Summary
The panzootic caused by A/goose/Guangdong/1/96‐lineage highly pathogenic avian influenza (HPAI) A(H5) viruses has occurred in multiple waves since 1996. From 2013 onwards, clade 2.3.4.4 viruses of subtypes A(H5N2), A(H5N6), and A(H5N8) emerged to cause panzootic waves of unprecedented magnitude among avian species accompanied by severe losses to the poultry industry around the world. Clade 2.3.4.4 A(H5) viruses have expanded in distinct geographical and evolutionary pathways likely via long distance migratory bird dispersal onto several continents and by poultry trade among neighboring countries. Coupled with regional circulation, the viruses have evolved further by reassorting with local viruses. As of February 2019, there have been 23 cases of humans infected with clade 2.3.4.4 H5N6 viruses, 16 (70%) of which had fatal outcomes. To date, no HPAI A(H5) virus has caused sustainable human‐to‐human transmission. However, due to the lack of population immunity in humans and ongoing evolution of the virus, there is a continuing risk that clade 2.3.4.4 A(H5) viruses could cause an influenza pandemic if the ability to transmit efficiently among humans was gained. Therefore, multisectoral collaborations among the animal, environmental, and public health sectors are essential to conduct risk assessments and develop countermeasures to prevent disease and to control spread. In this article, we describe an assessment of the likelihood of clade 2.3.4.4 A(H5) viruses gaining human‐to‐human transmissibility and impact on human health should such human‐to‐human transmission occur. This structured analysis assessed properties of the virus, attributes of the human population, and ecology and epidemiology of these viruses in animal hosts.
Keywords: avian influenza, zoonosis, zoonotic influenza
Abbreviations
- HPAI
Highly Pathogenic Avian Influenza
- LPAI
Low Pathogenicity Avian Influenza
- WHO
World Health Organization
- GIP
Global Influenza Programme
- TIPRA
Tool for Influenza Pandemic Risk Assessment
- IRAT
Influenza Risk Assessment Tool
- CDC
United States Centers for Disease Control and Prevention
- GISRS
WHO Global Influenza Surveillance and Response System
- OIE
World Organization for Animal Health
- FAO
Food and Agriculture Organization
- HA
Hemagglutinin
- ROK
Republic of Korea
- CVV
WHO Candidate Vaccine Virus
- USDA
United States Department of Agriculture
- ARDS
Acute Respiratory Distress Syndrome
- MOF
Multiple Organ Failure
- Sia‐α2,6Gal
Sialic Acid Linked to Galactose by an α2,6 linkage
- 3′SLeX
Sialyl Lewis X
- GOF
Gain‐Of‐Function
- GISAID
Global Initiative on Sharing All Influenza Data
- GSD
Genetic Sequence Data
1. INTRODUCTION
Influenza A viruses infect a wide spectrum of animal species precluding global eradication. Genetically diverse viruses circulate among wild aquatic birds, which are considered to be their natural reservoir and experience no or only mild signs of disease when infected. In birds, the viruses typically replicate in the intestinal and respiratory tracts and are shed in the environment where other hosts become infected. Viruses from the aquatic wild bird reservoir may infect other avian species including terrestrial poultry, such as chickens and quail, and domesticated waterfowl, such as ducks and geese. Following circulation in these densely populated host species, avian influenza viruses may then transmit to mammalian hosts, including humans, pigs, horses, dogs, and marine mammals. 1
Globalization and industrialization over the past decades have contributed to the emergence of novel influenza viruses that threaten animal and human health. Once they emerge and become transmissible between humans, influenza viruses can rapidly spread worldwide. Current vaccines which take 6 months to distribute from strain selection in the current influenza manufacturing cycle are unlikely to be available to contain the first wave of human infections of a pandemic. Therefore, it is strategically important to risk‐assess and prioritize animal influenza viruses with pandemic potential to initiate possible responses, including preparatory development of vaccines, and antiviral drug efficacy testing. The World Health Organization (WHO) Global Influenza Programme (GIP) developed a Tool for Influenza Pandemic Risk Assessment (TIPRA) 2 based on the Influenza Risk Assessment Tool (IRAT) 3 developed by the WHO Collaborating Centre at the United States Centers for Disease Control and Prevention (CDC) and in consultation with experts in the WHO Global Influenza Surveillance and Response System (GISRS) and other institutions and academia. Since TIPRA was launched in 2016, it has provided a framework for influenza A virus risk assessment through a standardized approach for evaluating the likelihood of pandemic emergence and associated impact of a novel virus. In this risk assessment process, the WHO, the World Organization for Animal Health (OIE), and the Food and Agriculture Organization (FAO) tripartite collaboration brings together multiple stakeholders worldwide, including public and animal health practitioners and influenza researchers from different sectors within the “One Health” concept, and strengthens interdisciplinary global collaboration. 4
Because the emergence of the highly pathogenic avian influenza (HPAI) A(H5) viruses of the A/goose/Guangdong/1/96 (gs/GD) hemagglutinin (HA) lineage, there have been 883 officially reported human infections by viruses of this lineage: 860 by A(H5N1) and 23 by A(H5N6) viruses. 5 The dominant HA clades of H5 viruses vary temporally and spatially, with some achieving a wide geographical spread. Infections among humans and other mammals,6, 7, 8 however, have been restricted to the initial index cases or a small number of close contacts. Because HPAI A(H5) viruses bearing an HA of clade 2.3.4 were identified in China in 2008, they have evolved into further subgroups including clade 2.3.4.4 9 and have acquired various neuraminidase subtypes, including N1, N2, N5, N6, and N8, by reassortment with other avian influenza viruses enzootic in different regions. In addition, the geographic spread of clade 2.3.4.4 A(H5) viruses has been unprecedented, resulting in regional epizootics in poultry, increasing the opportunities for avian‐to‐human transmission. Although human‐to‐human transmission of clade 2.3.4.4 A(H5) viruses has not been observed to date, the pandemic potential of these viruses remains unpredictable. Given the lack of population immunity to A(H5) subtype viruses, the ongoing evolution of clade 2.3.4.4 A(H5) viruses, and sporadic human infections, the pandemic potential of these viruses cannot be ignored. In this review, we focus on the three clade 2.3.4.4 subtypes, A(H5N2), A(H5N6), and A(H5N8), that have the greatest frequency of global detections, and describe their biological features and the use of TIPRA in risk asessment. 2
2. GLOBAL SPREAD OF HPAI CLADE 2.3.4.4 A(H5) VIRUSES
The clade 2.3.4.4 A(H5N8) viruses were first reported in migratory ducks and curlews in Shanghai, China in 2013 by retrospective surveillance, 10 followed by outbreaks in the Republic of Korea (ROK) in January of 2014.11, 12, 13, 14 During the outbreaks in ROK, two distinct genetic groups were identified: a group represented by A/broiler duck/Korea/Buan2/2014 and the WHO candidate vaccine virus (CVV) recommended by WHO, 15 A/gyrfalcon/Washington/41088‐6/2014 (referred to as “group A” by Lee et al 16 ), and another group represented by A/breeder duck/Korea/Gochang1/2014 14 and the CVV, A/Fujian‐Sanyuan/21099/2017 (referred to as “group B” by Lee et al 16 ) (Figure 1a). A group of viruses represented by A/gyrfalcon/Washington/41088‐6/2014 (hereby A/gyrfalcon/Washington/41088‐6/2014 group) likely spread eastwards, to North America via Beringia by long‐distance migratory birds17, 18, 19, 20, 21, 22 (Figure 1b). In November 2014, these viruses reassorted with avian influenza viruses from North American wild birds generating an A(H5N2) virus that was the cause of an outbreak in poultry farms in British Columbia. 23 From March through mid‐June of 2015, HPAI A(H5N2) viruses caused widespread outbreaks in commercial poultry flocks mainly in the Pacific, Western, and North Central regions of the United States. 24 The spread of the virus in the United States was accompanied by multiple reassortment events between HPAI A(H5) viruses and low pathogenicity avian influenza (LPAI) viruses from wild and domestic birds. 25 The United States Department of Agriculture (USDA) documented that during the outbreaks 50.4 million birds died or were culled in the 15 affected states.17, 20, 26, 27, 28 After the initial wave of outbreaks in North America, detections of the HPAI A(H5) virus declined; it has not been detected in poultry since June 16, 2015 or in wild birds since December 16, 2016 in North America25, 29, 30 (Supplementary Table 1). In parallel with the spread of clade 2.3.4.4 A(H5N8) viruses to North America, related A/gyrfalcon/Washington/41088‐6/2014‐group viruses had also moved into Europe and were widespread by the end of 2014.31, 32, 33, 34, 35 Nevertheless, it is noteworthy that sporadic outbreaks of A/gyrfalcon/Washington/41088‐6/2014‐group A(H5N2) and A(H5N8) viruses continue to be detected among poultry with the latest outbreak caused by A(H5N2) viruses in a chicken farm in April 2019. 36
Figure 1.
