. Yet the efficacy of polyclonal Treg transfer is not self-evident to date. Meanwhile, adoptive transfer of islet-specific Tregs outperformed polyclonal Tregs for blocking type 1 diabetes progression compared to polyclonal Tregs [173,176,177]. Unfortunately, diabetes is generally detected in patients when pancreas destruction is near complete, but other conditions may be more amenable to Treg based treatment in a clinical setting. Preclinical studies likewise suggest a superior efficacy of antigen-specific Tregs in transplantation procedures [144,[178], [179], [180]]. In different applications, antigen-specific CD19 CAR-T cells have been approved for clinical use by the Food and Drug administration (FDA) for large B-cell lymphoma and B-cell acute lymphoblastic leukemia [149]. It can be expected that antigen-specific CAR Tregs will meet with less side effects than CAR-T cell therapies in use for cancers [181]. Moreover, clinical protocols exist to counter cytokine release syndrome (CRS) and neurotoxicity after CAR Tregs infusion [152,174,175]. Thus, there is potential for antigen-specific Tregs to be prepared for the treatment of autoimmune skin diseases, which is expected to provide high efficacy while preventing non-specific immunosuppression.
A limitation to intravenous injection of antigen-specific Tregs might be that these much-needed immunosuppressive cells display a paucity at the desired site [177]. Should systemically applied Tregs not respond as required, the local injection might be needed, or the CCR4 Treg homing receptor ligand CCL22, can be introduced where Tregs are needed to attract systemically applied Tregs [128]. This leaves autoimmune diseases of the skin especially suited for adoptive treatment by antigen-specific Tregs when relevant antigens can be identified. To date, only alloantigen-reactive Tregs are currently being tested to prevent rejection after organ transplantation in clinical trials [177]. One of the other challenges of adoptive transfer is the cost and scalability of the technique. This has prompted the concept of developing off-the-shelf “Universal CAR Tregs” suitable for all patients [182].
Indeed, adoptive Treg treatment is expected to be most efficacious during active disease. In human patients however, progressive disease periods are interspersed with periods of inactivity, providing melanocyte stem cells with an opportunity to differentiate and repopulate the depigmented lesions For the intermittent treatment of vitiligo, it will be beneficial to store autologous CAR Tregs for later use [183]. By virtue of their antigen specificity, these Tregs might provide better safety profiles and decrease the risk of generalized immunosuppression.
10. Conclusion
The future of Treg-based therapy holds the potential for autoimmune skin diseases. Polyclonal Treg infusion was demonstrated to be safe and well-tolerated in patients. The results from currently ongoing clinical trials bring important insights regarding the Treg dose, efficacy and possible side effects. The safety of patients and well-understood protocols for managing possible side effects is crucial for the therapy. It is expected that the efficacy of Treg therapy will be enhanced if engineered as antigen-specific cells according to the several pre-clinical data. In vivo tracking will allow research groups to better understand the real potential of antigen-specific Tregs. The best practices for Treg-based therapy might be using antigen-specific Tregs accompanied by either IL-2 or rapamycin.
Funding sources
This work was supported in part by National Institutes of Health (grant numbers RO1s AR057643, CA191317) grants to I.C.L.P.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- [1].Chen W Tregs in immunotherapy: opportunities and challenges. Immunotherapy 2011;3:911–4. [DOI] [PubMed] [Google Scholar]
- [2].Famili F, Wiekmeijer AS, Staal FJ. The development of T cells from stem cells in mice and humans. Future Sci OA 2017;3:Fso186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gol-Ara M, Jadidi-Niaragh F, Sadria R, Azizi G, Mirshafiey A. The role of different subsets of regulatory T cells in immunopathogenesis of rheumatoid arthritis. Arthritis 2012;2012:805875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 2015;12:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gregori S, Roncarolo MG, Engineered T. Regulatory Type 1 cells for clinical application. Front Immunol 2018;9:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest 2016;126:1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014;20:62–8. [DOI] [PubMed] [Google Scholar]
