Abstract
Initially, direct oncolysis was thought to be the sole mechanism through which oncolytic viruses (OVs) exert their anti-tumor effect, and the immune system was perceived as the major obstacle in oncolytic virotherapy. Over the last decade, there has been a lot of debate on whether the immune system is a friend or foe of OVs. However, we are now at a stage where the initial thinking has been reversed as a result of compelling evidence that the immune system plays a critical role in the success of oncolytic virotherapy. In this review we discuss the importance of the involvement of innate and adaptive immunity for therapeutic efficacy of OVs, and the rational combination of OVs with other immunotherapies for further enhancement of overall therapeutic outcome.
Keywords: oncolytic virotherapy, anti-OV immunity, immunotherapy, immune modulation
Introduction - Oncolytic Viruses
Oncolytic virotherapy is a novel therapeutic approach that utilizes replication-competent viruses, which selectively replicate in and lyse cancer cells while leaving normal cells unharmed. During the course of cancer evolution, cancer cells accrue multiple mutations that allow them to grow in an uncontrolled manner [1]. The very same mutations that help cancer cells to thrive are targeted by naturally occurring OVs (e.g. reovirus [2,3] and vesicular stomatitis virus (VSV) [4]) or genetically engineered OVs (e.g. adenovirus [5,6] and herpesvirus [7]). After infecting cancer cells, OVs hijack the cell death machinery allowing death to occur only after cellular resources have been fully exploited for maximum production of progeny viruses [8]. As such, the complex cell death caused by oncolysis may not always fit into conventional cell death classifications: apoptosis, necrosis and autophagy [8,9]. Furthermore, unlike chemo- and radiation-therapy, OVs are self-amplifying therapeutics, whose therapeutic outcome is determined by a three-way race among tumor growth, virus replication, and immune activation [10].
Immunity and Cancer Therapy
The immune system’s role in cancer therapy has, historically, been neglected, which is evident from the fact that National Cancer Institute, USA, has used human xenografts in immune-deficient mice for testing oncologic drugs since 1976 [11,12]. Only recently the importance of immune system in cancer therapy is being appreciated. Indeed, recent studies suggest that the efficacy of chemo- and radiationtherapy, previously thought to exert their anti-tumor effect purely by direct cytotoxicity, depends on immune system involvement [13]. With the realization of the potent anti-tumor effect of appropriately activated immune system, the last two decades have seen a surge of interest in the field of cancer immunotherapy. Here we discuss the interactions among OVs, immune system and cancer, and the therapeutic outcome thereof.
OVs and Immune System
The innate immunity serves as a first line of defense against viruses, which limits the amplification and spread of viruses, whereas the adaptive immunity plays a major role against the virus during re-infection [14]. Antibodies could potentially neutralize OVs, greatly reducing the virus dose at the tumor site. This is a concern especially when delivering OVs systemically. Nevertheless, levels of neutralizing antibodies do not appear to correlate with efficacies of OVs in clinical studies [15,16]. Kim et al. reported ‘antibody-mediated complement-dependent cancer cell lysis’ as an important mechanism for therapeutic efficacy of the oncolytic virus JX-549 both in an animal model as well as in humans [17].
In the context of cellular innate immunity, natural killer (NK) cells are considered to have potent anti-tumor as well as anti-viral effect. Virus-infected cancer cells tend to down-regulate their class I major histocompatibility complex (MHC) making themselves a good target for NK cells [18,19]. Although NK cells may kill infected cancer cells and limit the amplification of OVs, studies have found that NK cells often have positive effects on therapeutic outcomes of OVs [18–24]. Furthermore, NK cells may play a role in the maturation of dendritic cells (DCs) and they can also induce differentiation of cancer stem cells as well as poorly differentiated cancer cells, through secretion of IFN-γ and TNF-α [25,26]. In this regard, one would expect the combination of NK cells with OVs to result in greater anti-tumor effect. Indeed, several studies have shown that the combination of NK cells with OVs can result into additive or synergistic anti-tumor effect [27,28].
Viruses can be taken up by antigen presenting cells directly through macropinocytosis or indirectly when OV-infected cells are engulfed, leading to presentation of viral antigens to T cells and ultimately activating the adaptive arm of the immune system against viruses [29]. Despite this possibility of anti-OV effect of adaptive immunity, most studies suggest that adaptive immunity enhances the therapeutic outcome of OVs [30,31].