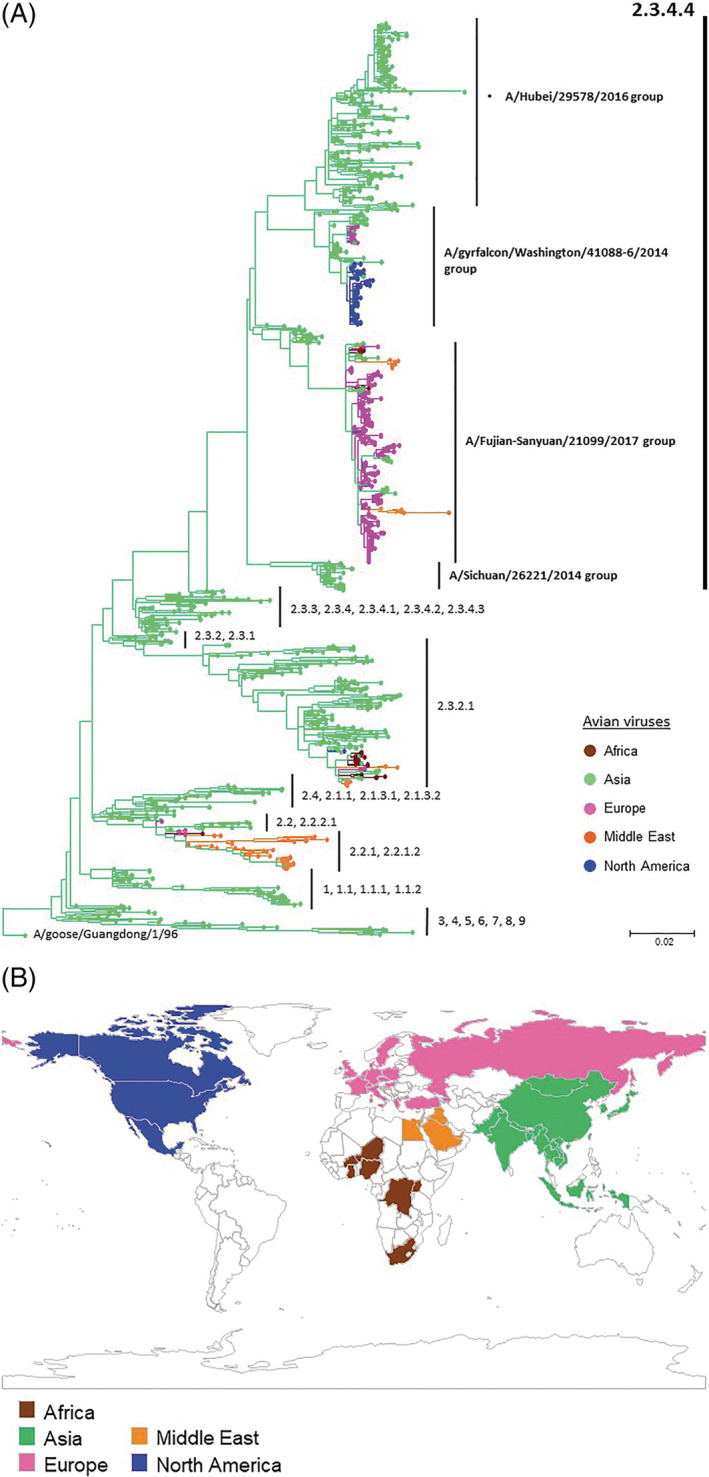
(a) Geographical regions in the world that confirmed to have isolated clade 2.3.4.4 A(H5) viruses from animals; mammals and avian species. Geographical regions colored in brown, Africa; green, Asia; pink, Europe; orange, Middle East; and blue, North and South America. (b) Phylogenetic relationships of HA genes of A(H5) highly pathogenic avian influenza viruses. Of 3685 HPAI A(H5) viruses isolated from animals including mammals and avian species available in Global Initiative on Sharing All Influenza Data (GISAID) and GenBank between 2013 and 2018, arbitrarily chosen 1134 strains were analyzed. The open reading frame of HA genes A(H5) virus was used for phylogenetic analysis. Multiple sequence alignment of A(H5) viruses was performed together with alignment of genetic sequence data (GSD) downloaded from GISAID using BioEdit 7.2. A maximum‐likelihood tree using the 1134 A(H5) HA genes and 242 representative A(H5) HA genes 135 rooted to A/goose/Guangdong/1/96 was constructed for MEGA 7 with 1000 replicate
Although the A/gyrfalcon/Washington/41088‐6/2014‐group viruses were disseminated from Asia to other continents, a group of viruses represented by A/Fujian‐Sanyuan/21099/2017 (hereby A/Fujian‐Sanyuan/21099/2017 group) did not initially appear to spread outside Asia. 37 However, this changed in mid‐2016 when these A(H5N8) viruses were detected in wild birds at Tyva Republic near Uvs‐Nuur Lake in Russian Federation38, 39, 40 and Qinghai Lake in China. 41 This group subsequently spread, presumably by wild birds, to many other countries19, 22, 42 in Africa,43, 44, 45 Asia,46, 47, 48, 49, 50 Europe,51, 52, 53, 54, 55, 56, 57 and the Middle East58, 59, 60, 61, 62 (Figure 1b). As was seen with the earlier spread of A(H5N8) viruses in North America, multiple reassortment events with local wild bird viruses occurred generating additional NA subtypes.63, 64 According to the OIE, between mid‐2016 and October 2018, 51 countries in Africa, Asia, and Europe reported clade 2.3.4.4 A(H5N8) viruses in either poultry or wild birds. 65 From 2017‐2018, A(H5N6) viruses with HA gene of the A/Fujian‐Sanyuan/21099/2017 group were isolated from birds in the Netherlands, 66 the United Kingdom, 67 Germany, Greece, Republic of Georgia, and Denmark and Eastern Asian countries48, 68, 69 (Supplementary Table 1).
In contrast to the A/gyrfalcon/Washington/41088‐6/2014‐group and A/Fujian‐Sanyuan/21099/2017‐group viruses, which were characterized by global spread, other genetic groups of clade 2.3.4.4 A(H5) viruses have to date remained more limited in geographic range. One group represented by the WHO CVVs, A/Hubei/29578/2016, A/chicken/Vietnam/NCVD‐15A59/2015, and A/duck/Hyogo/1/2016 (referred to as “group C” by Lee et al 16 and hereby A/Hubei/29578/2016 group), is comprised mainly A(H5N6) viruses that have been maintained among avian species since 2013 in China,70, 71 Japan,72, 73, 74 Lao People's Democratic Republic, 75 ROK, 76 and Vietnam 77 (Figure 1a and b). The viral ancestors to several groups of clade 2.3.4.4 A(H5) viruses described herein are represented by the WHO CVV, A/Sichuan/26221/2014 (referred to as “group D” by Lee et al 16 and hereby A/Sichuan/26221/2014 group). This group of primarily A(H5N6) viruses were identified in China as early as 2010,78, 79, 80, 81 but have not been detected since 2015.
3. INFECTIONS IN MAMMALS AND IN ANIMAL MODELS
Clade 2.3.4.4 A(H5N2), A(H5N6), and A(H5N8) with genetic groups have been identified from both poultry and wild birds.82, 83 Detections of A(H5N6) viruses in cats and pigs have been reported6, 7, 8; at least two of these had epidemiological links with infected poultry or an infected human.6, 7 In one instance, an A(H5N6) virus was detected from a dead cat found in proximity to the residence of a patient infected with an A/Sichuan/26221/2014‐group A(H5N6) virus in Sichuan province, China. 6 An A(H5N6) virus was also isolated from a nasal swab taken from a pig in Guangdong province, China, in 2014 and was found to be closely related to A(H5N6) viruses isolated from ducks in the area at the same time. 7 In addition, an A(H5N6) virus isolated from a cat carcass in Zhejiang province, China, in 2016 was found to share three gene segments, HA, NA, and PA, with A/Hubei/29578/2016‐group A(H5N6) viruses co‐circulating in eastern and southern China in 2013‐2016; the other five genes were closely related to A(H9N2) and A(H7N9) viruses. 8 In contrast to the A(H5N6) viruses, natural infections of mammalian species by clade 2.3.4.4 A(H5N2) and A(H5N8) viruses have not yet been detected.
Several studies have documented the enhanced virulence of some clade 2.3.4.4 A(H5) viruses in experimentally infected mammals.84, 85, 86, 87 An A(H5N6) virus isolated from a patient who had underlying medical conditions and recovered from severe pneumonia, A/Guangzhou/39715/2014, with the E627K substitution in the PB2 protein, produced severe pneumonia in ferrets inoculated intra‐tracheally with 106 TCID50 of the virus. 86 However, in several reports, clade 2.3.4.4 A(H5) viruses showed mild disease with no mortality in experimentally inoculated ferrets.88, 89, 90, 91 Two studies showed that the pathogenicity of clade 2.3.4.4 A(H5N8) viruses in ferrets were milder than control HPAI A(H5N1) viruses.91, 92 Although the ferret model has an advantage of displaying similar clinical manifestation of influenza virus infection to those of humans, there are limited experimental data available due to disadvantages such as high cost and laborious handling. Although susceptibility to influenza virus infection of mice varies with their genetic background and its clinical manifestations are dissimilar to those of typical influenza virus infection of humans, mice are an established model to assess the pathogenicity of influenza virus. An A(H5N8) A/Fujian‐Sanyuan/21099/2017‐group virus caused 100% mortality in mice when intra‐nasally inoculated at a dose of 106.0 EID50, despite the lack of the well characterized mammalian pathogenicity markers PB2 627K and 701N. 87 Although some clade 2.3.4.4 A(H5) viruses can cause severe disease in experimentally infected mammals, several studies showed considerable variation in pathogenicity.93, 94, 95 One A/Hubei/29578/2016‐group A(H5N6) virus showed enhanced virulence in mice with a mortality rate of 80%, whereas mice infected with three other viruses of the same subtype and group survived the 14‐day observation period. 94 Similarly, Zhao et al. showed that three A(H5N6) viruses exhibited different pathogenicity in mice following intra‐nasal inoculation with 106 EID50 of virus; two viruses caused 60% mortality, whereas the other was not lethal. 95 Dogs intra‐nasally inoculated with 106 EID50 of an A(H5N6) virus shed virus for 7 days with no mortality, similar to what was observed with a control HPAI A(H5N1) virus. 96 However, the extent to which common laboratory mammalian models can predict the pathogenicity of influenza viruses in humans or even the replication in human cells remains unclear. For example, Grund et al. demonstrated that a A/Fujian‐Sanyuan/21099/2017‐group A(H5N8) virus was highly pathogenic for mice without prior adaptation; however, the same virus replicated poorly in human lung explants. 97
4. HUMAN INFECTIONS WITH CLADE 2.3.4.4 A(H5N6) VIRUS
The first human infection caused by a clade 2.3.4.4 A(H5N6) virus was reported by China in April 2014.98, 99, 100, 101, 102 As of February 2019, a total of 23 human infections with A(H5N6) viruses were reported to WHO, 16 (70%) of which had fatal outcomes. Eighteen of the total human infections (78%) were reported in 2014‐2016, one in 2017, and four in 2018. Most infections occurred in the southern China provinces. According to the self‐reported exposure history of people infected with HPAI A(H5N6) virus, 19 of 23 had exposure to poultry, which therefore suggested that contact with poultry or contaminated poultry market environments was the source of infection.100, 103, 104, 105, 106, 107, 108 The hospitalized patients initially showed influenza‐like symptoms including fever, sore throat, headache, chills, cough, and myalgia, then developed into shortness of breath due to severe pneumonia and progressed to acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF) in the deceased patients.99, 100, 101, 103, 104, 105, 106, 107, 108, 109, 110 Bi et al. indicated that A(H5N6) patients were observed to have significantly higher levels of 11 cytokines and 5 chemokines among the 48 markers tested, compared to individuals with A(H7N9) or A(H1N1)pdm09 infections. 103 No human infections with A(H5N2) or A(H5N8) viruses have been reported to date.
Although virologic surveillance is typically not designed to detect cases that are not severe such as influenza‐like illness, serologic studies can estimate the frequencies of less severe and mild infections. Two studies have looked for evidence of seroconversion to clade 2.3.4.4 A(H5) viruses in poultry farmers.111, 112 In a study involving 523 farmers exposed to poultry during the 2016‐2017 ROK A(H5N6) outbreaks, no evidence for infection was found when using a microneutralization assay to detect seropositivity. 111 In another study, 61 of 760 sera from poultry farmers in the Russian Federation had hemagglutination inhibition titers greater than 20 against an A(H5N8) virus. 112 In terms of preexisiting antibodies to A(H5) viruses in the general population, Freidl et al. were unable to detect reactivity against A(H5N1) antigens both before and after the A(H1N1)pdm09 pandemic in 6896 blood samples collected from 11 countries in Asia, Europe, and North America as tested with an HA protein microarray. 113 Zhao et al. also showed that no neutralizing antibody against the A(H5N1) virus, A/Vietnam/1194/2004, was detected among 35 healthy volunteers in China. 114 These data support the premise that there is a lack of immunity in the general population, which constitutes a significant risk, should the clade 2.3.4.4 A(H5) virus gain efficient human‐to‐human transmissibility.
