Abstract
Multicenter, prospective, observational study to compare the relative bioavailability of once‐daily tacrolimus formulations in de novo kidney transplant recipients. De novo kidney transplant recipients who started a tacrolimus‐based regimen were included 14 days post‐transplant and followed up for 6 months. Data from 218 participants were evaluated: 129 in the LCPT group (Envarsus) and 89 in the PR‐Tac (Advagraf) group. Patients in the LCPT group exhibited higher relative bioavailability (Cmin /total daily dose [TDD]) vs. PR‐Tac (61% increase; P < .001) with similar Cmin and 30% lower TDD levels (P < .0001). The incidence of treatment failure was 3.9% in the LCPT group and 9.0% in the PR‐Tac group (P = .117). Study discontinuation rates were 6.2% in the LCPT group and 12.4% in the PR‐Tac group (P = .113). Adverse events, renal function and other complications were comparable between groups. The median accumulated dose of tacrolimus in the LCPT group from day 14 to month 6 was 889 mg. Compared to PR‐Tac, LCPT showed higher relative bioavailability, similar effectiveness at preventing allograft rejection, comparable effect on renal function, safety, adherence, treatment failure and premature discontinuation rates.
Keywords: bioavailability, clinical practice, pharmacokinetics, renal transplantation, tacrolimus, treatment failure
1. INTRODUCTION
The calcineurin inhibitor (CNI) tacrolimus is the mainstay of treatment to prevent allograft rejection after kidney and liver transplant. Due to its narrow therapeutic index, maintaining a proper balance of blood tacrolimus levels is essential to prevent organ rejection and minimize toxicity after kidney transplant, requiring individual dose titration and close drug monitoring. 1
Kidney transplant recipients require lifelong immunosuppression to maintain graft survival. This often results in a decline in adherence over time, with dramatic consequences for patients. 2 The poor and heterogeneous bioavailability of tacrolimus often results in high inter‐ and intra‐patient variability. 3 Thus, improving the convenience and pharmacokinetic profile of tacrolimus has been the focus of significant effort.
The immediate‐release formulation of tacrolimus was first developed for its administration twice daily (IR‐Tac and generics, Prograf, Astellas Pharma). 4 To improve treatment adherence, tacrolimus was formulated as a prolonged‐release once‐daily formulation (PR‐Tac, Advagraf, Astellas Pharma). 5 PR‐Tac was associated with improved adherence, non‐inferior efficacy and similar tolerability compared to IR‐Tac, 2 , 6 , 7 although lower tacrolimus exposure was achieved in the early post‐transplant period. 6 A novel once‐daily formulation of tacrolimus was developed based on the MeltDose drug delivery technology (LCPT, Envarsus, Chiesi), 8 a process that enhances the bioavailability of low water solubility drugs by decreasing its particle size, thereby controlling its release and allowing a more distal distribution of the drug within the gut. 9
Several studies compared the efficacy of LCPT and IR‐Tac both in stable 10 , 11 , 12 and de novo kidney transplant recipients, 13 reporting a similar safety profile and greater bioavailability of LCPT, with a 30% reduction of total daily dose (TDD) and a lower peak and less peak‐to‐trough fluctuation. 10 , 11 However, studies comparing the pharmacokinetic profile of once‐daily tacrolimus formulations are scarce, particularly in de novo patients. The crossover study conducted over 21 days in stable patients showed a 36% TDD reduction of LCPT after conversion from PR‐Tac. 14 The retrospective comparison pooling data from two randomized studies 13 , 15 also reported the higher bioavailability of LCPT versus PR‐Tac. 16 In a retrospective study, stable patients converted from IR‐Tac to LCPT showed an improved bioavailability and a 35% dose reduction versus those converted from IR‐Tac to PR‐Tac. 17 However, only a recent randomized study directly compared the pharmacokinetic profile of once‐daily formulations (PR‐Tac and LCPT) in de novo transplant recipients over 28 days, showing a 30% greater bioavailability and 40% lower dose of LCPT versus PR‐Tac. 18
Since comparative studies of once‐daily tacrolimus formulations in de novo kidney transplant recipients are limited to a retrospective comparison of two randomized clinical trials with a short follow‐up period, we set out to investigate the pharmacokinetic profile and other transplant‐related outcomes in de novo patients treated with LCPT and PR‐Tac.
2. METHODS
2.1. Study design
This multicenter, prospective, observational study was conducted at 15 Spanish transplant centers. The study adhered to the principles of the Declaration of Helsinki and was approved by the Independent Ethics Committees of participating centers and Spanish Health Authorities (protocol number: CHI‐TAC‐2016‐01). All participants provided written informed consent.
De novo kidney transplant recipients who started a tacrolimus‐based regimen were recruited 14 days after transplantation (baseline) and followed for 6 months under routine clinical practice conditions. Clinical and laboratory variables were evaluated at Visit 1 (14 days post‐transplant), Visit 2 (21 days post‐transplant), Visit 3 (30 days post‐transplant), Visit 4 (90 days post‐transplant), and Visit 5 (180 days post‐transplant). Pharmacokinetic variables (TDD, Cmin) were also monitored on days 1 and 7 to assess early post‐transplant levels, and across the visits.
Participants received PR‐Tac or LCPT according to routine clinical practice and their respective summary of product characteristics. The decision of which tacrolimus formulation was administered was completely dissociated from the inclusion in the study.
2.2. Study population
Predefined inclusion criteria were: (1) age ≥18 years, (2) de novo recipients of a deceased donor kidney transplant, and (3) post‐transplant immunosuppression treatment with any tacrolimus formulation in conjunction with mycophenolic acid derivatives and corticosteroids prior to enrolment in the study (within 14 days after transplant). Participants were excluded according to the following criteria: (1) prior solid organ transplant, (2) participation in a clinical trial within 30 days of enrolment, (3) any contraindication to study drugs, (4) cognitive impairment limiting the participation in the study, and (5) inability to comply with study follow‐ups or provide informed consent.
2.3. Study outcomes
The primary objective of the study was to compare the pharmacokinetic profile of each tacrolimus group by means of whole blood concentration levels (Cmin), TDD of tacrolimus, and normalized tacrolimus blood concentration (Cmin/TDD) throughout the study period.