- [8].Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? International Immunology 2009;21: 1105–11. [DOI] [PubMed] [Google Scholar]
- [9].Scott DW. Genetic engineering of T cells for immune tolerance. Molecular Therapy - Methods & Clinical Development 2020;16:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology 2008;124:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol 2012;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Le Poole IC, Mehrotra S, Replenishing Regulatory T. Cells to Halt depigmentation in vitiligo. J Investig Dermatol Symp Proc 2017;18:S38–s45. [DOI] [PubMed] [Google Scholar]
- [14].Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol 2017;18:716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Szilveszter KP, Nemeth T, Mocsai A. Tyrosine kinases in autoimmune and inflammatory skin diseases. Front Immunol 1862;10(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jain SV, Murrell DF. Psychosocial impact of inherited and autoimmune blistering diseases. Int J Womens Dermatol 2018;4:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee YW, Park EJ, Kwon IH, Kim KH, Kim KJ. Impact of psoriasis on quality of life: relationship between clinical response to therapy and change in health-related quality of life. Ann Dermatol 2010;22:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Radtke MA, Schafer I, Gajur A, Langenbruch A, Augustin M. Willingness-to-pay and quality of life in patients with vitiligo. Br J Dermatol 2009;161:134–9. [DOI] [PubMed] [Google Scholar]
- [19].Ceccarelli F, Agmon-Levin N, Perricone C. Genetic factors of autoimmune diseases. J Immunol Res 2017:2789242. [DOI] [PMC free article] [PubMed]
- [20].Ramos PS, Shedlock AM, Langefeld CD. Genetics of autoimmune diseases: insights from population genetics. Journal of Human Genetics 2015;60:657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jabri B, Terhorst C. Editorial overview: autoimmunity. Curr Opin Immunol 2014;31: v–vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kruger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol 2012;51:1206–12. [DOI] [PubMed] [Google Scholar]
- [23].van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 2009;129:2220–32. [DOI] [PubMed] [Google Scholar]
- [24].Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Lienard D, Lejeune F, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med 1999; 190:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Waterman EA, Kemp EH, Gawkrodger DJ, Watson PF, Weetman AP. Autoantibodies in vitiligo patients are not directed to the melanocyte differentiation antigen MelanA/MART1. Clin Exp Immunol 2002;129:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med 1998;188:1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu M, Wang J, Liu M. Hu X and Xu J, [Study of immunomodulatory function of exosomes derived from human umbilical cord mesenchymal stem cells]. Zhonghua Yi Xue Za Zhi 2015;95:2630–3. [PubMed] [Google Scholar]
- [28].Wang X, Shen H, He Q, Tian W, Xia A, Lu XJ. Exosomes derived from exhausted CD8 T cells impaired the anticancer function of normal CD8 T+ cells. J Med Genet 2019; 56:29–31. [DOI] [PubMed] [Google Scholar]
- [29].Wong PM, Yang L, Yang L, Wu H, Li W, Ma X, et al. New insight into the role of exosomes in vitiligo. Autoimmun Rev 2020;19:102664. [DOI] [PubMed] [Google Scholar]
- [30].Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res 2012;25:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mosenson JA, Eby JM, Hernandez C, Le Poole IC. A central role for inducible heat-shock protein 70 in autoimmune vitiligo. Exp Dermatol 2013;22:566–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res 2010;23:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eby JM, Kang HK, Klarquist J, Chatterjee S, Mosenson JA, Nishimura MI, et al. Immune responses in a mouse model of vitiligo with spontaneous epidermal de- and repigmentation. Pigment Cell Melanoma Res 2014;27:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One 2012;7:e37513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dwivedi M, Laddha NC, Arora P, Marfatia YS, Begum R. Decreased regulatory T-cells and CD4(+) /CD8(+) ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res 2013;26:586–91. [DOI] [PubMed] [Google Scholar]
- [36].Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001;193:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ben Ahmed M, Zaraa I, Rekik R, Elbeldi-Ferchiou A, Kourda N, Belhadj Hmida N, et al. Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res 2012;25:99–109. [DOI] [PubMed] [Google Scholar]