The 22 A(H5N6) viruses from human cases for which genetic sequence data (GSD) are available in the EpiFlu database of GISAID were all classified as clade 2.3.4.4 A(H5) viruses. Subgroups within clade 2.3.4.4 to which the human viruses belong have changed over time: a virus collected in February 2014 belonged to the A/Sichuan/26221/2014‐like group, 20 viruses collected between April 2014 and November 2017 belonged to the A/Hubei/29578/2016 group, and a virus in the A/Fujian‐Sanyuan/21099/2017 group was detected in 2017 (Table 1). GSD of the most recent viruses are not yet available. With the exception of the 2014 virus, of available data so far, all human viruses had a NA stalk deletion at amino acid positions 58‐68, which is known to be an adaptation to terrestrial poultry and has been associated with enhanced virulence in mice presumably by altering the HA‐NA balance of the virus. Although all human infections were with A(H5N6) viruses, a number of different genotypes were involved containing a variety of internal genes originating from A(H5N1) and A(H9N2) viruses circulating in poultry, as well as A(H3) viruses circulating in ducks.98, 102, 104, 115 Multiple amino acid substitutions associated with mammalian adaptation were found in viral proteins, particularly in internal proteins. Amino acid substitutions that confer oseltamivir resistance (H274Y and N294S by N2 numbering) were not found in the human A(H5N6) virus isolates, consistent with their low frequency in avian origin viruses. Some strains of A(H5N6) from human cases did, however, have the M2 S31N mutation associated with adamantine resistance.
Table 1.
Genomic characteristics of 22 human clade 2.3.4.4 A(H5N6) viruses
| Year of isolation | Virus strainsa , b | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sichuan/26221 | Changsha/1, Gz/39715, Gd/99710 | Yunnan/0127, Yunnan/DQ001, Yunnan/DQ002, Shenzhen/1/15, Shenzhen/TH001, Yunnan/14563, Yunnan/14564, Gd/SZ872, Gd/ZQ874 | Shenzhen/TH002, Shenzhen/TH003, Shenzhen/1/16 | Anhui/33162, Anhui/33163, Hubei/29578 | Hunan/55555, Guangxi/55726 | Fujian‐Sanyuan/21099 | |||
| 2014 | 2015 | 2016 | 2017 | ||||||
| Gene | Phenotype | Amino acid position | Amino acid substitution | ||||||
| HAc | Glycosylation site at 158 | N158D | N | N | N | N | N | N | N |
| T160A | A | A | A | A | S (Anhui/33162, Anhui/33163), A (Hubei/29578) | A | A | ||
| Receptor binding specificityd | N186K | N | N | N | N | N | N | N | |
| N193K | N | N | N | N | N | D | N | ||
| Q196R | K | K | K | K | K | K | K | ||
| N224 | N | N | N | N | N | N | N | ||
| Q226L | Q | Q | Q | Q | Q | Q | Q | ||
| G228S | G | G | G | G | G | G | G | ||
| S227N | R | R | S (Shenzhen1/15, Shenzhen/TH001, Gd/SZ872), R (the others) | S | S | R | R | ||
| S227R | |||||||||
| T318I | T | T | T | T | T | T | T | ||
| Cleavage site | 335‐348 | RERRRKR | RERRRKR | RERRRKR | RERRRKR | RERRRKR | RERRRKR | REKRRKR | |
| NAe | Oseltamivir resistance | H274Y | H | H | H | H | H | H | H |
| N294S | N | N | N | N | N | N | N | ||
| Stalk deletion | No | 58–68 deletion | |||||||
| PB2 | Increased pathogenicity in mice | E627K | E | E (Changsha/1), K (Gz/39715, Gd/99710) | E (Shenzhen/1/15, Shenzhen/TH001, Gd/SZ872, Gd/ZQ874), K (the others) | K | E (Hubei/29578), K (Anhui/33162, Anhui/33163) | E | E |
| D701N | N | D | D | D | D | D | D | ||
| Q591K | Q | Q | Q | Q | Q | Q | Q | ||
| T/I271A | T | T | T | T | T | T | T | ||
| 558 V | E | E | E | E | E | E | E | ||
| PB1 | Increased replication in mammalian cells | L473V | V | V | V | V | V | V | V |
| L598P | L | L | L | L | L | L | L | ||
| PB1‐F2 | Increased pathogenicity in mice | 57 | 11 | 11 (Gd/ZQ874), 90 (the others) | 90 (Shenzhen/TH002, Shenzhen/1/16), 76 (Shenzhen/TH003) | 34 (Hubei/29578), 90 (Anhui/33162, Anhui/33163) | 90 | 90 | |
| PA | Increased replication in mice | A36T | A | A | A | A | A | A | A |
| M1 | Increased pathogenicity in mice | N30D | N | N | N | N | N | N | N |
| T215A | A | A | A | A | A | A | A | ||
| M2 | Adamantane resistance | S31N | S | S | S (Gd/ZQ874), N (the others) | N | N | S | S |
| NS | Increased pathogenicity in mice | P42S | S | S | S | S | S | S | S |
| Deletion of aa 80‐84 | Yes | Yes | Yes (Gd/ZQ874), No (the others) | No | No | Yes | Yes | ||
| Increased pathogenicity in mice | D92E | E | E | E (Gd/ZQ874), D (the others) | D | D | E | D | |
| Presence of PDZ domain | Yes | Yes | Yes (Gd/ZQ874), No (the others) | No | No | Yes | Yes | ||
Font styles represent genetic groups to which the viruses tested belong; italic underline, A/Sichuan/26221/2014 group; italic, A/Hubei/29578/2016 group; underline, A/Fujian‐Sanyuan/21099/2017 group.
Sichuan/26221, A/Sichuan/26221/2014; Gd/99710, A/Guangdong/99710/2014; Gz/39715, A/Guangzhou/39715/2014; Changsha/1, A/Changsha/1/2014; Yunnan/0127, A/Yunnan/0127/2015; Yunnan/DQ001, A/Yunnan/DQ001/2015; Yunnan/DQ002, A/Yunnan/DQ002/2015; Shenzhen/1/15, A/Shenzhen/1/2015; ShenZhen/TH001, A/ShenZhen/TH001/2015; Yunnan/14563, A/Yunnan/14563/2015; Yunnan/1456, A/Yunnan/14564/2015; Gd/SZ872, A/_Guangdong_/SZ872/2015; Gd/ZQ874, A/_Guangdong_/ZQ874/2015; Shenzhen/1/16, A/Shenzhen/1/2016; Shenzhen/TH003, A/Shenzhen/TH003/2016; Shenzhen/TH002, A/Shenzhen/TH002/2016; Hunan/55555, A/Hunan/55555/2016; Guangxi/55726, A/Guangxi/55726/2016; Hubei/29578, A/Hubei/29578/2016; Anhui/33162, A/Anhui/33162/2016; Anhui/33163, A/Anhui/33163/2016; Fujian‐Sanyuan/21099, A/Fujian‐Sanyuan/21099/2017.
Amino acid positions of HA protein are designated by H3 numbering.
Amino acid substitution Q226L/G228S and N224K/Q226L in HA are responsible to increase the ability to bind to human‐type receptors in combination.
Amino acid positions of NA protein are designated by N2 numbering.
5. RECEPTOR BINDING PROPERTIES OF CLADE 2.3.4.4 A(H5) VIRUSES
The specificity of the viral HA for the host cell receptor molecule regulates virus entry into cells. Human influenza A viruses preferentially bind to receptors with sialic acid linked to galactose by an α2,6 linkage (Sia‐α2,6Gal), which is abundantly displayed in the upper respiratory tract of humans. 116 In contrast, most avian influenza A viruses have a binding preference for receptors with Sia‐α2,3Gal, which is sparse in the upper respiratory tract of humans, but abundant in the intestinal mucosa of birds. 116 The difference in receptor binding preference is considered to be one of the main reasons why avian viruses rarely infect and transmit poorly in humans and human influenza viruses do not replicate well in birds.
Among 1994 clade 2.3.4.4 A(H5) viruses isolated between January 2013 and October 2018 with GSD available in the EpiFlu database of GISAID, 1988 (99.7%) had an HA‐160A (H3 numbering) amino acid residue and 1295 (64.8%) had HA‐227R amino acid residue (Supplementary Table 2). The HA‐160A substitution results in lack of a glycosylation motif in combination with residues 158‐160 of HA1, which facilitate airborne transmission in ferrets (HA‐N158D by Imai et al. and HA‐T160A by Herfst et al. in H3 numbering).117, 118, 119 Amino acid residues at positions 222 and 227 play important roles in binding sialyl Lewis X (3′SLeX), which is abundant on the epithelial cells of the chicken trachea. 120 Overall, there was no notable difference in the GSD of the HA receptor binding site among A(H5N2), A(H5N6), and A(H5N8) viruses despite only A(H5N6) viruses being found in human infections (Supplementary Table 2). Among the studies that examined receptor binding specificity of clade 2.3.4.4 A(H5) viruses, 13 isolates including two A(H5N2), seven A(H5N6), and four A(H5N8) viruses had receptor binding specificity for both Sia‐α2,6Gal and Sia‐α2,3Gal (Table 2). In general, the viruses that exhibited affinity for human‐type receptors also maintained a high affinity for avian‐type receptors. It is thought that a human transmissible virus could only have low affinity for the avian‐type receptor. Most of the 13 viruses with dual receptor specificity had HA amino acids 128P, 137A, and 160A, but not all viruses possessing these amino acids had dual‐receptor specificity (Table 2). Additional amino acid substitutions needed to cross the species barrier likely vary with the makeup of the HA gene. Biophysical assays such as glycan arrays, solid‐phase binding assays, and HA assays using sialidase‐treated red blood cells have been mainstream methods to analyze the receptor binding specificity of influenza viruses. Virus tropism in ex vivo cultures of human bronchus has also been suggested to be an alternative experimental model to assess receptor binding of animal viruses to the human respiratory tract. 121 Only a few glycans present in glycan arrays are present on the human respiratory tract. 122 Similarly, A/environment/Korea/W541/2016 (H5N6), although not possessing known molecular markers associated with mammalian adaptation (namely PB2 627K, 271A, 590S, 591R, 147T, 339T, or 588T), replicated well in human NHBE cells and ex‐vivo lung tissues. 84 Moreover, A/Guangzhou/39715/2014 A(H5N6), which was shown to predominantly bind to Sia‐α2,3 Gal and possessed PB2 627K, grew comparably to an A(H1N1)pdm09 virus in ex vivo human bronchus and lung culture 123 (Table 2). A/Fujian‐Sanyuan/21099/2017‐like A(H5N8) viruses, in contrast, replicated poorly in ex vivo cultures of human lung explants. 97
Table 2.
