Abstract
Background:
Although atopic dermatitis (AD) often presents in infancy and persists into adulthood, comparative characterization of AD skin among different pediatric age groups is lacking.
Objective:
We sought to define skin biopsy profiles of lesional and nonlesional AD across different age groups (0-5-year-old infants with disease duration <6 months, 6-11-year-old children, 12-17-year-old adolescents, ≥18-year-old adults) versus age-appropriate controls.
Methods:
We performed gene expression analyses by RNA-sequencing and real-time PCR (RT-PCR) and protein expression analysis using immunohistochemistry.
Results:
TH2/TH22 skewing, including IL-13, CCL17/thymus and activation-regulated chemokine, IL-22, and S100As, characterized the common AD signature, with a global pathway-level enrichment across all ages. Nevertheless, specific cytokines varied widely. For example, IL-33, IL-1RL1/IL-33R, and IL-9, often associated with early atopic sensitization, showed greatest upregulations in infants. TH17 inflammation presented a 2-peak curve, with highest increases in infants (including IL-17A and IL-17F), followed by adults. TH1 polarization was uniquely detected in adults, even when compared with adolescents, with significant upregulation in adults of IFN-γ and CXCL9/CXCL10/CXCL11. Although all AD age groups had barrier abnormalities, only adults had significant decreases in filaggrin expression. Despite the short duration of the disease, infant AD presented robust downregulations of multiple barrier-related genes in both lesional and nonlesional skin. Clinical severity scores significantly correlated with TH2/TH22-related markers in all pediatric age groups.
Conclusions:
The shared signature of AD across ages is TH2/TH22-skewed, yet differential expression of specific TH2/TH22-related genes, other TH pathways, and barrier-related genes portray heterogenetic, age-specific molecular fingerprints.
Keywords: Atopic dermatitis, T(H)2, T(H)22, biomarkers, epidermal barrier, maturation, normal skin, pediatric
Atopic dermatitis (AD) is the most common inflammatory skin disease and its prevalence continues to increase worldwide.1,2 Although AD often presents in infancy and persists into adulthood,1 the evolution of the AD skin phenotype from infancy to adulthood has not yet been characterized. Because AD may be the window to the atopic march, understanding immune and barrier changes of AD across pediatric populations may provide insights into the initiation of other atopic comorbidities, such as food allergy, allergic rhinitis, and asthma.
Recently, we compared biopsied skin from infants and young children (<age 5 years) with AD within 6 months of disease initiation to skin from adults (age ≥18 years) with chronic AD.3,4 The early disease profile has substantial differences compared with adult chronic AD. Although both share robust TH2/TH22 skewing, infants have greater TH17 inflammation, lack TH1 polarization, and have different barrier abnormalities.3,4
Although data from blood of patients with AD in different age groups were recently published,5 skin of school-age children and adolescents (ages 5-18 years) with AD has not been investigated comprehensively. In blood, the immune fingerprint of AD was found to be age-specific, with the adult AD phenotype achieved only in adults.5 Because cutaneous analyses capture skin dysregulations more accurately and also allow assessment of barrier abnormalities, investigation of AD skin through development is imperative.
Current therapeutics for extensive AD are sparse,6-8 and therapeutic options available for pediatric patients with AD are even more limited.9,10 Narrow targeting agents for AD are emerging,11-13 including for pediatric patients.14-16 As new therapies that target different type 2 immune pathway components or other pathways are developed, characterization of immune and barrier abnormalities in different pediatric AD age groups may become more important in the decision making about optimal choice of therapeutic agent. A better understanding of age-specific disease markers will also potentially contribute to identifying novel age-specific treatment strategies.
To discover age-specific biomarkers, AD skin of different age groups (0-5, 6-11, 12-17, and ≥18 years) was compared with that of age-appropriate controls. Molecular dysregulations were assessed by RNA sequencing, real-time PCR (RT-PCR), and immunohistochemistry (IHC). Biomarker analyses showed different expression patterns of specific TH2-related cytokines and chemokines across the age spectrum, although TH2/TH22 skewing was shared among all pediatric groups and adults. TH1-related products progressively increased with age, including being significantly higher in adults versus adolescents. For TH17, despite global upregulations at all ages, a 2-curve peak of TH17-related polarization was captured, highest in infancy and less enriched in adulthood. These data portray the evolution of AD skin through maturation and improve our understanding of shared and age-specific disease mechanisms.
Methods
Clinical characteristics
Fifty-four patients with moderate to severe AD in different age groups (17 infants with disease duration of <6 months, 0-5 years old, mean age, 1.2 years; 10 children, 6-11 years old, mean age, 7.8 years; 14 adolescents, 12-17 years old, mean age, 14.8 years; 13 adults ≥ 18 years old, mean age, 45.4 years) were enrolled after parents and patients 12 years and older signed institutional review board–approved informed consents and assents (Table I; see Table E1 in this article’s Online Repository at www.jacionline.org). The cohort across age groups was composed primarily of European American patients. Sensitivity analyses on European American-only patients yielded similar results to analysis including subjects of all races (see Table E7 and Figs E15 and E16 in this article’s Online Repository at www.jacionline.org). Lesional and nonlesional skin biopsies were obtained, and exclusion criteria included recent use of systemic immunosuppressive treatment and phototherapy (<4 weeks), topical steroids or immunomodulators (<1 week), or moisturizers (<12 hours) before this study.
TABLE I.
Patient characteristics
| Characteristic | Infant AD (n = 17) |
Infant controls (n = 13) |
P value |
Child AD (n = 10) |
Child controls (n = 13) |
P value |
Adolescent AD (n = 14) |
Adolescent controls (n = 5) |
P value |
Adult AD (n = 13) |
Adult controls (n = 15) |
P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y), mean ± SD | 1.2 ± 1.2 | 1.2 ± 0.8 | 0.9 | 7.8 ± 2.1 | 7.9 ± 2.3 | 0.94 | 14.8 ± 1.7 | 14.2 ± 1.8 | 0.5 | 45.2 ± 14.6 | 35.6 ± 10.0 | 0.06 |
| Female sex, n (%) | 8 (47.0) | 7 (53.8) | >.99 | 6 (60.0) | 7 (53.8) | >.99 | 6 (42.8) | 3 (60.0) | 0.6 | 7 ± 53.8 | 8 ± 53.3 | >.99 |
| Clinical severity measures | ||||||||||||
| SCORAD, mean ± SD | 58.1 ± 12.4 | NA | NA | 64.3 ± 15.7 | NA | NA | 62.1 ± 20.0 | NA | NA | 65.1 ± 13.3 | NA | NA |
| EASI, mean ± SD | 15.3 ± 8.8 | NA | NA | 19.8 ± 12.6 | NA | NA | 27.4 ± 22.8 | NA | NA | NA | NA | NA |
| TEWL, lesional (g/h/m2), mean ± SD | 32.9 ± 13.8 | NA | NA | 36.8 ± 17.6 | NA | NA | 51.2 ± 25.9 | NA | NA | NA | NA | NA |
| TEWL, nonlesional (g/h/m2), mean ± SD | 16.2 ± 8.0 | NA | NA | 25.6 ± 10.5 | NA | NA | 25.6 ± 13.5 | NA | NA | NA | NA | NA |
| Pruritus ADQ, mean ± SD | 17.2 ± 9.3 | NA | NA | 26.8 ± 5.5 | NA | NA | 22.0 ± 9.4 | NA | NA | NA | NA | NA |
| IgE (IU/mL), mean ± SD | 5830.6 ± 858.3 | NA | NA | 1072 ± 915.8 | NA | NA | 6136 ± 5943.6 | NA | NA | 230 ± 291.0 | NA | NA |
ADQ, Atopic Dermatitis Quickscore; EASI, Eczema Area and Severity Index; NA, not applicable/available; TEWL, transepidermal water loss
Clinical severity was recorded using SCORing of AD (SCORAD), Eczema Area and Severity Index, and Atopic Dermatitis Quickscore in pediatric patients (≤18 years) and SCORAD in adults (≥18 years). IgE levels (IU/mL) were available for some patients with AD. In pediatric participants, measures of epidermal barrier function were captured using transepidermal water loss of lesional and nonlesional arm skin (AquaFlux Model AF200; Biox, London, United Kingdom).17
RNA sequencing
RNA was extracted from skin biopsy samples using Qiagen miRNeasy Mini kit, as previously described.18 Libraries were generated using TruSeq Stranded mRNA Library Prep kit (Illumina, San Diego, Calif). mRNA was first extracted from 240 ng of total RNA using oligo-dT magnetic beads and fragmented at high temperature. A complementary DNA library was then prepared by reverse transcription. Next-generation sequencing was performed on Illumina Hiseq 4000 (Illumina) with single-ended read 100 cycles. Our sequencing depth ranged between 35 and 45M reads, and the reference genome used was hg19 (GRCh37). Image analysis and bases calling was conducted in real-time by the Illumina analysis pipeline.