Secondary objectives included to compare between each tacrolimus group: (1) treatment failure and non‐biopsy proven rejection rates, (2) adherence and premature study discontinuation rates, (3) quality of life in tremor, (4) safety profile, findings of special interest (including the incidence of cytomegalovirus [CMV] and BK virus [BKV] infections) and laboratory values, (5) the evolution of renal function, and (6) the use of healthcare resources.
Treatment failure comprised any of the following events: death, graft failure, biopsy‐proven acute rejection (BPAR), or loss to follow‐up. Delayed graft function (DGF) was defined as the need for dialysis in the first post‐operative week. Adherence was measured with the Morisky‐Green test, a four‐item self‐reported adherence measure. 19 Premature study discontinuation comprised: loss to follow‐up, graft failure, effectiveness loss (non‐treated rejection), death, protocol deviations, and investigator decision.
The Quality of Life in Essential Tremor (QUEST) scale is a tremor‐specific questionnaire of quality of life subdivided into five scales: Physical (n = 9), Psychosocial (n = 9), Communication (n = 3), Hobbies/Leisure (n = 3), and Work/Finance (n = 6). 20 The QUEST was administered at Visits 1 and 5, with higher scores representing worse perceived quality of life. Safety was evaluated by the incidence of adverse events (AEs) and serious adverse events (SAEs). Findings of special interest included clinical laboratory measures, the incidence of post‐transplant diabetes mellitus (PTDM), infections (CMV and BKV), and tremor affecting daily activities. Renal function was evaluated by the evolution of the estimated glomerular filtration rate (eGFR), calculated with the Modification of Diet in Renal Disease‐4 (MDRD‐4 formula). 21 The use of healthcare resources comprised unscheduled hospitalizations, emergency department visits, outpatient visits, unscheduled laboratory tests, unscheduled explorations, and the cost per treatment.
2.4. Statistical analyses
Continuous variables were described by mean, standard deviation (SD), median, and extremes (Min, Max), and categorical variables by number and percentage. Comparisons between two independent groups for continuous variables were performed using the Student's t‐test for unpaired data or the Mann‐Whitney U test for non‐parametric comparisons. The Chi‐square test or the Fisher's exact test were used for categorical variables. The level of statistical significance was set at P < .05. Statistical analyses were performed using the SAS software (SAS Institute, Cary, SC, USA) for Windows, version 9.2.
The following factors were analyzed at the univariate level to assess potential determinants of tremor: (1) diabetes, (2) tacrolimus group, (3) magnesium values, and (4) Cmin levels. Because no correlation was found at the univariate level, the planned multivariate analysis (binary logistic regression) was not finally performed.
The sample size was calculated based on the primary objective (relative bioavailability). The studies of Budde et al. 13 and Rostaing et al. 22 showed a relative bioavailability of 1.6 ng/ml/mg for IR‐Tac and of 2.3 ng/ml/mg for LCPT, with differences of at least .6 ng/ml/mg between groups being considered clinically relevant. Based on these data, 212 participants (106 treated with PR‐Tac and 106 with LCPT), were required to detect statistically significant differences between groups with 90% power.
3. RESULTS
3.1. Study population
Between October 2016 and August 2017, 251 kidney transplant recipients were recruited from 15 centers. The safety population comprised 229 patients. Data from 218 participants were evaluated for effectiveness analyses: 129 treated with LCPT and 89 with PR‐Tac (Figure 1). Mean age in the overall study population was 56.9 years and 156/218 (72.0%) were male. Baseline and clinical characteristics were balanced between groups at baseline (Table 1). At baseline, 10/129 (7.8%) patients in the LCPT group and 10/89 (11.2%) in the PR‐Tac group were receiving antifungal treatments.
FIGURE 1.
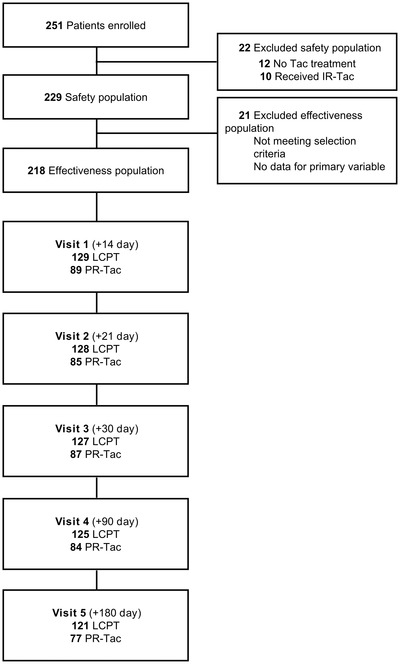
Flowchart showing the study design. Tac, tacrolimus
TABLE 1.
Demographic and clinic characteristics at baseline
| LCPT (N = 129) | PR‐Tac (N = 89) | |
|---|---|---|
| Age (years) | 57.5 ± 12.6 | 56.2 ± 12.4 |
| Gender (male) | 90 (69.8%) | 66 (74.2%) |
| Ethnic group (Caucasian) | 114 (88.4%) | 84 (94.4%) |
| BMI (kg/m2) | 26.7 ± 4.4 | 26.9 ± 4.8 |
| SBP (mm Hg) | 142.0 ± 18.6 | 143.2 ± 20.5 |
| DBP (mm Hg) | 79.8 ± 12.5 | 81.4 ± 14.0 |
| Pre‐Tx diabetes mellitus | 33 (25.6%) | 19 (21.3%) |
| Time from KTx to study inclusion (days) | 13.9 ± 1.8 | 13.8 ± 1.4 |
| Cold ischemia time (h) | 15.7 ± 6.6 | 16.5 ± 6.1 |
| Donor characteristics | ||
| Age (years) | 58.0 ± 15.1 | 54.5 ± 16.0 |
| Sex (male) | 74 (57.4%) | 55 (61.8%) |
| Type of donor | ||
| Brain death donor trauma | 8 (6.2%) | 7 (7.9%) |
| Brain death cerebrovascular | 65 (50.4%) | 51 (57.3%) |
| Brain death others | 16 (12.4%) | 13 (14.6%) |
| Donation after cardiac death Type II | 14 (10.9%) | 6 (6.7%) |
| Donation after cardiac death Type III | 26 (20.2%) | 12 (13.5%) |
| TDD tacrolimus (mg/day) | 7.18 ± 3.70 | 10.75 ± 4.89 |
| Induction therapy a | 100 | 70 |
| Basiliximab | 74 (57.4%) | 56 (62.9%) |
| Timoglobulin | 26 (20.2%) | 15 (16.9%) |
| Concomitant medication | ||
| Mycophenolic acid derivatives | 129 (100.0%) | 88 (98.9%) |
| Corticosteroids | ||
| Induction | ||
| Methylprednisolone | 56 (43.4%) | 35 (39.3%) |
| Maintenance | ||
| Prednisone | 125 (96.9%) | 88 (98.9%) |
Data are expressed as mean ± SD or n (%).