Receptor binding specificities of the clade 2.3.4.4 A(H5) virus characterized by biophysical assays
| Reference | Strain namec | Subtype | Method | Resultsb | Avian | Amino acid residue in H3 numbering | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 101 | 128 | 137 | 158 | 160 | 193 | 196 | 224 | 226 | 227 | 228 | 318 | |||||||
| Amino acid substitutiona | ||||||||||||||||||
| D | S | S | N | T | N | Q | N | Q | S | G | T | |||||||
| SA‐a2,6Gal | SA‐a2,3Gal | Human | N | P | A | D | A | K | R/H | K | L | N/R | S/A | I | ||||
| Li et al. 124 | A/duck/Eastern China/1111/2011 | H5N2 | Solid phase binding assay | ○ | ○ | N | P | A | A | K | R | |||||||
| A/goose/Eastern China/1112/2011 | ○ | ○ | N | P | A | A | K | R | ||||||||||
| Kaplan et al. 88 | A/Northern pintail/Washington/40964/2014 | × | ○ | T | P | A | A | K | ||||||||||
| Yang et al. 136 | Glycanarray | × | ○ | T | P | A | A | K | ||||||||||
| Kwon et al. 84 | A/environment/Korea/W541/2016 | H5N6 | Solid phase binding assay | × | ○ | N | P | A | A | K | Q | |||||||
| Sun et al. 94 | A/duck/Anhui/S4/2016 | ○ | ○ d | N | P | A | A | K | R | |||||||||
| A/goose/Yangzhou/YZ587/2016 | ○ | ○ d | N | P | A | A | K | R | ||||||||||
| A/Chicken/Guangdong/GD1602/2016 | ○ | ○ d | N | P | A | A | K | |||||||||||
| A/Chicken/Xuzhou/XZ6/2016 | ○ | ○ d | N | P | A | A | K | R | ||||||||||
| Liu et al. 137 | A/chicken/Anhui/MZ33/2016 | ○ | ○ | N | P | A | A | K | ||||||||||
| A/ chicken/Anhui/MZ34/2016 | ○ | ○ | N | P | A | A | K | |||||||||||
| A/chicken/Henan/YB0597/2016 | ○ | ○ | N | P | A | A | K | |||||||||||
| Herfst et al. 86 | A/Guangzhou/39715/2014 | Sialidase‐treated HA assay | × | ○ | N | P | A | K | ||||||||||
| Hui et al. 123 | × | ○ | ||||||||||||||||
| Yang et al. 136 | A/Sichuan/26221/2014 | Glycanarray | × | ○ | N | T | A | A | K | R | ||||||||
| Li et al. 124 | A/duck/Jiangsu/k1203/2010 | H5N8 | Solid phase binding assay | ○ | ○ | S | P | A | A | K | R | |||||||
| Kwon et al. 84 | A/Common teal/Korea/W555/2017 | × | ○ | S | P | A | A | K | Q | |||||||||
| Kaplan et al. 88 | A/mallard/Korea/W452/2014 | × | ○ | T | P | A | A | K | R | |||||||||
| A/chicken/Kumamoto/1–7/2014 | × | ○ | T | P | A | A | K | R | ||||||||||
| A/duck/England/36254/2014 | × | ○ | T | P | A | A | K | R | ||||||||||
| A/gyrfalcon/Washington/41088–6/2014 | × | ○ | T | P | A | A | K | |||||||||||
| Yang et al. 136 | Glycanarray | × | ○ | |||||||||||||||
| Li et al. 124 | A/duck/Shandong/Q1/2013 | Solid phase binding assay | ○ | ○ | S | P | A | A | K | R | ||||||||
| Fan et al. 87 | A/mallard duck/Shanghai/SH‐9/2013 | × | ○ | S | L | A | A | K | R | |||||||||
| Wang et al. 93 | A/goose/Eastern China/CZ/2013 | Sialidase‐treated HA assay | ○ | ○ | S | L | A | A | K | R | ||||||||
| A/duck/Eastern China/JY/2014 | ○ | ○ | S | P | A | A | K | R | ||||||||||
Dots represent amino acid residues that are conserved among avian species.
○ denotes the virus tested bound to SA‐a2,3Gal or SA‐a2,6Gal; × denotes the virus tested did not bind to SA‐a2,3Gal or SA‐a2,6Gal.
Font styles represent genetic groups to which the viruses tested belong; italic underline, A/Sichuan/26221/2014 group; bold, A/gyrfalcon/Washington/41088‐6/2014 group; italic, A/Hubei/29578/2016 group; underline, A/Fujian‐Sanyuan/21099/2017 group.
The virus tested showed the dual specificity both to SA‐a2,3Gal and SA‐a2,6Gal with stronger affinity to SA‐a2,3Gal than SA‐a2,6Gal.
6. ASSESSMENT OF THE TRANSMISSIBILITY OF THE CLADE 2.3.4.4 A(H5) VIRUSES IN ANIMAL MODELS
Several studies have been conducted to assess the transmissibility of the clade 2.3.4.4 A(H5) viruses. These included assessing direct contact and respiratory droplet transmission using multiple animal models, namely ferrets, pigs, guinea pigs, and dogs84, 86, 88, 89, 90, 91, 92, 95, 96, 97, 124, 125 (Table 3). Five clade 2.3.4.4 A(H5) viruses, including one of which preferentially bound to Sia‐α2,3 Gal 84 and one of which showed dual‐receptor specificity, 124 were transmitted via direct contact in guinea pig or ferret models84, 95, 124, 125 (Table 3). An A(H5N6) virus A/environment/Korea/W541/2016, which grew well in human cells despite having strong affinity to avian‐type receptors, transmitted to two of three ferrets co‐housed with infected animals. 84 The high proliferation competency of this virus strain in human NHBE cells and ex vivo lung tissues might have facilitated its transmission via direct contact. Herft et al. demonstrated that the HA of A/Guangzhou/39715/2014 A(H5N6) showed less acid stability than an A/Indonesia/5/2005 A(H5N1) virus adapted for airborne‐transmission between ferrets and an H3N2 seasonal influenza virus, A/Netherlands/213/2003. 86 Correspondingly, A/Guangzhou/39715/2014 A(H5N6), which exclusively bound to Sia‐α2,3 Gal, did not transmit among ferrets via respiratory droplets. The individual infected with this A(H5N6) virus had underlying disease and exposure to infected poultry which might have promoted virus replication competency in human cells and infection. Airborne or respiratory droplet transmission of clade 2.3.4.4 A(H5N2), A(H5N6), and A(H5N8) viruses has not been demonstrated in any animal model examined, which is consistent with the epidemiology of the virus in humans (showing no evidence of human‐to‐human spread) (Table 3).
Table 3.
Transmission of clade 2.3.4.4 A(H5) viruses in mammalian animal models
| Reference | Strain namec | Results of receptor binding propertya | Result of transmission studiesb | ||||
|---|---|---|---|---|---|---|---|
| Subtype | SA‐a2,6Gal | SA‐a2,3Gal | Animal model assessed | Direct contact | Respiratory droplet | ||
| Li et al. 124 | A/goose/Eastern China/1112/2011 | H5N2 | ○ | ○ | Guinea Pig | ○ | N/A |
| Pulit‐Penaloza et al. 89 | A/northern pintail/Washington/40964/2014 | × | ○ | Ferret | × | N/A | |
| Kaplan et al. 88 | × | ○ | Ferret | × | N/A | ||
| × | ○ | Pig | × | N/A | |||
| A/turkey/Minnesota/7172‐1/2015 | N/A | N/A | Pig | × | N/A | ||
| Sun et al. 125 | A/duck/Eastern China/S0711/2014 | H5N6 | N/A | N/A | Ferret | ○ | × |
| Kwon et al. 84 | A/Common teal/Korea/W555/2017 | × | ○ | Ferret | × | × | |
| Sun et al. 125 | A/goose/Eastern China/S0513/2013 | N/A | N/A | Ferret | ○ | × | |
| Noh et al. 90 | A/Mandarin duck/Korea/K16‐187‐3/2016 | N/A | N/A | Ferret | × | × | |
| Kwon et al. 84 | A/environment/Korea/W541/2016 | × | ○ | Ferret | ○ | × | |
| Herfst et al. 86 | A/Guangzhou/39715/2014 | × | ○ | Ferret | N/A | × | |
| Zhao et al. 95 | A/duck/Hubei/XY‐01/2016 | N/A | N/A | Guinea Pig | × | × | |
| A/chicken/Hubei/XY‐165/2016 | N/A | N/A | Guinea Pig | ○ | × | ||
| A/chicken/Hubei/XY‐918/2016 | N/A | N/A | Guinea Pig | × | × | ||
| Lyoo et al. 96 | A/chicken/VN/LangSon/P140450/2014 | N/A | N/A | Dogs | × | ||
| Pulit‐Penaloza et al. 89 | A/Gyrfalcon/Washington/41088‐6/2014 | H5N8 | × | ○ | Ferret | × | N/A |
| × | ○ | Pig | × | N/A | |||
| Kaplan et al. 88 | A/chicken/Kumamoto/1‐7/2014 | × | ○ | Ferret | × | N/A | |
| A/duck/England/36254/2014 | × | ○ | Ferret | × | N/A | ||
| Richard et al. 91 | A/Chicken/Netherlands/EMC‐3/2014 | N/A | N/A | Ferret | N/A | × | |
| Grund et al. 97 | A/tufted duck/Germany/AR8444‐L01987/2016 | N/A | N/A | Ferret | × | N/A | |
| Kaplan et al. 88 | A/mallard/Korea/W452/2014 | × | ○ | Ferret | × | N/A | |
| Kim et al. 92 | × | ○ | Ferret | N/A | × | ||
○ denotes the virus tested bound to SA‐a2,3Gal or SA‐a2,6Gal; × denotes the virus tested did not bind to SA‐a2,3Gal or SA‐a2,6Gal; N/A, not assessed.
○ denotes that transmission by direct contact or respiratory droplets; × denotes that transmission by direct contact or respiratory droplet was not observed; N/A, not assessed.
Font styles represent genetic groups to which the viruses tested belong; italic underline, A/Sichuan/26221/2014 group; bold, A/gyrfalcon/Washington/41088‐6/2014 group; italic, A/Hubei/29578/2016 group; underline, A/Fujian‐Sanyuan/21099/2017 group.