Several preclinical studies have demonstrated a prime role of immune system in the therapeutic efficacies of a wide range of OVs (Table 1) [32–37]. Prestwich et al., 2009, published one of the most compelling studies demonstrating the requirement of immune system in oncolytic virotherapy [38]. Their study reported that an oncolytic reovirus was able to purge lymph node and splenic metastases from the murine melanoma cell line B16Ova, a line that is extremely resistant to reovirus in vitro, in immune-competent C57BL/6 mice but not in severe combined immunodeficient mice. This study concluded that virus-mediated immune responses, rather than virus-mediated oncolysis, were critical for the anti-tumor efficacy of the reovirus [38]. Similarly, Apostolidis et al. found that locoregional administration of an oncolytic Newcastle disease virus (NDV) in immune-competent mice could significantly delay growth of tumors established from the murine colon cancer cell line CT26, despite these cells being very resistant to NDV in vitro [39]. In line with this, Diaz et al. showed that an oncolytic VSV that replicates extremely aggressively in B16Ova cells in vitro has no anti-tumor effect against B16Ova tumors, in mice, in the absence of CD8+ T cells or NK cells [21]. Furthermore, incorporation of immune-stimulatory genes such GM-CSF [40], IL-12 [41], IL-2 [42], IL-15 [43] and RANTES [44] in OVs has been shown to enhance therapeutic efficacy of OVs in immune-competent animal models. Importantly, in some instances, even non-replicating, heat or UV-inactivated viruses have been shown to eradicate established tumors in immune-competent animal models, underscoring the impact of immune system on virus-mediated anti-tumor effect [45,46].
Table 1.
Oncolytic viruses as immunotherapeutics
| Type of immunotherapy | Phase of Study (tumor model) | Immune component(s) involved | Therapeutic outcome | References |
|---|---|---|---|---|
| OVs | ||||
| VSV | Preclinical (Melanoma) | CD8+ T cells or NK cells | No regression of B16Ova tumors in mice lacking CD8+ T cells and/or NK cells | [21] |
| Reovirus | Preclinical (Melanoma) | CTLs | Purging of B16Ova metastases from spleen and lymph node in immune-competent mice but not in SCID mice | [38] |
| NDV | Preclinical (Colon cancer) | PBMCs | Significant delay in growth of CT26 tumors | [39] |
| HSV | Preclinical (Sarcoma) | High baseline level of neutrophils was associated with sensitivity to OV; resistance to OV correlated to high baseline level of TAMs |
Formation of protective antitumor immunity leading to rejection of subsequent tumor challenges | [33] |
| Oncolytic adenovirus (Delta24-RGD) | Preclinical, (Glioma) | Local rapid release of acute-phase cytokines (including IL-1b and IL-6), interferon gamma (IFNγ), CXCL10, MIP-1α; tumor infiltration by macrophages and CD8 + T cells; | Induction of an anti-tumor memory response, which prevented tumor growth upon reinjection of tumor cells | [32] |
| HSV-2 (FusOn-H2) | Preclinical (Breast cancer) | T cells | Regression of primary and metastatic tumors | [35] |
| ICP34.5-deleted HSV | Preclinical (Melanoma) | Cytotoxic T cell response; CD4+ and NK cells also implicated | Prolonged survival of mice bearing intracranial melanomas | [36] |
| OVs armed with immune-stimulatory genes | ||||
| HSV-1 encoding GM-CSF | Preclinical | Induction of IFNγ, memory T cells | Regression of OV injected and noninjected tumors, resistance to re-challenge formation | [40] |
| OncovexGM-CSF or T-VEC | Phase I clinical trial (Melanoma) | TILs | Regression of injected and uninjected tumors | [47] |
| Phase II and III clinical trials (Melanoma) | Local and systemic antigen-specific T cell responses, and significantly reduced immune-suppressive cells (Tregs and MDSCs) | Anti-tumor activities in both injected and uninjected distant lesions | [48,49][50] | |
| Oncolytic vaccinia virus JX-594 encoding GM-CSF | Phase I clinical trial (Liver cancer) | Activation of systemic immunity | Regression of both injected and uninjected tumors | [16] |
| Oncolytic adenovirus encoding GM-CSF (ONCOS-102) | Phase I (Ovarian cancer) | Infiltration of CD8+ T cells in tumor; systemic induction of tumorspecific CD8+ T cells | Local and systemic anti-tumor immune responses | [51] |
| Combination therapy | ||||
| Oncolytic adenovirus with PD-1 inhibitor | Preclinical (Lung cancer) | Neoantigen-directed T cell response | Synergistic anti-tumor effect | [58] |
| Oncolytic VSV plus recombinant adenovirus vaccine boost | Preclinical (Melanoma) | CD8+ T cells, effector and memory | Combination produced a synergistic increase in numbers of both effector and memory CD8+ T cells | [34] |
| Adoptive T cell therapy plus oncolytic VSV | Preclinical (Melanoma) | CD8+ T cells, CCR7hi | Autologous CCR7hi T cells destroyed metastatic cells within lymph nodes, spleen and other organs | [37] |
| Oncolytic Adenovirus encoding CCL20/IL-15 + NK cells + CD8+ T cells | Preclinical (Colorectal cancer) | NK and CD8+ T cells-mediated cytotoxicity | Enhanced anti-tumor activity | [27] |
| Oncolytic HSV-1 + bortezomib + NK cells | Preclinical (Glioma) | Bortezomib sensitized oHSV infected tumor cells to NK cells | Synergistic anti-tumor effect | [28] |
| T-VEC plus anti-PD-1 antibody | Phase Ib trial (Metastatic melanoma) | T cell infiltration into tumors | T-VEC increased efficacy of anti-PD-1 | [68] |
Notes: VSV, vesicular stomatitis virus; NDV, Newcastle disease virus; HSV, herpes simplex virus; CXCL10, chemokine (C-X-C) motif 10; GM-CSF, granulocyte-macrophagecolony-stimulating factor; MIP-1α, macrophage inflammatory protein-1α; NK, natural killer cells; TAMs, tumor-associated macrophages; TILs, tumor infiltrating lymphocytes.