Quantitative RT-PCR
For RT-PCR, reverse transcription to complementary DNA was carried out using the High Capacity complementary DNA reverse transcription (Thermo Fisher, Waltham, Mass). Preamplification was performed on all samples. TaqMan Low Density Array cards (Thermo Fisher) were used for quantitative RT-PCR (qRT-PCR). Primers are listed in Table E2 in this article’s Online Repository at www.jacionline.org. Hundred nanogram total RNA was used for PreAMP pool and TaqMan Low Density Array. Eukaryotic 18S recombinant RNA was used as an endogenous control. Expression values were normalized to Rplp0.
Immunohistochemistry
IHC was performed on frozen skin sections as previously described19,20 using antibodies as listed in Table E3 in this article’s Online Repository at www.jacionline.org. Epidermal thickness and cell counts were quantified using ImageJ V1.42 software (National Institutes of Health, Bethesda, Md).
Statistical analyses
Statistical analyses were performed using statistical language R (www.R-project.org). qRT-PCR data were profiled using TaqMan Low Density Array. Summaries of mean expression of all markers are summarized in a heatmap, where unsupervised clustering was performed using Euclidean distance and average agglomeration criteria. Threshold cycles (Ct) were normalized to Rplp0 by negatively transforming the Ct values to –dCt. The undetected expression values were estimated for each gene as 20% of the minimum unlogged expression across all samples. Log2-scale qRT-PCR expression data were modeled by a linear mixed-effect model, with biopsy type as a fixed effect and a random intercept for each patient. Means of each group were estimated using lsmeans, and comparisons of interest were tested using contrast. For correlation studies, unsupervised hierarchical clustering of variables or samples/patients was performed using the correlation coefficient as a distance metric with the average agglomeration algorithm.
RNA-sequencing data were preprocessed using standard pipeline incorporating quality control metrics, such as FastQC and MultiQC, sequence alignment based on STAR RNA-sequencing aligner, and sequencing reads assignment to genomic features by featureCounts and voom-transformed. Mixed-effect model on R’s limma framework was used, and P values were adjusted by Benjamini-Hochberg procedure. Differentially expressed genes (DEGs) were defined by fold change (FCH) more than 2.0 and false-discovery rate (FDR) less than 0.05. Gene set overexpression analysis was performed with XGR software using canonical/KEGG/REACTOME/BioCarta pathways.21
Results
Normal skin changes from infancy through adulthood
To better understand AD-related changes in different age groups, we evaluated changes in healthy skin throughout maturation, using RNA-seq data presented as heatmaps of previously reported immune and barrier gene set (see Figs E1 and E2 and Table E4 in this article’s Online Repository at www.jacionline.org). Comparisons between age groups were considered DEGs using a threshold of FCH more than 2 and FDR less than 0.05. Overall, although most immune- and barrier-related markers were comparable between age groups, a mixed expression pattern was detected, with the largest number of DEGs found in the adult versus infant comparison. A green box highlights genes that show a trend of increase with age, as visualized by a blue to red transition from infancy to adulthood (Fig E1). Among these, the negative regulatory marker IL-37 showed significant downregulation in infants compared with adults (FDR < 0.05). Pink boxes indicate clusters of genes that are most upregulated in infants. These include regulatory/regulatory T-cell markers such as CTLA4, IL-10, and TGFβ3, as well as TH17/TH22-related genes, such as CXCL2 and S100P/A7/A9 (FDR < 0.05). Using a gene set variation analysis curated from previously published data sets,22, 23, 24 we found that although most immune axes were comparable between age groups (TH1/TH2/TH22, P > .05), the TH17 pathway was significantly upregulated in infants (P < .05) (see Fig E3, A, in this article’s Online Repository at www.jacionline.org).
Barrier-related genes were slightly intensified in the older age groups, including terminal differentiation–related (late cornified envelope [LCE]1F/2B/2C/2D, filaggrin [FLG], and SCEL), tight junction–related (CLDN8/11/23), and lipid-related genes (ELOVL5, FADS1/2, FABP7, and SGPP1). Only few lipid-related markers were significantly increased in infants versus adults (fatty acid 2-hydroxylase, lipoprotein lipase, cell death inducing DFFA like effector C, and peroxisome proliferator activated receptor gamma) (FDR < 0.05) (Fig E2).
Spearman correlation between mRNA expressions (by RT-PCR) with age similarly showed that multiple TH17/TH22-related markers were inversely correlated with age (IL-20, IL-22, S100A7/9/12/8, CCL20, and PI3) (r ≤ −0.4, P < .05), whereas positive correlations with age were found for barrier-related markers (loricrin [LOR], CLDN1/CLDN23) and negative regulatory markers (IL-37, IL-34) (r > 0.4; P < .05) (see Table E5 in this article’s Online Repository at www.jacionline.org).
IHC evaluating for epidermal hyperplasia and immune cell infiltrates revealed that healthy infants had significantly thinner epidermis, with greater counts of TNF-related apoptosis-inducing ligand (TRAIL)+ dendritic cells (DCs), eosinophils (major basic protein [MBP]+ cells), and neutrophils (neutrophil elastase+ cells). However, older age groups showed greater counts of FcεR1+ DCs (Fig E3, B-F).
Shared and uniquely DEGs unfold the common versus age-specific features
Global molecular profiling of the age-specific AD transcriptomes by RNA-seq identified 9829 DEGs (3859 upregulated, 5970 downregulated) in lesional AD skin versus skin of similar-age healthy controls across all age groups (using the same threshold of FCH > 2 and FDR < 0.05) (E4, A, in this aricle's Online Repository at www.jacionline.org). Of these, 362 DEGs (3.7%) were shared among lesional AD skin in all age groups (154 upregulated, 208 downregulated). Lesional infant and adult AD had a larger number of uniquely expressed genes as compared with children and adolescents: 3060 uniquely expressed DEGs were detected in infants, 236 in children, 643 in adolescents, and 2766 in adults.
A principal-component analysis of all lesional and normal samples across ages using RNA-seq data shows that although the normal skin samples overlap, lesional AD samples present wider variations, with separation seen between adults and pediatric age groups (Fig E4, B). No clear trends were observed in nonlesional principal-component analysis (see Fig E4).
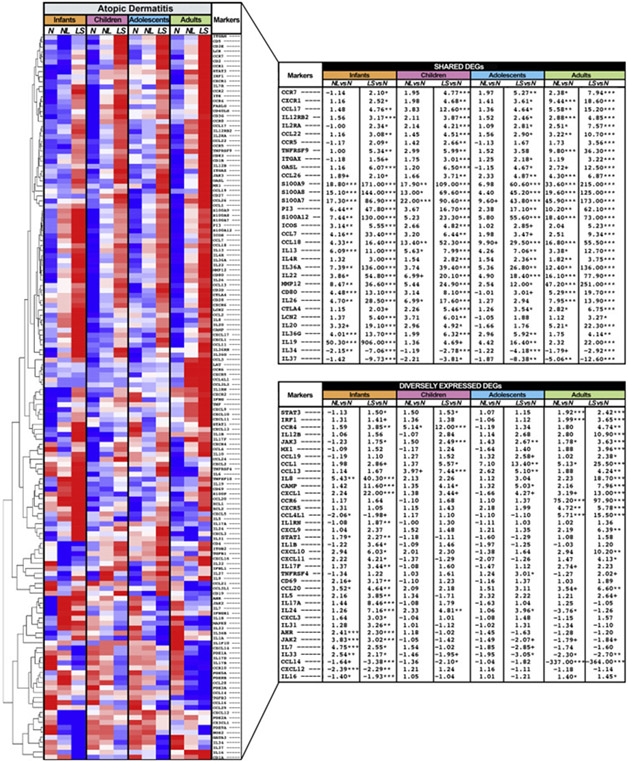
The global immune phenotype of AD across age groups in lesional skin presented key TH2-related markers (IL-13, CCL17/thymus and activation-regulated chemokine [TARC], CCL18/PARC, and IL-4R) and TH22-related markers (IL-22, S100A7/8/9/12) (Fig 1) (FDR < 0.05).25,26 Additional TH2-related products including TSLPR/CRLF2, CCL26, CCL7, and CCL27/CTACK were shared considering FDR less than 0.1 and FCH more than 2 criteria (Table E4). Other shared upregulated DEGs across ages in lesional AD included general inflammation (MMP12/25)27 and T-cell activation/migration/natural killer–cell (TNFRSF9, CCR7, GZMB, and CSF2) markers, whereas commonly downregulated DEGs included the negative regulators IL-34 and IL-37 (Fig 1 and Table E4) (FDR < 0.05).