Receptor binding affinity is a prerequisite, but insufficient alone to promote airborne transmission of A(H5) avian viruses. Several studies have shown that compensatory mutations in HA are required to counteract the HA instability caused by human‐type receptor‐binding mutations.117, 118, 126 Additional mutations are also involved to increase viral proliferation and transcription.117, 119 Identified compensatory mutations to enhance thermostability and facilitate membrane fusion at a lower pH are located in both the globular head and stalk regions of the HA.119, 127 Chen et al. suggested that optimization of HA, NA, and internal genes is a requirement for efficient transmission. 128 They demonstrated that an A(H5N1) reassortant virus with Sia‐α2,6 Gal preferential binding (amino acid substitutions Q196R, Q226L, G228S) coupled with the NA of a human seasonal A(H3N2) virus was transmitted via respiratory droplets among ferrets, whereas the same virus combined with the NA of the avian A(H5N1) virus was not transmitted. 128 Furthermore, internal genes also contribute undetermined functions that lead to efficient transmission. Zhang et al. demonstrated that the NS gene of the A(H1N1)pdm09 virus enabled a reassorted A(H5N1) virus to efficiently transmit among guinea pigs via respiratory droplets but the avian NS gene did not. 126 A scenario in which a clade 2.3.4.4 A(H5) virus reassorts with a human seasonal influenza virus may facilitate transmission among mammals, although further adaptations would likely be needed for optimal spread. Taken all together, and reassuringly, clade 2.3.4.4 A(H5N2), A(H5N6), and A(H5N8) viruses have so far shown limited ability to infect and transmit efficiently in mammalian species.
7. DISCUSSION
The experimental data generated to date has not detected differences in receptor binding specificity and transmission capability among mammals between clade 2.3.4.4 A(H5N2), A(H5N6), and A(H5N8) viruses despite that only the A(H5N6) subtype of the clade 2.3.4.4 A(H5) viruses among three has been found in humans. Dual‐receptor binding specificity, viruses that show equal binding in vitro to both human and avian receptor analogues, has been observed in viruses of all three subtypes, and some of the viruses were transmitted via direct contact among ferrets or guinea pigs. However, no studies have identified receptor binding profiles showing a preference for binding to human receptor analogues, or animal model transmission patterns, showing spread via the aerosol route, consistent with a virus adapted to transmit in humans. What is less clear is precisely which molecular changes would lead to such adaptation.
A/Hubei/29578/2016‐group clade 2.3.4.4 A(H5) viruses, which are primarily A(H5N6) viruses, have been confined to Asia. In contrast, A/gyrfalcon/Washington/41088‐6/2014‐group A(H5N2) and A(H5N8) viruses and A/Fujian‐Sanyuan/21099/2017‐group A(H5N8) viruses spread from Asia to North America, Europe, the Middle East, and the African continent and gave rise to numerous outbreaks among poultry and wild birds following reassortment with viruses from local avian species. Despite significant exposure to A(H5N2), A(H5N6) or A(H5N8) infected poultry, so far only the A(H5N6) subtype viruses have caused human infection. What then differentiates the zoonotic potential of the A(H5N6) viruses from that of the other two subtypes?
Several possible reasons can be considered here. First, biosecurity systems vary across countries. A/Fujian‐Sanyuan/21099/2017‐group A(H5N8) and A/gyrfalcon/Washington/41088‐6/2014‐group A(H5N2) and A(H5N8) viruses were detected in poultry in multiple places in the United States and in Europe, resulting in severe impacts on the poultry industries. The majority of affected countries executed a systematic stamping‐out strategy. 129 If outbreaks of the same magnitude as the A(H5N2) outbreaks in the United States during 2014‐2015 had happened in regions where biosecurity and precautionary strategies were less stringent, the risks of human infection might have been higher. Perhaps more importantly, live poultry markets, which are a significant source of human exposure in Asia, are rare in the United States and Europe, limiting highly contaminated environments inhabited by birds with humans in close contact. Second, more controversially, inadequate or improper vaccination in poultry can also complicate eradication of HPAI. Between 2002 and 2010, 15 countries implemented vaccination in poultry against HPAI A(H5N1) or A(H7) avian influenza viruses as food security and animal health measures within a long‐term control program. 130 In one example, when vaccines were antigenically similar with the targeted A(H5N1) viruses and were properly applied with production of a protective immune response in ≥60% of the poultry population, a reduction in virus infection and transmission was achieved and outbreaks declined. 131 When a protective immune response was produced in <60% of the poultry population or the vaccine was antigenically less similar to the field viruses, the A(H5N1) viruses were able to breakthrough vaccinated population and result in additional outbreaks. 131 In the latter scenario, A(H5N1) infected birds with no disease signs had been sent to market, resulting in infection and propagation of the virus within the market environment raising the risk of human infection. In 2017, after the emergence of the HPAI A(H7N9) variants, China added an H7 antigen to the existing monovalent H5 vaccines used in poultry. The bivalent H5/H7 vaccine was introduced in Guangdong and Guangxi provinces in July 2017, followed by introduction into other regions by the winter of 2017‐2018. The number of reported human A(H7N9) cases were reduced by 92% after the enhanced poultry vaccination campaign, and only one human H7N9 infection has been reported to WHO since 2019 to the present. 132 However, some countries lack the financial and human resources for a comprehensive stamping‐out program.
Last, although different exposures to infected poultry may have contributed to human infections with the specific subtype A(H5N6), it is also possible that biological features of A(H5N6) viruses also contributed to occurrence of human infections. In a study conducted by Chen et al., an A(H5N1) reassortant virus with several amino acid substitutions in HA and the NA gene of a human seasonal A(H3N2) virus (A/Brisbane/10/2007) was transmitted via respiratory droplets between ferrets, but an A(H5N1) reassortant virus with the same HA gene and the NA gene of a human seasonal A(H1N1) virus (A/Brisbane/59/2007) was not transmitted. 128 Additional studies, including gain‐of‐function (GOF) research, are instrumental to better elucidate the potential mechanism that allows some viruses to cross species barriers.
The importance of continued monitoring of the ecology and ongoing evolution of potentially zoonotic avian influenza viruses should not be underestimated. Some of the fundamental and important activities such as surveillance programs in diverse animal reservoirs, including wildlife, are not always a high priority and properly funded. Several studies on poultry outbreaks caused by clade 2.3.4.4 A(H5) viruses in the United States and the Netherlands suggested that the viruses were introduced from wild birds rather than farm‐to‐farm transmissions.29, 133 In a review, Morin et al. has warned that accelerated warming of the Arctic by climate change has the potential to affect migratory patterns, the timing of biological events, and habitats of migratory birds, resulting in the potential to impact virus transmission dynamics among avian species. 134 The surveillance activities should incorporate a component of how environmental changes may affect influenza virus hosts and the distribution and genomic constellations of influenza A viruses.
8. CONCLUSION
Because their emergence, the clade 2.3.4.4 HPAI A(H5) viruses have evolved through point mutations and reassortment with circulating local viruses following global expansion via distinct pathways. So far, these viruses have caused only sporadic human infections and are unable to transmit efficiently among humans. Studies have shown that some clade 2.3.4.4 A(H5) viruses have dual‐receptor specificity and can transmit between ferrets in direct contact. Furthermore, some A(H5N6) viruses isolated from humans have molecular signatures related to mammalian adaptation. It is uncertain what other changes are necessary for these viruses to become transmissible among humans. Their widespread distribution, ongoing evolution, and periodic infection of mammalian hosts increase the chances that efficient transmissibility is possible to be acquired. This calls for surveillance of influenza viruses in domestic and wild birds to be enhanced to allow for timely development and updating of veterinary and public health countermeasures and to reduce the threats of zoonotic and pandemic influenza.
9. GENOMIC ANALYSIS
HA genetic sequence data (GSD) of HPAI viruses isolated from animals, including mammals and avian species, that possessed multibasic amino acids at the HA cleavage site between 2013 and February 2019 and available in the EpiFlu database of Global Initiative on Sharing All Influenza Data (GISAID) were analyzed. Between 2013 and February 2019, 2553 A(H5) HPAI viruses were available. The open reading frame of the HA genes of A(H5) viruses was used for phylogenetic analysis. Multiple sequence alignment of H5 viruses was performed using BioEdit 7.2. A maximum‐likelihood tree was constructed for MEGA 7 with 1000 replicates. The 242 virus GSDs were used as the reference for the nomenclature of A(H5) HA systematized by World Health Organization/World Organization for Animal Health/Food and Agriculture Organization (WHO/OIE/FAO) H5 Evolution Working Group. 135 The phylogenetic tree is available upon request. Among the 2553 H5 viruses, 1994 H5 viruses, which belonged to clade 2.3.4.4, were used further analysis.
CONFLICT OF INTERESTS
JSMP has received research funding from Crucell NV and is ad‐hoc consultant for GlaxoSmithKline and Sanofi. YK has received speaker's honoraria from Toyama Chemical and Astellas; grant support from Chugai, Daiichi Sankyo, Toyama Chemicals, Tauns, Tsumura, and Denka Seiken and is a co‐founder of FluGen.
AUTHORS' CONTRIBUTIONS
RY drafted the manuscript and constructed the tables and figures with contributions from MDS, CTD, DES, DW, FYKW, JWM, JSMP, RJW, RAMF, YK, and WZ in reviewing results, reviewing the manuscript, and providing suggestions. RY, MDS, and WZ managed the project.
Supporting information
Supplementary Table 1 A(H5N6) viruses with the A/Fujian‐Sanyuan/21099/2017‐like HA gene for which sequences are available in the EpiFlu database of GISAID
Supplementary Table 2 Amino acid substitutions related to receptor binding specificity among clade 2.3.4.4 A(H5) avian viruses available from GISAID as of February 2019
ACKNOWLEDGEMENT
We thank all 21 technical experts: Alexander Ryzhikov (State Research Center of Virology and Biotechnology VECTOR), Bin Zhou (Centers for Disease Control and Prevention, USA), Chengjun Li (Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin, China), Cornelia Adlhoch (European Centre for Disease Prevention and Control), Gabrielle Nuemann (University of Wisconsin‐Madison), Gounalan Pavade (World Organization for Animal Health (OIE)), Ian G. Barr (WHO Collaborating Centre for Reference and Research on Influenza, Victorian Infectious Diseases Reference Laboratory, Peter Doherty Institute for Infection and Immunity, Australia), James Kile (Centers for Disease Control and Prevention, USA), Juliana Leite (Pan American Health Organization), Philip Gould (WHO South‐East Asia Region), Shu Yuelong (Chinese Center for Disease Control and Prevention, China, Sun Yat‐sen University, China), Pasi Penttinen (European Centre for Disease Prevention and Control), Sonja Olsen (WHO Regional Office for Europe), Sophie Von Dobschuetz (Food and Agriculture Organization of the United Nations (FAO), Italy), Sylvie van der Werf (Institut Pasteur, France), Tsutomu Kageyama (National Institute of Infectious Diseases, Japan), Yang Lei (Chinese Center for Disease Control and Prevention, Beijing, China), Yoshihiro Sakoda (Hokkaido University, Japan), Yuzo Arima (Infectious Disease Surveillance Center, Japan), Zhibin Peng (Chinese Center for Disease Control and Prevention, China), Zhu Wenfei (Chinese Center for Disease Control and Prevention, China) (listed alphabetically), who participated in the WHO risk assessment of clade 2.3.4.4 HPAI A(H5N6), A(H5N2), and A(H5N8) with the Tool for Influenza Pandemic Risk Assessment (TIPRA) between September and December 2018. We also acknowledge the Global Initiative on Sharing All Influenza Data (GISAID) for the EpiFlu database and other GSDs which were used to share gene sequences and associated information.