- [38].Abdallah M, Lotfi R, Othman W, Galal R. Assessment of tissue FoxP3+, CD4+ and CD8+ T-cells in active and stable nonsegmental vitiligo. Int J Dermatol 2014;53: 940–6. [DOI] [PubMed] [Google Scholar]
- [39].Ono S, Tanizaki H, Otsuka A, Endo Y, Koyanagi I, Kataoka TR, et al. Coexistent skin lesions of vitiligo and psoriasis vulgaris. Immunohistochemical analyses for IL-17A-producing cells and regulatory T cells. Acta Derm Venereol 2014;94:329–30. [DOI] [PubMed] [Google Scholar]
- [40].Zhou L, Li K, Shi YL, Hamzavi I, Gao TW, Henderson M, et al. Systemic analyses of immunophenotypes of peripheral T cells in non-segmental vitiligo: implication of defective natural killer T cells. Pigment Cell Melanoma Res 2012;25:602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Terras S, Gambichler T, Moritz RK, Altmeyer P, Lambert J. Immunohistochemical analysis of FOXP3+ regulatory T cells in healthy human skin and autoimmune dermatoses. Int J Dermatol 2014;53:294–9. [DOI] [PubMed] [Google Scholar]
- [42].Bala KK, Moudgil KD. Induction and maintenance of self tolerance: the role of CD4+CD25+ regulatory T cells. Arch Immunol Ther Exp (Warsz) 2006;54:307–21. [DOI] [PubMed] [Google Scholar]
- [43].Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25-precursors. J Immunol 2002; 169:4183–9. [DOI] [PubMed] [Google Scholar]
- [44].Khan R, Gupta S, Sharma A. Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J Am Acad Dermatol 2012:510–1. [DOI] [PubMed]
- [45].Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabiani M, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res 2002;15:87–92. [DOI] [PubMed] [Google Scholar]
- [46].Tembhre MK, Sharma VK, Sharma A, Chattopadhyay P, Gupta S. T helper and regulatory T cell cytokine profile in active, stable and narrow band ultraviolet B treated generalized vitiligo. Clin Chim Acta 2013;424:27–32. [DOI] [PubMed] [Google Scholar]
- [47].Ala Y, Pasha MK, Rao RN, Komaravalli PL, Jahan P. Association of IFN-γ : IL-10 cytokine ratio with nonsegmental vitiligo pathogenesis. Autoimmune Dis 2015: 423490. [DOI] [PMC free article] [PubMed]
- [48].Budinger L, Borradori L, Yee C, Eming R, Ferencik S, Grosse-Wilde H, et al. Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest 1998;102:2082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Izumi K, Bieber K, Ludwig RJ. Current clinical trials in pemphigus and pemphigoid. Front Immunol 2019;10:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Quaglino P, Antiga E, Comessatti A, Caproni M, Nardo T, Ponti R, et al. Circulating CD4+ CD25brightFOXP3+ regulatory T-cells are significantly reduced in bullous pemphigoid patients. Arch Dermatol Res 2012;304:639–45. [DOI] [PubMed] [Google Scholar]
- [51].Antiga E, Quaglino P, Volpi W, Pierini I, Del Bianco E, Bianchi B, et al. Regulatory T cells in skin lesions and blood of patients with bullous pemphigoid. J Eur Acad Dermatol Venereol 2014;28:222–30. [DOI] [PubMed] [Google Scholar]
- [52].Arakawa M, Dainichi T, Ishii N, Hamada T, Karashima T, Nakama T, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol 2011;20: 1022–4. [DOI] [PubMed] [Google Scholar]
- [53].Rensing-Ehl A, Gaus B, Bruckner-Tuderman L, Martin SF. Frequency, function and CLA expression of CD4+CD25+FOXP3 regulatory T cells in bullous pemphigoid. Exp Dermatol 2007;16:13–21. [DOI] [PubMed] [Google Scholar]
- [54].Bieber K, Sun S, Witte M, Kasprick A, Beltsiou F, Behnen M, et al. Cells suppress inflammation and blistering in pemphigoid diseases. Front Immunol 2017;8:1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sugiyama H, Matsue H, Nagasaka A, Nakamura Y, Tsukamoto K, Shibagaki N, et al. CD4+CD25high regulatory T cells are markedly decreased in blood of patients with pemphigus vulgaris. Dermatology 2007;214:210–20. [DOI] [PubMed] [Google Scholar]
- [56].Veldman C, Pahl A, Beissert S, Hansen W, Buer J, Dieckmann D, et al. Inhibition of the transcription factor Foxp3 converts desmoglein 3-specific type 1 regulatory T cells into Th2-Like cells. The Journal of Immunology 2006;176:3215–22. [DOI] [PubMed] [Google Scholar]
- [57].Yokoyama T, Matsuda S, Takae Y, Wada N, Nishikawa T, Amagai M, et al. Antigen-independent development of Foxp3+ regulatory T cells suppressing autoantibody production in experimental pemphigus vulgaris. Int Immunol 2011;23: 365–73. [DOI] [PubMed] [Google Scholar]