Fig 1.
Immune genes. Summary heatmap of immune genes from multiple pathways in lesional and nonlesional AD vs normal skin across age groups using RNA-seq, by criteria of FCH more than 2 and FDR less than 0.05. Samples are sorted by hierarchical clustering. Tables show DEGs presenting comparable (top) and differential (bottom) expression across AD age groups. LS, Lesional; N, normal; NL, nonlesional. ***FDR < 0.001, **FDR < 0.01, *FDR < 0.05, +FDR < 0.1.
Nevertheless, specific age groups also showed distinct phenotypes. In infant AD, uniquely/higher upregulated genes included those related to innate immunity (IL-1B and IL-8), and aryl hydrocarbon receptor (FDR < 0.05).28 Infant-specific DEGs also included TH2-related cytokines, including the itch cytokine IL-31,29 cytokines implicated in atopy initiation (IL-5 and IL-33),30,31 and multiple TH17-related markers, including the key TH17 cytokines IL-17A and IL-17F (Fig 1) (FDR < 0.05). These are in line with the dominant TH17-polarization characterizing AD in infants,4 and further define robust TH17-related upregulations as particularly characteristic of infant AD. Of note, many of these are significantly higher in infants versus children (eg, IL-17A, CXCL1, CCL20, and IL-19) (FDR < 0.05).
Except for IL-16 and CXCL12, both related with T-cell activation and recruitment (FDR < 0.05),32,33 significant upregulations of key immune DEGs were not found uniquely in child or adolescent AD. These age groups mostly exhibited comparable expression of many TH2- and TH22-related DEGs (eg, IL-13, CCL17/TARC, and IL-22), lower levels of TH17-related markers, as detailed above, or slightly higher levels of T-cell migration (CCL19 and CCR7), DCs (ITGAX/CD11c), and TH1-related markers (CCL3, CCL4, and CXCL16) compared with infants, but not to the levels found in adults.
Conversely, lesional adult AD presented numerous unique DEGs with intensification of the inflammatory tone across various immune axes. Specifically, multiple TH1-related markers were uniquely expressed or showed significantly higher expression levels in adults, including CXCL9/10, MX1, interferon regulatory factor-1, IL-12B/p40, and CCR1 (FDR < 0.05; Fig 1). Heatmaps with clustering analysis of nonnormalized gene expression results of lesional and nonlesional AD skin across age groups are presented in Figs E5 to E8 in this article’s Online Repository at www.jacionline.org and detailed in this article’s Online Repository at www.jacionline.org.
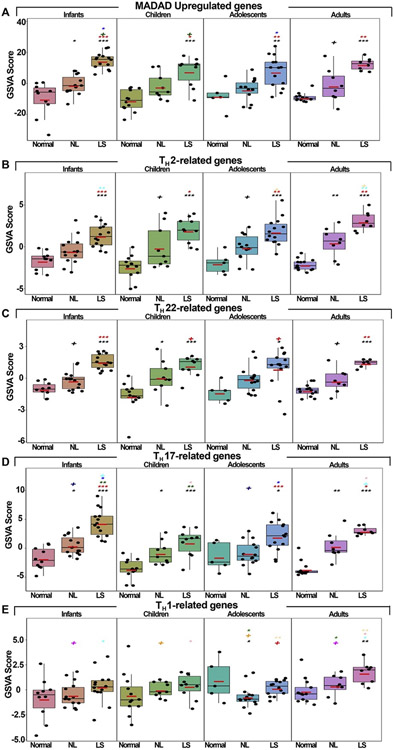
Using a gene set variation analysis on previously published AD-related pathways, including the Meta-Analysis Derived AD transcriptome,24 and key immune pathways,22,23 we observed significant enrichment across AD age groups in the Meta-Analysis Derived AD and TH2 and TH22 pathways compared with controls (Fig 2, A-D). However, the TH17 axis showed highest expression in infants, whereas the TH1 pathway significantly upregulated only in adults (P < .05; Fig 2).
Fig 2.
A-E, Gene set variation analyses for major TH immune pathways. Red bars represent means. Black symbols: significance of comparison to normal; red symbols: significance of comparison between lesional and nonlesional skin; symbols in other colors: significance of comparison between respective groups. LS, Lesional; NL, nonlesional. ***P < .001, **P < .01, *P < .05, +P < .1.
For a broader perspective on age-specific AD-related changes of gene expression, all DEGs in lesional AD versus controls by age groups were also analyzed using function-based pathway databases (canonical/KEGG/REACTOME/BioCarta pathways), which revealed significantly enriched pathways in all age groups (FDR < 0.05). Of these, some of the pathways were common to 2 and more age groups, and some unique for a specific age (see Table E6 in this article’s Online Repository at www.jacionline.org). Pathways that were significantly enriched across all ages including those related with cytokine and chemokine signaling, the JAK-signal transducer and activator of transcription pathway, and the adaptive immune system, whereas pathways enriched only in children, adolescents, and adults included those related with T-cytotoxic-cell, TH-cell, and other T-cell receptor signaling (FDR < 0.05) (see Fig E9 in this article’s Online Repository at www.jacionline.org). IFN-γ signaling showed gradual intensification with age, starting in children. The top upregulated pathways detected in the infant AD signature included various aspects of cell cycle and replication and were substantially enriched in that age group versus other age groups.
Barrier characteristics differ across AD age groups
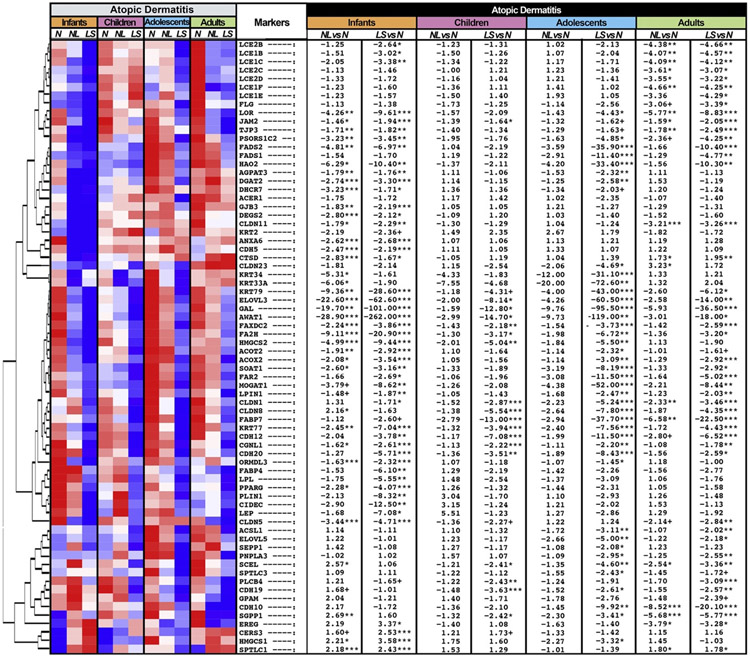
Because disrupted epidermal barrier is an important characteristic of AD, genes of epidermal barrier components, including epidermal differentiation, lipid biosynthesis/metabolism, tight and gap junctions, and keratins, were assessed using a previously reported epidermal barrier gene set (Fig 3; see Table E4).12,20,34,35
Fig 3.
Epidermal barrier-related genes. Summary heatmap of barrier-associated genes in lesional and nonlesional AD vs normal skin across age groups using RNA-seq, by criteria of FCH more than 2 and FDR less than 0.05. Samples are sorted by hierarchical clustering. LS, Lesional; N, normal; NL, nonlesional. ***FDR < .001, **FDR < .01, *FDR < .05, +FDR < .1.
The common signature of barrier impairment included key AD-associated lipid biosynthesis/metabolism-related genes, significantly downregulated across all age groups, including elongation of very long chain fatty acids protein 3,24 reported to improve with dupilumab treatment,12 acyl-CoA wax alcohol acyl transferase 1,36 and fatty acid 2-hydroxylase (FDR < 0.05). In addition, significant attenuation of tight junction–related genes in lesional skin, including cadherin (CDH20, CDH12),37 claudin (CLDN1),38 and paracingulin (CGNL1),39 was shared across age groups (FDR < 0.05). Epidermal differentiation genes that are considered a hallmark of the barrier dysregulation in AD were not commonly downregulated across age groups. Although in adults, multiple terminal differentiation genes showed significant downregulations versus controls (LCEs [LCE1B/1C/1E/1F/2C/2D], FLG, PSORC1C240) (FDR < 0.05), less significant or similar but nonsignificant trends were largely observed for other age groups.