Abbreviations: BMI, body mass index; KTx, kidney transplant; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; TDD, total daily dose.
Patients could have received more than one treatment.
3.2. Primary objective: pharmacokinetic profile
At initial time points (day 1 to 21), participants treated with LCPT exhibited higher Cmin levels and, thereafter, comparable levels were observed between groups (Figure 2A). The TDD was systematically lower in the LCPT group compared to the PR‐Tac group (Figure 2B). At baseline, mean TDD was 7.2 mg/day in the LCPT group and 10.8 mg/day in the PR‐Tac group. At the end of follow‐up, mean TDD was 4.1 mg/day in the LCPT group and 5.8 mg/day in the PR‐Tac group, representing a 29.3% lower dose in the LCPT group (P < .0001) (Figure 2B). Relative bioavailability, measured as the ratio of blood concentration levels and TDD (Cmin /TDD), was significantly higher in the LCPT group versus the PR‐Tac group throughout the entire follow‐up (P < .0001) (Figure 3). The Cmin/TDD ratio was 61% higher in the LCPT group versus the PR‐Tac group after 6 months of treatment (P < .001).
FIGURE 2.
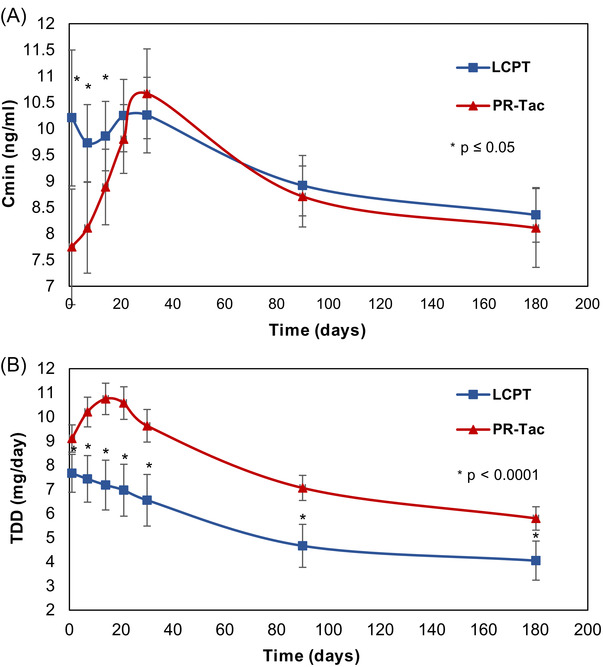
Trough levels and total daily doses over the study period in the LCPT and PR‐Tac groups. Graphs show mean ± SD levels for: A, Trough levels (Cmin), B, TDD (total daily dose). * P‐value < .05
FIGURE 3.
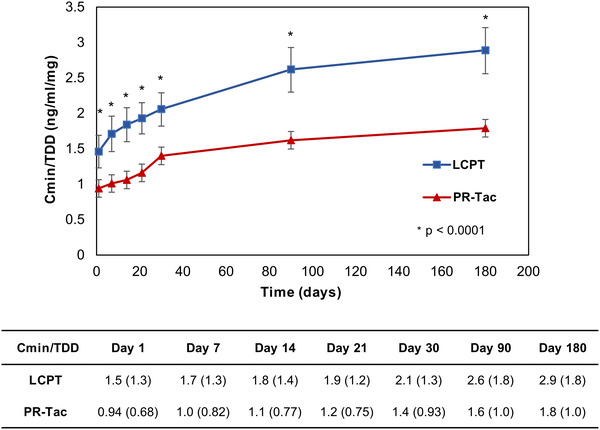
Relative bioavailability of tacrolimus over the study period in the LCPT and PR‐Tac groups. The graph shows mean ± SD for normalized blood tacrolimus levels (Cmin/TDD) over 6 months of follow‐up. * P‐value < .0001
3.3. Treatment failure
Thirteen out of 218 patients (6.0%) showed treatment failures throughout the study: 5/129 (3.9%) in the LCPT group and 8/89 (9.0%) in the PR‐Tac group. None of the causes of treatment failure showed statistically significant differences between groups (Table 2).
TABLE 2.
Treatment failure and non‐biopsy proven rejection rates in each tacrolimus group over the study period
| LCPT (N = 129) | PR‐Tac (N = 89) | P‐value a | |
|---|---|---|---|
| Treatment failure | 5 (3.9%) | 8 (9.0%) | .117 |
| Death | 1 (20.0%) | 3 (37.5%) | >.999 |
| Graft failure | 1 (20.0%) | 3 (37.5%) | >.999 |
| Lost to follow‐up | 3 (60%) | 1 (12.5%) | .217 |
| BPAR | 0 (.0%) | 1 (12.5%) | >.999 |
| Non‐biopsy‐proven rejection | 2 (1.6%) | 0 (.0%) | .200 |
Data are expressed as n (%) over the study period (Visit 1–Visit 5). The proportion of each cause of treatment failure was calculated relative to the total number of patients with treatment failure in each group.
Abbreviation: BPAR, biopsy‐proven acute rejection.
aStatistical significance was calculated using the Fisher exact test.
Four deaths were recorded during the study among 218 patients: one in the LCPT group and three in the PR‐Tac group (99.2% and 96.6% survival rate, respectively). The causes of death were cardiac infarction, infection, death of unknown cause and pulmonary thromboembolism. Among the causes of treatment failure, the highest incidence of graft failure was observed in the PR‐Tac group (3/8, 37.5% of the total treatment failures). Only one patient experienced BPAR in the PR‐Tac group (IA Banff BPAR), and two non‐biopsy‐proven rejections were registered, both in the LCPT group (Table 2).
3.4. Treatment adherence and premature discontinuation
No significant differences were observed between groups in the proportion of adherent patients. Treatment adherence was 97.8% (88/90) at month 1, 96.8% (92/95) at month 3 and 96.6% (85/88) at month 6 in the LCPT group and 95.1% (58/61) at month 1, 98.4% (62/63) at month 3 and 94.6% (53/56) at month 6 in the PR‐Tac group. The incidence of premature discontinuation was 6.2% (8/129) in the LCPT group and 12.4% (11/89) in the PR‐Tac group (Table 3).