Although there is not enough clinical data to conclude if the importance of immunity in the overall therapeutic efficacy of OVs in human patients will be similar to what has been observed in preclinical studies, there are some indications that immune system would favor oncolytic virotherapy in the clinical setting (Table 1). For example, in a phase I clinical trial, Talimogene laherparepvec or T-VEC, an oncolytic herpes simplex virus encoding human GM-CSF, was found to increase immune cell infiltration into OV-injected tumors, and 4 out of 30 patients showed extensive inflammation in uninjected tumors, suggestive of systemic anti-tumor immune responses [47]. T-VEC also showed anti-tumor activities in both injected and uninjected distant lesions including visceral metastases in melanoma patients in phase II and III clinical trials [48,49]. Analysis of immune cells in the patients revealed that intra-lesional injection of the virus induced local and systemic antigen-specific T cell responses, and significantly reduced immune-suppressive cells (Tregs and MDSCs) [50]. Likewise, an oncolytic vaccinia virus JX-594, which also encodes hGM-CSF was shown to regress both injected and uninjected liver tumors in a phase I clinical trial [16]. Regression of the uninjected tumors was thought to be due to activation of systemic anti-tumor immunity, although there was no direct evidence to prove this. Furthermore, in a case report of an ovarian cancer patient treated with an oncolytic adenovirus encoding hGM-CSF (ONCOS-102), progressive infiltration of CD8+ T cells in the tumor and concomitant systemic induction of tumor-specific CD8+ T cells were observed [51]. Taken together, these preclinical and clinical studies make a strong case for the critical role of immunity in the success of oncolytic virotherapy.
Mechanism of Anti-tumor Immune Modulation by OVs
In the last two decades the field of cancer immunotherapy has seen some major breakthroughs culminating into the FDA approval of several immunotherapeutics. While the approved immunotherapeutics, mostly immune checkpoint inhibitors (ICIs), have shown impressive and long-lasting responses in a subset of cancer patients, majority of patients fail to respond to these agents [52]. ICIs, such as anti-PD-1/PD-L-1 and anti-CTLA-4, act by restoring T cell function and rely on pre-existing tumor-specific T cells for therapeutic success [52,53]. Immunologically unresponsive or ‘cold’ tumors have one or more of the following characteristics: lack of tumor antigens, lack of T cells recognizing tumor antigens, heavy presence of immunosuppressive cells such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSC), M2 macrophages and immunosuppressive cytokines such as IL-10 and TGF-β [52,54], and are very likely to be resistant to ICIs [54].
OVs have the potential to convert immunologically ‘cold’ tumor into an inflamed, immunologically ‘hot’ tumor (Figure 1). There are a variety of mutually non-exclusive mechanisms through which OVs could modulate the tumor microenvironment (TME). First, oncolysis by OVs could cause the release of tumor associated/specific antigens and enhance cross-presentation of such antigens by dendritic cells (DCs), ultimately eliciting adaptive immunity against tumor [55–60]. Second, OVs are known to induce immunogenic cell death (ICD) [61], which plays an important role in the induction of anti-tumor adaptive immunity [62]. Discussion of the mechanisms through which OVs induce ICD is beyond the scope of this review; readers are encouraged to see an excellent review by Guo and Bartlett on this topic [63]. Third, some OVs such as reovirus [64] and VSV [65] can directly interact with DCs and enhance their antigen priming capability. Fourth, OVs have the ability to reduce immune-suppressive Tregs and MDSCs in the TME [50]. Lastly, OVs can modulate the cytokine milieu in the TME e.g., reovirus infection in human melanoma cells has been shown to abrogate the immunosuppressive cytokine IL-10 and enhance secretion of the proinflammatory cytokines IL-6, IL-8, RANTES and MIP-1α/β [66]. Likewise, infection with oncolytic VSV in murine melanoma cells rapidly induced proinflammatory cytokines including IL-6, type I IFN and TNF-α [67]. Interestingly, the cytokines/chemokines upregulated by these OVs are not only immunostimulatory in function but they can also kill residual uninfected cancer cells [67]. Perhaps the best evidence for the therapeutic potency of simply modulating TME through virus infection comes from a recent study by Dai et al. [46]. In this study the authors showed that repeated intra-tumoral injection of heat-inactivated modified vaccinia virus Ankara could eradicate tumors in different aggressive murine tumor models. Although the virus used in this study is not an oncolytic virus, this study provides an insight into the anti-tumor potency of virally-modulated TME. Taken together, these studies suggest that OVs have the potential to modulate TME from immunologically ‘cold’ to immunologically ‘hot’ status.