Many markers showed exclusive downregulation in infants with AD, but not in other AD age groups. These included lipid biosynthesis/metabolism-related genes, such as 7-dehydrocholesterol reductase and peroxisome proliferator activated receptor gamma, both also negatively associated with mast cell activation and hyper-degranulation,41,42 perpilin 1, suppressed by the topical drug adapalene, which causes sebum inhibition,43 CIDEC, associated with reduced skin surface lipids and dry skin,44 and leptin, reported to be lower in serum of children with AD versus children without AD (FDR < 0.05).45 Moreover, most of these genes were significantly downregulated in nonlesional infant AD.
RT-PCR studies further define AD age groups and developmental trajectories
To validate RNA-seq data and evaluate mRNA expressions of key inflammatory and epidermal differentiation markers in AD, some of which may be poorly detected by RNA-seq due to relatively low levels, we performed RT-PCR on a large panel of 71 genes (see Fig E10 in this article’s Online Repository at www.jacionline.org).
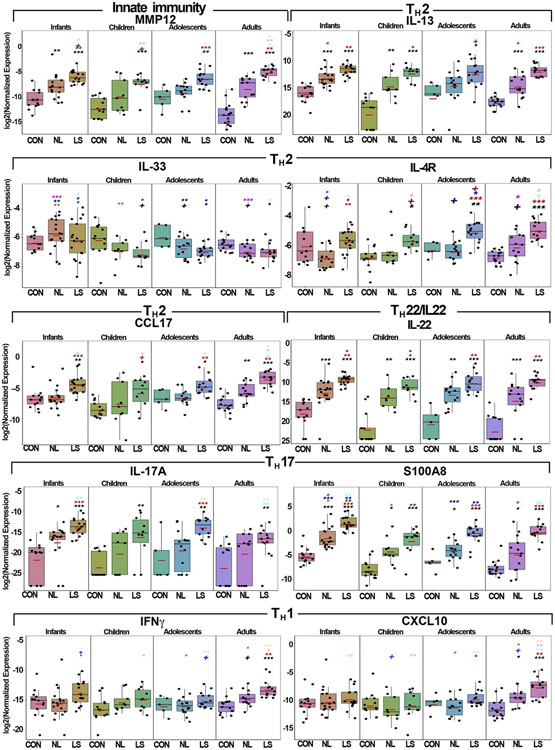
PCR was generally in line with the RNA-seq data, and further defined the common TH2/TH22 skewing across age groups as well as the unique inflammatory milieu of each one. Markers of general inflammation (ie, MMP12) were commonly upregulated across ages, with adults displaying the greatest upregulations (Fig 4). Some markers exhibited distinct expression patterns. For example, IL-17C, an epithelial marker and a potential therapeutic target for inflammatory skin disorders,46,47 was gradually increased starting in adolescence, whereas IL-8/CXCL8, associated with childhood asthma,48 was significantly highest in infants compared with other age groups (P < .05) (Fig E10).
Fig 4.
qRT-PCR analysis of selected inflammatory genes in skin of different AD age groups and age-appropriate controls. Values show log2 expression and are presented as means ± SEMs. Red bars represent means. Black symbols: significance of comparison to normal; red symbols: significance of comparison between lesional and nonlesional skin; symbols in other colors: significance of comparison between respective groups. CCL17, C-C motif chemokine ligand 17; CON, controls; LS, lesional; MMP12, matrix metallopeptidase 12; NL, nonlesional. ***P < .001, **P < .01, *P < .05, +P < .1.
Overall, TH2/TH22-related markers including IL-13, IL-31, CCL17/TARC, CCL18/PARC, CCL7, IL-26, and IL-22 showed highly significant upregulations in lesional AD across all age groups versus controls (P < .05) (Figs 4 and E10). Other TH2-related chemokines, including CCL13, CCL22, and CCL26, were upregulated in most AD age groups. IL-4R (targeted by dupilumab)49 showed significant upregulations in lesional AD skin across all age groups (for infants, significance was attained only for the nonlesional comparison, with increasing significance with age [P < .05; Figs 4 and E10]). In contrast, robust upregulations were found in infants compared with children in IL-9, IL-33 and its receptor IL-1RL1/IL-33R (in nonlesional skin), associated with early mite sensitization, food allergies, and asthma,50, 51, 52 and IL-5, associated with induction of eosinophilic inflammation30 (P < .05) (Figs 4 and E10). Infants also showed the highest expression levels of multiple TH17-related products, including IL-17A, LL37/CAMP, CCL20, PI3, DEFB4B, KYNU, and the TH17/TH22-regulated S100As (Figs 4 and E10).
Because overexpression of TH1-related markers is associated with chronicity in long-standing AD,26 multiple key TH1-related immune mediators were significantly upregulated only in adults, including IFN-γ, CXCL9/CXCL10/CXCL11, signal transducer and activator of transcription 1, and MX1 (P < .001) (Figs 4 and E10). Moreover, IFN-γ and CXCL9/CXCL10/CXCL11 were significantly upregulated in adult lesional skin, even as compared with adolescents, suggesting that the adult AD phenotype is achieved only in adulthood.
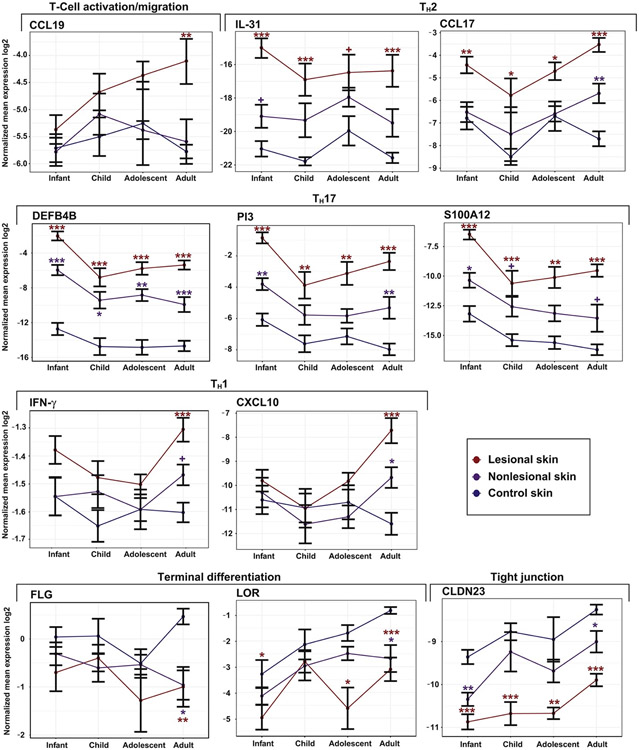
We then analyzed key immune and barrier markers in lesional and nonlesional AD in parallel with normal skin (Fig 5) to reflect developmental trajectories. The T-cell migration marker CCL19 presented upregulation with age only in lesional AD, whereas in nonlesional and normal skin expression levels remained similar across ages. The TH2 markers, IL-31 and CCL17, did not show a consistent trend in normal skin, but presented greater upregulation in specific ages (infants and adults, respectively), with upregulation versus normal skin detected across all ages. Although TH1-related markers were intensified in adults only in AD skin (IFN-γ and CXCL10), TH17-related markers were most upregulated in infants in both normal skin and AD (DEFB4B, PI3, and S100A12), with an increase toward adulthood in AD, but not in normal skin. Terminal differentiation (FLG and LOR) markers were most downregulated in adult AD versus controls, where normal skin presented greater expression level with age, whereas AD skin presented only modest increases in expression. The tight junction–related marker CLDN23 showed a comparable expression in AD versus normal skin across ages, with minor gradual upregulation with age in all tissues.
Fig 5.
qRT-PCR analysis of selected inflammatory and barrier genes of different AD and normal skin age groups presented as line charts, representing developmental trajectories. Values show log2 normalized expression and are presented as means ± SEMs. ***P < .001, **P < .01, *P < .05, +P < .1.