TABLE 3.
Treatment adherence and premature discontinuation in each tacrolimus group over the study period
| LCPT | PR‐Tac | P‐value a | |
|---|---|---|---|
| n | 88 | 56 | |
| Adherent patient | 85 (96.6%) | 53 (94.6%) | .678 |
| Non‐adherent patient | 3 (3.4%) | 3 (5.4%) | |
| n | 129 | 89 | |
| Premature discontinuation | 8 (6.2%) | 11 (12.4%) | .113 |
| Lost to follow‐up | 3 (37.5%) | 1 (9.1%) | |
| Graft failure | 0 b | 3 (27.3%) | |
| Efficacy loss | 0 | 0 | |
| Death | 1 (12.5%) | 3 (27.3%) | |
| Protocol deviation | 1 (12.5%) | 1 (9.1%) | |
| Investigator decision | 1 (12.5%) | 1 (9.1%) | |
| Other | 2 (25%) | 2 (18.2%) |
Data are expressed as n (%). % are calculated considering available data.
The proportion of adherent and non‐adherent patients was calculated using the Morisky‐Green test at Visit 5 (month 6). The proportion of causes of premature discontinuation was calculated relative to the total number of premature discontinuations in each group (Visit 2–Visit 5).
Statistical significance was calculated using the Fisher test for adherence or Chi‐square test for premature discontinuations.
One patient in the LCPT group had a graft failure at Visit 2 but completed the study and was not considered as study discontinuation.
Due to the observational nature of the study, four changes in prescribed tacrolimus formulations were registered during the follow‐up: one patient changed from LCPT to PR‐Tac, two patients from PR‐Tac to LCPT, and one patient from LCPT to IR‐Tac The immunosuppression regimen received at Visit 5 is shown in Table S1. The proportion of patients treated with mycophenolic acid derivatives throughout the follow‐up period was 100.0% (129/129) in the LCPT group and 98.9% (88/89) in the PR‐Tac group, 43.4% (56/129) in the LCPT group and 40.4% (36/89) in the PR‐Tac group for methylprednisolone, and 100% in both groups for prednisone.
3.5. Quality of life in essential tremor
At Visit 1, all the QUEST domains had lower scores (better quality of life) in the LCPT group vs. the PR‐Tac group, with statistically significant differences in the psychosocial domain (P = .043) (Figure 4A).
FIGURE 4.
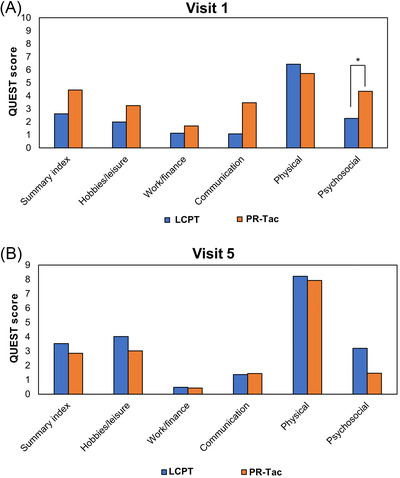
Evolution of quality of life in tremor. Bar graphs show QUEST scores at A, Visit 1 and B, Visit 5. * P‐value < .05
At Visit 5, no significant differences between groups were observed for any of the domains (Figure 4B). Likewise, the proportion of patients reporting a negative impact of tremor in daily habits was statistically comparable between LCPT and PR‐Tac groups (3.9% vs. 10.1%; P = .143) (Table 5).
TABLE 5.
Findings of special interest in each tacrolimus formulation over the study period
| LCPT (N = 129) | PR‐Tac (N = 89) | P‐value a | |
|---|---|---|---|
| DGF | 20 (15.5%) | 16 (18.0%) | .236 |
| PTDM | 11 (8.5%) | 12 (13.5%) | .242 |
| Tremor affecting daily activities | 5 (3.9%) | 9 (10.1%) | .143 |
| Infection by CMV b | 40 (37.0%) | 26 (36.1%) | .899 |
| Infection by BK b | 5 (5.7%) | 12 (16.7%) | .027 |
Data are expressed as n (%)
Abbreviations: DGF, delayed graft function; PTDM, post‐transplant diabetes mellitus; CMV, cytomegalovirus.
Statistical significance was calculated using the Fisher exact test or Chi‐square test.
Relative to patients with available data for PCR.
The univariate analysis revealed no statistically significant association between tremor and diabetes pre‐ and post‐transplantation, treatment group, Cmin, or magnesium levels. Since a significant association with tremor was found only for older patient's age, the planned multivariate analysis (binary logistic regression) was not performed.
3.6. Safety
The proportion of patients with AEs was similar between groups: 82/133 (61.7%) in the LCPT group and 55/96 (57.3%) in the PR‐Tac group. A similar proportion of patients with SAEs was observed between groups: 36/133 (27.1%) in the LCPT group and 26/96 (27.1%) in the PR‐Tac group (Table 4).
TABLE 4.
Safety profile of each tacrolimus formulation over the study period
| LCPT (N = 133) | PR‐Tac (N = 96) | |
|---|---|---|
| Patients with AEs | 82 (61.7%) | 55 (57.3%) |
| Number of AEs | 214 | 111 |
| System organ class | ||
| Infections and infestations | 52 (39.1%) | 24 (25%) |
| Injury, poisoning and procedural complications | 13 (9.8%) | 11 (11.5%) |
| Neoplasms benign, malignant, and unspecified | 2 (1.5%) | 0 |
| Cardiac disorders | 1 (.8%) | 1 (1.0%) |
| Congenital, familial, and genetic disorders | 1 (.8%) | 0 |
| Blood and lymphatic system disorders a | 1 (.8%) | 2 (2.1%) |
| Metabolism and nutrition disorders | 8 (6.0%) | 6 (6.3%) |
| Immune system disorders | 2 (1.5%) | 2 (2.1%) |
| Nervous system disorders | 42 (31.6%) | 22 (22.9%) |
| Gastrointestinal disorders | 16 (12%) | 13 (13.5%) |
| General disorders and administration site conditions b | 6 (4.5%) | 1 (1.0%) |
| Musculoskeletal and connective tissue disorders | 2 (1.5%) | 0 |
| Renal and urinary disorders | 11 (8.3%) | 3 (3.1%) |
| Vascular disorders | 7 (5.3%) | 3 (3.1%) |
| Patients with SAEs | 36 (27.1%) | 26 (27.1%) |
| Surgical complications c | 5 (6.1%) | 3 (5.5%) |
Data are expressed as n (%) of patients with AEs relative to the safety population.