- [58].Schmidt T, Willenborg S, Hunig T, Deeg CA, Sonderstrup G, Hertl M, et al. Induction of T regulatory cells by the superagonistic anti-CD28 antibody D665 leads to decreased pathogenic IgG autoantibodies against desmoglein 3 in a HLA-transgenic mouse model of pemphigus vulgaris. Exp Dermatol 2016;25:293–8. [DOI] [PubMed] [Google Scholar]
- [59].Chihara N, Madi A, Karwacz K, Awasthi A, Kuchroo VK. Differentiation and characterization of Tr1 cells. Current Protocols in Immunology 2016;113: 3.27.1–3.27.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Veldman C, Hohne A, Dieckmann D, Schuler G, Hertl M. Type I regulatory T cells specific for desmoglein 3 are more frequently detected in healthy individuals than in patients with pemphigus vulgaris. J Immunol 2004;172:6468–75. [DOI] [PubMed] [Google Scholar]
- [61].Albanesi C, De Pita O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol 2007;25:581–8. [DOI] [PubMed] [Google Scholar]
- [62].Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 2009;129:1339–50. [DOI] [PubMed] [Google Scholar]
- [63].Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol 2011;36:292–7. [DOI] [PubMed] [Google Scholar]
- [64].Das D, Akhtar S, Kurra S, Gupta S, Sharma A. Emerging role of immune cell network in autoimmune skin disorders: An update on pemphigus, vitiligo and psoriasis. Cytokine Growth Factor Rev 2019;45:35–44. [DOI] [PubMed] [Google Scholar]
- [65].Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol 2017;76:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tajalli M, Kabir S, Vance TM, Qureshi AA. Effective use of oral tofacitinib and phototherapy in a patient with concomitant alopecia areata, vitiligo, and plaque and inverse psoriasis. Clin Case Rep 2020:819–22. © 2020 They Authors. Clinical Case Reports published by John Wiley & Sons Ltd.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Beggs S, Short J, Rengifo-Pardo M, Ehrlich A. Applications of the Excimer Laser: A Review. Dermatol Surg 2015;41:1201–11. [DOI] [PubMed] [Google Scholar]
- [68].Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 2009;206:1983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Furue K, Yamamura K, Tsuji G, Mitoma C, Uchi H, Nakahara T, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol 2018;98:5–13. [DOI] [PubMed] [Google Scholar]
- [70].Karamehic J, Zecevic L, Resic H, Jukic M, Jukic T, Ridjic O, et al. Immunophenotype lymphocyte of peripheral blood in patients with psoriasis. Med Arch 2014;68:236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yun WJ, Lee DW, Chang SE, Yoon GS, Huh JR, Won CH, et al. Role of CD4CD25FOXP3 regulatory T cells in psoriasis. Ann Dermatol 2010;22:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol 2005;174:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yang L, Li B, Dang E, Jin L, Fan X, Wang G. Impaired function of regulatory T cells in patients with psoriasis is mediated by phosphorylation of STAT3. J Dermatol Sci 2016; 81:85–92. [DOI] [PubMed] [Google Scholar]
- [74].Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol 2011;131: 1853–60. [DOI] [PubMed] [Google Scholar]
- [75].Keijsers RR, van der Velden HM, van Erp PE, de Boer-van Huizen RT, Joosten I, Koenen HJ, et al. Balance of Treg vs. T-helper cells in the transition from symptomless to lesional psoriatic skin. Br J Dermatol 2013;168:1294–302. [DOI] [PubMed] [Google Scholar]
- [76].Hartwig T, Zwicky P, Schreiner B, Yawalkar N, Cheng P, Navarini A, et al. Regulatory T cells restrain pathogenic T helper cells during skin inflammation. Cell Rep 25 2018;e4:3564–72. [DOI] [PubMed] [Google Scholar]
- [77].Stockenhuber K, Hegazy AN, West NR, Ilott NE, Stockenhuber A, Bullers SJ, et al. Foxp3(+) T reg cells control psoriasiform inflammation by restraining an IFN-I-driven CD8(+) T cell response. J Exp Med 2018);215:1987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Leite Dantas R, Masemann D, Schied T, Bergmeier V, Vogl T, Loser K, et al. Macrophage-mediated psoriasis can be suppressed by regulatory T lymphocytes. J Pathol 2016;240:366–77. [DOI] [PubMed] [Google Scholar]
- [79].Wang H, Peters T, Sindrilaru A, Kess D, Oreshkova T, Yu XZ, et al. TGF-beta-dependent suppressive function of Tregs requires wild-type levels of CD18 in a mouse model of psoriasis. J Clin Invest 2008;118:2629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Paus R, Bertolini M. The role of hair follicle immune privilege collapse in alopecia areata: status and perspectives. J Investig Dermatol Symp Proc 2013;16:S25–7. [DOI] [PubMed] [Google Scholar]