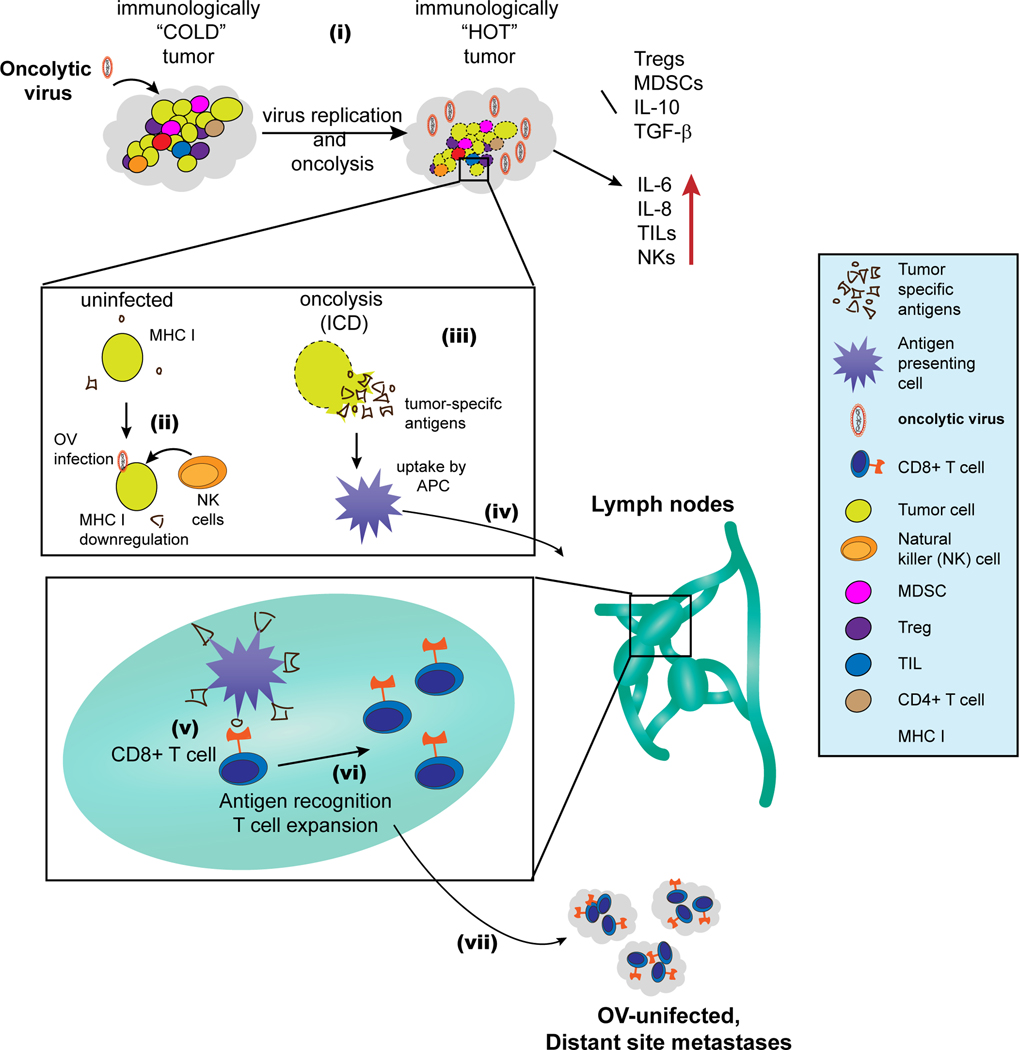
Figure 1. Modulation of the tumor microenvironment by OVs and elicitation of anti-tumor immunity.