Epidermal hyperplasia and compromised skin barrier characterize AD across ages
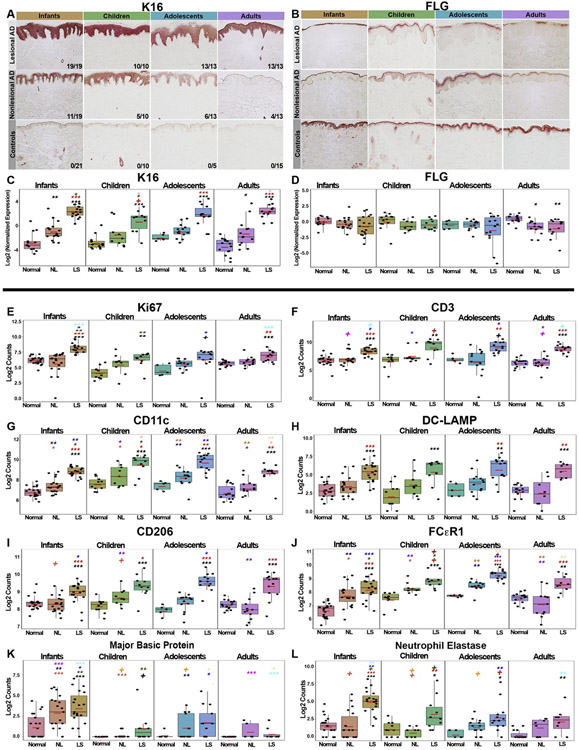
IHC was also performed to evaluate for protein expression of epidermal differentiation/proliferation markers, as well as immune cell infiltrates. All AD age groups showed significant increases in epidermal hyperplasia in lesional skin, assessed by thickness, Ki67+-cell counts, and K16 staining and mRNA expression (P < .01; Fig 6, A, C, and E; see Figs E11 and E12 in this article’s Online Repository at www.jacionline.org). Overall, the greatest abnormalities in nonlesional AD skin in terms of the proliferation marker K16 were seen in infants, with positive staining decreasing with age (14 of 19 in infants [74%], 5 of 10 in children [50%], 6 of 13 in adolescents [46%], and 4 of 13 in adults [31%]). Healthy controls were negative for K16 staining across all ages (Fig 6, A and C). Conversely, impairment of the key epidermal differentiation markers, FLG and LOR, was most significant in adult AD (P < .05; Fig 6, B and D, and Fig E11, B and D).
Fig 6.
IHC studies assessing AD across pediatric and adult age groups. A and B, Representative IHC images of keratin 16 (K16) and FLG staining at 10× magnification in AD age groups and age-appropriate controls. C and D, Corresponding mRNA expression levels by qRT-PCR. Values show log2 expression presented as means ± SEMs. Red bars represent means. E-L, Boxplots depicting cell counts of skin infiltrates in nonlesional/lesional AD and healthy skin across age groups. Values are presented as means ± SEMs. Black symbols: significance of comparison to normal; red symbols: significance of comparison between lesional and nonlesional skin; symbols in other colors: significance of comparison between respective groups. DC-LAMP, Dendritic cell - lysosomal associated membrane protein; LS, lesional; NL, nonlesional. ***P < .001, **P < .01, *P < .05, +P < .1.
We next assessed for inflammatory infiltrates including CD3+ T cells, CD11c+ myeloid DCs, CD206+, TRAIL+, and FcεR1+ inflammatory DCs, DC-LAMP+ mature DCs, CD1c+ resting DCs, as well as for polymorphonuclear infiltrates including eosinophils (MBP+ cells), neutrophils (neutrophil elastase+ cells), and mast cells (tryptase+ cells) (Fig 6; see Figs E11-E14 in this article’s Online Repository at www.jacionline.org). Overall, CD3+ T cells and all DC subsets were significantly increased in lesional AD across all age groups starting at disease initiation in infants. The highest counts of MBP+/eosinophils and neutrophil elastase+/neutrophils were found in infants, with MBP+-cell counts being higher in infant nonlesional AD than in lesional skin of other age groups (P < .01; Fig 6, K and L; see Fig E14).
TH2/TH22-related markers correlated with SCORAD across AD age groups
To define common AD as well as unique biomarkers in various age groups, we correlated mRNA expressions by RT-PCR with clinical variables (age, SCORAD, Eczema Area and Severity Index, pruritus AD) and transepidermal water loss, the functional barrier measure53 using Spearman correlations (Table II and Table E5).
TABLE II.
Spearman correlations of skin biomarkers with AD clinical severity based on SCORAD, r > 0.4/<20.4, and P < .05
| SCORAD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesional skin | Nonlesional skin | ||||||||||||||||
| Infants | Children | Adolescents | Infants | Children | Adolescents | ||||||||||||
| Marker | r |
P value |
Marker | r |
P value |
Marker | r |
P value |
Marker | r |
P value |
Marker | r |
P value |
Marker | r |
P value |
| IL-21 | 0.574 | .016 | CCL21 | 0.842 | .004 | CAMP | 0.697 | .007 | IL-26 | 0.493 | .044 | IL-9 | 0.870 | .002 | CXCL1 | 0.925 | <.001 |
| IL-34 | 20.575 | .016 | IL1B | 0.830 | .006 | K16 | 0.574 | .035 | IL-1RL1 | 0.485 | .049 | IL-19 | 0.850 | .006 | S100A12 | 0.859 | <.001 |
| PPL | 20.569 | .017 | IL31 | 0.827 | .003 | IL-23A | 0.560 | .040 | IL-37 | 20.483 | .049 | DEFB4B | 0.800 | .014 | DEFB4B | 0.842 | <.001 |
| LCN2 | 20.510 | .036 | IFNG | 0.77 | .014 | S100A8 | 0.552 | .044 | IL-17F | 0.749 | .020 | IL-1B | 0.789 | .001 | |||
| CCL22 | 20.499 | .041 | MMP12 | 0.745 | .018 | IL-4R | 0.538 | .050 | PPL | 0.700 | .043 | S100A8 | 0.771 | .002 | |||
| IL-22 | 0.733 | .021 | FLG | 20.622 | .020 | CLDN8 | 20.883 | .003 | CXCL11 | 0.758 | .003 | ||||||
| CXCL1 | 0.721 | .024 | CLDN1 | 20.833 | .008 | S100A9 | 0.754 | .003 | |||||||||
| IL-4R | 0.721 | .024 | IL33 | 20.783 | .017 | CCL17 | 0.754 | .003 | |||||||||
| IL-15 | 0.709 | .028 | IL34 | 20.717 | .037 | KRT16 | 0.749 | .003 | |||||||||
| CXCL11 | 0.697 | .031 | IL-20 | 0.745 | .003 | ||||||||||||
| OX40 | 0.697 | .031 | CAMP | 0.723 | .005 | ||||||||||||
| IL-10 | 0.697 | .031 | CCL19 | 0.719 | .005 | ||||||||||||
| IL-17A | 0.693 | .026 | IL-10 | 0.697 | .007 | ||||||||||||
| CCL18 | 0.685 | .035 | IL-22 | 0.697 | .007 | ||||||||||||
| CCL13 | 0.685 | .035 | IL-23A | 0.692 | .008 | ||||||||||||
| CCL7 | 0.673 | .039 | CXCL9 | 0.675 | .010 | ||||||||||||
| IL-13 | 0.661 | .044 | CCL7 | 0.666 | .011 | ||||||||||||
| FOXP3 | 0.648 | .049 | PI3 | 0.657 | .013 | ||||||||||||
| CLDN1 | 20.709 | .028 | IL-19 | 0.648 | .015 | ||||||||||||
| CCL20 | 0.644 | .015 | |||||||||||||||
| LCN2 | 0.644 | .015 | |||||||||||||||
| CXCL8 | 0.640 | .016 | |||||||||||||||
| MMP12 | 0.635 | .017 | |||||||||||||||
| IFNG | 0.631 | .018 | |||||||||||||||
| CCL13 | 0.626 | .019 | |||||||||||||||
| CXCL10 | 0.626 | .019 | |||||||||||||||
| KYNU | 0.607 | .021 | |||||||||||||||
| IL31 | 0.603 | .022 | |||||||||||||||
| S100A7 | 0.596 | .028 | |||||||||||||||
| IL-13 | 0.591 | .029 | |||||||||||||||
| CCL18 | 0.569 | .037 | |||||||||||||||
| CCL22 | 0.565 | .038 | |||||||||||||||
In all pediatric age groups, SCORAD correlated robustly (r > 0.4; P < .05) with key TH2 and TH17/TH22-related markers, but these markers differed between lesional and nonlesional samples as well as in different age groups, as presented in Table II. For example, IL-22 significantly correlated with SCORAD in nonlesional adolescent AD and in lesional child AD, and the TH17/TH22-related S100As correlated with severity in lesional and nonlesional adolescent AD. TH2-related products positively correlated with SCORAD included cytokines (IL-31 and IL-13), chemokines (CCL18, CCL13, CCL17, and CCL22), and cytokine receptors (IL-4R, IL-1RL1/IL-33R/ST2), all implicated in AD pathogenesis (r > 0.4; P < .05) (Table II).54-57
Robust positive correlations with age were found only in lesional skin. These included IL-17RA, which maintains skin barrier and immune homeostasis,58 CCL22/MDC, which was previously correlated with AD severity in serum,59 and the T-regulatory marker FOXP3 (r > 0.4, P < .05).60 Among markers negatively correlating with age was the TH17/TH2-regulated cytokine, IL-19 (in both lesional and nonlesional skin), previously shown to be highly and significantly increased in early AD in infants,3,4 and IL-33 (in lesional skin), associated with early allergy sensitization (r < −0.4; P < .05) (Table E5).61
Discussion
Despite the significant burden of AD in both pediatric and adult populations, the molecular signature of AD skin in different pediatric age groups has not been fully characterized. We performed comprehensive profiling of AD across age groups (infants/toddlers with disease duration of <6 months, 0-5 years old; children, 6-11 years old; adolescents, 12-17 years old; and adults ≥18 years old) to identify age-specific versus shared immune and barrier dysregulations, taking into account changes related to normal maturation in healthy individuals. Thus, our results portray the evolution of AD-related changes in the skin throughout life. Moreover, although reports on immune-aging and immune-senescence show upregulation of proinflammatory markers with aging even in response to nonpathogenic skin challenge,62-67 the molecular characteristics of normal skin throughout maturation are still elusive. Previous blood studies suggest that infants present overexpression of regulatory T cells68,69 and a greater TH17 lineage capacity versus adults,70 and the latter present a greater TH1-development potential.70 In lymphatic tissues, a developmental TH switch was postulated to occur after age 3 years.71 Our healthy control data provide a unique glimpse into these processes in human skin. In our youngest age groups, we found the greatest decrease in the negative regulators (ie, IL-37 and IL-34, both significantly correlated with age across all age groups), with the largest increase in regulatory T-cell–associated markers (IL-10, TGFβ3, and CTLA4) displayed in infants, simulating physiologic or pathogenic states of impaired immunity and inflammation.68,69,72-77 Infants also presented an enriched TH17 pathway compared with other age groups. These findings may reflect the developing cutaneous immune system in infants, with age-dependent changes in regulatory and protective or defensive mechanisms.68-70,78 Analysis presenting key immune and barrier markers as developmental trajectories in both normal and AD skin further reveals how AD alters normal skin development. For example, the greatest downregulation of terminal differentiation genes (FLG and LOR) in AD versus controls was detected in adults, a result of a gradual upregulation of these markers in normal skin but not in AD skin, where expression levels increases were less prominent.