- [81].Hoffmann R, Wenzel E, Huth A, van der Steen P, Schaufele M, Henninger HP, et al. Cytokine mRNA levels in Alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol 1994;103:530–3. [DOI] [PubMed] [Google Scholar]
- [82].Erb U, Freyschmidt-Paul P, Zoller M. Tolerance induction by hair-specific keratins in murine alopecia areata. J Leukoc Biol 2013;94:845–57. [DOI] [PubMed] [Google Scholar]
- [83].Zoller M, McElwee KJ, Engel P, Hoffmann R. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol 2002;118:983–92. [DOI] [PubMed] [Google Scholar]
- [84].McElwee KJ, Freyschmidt-Paul P, Hoffmann R, Kissling S, Hummel S, Vitacolonna M, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol 2005;124: 947–57. [DOI] [PubMed] [Google Scholar]
- [85].Speiser JJ, Mondo D, Mehta V, Marcial SA, Kini A, Hutchens KA. Regulatory T-cells in alopecia areata. J Cutan Pathol 2019;46:653–8. [DOI] [PubMed] [Google Scholar]
- [86].Hamed FN, Astrand A, Bertolini M, Rossi A, Maleki-Dizaji A, Messenger AG, et al. Alopecia areata patients show deficiency of FOXP3+CD39+ T regulatory cells and clonotypic restriction of Treg TCRbeta-chain, which highlights the immunopathological aspect of the disease. PLoS One 2019;14:e0210308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol 2014;150:748–51. [DOI] [PubMed] [Google Scholar]
- [88].Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010;466:113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tembhre MK, Sharma VK. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br J Dermatol 2013;169:543–8. [DOI] [PubMed] [Google Scholar]
- [90].Loh SH, Moon HN, Lew BL, Sim WY. Role of T helper 17 cells and T regulatory cells in alopecia areata: comparison of lesion and serum cytokine between controls and patients. J Eur Acad Dermatol Venereol 2018;32:1028–33. [DOI] [PubMed] [Google Scholar]
- [91].Shin BS, Furuhashi T, Nakamura M, Torii K, Morita A. Impaired inhibitory function of circulating CD4+CD25+ regulatory T cells in alopecia areata. J Dermatol Sci 2013; 70:141–3. [DOI] [PubMed] [Google Scholar]
- [92].Saigusa R, Asano Y, Sato S. Case of disseminated granuloma annulare with giant plaques. J Dermatol 2016;43:1443–4. [DOI] [PubMed] [Google Scholar]
- [93].Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol 2015;6:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mathian A, Parizot C, Dorgham K, Trad S, Arnaud L, Larsen M, et al. Activated and resting regulatory T cell exhaustion concurs with high levels of interleukin-22 expression in systemic sclerosis lesions. Ann Rheum Dis 2012;71:1227–34. [DOI] [PubMed] [Google Scholar]
- [95].Banica L, Besliu A, Pistol G, Stavaru C, Ionescu R, Forsea AM, et al. Quantification and molecular characterization of regulatory T cells in connective tissue diseases. Autoimmunity 2009;42:41–9. [DOI] [PubMed] [Google Scholar]
- [96].Liu X, Gao N, Li M, Xu D, Hou Y, Wang Q, et al. Elevated levels of CD4(+)CD25(+) FoxP3(+) T cells in systemic sclerosis patients contribute to the secretion of IL-17 and immunosuppression dysfunction. PLoS One 2013;8:e64531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fenoglio D, Battaglia F, Parodi A, Stringara S, Negrini S, Panico N, et al. Alteration of Th17 and Treg cell subpopulations co-exist in patients affected with systemic sclerosis. Clin Immunol 2011;139:249–57. [DOI] [PubMed] [Google Scholar]
- [98].Cordiali-Fei P, Mussi A, D’Agosto G, Trento E, Bordignon V, Trincone S, et al. Assessment of T regulatory cells and expanded profiling of autoantibodies may offer novel biomarkers for the clinical management of systemic sclerosis and undifferentiated connective tissue disease. Clin Dev Immunol 2013:390563. [DOI] [PMC free article] [PubMed]
- [99].Wang YY, Wang Q, Sun XH, Liu RZ, Shu Y, Kanekura T, et al. DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br J Dermatol 2014;171:39–47. [DOI] [PubMed] [Google Scholar]
- [100].Baraut J, Grigore EI, Jean-Louis F, Khelifa SH, Durand C, Verrecchia F, et al. Peripheral blood regulatory T cells in patients with diffuse systemic sclerosis (SSc) before and after autologous hematopoietic SCT: a pilot study. Bone Marrow Transplant 2014;49:349–54. [DOI] [PubMed] [Google Scholar]
- [101].Klein S, Kretz CC, Ruland V, Stumpf C, Haust M, Hartschuh W, et al. Reduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic scleroderma. Ann Rheum Dis 2011;70:1475–81. [DOI] [PubMed] [Google Scholar]
- [102].Antiga E, Quaglino P, Bellandi S, Volpi W, Del Bianco E, Comessatti A, et al. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol 2010;162:1056–63. [DOI] [PubMed] [Google Scholar]