Abbreviations: AE, adverse event; SAE, serious adverse event.
LCPT: one leucopenia, PR‐Tac: One anemia, one polycythemia.
LCPT: one edema, three peripheral edema, three pyrexia; PR‐Tac: one peripheral edema.
% calculated over the number of patients with AEs.
3.7. Delayed graft function
The overall incidence of DGF, defined as the need for dialysis in the first post‐transplant week, was 16.5% (36/218 patients), with 20/129 patients (15.5%) in the LCPT group and 16/89 (18.0%) in the PR‐Tac group (P = .236) (Table 5).
3.8. Infections
During the follow‐up, the frequency of CMV replication was similar amongst the study groups (40/108, 37.0%, in the LCPT group and 26/72, 36.1%, in the PR‐Tac group) (Table 5), with a higher frequency of donor CMV‐seropositive/receptor CMV‐seropositive (D+R+). Of note, 57/129 (44.2%) patients in the LCPT group and 32/89 (36.0%) in the PR‐Tac group received CMV prophylaxis (98.2% after transplant in the LCPT group and 96.9% in the PR‐Tac group). Virus BK replication was significantly lower in the LCPT group (5/88, 5.7%) compared to the PR‐Tac group (12/72, 16.7%; P = .027) (Table 5).
3.9. Renal function and laboratory values
Mean creatinine clearance (CrCl) levels were similar between patients treated with LCPT and PR‐Tac over the study period, with no significant differences between groups at any study visit (Figure 5). In the overall study population, only one patient developed proteinuria >.5 g/24 h in the LCPT group (1.1%) at Visit 3. Laboratory values showed minimal and comparable changes between groups from Visit 1 to 5 (Table 6).
FIGURE 5.
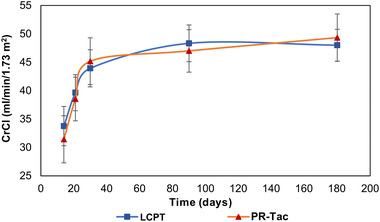
Evolution of renal function measured by creatinine clearance (CrCl) over the study period. No significant differences between groups were observed. The change in CrCl was statistically significant from Visit 2 to 4 in the LCPT group and from Visit 2 to 3 in the PR‐Tac group
TABLE 6.
Laboratory parameters in each tacrolimus group at Visit 1 and 5
| LCPT (N = 133) | PR‐Tac (N = 96) | |
|---|---|---|
| LDL cholesterol (mmol/L) | ||
| Visit 1 | 2.8 ± .8 | 3.1 ± 1.0 |
| Visit 5 | 2.8 ± 1.0 | 2.9 ± 1.0 |
| Total cholesterol (mmol/L) | ||
| Visit 1 | 4.7 ± 1.1 | 5.1 ± 1.3 |
| Visit 5 | 4.8 ± 1.0 | 5.0 ± 1.2 |
| Triglycerides (mmol/L) | ||
| Visit 1 | 1.9 ± .9 | 2.0 ± .8 |
| Visit 5 | 1.7 ± .8 | 1.7 ± .9 |
| Hemoglobin (g/L) | ||
| Visit 1 | 102.5 ± 14.0 | 99.5 ± 13.3 |
| Visit 5 | 129.1 ± 16.3 | 131.2 ± 17.7 |
| Leucocytes (x109/L) | ||
| Visit 1 | 11.0 ± 3.8 | 11.3 ± 4.2 |
| Visit 5 | 6.7 ± 2.5 | 7.4 ± 2.8 |
| Platelets (x109/L) | ||
| Visit 1 | 275.7 ± 86.6 | 282.0 ± 107.8 |
| Visit 5 | 210.1 ± 68.6 | 213.7 ± 67.3 |
| Magnesium (mmol/L) | ||
| Visit 1 | .95 ± .4 | .82 ± .3 |
| Visit 5 | .76 ± .2 | .76 ± .2 |
| HbA1c (%) | ||
| Visit 1 | 5.9 ± 1.1 | 5.5 ± .7 |
| Visit 5 | 6.2 ± 1.2 | 6.1 ± 1.2 |
Data are expressed as mean ± SD.
3.10. Healthcare resource use
Patients received a median accumulated (from day 14 to month 6) tacrolimus dose of 889 mg in the LCPT group and of 1267 mg in the PR‐Tac group, resulting in a 29.8% decrease in the LCPT group (Table 7). Considering the current cost of tacrolimus per mg in Spain (.768 €/mg for LCPT and 1.035 €/mg for PR‐Tac, 2018) 23 , the cost for the 6‐month treatment period of the study (median accumulated dose × cost per mg) was 682.8€ for LCPT vs. 1311.3€ for PR‐Tac, corresponding to a 47.9% cost reduction.
TABLE 7.
Healthcare use in each tacrolimus treatment over the study period
| LCPT (N = 129) | PR‐Tac (N = 89) | P‐value a | |
|---|---|---|---|
| Unscheduled hospitalizations | 40 (31.0%) | 29 (32.6%) | .806 |
| Days of hospitalization | 15.5 (13.6) | 12.6 (15.1) | |
| Emergency department visits | 41 (31.8%) | 28 (31.5%) | .960 |
| Unscheduled outpatient visits | 30 (23.3%) | 23 (25.8%) | .662 |
| Unscheduled laboratory tests | 52 (40.3%) | 32 (36.0%) | .516 |
| Unscheduled explorations | 46 (35.7%) | 30 (33.7%) | .764 |
| Accumulated tacrolimus dose (mg) | 1008.5 ± 566.4 | 1411.3 ± 736.2 | – |
| Median (IQR) | 889 (630; 1246) | 1267 (883; 1784) | |
| Cost per 6 months treatment (€) | 682.8 | 1311.3 | – |
Data are expressed as n (%), mean ± SD and median (IQR) over the study period (Visit 1‐Visit 5).
Abbreviations: IQR, interquartile range.
Statistical significance was calculated using the Mann‐Whitney U test for continuous variables or Chi‐square test for categorical variables.
No statistically significant differences were found between groups in the incidence of hospitalizations, emergency department visits, unscheduled outpatient visits, laboratory tests or explorations (Table 7).