(i) OVs convert immunologically “COLD” tumors to immunologically “HOT” tumors: tumors generally have high concentration of immunesuppressive cells and cytokines, which make them immunologically less responsive i.e. immunologically “COLD”. OVs induce inflammation and inhibit immunosuppressive cells (Tregs and MDSCs) and cytokines (IL-10 and TGF-β). Conversely, OVs also increase proinflammatory cytokines (IL-6 and IL-8) and foster tumor infiltration by NK cells and other TILs. This complex modulation of the TME by OVs converts the tumor to inflamed, immunologically “HOT”. (ii) OV infection increases NK cell-mediated killing of tumor cells: tumor cells tend to reduce their MHC I levels in response to virus infection. Reduction in MHC I allows recognition and killing of virus infected cancer cells by NK cells. (iii) Oncolysis by OVs causes the release of tumor-associated/specific antigens, and OVs also induce immunogenic cell death (ICD). (iv) Antigen-loaded APCs migrate to the lymph node, where (v) they cross-present tumor antigens to CD8+ T cells. (vi) Following activation, the tumor-specific CD8+ T cells undergo expansion. (vii) Finally, the tumor-specific T cells move to both OV injected and uninjected tumors (distant metastases) where they can exert anti-tumor effect.
Combination of OVs with Immunotherapeutics
Given the potential of OVs to modulate the immune landscape in TME, it would be logical to surmise that combination of OVs with immunotherapeutics may result in synergistic therapeutic effect. Indeed, a study by Woller et al. showed that localized tumor infection with an oncolytic adenovirus could overcome systemic tumor resistance to PD-1 inhibitor by broadening neoantigens-directed T cell responses in mice [58]. Interestingly, the tumor cells were found to upregulate their PD-L1 expression in response to virus infection. Therefore, both the therapeutics complemented each other and the outcome was a synergistic anti-tumor effect [58]. Very recently, Ribas et al. reported the findings of a phase Ib trial in which they studied the impact of T-VEC on therapeutic efficacy of anti-PD-1 antibody pembrolizumab in patients with metastatic melanoma [68]. The combination treatment was well tolerated; T-VEC was found to promote T cell infiltration into tumors and improved the overall therapeutic efficacy of pembrolizumab. This clinical trial essentially confirmed the findings from animal study that OVs can enhance the therapeutic efficacies of checkpoint inhibitors by converting immunologically ‘cold’ tumors into immunologically ‘hot’ tumors [54,58,60]. This study provides the hope that the benefits of checkpoint inhibitors may be harnessed in combination with OVs, even in tumor types that have previously shown very poor response to checkpoint inhibitors, such as breast, prostate and colon cancer [52].
Another logical combination of OVs would be with chimeric antigen receptor (CAR)-redirected T cells. CAR-T cells can recognize whole antigens (MHC unrestricted) on tumor cell surface, minimizing the probability of cancer cell escape by MHC I downregulation [69]. Several studies have shown the feasibility of using CAR-T cells for targeting virus-encoded antigens [70–72]. The combination of an OV encoding a unique tumor antigen with a CAR-T that recognizes the virus-encoded antigen should work synergistically potentially through: (i) tumor debulking by the OV, (ii) positive modulation of immunity in TME by OV for optimal function of CAR-T cells, and (iii) CAR-T cells should be able to kill infected residual cancer cells that may be resistant to the OV.
Conclusion
The success of oncolytic virotherapy depends on its ability to mobilize the host’s immune system against tumor. The approval of T-VEC has sparked great optimism in the field of oncolytic virotherapy with several more OVs currently being evaluated in the advanced phase of clinical trials. On the other hand, immunotherapeutics such as ICIs have shown unprecedented response rates in the clinic, bringing the field of immunotherapy into the main limelight of cancer therapy. However, both of these therapeutical platforms are still far from being adequate and more work needs to be done in order to expand the therapeutic benefits to broader population of cancer patients. Given the ability of oncolytic virotherapy and immunotherapy to complement each other, it would be reasonable to expect that their combination would be more effective in the battle against cancer.
Highlights.
Oncolytic viruses (OVs) elicit innate and adaptive immune responses against tumors
Although the immune system can react against OVs, a functional immune system is key to OV efficacy
OVs induce immunogenic cell death in cancer cells
OVs can change the immunologic landscape of tumor milieu from “cold” to “hot”
Combination of OV and immunotherapies may result in synergistic anti-tumor effect
Acknowledgements
The authors wish to thank Indra M. Newman, PhD, for assistance with manuscript editing and figure preparation.