- [103].Slobodin G, Ahmad MS, Rosner I, Peri R, Rozenbaum M, Kessel A, et al. Regulatory T cells (CD4(+)CD25(bright)FoxP3(+)) expansion in systemic sclerosis correlates with disease activity and severity. Cell Immunol 2010;261:77–80. [DOI] [PubMed] [Google Scholar]
- [104].Kataoka H, Yasuda S, Fukaya S, Oku K, Horita T, Atsumi T, et al. Decreased expression of Runx1 and lowered proportion of Foxp3(+) CD25(+) CD4(+) regulatory T cells in systemic sclerosis. Mod Rheumatol 2015;25:90–5. [DOI] [PubMed] [Google Scholar]
- [105].Ugor E, Simon D, Almanzar G, Pap R, Najbauer J, Nemeth P, et al. Increased proportions of functionally impaired regulatory T cell subsets in systemic sclerosis. Clin Immunol 2017;184:54–62. [DOI] [PubMed] [Google Scholar]
- [106].Papp G, Horvath IF, Barath S, Gyimesi E, Sipka S, Szodoray P, et al. Altered T-cell and regulatory cell repertoire in patients with diffuse cutaneous systemic sclerosis. Scand J Rheumatol 2011;40:205–10. [DOI] [PubMed] [Google Scholar]
- [107].Yang X, Yang J, Xing X, Wan L, Li M. Increased frequency of Th17 cells in systemic sclerosis is related to disease activity and collagen overproduction. Arthritis Res Ther 2014;16:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].MacDonald KG, Dawson NA, Huang Q, Dunne JV, Levings MK, Broady R. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J Allergy Clin Immunol 2015;135 (946–.e9). [DOI] [PubMed] [Google Scholar]
- [109].Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 2013;5:179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Arruda LCM, Malmegrim KCR, Lima-Junior JR, Clave E, Dias JBE, Moraes DA, et al. Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients. Blood Adv 2018;2:126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kamio K, Azuma A, Matsuda K, Usuki J, Inomata M, Morinaga A, et al. Resolution of bleomycin-induced murine pulmonary fibrosis via a splenic lymphocyte subpopulation. Respir Res 2018;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Boveda-Ruiz D, D’Alessandro-Gabazza CN, Toda M, Takagi T, Naito M, Matsushima Y, et al. Differential role of regulatory T cells in early and late stages of pulmonary fibrosis. Immunobiology 2013;218:245–54. [DOI] [PubMed] [Google Scholar]
- [113].Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest 2015;125:2795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mehrotra S, Al-Khami AA, Klarquist J, Husain S, Naga O, Eby JM, et al. A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. J Immunol 2012;189:1627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Oren R, Hod-Marco M, Haus-Cohen M, Thomas S, Blat D, Duvshani N, et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J Immunol 2014;193:5733–43. [DOI] [PubMed] [Google Scholar]
- [116].Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest 2008;118:3619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tenspolde M, Zimmermann K, Weber LC, Hapke M, Lieber M, Dywicki J, et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. Journal of Autoimmunity 2019;103: 102289. [DOI] [PubMed] [Google Scholar]
- [118].Yoon J, Schmidt A, Zhang AH, Konigs C, Kim YC, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood 2017;129:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Collison J Low-dose IL-2 therapy for autoimmune diseases. Nature Reviews Rheumatology 2019;15:2. [DOI] [PubMed] [Google Scholar]
- [120].Tahvildari M, Dana R. Low-Dose IL-2 therapy in transplantation, autoimmunity, and inflammatory diseases. The Journal of Immunology 2019;203:2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rosenzwajg M, Lorenzon R, Cacoub P, Pham HP, Pitoiset F, El Soufi K, et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann Rheum Dis 2019;78: 209–17. [DOI] [PubMed] [Google Scholar]
- [122].Wu R, Li N, Zhao X, Ding T, Xue H, Gao C, et al. Low-dose Interleukin-2: Biology and therapeutic prospects in rheumatoid arthritis. Autoimmun Rev 2020;19:102645. [DOI] [PubMed] [Google Scholar]
- [123].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A 2008;105:7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schwaiger T, van den Brandt C, Fitzner B, Zaatreh S, Kraatz F, Dummer A, et al. Autoimmune pancreatitis in MRL/Mp mice is a T cell-mediated disease responsive to cyclosporine A and rapamycin treatment. Gut 2014;63:494–505. [DOI] [PubMed] [Google Scholar]