Yamaji R, Saad MD, Davis CT, et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev Med Virol. 2020;30:e2099. 10.1002/rmv.2099
Funding information Centers for Disease Control and Prevention; World Health Organization
REFERENCES
- 1. Wahlgren J. Influenza A viruses: an ecology review. Infect Ecol Epidemiol. 2011;1. 10.3402/iee.v1i0.6004. Epub 2011 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organization WH. Tool for Influenza Pandemic Risk Assessment (TIPRA) . 2016; https://www.who.int/influenza/areas_of_work/human_animal_interface/tipra/en/. Accessed 18 February, 2019.
- 3. Centers for Disease Control and Prevention. Influenza Risk Assessment Tool (IRAT) . 2016; https://www.cdc.gov/flu/pandemic-resources/national-strategy/risk-assessment.htm. Accessed 4 March, 2019.
- 4. Anderson T, Capua I, Dauphin G, et al. FAO‐OIE‐WHO joint technical consultation on avian influenza at the human‐animal Interface. Influenza Other Respi Viruses. 2010;4(Suppl 1):1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Organization WH . Avian and other zoonotic influenza. https://www.who.int/influenza/human_animal_interface/en/. Accessed 21 Dec 2018.
- 6. Yu Z, Gao X, Wang T, et al. Fatal H5N6 avian influenza virus infection in a domestic cat and wild birds in China. Sci Rep. 2015;5:10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X, Fu Y, Yang J, et al. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infect Genet Evol. 2015;36:462‐466. [DOI] [PubMed] [Google Scholar]
- 8. Cao X, Yang F, Wu H, Xu L. Genetic characterization of novel reassortant H5N6‐subtype influenza viruses isolated from cats in eastern China. Arch Virol. 2017;162(11):3501‐3505. [DOI] [PubMed] [Google Scholar]
- 9. Gu M, Liu W, Cao Y, et al. Novel reassortant highly pathogenic avian influenza (H5N5) viruses in domestic ducks, China. Emerg Infect Dis. 2011;17(6):1060‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou LC, Liu J, Pei EL, et al. Novel avian influenza A(H5N8) viruses in migratory birds, China, 2013‐2014. Emerg Infect Dis. 2016;22(6):1121‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Si YJ, Choi WS, Kim YI, et al. Genetic characteristics of highly pathogenic H5N8 avian influenza viruses isolated from migratory wild birds in South Korea during 2014‐2015. Arch Virol. 2016;161(10):2749‐2764. [DOI] [PubMed] [Google Scholar]
- 12. Kim SH, Hur M, Suh JH, et al. Molecular characterization of highly pathogenic avian influenza H5N8 viruses isolated from Baikal teals found dead during a 2014 outbreak in Korea. J Vet Sci. 2016;17(3):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee YJ, Kang HM, Lee EK, et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis. 2014;20(6):1087‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeong J, Kang HM, Lee EK, et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet Microbiol. 2014;173(3–4):249‐257. [DOI] [PubMed] [Google Scholar]
- 15. Organization WH . Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. 2019; https://www.who.int/influenza/vaccines/virus/201902_zoonotic_vaccinevirusupdate.pdf?ua=1. Accessed 29 March, 2019.
- 16. Lee DH, Bertran K, Kwon JH, Swayne DE. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J Vet Sci. 2017;18(S1):269‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramey AM, Hill NJ, Cline T, et al. Surveillance for highly pathogenic influenza A viruses in California during 2014‐2015 provides insights into viral evolutionary pathways and the spatiotemporal extent of viruses in the Pacific Americas flyway. Emerg Microb Infect. 2017;6(9):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ip HS, Torchetti MK, Crespo R, et al. Novel Eurasian highly pathogenic avian influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg Infect Dis. 2015;21(5):886‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee DH, Torchetti MK, Winker K, Ip HS, Song CS, Swayne DE. Intercontinental spread of Asian‐origin H5N8 to North America through Beringia by migratory birds. J Virol. 2015;89(12):6521‐6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bevins SN, Dusek RJ, White CL, et al. Widespread detection of highly pathogenic H5 influenza viruses in wild birds from the Pacific flyway of the United States. Sci Rep. 2016;6:28980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verhagen JH, Herfst S, Fouchier RA. Infectious disease. How a virus travels the world. Science. 2015;347(6222):616‐6177. [DOI] [PubMed] [Google Scholar]
- 22. Global Consortium for HN, Related Influenza V . Role for migratory wild birds in the global spread of avian influenza H5N8. Science. 2016;354(6309):213‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pasick J, Berhane Y, Joseph T, et al. Reassortant highly pathogenic influenza A H5N2 virus containing gene segments related to Eurasian H5N8 in British Columbia, Canada, 2014. Sci Rep. 2015;5:9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee DH, Bahl J, Torchetti MK, et al. Highly pathogenic avian influenza viruses and generation of novel reassortants, United States, 2014‐2015. Emerg Infect Dis. 2016;22(7):1283‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krauss S, Stallknecht DE, Slemons RD, et al. The enigma of the apparent disappearance of Eurasian highly pathogenic H5 clade 2.3.4.4 influenza A viruses in North American waterfowl. Proc Natl Acad Sci U S A. 2016;113(32):9033‐9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jennelle CS, Carstensen M, Hildebrand EC, et al. Surveillance for highly pathogenic avian influenza in wild turkeys ( Meleagris gallopavo ) of Minnesota, USA during 2015 outbreaks in domestic poultry. J Wildl Dis. 2017;53(3):616‐620. [DOI] [PubMed] [Google Scholar]
- 27. Jennelle CS, Carstensen M, Hildebrand EC, et al. Surveillance for highly pathogenic avian influenza virus in wild birds during outbreaks in domestic poultry, Minnesota, 2015. Emerg Infect Dis. 2016;22(7):1278‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu W, Berhane Y, Dube C, et al. Epidemiological and evolutionary inference of the transmission network of the 2014 highly pathogenic avian influenza H5N2 outbreak in British Columbia, Canada. Sci Rep. 2016;6:30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee DH, Torchetti MK, Hicks J, et al. Transmission dynamics of highly pathogenic avian influenza virus A(H5Nx) clade 2.3.4.4, North America, 2014‐2015. Emerg Infect Dis. 2018;24(10):1840‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee DH, Torchetti MK, Killian ML, DeLiberto TJ, Swayne DE. Reoccurrence of avian influenza A(H5N2) virus clade 2.3.4.4 in wild birds, Alaska, USA, 2016. Emerg Infect Dis. 2017;23(2):365‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harder T, Maurer‐Stroh S, Pohlmann A, et al. Influenza a(H5N8) virus similar to strain in Korea causing highly pathogenic avian influenza in Germany. Emerg Infect Dis. 2015;21(5):860‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanna A, Banks J, Marston DA, Ellis RJ, Brookes SM, Brown IH. Genetic characterization of highly pathogenic avian influenza (H5N8) virus from domestic ducks, England, November 2014. Emerg Infect Dis. 2015;21(5):879‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bouwstra RJ, Koch G, Heutink R, et al. Phylogenetic analysis of highly pathogenic avian influenza A(H5N8) virus outbreak strains provides evidence for four separate introductions and one between‐poultry farm transmission in The Netherlands, November 2014. Euro Surveill. 2015;20(26):pii: 21174. [DOI] [PubMed] [Google Scholar]
- 34. Nunez A, Brookes SM, Reid SM, et al. Highly pathogenic avian influenza H5N8 clade 2.3.4.4 virus: equivocal pathogenicity and implications for surveillance following natural infection in breeder ducks in the United Kingdom. Transbound Emerg Dis. 2016;63(1):5‐9. [DOI] [PubMed] [Google Scholar]
- 35. Bouwstra R, Heutink R, Bossers A, Harders F, Koch G, Elbers A. Full‐genome sequence of influenza A(H5N8) virus in poultry linked to sequences of strains from Asia, The Netherlands, 2014. Emerg Infect Dis. 2015;21(5):872‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Council of Agriculture, Executive Yuan. 2019; https://ai.gov.tw/. Accessed 7 March, 2019.