Funding:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 2011, 144:646–674. [DOI] [PubMed] [Google Scholar]
- 2.Hashiro G, Loh PC, Yau JT: The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch Virol 1977, 54:307–315. [DOI] [PubMed] [Google Scholar]
- 3.Coffey MC, Strong JE, Forsyth PA, Lee PW: Reovirus therapy of tumors with activated Ras pathway. Science 1998, 282:1332–1334. [DOI] [PubMed] [Google Scholar]
- 4.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC: Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 2000, 6:821–825. [DOI] [PubMed] [Google Scholar]
- 5.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D: An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med 2000, 6:1134–1139. [DOI] [PubMed] [Google Scholar]
- 6.Sharon D, Schumann M, MacLeod S, McPherson R, Chaurasiya S, Shaw A, Hitt MM: 2-aminopurine enhances the oncolytic activity of an E1b-deleted adenovirus in hepatocellular carcinoma cells. PLoS One 2013, 8:e65222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong RJ, Joe JK, Kim SH, Shah JP, Horsburgh B, Fong Y: Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum Gene Ther 2002, 13:1213–1223. [DOI] [PubMed] [Google Scholar]
- 8.Thomson BJ: Viruses and apoptosis. Int J Exp Pathol 2001, 82:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell SJ, Peng KW, Bell JC: Oncolytic virotherapy. Nat Biotechnol 2012, 30:658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JT, Kirn DH, Wein LM: Analysis of a three-way race between tumor growth, a replication-competent virus and an immune response. Bull Math Biol 2004, 66:605–625. [DOI] [PubMed] [Google Scholar]
- 11.Kelland LR: Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer 2004, 40:827–836. [DOI] [PubMed] [Google Scholar]
- 12.Prendergast GC, Jaffee EM: Cancer immunologists and cancer biologists: why we didn’t talk then but need to now. Cancer Res 2007, 67:3500–3504. [DOI] [PubMed] [Google Scholar]
- 13.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G: Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008, 8:59–73. [DOI] [PubMed] [Google Scholar]
- 14.Mogensen TH: Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009, 22:240–273, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirn D: Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther 2001, 8:89–98. [DOI] [PubMed] [Google Scholar]
- 16.Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, Oh SY, Han SY, Yoon JH, Hong SH, et al. : Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phaseI trial. Lancet Oncol 2008, 9:533–542. [DOI] [PubMed] [Google Scholar]
- 17. Kim MK, Breitbach CJ, Moon A, Heo J, Lee YK, Cho M, Lee JW, Kim SG, Kang DH, Bell JC, et al. : Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med 2013, 5:185ra163. • First study to show ‘antibody-mediated complement-dependent cell lysis’ as one of the many mechanisms through which oncolytic poxviruses exert their anti-tumor effect. The effect was shown both in animal model (rabbit) and in humans.
- 18.Ogbomo H, Zemp FJ, Lun X, Zhang J, Stack D, Rahman MM, McFadden G, Mody CH, Forsyth PA: Myxoma virus infection promotes NK lysis of malignant gliomas in vitro and in vivo. PLoS One 2013, 8:e66825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat R, Dempe S, Dinsart C, Rommelaere J: Enhancement of NK cell antitumor responses using an oncolytic parvovirus. Int J Cancer 2011, 128:908–919. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Tai LH, Ilkow CS, Alkayyal AA, Ananth AA, de Souza CT, Wang J, Sahi S, Ly L, Lefebvre C, et al. : Maraba MG1 virus enhances natural killer cell function via conventional dendritic cells to reduce postoperative metastatic disease. Mol Ther 2014, 22:1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, Valdes M, Barber G, Vile RG: Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res 2007, 67:2840–2848. [DOI] [PubMed] [Google Scholar]
- 22.Gujar SA, Pan DA, Marcato P, Garant KA, Lee PW: Oncolytic virus-initiated protective immunity against prostate cancer. Mol Ther 2011, 19:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rintoul JL, Lemay CG, Tai LH, Stanford MM, Falls TJ, de Souza CT, Bridle BW, Daneshmand M, Ohashi PS, Wan Y, et al. : ORFV: a novel oncolytic and immune stimulating parapoxvirus therapeutic. Mol Ther 2012, 20:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai LH, de Souza CT, Belanger S, Ly L, Alkayyal AA, Zhang J, Rintoul JL, Ananth AA, Lam T, Breitbach CJ, et al. : Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res 2013, 73:97–107. [DOI] [PubMed] [Google Scholar]
- 25.Bhat R, Rommelaere J: Emerging role of Natural killer cells in oncolytic virotherapy. Immunotargets Ther 2015, 4:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlowska AK, Kaur K, Topchyan P, Jewett A: Novel strategies to target cancer stem cells by NK cells; studies in humanized mice. Front Biosci (Landmark Ed) 2017, 22:370–384. [DOI] [PubMed] [Google Scholar]
- 27.Ye JF, Qi WX, Liu MY, Li Y: The combination of NK and CD8+T cells with CCL20/IL15-armed oncolytic adenoviruses enhances the growth suppression of TERT-positive tumor cells. Cell Immunol 2017, 318:35–41. [DOI] [PubMed] [Google Scholar]
- 28.Yoo JY, Jaime-Ramirez AC, Bolyard C, Dai H, Nallanagulagari T, Wojton J, Hurwitz BS, Relation T, Lee TJ, Lotze MT, et al. : Bortezomib Treatment Sensitizes Oncolytic HSV-1-Treated Tumors to NK Cell Immunotherapy. Clin Cancer Res 2016, 22:5265–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M: Pathogen recognition and development of particulate vaccines: does size matter? Methods 2006, 40:1–9. [DOI] [PubMed] [Google Scholar]
- 30. Melcher A, Parato K, Rooney CM, Bell JC: Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther 2011, 19:1008–1016. •• This comprehensive review discusses the fine balace between anti-virus and anti-tumor activities of the immune system and provides evidence for beneficial role of immune system in oncolytic virotherapy. It also discusses several new strategies for combination of immunotherapeutics with oncolytic viruses to mount a multipronged biological attack against cancer.