- [125].Wong VW, You F, Januszyk M, Gurtner GC, Kuang AA. Transcriptional profiling of rapamycin-treated fibroblasts from hypertrophic and keloid scars. Ann Plast Surg 2014; 72:711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wei KC, Lai PC. Combination of everolimus and tacrolimus: a potentially effective regimen for recalcitrant psoriasis. Dermatol Ther 2015;28:25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Siklar Z, Karatas D, Dogu F, Hacihamdioglu B, Ikinciogullari A, Berberoglu M, et al. Vitamin D status in children with chronic autoimmune thyroiditis. J Clin Res Pediatr Endocrinol 2016;8:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Eby JM, Kang HK, Tully ST, Bindeman WE, Peiffer DS, Chatterjee S, et al. CCL22 to activate treg migration and suppress depigmentation in vitiligo. J Invest Dermatol 2015;135:1574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Henning SW, Fernandez MF, Mahon JP, Duff R, Azarafrooz F, Guevara-Patino JA, et al. HSP70iQ435A-encoding DNA repigments vitiligo lesions in sinclair swine. J Invest Dermatol 2018;138:2531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Klarquist J, Tobin K, Farhangi Oskuei P, Henning SW, Fernandez MF, Dellacecca ER, et al. Ccl22 diverts T regulatory cells and controls the growth of melanoma. Cancer research 2016;76:6230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Konieczna P, Akdis CA, Quigley EM, Shanahan F, O’Mahony L. Portrait of an immunoregulatory Bifidobacterium. Gut Microbes 2012;3:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, et al. L. O’Mahony, Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 2012;61:354–66. [DOI] [PubMed] [Google Scholar]
- [133].O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog 2008;4:e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Dellacecca ER, Cosgrove C, Mukhatayev Z, Akhtar S, Engelhard VH, Rademaker AW, et al. Antibiotics drive microbial imbalance and vitiligo development in mice. J Invest Dermatol 2019;140:676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten Brinke A, et al. Hurdles in therapy with regulatory T cells. Sci Transl Med 2015;7: 304ps18. [DOI] [PubMed] [Google Scholar]
- [136].Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen KL, McKenna DH, et al. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol Blood Marrow Transplant 2013;19:1271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 2004; 199:1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 2004;199:1467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol 2005;175:3053–9. [DOI] [PubMed] [Google Scholar]
- [140].Stephens LA, Malpass KH, Anderton SM. Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur J Immunol 2009;39:1108–17. [DOI] [PubMed] [Google Scholar]
- [141].Sanchez-Fueyo A, Sandner S, Habicht A, Mariat C, Kenny J, Degauque N, et al. Specificity of CD4+CD25+ regulatory T cell function in alloimmunity. J Immunol 2006;176:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol 2004;16: 1189–201. [DOI] [PubMed] [Google Scholar]
- [143].Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 2008;14:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood 2007;109:827–35. [DOI] [PubMed] [Google Scholar]
- [145].Boardman D, Maher J, Lechler R, Smyth L, Lombardi G. Antigen-specificity using chimeric antigen receptors: the future of regulatory T-cell therapy? Biochem Soc Trans 2016;44:342–8. [DOI] [PubMed] [Google Scholar]
- [146].Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 2014;257:107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 2009;126:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Harris DT, Kranz DM, Adoptive T. Cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends in pharmacological sciences 2016;37:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Salmikangas P, Kinsella N, Chamberlain P. Chimeric antigen receptor T-cells (CAR T-Cells) for cancer immunotherapy - moving target for industry? Pharm Res 2018;35: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhang J, Wang L. The emerging world of TCR-T cell trials against cancer: a systematic review. Technol Cancer Res Treat 2019;18 (1533033819831068). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol 2018;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015;7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant 2013;13:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Marek-Trzonkowska N, Mysliwiec M, Iwaszkiewicz-Grzes D, Gliwinski M, Derkowska I, Zalinska M, et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med 2016;14:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Mukhatayev Z, Cosgrove C, Shivde R, Jaishankar D, Pontarolo-Maag K, Eby JE, et al. Antigen specificity enhances disease control by Tregs in vitiligo. Front. Immunol 2020;11:581433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 2016;353:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ellebrecht CT, Payne AS. Setting the target for pemphigus vulgaris therapy. JCI Insight 2017;2:e92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene 2003;22:3035–41. [DOI] [PubMed] [Google Scholar]