4. DISCUSSION
In this prospective study, LCPT showed higher relative bioavailability, with lower BK replication and similar effectiveness at preventing allograft rejection, renal function and safety profile compared with PR‐Tac.
This study showed the improved relative bioavailability of LCPT compared to PR‐Tac throughout the study, supporting previous comparisons with these formulations in de novo patients. 13 , 16 , 18 Trough levels were higher in the LCPT group for the initial 21 days compared with the PR‐Tac formulation, as previously observed. 13 , 22 This result is of remarkable importance since, as indicated in the KDIGO guideline, achieving early tacrolimus levels is crucial to preventing acute rejection. 24 These higher initial trough levels were observed despite lower TDD at early time points, reinforcing the improved pharmacokinetic properties of LCPT in clinical practice. The pharmacokinetic profile at longer time points (from day 21 to month 6) was characterized by comparable blood concentrations with statistically lower TDD in the LCPT group. A 30% lower TDD was observed at month 6 in the LCPT group relative to the PR‐Tac group, in the range to the previously reported 40% lower TDD with LCPT vs. PR‐Tac achieved by day 28 18 and month 6. 16
The effectiveness of both once‐daily formulations was evident by the treatment failure rates (3.9% in the LCPT group and 9.0 in the PR‐Tac group). No statistically significant differences were observed between groups for the incidence of death, graft failure, loss to follow‐up, BPAR, and non‐biopsy proven rejection. Earlier studies support this comparable effectiveness and also show non‐significantly lower treatment failure rates in the LCPT group as compared to the IR‐Tac 13 , 22 and PR‐Tac groups. 16 Davis et al. observed that tacrolimus trough concentrations < 8 ng/ml were associated with de novo HLA donor‐specific antibodies and higher rejection rates by months 6 and 12 and, in our study, trough levels in the LCPT group were above 8 ng/ml throughout the follow‐up. 25 Another possible explanation for the low acute rejection rates in both groups is the fact that patients were recruited 14 days after transplantation, potentially biasing the results towards patients without clinical (infection, rejection, gastrointestinal problems) or surgical complications (thrombosis, uropathy).
The high and similar adherence rates between groups could be explained considering the short follow‐up period after transplantation (6 months) and that patients received a once‐daily formulation, since dosing regimen is one of the key contributors to adherence. The frequency of premature discontinuation was comparable in participants receiving LCPT (6.2%) vs. those treated with PR‐Tac (12.4%), contrasting with previous results of a phase III conversion study showing higher premature discontinuation in the LCPT group (12%) compared with the IR‐Tac group (5%). 12 Of remarkable importance are discontinuations resulting in death (one in the LCPT group vs. three in PR‐Tac group) or graft failure (none in the LCPT group vs. three in the PR‐Tac group), although these results require further confirmation.
One of the distinctive evaluations of this study was the longitudinal analysis of quality of life in tremor (QUEST), as tremor is one of the most common neurotoxic effects of tacrolimus. 26 , 27 To our knowledge, this is the first study showing the evolution of quality of life in the long term. An important finding was that the perception of quality of life in tremor differed significantly between groups across the visits, which could be explained by the tight relationship of neurotoxic effects with peak tacrolimus concentrations. 28 In this regard, LCPT is characterized by a flatter profile with lower peak and similar exposure compared to other tacrolimus formulations. 11 We observed that, although LCPT compared favorably to PR‐Tac for the psychosocial domain at Visit 1, differences between groups were not significant at Visit 5. Moreover, the proportion of patients reporting a negative impact of tremor in daily habits in our study was statistically similar in the LCPT and PR‐Tac groups (3.9% vs. 10.1%). In agreement with these results, LCPT was associated with improved hand tremor 14 days after the switch from IR‐Tac in a prospective phase IIIb study and in a recent retrospective study. 17 , 28
Once‐daily tacrolimus formulations exhibited a similar safety profile, as evidenced by the comparable incidence of AEs, SAEs, DGF and laboratory values recorded. As previously reported, the safety profile of LCPT was comparable to that of IR‐Tac, 12 , 13 PR‐Tac 18 or both 14 in de novo and conversion studies. Of note, DGF frequency ranged from 27.3% to 60.7% in different studies conducted in Spain. 29 , 30 , 31 One of the causes that could explain the lower incidence of DGF found in our study is the potential selection bias associated with participant recruitment 14 days post‐transplant.
Renal function improved in the overall population over the study period, regardless of the tacrolimus formulation received. Although nephrotoxicity caused by tacrolimus is known to depend on the Cmin/TDD ratio 32 , 33 and tacrolimus dosage, 34 we observed a comparable renal function despite the differential relative bioavailability between groups. Similar results were previously observed when comparing LCPT with PR‐Tac 18 and both IR‐Tac and PR‐Tac. 14 , 16
In this study, we observed a similar proportion of patients infected with CMV during the study and a lower incidence of BK infections in the LCPT group compared with the PR‐Tac group. 33 Of note, the comparable proportion of patients receiving mycophenolic acid derivatives at Visit 5 does not explain differences in BK infections. Compared with results from the Transform trial, tacrolimus exposure at month 6 was higher in our study (8.5 ng/ml vs. 4.6 ng/ml), but BK infection was similar (5.7% vs. 4.3%). 35 Conversely, in the Athena study, with higher tacrolimus levels than in the Transform study, the incidence of BK infection/year was 17% in the tacrolimus/everolimus group, 9.1% in the cyclosporine/everolimus group and 22.5% in the tacrolimus/mycophenolic acid group. Therefore, tacrolimus exposure in our study does not seem to explain the low incidence of BK infections. 36 However, the relatively short follow‐up period of the study (6 months) and the fact that not all patients had PCR results do not allow to draw reliable conclusions regarding infections.
The analysis of healthcare resources utilization revealed similar rates of unscheduled tests, emergency department visits, and hospitalizations. Patients received a 29.8% lower median cumulative dose of tacrolimus in the LCPT group vs. the PR‐Tac group (889 vs. 1267 mg), which corresponds to a 47.9% cost reduction. 23 However, these results were calculated based on Spanish costs and are not generalizable to other countries or formulations. Despite this, healthcare resources results agree with the lower treatment costs associated with LCPT treatment reported in a previous study. 16
This study is the first prospective study comparing once‐daily formulations of tacrolimus for up to 6 months in de novo kidney transplant patients. The study comprises a considerable sample size, as compared to other comparative studies, 14 , 16 and integrates data from a wide range of variables. Considering the limited number of studies comparing both once‐daily tacrolimus formulations, this study could be of great value for physicians managing kidney transplant patients.