- 31.Cassady KA, Haworth KB, Jackson J, Markert JM, Cripe TP: To Infection and Beyond: The Multi-Pronged Anti-Cancer Mechanisms of Oncolytic Viruses. Viruses 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleijn A, Kloezeman J, Treffers-Westerlaken E, Fulci G, Leenstra S, Dirven C, Debets R, Lamfers M: The therapeutic efficacy of the oncolytic virus Delta24-RGD in a murine glioma model depends primarily on antitumor immunity. Oncoimmunology 2014, 3:e955697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leddon JL, Chen CY, Currier MA, Wang PY, Jung FA, Denton NL, Cripe KM, Haworth KB, Arnold MA, Gross AC, et al. : Oncolytic HSV virotherapy in murine sarcomas differentially triggers an antitumor T-cell response in the absence of virus permissivity. Mol Ther Oncolytics 2015, 1:14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridle BW, Clouthier D, Zhang L, Pol J, Chen L, Lichty BD, Bramson JL, Wan Y: Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8+ T-cell responses to anticancer vaccines. Oncoimmunology 2013, 2:e26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Dutuor A, Fu X, Zhang X: Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J Gene Med 2007, 9:161–169. [DOI] [PubMed] [Google Scholar]
- 36.Miller CG, Fraser NW: Requirement of an integrated immune response for successful neuroattenuated HSV-1 therapy in an intracranial metastatic melanoma model. Mol Ther 2003, 7:741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, Thompson J, Ryno P, Barber GN, Chester J, et al. : Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med 2008, 14:37–44. [DOI] [PubMed] [Google Scholar]
- 38. Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, Thompson J, Galivo F, Harrington KJ, Pandha HS, et al. : Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res 2009, 15:4374–4381. • An early study that provided evidence for the requirement of host immune system in the success of oncolytic virus.
- 39.Apostolidis L, Schirrmacher V, Fournier P: Host mediated anti-tumor effect of oncolytic Newcastle disease virus after locoregional application. Int J Oncol 2007, 31:1009–1019. [PubMed] [Google Scholar]
- 40.Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, McGrath Y, Thomas SK, Thornton M, Bullock P, et al. : ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 2003, 10:292–303. [DOI] [PubMed] [Google Scholar]
- 41.Wong RJ, Patel SG, Kim S, DeMatteo RP, Malhotra S, Bennett JJ, St-Louis M, Shah JP, Johnson PA, Fong Y: Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Hum Gene Ther 2001, 12:253–265. [DOI] [PubMed] [Google Scholar]
- 42.Bai F, Niu Z, Tian H, Li S, Lv Z, Zhang T, Ren G, Li D: Genetically engineered Newcastle disease virus expressing interleukin 2 is a potential drug candidate for cancer immunotherapy. Immunol Lett 2014, 159:36–46. [DOI] [PubMed] [Google Scholar]
- 43.Tosic V, Thomas DL, Kranz DM, Liu J, McFadden G, Shisler JL, MacNeill AL, Roy EJ: Myxoma virus expressing a fusion protein of interleukin-15 (IL15) and IL15 receptor alpha has enhanced antitumor activity. PLoS One 2014, 9:e109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, O’Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, Thorne SH, Bartlett DL: Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther 2011, 19:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurooka M, Kaneda Y: Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res 2007, 67:227–236. [DOI] [PubMed] [Google Scholar]
- 46. Dai P, Wang W, Yang N, Serna-Tamayo C, Ricca JM, Zamarin D, Shuman S, Merghoub T, Wolchok JD, Deng L: Intratumoral delivery of inactivated modified vaccinia virus Ankara (iMVA) induces systemic antitumor immunity via STING and Batf3-dependent dendritic cells. Sci Immunol 2017, 2. •• This elegant study demonstrates robust activation of immune-system by viruses and the power of immune system to completely abrograte tumors when activated appropriately. The virus used in this study is a non-replicating poxvirus and the author showed that even heat or UV-inactivated virus can highly activate anti-tumor immunity ultimately causing abrogation of established tumors in mice.