- [159].Brusko TM, Koya RC, Zhu S, Lee MR, Putnam AL, McClymont SA, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One 2010;5:e11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Le Poole IC, Stennett LS, Bonish BK, Dee L, Robinson JK, Hernandez C, et al. Expansion of vitiligo lesions is associated with reduced epidermal CDw60 expression and increased expression of HLA-DR in perilesional skin. Br J Dermatol 2003;149:739–48. [DOI] [PubMed] [Google Scholar]
- [161].Di Ianni M, Del Papa B, Zei T, Iacucci Ostini R, Cecchini D, Cantelmi MG, et al. T regulatory cell separation for clinical application. Transfus Apher Sci 2012;47:213–6. [DOI] [PubMed] [Google Scholar]
- [162].Marín Morales JM, Münch N, Peter K, Freund D, Oelschlägel U, Hölig K, et al. Automated clinical grade expansion of regulatory T cells in a fully closed system. Front Immunol 2019;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol 2014;153:23–30. [DOI] [PubMed] [Google Scholar]
- [164].Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 2009;58: 652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Canavan JB, Scotta C, Vossenkamper A, Goldberg R, Elder MJ, Shoval I, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 2016;65:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 2014;124:638–44. [DOI] [PubMed] [Google Scholar]
- [167].Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS One 2011;6:e15868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Scottà C, Esposito M, Fazekasova H, Fanelli G, Edozie FC, Ali N, et al. Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4(+)CD25(+)FOXP3(+) T regulatory cell subpopulations. Haematologica 2013;98:1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Fraser H, Safinia N, Grageda N, Thirkell S, Lowe K, Fry LJ, et al. A Rapamycin-Based GMP-compatible process for the isolation and expansion of regulatory T cells for clinical trials. Mol Ther Methods Clin Dev 2018;8:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4 CD25 FOXP3 regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 2006;177:8338–47. [DOI] [PubMed] [Google Scholar]
- [171].Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 2009;9:324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Mathew JM, LeFever A, Konieczna I, Stratton C, He J, Huang X, et al. A phase I Clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep 2018;8:7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov 2019;18:749–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, et al. Administration of CD4+CD25highCD127-regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 2012;35: 1817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Marek-Trzonkowska N, Myśliwec M, Siebert J, Trzonkowski P. Clinical application of regulatory T cells in type 1 diabetes. Pediatr Diabetes 2013;14:322–32. [DOI] [PubMed] [Google Scholar]
- [176].Raffin C, Vo LT, Bluestone JA. T(reg) cell-based therapies: challenges and perspectives. Nat Rev Immunol 2020;20:158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bluestone JA, Tang Q. T(reg) cells-the next frontier of cell therapy. Science 2018;362: 154–5. [DOI] [PubMed] [Google Scholar]
- [178].Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest 2003;112:1688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med 2011;3:83ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Noyan F, Lee YS, Hardtke-Wolenski M, Knoefel AK, Taubert R, Baron U, et al. Donor-specific regulatory T cells generated on donor B cells are superior to CD4+CD25high cells in controlling alloimmune responses in humanized mice. Transplant Proc 2013;45:1832–7. [DOI] [PubMed] [Google Scholar]
- [181].Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev 2019;34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Koristka S, Kegler A, Bergmann R, Arndt C, Feldmann A, Albert S, et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J Autoimmun 2018;90:116–31. [DOI] [PubMed] [Google Scholar]
- [183].MacDonald KN, Ivison S, Hippen KL, Hoeppli RE, Hall M, Zheng G, et al. Cryopreservation timing is a critical process parameter in a thymic regulatory T-cell therapy manufacturing protocol. Cytotherapy 2019;21:1216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