The main limitation of the study is the fact that patients were recruited 14 days post‐transplant challenging the comparison with previous trials and potentially leading to unintended selection bias. Other limitations are mainly related to its observational and open‐label design, which cannot prove direct causality between treatments and observed effects. However, the results reported complement and agree with those observed in previous randomized clinical trials. The short follow‐up time for detecting viral infections (6 months) is also a drawback of the study, especially considering that patients might have received prophylaxis for CMV for 3–6 months.
5. CONCLUSION
Patients treated with LCPT showed higher tacrolimus relative bioavailability, similar allograft rejection rates, adherence, renal function and safety profile and a lower incidence of BK infections compared with those treated with PR‐Tac.
CONFLICT OF INTEREST
Constantino Fernández has received honoraria from Alexion, Novartis, Astelas, Chiesi, and Biotest. Amado Andres has received honoraria from Chiesi, Astellas, Novartis and Bristol‐Myers‐Squibb. Domingo Hernandez and Veronica López received honoraria from Chiesi. Francesc Moreso received honoraria from Chiesi, Astellas, Novartis and Bristol‐Myers‐Squibb. Edoardo Melilli received honoraria from Chiesi, Astellas, Novartis, Sandoz, Menarini. Gonzalo Gómez has received lecture fees from speaking on behalf of Novartis, and has been paid advisory boards by Alexion. Ana Sánchez Fructuoso has received honoraria from Chiesi. The rest of the authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
C.F.R. supervised the study, participated in research design and data analysis, and drafted the manuscript. M.C.R., J.L.P., J.P., M.C., G.G., S.C.P., J.P., R.L., M.P.M., F.M., M.P., A.A., E.G., A.F., A.M., B.F.C., A.S.F., N.C., A.S., G.B.B., A.O., M.C.R.F., E.M., N.M.P., A.R., B.F., V.L. and D.H. participated in research design, data analysis, and revised the manuscript. C.F.R., M.C.R., J.L.P., J.P., M.C., G.G., S.C.P., J.P., R.L., M.P.M., F.M., M.P., A.A., E.G., A.F., A.M., B.F.C., A.S.F., N.C., A.S., G.B.B., A.O., M.C.R.F., E.M., N.M.P., A.R., B.F., V.L. and D.H. contributed to data acquisition.
COLLABORATORS
The following investigators are members of the Better group: Inma Beltrán (University Hospital La Fe, Valencia); María José Pérez (University Hospital del Mar, Barcelona); Dolores Redondo (University Hospital del Mar, Barcelona); Ana Cristina Tugores Vázquez (University Hospital Son Espases, Palma de Mallorca); Fatima Alvaredo de Beas (University Hospital Son Espases, Palma de Mallorca); Álex Gutiérrez Dalmau (Hospital Miguel Servet, Zaragoza); Laura Cañas (University Hospital Germans Trias y Pujol, Badalona); Javier Juega (University Hospital Germans Trias y Pujol, Badalona); Joana Sellarés (University Hospital Vall d'Hebron, Barcelona); Daniel Serón (University Hospital Vall d'Hebron, Barcelona); Irina B. Torres (University Hospital Vall d'Hebron, Barcelona); Isabel Delgado (University Hospital Doce de Octubre, Madrid); Natalia Polanco (University Hospital Doce de Octubre, Madrid); Pilar Auñón (University Hospital Doce de Octubre, Madrid); Ana Hernández (University Hospital Doce de Octubre, Madrid); Cristina Galeano Alvarez (University Hospital Ramón y Cajal, Madrid); Sara Jiménez Alvaro (University Hospital Ramón y Cajal, Madrid); Sandra Elias Triviño (University Hospital Ramón y Cajal, Madrid); Ana Lucía Valencia Peláez (University Hospital Clínico de Valladolid, Valladolid); Guadalupe Rodríguez Portela (University Hospital Clínico de Valladolid, Valladolid); Isabel Pérez Flores (University Hospital Clínico San Carlos, Madrid); Angie Moreno de la Higuera (University Hospital Clínico San Carlos, Madrid); Beatriz Rodriguez Cubillo (University Hospital Clínico San Carlos, Madrid); Mercedes Cabello (University Hospital Regional, Málaga); Pedro Ruiz‐Esteban (University Hospital Regional, Málaga); Maria José Morales Lara (University Hospital Regional, Málaga); Cristina Jironda (University Hospital Regional, Málaga).
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors thank the following investigators: Marisa Mir (University Hospital del Mar, Barcelona); Anna Buxeda (University Hospital del Mar, Barcelona); Carla Burballa (University Hospital del Mar, Barcelona); Anna Faura (University Hospital del Mar, Barcelona); Berta Xargay (University Hospital del Mar, Barcelona); Sara Alvarez (University Hospital del Mar, Barcelona); Merce Espona (University Hospital del Mar, Barcelona); Carlos Arias (University Hospital del Mar, Barcelona); Natalia Allende Burgos (University Hospital Son Espases, Palma de Mallorca); María José Aladrén Regidor (Hospital Miguel Servet, Zaragoza); Lídia Hernandez Moreno (University Hospital Germans Trias y Pujol, Badalona); Andrea Collado Alsina (University Hospital Ramón y Cajal, Madrid); Miguel Angel Calleja (University Hospital Virgen de las Nieves, Granada); Agustín Martín (University Hospital Virgen de las Nieves, Granada); Josep Maria Cruzado (University Hospital Bellvitge). The authors thank Carla Granados of Trialance SCCL for providing medical writing assistance. The study described within the paper was sponsored by Chiesi España S.A.U.
Fernandez Rivera C, Calvo Rodríguez M, Poveda JL, et al. Bioavailability of once‐daily tacrolimus formulations used in clinical practice in the management of De Novo kidney transplant recipients: the better study. Clin Transplant. 2022;36:e14550. 10.1111/ctr.14550
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Nankivell BJ, PʼNg CH, OʼConnell PJ, Chapman JR. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology. Transplantation. 2016;100(8):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 2. Kuypers DRJ, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus oncE‐Daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333‐340. [DOI] [PubMed] [Google Scholar]
- 3. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623‐653. [DOI] [PubMed] [Google Scholar]
- 4. Prograf Summary of Product characteristics. Available at: https://www.medicines.org.uk/emc/product/6720/smpc. Last accessed: May 2021.