Generally, all AD age groups express a common genomic fingerprint, featuring robust TH2 and TH22 skewing. Common TH2-related markers included IL-13, CCL17/TARC, CCL18/PARC, and IL-4R. Common TH22-related markers included IL-22 and the S100As. The shared immune signature also included genes of general inflammation (eg, MMP12), T-cell activation/migration (eg, TNFRSF9 and CCR7), and negative regulators (IL-34 and IL-37). We also found positive and significant correlations of TH2/TH22-related markers with SCORAD in all pediatric age groups, further highlighting their pathogenic significance in AD regardless of age.
Despite these shared molecular abnormalities, major variations between age groups were detected. Overall, infant and adult AD skin displayed the most significant dysregulations when compared with other AD age groups. Although infants exhibit the greatest TH17-related enrichment (eg, IL-17A and IL-17F), chronic, long-standing adult AD reveal unique TH1 skewing (eg, IFN-γ and CXCL9/CXCL10/CXCL11). Analyzing publicly available function-based pathway databases,21 we found the greatest representation of genes related to cell growth and proliferation in infant AD versus controls, suggesting the rapid development of lesional AD in infancy. Thus, in agreement with findings recently published based on flow cytometry in AD blood,79 the adult skin phenotype of AD is achieved only at adulthood.
Although the levels of TH17-related products in infant AD skin are comparable to those found in psoriasis,3,4 we found these expression levels are not persistent in older pediatric age groups. In fact, enrichment of the TH17 pathway was also detected in normal infant skin, and even after normalization of AD skin by controls of the same age groups, these results were sustained. In addition, despite pathway-level upregulation versus controls, AD skin of children exhibits the lowest levels of TH17-related genes as compared with other AD age groups. These results in our youngest age group could be attributed to the role of TH17-related cytokines and chemokines in the developing immune system, first encountering multiple extrinsic pathogens early in life. TH17-cell–generated products such as IL-17A and IL-17F, which showed the greatest upregulations in infant AD, are of pivotal importance for antimicrobial defenses by recruiting and expanding immune response, both cellular and humoral.80
During early life and soon after AD starts to manifest, the initiation of other atopic diseases, including food allergy, allergic rhinitis, and asthma, also takes place and has been termed the atopic march.81 Indeed, in the infant AD age group, multiple markers associated with these atopic comorbidities were upregulated significantly versus children and older age groups. For example, IL-33 and IL-9 are associated with early mite and peanut allergic sensitization,50,51 IL-33 and its receptor IL-1RL1/IL-33R alter mast cell and basophil functions and induce asthma and allergic rhinitis,82 IL-5 plays a key role in asthma induction and eosinophilic inflammation,30,83 and IL-8 was suggested as an important mediator in childhood asthma.84
AD skin is also characterized by a disrupted epidermal barrier, with reported abnormalities of epidermal differentiation, keratinocyte junctions, and lipid composition.25,85 These components were downregulated in all AD age groups, most prominently in lesional skin. Although epidermal differentiation markers such as FLG and LCEs were most downregulated in adults, multiple barrier-related genes were most downregulated in lesional and nonlesional infant AD, despite the short duration of the disease in this age group. Positive staining of the entire epidermis by the epidermal hyperplasia marker K16 was most prevalent in nonlesional infant skin versus other age groups, supporting our gene expression findings. These findings argue that barrier impairment plays an important role at disease onset. Although our results cannot resolve the dispute of whether AD originates as immune versus barrier dysregulation (ie, inside-out vs the outside-in hypotheses),48 these findings suggest both are particularly robust very early in AD development.
Differences in expression levels of various therapeutic targets across ages, most of which are TH2-related markers, may potentially reflect differing responses to therapeutics based on age. For example, the itch cytokine IL-31, known to perpetuate the itch-scratch cycle of AD and other AD-related features, such as epidermal terminal differentiation disruption,86-90 is a target for inhibition by AD therapeutics. IL-31 antagonists have shown greater improvement in pruritus-related assessments than clinical scores in adult AD and are currently being tested in adolescents.91,92 The even greater increases that were found in infants and children versus adolescents and adults suggest additional benefit from IL-31 targeting in younger pediatric patients. However, IL-13 cytokine is consistently and significantly upregulated versus controls in all AD age groups. As such, IL-13 antagonists, which are in clinical trials,93,94 may benefit patients with AD across all ages. Nevertheless, these speculations need to be carefully investigated in age-specific clinical trials to establish whether age-related differences in efficacy exist and determine underlying mechanisms.
This study has a few limitations. Despite inclusion of different age groups, this is not a longitudinal, prospective study and reflects a single time-point measurement of different patients in different ages. FLG mutations were not assessed in our cohort, and different races were included. In addition, the pathogenicity of immune axes and specific cytokines and chemokines presented in different age groups cannot be further validated without future targeted clinical trials.
Conclusions
Our findings shed light on the development of AD skin throughout life and offer, for the first time, a broader view of the molecular dysregulations resulting in the AD phenotype during maturation. Moreover, our results provide a glimpse into the initiation of other atopic comorbidities as part of the atopic march. The molecular characterization of adult AD facilitated the development of AD-targeted treatments by an applicable bench-to-bedside approach. Our skin profiling portrays the immune and barrier abnormalities of different pediatric AD age groups and suggests age-related differences that may guide the development of targeted, age-specific therapies. Specific cytokine and barrier abnormalities characterize different AD age groups and should be carefully considered when future therapeutic agents are being developed or tested for younger patients with AD.
Supplementary Material
Clinical implications.
As targeted treatments are developing for pediatric atopic dermatitis, molecular skin profiling reveals specific immune and barrier signatures in different age groups, which may inform age-specific therapeutic choices.
Acknowledgments
This study was supported by a research grant from the LEO Foundation. Y.R.-Y. was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Rockefeller University (grant no. UL1TR001866). R.L. received salary support as a fellow from the National Psoriasis Foundation. Tissue collection was supported by the Northwestern University Skin Biology and Diseases Resource-based Center, National Institute of Arthritis and Musculoskeletal and Skin (grant no. #P30AR075049).