- 37. Kanehira K, Uchida Y, Takemae N, Hikono H, Tsunekuni R, Saito T. Characterization of an H5N8 influenza A virus isolated from chickens during an outbreak of severe avian influenza in Japan in April 2014. Arch Virol. 2015;160(7):1629‐1643. [DOI] [PubMed] [Google Scholar]
- 38. Lee DH, Sharshov K, Swayne DE, et al. Novel Reassortant clade 2.3.4.4 avian influenza A(H5N8) virus in wild aquatic birds, Russia, 2016. Emerg Infect Dis. 2017;23(2):359‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Voronina OL, Ryzhova NN, Aksenova EI, et al. Genetic features of highly pathogenic avian influenza viruses A(H5N8), isolated from the European part of The Russian Federation. Infect Genet Evol. 2018;63:144‐150. [DOI] [PubMed] [Google Scholar]
- 40. Marchenko VY, Susloparov IM, Komissarov AB, et al. Reintroduction of highly pathogenic avian influenza A/H5N8 virus of clade 2.3.4.4. in Russia. Arch Virol. 2017;162(5):1381‐1385. [DOI] [PubMed] [Google Scholar]
- 41. Li M, Liu H, Bi Y, et al. Highly pathogenic avian influenza A(H5N8) virus in wild migratory birds, Qinghai Lake, China. Emerg Infect Dis. 2017;23(4):637‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verhagen JH, van der Jeugd HP, Nolet BA, et al. Wild bird surveillance around outbreaks of highly pathogenic avian influenza A(H5N8) virus in The Netherlands, 2014, within the context of global flyways. Euro Surveill. 2015;20(12):pii: 21069. [DOI] [PubMed] [Google Scholar]
- 43. Wade A, Jumbo SD, Zecchin B, et al. Highly pathogenic avian influenza A(H5N8) virus, Cameroon, 2017. Emerg Infect Dis. 2018;24(7):1367‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Twabela AT, Tshilenge GM, Sakoda Y, et al. Highly pathogenic avian influenza A(H5N8) virus, Democratic Republic of the Congo, 2017. Emerg Infect Dis. 2018;24(7):1371‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ndumu D, Zecchin B, Fusaro A, et al. Highly pathogenic avian influenza H5N8 clade 2.3.4.4B virus in Uganda, 2017. Infect Genet Evol. 2018;66:269‐271. [DOI] [PubMed] [Google Scholar]
- 46. Nagarajan S, Kumar M, Murugkar HV, et al. Novel Reassortant highly pathogenic avian influenza (H5N8) virus in Zoos, India. Emerg Infect Dis. 2017;23(4):717‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu YP, Lee DH, Chen LH, et al. Detection of reassortant H5N6 clade 2.3.4.4 highly pathogenic avian influenza virus in a black‐faced spoonbill (Platalea minor) found dead, Taiwan, 2017. Infect Genet Evol. 2018;62:275‐278. [DOI] [PubMed] [Google Scholar]
- 48. Woo C, Kwon JH, Lee DH, et al. Novel reassortant clade 2.3.4.4 avian influenza A (H5N8) virus in a grey heron in South Korea in 2017. Arch Virol. 2017;162(12):3887‐3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ma L, Jin T, Wang H, et al. Two reassortant types of highly pathogenic H5N8 avian influenza virus from wild birds in Central China in 2016. Emerg Microb Infect. 2018;7(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee YN, Cheon SH, Kye SJ, et al. Novel reassortants of clade 2.3.4.4 H5N6 highly pathogenic avian influenza viruses possessing genetic heterogeneity in Republic of Korea in late 2017. J Vet Sci. 2018;19(6):850‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beerens N, Koch G, Heutink R, et al. Novel highly pathogenic avian influenza A(H5N6) virus in The Netherlands, December 2017. Emerg Infect Dis. 2018;24(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fusaro A, Monne I, Mulatti P, et al. Genetic diversity of highly pathogenic avian influenza A(H5N8/H5N5) viruses in Italy, 2016‐17. Emerg Infect Dis. 2017;23(9):1543‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pohlmann A, Starick E, Grund C, et al. Swarm incursions of reassortants of highly pathogenic avian influenza virus strains H5N8 and H5N5, clade 2.3.4.4b, Germany, winter 2016/17. Sci Rep. 2018;8(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beerens N, Heutink R, Bergervoet SA, Harders F, Bossers A, Koch G. Multiple Reassorted viruses as cause of highly pathogenic avian influenza A(H5N8) virus epidemic, The Netherlands, 2016. Emerg Infect Dis. 2017;23(12):1974‐1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Poen MJ, Bestebroer TM, Vuong O, et al. Local amplification of highly pathogenic avian influenza H5N8 viruses in wild birds in The Netherlands, 2016 to 2017. Euro Surveill. 2018;23(4). 10.2807/1560-7917.ES.2018.23.4.17-00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kleyheeg E, Slaterus R, Bodewes R, et al. Deaths among wild birds during highly pathogenic avian influenza A(H5N8) virus outbreak, The Netherlands. Emerg Infect Dis. 2017;23(12):2050‐2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mulatti P, Fusaro A, Scolamacchia F, et al. Integration of genetic and epidemiological data to infer H5N8 HPAI virus transmission dynamics during the 2016‐2017 epidemic in Italy. Sci Rep. 2018;8(1):18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salaheldin AH, El‐Hamid HS, Elbestawy AR, et al. Multiple introductions of influenza A(H5N8) virus into poultry, Egypt, 2017. Emerg Infect Dis. 2018;24(5). 10.3201/eid2405.171935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yehia N, Naguib MM, Li R, et al. Multiple introductions of reassorted highly pathogenic avian influenza viruses (H5N8) clade 2.3.4.4b causing outbreaks in wild birds and poultry in Egypt. Infect Genet Evol. 2018;58:56‐65. [DOI] [PubMed] [Google Scholar]
- 60. Ghafouri SA, GhalyanchiLangeroudi A, Maghsoudloo H, et al. Clade 2.3.4.4 avian influenza A (H5N8) outbreak in commercial poultry, Iran, 2016: the first report and update data. Tropl Anim Health Prod. 2017;49(5):1089‐1093. [DOI] [PubMed] [Google Scholar]
- 61. Selim AA, Erfan AM, Hagag N, et al. Highly pathogenic avian influenza virus (H5N8) clade 2.3.4.4 infection in migratory birds, Egypt. Emerg Infect Dis. 2017;23(6):1048‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Al‐Ghadeer H, Chu DKW, Rihan EMA, et al. Circulation of influenza A(H5N8) virus, Saudi Arabia. Emerg Infect Dis. 2018;24(10):1961‐1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pohlmann A, Starick E, Harder T, et al. Outbreaks among wild birds and domestic poultry caused by reassorted influenza A(H5N8) clade 2.3.4.4 viruses, Germany, 2016. Emerg Infect Dis. 2017;23(4):633‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim YI, Park SJ, Kwon HI, et al. Genetic and phylogenetic characterizations of a novel genotype of highly pathogenic avian influenza (HPAI) H5N8 viruses in 2016/2017 in South Korea. Infect Genet Evol. 2017;53:56‐67. [DOI] [PubMed] [Google Scholar]
- 65. Health WOfA . Update on avian influenza in animals (types H5 and H7). 2019; http://www.oie.int/animal-health-in-the-world/update-on-avian-influenza.
- 66. Poen MJ, Venkatesh D, Bestebroer TM, et al. Co‐circulation of genetically distinct highly pathogenic avian influenza A clade 2.3.4.4 (H5N6) viruses in wild waterfowl and poultry in Europe and East Asia, 2017‐18. Virus Evol 2019;5(1):vez004. 10.1093/ve/vez004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thornton AC, Parry‐Ford F, Tessier E, et al. Human exposures to H5N6 avian influenza, England, 2018. J Infect Dis. 2019;220(1):20‐22. 10.1093/infdis/jiz080 [DOI] [PubMed] [Google Scholar]
- 68. Lee EK, Lee YN, Kye SJ, et al. Characterization of a novel reassortant H5N6 highly pathogenic avian influenza virus clade 2.3.4.4 in Korea, 2017. Emerg Microb Infect. 2018;7(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kwon JH, Jeong S, Lee DH, et al. New reassortant clade 2.3.4.4b avian influenza A(H5N6) virus in wild birds, South Korea, 2017‐18. Emerg Infect Dis. 2018;24(10):1953‐1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ma MJ, Chen SH, Wang GL, et al. Novel highly pathogenic avian H5 influenza A viruses in live poultry markets, Wuxi City, China, 2013‐2014. Open Forum Infect Dis 2016;3(2):ofw054. 10.1093/ofid/ofw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yuan R, Wang Z, Kang Y, et al. Continuing reassortant of H5N6 subtype highly pathogenic avian influenza virus in Guangdong. Front Microbiol. 2016;7:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tsunekuni R, Yaguchi Y, Kashima Y, et al. Spatial transmission of H5N6 highly pathogenic avian influenza viruses among wild birds in Ibaraki prefecture, Japan, 2016‐2017. Arch Virol. 2018;163(5):1195‐1207. [DOI] [PubMed] [Google Scholar]
- 73. Okamatsu M, Ozawa M, Soda K, et al. Characterization of highly pathogenic avian influenza virus A(H5N6), Japan, November 2016. Emerg Infect Dis. 2017;23(4):691‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hiono T, Okamatsu M, Matsuno K, et al. Characterization of H5N6 highly pathogenic avian influenza viruses isolated from wild and captive birds in the winter season of 2016‐2017 in Northern Japan. Microbiol Immunol. 2017;61(9):387‐397. [DOI] [PubMed] [Google Scholar]
- 75. Wong FY, Phommachanh P, Kalpravidh W, et al. Reassortant highly pathogenic influenza A(H5N6) virus in Laos. Emerg Infect Dis. 2015;21(3):511‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee EK, Song BM, Lee YN, et al. Multiple novel H5N6 highly pathogenic avian influenza viruses, South Korea, 2016. Infect Genet Evol. 2017;51:21‐23. [DOI] [PubMed] [Google Scholar]
- 77. Thanh HD, Tran VT, Nguyen DT, Hung VK, Kim W. Novel reassortant H5N6 highly pathogenic influenza A viruses in Vietnamese quail outbreaks. Comp Immunol Microbiol Infect Dis. 2018;56:45‐57. [DOI] [PubMed] [Google Scholar]
- 78. Qi X, Cui L, Yu H, Ge Y, Tang F. Whole‐genome sequence of a Reassortant H5N6 avian influenza virus isolated from a live poultry market in China, 2013. Genome Announc. 2014;2(5):pii: e00706‐14. 10.1128/genomeA.00706-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu H, Meng F, Huang D, et al. Genomic and phylogenetic characterization of novel, recombinant H5N2 avian influenza virus strains isolated from vaccinated chickens with clinical symptoms in China. Viruses. 2015;7(3):887‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bi Y, Mei K, Shi W, et al. Two novel reassortants of avian influenza A (H5N6) virus in China. J Gen Virol. 2015;96(Pt 5):975‐981. [DOI] [PubMed] [Google Scholar]
- 81. Luo K, Zhang K, Liu L, et al. The genetic and phylogenetic analysis of a highly pathogenic influenza A H5N6 virus from a heron, southern China, 2013. Infect Genet Evol. 2018;59:72‐74. [DOI] [PubMed] [Google Scholar]
- 82. Globig A, Staubach C, Sauter‐Louis C, et al. Highly pathogenic avian influenza H5N8 clade 2.3.4.4b in Germany in 2016/2017. Front Vet Sci. 2017;4:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Saito T, Tanikawa T, Uchida Y, Takemae N, Kanehira K, Tsunekuni R. Intracontinental and intercontinental dissemination of Asian H5 highly pathogenic avian influenza virus (clade 2.3.4.4) in the winter of 2014‐2015. Rev Med Virol. 2015;25(6):388‐405. [DOI] [PubMed] [Google Scholar]
- 84. Kwon HI, Kim EH, Kim YI, et al. Comparison of the pathogenic potential of highly pathogenic avian influenza (HPAI) H5N6, and H5N8 viruses isolated in South Korea during the 2016‐2017 winter season. Emerg Microb Infect. 2018;7(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Marchenko V, Goncharova N, Susloparov I, et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia. Virology. 2018;525:216‐223. [DOI] [PubMed] [Google Scholar]