- 47. Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, Harrington KJ, James ND, Love CA, McNeish I, et al. : A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006, 12:6737–6747. • First study to show elicitation of anti-tumor immune responses in human patients. In this phase I clinical trial a subset of patients showed anti-tumor effect in both virusinjected and uninjected tumors.
- 48.Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, Gonzalez R, Glaspy J, Whitman E, Harrington K, et al. : Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 2009, 27:5763–5771. [DOI] [PubMed] [Google Scholar]
- 49.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. : Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015, 33:2780–2788. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S: Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 2010, 17:718–730. [DOI] [PubMed] [Google Scholar]
- 51.Vassilev L, Ranki T, Joensuu T, Jager E, Karbach J, Wahle C, Partanen K, Kairemo K, Alanko T, Turkki R, et al. : Repeated intratumoral administration of ONCOS-102 leads to systemic antitumor CD8+ T-cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. Oncoimmunology 2015, 4:e1017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A: Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168:707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012, 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haanen J: Converting Cold into Hot Tumors by Combining Immunotherapies. Cell 2017, 170:1055–1056. • A short discussion on the conversion of immunologically “cold” tumors to immunologically “hot” tumors based on the findings of the Phase Ib trial combining T-VEC with pembrolizumab.
- 55.Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F, Gregoire M: Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res 2008, 68:4882–4892. [DOI] [PubMed] [Google Scholar]
- 56.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C: Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005, 433:887–892. [DOI] [PubMed] [Google Scholar]
- 57.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF: Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 2003, 4:1009–1015. [DOI] [PubMed] [Google Scholar]
- 58. Woller N, Gurlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, Saborowski M, Geffers R, Manns MP, Wirth TC, et al. : Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol Ther 2015, 23:1630–1640. •• This preclinical study demonstrates that oncolytic viruses can generate neo-epitopes through oncolysis, which can lead to the conversion of tumors from checkpoint inhibitor-resistant to checkpoint inhibitor-sensitive. This study provides the mechanistic evidence for the sysnergistic anti-tumor effect often observed when oncolytic viruses are combined with checkpoint inhibitors in animal models.
- 59.Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T, Thompson J, Morrison EE, Harrington KJ, Pandha HS, et al. : Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res 2008, 14:7358–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woller N, Knocke S, Mundt B, Gurlevik E, Struver N, Kloos A, Boozari B, Schache P, Manns MP, Malek NP, et al. : Virusinduced tumor inflammation facilitates effective DC cancer immunotherapy in a Treg-dependent manner in mice. J Clin Invest 2011, 121:2570–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo ZS, Liu Z, Kowalsky S, Feist M, Kalinski P, Lu B, Storkus WJ, Bartlett DL: Oncolytic Immunotherapy: Conceptual Evolution, Current Strategies, and Future Perspectives. Front Immunol 2017, 8:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green DR, Ferguson T, Zitvogel L, Kroemer G: Immunogenic and tolerogenic cell death. Nat Rev Immunol 2009, 9:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guo ZS, Liu Z, Bartlett DL: Oncolytic Immunotherapy: Dying the Right Way is a Key to Eliciting Potent Antitumor Immunity. Front Oncol 2014, 4:74. •• An excellent review discussing the mechanisms through which oncolytic viruses induce immunogenic death in tumor cells. It also discusses different strategies to combine oncolytic viruses with other immunotherapeutics to break immune tolerance and enhance anti-tumor immunity.
- 64.Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, de Bono J, Selby P, Coffey M, Vile R, et al. : Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol 2008, 180:6018–6026. [DOI] [PubMed] [Google Scholar]
- 65.Boudreau JE, Bridle BW, Stephenson KB, Jenkins KM, Brunelliere J, Bramson JL, Lichty BD, Wan Y: Recombinant vesicular stomatitis virus transduction of dendritic cells enhances their ability to prime innate and adaptive antitumor immunity. Mol Ther 2009, 17:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L, Ilett LJ, Prestwich R, Pandha HS, Coffey M, et al. : Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther 2008, 15:1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, Vile RG: The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther 2009, 20:1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. : Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170:1109–1119 e1110. • First human trial combining an oncolytic virus with PD-1 inhibitor. This study clearly shows that the oncolytic virus T-VEC can improve efficacy of an anti-PD-1 antibody.
- 69.Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M: Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 2015, 28:415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, Leung K, Carrum G, Gee AP, Vera JF, et al. : Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014, 6:242ra283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. : Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008, 14:1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, Carrum G, Sasa G, Lulla P, Watanabe A, et al. : Off-theShelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol 2017:JCO2017730655. [DOI] [PMC free article] [PubMed] [Google Scholar]