Abbreviations used
- AD
Atopic dermatitis
- DEG
Differentially expressed gene
- FCH
Fold change
- FDR
False-discovery rate
- FLG
Filaggrin
- LCE
Late cornified envelope
- LOR
Loricrin
- MBP
Major basic protein
- qRT-PCR
Quantitative RT-PCR
- SCORAD
SCORing of AD
- TARC
Thymus and activation-regulated chemokine
Footnotes
Disclosure of potential conflict of interest:
E. Guttman-Yassky is an employee of Mount Sinai and has received research funds (grants paid to the institution) from AbbVie, Celgene, Eli Lilly, Janssen, Medimmune/Astra Zeneca, Novartis, Pfizer, Regeneron, Vitae, Glenmark, Galderma, Asana, Innovaderm, Dermira, and UCB; is also a consultant for Sanofi Aventis, Regeneron, Stiefel/GlaxoSmithKline, MedImmune, Celgene, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae, Leo Pharma, AbbVie, Eli Lilly, Kyowa, Mitsubishi Tanabe, Asana Biosciences, and Promius. J. G. Krueger has received research support (grants paid to his institution) and/or personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, BiogenIdec, Delenex, AbbVie, Sanofi, Baxter, Paraxel, Xenoport, and Kineta. A. S. Paller has received research support (grants paid to her institution) from AbbVie, Anaptysbio, Celgene, Eli Lilly, Galderma, Incyte, Leo, Janssen, Novartis, and Regeneron and has been a consultant for Almirall, Amgen, Asana, Boehringer-Ingelheim, Castle Creek, Celgene, Dermavant, Dermira, Eli Lilly, Exicure, Forte, Galderma, Lenus, Leo, Novan, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, and Sol Gel. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin 2017;35:283–9. [DOI] [PubMed] [Google Scholar]
- 2.Tsai TF, Rajagopalan M, Chu CY, Encarnacion L, Gerber RA, Santos-Estrella P, et al. Burden of atopic dermatitis in Asia. J Dermatol 2019;46:825–34. [DOI] [PubMed] [Google Scholar]
- 3.Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016;138:1639–51. [DOI] [PubMed] [Google Scholar]
- 4.Brunner PM, Israel A, Zhang N, Leonard A, Wen HC, Huynh T, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol 2018;141:2094–106. [DOI] [PubMed] [Google Scholar]
- 5.Czarnowicki T, He H, Canter T, Han J, Lefferdink R, Erickson T, et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J Allergy Clin Immunol 2020;145:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeki H, Nakahara T, Tanaka A, Kabashima K, Sugaya M, Murota H, et al. Clinical Practice Guidelines for the Management of Atopic Dermatitis 2016. J Dermatol 2016;43:1117–45. [DOI] [PubMed] [Google Scholar]
- 7.Brunner PM, Leung DYM, Guttman-Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol 2018; 120:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renert-Yuval Y, Guttman-Yassky E. Monoclonal antibodies for the treatment of atopic dermatitis. Curr Opin Allergy Clin Immunol 2018;18:356–64. [DOI] [PubMed] [Google Scholar]
- 9.Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions”. J Allergy Clin Immunol 2019;143:894–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glines KR, Stiff KM, Freeze M, Cline A, Strowd LC, Feldman SR. An update on the topical and oral therapy options for treating pediatric atopic dermatitis. Expert Opin Pharmacother 2019;20:621–9. [DOI] [PubMed] [Google Scholar]
- 11.de Bruin-Weller M, Thaci D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol 2018;178:1083–101. [DOI] [PubMed] [Google Scholar]
- 12.Guttman-Yassky E, Bissonnette R, Ungar B, Suarez-Farinas M, Ardeleanu M, Esaki H, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in atopic dermatitis patients. J Allergy Clin Immunol 2019;143:155–72. [DOI] [PubMed] [Google Scholar]
- 13.Renert-Yuval Y, Guttman-Yassky E. What’s new in atopic dermatitis. Dermatol Clin 2019;37:205–13. [DOI] [PubMed] [Google Scholar]
- 14.Cork MJ, Thaci D, Eichenfield LF, Arkwright PD, Hultsch T, Davis JD, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol 2020;182:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020;156:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Temimi MH, Griffee M, Enniss TM, Preston R, Vargo D, Overton S, et al. When is death inevitable after emergency laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement Program database. J Am Coll Surg 2012;215:503–11. [DOI] [PubMed] [Google Scholar]
- 17.Laudanska H, Reduta T, Szmitkowska D. Evaluation of skin barrier function in allergic contact dermatitis and atopic dermatitis using method of the continuous TEWL measurement. Rocz Akad Med Bialymst 2003;48:123–7. [PubMed] [Google Scholar]
- 18.Suarez-Farinas M, Ungar B, Noda S, Shroff A, Mansouri Y, Fuentes-Duculan J, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol 2015; 136:1277–87. [DOI] [PubMed] [Google Scholar]
- 19.Tintle S, Shemer A, Suarez-Farinas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol 2011;128: 583–93.e1-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011;127:954–64.e1-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang H, Knezevic B, Burnham KL, Knight JC. XGR software for enhanced interpretation of genomic summary data, illustrated by application to immunological traits. Genome Med 2016;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhingra N, Shemer A, Correa da Rosa J, Rozenblit M, Fuentes-Duculan J, Gittler JK, et al. Molecular profiling of contact dermatitis skin identifies allergendependent differences in immune response. J Allergy Clin Immunol 2014;134: 362–72. [DOI] [PubMed] [Google Scholar]
- 23.Guttman-Yassky E, Ungar B, Noda S, Suprun M, Shroff A, Dutt R, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol 2016;137:301–4. [DOI] [PubMed] [Google Scholar]
- 24.Ewald DA, Malajian D, Krueger JG, Workman CT, Wang T, Tian S, et al. Metaanalysis derived atopic dermatitis (MADAD) transcriptome defines a robust AD signature highlighting the involvement of atherosclerosis and lipid metabolism pathways. BMC Med Genomics 2015;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract 2014;2:371–9, quiz 80-1. [DOI] [PubMed] [Google Scholar]
- 26.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Sheshadri N, Zong WX. SERPINB3 and B4: from biochemistry to biology. Semin Cell Dev Biol 2017;62:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SH, Jayawickreme C, Rickard DJ, Nicodeme E, Bui T, Simmons C, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol 2017;137:2110–9. [DOI] [PubMed] [Google Scholar]
- 29.Kato A, Fujii E, Watanabe T, Takashima Y, Matsushita H, Furuhashi T, et al. Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J Dermatol Sci 2014;74:229–35. [DOI] [PubMed] [Google Scholar]
- 30.Pelaia C, Paoletti G, Puggioni F, Racca F, Pelaia G, Canonica GW, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol 2019;10:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, et al. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020;75: 1606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, et al. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity 2006;25:213–24. [DOI] [PubMed] [Google Scholar]
- 33.Laberge S, Ghaffar O, Boguniewicz M, Center DM, Leung DY, Hamid Q. Association of increased CD41 T-cell infiltration with increased IL-16 gene expression in atopic dermatitis. J Allergy Clin Immunol 1998;102:645–50. [DOI] [PubMed] [Google Scholar]
- 34.Guttman-Yassky E, Diaz A, Pavel AB, Fernandes M, Lefferdink R, Erickson T, et al. Use of tape strips to detect immune and barrier abnormalities in the skin of children with early-onset atopic dermatitis. JAMA Dermatol 2019;155:1358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanyal RD, Pavel AB, Glickman J, Chan TC, Zheng X, Zhang N, et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol 2019;122:99–110.e6. [DOI] [PubMed] [Google Scholar]
- 36.Turkish AR, Henneberry AL, Cromley D, Padamsee M, Oelkers P, Bazzi H, et al. Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. J Biol Chem 2005;280:14755–64. [DOI] [PubMed] [Google Scholar]
- 37.Charfeddine C, Dallali H, Abdessalem G, Ghedira K, Hamdi Y, Elouej S, et al. Identification of a CDH12 potential candidate genetic variant for an autosomal dominant form of transgrediens and progrediens palmoplantar keratoderma in a Tunisian family. J Hum Genet 2020;65:397–410. [DOI] [PubMed] [Google Scholar]
- 38.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol 2011;127:773–86.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasileva E, Citi S. The role of microtubules in the regulation of epithelial junctions. Tissue Barriers 2018;6:1539596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbas Zadeh S, Mlitz V, Lachner J, Golabi B, Mildner M, Pammer J, et al. Phylogenetic profiling and gene expression studies implicate a primary role of PSORS1C2 in terminal differentiation of keratinocytes. Exp Dermatol 2017;26: 352–8. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G, Yang J, Li P, Cao J, Nie H. Rosiglitazone inhibits HMC-1 cell migration and adhesion through a peroxisome proliferator-activated receptor gamma-dependent mechanism. Iran J Allergy Asthma Immunol 2014;13:11–8. [PubMed] [Google Scholar]