- 5. Advagraf Summary of Product characteristics. Available at: https://www.ema.europa.eu/en/documents/product‐information/advagraf‐epar‐product‐information_en.pdf. Last accessed: May 2021.
- 6. Sukkha S, Chindavijak B, Montakantikul P, Ingsathit A, Nosoongnoen W, Sumethkul V. Trough level from twice daily to once daily tacrolimus in early conversion kidney transplant recipients: a prospective study. Int J Clin Pharm. 2017;39(6):1298‐1303 [DOI] [PubMed] [Google Scholar]
- 7. Sańko‐Resmer J, Boillot O, Wolf P, Thorburn D. Renal function, efficacy and safety postconversion from twice‐ to once‐daily tacrolimus in stable liver recipients: an open‐label multicenter study. Transpl Int. 2012;25(3):283‐293. [DOI] [PubMed] [Google Scholar]
- 8. Envarsus Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product‐information/envarsus‐epar‐product‐information_en.pdf. Last accessed: May 2021.
- 9. Toblli JE, Di Gennaro F. Switching patients with non‐dialysis chronic kidney disease from oral iron to intravenous ferric carboxymaltose: Effects on erythropoiesis‐stimulating agent requirements, costs, hemoglobin and iron status. PLoS One. 2015;10(4):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alloway RR, Eckhoff DE, Washburn WK, Teperman LW. Conversion from twice daily tacrolimus capsules to once daily extended‐release tacrolimus (LCP‐Tacro): phase 2 trial of stable liver transplant recipients. Liver Transplant. 2014;20(5):564‐575. [DOI] [PubMed] [Google Scholar]
- 11. Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96(2):191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bunnapradist S, Ciechanowski K, West‐Thielke P, et al. Conversion from twice‐daily tacrolimus to once‐daily extended release tacrolimus (LCPT): The phase III randomized MELT trial. Am J Transplant. 2013;13(3):760‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Budde K, Bunnapradist S, Grinyo JM, et al. Novel once‐daily extended‐release tacrolimus (LCPT) versus twice‐daily tacrolimus in de novo kidney transplants: one‐year results of phase III, double‐blind, randomized trial. Am J Transplant. 2014;14(12):2796‐2806. [DOI] [PubMed] [Google Scholar]
- 14. Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady‐state head‐to‐head pharmacokinetic comparison of All FK‐506 (Tacrolimus) formulations (ASTCOFF): an open‐label, prospective, randomized, two‐arm, three‐period crossover study. Am J Transplant. 2017;17(2):432‐442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. https://clinicaltrials.gov/ct2/show/record/NCT01683331
- 16. Glander P, Waiser J, Kasbohm S, et al. Bioavailability and costs of once‐daily and twice‐daily tacrolimus formulations in de novo kidney transplantation. Clin Transplant. 2018;32(8):6‐12 [DOI] [PubMed] [Google Scholar]
- 17. Sánchez Fructuoso A, Ruiz JC, Franco A, et al. Effectiveness and safety of the conversion to MeltDose ® extended‐release tacrolimus from other formulations of tacrolimus in stable kidney transplant patients: a retrospective study. Clin Transplant. 2020;34(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamar N, Cassuto E, Piotti G, et al. Pharmacokinetics of prolonged‐release once‐daily formulations of tacrolimus in de novo kidney transplant recipients: a randomized, parallel‐group, open‐label, multicenter study. Adv Ther. 2019;36(2):462‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 20. Tröster AI, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in essential tremor questionnaire (QUEST): development and initial validation. Park Relat Disord. 2005;11(6):367‐373. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461‐470. [DOI] [PubMed] [Google Scholar]
- 22. Rostaing L, Bunnapradist S, Grinyó JM, et al. Novel once‐daily extended‐release tacrolimus versus twice‐daily tacrolimus in de novo kidney transplant recipients: two‐year results of phase 3, double‐blind, randomized trial. Am J Kidney Dis. 2016;67(4):648‐659. [DOI] [PubMed] [Google Scholar]
- 23. Orden SCB/1244/2018. https://www.boe.es/diario_boe/txt.php?id=BOE‐A‐2018‐16150. Last accessed: May 2021.
- 24. Journal A. Special issue: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1‐S155. [DOI] [PubMed] [Google Scholar]
- 25. Davis S, Gralla J, Klem P, et al. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor‐specific antibodies in the first year of kidney transplantation. Am J Transplant. 2018;18(4):907‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13(5):313‐326. [DOI] [PubMed] [Google Scholar]
- 27. Bulatova N, Yousef A‐M, Al‐Khayyat G, Qosa H. Adverse effects of tacrolimus in renal transplant patients from living donors. Curr Drug Saf. 2011;6(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 28. Langone A, Steinberg SM, Gedaly R, et al. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP‐TacrO (STRATO): an open‐label, multicenter, prospective phase 3b study. Clin Transplant. 2015;29(9):796‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arcos E, José Pérez‐Sáez M, Comas J, Lloveras J, Tort J, Pascual J. Assessing the limits in kidney transplantation: use of extremely elderly donors and outcomes in elderly recipients. Transplantation. 2020:176‐183. [DOI] [PubMed] [Google Scholar]
- 30. Ojo AO, Morales JM, González‐Molina M, et al. Comparison of the long‐term outcomes of kidney transplantation: USA versus Spain. Nephrol Dial Transplant. 2013;28(1):213‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torres A, Hernández D, Moreso F, et al. Randomized controlled trial assessing the impact of tacrolimus versus cyclosporine on the incidence of posttransplant diabetes mellitus. Kidney Int Reports. 2018;3(6):1304‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thölking G, Fortmann C, Koch R, et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS One. 2014;9(10):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thölking G, Gerth HU, Schuette‐Nuetgen K, Reuter S. Influence of tacrolimus metabolism rate on renal function after solid organ transplantation. World J Transplant. 2017;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuypers DRJ, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single‐nucleotide polymorphisms determine long‐term tacrolimus disposition and drug‐related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82(6):711‐725. [DOI] [PubMed] [Google Scholar]
- 35. Pascual J, Berger SP, Witzke O, et al. Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol. 2018;29(7):1979‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sommerer C, Suwelack B, Dragun D, et al. An open‐label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 2019;96(1):231‐244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author on reasonable request.