- 86. Herfst S, Mok CKP, van den Brand JMA, et al. Human clade 2.3.4.4 A/H5N6 influenza virus lacks mammalian adaptation markers and does not transmit via the airborne route between ferrets. mSphere. 2018;3(1):pii: e00405‐17. 10.1128/mSphere.00405-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fan S, Zhou L, Wu D, et al. A novel highly pathogenic H5N8 avian influenza virus isolated from a wild duck in China. Influenza Other Respi Viruses. 2014;8(6):646‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kaplan BS, Russier M, Jeevan T, et al. Novel highly pathogenic avian A(H5N2) and A(H5N8) influenza viruses of clade 2.3.4.4 from North America have limited capacity for replication and transmission in mammals. mSphere. 2016;1(2):pii: e00003‐16. 10.1128/mSphere.00003-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pulit‐Penaloza JA, Sun X, Creager HM, et al. Pathogenesis and transmission of novel highly pathogenic avian influenza H5N2 and H5N8 viruses in ferrets and mice. J Virol. 2015;89(20):10286‐10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Noh JY, Lee DH, Yuk SS, et al. Limited pathogenicity and transmissibility of Korean highly pathogenic avian influenza H5N6 clade 2.3.4.4 in ferrets. Transbound Emerg Dis. 2018;65(4):923‐926. [DOI] [PubMed] [Google Scholar]
- 91. Richard M, Herfst S, van den Brand JM, et al. Low virulence and lack of airborne transmission of the Dutch highly pathogenic avian influenza virus H5N8 in ferrets. PLoS One. 2015;10(6):e0129827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim YI, Pascua PN, Kwon HI, et al. Pathobiological features of a novel, highly pathogenic avian influenza A(H5N8) virus. Emerg Microb Infect. 2014;3(10):e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang X, Meng F, Wang D, et al. Characteristics of two highly pathogenic avian influenza H5N8 viruses with different pathogenicity in mice. Arch Virol. 2016;161(12):3365‐3374. [DOI] [PubMed] [Google Scholar]
- 94. Sun W, Li J, Hu J, et al. Genetic analysis and biological characteristics of different internal gene origin H5N6 reassortment avian influenza virus in China in 2016. Vet Microbiol. 2018;219:200‐211. [DOI] [PubMed] [Google Scholar]
- 95. Kang Y, Liu L, Feng M, et al. Highly pathogenic H5N6 influenza A viruses recovered from wild birds in Guangdong, southern China, 2014‐2015. Sci Rep. 2017;7:44410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lyoo KS, Na W, Phan LV, et al. Experimental infection of clade 1.1.2 (H5N1), clade 2.3.2.1c (H5N1) and clade 2.3.4.4 (H5N6) highly pathogenic avian influenza viruses in dogs. Transbound Emerg Dis. 2017;64(6):1669‐1675. [DOI] [PubMed] [Google Scholar]
- 97. Grund C, Hoffmann D, Ulrich R, et al. A novel European H5N8 influenza A virus has increased virulence in ducks but low zoonotic potential. Emerg Microb Infect. 2018;7(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen T, Zhang R. Symptoms seem to be mild in children infected with avian influenza A (H5N6) and other subtypes. J Infect. 2015;71(6):702‐703. [DOI] [PubMed] [Google Scholar]
- 99. Zhang R, Chen T, Ou X, et al. Clinical, epidemiological and virological characteristics of the first detected human case of avian influenza A(H5N6) virus. Infect Genet Evol. 2016;40:236‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pan M, Gao R, Lv Q, et al. Human infection with a novel, highly pathogenic avian influenza A (H5N6) virus: Virological and clinical findings. J Infect. 2016;72(1):52‐59. [DOI] [PubMed] [Google Scholar]
- 101. Gao R, Pan M, Li X, et al. Post‐mortem findings in a patient with avian influenza A (H5N6) virus infection. Clin Microbiol Infect. 2016;22(6):574 e1‐5. [DOI] [PubMed] [Google Scholar]
- 102. Zhang Z, Li R, Jiang L, et al. The complexity of human infected AIV H5N6 isolated from China. BMC Infect Dis. 2016;16(1):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bi Y, Tan S, Yang Y, et al. Clinical and immunological characteristics of human infections with H5N6 avian influenza virus. Clin Infect Dis. 2018;68(7):1100‐1109. 10.1093/cid/ciy681 [DOI] [PubMed] [Google Scholar]
- 104. Zhang Y, Chen M, Huang Y, et al. Human infections with novel reassortant H5N6 avian influenza viruses in China. Emerg Microb Infect. 2017;6(6):e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li K, Liu H, Yang Z, et al. Clinical and epidemiological characteristics of a patient infected with H5N6 avian influenza A virus. J Clin Virol. 2016;82:20‐26. [DOI] [PubMed] [Google Scholar]
- 106. Xu W, Li H, Jiang L. Human infection with a highly pathogenic avian influenza A (H5N6) virus in Yunnan province, China. Infect Dis. 2016;48(6):477‐482. [DOI] [PubMed] [Google Scholar]
- 107. Li T, Ma Y, Li K, Tang X, Wang M, Yang Z. Death of a very young child infected with influenza A (H5N6). J Infect. 2016;73(6):626‐627. [DOI] [PubMed] [Google Scholar]
- 108. Zhang YL, Yang SG, Li G, et al. Clinical and epidemiological characteristics of a case of avian influenza A H5N6 virus infection. J Infect. 2016;72(5):629‐631. [DOI] [PubMed] [Google Scholar]
- 109. Jiang H, Wu P, Uyeki TM, et al. Preliminary epidemiologic assessment of human infections with highly pathogenic avian influenza A(H5N6) virus, China. Clin Infect Dis. 2017;65(3):383‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang ZF, Mok CK, Peiris JS, Zhong NS. Human infection with a novel avian influenza A(H5N6) virus. N Engl J Med. 2015;373(5):487‐489. [DOI] [PubMed] [Google Scholar]
- 111. Ryu S, Kim CK, Kim K, Woo SH, Chun BC. Serosurveillance of avian influenza A/H5N6 virus infection in poultry farmers, Gyeonggi Province, Republic of Korea, 2016‐2017. Int J Infect Dis. 2018;75:49‐51. [DOI] [PubMed] [Google Scholar]
- 112. Ilyicheva TN, Durymanov AG, Svyatchenko SV, et al. Humoral immunity to influenza in an at‐risk population and severe influenza cases in Russia in 2016‐2017. Arch Virol. 2018;163(10):2675‐2685. 10.1007/s00705-018-3904-9 [DOI] [PubMed] [Google Scholar]
- 113. Freidl GS, van den Ham HJ, Boni MF, de Bruin E, Koopmans MP. Changes in heterosubtypic antibody responses during the first year of the 2009 A(H1N1) influenza pandemic. Sci Rep. 2016;6:20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhao M, Liu K, Luo J, et al. Heterosubtypic protections against human‐infecting avian influenza viruses correlate to biased cross‐T‐cell responses. MBio. 2018;9(4):pii: e01408‐18. 10.1128/mBio.01408-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fang S, Bai T, Yang L, et al. Sustained live poultry market surveillance contributes to early warnings for human infection with avian influenza viruses. Emerg Microb Infect. 2016;5(8):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312(5772):404‐410. [DOI] [PubMed] [Google Scholar]
- 117. Herfst S, Schrauwen EJ, Linster M, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Imai M, Watanabe T, Hatta M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486(7403):420‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Linster M, van Boheemen S, de Graaf M, et al. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell. 2014;157(2):329‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hiono T, Okamatsu M, Igarashi M, et al. Amino acid residues at positions 222 and 227 of the hemagglutinin together with the neuraminidase determine binding of H5 avian influenza viruses to sialyl Lewis X. Arch Virol. 2016;161(2):307‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hui KPY, Ching RHH, Chan SKH, et al. Tropism, replication competence, and innate immune responses of influenza virus: an analysis of human airway organoids and ex‐vivo bronchus cultures. Lancet Respir Med. 2018;6(11):846‐854. [DOI] [PubMed] [Google Scholar]
- 122. Walther T, Karamanska R, Chan RW, et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9(3):e1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hui KP, Chan LL, Kuok DI, et al. Tropism and innate host responses of influenza A/H5N6 virus: an analysis of ex vivo and in vitro cultures of the human respiratory tract. Eur Respir J. 2017;49(3):pii: 1601710. 10.1183/13993003.01710-2016 [DOI] [PubMed] [Google Scholar]
- 124. Li Q, Wang X, Gu M, et al. Novel H5 clade 2.3.4.6 viruses with both alpha‐2,3 and alpha‐2,6 receptor binding properties may pose a pandemic threat. Vet Res. 2014;45:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun H, Pu J, Wei Y, et al. Highly pathogenic avian influenza H5N6 viruses exhibit enhanced affinity for human type sialic acid receptor and in‐contact transmission in model ferrets. J Virol. 2016;90(14):6235‐6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhang Y, Zhang Q, Kong H, et al. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in Guinea pigs by respiratory droplet. Science. 2013;340(6139):1459‐1463. [DOI] [PubMed] [Google Scholar]
- 127. Hanson A, Imai M, Hatta M, et al. Identification of stabilizing mutations in an H5 hemagglutinin influenza virus protein. J Virol. 2015;90(6):2981‐2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen LM, Blixt O, Stevens J, et al. In vitro evolution of H5N1 avian influenza virus toward human‐type receptor specificity. Virology. 2012;422(1):105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Swayne DE, Spackman E. Current status and future needs in diagnostics and vaccines for high pathogenicity avian influenza. Dev Biol. 2013;135:79‐94. [DOI] [PubMed] [Google Scholar]
- 130. Swayne DE, Pavade G, Hamilton K, Vallat B, Miyagishima K. Assessment of national strategies for control of high‐pathogenicity avian influenza and low‐pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Rev Sci Tech. 2011;30(3):839‐870. [DOI] [PubMed] [Google Scholar]
- 131. Bouma A, Muljono AT, Jatikusumah A, et al. Field trial for assessment of avian influenza vaccination effectiveness in Indonesia. Rev Sci Tech. 2008;27(3):633‐642. [DOI] [PubMed] [Google Scholar]
- 132. Wu J, Ke C, Lau EHY, et al. Influenza H5/H7 virus vaccination in poultry and reduction of zoonotic infections, Guangdong Province, China, 2017‐18. Emerg Infect Dis. 2019;25(1):116‐118. 10.3201/eid2501.181259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Beerens N, Heutink R, Pritz‐Verschuren S, et al. Transbound Emerg Dis. 2019;66(3):1370‐1378. 10.1111/tbed.13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Morin CW, Stoner‐Duncan B, Winker K, et al. Avian influenza virus ecology and evolution through a climatic lens. Environ Int. 2018;119:241‐249. [DOI] [PubMed] [Google Scholar]
- 135. Smith GJ, Donis RO, World Health Organization/World Organisation for Animal HF, Agriculture Organization HEWG . Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013‐2014. Influenza Other Respi Viruses. 2015;9(5):271‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yang H, Carney PJ, Mishin VP, et al. Molecular characterizations of surface proteins hemagglutinin and neuraminidase from recent H5Nx avian influenza viruses. J Virol. 2016;90(12):5770‐5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liu K, Gu M, Hu S, et al. Genetic and biological characterization of three poultry‐origin H5N6 avian influenza viruses with all internal genes from genotype S H9N2 viruses. Arch Virol. 2018;163(4):947‐960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 A(H5N6) viruses with the A/Fujian‐Sanyuan/21099/2017‐like HA gene for which sequences are available in the EpiFlu database of GISAID
Supplementary Table 2 Amino acid substitutions related to receptor binding specificity among clade 2.3.4.4 A(H5) avian viruses available from GISAID as of February 2019