- 42.Kovarova M, Wassif CA, Odom S, Liao K, Porter FD, Rivera J. Cholesterol deficiency in a mouse model of Smith-Lemli-Opitz syndrome reveals increased mast cell responsiveness. J Exp Med 2006;203:1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato T, Akimoto N, Kitamura K, Kurihara H, Hayashi N, Ito A. Adapalene suppresses sebum accumulation via the inhibition of triacylglycerol biosynthesis and perilipin expression in differentiated hamster sebocytes in vitro. J Dermatol Sci 2013;70:204–10. [DOI] [PubMed] [Google Scholar]
- 44.Gao G, Chen FJ, Zhou L, Su L, Xu D, Xu L, et al. Control of lipid droplet fusion and growth by CIDE family proteins. Biochim Biophys Acta Mol Cell Biol Lipids 2017;1862:1197–204. [DOI] [PubMed] [Google Scholar]
- 45.Seo S, Yoon WS, Cho Y, Park SH, Choung JT, Yoo Y. Leptin and atopic dermatitis in Korean elementary school children. Iran J Allergy Asthma Immunol 2016;15: 138–44. [PubMed] [Google Scholar]
- 46.Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. Neutralization of IL-17C reduces skin inflammation in mouse models of psoriasis and atopic dermatitis. J Invest Dermatol 2018;138:1555–63. [DOI] [PubMed] [Google Scholar]
- 47.Guttman-Yassky E, Krueger JG. IL-17C: a unique epithelial cytokine with potential for targeting across the spectrum of atopic dermatitis and psoriasis. J Invest Dermatol 2018;138:1467–9. [DOI] [PubMed] [Google Scholar]
- 48.Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis 2015;96:359–61. [PubMed] [Google Scholar]
- 49.Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130–9. [DOI] [PubMed] [Google Scholar]
- 50.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 2013;131: 187–200.e1-e8. [DOI] [PubMed] [Google Scholar]
- 51.Brough HA, Cousins DJ, Munteanu A, Wong YF, Sudra A, Makinson K, et al. IL-9 is a key component of memory TH cell peanut-specific responses from children with peanut allergy. J Allergy Clin Immunol 2014;134:1329–38.e10. [DOI] [PubMed] [Google Scholar]
- 52.Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev 2018;281:154–68. [DOI] [PubMed] [Google Scholar]
- 53.Alexander H, Brown S, Danby S, Flohr C. Research techniques made simple: transepidermal water loss measurement as a research tool. J Invest Dermatol 2018;138: 2295–300.e1. [DOI] [PubMed] [Google Scholar]
- 54.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 2009;123:1244–52.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morita E, Takahashi H, Niihara H, Dekio I, Sumikawa Y, Murakami Y, et al. Stratum corneum TARC level is a new indicator of lesional skin inflammation in atopic dermatitis. Allergy 2010;65:1166–72. [DOI] [PubMed] [Google Scholar]
- 56.Kagami S, Saeki H, Komine M, Kakinuma T, Tsunemi Y, Nakamura K, et al. Interleukin-4 and interleukin-13 enhance CCL26 production in a human keratinocyte cell line, HaCaT cells. Clin Exp Immunol 2005;141:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005;202:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Floudas A, Saunders SP, Moran T, Schwartz C, Hams E, Fitzgerald DC, et al. IL-17 receptor A maintains and protects the skin barrier to prevent allergic skin inflammation. J Immunol 2017;199:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakazato J, Kishida M, Kuroiwa R, Fujiwara J, Shimoda M, Shinomiya N. Serum levels of Th2 chemokines, CCL17, CCL22, and CCL27, were the important markers of severity in infantile atopic dermatitis. Pediatr Allergy Immunol 2008;19:605–13. [DOI] [PubMed] [Google Scholar]
- 60.Ito Y, Adachi Y, Makino T, Higashiyama H, Fuchizawa T, Shimizu T, et al. Expansion of FOXP3-positive CD41CD251 T cells associated with disease activity in atopic dermatitis. Ann Allergy Asthma Immunol 2009;103:160–5. [DOI] [PubMed] [Google Scholar]
- 61.Yi L, Cheng D, Zhang K, Huo X, Mo Y, Shi H, et al. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol 2017;10:1491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L, Leonard A, Pavel AB, Malik K, Raja A, Glickman J, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2019;144:144–56. [DOI] [PubMed] [Google Scholar]
- 63.Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy 2017;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000;51:245–70. [DOI] [PubMed] [Google Scholar]
- 65.Giuliani N, Sansoni P, Girasole G, Vescovini R, Passeri G, Passeri M, et al. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol 2001;36:547–57. [DOI] [PubMed] [Google Scholar]
- 66.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol 2007;178:6912–22. [DOI] [PubMed] [Google Scholar]
- 67.Vukmanovic-Stejic M, Chambers ES, Suarez-Farinas M, Sandhu D, Fuentes-Duculan J, Patel N, et al. Enhancement of cutaneous immunity during aging by blocking p38 mitogen-activated protein (MAP) kinase-induced inflammation. J Allergy Clin Immunol 2018;142:844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010;330:1695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol 2012;42:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knolle J, Pierau M, Hebel K, Lampe K, Jorch G, Kropf S, et al. Children from the age of three show a developmental switch in T-cell differentiation. Front Immunol 2020;11:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan Y, Wen X, Hao D, Wang Y, Wang L, He G, et al. The role of IL-37 in skin and connective tissue diseases. Biomed Pharmacother 2020;122:109705. [DOI] [PubMed] [Google Scholar]
- 73.Esaki H, Ewald DA, Ungar B, Rozenblit M, Zheng X, Xu H, et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J Allergy Clin Immunol 2015;135:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okamura T, Sumitomo S, Morita K, Iwasaki Y, Inoue M, Nakachi S, et al. TGF-beta3-expressing CD41CD25(2)LAG31 regulatory T cells control humoral immune responses. Nat Commun 2015;6:6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujio K, Yamamoto K, Okamura T. Overview of LAG-3-expressing, IL-10-producing regulatory T cells. Curr Top Microbiol Immunol 2017;410:29–45. [DOI] [PubMed] [Google Scholar]
- 76.Lai C, August S, Behar R, Polak M, Ardern-Jones M, Theaker J, et al. Characteristics of immunosuppressive regulatory T cells in cutaneous squamous cell carcinomas and role in metastasis. Lancet 2015;385:S59. [DOI] [PubMed] [Google Scholar]
- 77.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol 2012; 189:4079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu R, Sun HF, Williams DW, Jones AV, Al-Hussaini A, Song B, et al. IL-34 suppresses Candida albicans induced TNFalpha production in M1 macrophages by downregulating expression of Dectin-1 and TLR2. J Immunol Res 2015;2015: 328146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Czarnowicki T, He H, Canter T, Han J, Lefferdink R, Erickson T, et al. Evolution of pathologic T-cell subsets in atopic dermatitis from infancy to adulthood. J Allergy Clin Immunol 2020;145:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol 2009;123:977–83, quiz 84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simpson EL, Eichenfield LF, Ellis CN, Mancini AJ, Paller AS. Current issues in atopic comorbidities and preventing the atopic march. Semin Cutan Med Surg 2012;31:S6–9. [DOI] [PubMed] [Google Scholar]
- 82.Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol 2015;63:80–5. [DOI] [PubMed] [Google Scholar]
- 83.Xu X, Xu JF, Zheng G, Lu HW, Duan JL, Rui W, et al. CARD9(S12N) facilitates the production of IL-5 by alveolar macrophages for the induction of type 2 immune responses. Nat Immunol 2018;19:547–60. [DOI] [PubMed] [Google Scholar]
- 84.Charrad R, Kaabachi W, Rafrafi A, Berraies A, Hamzaoui K, Hamzaoui A. IL-8 gene variants and expression in childhood asthma. Lung 2017;195:749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int 2013;62:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006;117:411–7. [DOI] [PubMed] [Google Scholar]
- 87.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 2004;5:752–60. [DOI] [PubMed] [Google Scholar]
- 88.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 2008;126: 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 2014;134: 769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Luscher-Firzlaff J, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol 2012;129:426–33.e1-8. [DOI] [PubMed] [Google Scholar]
- 91.Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol 2018;142:1121–30.e7. [DOI] [PubMed] [Google Scholar]
- 92.Mihara R, Kabashima K, Furue M, Nakano M, Ruzicka T. Nemolizumab in moderate to severe atopic dermatitis: an exploratory analysis of work productivity and activity impairment in a randomized phase II study. J Dermatol 2019;46: 662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taieb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol 2018;78:863–71.e11. [DOI] [PubMed] [Google Scholar]
- 94.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol 2019;143:135–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.